Abstract
The intestinal epithelial barrier is the largest exchange surface between the body and the external environment. Its functions are regulated by luminal, and also internal, components including the enteric nervous system. This review summarizes current knowledge about the role of the digestive “neuronal-glial-epithelial unit” on epithelial barrier function.
Introduction
To ensure its function in food digestion and nutrient absorption, the intestine has the difficult task to selectively import millions of molecules from the outside to the inside of the body, and to avoid, at the same time, invasion from pathogens or immune-reactive motives. From the lumen to the inner tissues, this semi-permeable barrier function has four major components: 1) the microbial biofilms, 2) the mucus layer, 3) the epithelial monolayer, and 4) the immune system (FIGURE 1). This review will focus on one of those components—the intestinal epithelial layer—and will discuss how this component of mucosal barrier is regulated by two cellular actors of the enteric nervous system: the neurons and the glia.
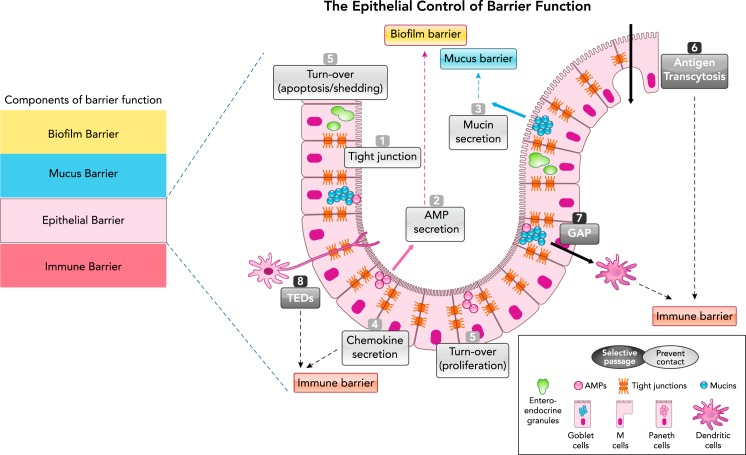
FIGURE 1.
Schematic representation of the epithelial barrier in the GI tract
Components of the intestinal barrier (left) and specifically the epithelial control of barrier function (right). The epithelial monolayer is composed of different cell types (represented here are Goblet cells, Paneth cells, and M cells), which exert barrier function either by preventing physical contact or by allowing selective passage of molecules. The physical barrier organized by the epithelium is composed of the tight-junction molecules (1), the secretion of antimicrobial peptides (AMPs; 2), the secretion of mucins (3), the secretion of chemokines that alert the immune barrier (4), cell turnover (equilibrium between shedding and proliferation; 5). Selective passage of antigens is ensured through M-cell transcytosis (6), Goblet cell antigen passages (GAP; 7), and transepithelial dendrite (TEDs) (8) pathways.
Studying the control of epithelial barrier function has been revealed to be of particular interest in pathological contexts such as inflammatory bowel diseases, irritable bowel syndrome, infection, food intolerance, or celiac disease (35). In all of these conditions, epithelial barrier function is affected, and enteric neurons and glia have been shown to play their part in such pathologies. Our understanding of the control of epithelial barrier function by neurons and glia would provide strategies to restore mucosal homeostasis.
Epithelial Barrier Control
The intestinal epithelium is composed of a monolayer of epithelial cells organized by a succession of finger-like protrusions called villi and invaginations called crypts. Although the villi in the small intestine provide an efficient surface for nutrient absorption, crypts create a protected niche for stem cells to constantly replace the epithelium all along the intestinal tract (47). The epithelium is composed of different specialized cell types: undifferentiated stem cells and progenitors, differentiated goblet cells, neuroendocrine cells, Paneth cells, Tuft cells, and enterocytes. In addition to all these epithelial subtypes, microfold (M) cells are specialized epithelial cells of the follicle-associated epithelium. In the intestine, all these cell types are organized in monolayers, forming both a physical and a chemical barrier (46).
Intestinal epithelial cell layers exert their barrier function through two major mechanisms, which could be considered somehow opposite, but which are indeed complementary. First, the epithelium prevents physical contacts between luminal content and the inner of the body, and in particular with immune cells. This physical barrier includes several components, which will be discussed below (FIGURE 1). Second, the epithelium allows the passage of some molecules in order for them to be presented to the immune system, which then decides to organize an immune barrier fighting off foreign antigens and microbial threats or to tolerate some of them. For both the physical barrier and the “sampling” exerted by intestinal epithelium, these mechanisms are remarkably controlled.
The physical barrier organized by the intestinal epithelium is achieved by a sophisticated organization, establishing a tightly regulated fence (FIGURE 1). First, the physical barrier is constituted by monolayered columnar cells connected with each other by intercellular junctions. These junctions include apical tight junction (TJ) molecules, subjacent adherens junctions, and desmosomes. The TJ form pores between epithelial cells, thereby controlling paracellular passage of molecules. The expression, organization, and function of junction molecules are regulated spatiotemporally and are altered by inflammatory insults. TJ proteins are issued from four different families of transmembrane proteins, including occludin, claudins, tricellulin, and the junctional adhesion molecules (JAM). The intracellular tail of TJ connects with cytosolic scaffold proteins such as the zonula occludens (ZO) protein family. A close interaction of all these proteins with a peri-junctional ring of myosin light chain (MLC) exists, and phosphorylation of this ring by MLC-kinase (MLCK) induces a contraction of the actin-myosin cytoskeleton that leads to TJ opening and paracellular leakage across epithelial monolayer. Transcellular passage across the epithelial cell is also tightly controlled, interplaying with paracellular transports (51). Second, specialized epithelial cells secrete factors that take an active part in barrier function. For instance, Paneth cells, and also Goblet cells, secrete antimicrobial molecules, which actively participate to form an anti-microbial barrier. Goblet cells are professional producers of mucins, which are secreted at the luminal surface, where they form a thick mucus layer preventing direct contact between luminal microbiota and epithelial cells.
The controlled passage of some antigens and their delivery to the immune system is ensured by different cell types present in the intestinal epithelial monolayer (FIGURE 1). M cells are differentiated epithelial cells present in the follicle-associated epithelium, and sometimes in the villous epithelium (54). Antigens introduced by M cells progress by transcytosis. M cells use pinocytosis, macropinocytosis, and receptor-mediated endocytosis to allow the passage of luminal antigens (54, 72). The type of antigens that are crossing through M cells ranges from small soluble particles to whole bacteria. M cells then deliver those antigens to dendritic cells and further to underneath lymphoid tissues, thereby supporting the production of immunoglobulin A. In vivo two-photon imaging has demonstrated that small antigenic proteins can enter Goblet cells and are transported to be presented to underneath dendritic cells of the lamina propria (72). This phenomenon, called Goblet-cell-associated antigen passages (GAPs), seems to be restricted to small molecules (not larger than 70 kDa) and serves peripheral tolerance. Finally, dendritic cells penetrate the epithelium with their dendrites to sample the molecules present in the lumen: this is recognized as the trans-epithelial dendrite (TED) pathway (87). By forming TEDs, dendritic cells are able to grab pathogens and contain them within the superficial mucosa, quickly initiating an inflammatory response that avoids barrier breaching. Through these different transport pathways present in the epithelium, luminal antigens are provided to dendritic cells and overall to the immune system, which then organizes appropriate handling of these motifs (tolerance or elimination), therefore participating in intestinal barrier homeostasis (61).
Epithelial barrier integrity is obviously challenged by inflammatory insults or infection, but it is also challenged by the high cell turnover rate and the cellular composition of the epithelium. Equilibrium between apoptosis and shedding of senescent epithelial cells and the generation of new epithelial cells at the basis of the crypt is fundamental to maintaining epithelial barrier integrity and homeostasis (FIGURE 1).
This review aims to summarize current knowledge about the control by neurons and glial cells of epithelial barrier integrity in all the aspects described above: the intercellular junctions, epithelial secretion of mucus and antimicrobial peptides, transport and presentation of antigens by epithelial components, and epithelial cell turnover (FIGURE 1). We will review the significance of neuronal and glial cell input in pathologies, and the future directions this field of research might take.
Neurons, Glia and Their Impact on Epithelial Barrier Function
The Enteric Nervous System
The gastrointestinal tract is innervated by a complex network of ganglia, named the enteric nervous system (ENS) (41) and constituted by neurons and glial cells bundled together, forming two main plexi, the submucosal (or Meissner’s) and the myenteric (or Auerbach’s) (41, 63). To accomplish the ENS task of controlling motor, sensory, absorptive, and secretory functions, neurons and glial cells tightly communicate with each other and with the other cells present in the gastrointestinal wall through paracrine and autocrine ways (64, 105, 113) (6, 9, 45, 50, 110). In the ENS, glial cells outnumber neurons, like astrocytes in the central nervous system, and the ratio of neurons to glia in the submucosal and myenteric plexus varies from 1:4 to 1:10, with an average of 1:7 (33, 52, 91). In this review, we will focus on the submucosal plexus, since it lays close to epithelial cells and directly receives and sends messages from and to the epithelium.
Enteric Neurons: The Master Regulators of Epithelial Barrier Function
Neuro-epithelial anatomy.
The ENS regulates various mucosal functions independently of central inputs. In this context, neurons in the submucosal plexus, rather than myenteric ones, are key controllers of the epithelium. In humans, the submucosal plexus is organized in three layers starting from the mucosa: the Meissner’s, the intermediary, and the Henle layers. These layers are not identifiable in small laboratory animals (98). The submucosal plexus comprises intrinsic primary afferent and effector neurons, which control mucosal functions, blood flow, and immune cell migration (4, 42). There are two key types of secretomotor neurons in the submucosal plexus: cholinergic neurons, immunoreactive for neuropeptide Y (NPY), and non-cholinergic neurons, immunoreactive for vasoactive intestinal peptide (VIP) (24, 58, 59). The neurochemical coding in the submucosal plexus allowed also the tracing of neurons projecting to the epithelium. The first functional demonstration of this innervation came by tracing choline acetyl transferase (ChAT) and VIP neuronal pathways in the guinea pig proximal colon (76). Descending neurons are VIP-positive and they predominantly innervate the mucosa, whereas ascending neurons are primarily ChA-positive. Both circuits are involved in neurally mediated ion secretion, through the release of acetylcholine (ascending) and VIP (descending), two potent secretagogues acting individually or sinergystically, potentiating secretion in epithelial cells. Actually, VIP signaling within the gut is more complex than simply linked to ChAT-positive neurons. It indeed implies the activation of alpha2-adrenoreceptors (81), and serotonergic and somatostatin receptors (39), whose expression and function on VIP-positive neurons has been demonstrated by pharmacological assays and biochemical techniques.
The strong innervation of the mucosa and the close anatomical relationship between enteric neurons and epithelial cells suggest active interplays of this neuro-epithelial unit. A large part of the neuro-epithelial interactions thus has consequences on epithelial barrier integrity (see Table 1 for the effects of neuromediators on barrier function). This has been particularly studied in the context of inflammation or infection-related epithelial distress (FIGURE 2).
Table 1.
List of neuronal mediators affecting epithelial barrier function
| Neuronal Mediator | Effect on Barrier Function | Mechanism | References |
|---|---|---|---|
| VIP | Preserve mucosal integrity | α2-Adrenoreceptors, 5-HT receptor, IL-1β, somatostatin receptors, TJ proteins, ERK1/2, p38MAP kinase, EPSP | 30, 34, 39, 74, 76, 78, 81, 99, 100 |
| Potentiate secretion | |||
| Decrease intestinal permeability | |||
| IL-8 production | |||
| Promote epithelial cell proliferation | |||
| ACh | Potentiate secretion | CRF-R2 | 43, 76, 83 |
| Increase epithelial permeability | |||
| ? | Promote epithelial cell proliferation | ? | 68 |
| NPY | Increase epithelial permeability | PI3K, TJ proteins | 15, 16 |
| ? | Increase epithelial permeability | Slow synaptic transmission | 53 |
VIP, vasoactive intestinal peptide; Ach, acetylcholine, NPY, neuropeptide Y; 5-HT, serotonin; TJ, tight junction; EPSP, excitatory postsynaptic potentials; CRF-R2, corticotrophin-releasing factor receptor 2; PI3K, phosphatidylinositol-3-kinase; ?, unknown.
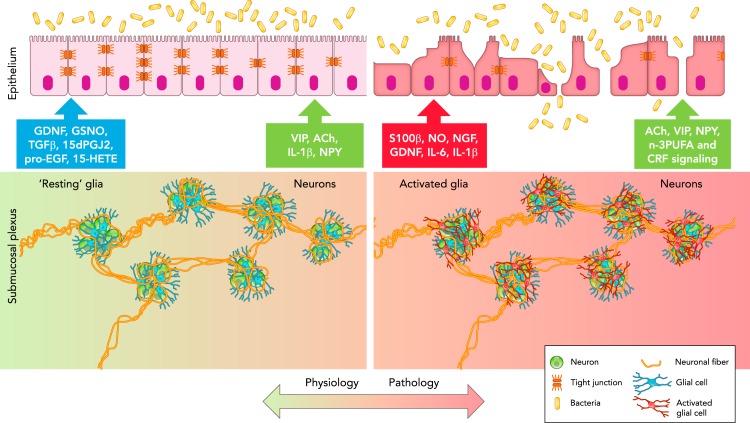
FIGURE 2.
Schematic representation of the neuronal-glial-epithelial unit in the GI tract
In physiological conditions (left), glial cells (blue) surround neurons (green) to form ganglia, which are interconnected through neuronal fibers (orange) to form the submucosal plexus. “Resting” glia communicate with epithelial cells via the release of GDNF, GSNO, TGFβ1, 15dPGJ2, pro-EGF, and 15-HETE to preserve barrier function, whereas submucosal neurons release VIP, ACh, IL-1β, and NPY. In pathological conditions (right), glial cells become activated (red) and release S100β, NO, NGF, GDNF, IL-6, and IL-1β, which alter epithelial barrier function, whereas submucosal neurons (green) release ACh, VIP, NPY, and n-3PUFA, and respond to CRF signaling, which affects epithelial barrier function.
In vivo evidences in infectious or inflammatory context.
The fine interplay between neurons and epithelial cells has been highlighted in animal models of gut infection, which have helped also in understanding what happens to the neuro-(immune-)epithelial unit in post-infectious gut disorders. Trichinella spiralis infection has been a good model (36) to study this unit. Studies have shown that the low-grade inflammation established after infection, causes a remodeling of the neuro-epithelial compartment, with secondary and long-lasting effects on barrier function. The ENS regulates fluid and electrolyte secretion during infection-evoked diarrhea in mice, and the involvement of a neuronal component was demonstrated using Tetrodotoxin (TTX), lidocaine (an L-type calcium channel blocker), or hexamethonium (an inhibitor of synaptic transmission that blocks nicotinic receptor) (69). Upregulation of NPY has been described in murine models of colitis: knockout mice for neuropeptide Y (NPY) developed less severe colitis and had improved barrier function (15, 16). Lomax et al. described submucosal plexus abnormalities during colitis in guinea pigs (67). Interestingly, Hons et al. reported that, in a model of colitis, slow synaptic transmission was altered in the ileum distant from the site of inflammation. This had consequences on neuron-mediated secretions in the ileum and, therefore, potentially on maintaining epithelial barrier function. However, the density of VIP-positive neurons was unchanged in the ileum (53).
Effects on tight junctions.
Direct evidence of the influence of VIPergic pathway on barrier function was obtained in coculture methods of epithelial cell lines exposed to human VIP-positive submucosal neurons, which were electrically stimulated (78). In that study, activation of submucosal neurons resulted in a reduction of macromolecule fluxes across epithelial monolayers, associated with a transcriptional downregulation of zonula occludens-1 (ZO-1) mRNA. The effects of submucosal neurons on epithelial monolayers were blocked by a VIP receptor antagonist, pointing to a direct role of VIP on ZO-1 tight-junction expression.
In vitro, exogenous application of NPY to cultured intestinal epithelial cells increased epithelial permeability through phosphatidyl-inositol-3-kinase-induced claudin-2 upregulation. Indeed, claudin-2 is a tight-junction protein known for inducing increased permeability when upregulated (15, 16).
Effects on epithelial cell secretions.
VIP is also known for its effect on other components of the barrier function. It induces the expression of the Trefoil factor-3 (TFF3), a peptide involved in the stabilization of the intestinal mucus layer (74). It stimulates mucus output and induces fluid movements (electrolyte transports) in vivo in perfused rat colon (34). It induces the production and release of interleukin-8, a chemokine produced by intestinal epithelial cells and responsible for the attraction of immune cells (100). This study extended the role of VIP from a key regulator of mucosal functions to the regulation of immune cell response, thereby contributing to the immune surveillance of intestinal epithelial barrier (100).
Effects on epithelial cell turnover.
Other studies have demonstrated that submucosal neuron activation regulated epithelial cell proliferation in a VIP-dependent manner, pointing to a key role for submucosal neurons in epithelial cell renewal and thereby in the efficacy of barrier function (99). This idea is supported by other studies that have shown that neurons and neuromodulators are essential for the renewal and migration of epithelial cells (68). Stimulation with capsaicin of afferent nerves innervating the mouse gut mucosa showed that cell renewal in the epithelium was boosted. Interestingly, the cell renewal was exclusively localized in the crypts. This finding confirmed very early studies targeting the myenteric plexus (60, 93) that pointed out the nervous control of intestinal stem cells. Although VIP appears as one of the mediators of neuronal effects on stem cells, other neuromediators might also exert their influence on intestinal stem cell biology and would have to be uncovered.
Enteric neurons, epithelium and stress.
In 2006, Gareau et al. (43) wondered whether stress-induced mucosal barrier dysfunction could be a consequence of altered neuronal signaling. They used the neonatal maternal separation mouse model to test their hypothesis and found that cholinergic input from submucosal neurons in the distal colon (ex vivo) was abnormal in pups separated from the dam compared with control pups. In an earlier study, the same authors showed that corticotropin-releasing factor (CRF), a key stress hormone, caused alteration in barrier function (43). Based on this finding, Gareau et al. demonstrated that, in their model of barrier dysfunction, CRF secretion and activation of its receptor 2 (CRF-R2) on submucosal neurons caused the release of acetylcholine (ACh) and the subsequent hypercholinergic state, stimulated enterochromaffin cells in the epithelium to increase permeability. Together with the release of ACh, CRF, once secreted, also induced the release of other mediators by surrounding cells (in particular mast cells), and this also had an impact on barrier function (83).
Enteric neurons, epithelium and diet.
Dietary lipids such as n-3 polyunsaturated fatty acids (n-3PUFA) are known to impact gut functions (30). N-3PUFA maternal diet increased intestinal paracellular permeability in newborn piglet tissues. This effect was inhibited by atropine or hexamethonium. In parallel, neuroplastic changes were observed with increased number of ChAT-immunoreactive neurons and a decreased number of VIP-positive neurons, suggesting an increment of the cholinergic vs. the VIPergic tone in the ENS. Surprisingly, the expression of tight-junction proteins (occludin, zonulin, or claudin) remained unchanged, leaving opened the question of the mechanism by which VIP might modify barrier function (30).
Future studies.
Direct and live imaging of human submucosal neurons is now commonly used in many laboratories and allows characterization of a number of ENS signals, particularly from the submucosal plexus (109). Similarly, culture of complex human epithelium, through stem cell or crypt isolation and further organoid cultures, now allows us to study epithelial monolayers in their full diversity and in three dimensions (89, 109). Cocultures of human submucosal neurons and intestinal organoids would constitute a model of choice to study neuro-epithelial interactions in general and the impact of neurons on epithelial barrier function. Indeed, only epithelial cell lines have been used so far in vitro for coculture studies, and the real effects of submucosal neurons on a complex and diverse epithelial monolayer still have to be studied. These new models could pave the way to a number of studies investigating the role of submucosal neurons on all aspects of epithelial barrier functions: not only intercellular junctions and epithelial secretion of mucus and antimicrobial peptides, but also transport and presentation of antigens by epithelial components, as well as epithelial cell turnover (FIGURE 1). Such studies could potentially discriminate the type of epithelial cells on which neuronal mediators exert the strongest control.
Enteric Glia: Friend or Foe of Epithelial Cells?
Glial cell-neuron interactions for the control of epithelium.
Glial cells in the ENS are small cells with a “star-like” appearance, like the brain counterpart astrocytes, and are called enteric glial cells (EGC) (37, 55, 56). EGC contain intracellular arrays of 10-nm filaments made up of glial fibrillary acidic protein (GFAP), which, together with S100 protein and Sox10, have been commonly used as specific glial markers (5, 52). Very recently, the proteolipid protein (Plp) 1, a marker expressed by myelinated cells, has been detected in virtually all EGC (85). In the submucosal plexus, EGC surround neuronal bodies and axons as well as blood microvessels. EGC also extend long processes into the mucosa (8, 57, 64). EGC release factors that are crucial for the development, survival, and differentiation of neurons (1, 2, 29). Through their role of support to neuronal networks, it is logical to think that EGC can indirectly affect neuron-controlled epithelial barrier function. The share of such indirect control is difficult to apprehend though. First, it is difficult to apprehend because in vivo studies do not allow clear discrimination between neuronal and glial output. Second, an increasing number of studies point to EGC in isolation as key regulators of intestinal homeostasis (23, 49, 77, 79, 94). EGC regulate enteric neuron functions through the release of a variety of soluble neuro-active factors. EGC also display dynamic responses to neuronal inputs (6, 40, 45). Adenosine tri phosphate (ATP) appears to be the main actor in neuro-glia communication; this has been observed in animal and human gut preparations (6, 11, 45, 50, 71, 88, 104). Recently, Fung et al. found that VIP signaling plays an unexpected role in the submucosal neuron-to-glia communication, regulating purine release in EGC (40). Although no link has been made between this neuron-to-glia communication and a potential effect on barrier function, this might constitute grounds for future studies.
Growth factors and the control of epithelial functions.
Assessment of intestinal epithelial cell proliferation after ablation of EGC demonstrated a significant increase in crypt hyperplasia, suggesting that EGC could directly, or indirectly, control epithelial cell proliferation (75). Coculture experiments showed that the presence of EGC induced a significant increase in epithelial cell surface area, without signs of cell atrophy and without modifying cell viability. These effects on cell density and surface area were mediated by a mechanism involving the release of transforming growth factor-beta-1 (TGF-β1) by EGC (75). Functional alterations in ECG might thereby modify intestinal barrier properties by altering intestinal epithelial cell proliferation and self-renewal.
If neuro-glia communication appears necessary to ensure ENS function in the gut, the interactions of EGC with epithelial cells and overall the role of EGC in mucosal integrity have proven to be equally essential (see Table 2 for ECG mediators signaling to epithelial cells). In transgenic mice ablated for glial cells by targeting GFAP, fulminant and fatal jejuno-ileitis was observed (13, 26). Since then, several studies have demonstrated that EGC regulate the expression of genes responsible for adhesion, differentiation, and proliferation of epithelial cells (12, 80, 95, 103). Among the different EGC-related mediators, glial-derived neurotrophic factor (GDNF) plays a fundamental role in the preservation of mucosal integrity. GDNF exerts anti-inflammatory effects via a dual mechanism: on the one hand, it inhibits EGC apoptosis in an autocrine manner; on the other hand, via a paracrine mechanism, it lowers the level of pro-inflammatory cytokines released during inflammation (96, 106). Interestingly, GDNF appears to favor the reconstitution and maturation of epithelial barrier even during mild inflammation, as demonstrated in biopsies from patients with functional dyspepsia (97). This finding correlates well with the observation that EGC undergo structural changes, as revealed by the overexpression of the protein S100B, in duodenal biopsies of patients with functional dyspepsia (19, 111). In vivo models of ischemia-reperfusion (IR) showed that glial network appears “distorted” after IR stimulation and that GDNF expression was increased, likely as a defense mechanism. This was confirmed in vitro in hypoxia reoxygenation conditions: GDNF protected from decreased ZO-1 and occludin expression and inhibited overall epithelial barrier defects (111). From a mechanistic point of view, both autocrine and paracrine effects of GDNF are dependent on cAMP/PKA and p38MAPK (73). GDNF seems to contribute to wound healing in a cAMP-/PKA-dependent manner and to promote barrier maturation by inactivation of p38MAPK signaling (73). Several studies suggest that EGC-derived GDNF enhanced tight-junction organization in intestinal epithelial cells (3, 73, 111). Morphine-stimulated EGC, however, lost their barrier protective effect, associating decreased GDNF mRNA and protein expression (3). It is important, however, to consider that EGC are not the sole source of GDNF; it is also expressed and released by eosinophils (10), neurons (108, 114), and epithelial cells themselves (73).
Table 2.
List of glial mediators affecting epithelial barrier function
| EGC Mediator | Effect on Barrier Function | Mechanism | References |
|---|---|---|---|
| GDNF | Preserve mucosal integrity | Activation of cAMP/PKA, p38 MAPK, TJ proteins | 73, 96, 97, 106, 111 |
| Anti-inflammatory effect | |||
| ProEGF | Induce barrier repair | Activation of FAK | 102 |
| GSNO | Reduce barrier lesions | Inhibition of NF-κB, TJ proteins | 17, 18, 27, 28, 38, 65, 90 |
| Preserve mucosal integrity | |||
| Anti-inflammatory effect | |||
| S100B | Pro-inflammatory effect | Secretion of NO, IL6, IL1β | 14, 20, 21, 22, 31, 32, 70, 101, 106, 107, 112, |
| Reduction of transepithelial resistance | |||
| Increase in permeability | |||
| 15-HETE | Preserve mucosal integrity | ? | 25, 84 |
| TGF-β1 | Promote epithelial cell proliferation | ? | 75 |
| 15-deoxy-Δ12,14-prostaglandin J2 | Modulate epithelial cell proliferation | Activation of PPAR-γ | 2 |
GDNF, glial-derived neurotrophic factor; ProEGF, pro-epidermal growth factor; GSNO, S-nitrosoglutathione; 15-HETE, 15-hydroxyeicosatetraenoic acid; TGF-β1, transforming growth factor-beta1; FAK, focal adhesion kinase; NF-κB, nuclear factor kappa B; NO, nitric oxide; PPAR-γ, peroxisome proliferator-activated receptor gamma.
If GDNF has been one of the first identified molecules participating in the glial-dependent maintenance of intestinal integrity and repair of mucosal barrier, it is not the only growth factor potentially involved. ProEGF secreted by EGC is able to enhance barrier repair in animal models of mucosal damage [dextran sodium sulfate (DSS), or diclofenac-induced damage]. This link was confirmed in vitro by evaluating epithelial cell spreading and wound healing after scratch (102). The authors of this study also identified focal adhesion kinase (FAK) as a signaling mechanism potentially involved in EGC-mediated actions. However, this last result would need to be confirmed upon the availability of better tools to study FAK signaling pathways.
GSNO (S-nitrosoglutathione) and α-7 nicotinic acetylcholine receptor.
In vitro, ex vivo, and in vivo data, generated in epithelial cell lines, mucosal human biopsies, and transgenic mice, respectively, have shown that EGC also control barrier functions via the release of GSNO (18, 38, 65, 90). This soluble molecule acts likely by increasing the expression of tight-junction proteins (ZO-1 and occludin), restoring in part mucosal integrity. The protective role of glia-derived GSNO has been highlighted not only in physiological conditions but also in pathologies. For example, by using Shigella flexneri as an infectious insult to alter barrier function, Flamant et al. demonstrated that the presence of EGC, or their derivative GSNO, was able to reduce barrier lesions (38). Another experimental model confirming the protective role of EGC on barrier integrity and the involvement of GSNO was set up by Costantini with the induction of intestinal injury by steam burn (27). In this study, EGC activation was induced by vagal nerve stimulation and measured in terms of GFAP expression. The protective effect exerted by EGC, as described in the study, was comparable to the exogenous administration of GSNO alone. It is well known that vagal nerve stimulation exerted anti-inflammatory action; however, the study by Costantini is the first evidence of a brain-gut signaling via EGC during intestinal injury. The same authors, trying to decipher the mechanism by which vagal nerve stimulation would protect barrier function, identified an important role for α-7 nicotinic acetylcholine receptor (nAChR) (28). They further demonstrated, in coculture systems, that nicotine was able to activate EGC, which express α-7 nAchR, and this activation provided protection from inflammation-induced increased intestinal epithelial cell permeability. No direct effect of nicotine on intestinal epithelial cells could be measured, but nicotine-activated EGC modulated barrier function through a NF-κB-dependent pathway (17). In the same model, GSNO prevented inflammation-induced epithelial barrier dysfunction, improving the expression and localization of occludin, ZO-1, and phosphorylated myosin-light chain (18). No link, however, has been established between α-7 nAchR and GSNO, and the mechanisms of action of GSNO still need to be addressed. In vivo studies, however, link the protective effects of GSNO to the inhibition of NF-κB pathway (65) but could not identify the cellular targets.
Nitric oxide.
The production of GSNO by EGC requires reactive nitric oxide (NO) intermediates, produced by NO synthase (NOS) isoforms. Studies in human biopsies and primary EGC showed that these cells contain L-arginine and express the inducible form of NOS, holding therefore the full machinery for the synthesis and release of NO (20, 22, 31). Indeed, in human EGC, the release of NO is busted by pro-inflammatory stimuli, i.e., lipopolysaccharide (LPS) and interferon-gamma (22), and by pathogens, i.e., entero-invasive Escherichia coli (101). Interestingly, S100B seems to have a prominent role during EGC activation, and its overexpression and release have been linked to NO production during intestinal inflammation (20, 22, 31). Once released, S100B acts on surrounding cells but also on EGC in an autocrine manner (20, 22). Studies have shown that, in gut inflammation, a large part of NO release might come from EGC (20, 22, 31, 32), and EGC-derived NO directly correlates with the integrity of epithelial barrier. In EGC-epithelium coculture model, when stimulated with LPS, EGC releases NO, which in turn causes a reduction of transepithelial resistance and an increase in permeability (112). More exhaustively, the link between glial-derived NO and epithelial barrier dysfunction has been characterized in three mouse models of colitis [trinitro benzene sulfonic acid (TNBS)-induced, DSS-induced, or genetic IL-10−/− models] and in colonic human submucosal biopsies (70). The authors discovered that EGC activation affects electrogenic ion transport, leading to altered barrier function, and that iNOS, mainly the one expressed in EGC, is involved in this effect. Indeed, inhibition of NO production and blockade of glial metabolism (by fluoroacetate) were able to restore ion transport during mouse colitis and prevented an EGC-mediated harmful process. These results were confirmed in human biopsies from patients with inflammatory bowel diseases (IBD).
PUFA metabolites.
EGC, like a number of other cells, possess the whole machinery to produce PUFA metabolites, and in particular one of them, the 15-hydroxyeicosatetraenoic acid (15-HETE) (84). Defects in EGC production of 15-HETE have an impact on intestinal epithelial cell permeability. The protective role of glial 15-HETE was corroborated by the observation that, in primary EGC from patients with Crohn’s disease (CD), 15-HETE expression was reduced compared with controls. Glial cells from CD patients were unable to stabilize barrier function in intestinal epithelial cells, but addition of 15-HETE restored barrier function. This study also demonstrated that 15-HETE regulates permeability by inhibiting an adenosine monophosphate-activated protein kinase and also by increasing ZO-1 expression. In EGC from CD patients, the expression of a number of PUFA metabolites was decreased, including 15-HETE, but also 18-hydroxyeicosapentaenoic acid (18-HEPE), 15-deoxy-delta-12,14-prostaglandin J2 (15dPGJ2), and 11-beta-prostaglandin F2 (11βPGF2), suggesting that other PUFA metabolites might control epithelial barrier functions (25). In line with this finding, studies aiming at confirming the capacity of EGC to modulate epithelial cell proliferation have identified 15-dPGJ2 as a molecule involved in peroxisome proliferator-activated receptor (PPAR)-gamma activation (2). 15-dPGJ2 is synthesized and released by EGC, as demonstrated in human submucosal and rat EGC, where it directly affects epithelial cell proliferation but not differentiation.
Control by EGC of immune barrier function.
EGC are able to discriminate between pathogens and probiotics via a different Toll-like receptor (TLR) expression, and this is tightly correlated to S100B protein upregulation (101). Together with TLRs, other receptors have been identified on human EGC, and these receptors tell us that EGC may act as antigen-presenting cells once activated. For example, in physiological conditions, EGC constitutively express major histocompatibility complex (MHC) class I molecules, while MHC class II molecules are almost undetectable (22). When an inflammatory scenario is set up, EGC express MHC class II molecules and c-fos, a marker of cell activation (22). Furthermore, during inflammation, EGC may also proliferate, undergoing reactive gliosis (similar to astrocytes in the brain) (14, 21). In these conditions, EGC morphology and activity appear profoundly altered and switch toward a so-called pro-inflammatory phenotype. This phenomenon is characterized by the release of glial factors and cytokines, which recruit immune cells like macrophages, neutrophils, and mast cells. More specifically, in response to external stimuli, EGC overexpress and release the protein S100B (22) and produce IL-6 and IL-1β, thereby activating an innate immune response participating in the immune mucosal barrier (106, 107) (FIGURE 2).
What is next with EGC?
During the last decade, our knowledge on EGC “tasks” in the context of modulation of barrier function could progress thanks to the development of optogenetic and microscopy tools (7). Application of high-resolution genetic marking technique in the mouse myenteric plexus, for example, unraveled the presence of four distinct populations of EGC, with a different response to glial activator (ATP) (8, 49). This finding suggested that EGC are highly specialized, and this specialization allows them to perform different activities in the gut. One captivating observation in Boesmans’ research article was about the identification, within the myenteric EGC population, of cells negative for the three classic markers used so far: S100, GFAP, and Sox10. This result was completed by the observation by Rao that virtually all EGC express proteolipid protein 1 (Plp1), a myelin protein that is normally present in oligodendrocytes in the central nervous system (85). Transcriptional profile also showed that other genes, necessary for the formation of myelin, are expressed in Plp1-positive EGC (85). Although this study was performed in myenteric plexus, one can hypothesize that submucosal EGC might also show a high population diversity. Recently, unexpected results, in conflict with the current dogma of “EGC have a protective role on barrier function,” have been generated (86). First, Rao and colleagues, inducing selective elimination of EGC (myenteric, submucosal, mucosal, and intramuscular) in mice by using the Plp1 promoter, observed that EGC are not required for the maintenance of the intestinal epithelium integrity (86). Second, Grubisic and Gulbransen, using transgenic mouse models to alter EGC activity (by the ablation of connexin-43), demonstrated that such an activity is not crucial to modulate barrier function (48). Fairly, we have to insist on the fact that the conflicting theories on the physiological role of EGC have been generated by using different approaches, in vitro vs. in vivo modulation of EGC activity, and different genetic models of EGC ablation, GFAP vs. Plp1. The beauty of this debate is that a new cursus toward the characterization of EGC morphology and function in physiological and pathophysiological situations has started. New and surprising revelations are most likely coming!
Conclusions on ENS Targeting for the Control of Epithelial Barrier Function in Diseases
Irritable bowel syndrome, inflammatory bowel diseases, gastrointestinal infections, celiac disease, and postoperative ileus are all associated with different grades of inflammation and intestinal barrier dysfunctions. Can we target the neuronal-glial-epithelial unit in such diseases to control barrier function? We have reviewed here current knowledge on submucosal neurons and EGC, which both synthesize and release mediators having either beneficial or detrimental effects on epithelial cells and thus on barrier function. Most of such mediators are ubiquitous and have multiple functions other than modulation of epithelial barrier functions. To define which mediator could constitute the best molecular target, a complete assessment of all the components of barrier function, i.e., intercellular junctions, epithelial secretion of mucus and antimicrobial peptides, transport and presentation of antigens, as well as epithelial cell turnover, is necessary.
Although neurons “simply” release neuromediators, EGC undergo functional and also morphological changes, they proliferate and become hypertrophic, they undergo structural distortion, and they act like true plastic elements of the gut (44). This plasticity of EGC might favor switches from a protective to a harmful role and vice-versa. Several and diverse evidences brought the hypothesis that EGC might even act as primum movens that initiates the inflammatory cascade in gut diseases (66, 82). This view is still far from being proven and needs novel technical approaches to be evaluated. Based on 1) EGC plasticity and 2) the notions we have on the neuron-to-epithelial and EGC-to-epithelial communications, the idea of targeting EGC appears more realistic than targeting neurons themselves. By modulating their activation during gut diseases, EGC and their specific mediators might represent better targets to diminish the entity of tissue damage and barrier function modification. However, based on the new and intriguing findings on Plp-1 and the subsequent controversial role of EGC in protecting barrier function (86), the journey is probably just beginning.
Acknowledgments
N.V. is supported by a grant from the European Research Council (ERC-310973 PIPE), the Région Midi-Pyrénées (now Occitanie). C.C. is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek (FWO, Belgium).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: N.V. conceived and designed research; N.V. and C.C. interpreted results of experiments; N.V. and C.C. prepared figures; N.V. and C.C. drafted manuscript; N.V. and C.C. edited and revised manuscript; N.V. and C.C. approved final version of manuscript.
References
- 1.Abdo H, Derkinderen P, Gomes P, Chevalier J, Aubert P, Masson D, Galmiche JP, Vanden Berghe P, Neunlist M, Lardeux B. Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J 24: 1082–1094, 2010. doi: 10.1096/fj.09-139519. [DOI] [PubMed] [Google Scholar]
- 2.Abdo H, Mahé MM, Derkinderen P, Bach-Ngohou K, Neunlist M, Lardeux B. The omega-6 fatty acid derivative 15-deoxy-Δ12,14-prostaglandin J2 is involved in neuroprotection by enteric glial cells against oxidative stress. J Physiol 590: 2739–2750, 2012. doi: 10.1113/jphysiol.2011.222935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauman BD, Meng J, Zhang L, Louiselle A, Zheng E, Banerjee S, Roy S, Segura BJ. Enteric glial-mediated enhancement of intestinal barrier integrity is compromised by morphine. J Surg Res 219: 214–221, 2017. doi: 10.1016/j.jss.2017.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertrand PP, Kunze WA, Bornstein JC, Furness JB. Electrical mapping of the projections of intrinsic primary afferent neurones to the mucosa of the guinea-pig small intestine. Neurogastroenterol Motil 10: 533–541, 1998. doi: 10.1046/j.1365-2982.1998.00128.x. [DOI] [PubMed] [Google Scholar]
- 5.Bishop AE, Carlei F, Lee V, Trojanowski J, Marangos PJ, Dahl D, Polak JM. Combined immunostaining of neurofilaments, neuron specific enolase, GFAP and S-100. A possible means for assessing the morphological and functional status of the enteric nervous system. Histochemistry 82: 93–97, 1985. doi: 10.1007/BF00502095. [DOI] [PubMed] [Google Scholar]
- 6.Boesmans W, Cirillo C, Van den Abbeel V, Van den Haute C, Depoortere I, Tack J, Vanden Berghe P. Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol Motil 25: e151–e160, 2013. doi: 10.1111/nmo.12065. [DOI] [PubMed] [Google Scholar]
- 7.Boesmans W, Hao MM, Vanden Berghe P. Optical tools to investigate cellular activity in the intestinal wall. J Neurogastroenterol Motil 21: 337–351, 2015. doi: 10.5056/jnm15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boesmans W, Lasrado R, Vanden Berghe P, Pachnis V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 63: 229–241, 2015. doi: 10.1002/glia.22746. [DOI] [PubMed] [Google Scholar]
- 9.Boesmans W, Martens MA, Weltens N, Hao MM, Tack J, Cirillo C, Vanden Berghe P. Imaging neuron-glia interactions in the enteric nervous system. Front Cell Neurosci 7: 183, 2013. doi: 10.3389/fncel.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun A, Lommatzsch M, Mannsfeldt A, Neuhaus-Steinmetz U, Fischer A, Schnoy N, Lewin GR, Renz H. Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am J Respir Cell Mol Biol 21: 537–546, 1999. doi: 10.1165/ajrcmb.21.4.3670. [DOI] [PubMed] [Google Scholar]
- 11.Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2: 77–91, 2016. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buac K, Watkins-Chow DE, Loftus SK, Larson DM, Incao A, Gibney G, Pavan WJ. A Sox10 expression screen identifies an amino acid essential for Erbb3 function. PLoS Genet 4: e1000177, 2008. doi: 10.1371/journal.pgen.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93: 189–201, 1998. doi: 10.1016/S0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 14.Capoccia E, Cirillo C, Gigli S, Pesce M, D’Alessandro A, Cuomo R, Sarnelli G, Steardo L, Esposito G. Enteric glia: a new player in inflammatory bowel diseases. Int J Immunopathol Pharmacol 28: 443–451, 2015. doi: 10.1177/0394632015599707. [DOI] [PubMed] [Google Scholar]
- 15.Chandrasekharan B, Bala V, Kolachala VL, Vijay-Kumar M, Jones D, Gewirtz AT, Sitaraman SV, Srinivasan S. Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. PLoS One 3: e3304, 2008. doi: 10.1371/journal.pone.0003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrasekharan B, Jeppsson S, Pienkowski S, Belsham DD, Sitaraman SV, Merlin D, Kokkotou E, Nusrat A, Tansey MG, Srinivasan S. Tumor necrosis factor-neuropeptide Y cross talk regulates inflammation, epithelial barrier functions, and colonic motility. Inflamm Bowel Dis 19: 2535–2546, 2013. doi: 10.1097/01.MIB.0000437042.59208.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheadle GA, Costantini TW, Bansal V, Eliceiri BP, Coimbra R. Cholinergic signaling in the gut: a novel mechanism of barrier protection through activation of enteric glia cells. Surg Infect (Larchmt) 15: 387–393, 2014. doi: 10.1089/sur.2013.103. [DOI] [PubMed] [Google Scholar]
- 18.Cheadle GA, Costantini TW, Lopez N, Bansal V, Eliceiri BP, Coimbra R. Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown. PLoS One 8: e69042, 2013. doi: 10.1371/journal.pone.0069042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cirillo C, Bessissow T, Desmet AS, Vanheel H, Tack J, Vanden Berghe P. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am J Gastroenterol 110: 1205–1215, 2015. doi: 10.1038/ajg.2015.158. [DOI] [PubMed] [Google Scholar]
- 20.Cirillo C, Sarnelli G, Esposito G, Grosso M, Petruzzelli R, Izzo P, Calì G, D’Armiento FP, Rocco A, Nardone G, Iuvone T, Steardo L, Cuomo R. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol Motil 21: 1209–e112, 2009. doi: 10.1111/j.1365-2982.2009.01346.x. [DOI] [PubMed] [Google Scholar]
- 21.Cirillo C, Sarnelli G, Esposito G, Turco F, Steardo L, Cuomo R. S100B protein in the gut: the evidence for enteroglial-sustained intestinal inflammation. World J Gastroenterol 17: 1261–1266, 2011. doi: 10.3748/wjg.v17.i10.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cirillo C, Sarnelli G, Turco F, Mango A, Grosso M, Aprea G, Masone S, Cuomo R. Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol Motil 23: e372–e382, 2011. doi: 10.1111/j.1365-2982.2011.01748.x. [DOI] [PubMed] [Google Scholar]
- 23.Coelho-Aguiar JM, Bon-Frauches AC, Gomes AL, Veríssimo CP, Aguiar DP, Matias D, Thomasi BB, Gomes AS, Brito GA, Moura-Neto V. The enteric glia: identity and functions. Glia 63: 921–935, 2015. doi: 10.1002/glia.22795. [DOI] [PubMed] [Google Scholar]
- 24.Cooke HJ, Carey HV. Pharmacological analysis of 5-hydroxytryptamine actions on guinea-pig ileal mucosa. Eur J Pharmacol 111: 329–337, 1985. doi: 10.1016/0014-2999(85)90639-9. [DOI] [PubMed] [Google Scholar]
- 25.Coquenlorge S, Van Landeghem L, Jaulin J, Cenac N, Vergnolle N, Duchalais E, Neunlist M, Rolli-Derkinderen M. The arachidonic acid metabolite 11β-ProstaglandinF2α controls intestinal epithelial healing: deficiency in patients with Crohn’s disease. Sci Rep 6: 25203, 2016. doi: 10.1038/srep25203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn’s disease? Proc Natl Acad Sci USA 98: 13306–13311, 2001. doi: 10.1073/pnas.231474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costantini TW, Bansal V, Krzyzaniak M, Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP, Coimbra R. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol 299: G1308–G1318, 2010. doi: 10.1152/ajpgi.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costantini TW, Krzyzaniak M, Cheadle GA, Putnam JG, Hageny AM, Lopez N, Eliceiri BP, Bansal V, Coimbra R. Targeting α-7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury. Am J Pathol 181: 478–486, 2012. doi: 10.1016/j.ajpath.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 29.De Giorgio R, Giancola F, Boschetti E, Abdo H, Lardeux B, Neunlist M. Enteric glia and neuroprotection: basic and clinical aspects. Am J Physiol Gastrointest Liver Physiol 303: G887–G893, 2012. doi: 10.1152/ajpgi.00096.2012. [DOI] [PubMed] [Google Scholar]
- 30.De Quelen F, Chevalier J, Rolli-Derkinderen M, Mourot J, Neunlist M, Boudry G. n-3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J Physiol 589: 4341–4352, 2011. doi: 10.1113/jphysiol.2011.214056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esposito G, Cirillo C, Sarnelli G, De Filippis D, D’Armiento FP, Rocco A, Nardone G, Petruzzelli R, Grosso M, Izzo P, Iuvone T, Cuomo R. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology 133: 918–925, 2007. doi: 10.1053/j.gastro.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Esposito G, De Filippis D, Cirillo C, Sarnelli G, Cuomo R, Iuvone T. The astroglial-derived S100beta protein stimulates the expression of nitric oxide synthase in rodent macrophages through p38 MAP kinase activation. Life Sci 78: 2707–2715, 2006. doi: 10.1016/j.lfs.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Esposito G, Sarnelli G, Capoccia E, Cirillo C, Pesce M, Lu J, Calì G, Cuomo R, Steardo L. Autologous transplantation of intestine-isolated glia cells improves neuropathology and restores cognitive deficits in β amyloid-induced neurodegeneration. Sci Rep 6: 22605, 2016. doi: 10.1038/srep22605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farack UM, Reiter J, Gross M, Moroder L, Wünsch E, Loeschke K. Influence of vasoactive intestinal peptide, secretin, and Ala4, Val5-secretin on the net movements of electrolytes, fluid, and mucus in the rat colon in vivo. Scand J Gastroenterol Suppl 139: 32–36, 1987. doi: 10.3109/00365528709089772. [DOI] [PubMed] [Google Scholar]
- 35.Farré R, Vicario M. Abnormal barrier function in gastrointestinal disorders. Handb Exp Pharmacol 239: 193–217, 2017. doi: 10.1007/164_2016_107. [DOI] [PubMed] [Google Scholar]
- 36.Fernández-Blanco JA, Barbosa S, Sánchez de Medina F, Martínez V, Vergara P. Persistent epithelial barrier alterations in a rat model of postinfectious gut dysfunction. Neurogastroenterol Motil 23: e523–e533, 2011. doi: 10.1111/j.1365-2982.2011.01777.x. [DOI] [PubMed] [Google Scholar]
- 37.Ferri GL, Probert L, Cocchia D, Michetti F, Marangos PJ, Polak JM. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature 297: 409–410, 1982. doi: 10.1038/297409a0. [DOI] [PubMed] [Google Scholar]
- 38.Flamant M, Aubert P, Rolli-Derkinderen M, Bourreille A, Neunlist MR, Mahé MM, Meurette G, Marteyn B, Savidge T, Galmiche JP, Sansonetti PJ, Neunlist M. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut 60: 473–484, 2011. doi: 10.1136/gut.2010.229237. [DOI] [PubMed] [Google Scholar]
- 39.Foong JP, Parry LJ, and Bornstein JC. Activation of neuronal SST1 and SST2 receptors decreases neurogenic secretion in the guinea-pig jejunum. Neurogastroenterol Motil 22: 1209–16,e317, 2010. doi: 10.1111/j.1365-2982.2010.01566.x. [DOI] [PubMed] [Google Scholar]
- 40.Fung C, Boesmans W, Cirillo C, Foong JPP, Bornstein JC, Vanden Berghe P. VPAC Receptor Subtypes Tune Purinergic Neuron-to-Glia Communication in the Murine Submucosal Plexus. Front Cell Neurosci 11: 118, 2017. doi: 10.3389/fncel.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286–294, 2012. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 42.Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol 54: 1–18, 1998. doi: 10.1016/S0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 43.Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res 59: 83–88, 2006. doi: 10.1203/01.pdr.0000190577.62426.45. [DOI] [PubMed] [Google Scholar]
- 44.Giaroni C, De Ponti F, Cosentino M, Lecchini S, Frigo G. Plasticity in the enteric nervous system. Gastroenterology 117: 1438–1458, 1999. doi: 10.1016/S0016-5085(99)70295-7. [DOI] [PubMed] [Google Scholar]
- 45.Gomes P, Chevalier J, Boesmans W, Roosen L, van den Abbeel V, Neunlist M, Tack J, Vanden Berghe P. ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol Motil 21: 870–e62, 2009. doi: 10.1111/j.1365-2982.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- 46.González-Castro AM, Martínez C, Salvo-Romero E, Fortea M, Pardo-Camacho C, Pérez-Berezo T, Alonso-Cotoner C, Santos J, Vicario M. Mucosal pathobiology and molecular signature of epithelial barrier dysfunction in the small intestine in irritable bowel syndrome. J Gastroenterol Hepatol 32: 53–63, 2017. doi: 10.1111/jgh.13417. [DOI] [PubMed] [Google Scholar]
- 47.Greenwood-Van Meerveld B, Johnson AC, Grundy D. Gastrointestinal physiology and function. Handb Exp Pharmacol 239: 1–16, 2017. doi: 10.1007/164_2016_118. [DOI] [PubMed] [Google Scholar]
- 48.Grubišić V, Gulbransen BD. Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J Physiol 595: 3409–3424, 2017. doi: 10.1113/JP273492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9: 625–632, 2012. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 50.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136: 1349–1358, 2009. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 51.Herrmann JR, Turner JR. Beyond Ussing’s chambers: contemporary thoughts on integration of transepithelial transport. Am J Physiol Cell Physiol 310: C423–C431, 2016. doi: 10.1152/ajpcell.00348.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoff S, Zeller F, von Weyhern CW, Wegner M, Schemann M, Michel K, Rühl A. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J Comp Neurol 509: 356–371, 2008. doi: 10.1002/cne.21769. [DOI] [PubMed] [Google Scholar]
- 53.Hons IM, Burda JE, Grider JR, Mawe GM, Sharkey KA. Alterations to enteric neural signaling underlie secretory abnormalities of the ileum in experimental colitis in the guinea pig. Am J Physiol Gastrointest Liver Physiol 296: G717–G726, 2009. doi: 10.1152/ajpgi.90472.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, Rennert PD, Hiroi T, Tamagawa H, Iijima H, Kunisawa J, Yuki Y, Kiyono H. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA 101: 6110–6115, 2004. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 286: 736–737, 1980. doi: 10.1038/286736a0. [DOI] [PubMed] [Google Scholar]
- 56.Jessen KR, Thorpe R, Mirsky R. Molecular identity, distribution and heterogeneity of glial fibrillary acidic protein: an immunoblotting and immunohistochemical study of Schwann cells, satellite cells, enteric glia and astrocytes. J Neurocytol 13: 187–200, 1984. doi: 10.1007/BF01148114. [DOI] [PubMed] [Google Scholar]
- 57.Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S, Pachnis V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 85: 289–295, 2015. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keast JR, Furness JB, Costa M. Different substance P receptors are found on mucosal epithelial cells and submucous neurons of the guinea-pig small intestine. Naunyn Schmiedebergs Arch Pharmacol 329: 382–387, 1985. doi: 10.1007/BF00496372. [DOI] [PubMed] [Google Scholar]
- 59.Keast JR, Furness JB, Costa M. Investigations of nerve populations influencing ion transport that can be stimulated electrically, by serotonin and by a nicotinic agonist. Naunyn Schmiedebergs Arch Pharmacol 331: 260–266, 1985. doi: 10.1007/BF00634247. [DOI] [PubMed] [Google Scholar]
- 60.Kennedy MF, Tutton PJ, Barkla DH. Adrenergic factors involved in the control of crypt cell proliferation in jejunum and descending colon of mouse. Clin Exp Pharmacol Physiol 10: 577–586, 1983. doi: 10.1111/j.1440-1681.1983.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 61.Knoop KA, Miller MJ, Newberry RD. Transepithelial antigen delivery in the small intestine: different paths, different outcomes. Curr Opin Gastroenterol 29: 112–118, 2013. doi: 10.1097/MOG.0b013e32835cf1cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laranjeira C, Pachnis V. Enteric nervous system development: Recent progress and future challenges. Auton Neurosci 151: 61–69, 2009. doi: 10.1016/j.autneu.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Lasrado R, Boesmans W, Kleinjung J, Pin C, Bell D, Bhaw L, McCallum S, Zong H, Luo L, Clevers H, Vanden Berghe P, Pachnis V. Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science 356: 722–726, 2017. doi: 10.1126/science.aam7511. [DOI] [PubMed] [Google Scholar]
- 65.Li Z, Zhang X, Zhou H, Liu W, Li J. Exogenous S-nitrosoglutathione attenuates inflammatory response and intestinal epithelial barrier injury in endotoxemic rats. J Trauma Acute Care Surg 80: 977–984, 2016. doi: 10.1097/TA.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 66.Liñán-Rico A, Turco F, Ochoa-Cortes F, Harzman A, Needleman BJ, Arsenescu R, Abdel-Rasoul M, Fadda P, Grants I, Whitaker E, Cuomo R, Christofi FL. Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype: implications for GI infection, IBD, POI, neurological, motility, and GI disorders. Inflamm Bowel Dis 22: 1812–1834, 2016. doi: 10.1097/MIB.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol 564: 863–875, 2005. doi: 10.1113/jphysiol.2005.084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lundgren O, Jodal M, Jansson M, Ryberg AT, Svensson L. Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PLoS One 6: e16295, 2011. doi: 10.1371/journal.pone.0016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lundgren O, Peregrin AT, Persson K, Kordasti S, Uhnoo I, Svensson L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 287: 491–495, 2000. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- 70.MacEachern SJ, Patel BA, Keenan CM, Dicay M, Chapman K, McCafferty DM, Savidge TC, Beck PL, MacNaughton WK, Sharkey KA. Inhibiting inducible nitric oxide synthase in enteric glia restores electrogenic ion transport in mice with colitis. Gastroenterology 149: 445–55.e3, 2015. doi: 10.1053/j.gastro.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McClain J, Grubišić V, Fried D, Gomez-Suarez RA, Leinninger GM, Sévigny J, Parpura V, Gulbransen BD. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146: 497–507.e1, 2014. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483: 345–349, 2012. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meir M, Flemming S, Burkard N, Bergauer L, Metzger M, Germer CT, Schlegel N. Glial cell line-derived neurotrophic factor promotes barrier maturation and wound healing in intestinal epithelial cells in vitro. Am J Physiol Gastrointest Liver Physiol 309: G613–G624, 2015. doi: 10.1152/ajpgi.00357.2014. [DOI] [PubMed] [Google Scholar]
- 74.Moro F, Levenez F, Durual S, Plaisancié P, Thim L, Giraud AS, Cuber JC. Secretion of the trefoil factor TFF3 from the isolated vascularly perfused rat colon. Regul Pept 101: 35–41, 2001. doi: 10.1016/S0167-0115(01)00257-9. [DOI] [PubMed] [Google Scholar]
- 75.Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, Naveilhan P, Ruhl A, Lardeux B, Savidge T, Paris F, Galmiche JP. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am J Physiol Gastrointest Liver Physiol 292: G231–G241, 2007. doi: 10.1152/ajpgi.00276.2005. [DOI] [PubMed] [Google Scholar]
- 76.Neunlist M, Frieling T, Rupprecht C, Schemann M. Polarized enteric submucosal circuits involved in secretory responses of the guinea-pig proximal colon. J Physiol 506: 539–550, 1998. doi: 10.1111/j.1469-7793.1998.539bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neunlist M, Rolli-Derkinderen M, Latorre R, Van Landeghem L, Coron E, Derkinderen P, De Giorgio R. Enteric glial cells: recent developments and future directions. Gastroenterology 147: 1230–1237, 2014. doi: 10.1053/j.gastro.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 78.Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol 285: G1028–G1036, 2003. doi: 10.1152/ajpgi.00066.2003. [DOI] [PubMed] [Google Scholar]
- 79.Neunlist M, Van Landeghem L, Mahé MM, Derkinderen P, des Varannes SB, Rolli-Derkinderen M. The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol 10: 90–100, 2013. doi: 10.1038/nrgastro.2012.221. [DOI] [PubMed] [Google Scholar]
- 80.Ngan ES, Shum CK, Poon HC, Sham MH, Garcia-Barcelo MM, Lui VC, Tam PK. Prokineticin-1 (Prok-1) works coordinately with glial cell line-derived neurotrophic factor (GDNF) to mediate proliferation and differentiation of enteric neural crest cells. Biochim Biophys Acta 1783: 467–478, 2008. doi: 10.1016/j.bbamcr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 81.North RA, Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol 358: 17–33, 1985. doi: 10.1113/jphysiol.1985.sp015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ochoa-Cortes F, Turco F, Linan-Rico A, Soghomonyan S, Whitaker E, Wehner S, Cuomo R, Christofi FL. Enteric glial cells: a new frontier in neurogastroenterology and clinical target for inflammatory bowel diseases. Inflamm Bowel Dis 22: 433–449, 2016. doi: 10.1097/MIB.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One 7: e39935, 2012. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pochard C, Coquenlorge S, Jaulin J, Cenac N, Vergnolle N, Meurette G, Freyssinet M, Neunlist M, Rolli-Derkinderen M. Defects in 15-HETE production and control of epithelial permeability by human enteric glial cells from patients with Crohn’s disease. Gastroenterology 150: 168–180, 2016. doi: 10.1053/j.gastro.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 85.Rao M, Nelms BD, Dong L, Salinas-Rios V, Rutlin M, Gershon MD, Corfas G. Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rao M, Rastelli D, Dong L, Chiu S, Setlik W, Gershon MD, Corfas G. Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153: 1068–1081.e7, 2017. doi: 10.1053/j.gastro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2: 361–367, 2001. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 88.Sarosi GA, Barnhart DC, Turner DJ, Mulholland MW. Capacitative Ca2+ entry in enteric glia induced by thapsigargin and extracellular ATP. Am J Physiol 275: G550–G555, 1998. [DOI] [PubMed] [Google Scholar]
- 89.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772, 2011. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 90.Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132: 1344–1358, 2007. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 91.Savidge TC, Sofroniew MV, Neunlist M. Starring roles for astroglia in barrier pathologies of gut and brain. Lab Invest 87: 731–736, 2007. doi: 10.1038/labinvest.3700600. [DOI] [PubMed] [Google Scholar]
- 93.See NA, Epstein ML, Dahl JL, Bass P. The myenteric plexus regulates cell growth in rat jejunum. J Auton Nerv Syst 31: 219–229, 1990. doi: 10.1016/0165-1838(90)90188-O. [DOI] [PubMed] [Google Scholar]
- 94.Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest 125: 918–925, 2015. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sofroniew MV, Bush TG, Blumauer N, Lawrence Kruger, Mucke L, Johnson MH. Genetically-targeted and conditionally-regulated ablation of astroglial cells in the central, enteric and peripheral nervous systems in adult transgenic mice. Brain Res 835: 91–95, 1999. doi: 10.1016/S0006-8993(99)01639-X. [DOI] [PubMed] [Google Scholar]
- 96.Steinkamp M, Geerling I, Seufferlein T, von Boyen G, Egger B, Grossmann J, Ludwig L, Adler G, Reinshagen M. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology 124: 1748–1757, 2003. doi: 10.1016/S0016-5085(03)00404-9. [DOI] [PubMed] [Google Scholar]
- 97.Tanaka F, Tominaga K, Fujikawa Y, Nagami Y, Kamata N, Yamagami H, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Arakawa T. Concentration of glial cell line-derived neurotrophic factor positively correlates with symptoms in functional dyspepsia. Dig Dis Sci 61: 3478–3485, 2016. doi: 10.1007/s10620-016-4329-5. [DOI] [PubMed] [Google Scholar]
- 98.Timmermans JP, Adriaensen D, Cornelissen W, and Scheuermann DW. Structural organization and neuropeptide distribution in the mammalian enteric nervous system, with special attention to those components involved in mucosal reflexes. Comp Biochem Physiol A Physiol 118: 331–340, 1997. [DOI] [PubMed] [Google Scholar]
- 99.Toumi F, Neunlist M, Cassagnau E, Parois S, Laboisse CL, Galmiche JP, Jarry A. Human submucosal neurones regulate intestinal epithelial cell proliferation: evidence from a novel co-culture model. Neurogastroenterol Motil 15: 239–242, 2003. doi: 10.1046/j.1365-2982.2003.00409.x. [DOI] [PubMed] [Google Scholar]
- 100.Toumi F, Neunlist M, Denis MG, Oreshkova T, Laboisse CL, Galmiche JP, Jarry A. Vasoactive intestinal peptide induces IL-8 production in human colonic epithelial cells via MAP kinase-dependent and PKA-independent pathways. Biochem Biophys Res Commun 317: 187–191, 2004. doi: 10.1016/j.bbrc.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 101.Turco F, Sarnelli G, Cirillo C, Palumbo I, De Giorgi F, D’Alessandro A, Cammarota M, Giuliano M, Cuomo R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 63: 105–115, 2014. doi: 10.1136/gutjnl-2012-302090. [DOI] [PubMed] [Google Scholar]
- 102.Van Landeghem L, Chevalier J, Mahé MM, Wedel T, Urvil P, Derkinderen P, Savidge T, Neunlist M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol 300: G976–G987, 2011. doi: 10.1152/ajpgi.00427.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Landeghem L, Mahé MM, Teusan R, Léger J, Guisle I, Houlgatte R, Neunlist M. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics 10: 507, 2009. doi: 10.1186/1471-2164-10-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vanderwinden JM, Timmermans JP, Schiffmann SN. Glial cells, but not interstitial cells, express P2X7, an ionotropic purinergic receptor, in rat gastrointestinal musculature. Cell Tissue Res 312: 149–154, 2003. doi: 10.1007/s00441-003-0716-2. [DOI] [PubMed] [Google Scholar]
- 105.Verheijden S, Boeckxstaens GE. Neuroimmune interaction and the regulation of intestinal immune homeostasis. Am J Physiol Gastrointest Liver Physiol 314: G75–G80, 2018. doi: 10.1152/ajpgi.00425.2016. [DOI] [PubMed] [Google Scholar]
- 106.von Boyen GB, Steinkamp M, Geerling I, Reinshagen M, Schäfer KH, Adler G, Kirsch J. Proinflammatory cytokines induce neurotrophic factor expression in enteric glia: a key to the regulation of epithelial apoptosis in Crohn’s disease. Inflamm Bowel Dis 12: 346–354, 2006. doi: 10.1097/01.MIB.0000219350.72483.44. [DOI] [PubMed] [Google Scholar]
- 107.von Boyen GB, Steinkamp M, Reinshagen M, Schäfer KH, Adler G, Kirsch J. Nerve growth factor secretion in cultured enteric glia cells is modulated by proinflammatory cytokines. J Neuroendocrinol 18: 820–825, 2006. doi: 10.1111/j.1365-2826.2006.01478.x. [DOI] [PubMed] [Google Scholar]
- 108.Worley DS, Pisano JM, Choi ED, Walus L, Hession CA, Cate RL, Sanicola M, Birren SJ. Developmental regulation of GDNF response and receptor expression in the enteric nervous system. Development 127: 4383–4393, 2000. [DOI] [PubMed] [Google Scholar]
- 109.Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA, Valdez-Morales EE, Nasser Y, Van Veldhoven PP, Vanbrabant W, Van der Merwe S, Mols R, Ghesquiere B, Cirillo C, Kortekaas I, Carmeliet P, Peetermans WE, Vermeire S, Rutgeerts P, Augustijns P, Hellings PW, Belmans A, Vanner S, Bulmer DC, Talavera K, Vanden Berghe P, Liston A, and Boeckxstaens GE. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 150: 875–87.e9, 2016. doi: 10.1053/j.gastro.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 110.Wunderlich JE, Needleman BJ, Chen Z, Yu JG, Wang Y, Grants I, Mikami DJ, Melvin WS, Cooke HJ, Christofi FL. Dual purinergic synaptic transmission in the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol 294: G554–G566, 2008. doi: 10.1152/ajpgi.00500.2007. [DOI] [PubMed] [Google Scholar]
- 111.Xiao W, Wang W, Chen W, Sun L, Li X, Zhang C, Yang H. GDNF is involved in the barrier-inducing effect of enteric glial cells on intestinal epithelial cells under acute ischemia reperfusion stimulation. Mol Neurobiol 50: 274–289, 2014. doi: 10.1007/s12035-014-8730-9. [DOI] [PubMed] [Google Scholar]
- 112.Xiao WD, Chen W, Sun LH, Wang WS, Zhou SW, Yang H. The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation. Mol Cell Neurosci 46: 527–534, 2011. doi: 10.1016/j.mcn.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 113.Yoo BB, Mazmanian SK. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 46: 910–926, 2017. doi: 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF. GDNF is a chemoattractant for enteric neural cells. Dev Biol 229: 503–516, 2001. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]