Abstract
Advances in our understanding of brain mechanisms for the hypoxic ventilatory response, coordinated changes in blood pressure, and the long-term consequences of chronic intermittent hypoxia as in sleep apnea, such as hypertension and heart failure, are giving impetus to the search for therapies to “erase” dysfunctional memories distributed in the carotid bodies and central nervous system. We review current network models, open questions, sex differences, and implications for translational research.
Introduction
Carotid body chemoreceptors are sensors of arterial O2, CO2, and pH (31, 123, 143, 236). These peripheral receptors operate cooperatively with central chemoreceptors in the brain that monitor CO2-pH or O2 (22, 43, 78, 87, 180, 195, 211, 259). Carotid chemoreceptors contribute to the drive to breathe. Their activity is enhanced during hypoxemia, a reduction in the Po2 in the blood that commonly occurs at high altitude, during apneas associated with sleep disordered breathing or dysautonomia (80, 107, 214), with ventilation-perfusion mismatching in the lungs, or as a consequence of other disorders (201, 255). Numerous medical conditions are associated with altered carotid body function (see discussion in Refs. 143, 154).
Hypoxia stimulates oxygen-sensing mechanisms in the carotid bodies, leading to reduced potassium currents in type I glomus cells (29, 30, 123, 141–143, 208, 212). The resulting depolarization and transmitter release evoke action potentials in the glossopharyngeal nerve, exciting neurons in the nucleus of the solitary tract (NTS). The brain stem circuits targeted by these NTS neurons generate a ventilatory response, expressed as increases in tidal volume and breathing frequency, along with coordinated changes in sympathetic and parasympathetic outflows that increase cardiac output and vascular tone to maintain or reestablish tissue Po2 sufficient to meet metabolic demands (114, 152, 209, 236, 249, 260). Reciprocal interactions between the respiratory and cardiovascular control systems through brain networks and via sensory systems mediate this “cardiorespiratory coupling” (14, 56, 91, 165, 265).
Carotid chemoreceptor activity evoked by hypoxia is sufficient to cause the reemergence of breathing during hypocapnic apneas (69, 185) and to maintain ventilation under conditions associated with the congenital loss of central chemoreceptors (213). Repeated episodes of transient hypoxia, as during sleep apneas, can induce a distributed “memory” in circuits of the brain stem and spinal cord, termed “long-term facilitation” or LTF, expressed as increases in respiratory drive and blood pressure that persist well beyond the evoking perturbations (17, 51, 77, 113, 160, 161, 171, 172). Parallel mechanisms evoked by disruption of vagal feedback during repeated obstructive apneas, but independent of hypoxia, can also trigger potentiated hypoglossal motor neuron activity (243, 248).
Chronic intermittent hypoxia, depending on its severity, duration, and patterning, can trigger a time-dependent and escalating cascade of physiological responses (206, 235) that, although initially adaptive, can cause or exacerbate hypertension, heart failure, and other disorders (143, 207, 209, 256). There is a growing body of preclinical and clinical research targeting the carotid bodies with the goal of developing therapies for these disorders (45, 106, 153, 179, 237). Recent papers have described hypoxia-sensing transduction mechanisms in the carotid bodies (143, 208, 212, 236), the ventilatory response to hypoxia (192, 249), adaptations to chronic sustained or intermittent hypoxia (17, 72, 82, 84, 101, 143, 193, 196, 205, 220, 222), and cardiorespiratory coupling (56, 91), including pulmonary receptor-evoked cardiovascular effects triggered by air pollution (12, 103).
This review focuses on the brain stem network through which the carotid bodies enhance the drive to breathe and blood pressure, and induce LTF. Some open questions on mechanisms, sex differences, and translational research are also considered. The models and hypotheses reviewed are based on results from many laboratories and diverse experimental and computational approaches, as described in the cited literature and in other recent complementary reviews (17, 56, 80, 81, 91, 131, 162, 164, 216, 265).
Brain Stem Circuits for the Integrated Cardiorespiratory Response to Transient Hypoxia
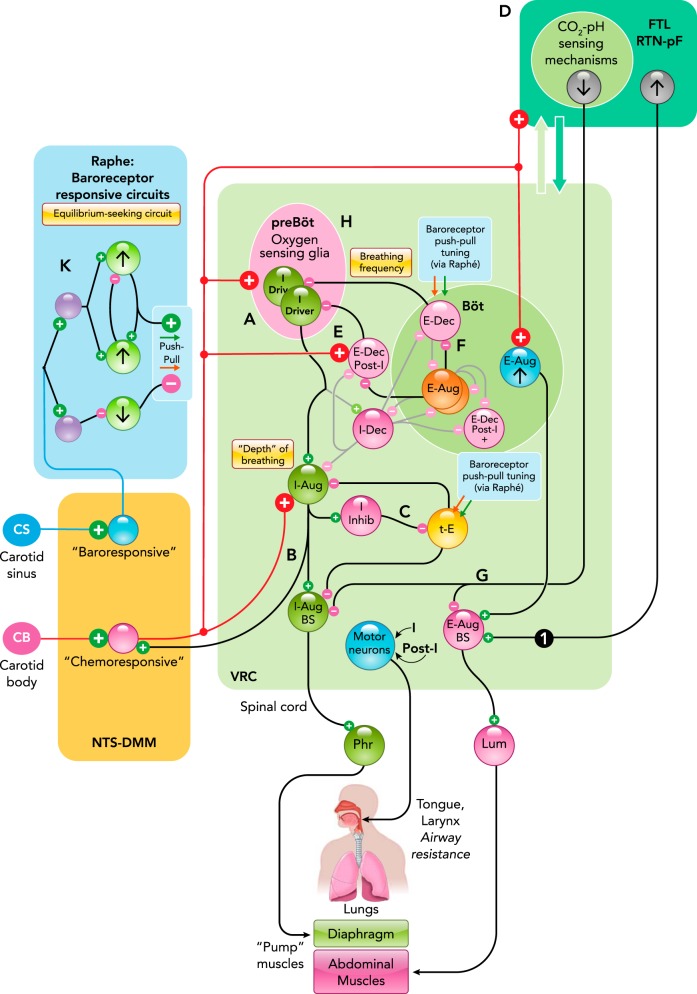
Neurons in the NTS and adjacent medial medulla have heterogeneous phenotypes and diverse responses to carotid body stimulation (2, 4, 36, 116, 120, 157, 194, 265). These “chemoresponsive” neurons operate through multiple circuit pathways to regulate the depth and frequency of breathing and, concurrently, cardiac output and vascular tone (FIGURE 1) (91, 177).
FIGURE 1.
Continued
Inspiratory Drive and the Hypoxic Ventilatory Response
A rostral cluster of pre-inspiratory “I-Driver” neurons in the pre-Bötzinger complex (128, 131, 162, 229, 231, 240) of the ventral respiratory column (VRC) excites a downstream “chain” of premotor and motor neurons for the diaphragm, other inspiratory pump muscles, and muscles that influence airway resistance, such as those of the tongue and oropharynx (FIGURE 1A) (19, 67, 73, 83, 246). Carotid chemoreceptor stimulation can shorten the inspiratory burst duration of some I-Driver neurons, which otherwise exhibit little or no change in their firing rate, whereas their targets, including bulbospinal premotor neurons, exhibit larger rate increases, enhancing inspiratory drive. These observations led to a model with parallel circuit mechanisms for tuning tidal volume and breathing frequency (173, 177).
Chemoresponsive NTS neurons operate on a subset of rostral I-Driver neurons and at multiple sites along the inspiratory neuron chain to increase neuronal firing rates (11, 171, 173). Evidence for excitatory synaptic interactions between some responsive NTS and VRC neurons supports a model for inspiratory drive amplification that includes positive feedback circuits constrained by synaptic depression in the recurrent arm of the loop (FIGURE 1B; see Fig. 11B in Ref. 177). This model is consistent with the observation that some chemoresponsive NTS neurons have an evoked or enhanced inspiratory-modulated firing pattern during carotid body stimulation (194).
A second stage of amplification mediated by a disinhibitory microcircuit downstream from the pre-Bötzinger complex has also been proposed (233). An efference copy of inspiratory drive excites inhibitory inspiratory neurons, which in turn inhibit pericolumnar tonic expiratory (t-E) neurons. This sequence results in disinhibition of their excitatory inspiratory neuron targets (FIGURE 1C). These t-E neurons operate as network “hubs” for integration of convergent baroreceptor, peripheral and central chemoreceptor, and motor efference copy inputs that cooperatively tune inspiratory drive and, as considered subsequently, autonomic influences on cardiovascular function (135, 177, 190, 233). During exercise, carotid chemoreceptors contribute to tonic vasoconstriction and locomotor blood flow; their role in the enhancement of sympathetic activity and their interactions with other afferent and feed-forward central command mechanisms remain incompletely understood (46). The convergence of chemoreceptor and efference copy motor influences at VRC t-E hub neurons during rhythmic behaviors like breathing and coughing suggests that these hubs may have a role in the generation of hyperpnea and other physiological responses to exercise (186, 234).
Via NTS neurons, carotid chemoreceptors also cooperate with central CO2-pH chemoreceptors in the retrotrapezoid nucleus-parafacial region (FIGURE 1D) (22, 89, 91, 94, 253, 259). Recent evidence suggests that chemoresponsive neurons in this area influence inspiratory drive via multi-path modulation of t-E neuron nodes, supporting a complex, partly hierarchical architecture for cooperative interactions between peripheral and central chemoreceptor drives and a quasi-periodic tuning of VRC circuits by coordinated clusters of adjacent tegmental field neurons (177, 189, 190, 233).
FIGURE 1.
Medullary circuit mechanisms in contemporary models of carotid chemoreceptor and baroreceptor modulation of breathing and cardiovascular functions
A–K: schematic representations of specific functional interactions described in the text. Neuron populations are grouped by brain stem location and labeled by their respiratory modulated firing rates: inspiratory (I), post-inspiratory (post-I), or expiratory (E), according to the phase of greatest average activity, and as decrementing (Dec) or augmenting (Aug) if the average peak firing rate occurs during the first or second half of the phase, respectively. I-Driver neuron activity begins slightly before phrenic nerve discharge, with firing rates that peak in the early I phase and then slowly decrease before abruptly decreasing at the end of inspiration. Tonic E cells (t-E) are active throughout the respiratory cycle, with their greatest rate during expiration. Black lines and “synapses” indicate inferred functional connectivity discussed in the text. 1, Cross-correlogram with offset peak consistent with RTN-pF region neuron exciting a putative premotor caudal VRC augmenting expiratory neuron. Figure adapted from Fig. 5D in Ref. 190 with permission from Journal of Neurophysiology. Both neurons responded to transient hypoxia with increased firing rates (177). Binwidth = 15.5 ms, 71,132 trigger neuron spikes, 25,541 target neuron spikes. Böt, Bötzinger complex; BS, bulbospinal; FTL/RTN-pF, lateral tegmental field/retrotrapezoid n.-parafacial n; IML, intermediolateral column of the spinal cord; IVLM, intermediate ventrolateral medulla; Lum, lumbar nerve; NA, n. ambiguus; NTS-DMM, n. tractus solitarius-dorsal medial medulla; Phr, phrenic nerve; pre-Böt, pre-Bötzinger complex; RVLM, rostral ventrolateral medulla; VRC, ventral respiratory column.
Tuning Breathing Rate
Chemoresponsive NTS neurons evoke changes in breathing frequency through mechanisms that reduce or limit the durations of both inspiratory and expiratory phases (Ti and Te, respectively). Truncation of I-Driver activity and the inspiratory phase is mediated in part by enhanced post-inspiratory (decrementing expiratory) neuron inhibition caused by carotid body stimulation (FIGURE 1E) (125, 128, 138, 139, 173, 177, 216, 226). Teasing apart the physiological contexts and respective contributions of inhibition and intrinsic mechanisms for the I-Driver “off switch” remains an active and controversial area of research, in part because of the different preparations and model systems studied and the complex consequences of pharmacological manipulations used (7, 10, 37, 150, 216, 225).
A shortened expiratory phase, although not obligatory, when present, may reflect enhanced rostral Bötzinger complex augmenting expiratory neuron inhibition of the relevant inhibitory decrementing expiratory (E-Dec) cells (FIGURE 1F) (129, 139, 140), overcoming chemoreceptor-driven enhancement of E-Dec neuron activity (11, 96, 170, 173, 187, 238). Regulation of the expiratory phase of the respiratory cycle remains an area of active research, and additional network mechanisms proposed to tune breathing frequency, including circuit loops through the pons, are considered subsequently.
Enhancement of Expiratory Drive
Mechanisms for the enhanced expiratory effort associated with hypoxia are less well understood. Active expiration, including excitation of abdominal and intercostal expiratory muscles, is present during resting breathing under some conditions (1, 35, 42, 105, 185, 219) and dramatically enhanced during hypoxia (140). It remains to be determined whether this increased expiratory drive reflects, in part, NTS chemoresponsive neurons acting directly on caudal bulbospinal expiratory premotor neurons. However, there is evidence for the recruitment of both Bötzinger and adjacent RTN-parafacial neurons during chemoreceptor stimulation (75, 108, 109, 191). The parafacial-lateral tegmental field region, a site with complex neuronal interactions (177, 189, 190) and where peripheral and central chemoreceptor influences converge, as noted above, has been strongly implicated as a source of expiratory drive (20, 94, 131, 164). Hypercapnia leads to disinhibition of non-chemosensitive late-E neurons proposed to excite downstream expiratory neurons (41). This observation and other recent results collectively suggest parallel push-pull excitatory and disinhibitory control circuits for expiratory drive tuning, including tightly coordinated control of premotor inspiratory and expiratory drives by shared RTN-pF region neurons (FIGURE 1G) (104, 162, 177, 189, 190, 233).
Central Oxygen Sensing and Gasping
Selective stimulation of carotid chemoreceptors may evoke somewhat different motor patterns and autonomic responses than transient systemic hypoxia, which stimulates oxygen sensors within the carotid bodies and in the brain, all of which can potentially contribute to the hypoxic ventilatory response via parallel actions on pre-Bötzinger complex I-Driver neurons (FIGURE 1H) and other elements of the brain stem respiratory network (87, 236).
Under conditions of severe hypoxia, breathing is first enhanced and then depressed until apnea or the cessation of breathing (217, 218), followed by the emergence of a simplified reconfigured respiratory network that generates an autoresuscitative gasping motor pattern. The network states and biophysical processes required for this behavior remain incompletely understood, as does the extent to which efferent copies of this drive engage autonomic circuits (57, 64, 82, 122, 197, 226, 245, 251). Gasping is common in patients in cardiac arrest with ventricular fibrillation and is associated with successful resuscitation (61).
Efference Copy and Hypoxia-Evoked Changes in Autonomic Activity
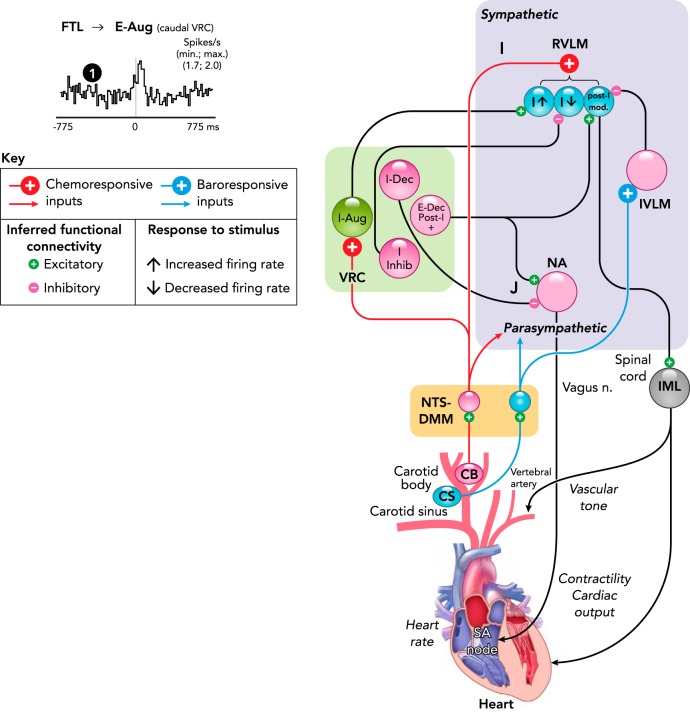
The brain stem respiratory network has a profound influence on the patterns (121) and magnitude (8) of autonomic nervous system signaling that modulates cardiac output and blood pressure in response to hypoxia (FIGURE 1I). Carotid chemoreceptors evoke coordinated changes through other routes, including direct NTS neuron projections to pre-sympathetic C1 and non-C1 neurons in the rostral-ventrolateral medulla (RVLM) (116, 120). These RVLM neurons excite spinal pre-ganglionic sympathetic neurons that drive vasoconstriction and stimulate the heart in response to hypoxia (93, 156, 188, 265). The RVLM C1 and non-C1 neurons are phenotypically heterogeneous, with attributes including three distinct classes of respiratory modulated discharge patterns: inspiratory-excited [presumably excited by pre-Bötzinger complex I-Driver neurons or downstream inspiratory chain follower neurons (166)], inspiratory-inhibited, and those with a post-inspiratory modulation (166, 168). C1 neurons are involved in inspiratory modulation of sympathetic activity enhanced by carotid chemoreceptors (167). The post-inspiratory subset includes neurons that also exhibit late expiratory neuron excitation following chronic intermittent hypoxia, an example of plasticity considered further in a subsequent section of this review (168). RVLM neurons are modulated by baroreceptors indirectly through inhibitory neurons (FIGURE 1I) in the intermediate ventrolateral medulla (IVLM, “alias” caudal ventrolateral medulla) (93), which also receive post-inspiratory excitation during hypoxia (168).
Parasympathetic cardiac vagal pre-ganglionic neurons in the nucleus ambiguus (NA) receive convergent carotid chemoreceptor and baroreceptor influences via the NTS; they are inhibited during inspiration, presumably by neurons in the VRC (FIGURE 1J). The resulting respiratory sinus arrhythmia (RSA) caused by reduced vagal inhibition of the heart can contribute to increased cardiac output (85, 88). Moderate hypoxia diminishes the magnitude of the RSA (28, 60, 254, 262, 263). The extent to which changes in the breathing pattern and autonomic modulation of the heart and circulation influence venous return and cardiac output depends on various factors (147). Left ventricular stroke volume decreases during inspiration, although right ventricular filling pressure increases (221). Moreover, venous flow from the legs during inspiration varies, depending on the relative contributions of the diaphragm and intercostal and abdominal muscle activity (258). Respiratory sinus arrhythmia does not appear to improve gas exchange efficiency, but rather minimizes the work done by the heart while maintaining physiological levels of arterial carbon dioxide or Paco2 (23, 24). An enhancement of firing rate during the post-inspiratory interval also has been observed in vivo, supporting the hypothesis that slow breathing, which increases the post-inspiratory phase duration, slows the heart rate by increasing the firing rate of cardiac vagal pre-ganglionic neurons (65, 88).
Cardiorespiratory coupling is also apparent in the arterial pulse modulation of respiratory premotor and motor neurons (52, 74). Baroreceptors provide a major coordinating influence on cardiorespiratory coupling during hypoxia through their actions on breathing and via coordinated feedback regulation of cardiovascular function. Functional connectivity of medullary raphe circuits supports baroreceptor modulation of inspiratory drive and expiratory phase duration via push-pull tuning of VRC t-E and E-Dec neurons, respectively (9, 135). Raphe circuits are dynamically reconfigured over the course of the respiratory cycle and baroreceptor and carotid chemoreceptor stimulation (6, 32, 127, 172). These circuits (FIGURE 1K) have been proposed to incorporate internal equilibrium-seeking mechanisms that help to stabilize breathing and regulate reflex gain during perturbations of blood pressure, for example, during cough (130, 134, 135, 202), and to contribute to the respiratory modulation of the sympathetic baroreceptor reflex (9).
The Role of the Pons in the Hypoxic Ventilatory Response and Cardiorespiratory Coupling
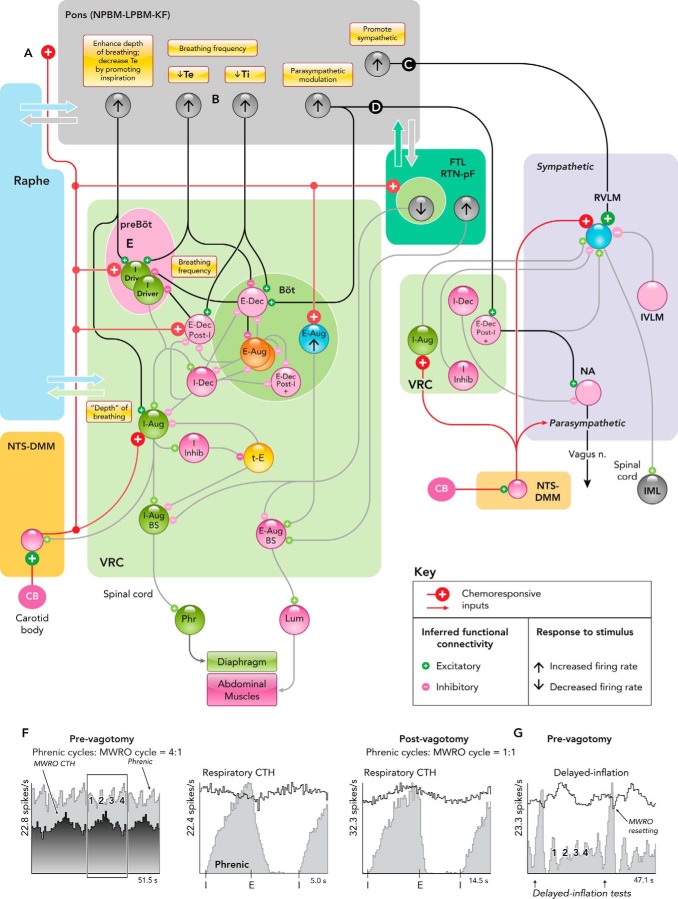
The Kölliker-Fuse/parabrachial complex of the dorsolateral pons receives inputs from NTS neurons that relay signals from carotid body afferents (FIGURE 2A) (98, 244). Neurons of the Kölliker-Fuse (K-F) region contribute to cardiorespiratory coupling and are activated during hypoxia and carotid sinus nerve stimulation (63). They project to several brain stem sites that participate in the control of tidal volume and breathing frequency and directly to brain stem sympathetic circuits that regulate the cardiovascular network (13, 15, 16, 53, 59). Pharmacological perturbations of K-F neurons disrupt the hypoxic ventilatory response (38), possibly, in part, through reciprocal connections with the RTN and parafacial region (59, 223, 239).
FIGURE 2.
Simplified functional connectivity of hypoxia-responsive pontine neurons
A–E: interactions inferred from various experimental approaches reviewed in the text that contribute to the hypoxic ventilatory response and changes in cardiorespiratory coupling during hypoxia in current models. F and G: MWRO-triggered firing rate histograms of a pontine neuron before and after bilateral vagotomy and phase resetting of MWROs by withholding lung inflation in vagus-intact cat. Figure derived from Fig. 5A in Ref. 176 with permission. See text for details. KF, Kölliker-Fuse n.; LPBM, lateral parabrachial n.; MWRO, Mayer wave-related oscillations; NPBM, medial parabrachial n.; Ti (Te), duration of inspiratory (expiratory) phase.
The dorsolateral pons influences breathing frequency during hypoxia through inspiratory-expiratory phase switching mechanisms. These circuits remain incompletely understood; models (5, 17, 178, 203) include pontine neurons that operate to shorten inspiration and expiration (e.g., FIGURE 2B). Inhibition of the K-F region with muscimol, a GABA-A receptor agonist, reduces the enhancement of phrenic and sympathetic drive (FIGURE 2C) and breathing frequency responses to hypoxia (39). The Kölliker-Fuse nucleus also provides a pathway for post-inspiratory modulation of cardiac vagal parasympathetic activity (FIGURE 2D) (65). K-F connectivity to post-inspiratory or E-Dec neurons could also contribute to modulation of augmenting or late expiratory neuron activity and the enhancement of active expiration with increased chemical drive (18).
Neurons of the lateral parabrachial nucleus (LPBN) may also enhance breathing frequency during hypoxia by reducing expiratory phase duration (242). Bilateral lesions of K-F nuclei provoke apnea, suggesting that neurons there promote inspiration (204). This conclusion is supported by the finding that both pharmacological and patterned electrical microstimulation within the pontine medial parabrachial nucleus promote inspiratory burst onset (FIGURE 2E) and can evoke a premature onset of the next inspiratory phase, thereby shortening Te and increasing breathing frequency (266).
The A5 cell group of noradrenergic and glutamatergic neurons in the ventrolateral pons modulates breathing frequency and cardiovascular function during and following hypoxia (118, 119). Neurons in the A5 group project to the VRC, have post-inspiratory and expiratory modulated firing patterns, and are responsive to hypoxia (50, 59, 92). Micro-injections of muscimol into the ventrolateral pons prolongs inspiration (110), whereas application of glutamate prolongs expiration (111). This expiratory-facilitating function is apparent during and following episodes of transient severe hypoxia: respiratory frequency first increases, then slows in a “post-hypoxic frequency decline,” followed by a gradual return to control levels. A similar sequence is observed during and following carotid sinus nerve stimulation under hyperoxic conditions, suggesting that the frequency decline is of central origin (97).
Raphe-Pontine Circuits, Slow Breathing, and Mayer Wave-Related Oscillations
Slow (~0.1 Hz) oscillations in respiratory drive and blood pressure, termed Mayer waves, can be triggered by hypoxia or hemorrhage, conditions that increase carotid body chemoreceptor activity and sympathetic drive (3, 33, 68, 124, 262). Oscillations evoked by carotid artery occlusion with hemorrhage can be eliminated by section of the carotid sinus nerves or destruction of carotid body chemoreceptors, suggesting that hypoxia or under-perfusion of the carotid body can evoke this category of cardiorespiratory coupling (3).
Bilateral vagotomy commonly results in a prolongation of the inspiratory phase. The loss of the Breuer-Hering reflex (96) together with the aforementioned inspiratory off-switch function of the pons (55) contributes to this slowing of breathing frequency. A second mechanism involving cardiorespiratory coupling has been proposed to play a role in the generation of the slower breathing rhythm. The VRC is embedded in a highly interconnected pontomedullary network with medullary raphe circuits providing parallel signaling routes between the pons and the VRC (184, 230, 232). Pontine and raphe neurons with Mayer wave-related oscillations (MWROs) can become synchronized 1:1 with the breathing rhythm after vagotomy (FIGURE 2F) (176). Computational models show that neurons with inspiratory efference copy input and feed-forward inhibition produce similar rate profiles (55). Prior to vagotomy, withholding lung inflation during inspiration both prolongs the inspiratory phase and resets the MWROs (FIGURE 2G) (176). Increased cardiorespiratory coupling can persist following slow deep breathing in human subjects (54). Functional connectivity inferred from multi-array recordings suggests that pontine-raphe circuits contribute to this aspect of cardiorespiratory coupling (176).
Distributed Memories Induced by Intermittent Hypoxia
In 1980, David Millhorn and colleagues reported that episodic stimulation of carotid body chemoreceptors can induce a respiratory memory expressed as an increase in phrenic nerve inspiratory burst amplitude and breathing frequency. This “long-term facilitation” (LTF) persists well beyond the period of stimulation (160, 161) and can also be evoked by direct electrical stimulation of n. raphe obscurus but not by central CO2 chemoreceptors; it is prevented or attenuated by serotonin (5-HT) antagonists (76, 159, 160). Hypoxia and electrical stimulation of the carotid sinus nerve also induces Fos-like immunoreactivity in serotoninergic and catecholaminergic neurons at multiple sites within the brain stem (63). Raphe stimulation releases 5-HT in the cervical ventral horn of the spinal cord, the site of phrenic motor neurons (27, 215). Subsequent research has identified stimulus parameters and conditions necessary for enhancement of specific motor and autonomic outflows by intermittent hypoxia (76, 117, 169, 257), along with insights into the network, and cellular and molecular mechanisms for a memory system distributed among multiple sites in the brain stem (26, 77, 126, 137, 174, 175), spinal cord (34, 70, 247), and carotid bodies (236). These multi-scale mechanisms for adaptive physiological responses alter cardiorespiratory coordination and can exacerbate or result in various pathophysiological conditions (143, 207, 209, 256, 261).
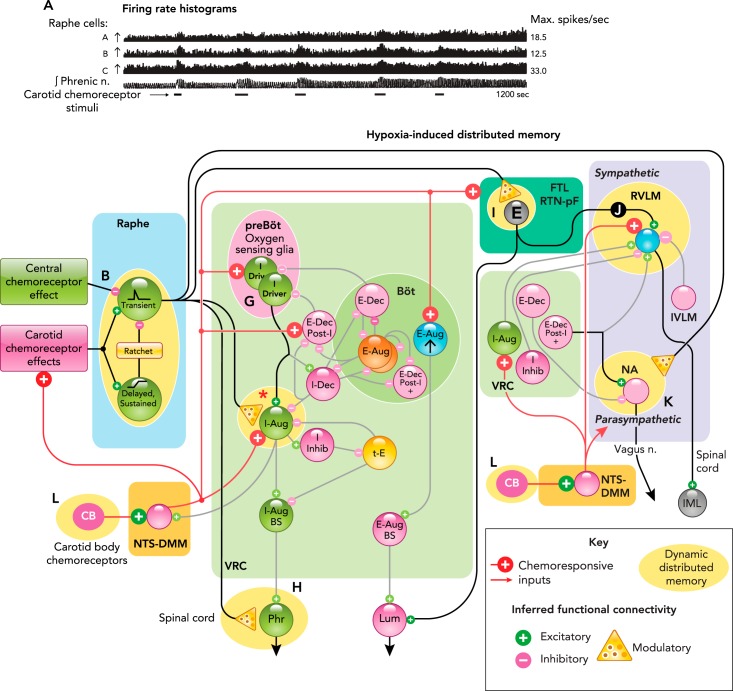
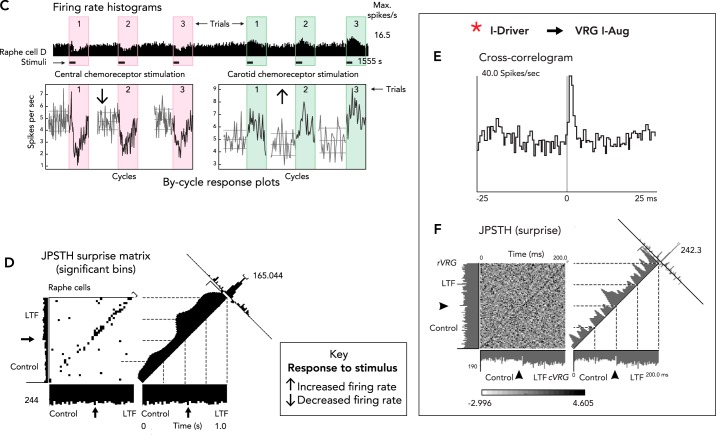
Cardiorespiratory-related neurons of the medullary raphe nuclei have dynamic functional associations and interact with the VRC, pons, RTN, and other brain stem sites for the coordinated regulation of breathing and the cardiovascular system (6, 32, 91, 132–134, 136, 172, 173, 177, 184, 210). Some raphe neurons respond to repeated stimulation of the carotid chemoreceptors with step-like increases in firing rate (FIGURE 3A). A “ratchet-like” circuit mechanism has been proposed to contribute to this incremental induction of respiratory LTF: periods of evoked high firing rate in some neurons are limited by the delayed inhibitory actions of other neurons (FIGURE 3B), until the firing rates of the early responders increase and “saturate” (149, 171, 172). Some raphe neurons excited by peripheral carotid chemoreceptor stimulation are functionally inhibited by central chemoreceptor activation (FIGURE 3C) (183), a result consistent with the inability of central chemoreceptor stimulation to evoke LTF (159). Indeed, repeated episodes of hypercapnia have been shown to induce a long-term depression of phrenic nerve activity (115) through processes involving activation of 5-HT1A and α2-adrenergic receptors (227).
FIGURE 3.
Distributed network sites for induction and expression of carotid body- and intermittent hypoxia-induced long-term facilitation
A: responses of raphe neurons and integrated phrenic nerve activity during repeated selective stimulation of carotid chemoreceptors and induction of long-term facilitation (LTF). Figure is derived from Ref. 174 with permission from Respiration Physiology. B: raphe circuit mechanism proposed to contribute to ratchet-like incremental induction of LTF (171, 172). C: firing rate histogram and by-cycle rate plots show opposite responses of a raphe neuron to selective stimulation of central and peripheral carotid chemoreceptors. Figure derived from Fig. 2A, C in Ref. 183 with permission from The Royal Society. D: increased number of black bins in the top right quadrant along the diagonal of the joint peri-stimulus time histogram (JPSTH) documents a significant increase in spike synchrony and effective connectivity for two raphe cells following induction of LTF. Figure adapted from Fig. 4A in Ref. 171 with permission from Journal of Physiology. E: offset cross-correlogram peak shows effective connectivity between a pre-Bötzinger I-Driver neuron and a VRG inspiratory target cell; binwidth = 0.5 ms, 8,216 trigger neuron spikes, 4,569 target neuron spikes. F: non-uniform distribution of statistically significant black bins along the diagonal of the JPSTH for the same neurons shown in E documents enhanced effective connectivity between this pair of neurons following induction of LTF. Figure derived from Fig. 3, B and C, in Ref. 171 with permission from Journal of Physiology. G–L: additional sites for distributed LTF expression following repeated episodes of intermittent hypoxia. See text for details.
Intermittent or sustained hypercapnia can have a profound effect on the responses to intermittent hypoxia (155). Although intermittent hypercapnia may depress ventilatory LTF, sustained hypercapnia facilitates it (90, 95). Sustained hypocapnia prevents the occurrence of LTF, hence LTF can be difficult to observe during sleep-disordered breathing. Such breathing is made up of cycles of hyperventilation followed by apneic hypoxic hypercapnia followed by arousal, hyperventilation, etc. (155).
In rats, both intermittent and sustained hypoxia increase sympathetic nerve activity after 2 wk, but they affect sympatho-respiratory coupling differentially (56). Intermittent hypoxia enhances sympatho-respiratory coupling and decreases ventilatory variability. Constant hypobaric hypoxia replaces normal 1-to-1 coupling between bursts of sympathetic and phrenic nerve activity with 2-to-3 coupling with increased ventilatory variability. Even a single session of hypoxic exposure is capable of increasing tonic sympathetic nerve output (sympathetic long-term facilitation) and altering chemo- and baroreflexes (260).
Several lines of evidence collectively support an important role for distributed brain stem circuits in the induction and expression of LTF, including carotid chemoreceptor-evoked responses, changes in functional connectivity among raphe neurons (FIGURE 3D), and evidence of increased effective connectivity among I-Driver neurons and their premotor targets (FIGURE 3, E–G) (17, 62, 79, 127, 171, 172, 257). How chronic intermittent hypoxia impacts different states of inspiratory activity in the pre-Bötzinger complex remains an active area of research (81). Cell signaling pathways for LTF of inspiratory drive have been most thoroughly investigated in the spinal cord (FIGURE 3H), where serotonin-dependent induction of facilitation in phrenic motor neurons depends on competing cell signaling mechanisms that interact conditionally with adenosine-dependent pathways for motor facilitation (47–49, 71, 77, 200, 252).
FIGURE 3.
Continued
Serotonergic mechanisms are also implicated in the induction of expiratory LTF (126) at sites in the RTN-pF respiratory group (FIGURE 3I) (91). This enhanced expiratory drive, together with sensitization to central CO2 chemoreception with chronic intermittent hypoxia, contribute to increased sympathetic nerve activity (51, 163, 264). A subset of non-C1 RVLM presympathetic neurons (FIGURE 3J) has been implicated as a source of this enhancement (168). Recruitment of 5-HT systems by hypoxia and hypercapnia also modulates the excitability of parasympathetic cardiac vagal neurons (FIGURE 3K) (113). An altered balance of hypoxia-inducible factor-α isoforms and redox state were identified in the NTS, RVLM, and adrenal medulla of rats exposed to chronic intermittent hypoxia. These changes were prevented by carotid body ablation, suggesting that carotid-evoked changes in neural activity lead to these alterations and the associated development of hypertension and elevated sympathetic activity (199).
Chronic intermittent hypoxia enhances the sensitivity and gain of carotid body chemoreceptors in an age- and history-dependent manner (FIGURE 3L). Hypoxic sensitization requires ~10 times the number of episodes of intermittent hypoxia in adult compared with neonatal rats. Moreover, subsequent acute intermittent hypoxia evokes LTF in the carotid body of previously exposed adults but not of neonates (196, 198). Hypoxia-induced changes in the redox state of the carotid bodies via an altered balance of HIF-1α-dependent pro-oxidant and HIF-2α-dependent anti-oxidant activities may play a role in the induction of these changes (235, 236); a recent study reports that HIF-2α is essential for carotid body function and development (146). Acute hypoxia with concurrent hypercapnia also evokes sensory LTF in carotid bodies (224).
The hypoxemic carotid body expands far beyond its normal size (142). Adult neural stem cells contribute to carotid body growth during hypoxia acclimatization, and these new cells contain voltage-dependent ion channels (193). This adaptation to chronic hypoxia requires HIF-2α for oxygen-sensitive glomus cells to develop and survive within the carotid body (146) and for normal responses to hypoxia (101). Contrary to maladaptive responses outlined previously, other studies have suggested therapeutic benefits of hypoxic or hypercapnic regimes in spinal cord injury (34, 181), obstructive sleep apnea (153), and other cardiovascular, respiratory, cognitive, and metabolic consequences of intermittent hypoxia (154).
Future Directions for Basic and Translational Science
Sex Differences in Responses to Hypoxia
Further research is needed to better understand sex differences in the hypoxic ventilatory response and their impact during development. Prenatal nicotine exposure alters the postnatal heart rate response to hypoxia and respiratory sinus arrhythmias in young male but not female rats (25). The hypoxic ventilatory response is greater in men than in women (86), and is differentially depressed more in women by morphine (228). Age- and sex-dependent differences in serotonin-dependent hypoxia-evoked neural plasticity have also been identified: LTF increases with age in female rats but declines in male rats (21).
The “Erasure” of Dysfunctional Cardiorespiratory Network Memories
An emerging goal of contemporary translational research is to identify safe and effective therapeutic strategies for management and treatment of the long-term consequences of adaptations to chronic intermittent hypoxia (106, 143, 153, 236). Pharmacological and immunological approaches and gene therapy are being actively considered (145, 148, 182, 236, 243). Ablation or denervation of the carotid bodies for resistant hypertension is also being evaluated in preclinical research (106). A prospective human feasibility and safety trial on unilateral ablation has been reported (179), and similar approaches for heart failure are being explored (44, 151).
The ablation approach is controversial. Carotid body ablation was used in humans as an experimental treatment for bronchial asthma and chronic obstructive pulmonary disease but did not produce significant benefits for patients (112). Patients with previous bilateral carotid body ablations to treat asthma were subsequently evaluated for responses to hypoxia and hypercapnia; whereas ventilation in room air was normal, hyperpnea in response to hypoxia was absent, and the response to hypercapnia was reduced (144). These observations suggest that central hypoxia-sensing mechanisms cannot fully compensate for the loss of carotid O2 chemoreception (78, 87), and it remains an open question as to whether central mechanisms can “appropriate” network mechanisms used by carotid chemoreceptors to reestablish adaptive (or maladaptive) set points and system gains (100, 158, 241). Several recent reviews and perspectives (112, 143) and preclinical studies (58, 66, 102, 237) have noted potential risks and adverse consequences of carotid body removal, including baroreceptor failure, exacerbation of co-morbidities, altered blood glucose regulation, reduced ability for acclimatization to high altitude, and irregular breathing rhythms.
Significant gaps remain in our understanding of brain networks and cell signaling mechanisms for cardiorespiratory regulation and hypoxia sensing. The development, organization, plasticity, and state-dependent functionality of these systems and the integration of peripheral and central chemoreceptor influences are areas of active investigation (259). These lines of inquiry also have a broader relevance beyond cardiorespiratory integration with the growing appreciation that other brain regions, rhythms, and functions are influenced by breathing (40, 99, 233, 250).
Acknowledgments
Work from the authors' laboratory reviewed herein was supported by National Institute of Neurological Disorders and Stroke Grants R01/37 NS-019814 and R01 NS-046062 as part of the NSF/NIH Collaborative Research in Computational Neuroscience (CRCNS) Program.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: B.G.L. and L.S.S. prepared figures; B.G.L., S.C.N., and L.S.S. drafted manuscript; B.G.L., S.C.N., L.S.S., and K.F.M. edited and revised manuscript; B.G.L., S.C.N., L.S.S., and K.F.M. approved final version of manuscript.
References
- 1.Abe T, Kusuhara N, Yoshimura N, Tomita T, Easton PA. Differential respiratory activity of four abdominal muscles in humans. J Appl Physiol (1985) 80: 1379–1389, 1996. doi: 10.1152/jappl.1996.80.4.1379. [DOI] [PubMed] [Google Scholar]
- 2.Accorsi-Mendonça D, Machado BH. Synaptic transmission of baro- and chemoreceptors afferents in the NTS second order neurons. Auton Neurosci 175: 3–8, 2013. doi: 10.1016/j.autneu.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Andersson B, Kenney RA, Neil E. The role of the chemoceptors of the carotid and aortic regions in the production of the Mayer waves. Acta Physiol Scand 20: 203–220, 1950. doi: 10.1111/j.1748-1716.1950.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 4.Andresen MC, Kunze DL. Nucleus tractus solitarius--gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 5.Arata A, Tanaka I, Fujii M, Ezure K. Active inspiratory-expiratory phase switching mechanism exists in the neonatal nucleus parabrachialis. In: New Frontiers in Respiratory Control. Advances in Experimental Medicine and Biology, edited by Homma I, Onimaru H, Fukuchi Y. New York, NY: Springer New York, 2010, p. 135–138. doi: 10.1007/978-1-4419-5692-7_27. [DOI] [PubMed] [Google Scholar]
- 6.Arata A, Hernandez YM, Lindsey BG, Morris KF, Shannon R. Transient configurations of baroresponsive respiratory-related brainstem neuronal assemblies in the cat. J Physiol 525: 509–530, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacak BJ, Kim T, Smith JC, Rubin JE, Rybak IA. Mixed-mode oscillations and population bursting in the pre-Bötzinger complex. eLife 5: e13403, 2016. doi: 10.7554/eLife.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badrov MB, Barak OF, Mijacika T, Shoemaker LN, Borrell LJ, Lojpur M, Drvis I, Dujic Z, Shoemaker JK. Ventilation inhibits sympathetic action potential recruitment even during severe chemoreflex stress. J Neurophysiol 118: 2914–2924, 2017. doi: 10.1152/jn.00381.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baekey DM, Molkov YI, Paton JFR, Rybak IA, Dick TE. Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory-sympathetic interactions. Respir Physiol Neurobiol 174: 135–145, 2010. doi: 10.1016/j.resp.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baertsch NA, Baertsch HC, Ramirez JM. The interdependence of excitation and inhibition for the control of dynamic breathing rhythms. Nat Commun 9: 843, 2018. doi: 10.1038/s41467-018-03223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balis UJ, Morris KF, Koleski J, Lindsey BG. Simulations of a ventrolateral medullary neural network for respiratory rhythmogenesis inferred from spike train cross-correlation. Biol Cybern 70: 311–327, 1994. doi: 10.1007/BF00200329. [DOI] [PubMed] [Google Scholar]
- 12.Bantikyan A, Song G, Feinberg-Zadek P, Poon C-S. Intrinsic and synaptic long-term depression of NTS relay of nociceptin- and capsaicin-sensitive cardiopulmonary afferents hyperactivity. Pflugers Arch 457: 1147–1159, 2009. doi: 10.1007/s00424-008-0571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barman SM, Gebber GL. Classification of caudal ventrolateral pontine neurons with sympathetic nerve-related activity. J Neurophysiol 80: 2433–2445, 1998. doi: 10.1152/jn.1998.80.5.2433. [DOI] [PubMed] [Google Scholar]
- 14.Barman SM, Yates BJ. Deciphering the neural control of sympathetic nerve activity: Status report and directions for future research. Front Neurosci 11: 730, 2017. doi: 10.3389/fnins.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barman SM, Gebber GL, Kitchens H. Rostral dorsolateral pontine neurons with sympathetic nerve-related activity. Am J Physiol Heart Circ Physiol 276: H401–H412, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Barman SM, Kitchens HL, Leckow AB, Gebber GL. Pontine neurons are elements of the network responsible for the 10-Hz rhythm in sympathetic nerve discharge. Am J Physiol Heart Circ Physiol 273: H1909–H1919, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Barnett WH, Abdala AP, Paton JFR, Rybak IA, Zoccal DB, Molkov YI. Chemoreception and neuroplasticity in respiratory circuits. Exp Neurol 287: 153–164, 2017. doi: 10.1016/j.expneurol.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett WH, Jenkin SEM, Milsom WK, Paton JFR, Abdala AP, Molkov YI, Zoccal DB. The Kölliker-Fuse nucleus orchestrates the timing of expiratory abdominal nerve bursting. J Neurophysiol 119: 401–412, 2018. doi: 10.1152/jn.00499.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartlett D., Jr Effects of hypercapnia and hypoxia on laryngeal resistance to airflow. Respir Physiol 37: 293–302, 1979. doi: 10.1016/0034-5687(79)90076-8. [DOI] [PubMed] [Google Scholar]
- 20.Basting TM, Burke PGR, Kanbar R, Viar KE, Stornetta DS, Stornetta RL, Guyenet PG. Hypoxia silences retrotrapezoid nucleus respiratory chemoreceptors via alkalosis. J Neurosci 35: 527–543, 2015. doi: 10.1523/JNEUROSCI.2923-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behan M, Zabka AG, Mitchell GS. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol 131: 65–77, 2002. doi: 10.1016/S1569-9048(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 22.Beltrán-Castillo S, Olivares MJ, Contreras RA, Zúñiga G, Llona I, von Bernhardi R, Eugenín JL. D-serine released by astrocytes in brainstem regulates breathing response to CO2 levels. Nat Commun 8: 838, 2017. doi: 10.1038/s41467-017-00960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Tal A, Shamailov SS, Paton JFR. Evaluating the physiological significance of respiratory sinus arrhythmia: looking beyond ventilation-perfusion efficiency. J Physiol 590: 1989–2008, 2012. doi: 10.1113/jphysiol.2011.222422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Tal A, Shamailov SS, Paton JFR. Central regulation of heart rate and the appearance of respiratory sinus arrhythmia: new insights from mathematical modeling. Math Biosci 255: 71–82, 2014. doi: 10.1016/j.mbs.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boychuk CR, Hayward LF. Prenatal nicotine exposure alters postnatal cardiorespiratory integration in young male but not female rats. Exp Neurol 232: 212–221, 2011. doi: 10.1016/j.expneurol.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braegelmann KM, Streeter KA, Fields DP, Baker TL. Plasticity in respiratory motor neurons in response to reduced synaptic inputs: A form of homeostatic plasticity in respiratory control? Exp Neurol 287: 225–234, 2017. doi: 10.1016/j.expneurol.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodin E, Linderoth B, Goiny M, Yamamoto Y, Gazelius B, Millhorn DE, Hökfelt T, Ungerstedt U. In vivo release of serotonin in cat dorsal vagal complex and cervical ventral horn induced by electrical stimulation of the medullary raphe nuclei. Brain Res 535: 227–236, 1990. doi: 10.1016/0006-8993(90)91605-G. [DOI] [PubMed] [Google Scholar]
- 28.Brown SJ, Barnes MJ, Mündel T. Effects of hypoxia and hypercapnia on human HRV and respiratory sinus arrhythmia. Acta Physiol Hung 101: 263–272, 2014. doi: 10.1556/APhysiol.101.2014.3.1. [DOI] [PubMed] [Google Scholar]
- 29.Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol 498: 649–662, 1997. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckler KJ. TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing. Pflugers Arch 467: 1013–1025, 2015. doi: 10.1007/s00424-015-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckler KJ, Turner PJ. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J Physiol 591: 3549–3563, 2013. doi: 10.1113/jphysiol.2013.257741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang EY, Morris KF, Shannon R, Lindsey BG. Repeated sequences of interspike intervals in baroresponsive respiratory related neuronal assemblies of the cat brain stem. J Neurophysiol 84: 1136–1148, 2000. doi: 10.1152/jn.2000.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 33.Cherniack NS, Edelman NH, Fishman AP. Pattern of discharge of respiratory neurons during systemic vasomotor waves. Am J Physiol 217: 1375–1383, 1969. [DOI] [PubMed] [Google Scholar]
- 34.Christiansen L, Urbin MA, Mitchell GS, Perez MA. Acute intermittent hypoxia enhances corticospinal synaptic plasticity in humans. eLife 7: e34304, 2018. doi: 10.7554/eLife.34304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen MI, Feldman JL, Sommer D. Caudal medullary expiratory neurone and internal intercostal nerve discharges in the cat: effects of lung inflation. J Physiol 368: 147–178, 1985. doi: 10.1113/jphysiol.1985.sp015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruz JC, Bonagamba LG, Stern JE, Machado BH. Fos expression in the NTS in response to peripheral chemoreflex activation in awake rats. Auton Neurosci 152: 27–34, 2010. doi: 10.1016/j.autneu.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Cui Y, Kam K, Sherman D, Janczewski WA, Zheng Y, Feldman JL. Defining preBötzinger complex rhythm and pattern generating neural microcircuits in vivo. Neuron 91: 602–614, 2016. doi: 10.1016/j.neuron.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Damasceno RS, Takakura AC, Moreira TS. Regulation of the chemosensory control of breathing by Kölliker-Fuse neurons. Am J Physiol Regul Integr Comp Physiol 307: R57–R67, 2014. doi: 10.1152/ajpregu.00024.2014. [DOI] [PubMed] [Google Scholar]
- 39.Damasceno RS, Takakura AC, Moreira TS. Respiratory and sympathetic chemoreflex regulation by Kölliker-Fuse neurons in rats. Pflugers Arch 467: 231–239, 2015. doi: 10.1007/s00424-014-1525-z. [DOI] [PubMed] [Google Scholar]
- 40.Davenport PW, Shannon R, Mercak A, Reep RL, Lindsey BG. Cerebral cortical evoked potentials elicited by cat intercostal muscle mechanoreceptors. J Appl Physiol (1985) 74: 799–804, 1993. doi: 10.1152/jappl.1993.74.2.799. [DOI] [PubMed] [Google Scholar]
- 41.de Britto AA, Moraes DJA. Non-chemosensitive parafacial neurons simultaneously regulate active expiration and airway patency under hypercapnia in rats. J Physiol 595: 2043–2064, 2017. doi: 10.1113/JP273335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Troyer A, Estenne M, Ninane V, Van Gansbeke D, Gorini M. Transversus abdominis muscle function in humans. J Appl Physiol (1985) 68: 1010–1016, 1990. doi: 10.1152/jappl.1990.68.3.1010. [DOI] [PubMed] [Google Scholar]
- 43.Dean JB, Mulkey DK, Henderson RA III, Potter SJ, Putnam RW. Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brain stem neurons. J Appl Physiol (1985) 96: 784–791, 2004. doi: 10.1152/japplphysiol.00892.2003. [DOI] [PubMed] [Google Scholar]
- 44.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62: 2422–2430, 2013. doi: 10.1016/j.jacc.2013.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Del Rio R, Iturriaga R, Schultz HD. Editorial: Carotid body: a new target for rescuing neural control of cardiorespiratory balance in disease. Front Physiol 6: 181, 2015. doi: 10.3389/fphys.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dempsey JA. New perspectives concerning feedback influences on cardiorespiratory control during rhythmic exercise and on exercise performance. J Physiol 590: 4129–4144, 2012. doi: 10.1113/jphysiol.2012.233908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devinney MJ, Nichols NL, Mitchell GS. Sustained hypoxia elicits competing spinal mechanisms of phrenic motor facilitation. J Neurosci 36: 7877–7885, 2016. doi: 10.1523/JNEUROSCI.4122-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann N Y Acad Sci 1279: 143–153, 2013. doi: 10.1111/nyas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS. Phrenic long-term facilitation requires PKCθ activity within phrenic motor neurons. J Neurosci 35: 8107–8117, 2015. doi: 10.1523/JNEUROSCI.5086-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respir Physiol 121: 87–100, 2000. doi: 10.1016/S0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- 51.Dick TE, Hsieh Y-H, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol 92: 87–97, 2007. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- 52.Dick TE, Hsieh YH, Morrison S, Coles SK, Prabhakar N. Entrainment pattern between sympathetic and phrenic nerve activities in the Sprague-Dawley rat: hypoxia-evoked sympathetic activity during expiration. Am J Physiol Regul Integr Comp Physiol 286: R1121–R1128, 2004. doi: 10.1152/ajpregu.00485.2003. [DOI] [PubMed] [Google Scholar]
- 53.Dick TE, Baekey DM, Paton JFR, Lindsey BG, Morris KF. Cardio-respiratory coupling depends on the pons. Respir Physiol Neurobiol 168: 76–85, 2009. doi: 10.1016/j.resp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Dick TE, Mims JR, Hsieh Y-H, Morris KF, Wehrwein EA. Increased cardio-respiratory coupling evoked by slow deep breathing can persist in normal humans. Respir Physiol Neurobiol 204: 99–111, 2014. doi: 10.1016/j.resp.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J Physiol 586: 4265–4282, 2008. doi: 10.1113/jphysiol.2008.152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dick TE, Hsieh YH, Dhingra RR, Baekey DM, Galán RF, Wehrwein E, Morris KF. Cardiorespiratory coupling: common rhythms in cardiac, sympathetic, and respiratory activities. Prog Brain Res 209: 191–205, 2014. doi: 10.1016/B978-0-444-63274-6.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diekman CO, Thomas PJ, Wilson CG. Eupnea, tachypnea, and autoresuscitation in a closed-loop respiratory control model. J Neurophysiol 118: 2194–2215, 2017. doi: 10.1152/jn.00170.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donnelly DF, Haddad GG. Prolonged apnea and impaired survival in piglets after sinus and aortic nerve section. J Appl Physiol (1985) 68: 1048–1052, 1990. doi: 10.1152/jappl.1990.68.3.1048. [DOI] [PubMed] [Google Scholar]
- 59.Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol 2: 2443–2469, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eckberg DL, Bastow H III, Scruby AE. Modulation of human sinus node function by systemic hypoxia. J Appl Physiol Respir Environ Exerc Physiol 52: 570–577, 1982. [DOI] [PubMed] [Google Scholar]
- 61.Eisenberg MS. Incidence and significance of gasping or agonal respirations in cardiac arrest patients. Curr Opin Crit Care 12: 204–206, 2006. doi: 10.1097/01.ccx.0000224862.48087.66. [DOI] [PubMed] [Google Scholar]
- 62.ElMallah MK, Stanley DA, Lee K-Z, Turner SM, Streeter KA, Baekey DM, Fuller DD. Power spectral analysis of hypoglossal nerve activity during intermittent hypoxia-induced long-term facilitation in mice. J Neurophysiol 115: 1372–1380, 2016. doi: 10.1152/jn.00479.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol 348: 161–182, 1994. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- 64.Erickson JT, Sposato BC. Autoresuscitation responses to hypoxia-induced apnea are delayed in newborn 5-HT-deficient Pet-1 homozygous mice. J Appl Physiol (1985) 106: 1785–1792, 2009. doi: 10.1152/japplphysiol.90729.2008. [DOI] [PubMed] [Google Scholar]
- 65.Farmer DGS, Dutschmann M, Paton JFR, Pickering AE, McAllen RM. Brainstem sources of cardiac vagal tone and respiratory sinus arrhythmia. J Physiol 594: 7249–7265, 2016. doi: 10.1113/JP273164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fatemian M, Nieuwenhuijs DJF, Teppema LJ, Meinesz S, van der Mey AG, Dahan A, Robbins PA. The respiratory response to carbon dioxide in humans with unilateral and bilateral resections of the carotid bodies. J Physiol 549: 965–973, 2003. doi: 10.1113/jphysiol.2003.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci 5: 1993–2000, 1985. doi: 10.1523/JNEUROSCI.05-08-01993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferretti R, Cherniack NS, Longobardo G, Levine OR, Morkin E, Singer DH, Fishman AP. Systemic and pulmonary vasomotor waves. Am J Physiol 209: 37–50, 1965. [DOI] [PubMed] [Google Scholar]
- 69.Fiamma M-N, O’Connor ET, Roy A, Zuna I, Wilson RJ. The essential role of peripheral respiratory chemoreceptor inputs in maintaining breathing revealed when CO2 stimulation of central chemoreceptors is diminished. J Physiol 591: 1507–1521, 2013. doi: 10.1113/jphysiol.2012.247304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fields DP, Mitchell GS. Spinal metaplasticity in respiratory motor control. Front Neural Circuits 9: 2, 2015. doi: 10.3389/fncir.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fields DP, Mitchell GS. Divergent cAMP signaling differentially regulates serotonin-induced spinal motor plasticity. Neuropharmacology 113: 82–88, 2017. doi: 10.1016/j.neuropharm.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flor KC, Silva EF, Menezes MF, Pedrino GR, Colombari E, Zoccal DB. Short-term sustained hypoxia elevates basal and hypoxia-induced ventilation but not the carotid body chemoreceptor activity in rats. Front Physiol 9: 134, 2018. doi: 10.3389/fphys.2018.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fogarty MJ, Mantilla CB, Sieck GC. Breathing: Motor control of diaphragm muscle. Physiology (Bethesda) 33: 113–126, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ford TW, Kirkwood PA. Cardiac modulation of alpha motoneuron discharges. J Neurophysiol 119: 1723–1730, 2018. doi: 10.1152/jn.00025.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fortuna MG, West GH, Stornetta RL, Guyenet PG. Botzinger expiratory-augmenting neurons and the parafacial respiratory group. J Neurosci 28: 2506–2515, 2008. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fuller DD, Mitchell GS. Respiratory neuroplasticity - Overview, significance and future directions. Exp Neurol 287: 144–152, 2017. doi: 10.1016/j.expneurol.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 78.Funk GD, Rajani V, Alvares TS, Revill AL, Zhang Y, Chu NY, Biancardi V, Linhares-Taxini C, Katzell A, Reklow R. Neuroglia and their roles in central respiratory control; an overview. Comp Biochem Physiol A Mol Integr Physiol 186: 83–95, 2015. doi: 10.1016/j.cbpa.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 79.Galán RF, Dick TE, Baekey DM. Analysis and modeling of ensemble recordings from respiratory pre-motor neurons indicate changes in functional network architecture after acute hypoxia. Front Comput Neurosci 4: 131, 2010. doi: 10.3389/fncom.2010.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garcia AJ III, Koschnitzky JE, Dashevskiy T, Ramirez J-M. Cardiorespiratory coupling in health and disease. Auton Neurosci 175: 26–37, 2013. doi: 10.1016/j.autneu.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia AJ III, Dashevskiy T, Khuu MA, Ramirez J-M. Chronic intermittent hypoxia differentially impacts different states of inspiratory activity at the level of the preBötzinger complex. Front Physiol 8: 571, 2017. doi: 10.3389/fphys.2017.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garcia AJ III, Zanella S, Dashevskiy T, Khan SA, Khuu MA, Prabhakar NR, Ramirez J-M. Chronic intermittent hypoxia alters local respiratory circuit function at the level of the preBötzinger complex. Front Neurosci 10: 4, 2016. doi: 10.3389/fnins.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghali MGZ. Phrenic motoneurons: output elements of a highly organized intraspinal network. J Neurophysiol 119: 1057–1070, 2018. doi: 10.1152/jn.00705.2015. [DOI] [PubMed] [Google Scholar]
- 84.Gilbert-Kawai ET, Milledge JS, Grocott MPW, Martin DS. King of the mountains: Tibetan and Sherpa physiological adaptations for life at high altitude. Physiology (Bethesda) 29: 388–402, 2014. [DOI] [PubMed] [Google Scholar]
- 85.Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. J Physiol 356: 65–78, 1984. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goldberg S, Ollila HM, Lin L, Sharifi H, Rico T, Andlauer O, Aran A, Bloomrosen E, Faraco J, Fang H, Mignot E. Analysis of hypoxic and hypercapnic ventilatory response in healthy volunteers. PLoS One 12: e0168930, 2017. doi: 10.1371/journal.pone.0168930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gourine AV, Funk GD. On the existence of a central respiratory oxygen sensor. J Appl Physiol (1985) 123: 1344–1349, 2017. doi: 10.1152/japplphysiol.00194.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gourine AV, Machhada A, Trapp S, Spyer KM. Cardiac vagal preganglionic neurones: An update. Auton Neurosci 199: 24–28, 2016. doi: 10.1016/j.autneu.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Griffin HS, Pugh K, Kumar P, Balanos GM. Long-term facilitation of ventilation following acute continuous hypoxia in awake humans during sustained hypercapnia. J Physiol 590: 5151–5165, 2012. doi: 10.1113/jphysiol.2012.236109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guyenet PG, Koshiya N, Huangfu D, Verberne AJ, Riley TA. Central respiratory control of A5 and A6 pontine noradrenergic neurons. Am J Physiol Regul Integr Comp Physiol 264: R1035–R1044, 1993. [DOI] [PubMed] [Google Scholar]
- 93.Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PGR, Abbott SBG. C1 neurons: the body’s EMTs. Am J Physiol Regul Integr Comp Physiol 305: R187–R204, 2013. doi: 10.1152/ajpregu.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guyenet PG, Bayliss DA, Stornetta RL, Kanbar R, Shi Y, Holloway BB, Souza GMPR, Basting TM, Abbott SBG, Wenker IC. Interdependent feedback regulation of breathing by the carotid bodies and the retrotrapezoid nucleus. J Physiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 291: R1111–R1119, 2006. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- 96.Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. J Neurosci 16: 6526–6536, 1996. doi: 10.1523/JNEUROSCI.16-20-06526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol 265: R811–R819, 1993. [DOI] [PubMed] [Google Scholar]
- 98.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 99.Herrero JL, Khuvis S, Yeagle E, Cerf M, Mehta AD. Breathing above the brain stem: volitional control and attentional modulation in humans. J Neurophysiol 119: 145–159, 2018. doi: 10.1152/jn.00551.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hodges MR, Forster HV. Respiratory neuroplasticity following carotid body denervation: central and peripheral adaptations. Neural Regen Res 7: 1073–1079, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hodson EJ, Nicholls LG, Turner PJ, Llyr R, Fielding JW, Douglas G, Ratnayaka I, Robbins PA, Pugh CW, Buckler KJ, Ratcliffe PJ, Bishop T. Regulation of ventilatory sensitivity and carotid body proliferation in hypoxia by the PHD2/HIF-2 pathway. J Physiol 594: 1179–1195, 2016. doi: 10.1113/JP271050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hofer MA. Role of carotid sinus and aortic nerves in respiratory control of infant rats. Am J Physiol Regul Integr Comp Physiol 251: R811–R817, 1986. [DOI] [PubMed] [Google Scholar]
- 103.Hooper JS, Hadley SH, Morris KF, Breslin JW, Dean JB, Taylor-Clark TE. Characterization of cardiovascular reflexes evoked by airway stimulation with allylisothiocyanate, capsaicin, and ATP in Sprague-Dawley rats. J Appl Physiol (1985) 120: 580–591, 2016. doi: 10.1152/japplphysiol.00944.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huckstepp RTR, Henderson LE, Cardoza KP, Feldman JL. Interactions between respiratory oscillators in adult rats. eLife 5: e14203, 2016. doi: 10.7554/eLife.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iscoe S. Control of abdominal muscles. Prog Neurobiol 56: 433–506, 1998. doi: 10.1016/S0301-0082(98)00046-X. [DOI] [PubMed] [Google Scholar]
- 106.Iturriaga R. Translating carotid body function into clinical medicine. J Physiol. In press. doi: 10.1113/JP275335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol 3: 141–163, 2013. doi: 10.1002/cphy.c110057. [DOI] [PubMed] [Google Scholar]
- 108.Jenkin SEM, Milsom WK. Expiration: breathing’s other face. Prog Brain Res 212: 131–147, 2014. doi: 10.1016/B978-0-444-63488-7.00008-2. [DOI] [PubMed] [Google Scholar]
- 109.Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Bötzinger complex in the cat. Exp Brain Res 81: 639–648, 1990. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- 110.Jodkowski JS, Coles SK, Dick TEA. A ‘pneumotaxic centre’ in rats. Neurosci Lett 172: 67–72, 1994. doi: 10.1016/0304-3940(94)90664-5. [DOI] [PubMed] [Google Scholar]
- 111.Jodkowski JS, Coles SK, Dick TE. Prolongation in expiration evoked from ventrolateral pons of adult rats. J Appl Physiol (1985) 82: 377–381, 1997. doi: 10.1152/jappl.1997.82.2.377. [DOI] [PubMed] [Google Scholar]
- 112.Johnson BD, Joyner MJ. Carotid body denervation: too soon to get breathless about heart failure? J Am Coll Cardiol 62: 2431–2432, 2013. doi: 10.1016/j.jacc.2013.08.718. [DOI] [PubMed] [Google Scholar]
- 113.Kamendi HW, Cheng Q, Dergacheva O, Frank JG, Gorini C, Jameson HS, Pinol RA, Wang X, Mendelowitz D. Recruitment of excitatory serotonergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus post hypoxia and hypercapnia. J Neurophysiol 99: 1163–1168, 2008. doi: 10.1152/jn.01178.2007. [DOI] [PubMed] [Google Scholar]
- 114.Kim SJ, Fong AY, Pilowsky PM, Abbott SBG. Sympathoexcitation following intermittent hypoxia in rat is mediated by circulating angiotensin II acting at the carotid body and subfornical organ. J Physiol. In press. doi: 10.1113/JP275804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol 130: 207–218, 2001. doi: 10.1016/S1095-6433(01)00393-2. [DOI] [PubMed] [Google Scholar]
- 116.Kline DD, King TL, Austgen JR, Heesch CM, Hasser EM. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience 167: 510–527, 2010. doi: 10.1016/j.neuroscience.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koehle M, Sheel W, Milsom W, McKenzie D. The effect of two different intermittent hypoxia protocols on ventilatory responses to hypoxia and carbon dioxide at rest. In: Integration in Respiratory Control: From Genes to Systems, edited by Poulin MJ, Wilson RJA. New York: Springer New York, 2008, p. 218–223. doi: 10.1007/978-0-387-73693-8_38. [DOI] [PubMed] [Google Scholar]
- 118.Koshiya N, Guyenet PG. Role of the pons in the carotid sympathetic chemoreflex. Am J Physiol Regul Integr Comp Physiol 267: R508–R518, 1994. [DOI] [PubMed] [Google Scholar]
- 119.Koshiya N, Guyenet PG. A5 noradrenergic neurons and the carotid sympathetic chemoreflex. Am J Physiol Regul Integr Comp Physiol 267: R519–R526, 1994. [DOI] [PubMed] [Google Scholar]
- 120.Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 270: R1273–R1278, 1996. [DOI] [PubMed] [Google Scholar]
- 121.Koshiya N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. J Physiol 491: 859–869, 1996. doi: 10.1113/jphysiol.1996.sp021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krause A, Nowak Z, Srbu R, Bell HJ. Respiratory autoresuscitation following severe acute hypoxemia in anesthetized adult rats. Respir Physiol Neurobiol 232: 43–53, 2016. doi: 10.1016/j.resp.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 123.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2: 141–219, 2012. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lahiri S, Hsiao C, Zhang R, Mokashi A, Nishino T. Peripheral chemoreceptors in respiratory oscillations. J Appl Physiol (1985) 58: 1901–1908, 1985. doi: 10.1152/jappl.1985.58.6.1901. [DOI] [PubMed] [Google Scholar]
- 125.Lawson EE, Richter DW, Ballantyne D, Lalley PM. Peripheral chemoreceptor inputs to medullary inspiratory and postinspiratory neurons of cats. Pflugers Arch 414: 523–533, 1989. doi: 10.1007/BF00580987. [DOI] [PubMed] [Google Scholar]
- 126.Lemes EV, Aiko S, Orbem CB, Formentin C, Bassi M, Colombari E, Zoccal DB. Long-term facilitation of expiratory and sympathetic activities following acute intermittent hypoxia in rats. Acta Physiol (Oxf) 217: 254–266, 2016. doi: 10.1111/apha.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Z, Morris KF, Baekey DM, Shannon R, Lindsey BG. Multimodal medullary neurons and correlational linkages of the respiratory network. J Neurophysiol 82: 188–201, 1999. doi: 10.1152/jn.1999.82.1.188. [DOI] [PubMed] [Google Scholar]
- 128.Lindsey BG, Segers LS, Shannon R. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. II. Evidence for inhibitory actions of expiratory neurons. J Neurophysiol 57: 1101–1117, 1987. doi: 10.1152/jn.1987.57.4.1101. [DOI] [PubMed] [Google Scholar]
- 129.Lindsey BG, Segers LS, Shannon R. Discharge patterns of rostrolateral medullary expiratory neurons in the cat: regulation by concurrent network processes. J Neurophysiol 61: 1185–1196, 1989. doi: 10.1152/jn.1989.61.6.1185. [DOI] [PubMed] [Google Scholar]
- 130.Lindsey BG, Hernandez YM, Shannon R. Cooperativity in Distributed Respiratory and Cardiovascular-Related Brainstem Neural Assemblies: Insights from Many-Neuron Recordings. Berlin: Springer Berlin Heidelberg, 1991, p. 131–137. [Google Scholar]
- 131.Lindsey BG, Rybak IA, Smith JC. Computational models and emergent properties of respiratory neural networks. Compr Physiol 2: 1619–1670, 2012. doi: 10.1002/cphy.c110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lindsey BG, Hernandez YM, Morris KF, Shannon R. Functional connectivity between brain stem midline neurons with respiratory-modulated firing rates. J Neurophysiol 67: 890–904, 1992. doi: 10.1152/jn.1992.67.4.890. [DOI] [PubMed] [Google Scholar]
- 133.Lindsey BG, Hernandez YM, Morris KF, Shannon R, Gerstein GL. Dynamic reconfiguration of brain stem neural assemblies: respiratory phase-dependent synchrony versus modulation of firing rates. J Neurophysiol 67: 923–930, 1992. doi: 10.1152/jn.1992.67.4.923. [DOI] [PubMed] [Google Scholar]
- 134.Lindsey BG, Hernandez YM, Morris KF, Shannon R, Gerstein GL. Respiratory-related neural assemblies in the brain stem midline. J Neurophysiol 67: 905–922, 1992. doi: 10.1152/jn.1992.67.4.905. [DOI] [PubMed] [Google Scholar]
- 135.Lindsey BG, Arata A, Morris KF, Hernandez YM, Shannon R. Medullary raphe neurones and baroreceptor modulation of the respiratory motor pattern in the cat. J Physiol 512: 863–882, 1998. doi: 10.1111/j.1469-7793.1998.863bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lindsey BG, Segers LS, Morris KF, Hernandez YM, Saporta S, Shannon R. Distributed actions and dynamic associations in respiratory-related neuronal assemblies of the ventrolateral medulla and brain stem midline: evidence from spike train analysis. J Neurophysiol 72: 1830–1851, 1994. doi: 10.1152/jn.1994.72.4.1830. [DOI] [PubMed] [Google Scholar]
- 137.Ling L. Serotonin and NMDA receptors in respiratory long-term facilitation. Respir Physiol Neurobiol 164: 233–241, 2008. doi: 10.1016/j.resp.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lipski J, Voss MD. “Gating” of peripheral chemoreceptor input to medullary inspiratory neurons: role of Bötzinger complex neurons. In: Chemoreceptors and Chemoreceptor Reflexes, edited by Acker H, Trzebski A, O’Regan RC. New York: Plenum Press, 1990, p. 323–329. doi: 10.1007/978-1-4684-8938-5_47. [DOI] [Google Scholar]
- 139.Lipski J, McAllen RM, Spyer KM. The carotid chemoreceptor input to the respiratory neurones of the nucleus of tractus solitarus. J Physiol 269: 797–810, 1977. doi: 10.1113/jphysiol.1977.sp011930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lipski J, Trzebski A, Chodobska J, Kruk P. Effects of carotid chemoreceptor excitation on medullary expiratory neurons in cats. Respir Physiol 57: 279–291, 1984. doi: 10.1016/0034-5687(84)90077-X. [DOI] [PubMed] [Google Scholar]
- 141.López-Barneo J, López-López JR, Ureña J, González C. Chemotransduction in the carotid body: K+ current modulated by Po2 in type I chemoreceptor cells. Science 241: 580–582, 1988. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- 142.López-Barneo J, González-Rodríguez P, Gao L, Fernández-Agüera MC, Pardal R, Ortega-Sáenz P. Oxygen sensing by the carotid body: mechanisms and role in adaptation to hypoxia. Am J Physiol Cell Physiol 310: C629–C642, 2016. doi: 10.1152/ajpcell.00265.2015. [DOI] [PubMed] [Google Scholar]
- 143.López-Barneo J, Ortega-Sáenz P, González-Rodríguez P, Fernández-Agüera MC, Macías D, Pardal R, Gao L. Oxygen-sensing by arterial chemoreceptors: Mechanisms and medical translation. Mol Aspects Med 47-48: 90–108, 2016. doi: 10.1016/j.mam.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 144.Lugliani R, Whipp BJ, Seard C, Wasserman K. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. N Engl J Med 285: 1105–1111, 1971. doi: 10.1056/NEJM197111112852002. [DOI] [PubMed] [Google Scholar]
- 145.MacFarlane PM, Vinit S, Mitchell GS. Enhancement of phrenic long-term facilitation following repetitive acute intermittent hypoxia is blocked by the glycolytic inhibitor 2-deoxyglucose. Am J Physiol Regul Integr Comp Physiol 314: R135–R144, 2018. doi: 10.1152/ajpregu.00306.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Macias D, Cowburn AS, Torres-Torrelo H, Ortega-Sáenz P, López-Barneo J, Johnson RS. HIF-2α is essential for carotid body development and function. eLife 7: e34681, 2018. doi: 10.7554/eLife.34681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Magder S, Scharf SM. Venous return. In: Respiratory-Circulatory Interactions in Health and Disease, edited by Scharf SM, Magder S, Pinsky MR. New York: Marcel Dekker, Inc, 2001, p. 93–112. [Google Scholar]
- 148.Mantilla CB. Gene therapy and respiratory neuroplasticity. Exp Neurol 287: 261–267, 2017. doi: 10.1016/j.expneurol.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 149.Manzke T, Dutschmann M, Schlaf G, Mörschel M, Koch UR, Ponimaskin E, Bidon O, Lalley PM, Richter DW. Serotonin targets inhibitory synapses to induce modulation of network functions. Philos Trans R Soc Lond B Biol Sci 364: 2589–2602, 2009. doi: 10.1098/rstb.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marchenko V, Koizumi H, Mosher B, Koshiya N, Tariq MF, Bezdudnaya TG, Zhang R, Molkov YI, Rybak IA, Smith JC. Perturbations of respiratory rhythm and pattern by disrupting synaptic inhibition within Pre-Bötzinger and Bötzinger complexes. eNeuro 3: ENEURO.0011-16.2016, 2016. doi: 10.1523/ENEURO.0011-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Marcus NJ, Del Rio R, Schultz EP, Xia X-H, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol 592: 391–408, 2014. doi: 10.1113/jphysiol.2013.266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev 74: 543–594, 1994. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- 153.Mateika JH, Komnenov D. Intermittent hypoxia initiated plasticity in humans: A multipronged therapeutic approach to treat sleep apnea and overlapping co-morbidities. Exp Neurol 287: 113–129, 2017. doi: 10.1016/j.expneurol.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 154.Mateika JH, El-Chami M, Shaheen D, Ivers B. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. J Appl Physiol (1985) 118: 520–532, 2015. doi: 10.1152/japplphysiol.00564.2014. [DOI] [PubMed] [Google Scholar]
- 155.Mateika JH, Panza G, Alex R, El-Chami M. The impact of intermittent or sustained carbon dioxide on intermittent hypoxia initiated respiratory plasticity. What is the effect of these combined stimuli on apnea severity? Respir Physiol Neurobiol S1569-9048(17)30258-6. In press. [DOI] [PubMed] [Google Scholar]
- 156.McAllen RM. Actions of carotid chemoreceptors on subretrofacial bulbospinal neurons in the cat. J Auton Nerv Syst 40: 181–188, 1992. doi: 10.1016/0165-1838(92)90199-Q. [DOI] [PubMed] [Google Scholar]
- 157.McDougall SJ, Andresen MC. Independent transmission of convergent visceral primary afferents in the solitary tract nucleus. J Neurophysiol 109: 507–517, 2013. doi: 10.1152/jn.00726.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miller JR, Neumueller S, Muere C, Olesiak S, Pan L, Hodges MR, Forster HV. Changes in neurochemicals within the ventrolateral medullary respiratory column in awake goats after carotid body denervation. J Appl Physiol (1985) 115: 1088–1098, 2013. doi: 10.1152/japplphysiol.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Millhorn DE. Stimulation of raphe (obscurus) nucleus causes long-term potentiation of phrenic nerve activity in cat. J Physiol 381: 169–179, 1986. doi: 10.1113/jphysiol.1986.sp016320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol 42: 171–188, 1980. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- 161.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol 41: 87–103, 1980. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- 162.Molkov YI, Rubin JE, Rybak IA, Smith JC. Computational models of the neural control of breathing. Wiley Interdiscip Rev Syst Biol Med 9: e1371, 2017. doi: 10.1002/wsbm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Molkov YI, Zoccal DB, Moraes DJA, Paton JFR, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol 105: 3080–3091, 2011. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Molkov YI, Shevtsova NA, Park C, Ben-Tal A, Smith JC, Rubin JE, Rybak IA. A closed-loop model of the respiratory system: focus on hypercapnia and active expiration. PLoS One 9: e109894, 2014. doi: 10.1371/journal.pone.0109894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Molkov YI, Zoccal DB, Baekey DM, Abdala APL, Machado BH, Dick TE, Paton JFR, Rybak IA. Physiological and pathophysiological interactions between the respiratory central pattern generator and the sympathetic nervous system. Prog Brain Res 212: 1–23, 2014. doi: 10.1016/B978-0-444-63488-7.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moraes DJA, Machado BH, Paton JFR. Specific respiratory neuron types have increased excitability that drive presympathetic neurones in neurogenic hypertension. Hypertension 63: 1309–1318, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02283. [DOI] [PubMed] [Google Scholar]
- 167.Moraes DJA, Bonagamba LGH, da Silva MP, Paton JFR, Machado BH. Role of ventral medullary catecholaminergic neurons for respiratory modulation of sympathetic outflow in rats. Sci Rep 7: 16883, 2017. doi: 10.1038/s41598-017-17113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moraes DJA, da Silva MP, Bonagamba LGH, Mecawi AS, Zoccal DB, Antunes-Rodrigues J, Varanda WA, Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci 33: 19223–19237, 2013. doi: 10.1523/JNEUROSCI.3041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Morris KF, Gozal D. Persistent respiratory changes following intermittent hypoxic stimulation in cats and human beings. Respir Physiol Neurobiol 140: 1–8, 2004. doi: 10.1016/j.resp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 170.Morris KF, Shannon R, and Lindsey BG. Parallel processing of carotid chemoreceptor inputs: In vivo experiments and network simulations, In: IFAC Proceedings Volumes. 1994: Galveston, TX USA. p. 573–574. [Google Scholar]
- 171.Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol 532: 483–497, 2001. doi: 10.1111/j.1469-7793.2001.0483f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morris KF, Arata A, Shannon R, Lindsey BG. Long-term facilitation of phrenic nerve activity in cats: responses and short time scale correlations of medullary neurones. J Physiol 490: 463–480, 1996. doi: 10.1113/jphysiol.1996.sp021158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Morris KF, Arata A, Shannon R, Lindsey BG. Inspiratory drive and phase duration during carotid chemoreceptor stimulation in the cat: medullary neurone correlations. J Physiol 491: 241–259, 1996. doi: 10.1113/jphysiol.1996.sp021212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morris KF, Baekey DM, Shannon R, Lindsey BG. Respiratory neural activity during long-term facilitation. Respir Physiol 121: 119–133, 2000. doi: 10.1016/S0034-5687(00)00123-7. [DOI] [PubMed] [Google Scholar]
- 175.Morris KF, Baekey DM, Nuding SC, Dick TE, Shannon R, Lindsey BG. Plasticity in respiratory motor control. Invited review: Neural network plasticity in respiration control. J Appl Physiol 94: 1242–1252, 2003. doi: 10.1152/japplphysiol.00715.2002. [DOI] [PubMed] [Google Scholar]
- 176.Morris KF, Nuding SC, Segers LS, Baekey DM, Shannon R, Lindsey BG, Dick TE. Respiratory and Mayer wave-related discharge patterns of raphé and pontine neurons change with vagotomy. J Appl Physiol (1985) 109: 189–202, 2010. doi: 10.1152/japplphysiol.01324.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Morris KF, Nuding SC, Segers LS, Iceman KE, O’Connor R, Dean JB, Ott MM, Alencar PA, Shuman D, Horton K-K, Taylor-Clark TE, Bolser DC, Lindsey BG. Carotid chemoreceptors tune breathing via multipath routing: reticular chain and loop operations supported by parallel spike train correlations. J Neurophysiol 119: 700–722, 2018. doi: 10.1152/jn.00630.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mörschel M, Dutschmann M. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos Trans R Soc Lond B Biol Sci 364: 2517–2526, 2009. doi: 10.1098/rstb.2009.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Narkiewicz K, Ratcliffe LEK, Hart EC, Briant LJB, Chrostowska M, Wolf J, Szyndler A, Hering D, Abdala AP, Manghat N, Burchell AE, Durant C, Lobo MD, Sobotka PA, Patel NK, Leiter JC, Engelman ZJ, Nightingale AK, Paton JFR. Unilateral carotid body resection in resistant hypertension: A safety and feasibility trial. JACC Basic Transl Sci 1: 313–324, 2016. doi: 10.1016/j.jacbts.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nattie E, Li A. Central chemoreceptors: locations and functions. Compr Physiol 2: 221–254, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Navarrete-Opazo A, Vinit S, Dougherty BJ, Mitchell GS. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp Neurol 266: 1–10, 2015. doi: 10.1016/j.expneurol.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med 215: 21–33, 2018. doi: 10.1084/jem.20171773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nuding SC, Segers LS, Shannon R, O’Connor R, Morris KF, Lindsey BG. Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphé-pontomedullary respiratory network. Philos Trans R Soc Lond B Biol Sci 364: 2501–2516, 2009. doi: 10.1098/rstb.2009.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nuding SC, Segers LS, Baekey DM, Dick TE, Solomon IC, Shannon R, Morris KF, Lindsey BG. Pontine-ventral respiratory column interactions through raphe circuits detected using multi-array spike train recordings. J Neurophysiol 101: 2943–2960, 2009. doi: 10.1152/jn.91305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nuding SC, Segers LS, Iceman KE, O’Connor R, Dean JB, Bolser DC, Baekey DM, Dick TE, Shannon R, Morris KF, Lindsey BG. Functional connectivity in raphé-pontomedullary circuits supports active suppression of breathing during hypocapnic apnea. J Neurophysiol 114: 2162–2186, 2015. doi: 10.1152/jn.00608.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.O’Connor R, Segers LS, Morris KF, Nuding SC, Pitts T, Bolser DC, Davenport PW, Lindsey BG. A joint computational respiratory neural network-biomechanical model for breathing and airway defensive behaviors. Front Physiol 3: 264, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Oku Y, Hülsmann S. A computational model of the respiratory network challenged and optimized by data from optogenetic manipulation of glycinergic neurons. Neuroscience 347: 111–122, 2017. doi: 10.1016/j.neuroscience.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 188.Oshima N, Kumagai H, Onimaru H, Kawai A, Pilowsky PM, Iigaya K, Takimoto C, Hayashi K, Saruta T, Itoh H. Monosynaptic excitatory connection from the rostral ventrolateral medulla to sympathetic preganglionic neurons revealed by simultaneous recordings. Hypertens Res 31: 1445–1454, 2008. doi: 10.1291/hypres.31.1445. [DOI] [PubMed] [Google Scholar]
- 189.Ott MM, Nuding SC, Segers LS, Lindsey BG, Morris KF. Ventrolateral medullary functional connectivity and the respiratory and central chemoreceptor-evoked modulation of retrotrapezoid-parafacial neurons. J Neurophysiol 105: 2960–2975, 2011. doi: 10.1152/jn.00262.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ott MM, Nuding SC, Segers LS, O’Connor R, Morris KF, Lindsey BG. Central chemoreceptor modulation of breathing via multipath tuning in medullary ventrolateral respiratory column circuits. J Neurophysiol 107: 603–617, 2012. doi: 10.1152/jn.00808.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci 31: 2895–2905, 2011. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pamenter ME, Powell FL. Time domains of the hypoxic ventilatory response and their molecular basis. Compr Physiol 6: 1345–1385, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pardal R, Ortega-Sáenz P, Durán R, López-Barneo J. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell 131: 364–377, 2007. doi: 10.1016/j.cell.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 194.Paton JFR, Deuchars J, Li YW, Kasparov S. Properties of solitary tract neurones responding to peripheral arterial chemoreceptors. Neuroscience 105: 231–248, 2001. doi: 10.1016/S0306-4522(01)00106-3. [DOI] [PubMed] [Google Scholar]
- 195.Paula-Ribeiro M, Rocha A. The peripheral-central chemoreflex interaction: where do we stand and what is the next step? J Physiol 594: 1527–1528, 2016. doi: 10.1113/JP271901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pawar A, Peng Y-J, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol (1985) 104: 1287–1294, 2008. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Peña F. Neuronal network properties underlying the generation of gasping. Clin Exp Pharmacol Physiol 36: 1218–1228, 2009. doi: 10.1111/j.1440-1681.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- 198.Peng Y-J, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Peng Y-J, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL, Prabhakar NR. Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J Physiol 592: 3841–3858, 2014. doi: 10.1113/jphysiol.2014.273789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Perim RR, Fields DP, Mitchell GS. Cross-talk inhibition between 5-HT2B and 5-HT7 receptors in phrenic motor facilitation via NADPH oxidase and PKA. Am J Physiol Regul Integr Comp Physiol 314: R709–R715, 2018. doi: 10.1152/ajpregu.00393.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Phillips DB, Steinback CD, Collins SÉ, Fuhr DP, Bryan TL, Wong EY, Tedjasaputra V, Bhutani M, Stickland MK. The carotid chemoreceptor contributes to the elevated arterial stiffness and vasoconstrictor outflow in COPD. J Physiol. In press. doi: 10.1113/JP275762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Poliaček I, Morris KF, Lindsey BG, Segers LS, Rose MJ, Corrie LW-C, Wang C, Pitts TE, Davenport PW, Bolser DC. Blood pressure changes alter tracheobronchial cough: computational model of the respiratory-cough network and in vivo experiments in anesthetized cats. J Appl Physiol (1985) 111: 861–873, 2011. doi: 10.1152/japplphysiol.00458.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Poon C-S, Song G. Bidirectional plasticity of pontine pneumotaxic postinspiratory drive: implication for a pontomedullary respiratory central pattern generator. Prog Brain Res 209: 235–254, 2014. doi: 10.1016/B978-0-444-63274-6.00012-6. [DOI] [PubMed] [Google Scholar]
- 204.Song G, Tin C, Poon CS. Bilateral lesions of pontine Kölliker-Fuse nuclei provoke apnea instead of apneusis in anesthetized adult rats. Adv Exp Med Biol 669: 185–188, 2010. doi: 10.1007/978-1-4419-5692-7_37. [DOI] [PubMed] [Google Scholar]
- 205.Powell FL, Fu Z. HIF-1 and ventilatory acclimatization to chronic hypoxia. Respir Physiol Neurobiol 164: 282–287, 2008. doi: 10.1016/j.resp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. doi: 10.1016/S0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 207.Prabhakar NR. Carotid body chemoreflex: a driver of autonomic abnormalities in sleep apnoea. Exp Physiol 101: 975–985, 2016. doi: 10.1113/EP085624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Prabhakar NR, Semenza GL. Oxygen sensing and homeostasis. Physiology (Bethesda) 30: 340–348, 2015. doi: 10.1152/physiol.00022.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Prabhakar NR, Peng Y-J, Kumar GK, Nanduri J. Peripheral chemoreception and arterial pressure responses to intermittent hypoxia. Compr Physiol 5: 561–577, 2015. doi: 10.1002/cphy.c140039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rajani V, Zhang Y, Jalubula V, Rancic V, SheikhBahaei S, Zwicker JD, Pagliardini S, Dickson CT, Ballanyi K, Kasparov S, Gourine AV, Funk GD. Release of ATP by pre-Bötzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca2+-dependent P2Y1 receptor mechanism. J Physiol. In press. doi: 10.1113/jp274727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rakoczy RJ, Wyatt CN. Acute oxygen sensing by the carotid body: a rattlebag of molecular mechanisms. J Physiol. In press. doi: 10.1113/JP274351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ramanantsoa N, Hirsch M-R, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault P-L, Matrot B, Fortin G, Brunet J-F, Gallego J, Goridis C. Breathing without CO(2) chemosensitivity in conditional Phox2b mutants. J Neurosci 31: 12880–12888, 2011. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ramirez J-M, Garcia AJ III, Anderson TM, Koschnitzky JE, Peng Y-J, Kumar GK, Prabhakar NR. Central and peripheral factors contributing to obstructive sleep apneas. Respir Physiol Neurobiol 189: 344–353, 2013. doi: 10.1016/j.resp.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rice CD, Lois JH, Kerman IA, Yates BJ. Localization of serotoninergic neurons that participate in regulating diaphragm activity in the cat. Brain Res 1279: 71–81, 2009. doi: 10.1016/j.brainres.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology (Bethesda) 29: 58–71, 2014. doi: 10.1152/physiol.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Richter DW, Bischoff A, Anders K, Bellingham M, Windhorst U. Response of the medullary respiratory network of the cat to hypoxia. J Physiol 443: 231–256, 1991. doi: 10.1113/jphysiol.1991.sp018832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol 514: 567–578, 1999. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Road JD, Ford TW, Kirkwood PA. Connections between expiratory bulbospinal neurons and expiratory motoneurons in thoracic and upper lumbar segments of the spinal cord. J Neurophysiol 109: 1837–1851, 2013. doi: 10.1152/jn.01008.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Robbins PA. Role of the peripheral chemoreflex in the early stages of ventilatory acclimatization to altitude. Respir Physiol Neurobiol 158: 237–242, 2007. doi: 10.1016/j.resp.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 221.Robotham JL, Lixfeld W, Holland L, MacGregor D, Bryan AC, Rabson J. Effects of respiration on cardiac performance. J Appl Physiol Respir Environ Exerc Physiol 44: 703–709, 1978. [DOI] [PubMed] [Google Scholar]
- 222.Ronen R, Zhou D, Bafna V, Haddad GG. The genetic basis of chronic mountain sickness. Physiology (Bethesda) 29: 403–412, 2014. doi: 10.1152/physiol.00008.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499: 64–89, 2006. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- 224.Roy A, Farnham MMJ, Derakhshan F, Pilowsky PM, Wilson RJA. Acute intermittent hypoxia with concurrent hypercapnia evokes P2X and TRPV1 receptor-dependent sensory long-term facilitation in naïve carotid bodies. J Physiol. In press. doi: 10.1113/JP275001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rybak IA, Molkov YI, Jasinski PE, Shevtsova NA, Smith JC. Rhythmic bursting in the pre-Bötzinger complex: mechanisms and models. Prog Brain Res 209: 1–23, 2014. doi: 10.1016/B978-0-444-63274-6.00001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rybak IA, O’Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol 100: 1770–1799, 2008. doi: 10.1152/jn.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Safic IS, Pecotic R, Dodig IP, Dogas Z, Valic Z, Valic M. Phrenic long term depression evoked by intermittent hypercapnia is modulated by serotonergic and adrenergic receptors in raphe nuclei. J Neurophysiol. In press. doi: 10.1152/jn.00776.2017. [DOI] [PubMed] [Google Scholar]
- 228.Sarton E, Teppema L, Dahan A. Sex differences in morphine-induced ventilatory depression reside within the peripheral chemoreflex loop. Anesthesiology 90: 1329–1338, 1999. doi: 10.1097/00000542-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 229.Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol 73: 1452–1461, 1995. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- 230.Segers LS, Shannon R, Lindsey BG. Interactions between rostral pontine and ventral medullary respiratory neurons. J Neurophysiol 54: 318–334, 1985. doi: 10.1152/jn.1985.54.2.318. [DOI] [PubMed] [Google Scholar]
- 231.Segers LS, Shannon R, Saporta S, Lindsey BG. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. I. Evidence for excitatory and inhibitory actions of inspiratory neurons. J Neurophysiol 57: 1078–1100, 1987. doi: 10.1152/jn.1987.57.4.1078. [DOI] [PubMed] [Google Scholar]
- 232.Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF, Lindsey BG. Functional connectivity in the pontomedullary respiratory network. J Neurophysiol 100: 1749–1769, 2008. doi: 10.1152/jn.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Segers LS, Nuding SC, Ott MM, Dean JB, Bolser DC, O’Connor R, Morris KF, Lindsey BG. Peripheral chemoreceptors tune inspiratory drive via tonic expiratory neuron hubs in the medullary ventral respiratory column network. J Neurophysiol 113: 352–368, 2015. doi: 10.1152/jn.00542.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Segers LS, Nuding SC, Vovk A, Pitts T, Baekey DM, O’Connor R, Morris KF, Lindsey BG, Shannon R, Bolser DC. Discharge identity of medullary inspiratory neurons is altered during repetitive fictive cough. Front Physiol 3: 223, 2012. doi: 10.3389/fphys.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Semenza GL, Prabhakar NR. The role of hypoxia-inducible factors in carotid body (patho) physiology. J Physiol. In press. doi: 10.1113/JP275696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sheikhbahaei S, Gourine AV, Smith JC. Respiratory rhythm irregularity after carotid body denervation in rats. Respir Physiol Neurobiol 246: 92–97, 2017. doi: 10.1016/j.resp.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sherman D, Worrell JW, Cui Y, Feldman JL. Optogenetic perturbation of preBötzinger complex inhibitory neurons modulates respiratory pattern. Nat Neurosci 18: 408–414, 2015. doi: 10.1038/nn.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Silva JN, Lucena EV, Silva TM, Damasceno RS, Takakura AC, Moreira TS. Inhibition of the pontine Kölliker-Fuse nucleus reduces genioglossal activity elicited by stimulation of the retrotrapezoid chemoreceptor neurons. Neuroscience 328: 9–21, 2016. doi: 10.1016/j.neuroscience.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 240.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Smith PG, Mills E. Restoration of reflex ventilatory response to hypoxia after removal of carotid bodies in the cat. Neuroscience 5: 573–580, 1980. doi: 10.1016/0306-4522(80)90054-8. [DOI] [PubMed] [Google Scholar]
- 242.Song G, Poon C-S. Lateral parabrachial nucleus mediates shortening of expiration and increase of inspiratory drive during hypercapnia. Respir Physiol Neurobiol 165: 9–12, 2009. doi: 10.1016/j.resp.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Song G, Poon C-S. α2-Adrenergic blockade rescues hypoglossal motor defense against obstructive sleep apnea. JCI Insight 2: e91456, 2017. doi: 10.1172/jci.insight.91456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Song G, Xu H, Wang H, Macdonald SM, Poon CS. Hypoxia-excited neurons in NTS send axonal projections to Kölliker-Fuse/parabrachial complex in dorsolateral pons. Neuroscience 175: 145–153, 2011. doi: 10.1016/j.neuroscience.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.St-John WM, Li A, Leiter JC. Genesis of gasping is independent of levels of serotonin in the Pet-1 knockout mouse. J Appl Physiol (1985) 107: 679–685, 2009. doi: 10.1152/japplphysiol.91461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.St John WM, Bianchi AL. Responses of bulbospinal and laryngeal respiratory neurons to hypercapnia and hypoxia. J Appl Physiol (1985) 59: 1201–1207, 1985. doi: 10.1152/jappl.1985.59.4.1201. [DOI] [PubMed] [Google Scholar]
- 247.Streeter KA, Sunshine MD, Patel S, Gonzalez-Rothi EJ, Reier PJ, Baekey DM, Fuller DD. Intermittent hypoxia enhances functional connectivity of midcervical spinal interneurons. J Neurosci 37: 8349–8362, 2017. doi: 10.1523/JNEUROSCI.0992-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tadjalli A, Duffin J, Peever J. Identification of a novel form of noradrenergic-dependent respiratory motor plasticity triggered by vagal feedback. J Neurosci 30: 16886–16895, 2010. doi: 10.1523/JNEUROSCI.3394-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 90: 675–754, 2010. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- 250.Tort ABL, Brankačk J, Draguhn A. Respiration-entrained brain rhythms are global but often overlooked. Trends Neurosci 41: 186–197, 2018. doi: 10.1016/j.tins.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 251.Tryba AK, Peña F, Ramirez J-M. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci 26: 2623–2634, 2006. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Turner S, Streeter KA, Greer J, Mitchell GS, Fuller DD. Pharmacological modulation of hypoxia-induced respiratory neuroplasticity. Respir Physiol Neurobiol S1569-9048(17)30279-3. In press. doi: 10.1016/j.resp.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Turovsky E, Theparambil SM, Kasymov V, Deitmer JW, Del Arroyo AG, Ackland GL, Corneveaux JJ, Allen AN, Huentelman MJ, Kasparov S, Marina N, Gourine AV. Mechanisms of CO2/H+ sensitivity of astrocytes. J Neurosci 36: 10750–10758, 2016. doi: 10.1523/JNEUROSCI.1281-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Tzeng YC, Larsen PD, Galletly DC. Effects of hypercapnia and hypoxemia on respiratory sinus arrhythmia in conscious humans during spontaneous respiration. Am J Physiol Heart Circ Physiol 292: H2397–H2407, 2007. doi: 10.1152/ajpheart.00817.2006. [DOI] [PubMed] [Google Scholar]
- 255.West JB. Causes of and compensations for hypoxemia and hypercapnia. Compr Physiol 1: 1541–1553, 2011. [DOI] [PubMed] [Google Scholar]
- 256.White DP. Long-term facilitation (LTF) and obstructive sleep apnea. Respir Physiol Neurobiol 158: 112–113, 2007. doi: 10.1016/j.resp.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wilkerson JER, Devinney M, Mitchell GS. Intermittent but not sustained moderate hypoxia elicits long-term facilitation of hypoglossal motor output. Respir Physiol Neurobiol S1569-9048(17)30221-5. In press. doi: 10.1016/j.resp.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Willeput R, Rondeux C, De Troyer A. Breathing affects venous return from legs in humans. J Appl Physiol Respir Environ Exerc Physiol 57: 971–976, 1984. [DOI] [PubMed] [Google Scholar]
- 259.Wilson RJA, Teppema LJ. Integration of central and peripheral respiratory chemoreflexes. Compr Physiol 6: 1005–1041, 2016. doi: 10.1002/cphy.c140040. [DOI] [PubMed] [Google Scholar]
- 260.Xing T, Pilowsky PM, Fong AY. Mechanism of sympathetic activation and blood pressure elevation in humans and animals following acute intermittent hypoxia. Prog Brain Res 209: 131–146, 2014. doi: 10.1016/B978-0-444-63274-6.00007-2. [DOI] [PubMed] [Google Scholar]
- 261.Yamamoto K, Lalley P, Mifflin S. Acute intermittent optogenetic stimulation of nucleus tractus solitarius neurons induces sympathetic long-term facilitation. Am J Physiol Regul Integr Comp Physiol 308: R266–R275, 2015. doi: 10.1152/ajpregu.00381.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yasuma F, Hayano JI. Impact of acute hypoxia on heart rate and blood pressure variability in conscious dogs. Am J Physiol Heart Circ Physiol 279: H2344–H2349, 2000. doi: 10.1152/ajpheart.2000.279.5.H2344. [DOI] [PubMed] [Google Scholar]
- 263.Yasuma F, Hirai M, Hayano JI. Differential effects of hypoxia and hypercapnia on respiratory sinus arrhythmia in conscious dogs. Jpn Circ J 65: 738–742, 2001. doi: 10.1253/jcj.65.738. [DOI] [PubMed] [Google Scholar]
- 264.Zoccal DB, Machado BH. Sympathetic overactivity coupled with active expiration in rats submitted to chronic intermittent hypoxia. Respir Physiol Neurobiol 174: 98–101, 2010. doi: 10.1016/j.resp.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 265.Zoccal DB, Furuya WI, Bassi M, Colombari DSA, Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front Physiol 5: 238, 2014. doi: 10.3389/fphys.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zuperku EJ, Stucke AG, Hopp FA, Stuth EAE. Characteristics of breathing rate control mediated by a subregion within the pontine parabrachial complex. J Neurophysiol 117: 1030–1042, 2017. doi: 10.1152/jn.00591.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]