Abstract
Hypoxia is one of the most common and severe challenges to the maintenance of homeostasis. Oxygen sensing is a property of all tissues, and the response to hypoxia is multidimensional involving complicated intracellular networks concerned with the transduction of hypoxia-induced responses. Of all the stresses to which the fetus and newborn infant are subjected, perhaps the most important and clinically relevant is that of hypoxia. Hypoxia during gestation impacts both the mother and fetal development through interactions with an individual’s genetic traits acquired over multiple generations by natural selection and changes in gene expression patterns by altering the epigenetic code. Changes in the epigenome determine “genomic plasticity,” i.e., the ability of genes to be differentially expressed according to environmental cues. The genomic plasticity defined by epigenomic mechanisms including DNA methylation, histone modifications, and noncoding RNAs during development is the mechanistic substrate for phenotypic programming that determines physiological response and risk for healthy or deleterious outcomes. This review explores the impact of gestational hypoxia on maternal health and fetal development, and epigenetic mechanisms of developmental plasticity with emphasis on the uteroplacental circulation, heart development, cerebral circulation, pulmonary development, and the hypothalamic-pituitary-adrenal axis and adipose tissue. The complex molecular and epigenetic interactions that may impact an individual’s physiology and developmental programming of health and disease later in life are discussed.
I. INTRODUCTION
Hypoxia is a state of insufficient oxygen availability, which may occur in the whole body or a part of the body. It is one of the most frequent and severe stresses to an organism’s homeostatic mechanisms. Oxygen sensing is a property of all tissues, and the response to hypoxia is multidimensional involving complicated intracellular networks concerned with the transduction of hypoxia-induced responses. Similar to stresses like hypovolemia and hyperthermia, hypoxia incites a multitude of systemic and cell/tissue specific hormonal and molecular homeostatic responses in the organism. Among different conditions in which humans may experience hypoxia, worldwide, over 140 million people live at a risk of hypoxia in high altitude. High altitude is defined as elevations >2,500 m (~8,200 ft), as this is the altitude above which a number of acclimatization responses occur (1050). Acclimatization is the process of becoming accustomed to a new environment. In regards to high altitude, this process happens in an environment with a relatively low O2 tension, as ambient Po2 is inversely related to altitude. In the adult, acclimatization to prolonged hypoxia involves hyperventilation, polycythemia, a rightward shift in the oxyhemoglobin saturation curve, pulmonary vasoconstriction, and increased capillary density and includes a number of poorly understood physiological processes (1050). It occurs over a period of days to weeks but is believed to be virtually complete within 6 wk. Existing at high altitude can be a significant threat to adults and children because of a complex of illnesses including acute mountain sickness, high-altitude cerebral edema, and high-altitude pulmonary edema. Although the latter two conditions can be life threatening, little is known of their pathophysiology. A large body of work has been devoted to describing the cardiac, pulmonary, hematologic, and other responses to high-altitude, long-term hypoxia in the adult. Unlike acclimatization, the native highlanders adapt to the hypoxic environment by natural selection of genetic adaptation to boost oxygen usability with a limited increase in hemoglobin levels (401, 1112).
Compared with the adult, the fetus in utero lives in a much lower oxygen environment, and the state of fetal oxygenation was compared with “Mount Everest in Utero” (244). Fetal arterial O2 tension is relatively low (~25 mmHg) as compared with that of adult (~95 mmHg). Although physiologically “normal” hypoxia (lower oxygen tension in the fetus as compared with the adult) is critical for embryonic and fetal development, pathophysiological hypoxia (lower than normal fetal oxygen tension) during gestation has profound adverse effects on developmental plasticity. Indeed, of all the stresses to which the fetus and newborn infant are subjected, perhaps the most significant and clinically relevant is that of hypoxia. In addition to pregnancy at high altitude that adversely impacts maternal health and fetal development, the fetus may experience in utero hypoxia under many other conditions. Importantly, the fetuses of women who smoke during pregnancy and those exposed to environmental pollution with carbon monoxide are subjected to prolonged hypoxemia. There exist a large number of other clinical conditions in which the fetus may experience prolonged hypoxemic stress. These include women who are anemic; those who are malnourished; those with pregnancy complications such as preeclampsia, placental insufficiency, heart, lung and kidney disease, or with a hemoglobinopathy; and those who engage in strenuous physical exercise or work. Thus the problem of long-term hypoxia impacting developmental plasticity is of great importance for many reasons in addition to pregnancy at high altitude. Among many other effects, gestational hypoxia is often associated with increases in the incidence of maternal complications of preeclampsia and fetal intrauterine growth restriction (312, 430, 474, 678, 680, 736, 758, 1134, 1143). In addition to increased maternal complications and perinatal morbidity and mortality resulting from gestational hypoxia, an important consideration concerns the issue of maternal/fetal stress and developmental programming of chronic disease later in life. As first articulated by the late David J. Barker (1938–2013), many chronic diseases including, but not limited to, hypertension, cardiovascular disease, obesity, type II diabetes, insulin resistance, dyslipidemia, and cognitive and behavioral disorders have their roots in fetal and early childhood development of intrauterine stress (58, 64, 253a, 315, 645).
Hypoxic-mediated responses are highly integrated across many cell types; nonetheless, they are tissue specific. In many respects, these responses differ significantly between fetus and adult, as well as between nonpregnant and pregnant states. Recent findings suggest exciting epigenetic-mediated mechanisms of DNA methylation, active demethylation, histone modifications, and micro RNAs in regulating the expression and function of several important genes including ion channels, membrane receptors, key enzymes and signaling proteins in maternal and fetal organs and tissues in response to chronic hypoxia during gestation (FIGURE 1). In this review, we will first briefly address the impact of gestational hypoxia on maternal health and fetal development, and describe the concepts of epigenetic mechanisms and developmental programming of health and disease. We will then turn our focus on the effects of gestational hypoxia impacting epigenetic-mediated molecular modifications of systemic and cellular and subcellular responses in the mother and her fetus. The following specific areas will be discussed: 1) uteroplacental circulation and preeclampsia; 2) heart development and programming of cardiac disease; 3) cerebral circulation and brain development and programming of neurological disease; 4) pulmonary circulation and lung development and persistent pulmonary hypertension; 5) hypothalamic-pituitary-adrenal axis and adipose tissue programming; and 6) directions of future research in the areas.
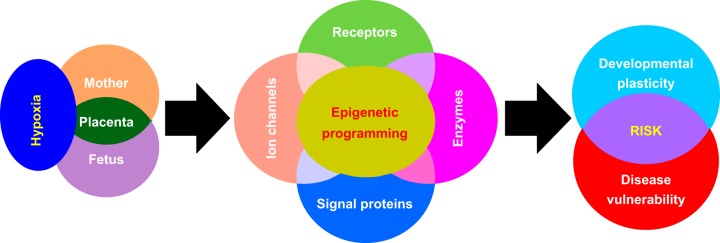
FIGURE 1.
Hypoxia and developmental origins of health and disease. Gestational hypoxia is associated with women residing at high altitude, for whom having preeclampsia and placental insufficiency imposes a significant challenge to both the mother and the developing fetus. To cope with such an adverse environmental condition, the mother and her fetus as well as the placenta undergo significant changes to achieve a certain degree of adaptation to ensure reproductive success and to sustain life before birth. Notably, the expression patterns of suites of genes encoding receptors, ion channels, enzymes, and other signal proteins are altered via epigenetic modifications. However, certain trade-offs occur along with this adaptation and result in permanent changes in phenotypic plasticity and cell/tissue function, leading to increased risk of disease later in life.
II. GESTATIONAL HYPOXIA IMPACTS MATERNAL HEALTH AND FETAL DEVELOPMENT
During normal gestation, maternal hemodynamic adaptations are essential both for fetal growth and survival as well as the cardiovascular well-being of the mother. These adaptations include a decrease in systemic vascular resistance, expansion of blood volume, an increase in cardiac output, and the development of low-resistance uteroplacental circulation. Because plasma volume expansion exceeds the increase in red blood cells, pregnancy is a state of physiological anemia. The reductions in hematocrit and hence blood viscosity contribute to the decreased systemic vascular resistance and help facilitate blood flow in the placental circulation. Hypoxia during gestation has adverse impact on maternal cardiovascular adaptations. In humans, pregnancy at high altitude is associated with maladaptation of maternal circulatory adjustments to pregnancy, including decreases in blood volume expansion, lower stroke volume and cardiac output, and increases in hematocrit, blood viscosity, and peripheral resistance (460, 634) (TABLE 1).
Table 1.
Pregnant and nonpregnant women response to high altitude hypoxia
|
Nonpregnant |
Pregnant |
|||
|---|---|---|---|---|
| Variables | Sea level | High altitude | Sea level | High altitude |
| Blood gases | ||||
| Po2, mmHg | 93 (9) | 48 (4)* | 98.5 (10) | 53 (3)* |
| Pco2, mmHg | 40 (2.5) | 27 (2.0)* | 32 (3.0) | 23 (1.6)* |
| pH | 7.43 (0.02) | 7.48 (0.03)* | 7.45 (0.02) | 7.495 (0.03)* |
| Saturation, % | 98 (0.8) | 88 (3.0)* | 98.5 (0.7) | 89.9 (2.4)* |
| Hemoglobin, g/dl | 14 (1.6) | 16 (1.7)* | 11.8 (1.4) | 14.3 (1.5)* |
| HCO3, mM | 25.3 (1.2) | 19.9 (1.3)* | 21.7 (1.6) | 17.5 (1.2)* |
| Base excess | 1.37 (0.9) | −0.7 (1.4)* | −0.69 (1.3) | −2.06 (1.3)* |
| O2 content, ml/100 ml whole blood | 1.82 (0.2) | 1.89 (0.2) | 1.58 (0.2) | 1.75 (0.2)* |
| Minute ventilation, l/min | 10.5 (4) | 12.4 (4) | 13.3 (3.8) | 16.7 (7)* |
| Respiratory rate/min | 15.7 (4.8) | 18.9 (5.3) | 18.6 (5.9) | 20.9 (6.6)† |
| Tidal volume, l | 0.7 (0.3) | 0.7 (0.2) | 0.8 (0.3) | 0.8 (0.4) |
| Cardiovascular parameters | ||||
| Cardiac output, l/min | 5.08 (1.04)§ | 4.68 (0.78) | 6.89 (1.04) | 5.60 (1.04)§ |
| Stroke volume, ml | 71.64 (13.78)§ | 70.49 (9.34) | 86.25 (10.73) | 77.74 (12.29)§ |
| Left atrial diameter, mm | 30.70 (1.98)§ | 31.83 (5.10) | 38.24 (4.03) | 35.84 (4.91)§ |
| Heart rate, beats/min | 71.22 (7.85)§ | 66.68 (8.82) | 80.79 (9.31) | 72.32 (10.25)§ |
| LV intraventricular septum during diastole/BSA, 10−3 × m−1 | 4.09 (0.37) | 4.11 (0.67) | 4.14 (0.50) | 4.38 (0.43) |
| LV end-diastolic diameter/BSA, 10−3 × m−1 | 29.81 (2.21) | 28.91 (2.04) | 31.16 (2.84) | 29.93 (2.73) |
| LV posterior wall diameter during diastole/BSA, 10−3 × m−1 | 4.20 (0.37) | 4.17 (0.43) | 4.16 (0.48) | 4.29 (0.50) |
| LV intraventricular septum during systole/BSA, 10−3 × m−1 | 6.50 (0.67)§ | 5.69 (0.70)‡ | 6.08 (0.62) | 6.29 (0.83)§ |
| LV end-systolic diameter/BSA, 10−3 × m−1 | 18.47 (1.63)§ | 19.83 (1.72)‡ | 19.93 (2.05) | 19.17 (2.14) |
| LV posterior wall diameter during systole/BSA, 10−3 × m−1 | 7.28 (0.66) | 6.28 (0.67)‡ | 7.18 (1.02) | 7.19 (0.74)§ |
| Fractional shortening | 0.38 (0.04) | 0.31 (0.03)‡ | 0.36 (0.04) | 0.36 (0.04)§ |
| Ejection fraction | 0.68 (0.05) | 0.59 (0.05)‡ | 0.65 (0.02) | 0.64 (0.05)§ |
| Mean arterial pressure, mmHg | 96.32 (10.42)§ | 92.33 (8.95) | 91.28 (7.51) | 84.81 (6.77)§ |
| Total vascular resistance, dyn·s−1·cm−5 | 1579.36 (272.13) | 1550.99 (207.37) | 1081.95 (168.09)§ | 1247.20 (235.55)§ |
| Global long axis shortening, mm | 15.37 (1.16) | 14.48 (1.21)‡ | 15.67 (1.52) | 15.63 (1.40)§ |
| Global time to shortening, ms | 78.57 (8.65) | 91.42 (13.74)‡ | 80.65 (10.42) | 83.07 (12.15)§ |
| LV mass/BSA, g/m2 | 71.11 (9.16)§ | 66.47 (13.24) | 78.49 (10.49) | 72.71 (13.21) |
P < 0.01. †P < 0.05. ‡Denotes statistically significant difference (P < 0.05) between nonpregnant controls at altitude and sea level. §Denotes statistically significant difference (P < 0.05) between pregnant women and nonpregnant controls at their respective altitude. [Table adapted from Kametas et al. (460) and McAuliffe et al. (634).]
Perhaps the most significant and clinically relevant pregnancy complications caused by gestational hypoxia are the increased risk of maternal preeclampsia and fetal intrauterine growth restriction (312, 430, 474, 678, 680, 736, 758, 1134, 1143). Preeclampsia occurs in 5–8% of pregnancies worldwide and is a leading cause of maternal and fetal morbidity or mortality. Pregnant women at high altitude have a two- to fourfold increase in the incidence of preeclampsia, which is often proceeded by a decrease in uteroplacental blood flow and is associated with growth-restricted infants (1144, 1145). The extent of these changes is associated with elevation above sea level, as well as with maternal ventilatory rate and arterial O2 content. The most striking hemodynamic change in the maternal circulation during pregnancy is the increase in uterine blood flow that in humans reaches >30-fold of the nonpregnant value. This adaptation is of critical importance for the optimal growth and development of the fetus. The increased uterine blood flow during gestation is mainly achieved by the remodeling of uterine vasculature, vasodilatation, and the development of low-resistance placental circulation. Extensive human and animal studies indicate that chronic hypoxia attenuates pregnancy-induced adaptation of uteroplacental blood flow, which is important in the increased incidence of preeclampsia and fetal intrauterine growth restriction (18, 450, 758, 1144, 1145). Many questions remain unanswered, yet several recent findings provide valuable leads to explore in greater detail the molecular and epigenetic mechanisms in the understanding of steroid hormone-induced dynamic regulation of large-conductance Ca2+-activated K+ (BKCa) channels in uterine vascular adaptation to pregnancy and chronic hypoxia.
Central to many pregnancy complications and abnormal fetal development is placental dysfunction. The placenta interfaces between the mother and fetus and transfers nutrients and oxygen from the mother to her fetus. Placental structure and function, the effect of hypoxia on the placenta, and placental dysfunction as origins in developmental programming of chronic disease later in life have been reviewed recently (120). Hypoxia has variable effects on the placenta, including the weight, metabolism, nutrient transport, vascular remodeling, and placental blood flow. Placental hypoxia and chronically elevated hypoxia-inducible factor (HIF)-1α and its downstream target microRNA 210 are believed to play a major role in placental dysfunction and in the pathogenesis of preeclampsia (2, 413, 414, 603, 700). Studies in both humans and animals have demonstrated that the placenta undergoes multiple morphological and aberrant global gene expression changes in response to prolonged hypoxia (120). However, the findings that placentas from preeclamptic pregnancies at high altitude of 3,100 m had little morphometric differences from those of normotensive pregnant women at the same altitude suggest that the pathogenesis of late-onset preeclampsia at altitude may be somewhat different and may depend more on maternal stress other than poor placental development at high altitude (1006).
In addition to maternal complications, lower birth weight is a consistent finding in pregnancy at high altitude, and birth weight decreases 100 g per 1,000 m increase in elevation above 2,500 m. However, the high-altitude native residences of multigenerations are protected from low birth weight and infant mortality by genetic selection of genotypes for high oxygen saturation of hemoglobin (401, 1112). Although fetal arterial O2 tension is relatively low as compared with the adult, fetal arterial O2 content (~12 ml/dl) is only slightly lower than that of the adult (~15 ml/dl). This is as a result of its higher hemoglobin concentrations and increased affinity of hemoglobin for oxygen (586). Nonetheless, with its relatively low arterial O2 tension and steep oxyhemoglobin saturation curve, the fetus is particularly vulnerable to hypoxia (586, 588). In addition to hypoxia, the fetus at high altitude may also experience a decrease in nutrients transported from the placenta, particularly glucose (1138). The fetus responds to acute hypoxia in utero by an increase in blood pressure, bradycardia, and redistribution of cardiac output away from peripheral towards essential circulations of the brain and heart (309). Although the fetal response to chronic hypoxia is less clear, it appears that the adaptation of cardiac output redistribution to the brain and heart persists (22, 372). Some specific physiological responses in pregnant sheep and near-term fetal lambs acclimatized for ~110 days (full gestation of 150 days) to high altitude (3,801 m) are listed in TABLE 2. In addition to intrauterine growth restriction that occurs in 7–10% of all pregnancies and is particularly common at high altitude, chronic hypoxia during gestation is associated with serious problems in the fetal development. Conditions such as dysregulation of cerebral blood flow with associated intraventricular and germinal matrix hemorrhage make the fetus susceptible to neurological and other developmental handicaps, as well as persistent fetal circulation, persistent pulmonary hypertension of the newborn, congenital heart anomalies, necrotizing enterocolitis, and other conditions associated with increased perinatal morbidity and mortality (466, 563, 637, 964).
Table 2.
Sheep maternal and fetal response to high altitude hypoxia
| Physiological Responses | Normoxia | Hypoxemia | %Change |
|---|---|---|---|
| Mother | |||
| Po2, mmHg | 98 ± 2 | 64 ± 2* | −37.1 |
| Pco2, mmHg | 35 ± 1 | 29 ± 1* | −17.9 |
| pH | 7.44 ± 0.01 | 7.46 ± 0.01 | 0.3 |
| [Hb], g/dl | 8.7 ± 0.3 | 10.5 ± 0.4* | 20.7 |
| BP, mmHg | 81 ± 3 | 88 ± 4 | 8.6 |
| Fetus | |||
| Weight (at 140 days gestation), g | 4,640 ± 180 | 4,862 ± 300 | |
| Po2, mmHg | 25 ± 1 | 19 ± 1* | −24 |
| [HbO2], % | 59 ± 3 | 50 ± 3† | −15.9 |
| [Hb], g/dl | 10.1 ± 0.7 | 12.6 ± 0.6* | 24.7 |
| O2 content, ml/dl | 12.0 ± 0.5 | 9.0 ± 0.5 | −25 |
| Pco2, mmHg | 48 ± 1 | 40 ± 1* | −18.4 |
| pH | 7.36 ± 0.01 | 7.37 ± 0.01 | 0.1 |
| Lactate, mg/dl | 13.1 ± 0.7 | 14.4 ± 1 | 9.9 |
| Heart rate, beats/min | 168 ± 5 | 165 ± 5 | −1.6 |
| Arterial pressure, mmHg | 44 ± 1 | 52 ± 1* | 17.1 |
| Right ventricular output, ml·min−1·kg−1 | 276 ± 10 | 183 ± 10* | −33.6 |
| Left ventricular output, ml·min−1·kg−1 | 166 ± 16 | 142 ± 16 | −14.5 |
| Right stroke volume, ml/kg | 1.66 ± 0.05 | 1.11 ± 0.05* | −33.1 |
| Left stroke volume, ml/kg | 0.97 ± 0.09 | 0.84 ± 0.08 | −13.4 |
| Combined ventricular output, ml·min−1·kg−1 | 441 ± 23 | 335 ± 28† | −24.1 |
| Breathing incidence, min/h | 25 | 25 | |
| Norepinephrine, pg/ml | 553 ± 55 | 635 ± 65 | 14.8 |
| Epinephrine, pg/ml | 81 ± 19 | 113 ± 12 | 39.5 |
| ACTH, pM | 4.1 ± 0.9 | 8.2 ± 2.7* | 200 |
| Cortisol, ng/ml | 10.9 ± 1.2 | 12.3 ± 1.6 | 13 |
III. HYPOXIA AND EPIGENETIC MECHANISMS OF DEVELOPMENTAL PLASTICITY
A. Developmental Programming of Health and Disease
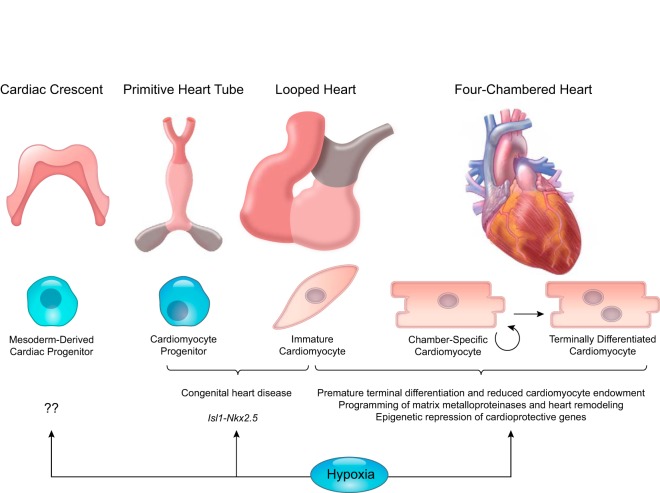
Organism development is a well-orchestrated process. The genetic information is passed down from parents to their offspring encoding in the sequence of DNA in the genome. The genotype of an organism provides stability and accurate heritability from generation to generation. The faithful translation of a genotype to a phenotype may be affected by multiple environmental factors, particularly during the early developmental period. The ability of an organism to change the gene regulation and expression patterns to provide phenotypic alterations to cope with the changing environment is known as developmental plasticity. Large epidemiological studies in humans and experimental studies in laboratory animals have demonstrated a relationship between the intrauterine fetal stress resulting from multiple factors including maternal hypoxia, food deprivation, drug addiction, alcohol, emotional stress, etc., and an increased risk of diseases later in life (FIGURE 2) (58, 64, 253a, 315, 645). The concept of “Developmental (or Fetal) Origins of Adult Health and Disease” has been receiving much attention for over two decades and has been supported by numerous studies from many countries and cultures worldwide. Among other environmental factors that affect fetal development, gestational hypoxia is an important cause of fetal stress impacting developmental plasticity. Extensive studies in experimental animals and some in humans indicate that antenatal hypoxia results in developmental programming of phenotypic changes that predispose offspring to various dysfunctions and diseases, including cardiac dysfunction and ischemic heart disease, hypertension and pulmonary hypertension, endothelial dysfunction and atherosclerosis, metabolic disease, neurological disorders, and other conditions (TABLE 3). Evidence shows that individual’s developmental plasticity, postnatal growth, and physiological function can be determined even in the preimplantation period of development by both maternal and paternal stressors.
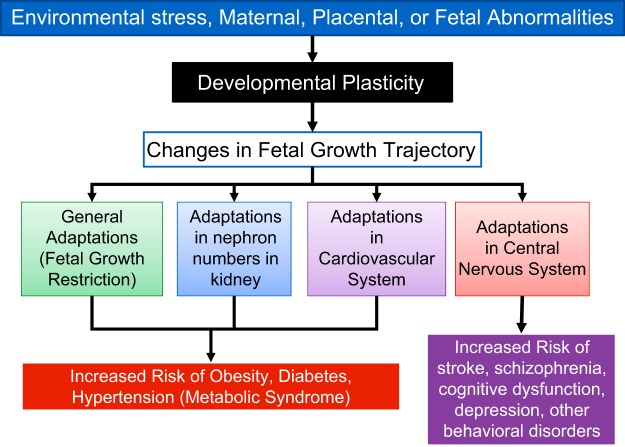
FIGURE 2.
Illustration of the interplay of external factors on developmental plasticity and the downstream effects on general and system specific adaptations in the developing fetus. Also shown are the known disorders associated with such interactions.
Table 3.
Antenatal hypoxia and developmental plasticity
| Species | Offspring Phenotypes of Disorders and Diseases | Reference Nos. |
|---|---|---|
| Human | Pulmonary vascular dysfunction and hypertension | 430, 451, 882 |
| Congenital heart disease | 574 | |
| Increased microvessel density | 298 | |
| Schizophrenia | 129, 357, 734, 1004 | |
| Autism | 118 | |
| Activation of the magnocellular neuroendocrine neurons | 757 | |
| Decreased verbal IQ | 32 | |
| Sheep | Pulmonary vascular dysfunction and hypertension | 370, 371, 570 |
| Systemic vascular dysfunction | 370, 684 | |
| Decreased cardiomyocyte endowment | 96 | |
| Coronary tree remodeling with increased conductance | 108, 209 | |
| Rodent | Increased ischemic heart injury | 552, 553, 784, 785, 838, 1089, 1100, 1104, 1108 |
| Chronic cardiopulmonary dysfunction | 862 | |
| Congenital heart disease | 126, 913, 1126 | |
| Decreased cardiac output | 359 | |
| Decreased ventricular stiffness | 358 | |
| Decreased coronary flow reserve | 360 | |
| Myocardial thinning | 828 | |
| Decreased cardiomyocyte endowment | 47, 49, 553, 767, 985 | |
| Matrix metalloproteinases and cardiac remodeling | 986 | |
| Increased blood pressure | 794, 851, 859, 945, 1078, 1080, 1083, 1086 | |
| Endothelial dysfunction and aging | 23, 99, 184, 310, 365, 690, 839, 1021 | |
| Systemic vascular dysfunction | 1061, 1062, 1075, 1079, 1081, 1087 | |
| Atherosclerosis | 1037, 1153 | |
| Pulmonary vascular dysfunction and hypertension | 445, 621, 797 | |
| Metabolic dysfunction | 127, 412, 479, 754, 861 | |
| Renal vascular dysfunction | 957 | |
| Kidney development defects | 321, 1057, 1071 | |
| Reduced bone mass and density | 537 | |
| Liver enzyme dysfunction and fatty liver disease | 754, 938 | |
| Retinal dysfunction | 100 | |
| Changed salt appetite and body fluid regulations | 1117 | |
| Impaired respiratory behavior | 795, 796 | |
| Altered adrenal medulla maturation | 617 | |
| Increased sensitivity to ischemic brain injury | 322, 558, 559 | |
| Alzheimer's disease | 1157 | |
| Schizophrenia | 385 | |
| Sensitized HPA reactivity to stress and anxiety-like behavior | 259, 819, 869, 1033 | |
| Impaired circadian synchronization and biological clock to light | 448 | |
| Cognitive dysfunctions | 231, 318, 963 | |
| Cerebral white matter damage | 1024 |
B. Epigenetic Mechanisms of Developmental Plasticity
Epigenetic mechanisms are essential for development and differentiation, allowing an organism to respond to the environment through alterations in gene expression patterns. Growing evidence suggests that the epigenetic regulation of gene expression patterns play a crucial role in fetal stress and developmental plasticity (315). Epigenetic modifications relate to relatively stable and heritable patterns of gene expression that do not involve changes in DNA sequence and mainly are mediated by DNA methylation, histone modifications, and noncoding RNAs (ncRNAs) such as long noncoding RNA (lncRNA) and microRNAs (FIGURE 3). DNA methylation is a principal mechanism in epigenetic modification of gene expression patterns and occurs at cytosine of the dinucleotide sequence CpG. Newly discovered adenine methylation in DNA expands the complexity of the epigenome in the development of cells into the multitude of phenotypes that make up different tissues (601). Methylation in gene promoter regions is typically associated with transcription repression of the associated genes. A recent exciting finding is that changes in DNA methylation and demethylation are highly dynamically regulated. Several studies have suggested a robust mechanism of ten-eleven translocation 1–3 (TET1–3) proteins in active DNA demethylation through conversion of 5-methylcytosine (5mC) into 5-hydroxymethylcytosine (5hmC), followed by deamination into 5-hydroxyluracil (5hmU) (81, 951). 5hmU is excised by 5hmU glycosylases and repaired by the base excision repair pathway with unmethylated cytosine. TET1-catalyzed 5mC hydroxylation to 5hmC is a key initiating step in DNA excision repair-based active DNA demethylation, resulting in increased expression of associated genes in mammalian cells both in vitro and in vivo. In addition to DNA methylation/demethylation, histone modifications are crucial for the regulation of gene expression by modulating chromatin structure of euchromatin or heterochromatin that determine the accessibility and the sequential recruitment of regulatory factors to underlying DNA (939). Histones undergo a variety of reversible posttranslational modifications, including acetylation and methylation of conserved lysine and arginine residues on the amino-terminal tail domain, phosphorylation of serine and threonine, ubiquitinylation and ADP-ribosylation. While DNA methylation and histone modifications are important epigenetic mechanisms in regulating gene transcription, microRNAs, a class of small noncoding RNAs, are also important players in the epigenetic control of gene expression patterns by targeting mRNAs and leading to degradation of mRNAs or translational suppression of the target genes (245, 924). The interactions of DNA methylation, histone modifications, and microRNAs may serve as a self-reinforcing network to regulate gene expression patterns in a highly sophisticated feedback manner.
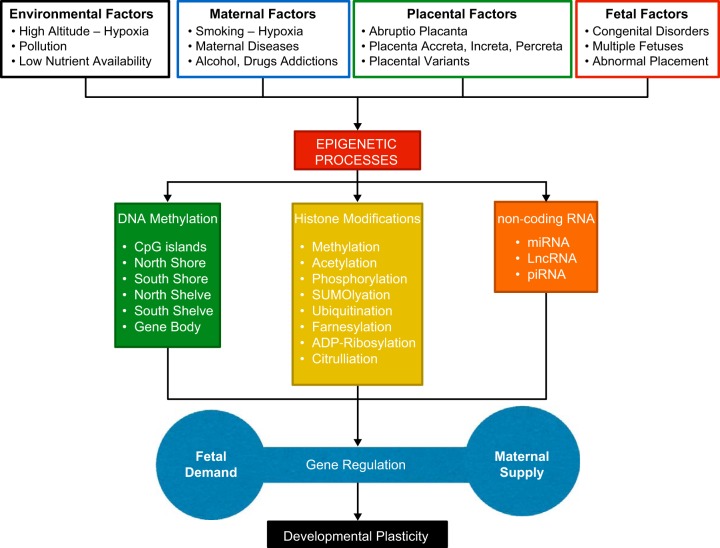
FIGURE 3.
Illustration of the effect of various environmental, maternal, placental, and fetal factors on the known alterations in the three major epigenetic processes (DNA methylation, histone modifications, and noncoding RNA) and downstream effect on developmental plasticity.
Undoubtedly, epigenetic mechanisms play a key role in the regulation of developmental plasticity of an organism, although a recent study shows life-long associations in shared genetic effects between early growth phenotypes and adult cardiometabolic disease (382). The epigenome with its plasticity is the basis for phenotypic information beyond that of the genome per se in determining what and who we are, and the modification of epigenetic process by in utero stress is now regarded as the major factor in several chronic disorders of adult life. Reprogramming of the epigenome during the early development of an organism is a highly complex and well-orchestrated process. It occurs through the interaction of molecular modifications to DNA and associated histone proteins (202, 576), which determines the balance of epigenetic memory to maintain a subset of genes expressed in each cell type to the next generation versus the flexibility or plasticity for adaptation to changing environments (528, 1034). Environmental impact during the early development can leave its marks on epigenome reprogramming and influence an individual’s lifelong health. Whether epigenetic variations and acquired traits at the F1 generation can be maintained and inherited in a manner of true transgenerational epigenetic inheritance at F3 individuals remains unclear because of germline reprogramming and erase of epigenetic signatures imposed by the environment at the F2 generation (362). However, several lines of evidence show that certain environment-induced epigenetic marks can be retained and transmitted to F4 generations (38, 822), indicating that germline reprogramming can fail, thus allowing transgenerational inheritance of epigenetic variation and acquired traits resulting from environmental cues to occur.
C. Hypoxia and Epigenetic Programming
A question of great relevance is the extent to which hypoxic stress during gestation affects epigenetic programming impacting developmental plasticity of health and disease. Of critical importance, epigenetic-mediated regulations play a central role in the cell’s response pathways that are crucial for adaptation to hypoxia (593, 717, 1039). Epigenetic-related mechanisms of DNA methylation, histone modifications, and microRNAs have all been shown to be important in maternal, placental, and fetal responses to hypoxia in gestation (156, 183, 212, 305, 616, 691, 754, 1129). Recent studies in several animal models including gestational hypoxia have shown significant changes in gene expression patterns in the placenta and in fetal organs/tissues, including brain, cerebral artery, pulmonary artery, lung, heart, and liver. These are associated with altered patterns of DNA methylation, histone modifications, and microRNAs (164, 304, 305, 322, 327, 534, 553, 656, 784, 785, 1074, 1089, 1090, 1104, 1114, 1151).
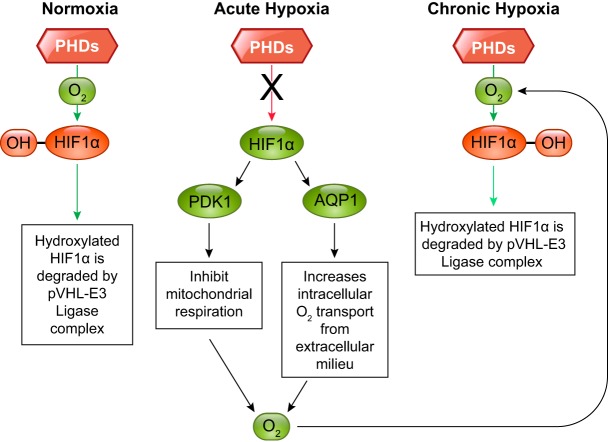
Under hypoxia, cells undergo several changes to survive, mainly through the accumulation and activation of HIF. HIF exists in five active isoforms (FIGURE 4). However, the transcription factor HIF-1α is an essential regulator responsible for modulating a large set of genes that facilitate adaptation and survival of cells/organism from normoxia to hypoxia. HIF-1 binds to a hypoxic response element (HRE; 5′-A/GCGTG-3′) under hypoxic conditions as a heterodimeric complex consisting of a subunit HIF-1α and HIF-1β. HIF-1β is also known as the aryl hydrocarbon nuclear translocator (ARNT), originally identified as a binding partner of the aryl hydrocarbon receptor. These proteins belong to the basic helix-loop-helix–Per-ARNT-Sim (bHLH–PAS) protein family. The bHLH and PAS motifs are essential for heterodimer formation between the HIF-1α and HIF-1β subunits and binding to HRE. The COOH-terminal of HIF-1α contains two transactivation (stimulation of transcription) domains, NH2 terminal (N-TAD) and COOH terminal (C-TAD). Of significance, HIF-1α also contains an oxygen-dependent degradation domain (ODDD) that mediates oxygen-regulated stability. In the presence of oxygen, prolyl hydroxylase dioxygenases (PHD) hydroxylate HIF-1α and mark it for degradation. Under the hypoxic condition, PHD becomes deactivated and HIF-1α accumulates. HIF-1α is a transcriptional regulator and its accumulation increases the expression of several genes involved in anaerobic glycolysis and those involved in reducing cellular oxygen consumption. Consequently, there is a relative increase in cellular oxygen, leading to reactivation of PHD and despite continued hypoxia, HIF-1α returns to the basal (normoxic) levels (FIGURE 5). Thus it appears that HIF-1α rises initially with hypoxia, but it degrades with continued hypoxia while the cell is still able to survive. Other molecules take over the role of protecting the cell under sustained hypoxia. Nevertheless, HIF-1α is critical for cell survival in the initial phase of hypoxia.
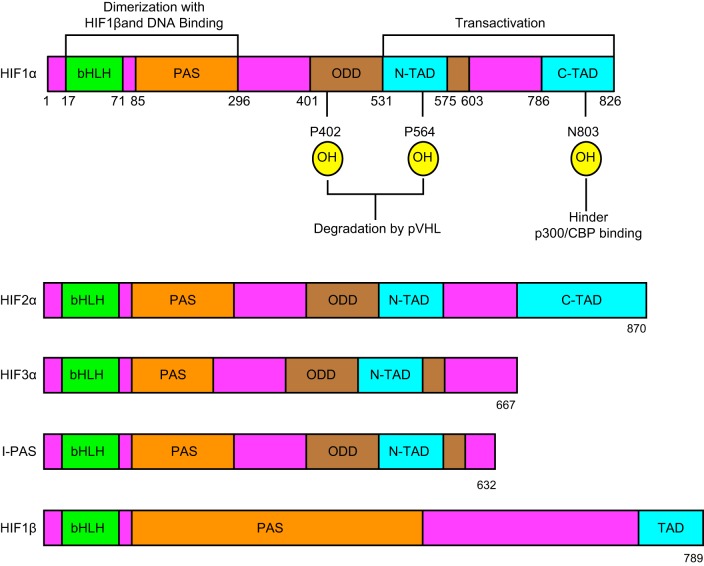
FIGURE 4.
Illustration of the structure of the major hypoxia inducible factor (HIF) isoforms. Shown are the presence and absence of different domains on each specific HIF isoform and the amino acid numbers demarcating these domains. Dimerization and DNA binding domains (bHLB-PAS) are present in all the isoforms. Oxygen detecting domains (ODD) are not present in HIF-1β, whereas transactivation domain, which is made up of NH2-terminal domain (N-TAD) and COOH-terminal domain (C-TAD), is incomplete in HIF-3α, I-PAS, and HIF-1β isoforms.
FIGURE 5.
Illustration of prolyl hydroxylase-mediated hydroxylation of HIF-1α in normoxic, acute hypoxic, and chronic hypoxic conditions. With acute hypoxia, HIF-1α is not hydroxylated; however, with continued hypoxia there is an increased intracellular oxygen availability and hydroxylation of HIF-1α.
HIF-1α is mainly regulated posttranslationally by protein degradation via either oxygen-dependent or oxygen-independent proteasomal degradation. However, several studies have suggested an important role of DNA methylation in the regulation of HIF-1α gene expression at the transcription level (731, 1019). The HIF-1α promoter contains high abundance of CpG dinucleotides and harbors several HREs in both humans and rats. The HRE-152 (5′-RCGTG-3′) is identified as the HIF-1α binding site responsible for the robust induction of the HIF-1α promoter activity in humans (506). Importantly, it is demonstrated that the CpG at the HRE-152 is heavily methylated, and CpG demethylation by 5-aza-2′-deoxycytidine stimulates positive autoregulation of HIF-1α expression (506). The HRE located on the proximal HIF-1α promoter appears highly conserved across species, and rat HIF-1α promoter harbors an HRE at positions −146 to −142 (5′-RCGTG-3′). In addition to the HRE, SP1 binding sites are also identified in the proximal HIF-1α promoter in both humans and rats. CpG methylation at the SP1 binding site plays an important role in the regulation of promoter activities. Fetal hypoxia results in global hypomethylation and a sustained increase in HIF-1α mRNA and protein abundance in the brains of both fetuses and rat pups 12 days after birth, and increases brain injury in response to hypoxia and ischemia in neonatal rats (322, 558). Notably, inhibition of DNA methylation at a critical window in the brain development significantly increases the brain susceptibility to hypoxic-ischemic injury and worsens the adverse outcomes of long-term neurobehavioral function in an HIF-1α-dependent manner (557). These findings reveal a new epigenetic mechanism of promoter methylation in regulating HIF-1α expression in fetal hypoxia-induced programming of ischemic sensitive phenotype in the developing brain.
1. Hypoxia and DNA methylation
Genomic regions are differentially methylated during organismal development. Following fertilization, the sperm-derived pronucleus undergoes genome-wide demethylation. This is followed by a second wave of demethylation during primordial germ cell development. Subsequently, the genome undergoes de novo remethylation process to establish the basic bimodal methylation pattern observed at the time of implantation. This methylation organizes a fixed expression pattern in the genome, by which tissue/cell specific genes are globally repressed, while housekeeping genes are active in all types of the cells. DNA methylation plays an important role in several cellular and molecular mechanisms that control the establishment of cellular identity, silencing of transposon elements, parental imprinting, X-chromosome inactivation, and cellular differentiation (74) and is a key factor in developmental plasticity.
DNA methylation plays an important role in placental trophoblast differentiation and function. Genome-wide DNA methylation analysis reveals that a subset of functionally relevant genes undergoes hypomethylation in the differentiation from villous cytotrophoblasts into multinucleated syncytiotrophoblasts in human placentas (1129). Importantly, hypoxia counteracts and induces hypermethylation in ~50% of CpGs that would otherwise become hypomethylated upon trophoblast differentiation (1129). Interestingly, widespread DNA hypomethylation at gene enhancer regions is observed in placentas associated with early-onset preeclampsia (87, 1130). A large differentially methylated region (DMR) in the gene body of death domain-associated has been identified as a common feature of placental trophoblast differentiation, preeclampsia, and response to hypoxia (87, 741, 1129).
A number of studies have highlighted the diverse manner in which the developing fetus responds to hypoxic stress, and many have investigated gene expression differences in response to hypoxia at the global level. The rat embryo responds to hypoxia by the upregulation of glycolysis-related, calcium homeostasis-related, and inflammatory genes (particularly as related to oxidative stress), while cell growth-related genes are downregulated (397). In general, the murine embryo appears to respond to hypoxia through several adaptive mechanisms, including upregulation of genes involved in erythropoiesis, as well as heme and iron metabolism, and genes involved in proteolysis and peptidolysis. These varied responses suggest that the developing organism responds by raising its oxygen carrying ability, as well as increasing metabolic and antioxidant responses, and initiating tissue growth, turnover, and remodeling. Moreover, epigenetic changes involved in extracellular matrix remodeling, the modulation of apoptosis, and altered cellular metabolism, seem to be crucial steps in the physiological adjustments in response to hypoxia. Gestational hypoxia reduces global DNA methylation levels in the developing brain and increases hypoxia-ischemia-induced brain injury after birth in rat pups (558). Moreover, inhibition of DNA methyltransferases by 5-aza-2′-deoxycytidine during postnatal days 1–3 has been shown to disrupt neurobehavioral profiles and inhibit sex differences of neurobehavioral phenotypes in the adult (557). In addition to the changes in global DNA methylation, several studies in rats have revealed hypoxia-induced gene-specific DNA hypermethylation in developmental programming of ischemic-sensitive phenotype in the heart and brain (322, 534, 560, 656, 784, 785, 1080, 1089, 1090, 1104, 1151). In the developing heart and brain, fetal hypoxia induces promoter hypermethylation of the glucocorticoid receptor (GR) gene and epigenetic repression of the GR in the offspring (322, 1089, 1104). This leads to an increase in heart and brain vulnerability to hypoxic-ischemic injury (322, 1089). The GR gene (NR3C1) structure is highly conserved between the human and rodent, which contains multiple 5′-untranslated regions (5′UTR) (1091). Differential methylation of untranslated exon 1s at 5′UTR provides a rigorous epigenetic regulation in fine-tuning tissue/cell specific GR expression patterns, and plays an important role in stress-mediated programming of GR expression and function later in life (322, 640, 1089). Another example of gene-specific hypermethylation involved in fetal hypoxia and increased heart susceptibility to ischemia-reperfusion injury in offspring is protein kinase C-ε (PKCε) gene. A clear and cause-and-effect relationship has been demonstrated between PKCε gene repression by promoter hypermethylation and fetal hypoxia-induced programming of ischemic-sensitive phenotype in the heart (784, 785, 1108). Taken together, these findings in animal models reveal a direct and causal effect of gestational hypoxia in the regulation of gene-specific DNA methylation in the developing fetus and pathophysiological consequences in the heart and brain in offspring.
DNA methylation is catalyzed by DNA methyltransferases (DNMTs). There are three major DNMTs with methyltransferase activity and are generally grouped into maintenance DNMTs (DNMT1) and de novo DNMTs (DNMT3a and DNMT3b). Importantly, DNMT1 knockdown is embryonic lethal in both heterozygous and homozygous mice (551). However, both Dnmt3a+/− and Dnmt3b+/− heterozygous mice are grossly normal and fertile. Of vital importance, homozygous Dnmt3a−/− mice appear normal at birth, but growth is stunted postnatally, and animals die at ~4 wk of age (750). In contrast, homozygous Dnmt3b−/− mice die at embryonic day 9.5 (750). Importantly, hypoxia is known to upregulate DNMT expression (389, 718, 1038); however, whether and to what extent hypoxia-mediated developmental programming is regulated by DNMTs remain to be determined.
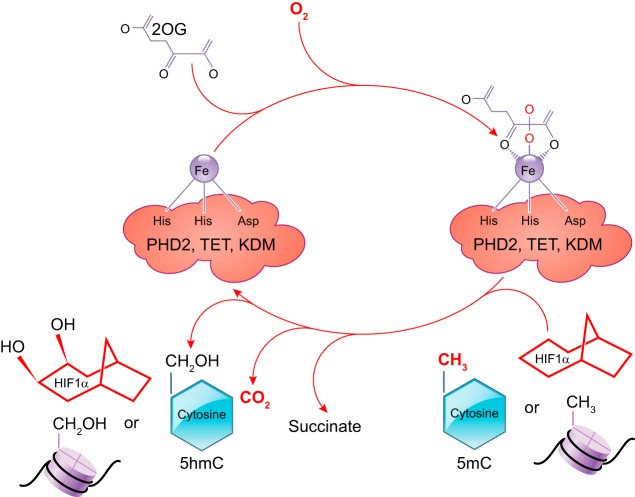
DNA methylation is dynamically regulated by both DNMTs and TETs. TET-mediated DNA demethylation plays an important role in embryonic stem cell maintenance and inner cell mass specification (416). TET1 or TET2 knockout mice are viable; however, TET1 knockout mice are small in body size (211). Double knockout of both TET1 and TET2 in mice results in an increase in DNA methylation at various imprinted loci, and a small number of these mice show mid-gestation abnormalities with perinatal lethality and others are viable and normal (211). TET3 knockout studies demonstrated that it localizes in both the maternal and paternal pronucleus and plays a role in active demethylation; however, the gene knockout has no significant effect on fertilization or other aspects of embryonic and fetal development, but it results in neonatal lethality. Although individual knockdown of TETs did not produce much effect on in utero development, the triple knockout of all the three TETs led to impaired embryonic stem cell differentiation and complete arrest of embryonic development (210). Thus these studies demonstrate the redundant nature of these isozymes involved in the embryonic and fetal development. TETs are 2-oxoglutarate-dependent dioxygenases and their activity is oxygen dependent (780, 1120). Hypoxia causes a potent inhibition of the TET activity (970). Of great interest, the enzyme prolyl hydroxylase dioxygenases (PHD) that hydroxylates HIF-1α under normoxia is also a 2-oxoglutarate-dependent dioxygenase, and its activity is dependent on oxygen and 2-oxoglutarate. Thus it is entirely possible that under hypoxia TET-mediated epigenetic modification and HIF stabilization act together and coordinate in the regulation of hypoxia-inducible genes (FIGURE 6). Indeed, this notion is supported by a recent finding that TET1 or TET3 silencing differentially regulated HIF target genes (531).
FIGURE 6.
Illustration of the mechanisms of the three oxygen-dependent dioxygenases: prolyl hydroxylase 2 (PHD), ten-eleven translocation methylcytosine (TET), and lysine demethylase (KDM)-mediated modifications of HIF, cytosine on DNA, and histone tails, respectively.
2. Hypoxia and histone modifications
Four major histone variants (H2A, H2B, H3, and H4) form the nucleosome core, around which 146 base pairs of DNA are wrapped. Of note, histones H3 and H4 have long tails that protrude out from the nucleosomes. However, the wrapping of positive charged DNA around the negative histones can be loosened or tightened by altering the charges of the amino acid residues of histones by chemical modifications such as acetylation that neutralizes the nitrogen charge of the lysine residues at the histone tail and hence loosens the histone-DNA interaction. Moreover, gene transcription is regulated by several other histone modifications such as methylation, phosphorylation, ubiquitination, SUMOylation, farnesylation, citrullination, and ADP-ribosylation. These changes constitute a “histone code” in regulating gene expression and splicing. The two major histone marks that have been extensively studied are acetylation and methylation. Depending on the type of modification and the specific amino acid at a particular position, histone modifications can activate or inhibit the gene expression. For instance, H3K4me3 is known to activate gene transcription, whereas H3k27me3 inhibits transcription (811). Importantly, histone demethylation is also regulated by 2-oxoglutarte dioxygenase enzymes that are activated by oxygen (875) (FIGURE 6). Many studies have demonstrated that histone methylation is significantly altered by hypoxic stress (441, 811). There are six classes of these dioxygenase enzymes with histone lysine demethylase activity (KDM2–7), which can remove both activating and repressing methyl groups from the chromatin (440). Several studies have demonstrated that hypoxia increases the expression of KDM3 gene, which removes methyl groups from the H3K9 repressive site and activates gene expression (804, 879). Of note, H3K9 methylation has also been observed throughout the meiotic maturation of human oocytes and the embryo development (816). Additionally, H3K4 methylation has been demonstrated to be crucially involved in the heart development (35). Moreover, it has been shown that methylation of H3K27 is a key factor in B cell development (816). The differential regulation of gene activation and repression by H3K4 and H3K27 have been shown to keep the key developmental genes repressed in embryonic stem cells but ready for later activation upon differentiation stimuli to different lineage cells. Additionally, histone H3 lysine 9 trimethylation (H3K9me3) of donor cell genome as a significant barrier for efficient reprogramming by somatic cell nuclear transfer and use of donor somatic cell nuclei depleted of H3K9 methyltransferases significantly improved the nuclei transfer (629). Thus modifications of these genes by chronic hypoxia during development may have long-term consequences on gene expression and cell lineage differentiation (849).
Another important histone modification, histone acetylation also has been shown to mediate the transgenerational effect of hypoxia on gene expression as well as acclimatization responses (521) and be involved in development (25, 117). Histones are acetylated by enzyme histone acetyl transferases (HATs) that are grouped into different families and work as multi-subunit complexes with other proteins, of which p300/CBP, PCAF, SRC are known to associate with HIF-1α and directly regulate its transcriptional activity (39, 441, 1039). Importantly, p300/CBP has been shown to be crucial for HIF-1α-mediated transcriptional activation (39). HIF-1α binds to p300/CBP affecting chromatin remodeling and gene transcription. Other than HATs, the group of enzymes which remove acetyl marks are known as histone deacetylases (HDACs). HDAC inhibitors have been shown to provide neuroprotection following hypoxic insult (429) and also provide protection against hypoxia-induced vascular remodeling (956). Histone acetylation has also been implicated to play a major role in nervous system (175), cardiovascular system (1028), and skeletal muscle development (685). Hypoxia is known to regulate histone acetylation (803) and have a profound role in developmental plasticity. In a rat uterine artery ligation model of intrauterine ischemia/hypoxia, histone acetylation was dramatically altered, decreasing expression of genes associated with the development of the pancreas, with associated decrease in hormonal neurotrophic factors, and development of pulmonary hypertension (801, 1097). Importantly, histone acetylation/methylation precedes DNA methyltransferase or TET methylcytosine dioxygenase binding and promoter methylation/demethylation (1159), and these mechanisms function cooperatively. These studies emphasize the role of multiple trophic inputs to development, many of which are as yet unappreciated. In addition, they add emphasis to the urgency to uncover the molecular basis of hypoxia-mediated epigenetic regulation in the development.
3. Hypoxia and noncoding RNAs
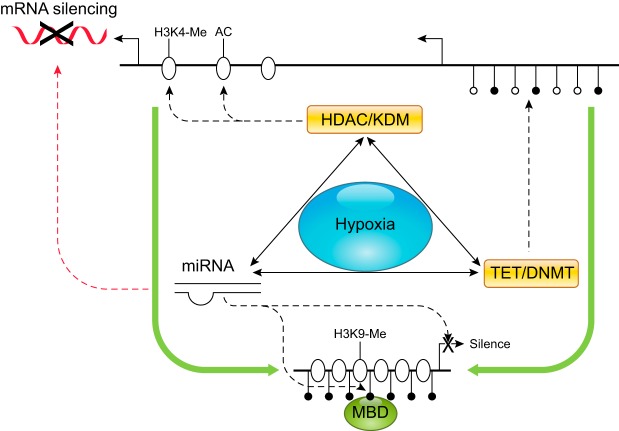
In addition to DNA methylation and histone modification that modulate gene transcription, regulatory noncoding RNAs including microRNAs (miRNAs), lncRNAs, small nucleolar RNAs, and circular RNAs function to fine-tune gene expression primarily at the translation level. MiRNAs are important players in the epigenetic control of gene expression, and silence gene expression by binding to the 3′-untranslated region (3′UTR) of transcripts via their seed sequences at 5′ ends (nucleotides 2–8), resulting in transcript degradation or translational inhibition of the target genes. More than 1,000 miRNAs have been identified in the human genome. A number of miRNAs are regulated by hypoxia and are termed “Hypoxamirs” (141, 142). Among them, miRNA-210 is The Master Hypoxamir and its de novo synthesis is upregulated by hypoxia in all species and cell types that have been studied, including humans and rodents. Mature miRNA-210 of 22 nt is highly homologous and is identical among the human, ovine, bovine, and rodent. The proximal promoter immediately upstream of miRNA-210 stem-loop structure harbors four HREs that are highly conserved across species and are responsible for the robust induction of miRNA-210 promoter by hypoxia (142, 626). It has been shown that miRNA-210 is essential to regulate ischemic-sensitive phenotype in the developing brain and heart (605, 626, 1025). Importantly, miRNA-210 appears to be a common mechanism in preeclampsia and gestational hypoxia. Elevated placental expression of miRNA-210 and circulating miRNA-210 levels have been demonstrated in both preeclampsia and pregnancy at high altitude (37, 183, 348, 391, 538, 700, 800, 1096). Of great interest, TET1 and TET2 are miRNA-210 target genes and are suppressed by miRNA-210 (391). This provides an example of interactions between miRNAs, DNA methylation, and histone modification in a highly sophisticated manner in hypoxia-mediated epigenetic regulation of gene expression (FIGURE 7). Additionally, many miRNAs have been discovered which regulate various aspects of embryonic and fetal development (437, 924). A recent study examined the role of miRNAs in embryonic development under hypoxic environment and identified ~22 miRNA families that have the ability to control embryonic survival under hypoxia (457). Of further relevance, six miRNAs were discovered circulating in maternal blood with pregnancies complicated with fetal hypoxia (1054). The authors suggested that these miRNAs may be used to identify fetal stress as a consequence of in utero hypoxia. However, much has yet to be discovered about the precise role of these miRNAs in hypoxic stress and fetal development.
FIGURE 7.
Cross-talks among DNA methylation, histone modifications, and micro RNA (miRNA) under hypoxia. Hypoxia impacts gene activities through altering DNA methylation and histone modification machineries and miRNAs. DNA methylation-induced chromatin silencing can be facilitated by the coupling action of covalent modifications of histone and vice versa. Interactions among histone deacetylases (HDACs), histone methyltransferases (HMTs), and methyl CpG binding proteins (MBD) promote the recruitment of DNA methyltransferases (DNMTs). Histone lysine demethylases (KDMs) and ten-eleven translocation enzymes (TETs) regulate gene expression by catalyzing the removal of methyl moiety from histone lysine residues and cytosine residues, respectively. miRNAs usually suppress protein expression by increasing the degradation or inhibiting the translation of mRNAs. In addition, miRNAs can modulate gene transcriptional activities via their regulation of HDACs, HMTs, TETs, and DNMTs. Furthermore, the expression of miRNAs is subject to regulation by other epigenetic mechanisms such as DNA methylation. AC, acetylation; H3K4, histone H3 lysine 4; H3K9, histone H3 lysine 9; Me, methylation.
Unlike miRNAs, lncRNAs have the ability to fold into complex secondary and tertiary structure and may serve as sponges for miRNAs or provide a scaffold for proteins to form regulatory complexes (844). Several lines of evidence suggest that lncRNAs also play a significant role in organismal development. For instance, lincRNA-RoR regulates reprogramming of human induced pluripotent stem cells (582). Similarly, lincRNA ES1, ES2, and ES3 promote pluripotency and neuronal differentiation indicating a vital role in human brain development (728). In addition, linc-MD1 regulates muscle differentiation by acting as competing-endogenous RNA in mouse and human myoblasts (135). Linc-MD1 “sponges” miRNA-133 and -135 to regulate the expression of transcription factors MAML1 and MEF2C that activate muscle-specific gene expression (135). LncRNAs have been implicated in cardiovascular development (518). Initial reports of stem cell-based studies have highlighted the involvement of lncRNA Braveheart (AK143260; Bvht) in cardiac development (491). Similarly, Kcnq1 is reported to play a pivotal role in heart development during embryogenesis (504). Additionally, lncRNA-Fendrr, a lateral-mesoderm specific lncRNA, has been shown to play a functional role in chromatin modifications and is an essential regulator of heart and body wall development in a mouse knockout study (338). Of critical importance, recent evidence suggests that lncRNAs are induced by hypoxia (658), providing new leads regarding their role in several physiological and pathological conditions where hypoxia is an associated factor. However, further studies are needed to identify the role of lncRNAs in hypoxia-induced developmental plasticity.
D. Perspective
The impact of gestational hypoxia in maternal health and developmental plasticity is a multifaceted process of cellular responses and is a function of genotype, “epigenotype,” cell lineage, developmental age, metabolic state, and other factors. Although it is clear that epigenetic modification is essential in fetal stress-mediated developmental plasticity, many questions remain unanswered. Emerging evidence suggests the crosstalk among various epigenetic regulators in hypoxia (or any other stress) during in utero life, yet the challenge is to identify the pathways that involve the three major players, DNA methylation, histone modification, and microRNA in determining the “epigenotype.” In addition, the rapidly evolving developments in bioinformatics and analytical technologies, and recent release of human proteome database (484, 1055) hold a great promise in the omics approaches of transcriptome, proteome, and metabolome to understand the ultimate phenotype and physiological and pathological consequences in developmental programming of health and disease (FIGURE 8).
FIGURE 8.
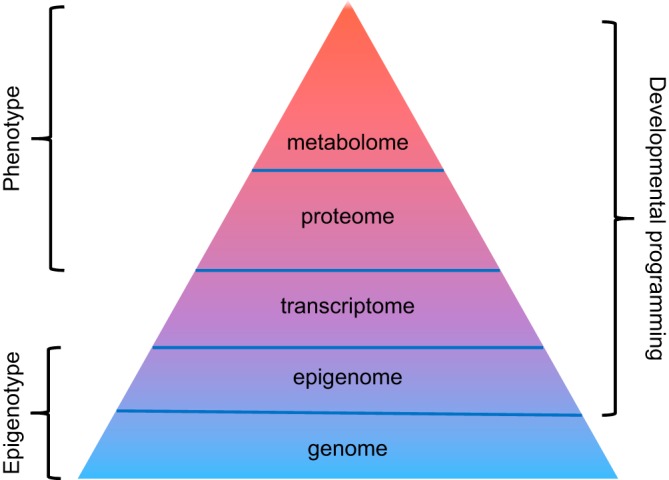
A journey from epigenotype to phenotype. The function of an organism is guided by genome that contains the organism’s complete set of genetic information (i.e., DNA). The epigenome, consisting of chemical modification of DNA and histones, regulates the expression of genes within the genome. Whereas the genome is relatively static, the epigenome is dynamic and can be altered by environmental factors. The transcriptome comprises the complete set of messenger RNA (mRNA). The proteome (the complete set of proteins expressed by a genome) is constructed by translation of mRNAs, whereas the metabolome represents the collection of all low-molecular-weight metabolites in a biological sample. Both endogenous and exogenous factors exert their influence on a living organism by altering levels of proteins and especially metabolites. Accordingly, both proteome and metabolome provide direct functional readouts of cellular activity and physiological status and serve as surrogates to the phenotype. Thus epigenome, proteome, and metabolome all contribute to developmental programming of adult diseases.
IV. HIGH-ALTITUDE HYPOXIA AND UTEROPLACENTAL CIRCULATION
A. Humans and High Altitude
The planet Earth is surrounded by the atmosphere, a gaseous mixture consisting of nitrogen (78%), oxygen (21%), and other gases (1%). At sea level, the atmospheric (barometric) pressure is 760 mmHg (101 kPa) and the partial pressure of oxygen (Po2) is 160 mmHg (21.3 kPa). Barometric pressure and Po2 decrease as altitude increases. At an altitude of 4,000 m, an average elevation of the Tibetan plateau, the barometric pressure is ~475 mmHg (63 kPa) and Po2 is ~100 mmHg (13.3 kPa), suggesting that oxygen availability at this altitude is only ~60% of that at sea level. The fall in Po2 reduces the driving pressure for gas exchange in the lung because the transfer of oxygen from alveoli to pulmonary capillaries is primarily determined by the partial pressure gradient. Not surprisingly, maternal arterial Po2 decreases from 91 mmHg at 400 m to 55 mmHg at 3,600 m.
Approximately 140 million people permanently live at altitudes higher than 2,500 m worldwide, mainly on the Tibetan, the Andean, and the East African plateaus. The highest permanent residence is a mining village of over 7,000 people in La Rinconada, Peru at 5,100 m. Tibetan and Andean habitations started ∼25,000 and ∼12,000 yr ago. In contrast, the residence of Europeans and Hans in Andes and Tibet has been only for ~400 and ~70 yr, respectively. The primary challenge to reside at high altitude is decreased Po2, although high altitude is also associated with low temperature, aridity, and ultraviolet radiation. Despite these stresses, humans have successfully lived and reproduced at high altitude for hundreds of generations, although the adaptation varies among the populations. Tibetans and Andeans appear to be well adapted to high-altitude environments through natural selection.
B. Pregnancy at High Altitude
Gestational stresses have enormous impacts on both maternal and fetal health. Intrauterine growth restriction (IUGR) [also termed fetal growth restriction (FGR)] is the failure of the fetus to achieve its growth potential and is defined as newborn weight below the 10th percentile. IUGR is one of the leading causes of perinatal mortality and morbidity and is also associated with an increased risk for cardiovascular and metabolic diseases later in life (722, 930). The occurrence of IUGR is threefold higher at high altitude, and infant birthweight is lower in all high-altitude populations (452, 474, 661, 929, 1000). IUGR primarily occurs in the third trimester, a period that the fetus markedly increases in size and weight (511, 1000). It is estimated that birthweight decreases at a rate of ~100 g/1,000 m elevation above 2,500 m; however, the frequency and magnitude of this birthweight reduction depend on the duration of acclimatization to high altitude. The incidence of IUGR is fivefold higher in Europeans than in Andeans at high altitude (449). Moreover, high altitude-induced birthweight reduction in indigenous high-altitude residents Tibetans and Andeans is significantly less than that seen in nonindigenous high-altitude Han and European dwellers. At 4,000 m, birthweight reduction is ~300 g for both Tibetan and Andean infants, whereas the reduction is >400 g and >700 g for European and Han newborns, respectively (735, 1146). At altitudes of 2,700–3,800 m, Tibetan newborns are 300–530 g heavier than Han newborns (682). Similarly, Andean infants weigh 253 g more than European infants at 3,100–4,100 m (454).
Pregnant women at high altitude are more prone to develop preeclampsia. Preeclampsia is a pregnancy-specific syndrome characterized by the new onset of hypertension (>140 mmHg systolic or >90 mmHg diastolic) after 20 wk of gestation. It is a leading cause of maternal and fetal morbidity and mortality. The incidence of preeclampsia was approximately two- to threefold higher in pregnant women at high altitude than their counterparts at low altitude (474, 679, 758). Notably, the rate of preeclampsia in Tibetan women was approximately half of that in Han women (5.9 vs. 10.3%) (662). However, the incidence of preeclampsia in native Tibetans and Andeans at high altitude are still higher than their counterparts at low altitude (474, 661). The increased incidence of preeclampsia is a significant contributor to high altitude-induced IUGR (109, 474). High-altitude Andeans with preeclampsia/gestational hypertension had 6.3-fold higher frequency of low-birth weight infants, and infants born to Andeans with preeclampsia and gestational hypertension are ~650 g lighter than newborns to normotensive Andeans at high altitude (109, 110).
Both preeclampsia and IUGR have a profound impact on both maternal and offspring health. Preeclampsia is associated with increased risk of cardiovascular diseases later in life (368) as well as in offspring (302). Offspring born to mothers with preeclampsia at high altitude developed pulmonary artery hypertension, showing higher pulmonary artery pressure and lower flow-mediated dilation (430). Impaired fetal growth at high altitude is associated with higher mortality (637, 1000). Moreover, IUGR is also shown to program an increased risk for cardiovascular disease late in life (19, 977).
In a sheep model of high-altitude pregnancy, Parraguez et al. (773) demonstrated that high-altitude impaired fetal sheep growth, leading to reduced fetal weight, although Penninga and Longo (792) observed reduced fetal weight only in twin pregnancy but not in singleton pregnancy at high altitude. Pregnancy induced a significant decrease in mean arterial pressure in low-altitude sheep, and this adaptation was absent in animals acclimatized to high-altitude hypoxia, leading to increased mean arterial pressure in high-altitude pregnant sheep relative to low-altitude animals (391). Moreover, induction of chronic hypoxia during pregnancy, which simulates high-altitude pregnancy, has been conducted in animal models such as mice, rats, guinea pigs, and sheep (106, 423, 864, 976, 1076, 1164). In these sets of experiments, animals were exposed to hypoxia (10–13% O2) for various durations (between 6 and 21 days of gestation for rodents and between 30 and 138 days of gestation for sheep). Gestational hypoxia in both rodents and sheep reduced fetal and birth weight (106, 423, 864, 976, 1076), and induced preeclampsia-like symptoms (21, 864, 976, 1164), supporting the notion that reduced oxygen availability at high altitude is a causative factor in IUGR and preeclampsia.
C. Uteroplacental Circulation in Normal Pregnancy
Fetal growth and development require continuous supplies of nutrient and oxygen. Successful pregnancy is dependent on adequate perfusion of the placenta via uterine circulation. Changes in uterine and placental circulation contribute to either successful adaptation or maladaptation. Both human and animal studies revealed a causative role of higher uterine vascular tone and lowered uterine blood flow in IUGR and preeclampsia (109, 110, 500, 527).
To accommodate the growth and development of the fetus, pregnancy is accompanied by significant physiological changes of the cardiovascular system. Blood volume increases by 40–50%. Cardiac output also progressively increases in the first two trimesters owing to increased heart rate and stroke volume, reaching an ~50% increase at week 20 of pregnancy and remains elevated until term. Mean arterial pressure slightly falls at the second trimester and then slowly returns to prepregnancy level. However, the most dramatic changes occur in the uterine circulation, and adequate adaptation of uterine hemodynamics is essential for successful pregnancy.
In the human and other mammals, blood supply to the uterus is mainly provided bilaterally by uterine arteries, with a minor contribution from ovarian arteries. More than 80% of total uterine blood perfusion is provided by uterine arteries and the remainder is supplied by ovarian arteries in both rhesus monkey and sheep (858, 1048). The anatomy of uteroplacental circulation and remodeling of uterine arteries have been comprehensively reviewed elsewhere (119, 214, 753). A brief description of uteroplacental circulation is given as follows. Uterine arteries arise from internal iliac arteries. At the outer layer of the uterus, uterine arteries branch into arcuate arteries that enter the myometrium and ramify to radial arteries. At the myoendometrial border, radial arteries split into basal and spiral arteries. Whereas basal arteries extend laterally and supply the basal layer of the endometrium and adjoining myometrium, spiral arteries exclusively supply the endometrium. Main uterine arteries, arcuate and radial arteries during pregnancy undergo remodeling to increase their lumen sizes. The diameter of main uterine arteries doubles during human pregnancy. Similar changes have also been observed in other species including sheep and rodents. Uterine arteries also elongate during pregnancy in part due to the fetal development-induced enlargement of the uterus. Spiral arteries undergo remodeling following placentation. Extravillous cytotrophoblast cells invade into the lumen of spiral arteries and subsequently replace endothelial and vascular smooth muscle cells from the arterial wall. The diameter of spiral arteries increases from 200 μm in the nonpregnant state to 500 μm in the pregnant state (214). At term, the diameter of the vessel at the myometrial-endometrial boundary could reach up to 2.4 mm (121). According to Poiseuielle’s law, blood flow (Q) is directly related to the pressure difference between the two ends of a vessel (∆P) and radius of the vessel (r) and inversely related to the viscosity of the blood (η) and vessel length (L): . It is obvious that artery caliber is a very powerful determinant of blood flow. Therefore, the remodeling of spiral arteries leads to the establishment of widened, low-resistance vascular channels that carry the dramatically increased maternal blood flow to the placenta.
The placenta is a temporary organ developed during pregnancy to connect the fetus to the mother. Placenta structure and development have been recently reviewed (120). Structurally, the human placenta is an interface between the maternal and fetal circulation, composed of a basal plate (maternal surface) and anchoring villi, terminal villous unit for gas and nutrient exchange, the chorionic plate (fetal-side surface) and stem villi. Villi are formed from trophoblast through vasculogenesis and angiogenesis. The number, volume, and surface area of the capillary profiles within villi gradually increase in the first and second trimester, and this increase accelerates in the third trimester. In the sheep placenta, capillary growth and total capillary volume increase dramatically after day 18 of pregnancy, and this growth lasts the whole pregnancy (840). Through spiral arteries, maternal blood enters the placenta to perfuse intervillous spaces and to stream around the villi where transfer of oxygen and nutrients to the fetus and removal of waste products from fetal blood occur.
Uterine blood flow in the nonpregnant state is relatively low. Measurements in nonpregnant women and sheep reveal that blood flows to the uterus at a rate of 20–50 ml/min, corresponding to 1–3% of the cardiac output (79, 858). Uterine blood flow at near term pregnancy rises to 800–1,000 ml/min in the human (501, 759) and ≥1,000 ml/min in sheep (609, 858), and approximate 20% cardiac output perfuse the uterus in human and sheep at term (759, 854). As mentioned above, the establishment of placenta and remodeling of uterine arteries play a role in lowering uterine vascular tone and increasing uterine blood flow. Other factors also contribute to reduced uterine vascular resistance. Uterine vasopressor responses are blunted during pregnancy (369, 713). Local release of endothelium-derived vasodilators such as nitric oxide (NO) and prostacyclin increase as pregnancy advances (346, 618). Importantly, the myogenic tone of uterine arteries decreases in pregnancy (1073). This is primarily achieved by the upregulation of the estrogen receptor-α (ERα) and BKCa channel in the vessel. The BKCa channel is a major regulator of vascular tone (536). Many studies have established important roles of ERα and BKCa channels in the regulation of uterine circulation (162, 163, 393, 480, 610, 856). The expression of ERα and BKCa channel β1 subunit in uterine arteries is governed by the methylation status of their gene promoters (162, 163, 203), which will be discussed later.
Uterine blood flow to the placenta is limited in the first trimester, but increases markedly starting at ~12 wk of gestation (428). A pressure gradient exists in the uteroplacental circulation, and the blood pressure is 80–100 mmHg in uterine arteries, 70 mmHg in spiral arteries, and only 10 mmHg within intervillous space (1036). Studies in sheep and rodents revealed that 80–90% of total uterine blood flow perfused the placenta at term (789, 858). Maternal-fetal exchange takes place as blood passes over the surface of the placental villi.
D. Uteroplacental Circulation in Pregnancy at High-Altitude Hypoxia
Accumulating evidence suggests that long-term high altitude has immense effects on cardiovascular function. Compared with their counterparts at low altitude, pregnant women residing at high altitude had 31% lower blood volume and 16% lower cardiac output (460, 1143). Mean arterial blood pressure during pregnancy decreased at 1,260 m but not at 3,100 m in Colorado (758). The pregnancy-associated decrease in mean arterial blood pressure was also absent in high-altitude sheep (391). Preeclampsia at 3,100 m caused an additional 14% decrease in blood volume and a dramatic increase in mean arterial blood pressure compared with normotensive pregnancy (1144).
Uteroplacental circulation is greatly impacted by high altitude. Studies conducted in Colorado revealed that pregnant women at 3,100 m had 26% smaller uterine artery diameter and 14% lower uterine blood flow velocity than their counterparts at 1,600 m (1145). As volumetric blood flow is the product of the cross-sectional area of the vessel and blood flow velocity, these changes result in one-third lower volumetric uterine artery blood flow at high altitude. Similar patterns of reduction in both uterine artery diameter and volumetric uterine blood flow were reported in Han pregnant women at high altitude in Tibet (158). Unlike normotensive pregnancy, uterine blood flow velocity was not increased in near-term women with late-onset preeclampsia at high altitude (1144). Intriguingly, the impacts of high altitude on uteroplacental circulation appear to be ancestry specific. Andean women at 3,100–4,100 m in Bolivia had 16% greater uterine artery diameter and 34% greater cross-sectional area than European women, leading to an approximately twofold increase in volumetric uterine blood flow near term despite similar uterine blood flow velocity in both populations (454, 1063). Similar findings were also observed in Andean and European women at 3,600 m in Bolivia (1146). Pregnant Tibetans had 16% higher uterine blood flow velocity than Han women at a similar elevation (683). The reduction of uterine blood flow occurred before the development of late-onset preeclampsia at high altitude in Colorado (1144). Among high-altitude Andeans, the early-onset preeclamptic women had ~50% lower uterine blood flow velocity, 2.7-fold higher uterine vascular resistance, and >60% lower volumetric uterine blood flow than normotensive women despite similar uterine artery diameter in both normotensive and preeclamptic women, whereas volumetric uterine blood flow was not changed in the late-onset preeclampsia compared with normotensive Andeans (110). Whereas the reduction in uterine blood flow in non-native high-altitude inhabitants contributed to the lower birth weight, the greater uterine blood flow in Andeans and Tibetans partially protected against hypoxia-associated reductions in fetal growth at high altitude (110, 454, 683, 1063, 1145, 1146). However, the hemodynamic changes in native high-altitude residents Andeans or Tibetans do not reduce the incidence of preeclampsia, and its occurrence at high altitude is still two- to fourfold higher than at low altitude (474, 661). These observations suggest that different etiologies may account for IUGR and preeclampsia associated with high-altitude hypoxia.
Blood flow (Q) is also a function of the pressure gradient of inflow and outflow of a vessel (∆P) and vascular resistance (R), which can be described by modifying Ohm’s law: .Vascular tone is a major determinant of the resistance to blood flow. An increase in uterine vascular tone contributes to reduced uterine blood flow observed in high-altitude pregnancy. Indeed, high-altitude Andeans with preeclampsia had higher vascular resistance in uterine arterioles (110). This finding was reinforced by observations in an animal model. Pregnancy, through the actions of steroid hormones, suppresses pressure-dependent myogenic response that ultimately leads to reduced uterine vascular tone in sheep (1073, 1082). However, uterine vascular tone increased in pregnant sheep residing at 3,820 m for 110 days beginning on day 30 of gestation, and steroid hormone- and pregnancy-induced attenuation of uterine vascular tone was absent in high-altitude sheep (148, 149). These changes are translated into increased uterine vascular resistance in high-altitude pregnant sheep (391). Similarly, gestational hypoxia also increased uterine vascular resistance in guinea pig (995). High altitude also impairs remodeling of uteroplacental arteries. Approximate 67% of decidual portion of myometrial arteries were completely remodeled at 1,600 m, and only ~29% of the vessels were remolded at 3,100 m, representing >50% decrease in remodeling (979). Thompson et al. (976) also demonstrated that gestational hypoxia impaired the invasion of spiral arteries by trophoblasts in guinea pigs. Both preeclampsia and IUGR at sea level or low altitude were associated with incomplete remodeling of spiral arteries (470, 604). The vessels retained medial wall and were still sensitive to vasoactive factors (1005) with impaired flow-mediated dilation (515). Consequently, inadequate remodeling of spiral arteries would also lead to high uteroplacental vascular resistance and inadequate placental perfusion (1013).
Increased, similar, or decreased placental size and weight were reported at high-altitude pregnancy in both human and animal models (477, 514, 833, 979). There is also great variability in placenta morphology regarding the impact of high altitude. Augmented villous vascularization and increased capillary diameter and length densities were frequently (477, 775, 979), but not always (1006), observed in high-altitude placenta. Thus increased angiogenesis appears to be an important adaptive mechanism to hypoxia. High altitude was also reported to cause thinning and increased morphometric diffusing capacity of the villous membrane (421, 833), which is thought to facilitate maternal-fetal exchange of gas and nutrients (1137). However, it was also reported that the thickness of the villous membrane was not altered by altitude (1006). High-altitude placenta appeared to have reduced villous volume (422, 1006) and decreased villous surface area (1006). Overall, these changes represent incomplete adaptation of placenta to high-altitude hypoxia. Intriguingly, placentas from normotensive and preeclamptic pregnancy were not morphometrically different, and they had a similar villous surface area, villous membrane thickness, and unaltered morphometric diffusion capacity (979).
E. Maternal and Placental Factors on Uteroplacental Circulation at High Altitude
1. Angiogenic/antiangiogenic factors
Angiogenesis is essential for a successful pregnancy. Increased angiogenesis is a major factor that leads to increased uteroplacental blood flow during pregnancy. Vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) are two prominent proangiogenic factors that bind to tyrosine kinase receptors flt-1 (VEGFR-1) and flk-1 (VEGFR-2) to regulate vasculogenesis and angiogenesis. In addition, VEGF plays an important role in the maintenance of endothelial cell functions including NO synthesis and permeability. On the other hand, soluble fms-like tyrosine kinase-1 (sFlt-1, or sVEGFR-1) is an antiangiogenic factor that is an alternatively spliced variant of VEGFR-1 without transmembrane and cytoplasmic domains. sFlt-1 binds to VEGF and PlGF and functions as a scavenger to reduce their bioavailability, thus blocking the effects of VEGF and PlGF. The primary source of PlGF and sFlt-1 in pregnancy is the placenta. Angiogenic imbalance in circulation plays a critical role in the pathogenesis of preeclampsia (468).
It appears that free VEGF in maternal circulation is barely detectable (1140). Pregnant women at 1,600 and 3,100 m in Colorado had similar levels of total VEGF, circulating PlGF, and sFlt-1, and there was no altitude-associated alteration of sFlt-1/PlGF ratio (450, 1147). There were no associations between sFlt-1 or PlGF and uterine blood flow or birth weight at both altitudes. Altitude did not alter circulating PlGF and sFlt-1 levels in either normotensive or preeclamptic Andeans living in Bolivia (1139). However, lower PlGF and higher sFlt-1 were detected in preeclamptic women at both low and high altitudes (1139), suggesting higher sFlt-1/PlGF ratio in preeclampsia. Similarly, pregnancy with preeclampsia or IUGR exhibited higher sFlt-1/PlGF ratio in a high-altitude Ecuadorian population (482). In a rat model of growth restriction, chronic hypoxia also decreased maternal circulating PlGF and increased sFlt-1/PlGF ratio without affecting VEGF (50). Of interest, compared with Europeans at high altitude, Andeans had lower plasma sFlt-1 and sFlt-1/PlGF ratio at 20 and 36 wk of pregnancy (206). There were negative associations between sFlt-1/PlGF ratio and uterine artery diameter and between sFlt-1 and birth weight in high-altitude Europeans. Preeclampsia at low altitude was associated with higher sFlt-1 and sFlt-1/PlGF ratio as well as increased uterine arterial resistance (960). Increased expression of sFlt-1 was observed in placentas from high-altitude pregnancy and from sea-level preeclampsia as well as placentas treated with ex vivo hypoxia (726). High altitude increased VEGF expression in sheep placenta (776), which may contribute to the increased angiogenesis in the placenta described in the previous section. VEGF was also shown to upregulate endothelial nitric oxide synthase (eNOS) in endothelial cells (97) and functioned as a potent vasodilator of uterine arteries (733). Locally generated, but not circulating VEGF, might account for the increased eNOS in uterine arteries during low- and high-altitude pregnancies (733, 1072). Like VEGF, PlGF also produced very potent vasodilation of uterine arteries (752), and decreased PlGF level may lead to reduced uterine blood flow. Together, it appears that imbalance of proangiogenic and antiangiogenic factors contributes to the pathogenesis of preeclampsia regardless of altitudes. Moreover, lower sFlt-1 level and sFlt-1/PlGF ratio may contribute to greater uterine blood flow and diameters in Andeans, leading to improved fetal growth.
2. Inflammation/cytokines
Normal pregnancy is a state of mild inflammation. The pregnancy-associated inflammation is exaggerated in preeclampsia. Preeclamptic women exhibited elevated cytokines in maternal circulation and in the placenta (70, 948). Circulating syncytiotrophoblast microparticles were found to bind to monocytes, promoting the production of inflammatory cytokines. In vitro studies demonstrated that hypoxia stimulated proinflammatory and suppressed anti-inflammatory cytokines production in villous explants of human placenta (69). Near-term pregnant women residing at high altitude in Colorado exhibited higher tumor necrosis factor (TNF)-α and interleukin (IL)-6 and lower IL-10 in circulation compared with counterparts living at mild altitude (193). In contrast, both TNF-α and IL-6 were lower in Andeans and Europeans at high altitude than at low altitude (207). At high altitude, preeclamptic Andeans had elevated proinflammatory cytokines IL-6 and IL-8 compared with normotensive counterparts, whereas proinflammatory TNF-α and anti-inflammatory IL-10 were similar in both normotensive and preeclamptic women (205). Birth weight was negatively associated with IL-6 levels. Cytokines are believed to interact with endothelial cells and vascular smooth muscle cells as well as matrix metalloproteinases to cause vascular dysfunction. In a rat model, inflammation, through the action of TNF-α, impaired trophoblast invasion and spiral remodeling (190). Thus altitude-induced cytokines could elevate myometrial artery vascular resistance by impairing remodeling, leading to IUGR and preeclampsia.
3. Oxidative stress and endoplasmic reticulum stress
Imbalance of oxidant-antioxidant leads to oxidative stress. Hypoxia-induced oxidative stress has been implicated in a variety of diseases including hypertension (799). Like inflammation, mild oxidative stress in normal pregnancy is augmented in preeclampsia (467). Unexpectedly, human placental antioxidant (such as superoxide dismutase, glutathione peroxidase, and thioredoxin reductase) activity was lower, whereas lipid peroxidation and protein carbonylation were also reduced at high altitude in Colorado, leading to reduced placental oxidative stress (1141). Similarly, antioxidants taurine and inositol were higher in high-altitude human placenta (981). However, Yung et al. (1134) demonstrated that high altitude heightened oxidative stress in placentas as evidenced by increased lipid peroxidation product 4-hydroxynonenal, elevated phosphorylation of p38 mitogen-activated protein kinases, and heat shock protein 27. Placental NADPH oxidase (Nox) 4 expression was increased in preeclamptic pregnancy at low altitude (519). Intriguingly, high altitude increased placental Nox4 expression at protein level in normal pregnancy, but decreased it in preeclamptic pregnancy (519). Pregnancy and high altitude also increased catalase and superoxide dismutase activity in both Andeans and Europeans but with a greater increase in Andeans (453). Relatively short-term (3 mo) exposure to high altitude decreased but long-term (13 mo) high-altitude acclimatization enhanced plasma total antioxidant status in human (1014). It appears that elevated antioxidant capacity is an adaptive feature to high-altitude hypoxia. The improved antioxidant status in Andeans likely contributes to increased uterine blood flow and birth weight as antioxidants are able to lower uterine vascular tone as discussed below. It should be noted that placentas from term are mostly studied and sampling methods such as vaginal delivery and caesarean section may have different effects on the oxidant-antioxidant system. The status of oxidants and antioxidants in the placenta during early pregnancy at both low and high altitude is largely unknown.
Unlike the human, experimental animals develop oxidative stress when exposed to high altitude and chronic hypoxia. Plasma urate and l-cysteine concentrations and placental levels of 4-hydroxynonenal and heat shock protein 70 were increased in pregnant rats following exposure to chronic hypoxia (843). Vitamin C treatment prevented oxidative stress and increased fetal weight. Both high-altitude native and newcomer ewes displayed higher plasma carbonyls and malondialdehyde, lower plasma 17β-estradiol levels, lower lamb birth weight compared with low-altitude animals (774, 778). Supplement with vitamins C and E lowered oxidative stress, elevated 17β-estradiol levels, and improved birth weight. In sheep, both high altitude and ex vivo chronic hypoxia treatment promoted oxidative stress in uterine arteries, and increased expression of Nox2 was probably responsible for the heightened oxidative stress (392, 1077). Hypoxia-induced oxidative stress triggered downregulation of BKCa channel β1 subunit and suppressed both channel activity and function, leading to the elevated myogenic tone of uterine arteries (392, 1077, 1169). The impaired BKCa channel expression, activity, and function in uterine arteries could be modified by antioxidants.
The endoplasmic reticulum (ER) is a specialized cell organelle for synthesis, folding, modification, and transport of proteins. Many pathophysiological conditions disturb the ER function, leading to an accumulation of misfolded proteins in the ER that activates unfolded protein response (UPR) (292). Prolonged ER stress could lead to apoptosis (949). ER stress has been implicated in various cardiovascular disorders (665). Hypoxia induced ER stress in human umbilical vein endothelial cells (1111). Dilation of ER cisternae, increased phosphorylation of eukaryotic initiation factor 2 subunit α (P-eIF2α), reduced AKT phosphorylation, and reduced P-4E-BP1 were observed in high-altitude placentas (1134), suggesting the occurrence of ER stress and protein synthesis inhibition. Relevantly, ER stress was found to contribute to the pathophysiology of IUGR and preeclampsia with IUGR. Increased P-eIF2α was observed in placentas from both IUGR and preeclamptic pregnancies complicated with IUGR (1133). The downregulation of AKT protein by increased P-eIF2α suppresses mTOR signaling and reduces placental growth, ultimately leading to impaired fetal growth. Of interest, activation of UPR pathways was observed only in early-onset, but not in late-onset preeclamptic placentas (1132), suggesting that early-onset preeclampsia, but not late-onset preeclampsia, is originated from placenta ER dysfunction. PlGF was found to be regulated by the UPR transcription factors ATF4 and ATF6β and elevated ER stress suppressed PlGF expression in the placenta (672). ER stress also resulted in endothelial dysfunction by impairing intermediate- and small-conductance Ca2+-activated K+ (IKCa and SKCa) channel activity (1035). ER and oxidative stresses are closely linked events (615). The interplay between them probably plays a major role in the altitude-induced dysfunction of BKCa and SKCa in uterine arteries (392, 1077, 1169).
4. Estrogen and progesterone
Estrogen levels in circulation rise progressively during pregnancy. Estrogen and its metabolites are critical for uterine vascular development and uteroplacental circulation (147, 781). Hypoxia suppressed the expression of aromatase, an enzyme that catalyzes the aromatization of androgens into estrogens, in human trophoblast cells in culture (434). Reduced levels of plasma 17β-estradiol and estriol were originally reported in pregnant women at high altitude (925, 1142). However, these findings were not corroborated by a recent study (152). Circulating 17β-estradiol levels during pregnancy increased in Andeans but remained unchanged in Europeans at high altitude. Moreover, 17β-estradiol levels were higher in Andean than European high-altitude residents of late pregnancy, which was associated with greater uterine artery diameter and uterine blood flow in Andeans. Plasma 17β-estradiol levels in pregnant ewes decreased at high altitude, and native high-altitude pregnant sheep had higher circulating 17β-estradiol than newcomer high-altitude sheep (777). Circulating levels of 17β-estradiol were lower in preeclamptic women (439). To our knowledge, no data have been published yet regarding the estrogen level in altitude-induced preeclampsia and IUGR. 2-Methoxyestradiol, an estrogen metabolite synthesized by catechol-O-methyltransferase (COMT), is implicated in the pathogenesis of preeclampsia (462). Circulating 2-methoxyestradiol and placental COMT levels were low in preeclampsia. The effect of altitude on circulating 2-methoxyestradiol and COMT expression in the placenta remains to be determined.
It should be noted that estrogen needs to interact with its receptors to elicit biological responses. Even though circulating levels of estrogen may not be altered by high altitude, impairment of estrogen receptors by gestational hypoxia could still disrupt estrogen signaling pathways. Recent studies demonstrated that high-altitude hypoxia suppressed ERα expression and ERα-associated regulation of uterine vascular functions. Thus high-altitude hypoxia repressed ERα coding gene ESR1 via hypermethylation of two transcription factor binding sites, USF-15 and Sp1-520, in the promoter region of the gene (203). Estrogen-mediated upregulation of nitric oxide synthase (NOS), ERK ½, and BKCa channel β1 subunit are of critical importance in lowering uterine vascular tone and increasing uterine blood flow during pregnancy (390, 393, 611, 855, 857, 1082). The consequence of downregulation of ERα is diminution of estrogen-mediated upregulation of MAPK3/MAPK1 (encoding ERK ½) and KCNMB1 (encoding BKCa channel β1 subunit) in uterine arteries during pregnancy, leading to increased uterine vascular tone (149, 163, 394, 1084, 1169). Additionally, estrogen-induced upregulation of SK3 channel expression and function in uterine arteries during pregnancy was ablated by gestational hypoxia, probably being the result of ESR1 repression (1168, 1169). Estrogen also participated in remodeling of uterine vessels by stimulating DNA synthesis and proliferation of endothelial and vascular smooth muscle cells through interacting with ERα and/or ERβ (438, 475, 476). Downregulation of ERα is probably an important contributing factor for altitude-induced reduction in uterine artery diameter (1145).
Maternal plasma 17β-estradiol levels in Andeans were 1.5-fold of that in Europeans at high altitude, although there was no difference between ancestries at low altitude (152). Interestingly, uterine blood flow in pregnant Andeans was approximately twofold higher than Europeans at high altitude (454). Uterine blood flow positively correlated with circulating estrogen levels during gestation (134). The high uterine blood flow in Andeans was highly likely the result of estrogen-induced upregulation of NOS and BKCa channel β1 subunit in uterine arteries (390, 393, 611, 857, 1082). The direct vasodilation of uteroplacental vessels by estrogen via releasing NO and activating BKCa channels may also contribute to the increase in uterine blood flow (856).
Maternal plasma levels of progesterone steadily increase during pregnancy. However, conflicting observations have been reported regarding the plasma level of progesterone in preeclampsia. Parker et al. (771) found no difference in plasma progesterone and 5-α-pregnane-3,20-dione (5-α-dihydroprogesterone) between normotensive pregnancy and pregnancy-induced hypertension. Circulating progesterone was later reported to be increased in preeclamptic women (874). However, recent studies revealed a decrease in plasma progesterone in preeclampsia (488, 996). Interestingly, increased serum progesterone was observed in pregnant women at high altitude (1142). Parraguez’s group measured and compared plasma progesterone in low-altitude, naive high-altitude, and native high-altitude sheep. They found lower plasma progesterone in naive high-altitude sheep than in other sheep groups at days 17 and 21 of gestation (777). Moreover, plasma progesterone in the second half of gestation in native high-altitude pregnant sheep was lower than in low-altitude sheep brought to high altitude at 20 days of gestation (778). Although 17-α-hydroxyprogesterone, a synthetic metabolite of progesterone, improved preeclamptic symptoms and pup weight in a reduced uterine perfusion pressure rat model (28, 29), human trials showed no clear benefit of progesterone in preventing preeclampsia (652). Thus the role of progesterone in the pathogenesis of preeclampsia and gestational hypoxia-associated complications remains uncertain.
5. Endothelium and endothelium-derived factors
The endothelium is an integrated part of blood vessels and participates in regulating vascular tone via releasing vasoactive factors [i.e., NO, endothelin-1 (ET-1), and endothelium-derived hyperpolarizing factor (EDHF)] and/or activation of ion channels in endothelial cells. Moore’s group demonstrated that high altitude did not alter plasma ET-1 levels but decreased circulating nitric oxide metabolites (NOx), thus resulting in a greater ET-1/NOx ratio that was negatively associated with birth weight (450). Similarly, plasma and placental NO levels were not altered by altitude in both normotensive and preeclamptic women (969). However, Europeans at high altitude had higher ET-1 levels without normal pregnancy-associated decline of ET-1 observed at low altitude (681). In contrast, Andeans appear to maintain the ability of pregnancy-associated ET-1 decrease at high altitude and show a lower ET-1 level than Europeans.
Conflicting results have been observed in animal studies. High-altitude increased plasma nitrate concentration (1152) and expression of endothelial nitric oxide synthase (eNOS or NOS3) in uterine arteries and placenta of pregnant ewe (776, 1072). However, the finding that inhibition of NOS with NG-nitro-l-arginine had a less inhibitory effect on the relaxation induced by acetylcholine in uterine arteries from guinea pig exposed to chronic hypoxia suggests impaired NO-mediated relaxation in hypoxic animals (1053). Chronic hypoxia also suppressed endothelium-dependent relaxation of rat uterine arteries (21). Similarly, flow-mediated dilatation of uterine arteries from chronically hypoxic pregnant guinea pig was reduced (627). In a rat model, chronic hypoxia in gestation increased plasma endothelin-1 levels and the expression of prepro-endothelin-1 mRNA and ET-1 type A receptor in kidney and placenta (1164). Pregnant rats exposed to hypoxia had higher mean arterial pressure, proteinuria, and fetal growth restriction. Together, these findings suggest that the role of NO in altitude-induced dysfunction of uteroplacental circulation is probably species-dependent and that ET-1 may be a potential mediator of aberrant uteroplacental vascular function in response to hypoxia. Chronic hypoxia also impaired the activity of SKCa channel in ovine uterine arterial endothelial cells. Hypoxic treatment of uterine arteries from normoxic pregnant sheep decreased SKCa channel-mediated relaxation, and the impaired relaxation was restored by N-acetylcysteine in endothelium-intact, but not endothelium-denuded arteries (1169). In addition to oxidative stress, ER stress may also have a role in impaired SKCa function as aforementioned. SKCa channel dysfunction is a major contributor to the development of hypertension (296).
6. Hypoxia response factors
Hypoxia is the prime environmental factor encountered by living organisms at high altitude. HIF-1 is a transcription factor produced by cells in response to hypoxia, functioning as a master regulator of oxygen homeostasis. Placental HIF-1α was overexpressed in preeclampsia (519, 821). Similarly, placentas from high-altitude pregnancy also exhibited higher HIF-1α (519, 1147). However, no change in placental HIF-1α at high altitude was also reported (980). Consistently, high altitude increased placental transforming growth factor beta 3 (TGF-β3) and total circulating VEGF, two products of HIF-1-targeted genes (1147). Locally elevated VEGF was probably responsible for increased angiogenesis in the placenta of high-altitude pregnancy (775, 980). TGF-β3 was shown to impair placental explant trophoblast differentiation and invasion (128). Given that myometrial artery remodeling was impaired at high altitude (979), it is possible that TGF-β3 may contribute to this inadequate remodeling. A recent study involving both normotensive and preeclamptic pregnancy showed that, relative to low altitude, high altitude decreased placental HIF-1α in preeclampsia (519). The same study also demonstrated that placental HIF-1α in preeclampsia was lower than normotensive pregnancy at high altitude. These findings are puzzling and may suggest that etiology of preeclampsia at high altitude is different from that at low altitude. Hypoxia enhanced ET-1 expression in endothelial cells in a HIF-1-dependent way (666). The upregulation of ET-1 in endothelial cells by hypoxia probably contributed to the elevated circulating ET-1 in chronically hypoxic pregnant rats (1164). Similarly, high altitude increased circulating ET-1 or ET-1/NOx ratio during pregnancy (450, 681). Locally produced ET-1 may play an important role in regulating uteroplacental blood flow. It is not clear whether high-altitude hypoxia would alter local synthesis and release of ET-1 in uteroplacental circulation. High-altitude increased HIF-1α in ovine uterine arteries (1077). Concomitantly, expression of DNMT 3b, a product of HIF-1α-targeted genes, was also boosted by high altitude in the uterine artery (389). Reactive oxygen species (ROS) have been shown to stabilize HIF-1α under hypoxia (145) and to promote hypermethylation of gene promoters (664). Hypoxia increased production of ROS in uterine arteries in vivo and ex vivo (389). Hence, HIF-1 could be a potential mediator of oxidative stress-induced impairment of uterine vascular dysfunction.
MicroRNAs (miRs) are short noncoding RNAs silencing gene expression by targeting the 3′-untranslated region (3′UTR) of the transcript. MiR-210 is prominently upregulated by hypoxia in a HIF-dependent manner (516). Accumulating evidence suggests that miR-210 functions as a key element in cellular responses to hypoxia (141). Circulating levels and uteroplacental expression of HIF-responsive miR-210 were increased in preeclamptic (37, 538, 700, 800, 1096) and high-altitude pregnancy (183, 348, 391). MiR-210 probably contributes to the dysfunction of uteroplacental circulation via acting a variety of targets, which will be discussed in detail in the following section.
7. Single nucleotide polymorphisms and high-altitude adaptation
Efforts to identify genetic adaptations have been recently intensified. Studying single nucleotide polymorphisms (SNPs) revealed several candidate genes associated with high-altitude adaptation of Tibetans and Andeans: EPAS1 (encoding HIF-2α), EGLN1 (encoding PHD2), and PPARA (encoding peroxisome proliferator-activated receptor α) in Tibetans (65, 1122) and PRKAA1 (encoding protein kinase AMP-activated catalytic subunit α1) and EDNRA (encoding endothelin receptor type A) in Andeans (83). SNPs representing EPAS1, EGLN1, and PPARA contributed to low hemoglobin levels in Tibetans at high altitude (65, 921, 1122). PHD2 targets HIF-α subunits for degradation. A high frequency of EGLN1 SNPs, D4E and C127S, was found in Tibetans (592, 962). These two variants possess gain-of-function and would increase HIF degradation, leading to decreased erythropoiesis in Tibetans. In Andeans, maternal genotypes for PRKAA1 are associated with uterine artery diameter, whereas both PRKAA1 and EDNRA are associated with birth weight (83). Protein kinase AMP-activated catalytic subunit α1 is the catalytic subunit of 5′-AMP-activated protein kinase (AMPK). Skeffington et al. (923) recently demonstrated that high-altitude hypoxia increased AMPK levels in human placenta and in near-term murine uterine arteries and placenta. Activation of AMPK produced vasodilation of uterine artery, which may be an attributing factor to higher uterine blood flow and greater birth weight in Andeans at high altitude. SNPs of EGLN1, EPAS1, and PPARA and their potential roles in uteroplacental circulation have yet to be determined.
F. Epigenetic Mechanisms in the Regulation of Uteroplacental Circulation
1. Epigenetics in normal uteroplacental adaptation during pregnancy
Among other mechanisms, ERα encoded by ESR1 and BKCa channel β1 subunit encoded by KCNMB1 are primary mediators of pregnancy-induced adaptive changes in the uterine vasculature during pregnancy (147, 149, 393, 857). Their expression is governed by the methylation status of their gene promoters (162, 163, 203). CpG dinucleotides of the transcription factor binding sites Sp1-520 in the ESR1 promoter, and Sp1-380 in the KCNMB1 promoter were highly methylated in uterine arteries of nonpregnant sheep (162, 203). The hypermethylated Sp1 binding sites precluded transcription factor binding and repressed promoter activities. Consequently, the expression of ERα and BKCa channel β1 subunit was restrained, leading to low abundance of them in uterine arteries of nonpregnant sheep. Pregnancy is associated with significantly increased expression of ERα and BKCa channel β1 subunit in uterine arteries (149, 393, 857). Estrogen functions as an initiator of pregnancy-induced upregulation of ERα and BKCa channel β1 subunit in uterine arteries by promoting demethylation of ESR1 and KCNMB1 promoters (163, 203). Estrogen stimulated the expression of TET1 and TET2 (390), which in turn promoted active demethylation of Sp1-380 binding site in the KCNMB1 promoter (possibly Sp1-520 in ESR1) (FIGURE 9). Thus Sp1-520 in the ESR1 promoter and Sp1-380 in the KCNMB1 promoter became hypomethylated in uterine arteries during pregnancy (162, 163, 203, 390). Consequently, Sp1 and/or ERα binding to Sp1-520 or Sp1-380 increased, resulting in increased expression of ERα and BKCa channel β1 subunit (149, 390). The upregulation of ESR1 and KCNMB1 has been shown to contribute to reduced uterine vascular tone and increased uterine blood flow during pregnancy (149, 390, 393, 857).
FIGURE 9.
Epigenetic regulation of the large-conductance Ca2+-activated K+ (BKCa) channel in the uterine artery. The BKCa channel in vascular smooth muscle cells is a heteromer composed of four pore-forming α subunits and up to four auxiliary β1 subunits. It is primarily activated by Ca2+ sparks due to Ca2+ release from the endoplasmic (sarcoplasmic) reticulum via ryanodine receptors. The β1 subunit increases Ca2+ sensitivity of the channel. Opening of the BKCa channel allows K+ efflux that subsequently hyperpolarizes the cell membrane, leading to reduced global Ca2+ concentrations and vascular tone. In normal (normoxic) pregnancy, estrogen upregulates ten-eleven translocation methylcytosine dioxygenase 1 (TET1) in the uterine artery. The upregulation of TET1 promotes demethylation of ESR1 (encoding estrogen receptor α, ERα) and KCNMB1 (encoding BKCa β1 subunit) and increases their expression, resulting in attenuated uterine vascular tone. However, hypoxia during gestation elevates hypoxia-inducible factor 1α (HIF-1α) and stimulates HIF-1α-dependent upregulation of both microRNA-210 (miR-210) and DNA methyltransferases (DNMTs). The elevated miR-210 in turn instigates downregulation of TET1. Consequently, gestational hypoxia triggers hypermethylation of ESR1 and KCNMB1 by suppressing TET1-mediated demethylation and promoting DNMT-mediated methylation, resulting in repression of ESR1 and KCNMB1 and increased uterine vascular tone.
2. Epigenetics and uteroplacental maladaptation in preeclampsia and high-altitude pregnancy
High-altitude pregnancy and preeclampsia share familiar features, and there is great similarity in gene expression patterns in high-altitude and preeclamptic placentas as well as hypoxia-treated placenta explants (926). The following is a brief summary of epigenetic modifications in the uteroplacental circulation that are commonly observed in both preeclampsia and high-altitude pregnancy. Preeclampsia is associated with upregulation of DNMTs (287, 697) and downregulation of TETs (568, 940) in the placenta. Similar findings were observed for DNMT3b (389) and TET1 (391) in uterine arteries from high-altitude pregnant sheep and hypoxia-treated uterine arteries of low-altitude animals. The changes of DNA methylation-demethylation machineries induced by high-altitude acclimatization promoted hypermethylation of USF-15 and Sp1-520 in ESR1 promoter and Sp1-380 in KCNMB1 promoter, and impaired transcription factor binding, resulting in ESR1 and KCNMB1 repression (162, 163, 203, 389). The downregulation of ESR1 and KCNMB1 due to hypermethylation ultimately led to increased uterine vascular tone (163, 389).
In addition to changes in DNA methylation, the expression of HIF-1α and HIF-responsive miR-210 in the placenta increased in high-altitude pregnancy and pregnancy complicated with preeclampsia (183, 503, 800, 1096, 1158, 1170). In vitro hypoxia treatment also induced a similar increase in miR-210 expression in trophoblasts (183, 538, 1158). MiR-210 impaired trophoblast invasion by targeting iron-sulfur cluster scaffold (ISCU) protein, potassium channel modulatory factor 1, and thrombospondin type I domain containing 7A (37, 538, 602, 603, 700). Impaired trophoblast invasion is one of the major features of IUGR and preeclampsia as well as high-altitude pregnancy (470, 979). In addition, MiR-210 activated Notch1 signaling and downregulated receptor tyrosine kinase ligand ephrin-A3 (266, 595). Consequently, miR-210 induced human umbilical vein endothelial cell migration, which probably attributed to the enhanced angiogenesis in high-altitude placenta. Moreover, miR-210 repressed HSD17B1 in preeclamptic placenta (414). Hydroxysteroid (17β) dehydrogenase 1 encoded by HSD17B1 catalyzes the conversion of estrone to 17β-estradiol. The reduced circulating 17β-estradiol in preeclampsia (439) probably results from the repression of HSD17B1 by miR-210. Intriguingly, miR-210 also functions as an epi-miR to control DNA methylation. TET1 was found to be the direct target of miR-210 and miR-210 binding to TET1 mRNA 3′UTR downregulated TET1 (391). Thus high-altitude acclimatization heightened methylation of ESR1 and KCNMB1 via HIF-1-dependent DNMT3b upregulation and miR-210-dependent TET1 downregulation, leading to increased uterine vascular tone in pregnancy (FIGURE 9).
G. Intervention
There are currently no effective treatments to improve IUGR, and the only cure available for preeclampsia is delivering the fetus. Despite the fact that significant progress has been made in understanding both complications, development of effective therapies is still lagging behind. Theoretically, any factors contributing to the pathogenesis of preeclampsia and IUGR could be potential targets of therapy. Several therapeutic approaches have been tested in animal models for the treatment of preeclampsia and IUGR. One approach is to increase maternal antioxidant capacity. Reducing oxidative stress with supplementation of vitamins C and E increased lamb birthweight and improved placental weight and number of cotyledons at high altitude (774). However, antioxidant administration failed to reduce the incidence of preeclampsia and IUGR (866). Enhancement of NO-mediated responses has also been tested as a therapeutic option for preeclampsia and IUGR. Administration of sildenafil increased fetal growth in pregnancies complicated by IUGR and animal models of IUGR (932, 1016). HIF-1 is also a target for remedying preeclampsia. Ouabain reduced mean arterial pressure in a rat model of preeclampsia by downregulating HIF-1α in the placenta (825). Other approaches include ameliorating the imbalance of angiogenesis. For example, delivery of VEGF-121 and adenovirus carrying VEGF were shown to reduce systemic blood pressure and to mend the renal damage in rodent models of preeclampsia (628). In addition, VEGF-121 administration also increased fetal weight (628). As uteroplacental blood flow is the major determinant of fetal growth, uteroplacental vasculature is thus an ideal target for improving altitude-associated fetal growth restriction. Local expression of VEGF in the uterine arteries increased uterine blood flow and improved fetal growth in growth-restricted sheep pregnancy (130, 653). The same approach also resulted in increased fetal growth in a guinea pig model of fetal growth restriction (947). These findings are promising and provide an important information for prospective clinical trials.
H. Perspective
Living at high altitude is a momentous challenge for a pregnant woman who breathes for herself and her fetus. High-altitude hypoxia has been shown to instigate adverse pregnancy outcomes in individuals who are either not accustomed to or not properly acclimated to such an environment. The incidence of preeclampsia and IUGR increases as altitude rises. Preeclampsia and IUGR have long been considered to be originated from placental insufficiency. Long-term adaptation enables native highlanders to be fairly protected from IUGR but not necessarily preeclampsia at high altitude. Intriguingly, preeclampsia itself shows signs of heterogeneity. Uterine blood flow decreases in Andeans with early-onset preeclampsia but not in those with late-onset preeclampsia. One should bear in mind that IUGR and preeclampsia are multifactorial disorders. The etiologies of these two pregnancy complications have not been fully established. On the basis of the discussion above, it is clear that networks of signaling pathways are involved in the pathogenesis of these complications. The pathogenic factors may contribute differently at a given stage of these disorders, and more importantly, it is highly likely that their interactions in the uteroplacental circulation system lead to increased uteroplacental vascular resistance and reduced blood flow. Research on both maladaptation and adaptation of uteroplacental circulation to high altitude is equally important, which will help to reveal how pregnancy complications and adaptation occur. Undoubtedly, knowledge from these studies will advance our understanding of etiologies of preeclampsia and IUGR at high altitude and facilitate the translation of research findings into clinical applications.
V. HEART DEVELOPMENT AND PROGRAMMING OF CARDIAC DISEASE
The heart is the first organ to develop and to function as the embryonic and fetal development requires a constant supply of oxygen and nutrients. Cardiogenesis begins shortly after implantation and the tubular heart starts to beat and pump blood at 22 days in human and 8 days in mice after conception. Normal heart development in utero depends on coordinated cell specification, differentiation, and proliferation. Notably, cells at the embryonic and fetal stages exhibit great plasticity and are vulnerable to adverse environments. Early epidemiological studies revealed a link between ischemic heart disease and fetal growth restriction (58), which set a basis for the later developed concept of “Developmental Programming of Health and Disease.” In this section, we provide an overview of the current knowledge on the impacts of fetal stress including hypoxia on heart development in utero and programming of the ischemic-sensitive phenotype of the heart later in life. We will discuss some mechanistic insights, especially the epigenetic mechanisms underlying this programming process. In addition, the potential of cardiomyocyte regeneration as a therapeutic approach for heart ischemic injury will also be briefly discussed.
A. Embryonic Heart Development
The formation of the heart is a complex process that has been extensively reviewed elsewhere (250, 625). The embryonic heart development is temporally regulated by oxygen levels. Relatively low oxygen (physiological hypoxia) is critical for normal cardiac morphogenesis. Hypoxia-induced accumulation of HIF-1α is important for the expression of the TBX5, MEF2C, and titin genes between embryonic day E8.5 and E10.0 in mice, and restricted inactivation of HIF-1α in ventricular cardiomyocytes impaired looping morphogenesis (513). HIF-1α inactivation at E10.5, but not E13.5, caused septation and conotruncal heart defects in mice (471). A recent study revealed that the mouse heart tube was hypoxic, whereas cardiac progenitor cells expressing Islet 1 (ISL1) in the secondary heart field were normoxic relative to the heart tube (1126). Excessive hypoxia during early heart development (E7.5) resulted in congenital heart disease by inhibiting Isl1 in second heart field but increasing NK2 homeobox 5 (Nkx2.5) expression in first heart field. The Isl1 repression was caused by forming the HIF-1α-Notch effector hes family bHLH transcription factor 1 (HES1)-protein deacetylase sirtuin 1 (SIRT1) complex at the gene.
B. Fetal Stress and Premature Terminal Differentiation of Cardiomyocyte
Cardiomyocytes undergo rapid proliferation, and the increase in cardiac size is primarily achieved through an increase in myocyte number during fetal development. In most mammals, cardiomyocytes become terminally differentiated due to withdrawal from the cell cycle, which can be either mononucleated or binucleated. In late pregnancy and/or shortly after birth, karyokinesis (nuclear division) occurs without subsequent cytokinesis (cytoplasm division) in a fraction of cardiomyocytes, leading to binucleation. The occurrence of cardiomyocyte binucleation varies among species. In humans, binucleation appears at week 32 of pregnancy. At birth, binucleated cardiomyocytes account for ~10% of entire myocyte population and increase to ~50% in the first year. Binucleation in sheep begins at day 77 of gestation, reaching 50% at 135 days of gestation and 90% at 4–6 wk after birth. In rodents, the transition from mononucleate to binucleate cardiomyocytes takes place within the first 2 wk after birth: starting from day 2 for mice and day 4 for rats and attaining ~90% binucleation by day 12.
Heart growth switches to predominantly hypertrophic growth upon terminal differentiation by increasing size but not number of cardiomyocytes, although recent studies suggest that cardiomyocytes can proliferate in the adult heart with very low frequencies (<1%/yr) (75). Therefore, cardiomyocyte endowment is essentially determined by myocyte proliferation in utero and around birth. Accumulating evidence suggests that perinatal phase is a period of vulnerability during which a range of insults has been found to affect the heart development (768, 786, 978).
The mechanisms underlying the terminal differentiation are not fully understood. The cyclins and cyclin-dependent kinases (CDKs) participate in cell cycle progression, and mitotic arrest due to inhibition of the cyclin-CDK complex is believed to contribute to the withdrawal from the cell cycle (577). ROS may also contribute to cardiomyocyte cell cycle arrest, but conflicting findings have been reported. Puente et al. (814) observed that the transition from in utero hypoxic environment to postnatal oxygen-rich environment elevated ROS in mouse cardiomyocytes. These authors demonstrated that reducing oxidative stress by scavenging ROS or by targeted expression of a mitochondrial-specific catalase, an enzyme that breaks down H2O2, in cardiomyocytes prolonged cardiomyocyte proliferation in postnatal animals, whereas ROS generators promoted early exit from the cell cycle. However, cardiac overexpression of NADPH oxidase 4 (Nox4), an enzyme that produces ROS, extended cell cycle activity in postnatal mice by increasing cyclin D2 expression (701). The transcription factor MEIS1 is another potential regulator of cardiomyocyte differentiation. Genetic deletion of MEIS1 in mouse cardiomyocytes delayed cell cycle withdrawal after birth, whereas overexpression of MEIS1 suppressed neonatal cardiomyocyte proliferation (613).
1. Hypoxia
Intrauterine hypoxia presents a significant challenge to the developing fetus. Maternal hypoxia increased the percentage and size of binucleated myocytes in fetal rat hearts (47). In vitro hypoxic treatment of cultured fetal rat cardiomyocytes tended to increase binucleation (766). Similarly, ex vivo hypoxia suppressed cardiomyocyte proliferation in fetal rat heart (985). Concomitantly, both maternal hypoxia and ex vivo hypoxia decreased cyclin D2 and increased the CDK inhibitor p27 expression in the fetal heart (766, 767). Endothelin-1 appeared to be a mediator of hypoxia-induced inhibition of rat cardiomyocyte proliferation. Likewise, intrauterine hypoxia by induction of anemia increased both heart weight and binucleation in the right ventricle of fetal sheep (447). However, fetal hypoxia induced by placental embolization or placental restriction by removing maternal endometrial caruncles before conception decreased binucleation (113, 596) and increased mononucleated cardiomyocytes (687) in fetal sheep. Intermittent maternal hypoxia increased the number of mononucleated cells by ~150% in the left ventricle and ~37% in the right ventricle of rats on postnatal day 60 (1171).
Exposure of newborn rats to intermittent anoxia from postnatal day 1 to 3 decreased cardiomyocyte proliferation without altering binucleation, resulting in a significant reduction of cardiomyocyte number in the heart of postnatal day 14 neonates (767). However, exposure of neonate mice to mild hypoxia (15% O2) delayed cardiomyocyte cell cycle exit (814). In a transgenic mouse model expressing a chimeric protein in which the oxygen-dependent degradation domain of HIF-1α was fused to the tamoxifen-inducible CreERT2 recombinase, tamoxifen induction led to generation of a small population of hypoxic cardiomyocytes that displayed neonatal proliferative feature such as smaller size and mononucleation (486). These myocytes contributed to new cardiomyocyte generation in the adult heart at a rate of ~1% annually. Of interest, exposure to gradual systemic hypoxia induced reentry of terminally differentiated cardiomyocytes into cell cycle (714). Consistently, transgenic mice overexpressing HIF-1α displayed attenuated infarct size and improved cardiac function after myocardial infarction (481).
2. Nutrition
Both nutritional deprivation and excess have great impact on fetal development and increase risk of disease later in life (624). Maternal protein restriction reduced heart weight and the number of cardiomyocytes in newborn rats without altering binucleation (187). Similar treatment in mice resulted in smaller fetal hearts and decreased expression of cyclin G1 (43). However, maternal undernutrition led to increased heart weight-to-body weight ratio in adult rat offspring (846). Rat offspring from protein-restricted mother had increased heart volume with similar total number of cardiomyocytes (565), suggesting cardiac hypertrophy. In a sheep model, fetal heart weight is not altered by maternal undernutrition, but increased by overnutrition (228). However, maternal nutrient restriction in sheep slightly but significantly increased heart weight in adult offspring (301). Thus maternal undernutrition in general suppresses fetal cardiac hyperplasia in rodents but enhances hypertrophy in adult offspring in both rodents and sheep. The heart hypertrophy in offspring born to mothers experiencing undernutrition probably compensates the loss of cardiomyocytes during the fetal development.
3. Glucocorticoids
Glucocorticoids play an important role in fetal development and are critical for the development and maturation of fetal lung and heart (675, 842, 1091). Glucocorticoids regulate heart development by activating glucocorticoid (GR) and mineralocorticoid (MR) receptors, often leading to opposite effects. Cardiomyocyte-specific deletion of the GR impaired fetal heart maturation and left ventricular systolic function (745, 848). However, excessive exposure to glucocorticoids has been shown to negatively affect the development of the heart (744, 842). In preterm piglet, maternal glucocorticoid treatment promoted cardiomyocyte maturation as evidenced by increased binucleation (485). Rudolph et al. (860) reported that fetal cortisol infusion inhibited cardiomyocyte proliferation in sheep fetus. High levels of cortisol promoted heart hypertrophy of sheep fetal hearts (600). The dexamethasone treatment increased expression of maturation markers in cultured fetal cardiomyocytes (847) and hypertrophic markers in cultured neonate cardiomyocytes (831) via activation of the GR. However, studies from other groups demonstrated that fetal heart size and cell proliferation in the late-gestation fetal sheep heart were all increased by maternal or fetal cortisol infusion via activation of the MR without altering myocyte size and binucleation, suggesting increased proliferation (269, 830). Interestingly, cardiomyocyte-specific inactivation of the MR encoding gene NR3C2 triggered mild cardiac hypertrophy (594). Prenatal treatment with dexamethasone increased proliferation, leading to greater heart-to-body weight ratio in neonatal rats (988). In addition, neonatal dexamethasone treatment decreased total cardiomyocyte number and increased both myocyte size and binucleation (51, 300). Reduced cyclin D2 may contribute to dexamethasone-induced cardiac hypertrophy in neonates (299, 300). These findings suggest that cardiac actions of glucocorticoids are development stage- and receptor type-dependent.
4. Other stresses
Maternal lifestyles such as alcohol consumption and cigarette smoking also affect fetal heart development in utero (270). In a rat model, prenatal alcohol exposure increased cardiomyocyte binucleation in neonates (27), and the neonates developed left ventricle hypertrophy (732). Maternal exposure to ethanol was associated with left ventricular hypertrophy in fetal sheep by increasing cardiomyocyte size and promoting binucleation of cardiomyocytes (317). Exposing cultured cardiomyocytes to ethanol also increased cell maturation by increasing the number of cells in the G0/G1 phase of the cell cycle (5). The increased cardiomyocyte size was accompanied by an increased insulin-like growth factor I (IGF-I) expression; thus IGF-I is a potential mediator of alcohol-induced hypertrophy (317). Nicotine is the major component of tobacco smoke. Maternal nicotine exposure downregulated Tbx5 and Gata4 expression in rat offspring, resulting in cardiac malfunction (435). Maternal smoking also induced cardiomyocyte hypertrophy in the first to third week postnatal heart as evidenced by increased size and decreased number of cardiomyocytes (177).
C. Fetal Stress and Programming of Matrix Metalloproteinases in the Heart
The extracellular matrix (ECM) is composed of extracellular molecules secreted locally by cells, and the main components include collagen, gelatin, proteoglycans, and polysaccharides. The collagen types I and III are predominant interstitial collagens in the heart, accounting for 85 and 11% of total collagen. The primary function of ECM is to form physical scaffolding to support surrounding cells. In addition, ECM also participates in cellular differentiation. The degradation of ECM is achieved by actions of matrix metalloproteinases (MMPs), a family of Zn2+-dependent endopeptidases that include collagenases, gelatinases, stromelysins, matrilysins, metalloelastases, and membrane-type matrix metalloproteinases (MT-MMPs). MMPs are synthesized and secreted as inactive proenzymes. In addition to being regulated by transcription and zymogen activation, these enzymes are also inhibited by endogenous tissue inhibitors of metalloproteinases (TIMPs). The balance of production and proteolysis of ECM components is essential for heart development and function. Disruption of this balance leads to cardiac malformation and dysfunction (580, 987). Using chick embryos, Linask et al. (567) demonstrated that either specifically inactivating MMP-2 with antibody or generally inhibiting MMPs with an inhibitor causes heart tube defects by interrupting looping process. Aberrant expression of MMPs and TIMPs has been associated with heart defects (619).
In rats, the heart collagen content increases upon the fetus to neonate transition (986). Both collagen type I and III were expressed in 12-wk-old human fetal myocardium (420). Studying human hearts from 20 wk of gestation to preadolescence, Marijianowski et al. (620) observed that the ratio of collagen I to collagen III was high in the fetus and neonate. The ratio gradually decreased with age and the 12-yr-old heart had only approximate half of that in fetus/neonates. Given that collagen type I is rigid and type III elastic in nature, this change may represent an adaptive remodeling. Disproportionate collagen deposition in the heart is detrimental to cardiac function, and aberrant cardiac remodeling is associated with cardiac dysfunction (667). Collagenases could be detected in 11.5 or 12.5 embryonic day rat hearts (715). Collagenase-3 (MMP-13), stromelysin (MMP-3), and gelatinases A and B (MMP-2 and -9) were expressed in prenatal rat hearts (827). MMP-9 activity was only observed between E16 and E18, whereas MMP-13 activity gradually increased from E14, reaching peak between E16 and E21. Highest MMP-2 activity was detected between E13 and E16, the period in which the heart tube undergoes remodeling to form heart chambers.
1. Hypoxia
In fetal hearts of guinea pig and sheep, gestational hypoxia increased collagen deposition (254, 972). Both procollagen I and III mRNA levels were elevated in the hypoxic ovine fetal heart. The effect of maternal hypoxia on rat heart collagen deposition is dependent on developmental stages. Maternal hypoxia increased total heart collagen in the neonate rat heart but not in the fetal heart (986). Collagen I expression at these two development stages was also differently affected by maternal hypoxia: a decrease in the fetal heart and an increase in the neonate heart. Given that hypoxia did not alter heart collagen III expression in both the fetus and neonate, the ratio of collagen I to collagen III was thus lower in the fetal heart and higher in the neonate heart. Prenatal hypoxia increased both collagen I and III in 4- and 7-mo rat offspring hearts (1100). Intermittent maternal hypoxia exposure starting from early and middle pregnancy to term, but not during late pregnancy, increased both collagen I and III in adult rat offspring hearts (1026).
Heart MMPs were also differently affected by maternal hypoxia (986). In response to maternal hypoxia, MMP-1 decreased, whereas MMP-13 and MT1-MMP increased in the fetal rat heart. However, maternal hypoxia increased both MMP-1 and MMP-13 in the neonate rat heart. Hypoxia also promoted expression of MMP-9 in the fetal guinea pig heart (254) and MMP-2 and MT1-MMP in the ovine fetal heart (972). However, the levels and activities of active MMP-2 and MMP-9 in fetal rat neonatal hearts were not altered by maternal hypoxia (986). The activity of MMP-2 in rat offspring was suppressed by prenatal hypoxia exposure (1100). Maternal hypoxia-induced MMP-9 expression was probably stimulated by oxidative stress as the upregulation of MMP-9 was ablated by the antioxidant N-acetylcysteine (254). The parallel increases in TNF-α, IL-6, transforming growth factor (TGF)-β, MMP-2, and MMP-9 suggest that hypoxia-triggered cytokine release may also participate in regulating MMP-9 expression in fetal guinea pig heart (746, 972). Therefore, inflammatory cytokines and oxidative stress may exert their detrimental effects on the heart by altering ECM catabolism. Furthermore, maternal hypoxia also altered expression of TIMPs in the developing heart. Tong and co-workers (985, 986) demonstrated that expression of TIMP-3 and TIMP-4 was increased by maternal hypoxia in both fetal and neonatal hearts. Interestingly, knockdown of TIMP-3, but not TIMP-4, increased cyclin D2 expression, whereas TIMP-3 and TIMP-4 knockdown partially or completely abrogated hypoxia-mediated inhibition of cyclin D2 expression. These findings implicate a role of TIMP-3 in normal cardiomyocyte proliferation and TIMP-3/TIMP-4 in hypoxia-induced proliferation inhibition. Indeed, Hammoud et al. (351) demonstrated that cardiomyocyte proliferation was boosted in TIMP-3 knockout mouse. In a rat model, maternal hypoxia-induced alterations of ECM/MMPs contributed to diastolic dysfunction and increased sensitivity to ischemic injury in the adult offspring (1100).
2. Nutrition
In addition to hypoxia, maternal nutrition restriction also affected ECM/MMPs in the neonate rat heart. Maternal undernutrition suppressed MMP-2 activity and reduced collagen I and collagen III levels in the neonate heart but not in the fetal heart (1100). Increased fetal sheep heart connective tissue accumulation was observed in maternal obesity (399). The deposition of collagen and expression of TGF-β in the fetal heart, but not the ratio of collagen I to collagen III, were increased by maternal obesity. Moreover, maternal obesity increases MMP-9 and TIMP-3 in the fetal sheep heart.
3. Other stresses
Prenatal dexamethasone treatment reduced ECM in fetal rat hearts (988), probably by interfering collagen synthesis (181, 1049). Maternal nicotine exposure modified cardiac remodeling by increasing collagen deposition and MMP-2 expression in fetal guinea pig hearts (975). Heightened oxidative stress possibly mediated nicotine-induced increase in MMP-2 and collagen in the heart as these effects could be rescinded by N-acetylcysteine. In addition, maternal nicotine also boosted collagen deposition and TGF-β1 expression in rat offspring hearts (177). Prenatal nicotine exposure was found to increase collagen I and decrease collagen III in offspring hearts, resulting in increased ratio of collagen I to collagen III (1124). TGF-β contributes to fibrosis of various organs by stimulating myofibroblast transdifferentiation (984). Thus the alternations of collagen and TGF-β expression by nicotine could lead to myocardial stiffening and loss of myocardial compliance.
D. Fetal Stress and Programming of Ischemic-Sensitive Phenotype in the Heart
Myocardial infarction is a major cause of morbidity and mortality. Impaired coronary artery blood flow results in myocardial ischemia, and myocardial ischemia reperfusion injury promotes cell death that contributes up to ~50% of the final size of myocardial infarct. Myocardial infarction frequently occurs as the result of coronary heart disease, which accounts for more than 7 million deaths worldwide and 2.4 million death in the United States annually. The intrauterine environment has been known to be important for cardiovascular health and diseases in adult life for over three decades (58).
1. Hypoxia
Although epidemiological studies suggested a link between in utero stress and increased risk of ischemic heart disease later in life, the first animal study using a rat model in 2003 provided clear causative evidence that mild fetal hypoxia resulted in programming of ischemic-sensitive phenotype in the heart and increased myocardial damage after ischemia and reperfusion (I/R) and reduced postischemic recovery of left ventricle function in adult offspring (553). Of particular interest, the study demonstrated that moderate fetal stress did not affect baseline cardiac function in offspring but made the heart less adaptable and more vulnerable for life to challenge by a stressor; thus it revealed a novel phenomenon of “second hit model.” These original findings have been subsequently corroborated in both rat and sheep models by different laboratories (552, 863, 1100, 1108, 1113). The “second hit” phenomenon, i.e., programming of an organ adaptive capability and vulnerability to a late-life stressor, appears common for developmental programming of health and disease. This suggests that some genes and/or signaling elements/pathways programmed during fetal stress are hibernated or silent (i.e., dispensable) in adulthood under physiological conditions. However, they can be activated in later life by certain pathophysiological stresses, leading to increased organ damage and dysfunction. The other interesting feature is a gender-dependent response to ischemic injury in adult offspring following prenatal hypoxia. Female adult offspring seemed to have developed certain strategies to tolerate, but not to prevent, I/R injury of the heart. Netuka et al. (725) reported that unlike the male counterpart, female adult offspring had reduced incidence and severity of ischemic ventricular arrhythmias in response to I/R injury. Similarly, the reperfusion-induced increase in left ventricle end-diastolic pressure and myocardial infarct size seen in the male rats were absent in the female rats (1108). Moreover, in contrast to impaired postischemic recovery of left ventricle function by prenatal hypoxia in the male rats, the female rats retained a similar capability to recover from I/R injury as the normoxic control animals.
Myocardial infarction results from the death of cardiomyocytes with a significant contribution from apoptosis. Apoptosis is usually initiated by activation of caspases mediated by two pathways. Whereas the extrinsic pathway requires activating membrane receptors by Fas ligand and tumor necrosis factor-α, the intrinsic pathway involves release of cytochrome c from the mitochondria (284). The activation of the initiator caspases such as caspase-8 by the extrinsic pathway or caspase-9 by the intrinsic pathway activates the executioner caspase-3, leading to apoptosis. Apoptosis is subject to regulation by various pro-apoptoptic and anti-apoptoptic factors such as Bcl-2 family proteins and heat shock protein (Hsp) 70 (505). A number of investigations have been conducted to gain insights into the mechanisms underlying the susceptibility and tolerance to cardiac ischemia in adults following prenatal hypoxia exposure. Prenatal hypoxia increased caspase-3 activity, cytochrome c, and Fas, whereas it decreased Bcl-2 and Hsp70 in the fetal heart (47, 1076). Consistently, apoptosis was relatively low in the normoxic fetal heart and was doubled (from 1.4 to 2.7%) by hypoxia treatment (47). Maternal hypoxia increased active caspase-3 and decreased Hsp70 in the heart of adult offspring following I/R (552, 553). Consistently, I/R-induced apoptosis was 44% higher in the prenatal hypoxia-primed adult offspring hearts than in the normoxic hearts. In addition, a recent study revealed heightened oxidative stress after I/R injury in hearts of both male and female offspring exposed to prenatal hypoxia (908). Resveratrol supplementation after weaning for 9 wk ablated the elevated oxidative stress and improved cardiac recovery from I/R injury. These findings indicate an important role of oxidative stress in programming of myocardial infarction by prenatal hypoxia.
2. Nutrition
Accumulating evidence suggests that maternal nutrition status also affects the susceptibility of offspring to I/R injury. Xu et al. (1100) demonstrated that maternal nutrient restriction increased susceptibility of adult male offspring to I/R injury. Subsequently, studies from the Langley-Evans laboratory revealed that maternal protein restriction also resulted in gender-dependent effect on recovery of left ventricle function from I/R injury in a rat model without altering baseline cardiac function (247, 248). The recovery was impaired only in male, but not in female, offspring compared with control fed dams. It appears that maternal undernutrition predetermined the heart’s capacity to buffer I/R-induced oxidants. Maternal protein restriction increased ROS production and decreased enzymatic antioxidant capacity in heart of rat offspring (719). Recovery of cardiac function from I/R injury was improved by the ROS scavenger/glutathione precursor N-acetylcysteine and diminished by the glutathione depleting agent diethylmaleic acid in the offspring of maternal undernutrition (249). In contrast, Lawrence et al. (535) demonstrated that maternal food restriction improved postischemic recovery of left ventricle function in male offspring hearts. This notion was confirmed by a later study from the Langley-Evans laboratory (868). They observed that male offspring from maternal protein restriction exhibited improved recovery of cardiac function following I/R compared with control fed counterparts. The cause(s) for this inconsistency is currently unclear.
Gender-specific phenomenon was also observed in maternal overnutrition. Feeding pregnant rats with a high-fat diet also increased heart susceptibility to I/R injury in adult male, but not female, offspring (1103). Consistently, using a mouse model of maternal obesity, Calvert et al. (125) also demonstrated an increased susceptibility to I/R injury in adult offspring, evidenced by increased infarct size following I/R injury only in the male, but not the female, offspring. High-fat diet-exposed offspring showed elevated lipid peroxidation in cardiomyocytes (647). This elevated oxidative stress may contribute to increased cardiac sensitivity to I/R injury following maternal overnutrition exposure.
3. Other stresses
Prenatal nicotine exposure in a rat model resulted in increased left ventricle infarct size and reduced postischemic recovery of left ventricle function in both male and female offspring (535, 1085). Unlike maternal hypoxia and cocaine exposure, prenatal nicotine did not alter the abundance of caspases such as caspase-3, caspase-8, and caspase-9 in adult offspring hearts. In addition, prenatal nicotine led to oxidative stress in adult offspring hearts, antenatal administration of the antioxidant N-acetylcysteine restored PRKCE expression, and annulled perinatal nicotine-induced increase in susceptibility to I/R injury (1085).
Increased susceptibility to I/R injury following maternal cocaine exposure also occurred in rats. Maternal cocaine exposure increased infarct size and diminished postischemic recovery of left ventricle function only in male adult offspring (46, 49). Prenatal cocaine induced apoptosis in the juvenile hearts by decreasing antiapoptotic protein Bcl-2 and increasing pro-apoptotic protein Bax (49). In addition, prenatal cocaine promoted apoptosis in the fetal heart as the results of increased activities of caspase-3, caspase-8, and caspase-9 in the heart in addition to increased Bax and decreased Bcl-2 (1088).
4. Fetal stress and programming of cardioprotective genes
PKCε has been shown to be cardioprotective against I/R injury (161, 408). Prenatal hypoxia suppressed expression of PKCε in male, but not female, fetal and adult hearts (552, 784, 1108). Female adult rats are relatively protected against I/R injury with PKCε being a major contributor (48). The distinct patterns of gender-dependent PKCε expression in response to maternal hypoxia could explain different susceptibility to I/R injury and postischemic recovery of left ventricle function in male and female adult offspring (725, 1108). A causative effect of PKCε gene repression in fetal hypoxia-induced programming of ischemic-sensitive phenotype in the heart has been demonstrated (784, 785). Similar to the effect of hypoxia, maternal cocaine exposure downregulated PKCε in male but not female hearts and increased heart vulnerability to I/R injury in adult male offspring (48). The cardioprotective effect by PKCε is probably due to its inhibition of apoptosis and activation of the mitochondrial ATP-sensitive K channel (569, 747). Fetal nicotine exposure decreased PKCε in both adult male and female hearts as well as in fetal hearts (534, 535). Prenatal nicotine exposure has been shown to increase the release of the sympathetic neurotransmitter norepinephrine (1124), and norepinephrine in turn could repress PRKCE via epigenetic modifications (534, 1090). Interestingly, prenatal methamphetamine only increased infarct size and impaired recovery from ischemia in female, but not male, hearts (852). Consistently, maternal methamphetamine exposure downregulated PKCε in female, but not male, hearts (852). Cocaine and methamphetamine could lead to catecholamine accumulation by inhibiting uptake and promoting release of catecholamines, which in turn repress PKCε via DNA methylation. Prenatal cocaine exposure also ablated ischemic preconditioning increased phospho-PKCε level and cardioprotection in the male, but not the female, offspring heart (657).
NO is another potential cardioprotective factor during I/R (263), and I/R injury was attenuated in transgenic mice overexpressing eNOS (446). Prenatal hypoxia also differentially regulated eNOS expression in a sex-specific manner. eNOS expression was reduced in male offspring hearts (553) but increased in female offspring hearts (358), which could also contribute to distinct susceptibility to I/R injury in adult male and female rats. The antiapoptotic protein Bcl-2 expression in heart was increased by NO donors, suggesting the cardioprotection by NO is in part mediated by inhibiting apoptosis (1022). Interestingly, eNOS was found to decrease in fetal hearts but increase in adult offspring hearts following exposure of pregnant guinea pigs to hypoxia (974).
The angiotensin type II (AT2) receptor also contributed to fetal hypoxia-induced increase in susceptibility to I/R injury. Prenatal hypoxia exposure increased AT2 receptor expression in the adult offspring heart (1104). AT2 receptor inhibition increased postischemic recovery of left ventricular function and amended prenatal hypoxia-induced ischemic vulnerability in adult male hearts. Feeding pregnant rats with a high-fat diet also increased the expression of AT2 receptor only in adult male offspring heart (1103), implicating the AT2 receptor as a potential mediator of maternal overnutrition-induced reprogramming in adult offspring heart. The upregulation of AT2 receptor was due to the loss of the regulatory GR that normally repressed the AT2 receptor encoding gene AGTR2 by binding to glucocorticoid response elements in the gene promoter.
Among other mechanisms, glucocorticoids play a center role in the response to stress. Timing and level of prenatal glucocorticoid exposure are key determinants of cardiovascular disease risk in later life. The glucocorticoid receptor (GR) is abundantly expressed in fetal cardiomyocytes. Maternal hypoxia during gestation resulted in a decrease in GR mRNA and protein abundance in fetal hearts, which persists in adult offspring (1107). The pathophysiological significance of decreased GR expression levels in the heart is highlighted by the findings that demonstrated the cardioprotective effects of glucocorticoids in the acute setting of myocardial ischemia and reperfusion injury both in humans and in animals (350, 562, 983). Relevantly, the recent study has demonstrated that restoration of GR expression prevents fetal hypoxia-mediated increase in heart I/R injury in offspring (1089).
The β2-adrenergic receptor also plays an important cardioprotective role (258). Prenatal hypoxia increased β2-adrenergic receptor expression and Gsα/Giα ratio in offspring hearts (553). The contribution of β2-adrenergic receptor to hypoxia-induced increases the susceptibility of adult heart to I/R injury is not clear, but it may serve as a compensatory mechanism. However, the observation of increased expression of β2-adrenergic receptors in the female heart of maternal low-protein fed offspring (248) suggests that in utero undernutrition may program the offspring’s response to I/R injury via regulating the expression of β2-adrenergic receptors in the heart in a sex-dependent manner.
Another cardioprotective protein is Hsp70 that is induced by a variety of stress stimuli including the heat (673). Hsp70 is a negative regulator of apoptosis (67). The expression of Hsp70 was reduced in fetal hearts following maternal hypoxia (47). Interestingly, in contrast to the normoxic control, adult offspring rats of prenatal hypoxia were unable to produce Hsp 70 in response to heat stress (552), resulting in loss of an antiapoptotic mechanism.
Estrogen is likely to be the key player in gender-dependent cardioprotection. The expression of both PKCε and eNOS in hearts appeared to be regulated by estrogen. Ovariectomy reduced the expression of PKCε in rat hearts, which was accompanied by increased myocardial infarction and decreased postischemic recovery of left ventricular function (404, 1107). The impaired cardioprotection in ovariectomized rats could be restored by estrogen supplement (1099, 1107). The ischemic preconditioning-mediated cardioprotection via increase in phospho-PKCε level in female rats appeared to be mediated by estrogen, as this cardioprotective effect was not observed in ovariectomized rats (916). Moreover, estrogen replacement was able to amend both reduced cardiac eNOS expression in ovariectomized rats (1032) and I/R-induced increase in TNF-α in the left ventricle (1099). Thus estrogen plays an essential role in sex-specific cardioprotection by regulating the expression of PKCε and eNOS in the heart.
E. Epigenetic Mechanisms in Heart Development and Programming of Heart Disease
1. Terminal differentiation of cardiomyocyte
Histone acetylation/deacetylation dynamics is critical for gene regulation. Histone acetylation catalyzed by histone acetyltransferases facilitates gene activation. Chromatin consists of euchromatin and heterochromatin regions, and the heterochromatin is transcriptionally inactive. In embryonic cardiomyocytes, chromatin is hyperacetylated. During the transition to adult cardiomyocytes, heterochromatin increased with hypoacetylated histones and high trimethylation of lysine 27 on histone H3 (H3K27me3) (896). Adult cardiomyocytes also displayed elevation in retinoblastoma (Rb) tumor suppressor and p130, which participated in H3K9me3 and heterochromatin formation through their interaction with heterochromatin protein 1-γ (896). These changes silenced proliferation-promoting genes and convert cardiomyocytes from proliferative phenotype in the fetus to postmitotic phenotype in the adult.
DNA methylation is also of critical importance in cardiomyocyte terminal differentiation. The heart development in rat neonates was accompanied by upregulation of DNMT1 and increased global methylation (508). ET-1 was the mediator of hypoxia-induced inhibition of cardiomyocyte proliferation (767). Subsequent studies revealed that ET-1 dramatically increased binucleation in cultured cardiomyocytes with a concomitant globe hypermethylation (766). ET-1-induced binucleation and methylation were ablated by the DNA methyltransferase inhibitor 5-aza-2'-deoxycytidine. Similarly, the suppression of cardiomyocyte proliferation and promotion of terminal differentiation in neonate hearts by dexamethasone was also inhibited by 5-aza-2'-deoxycytidine (299). Maternal nicotine exposure upregulated expression of DNMTs, which in turn repressed Tbx5 and Gata4 expression in rat offspring hearts by inducing hypermethylation of promoters of these two genes (435). Tbx5 and Gata4 were found to regulate Cdk4 and Cdk2 expression, and disruption of genes coding Tbx5 and Gata4 led to cardiac septation defects (669). These observations implicate a critical role of DNA methylation in cardiomyocyte endowment.
In addition, a variety of miRs have been identified to regulate cardiomyocyte maturation (625, 1110). Some of them appear to be proproliferative, and the other antiproliferative. In late gestation, the expression of miR-133a in fetal cardiomyocytes increased with a concomitant decrease in the expression of its target gene IGF-I receptor, concurring the time frame of the suppression of cardiomyocyte proliferation (688). MiR-133 was also shown to inhibit cardiomyocyte proliferation by repressing cyclin D2 (573). Similarly, cardiomyocyte proliferation is negatively regulated by miR-1 due to downregulation of the transcription factor heart- and neural crest derivatives-expressed protein 2 (Hand2) (1163). Studying 1- and 10-day-old mouse hearts revealed developmental upregulation of miR-195 (a member of the miR-15 family) (807). MiR-195 inhibited cardiomyocyte proliferation in neonates by suppressing various cell cycle genes including checkpoint kinase 1 (807). In contrast, overexpression of miR-199a and miR-590 increased cardiomyocyte proliferation in neonatal hearts (253). MiR-17–92 promoted cardiomyocyte proliferation via downregulating phosphatase and tensin homolog (PTEN), and cardiac-specific knockout of miR-17–92 reduced both heart size and proliferating cardiomyocytes in postnatal hearts (160). Together, evidence available suggests that cardiomyocyte differentiation and proliferation are probably regulated by the balance of actions of various miRs.
2. Matrix metalloproteinases
There is scant information regarding the epigenetic regulation of MMPs and TIMPs in the developing heart. In utero PM2.5 exposure increased collagen deposition and expression of DNMTs in adult mouse offspring (959). The following observations were primarily obtained from adult cardiomyocytes/hearts. The expression of TIMP-4 was repressed in cardiomyocytes in a rat model of heart failure, which was accompanied by increased expression of DNMT1 and hypermethylation in the promoter region of the TIMP-4 gene (154). Upregulated miR-122a might also contribute to TIMP-4 repression (153). In diabetic hearts, upregulation of miR-29b and miR-455 was associated with downregulation of MMP-9 (153). MMP-2 was also a direct target of miR-29b (261). Expression of class I histone deacetylases, MMP-2 and MMP-9, in heart increased after myocardial infarction (619). Inhibition of histone deacetylases markedly diminished MMP-2 and MMP-9 increase after myocardial infarction, suggesting that the expression of MMP-2 and MMP-9 in myocardial infarction was in part mediated by class I histone deacetylases. Together, epigenetic modifications of MMPs and TIMPs could play important roles in remodeling of the heart and cardiac dysfunction, but yet to be determined.
3. Ischemic sensitivity
As discussed above, exposure to several intrauterine stresses such as hypoxia, nicotine, cocaine, and methamphetamine have been shown to trigger sex-specific downregulation of PKCε in the developing hearts, leading to distinct tolerance to I/R injury in the male and female offspring (535, 552, 852, 1088, 1108). A series of investigations revealed that epigenetic modification attributed to the distinct patterns of PKCε expression in fetal stress-programmed male and female adult offspring hearts. Two specificity protein 1 (SP1) binding sites and one early growth response factor 1 (Egr1) binding site in PRKCE promoter of the male offspring hearts displayed higher methylation than the female offspring hearts following prenatal hypoxia (164, 784). Similarly, fetal cocaine exposure led to greater methylation at these two SP1 binding sites at the PRKCE promoter in male fetal and offspring hearts (656, 1149, 1151). In addition, maternal nicotine treatment also induced hypermethylation at the Egr-1 binding site in both fetal and adult hearts (534). Notably, methylation of these binding sites retarded the binding of congruent transcript factors to them, leading to PRKCE repression in male offspring hearts. Moreover, cardiac ERα and ERβ levels were higher in female than in male fetal hearts (164, 784). Both ERα and ERβ were able to bind to SP1 and Egr1 sites with greater capacity in the female hearts to protect and transactivate PRKCE expression. These distinct patterns of epigenetic modification would then lead to gender-dependent tolerance to I/R injury.
Prenatal exposure to hypoxia, nicotine, cocaine, and methamphetamine all elevated catecholamine levels in the fetus and offspring. Xiong et al. (1090) demonstrated that norepinephrine induced oxidative stress in fetal hearts by upregulating NADPH oxidase 1 expression. Importantly, other prenatal stresses such as hypoxia and undernutrition also promoted ROS production in the developing hearts (719, 785). Oxidative stress plays an important role in fetal programming (973). The increased ROS level repressed the PRKCE gene by increasing methylation at the Egr1 and Sp1 binding sites in the promoter (785, 1090). ROS scavengers blocked hypoxia-mediated promoter methylation and restored PKC expression in the fetal heart. More importantly, inhibition of ROS reversed fetal hypoxia-induced ischemic-sensitive phenotype of the heart in offspring (785). These studies implicate ROS as common mediators for epigenetic programming of cardioprotection gene repression induced by prenatal stresses such as hypoxia, undernutrition, nicotine, cocaine, and methamphetamine.
Another example of cardioprotection gene repressed by hypermethylation in the developing heart is the GR. The GR encoding gene NR3C1 contains multiple untranslated exon 1s at 5′UTR, and it is rigorously regulated by methylation during the development (1091). Xiong et al. (1089) revealed that prenatal hypoxia repressed the GR encoding gene NR3C1 by increasing methylation of cAMP-response elements (CREs) and Sp1 binding sites at NR3C1 untranslated exon 1 promoters. This epigenetic modification blocked CREB and Sp1 binding to the NR3C1 promoter, repressing the gene in the developing hearts. The GR is a repressor of the AT2 receptor, and the downregulation of the GR resulted in the upregulation of the AT2 receptor in the heart, which also contributed to the increased heart vulnerability to I/R injury in adult rats (1104).
Cardiomyocyte apoptosis is also subject to the regulation by miRs. MiR-15b was upregulated after I/R in porcine hearts, and inhibition of miR-15b reduced infarct size by inhibiting apoptosis and improved cardiac function (403). I/R promoted downregulation of miR-133a in rat hearts, and administration of miR-133a mimic reduced I/R-induced apoptosis (361). In cultured cardiomyocytes, nicotine-induced downregulation of miR-133 resulted in increased caspase-3 level that subsequently promoted apoptosis (1027). Overexpression of miR-133 led to downregulation of caspase-9 and caspase-3 by lowering oxidative stress and protected cardiomyocytes from apoptosis (361, 1092). Caspase-3 is also a direct target of miR-378 and myocardial ischemia downregulated miR-378 (260). Overexpression of miR-378 diminished hypoxia-induced apoptosis in vitro. Transgenic mice overexpressing miR-1 exhibited increased infarct area and elevated caspase-3 following I/R (760). In a rat model, I/R increased miR-1, which in turn triggered posttranscriptional repression of Bcl-2 (958). Maternal hypoxia concurrently increased miR-210 and decreased the GR in fetal heart (626). Further studies revealed that the upregulation of miR-210 was HIF-1α-dependent and the upregulated miR-210 in turn downregulated the GR by binding to 3′UTR of NR3C1. Consequently, the GR suppression by maternal hypoxia promoted apoptosis. A vast amount of information has accumulated on the regulation of cardiac I/R injury by miRs, and the readers are referred to recent reviews on this topic (927, 1121).
F. Omic Approaches in Heart Development and Disease
Lineage-specific and spatiotemporal heart development was characterized by single-cell RNA-Seq in cells isolated at different developmental stages from embryonic day 9.5 (primordial heart tube) to postnatal day 21 (mature heart) (217). Analysis of transcriptomes in the hearts of postnatal day 2 (cell cycle active) and postnatal day 13 (cell cycle inactive) neonatal mice revealed that 3,273 transcripts were differentially expressed between the 2- and 13-day-old hearts with 1,768 of them being upregulated and 1,505 downregulated (286). Importantly, several sets of genes including genes for cyclin and CDKs were among those being repressed, consistent with cardiomyocyte cell cycle withdrawal in the 13-day-old heart. In addition, 2,545 DMRs from postnatal day 1 to day 14 in the mouse hearts were identified (920). Approximately 80% of DMRs were hypermethylated between day 1 and day 14, coinciding with the silence of critical developmental factors and signaling pathways including Hedgehog, TGF-β, BMPs, and Wnt/β-catenin signaling. Similarly, single-cell transcriptome and whole-genome DNA methylome analyses of mouse cardiac progenitor cells (mCPCs) revealed various DMRs, either hypermethylated or hypomethylated, which were associated with downregulation of genes encoding cardiac structure and function proteins and upregulation of genes for cell cycle and proliferation (167). The work from the Ping laboratory quantified in vivo half-life of 3,228 and the expression of 8,064 cardiac proteins with mass spectrometry in six mouse genetic strains under physiological and pathophysiological conditions (530). Sabatine et al. (870) examined the impact of myocardial ischemia on metabolome in human blood samples and demonstrated that γ-aminobutyric acid and MET 288 increased, whereas oxaloacetate, citrulline, and argininosuccinate decreased in ischemic patients but not in the control subjects. Measurement of hypoxia-induced metabolic changes in fetal rat hearts and hearts of adult offspring via metabolomics approach revealed that hypoxic fetal hearts displayed signs of stress and some persisted into adulthood, and suggested changes in AMPK activity and pathogenic glycogen storage phenotype in hearts from hypoxic adult males (unpublished observations). Clearly, the application of omics techniques has increased and will continue to enhance our understanding of the molecular mechanisms of heart development and heart disease. The integration of changes in genes, mRNAs, proteins and metabolites will facilitate the identification of multiple branches of signaling networks attributed to the developmental programming of heart disease and to the design of effective therapeutic strategies.
G. Hypoxia and Cardiac Regeneration
The majority of mammalian cardiomyocytes complete the transition from proliferation to terminal differentiation shortly after birth. Although the human heart maintains a limited cardiomyocyte turnover in adulthood (at a rate of ~1% per year at the age of 25 and gradually decreasing to 0.45% at the age of 75) (75), this capacity is insufficient to compensate the loss of cardiomyocytes after the heart injury. Consequently, the loss of cardiomyocytes results in formation of fibrotic scar, which in turn leads to electrical uncoupling from the rest of the myocardium and eventually to heart failure. The induction of cardiac regeneration is thus a promising strategy to treat heart failure. Multiple approaches have been established to stimulate cardiomyocyte regeneration and proliferation. In general, they fall into two major categories: 1) cell transplantation of cardiomyocytes generated from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) and 2) stimulation of endogenous regenerative mechanisms such as differentiation of resident cardiac progenitor cells (CPCs) and reactivation of cardiomyocyte proliferation.
Notably, oxygen levels play an important role in regulating cell cycle within the heart. A study from the Sadek laboratory demonstrated that the transition of hypoxia to normoxia after birth was responsible for the cell cycle arrest in the adult heart (814). The authors showed that exposure of newborn mice to mild hypoxia (15% O2) delayed the exit from the cell cycle. To test the hypothesis that hypoxia could revive adult cardiomyocyte proliferation, Kimura et al. (486) labeled hypoxic cardiomyocytes in the adult heart with stabilized HIF-1α and the fluorescent protein tdTomato by crossbreeding the transgenic mice expressing the oxygen-dependent degradation (ODD) domain of HIF-1α fused to the tamoxifen-inducible CreERT2 recombinase with mice expressing Rosa26 floxed-stop tdTomato (R26R/tdTomato) reporter mice. When driven by either the ubiquitous CAG promoter or the cardiomyocyte-specific α myosin heavy chain promoter, a set of hypoxic cells was found to proliferate with a turnover rate of ~1%. The same laboratory recently demonstrated that chronic exposure to systemic hypoxia (from 20.9 to 7% O2 over 2 wk followed by exposure to 7% O2 for another 2 wk) promoted cardiomyocyte proliferation in adult hearts by reducing ROS (714). Myocardial infarction is caused by cardiomyocyte death due to prolonged ischemia. Paradoxically, hypoxia can induce cardiac regeneration after myocardial infarct injury. Hypoxia exposure induced a robust cardiomyocyte regeneration with decreased myocardial fibrosis and improved left ventricular function after myocardial infarction (486, 714). The newly formed cardiomyocytes were derived from preexisting cardiomyocytes, but not from c-Kit+ or Sca-1+ CPCs. Consistently, transgenic mice overexpressing HIF1-α displayed attenuated infarct size and improved cardiac function after myocardial infarction (481).
H. Interventions: Targeting Epigenetic Mechanisms and Other Pathways to Treat Cardiovascular Disease
As discussed above, a number of fetal stresses disrupt heart development and increase the susceptibility to I/R injury later in life. Given that ischemic heart disease is a leading cause of death worldwide, significant efforts have been made to explore potential preventative and therapeutic strategies during the last decade.
Epigenetic modifications are potential targets for preventing and repairing I/R injury. Downregulation of PKCε by hypermethylation of PRKCE gene is critical in programing cardiac dysfunction in response to fetal stresses. Thus targeting DNA methylation to restore PKCε expression provides a plausible intervention. In vitro and ex vivo studies demonstrated that the DNA methyltransferase inhibitor 5-aza-2′-deoxycytodine blocked hypoxia-, nicotine-, and cocaine-induced increase in methylation of both SP1 binding sites at PRKCE and restored PKCε expression in embryonic ventricular myocyte H9c2 cell line and intact isolated rat hearts (534, 656, 784). 5-Aza-2′-deoxycytidine is approved by the United States Food and Drug Administration (FDA) for treatment of childhood acute myeloid leukemia (155). Thus 5-aza-2′-deoxycytodine or other methylation inhibitors might have great potential for preventing cardiac dysfunction induced by in utero stresses. Results from animal studies in preventing I/R injury with miR mimics or anti-miRs (antagomirs) are also promising. Intramyocardial injections with minicircle vector carrying miR-210 precursor reduced apoptosis in part by inhibiting caspase activity and improved cardiac function in a murine model of myocardial infarction (388). In addition, application of antagomir-92a (36 or antagomir-15) (403) reduced infarct size and promoted recovery of damaged tissue in rodents and pigs. Of interest, Eulalio et al. (253) demonstrated that miR-590 and miR-199a promoted reentry of adult cardiomyocytes into the cell cycle, leading to cardiomyocyte proliferation. Furthermore, they also demonstrated that adeno-associated virus vector delivery of these miRs to the heart promoted cardiac regeneration following myocardial infarction in mice.
Accumulating evidence has implicated oxidative stress in the programming of cardiac dysfunction in the adult by prenatal stresses. Oxidative stress results from imbalance between the production of ROS and antioxidant defenses, and it is conceivable that restoring the oxidant-antioxidant balance would be a promising approach. Patterson et al. (785) reported that maternal administration of N-acetylcysteine or tempol to scavenge ROS was able to ablate prenatal hypoxia-induced increase in the susceptibility of the rat offspring heart to I/R injury by lessening hypoxia-induced CpG methylation of the SP1-binding sites at PRKCE and restoring PKCε expression. In addition, Giussani et al. (310) also demonstrated that maternal hypoxia resulted in aortic thickening and oxidative stress in near-term fetal rat hearts and increased myocardial contractility in the offspring. These changes were reversed by maternal vitamin C supplement. Similarly, melatonin treatment of hypoxic chick embryo rescued cardiac function impaired by hypoxia in part by increasing catalase activity and restoring cardiac expression of glutathione peroxidase (415). Moreover, maternal supplementation of N-acetylcysteine diminished prenatal nicotine-induced I/R injury and improved postischemic recovery of cardiac function in the offspring, and these beneficial effects were the results of reducing ROS and reinstating PKCε expression (1085). Administration of N-acetylcysteine to mice starting after birth improved systolic function following I/R at postnatal day 21 with increased cardiomyocyte number and reduced scar formation relative to control animals (814). Interestingly, application of antioxidants in the offspring exposed to fetal stresses still exhibited cardioprotection. Administration of N-acetylcysteine for 48 h at 6 mo of age reduced the degree of infarction and improved the recovery of left ventricular function in offspring of maternal protein restriction (247). Resveratrol supplementation for 9 wk after weaning lowered oxidative stress, leading to increased cardiac recovery from I/R injury (908). Together, these observations suggest that application of antioxidants antenatally and postnatally could produce cardioprotective effects by halting the programing process initiated by fetal stresses.
It is conceivable that inhibition of apoptosis could be another potential strategy to reduce I/R injury. Caspase inhibitors have been shown to reduce infarct size and improve postischemic recovery following I/R injury (510, 1119). Other strategies might also be beneficial in cardioprotection against I/R injury. For example, various studies have shown that dexamethasone has a cardioprotective effect against I/R injury (1002, 1008, 1106). A variety of elements/pathways appear to be involved in the glucocorticoid-mediated cardioprotection, such as upregulating PKCε (1106), Mn-superoxide dismutase (SOD2) and glutathione reductase (44), heat shock protein 72 (1002), and lipocalin-type prostaglandin D synthase (983), downregulating AT2 receptors (1106) and inhibiting cytochrome c release (1008). However, one should be cautious when translating these finding into clinical trials. Mihailidou et al. (659) demonstrated that the heart infraction was aggravated as the result of activating the mineralocorticoid receptor by cortisol and the GR by dexamethasone. Instead, low-dose mineralocorticoid receptor antagonist spironolactone reduced infarct size and apoptosis by suppressing I/R-induced activation of caspases 2, 3, and 9 (579). Additionally, Xue et al. (1104) reported that inhibition of AT2 receptor improved postischemic recovery of left ventricular function in offspring exposed to prenatal hypoxia.
I. Perspective
In summary, cardiomyocyte proliferation, differentiation, and gene expression patterns are affected by adverse in utero environments, which may program heath and disease that extend beyond birth, leaving an imprint(s) in the heart with a long-term cardiac consequence in childhood and adulthood (FIGURE 10). A large body of evidence clearly suggests that the disturbed proliferation and differentiation of cardiomyocytes and altered gene expression patterns due to epigenetic modifications contribute to the fetal programming of heart disease. These alterations in turn limit the heart’s capacity to respond to new stimuli later in life. Regeneration of cardiomyocytes from stem cells and/or stimulation of preexisting cardiomyocyte proliferation hold great promise for cardiac repair. Despite significant progresses, we are still far from fully understanding of developmental programming of cardiac diseases. No effective cures are available currently for cardiac remodeling and injury. Gaining more mechanistic insights into adult heart disease programmed by prenatal stresses with new technologies such as omics and systems biology will help to identify therapeutic strategies to reverse cardiac programming and to prevent or to ameliorate the risk of cardiac diseases in later life.
FIGURE 10.
Fetal hypoxia impairs heart development. Mesodermal cells migrate to the cardiogenic region to develop into the cardiac crescent which later forms the primitive heart tube. Cardiac looping ensures proper orientation of the future atria, ventricles, and outflow tract. The development of the conduction and valvular systems, as well as septation of atria, ventricles, and outflow tract gives rise to the four-chambered heart. Cardiomyocyte terminal differentiation occurring well after the formation of the four-chambered heart determines cardiomyocyte endowment. The formation and maturation of the heart are tightly regulated by oxygen levels. Although the heart development occurs in a “physiologically” hypoxic microenvironment, excessive “pathologically” hypoxia impacts the heart development by modifying gene expression, leading to detrimental consequences such as congenital heart defects and increased vulnerability of heart diseases later in life.
VI. DEVELOPMENT, CEREBRAL CIRCULATION, AND HYPOXIC PROGRAMMING
A. Brain Development
1. Neuronal and glial development
Stressful early life experiences long have been associated with increased risk for psychological and/or neurological disorders in adults. Considerable evidence has demonstrated that complex interactions between genes and environmental factors such as diet, stress, and hypoxia can directly affect long-term neuronal function and plasticity. Studies of these interactions in humans and animals have given rise to the concept of “fetal programming.” This concept associates intrauterine stress of immature fetal systems with an increased risk of undesirable lifelong consequences for adult health and vitality. Correspondingly, the unique patterns of growth and development characteristic of the fetal brain render it particularly susceptible to the influences of an adverse intrauterine environment. Neuron number is typically established by mid to late gestation in the fetus, with little postnatal neurogenesis other than in the hippocampus. Associated glial cells follow a similar course, with perhaps a 1- to 2-wk delay depending on species and brain region. The simultaneous processes of neurogenesis, migration, axon and dendrite formation, differentiation, functional specialization, establishment of billions of synaptic connections, and in some cases selective cell death (apoptosis), together confer great complexity to overall brain development. This complexity, in turn, renders the fetal brain particularly sensitive to environmental and other factors that may impede its normal pattern of growth and development and result in increased frequencies of learning and neurodevelopmental disorders. As has been recognized for many years, compromised cerebral development can have both short- and long-term consequences for the conceptus. Despite remarkable advances in contemporary neuroscience during the 1990s “Decade of the Brain” (319) and the “BRAIN initiative” (Obama Administration, 2013), the neuroscience community remains in the earliest stages of understanding the mechanisms that govern and guide cerebral development and function (11, 342, 433, 810). At the other end of the ever-lengthening human lifespan, improved diagnostic methods have revealed growing numbers of cases of cognitive and neuropsychiatric disturbances, including Alzheimer’s disease, senile dementia, schizophrenia (550), bipolar disorder (91), and others. Adult mood disorders such as depression and addiction also appear to be increasingly prevalent, particularly among those who smoke, have a sedentary lifestyle, or have a high body-mass index. It is important to note, however, that many individuals with neuropsychiatric disorders have few risk factors for cardiovascular or metabolic disease, which suggests that numerous unrecognized and underappreciated factors contribute to the genesis of these pathologies. One important possibility is that many of these pathologies arise from epigenetic origins, put in motion during fetal development. Although early fetal responses to stress may constitute a recruitment of developmental plasticity to mount an appropriate biological defense, the long-term effects of these responses may be detrimental to adult neurological health.
Brain development proceeds during both the prenatal and postnatal periods. In all mammals, brain growth follows a sigmoid trajectory when weight is plotted against age; the transit period of rapid growth is known as the “brain growth spurt” (223, 224). The timing of this growth spurt varies among species, with that in precocial species (guinea pig, monkey, sheep) occurring prenatally, and that in nonprecocial species (rat, rabbit, pig) occurring following birth. For humans, this growth spurt is intermediate or perinatal, occurring during late gestation and early neonatal life (223, 224). At the time of birth, the fetal brain weighs ~350 g and accounts for about one-quarter of basal metabolic rate (378). During fetal life, the brain-to-body weight ratio is relatively larger than that of the adult, by ~27% of adult brain-to-body weight ratio, with brain mass continuing to increase in the infant and child, largely as a result of myelin deposition (223, 224). Due to multiple physiological adaptations to hypoxia that protect the developing brain, in an infant with fetal growth restriction (FGR), the size of the brain and head are relatively preserved in relation to overall body size and girth. These adaptations also help ensure that the development of the cerebral cortex, subcortical nuclei, white matter tracts, and other structures proceed according to precisely orchestrated and critically timed patterns of cell migration and differentiation. Owing to the enormous number of anatomic, metabolic, and behavioral events associated with brain growth spurts, these periods of developmental plasticity are times of particularly enhanced vulnerability to maternal hypoxia or other stresses.
A significant effort has been made to more clearly understand brain development at the genomic level. Recent studies have begun to examine gene expression patterns in the developing brain of rodents and other species at various pre- and postnatal ages, to test hypotheses related to the up- or downregulation of specific genes and their functional roles at different developmental stages (903). Within this context, multiple microarray studies have identified nuanced patterns of gene expression involved in the development of the hippocampus (674), cerebellum (564), prefrontal cortex (903), and ventral mesencephalon (1123). In vitro cultures of human neurons have also implicated several distinct gene classes that are strongly up- and downregulated in the course of differentiation (201). In developing mouse hippocampal cells, microarray analyses with gene clustering demonstrated significant increases up to postnatal day 30 of genes involved in neurogenesis, differentiation, and synapse formation (674). Similarly, in developing mouse prefrontal cortex, the basic laminar structure was first established in utero and then extensively remodeled through adolescence. mRNA transcripts that demonstrated significant changes between weeks 2 and 4 of life included those involved with cell growth, membrane expansion, extracellular matrix modifications, as well as transcription factors, zinc finger proteins, DNA methylation, and translational regulation (903). Developing cerebellum exhibited particularly striking upregulation of genes for ion transport channels, gamma-aminobutyric acid (GABA) and N-methyl-d-aspartate (NMDA) receptors, and synaptic components (564). At 2–4 wk of age, several categories of functional genes were overrepresented, including genes involved in blood vessel development and angiogenesis, morphogenesis, organogenesis, lipid metabolism and transport, cell cycle control, cell growth, and maintenance. Studies of the developing hindbrain in wild-type and transcription factor Pax6 homozygous mutant Sprague-Dawley rats have demonstrated that Pax6 regulates anterior-posterior patterning via activation of several genes, including Cyp26b1, an enzyme that metabolizes retinoic acid (743).
2. Hypoxic modulation of neuronal and glial development
Within the context of gestational hypoxia, a broad variety of studies have shown that antenatal maternal hypoxia potently modulates patterns of expression for many genes and can precipitate not only growth restriction but also can lead to neuronal cell death and/or induce neurological disorders in the newborn (91, 532, 550). As for all cell types, oxygen is a critical regulator of neuronal development, and hypoxic stress can cause epigenetic changes of the renin-angiotensin system (RAS) (324, 325). In addition to the well-recognized role of the renin-angiotensin cascade in the systemic circulation, it also functions locally in multiple independent tissues and organ systems, including the brain (323, 533, 546, 642, 730). In addition, the brain renin-angiotensin cascade can be altered in numerous CNS disorders including depression, neurodegenerative disease, Alzheimer's disease, and hypertension and stroke (885, 944). The brain renin-angiotensin cascade is also involved in learning, memory, behavior, osmoregulation, and thermoregulation (642, 885). This diversity in involvement of the brain renin-angiotensin cascade reflects the marked functional heterogeneity among different regions and cell types within the brain. Correspondingly, the responses of developing brain cells to hypoxia are also remarkably heterogeneous and depend on both age and duration of exposure. In rat embryos exposed to hypoxia at both 24 h and 11 days of age, glycolysis-related genes, calcium homeostasis-related genes, and inflammatory genes (specifically as related to oxidative stress) were upregulated, while cell growth-related genes were downregulated (397). These and other results emphasize that the developing murine brain responds to hypoxia through multiple adaptive mechanisms, including upregulation of genes associated with erythropoiesis, proteolysis, and peptidolysis. Through these adaptations to hypoxia, the mouse brain increases blood oxygen-carrying capacity and oxygen delivery to the brain while also increasing its metabolic flexibility, antioxidant reactivity, and tissue remodeling. Whereas the exact mechanisms that coordinate these responses remain unknown, it is clear that extracellular matrix remodeling, altered cellular metabolism, regulation of apoptosis, and epigenetic changes all appear to be vital steps in the physiological adaptations to hypoxia.
Laboratory animal models have provided detailed insights into roles of specific stressors in neurodevelopmental disorders and the mechanisms of their genesis. From these studies, it is apparent that sustained stress promotes neurological changes associated with depression and chronic anxiety disorders (639). Stress can also induce heterogeneous changes in neuroanatomical structure: pyramidal neurons in the CA-3 region and dentate gyrus of the hippocampus demonstrated attenuated dendritic length and synaptic branching, with opposite findings in basolateral amygdala and medial prefrontal cortex (608, 670, 1018). Given the importance of hippocampal neurons for release of excitatory amino acids (aspartate and glutamate) and activation of NMDA receptors, these findings show that stress can potently modulate learning, memory, and behavior (670, 1068). Importantly, these effects also appear to be gender-dependent (599). In male offspring of mice (694) and guinea pigs (464), prenatal stress can increase behavioral stress sensitivity, a hallmark of many neuropsychiatric disorders including depression (724), and a phenomenon associated with changes in corticotrophin-releasing hormone and glucocorticoid receptor (723). Importantly, variations in maternal care, which can attenuate neonatal stress, can permanently affect “context-dependent” neurodevelopment (196–199) and can induce changes in epigenetic programming that dynamically influence integrated CNS function and behavior throughout life (648, 649, 950). Conversely, sustained increases in maternal corticosteroids and subsequent transfer across the placenta to the developing conceptus can also induce potentially detrimental, gene specific patterns of epigenetic programming (898).
B. Cerebrovascular Development
Formation of the cerebrovasculature is governed by highly dynamic processes that continue throughout adult life. At all ages, cerebral vessels continuously undergo angiogenesis and differentiation, and in response to stress or injury, also can undergo dedifferentiation. The plasticity of the adult cerebrovasculature, however, relies directly on modulation of existing vasculature formed during embryonic vasculogenesis.
1. Vasculogenesis and angiogenesis
Human cerebral blood vessels first form as a leptomeningeal plexus at 24 days of embryonic age and then later develop into individual arteries, veins, and capillaries by 28 days (308). The leptomeningeal plexus undergoes waves of differentiation that originate at the base of the brain and propagate towards the midbrain convexity. By 44 days of embryonic age, the internal carotid arteries have formed from mergers of branches of the first and third aortic arch and dorsal aorta. At about the same time, vessels of the Circle of Willis become recognizable. Then, leptomeningeal vasculature rapidly arborizes to vascularize the developing cortex (841). This vascularization continues into the third month of postnatal life (355) and includes a sustained expansion of capillary density that is not complete in cerebral gray matter until the third or fourth year of life (755). Owing to the prolonged phase of cerebral vasculogenesis, the fetal and neonatal cerebrovasculature is highly vulnerable virtually to all varieties of metabolic (hypoxic) and mechanical injury.
In contrast to vasculogenesis, which arises from proliferation and migration of mesodermal angioblasts that differentiate into endothelium and then form the initial vasculature, angiogenesis produces new vessels from vasculature already formed (540). This process tightly matches vessel density to regional metabolic activity; increased metabolism and/or decreased oxygen delivery drive down tissue oxygen tension. This, in turn, stimulates multiple cell types to produce and release HIF-1α, a transcription factor that when translocated to the nucleus will bind its coactivator (HIF-1β) (902). The complete HIF complex then binds HREs in the promoters of numerous genes that promote angiogenesis, including the genes coding for erythropoietin and VEGF (900). The combined influences of these factors, along with other angiogenic factors such as TGF-β (1131), Wnts (935), and angiopoietin (943) determine the pattern of new vessel growth. The direction in which these new vessels grow is guided by a variety of multipurpose axonal growth cues including semaphorins and ephrins that act in concert with regionally produced neurotrophins such as brain-derived neurotrophic factor, nerve growth factor, and neurotensin-3 (540). This broad diversity of factors that govern angiogenesis is quite robust owing to numerous redundancies that help guarantee tight coupling of local vascular density to metabolic demand, which is particularly important during the rapid growth and expansion of the immature mammalian brain.
2. Smooth muscle differentiation
Detailed studies of gene expression in smooth muscle have identified a complex family of molecular factors that govern its migration, proliferation, and differentiation (756). Early studies relied on morphological characteristics to categorize smooth muscle as either contractile or synthetic (140). However, the development of highly selective antibodies capable of detecting both contractile and noncontractile smooth muscle proteins, together with the broad availability of high-resolution confocal microscopy, has helped establish that smooth muscle exists in a wide continuum of various cell types. These are characterized by diverse variations in the capacity for proliferation, migration, secretion, and contraction (756, 832). In addition, the different phenotypes of smooth muscle are highly plastic and can be interconverted depending on the local availability of growth factors including VEGF, platelet-derived growth factor (PDGF), fibroblast growth factors (FGFs), and angiotensin II among others. Somewhat unconventionally, receptors for these growth factors include both receptor tyrosine kinases (RTKs) as well as G protein-coupled receptors (GPCRs), which interact via multiple signaling pathways within the smooth muscle cell cytoplasm (crosstalk) to influence phosphorylation and nuclear translocation of transcription factors that govern smooth muscle phenotype. Among these transcription factors, some of the most important are serum response factor (SRF), ternary complex factor (TCF), Elk-1, and Myocardin (772). The genes these transcription factors activate act in concert to determine the phenotypic characteristics of all cerebrovascular smooth muscle and, in turn, cerebral artery structure and function.
A key feature of smooth muscle phenotype in cerebral arteries is its heterogeneity; within the same artery, adjacent smooth muscle cells can exhibit very different phenotypes. Such heterogeneity is particularly prominent in immature cerebral arteries, which express both non-muscle myosin heavy chain (NM-MHC), a marker for synthetic smooth muscle, and smooth muscle myosin heavy chain (SM-MHC), a marker for fully differentiated contractile smooth muscle (400). As fetal cerebral arteries age and mature, the NM-MHC is gradually replaced by SM-MHC, and correspondingly, contractile capacity improves toward adult levels. Importantly, as demonstrated for fibroblasts by Yamanaka’s Nobel-winning work (952), contractile smooth muscle also is not terminally differentiated. In response to injury or inflammation, contractile smooth muscle can dedifferentiate back into synthetic, proliferative, or migratory phenotypes depending on local conditions. This plasticity ensures that fetal and neonatal cerebrovascular smooth muscle are highly adaptive and responsive to both physiological stimuli, such as changes in local concentrations of vasotrophic factors, or pathophysiological stimuli, such as infection, inflammation, ischemia, or hypoxia (320, 364).
3. Hypoxic modulation of cerebrovascular structure and function
Fetal cerebral arteries undergo a near-continuous process of remodeling that dynamically matches the structure and function of immature arteries to regional conditions. Among the factors that influence fetal cerebrovascular remodeling, one of the most important is hypoxia (787, 919). As occurs in adult arteries, hypoxia mobilizes the release of HIF-1α, which in turn can stimulate angiogenesis, increase capillary density and permeability, and reduce intercapillary distances over which oxygen must diffuse with the brain parenchyma (94, 461, 525, 526, 554, 654, 663, 783, 1095). Many of these same mechanisms are also recruited in response to local ischemia (192). In addition, hypoxia also potently influences fetal cerebral artery composition that contributes to increases in protein content and wall thickness (587, 589, 787, 1060). Importantly, all of these effects depend not only on the severity and duration of hypoxia but also on age, which is a critical determinant of remodeling responses to hypoxia from fetal through adult life (114, 122, 720). These age-dependent influences, in turn, arise directly from changes in each of the three main families of factors that govern overall vascular remodeling including oxygen-sensitive transcription factors such as HIF-1α (902), RTK-dependent growth factors such as VEGF (400), and GPCR-dependent vasotrophic factors such as ET-1 (918, 919) (FIGURE 11).
FIGURE 11.
Hypoxia acts through the HIF family of transcription factors to influence the expression of numerous genes, one of the most important of which is vascular endothelial growth factor (VEGF). In addition to its well-documented angiogenic ability to increase capillary density, VEGF also exerts multiple effects on the main cell types that make up cerebral arteries. Through direct effects on Flk (VEGFR-2)/Flt (VEGFR-1) receptors expressed by vascular smooth muscle, VEGF can promote contractile dedifferentiation. Through action on Flk/Flt receptors expressed in vascular endothelium, VEGF can promote the synthesis and release of NO and endothelin-1. In turn, NO can act through cGMP and protein kinase G to promote contractile differentiation. Similarly, endothelin-1 can act through ET-A receptors also to promote contractile differentiation. Through action on Flk/Flt receptors expressed in perivascular adrenergic nerves, VEGF can promote the release of norepinephrine and neuropeptide Y, both of which can activate specific receptors to promote contractile differentiation. Although this diagram lists only a few of the pathways through which VEGF can influence arterial contractility, it emphasizes that hypoxia works through VEGF and other downstream molecules to modulate arterial function in the fetal cerebral circulation.
Hypoxia-induced changes in fetal cerebrovascular structure and composition have a broad variety of effects on the contractile capacity of fetal cerebral arteries. In general, chronic hypoxia depresses contractility, largely through decreased densities of cell-surface receptors that drive contraction (587, 997), decreased inositol trisphosphate (IP3) mobilization and IP3 receptor density (1165). Chronic hypoxia also attenuates the ability of calcium to stimulate myosin light chain phosphorylation, but this effect is offset by upregulation of the ability of phosphorylated myosin light chain to produce force, with the result that overall myofilament calcium sensitivity is increased in fetal cerebral arteries (720). Other effects further enhance contractility in hypoxic fetal cerebral arteries, including increased binding affinity of serotonergic receptors (966), and a reduced ability of ATP-sensitive and calcium-sensitive potassium channels to promote vasorelaxation (585). The net result of these diverse changes is that contractile tone appears to be more delicately balanced between contraction and relaxation in hypoxic than in normoxic fetal cerebral arteries. Although it remains uncertain if this rearrangement of pro-contractile and pro-relaxant influences confers some homeostatic benefit to hypoxic fetal cerebral arteries, it is clear that contraction is more fragile, and probably more vulnerable to perturbation in hypoxic compared with normoxic fetal arteries.
External to the smooth muscle, chronic hypoxia also reduces expression of neuronal NOS in fetal cerebrovascular perivascular nitridergic nerves (633). Owing to the ability of NO released from these nerves to enhance norepinephrine release from adjacent adrenergic nerves, chronic hypoxia also decreases perivascular norepinephrine release (114, 115). Conversely, chronic hypoxia appears to upregulate the ability of adrenergic stimulation to initiate contraction in fetal cerebral arteries (307, 589, 590). Increased activity of perivascular norepinephrine also influences phenotypic differentiation in cerebrovascular smooth muscle, and this mechanism contributes significantly to the phenotypic changes typical in hypoxic fetal cerebral arteries (3).
In addition to its direct effects on cerebrovascular smooth muscle and perivascular nerves, chronic hypoxia also induces a broad variety of secondary effects with important influences on cerebrovascular, as well as neuronal and glial, development. Important among this is the ability of chronic hypoxia to modulate the hypothalamic-pituitary-adrenal (HPA) axis, and possibly alter glucocorticoid stimulation of fetal cerebrovascular maturation (706, 727). Chronic hypoxia also disturbs anabolic metabolism and can restrict fetal growth (703). Changes in the availability of either glucocorticoids or nutrients can program the fetal cerebrovasculature, and produce long-term changes in cerebrovascular structure and function that include depression of myogenic contractility and cytosolic calcium regulation (243).
4. Endothelial differentiation and the blood-brain barrier
The fetal cerebrovascular endothelium serves four primary functions that are vital for 1) angiogenesis, 2) hemostasis, 3) blood-brain barrier function, and 4) vascular tone. The ability to initiate capillary angiogenesis is among the first of these functions to mature, and the fetal endothelium can release FGFs, PDGFs, IGFs, and thrombospondin, all of which are proangiogenic (409). In counterbalance, fetal endothelium also releases angiostatic factors such as heparin, heparan sulfate, TGF-β, NO, and prostacyclin. Together, this arsenal of factors enables the fetal cerebrovascular endothelium to precisely control the rapid rates of vascular expansion required to support brain growth during late fetal and early postnatal life.
Healthy and intact fetal cerebrovascular endothelium continuously releases NO and prostacyclin, which inhibit local initiation of hemostasis (34). Upon injury, however, the fetal cerebrovascular endothelium can release platelet activating factor, factor V, factor X, von Willebrand factor, and tissue factor (thromboplastin) (34), all of which promote hemostasis. Whereas the relative amounts of each of these pro- and anticoagulant factors released from the vascular endothelium change throughout development and postnatal maturation (89), beginning in the third trimester the fetal cerebrovascular endothelium is functionally competent in regards to its role in hemostasis.
Blood-brain barrier function is a unique aspect of the cerebrovascular endothelium that efficiently restricts or mediates the transfer of blood-borne molecules into and out of the brain parenchyma. Its properties are attributable largely to presence of tight junctions between adjacent endothelial cells, together with the expression of occludin, an integral membrane protein that spans intracellular gaps between adjacent endothelial cells and physically binds the cells together (375). Cell adhesion molecules, including claudins, bind together adjacent cerebrovascular endothelial cells and increase hydraulic and electrical resistance across the endothelial cell layer (356, 540). At term, the fetal blood-brain barrier is not yet fully functional, and its permeability progressively falls throughout the postnatal period (116, 845, 937). A key factor limiting the tightening of the fetal blood-brain barrier is the high rate of angiogenesis characteristic of the fetal brain; newly formed vessels require several days to synthesize and position the claudins, occludins, and other proteins required for low endothelial permeability. Astrocyte maturation also is not complete at birth, which may further retard attainment of a fully functional blood-brain barrier, given that astrocytes envelop, and interact metabolically with, cerebral capillary endothelium (290, 676). A fully intact blood-brain barrier also requires the presence of subendothelial pericytes, and these cells are not fully present and appropriately distributed near the fetal cerebrovascular endothelium, which may further limit the tightening of the immature blood-brain barrier (540). Together, this pattern of structural and functional maturation of the fetal blood-brain barrier emphasizes that it is vulnerable to perturbation and injury throughout the perinatal period.
In addition to their roles in angiogenesis, hemostasis, and barrier function, endothelial cells also release a broad variety of factors that influence local vascular tone. One of the most important of these is NO, which has a half-life just long enough to allow it to diffuse into adjacent smooth muscle, bind to the heme moiety of soluble guanylate cyclase, and activate the synthesis of the potent vasodilator, cGMP (677). Immature endothelium appears to be less sensitive to shear stress and releases less NO than mature vascular endothelium (561, 1052, 1059). In contrast to NO, the pathways that mediate the release of the vasodilator prostacyclin (817) appear to be fully developed in immature cerebrovascular endothelium (770). The ability of the fetal cerebrovascular endothelium to release other vasodilators, such as endothelium-derived hyperpolarizing factor (297), and other vasoconstrictors, such as superoxide, thromboxane A2, and endothelin (1007), remains largely unexplored. Nonetheless, the functional and structural immaturity of the fetal blood-brain barrier suggests that the release of vasoactive molecules also is attenuated in fetal, relative to adult, cerebrovascular endothelium.
5. Hypoxic modulation of cerebrovascular endothelial function
Chronic hypoxia exerts several important effects on the cerebrovascular endothelium, including dysregulation of vasculogenesis and development of the cerebral vascular bed due to increased VEGF activity. Correspondingly, gestational hypoxia increases blood-brain barrier permeability in human neonates (886). Chronic hypoxia also attenuates the magnitude of endothelium-dependent vasodilatation in fetal, but not adult, cerebral arteries (587), and this effect seems to be highly conserved even in nonmammalian species (865). Hypoxic attenuation of endothelial vasodilatory capacity appears to be attributable to depression of eNOS mRNA and protein levels (10), along with simultaneous reduction in smooth muscle expression of soluble guanylate cyclase, which is the primary vascular target for NO released from the endothelium (788). Although NO is not the only vasoactive molecule released from the vascular endothelium, the effects of chronic hypoxia on the release of these other factors remain largely unexplored, particularly in the fetal cerebrovascular endothelium.
C. Epigenetics and Gene Expression in the Developing Brain
Epigenetic changes do not alter genomic base sequences but modify the expression of these sequences by 1) methylation of DNA; 2) modification of DNA-associated histone proteins by acetylation, methylation, or phosphorylation; and 3) expression of nontranslated RNA, including miRNA, lncRNA, piRNA, circular RNA and other ncRNA mechanisms (268, 386, 1070). Epigenetic changes play a critical role in the development and differentiation of brain neurons, and also play important roles in the normal function of many different cell types (230, 419, 820, 829, 884). Examples include X-chromosome inactivation in female organisms, and genomic imprinting in which one parental allele is methylated resulting in its silencing, or random modification of gene transcription (1058). For brain and other tissues, these epigenetic alterations may be associated with distinct sequelae such as abnormal cell proliferation, cell dysfunction and loss, and alteration of cell state (442). Evidence from multiple laboratories, including our own, indicates that epigenetic states can be disrupted antenatally by maternal influences such as hypoxia (305, 324, 325), protein deprivation (323, 326), and caloric excess. These and other stressors can alter DNA methylation, modify histones, and/or change patterns of ncRNA expression (305, 323, 328) to produce life-long consequences for organismal health and vitality.
As of the time of this writing, more than 2,500 published studies have focused on epigenetic mechanisms in the fetus. The vast majority of these have focused on the mechanisms whereby intrauterine stress translates into epigenetic modification of the fetal genome (61, 267, 898). Among these diverse studies, only ~72 have explored the involvement of epigenetic mechanisms in fetal responses to hypoxia, and of these more than three-quarters have focused on either fetal growth or placental function. A few other studies have explored the effects of hypoxia on epigenetic mechanisms in the immature pulmonary circulation (241, 883). Studies of the fetal brain suggest that hypoxic stress activates multiple epigenetic mechanisms that increase the likelihood for neurological and neurobehavioral disturbances in adult offspring (255, 256, 278, 606, 809), increase vulnerability to hypoxic-ischemic insults (194, 322, 556, 559), disrupt normal endocrine regulation (1067, 1091), and increase risks for adult cardiopulmonary disease (502). Although no studies have yet directly examined the effects of hypoxia on epigenetic programming of the fetal cerebrovasculature, the range of epigenetic effects elicited in the fetal brain by hypoxia suggests that such effects are likely and should be pursued in future studies.
1. DNA methylation and gene expression in the developing brain
DNA methylation/demethylation is a highly dynamic process that modulates synaptic plasticity and memory (427, 548, 549, 598, 813, 835, 836). Epigenetic polymorphisms in methyl-group metabolism via methyl donors such as choline (1148) or folic acid (934) also may have profound effects on neural development. For example, decreased DNA methylation within the hippocampus gene promoter regions of reelin and brain-derived neurotrophic factor can alter synaptic plasticity. Reelin is vital for cortical development including neuronal migration of laminated structures and synaptic plasticity and displays prominent epigenetic regulation (168, 226). This is of particular importance as reelin dysregulation has been implicated in the pathogenesis of autism (33, 1150), schizophrenia (189, 226), and Alzheimer’s disease (242), among other diseases. In adult mice, administration of l-methionine, an essential amino acid and methyl group donor, hypermethylates the reelin promoter and several other genes to reduce its mRNA levels. Of significance, these mice demonstrate altered learning and memory, as well as altered sociability, suggesting the importance of this protein and its signaling pathway for learning and behavior (168, 226). The effects of l-methionine on reelin mRNA expression can be blocked by antisense oligonucleotides (739). Reelin expression in the adult mouse brain also can be manipulated by pharmacological intervention (227, 739). Together, these findings suggest that reelin is a potential therapeutically accessible target for a variety of neurological diseases with etiologies that involve epigenetic mechanisms.
Multiple neurodevelopmental disorders have been suggested to be a consequence of a “double whammy” effect, with a “first hit” delivered by an antenatal insult and a “second hit” occurring in adulthood (1069). Relevant to such patterns of injury, methionine-treated fetal mice mimic increased incidence of adult behavioral and neurochemical aspects of schizophrenia (990). Consistent with this idea, glucocorticoid receptor activation plays a role in DNA methylation within an enhancer of the tyrosine aminotransferase gene (971), such that antenatal stress in the pregnant mother may play a pivotal role in fetal epigenetic changes with consequences later in life. Altered DNA methylation following stress also may alter expression of D-2 dopamine receptor variants with implications for epigenetic responses (24). Adult offspring who received an intracerebroventricular infusion of methyl donors (l-methionine) showed a reversal of the effects of maternal care on open-field behavior, as well as hippocampal gene expression (1043). Stress-reducing postnatal experiences during the first week of life (licking/grooming by the mother) also can alter significantly the DNA methylation pattern of the glucocorticoid receptor in the neonatal hippocampus (648, 649). With neuronal terminal differentiation and low proliferative activity being maintained, the process of methylation/demethylation decreases, although probably continuing at a low level in the periventricular area. Together, these findings emphasize the important interactions between fetal stress and genomic methylation in the fetal brain.
In relation to fetal hypoxia, studies of DNA methylation have just begun and total <20 at the time of this writing. Of these, fetal hypoxia has been shown to act through altered DNA methylation to compromise placental function (305, 347, 1129), fetal pancreatic function (251), fetal pulmonary artery function (1114), and whole body metabolism in adult offspring (478). In fetal heart, hypoxia modified DNA methylation and programming of the cardiac PKCε gene, which compromised heart function in adult offspring (784, 785). In fetal brain, hypoxia increased DNA methylation of the gene coding for the glucocorticoid receptor, reduced the expression of this receptor, which in turn increased vulnerability to a cerebral hypoxic-ischemic insult (322). The extent to which this effect influenced the cerebrovascular remains unexplored. Indeed, the role of DNA methylation in stress-related changes in fetal cerebral vasculogenesis, neurogenesis, differentiation, and function remain completely unexplored and offer a promising area for future investigation.
2. Histone modifications and gene expression in the developing brain
Gene expression is regulated by the biochemical organization of the histones in the nucleosomes around which double-stranded DNA is wrapped (542). DNA with accompanying histones (known as chromatin) are packaged in nucleosomes, the core of which contains an octamer of histone proteins. Four primary forms of histones (H2A, H2B, H3, and H4, in addition to minor variants) are encircled by 146 base pairs of DNA (132), and a fifth histone H1 serves as a linker protein (78). Several different posttranslational modifications may occur on the NH2-terminal amino acids of the histone proteins, and these modify histone interactions with DNA and other nuclear proteins. These posttranslational modifications include acetylation, methylation, phosphorylation, ubiquitination, and sumoylation, either alone or in combination, and play a key role in making specific regions of the genome accessible to the transcriptional machinery and thereby influence regulation by expression or repression of contiguous genes (432). Together with DNA methylation, these histone modifications increase the regulatory capacity of each nucleosome and enable regulation of specific functions such as gene activation and DNA repair (881). Mechanistically, histone modifications can regulate gene expression several ways, including 1) regulation of chromatin structure, altering accessibility to transcriptional machinery; 2) integration of multiple signaling cascade/networks to modulate activity of transcription promoters or repressors; and 3) mediation of epigenetic changes in gene expression to alter cellular function (73, 509). Acetylated and methylated histones may also function as epigenetic “readers” of a nuanced chromatin “language” (72).
Enzymes associated with nucleosomal modifications of histone proteins include HAT, HDAC, histone methyltransferase (HMT), histone demethylases (HDM), and others (225, 494, 994). The most widely studied histone modification is acetylation of lysine residues, which induces structural and electrostatic changes that decrease interaction between the negatively charged DNA backbone and the positively charged histone tail (432). In the mouse hippocampus, deregulation of histone H4 lysine 12 acetylation is associated with impairment of learning and memory; restoration of histone H4 lysine 12 acetylation rescues the expression of genes leading to the recovery of cognitive abilities (790). The regulation of histone acetylation, methylation, and/or other modifications constitutes a poorly understood “histone code” whereby selective histone modifications regulate the expression of select genes in a highly cell-type specific manner and may serve as a form of cellular memory (72, 85, 442). Importantly, administration or removal of methionine can influence histone deacetyltransferase inhibition and modulate the regulation of expression of reelin and other neuronal proteins, suggesting an interplay of histone acetylation and DNA methylation in the CNS (227, 739, 990). The importance of interaction of DNA methylation and histone modification has been suggested by a study in which choline deficiency during intrauterine development altered neuronal gene expression. Choline deficiency also decreased progenitor cell proliferation, increased hippocampal apoptosis, and produced several behavioral alterations (651).
Whereas studies of the roles of histone modification in epigenetic regulation have been the focus of more than 38,000 published studies to date, <800 of these have examined fetal tissues of any kind. Among the studies of histone modifications in fetal tissues, the vast majority have studied fetal growth, metabolism, and placental function (883, 1023). Less than a dozen studies have yet explored any aspect of fetal hypoxia. Of these the majority have detailed the effects of hypoxia on both physiological (68) and pathological (837, 1097, 1098, 1114) responses of the immature lung. A few other studies have also examined pathophysiological interactions between fetal hypoxia and histone modifications in liver (754) and placenta (544). In the brain, mutations in enzymes governing histone modification can increase sensitivity to hypoxia and contribute to congenital malformations and mental retardation (581). A recent review by Ma and Zhang (607) nicely summarizes the general importance of histone modifications in both endothelial and smooth muscle genes during normal vascular development, but cites no studies of interactions between fetal hypoxia and histone modification of vascular genes in any vascular bed. Such studies have yet to be performed, and in the fetal cerebral vasculature offer the promise of better understanding of cerebrovascular malformations and the mechanisms mediating vascular injury in hypoxic-ischemic encephalopathy.
3. MicroRNAs and gene expression in the developing brain
An entirely new subdiscipline in genomics has been established by the discovery that small noncoding RNA molecules (the stuff of “junk” DNA) play critical roles in gene expression. Specifically, miRNA, which are small, noncoding RNA molecules 21–25 nucleotides in length, have emerged as important players in pre- and posttranscriptional gene regulation. These miRNAs can base pair with mRNA to regulate translation and/or degradation, can fine-tune promoters/repressors of gene expression, and can influence proximate genes during development and differentiation, without changes in the nucleic acid code per se (623, 1064). Following the discovery of the first miRNA, “lin4” in 1993 as a small temporal RNA (543), there has been enormous growth in this family, with the identification of their targets. Targeting to the 3′UTRs of mRNAs with which they share partial sequence complementarity, miRNA can silence posttranscriptional gene translation without degrading the target mRNA. In this way, cells can upregulate or downregulate miRNA production to decrease or increase gene expression according to developmental need, producing desired, phenotypic, morphological, and/or physiological change. The use of miRNA microarrays to assay both rat and monkey miRNA libraries has demonstrated a temporal wave of expression of sequential classes of miRNAs with brain development (668). Specifically, miRNA-124 has been shown to regulate several aspects of neuronal growth and differentiation, including the cytoskeletal elements actin and tubulin (1125). Other classes of regulatory RNAs include small interfering RNA (siRNA), long-stranded RNAs (lsRNAs), and others. Clearly, further studies will be vital to better understand the complex roles of miRNAs in cerebral genetic regulation, and the contributions of these molecules to fetal and adult brain health and disease.
The dynamic remodeling of neuronal stem cells into mature differentiated neurons requires a host of epigenetic events including DNA methylation, histone modifications, and miRNA regulation (922). Recent reviews of the molecular regulation of DNA methylation, histone modification, and the regulatory influences of miRNA during embryogenesis and fetal development reveal that these processes are dynamically integrated and reflect an unprecedented complexity of gene regulation (85, 424, 425, 444, 877). The combination of the epigenetic modifications of coding as well as noncoding genes (sequences), known as the “epigenome” or “epigenotype,” determine the activation or repression status of the gene and influences the phenotype at birth. By integrating both genetic and epigenetic information, more precise insights can be obtained with regards to regulation of gene expression and changes in biological phenotype.
Despite the impressive scientific interest in noncoding RNAs, of the more than 130,000 publications on this topic as of the writing of this review, only ~2,200 have focused on fetal tissues of any kind. As for the other mechanisms of epigenetic regulation, the majority of these have examined fetal growth, metabolism, and placental function. Only ~70 publications have yet been published that focus on interactions between fetal hypoxia and miRNA regulation. Again, the majority have focused on the roles of miRs in placental pathophysiology (15) including miR-21 (178), miR-34a (229), miR-365 (686), miR-424 (396, 692), miR-455 (524), and miR-517 (36) among others (1054). In heart cells, attention has focused on miRs with important physiological roles, including miR-199 (928), miR-210 (45), miR-378 (495), as well as lncRNAs (793). In relation to hypoxic effects, miR-210 is perhaps one of the most widely studied miRs, as it is highly sensitive to oxygen levels and helps reinforce many of the actions of HIF-1α (257, 699, 700, 704). In the brain, miRs 23a/b and 27a/b have attracted considerable attention owing to their involvement in hypoxia-induced apoptosis (123, 165, 166). Another miR that appears to be critical for neural tube development, miR-30b, has also been the focus of several studies, at least in part because it targets Sirt1, a multifunctional protein that interacts with p53, inhibits nuclear factor κB (NFκB) expression, and enhances insulin sensitivity (824). Other miRs, such as miR-347a, have been studied for their potential to serve as biomarkers for cerebral injury and/or hypoxic-ischemic encephalopathy (330, 591). Within the vasculature, several miRs have been found to be proangiogenic under hypoxic conditions, including miR-21 (1167), miR-31 (170), and miR-486, which upregulates VEGF (914). In contrast, miRs 221 and 222 exhibit an ability to attenuate hypoxic angiogenesis (150), demonstrating that multiple miRs also participate in the delicate balance between proangiogenic and antiangiogenic factors in both normoxic and hypoxic fetal arteries. Although no studies have yet examined the roles of miRs in responses of the fetal cerebrovasculature to hypoxia, the numerous miRs that respond to hypoxia in fetal brain parenchymal cells, and in systemic vascular cells, provide a promising list of miR candidates to explore in this regard.
D. Role of Epigenetics in Human Correlates of Mental Health and Disease
The most common heritable disorder causing mental retardation is fragile X syndrome (severe mental retardation with a high forehead, enlarged jaws, ears, and testes in males, mild menstrual retardation in heterozygous females). This disorder is associated with the expansion of CGG triplet repeat sequences in the promoter region of the fragile X mental retardation gene (FMR1) in brain cells with triplet repeats expanding from normal to several thousand leading to methylation and gene silencing (1029). In addition, mental retardation in some other cases is also a consequence of mutations in genes that encode regulators of epigenetic mechanisms. Rett syndrome for example, is a progressive developmental disorder in females of cerebral cortical gray matter, characterized by autistic behavior, ataxia, dementia, seizures, loss of purposeful movement of hands, cerebral atrophy, mild hyperammonemia, and decreased levels of biogenic amines. This disease is a consequence of mutations in the X-linked gene MECP2 that encodes a methylated CpG binding protein linking DNA methylation to chromatin complexes, which alter expression of other genes (31, 136). Discovery of these epigenetic-associated syndromes of mental retardation have helped to promote exploration of the role of epigenetics in other neurological disorders with as yet undefined etiologies, and in which prolonged stress is believed to be a factor. An example is that of autism, a pervasive disorder with onset early in life, characterized by impairment of reciprocal social interactions, verbal and nonverbal communication, and capacity for other activities and interests, hypo- or hyperreactivity to certain stimuli, stereotypic behavior, and other problems. Considerable evidence suggests that the so-called autism spectrum disorders (ASDs) display features of autism in a broad range of phenotypes with the involvement of both genetic and epigenetic origins in their pathogenesis (329, 769, 826, 890). Specifically, these have been shown to be associated with alterations in DNA methylation of genes vital to neuronal development such as BCL2 and the retinoic acid-related orphan receptor in both lymphoblastoid cell lines and the brain itself (729). The vascular components of these diseases remain completely unexplored.
E. Perspective
Successful development of the brain, its many nuclei, and vasculature is vital for growth, maturation, development, and survival of the embryo through adulthood. Numerous epidemiological and experimental studies demonstrate the profound influence of intrauterine environment on both physiological and pathophysiological neuronal and cerebrovascular function. In large part, maternal influences such as hypoxia, protein or caloric deficiency/excess, and drug abuse are perceived as stressors by the developing fetus. All of these stressors, and hypoxia, in particular, induce not only developmental changes in the fetal brain and its vasculature but also epigenetic changes that can persist a lifetime and influence adult health. These epigenetic changes include DNA methylation, histone modification, and miRNA regulation but are only in the earliest stages of study, particularly in humans. Without doubt, a great deal of work lies ahead, but the potential of future studies of epigenetic mechanisms in the fetal brain offer tremendous promise not only for understanding the normal physiology of brain development, but also the mechanisms whereby dysregulation, injury, and disease occur and hopefully, be treated.
VII. HYPOXIA PROGRAMMING OF THE LUNG
Pulmonary hypertension in the newborn is a devastating disease that is marked by aberrant lung development. The origins for pulmonary hypertension of the newborn encompass a wide range of different stressors. These include bronchopulmonary dysplasia (BPD), preeclampsia, smoking, toxin exposure, drug use, and long-term maternal hypoxia due to living at high altitude. Disease manifestation and progression can be unique depending on the genetic background and type of stress to which the mother, fetus, as well as the newborn are exposed. The characteristics of pulmonary hypertension in the newborn are well described for many different disease etiologies, but the fundamental mechanisms important to the disease process remain poorly understood.
This section concentrates on the development of pulmonary hypertension that occurs in the fetus and newborn due to the effects of low oxygenation and the involvement of inflammation and oxidative stress pathways. A number of low oxygen models are used to induce pulmonary hypertension in newborn animals. These include exposing rats, mice, or guinea pigs to half partial pressure (10.5% O2) during gestation or immediately after birth (17, 82, 426, 646, 1116). Although cattle are not used as often, they are relevant as they are prone to getting brisket disease at moderately high altitudes (>2,133 m), which includes severe pulmonary hypertension and right ventricular hypertrophy (815). Piglets exposed to 10–12% O2 immediately after birth and up to 10 days of age also exhibit pulmonary hypertension (41, 219, 272, 273, 275). Several sheep models are also used to induce pulmonary hypertension. These include a preeclampsia model where the uterine arteries are ligated to restrict fetal nutrient and oxygen supply (216, 306). Ligation of the ductus arteriosus in fetal lambs places added pressure on the pulmonary vasculature, reduces oxygenation, and induces morphological and functional changes in the lung that emulate BPD (6, 7, 216, 306, 497–499, 523, 968). Placing a shunt between the systemic and pulmonary vasculature causes high pulmonary blood flow and reduces oxygenation (8, 9, 748, 911, 941). Our research group and a Chilean group each induce hypoxic stress by housing pregnant sheep at high-altitude field stations (3,600 and 3,800 m) during gestation (90, 370, 764, 989). This high-altitude exposure causes roughly a 35% reduction in O2 supply and is representative of cities in the Himalayas and Andes where millions of people live (455).
A. Morphological and Functional Problems
Newborns with pulmonary hypertension can have defects that lead to both structural and functional defects. This includes impaired lung growth that reduces alveolarization and surface area as well as inappropriate lung septation and alveolar arborization during late-stage development, as recently reviewed (762). Depending on the type of animal model examined, there can be increased pulmonary arterial medial wall thickness, development of plexiform lesions, poor angiogenesis and vascularization, reduced vasodilatory capacity, and enhanced arterial reactivity. There is a wide range of responsiveness to low oxygen ranging from mild responses in llamas and other high-altitude-adapted animals to cattle, which succumb to congestive heart failure (578, 763, 815, 936). The vast variation in responses illustrates that care must be taken when extrapolating findings from animal models to our understanding of the development of disease in human infants. Even still, the malformations due to the development of pulmonary hypertension in newborn infants restrict lung blood flow and impair gas exchange. Loss of lung function causes hypoxemia and increases pulmonary arterial pressures, and the infants develop right ventricular hypertrophy and are unable to thrive (762, 763, 1046).
The diverse stressors that cause pulmonary hypertension in the newborn are due to overlapping as well as distinct molecular mechanisms. For many forms of pulmonary hypertension in the newborn, the sequence of events that lead to disease start with activation of inflammatory processes (FIGURE 12). The ensuing cellular and tissue inflammation impacts metabolism and induces oxidative stress, which influences other intertwined signaling pathways. The dysfunctions in multiple cellular and molecular pathways then contribute to the subsequent structural and functional malformations. There can be up- or downregulation in the expression of genes due to activation or repression of transcriptional modulators, by DNA methylation, or histone acetylation, details of which are covered in the first two sections of the review. There can also be induction of select gene isoforms that ultimately lead to modifications in the function of target proteins. Protein translation can be modified via changes in protein production or degradation involving modulation by miR, protein ubiquitination, or the like. Lastly, there can be other posttranscriptional modifications mediated by alterations in the regulation of processes involving protein phosphorylation, hydroxylation, and S-acylation.
FIGURE 12.
Tissue inflammation and reactive species are important to long-term hypoxia induced pulmonary hypertension. Hypoxic stress leads to the production of reactive species including O2−, OH, H2O2, as well as ONOO−. These species are generated in many cell types associated with the pulmonary vasculature ranging from endothelial and smooth muscle cells to macrophages and other immune cells important to tissue inflammation. The reactive species then influence mRNA transcription, protein translation, and posttranslational processes. The combination of tissue inflammation and reactive species production leads to pulmonary hypertension though structural alterations that result in vascular remodeling, as well as regulatory and functional changes that lead to enhanced vasoconstriction, and depressed in vasodilatory capacity.
B. Inflammatory Processes Associated With Tissue Hypoxia in the Fetal Lung
Chronic hypoxia induces inflammation in the adult lung that elicits multiple changes in lung structure and function (FIGURE 12), recently reviewed in Refs. 402, 815. However, its effects on the immature lung remain less clear. Given that the focus of this review is on prenatal programming and the impact of hypoxia on the fetal and neonatal lung, the process of inflammation in the lung will be described in brief and then compared with what is known to occur in the fetus.
Alveolar macrophages that are intrinsic to the adult lung are activated by hypoxic stress (151, 1012). The resident alveolar macrophages release a different set of cytokines in response to tissue hypoxia as compared with the peripheral macrophages that infiltrate the lung during stress (151). In particular, alveolar macrophages release proinflammatory mediators as the supernatant from hypoxia-treated alveolar macrophages induces pulmonary arterial myocyte proliferation (151). Cytokines released by the activated macrophages, including IL-6, act as chemical attractants. This prompts monocyte recruitment into the perivascular space (899) and induces mast cell degranulation (151), all of which compounds tissue inflammation. These inflammatory signals modulate endothelial, smooth muscle, fibroblast, and stem cell populations of the vasculature, which causes changes in lung morphology and function.
The impact of hypoxia on inflammatory processes in the immature lung is far less well studied relative to the adult. There is a progressive development of innate immunity during gestation, and this process is influenced by stress (86, 612, 812). In general, the fetus has an underdeveloped immunological system, which likely dampens immune responsiveness. Alveolar macrophages are not fully developed in fetal mice until after birth (343), even still, resident macrophages in the fetal lung will respond to stimuli (86). Infants with chronic lung disease have high levels of chemoattractants including IL-8 that is produced by macrophages, endothelial cells, epithelial cells, as well as airway smooth muscle (507). IL-8 is important as it attracts neutrophils and promotes lung inflammation. However, the anti-inflammatory complement protein CD36 is also important during hypoxia. CD36 serves a protective role in the lung because it scavenges fatty acids at the membrane. This complement protein is induced by 21 days of 10% O2 in pulmonary arteries of mice (520) as well as human pulmonary arterial smooth muscle cells cultured in 2% O2 for 24 h (702). A recent study also showed that lavage samples from preterm infants with respiratory distress syndrome had a greater proportion of CD36+ immature macrophages as opposed to infants with other forms of chronic lung disease (812). Beyond the role of CD36 in macrophages and myocytes, the complement protein is also important to peroxide-mediated Ca2+ responses in pulmonary microvascular endothelial cells through activation of TRPV4 channels (942). These findings are far from definitive but leave open the possibility that macrophages in the fetal lung become more susceptible to activation after long-term hypoxia. The picture that also is emerging is that the level of hypoxic stress is important as to whether the macrophages become either pro- or anti-inflammatory.
C. ROS and Association With Hypoxia-Induced Disease
Hypoxemia transforms pulmonary vascular function through the direct or indirect modification of the activity of various cellular proteins (894, 1041, 1045). Many of these proteins are involved in transducing signals and thereby influencing function through reactive oxygen species (FIGURE 12). Some of the more relevant proteins include eNOS that produces NO, NADPH oxidases (NOX) that are important for energy metabolism, as well as transcriptional regulators including HIF, nuclear respiratory factor (NRF), and cAMP response element binding protein (CREB). Among the transcriptional factors, HIFs are considered the master regulators of transcriptional control in response to hypoxia (915, 1045). Even though many proteins, such as eNOS, are not directly coupled to transcriptional regulation, the associated signaling pathways cause eNOS to have important roles in processes that acutely modulate arterial reactivity and also have long-lived influences on transcriptional responses.
The oxygen levels in the fetus are important because a comprehensive analysis of the effects of oxygen tension on various enzyme activity has only been examined in depth in the adult. At an altitude of 355 m, the oxygen tension in our fetal sheep is roughly 23 mmHg as compared with the mother, whose Po2 is between 90 and 100 mmHg (reviewed in Ref. 762). Placing pregnant sheep at 3,801 m reduces the fetal sheep Po2 by ~20% to 19 mmHg, which presents a substantial fetal stress. Lowering Po2 to half partial pressure such as is routinely done in rodent studies would reduce fetal Po2 further still. Interestingly, breathing 21% O2 at sea level with a PiO2 of ~147 mmHg O2 is considered a normoxic atmosphere. However, during the transition at birth, raising the intrauterine arterial oxygen tension from 23 mmHg up toward that of an adult (100 mmHg) presents a substantial hyperoxic challenge to the newborn. The rapid increase in oxygen tension when the newborn begins to breathe leads to a respiratory burst and increases mitochondrial ROS generation in cells of the fetal lung, including pulmonary arterial endothelial and smooth muscle cells from fetal sheep (265).
The impact of oxidative stress on lung function due to low oxygenation is dependent not only on the amount and types of ROS that are generated but also on the cellular compartment where the ROS is produced. In this regard, there is cellular compartmentalization of the influences of oxidative stress, both in terms of where the ROS is generated as well as where it is scavenged (reviewed in Ref. 1040). In the fetal lung, there is, unfortunately, scant information regarding the impact of long-term hypoxia on oxidative stress. However, mitochondrial-derived ROS are associated with the development of pulmonary hypertension in newborns (4, 6, 9, 17, 77, 179). The fetal lung, therefore, needs to be well prepared for the respiratory burst at birth and corresponding ROS generation. The fetus accomplishes this by having reasonably well-developed ROS scavenging systems. When comparing human fetal to adult lung tissues, the expression of MnSOD (mitochondrial), CuZnSOD (cytosolic), and catalase all increased, while glutathione peroxidase remained unchanged, although enzyme activity does not necessarily follow suit as only catalase activity levels increased (42).
The respiratory burst associated with the newborn transitioning to breathing air at birth is important (208, 381). During these first moments outside the womb, the newborn infant goes from having lungs filled with amniotic fluid to breathing air, where the lung is suddenly required to exchange gasses properly (381). A series of studies published over a number of years illustrate how 3–10 days of continuous hypoxia in newborn piglets induces oxidative stress (41, 219, 272, 273, 275). These studies have provided evidence that key enzymes involved with the respiratory burst become dysfunctional and that exogenous treatments can counterbalance this stress.
Superoxide and peroxide are the two primary ROS of interest during hypoxia, and they modify the function of many proteins and lipids. Secondarily, superoxide rapidly reacts with available NO to form peroxynitrite, which causes protein nitrosylation that also influences cellular function (6, 218, 721, 968). Major components important to ROS generation and scavenging are individually discussed below with specific reference to changes in the pulmonary vasculature.
The mitochondria are a critical source of ROS primarily being generated through complexes of the electron transport chain. Complex I is the first component of the mitochondrial electron transport chain and is a major producer of superoxide. Superoxide from complex I is generated within the mitochondrial matrix, where it is converted to peroxide by manganese superoxide dismutase that is expressed in the mitochondria (MnSOD). Recent pharmacological studies in pulmonary arterial myocytes isolated from lung resections of human patients provide important insights into its function (1118). The studies show that complex I as well as II and the Q(0) site of complex III are important to hypoxic pulmonary vasoconstriction. Unfortunately, there is no direct information regarding the potential for changes in complex I during lung development, nor changes due to long-term hypoxia.
Complex III also generates significant superoxide. Complex III, like complex I, is a component of the electron transport chain and is located at the inner mitochondrial membrane (88, 377). Complex III generates superoxide as a byproduct of its electron transport function into the leaflet between the inner and outer mitochondrial membranes where it is converted into peroxide (reviewed in Refs. 88, 893, 894, 1041). Unfortunately, there is very little information regarding the role of complex III to superoxide production in the fetal lung. What is known, however, is that as the oxygen tension falls there is a decrease in oxidative phosphorylation and function of complex III (377). Notably, oxidative phosphorylation is suppressed by the low oxygen tensions in the fetal lung (143, 144). However, low oxygen tensions can increase superoxide production at complex III (345), which leads to the possibility that long-term hypoxia may augment ROS production at complex III in the fetus.
NADPH oxidases are multisubunit enzymes that have catalytic and regulatory subunits distinct from the cytochrome complexes, which use oxygen to generate superoxide (892). These oxidases are important for vascular homeostasis and disease and are expressed on the plasma membrane as well as endosomes (9, 336, 529, 892, 1003). There are multiple NADPH oxidases, NOX1–5 and NOX/Dual oxidase (DUOX 1 and 2), which are comprised of phagocytotic oxidase subunits along with the small Rho GTPase, Rac. A number of oxidases are important to the pulmonary vasculature and responsive to tissue hypoxia. The functions of several key oxidases are detailed. NOX 1 is often associated with vascular smooth muscle and endothelial cells and is functionally important for angiogenesis, cell growth, and motility (recently reviewed in Ref. 892). In newborn piglets exposed to 10–12% O2 NOX1 protein expression was increased at 3 and 10 days (219), illustrative of enhanced ROS generation. However, the findings in adult mice are distinct as 10% O2 for 21 days failed to alter NOX1 expression, even though the mice had vascular remodeling and pulmonary hypertension (671). Interestingly, neonatal mice exposed to hyperoxia (75%) for several days had increased NOX1 expression that was dependent on mitochondrial ROS production (204). These authors have also begun to link early prenatal hyperoxia and increased NOX1 to suppression in lung septation and alveolarization as mitochondrial targeted tempol, which catalyzes superoxide, prevented disease progression (204). In comparison, 9- to 18-wk-old NOX1 knockout mice spontaneously develop right ventricular hypertrophy and pulmonary artery thickening, indicative of pulmonary hypertension (417). The increase in pulmonary arterial thickening is coupled to a reduction in cellular apoptosis without changes in HIFs or ET-1. Moreover, when NOX1 null mice were crossed with those overexpressing NOX1, it alleviated the phenotypic changes. Altogether, the data illustrate the complexity with regards to the role of NOX1 in the pulmonary vasculature. The picture emerging from these studies is that either reduced or accentuated NOX1 expression have the potential to be involved with the development of pulmonary hypertension.
NOX2 also contributes significantly to the generation of ROS and is found in the pulmonary vascular endothelium, as well as in alveolar macrophages. In the pulmonary vascular endothelium NOX2 generates superoxide that induces proinflammatory cytokines important for cell migration, angiogenesis, and remodeling, and this isoform is important to barrier function (reviewed in Ref. 336). Hypoxia-induced superoxide generation via activation of NOX2 is associated with pulmonary arterial hypertension, right ventricular hypertrophy, and pulmonary vascular remodeling in adult mice (572). Recent work illustrates that the effects of chronic hypoxia, as well as chronic intermittent hypoxia, are reduced in NOX2 (gp91phox) knockout mice (738) and that NOX2 is important to endothelial disruption in pulmonary arteries of chronic hypoxic mice (281). Even though the role of NOX2 in oxidative stress is well studied and it is involved with pulmonary hypertension, there are few details regarding NOX2 in the fetal pulmonary vasculature. Ductal ligation in fetal sheep increased NOX2 mRNA expression as well as mRNA for the regulatory subunits RAC1 and p47phox and protein expression for RAC1 and P67phox (967).
NOX3 is another important NOX enzyme, and its expression is highest in pneumocytes, endothelial cells, and macrophages in the lungs of adult humans (989) and is also expressed in mouse lung (1160). Like most other NOX enzymes it generates superoxide and is a likely sensor of the oxygen tension. Recent work has illustrated that NOX3 may be important for acute lung injury and is suppressed by TLR4 activation in mouse lung endothelial cells (1160). However, there is no information about NOX3 in the fetal lung, and it has not been as widely studied as other NOX isoforms.
NOX4 is mainly expressed in pneumocytes and endothelial cells of the human lung (998). Ductal ligation in fetal sheep increased NOX4 mRNA in parallel with NOX2 and the regulatory domains detailed previously (967). Additional information regarding NOX4 in the lung is derived from studies in adult mice and cultured cells. There is strong evidence from the Hart group relating to the contribution of NOX4 to the proliferation of human pulmonary endothelial and smooth muscle cells (334, 597). This is based on studies where cultured cells were exposed to 1% O2 for 72 h or mice were exposed to chronic hypoxia of 10% O2 for 3 wk. In cultured cells, NOX4 inhibition with GKT137831 blocked hypoxia-induced reductions in peroxisome proliferator-activated receptor γ (PPARγ) as well as prevented TGF-β increases and cell proliferation (334). In vivo experiments show NOX4 inhibition stunted hypoxia-induced right ventricular hypertrophy and prevented thickening of the pulmonary arterial wall. However, NOX4 inhibition did not correct the increase in right ventricular systolic pressures or prevent muscularization of small arterioles. Notably, NOX4 is coupled to the reduction in PPARγ via regulation of ERK and NFκB (597). PPARγ may serve an important role since reducing PPARγ in human PASMCs enhanced NOX4 in an NFκB-dependent manner, and this effect was associated with increased cell proliferation (84). The work is controversial, however, as recent studies from another research group using total and inducible NOX4 knockout mice differ substantially. The studies with the knockout animals failed to prevent the dysregulation in right ventricular function and structure as well as pulmonary vascular structure and function described by the Hart group in their pharmacological studies (1011). Given the complexity of NOX signaling, addressing the differences between the findings of these groups will require more refined in vitro as well as in vivo studies. Overall, additional work is needed to resolve the impact of variation in NOX expression levels along with species- and age-dependent effects regarding the influence of hypoxia and the role of the various NOX isoforms in the development of pulmonary hypertension in the newborn.
eNOS produces the potent vasodilator NO through reduction of l-arginine to l-citrulline, and thus it is not generally thought of being important for ROS production. However, when the oxygen tension falls, the NOS becomes uncoupled. This skews the synthase to produce superoxide instead of NO. However, eNOS function is governed not only by the substrates but also by a multiplicity of cofactors, many of which are influenced by chronic hypoxia. Calcium signals activate eNOS to produce NO. This is mediated through calmodulin-dependent phosphorylation of Ser 1177. Recent studies from our group show that eNOS-related bradykinin-dependent relaxation is upregulated by long-term maternal hypoxia in fetal lambs, but maturation of the relaxation response is blunted in long-term hypoxic 2-wk-old newborn sheep (90). Yet, our group previously showed eNOS expression is unaltered by maternal long-term hypoxia in fetal pulmonary arteries (1105). Overall, the data suggest that changes in eNOS regulation enhance bradykinin-dependent relaxation in the fetus. Even still, we do not yet know the eNOS expression levels in newborn, the phosphorylation status of eNOS, or interaction with regulatory components. Maternal long-term hypoxia, however, enhanced pulmonary arterial relaxation in the sheep fetus without any substantial changes in the calcium signal (90). In comparison, maternal long-term hypoxia augmented bradykinin-induced calcium signals in the newborn lamb even though eNOS-dependent relaxation was decreased. Together these findings suggest maternal long-term hypoxia disrupts components important to eNOS activation, changing the gain between the calcium signal and the generation of NO and suggesting that there are modifications in the cofactors that regulate eNOS activity. This is significant as these effects may be related to eNOS uncoupling and an increase in ROS production (498).
The influence of hypoxia on the regulators of eNOS in the fetus or neonate and the relationships to oxidative stress are not as well understood as in the adult, though they are critical to their function. Tetrahydrobiopterin (BH4) is sensitive to oxidation and reduction and is one of the most well-studied eNOS cofactors (240). Interestingly, bovine eNOS Ser 1179 phosphorylation, akin to human Ser 1177, also enhances superoxide production and retards BH4 inhibition of superoxide production (240). In parallel with this finding, BH4 treatment recoupled eNOS and improved vasodilation in chronic hypoxic newborn piglets (222). Arginase also is important to eNOS uncoupling, where increased arginase expression enhances l-arginine degradation. l-Arginine breakdown reduces substrate availability and prevents NO generation, which ultimately impedes vasorelaxation. A recent study shows that long-term hypoxia increased arginase expression and activity in cultured human PASMCs (1102). However, the impact of maternal long-term hypoxia on arginase activity is unknown. Such enhanced arginase activity and decreased l-arginine availability would compromise eNOS function and vasodilation but would not necessarily increase superoxide production. In addition to l-arginine breakdown by arginase, the concentration of asymmetric dimethylarginine (ADMA) is also important as it interferes with normal l-arginine binding to eNOS and NO production. ADMA is a metabolic byproduct of protein methylation, and its levels are dependent on the activity of dimethylarginine dimethylaminohydrolase 1 (DDAH1), a key enzyme that degrades it. Interfaced with this, evidence indicates that ADMA levels may be linked to oxidative stress through DDAH1 (1161). Overall, there are quite a few different regulatory components to eNOS, any of which could be modified by long-term hypoxia and could impact production of NO and superoxide.
Xanthine oxidases (XO), like the NADPH oxidases and eNOS, are also sources of ROS. These oxidases break down purines into uric acid and as a byproduct generate superoxide. XO increases during development and after birth in newborn lambs, but in lambs with high pulmonary blood flow, these increases are blunted (911). There is evidence, however, that chronic hypoxia increased XO expression in neonatal rats at 4 days, but this expression subsided to control levels by 7 days (426). Oxidative stress due to chronic hypoxia was also corrected with the antioxidants tempol or U74389G or the XO inhibitor allopurinol (426). These studies are certainly promising and suggest XO is important to chronic hypoxia-induced pulmonary hypertension.
D. Oxidative Stress Scavengers
While in the womb the fetus is in a low oxygen environment, the level of oxidative stress is suppressed. Breathing room air, therefore, presents a relative hyperoxic challenge. When the baby begins to breathe, the tissue oxygenation causes a respiratory burst, which elicits an oxidative stress response. As such, during gestation the fetal lung is preparing for birth in an oxygen-enriched environment. The antioxidant pathways become primed late in fetal life to handle the increased load of reactive oxygen species that occur with birth. Expression levels of endogenous antioxidants increase dramatically during the third trimester and extending post-term (76, 77, 910). The lack of fetal antioxidant systems in earlier stages of development is exemplified in prenatal mouse lung slices that are poorly tolerant to hyperoxic conditions as compared with adults (77). Studies from the Fike group further illustrate issues associated with poorly developed pulmonary vascular antioxidant systems before birth (41, 219, 272–275) while work from other laboratories provides additional evidence regarding the importance of antioxidants (264, 426, 487, 498, 522, 989). Key findings are outlined below:
Superoxide dismutases (SODs) are primary antioxidants of ROS generated by NOX, XO, and through other mechanisms. There are three different SOD isoforms including SOD1, SOD2, and SOD3. All isoforms of the enzyme break down two molecules of superoxide into hydrogen peroxide, also a reactive molecule, and oxygen. The three isoforms are regulated somewhat differently and expressed in different locations within the cell which influences their function. SOD1 (CuZnSOD) is expressed in the cytosol where it scavenges superoxide. Previous studies show that piglets exposed to half partial pressure O2 have a decrease in SOD1 expression as soon as 3 days, which persists after 10 days of chronic hypoxia (219). Chronic hypoxia in adult rats also decreased lung SOD1 expression, which was linked to redox-sensitive activation of ASIC1 and store-operated calcium entry (802). However, in shunted lambs with high pulmonary blood flow, SOD1 expression and activity were no different relative to control (910). These studies suggest that in utero or postnatal chronic hypoxia may suppress SOD1 expression but that there is variation depending on the type of stress or species examined. The data also lead to the prediction that the redox-dependent changes in cell function are coupled to complex signaling cascades and that there may be distinct changes depending on how pulmonary hypertension is induced.
SOD2 (MnSOD) is distinct from SOD1 as it is expressed in the mitochondria and scavenges superoxide generated in the inner mitochondrial matrix by complex I. In the newborn piglet, SOD2 expression was unchanged after 3 or 10 days of chronic hypoxia (219). In comparison, in shunted lambs with high pulmonary blood flow, developmental increases in SOD2 expression and activity are blunted at 2 wk but not at 4 or 8 wk (910). Similarly, SOD2 expression and activity are blunted by ductal ligation while adenoviral expression of SOD2 enhances vascular dilation to ATP as well as catalase and eNOS expression (6). There is also decreased SOD2 expression in adult rats (716) and mice (20) following 21 days of 10% O2. Overall, these studies are suggestive that impaired SOD2 contributes to multiple components of pulmonary hypertension but may not be involved with hypoxia-related tissue remodeling.
SOD3 like the two other isoforms also scavenges superoxide, although it is expressed on the extracellular surface and helps reduce plasma membrane oxidation. There is high SOD3 expression in the vasculature (622), which has led investigators to examine its importance in pulmonary vascular hypertension. SOD3 expression is stable through the first 2 mo of life, and lambs with a high flow shunt did not have any change in enzyme expression (910). In conditional and total SOD3 knockout mice, reduced SOD3 expression exacerbated hypoxia-induced right ventricular hypertrophy as determined by a worsening in the Fulton index and elevated right ventricular pressures (742, 1093). Furthermore, studies show SOD3 serves a protective role in NO signaling, where knockdown of SOD3 decreased eNOS function (742). A loss-of-function mutation in SOD3 in rats worsened pulmonary arterial hypertension due to monocrotaline (1093). SOD3 was also decreased following 21 days of chronic hypoxia in mice and was associated with the development of pulmonary hypertension (782). SOD3 expression decreased in a ductal ligation lamb model of pulmonary hypertension (1044) while exogenous treatment with recombinant SOD to scavenge superoxide improved oxygenation in these animals. Treatment also reduced vessel contractility in response to membrane depolarization with high extracellular potassium and adrenergic receptor stimulation with norepinephrine (522). In separate studies using the ductal ligation model, treatment with the scavenger Tiron improved ATP-dependent pulmonary arterial vasorelaxation (498). Recombinant SOD also indirectly improved arterial function in lambs with ductal ligation through improvements in NO signaling, including increased eNOS expression and BH4 levels (264). The available evidence suggests that extracellular SOD is disrupted in some forms of pulmonary hypertension of the newborn, although whether or not SOD3 is decreased following chronic hypoxia in the newborn has yet to be determined. Available evidence, however, raises the potential that there are important and interactive influences between the various sources and sinks associated with ROS signaling. Moreover, the interconversion of superoxide to peroxide outside the cell is important not just for acute signaling but also to the regulation of other pathways that are vital to lung function.
Catalase is a well-studied enzyme that degrades hydrogen peroxide in the cytosol to water and oxygen and thus works cooperatively with the various SOD isoforms to regulate cellular ROS. However, only a few studies have examined its role in pulmonary hypertension due to chronic hypoxia in the newborn. Catalase expression increases within the first 2 mo after birth in lambs (910). Ductal ligation may accelerate this developmental process as these animals had increased expression in pulmonary arterial endothelial cells (6). This differs from the lack of change in expression at 3 or 10 days in pulmonary arteries isolated from chronic hypoxic newborn piglets (219) or the depression in catalase activity but not expression observed in lambs with high pulmonary blood flow (517, 910). The variability in the changes in catalase between models is illustrative that the etiology of disease is a factor when evaluating the underlying mechanisms.
Glutathione peroxidase and peroxiredoxins are enzymes expressed in the cytosol, and similar to catalase reduce peroxide. However, they differ from catalase in that they act to reduce peroxide along with two molecules of glutathione to glutathione disulfide. The ratio of glutathione to glutathione disulfide is therefore often used as a biomarker of cellular health. The process of glutathione conversion is dynamic, and it acts to preferentially scavenge peroxide. The importance of glutathione in oxidative stress is well understood, and two laboratories have shown that 10% O2 exposure in rats increased glutathione peroxidase expression and activity by 21 days (716, 802). However, another group showed a decrease in expression at 6 h (880), suggesting expression is labile and dependent on the length of exposure. Even still the studies did not show any change in the expression or activity of glutathione peroxidase in lambs with ductal ligation (1047). Overall, while there is good evidence regarding the importance of the peroxidases during pulmonary hypertension, there has yet to be any conclusive data linking peroxidases to hypoxia-induced pulmonary hypertension of the newborn.
E. Transcriptional Regulation
Mechanistic changes and functional abnormalities in pulmonary vascular function associated with the development of disease are often due to changes in gene expression as described in FIGURE 12. These were discussed extensively in the first two sections of the review and thus are only presented here with particular relevance to the pulmonary vasculature. A number of research groups have performed gene arrays to examine the changes in mRNA associated with chronic hypoxia in the lung. Many of the genes are linked to cellular inflammation and are activated by a few key transcriptional regulators directly or indirectly regulated by hypoxia.
Hypoxia-inducible factors including HIF-1α are important to morphological changes in the lung and underlie many aspects related to the development of pulmonary hypertension by low oxygen (271, 483, 592, 915, 1045). When considering the perinatal condition, long-term maternal hypoxia stimulates erythropoiesis in sheep, which is indicative of HIF-1α activation (592). Other changes due to long-term maternal hypoxia are also linked to HIF-1α, including medial wall thickening and small vessel muscularization (915). Interestingly, chronic hypoxia (5% O2) in cultured pulmonary arterial myocytes from near-term fetal sheep did not increase HIF-1α protein levels, although it did increase mRNA synthesis (834). This is partially explained because 5% O2 increased the expression of CREB-binding protein/p300 interacting transactivator with ED-rich tail (CITED2) (834), which limits HIF-1 activation by blocking the transactivation domain.
HIF-2α complements HIF-1α and is encoded by the endothelial PAS domain protein 1 (EPAS1). The consequences of HIF-2α activation also feed into pathways that increase red blood cell production. Notably, EPAS1 is implicated in the adaptations due to high altitude in Tibetans (387, 1112) who express a number of gene variants depending on their altitude of residence (62).
Nuclear factor erythroid 2-related factor 2 (NRF2) is another transcriptional regulator important to hypoxia related pulmonary vascular disease. NRF2 is central because it regulates the expression of antioxidant enzymes and is activated in response to oxidative stress (reviewed in Ref. 176). Enhanced function of various antioxidant pathways counterbalances oxidative stress issues associated with low oxygen tensions. In a murine hyperoxia model of preterm birth, NRF2-related gene products were increased (806). However, the importance of NRF2 to long-term hypoxia in the fetal lung is less well understood, and whether or not maternal hypoxia influences the expression of NRF2 in the lung is presently unknown.
CREB is a well-described transcription factor; however, there is very little information regarding its role during pulmonary vascular development or during hypoxic stimulation of the developing lung. Previous work shows that CREB is activated during smooth muscle differentiation, and its expression decreases during cellular proliferation (493). Chronic hypoxia in adult rats for 21 days (~11% O2) depressed CREB expression, and this was associated with tissue remodeling (492); processes that were reversed by treating with the SOD mimetic tempol. Hypoxia-related remodeling is also tied to loss of CREB via Akt signaling pathways (289). The duration of chronic hypoxia is important as 10% O2 exposure increased CREB activity within hours in C57Bl6 mice, which subsided to control levels over a period of days (547). Furthermore, CREB activity increased with exposure to 5% O2 in cultured human lung microvascular endothelial cells, resulting in increased expression of ATF2, EDN-1, and VEGFR-1 (547).
NFκB is a family of transcription factors that are often associated with immune or inflammatory activation and thus are coordinated with the activation of those that are hypoxia dependent. There is little known regarding their role in hypoxia induction of pulmonary hypertension in the newborn. However, NFκB is involved with VEGF activation of cell growth, which ties them to HIF-1-dependent signaling (reviewed in Ref. 823). Secondarily, hypoxia induced the inflammatory cytokine IL-8 in human endothelial cells, in what may be an NFκB-dependent manner (465). Such a finding has been linked to pulmonary edema (880) as well as inflammatory-mediated processes involved in the development of pulmonary hypertension (282). However, NFκB has not yet been directly linked to the development of hypoxia-related pulmonary hypertension in the newborn.
Nuclear factor of activated T cells (NFAT) is not directly activated by hypoxia but has come under scrutiny for its importance in the development of pulmonary hypertension due to chronic hypoxia (82). Recent evidence illustrates that NFATc3 is essential to vascular remodeling due to chronic hypoxia in neonatal as well as adult mice (82). As the name implies, NFAT is critical for regulation of cell growth and proliferation, and thus is significant to medial wall thickening and changes in the expression of genes important to vessel reactivity.
Activator protein-1 (AP-1) is a transcriptional regulator that is a dimer of c-FOS and c-JUN and is activated by numerous stimuli including hypoxia. AP-1, therefore, serves diverse roles in cell growth and proliferation, apoptosis, and cellular differentiation. There has not been much specific work with regards to tissue hypoxia and activation of AP-1 in the fetal and newborn lung. However, there is substantial evidence that AP-1 is involved in hypoxia-related cell growth and differentiation as summarized in Reference 200. For example, AP-1 works with other transcription factors to choreograph TGF-β1-mediated myofibroblast differentiation into mature smooth muscle (395). There is also evidence for a role of AP-1 in the lung, where acute hypoxia (9% O2 for 6 h) increased c-FOS and c-JUN expression in rat lung (303). Similarly, AP-1 is induced by lowering the oxygen to 1% O2 for 48 h in cultured human lung fibroblasts (171). Hypoxia also activates AP-1 in cultured human pulmonary arterial endothelial cells (262).
The mitogen-activated protein kinase (MAPK) pathway, like that of AP-1, can be activated by various mitogens and hypoxia. The MAPK pathway is implicated in VEGF signaling along with HIF-1α and thus is important to vascular remodeling associated with chronic hypoxia in the lung (171, 395). However, very little is known regarding the importance of MAPK to hypoxia-induced changes in the fetal lung.
DNA methylation is a key epigenetic mechanism essential to the development of pulmonary hypertension, reviewed in Reference 483 and discussed at more length in section III of this review. Chronic maternal hypoxia causes global reductions in DNA methylation of fetal sheep pulmonary arteries (1114), which is presumed to relate to methylation of specific genes. Hypoxic neonatal mice also have differential DNA methylation around the promoter region of IGF-I, which is important to AKT activation and development of pulmonary hypertension (1115).
F. Translational Modifications
miR is a key mechanism of regulating protein translation (FIGURE 12) that is an emerging area in the field of pulmonary hypertension and was discussed more fully in section III. These are short noncoding RNA strands of ~21–25 nucleotides that complement mRNA strands. When the miR binds to the messenger strand, it inhibits RNA translation. This section will cover what is specifically known with regards to prenatal hypoxia and modulation of protein translation by miR in the lung.
The expression of many miR in the lung changes with tissue hypoxia, and miR-210a is generally regarded as a master hypoxamir (142, 717). The adaptations in miR expression and function with regards to hypoxia are a developing area of research, and there is still much to understand about miR in the neonatal vasculature. There are only a few studies that have examined changes in miR in hypoxic fetal or neonatal lungs (324, 353, 405, 1101, 1115). The most comprehensive gestational hypoxia study to date that has examined miR in the lung is that of Huo et al. in 2014 (405). They exposed pregnant Sprague-Dawley rats at E19 to 10.5% O2 for the remainder of gestation, which equates to the cannicular and saccular stages of human lung development, periods that are marked by rapid tissue growth (762). Gene arrays were performed on lung isolates to examine changes in expression of various miRs, and a number of miRs increased while others decreased.
The largest changes in miR that were found in the Huo study during fetal hypoxia were not in the expression of what are considered normal hypoxia-related miR. Instead, there was upregulation of miR-465 (~25-fold), and miR-377 (~20-fold), while miR-210 that is classically upregulated by hypoxia was only modestly increased by hypoxia (405). miR-327 was downregulated nearly 10-fold, while miR-338 was depressed ~5 fold. Interestingly, miR-338 is also downregulated in chronic obstructive pulmonary disease patients, suggesting that there may be some overlap in the changes in miR between various forms of pulmonary hypertension (1030). We were unable to find any information regarding the function of miR-327. miR-338 appears to target HIF-1α (909), suggesting this miR may be important to morphological changes associated with enhanced HIF-1α activity.
Unraveling the myriad functional impacts of the changes in miR expression will require additional work. Gestational hypoxia is expected to induce large-scale changes in networks of genes that influence various cellular processes. miR are likely central to the morphological and functional consequences that occur.
G. Posttranslational Modifications
Posttranslational modifications are necessary for the functional regulation of expressed proteins as delineated in FIGURE 12. This encompasses phosphorylation, hydroxylation, acetylation, S-acylation, oxidation, as well as ubiquitination. Such modifications represent the integrative influences of cellular signaling and responses to hypoxic stress. This includes altered activation of enzymatic and ionic signaling due to changes in the expression or function of key proteins.
Protein phosphorylation is a widely studied method of posttranslational modification with changes in individual proteins being observed in numerous forms of pulmonary hypertension. However, only a handful of studies have examined changes in phosphorylation in the pulmonary vasculature following gestational long-term hypoxia. Most of this work was performed by Dr. Raj. Her studies have focused on changes in phosphorylation due to Rho kinase and protein kinase G as well as platelet activating factor (406, 1166). The studies on Rho kinase and protein kinase G show that in pulmonary arteries of hypoxic fetal lambs type II ROCK expression is increased as is the phosphorylation of Thr696 and Thr850 of MYPT1, a regulatory subunit of myosin light-chain phosphatase (MLCP) (288). Phosphorylation at these sites suppresses MLCP activity and impairs vessel relaxation. In addition to this, cGMP failed to prevent phosphorylation of MYPT1 by ROCK and is suggestive of impaired PKG function. Similar work from Xu et al. (1094) shows that rat pups exposed to 13% O2 for 21 days since birth had increased MLC-P/MLC ratio (phosphorylated to total myosin light chain) and MYPT1-P/MYPT-1 ratio (ration of phosphorylated to total MYPT1) and this was reversed by Rho-kinase inhibition. However, pulmonary veins from chronic hypoxic fetal lambs had responses that were distinct from the arteries (288). The activity of PKG was increased while the phosphorylation of MYPT1 at SER695 by 8-BrcGMP was decreased. Similarly, there was less ET-1-mediated phosphorylation of the ROCKII site, Thr696, on MYPT1. Secondarily, previous work illustrates that high-altitude gestation enhanced PAF-r phosphorylation in fetal sheep pulmonary veins (406). This likely contributes to enhanced IP3 generated Ca2+ signals and venous reactivity. Unfortunately, we do not know much more than this regarding changes in the phosphorylation status of various proteins following gestational hypoxia or potential linkages to ROS. However, based on the work of various groups showing changes in expression and interaction of various proteins, there is certainly much to uncover (41, 90, 273, 275, 288, 646, 721, 1105).
Protein hydroxylation is a major mechanism of protein regulation in response to hypoxia (901) that operates in tandem with other methods that induce posttranslational modification. Hydroxyl ions are highly reactive, and the process of hydroxylation is governed by multisubunit enzymes. This includes prolyl-hydroxylases, PHD1, PHD2, and PHD3 that are important for regulating HIF. Importantly, the PHD2 gene is encoded for by EGLn1, which is part of the genetic high-altitude adaptation among Tibetans (592). Hypoxia generally leads to inhibition of prolyl hydroxylases, which in turn stabilizes HIF-1α and prevents its degradation. However, PHD2 expression levels are enhanced by chronic hypoxia in cultured fetal PASMC (834), which would counteract the actions of HIF-1α. Beyond the prolyl-hydroxylases, ductal ligation increased the expression of tryptophan hydroxylase 1 in the lung, which likely contributes to the enhanced role for serotonin in the development of persistent pulmonary hypertension in lambs (216). These sheep and human studies suggest that epigenetic programming in the fetus due to maternal high-altitude exposures may follow a similar pattern as multigenerational adaptations among resident populations.
Acetylation is similar to DNA methylation in that it is one of several processes important to epigenetic modification. There is also significant evidence that acetylation processes are important to the development of pulmonary hypertension (recently reviewed in Ref. 483). Reduction in histone acetylation is important to pulmonary arterial smooth muscle cell migration and proliferation in fetal lambs exposed to gestational hypoxia (1114). In idiopathic pulmonary hypertension in humans and in rats with chronic hypoxia-induced pulmonary hypertension, the decrease in histone acetylation is dependent on an increase in the activity of histone deacetylase (1162). In addition to this, deacetylase inhibition decreases fetal sheep pulmonary arterial smooth muscle cell migration and proliferation (1114). In neonatal mice exposed to chronic hypoxia, deacetylases are tied to IGF induction, which in turn is linked to ET-1 (1115). Interestingly, in a rat monocrotaline pulmonary hypertension model, multiple HDAC isoforms are upregulated in pulmonary arteries (159). Inhibition of deacetylases in these monocrotaline-treated rats decreased the right ventricular thickness, improved the Fulton index, and reduced pulmonary arterial stiffness along with the expression of Nox2 and Nox4. The researchers also found that inhibition of acetylation suppressed fibrotic markers including fibronectin, FN1, and periostin and reduced CD45, an inflammatory marker.
S-acylation is a well-known process that regulates protein function through a variety of different target residues (139). However, such regulation is an emerging field in the realm of hypoxia-induced pulmonary hypertension. The one recent study that examined S-acylation following hypoxia-related pulmonary hypertension focused on the effects on thromboxane signaling (917). They determined that S-acylation of the Gαq subunit enhanced the coupling to phospholipase C and generation of Ca2+ responses. Other reports illustrate that eNOS targeting to caveolae in endothelial cells is regulated by S-acylation (291). This opens the possibility that S-acylation may be important to the suppression of eNOS-mediated relaxation by hypoxia. More globally, inflammatory processes leading to endothelial dysfunction are dependent on DHHC-containing palmitoyl acyltransferase function (66).
Ubiquitination is another important mechanism that regulates protein function. Ubiquitination is unlike other methods that directly regulate protein function because the process places a recognition marker on proteins that target them for trafficking and degradation. With regards to hypoxia and the lung, it is well known that degradation of HIF-1α is dependent on ubiquitination following hydroxylation by PHDs (901). Even still, the role of ubiquitination in the fetal lung during hypoxia remains largely unresolved but is likely to be important to regulation of the expression of many different proteins.
H. Perspective
Studies over the past several decades have brought to light the idea that chronic hypoxic intrauterine stress impairs lung function in the newborn, effects that can persist throughout life. Recent evidence has linked detriments in pulmonary vascular function to enhanced oxidative stress. There is good supportive evidence for the involvement of increased oxidative stress and decreased antioxidants to the development of pulmonary vascular disease in the newborn. However, studies detailing the extent of changes; linkages to transcriptional, translational, and posttranslational processing; as well as the consequences of those changes in the function of relevant pathways are needed as our knowledge regarding these interactions is far from complete.
VIII. HYPOTHALAMIC-PITUITARY-ADRENAL AXIS AND PROGRAMMING OF ADIPOSE TISSUE
The hypothalamic-pituitary-adrenal (HPA) axis, through its regulation of glucocorticoid secretion, is a vital element in the stress response and is acutely susceptible to adverse prenatal conditions (649). Appropriate basal glucocorticoid concentrations are crucial to normal fetal growth and development. Regulation not only determines differentiation and maturation of critical organ systems but also governs fundamental metabolic processes regulating glucose, proteins, and lipids (138, 650, 698). However, since chronic elevations in glucocorticoids can inhibit fetal growth, prevention of long-term elevation is crucial to maintaining appropriate growth and development. Furthermore, in species like the sheep, it is imperative that the mechanisms involved in triggering parturition are somehow attenuated so that the process is not stimulated in response to chronic stress. At the same time, the fetus must balance this aspect of development with the ability to respond to a secondary, acute stressor.
Despite the vast amount of data on the effects of acute hypoxia on the fetal HPA, information on the mechanisms by which the fetus adapts to conditions of chronic or long-term hypoxia (LTH) is lacking. This section will summarize what is currently known regarding the effects of hypoxic stress on the HPA axis and how this may program alterations in HPA function in the late prepartum period as well as in extrauterine life. A further effort will be made to integrate the dramatic interplay between the HPA axis and adipose tissue that program changes in metabolism later in life.
A. Stress and the Fetal HPA Axis
The fetus has the uncanny ability to respond and potentially adapt to intrauterine stressors that permits development and completion of gestation to term. In longer gestation mammalian species (i.e., primates and ruminants), the HPA axis undergoes maturation in late gestation, thus facilitating the ability to respond to stress in utero. During the final few weeks of pregnancy, acute hypoxia results in enhanced production of cortisol. The magnitude of this response is dependent on both the duration and severity of the hypoxic insult, as well as the degree of maturation of the adrenal cortex as term approaches (103, 137, 138, 632). The adaptive responses to stress allow the fetus to overcome an adverse intrauterine environment; however, the same adaptations can become maladaptive over time. In contrast, in altricial species like the rodent, maturation does not occur until after birth (26, 463, 636, 878).
As described in detail earlier, epigenetic imprinting at critical windows of development can result in enhanced susceptibility to cardiovascular, metabolic and behavioral dysfunction later in life (54–56, 853). As previously described, the original “Barker hypothesis” evolved primarily from epidemiological studies following malnutrition or undernutrition during pregnancy. Additional studies have also shown a clear programming effect from an abundance of other intrauterine stressors (124, 316). Follow-up studies in animal models have confirmed and expanded these findings (80, 946, 1017).
1. Role of glucocorticoids
The regulation of glucocorticoid synthesis during development is essential in determining a normal trajectory of growth and development. Moreover, prenatal (and in some cases, postnatal) glucocorticoid exposure can have profound effects on development into adulthood. The cornerstone to this regulation is the HPA axis, which is rigidly regulated by a complex, multitissue negative-feedback system. Specific details of this homeostatic pathway are reviewed in detail (999). Described briefly, both corticotropin releasing hormone (CRH) and arginine vasopressin (AVP) are secreted from the paraventricular nucleus (PVN) into the hypophysial portal circulation where these peptides reach the pituitary and bind to specific receptors. This ligand binding enhances the synthesis and processing of proopiomelanocortin (POMC) to ACTH. After release into the systemic circulation, ACTH binds to receptors in the adrenal cortex, initiating the synthesis of glucocorticoids (corticosterone in rodents and cortisol in humans and sheep).
Circulating glucocorticoids exert a negative feedback of the HPA axis at the level of both the brain and pituitary with specific inhibition from neurons in the hippocampus that express glucocorticoid receptors (GR). Binding of glucocorticoids, in turn, inhibits the release of neurohormones like CRH and AVP. Inhibition also occurs in the pituitary by binding to GR at the level of the pituitary and both GR and mineralocorticoid receptors in the hypothalamus (761). Exposure to stress during development reduces hippocampal GR expression resulting in the loss of inhibitory feedback leading to a hyperactive HPA axis (191). In contrast, synthetic glucocorticoids bind principally to the GR as opposed to the MR, and the affinity of the GR is greater for the synthetic form (965). There is a significant impact of exogenous glucocorticoid administration during pregnancy, and a large literature base has been developed from both human and animal studies demonstrating dramatic programming effects (for reviews, see Refs. 131, 630). Although we may draw inferences from such studies, they will not be discussed in detail, and the focus will be on fetal/neonatal exposure to endogenous glucocorticoids as occurs in fetal stress.
2. HPA axis and glucocorticoids
The HPA axis, therefore, operates under a well-defined negative-feedback system to maintain glucocorticoid concentrations within a relatively narrow range. However, in response to a stressor, the system becomes upregulated, and glucocorticoid biosynthesis is enhanced. This imbalance in glucocorticoid exposure during critical periods of development is the key to the potential programming of the hypothalamus and potentially the entire HPA. Indeed, glucocorticoids have been referred to as the “gatekeepers” of fetal/neonatal programming (279). Enhanced exposure to glucocorticoids during fetal life is associated with potential lifelong changes in HPA axis regulation. These alterations can be manifested by altered responses to stress, metabolic changes, and behavioral disorders. Such changes have been observed in both humans and animals, and in animal models, overexposure to glucocorticoids is linked to transcriptomic and epigenomic alterations that impact HPA function (185, 186, 191, 897).
Since the perinatal period of development is characterized by heightened neuroplasticity, the intrauterine environment can play a significant role in programming the HPA axis. In the rodent, a wide variety of prenatal stress models have been utilized which have revealed dramatic programming effects. Stress during pregnancy has been associated with stress axis deregulation and altered social behavior (496), compromised neural development and spatial memory (545, 1109), as well as other metabolic disorders like insulin resistance and diet-induced obesity (737, 955). It is critical to emphasize that the overall effect of prenatal stress on programming of the HPA axis is intimately linked to the severity of the stress, the developmental window in which the stress occurred and the sex of the offspring (111). One factor that does appear to be relatively consistent in the rodent model is enhanced responses to a secondary stressor in adults exposed to perinatal stress (112, 953, 954). Furthermore, it appears the normal negative-feedback system is dysfunctional since the exaggerated responses (enhanced ACTH and corticosterone levels) are longer in duration compared with control animals (53, 112). This may occur through observed reductions in GR expression in the hippocampus of adult offspring (1172). Stress-induced increases in glucocorticoids during fetal development may also increase sensitivity of the neonatal brain to hypoxic-ischemic brain damage (556).
B. Epigenetic Regulation of the HPA Axis
Whether the cause of elevated glucocorticoid exposure in the prenatal period is the result of a fetal stressor like hypoxia or other elements of a hostile intrauterine environment, the programming effects appear to be similar, and multiple mechanisms appear to be involved. Although glucocorticoids can have a direct effect on gene transcription following binding to the GR and subsequent binding to the glucocorticoid response element (GRE) (635), key epigenetic mechanisms also appear to affect the HPA axis. As described in section IIIB, increasing evidence suggests that the long-term influences of stress on the HPA axis during development is the result of changes in DNA methylation and chromatin remodeling (436, 473, 1042). The most widely studied of these mechanisms is DNA methylation. This is a fluid process that can occur over a varied period of minutes to hours and is the result of methylation of the fifth carbon of a cytosine pyrimidine ring in a cytosine-phosphate-guanine nucleotide thus “CpG methylation” (982).
Despite that a significant portion of the studies on stress altering DNA methylation in the brain was generated using a postnatal stress model, it appears that a valid parallel can be drawn to intrauterine stress. In the postnatal stress experiments, altered DNA methylation was observed in the hippocampus of the gene encoding the GR, specifically at the exon 17 region. This encompasses a transcription factor-binding site for nerve growth inducible factor A (NGFIA) (641, 1155). In a prenatal stress model, Mueller and Bale (694) showed a similar effect at the level of the hypothalamus with enhanced methylation of the exon 17 promoter region. As described above, regulation of the GR is essential to control of negative feedback at the level of the hypothalamus.
Although there are no studies on the direct effect of hypoxia on epigenetic regulation of the HPA axis in humans, a number of studies have shown dramatic effects of other prenatal stressors. Methylation of genes encoding the GR receptor in the area of NGFIA binding site in the promoter region of NR3C1 has been observed in neonatal cord blood following gestational maternal anxiety or relationship violence (379, 818). To further assess the role of prenatal stress on programming of HPA axis genes, Kertes et al. (472) examined epigenetic alterations in multiple genes from pregnancies in which the mothers were exposed to chronic, war-related stress. Significant methylation was noted in multiple CRH and NR3C1 sites and was predictive of body weight enforcing the concept of prenatal stress exerting epigenetic influences in development.
Prenatal stressors like hypoxia, both acute and chronic, represent a potential threat to the developing fetus and, as such, are a potent stimulator of a fetal stress response. Indeed, acute hypoxia has been widely used as a physiological stressor in studies of the fetal sheep HPA stress axis. Similar to the adult, activation of the HPA axis is a normal homeostatic mechanism for fetal survival in response to a hypoxic insult. During the final few weeks of gestation, acute hypoxia results in enhanced production of cortisol. The magnitude of this response is dependent on both the duration and severity of the hypoxic insult, as well as the degree of maturation of the adrenal cortex as term approaches. Fetal hypoxia is a powerful stressor that commonly occurs during pregnancy. It can be the result of different situations such as maternal obesity, preterm labor, malnutrition, heart or lung disease, or residing at high altitude (57, 180, 232, 349, 474, 740, 873).
The programming effects of hypoxia on the HPA axis can also occur during the postnatal period. Rat pups exposed to intermittent hypoxia during the first 2 wk of life exhibited increased plasma ACTH and corticosterone levels. There was also increased adrenal mRNA expression of StAR and LdLR, genes involved in corticosterone biosynthesis. Enhancing the link between the HPA axis and metabolism (as discussed below), hyperinsulinemia and hyperglycemia were also noted (173, 174). Additional studies with the same model indicated that the observed changes were not just acute but a programmed response. As adults, these animals demonstrated enhanced ACTH secretion and a protracted corticosterone response to restraint-induced stress (174). This was coupled with decreased pituitary expression of POMC, Crhr1 and Hif1a mRNA leading to the conclusion that intermittent hypoxia in the neonatal period heightens the sensitivity of the HPA axis to stressors in adulthood. Additional studies also demonstrated that neonatal hypoxia resulted in enhanced basal corticosterone levels in adults but a decrease in the HPA response to forced swimming (660).
The rodent model has proven quite useful in the study of programming effects of stress not only on the HPA axis but also on the relationship to metabolic changes, particularly in adipose tissue as discussed below. It is imperative, however, to underscore that the rodent model has limitations when it comes to clinical translatability of findings. The rodent is quite different from the human when considering the timing of development of the HPA axis. Maturation of the hypothalamic nuclei, particularly those governing appetite, expression of orexigenic and anorexigenic neuropeptides, and ARH connections to appetitive CNS centers, develop after birth in the rodent whereas in the human, development occurs in utero. Fortunately, the ovine model of fetal development shares a commonality with the human regarding these key areas. Because of its relative size, similar endocrine responses and ability to implant catheters (946), the ovine fetus has served as a useful model to elucidate HPA responses to stressors like hypoxia as key changes related to alterations in programming.
C. Hypoxia and the HPA Axis
1. Acute hypoxia
As studied in the ovine model, hypoxia causes substantial changes in endocrine as well as cardiovascular responses. Following acute hypoxia, there are classically documented increases in plasma concentrations of epinephrine, norepinephrine (340, 443), AVP (867), as well as ACTH and cortisol (92, 93). Additionally, hypoxia increases blood pressure and decreases heart rate in the ovine fetus (313). All of these responses are assumed to aid the fetus in surviving intrauterine stress. The magnitude of these responses, however, is dependent on both the duration and severity of the hypoxic stress, as well as the stage of development. Furthermore, underlying adverse intrauterine conditions may alter the ability of the fetus to respond to a secondary hypoxic episode (293). In an experimental setting, acute fetal hypoxia can be induced by a number of different manipulations. These include maternal hypoxia (16, 182, 311) reducing uterine and placental blood flow on the maternal side (1056), or using cord compression to restrict umbilical blood flow (314, 431, 1001). Fetal hypoxemia can also be initiated for more prolonged periods by repeated umbilical cord compression (294, 295, 1001) as well as placental embolization (102, 285) and restriction of uterine blood flow (798).
Experimentally induced, fetal hypoxia during the latter third of gestation in sheep results in activation of the fetal HPA axis, elevating fetal plasma immunoreactive ACTH (IR-ACTH) and cortisol. The duration and magnitude of the fetal HPA response to hypoxia are proportional to the degree and duration of the hypoxic insult and increases with gestational age, indicative of maturation of the HPA axis during the final third of gestation. In the late gestation sheep fetus, ACTH and cortisol increase in a typical stress-response during the first hours following the initiation of hypoxia. If the hypoxia is repeated or sustained (days), then basal levels of IR-ACTH and cortisol increase, and if the hypoxic insult is sustained, then delivery occurs in response to the sustained activation of the fetal HPA axis.
Acute hypoxia (6 h) increased CRH mRNA in the PVN at 135 days of gestation (631). Green et al. (335) found that POMC mRNA in the pars distalis of ovine fetuses increased 2.5-fold following repeated hypoxic insults. Furthermore, 48 h of hypoxia (105) at either 126–130 or 134–136 days of gestation resulted in increased POMC mRNA in the anterior pituitary as well as an increase in plasma IR-ACTH. In the latter study, IR-ACTH returned to baseline by 12–24 h post-initiation of hypoxia. Cortisol increased at both ages in response to hypoxia; however, in the older fetuses, plasma cortisol remained elevated even after IR-ACTH had decreased to baseline. Wood et al. (1066) showed that even transient hypoxia can induce significant genomic alterations in the ovine fetal hypothalamus related to changes in cellular metabolism in response to reduced oxygen tension.
At the level of the adrenal gland, fetal hypoxia for 48 h resulted in enhanced levels of mRNAs encoding 3β-hydroxysteroid dehydrogenase, P450scc and P450C21 (CYP21) but not P450C17 (CYP17) (104). Furthermore, studies by Fraser et al. (280) demonstrated that sustained hypoxia upregulated ACTH receptor mRNA. POMC processing to ACTH is clearly affected by fetal stress (133). Braems (103) also showed that 48-h hypoxia increased the bioactivity of fetal plasma ACTH coupled with increased adrenal steroidogenic enzymes and cortisol production. Since PVN lesions prevent the increase in plasma ACTH and cortisol in response to acute hypoxia (638), it is likely that the hypoxic modulation of the anterior pituitary and adrenal cortex occurs through specific activation of CRH and/or AVP neurons in a classic stress response manner. Thus hypoxia ranging from hours to days during the final weeks of gestation clearly has an activating effect on the ovine fetus. This activation represents a logical adaptive response allowing normal development in a potentially hazardous intrauterine environment.
2. Chronic hypoxia
Like acute hypoxia, chronic or long-term hypoxia (LTH) can have profound effects on the fetus. However, unlike acute hypoxia, the fetus has a tremendous capacity to adapt to the challenges of chronic hypoxia. A very effective animal model has been developed by our group to mimic LTH using the pregnant ewe maintained at high altitude (3,820 m). The animals are at altitude from ~40 days of gestational age (dGA) onward resulting in a maintained fetal Po2 of ~18 mmHg (normal ~23–25 mmHg) at ~140 dGA (term ~148 dGA) (1, 235, 707). In marked contrast to this activational function of acute or prolonged hypoxia when initiated during the final third of gestation, the HPA axis of the fetus adapts divergently when development occurs under conditions of LTH from day 40 of gestation onward (235, 709, 712). Indeed, LTH fetal sheep do not deliver early and are not growth restricted, reflective of the ability to maintain normal basal cortisol production. However, even though basal plasma cortisol production in the LTH fetal sheep is not different compared with control normoxic fetuses between 136 and 141 days of gestation, critical aspects of HPA axis function are clearly altered. These findings include 1) both basal and stimulated plasma ACTH1–39 were significantly elevated in LTH fetuses (407, 709, 1010); 2) while cortisol production was greater in the LTH fetus when exposed to a secondary stress, expression of key enzymes fundamental for glucocorticoid synthesis, CYP17 (cytochrome P-450 17α-hydroxylase) and CYP11A1 (cytochrome P-450 side-chain cleavage) were significantly lower in the adrenal cortex (1, 407, 705); 3) expression of the ACTH receptor was also lower in the LTH adrenal gland (235); and 4) POMC processing/release was enhanced (705). Undoubtedly, the ability of elevated basal plasma ACTH in the LTH fetuses to increase expression of target genes in the adrenal cortex has been tempered, thus ensuring that premature maturation of glucocorticoid synthetic capacity does not occur.
Although the ability of ACTH to stimulate short-term cortisol output in response to an acute stress is well established, there are experimental situations where ACTH and adrenal cortisol production are not well-correlated (512). Studies by Wood (1065) indicated the involvement of the sinoaortic chemo- and/or baroreceptors in the fetal stress response. In near term fetal sheep, sinoaortic denervation (311) or splanchnic nerve section (710) altered the cortisol response to acute hypoxia without affecting the increase in ACTH. However, the mechanism(s) of neurally mediated influences on adrenocortical function remain unclear. Based on studies in the ovine LTH models, the enhanced cortisol response to a secondary stressor following LTH does not appear to be mediated by this pathway as LTH denervated fetuses responded like the intact LTH group (469). A key question is what is the mechanism that mediates the observed dissociation between elevated ACTH and suppressed adrenal steroidogenic gene expression under when development occurs under LTH?
D. Leptin as a Modulator of the HPA Axis
Leptin is a fascinating potential link to reprogramming the ovine HPA axis to adapt to conditions of chronic hypoxia. This 16-kDa polypeptide is synthesized and secreted primarily by adipose tissue. The product of the obese (ob) gene, this hormone has a broad range of physiological functions including regulation of energy homeostasis, bone formation, reproduction, and effects on the cardiovascular system (12) as well as significant effects on neuroendocrine function (14). The most studied effect of leptin is its regulatory role in energy balance. In the adult, it acts at the level of the hypothalamus to signal adequacy of nutritional status, thereby regulating energy intake and expenditure, thus weight gain and fat deposition (871, 872). Leptin exerts its effect through the leptin receptor (OB-R). Different splice variants of the leptin receptor are known. However, the OB-Rb or “long form” of the receptor is the biologically active form, while a short isoform (OB-Ra) represents the other major form. Also, in a species-dependent manner, several other short, inactive forms other than Ob-Ra have been described. All isoforms of the OB-R are generated by alternative splicing of the primary transcript. Signal transduction via the OB-Rb has best been characterized in the hypothalamus (696, 872), although other tissues appear to utilize a similar pathway. The JAK-STAT pathway has been established as the primary pathway of leptin signal transduction in the hypothalamus (961).
The hypothalamus is a critical site of leptin action and has high levels of expression of the OB-Rb (98, 696, 871). The OB-Rb receptor is primarily observed in hypothalamic neurons that express neuropeptide Y (NPY), agouti-related peptide (AgRP), POMC, and cocaine- and amphetamine-regulated transcript (CART) (246, 655, 850). CART is abundant in hypothalamic nuclei controlling anterior pituitary function. In the PVN, CART mRNA is colocalized with vasopressin and CRF-containing neurons and central CART infusion enhanced ACTH secretion in the rat in vivo while in vitro studies also demonstrated increased hypothalamic release of CRF by CART (933). Therefore, leptin binding to the OB-Rb may act on CART neurons to regulate hypothalamic neuroendocrine functions. Further studies in adult sheep showed that intracerebroventricular leptin infusion resulted in diminished expression of mRNA for NPY in the hypothalamic arcuate nucleus (366). These findings are consistent with the isolation of OB-Rb in 60% of NPY containing cells in sheep hypothalamus (411). In the ovine hypothalamus, NPY can also regulate ACTH secretagogues AVP and CRF (571). Furthermore, OB-R has been localized in the PVN, indicating that leptin can have direct effects on CRH/AVP neurons as well (14). Leptin also blocks restraint-related stimulation of the HPA in the adult rat (363). Furthermore, chronic administration of leptin to ob/ob mice decreased plasma corticosterone levels, suggesting that the adipose hormone is involved in regulation of the HPA axis (13). At present, however, data on the mechanisms of leptin action on fetal HPA function are lacking.
In addition to the signaling cascades previously described, it seems that another layer of gene regulation can also be involved. This level of epigenetic influence appears to be accomplished in part by miRNAs, short noncoding RNAs that interfere with target gene expression posttranscriptionally by binding to their respective RNAs. When the RNase III ribonuclease Dicer that regulates miRNA maturation was deleted from POMC-expressing neurons, loss of POMC neurons and subsequent development of obesity was observed (891). More recent studies demonstrated that leptin downregulated expression of miRNAs that target POMC 3′UTR, which affects energy homeostasis (220).
In the adult, the function(s) of leptin has been widely studied. In marked contrast, there is little information on the functional role of leptin in the fetus. We do know however that leptin is found in the fetal circulation and is expressed in fetal adipocytes as well as in placental trophoblast tissue (367, 644, 1127). Forhead et al. (277) found that cortisol and leptin both increase as term approaches in fetal sheep and adrenalectomy caused a reduction in leptin after day 136, consistent with a known adipogenic role for glucocorticoids in adipocyte differentiation. Thus these results are in agreement with data in adult adipocytes where glucocorticoids stimulate both leptin gene expression and secretion (213). It appears that there is a positive relationship between fetal HPA activation and leptin. In contrast, developmental studies in the sheep fetus demonstrated a decrease in leptin mRNA expression in adipose tissue from days 75–110 to term (221). Furthermore, intracerebral infusion of leptin depressed the size of the increase in amplitude and mean value of plasma ACTH as well as cortisol pulses near term (384). Intravenous leptin infusions blocked the prepartum rise in both ACTH and cortisol. Infusion after day 144 did not affect ACTH, but there was still marked suppression of cortisol up to 3 days before delivery. In contrast to the studies by Forhead et al. (277), these studies did not demonstrate an increase in plasma leptin associated with the prepartum cortisol surge. Based on these studies, it appears that leptin has a negative-feedback effect on the HPA axis. Together, these data also showed that the regulatory effects of leptin seem to differ at various gestational ages.
1. Leptin and LTH
So how does leptin play a role in altering the response to LTH? Interestingly, the OB-Rb is found in the adrenal cortex in rodents (614), humans (808), and ruminants (157). In studies using the ovine LTH model, Ducsay et al. (234) showed dramatic increases in both plasma leptin and adipose leptin gene expression in hypoxic fetuses. Additionally, there was an enhanced expression of the OB-Rb in the adrenal gland. Although the function of leptin at the level of the adrenal has not been fully elucidated, it appears that it may act to inhibit adrenal ACTH stimulation of adrenocortical function directly. Leptin has also been demonstrated to inhibit ACTH-stimulated cortisol secretion in isolated human adrenal cortical tissue (808). Taken together, it appears that leptin reduces cortisol synthesis in the adrenal cortex by downregulating key steroidogenic enzymes. In addition to downregulation of steroidogenic enzymes, leptin also seems to inhibit adrenal glucocorticoid secretion by an additional mechanism. Steroidogenic acute regulatory protein (StAR) and the peripheral benzodiazepine receptor (PBR) move cholesterol to the inner mitochondrial membrane and are thus essential for steroidogenesis. Experiments in rats showed quite convincingly that leptin inhibition of corticosterone secretion occurs through a rapid suppression of both StAR and PBR protein expression (172, 876). In studies using the LTH sheep model, there was a marked reduction in adrenal CYP-17, CYP11A1, as well as the ACTH receptor (MC2R) and StAR (709). The observed enhancement of leptin and adrenal leptin receptors strongly suggests that leptin is indeed decreasing the capacity of the adrenal to respond to the augmented levels of ACTH. Additional studies demonstrating restoration of adrenal steroidogenic enzymes in LTH fetuses following the infusion of a specific leptin antagonist further strengthen this concept (233).
In addition to elevated OB-Rb in the LTH fetal adrenal (234), the ovine fetal and neonatal adrenal gland robustly expresses PDE3B, STAT3, SF-1, and SOCS3 (233, 1154), principal components of the leptin signaling pathway. Additional data show that leptin attenuates, at physiological levels of leptin and ACTH, ACTH-induced CYP17 expression in ovine fetal adrenocortical cells (895) and cortisol production in vivo (1128). Additional studies in the sheep model were the first to demonstrate that leptin levels are elevated in the fetus following hypoxic exposure (234). Through activation of PDE3B, leptin could attenuate the ACTH-induced cAMP signal necessary for both acute cortisol production and ACTH-induced expression of the primary steroidogenic genes. Leptin could also inhibit ACTH-induced gene expression through activation of the ERK signaling pathway. Inhibition of ERK mimics ACTH-induced expression of CYP17 and that ERK phosphorylation of SF-1 in adrenocortical cells inhibits its activity (904–907). Since leptin impacts both fetal metabolism and the HPA axis, such information is critical to further our understanding of the effects of LTH on fetal development as well as potential programming effects (643). Exogenous leptin can modulate the HPA axis at the level of the hypothalamus in normoxic fetuses (1128). However, more recent studies using a selective leptin antagonist demonstrate that another site of action of leptin in the adaptive response to LTH is at the level of the adrenal gland (233), altering the capacity of adrenocortical cells to respond to the elevation in hypothalamic-anterior pituitary function observed in the LTH fetus.
Data suggest that leptin production could be stimulated by angiogenic stimuli such as hypoxia (341). Indeed, this concept is supported by elevated leptin levels in individuals following hypobaric hypoxia (992, 993), or in patients with sleep apnea (410). Studies in mice exposed to intermittent hypoxia also demonstrated an upregulation of leptin mRNA expression (805). Hypoxia in adipocytes dramatically enhances expression of leptin, MMP, and VEGF as well as stimulating the HIF-1 pathway (583). Therefore, if hypoxia occurs in adipose tissue, leptin may modulate the angiogenic process. Furthermore, agents that mimic hypoxia (DFO or CoCl2) stimulate HIF-1 and leptin in cultured adipose cells (337). As previously described, HIF-1 is a transcriptional activator produced in response to cellular hypoxia and mediates a number of homeostatic responses to hypoxia at both the cellular and systemic level (30, 418). Since HIF-1 appears to be almost instantly degraded with the end of the hypoxic stimulus (380), it is sometimes difficult to accurately assess changes in HIF-1 upregulation in relation to hypoxia-induced changes in leptin.
The intimate relationship between leptin and programming of the HPA axis is critical to our understanding of the “give and take” that occurs and how an intrauterine stress like hypoxia can have long-term metabolic consequences with the resultant development of obesity and other comorbidities. This relationship of leptin to the HPA axis also draws attention to the potential impact of stressors during development can affect adipose tissue.
E. Adipose-Stress Axis
Typically, adipose tissue is categorized as either white adipose tissue (WAT) or brown adipose tissue (BAT). WAT is associated with storage while BAT is characterized by energy dissipation as heat. In 1979, Himms-Hagen et al. (373) proposed that a deficit in BAT is a potential major contributor to obesity. Another population of adipose cells beige/BRITE (‟brown-in-whiteˮ) were also found to express the thermogenic regulatory uncoupling protein-1 (UCP-1) (71, 354). It was also noted that these UCP-1 positive adipocytes found in certain WAT deposits of rodents could be expanded by prolonged exposure to cold or extended β-agonist treatment (either general β-agonist or selective β3) (52, 374, 1015). Upon stimulation, beige or BRITE adipocytes undergo a molecular program similar to BAT cells culminating with the expression of UCP1 equal to that of BAT and dissipate energy as heat when activated. In adult rodents, ‟beigingˮ of WAT aids in protection against diet-induced obesity, and increased formation of beige/BRITE fat has been demonstrated to exert antiobesity and antidiabetic actions in rodent models (188, 354). Thus beige/BRITE adipose represents a novel targetable mechanism for attenuating obesity and associated metabolic disorders.
While genetic manipulation of the BRITE adipocyte population has been achieved (276, 283), there are virtually no studies focusing on the in utero programming of the BRITE adipocyte population in WAT deposits in rodents or larger animal species where adipose tissue develops during fetal life. In rodents, while true BAT differentiates during fetal development, abdominal and subcutaneous adipose and importantly, BRITE adipocytes, develop after birth, thus largely eliminating these species from studying in utero programming of these deposits. Both sheep and human fetuses have considerable adipose expansion during the final half to third of gestation. Like sheep, human perirenal adipose is multilocular fat with characteristics of BAT.
In the fetus, perirenal fat (PRF) classically is considered a ‟brownˮ adipose tissue (BAT), expressing the thermogenic mediator UCP-1 and genes that support UCP-1 expression. In the late gestation fetus, the PRF BAT phenotype is driven by glucocorticoids and thyroid hormones. Unlike actual BAT (e.g., supraclavicular), PRF rapidly in both humans and sheep loses the BAT phenotype after birth, while retaining/expanding the WAT phenotype into abdominal fat. This transformation is not due to apoptosis, but rather the loss of the molecular program of gene expression necessary for BAT function (UCP1, PGC1α, PRDM16, DIO2). Interestingly, beige adipocytes have been characterized that reside in WAT of adults. In adult rodents, the molecular program of gene expression supporting BAT function in BRITE adipocytes is induced through sympathetic nervous system (SNS) activation as well as via hepatic FGF21 (an endocrine member of the FGF family). However, in the sheep and human, the regulation of “beiging” is not well defined.
In the ovine fetus, moderate gestational hypoxia significantly impacts the developing perirenal/abdominal fat (234, 706, 708) with potential long-term ramifications for susceptibility to obesity. The PRF of the late gestation LTH ovine fetus exhibits increased expression of the BAT program of gene expression (UCP1, DIO2, PRDM16, PGC1α) (234, 706). The primary drivers of the BAT phenotype of fetal PRF are glucocorticoids and T3. LTH results in a significant increase in HSD11B1 (cortisone to cortisol conversion) with no effect on HSD11B2 expression (cortisol to cortisone inactivation). This information, coupled with a significant elevation in DIO2 expression (T4 to T3 conversion) in PRF, underscores a local increase in these endocrine factors driving the increased BAT phenotype in the LTH fetal PRF. Furthermore, HSD11B1 expression was an order of magnitude greater than HSD11B2 in fetal PRF, further favoring local cortisol production in PRF. Collectively, this provides an essential mechanism for enhanced local production of cortisol in the PRF of LTH fetuses (708) despite normal ontogenic levels of plasma cortisol that was observed in this group (237, 709). FGF21 may also play a role in the increased BAT phenotype of fetal PRF in the LTH fetus since there was an observed increase in hepatic FGF21 expression coupled with the enhanced expression of FGF21 receptors in the LTH fetal PRF (711). However, by postnatal day 14, LTH lambs exhibit a significant decrease in the BAT/beige molecular program compared with control lambs. This change occurs while retaining and/or amplifying genes of the WAT phenotype (e.g., RIP140, a key co-repressor of BAT genes as well as leptin and adiponectin) (238).
1. Potential mechanisms programming beige adipose induced by hypoxia
The impact of the intrauterine environment on programming of adipose remains poorly understood. More specifically, there is little information on the epigenetic mechanisms of hypoxia-induced fetal programming of adipose. From a more general perspective, it is quite clear that epigenetic alterations in adipose can occur by a number of different means. In models of obesity, for example, offspring of obese dams demonstrated enhanced expression of adipogenic regulatory factors that were linked to changes in DNA methylation and enhanced white adipose tissue differentiation (95). A recent study in offspring of women from diabetic pregnancies found increases in adiponectin DNA methylation (383). DNA methylation has also been linked to adipose differentiation in BAT (541, 555). miRNAs have also been shown to regulate of adipogenesis (252, 458, 1031) and differentiation (539, 566).
The post-birth loss of the ‟beigeˮ/brown phenotype (UCP-1) in the LTH offspring at PN14 is likely mediated at the intracellular level by the decreased expression of PGC1α, PRDM16, and DIO2 in the LTH offspring PRF, all of which converge to maintain UCP-1 expression and the “BAT” phenotype. Also, expression of RIP140 is elevated in the LTH offspring PRF; RIP140 is a co-repressor that interacts with PPARγ to support the WAT phenotype while repressing the BAT program of gene expression. The increase in RIP140 is thus consistent with the upregulation of leptin and adiponectin expression in the LTH offspring PRF. Preliminary data also show that that SNS innervation is decreased in the LTH offspring at PN14. Our group has also shown a programming effect of gestational hypoxia on β adrenoreceptors (β-AR) where chronic hypoxia differentially regulated β-AR subtypes with an increase in β1 in the fetal heart (47). The loss of DIO2 likely also plays a role in the loss of the beige phenotype in the LTH offspring.
Another possible mechanism is miRNAs. These short, noncoding RNAs have been shown to play a role in the transcriptional and posttranscriptional regulation of gene expression in beiging of adipose tissue (40, 169, 376, 575). miR-133a has been implicated in the beiging process (575), and a BLAST search for ovine 133a identified a match for RIP140. RIP140 has also been shown to be regulated by miR-346 in a positive manner (407, 705, 991, 1009). In the LTH PN14 lamb, RIP140 is upregulated (and associated with a strong trend for increased miR-346), which is a binding partner of PPARγ and acts to repress the PPARγ/PGC1α beiging process, thus serving as a dominant negative in adipose tissue. Furthermore, in the LTH PN14, expression of miR-133 is significantly reduced (which targets and decreases RIP140), suggesting a dual regulation of this critical repressor of the BAT/BRITE phenotype (236).
Even though the generation of BRITE adipocytes during fetal development remains largely unexplored, the preliminary evidence from the ovine model indicates that LTH can either limit their formation during development or the capacity for the beiging program to be activated in early infant life. It seems reasonable to speculate that losing the BAT/beige phenotype while upregulating WAT gene expression after birth in the LTH offspring will promote obesity due to decreased adipose energy expenditure, favoring fat deposition. This would profoundly increase the propensity for adipogenesis in these offspring.
F. Appetite and Hypoxia
Not only does the hypothalamus play a pivotal role in the ability to respond to stress (as part of the HPA axis), but it is also critical in the regulation of appetite. Animal studies have utilized a wide variety of maternal manipulations to induce offspring obesity (maternal undernutrition, protein restriction, obesity, synthetic glucocorticoids), and these studies mainly support epidemiological studies on the intrauterine programming of adult obesity. However, few studies have focused on fetal origins of childhood obesity or, equally important, early changes in appetite that can predispose an individual to obesity, metabolic disorders, and cardiovascular disease. It is apparent that not all children born from obese women will develop obesity (751, 1051), and a significant population of children from nonobese/nondiabetic mothers are at high risk or will develop childhood obesity. This emphasizes that other factors contribute to the programming of childhood obesity. Therefore, it seems likely that fetal hypoxia predisposes the offspring to excess caloric intake leading to excess fat deposition by its impact on developing appetitive neurocircuitry. Sustained fetal hypoxia is a highly relevant fetal perturbation associated with a number of pregnancy complications. These include preeclampsia (749, 1136), smoking tobacco during pregnancy (331, 1020) (1 in 5 pregnancies in the United States), and high altitude (449, 1136, 1145). Importantly, further linking hypoxia and obesity, smoking during gestation is related to childhood obesity in the offspring (331, 1051), and obesity itself is associated with fetal hypoxia (912).
In addition to its role in the crucial response to stress, the neural networks in the hypothalamus are a critical element in the regulation of energy homeostasis (689). Specifically, POMC neurons within the arcuate nucleus of the hypothalamus (AH) are crucial to the regulation of glucose and energy balance (693, 779). An important regulatory component of hypothalamic gene expression is leptin (689) which can act through different signaling pathways already discussed.
Although there is considerable variation among different species regarding the development of food intake pathways, these neuronal networks can be influenced extensively by the environment (195). This occurs through developmental plasticity and the impact of epigenetic alterations (333, 339, 931). Interestingly, the regulation of appetitive pathways is intimately linked to nutritional status as well as energy output forming the hypothalamic-adipose axis (107). As an example, using a rodent model of intermittent hypoxia during gestation, Khalyfa et al. (478) demonstrated an increased propensity for metabolic disorders in adulthood including food intake, body weight, adiposity, and cholesterol levels. DNA methylation profiling and microarray analysis revealed a significant number of DMRs associated with genes responsible for metabolic regulation and inflammation. While these patterns were only observed in male offspring, the data revealed the epigenomic effects of intrauterine hypoxia on adipose tissue function.
As reviewed by Grove and colleagues (333), there is a lack information on the impact of an adverse intrauterine environment and leptin per se on development of the critical hypothalamic systems governing food intake in a long-gestation species in which these systems develop in the fetus in utero. Presently, nearly all of the research available has used the rodent model which has some strengths including the short duration of gestation and the ability to follow subsequent generations in a relatively brief timeframe. However, this might not be the most appropriate translational model. It is important to recognize that in altricial species like mice and rats, the appetitive neuroendocrine pathways develop largely during postnatal life. This is in marked contrast to precocial species like the sheep and, more importantly, the human where these pathways develop primarily during the fetal period (98, 332, 695, 696).
Hyperphagia is recognized as a major factor contributing to obesity (459). The adipose hormone leptin is a key regulator of appetite acting on its receptor (ObRb) in specific hypothalamic nuclei that govern food intake (63, 459, 887). The effect of leptin at the level of the hypothalamus has recently been shown to be mediated by histone deacetylase 5 (456). In adults, loss of leptin regulation of appetite (ObRb desensitization) is a hallmark of diet-induced obesity (DIO; Refs. 63, 459, 887). Recent studies performed nearly exclusively in rodents have shown that leptin also exerts key developmental roles in the hypothalamus (98, 333). During the first 2 wk after birth in rodents, a so-called ‟surgeˮ in plasma leptin occurs concurrently with the early expansion of WAT. Paradoxically, experimental paradigms that either increase (e.g., maternal obesity) or decrease (e.g., maternal nutritional restriction) plasma leptin during this neonatal period program offspring obesity (146, 215, 344, 1135). These paradigms alter expression of the principal neuropeptides (NPY and α-MSH) in the arcuate nucleus of the hypothalamus (ARH) as well as disrupt the proper formation of ARH projections to other nuclei in the appetitive neurocircuitry.
In longer gestation precocial species including humans, non-human primates, and sheep, the appetitive neuroendocrine system develops during pregnancy (98). In both humans and sheep, adipose differentiation and expansion initiate just before mid-gestation and are accompanied by increased fetal plasma leptin. In the ovine fetus, plasma leptin and adipose leptin expression can be affected by a variety of maternal-fetal perturbations such as placental restriction (239) and maternal obesity (584). Furthermore, in our model of long-term moderate gestational hypoxia (LTH), late gestation ovine fetuses exhibit an approximately three- to fourfold increase in fetal plasma leptin driven by increased adipose leptin expression (234).
Preliminary unpublished data from our group showed that mRNA for POMC and the melanocortin 4 receptor (MC4R) in the hypothalamus are decreased (P < 0.01) while NPY exhibited a trend for being elevated in late gestation LTH fetuses. POMC is the precursor for the anorexigenic peptide αMSH, which mediates its actions via the MC4R, while NPY is orexigenic, supporting an impact of LTH on the appetite neuroendocrine system in utero. These data suggest that gestational LTH in sheep alters the development of the hypothalamic neuroendocrine system governing appetite behavior rendering the lamb susceptible to obesity via hyperphagia, resulting in increased adipose deposition. This effect on the development of the appetitive neuroendocrine system may be mediated by the observed increased fetal leptin, ultimately leading to leptin desensitization in the ARH. There could also be direct effects of hypoxia on the maturation of this system.
As delineated throughout this section, there is an intimate relationship between the brain and adipose tissue. In addition to effects on the adrenal gland, leptin from adipose exerts a significant programming effect on the hypothalamus. However, it is important to indicate that there also appears to be a direct input from the hypothalamus and hippocampus to adipose tissue. Both BAT and WAT are innervated by the SNS (59, 60, 101). NPY enhances adipogenesis while inhibiting lipolysis in adipose tissue (1156).
Schäffler and co-workers (888, 889) even take this concept further to include the pituitary in this complicated relationship. It is clear that products of adipose tissue “adipokines” signal the brain as to energy status, but do products of the hypothalamus/pituitary impact adipose tissue? Although the data are correlative, the studies unmistakably demonstrated the presence of receptors in adipose tissue for anterior pituitary hormones like ACTH, GH, TSH, etc. as well as for the hypothalamic peptide AVP (888). Based on the dramatic effects of hypoxia (and other stressors) on the developing HPA axis and adipose tissue as outlined in detail above, it is not unreasonable to anticipate significant alterations in the hypothalamic-adipose axis. Indeed, data from the LTH sheep studies above clearly indicate effects on this axis with major hypoxia-induced changes in both the HPA axis and adipose tissue.
G. Perspective
The HPA axis plays a critical role in the regulation of glucocorticoids and is highly sensitive to hypoxia both acute and chronic. It is the exposure to chronic hypoxia, however, that makes it a target for epigenetic regulation. In addition to its role in the critical response to stress, the neural networks in the hypothalamus are an essential component in the regulation of energy homeostasis (689). However, the short-term adaptations to a chronic stress may have unintended consequences of altered stress responses, alterations in metabolism, and obesity. Further complicating the picture is the intimate relationship between the HPA axis and adipose tissue. The effects of hypoxia-induced leptin concentrations not only impact HPA function and programming but also point to hypoxic regulation of adipose, particularly concerning maintenance or loss of the BAT phenotype in beige adipose tissue. This intricate relationship and the influence of hypoxia are illustrated in FIGURE 13. Although species differences are evident, data from the ovine model suggest that LTH has a dual impact on programming. Not only does it alter adipose development to favor the WAT phenotype, but it also changes the appetitive pathways to potentially enhance food intake, thus dramatically increasing the likelihood of the development of obesity. Whether this same phenomenon occurs in humans is yet unproven. However, the potential to manipulate the thermogenic activity of adipose cells has significant potential to treat programmed metabolic disorders.
FIGURE 13.
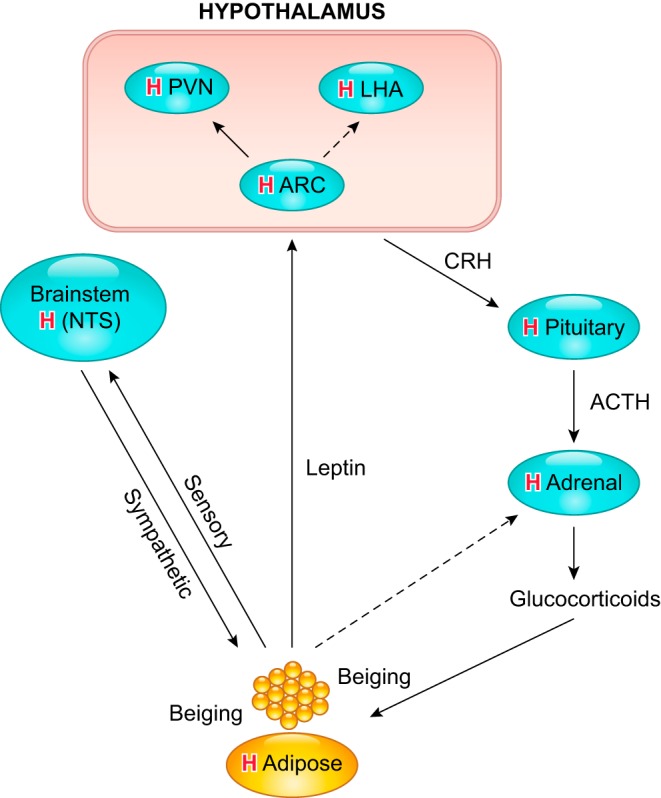
The complex interplay between the HPA axis and adipose tissue during development. H indicates that hypoxia can play a significant role in altering the plasticity of all of these components. Solid lines represent stimulatory pathways, whereas dashed lines are indicative of inhibitory pathways. ACTH, adrenocorticotropic hormone; ARC, arcuate nucleus; CRH, corticotropin-releasing hormone; LHA, lateral hypothalamic area; NTS, nucleus tractus solitarius; PVN, paraventricular nucleus.
IX. CONCLUSIONS AND FUTURE DIRECTIONS
Hypoxia during gestation impacts both the mother and fetal development through interactions with an individual’s genetic traits acquired over multiple generations by natural selection and changes in gene expression patterns by altering the histone code (epigenome). Changes in the epigenome determine “genomic plasticity,” i.e., the ability of genes to be differentially expressed according to environmental cues. The genomic plasticity defined by epigenomic mechanisms including DNA methylation, histone modifications, and noncoding RNAs during development is the mechanistic substrate for phenotypic programming that determines physiological response and risks for the organism’s healthy or deleterious outcomes (FIGURE 14).
FIGURE 14.
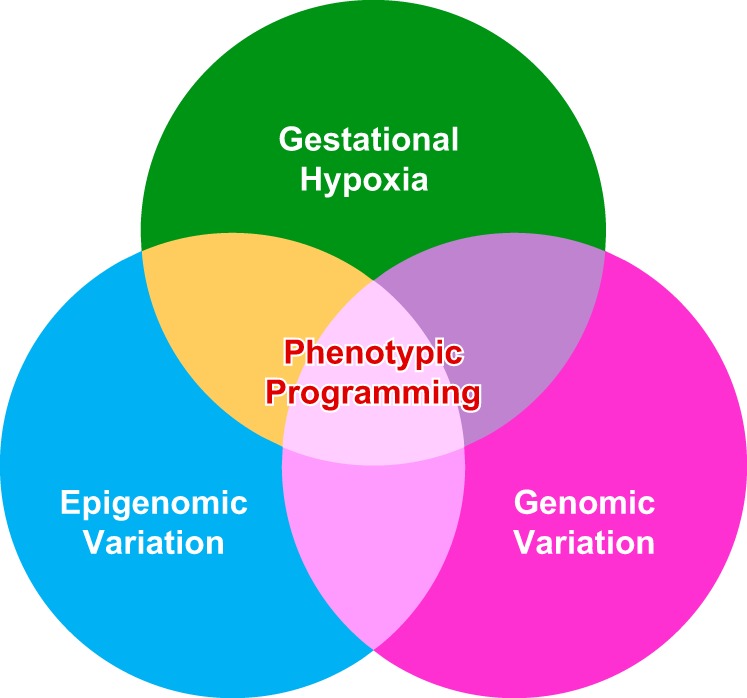
Hypoxia during gestation interplays with genomic and epigenomic variations resulting in phenotypic programming that determines an individual’s healthy and disease later in life.
The future direction in this ever-expanding field centers on the feasibility of preventing or at least treating these changes to preserve normal outcomes. The identification of interventions that have the potential to reverse epigenetic alterations holds great promise. An understanding of the specific hypoxia-induced environmental and epigenetic adaptations linked to specific organ systems will enhance the development of target-specific inhibition of DNA methylation, histone modifications, and noncoding RNAs that underlie hypoxia-induced phenotypic programming of disease vulnerability later in life. Measures to reduce oxidative stress and changes in the fetal steroid milieu may also play a critical role in reducing the potent effects of hypoxia on programming of disease. A potential stumbling block to these efforts, however, relates to timing of the intervention. The greatest potential effect would be accomplished at the critical period in development for which the genomic plasticity is at its peak, thus ameliorating the influence of hypoxia or other stressors. However, more information is obviously needed since a number of programming changes in the fetus may be crucial for the ability to respond to hypoxic stress. It is not until later in life that the “unintended consequences” of such changes may become evident. With future developments, it may even become possible to intervene before conception, before the genetic determinants of the risk of developing programmed disease are established (352).
GRANTS
We thank the National Institute of Child Health and Human Development and National Heart, Lung, and Blood Institute that have supported our research over four decades, including Program Project Grants P01 HD31226 and P01 HD083132 as well as many other grants.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
This review is in honor of the memory of Lawrence D. Longo, M.D., Dr.H.C. (hon), FACOG, FRCOG, a friend, mentor, and colleague.
We thank Jimin Suh for editorial assistance.
Address for reprint requests and other correspondence: L. Zhang, The Lawrence D. Longo, MD Center for Perinatal Biology, Dept. of Basic Sciences, Loma Linda University School of Medicine, Loma Linda, CA 92350 (e-mail: lzhang@llu.edu).
REFERENCES
- 1.Adachi K, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters ovine fetal endocrine and physiological responses to hypotension. Am J Physiol Regul Integr Comp Physiol 287: R209–R217, 2004. doi: 10.1152/ajpregu.00701.2003. [DOI] [PubMed] [Google Scholar]
- 2.Adel S, Mansour A, Louka M, Matboli M, Elmekkawi SF, Swelam N. Evaluation of MicroRNA-210 and protein tyrosine phosphatase, non-receptor type 2 in pre-eclampsia. Gene 596: 105–109, 2017. doi: 10.1016/j.gene.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Adeoye OO, Silpanisong J, Williams JM, Pearce WJ. Role of the sympathetic autonomic nervous system in hypoxic remodeling of the fetal cerebral vasculature. J Cardiovasc Pharmacol 65: 308–316, 2015. doi: 10.1097/FJC.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adesina SE, Kang BY, Bijli KM, Ma J, Cheng J, Murphy TC, Michael Hart C, Sutliff RL. Targeting mitochondrial reactive oxygen species to modulate hypoxia-induced pulmonary hypertension. Free Radic Biol Med 87: 36–47, 2015. doi: 10.1016/j.freeradbiomed.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adickes ED, Mollner TJ, Makoid MC. Ethanol-induced teratogenic alterations in developing cardiomyocytes in culture. Alcohol Alcohol Suppl 2: 283–288, 1993. [PubMed] [Google Scholar]
- 6.Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, Konduri GG. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 303: L870–L879, 2012. doi: 10.1152/ajplung.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afolayan AJ, Teng RJ, Eis A, Rana U, Broniowska KA, Corbett JA, Pritchard K, Konduri GG. Inducible HSP70 regulates superoxide dismutase-2 and mitochondrial oxidative stress in the endothelial cells from developing lungs. Am J Physiol Lung Cell Mol Physiol 306: L351–L360, 2014. doi: 10.1152/ajplung.00264.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal S, Gross C, Fineman JR, Black SM. Oxidative stress and the development of endothelial dysfunction in congenital heart disease with increased pulmonary blood flow: lessons from the neonatal lamb. Trends Cardiovasc Med 20: 238–246, 2010. doi: 10.1016/j.tcm.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal S, Gross CM, Sharma S, Fineman JR, Black SM. Reactive oxygen species in pulmonary vascular remodeling. Compr Physiol 3: 1011–1034, 2013. doi: 10.1002/cphy.c120024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguan K, Murotsuki J, Gagnon R, Thompson LP, Weiner CP. Effect of chronic hypoxemia on the regulation of nitric-oxide synthase in the fetal sheep brain. Brain Res Dev Brain Res 111: 271–277, 1998. doi: 10.1016/S0165-3806(98)00145-X. [DOI] [PubMed] [Google Scholar]
- 11.Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 467: 323–327, 2010. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahima RS, Flier JS. Leptin. Annu Rev Physiol 62: 413–437, 2000. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 13.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 14.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21: 263–307, 2000. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed A, Rezai H, Broadway-Stringer S. Evidence-Based Revised View of the Pathophysiology of Preeclampsia. Adv Exp Med Biol 956: 355–374, 2017. doi: 10.1007/5584_2016_168. [DOI] [PubMed] [Google Scholar]
- 16.Akagi K, Challis JR. Threshold of hormonal and biophysical responses to acute hypoxemia in fetal sheep at different gestational ages. Can J Physiol Pharmacol 68: 549–555, 1990. doi: 10.1139/y90-080. [DOI] [PubMed] [Google Scholar]
- 17.Al-Hasan YM, Evans LC, Pinkas GA, Dabkowski ER, Stanley WC, Thompson LP. Chronic hypoxia impairs cytochrome oxidase activity via oxidative stress in selected fetal guinea pig organs. Reprod Sci 20: 299–307, 2013. doi: 10.1177/1933719112453509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander BT, Bennett WA, Khalil RA, Granger JP. Preeclampsia: linking placental ischemia with cardiovascular-renal dysfunction. News Physiol Sci 16: 282–286, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Alexander BT, Dasinger JH, Intapad S. Fetal programming and cardiovascular pathology. Compr Physiol 5: 997–1025, 2015. doi: 10.1002/cphy.c140036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alhawaj R, Patel D, Kelly MR, Sun D, Wolin MS. Heme biosynthesis modulation via δ-aminolevulinic acid administration attenuates chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L719–L728, 2015. doi: 10.1152/ajplung.00155.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aljunaidy MM, Morton JS, Cooke CL, Davidge ST. Maternal vascular responses to hypoxia in a rat model of intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 311: R1068–R1075, 2016. doi: 10.1152/ajpregu.00119.2016. [DOI] [PubMed] [Google Scholar]
- 22.Allison BJ, Brain KL, Niu Y, Kane AD, Herrera EA, Thakor AS, Botting KJ, Cross CM, Itani N, Skeffington KL, Beck C, Giussani DA. Fetal in vivo continuous cardiovascular function during chronic hypoxia. J Physiol 594: 1247–1264, 2016. doi: 10.1113/JP271091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allison BJ, Kaandorp JJ, Kane AD, Camm EJ, Lusby C, Cross CM, Nevin-Dolan R, Thakor AS, Derks JB, Tarry-Adkins JL, Ozanne SE, Giussani DA. Divergence of mechanistic pathways mediating cardiovascular aging and developmental programming of cardiovascular disease. FASEB J 30: 1968–1975, 2016. doi: 10.1096/fj.201500057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almli LM, Fani N, Smith AK, Ressler KJ. Genetic approaches to understanding post-traumatic stress disorder. Int J Neuropsychopharmacol 17: 355–370, 2014. doi: 10.1017/S1461145713001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almouzni G, Khochbin S, Dimitrov S, Wolffe AP. Histone acetylation influences both gene expression and development of Xenopus laevis. Dev Biol 165: 654–669, 1994. doi: 10.1006/dbio.1994.1283. [DOI] [PubMed] [Google Scholar]
- 26.Altman J, Bayer SA. The development of the rat hypothalamus. Adv Anat Embryol Cell Biol 100: 1–178, 1986. doi: 10.1007/978-3-642-71301-9_1. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez MR, Clark LC, Moore JA, Morales MC, Gerdes AM. Results of prenatal alcohol exposure on the dimensions and binucleation of cardiac myocytes in neonatal and weanling rats. Teratology 44: 395–404, 1991. doi: 10.1002/tera.1420440406. [DOI] [PubMed] [Google Scholar]
- 28.Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin JN Jr, LaMarca B. 17-Hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension 65: 225–231, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amaral LM, Faulkner JL, Elfarra J, Cornelius DC, Cunningham MW, Ibrahim T, Vaka VR, McKenzie J, LaMarca B. Continued Investigation Into 17-OHPC: Results From the Preclinical RUPP Rat Model of Preeclampsia. Hypertension 70: 1250–1255, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambrosini G, Nath AK, Sierra-Honigmann MR, Flores-Riveros J. Transcriptional activation of the human leptin gene in response to hypoxia. Involvement of hypoxia-inducible factor 1. J Biol Chem 277: 34601–34609, 2002. doi: 10.1074/jbc.M205172200. [DOI] [PubMed] [Google Scholar]
- 31.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23: 185–188, 1999. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 32.Anastario M, Salafia CM, Fitzmaurice G, Goldstein JM. Impact of fetal versus perinatal hypoxia on sex differences in childhood outcomes: developmental timing matters. Soc Psychiatry Psychiatr Epidemiol 47: 455–464, 2012. doi: 10.1007/s00127-011-0353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andres C. Molecular genetics and animal models in autistic disorder. Brain Res Bull 57: 109–119, 2002. doi: 10.1016/S0361-9230(01)00642-6. [DOI] [PubMed] [Google Scholar]
- 34.Andrew M, Paes B, Johnston M. Development of the hemostatic system in the neonate and young infant. Am J Pediatr Hematol Oncol 12: 95–104, 1990. doi: 10.1097/00043426-199021000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Ang SY, Uebersohn A, Spencer CI, Huang Y, Lee JE, Ge K, Bruneau BG. KMT2D regulates specific programs in heart development via histone H3 lysine 4 di-methylation. Development 143: 810–821, 2016. doi: 10.1242/dev.132688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anton L, Olarerin-George AO, Hogenesch JB, Elovitz MA. Placental expression of miR-517a/b and miR-517c contributes to trophoblast dysfunction and preeclampsia. PLoS One 10: e0122707, 2015. doi: 10.1371/journal.pone.0122707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anton L, Olarerin-George AO, Schwartz N, Srinivas S, Bastek J, Hogenesch JB, Elovitz MA. miR-210 inhibits trophoblast invasion and is a serum biomarker for preeclampsia. Am J Pathol 183: 1437–1445, 2013. doi: 10.1016/j.ajpath.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308: 1466–1469, 2005. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA 93: 12969–12973, 1996. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arias N, Aguirre L, Fernández-Quintela A, González M, Lasa A, Miranda J, Macarulla MT, Portillo MP. MicroRNAs involved in the browning process of adipocytes. J Physiol Biochem 72: 509–521, 2016. doi: 10.1007/s13105-015-0459-z. [DOI] [PubMed] [Google Scholar]
- 41.Aschner JL, Zeng H, Kaplowitz MR, Zhang Y, Slaughter JC, Fike CD. Heat shock protein 90-eNOS interactions mature with postnatal age in the pulmonary circulation of the piglet. Am J Physiol Lung Cell Mol Physiol 296: L555–L564, 2009. doi: 10.1152/ajplung.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asikainen TM, Raivio KO, Saksela M, Kinnula VL. Expression and developmental profile of antioxidant enzymes in human lung and liver. Am J Respir Cell Mol Biol 19: 942–949, 1998. doi: 10.1165/ajrcmb.19.6.3248. [DOI] [PubMed] [Google Scholar]
- 43.Asopa S, Cagampang FR, Anthony FW, Lanham SA, Schneider JE, Ohri SK, Hanson MA. Effect of a low-protein diet during pregnancy on expression of genes involved in cardiac hypertrophy in fetal and adult mouse offspring. J Dev Orig Health Dis 1: 371–375, 2010. doi: 10.1017/S2040174410000541. [DOI] [PubMed] [Google Scholar]
- 44.Atanasova S, Wieland E, Schlumbohm C, Korecka M, Shaw L, von Ahsen N, Fuchs E, Oellerich M, Armstrong V. Prenatal dexamethasone exposure in the common marmoset monkey enhances gene expression of antioxidant enzymes in the aorta of adult offspring. Stress 12: 215–224, 2009. doi: 10.1080/10253890802305075. [DOI] [PubMed] [Google Scholar]
- 45.Azzouzi HE, Leptidis S, Doevendans PA, De Windt LJ. HypoxamiRs: regulators of cardiac hypoxia and energy metabolism. Trends Endocrinol Metab 26: 502–508, 2015. doi: 10.1016/j.tem.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Bae S, Gilbert RD, Ducsay CA, Zhang L. Prenatal cocaine exposure increases heart susceptibility to ischaemia-reperfusion injury in adult male but not female rats. J Physiol 565: 149–158, 2005. doi: 10.1113/jphysiol.2005.082701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol 285: H983–H990, 2003. doi: 10.1152/ajpheart.00005.2003. [DOI] [PubMed] [Google Scholar]
- 48.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther 315: 1125–1135, 2005. doi: 10.1124/jpet.105.090803. [DOI] [PubMed] [Google Scholar]
- 49.Bae S, Zhang L. Prenatal cocaine exposure increases apoptosis of neonatal rat heart and heart susceptibility to ischemia-reperfusion injury in 1-month-old rat. Br J Pharmacol 144: 900–907, 2005. doi: 10.1038/sj.bjp.0706129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bahtiyar MO, Buhimschi C, Ravishankar V, Copel J, Norwitz E, Julien S, Guller S, Buhimschi IA. Contrasting effects of chronic hypoxia and nitric oxide synthase inhibition on circulating angiogenic factors in a rat model of growth restriction. Am J Obstet Gynecol 196: 72.e1–72.e6, 2007. doi: 10.1016/j.ajog.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 51.Bal MP, de Vries WB, Steendijk P, Homoet-van der Kraak P, van der Leij FR, Baan J, van Oosterhout MF, van Bel F. Histopathological changes of the heart after neonatal dexamethasone treatment: studies in 4-, 8-, and 50-week-old rats. Pediatr Res 66: 74–79, 2009. doi: 10.1203/PDR.0b013e3181a283a0. [DOI] [PubMed] [Google Scholar]
- 52.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298: E1244–E1253, 2010. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 53.Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci 16: 3943–3949, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol 49: 270–283, 2006. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Barker DJ. The fetal and infant origins of adult disease. BMJ 301: 1111, 1990. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 95: 115–128, 1998. doi: 10.1042/cs0950115. [DOI] [PubMed] [Google Scholar]
- 57.Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod 2: 105–112, 1997. doi: 10.1530/ror.0.0020105. [DOI] [PubMed] [Google Scholar]
- 58.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 327: 1077–1081, 1986. doi: 10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 59.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 318: 34–43, 2010. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes 34, Suppl 1: S36–S42, 2010. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartolomei MS, Tilghman SM. Genomic imprinting in mammals. Annu Rev Genet 31: 493–525, 1997. doi: 10.1146/annurev.genet.31.1.493. [DOI] [PubMed] [Google Scholar]
- 62.Basang Z, Wang B, Li L, Yang L, Liu L, Cui C, Lanzi G, Yuzhen N, Duo J, Zheng H, Wang Y, Xu S, Jin L, Wang X. HIF2A Variants Were Associated with Different Levels of High-Altitude Hypoxia among Native Tibetans. PLoS One 10: e0137956, 2015. doi: 10.1371/journal.pone.0137956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bates SH, Myers MG Jr. The role of leptin receptor signaling in feeding and neuroendocrine function. Trends Endocrinol Metab 14: 447–452, 2003. doi: 10.1016/j.tem.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature 430: 419–421, 2004. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 65.Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P, Zheng YT. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA 107: 11459–11464, 2010. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beard RS Jr, Yang X, Meegan JE, Overstreet JW, Yang CG, Elliott JA, Reynolds JJ, Cha BJ, Pivetti CD, Mitchell DA, Wu MH, Deschenes RJ, Yuan SY. Palmitoyl acyltransferase DHHC21 mediates endothelial dysfunction in systemic inflammatory response syndrome. Nat Commun 7: 12823, 2016. doi: 10.1038/ncomms12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beere HM, Green DR. Stress management - heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol 11: 6–10, 2001. doi: 10.1016/S0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- 68.Benlhabib H, Mendelson CR. Epigenetic regulation of surfactant protein A gene (SP-A) expression in fetal lung reveals a critical role for Suv39h methyltransferases during development and hypoxia. Mol Cell Biol 31: 1949–1958, 2011. doi: 10.1128/MCB.01063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab 82: 1582–1588, 1997. [DOI] [PubMed] [Google Scholar]
- 70.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab 86: 2505–2512, 2001. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 71.Beranger GE, Karbiener M, Barquissau V, Pisani DF, Scheideler M, Langin D, Amri EZ. In vitro brown and “brite”/“beige” adipogenesis: human cellular models and molecular aspects. Biochim Biophys Acta 1831: 905–914, 2013. doi: 10.1016/j.bbalip.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Berger SL. The complex language of chromatin regulation during transcription. Nature 447: 407–412, 2007. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 73.Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev 23: 781–783, 2009. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergman Y, Cedar H. DNA methylation dynamics in health and disease. Nat Struct Mol Biol 20: 274–281, 2013. doi: 10.1038/nsmb.2518. [DOI] [PubMed] [Google Scholar]
- 75.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science 324: 98–102, 2009. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berkelhamer SK, Farrow KN. Developmental regulation of antioxidant enzymes and their impact on neonatal lung disease. Antioxid Redox Signal 21: 1837–1848, 2014. doi: 10.1089/ars.2013.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berkelhamer SK, Kim GA, Radder JE, Wedgwood S, Czech L, Steinhorn RH, Schumacker PT. Developmental differences in hyperoxia-induced oxidative stress and cellular responses in the murine lung. Free Radic Biol Med 61: 51–60, 2013. doi: 10.1016/j.freeradbiomed.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell 128: 669–681, 2007. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 79.Bernstein IM, Ziegler WF, Leavitt T, Badger GJ. Uterine artery hemodynamic adaptations through the menstrual cycle into early pregnancy. Obstet Gynecol 99: 620–624, 2002. [DOI] [PubMed] [Google Scholar]
- 80.Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull 60: 103–121, 2001. doi: 10.1093/bmb/60.1.103. [DOI] [PubMed] [Google Scholar]
- 81.Bhutani N, Burns DM, Blau HM. DNA demethylation dynamics. Cell 146: 866–872, 2011. doi: 10.1016/j.cell.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bierer R, Nitta CH, Friedman J, Codianni S, de Frutos S, Dominguez-Bautista JA, Howard TA, Resta TC, Bosc LV. NFATc3 is required for chronic hypoxia-induced pulmonary hypertension in adult and neonatal mice. Am J Physiol Lung Cell Mol Physiol 301: L872–L880, 2011. doi: 10.1152/ajplung.00405.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bigham AW, Julian CG, Wilson MJ, Vargas E, Browne VA, Shriver MD, Moore LG. Maternal PRKAA1 and EDNRA genotypes are associated with birth weight, and PRKAA1 with uterine artery diameter and metabolic homeostasis at high altitude. Physiol Genomics 46: 687–697, 2014. doi: 10.1152/physiolgenomics.00063.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bijli KM, Kleinhenz JM, Murphy TC, Kang BY, Adesina SE, Sutliff RL, Hart CM. PPARγ depletion stimulates Nox4 expression and human pulmonary artery smooth muscle cell proliferation. Free Radic Biol Med 80: 111–120, 2015. doi: 10.1016/j.freeradbiomed.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bird A. Perceptions of epigenetics. Nature 447: 396–398, 2007. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 86.Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, Yull FE, Prince LS. NF-κB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol 187: 2740–2747, 2011. doi: 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blair JD, Yuen RK, Lim BK, McFadden DE, von Dadelszen P, Robinson WP. Widespread DNA hypomethylation at gene enhancer regions in placentas associated with early-onset pre-eclampsia. Mol Hum Reprod 19: 697–708, 2013. doi: 10.1093/molehr/gat044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bleier L, Dröse S. Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochim Biophys Acta 1827: 1320–1331, 2013. doi: 10.1016/j.bbabio.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 89.Bleyer WA, Hakami N, Shepard TH. The development of hemostasis in the human fetus and newborn infant. J Pediatr 79: 838–853, 1971. doi: 10.1016/S0022-3476(71)80405-5. [DOI] [PubMed] [Google Scholar]
- 90.Blum-Johnston C, Thorpe RB, Wee C, Romero M, Brunelle A, Blood Q, Wilson R, Blood AB, Francis M, Taylor MS, Longo LD, Pearce WJ, Wilson SM. Developmental acceleration of bradykinin-dependent relaxation by prenatal chronic hypoxia impedes normal development after birth. Am J Physiol Lung Cell Mol Physiol 310: L271–L286, 2016. doi: 10.1152/ajplung.00340.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS. Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Ann N Y Acad Sci 1021: 376–383, 2004. doi: 10.1196/annals.1308.048. [DOI] [PubMed] [Google Scholar]
- 92.Bocking AD, McMillen IC, Harding R, Thorburn GD. Effect of reduced uterine blood flow on fetal and maternal cortisol. J Dev Physiol 8: 237–245, 1986. [PubMed] [Google Scholar]
- 93.Boddy K, Jones CT, Mantell C, Ratcliffe JG, Robinson JS. Changes in plasma ACTH and corticosteroid of the maternal and fetal sheep during hypoxia. Endocrinology 94: 588–591, 1974. doi: 10.1210/endo-94-2-588. [DOI] [PubMed] [Google Scholar]
- 94.Boero JA, Ascher J, Arregui A, Rovainen C, Woolsey TA. Increased brain capillaries in chronic hypoxia. J Appl Physiol (1985) 86: 1211–1219, 1999. doi: 10.1152/jappl.1999.86.4.1211. [DOI] [PubMed] [Google Scholar]
- 95.Borengasser SJ, Zhong Y, Kang P, Lindsey F, Ronis MJ, Badger TM, Gomez-Acevedo H, Shankar K. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 154: 4113–4125, 2013. doi: 10.1210/en.2012-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Botting KJ, McMillen IC, Forbes H, Nyengaard JR, Morrison JL. Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes. J Am Heart Assoc 3: e000531, 2014. doi: 10.1161/JAHA.113.000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bouloumié A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res 41: 773–780, 1999. doi: 10.1016/S0008-6363(98)00228-4. [DOI] [PubMed] [Google Scholar]
- 98.Bouret SG, Simerly RB. Minireview: leptin and development of hypothalamic feeding circuits. Endocrinology 145: 2621–2626, 2004. doi: 10.1210/en.2004-0231. [DOI] [PubMed] [Google Scholar]
- 99.Bourque SL, Gragasin FS, Quon AL, Mansour Y, Morton JS, Davidge ST. Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male, but not female, offspring. Hypertension 62: 753–758, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01516. [DOI] [PubMed] [Google Scholar]
- 100.Bourque SL, Kuny S, Reyes LM, Davidge ST, Sauvé Y. Prenatal hypoxia is associated with long-term retinal dysfunction in rats. PLoS One 8: e61861, 2013. doi: 10.1371/journal.pone.0061861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bowers RR, Festuccia WT, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol 286: R1167–R1175, 2004. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- 102.Boyle JW, Lotgering FK, Longo LD. Acute embolization of the uteroplacental circulation: uterine blood flow and placental CO diffusing capacity. J Dev Physiol 6: 377–386, 1984. [PubMed] [Google Scholar]
- 103.Braems G. Fetal hypoxemia on a molecular level: adaptive changes in the hypothalamic-pituitary-adrenal (HPA) axis and the lungs. Eur J Obstet Gynecol Reprod Biol 110, Suppl 1: S63–S69, 2003. doi: 10.1016/S0301-2115(03)00174-X. [DOI] [PubMed] [Google Scholar]
- 104.Braems GA, Han VK, Challis JR. Gestational age-dependent changes in the levels of mRNAs encoding cortisol biosynthetic enzymes and IGF-II in the adrenal gland of fetal sheep during prolonged hypoxemia. J Endocrinol 159: 257–264, 1998. doi: 10.1677/joe.0.1590257. [DOI] [PubMed] [Google Scholar]
- 105.Braems GA, Matthews SG, Challis JR. Differential regulation of proopiomelanocortin messenger ribonucleic acid in the pars distalis and pars intermedia of the pituitary gland after prolonged hypoxemia in fetal sheep. Endocrinology 137: 2731–2738, 1996. doi: 10.1210/endo.137.7.8770892. [DOI] [PubMed] [Google Scholar]
- 106.Brain KL, Allison BJ, Niu Y, Cross CM, Itani N, Kane AD, Herrera EA, Giussani DA. Induction of controlled hypoxic pregnancy in large mammalian species. Physiol Rep 3: e12614, 2015. doi: 10.14814/phy2.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Breton C. Role of maternal nutrition in programming adiposity in the offspring: potential implications of glucocorticoids. Horm Mol Biol Clin Investig 14: 33–47, 2013. doi: 10.1515/hmbci-2013-0010. [DOI] [PubMed] [Google Scholar]
- 108.Broberg CS, Giraud GD, Schultz JM, Thornburg KL, Hohimer AR, Davis LE. Fetal anemia leads to augmented contractile response to hypoxic stress in adulthood. Am J Physiol Regul Integr Comp Physiol 285: R649–R655, 2003. doi: 10.1152/ajpregu.00656.2002. [DOI] [PubMed] [Google Scholar]
- 109.Browne VA, Julian CG, Toledo-Jaldin L, Cioffi-Ragan D, Vargas E, Moore LG. Uterine artery blood flow, fetal hypoxia and fetal growth. Philos Trans R Soc Lond B Biol Sci 370: 20140068, 2015. doi: 10.1098/rstb.2014.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Browne VA, Toledo-Jaldin L, Davila RD, Lopez LP, Yamashiro H, Cioffi-Ragan D, Julian CG, Wilson MJ, Bigham AW, Shriver MD, Honigman B, Vargas E, Roach R, Moore LG. High-end arteriolar resistance limits uterine artery blood flow and restricts fetal growth in preeclampsia and gestational hypertension at high altitude. Am J Physiol Regul Integr Comp Physiol 300: R1221–R1229, 2011. doi: 10.1152/ajpregu.91046.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brunton PJ, Russell JA. Neuroendocrine control of maternal stress responses and fetal programming by stress in pregnancy. Prog Neuropsychopharmacol Biol Psychiatry 35: 1178–1191, 2011. doi: 10.1016/j.pnpbp.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 112.Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol 22: 258–271, 2010. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- 113.Bubb KJ, Cock ML, Black MJ, Dodic M, Boon WM, Parkington HC, Harding R, Tare M. Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J Physiol 578: 871–881, 2007. doi: 10.1113/jphysiol.2006.121160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buchholz J, Duckles SP. Chronic hypoxia alters prejunctional alpha(2)-receptor function in vascular adrenergic nerves of adult and fetal sheep. Am J Physiol Regul Integr Comp Physiol 281: R926–R934, 2001. doi: 10.1152/ajpregu.2001.281.3.R926. [DOI] [PubMed] [Google Scholar]
- 115.Buchholz J, Edwards-Teunissen K, Duckles SP. Impact of development and chronic hypoxia on NE release from adrenergic nerves in sheep arteries. Am J Physiol Regul Integr Comp Physiol 276: R799–R808, 1999. [DOI] [PubMed] [Google Scholar]
- 116.Buckley NM. Maturation of circulatory system in three mammalian models of human development. Comp Biochem Physiol A 83: 1–7, 1986. doi: 10.1016/0300-9629(86)90080-0. [DOI] [PubMed] [Google Scholar]
- 117.Burdick CJ, Taylor BA. Histone acetylation during early stages of sea urchin (Arbacia punctulata) development. Exp Cell Res 100: 428–433, 1976. doi: 10.1016/0014-4827(76)90174-9. [DOI] [PubMed] [Google Scholar]
- 118.Burstyn I, Wang X, Yasui Y, Sithole F, Zwaigenbaum L. Autism spectrum disorders and fetal hypoxia in a population-based cohort: accounting for missing exposures via Estimation-Maximization algorithm. BMC Med Res Methodol 11: 2, 2011. doi: 10.1186/1471-2288-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burton GJ, Charnock-Jones DS, Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction 138: 895–902, 2009. doi: 10.1530/REP-09-0092. [DOI] [PubMed] [Google Scholar]
- 120.Burton GJ, Fowden AL, Thornburg KL. Placental Origins of Chronic Disease. Physiol Rev 96: 1509–1565, 2016. doi: 10.1152/physrev.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30: 473–482, 2009. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Butler SM, Abrassart JM, Hubbell MC, Adeoye O, Semotiuk A, Williams JM, Mata-Greenwood E, Khorram O, Pearce WJ. Contributions of VEGF to age-dependent transmural gradients in contractile protein expression in ovine carotid arteries. Am J Physiol Cell Physiol 301: C653–C666, 2011. doi: 10.1152/ajpcell.00413.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cai Q, Wang T, Yang WJ, Fen X. Protective mechanisms of microRNA-27a against oxygen-glucose deprivation-induced injuries in hippocampal neurons. Neural Regen Res 11: 1285–1292, 2016. doi: 10.4103/1673-5374.189194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Calkins K, Devaskar SU. Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care 41: 158–176, 2011. doi: 10.1016/j.cppeds.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Calvert JW, Lefer DJ, Gundewar S, Poston L, Coetzee WA. Developmental programming resulting from maternal obesity in mice: effects on myocardial ischaemia-reperfusion injury. Exp Physiol 94: 805–814, 2009. doi: 10.1113/expphysiol.2009.047183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Camm EJ, Hansell JA, Kane AD, Herrera EA, Lewis C, Wong S, Morrell NW, Giussani DA. Partial contributions of developmental hypoxia and undernutrition to prenatal alterations in somatic growth and cardiovascular structure and function. Am J Obstet Gynecol 203: 495.e24–495.e34, 2010. doi: 10.1016/j.ajog.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 127.Camm EJ, Martin-Gronert MS, Wright NL, Hansell JA, Ozanne SE, Giussani DA. Prenatal hypoxia independent of undernutrition promotes molecular markers of insulin resistance in adult offspring. FASEB J 25: 420–427, 2011. doi: 10.1096/fj.10-158188. [DOI] [PubMed] [Google Scholar]
- 128.Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest 105: 577–587, 2000. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cannon TD, van Erp TG, Rosso IM, Huttunen M, Lönnqvist J, Pirkola T, Salonen O, Valanne L, Poutanen VP, Standertskjöld-Nordenstam CG. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 59: 35–41, 2002. doi: 10.1001/archpsyc.59.1.35. [DOI] [PubMed] [Google Scholar]
- 130.Carr DJ, Wallace JM, Aitken RP, Milne JS, Martin JF, Zachary IC, Peebles DM, David AL. Peri- and Postnatal Effects of Prenatal Adenoviral VEGF Gene Therapy in Growth-Restricted Sheep. Biol Reprod 94: 142, 2016. doi: 10.1095/biolreprod.115.133744. [DOI] [PubMed] [Google Scholar]
- 131.Carson R, Monaghan-Nichols AP, DeFranco DB, Rudine AC. Effects of antenatal glucocorticoids on the developing brain. Steroids 114: 25–32, 2016. doi: 10.1016/j.steroids.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carter CW., Jr Histone packing in the nucleosome core particle of chromatin. Proc Natl Acad Sci USA 75: 3649–3653, 1978. doi: 10.1073/pnas.75.8.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Castro MI, Valego NK, Zehnder TJ, Rose JC. Bioactive-to-immunoreactive ACTH activity changes with severity of stress in late-gestation ovine fetus. Am J Physiol Endocrinol Metab 265: E68–E73, 1993. [DOI] [PubMed] [Google Scholar]
- 134.Caton D, Kalra PS. Endogenous hormones and regulation of uterine blood flow during pregnancy. Am J Physiol Regul Integr Comp Physiol 250: R365–R369, 1986. [DOI] [PubMed] [Google Scholar]
- 135.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147: 358–369, 2011. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320: 1224–1229, 2008. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Challis JR, Brooks AN. Maturation and activation of hypothalamic-pituitary adrenal function in fetal sheep. Endocr Rev 10: 182–204, 1989. doi: 10.1210/edrv-10-2-182. [DOI] [PubMed] [Google Scholar]
- 138.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21: 514–550, 2000. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 139.Chamberlain LH, Shipston MJ. The physiology of protein S-acylation. Physiol Rev 95: 341–376, 2015. doi: 10.1152/physrev.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev 59: 1–61, 1979. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 141.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle 9: 1072–1083, 2010. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation 19: 215–223, 2012. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chandel N, Budinger GR, Kemp RA, Schumacker PT. Inhibition of cytochrome-c oxidase activity during prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 268: L918–L925, 1995. [DOI] [PubMed] [Google Scholar]
- 144.Chandel NS, Budinger GR, Schumacker PT. Molecular oxygen modulates cytochrome c oxidase function. J Biol Chem 271: 18672–18677, 1996. doi: 10.1074/jbc.271.31.18672. [DOI] [PubMed] [Google Scholar]
- 145.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275: 25130–25138, 2000. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 146.Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci 28: 12107–12119, 2008. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chang K, Lubo Zhang. Review article: steroid hormones and uterine vascular adaptation to pregnancy. Reprod Sci 15: 336–348, 2008. doi: 10.1177/1933719108317975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chang K, Xiao D, Huang X, Longo LD, Zhang L. Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: role of ERK/PKC pathway. Am J Physiol Heart Circ Physiol 296: H1840–H1849, 2009. doi: 10.1152/ajpheart.00090.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chang K, Xiao D, Huang X, Xue Z, Yang S, Longo LD, Zhang L. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension 56: 750–757, 2010. doi: 10.1161/HYPERTENSIONAHA.110.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chang TY, Huang TS, Wang HW, Chang SJ, Lo HH, Chiu YL, Wang YL, Hsiao CD, Tsai CH, Chan CH, You RI, Wu CH, Tsai TN, Cheng SM, Cheng CC. miRNome traits analysis on endothelial lineage cells discloses biomarker potential circulating microRNAs which affect progenitor activities. BMC Genomics 15: 802, 2014. doi: 10.1186/1471-2164-15-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chao J, Wood JG, Blanco VG, Gonzalez NC. The systemic inflammation of alveolar hypoxia is initiated by alveolar macrophage-borne mediator(s). Am J Respir Cell Mol Biol 41: 573–582, 2009. doi: 10.1165/rcmb.2008-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Charles SM, Julian CG, Vargas E, Moore LG. Higher estrogen levels during pregnancy in Andean than European residents of high altitude suggest differences in aromatase activity. J Clin Endocrinol Metab 99: 2908–2916, 2014. doi: 10.1210/jc.2013-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chaturvedi P, Kalani A, Medina I, Familtseva A, Tyagi SC. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J Cell Mol Med 19: 2153–2161, 2015. doi: 10.1111/jcmm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chaturvedi P, Tyagi SC. Epigenetic silencing of TIMP4 in heart failure. J Cell Mol Med 20: 2089–2101, 2016. doi: 10.1111/jcmm.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cheishvili D, Boureau L, Szyf M. DNA demethylation and invasive cancer: implications for therapeutics. Br J Pharmacol 172: 2705–2715, 2015. doi: 10.1111/bph.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chelbi ST, Vaiman D. Genetic and epigenetic factors contribute to the onset of preeclampsia. Mol Cell Endocrinol 282: 120–129, 2008. doi: 10.1016/j.mce.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 157.Chelikani PK, Glimm DR, Kennelly JJ. Short communication: tissue distribution of leptin and leptin receptor mRNA in the bovine. J Dairy Sci 86: 2369–2372, 2003. doi: 10.3168/jds.S0022-0302(03)73830-2. [DOI] [PubMed] [Google Scholar]
- 158.Chen D, Zhou X, Zhu Y, Zhu T, Wang J. [Comparison study on uterine and umbilical artery blood flow during pregnancy at high altitude and at low altitude]. Zhonghua Fu Chan Ke Za Zhi 37: 69–71, 2002. [PubMed] [Google Scholar]
- 159.Chen F, Li X, Aquadro E, Haigh S, Zhou J, Stepp DW, Weintraub NL, Barman SA, Fulton DJR. Inhibition of histone deacetylase reduces transcription of NADPH oxidases and ROS production and ameliorates pulmonary arterial hypertension. Free Radic Biol Med 99: 167–178, 2016. doi: 10.1016/j.freeradbiomed.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, Hu X, Wang G, Lin Z, Wang S, Pu WT, Liao R, Wang DZ. mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res 112: 1557–1566, 2013. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW II, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA 98: 11114–11119, 2001. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen M, Dasgupta C, Xiong F, Zhang L. Epigenetic upregulation of large-conductance Ca2+-activated K+ channel expression in uterine vascular adaptation to pregnancy. Hypertension 64: 610–618, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen M, Xiao D, Hu XQ, Dasgupta C, Yang S, Zhang L. Hypoxia Represses ER-α Expression and Inhibits Estrogen-Induced Regulation of Ca2+-Activated K+ Channel Activity and Myogenic Tone in Ovine Uterine Arteries: Causal Role of DNA Methylation. Hypertension 66: 44–51, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen M, Xiong F, Zhang L. Promoter methylation of Egr-1 site contributes to fetal hypoxia-mediated PKCε gene repression in the developing heart. Am J Physiol Regul Integr Comp Physiol 304: R683–R689, 2013. doi: 10.1152/ajpregu.00461.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen Q, Xu J, Li L, Li H, Mao S, Zhang F, Zen K, Zhang CY, Zhang Q. MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis 5: e1132, 2014. doi: 10.1038/cddis.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen Q, Zhang F, Wang Y, Liu Z, Sun A, Zen K, Zhang CY, Zhang Q. The transcription factor c-Myc suppresses MiR-23b and MiR-27b transcription during fetal distress and increases the sensitivity of neurons to hypoxia-induced apoptosis. PLoS One 10: e0120217, 2015. doi: 10.1371/journal.pone.0120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen X, Chakravarty T, Zhang Y, Li X, Zhong JF, Wang C. Single-cell transcriptome and epigenomic reprogramming of cardiomyocyte-derived cardiac progenitor cells. Sci Data 3: 160079, 2016. doi: 10.1038/sdata.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen Y, Sharma RP, Costa RH, Costa E, Grayson DR. On the epigenetic regulation of the human reelin promoter. Nucleic Acids Res 30: 2930–2939, 2002. doi: 10.1093/nar/gkf401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen Y, Siegel F, Kipschull S, Haas B, Fröhlich H, Meister G, Pfeifer A. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun 4: 1769, 2013. doi: 10.1038/ncomms2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cheng CC, Lo HH, Huang TS, Cheng YC, Chang ST, Chang SJ, Wang HW. Genetic module and miRNome trait analyses reflect the distinct biological features of endothelial progenitor cells from different anatomic locations. BMC Genomics 13: 447, 2012. doi: 10.1186/1471-2164-13-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cheng Y, Lin CH, Chen JY, Li CH, Liu YT, Chen BC. Induction of Connective Tissue Growth Factor Expression by Hypoxia in Human Lung Fibroblasts via the MEKK1/MEK1/ERK1/GLI-1/GLI-2 and AP-1 Pathways. PLoS One 11: e0160593, 2016. doi: 10.1371/journal.pone.0160593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cherradi N, Capponi AM, Gaillard RC, Pralong FP. Decreased expression of steroidogenic acute regulatory protein: a novel mechanism participating in the leptin-induced inhibition of glucocorticoid biosynthesis. Endocrinology 142: 3302–3308, 2001. doi: 10.1210/endo.142.8.8341. [DOI] [PubMed] [Google Scholar]
- 173.Chintamaneni K, Bruder ED, Raff H. Effects of age on ACTH, corticosterone, glucose, insulin, and mRNA levels during intermittent hypoxia in the neonatal rat. Am J Physiol Regul Integr Comp Physiol 304: R782–R789, 2013. doi: 10.1152/ajpregu.00073.2013. [DOI] [PubMed] [Google Scholar]
- 174.Chintamaneni K, Bruder ED, Raff H. Programming of the hypothalamic-pituitary-adrenal axis by neonatal intermittent hypoxia: effects on adult male ACTH and corticosterone responses are stress specific. Endocrinology 155: 1763–1770, 2014. doi: 10.1210/en.2013-1736. [DOI] [PubMed] [Google Scholar]
- 175.Cho B, Kim HJ, Kim H, Sun W. Changes in the Histone Acetylation Patterns during the Development of the Nervous System. Exp Neurobiol 20: 81–84, 2011. doi: 10.5607/en.2011.20.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cho H-Y, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 8: 76–87, 2006. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 177.Chou HC, Chen CM. Maternal nicotine exposure during gestation and lactation induces cardiac remodeling in rat offspring. Reprod Toxicol 50: 4–10, 2014. doi: 10.1016/j.reprotox.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 178.Cindrova-Davies T, Herrera EA, Niu Y, Kingdom J, Giussani DA, Burton GJ. Reduced cystathionine γ-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: hydrogen sulfide as a placental vasodilator. Am J Pathol 182: 1448–1458, 2013. doi: 10.1016/j.ajpath.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cloonan SM, Choi AM. Mitochondria in lung disease. J Clin Invest 126: 809–820, 2016. doi: 10.1172/JCI81113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cnattingius S, Bergström R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med 338: 147–152, 1998. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 181.Cockayne D, Sterling KM Jr, Shull S, Mintz KP, Illeyne S, Cutroneo KR. Glucocorticoids decrease the synthesis of type I procollagen mRNAs. Biochemistry 25: 3202–3209, 1986. doi: 10.1021/bi00359a018. [DOI] [PubMed] [Google Scholar]
- 182.Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol 120: 817–824, 1974. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- 183.Colleoni F, Padmanabhan N, Yung HW, Watson ED, Cetin I, Tissot van Patot MC, Burton GJ, Murray AJ. Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: a role for miRNA-210 and protein synthesis inhibition. PLoS One 8: e55194, 2013. doi: 10.1371/journal.pone.0055194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Coney AM, Marshall JM. Effects of maternal hypoxia on muscle vasodilatation evoked by acute systemic hypoxia in adult rat offspring: changed roles of adenosine and A1 receptors. J Physiol 588: 5115–5125, 2010. doi: 10.1113/jphysiol.2010.198275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Constantinof A, Moisiadis VG, Matthews SG. Programming of stress pathways: a transgenerational perspective. J Steroid Biochem Mol Biol 160: 175–180, 2016. doi: 10.1016/j.jsbmb.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 186.Correia-Branco A, Keating E, Martel F. Maternal undernutrition and fetal developmental programming of obesity: the glucocorticoid connection. Reprod Sci 22: 138–145, 2015. doi: 10.1177/1933719114542012. [DOI] [PubMed] [Google Scholar]
- 187.Corstius HB, Zimanyi MA, Maka N, Herath T, Thomas W, van der Laarse A, Wreford NG, Black MJ. Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr Res 57: 796–800, 2005. doi: 10.1203/01.PDR.0000157726.65492.CD. [DOI] [PubMed] [Google Scholar]
- 188.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149: 6018–6027, 2008. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 189.Costa E, Chen Y, Davis J, Dong E, Noh JS, Tremolizzo L, Veldic M, Grayson DR, Guidotti A. REELIN and schizophrenia: a disease at the interface of the genome and the epigenome. Mol Interv 2: 47–57, 2002. doi: 10.1124/mi.2.1.47. [DOI] [PubMed] [Google Scholar]
- 190.Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, Graham CH. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med 211: 165–179, 2014. doi: 10.1084/jem.20130295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 3: 19, 2009. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Coulson RJ, Chesler NC, Vitullo L, Cipolla MJ. Effects of ischemia and myogenic activity on active and passive mechanical properties of rat cerebral arteries. Am J Physiol Heart Circ Physiol 283: H2268–H2275, 2002. doi: 10.1152/ajpheart.00542.2002. [DOI] [PubMed] [Google Scholar]
- 193.Coussons-Read ME, Mazzeo RS, Whitford MH, Schmitt M, Moore LG, Zamudio S. High altitude residence during pregnancy alters cytokine and catecholamine levels. Am J Reprod Immunol 48: 344–354, 2002. doi: 10.1034/j.1600-0897.2002.01078.x. [DOI] [PubMed] [Google Scholar]
- 194.Cox-Limpens KE, Vles JS, Schlechter J, Zimmermann LJ, Strackx E, Gavilanes AW. Fetal brain genomic reprogramming following asphyctic preconditioning. BMC Neurosci 14: 61, 2013. doi: 10.1186/1471-2202-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Crespi EJ, Unkefer MK. Development of food intake controls: neuroendocrine and environmental regulation of food intake during early life. Horm Behav 66: 74–85, 2014. doi: 10.1016/j.yhbeh.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 196.Crews D. Epigenetics and its implications for behavioral neuroendocrinology. Front Neuroendocrinol 29: 344–357, 2008. doi: 10.1016/j.yfrne.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Crews D. Epigenetics, brain, behavior, and the environment. Hormones (Athens) 9: 41–50, 2010. doi: 10.14310/horm.2002.1251. [DOI] [PubMed] [Google Scholar]
- 198.Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA 104: 5942–5946, 2007. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Crews D, Lou W, Fleming A, Ogawa S. From gene networks underlying sex determination and gonadal differentiation to the development of neural networks regulating sociosexual behavior. Brain Res 1126: 109–121, 2006. doi: 10.1016/j.brainres.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch 450: 363–371, 2005. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- 201.Dabrowski M, Aerts S, Van Hummelen P, Craessaerts K, De Moor B, Annaert W, Moreau Y, De Strooper B. Gene profiling of hippocampal neuronal culture. J Neurochem 85: 1279–1288, 2003. doi: 10.1046/j.1471-4159.2003.01753.x. [DOI] [PubMed] [Google Scholar]
- 202.Dahl JA, Jung I, Aanes H, Greggains GD, Manaf A, Lerdrup M, Li G, Kuan S, Li B, Lee AY, Preissl S, Jermstad I, Haugen MH, Suganthan R, Bjørås M, Hansen K, Dalen KT, Fedorcsak P, Ren B, Klungland A. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature 537: 548–552, 2016. doi: 10.1038/nature19360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dasgupta C, Chen M, Zhang H, Yang S, Zhang L. Chronic hypoxia during gestation causes epigenetic repression of the estrogen receptor-α gene in ovine uterine arteries via heightened promoter methylation. Hypertension 60: 697–704, 2012. doi: 10.1161/HYPERTENSIONAHA.112.198242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Datta A, Kim GA, Taylor JM, Gugino SF, Farrow KN, Schumacker PT, Berkelhamer SK. Mouse lung development and NOX1 induction during hyperoxia are developmentally regulated and mitochondrial ROS dependent. Am J Physiol Lung Cell Mol Physiol 309: L369–L377, 2015. doi: 10.1152/ajplung.00176.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dávila RD, Julian CG, Browne VA, Toledo-Jaldin L, Wilson MJ, Rodriguez A, Vargas E, Moore LG. Role of cytokines in altitude-associated preeclampsia. Pregnancy Hypertens 2: 65–70, 2012. doi: 10.1016/j.preghy.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dávila RD, Julian CG, Wilson MJ, Browne VA, Rodriguez C, Bigham AW, Shriver MD, Vargas E, Moore LG. Do anti-angiogenic or angiogenic factors contribute to the protection of birth weight at high altitude afforded by Andean ancestry? Reprod Sci 17: 861–870, 2010. doi: 10.1177/1933719110372418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dávila RD, Julian CG, Wilson MJ, Browne VA, Rodriguez C, Bigham AW, Shriver MD, Vargas E, Moore LG. Do cytokines contribute to the Andean-associated protection from reduced fetal growth at high altitude? Reprod Sci 18: 79–87, 2011. doi: 10.1177/1933719110380061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med 15: 191–195, 2010. doi: 10.1016/j.siny.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 209.Davis L, Roullet JB, Thornburg KL, Shokry M, Hohimer AR, Giraud GD. Augmentation of coronary conductance in adult sheep made anaemic during fetal life. J Physiol 547: 53–59, 2003. doi: 10.1113/jphysiol.2002.023283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dawlaty MM, Breiling A, Le T, Barrasa MI, Raddatz G, Gao Q, Powell BE, Cheng AW, Faull KF, Lyko F, Jaenisch R. Loss of Tet enzymes compromises proper differentiation of embryonic stem cells. Dev Cell 29: 102–111, 2014. doi: 10.1016/j.devcel.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dawlaty MM, Breiling A, Le T, Raddatz G, Barrasa MI, Cheng AW, Gao Q, Powell BE, Li Z, Xu M, Faull KF, Lyko F, Jaenisch R. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev Cell 24: 310–323, 2013. doi: 10.1016/j.devcel.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.De Lella Ezcurra AL, Bertolin AP, Melani M, Wappner P. Robustness of the hypoxic response: another job for miRNAs? Dev Dyn 241: 1842–1848, 2012. doi: 10.1002/dvdy.23865. [DOI] [PubMed] [Google Scholar]
- 213.De Vos P, Lefebvre AM, Shrivo I, Fruchart JC, Auwerx J. Glucocorticoids induce the expression of the leptin gene through a non-classical mechanism of transcriptional activation. Eur J Biochem 253: 619–626, 1998. doi: 10.1046/j.1432-1327.1998.2530619.x. [DOI] [PubMed] [Google Scholar]
- 214.Degner K, Magness RR, Shah DM. Establishment of the Human Uteroplacental Circulation: A Historical Perspective. Reprod Sci 24: 753–761, 2017. doi: 10.1177/1933719116669056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology 149: 470–475, 2008. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- 216.Delaney C, Gien J, Roe G, Isenberg N, Kailey J, Abman SH. Serotonin contributes to high pulmonary vascular tone in a sheep model of persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 304: L894–L901, 2013. doi: 10.1152/ajplung.00043.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.DeLaughter DM, Bick AG, Wakimoto H, McKean D, Gorham JM, Kathiriya IS, Hinson JT, Homsy J, Gray J, Pu W, Bruneau BG, Seidman JG, Seidman CE. Single-Cell Resolution of Temporal Gene Expression during Heart Development. Dev Cell 39: 480–490, 2016. doi: 10.1016/j.devcel.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Demiryürek AT, Karamsetty MR, McPhaden AR, Wadsworth RM, Kane KA, MacLean MR. Accumulation of nitrotyrosine correlates with endothelial NO synthase in pulmonary resistance arteries during chronic hypoxia in the rat. Pulm Pharmacol Ther 13: 157–165, 2000. doi: 10.1006/pupt.2000.0238. [DOI] [PubMed] [Google Scholar]
- 219.Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L596–L607, 2009. doi: 10.1152/ajplung.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Derghal A, Djelloul M, Airault C, Pierre C, Dallaporta M, Troadec JD, Tillement V, Tardivel C, Bariohay B, Trouslard J, Mounien L. Leptin is required for hypothalamic regulation of miRNAs targeting POMC 3'UTR. Front Cell Neurosci 9: 172, 2015. doi: 10.3389/fncel.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Devaskar SU, Anthony R, Hay W Jr. Ontogeny and insulin regulation of fetal ovine white adipose tissue leptin expression. Am J Physiol Regul Integr Comp Physiol 282: R431–R438, 2002. doi: 10.1152/ajpregu.2002.282.2.R431. [DOI] [PubMed] [Google Scholar]
- 222.Dikalova A, Aschner JL, Kaplowitz MR, Summar M, Fike CD. Tetrahydrobiopterin oral therapy recouples eNOS and ameliorates chronic hypoxia-induced pulmonary hypertension in newborn pigs. Am J Physiol Lung Cell Mol Physiol 311: L743–L753, 2016. doi: 10.1152/ajplung.00238.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev 3: 79–83, 1979. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 224.Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child 48: 757–767, 1973. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dodd IB, Micheelsen MA, Sneppen K, Thon G. Theoretical analysis of epigenetic cell memory by nucleosome modification. Cell 129: 813–822, 2007. doi: 10.1016/j.cell.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 226.Dong E, Agis-Balboa RC, Simonini MV, Grayson DR, Costa E, Guidotti A. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc Natl Acad Sci USA 102: 12578–12583, 2005. doi: 10.1073/pnas.0505394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc Natl Acad Sci USA 104: 4676–4681, 2007. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dong F, Ford SP, Nijland MJ, Nathanielsz PW, Ren J. Influence of maternal undernutrition and overfeeding on cardiac ciliary neurotrophic factor receptor and ventricular size in fetal sheep. J Nutr Biochem 19: 409–414, 2008. doi: 10.1016/j.jnutbio.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Doridot L, Houry D, Gaillard H, Chelbi ST, Barbaux S, Vaiman D. miR-34a expression, epigenetic regulation, and function in human placental diseases. Epigenetics 9: 142–151, 2014. doi: 10.4161/epi.26196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Drake AJ, Walker BR. The intergenerational effects of fetal programming: non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. J Endocrinol 180: 1–16, 2004. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- 231.Dubrovskaya NM, Zhuravin IA. Ontogenetic characteristics of behavior in rats subjected to hypoxia on day 14 or day 18 of embryogenesis. Neurosci Behav Physiol 40: 231–238, 2010. doi: 10.1007/s11055-009-9235-2. [DOI] [PubMed] [Google Scholar]
- 232.Ducsay CA. Fetal and maternal adaptations to chronic hypoxia: prevention of premature labor in response to chronic stress. Comp Biochem Physiol A Mol Integr Physiol 119: 675–681, 1998. doi: 10.1016/S1095-6433(98)01004-6. [DOI] [PubMed] [Google Scholar]
- 233.Ducsay CA, Furuta K, Vargas VE, Kaushal KM, Singleton K, Hyatt K, Myers DA. Leptin receptor antagonist treatment ameliorates the effects of long-term maternal hypoxia on adrenal expression of key steroidogenic genes in the ovine fetus. Am J Physiol Regul Integr Comp Physiol 304: R435–R442, 2013. doi: 10.1152/ajpregu.00377.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ducsay CA, Hyatt K, Mlynarczyk M, Kaushal KM, Myers DA. Long-term hypoxia increases leptin receptors and plasma leptin concentrations in the late-gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 291: R1406–R1413, 2006. doi: 10.1152/ajpregu.00077.2006. [DOI] [PubMed] [Google Scholar]
- 235.Ducsay CA, Hyatt K, Mlynarczyk M, Root BK, Kaushal KM, Myers DA. Long-term hypoxia modulates expression of key genes regulating adrenomedullary function in the late gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 293: R1997–R2005, 2007. doi: 10.1152/ajpregu.00313.2007. [DOI] [PubMed] [Google Scholar]
- 236.Ducsay CA, Kaushal KM, Zhang L, Dasgupta C, Myers DA. Long term hypoxia (LTH) alters microRNA (miR) and gene expression affecting beige phenotype in perinatal adipose tissue in the transition fro fetus to lamb. Reprod Sci 22: 1A–54A, 2015. doi: 10.1177/1933719115579630. [DOI] [Google Scholar]
- 237.Ducsay CA, Myers DA. eNOS activation and NO function: differential control of steroidogenesis by nitric oxide and its adaptation with hypoxia. J Endocrinol 210: 259–269, 2011. doi: 10.1530/JOE-11-0034. [DOI] [PubMed] [Google Scholar]
- 238.Ducsay CA, Newby EA, Cato C, Singleton K, Myers DA. Long term hypoxia during gestation alters perirenal adipose tissue in the lamb: a trigger for adiposity? J Dev Orig Health Dis 4: 1194, 2013. [Google Scholar]
- 239.Duffield JA, Vuocolo T, Tellam R, Yuen BSJ, Muhlhausler BS, McMillen IC. Placental restriction of fetal growth decreases IGF1 and leptin mRNA expression in the perirenal adipose tissue of late gestation fetal sheep. Am J Physiol Regul Integr Comp Physiol 294: R1413–R1419, 2008. doi: 10.1152/ajpregu.00787.2007. [DOI] [PubMed] [Google Scholar]
- 240.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci USA 104: 15081–15086, 2007. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dunham-Snary KJ, Wu D, Sykes EA, Thakrar A, Parlow LR, Mewburn JD, Parlow JL, Archer SL. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 151: 181–192, 2017. doi: 10.1016/j.chest.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Durakoglugil MS, Chen Y, White CL, Kavalali ET, Herz J. Reelin signaling antagonizes beta-amyloid at the synapse. Proc Natl Acad Sci USA 106: 15938–15943, 2009. doi: 10.1073/pnas.0908176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Durrant LM, Khorram O, Buchholz JN, Pearce WJ. Maternal food restriction modulates cerebrovascular structure and contractility in adult rat offspring: effects of metyrapone. Am J Physiol Regul Integr Comp Physiol 306: R401–R410, 2014. doi: 10.1152/ajpregu.00436.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Eastman NJ. Mount Everest in utero. Am J Obstet Gynecol 67: 701–711, 1954. doi: 10.1016/0002-9378(54)90098-8. [DOI] [PubMed] [Google Scholar]
- 245.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell 149: 515–524, 2012. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23: 775–786, 1999. doi: 10.1016/S0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 247.Elmes MJ, Gardner DS, Langley-Evans SC. Fetal exposure to a maternal low-protein diet is associated with altered left ventricular pressure response to ischaemia-reperfusion injury. Br J Nutr 98: 93–100, 2007. doi: 10.1017/S000711450769182X. [DOI] [PubMed] [Google Scholar]
- 248.Elmes MJ, Haase A, Gardner DS, Langley-Evans SC. Sex differences in sensitivity to beta-adrenergic agonist isoproterenol in the isolated adult rat heart following prenatal protein restriction. Br J Nutr 101: 725–734, 2009. doi: 10.1017/S0007114508025075. [DOI] [PubMed] [Google Scholar]
- 249.Elmes MJ, McMullen S, Gardner DS, Langley-Evans SC. Prenatal diet determines susceptibility to cardiac ischaemia-reperfusion injury following treatment with diethylmaleic acid and N-acetylcysteine. Life Sci 82: 149–155, 2008. doi: 10.1016/j.lfs.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 250.Epstein JA. Franklin H. Epstein Lecture. Cardiac development and implications for heart disease. N Engl J Med 363: 1638–1647, 2010. doi: 10.1056/NEJMra1003941. [DOI] [PubMed] [Google Scholar]
- 251.Ergaz Z, Neeman-Azulay M, Weinstein-Fudim L, Weksler-Zangen S, Shoshani-Dror D, Szyf M, Ornoy A. Diabetes in the Cohen Rat Intensifies the Fetal Pancreatic Damage Induced by the Diabetogenic High Sucrose Low Copper Diet. Birth Defects Res B Dev Reprod Toxicol 107: 21–31, 2016. doi: 10.1002/bdrb.21169. [DOI] [PubMed] [Google Scholar]
- 252.Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, Jain R, Dean NM, Freier SM, Bennett CF, Lollo B, Griffey R. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem 279: 52361–52365, 2004. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 253.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492: 376–381, 2012. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 253a.Eunice Kennedy Shriver National Institute of Child Health and Human Development New White Paper: Scientific Vision Workshop on Developmental Origins of Health and Disease. Bethesda, MD: NICHD, 2011. [Google Scholar]
- 254.Evans LC, Liu H, Pinkas GA, Thompson LP. Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts. Pediatr Res 71: 25–31, 2012. doi: 10.1038/pr.2011.10. [DOI] [PubMed] [Google Scholar]
- 255.Faa G, Manchia M, Pintus R, Gerosa C, Marcialis MA, Fanos V. Fetal programming of neuropsychiatric disorders. Birth Defects Res C Embryo Today 108: 207–223, 2016. doi: 10.1002/bdrc.21139. [DOI] [PubMed] [Google Scholar]
- 256.Faa G, Marcialis MA, Ravarino A, Piras M, Pintus MC, Fanos V. Fetal programming of the human brain: is there a link with insurgence of neurodegenerative disorders in adulthood? Curr Med Chem 21: 3854–3876, 2014. doi: 10.2174/0929867321666140601163658. [DOI] [PubMed] [Google Scholar]
- 257.Fabbri E, Manicardi A, Tedeschi T, Sforza S, Bianchi N, Brognara E, Finotti A, Breveglieri G, Borgatti M, Corradini R, Marchelli R, Gambari R. Modulation of the biological activity of microRNA-210 with peptide nucleic acids (PNAs). ChemMedChem 6: 2192–2202, 2011. doi: 10.1002/cmdc.201100270. [DOI] [PubMed] [Google Scholar]
- 258.Fajardo G, Zhao M, Powers J, Bernstein D. Differential cardiotoxic/cardioprotective effects of beta-adrenergic receptor subtypes in myocytes and fibroblasts in doxorubicin cardiomyopathy. J Mol Cell Cardiol 40: 375–383, 2006. doi: 10.1016/j.yjmcc.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Fan JM, Chen XQ, Jin H, Du JZ. Gestational hypoxia alone or combined with restraint sensitizes the hypothalamic-pituitary-adrenal axis and induces anxiety-like behavior in adult male rat offspring. Neuroscience 159: 1363–1373, 2009. doi: 10.1016/j.neuroscience.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 260.Fang J, Song XW, Tian J, Chen HY, Li DF, Wang JF, Ren AJ, Yuan WJ, Lin L. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis 17: 410–423, 2012. doi: 10.1007/s10495-011-0683-0. [DOI] [PubMed] [Google Scholar]
- 261.Fang JH, Zhou HC, Zeng C, Yang J, Liu Y, Huang X, Zhang JP, Guan XY, Zhuang SM. MicroRNA-29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology 54: 1729–1740, 2011. doi: 10.1002/hep.24577. [DOI] [PubMed] [Google Scholar]
- 262.Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JX. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 285: L1233–L1245, 2003. doi: 10.1152/ajplung.00445.2002. [DOI] [PubMed] [Google Scholar]
- 263.Farah C, Reboul C. NO Better Way to Protect the Heart during Ischemia-Reperfusion: To be in the Right Place at the Right Time. Front Pediatr 3: 6, 2015. doi: 10.3389/fped.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L979–L987, 2008. doi: 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Farrow KN, Lee KJ, Perez M, Schriewer JM, Wedgwood S, Lakshminrusimha S, Smith CL, Steinhorn RH, Schumacker PT. Brief hyperoxia increases mitochondrial oxidation and increases phosphodiesterase 5 activity in fetal pulmonary artery smooth muscle cells. Antioxid Redox Signal 17: 460–470, 2012. doi: 10.1089/ars.2011.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 283: 15878–15883, 2008. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet 13: 97–109, 2012. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- 268.Feng J, Fouse S, Fan G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res 61: 58R–63R, 2007. doi: 10.1203/pdr.0b013e3180457635. [DOI] [PubMed] [Google Scholar]
- 269.Feng X, Reini SA, Richards E, Wood CE, Keller-Wood M. Cortisol stimulates proliferation and apoptosis in the late gestation fetal heart: differential effects of mineralocorticoid and glucocorticoid receptors. Am J Physiol Regul Integr Comp Physiol 305: R343–R350, 2013. doi: 10.1152/ajpregu.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Feng Y, Yu D, Yang L, Da M, Wang Z, Lin Y, Ni B, Wang S, Mo X. Maternal lifestyle factors in pregnancy and congenital heart defects in offspring: review of the current evidence. Ital J Pediatr 40: 85, 2014. doi: 10.1186/s13052-014-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Fijalkowska I, Xu W, Comhair SAA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, Tuder RM. Hypoxia inducible-factor1α regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol 176: 1130–1138, 2010. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Fike CD, Aschner JL, Kaplowitz MR, Zhang Y, Madden JA. Reactive oxygen species scavengers improve voltage-gated K(+) channel function in pulmonary arteries of newborn pigs with progressive hypoxia-induced pulmonary hypertension. Pulm Circ 3: 551–563, 2013. doi: 10.1086/674307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Fike CD, Aschner JL, Slaughter JC, Kaplowitz MR, Zhang Y, Pfister SL. Pulmonary arterial responses to reactive oxygen species are altered in newborn piglets with chronic hypoxia-induced pulmonary hypertension. Pediatr Res 70: 136–141, 2011. doi: 10.1203/PDR.0b013e3182207ce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fike CD, Dikalova A, Kaplowitz MR, Cunningham G, Summar M, Aschner JL. Rescue Treatment with l-Citrulline Inhibits Hypoxia-Induced Pulmonary Hypertension in Newborn Pigs. Am J Respir Cell Mol Biol 53: 255–264, 2015. doi: 10.1165/rcmb.2014-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fike CD, Dikalova A, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species-reducing strategies improve pulmonary arterial responses to nitric oxide in piglets with chronic hypoxia-induced pulmonary hypertension. Antioxid Redox Signal 18: 1727–1738, 2013. doi: 10.1089/ars.2012.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26: 271–281, 2012. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Forhead AJ, Thomas L, Crabtree J, Hoggard N, Gardner DS, Giussani DA, Fowden AL. Plasma leptin concentration in fetal sheep during late gestation: ontogeny and effect of glucocorticoids. Endocrinology 143: 1166–1173, 2002. doi: 10.1210/endo.143.4.8762. [DOI] [PubMed] [Google Scholar]
- 278.Forsyth JK, Ellman LM, Tanskanen A, Mustonen U, Huttunen MO, Suvisaari J, Cannon TD. Genetic risk for schizophrenia, obstetric complications, and adolescent school outcome: evidence for gene-environment interaction. Schizophr Bull 39: 1067–1076, 2013. doi: 10.1093/schbul/sbs098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 21: 29–37, 2006. doi: 10.1152/physiol.00050.2005. [DOI] [PubMed] [Google Scholar]
- 280.Fraser M, Braems GA, Challis JR; CIHR Group in Fetal and Neonatal Health and Development . Developmental regulation of corticotrophin receptor gene expression in the adrenal gland of the ovine fetus and newborn lamb: effects of hypoxia during late pregnancy. J Endocrinol 169: 1–10, 2001. doi: 10.1677/joe.0.1690001. [DOI] [PubMed] [Google Scholar]
- 281.Fresquet F, Pourageaud F, Leblais V, Brandes RP, Savineau JP, Marthan R, Muller B. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. Br J Pharmacol 148: 714–723, 2006. doi: 10.1038/sj.bjp.0706779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Fu J, Chen YF, Zhao X, Creighton JR, Guo Y, Hage FG, Oparil S, Xing DD. Targeted delivery of pulmonary arterial endothelial cells overexpressing interleukin-8 receptors attenuates monocrotaline-induced pulmonary vascular remodeling. Arterioscler Thromb Vasc Biol 34: 1539–1547, 2014. doi: 10.1161/ATVBAHA.114.303821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Fu T, Seok S, Choi S, Huang Z, Suino-Powell K, Xu HE, Kemper B, Kemper JK. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Mol Cell Biol 34: 4130–4142, 2014. doi: 10.1128/MCB.00596-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol 16: 329–344, 2015. doi: 10.1038/nrm3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Gagnon R, Murotsuki J, Challis JR, Fraher L, Richardson BS. Fetal sheep endocrine responses to sustained hypoxemic stress after chronic fetal placental embolization. Am J Physiol Endocrinol Metab 272: E817–E823, 1997. [DOI] [PubMed] [Google Scholar]
- 286.Gan J, Sonntag HJ, Tang MK, Cai D, Lee KK. Integrative Analysis of the Developing Postnatal Mouse Heart Transcriptome. PLoS One 10: e0133288, 2015. doi: 10.1371/journal.pone.0133288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gao WL, Li D, Xiao ZX, Liao QP, Yang HX, Li YX, Ji L, Wang YL. Detection of global DNA methylation and paternally imprinted H19 gene methylation in preeclamptic placentas. Hypertens Res 34: 655–661, 2011. doi: 10.1038/hr.2011.9. [DOI] [PubMed] [Google Scholar]
- 288.Gao Y, Portugal AD, Negash S, Zhou W, Longo LD, Usha Raj J. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high altitude hypoxia. Am J Physiol Lung Cell Mol Physiol 292: L678–L684, 2007. doi: 10.1152/ajplung.00178.2006. [DOI] [PubMed] [Google Scholar]
- 289.Garat CV, Crossno JT Jr, Sullivan TM, Reusch JE, Klemm DJ. Inhibition of phosphatidylinositol 3-kinase/Akt signaling attenuates hypoxia-induced pulmonary artery remodeling and suppresses CREB depletion in arterial smooth muscle cells. J Cardiovasc Pharmacol 62: 539–548, 2013. doi: 10.1097/FJC.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Garcia CM, Darland DC, Massingham LJ, D’Amore PA. Endothelial cell-astrocyte interactions and TGF beta are required for induction of blood-neural barrier properties. Brain Res Dev Brain Res 152: 25–38, 2004. doi: 10.1016/j.devbrainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 291.García-Cardeña G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA 93: 6448–6453, 1996. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol 5: a013169, 2013. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gardner DS, Fletcher AJ, Bloomfield MR, Fowden AL, Giussani DA. Effects of prevailing hypoxaemia, acidaemia or hypoglycaemia upon the cardiovascular, endocrine and metabolic responses to acute hypoxaemia in the ovine fetus. J Physiol 540: 351–366, 2002. doi: 10.1113/jphysiol.2001.013434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gardner DS, Fletcher AJ, Fowden AL, Giussani DA. A novel method for controlled and reversible long term compression of the umbilical cord in fetal sheep. J Physiol 535: 217–229, 2001. doi: 10.1111/j.1469-7793.2001.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Gardner DS, Fletcher AJ, Fowden AL, Giussani DA. Plasma adrenocorticotropin and cortisol concentrations during acute hypoxemia after a reversible period of adverse intrauterine conditions in the ovine fetus during late gestation. Endocrinology 142: 589–598, 2001. doi: 10.1210/endo.142.2.7980. [DOI] [PubMed] [Google Scholar]
- 296.Garland CJ. Compromised vascular endothelial cell SK(Ca) activity: a fundamental aspect of hypertension? Br J Pharmacol 160: 833–835, 2010. doi: 10.1111/j.1476-5381.2010.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Garland CJ, Hiley CR, Dora KA. EDHF: spreading the influence of the endothelium. Br J Pharmacol 164: 839–852, 2011. doi: 10.1111/j.1476-5381.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Gassmann NN, van Elteren HA, Goos TG, Morales CR, Rivera-Ch M, Martin DS, Cabala Peralta P, Passano Del Carpio A, Aranibar Machaca S, Huicho L, Reiss IK, Gassmann M, de Jonge RC. Pregnancy at high altitude in the Andes leads to increased total vessel density in healthy newborns. J Appl Physiol (1985) 121: 709–715, 2016. doi: 10.1152/japplphysiol.00561.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Gay MS, Dasgupta C, Li Y, Kanna A, Zhang L. Dexamethasone Induces Cardiomyocyte Terminal Differentiation via Epigenetic Repression of Cyclin D2 Gene. J Pharmacol Exp Ther 358: 190–198, 2016. doi: 10.1124/jpet.116.234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gay MS, Li Y, Xiong F, Lin T, Zhang L. Dexamethasone Treatment of Newborn Rats Decreases Cardiomyocyte Endowment in the Developing Heart through Epigenetic Modifications. PLoS One 10: e0125033, 2015. doi: 10.1371/journal.pone.0125033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ge W, Hu N, George LA, Ford SP, Nathanielsz PW, Wang XM, Ren J. Maternal nutrient restriction predisposes ventricular remodeling in adult sheep offspring. J Nutr Biochem 24: 1258–1265, 2013. doi: 10.1016/j.jnutbio.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 302.Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, Nelson SM, Lawlor DA. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation 122: 1192–1199, 2010. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Gess B, Wolf K, Pfeifer M, Riegger GA, Kurtz A. In vivo carbon monoxide exposure and hypoxic hypoxia stimulate immediate early gene expression. Pflugers Arch 434: 568–574, 1997. doi: 10.1007/s004240050437. [DOI] [PubMed] [Google Scholar]
- 304.Gheorghe CP, Goyal R, Holweger JD, Longo LD. Placental gene expression responses to maternal protein restriction in the mouse. Placenta 30: 411–417, 2009. doi: 10.1016/j.placenta.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Gheorghe CP, Goyal R, Mittal A, Longo LD. Gene expression in the placenta: maternal stress and epigenetic responses. Int J Dev Biol 54: 507–523, 2010. doi: 10.1387/ijdb.082770cg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Gien J, Tseng N, Seedorf G, Roe G, Abman SH. Endothelin-1 impairs angiogenesis in vitro through Rho-kinase activation after chronic intrauterine pulmonary hypertension in fetal sheep. Pediatr Res 73: 252–262, 2013. doi: 10.1038/pr.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Gilbert RD, Pearce WJ, Longo LD. Fetal cardiac and cerebrovascular acclimatization responses to high altitude, long-term hypoxia. High Alt Med Biol 4: 203–213, 2003. doi: 10.1089/152702903322022802. [DOI] [PubMed] [Google Scholar]
- 308.Gilles FH, Leviton A, Dooling EC. The Developing Human Brain: Growth and Epidemiologic Neuropathology. Littleton, MA: John Wright, 1983, p. 343. [Google Scholar]
- 309.Giussani DA. The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol 594: 1215–1230, 2016. doi: 10.1113/JP271099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FB, Cross CM, Herrera EA. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One 7: e31017, 2012. doi: 10.1371/journal.pone.0031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Giussani DA, McGarrigle HH, Moore PJ, Bennet L, Spencer JAD, Hanson MA. Carotid sinus nerve section and the increase in plasma cortisol during acute hypoxia in fetal sheep. J Physiol 477: 75–80, 1994. doi: 10.1113/jphysiol.1994.sp020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Giussani DA, Phillips PS, Anstee S, Barker DJ. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res 49: 490–494, 2001. doi: 10.1203/00006450-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 313.Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol 461: 431–449, 1993. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Giussani DA, Unno N, Jenkins SL, Wentworth RA, Derks JB, Collins JH, Nathanielsz PW. Dynamics of cardiovascular responses to repeated partial umbilical cord compression in late-gestation sheep fetus. Am J Physiol Heart Circ Physiol 273: H2351–H2360, 1997. [DOI] [PubMed] [Google Scholar]
- 315.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359: 61–73, 2008. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr 4, 2B: 611–624, 2001. doi: 10.1079/PHN2001145. [DOI] [PubMed] [Google Scholar]
- 317.Goh JM, Bensley JG, Kenna K, Sozo F, Bocking AD, Brien J, Walker D, Harding R, Black MJ. Alcohol exposure during late gestation adversely affects myocardial development with implications for postnatal cardiac function. Am J Physiol Heart Circ Physiol 300: H645–H651, 2011. doi: 10.1152/ajpheart.00689.2010. [DOI] [PubMed] [Google Scholar]
- 318.Golan H, Huleihel M. The effect of prenatal hypoxia on brain development: short- and long-term consequences demonstrated in rodent models. Dev Sci 9: 338–349, 2006. doi: 10.1111/j.1467-7687.2006.00498.x. [DOI] [PubMed] [Google Scholar]
- 319.Goldstein M. Decade of the brain. An agenda for the nineties. West J Med 161: 239–241, 1994. [PMC free article] [PubMed] [Google Scholar]
- 320.Gomis P, Kacem K, Sercombe C, Seylaz J, Sercombe R. Confocal microscopic evidence of decreased alpha-actin expression within rabbit cerebral artery smooth muscle cells after subarachnoid haemorrhage. Histochem J 32: 673–678, 2000. doi: 10.1023/A:1004115432660. [DOI] [PubMed] [Google Scholar]
- 321.Gonzalez-Rodriguez P Jr, Tong W, Xue Q, Li Y, Hu S, Zhang L. Fetal hypoxia results in programming of aberrant angiotensin ii receptor expression patterns and kidney development. Int J Med Sci 10: 532–538, 2013. doi: 10.7150/ijms.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Gonzalez-Rodriguez PJ, Xiong F, Li Y, Zhou J, Zhang L. Fetal hypoxia increases vulnerability of hypoxic-ischemic brain injury in neonatal rats: role of glucocorticoid receptors. Neurobiol Dis 65: 172–179, 2014. doi: 10.1016/j.nbd.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Goyal R, Goyal D, Leitzke A, Gheorghe CP, Longo LD. Brain renin-angiotensin system: fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod Sci 17: 227–238, 2010. doi: 10.1177/1933719109351935. [DOI] [PubMed] [Google Scholar]
- 324.Goyal R, Leitzke A, Goyal D, Gheorghe CP, Longo LD. Antenatal maternal hypoxic stress: adaptations in fetal lung Renin-Angiotensin system. Reprod Sci 18: 180–189, 2011. doi: 10.1177/1933719110385134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Goyal R, Lister R, Leitzke A, Goyal D, Gheorghe CP, Longo LD. Antenatal maternal hypoxic stress: adaptations of the placental renin-angiotensin system in the mouse. Placenta 32: 134–139, 2011. doi: 10.1016/j.placenta.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Goyal R, Longo LD. Acclimatization to long-term hypoxia: gene expression in ovine carotid arteries. Physiol Genomics 46: 725–734, 2014. doi: 10.1152/physiolgenomics.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Goyal R, Van Wickle J, Goyal D, Matei N, Longo LD. Antenatal maternal long-term hypoxia: acclimatization responses with altered gene expression in ovine fetal carotid arteries. PLoS One 8: e82200, 2013. doi: 10.1371/journal.pone.0082200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Goyal R, Wong C, Van Wickle J, Longo LD. Antenatal maternal protein deprivation: sexually dimorphic programming of the pancreatic renin-angiotensin system. J Renin Angiotensin Aldosterone Syst 14: 137–145, 2013. doi: 10.1177/1470320312456329. [DOI] [PubMed] [Google Scholar]
- 329.Grafodatskaya D, Chung B, Szatmari P, Weksberg R. Autism spectrum disorders and epigenetics. J Am Acad Child Adolesc Psychiatry 49: 794–809, 2010. doi: 10.1016/j.jaac.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 330.Graham EM, Burd I, Everett AD, Northington FJ. Blood Biomarkers for Evaluation of Perinatal Encephalopathy. Front Pharmacol 7: 196, 2016. doi: 10.3389/fphar.2016.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Grangé G, Pannier E. [Effect of smoking on mode of delivery and per partum hypoxia and acidosis]. J Gynecol Obstet Biol Reprod (Paris) 34, Spec No 1: S146–S151, 2005. [PubMed] [Google Scholar]
- 332.Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience 143: 975–986, 2006. doi: 10.1016/j.neuroscience.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 333.Grayson BE, Kievit P, Smith MS, Grove KL. Critical determinants of hypothalamic appetitive neuropeptide development and expression: species considerations. Front Neuroendocrinol 31: 16–31, 2010. doi: 10.1016/j.yfrne.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 47: 718–726, 2012. doi: 10.1165/rcmb.2011-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Green LR, Kawagoe Y, Fraser M, Challis JR, Richardson BS. Activation of the hypothalamic-pituitary-adrenal axis with repetitive umbilical cord occlusion in the preterm ovine fetus. J Soc Gynecol Investig 7: 224–232, 2000. doi: 10.1177/107155760000700406. [DOI] [PubMed] [Google Scholar]
- 336.Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, Thannickal VJ. NOX enzymes and pulmonary disease. Antioxid Redox Signal 11: 2505–2516, 2009. doi: 10.1089/ars.2009.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Grosfeld A, Zilberfarb V, Turban S, André J, Guerre-Millo M, Issad T. Hypoxia increases leptin expression in human PAZ6 adipose cells. Diabetologia 45: 527–530, 2002. doi: 10.1007/s00125-002-0804-y. [DOI] [PubMed] [Google Scholar]
- 338.Grote P, Wittler L, Hendrix D, Koch F, Währisch S, Beisaw A, Macura K, Bläss G, Kellis M, Werber M, Herrmann BG. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell 24: 206–214, 2013. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Grove KL, Grayson BE, Glavas MM, Xiao XQ, Smith MS. Development of metabolic systems. Physiol Behav 86: 646–660, 2005. doi: 10.1016/j.physbeh.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 340.Gu W, Jones CT, Parer JT. Metabolic and cardiovascular effects on fetal sheep of sustained reduction of uterine blood flow. J Physiol 368: 109–129, 1985. doi: 10.1113/jphysiol.1985.sp015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Guerre-Millo M, Grosfeld A, Issad T. Leptin is a hypoxia-inducible gene. Obes Res 10: 856–858, 2002. doi: 10.1038/oby.2002.116. [DOI] [PubMed] [Google Scholar]
- 342.Guillemot F, Molnár Z, Tarabykin V, Stoykova A. Molecular mechanisms of cortical differentiation. Eur J Neurosci 23: 857–868, 2006. doi: 10.1111/j.1460-9568.2006.04626.x. [DOI] [PubMed] [Google Scholar]
- 343.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210: 1977–1992, 2013. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Gupta A, Srinivasan M, Thamadilok S, Patel MS. Hypothalamic alterations in fetuses of high fat diet-fed obese female rats. J Endocrinol 200: 293–300, 2009. doi: 10.1677/JOE-08-0429. [DOI] [PubMed] [Google Scholar]
- 345.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 91: 807–819, 2006. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 346.Habermehl DA, Janowiak MA, Vagnoni KE, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. IV. Cyclooxygenase isoform expression during the ovarian cycle and pregnancy in sheep. Biol Reprod 62: 781–788, 2000. doi: 10.1095/biolreprod62.3.781. [DOI] [PubMed] [Google Scholar]
- 347.Hahn S, Rusterholz C, Hösli I, Lapaire O. Cell-free nucleic acids as potential markers for preeclampsia. Placenta 32, Suppl: S17–S20, 2011. doi: 10.1016/j.placenta.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 348.Hale A, Lee C, Annis S, Min PK, Pande R, Creager MA, Julian CG, Moore LG, Mitsialis SA, Hwang SJ, Kourembanas S, Chan SY. An Argonaute 2 switch regulates circulating miR-210 to coordinate hypoxic adaptation across cells. Biochim Biophys Acta 1843: 2528–2542, 2014. doi: 10.1016/j.bbamcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Hameed A, Karaalp IS, Tummala PP, Wani OR, Canetti M, Akhter MW, Goodwin I, Zapadinsky N, Elkayam U. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol 37: 893–899, 2001. doi: 10.1016/S0735-1097(00)01198-0. [DOI] [PubMed] [Google Scholar]
- 350.Hammerman H, Kloner RA, Hale S, Schoen FJ, Braunwald E. Dose-dependent effects of short-term methylprednisolone on myocardial infarct extent, scar formation, and ventricular function. Circulation 68: 446–452, 1983. doi: 10.1161/01.CIR.68.2.446. [DOI] [PubMed] [Google Scholar]
- 351.Hammoud L, Burger DE, Lu X, Feng Q. Tissue inhibitor of metalloproteinase-3 inhibits neonatal mouse cardiomyocyte proliferation via EGFR/JNK/SP-1 signaling. Am J Physiol Cell Physiol 296: C735–C745, 2009. doi: 10.1152/ajpcell.00246.2008. [DOI] [PubMed] [Google Scholar]
- 352.Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr 94, Suppl_6: 1754S–1758S, 2011. doi: 10.3945/ajcn.110.001206. [DOI] [PubMed] [Google Scholar]
- 353.Hao R, Hu X, Wu C, Li N. Hypoxia-induced miR-15a promotes mesenchymal ablation and adaptation to hypoxia during lung development in chicken. PLoS One 9: e98868, 2014. doi: 10.1371/journal.pone.0098868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 19: 1252–1263, 2013. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 355.Harnarine-Singh D, Hyde JB. Post-natal growth of the arterial net in the human cerebral pia mater. Nature 225: 86–87, 1970. doi: 10.1038/225086a0. [DOI] [PubMed] [Google Scholar]
- 356.Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE. Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin Cell Dev Biol 38: 16–25, 2015. doi: 10.1016/j.semcdb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 357.Haukvik UK, McNeil T, Lange EH, Melle I, Dale AM, Andreassen OA, Agartz I. Pre- and perinatal hypoxia associated with hippocampus/amygdala volume in bipolar disorder. Psychol Med 44: 975–985, 2014. doi: 10.1017/S0033291713001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Hauton D. Hypoxia in early pregnancy induces cardiac dysfunction in adult offspring of Rattus norvegicus, a non-hypoxia-adapted species. Comp Biochem Physiol A Mol Integr Physiol 163: 278–285, 2012. doi: 10.1016/j.cbpa.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 359.Hauton D, Al-Shammari A, Gaffney EA, Egginton S. Maternal hypoxia decreases capillary supply and increases metabolic inefficiency leading to divergence in myocardial oxygen supply and demand. PLoS One 10: e0127424, 2015. doi: 10.1371/journal.pone.0127424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Hauton D, Ousley V. Prenatal hypoxia induces increased cardiac contractility on a background of decreased capillary density. BMC Cardiovasc Disord 9: 1, 2009. doi: 10.1186/1471-2261-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.He B, Xiao J, Ren AJ, Zhang YF, Zhang H, Chen M, Xie B, Gao XG, Wang YW. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J Biomed Sci 18: 22, 2011. doi: 10.1186/1423-0127-18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157: 95–109, 2014. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology 138: 3859–3863, 1997. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- 364.Hellstrand P, Albinsson S. Stretch-dependent growth and differentiation in vascular smooth muscle: role of the actin cytoskeleton. Can J Physiol Pharmacol 83: 869–875, 2005. doi: 10.1139/y05-061. [DOI] [PubMed] [Google Scholar]
- 365.Hemmings DG, Williams SJ, Davidge ST. Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol 289: H674–H682, 2005. doi: 10.1152/ajpheart.00191.2005. [DOI] [PubMed] [Google Scholar]
- 366.Henry BA, Goding JW, Alexander WS, Tilbrook AJ, Canny BJ, Dunshea F, Rao A, Mansell A, Clarke IJ. Central administration of leptin to ovariectomized ewes inhibits food intake without affecting the secretion of hormones from the pituitary gland: evidence for a dissociation of effects on appetite and neuroendocrine function. Endocrinology 140: 1175–1182, 1999. doi: 10.1210/endo.140.3.6604. [DOI] [PubMed] [Google Scholar]
- 367.Henson MC, Castracane VD. Leptin in pregnancy. Biol Reprod 63: 1219–1228, 2000. doi: 10.1095/biolreprod63.5.1219. [DOI] [PubMed] [Google Scholar]
- 368.Hermes W, Franx A, van Pampus MG, Bloemenkamp KW, Bots ML, van der Post JA, Porath M, Ponjee GA, Tamsma JT, Mol BW, de Groot CJ. Cardiovascular risk factors in women who had hypertensive disorders late in pregnancy: a cohort study. Am J Obstet Gynecol 208: 474.e1–474.e8, 2013. doi: 10.1016/j.ajog.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 369.Hermsteiner M, Zoltan DR, Künzel W. The vasoconstrictor response of uterine and mesenteric resistance arteries is differentially altered in the course of pregnancy. Eur J Obstet Gynecol Reprod Biol 100: 29–35, 2001. doi: 10.1016/S0301-2115(01)00428-6. [DOI] [PubMed] [Google Scholar]
- 370.Herrera EA, Pulgar VM, Riquelme RA, Sanhueza EM, Reyes RV, Ebensperger G, Parer JT, Valdéz EA, Giussani DA, Blanco CE, Hanson MA, Llanos AJ. High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol 292: R2234–R2240, 2007. doi: 10.1152/ajpregu.00909.2006. [DOI] [PubMed] [Google Scholar]
- 371.Herrera EA, Riquelme RA, Ebensperger G, Reyes RV, Ulloa CE, Cabello G, Krause BJ, Parer JT, Giussani DA, Llanos AJ. Long-term exposure to high-altitude chronic hypoxia during gestation induces neonatal pulmonary hypertension at sea level. Am J Physiol Regul Integr Comp Physiol 299: R1676–R1684, 2010. doi: 10.1152/ajpregu.00123.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Herrera EA, Rojas RT, Krause BJ, Ebensperger G, Reyes RV, Giussani DA, Parer JT, Llanos AJ. Cardiovascular function in term fetal sheep conceived, gestated and studied in the hypobaric hypoxia of the Andean altiplano. J Physiol 594: 1231–1245, 2016. doi: 10.1113/JP271110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Himms-Hagen J, Cui J, Danforth E Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol Regul Integr Comp Physiol 266: R1371–R1382, 1994. [DOI] [PubMed] [Google Scholar]
- 374.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 279: C670–C681, 2000. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 375.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci 110: 1603–1613, 1997. [DOI] [PubMed] [Google Scholar]
- 376.Ho L, Crabtree GR. Chromatin remodelling during development. Nature 463: 474–484, 2010. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Hoffman DL, Brookes PS. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions. J Biol Chem 284: 16236–16245, 2009. doi: 10.1074/jbc.M809512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Holliday MA. Metabolic rate and organ size during growth from infancy to maturity and during late gastation and early infancy. Pediatrics 47, Suppl 2: 2, 1971. [PubMed] [Google Scholar]
- 379.Hompes T, Izzi B, Gellens E, Morreels M, Fieuws S, Pexsters A, Schops G, Dom M, Van Bree R, Freson K, Verhaeghe J, Spitz B, Demyttenaere K, Glover V, Van den Bergh B, Allegaert K, Claes S. Investigating the influence of maternal cortisol and emotional state during pregnancy on the DNA methylation status of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood. J Psychiatr Res 47: 880–891, 2013. doi: 10.1016/j.jpsychires.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 380.Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW, Maxwell PH, Ratcliffe PJ, Stuart DI, Jones EY. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature 417: 975–978, 2002. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 381.Hooper SB, Te Pas AB, Kitchen MJ. Respiratory transition in the newborn: a three-phase process. Arch Dis Child Fetal Neonatal Ed 101: F266–F271, 2016. doi: 10.1136/archdischild-2013-305704. [DOI] [PubMed] [Google Scholar]
- 382.Horikoshi M, Beaumont RN, Day FR, Warrington NM, Kooijman MN, Fernandez-Tajes J, Feenstra B, van Zuydam NR, Gaulton KJ, Grarup N, Bradfield JP, Strachan DP, Li-Gao R, Ahluwalia TS, Kreiner E, Rueedi R, Lyytikäinen LP, Cousminer DL, Wu Y, Thiering E, Wang CA, Have CT, Hottenga JJ, Vilor-Tejedor N, Joshi PK, Boh ETH, Ntalla I, Pitkänen N, Mahajan A, van Leeuwen EM, Joro R, Lagou V, Nodzenski M, Diver LA, Zondervan KT, Bustamante M, Marques-Vidal P, Mercader JM, Bennett AJ, Rahmioglu N, Nyholt DR, Ma RCW, Tam CHT, Tam WH, Ganesh SK, van Rooij FJ, Jones SE, Loh PR, Ruth KS, Tuke MA, Tyrrell J, Wood AR, Yaghootkar H, Scholtens DM, Paternoster L, Prokopenko I, Kovacs P, Atalay M, Willems SM, Panoutsopoulou K, Wang X, Carstensen L, Geller F, Schraut KE, Murcia M, van Beijsterveldt CE, Willemsen G, Appel EVR, Fonvig CE, Trier C, Tiesler CM, Standl M, Kutalik Z, Bonas-Guarch S, Hougaard DM, Sánchez F, Torrents D, Waage J, Hollegaard MV, de Haan HG, Rosendaal FR, Medina-Gomez C, Ring SM, Hemani G, McMahon G, Robertson NR, Groves CJ, Langenberg C, Luan J, Scott RA, Zhao JH, Mentch FD, MacKenzie SM, Reynolds RM, Lowe WL Jr, Tönjes A, Stumvoll M, Lindi V, Lakka TA, van Duijn CM, Kiess W, Körner A, Sørensen TI, Niinikoski H, Pahkala K, Raitakari OT, Zeggini E, Dedoussis GV, Teo YY, Saw SM, Melbye M, Campbell H, Wilson JF, Vrijheid M, de Geus EJ, Boomsma DI, Kadarmideen HN, Holm JC, Hansen T, Sebert S, Hattersley AT, Beilin LJ, Newnham JP, Pennell CE, Heinrich J, Adair LS, Borja JB, Mohlke KL, Eriksson JG, Widén EE, Kähönen M, Viikari JS, Lehtimäki T, Vollenweider P, Bønnelykke K, Bisgaard H, Mook-Kanamori DO, Hofman A, Rivadeneira F, Uitterlinden AG, Pisinger C, Pedersen O, Power C, Hyppönen E, Wareham NJ, Hakonarson H, Davies E, Walker BR, Jaddoe VW, Jarvelin MR, Grant SF, Vaag AA, Lawlor DA, Frayling TM, Davey Smith G, Morris AP, Ong KK, Felix JF, Timpson NJ, Perry JR, Evans DM, McCarthy MI, Freathy RM; CHARGE Consortium Hematology Working Group . Genome-wide associations for birth weight and correlations with adult disease. Nature 538: 248–252, 2016. doi: 10.1038/nature19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Houshmand-Oeregaard A, Hansen NS, Hjort L, Kelstrup L, Broholm C, Mathiesen ER, Clausen TD, Damm P, Vaag A. Differential adipokine DNA methylation and gene expression in subcutaneous adipose tissue from adult offspring of women with diabetes in pregnancy. Clin Epigenetics 9: 37, 2017. doi: 10.1186/s13148-017-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Howe DC, Gertler A, Challis JR. The late gestation increase in circulating ACTH and cortisol in the fetal sheep is suppressed by intracerebroventricular infusion of recombinant ovine leptin. J Endocrinol 174: 259–266, 2002. doi: 10.1677/joe.0.1740259. [DOI] [PubMed] [Google Scholar]
- 385.Howell KR, Pillai A. Effects of prenatal hypoxia on schizophrenia-related phenotypes in heterozygous reeler mice: a gene × environment interaction study. Eur Neuropsychopharmacol 24: 1324–1336, 2014. doi: 10.1016/j.euroneuro.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Hsieh J, Eisch AJ. Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind. Neurobiol Dis 39: 73–84, 2010. doi: 10.1016/j.nbd.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Hu H, Petousi N, Glusman G, Yu Y, Bohlender R, Tashi T, Downie JM, Roach JC, Cole AM, Lorenzo FR, Rogers AR, Brunkow ME, Cavalleri G, Hood L, Alpatty SM, Prchal JT, Jorde LB, Robbins PA, Simonson TS, Huff CD. Evolutionary history of Tibetans inferred from whole-genome sequencing. PLoS Genet 13: e1006675, 2017. doi: 10.1371/journal.pgen.1006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, Robbins RC, Wu JC. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 122, Suppl: S124–S131, 2010. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Hu XQ, Chen M, Dasgupta C, Xiao D, Huang X, Yang S, Zhang L. Chronic hypoxia upregulates DNA methyltransferase and represses large conductance Ca2+-activated K+ channel function in ovine uterine arteries. Biol Reprod 96: 424–434, 2017. doi: 10.1095/biolreprod.116.145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Hu XQ, Dasgupta C, Xiao D, Huang X, Yang S, Xu Z, Zhang L. Pregnancy reprograms large-conductance Ca2+-activated K+ channel in uterine arteries: roles of TET-mediated active demethylation. Hypertension 69: 1181–1191, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Hu XQ, Dasgupta C, Xiao D, Huang X, Yang S, Zhang L. Micro RNA-210 targets TET1 and suppresses pregnancy-mediated adaptation of BKCa channel expression and function in ovine uterine arteries. Hypertension 70: 601–612, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Hu XQ, Huang X, Xiao D, Zhang L. Direct effect of chronic hypoxia in suppressing large conductance Ca(2+)-activated K(+) channel activity in ovine uterine arteries via increasing oxidative stress. J Physiol 594: 343–356, 2016. doi: 10.1113/JP271626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L. Pregnancy upregulates large-conductance Ca(2+)-activated K(+) channel activity and attenuates myogenic tone in uterine arteries. Hypertension 58: 1132–1139, 2011. doi: 10.1161/HYPERTENSIONAHA.111.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson SM, Zhang L. Chronic hypoxia suppresses pregnancy-induced upregulation of large-conductance Ca2+-activated K+ channel activity in uterine arteries. Hypertension 60: 214–222, 2012. doi: 10.1161/HYPERTENSIONAHA.112.196097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Hu Y, Peng J, Feng D, Chu L, Li X, Jin Z, Lin Z, Zeng Q. Role of extracellular signal-regulated kinase, p38 kinase, and activator protein-1 in transforming growth factor-beta1-induced alpha smooth muscle actin expression in human fetal lung fibroblasts in vitro. Lung 184: 33–42, 2006. doi: 10.1007/s00408-005-2560-5. [DOI] [PubMed] [Google Scholar]
- 396.Huang L, Shen Z, Xu Q, Huang X, Chen Q, Li D. Increased levels of microRNA-424 are associated with the pathogenesis of fetal growth restriction. Placenta 34: 624–627, 2013. doi: 10.1016/j.placenta.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 397.Huang ST, Vo KC, Lyell DJ, Faessen GH, Tulac S, Tibshirani R, Giaccia AJ, Giudice LC. Developmental response to hypoxia. FASEB J 18: 1348–1365, 2004. doi: 10.1096/fj.03-1377com. [DOI] [PubMed] [Google Scholar]
- 399.Huang Y, Yan X, Zhao JX, Zhu MJ, McCormick RJ, Ford SP, Nathanielsz PW, Ren J, Du M. Maternal obesity induces fibrosis in fetal myocardium of sheep. Am J Physiol Endocrinol Metab 299: E968–E975, 2010. doi: 10.1152/ajpendo.00434.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Hubbell MC, Semotiuk AJ, Thorpe RB, Adeoye OO, Butler SM, Williams JM, Khorram O, Pearce WJ. Chronic hypoxia and VEGF differentially modulate abundance and organization of myosin heavy chain isoforms in fetal and adult ovine arteries. Am J Physiol Cell Physiol 303: C1090–C1103, 2012. doi: 10.1152/ajpcell.00408.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Huerta-Sánchez E, Jin X, Asan, Bianba Z, Peter BM, Vinckenbosch N, Liang Y, Yi X, He M, Somel M, Ni P, Wang B, Ou X, Huasang, Luosang J, Cuo ZX, Li K, Gao G, Yin Y, Wang W, Zhang X, Xu X, Yang H, Li Y, Wang J, Wang J, Nielsen R. Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature 512: 194–197, 2014. doi: 10.1038/nature13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Huetsch JC, Suresh K, Bernier M, Shimoda LA. Update on novel targets and potential treatment avenues in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 311: L811–L831, 2016. doi: 10.1152/ajplung.00302.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C, Latimer PA, Hare JM, Olson EN, van Rooij E. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res 110: 71–81, 2012. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen deficiency decreases ischemic tolerance in the aged rat heart: roles of PKCdelta, PKCepsilon, Akt, and GSK3beta. Am J Physiol Regul Integr Comp Physiol 292: R800–R809, 2007. doi: 10.1152/ajpregu.00374.2006. [DOI] [PubMed] [Google Scholar]
- 405.Huo H, Luo Z, Wang M, Yu X, Liao Z, Zhou X, Yue S. MicroRNA expression profile in intrauterine hypoxia-induced pulmonary hypoplasia in rats. Exp Ther Med 8: 747–753, 2014. doi: 10.3892/etm.2014.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Ibe BO, Portugal AM, Chaturvedi S, Raj JU. Oxygen-dependent PAF receptor binding and intracellular signaling in ovine fetal pulmonary vascular smooth muscle. Am J Physiol Lung Cell Mol Physiol 288: L879–L886, 2005. doi: 10.1152/ajplung.00341.2004. [DOI] [PubMed] [Google Scholar]
- 407.Imamura T, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters endocrine and physiologic responses to umbilical cord occlusion in the ovine fetus. J Soc Gynecol Investig 11: 131–140, 2004. doi: 10.1016/j.jsgi.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 408.Inagaki K, Begley R, Ikeno F, Mochly-Rosen D. Cardioprotection by epsilon-protein kinase C activation from ischemia: continuous delivery and antiarrhythmic effect of an epsilon-protein kinase C-activating peptide. Circulation 111: 44–50, 2005. doi: 10.1161/01.CIR.0000151614.22282.F1. [DOI] [PubMed] [Google Scholar]
- 409.Inagami T, Naruse M, Hoover R. Endothelium as an endocrine organ. Annu Rev Physiol 57: 171–189, 1995. doi: 10.1146/annurev.ph.57.030195.001131. [DOI] [PubMed] [Google Scholar]
- 410.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest 118: 580–586, 2000. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 411.Iqbal J, Pompolo S, Murakami T, Grouzmann E, Sakurai T, Meister B, Clarke IJ. Immunohistochemical characterization of localization of long-form leptin receptor (OB-Rb) in neurochemically defined cells in the ovine hypothalamus. Brain Res 920: 55–64, 2001. doi: 10.1016/S0006-8993(01)02932-8. [DOI] [PubMed] [Google Scholar]
- 412.Iqbal W, Ciriello J. Effect of maternal chronic intermittent hypoxia during gestation on offspring growth in the rat. Am J Obstet Gynecol 209: 564.e1–564.e9, 2013. doi: 10.1016/j.ajog.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 413.Iriyama T, Wang W, Parchim NF, Song A, Blackwell SC, Sibai BM, Kellems RE, Xia Y. Hypoxia-independent upregulation of placental hypoxia inducible factor-1α gene expression contributes to the pathogenesis of preeclampsia. Hypertension 65: 1307–1315, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Ishibashi O, Ohkuchi A, Ali MM, Kurashina R, Luo SS, Ishikawa T, Takizawa T, Hirashima C, Takahashi K, Migita M, Ishikawa G, Yoneyama K, Asakura H, Izumi A, Matsubara S, Takeshita T, Takizawa T. Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: a novel marker for predicting preeclampsia. Hypertension 59: 265–273, 2012. doi: 10.1161/HYPERTENSIONAHA.111.180232. [DOI] [PubMed] [Google Scholar]
- 415.Itani N, Skeffington KL, Beck C, Niu Y, Giussani DA. Melatonin rescues cardiovascular dysfunction during hypoxic development in the chick embryo. J Pineal Res 60: 16–26, 2016. doi: 10.1111/jpi.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466: 1129–1133, 2010. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Iwata K, Ikami K, Matsuno K, Yamashita T, Shiba D, Ibi M, Matsumoto M, Katsuyama M, Cui W, Zhang J, Zhu K, Takei N, Kokai Y, Ohneda O, Yokoyama T, Yabe-Nishimura C. Deficiency of NOX1/nicotinamide adenine dinucleotide phosphate, reduced form oxidase leads to pulmonary vascular remodeling. Arterioscler Thromb Vasc Biol 34: 110–119, 2014. doi: 10.1161/ATVBAHA.113.302107. [DOI] [PubMed] [Google Scholar]
- 418.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12: 149–162, 1998. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Jablonka E, Lamb MJ. The changing concept of epigenetics. Ann N Y Acad Sci 981: 82–96, 2002. doi: 10.1111/j.1749-6632.2002.tb04913.x. [DOI] [PubMed] [Google Scholar]
- 420.Jackson M, Connell MG, Smith A. Development of the collagen network of the human fetal myocardium: an immunohistochemical study. Int J Cardiol 41: 77–86, 1993. doi: 10.1016/0167-5273(93)90139-8. [DOI] [PubMed] [Google Scholar]
- 421.Jackson MR, Mayhew TM, Haas JD. On the factors which contribute to thinning of the villous membrane in human placentae at high altitude. II. An increase in the degree of peripheralization of fetal capillaries. Placenta 9: 9–18, 1988. doi: 10.1016/0143-4004(88)90068-9. [DOI] [PubMed] [Google Scholar]
- 422.Jackson MR, Mayhew TM, Haas JD. The volumetric composition of human term placentae: altitudinal, ethnic and sex differences in Bolivia. J Anat 152: 173–187, 1987. [PMC free article] [PubMed] [Google Scholar]
- 423.Jacobs R, Robinson JS, Owens JA, Falconer J, Webster ME. The effect of prolonged hypobaric hypoxia on growth of fetal sheep. J Dev Physiol 10: 97–112, 1988. [PubMed] [Google Scholar]
- 424.Jaenisch R. DNA methylation and imprinting: why bother? Trends Genet 13: 323–329, 1997. doi: 10.1016/S0168-9525(97)01180-3. [DOI] [PubMed] [Google Scholar]
- 425.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33, Suppl: 245–254, 2003. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 426.Jankov RP, Kantores C, Pan J, Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol 294: L233–L245, 2008. doi: 10.1152/ajplung.00166.2007. [DOI] [PubMed] [Google Scholar]
- 427.Jarome TJ, Lubin FD. Epigenetic mechanisms of memory formation and reconsolidation. Neurobiol Learn Mem 115: 116–127, 2014. doi: 10.1016/j.nlm.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 157: 2111–2122, 2000. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Jaworska J, Ziemka-Nalecz M, Sypecka J, Zalewska T. The potential neuroprotective role of a histone deacetylase inhibitor, sodium butyrate, after neonatal hypoxia-ischemia. J Neuroinflammation 14: 34, 2017. doi: 10.1186/s12974-017-0807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Jayet PY, Rimoldi SF, Stuber T, Salmòn CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, Nicod P, Villena M, Allemann Y, Scherrer U, Sartori C. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation 122: 488–494, 2010. doi: 10.1161/CIRCULATIONAHA.110.941203. [DOI] [PubMed] [Google Scholar]
- 431.Jensen EC, Bennet L, Fraser M, Power GG, Hunter CJ, Gunn AJ. Adenosine A(1) receptor mediated suppression of adrenal activity in near-term fetal sheep. Am J Physiol Regul Integr Comp Physiol 298: R700–R706, 2010. doi: 10.1152/ajpregu.00474.2009. [DOI] [PubMed] [Google Scholar]
- 432.Jenuwein T, Allis CD. Translating the histone code. Science 293: 1074–1080, 2001. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 433.Jessell TM, Sanes JR. Development. The decade of the developing brain. Curr Opin Neurobiol 10: 599–611, 2000. doi: 10.1016/S0959-4388(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 434.Jiang B, Kamat A, Mendelson CR. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2). Mol Endocrinol 14: 1661–1673, 2000. doi: 10.1210/mend.14.10.0539. [DOI] [PubMed] [Google Scholar]
- 435.Jiang XY, Feng YL, Ye LT, Li XH, Feng J, Zhang MZ, Shelat HS, Wassler M, Li Y, Geng YJ, Yu XY. Inhibition of Gata4 and Tbx5 by Nicotine-Mediated DNA Methylation in Myocardial Differentiation. Stem Cell Rep 8: 290–304, 2017. doi: 10.1016/j.stemcr.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Jiang Y, Langley B, Lubin FD, Renthal W, Wood MA, Yasui DH, Kumar A, Nestler EJ, Akbarian S, Beckel-Mitchener AC. Epigenetics in the nervous system. J Neurosci 28: 11753–11759, 2008. doi: 10.1523/JNEUROSCI.3797-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Jin S, Wang J, Chen H, Xiang B. Differential miRNA expression analysis during late stage terminal hindgut development in fetal rats. J Pediatr Surg 52: 1516–1519, 2017. doi: 10.1016/j.jpedsurg.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 438.Jobe SO, Ramadoss J, Koch JM, Jiang Y, Zheng J, Magness RR. Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension 55: 1005–1011, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension 61: 480–487, 2013. doi: 10.1161/HYPERTENSIONAHA.111.201624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Johansson C, Tumber A, Che K, Cain P, Nowak R, Gileadi C, Oppermann U. The roles of Jumonji-type oxygenases in human disease. Epigenomics 6: 89–120, 2014. doi: 10.2217/epi.13.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Johnson AB, Denko N, Barton MC. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat Res 640: 174–179, 2008. doi: 10.1016/j.mrfmmm.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Johnstone SE, Baylin SB. Stress and the epigenetic landscape: a link to the pathobiology of human diseases? Nat Rev Genet 11: 806–812, 2010. doi: 10.1038/nrg2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Jones CT, Robinson RO. Plasma catecholamines in foetal and adult sheep. J Physiol 248: 15–33, 1975. doi: 10.1113/jphysiol.1975.sp010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science 293: 1068–1070, 2001. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 445.Jones RD, Morice AH, Emery CJ. Effects of perinatal exposure to hypoxia upon the pulmonary circulation of the adult rat. Physiol Res 53: 11–17, 2004. [PubMed] [Google Scholar]
- 446.Jones SP, Greer JJ, Kakkar AK, Ware PD, Turnage RH, Hicks M, van Haperen R, de Crom R, Kawashima S, Yokoyama M, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol 286: H276–H282, 2004. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 447.Jonker SS, Giraud MK, Giraud GD, Chattergoon NN, Louey S, Davis LE, Faber JJ, Thornburg KL. Cardiomyocyte enlargement, proliferation and maturation during chronic fetal anaemia in sheep. Exp Physiol 95: 131–139, 2010. doi: 10.1113/expphysiol.2009.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Joseph V, Mamet J, Lee F, Dalmaz Y, Van Reeth O. Prenatal hypoxia impairs circadian synchronisation and response of the biological clock to light in adult rats. J Physiol 543: 387–395, 2002. doi: 10.1113/jphysiol.2002.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Julian CG. High altitude during pregnancy. Clin Chest Med 32: 21–31, 2011. doi: 10.1016/j.ccm.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 450.Julian CG, Galan HL, Wilson MJ, Desilva W, Cioffi-Ragan D, Schwartz J, Moore LG. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol 295: R906–R915, 2008. doi: 10.1152/ajpregu.00164.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Julian CG, Gonzales M, Rodriguez A, Bellido D, Salmon CS, Ladenburger A, Reardon L, Vargas E, Moore LG. Perinatal hypoxia increases susceptibility to high-altitude polycythemia and attendant pulmonary vascular dysfunction. Am J Physiol Heart Circ Physiol 309: H565–H573, 2015. doi: 10.1152/ajpheart.00296.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed 92: F372–F377, 2007. doi: 10.1136/adc.2006.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Julian CG, Vargas E, Browne VA, Wilson MJ, Bigham AW, Rodriguez C, McCord JM, Moore LG. Potential role for elevated maternal enzymatic antioxidant status in Andean protection against altitude-associated SGA. J Matern Fetal Neonatal Med 25: 1233–1240, 2012. doi: 10.3109/14767058.2011.636102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, Shriver MD, Rodriguez C, Vargas E, Moore LG. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol 296: R1564–R1575, 2009. doi: 10.1152/ajpregu.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Julian CG, Wilson MJ, Moore LG. Evolutionary adaptation to high altitude: a view from in utero. Am J Hum Biol 21: 614–622, 2009. doi: 10.1002/ajhb.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Kabra DG, Pfuhlmann K, García-Cáceres C, Schriever SC, Casquero García V, Kebede AF, Fuente-Martin E, Trivedi C, Heppner K, Uhlenhaut NH, Legutko B, Kabra UD, Gao Y, Yi CX, Quarta C, Clemmensen C, Finan B, Müller TD, Meyer CW, Paez-Pereda M, Stemmer K, Woods SC, Perez-Tilve D, Schneider R, Olson EN, Tschöp MH, Pfluger PT. Hypothalamic leptin action is mediated by histone deacetylase 5. Nat Commun 7: 10782, 2016. doi: 10.1038/ncomms10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Kagias K, Pocock R. microRNA regulation of the embryonic hypoxic response in Caenorhabditis elegans. Sci Rep 5: 11284, 2015. doi: 10.1038/srep11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Kajimoto K, Naraba H, Iwai N. MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA 12: 1626–1632, 2006. doi: 10.1261/rna.7228806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 20: 68–100, 1999. [DOI] [PubMed] [Google Scholar]
- 460.Kametas NA, McAuliffe F, Krampl E, Chambers J, Nicolaides KH. Maternal cardiac function during pregnancy at high altitude. BJOG 111: 1051–1058, 2004. doi: 10.1111/j.1471-0528.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 461.Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol 290: R1105–R1114, 2006. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- 462.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 453: 1117–1121, 2008. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 463.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol 572: 31–44, 2006. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Kapoor A, Leen J, Matthews SG. Molecular regulation of the hypothalamic-pituitary-adrenal axis in adult male guinea pigs after prenatal stress at different stages of gestation. J Physiol 586: 4317–4326, 2008. doi: 10.1113/jphysiol.2008.153684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Karakurum M, Shreeniwas R, Chen J, Pinsky D, Yan SD, Anderson M, Sunouchi K, Major J, Hamilton T, Kuwabara K. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J Clin Invest 93: 1564–1570, 1994. doi: 10.1172/JCI117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Karch D. [Hypoxia during the perinatal period and the formation of cerebral lesions]. Klin Wochenschr 60: 1427–1434, 1982. doi: 10.1007/BF01720989. [DOI] [PubMed] [Google Scholar]
- 467.Karowicz-Bilinska A, Kedziora-Kornatowska K, Bartosz G. Indices of oxidative stress in pregnancy with fetal growth restriction. Free Radic Res 41: 870–873, 2007. doi: 10.1080/10715760701291647. [DOI] [PubMed] [Google Scholar]
- 468.Karumanchi SA. Angiogenic Factors in Preeclampsia: From Diagnosis to Therapy. Hypertension 67: 1072–1079, 2016. doi: 10.1161/HYPERTENSIONAHA.116.06421. [DOI] [PubMed] [Google Scholar]
- 469.Kato A, Umezaki H, Imamura T, Kaushal KM, Mlynarczyk M, Gilbert RD, Bucholz J, Longo LD, Ducsay CA. Catecholamine and cardiovascular responses to superimposed hypoxia following carotid body denervation in the long-term hypoxemic ovine fetus. J Soc Gynecol Investig 9: 21, 2002. [Google Scholar]
- 470.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 69: 1–7, 2003. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 471.Kenchegowda D, Liu H, Thompson K, Luo L, Martin SS, Fisher SA. Vulnerability of the developing heart to oxygen deprivation as a cause of congenital heart defects. J Am Heart Assoc 3: e000841, 2014. doi: 10.1161/JAHA.114.000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Kertes DA, Kamin HS, Hughes DA, Rodney NC, Bhatt S, Mulligan CJ. Prenatal Maternal Stress Predicts Methylation of Genes Regulating the Hypothalamic-Pituitary-Adrenocortical System in Mothers and Newborns in the Democratic Republic of Congo. Child Dev 87: 61–72, 2016. doi: 10.1111/cdev.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Keverne EB, Pfaff DW, Tabansky I. Epigenetic changes in the developing brain: Effects on behavior. Proc Natl Acad Sci USA 112: 6789–6795, 2015. doi: 10.1073/pnas.1501482112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res 54: 20–25, 2003. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- 475.Keyes LE, Majack R, Dempsey EC, Moore LG. Pregnancy stimulation of DNA synthesis and uterine blood flow in the guinea pig. Pediatr Res 41: 708–715, 1997. doi: 10.1203/00006450-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 476.Keyes LE, Moore LG, Walchak SJ, Dempsey EC. Pregnancy-stimulated growth of vascular smooth muscle cells: importance of protein kinase C-dependent synergy between estrogen and platelet-derived growth factor. J Cell Physiol 166: 22–32, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 477.Khalid ME, Ali ME, Ali KZ. Full-term birth weight and placental morphology at high and low altitude. Int J Gynaecol Obstet 57: 259–265, 1997. doi: 10.1016/S0020-7292(97)00067-2. [DOI] [PubMed] [Google Scholar]
- 478.Khalyfa A, Cortese R, Qiao Z, Ye H, Bao R, Andrade J, Gozal D. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. J Physiol 595: 2551–2568, 2017. doi: 10.1113/JP273570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Khan LH, Rosenfeld CR, Liu XT, Magness RR. Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels in uterine arteries during the ovine ovarian cycle. Am J Physiol Endocrinol Metab 298: E222–E228, 2010. doi: 10.1152/ajpendo.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol 46: 2116–2124, 2005. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 482.Kienast C, Moya W, Rodriguez O, Jijón A, Geipel A. Predictive value of angiogenic factors, clinical risk factors and uterine artery Doppler for pre-eclampsia and fetal growth restriction in second and third trimester pregnancies in an Ecuadorian population. J Matern Fetal Neonatal Med 29: 537–543, 2016. doi: 10.3109/14767058.2015.1012063. [DOI] [PubMed] [Google Scholar]
- 483.Kim GH, Ryan JJ, Marsboom G, Archer SL. Epigenetic mechanisms of pulmonary hypertension. Pulm Circ 1: 347–356, 2011. doi: 10.4103/2045-8932.87300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S, Thomas JK, Muthusamy B, Leal-Rojas P, Kumar P, Sahasrabuddhe NA, Balakrishnan L, Advani J, George B, Renuse S, Selvan LD, Patil AH, Nanjappa V, Radhakrishnan A, Prasad S, Subbannayya T, Raju R, Kumar M, Sreenivasamurthy SK, Marimuthu A, Sathe GJ, Chavan S, Datta KK, Subbannayya Y, Sahu A, Yelamanchi SD, Jayaram S, Rajagopalan P, Sharma J, Murthy KR, Syed N, Goel R, Khan AA, Ahmad S, Dey G, Mudgal K, Chatterjee A, Huang TC, Zhong J, Wu X, Shaw PG, Freed D, Zahari MS, Mukherjee KK, Shankar S, Mahadevan A, Lam H, Mitchell CJ, Shankar SK, Satishchandra P, Schroeder JT, Sirdeshmukh R, Maitra A, Leach SD, Drake CG, Halushka MK, Prasad TS, Hruban RH, Kerr CL, Bader GD, Iacobuzio-Donahue CA, Gowda H, Pandey A. A draft map of the human proteome. Nature 509: 575–581, 2014. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Kim MY, Eiby YA, Lumbers ER, Wright LL, Gibson KJ, Barnett AC, Lingwood BE. Effects of glucocorticoid exposure on growth and structural maturation of the heart of the preterm piglet. PLoS One 9: e93407, 2014. doi: 10.1371/journal.pone.0093407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM, Richardson JA, Ashour AM, Asaithamby A, Liang H, Xing C, Lu Z, Zhang CC, Sadek HA. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature 523: 226–230, 2015. doi: 10.1038/nature14582. [DOI] [PubMed] [Google Scholar]
- 487.Kinsella JP, Parker TA, Davis JM, Abman SH. Superoxide dismutase improves gas exchange and pulmonary hemodynamics in premature lambs. Am J Respir Crit Care Med 172: 745–749, 2005. doi: 10.1164/rccm.200501-146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Kiprono LV, Wallace K, Moseley J, Martin J Jr, Lamarca B. Progesterone blunts vascular endothelial cell secretion of endothelin-1 in response to placental ischemia. Am J Obstet Gynecol 209: 44.e1–44.e6, 2013. doi: 10.1016/j.ajog.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Kitanaka T, Alonso JG, Gilbert RD, Siu BL, Clemons GK, Longo LD. Fetal responses to long-term hypoxemia in sheep. Am J Physiol Regul Integr Comp Physiol 256: R1348–R1354, 1989. [DOI] [PubMed] [Google Scholar]
- 490.Kitanaka T, Gilbert RD, Longo LD. Maternal responses to long-term hypoxemia in sheep. Am J Physiol Regul Integr Comp Physiol 256: R1340–R1347, 1989. [DOI] [PubMed] [Google Scholar]
- 491.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, Boyer LA. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 152: 570–583, 2013. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Klemm DJ, Majka SM, Crossno JT Jr, Psilas JC, Reusch JE, Garat CV. Reduction of reactive oxygen species prevents hypoxia-induced CREB depletion in pulmonary artery smooth muscle cells. J Cardiovasc Pharmacol 58: 181–191, 2011. doi: 10.1097/FJC.0b013e31821f2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Klemm DJ, Watson PA, Frid MG, Dempsey EC, Schaack J, Colton LA, Nesterova A, Stenmark KR, Reusch JE-B. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J Biol Chem 276: 46132–46141, 2001. doi: 10.1074/jbc.M104769200. [DOI] [PubMed] [Google Scholar]
- 494.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet 7: 715–727, 2006. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 495.Knezevic I, Patel A, Sundaresan NR, Gupta MP, Solaro RJ, Nagalingam RS, Gupta M. A novel cardiomyocyte-enriched microRNA, miR-378, targets insulin-like growth factor 1 receptor: implications in postnatal cardiac remodeling and cell survival. J Biol Chem 287: 12913–12926, 2012. doi: 10.1074/jbc.M111.331751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res 156: 251–261, 2005. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 497.Konduri GG, Afolayan AJ, Eis A, Pritchard KA Jr, Teng RJ. Interaction of endothelial nitric oxide synthase with mitochondria regulates oxidative stress and function in fetal pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 309: L1009–L1017, 2015. doi: 10.1152/ajplung.00386.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Konduri GG, Bakhutashvili I, Eis A, Pritchard K Jr. Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 292: H1812–H1820, 2007. doi: 10.1152/ajpheart.00425.2006. [DOI] [PubMed] [Google Scholar]
- 499.Konduri GG, Ou J, Shi Y, Pritchard KA Jr. Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 285: H204–H211, 2003. doi: 10.1152/ajpheart.00837.2002. [DOI] [PubMed] [Google Scholar]
- 500.Konje JC, Howarth ES, Kaufmann P, Taylor DJ. Longitudinal quantification of uterine artery blood volume flow changes during gestation in pregnancies complicated by intrauterine growth restriction. BJOG 110: 301–305, 2003. doi: 10.1046/j.1471-0528.2003.t01-1-02163.x. [DOI] [PubMed] [Google Scholar]
- 501.Konje JC, Kaufmann P, Bell SC, Taylor DJ. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am J Obstet Gynecol 185: 608–613, 2001. doi: 10.1067/mob.2001.117187. [DOI] [PubMed] [Google Scholar]
- 502.Koos BJ, Rajaee A. Fetal breathing movements and changes at birth. Adv Exp Med Biol 814: 89–101, 2014. doi: 10.1007/978-1-4939-1031-1_8. [DOI] [PubMed] [Google Scholar]
- 503.Korkes HA, De Oliveira L, Sass N, Salahuddin S, Karumanchi SA, Rajakumar A. Relationship between hypoxia and downstream pathogenic pathways in preeclampsia. Hypertens Pregnancy 36: 145–150, 2017. doi: 10.1080/10641955.2016.1259627. [DOI] [PubMed] [Google Scholar]
- 504.Korostowski L, Sedlak N, Engel N. The Kcnq1ot1 long non-coding RNA affects chromatin conformation and expression of Kcnq1, but does not regulate its imprinting in the developing heart. PLoS Genet 8: e1002956, 2012. doi: 10.1371/journal.pgen.1002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Korsmeyer SJ. Regulators of cell death. Trends Genet 11: 101–105, 1995. doi: 10.1016/S0168-9525(00)89010-1. [DOI] [PubMed] [Google Scholar]
- 506.Koslowski M, Luxemburger U, Türeci O, Sahin U. Tumor-associated CpG demethylation augments hypoxia-induced effects by positive autoregulation of HIF-1α. Oncogene 30: 876–882, 2011. doi: 10.1038/onc.2010.481. [DOI] [PubMed] [Google Scholar]
- 507.Kotecha S, Chan B, Azam N, Silverman M, Shaw RJ. Increase in interleukin-8 and soluble intercellular adhesion molecule-1 in bronchoalveolar lavage fluid from premature infants who develop chronic lung disease. Arch Dis Child Fetal Neonatal Ed 72: F90–F96, 1995. doi: 10.1136/fn.72.2.F90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Kou CY, Lau SL, Au KW, Leung PY, Chim SS, Fung KP, Waye MM, Tsui SK. Epigenetic regulation of neonatal cardiomyocytes differentiation. Biochem Biophys Res Commun 400: 278–283, 2010. doi: 10.1016/j.bbrc.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 509.Kouzarides T. Chromatin modifications and their function. Cell 128: 693–705, 2007. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 510.Kovacs P, Bak I, Szendrei L, Vecsernyes M, Varga E, Blasig IE, Tosaki A. Non-specific caspase inhibition reduces infarct size and improves post-ischaemic recovery in isolated ischaemic/reperfused rat hearts. Naunyn Schmiedebergs Arch Pharmacol 364: 501–507, 2001. doi: 10.1007/s002100100483. [DOI] [PubMed] [Google Scholar]
- 511.Krampl E, Lees C, Bland JM, Espinoza Dorado J, Moscoso G, Campbell S. Fetal biometry at 4300 m compared to sea level in Peru. Ultrasound Obstet Gynecol 16: 9–18, 2000. doi: 10.1046/j.1469-0705.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- 512.Krieger DT, Liotta AS, Hauser H, Brownstein MJ. Effect of stress, adrenocorticotropin or corticosteroid treatment, adrenalectomy, or hypophysectomy on hypothalamic immunoreactive adrenocorticotropin concentrations. Endocrinology 105: 737–742, 1979. doi: 10.1210/endo-105-3-737. [DOI] [PubMed] [Google Scholar]
- 513.Krishnan J, Ahuja P, Bodenmann S, Knapik D, Perriard E, Krek W, Perriard JC. Essential role of developmentally activated hypoxia-inducible factor 1alpha for cardiac morphogenesis and function. Circ Res 103: 1139–1146, 2008. doi: 10.1161/01.RES.0000338613.89841.c1. [DOI] [PubMed] [Google Scholar]
- 514.Krüger H, Arias-Stella J. The placenta and the newborn infant at high altitudes. Am J Obstet Gynecol 106: 586–591, 1970. doi: 10.1016/0002-9378(70)90045-1. [DOI] [PubMed] [Google Scholar]
- 515.Kublickiene KR, Lindblom B, Krüger K, Nisell H. Preeclampsia: evidence for impaired shear stress-mediated nitric oxide release in uterine circulation. Am J Obstet Gynecol 183: 160–166, 2000. doi: 10.1016/S0002-9378(00)41620-0. [DOI] [PubMed] [Google Scholar]
- 516.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol 27: 1859–1867, 2007. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Kumar S, Sud N, Fonseca FV, Hou Y, Black SM. Shear stress stimulates nitric oxide signaling in pulmonary arterial endothelial cells via a reduction in catalase activity: role of protein kinase C δ. Am J Physiol Lung Cell Mol Physiol 298: L105–L116, 2010. doi: 10.1152/ajplung.00290.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Kurian L, Aguirre A, Sancho-Martinez I, Benner C, Hishida T, Nguyen TB, Reddy P, Nivet E, Krause MN, Nelles DA, Esteban CR, Campistol JM, Yeo GW, Belmonte JCI. Identification of novel long noncoding RNAs underlying vertebrate cardiovascular development. Circulation 131: 1278–1290, 2015. doi: 10.1161/CIRCULATIONAHA.114.013303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Kurlak LO, Mistry HD, Cindrova-Davies T, Burton GJ, Broughton Pipkin F. Human placental renin-angiotensin system in normotensive and pre-eclamptic pregnancies at high altitude and after acute hypoxia-reoxygenation insult. J Physiol 594: 1327–1340, 2016. doi: 10.1113/JP271045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Kwapiszewska G, Wilhelm J, Wolff S, Laumanns I, Koenig IR, Ziegler A, Seeger W, Bohle RM, Weissmann N, Fink L. Expression profiling of laser-microdissected intrapulmonary arteries in hypoxia-induced pulmonary hypertension. Respir Res 6: 109, 2005. doi: 10.1186/1465-9921-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Lai KP, Li JW, Chan CY, Chan TF, Yuen KW, Chiu JM. Transcriptomic alterations in Daphnia magna embryos from mothers exposed to hypoxia. Aquat Toxicol 177: 454–463, 2016. doi: 10.1016/j.aquatox.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 522.Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 174: 1370–1377, 2006. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Lakshminrusimha S, Swartz DD, Gugino SF, Ma CX, Wynn KA, Ryan RM, Russell JA, Steinhorn RH. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res 66: 539–544, 2009. doi: 10.1203/PDR.0b013e3181bab0c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Lalevée S, Lapaire O, Bühler M. miR455 is linked to hypoxia signaling and is deregulated in preeclampsia. Cell Death Dis 5: e1408, 2014. doi: 10.1038/cddis.2014.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol 207: 3163–3169, 2004. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- 526.LaManna JC, Vendel LM, Farrell RM. Brain adaptation to chronic hypobaric hypoxia in rats. J Appl Physiol (1985) 72: 2238–2243, 1992. doi: 10.1152/jappl.1992.72.6.2238. [DOI] [PubMed] [Google Scholar]
- 527.Lang U, Baker RS, Braems G, Zygmunt M, Künzel W, Clark KE. Uterine blood flow–a determinant of fetal growth. Eur J Obstet Gynecol Reprod Biol 110, Suppl 1: S55–S61, 2003. doi: 10.1016/S0301-2115(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 528.Laprell F, Finkl K, Müller J. Propagation of Polycomb-repressed chromatin requires sequence-specific recruitment to DNA. Science 356: 85–88, 2017. doi: 10.1126/science.aai8266. [DOI] [PubMed] [Google Scholar]
- 529.Lassègue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30: 653–661, 2010. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Lau E, Cao Q, Ng DC, Bleakley BJ, Dincer TU, Bot BM, Wang D, Liem DA, Lam MP, Ge J, Ping P. A large dataset of protein dynamics in the mammalian heart proteome. Sci Data 3: 160015, 2016. doi: 10.1038/sdata.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Laukka T, Mariani CJ, Ihantola T, Cao JZ, Hokkanen J, Kaelin WG Jr, Godley LA, Koivunen P. Fumarate and Succinate Regulate Expression of Hypoxia-inducible Genes via TET Enzymes. J Biol Chem 291: 4256–4265, 2016. doi: 10.1074/jbc.M115.688762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Laviola G, Adriani W, Rea M, Aloe L, Alleva E. Social withdrawal, neophobia, and stereotyped behavior in developing rats exposed to neonatal asphyxia. Psychopharmacology (Berl) 175: 196–205, 2004. doi: 10.1007/s00213-004-1800-3. [DOI] [PubMed] [Google Scholar]
- 533.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system–an endocrine and paracrine system. Endocrinology 144: 2179–2183, 2003. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 534.Lawrence J, Chen M, Xiong F, Xiao D, Zhang H, Buchholz JN, Zhang L. Foetal nicotine exposure causes PKCε gene repression by promoter methylation in rat hearts. Cardiovasc Res 89: 89–97, 2011. doi: 10.1093/cvr/cvq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Lawrence J, Xiao D, Xue Q, Rejali M, Yang S, Zhang L. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J Pharmacol Exp Ther 324: 331–341, 2008. doi: 10.1124/jpet.107.132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–78, 2006. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 537.Lee AM, Morrison JL, Botting KJ, Shandala T, Xian CJ. Effects of Maternal Hypoxia during Pregnancy on Bone Development in Offspring: A Guinea Pig Model. Int J Endocrinol 2014: 916918, 2014. doi: 10.1155/2014/916918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Lee DC, Romero R, Kim JS, Tarca AL, Montenegro D, Pineles BL, Kim E, Lee J, Kim SY, Draghici S, Mittal P, Kusanovic JP, Chaiworapongsa T, Hassan SS, Kim CJ. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am J Pathol 179: 590–602, 2011. doi: 10.1016/j.ajpath.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim MM, Srikantan S, Martindale JL, Hutchison ER, Kim HH, Marasa BS, Selimyan R, Egan JM, Smith SR, Fried SK, Gorospe M. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol 31: 626–638, 2011. doi: 10.1128/MCB.00894-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Lee HS, Han J, Bai HJ, Kim KW. Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. FEBS J 276: 4622–4635, 2009. doi: 10.1111/j.1742-4658.2009.07174.x. [DOI] [PubMed] [Google Scholar]
- 541.Lee JE, Ge K. Transcriptional and epigenetic regulation of PPARγ expression during adipogenesis. Cell Biosci 4: 29, 2014. doi: 10.1186/2045-3701-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Lee JS, Smith E, Shilatifard A. The language of histone crosstalk. Cell 142: 682–685, 2010. doi: 10.1016/j.cell.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843–854, 1993. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 544.Lee SA, Ding C. The dysfunctional placenta epigenome: causes and consequences. Epigenomics 4: 561–569, 2012. doi: 10.2217/epi.12.49. [DOI] [PubMed] [Google Scholar]
- 545.Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA 97: 11032–11037, 2000. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol 18: 383–439, 1997. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 547.Leonard MO, Howell K, Madden SF, Costello CM, Higgins DG, Taylor CT, McLoughlin P. Hypoxia selectively activates the CREB family of transcription factors in the in vivo lung. Am J Respir Crit Care Med 178: 977–983, 2008. doi: 10.1164/rccm.200712-1890OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci 6: 108–118, 2005. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- 549.Levenson JM, Sweatt JD. Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell Mol Life Sci 63: 1009–1016, 2006. doi: 10.1007/s00018-006-6026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann N Y Acad Sci 1021: 64–76, 2004. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- 551.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69: 915–926, 1992. doi: 10.1016/0092-8674(92)90611-F. [DOI] [PubMed] [Google Scholar]
- 552.Li G, Bae S, Zhang L. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am J Physiol Heart Circ Physiol 286: H1712–H1719, 2004. doi: 10.1152/ajpheart.00898.2003. [DOI] [PubMed] [Google Scholar]
- 553.Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig 10: 265–274, 2003. doi: 10.1016/S1071-55760300074-1. [DOI] [PubMed] [Google Scholar]
- 554.Li L, Welser JV, Milner R. Absence of the alpha v beta 3 integrin dictates the time-course of angiogenesis in the hypoxic central nervous system: accelerated endothelial proliferation correlates with compensatory increases in alpha 5 beta 1 integrin expression. J Cereb Blood Flow Metab 30: 1031–1043, 2010. doi: 10.1038/jcbfm.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Li X, Wang J, Jiang Z, Guo F, Soloway PD, Zhao R. Role of PRDM16 and its PR domain in the epigenetic regulation of myogenic and adipogenic genes during transdifferentiation of C2C12 cells. Gene 570: 191–198, 2015. doi: 10.1016/j.gene.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 556.Li Y, Gonzalez P, Zhang L. Fetal stress and programming of hypoxic/ischemic-sensitive phenotype in the neonatal brain: mechanisms and possible interventions. Prog Neurobiol 98: 145–165, 2012. doi: 10.1016/j.pneurobio.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Li Y, Ma Q, Dasgupta C, Halavi S, Hartman RE, Xiao D, Zhang L. Inhibition of DNA Methylation in the Developing Rat Brain Disrupts Sexually Dimorphic Neurobehavioral Phenotypes in Adulthood. Mol Neurobiol 54: 3988–3999, 2017. doi: 10.1007/s12035-016-9957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Li Y, Ma Q, Halavi S, Concepcion K, Hartman RE, Obenaus A, Xiao D, Zhang L. Fetal stress-mediated hypomethylation increases the brain susceptibility to hypoxic-ischemic injury in neonatal rats. Exp Neurol 275: 1–10, 2016. doi: 10.1016/j.expneurol.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Li Y, Xiao D, Dasgupta C, Xiong F, Tong W, Yang S, Zhang L. Perinatal nicotine exposure increases vulnerability of hypoxic-ischemic brain injury in neonatal rats: role of angiotensin II receptors. Stroke 43: 2483–2490, 2012. doi: 10.1161/STROKEAHA.112.664698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Li Y, Xiao D, Yang S, Zhang L. Promoter methylation represses AT2R gene and increases brain hypoxic-ischemic injury in neonatal rats. Neurobiol Dis 60: 32–38, 2013. doi: 10.1016/j.nbd.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Liang Y, Fang M, Li J, Yew DT. Immunohistochemical localization of endothelial isoform (eNOS) in human cerebral arteries and the aorta. Int J Neurosci 116: 1403–1417, 2006. doi: 10.1080/00207450500514375. [DOI] [PubMed] [Google Scholar]
- 562.Libby P, Maroko PR, Bloor CM, Sobel BE, Braunwald E. Reduction of experimental myocardial infarct size by corticosteroid administration. J Clin Invest 52: 599–607, 1973. doi: 10.1172/JCI107221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Lichty JA, Ting RY, Bruns PD, Dyar E. Studies of babies born at high altitudes. I. Relation of altitude to birth weight. AMA J Dis Child 93: 666–669, 1957. doi: 10.1001/archpedi.1957.02060040668009. [DOI] [PubMed] [Google Scholar]
- 564.Lim CR, Fukakusa A, Matsubara K. Gene expression profiling of mouse postnatal cerebellar development using cDNA microarrays. Gene 333: 3–13, 2004. doi: 10.1016/j.gene.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 565.Lim K, Zimanyi MA, Black MJ. Effect of maternal protein restriction during pregnancy and lactation on the number of cardiomyocytes in the postproliferative weanling rat heart. Anat Rec (Hoboken) 293: 431–437, 2010. doi: 10.1002/ar.21084. [DOI] [PubMed] [Google Scholar]
- 566.Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z. A role of miR-27 in the regulation of adipogenesis. FEBS J 276: 2348–2358, 2009. doi: 10.1111/j.1742-4658.2009.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Linask KK, Han M, Cai DH, Brauer PR, Maisastry SM. Cardiac morphogenesis: matrix metalloproteinase coordination of cellular mechanisms underlying heart tube formation and directionality of looping. Dev Dyn 233: 739–753, 2005. doi: 10.1002/dvdy.20377. [DOI] [PubMed] [Google Scholar]
- 568.Liu H, Tang Y, Liu X, Zhou Q, Xiao X, Lan F, Li X, Hu R, Xiong Y, Peng T. 14-3-3 tau (YWHAQ) gene promoter hypermethylation in human placenta of preeclampsia. Placenta 35: 981–988, 2014. doi: 10.1016/j.placenta.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 569.Liu H, Zhang HY, Zhu X, Shao Z, Yao Z. Preconditioning blocks cardiocyte apoptosis: role of K(ATP) channels and PKC-epsilon. Am J Physiol Heart Circ Physiol 282: H1380–H1386, 2002. doi: 10.1152/ajpheart.00348.2001. [DOI] [PubMed] [Google Scholar]
- 570.Liu J, Gao Y, Negash S, Longo LD, Raj JU. Long-term effects of prenatal hypoxia on endothelium-dependent relaxation responses in pulmonary arteries of adult sheep. Am J Physiol Lung Cell Mol Physiol 296: L547–L554, 2009. doi: 10.1152/ajplung.90333.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Liu JP, Clarke IJ, Funder JW, Engler D. Studies of the secretion of corticotropin-releasing factor and arginine vasopressin into the hypophysial-portal circulation of the conscious sheep. II. The central noradrenergic and neuropeptide Y pathways cause immediate and prolonged hypothalamic-pituitary-adrenal activation. Potential involvement in the pseudo-Cushing’s syndrome of endogenous depression and anorexia nervosa. J Clin Invest 93: 1439–1450, 1994. doi: 10.1172/JCI117121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 573.Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev 22: 3242–3254, 2008. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Liu S, Liu J, Tang J, Ji J, Chen J, Liu C. Environmental risk factors for congenital heart disease in the Shandong Peninsula, China: a hospital-based case-control study. J Epidemiol 19: 122–130, 2009. doi: 10.2188/jea.JE20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Liu W, Bi P, Shan T, Yang X, Yin H, Wang YX, Liu N, Rudnicki MA, Kuang S. miR-133a regulates adipocyte browning in vivo. PLoS Genet 9: e1003626, 2013. doi: 10.1371/journal.pgen.1003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Liu X, Wang C, Liu W, Li J, Li C, Kou X, Chen J, Zhao Y, Gao H, Wang H, Zhang Y, Gao Y, Gao S. Distinct features of H3K4me3 and H3K27me3 chromatin domains in pre-implantation embryos. Nature 537: 558–562, 2016. doi: 10.1038/nature19362. [DOI] [PubMed] [Google Scholar]
- 577.Liu Z, Yue S, Chen X, Kubin T, Braun T. Regulation of cardiomyocyte polyploidy and multinucleation by CyclinG1. Circ Res 106: 1498–1506, 2010. doi: 10.1161/CIRCRESAHA.109.211888. [DOI] [PubMed] [Google Scholar]
- 578.Llanos AJ, Ebensperger G, Herrera EA, Reyes RV, Pulgar VM, Serón-Ferré M, Díaz M, Parer JT, Giussani DA, Moraga FA, Riquelme RA. Fetal and postnatal pulmonary circulation in the Alto Andino. Placenta 32, Suppl 2: S100–S103, 2011. doi: 10.1016/j.placenta.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 579.Loan Le TY, Mardini M, Howell VM, Funder JW, Ashton AW, Mihailidou AS. Low-dose spironolactone prevents apoptosis repressor with caspase recruitment domain degradation during myocardial infarction. Hypertension 59: 1164–1169, 2012. doi: 10.1161/HYPERTENSIONAHA.111.190488. [DOI] [PubMed] [Google Scholar]
- 580.Lockhart M, Wirrig E, Phelps A, Wessels A. Extracellular matrix and heart development. Birth Defects Res A Clin Mol Teratol 91: 535–550, 2011. doi: 10.1002/bdra.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Loenarz C, Ge W, Coleman ML, Rose NR, Cooper CD, Klose RJ, Ratcliffe PJ, Schofield CJ. PHF8, a gene associated with cleft lip/palate and mental retardation, encodes for an Nepsilon-dimethyl lysine demethylase. Hum Mol Genet 19: 217–222, 2010. doi: 10.1093/hmg/ddp480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, Manos PD, Datta S, Lander ES, Schlaeger TM, Daley GQ, Rinn JL. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet 42: 1113–1117, 2010. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Lolmède K, Durand de Saint Front V, Galitzky J, Lafontan M, Bouloumié A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int J Obes Relat Metab Disord 27: 1187–1195, 2003. doi: 10.1038/sj.ijo.0802407. [DOI] [PubMed] [Google Scholar]
- 584.Long NM, Ford SP, Nathanielsz PW. Maternal obesity eliminates the neonatal lamb plasma leptin peak. J Physiol 589: 1455–1462, 2011. doi: 10.1113/jphysiol.2010.201681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Long W, Zhang L, Longo LD. Fetal and adult cerebral artery K(ATP) and K(Ca) channel responses to long-term hypoxia. J Appl Physiol (1985) 92: 1692–1701, 2002. doi: 10.1152/japplphysiol.01110.2001. [DOI] [PubMed] [Google Scholar]
- 586.Longo LD. Respiratory Gas Exchange in the Placenta. In: Handbook of Physiology. The Respiratory System. Gas Exchange. Bethesda, MD: Am. Physiol. Soc, 1987, sect. 3, vol. IV, p. 351–401. [Google Scholar]
- 587.Longo LD, Hull AD, Long DM, Pearce WJ. Cerebrovascular adaptations to high-altitude hypoxemia in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol 264: R65–R72, 1993. [DOI] [PubMed] [Google Scholar]
- 588.Longo LD, Packianathan S. Hypoxia-ischaemia and the developing brain: hypotheses regarding the pathophysiology of fetal-neonatal brain damage. Br J Obstet Gynaecol 104: 652–662, 1997. doi: 10.1111/j.1471-0528.1997.tb11974.x. [DOI] [PubMed] [Google Scholar]
- 589.Longo LD, Pearce WJ. Fetal cerebrovascular acclimatization responses to high-altitude, long-term hypoxia: a model for prenatal programming of adult disease? Am J Physiol Regul Integr Comp Physiol 288: R16–R24, 2005. doi: 10.1152/ajpregu.00462.2004. [DOI] [PubMed] [Google Scholar]
- 590.Longo LD, Pearce WJ. High altitude, hypoxic-induced modulation of noradrenergic-mediated responses in fetal and adult cerebral arteries. Comp Biochem Physiol A Mol Integr Physiol 119: 683–694, 1998. doi: 10.1016/S1095-6433(98)01006-X. [DOI] [PubMed] [Google Scholar]
- 591.Looney AM, Walsh BH, Moloney G, Grenham S, Fagan A, O’Keeffe GW, Clarke G, Cryan JF, Dinan TG, Boylan GB, Murray DM. Downregulation of Umbilical Cord Blood Levels of miR-374a in Neonatal Hypoxic Ischemic Encephalopathy. J Pediatr 167: 269–73.e2, 2015. doi: 10.1016/j.jpeds.2015.04.060. [DOI] [PubMed] [Google Scholar]
- 592.Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, Khan TM, Koul PA, Guchhait P, Salama ME, Xing J, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorde LB, Kaelin WG Jr, Koivunen P, Prchal JT. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet 46: 951–956, 2014. doi: 10.1038/ng.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Loscalzo J. The cellular response to hypoxia: tuning the system with microRNAs. J Clin Invest 120: 3815–3817, 2010. doi: 10.1172/JCI45105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Lother A, Berger S, Gilsbach R, Rösner S, Ecke A, Barreto F, Bauersachs J, Schütz G, Hein L. Ablation of mineralocorticoid receptors in myocytes but not in fibroblasts preserves cardiac function. Hypertension 57: 746–754, 2011. doi: 10.1161/HYPERTENSIONAHA.110.163287. [DOI] [PubMed] [Google Scholar]
- 595.Lou YL, Guo F, Liu F, Gao FL, Zhang PQ, Niu X, Guo SC, Yin JH, Wang Y, Deng ZF. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol Cell Biochem 370: 45–51, 2012. doi: 10.1007/s11010-012-1396-6. [DOI] [PubMed] [Google Scholar]
- 596.Louey S, Jonker SS, Giraud GD, Thornburg KL. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J Physiol 580: 639–648, 2007. doi: 10.1113/jphysiol.2006.122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Lu X, Bijli KM, Ramirez A, Murphy TC, Kleinhenz J, Hart CM. Hypoxia downregulates PPARγ via an ERK1/2-NF-κB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells. Free Radic Biol Med 63: 151–160, 2013. doi: 10.1016/j.freeradbiomed.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Lubin FD, Gupta S, Parrish RR, Grissom NM, Davis RL. Epigenetic mechanisms: critical contributors to long-term memory formation. Neuroscientist 17: 616–632, 2011. doi: 10.1177/1073858410386967. [DOI] [PubMed] [Google Scholar]
- 599.Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol 19: 743–751, 2007. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 600.Lumbers ER, Boyce AC, Joulianos G, Kumarasamy V, Barner E, Segar JL, Burrell JH. Effects of cortisol on cardiac myocytes and on expression of cardiac genes in fetal sheep. Am J Physiol Regul Integr Comp Physiol 288: R567–R574, 2005. doi: 10.1152/ajpregu.00556.2004. [DOI] [PubMed] [Google Scholar]
- 601.Luo GZ, Blanco MA, Greer EL, He C, Shi Y. DNA N(6)-methyladenine: a new epigenetic mark in eukaryotes? Nat Rev Mol Cell Biol 16: 705–710, 2015. doi: 10.1038/nrm4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Luo R, Shao X, Xu P, Liu Y, Wang Y, Zhao Y, Liu M, Ji L, Li YX, Chang C, Qiao J, Peng C, Wang YL. MicroRNA-210 contributes to preeclampsia by downregulating potassium channel modulatory factor 1. Hypertension 64: 839–845, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03530. [DOI] [PubMed] [Google Scholar]
- 603.Luo R, Wang Y, Xu P, Cao G, Zhao Y, Shao X, Li YX, Chang C, Peng C, Wang YL. Hypoxia-inducible miR-210 contributes to preeclampsia via targeting thrombospondin type I domain containing 7A. Sci Rep 6: 19588, 2016. doi: 10.1038/srep19588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension 62: 1046–1054, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01892. [DOI] [PubMed] [Google Scholar]
- 605.Ma Q, Dasgupta C, Li Y, Bajwa NM, Xiong F, Harding B, Hartman R, Zhang L. Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol Dis 89: 202–212, 2016. doi: 10.1016/j.nbd.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Ma Q, Xiong F, Zhang L. Gestational hypoxia and epigenetic programming of brain development disorders. Drug Discov Today 19: 1883–1896, 2014. doi: 10.1016/j.drudis.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Ma Q, Zhang L. Epigenetic programming of hypoxic-ischemic encephalopathy in response to fetal hypoxia. Prog Neurobiol 124: 28–48, 2015. doi: 10.1016/j.pneurobio.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Magariños AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci USA 94: 14002–14008, 1997. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Magness RR, Phernetton TM, Gibson TC, Chen DB. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J Physiol 565: 71–83, 2005. doi: 10.1113/jphysiol.2005.086439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Magness RR, Phernetton TM, Zheng J. Systemic and uterine blood flow distribution during prolonged infusion of 17beta-estradiol. Am J Physiol Heart Circ Physiol 275: H731–H743, 1998. [DOI] [PubMed] [Google Scholar]
- 611.Magness RR, Shaw CE, Phernetton TM, Zheng J, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am J Physiol Heart Circ Physiol 272: H1730–H1740, 1997. [DOI] [PubMed] [Google Scholar]
- 612.Maheshwari A, Voitenok NN, Akalovich S, Shaik SS, Randolph DA, Sims B, Patel RP, Killingsworth CR, Fallon MB, Ohls RK. Developmental changes in circulating IL-8/CXCL8 isoforms in neonates. Cytokine 46: 12–16, 2009. doi: 10.1016/j.cyto.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 497: 249–253, 2013. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Malendowicz LK, Neri G, Markowska A, Hochol A, Nussdorfer GG, Majchrzak M. Effects of leptin and leptin fragments on steroid secretion of freshly dispersed rat adrenocortical cells. J Steroid Biochem Mol Biol 87: 265–268, 2003. doi: 10.1016/j.jsbmb.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 615.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2294, 2007. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 616.Maltepe E, Krampitz GW, Okazaki KM, Red-Horse K, Mak W, Simon MC, Fisher SJ. Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development 132: 3393–3403, 2005. doi: 10.1242/dev.01923. [DOI] [PubMed] [Google Scholar]
- 617.Mamet J, Peyronnet J, Roux JC, Perrin D, Cottet-Emard JM, Pequignot JM, Lagercrantz H, Dalmaz Y. Long-term prenatal hypoxia alters maturation of adrenal medulla in rat. Pediatr Res 51: 207–214, 2002. doi: 10.1203/00006450-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 618.Mandalà M, Gokina N, Osol G. Contribution of nonendothelial nitric oxide to altered rat uterine resistance artery serotonin reactivity during pregnancy. Am J Obstet Gynecol 187: 463–468, 2002. doi: 10.1067/mob.2002.123894. [DOI] [PubMed] [Google Scholar]
- 619.Mani SK, Kern CB, Kimbrough D, Addy B, Kasiganesan H, Rivers WT, Patel RK, Chou JC, Spinale FG, Mukherjee R, Menick DR. Inhibition of class I histone deacetylase activity represses matrix metalloproteinase-2 and -9 expression and preserves LV function postmyocardial infarction. Am J Physiol Heart Circ Physiol 308: H1391–H1401, 2015. doi: 10.1152/ajpheart.00390.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Marijianowski MM, van der Loos CM, Mohrschladt MF, Becker AE. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J Am Coll Cardiol 23: 1204–1208, 1994. doi: 10.1016/0735-1097(94)90612-2. [DOI] [PubMed] [Google Scholar]
- 621.Marino M, Bény JL, Peyter AC, Diaceri G, Tolsa JF. Perinatal hypoxia enhances cyclic adenosine monophosphate-mediated BKCa channel activation in adult murine pulmonary artery. J Cardiovasc Pharmacol 57: 154–165, 2011. doi: 10.1097/FJC.0b013e3182016adf. [DOI] [PubMed] [Google Scholar]
- 622.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J 222: 649–655, 1984. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 6: 838–849, 2005. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 624.Martin-Gronert MS, Ozanne SE. Maternal nutrition during pregnancy and health of the offspring. Biochem Soc Trans 34: 779–782, 2006. doi: 10.1042/BST0340779. [DOI] [PubMed] [Google Scholar]
- 625.Martinez SR, Gay MS, Zhang L. Epigenetic mechanisms in heart development and disease. Drug Discov Today 20: 799–811, 2015. doi: 10.1016/j.drudis.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Martinez SR, Ma Q, Dasgupta C, Meng X, Zhang L. MicroRNA-210 suppresses glucocorticoid receptor expression in response to hypoxia in fetal rat cardiomyocytes. Oncotarget 8: 80249–80264, 2017. doi: 10.18632/oncotarget.17801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Mateev S, Sillau AH, Mouser R, McCullough RE, White MM, Young DA, Moore LG. Chronic hypoxia opposes pregnancy-induced increase in uterine artery vasodilator response to flow. Am J Physiol Heart Circ Physiol 284: H820–H829, 2003. doi: 10.1152/ajpheart.00701.2002. [DOI] [PubMed] [Google Scholar]
- 628.Mateus J, Bytautiene E, Lu F, Tamayo EH, Betancourt A, Hankins GD, Longo M, Saade GR. Endothelial growth factor therapy improves preeclampsia-like manifestations in a murine model induced by overexpression of sVEGFR-1. Am J Physiol Heart Circ Physiol 301: H1781–H1787, 2011. doi: 10.1152/ajpheart.00373.2011. [DOI] [PubMed] [Google Scholar]
- 629.Matoba S, Liu Y, Lu F, Iwabuchi KA, Shen L, Inoue A, Zhang Y. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell 159: 884–895, 2014. doi: 10.1016/j.cell.2014.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Matthews SG. Antenatal glucocorticoids and the developing brain: mechanisms of action. Semin Neonatol 6: 309–317, 2001. doi: 10.1053/siny.2001.0066. [DOI] [PubMed] [Google Scholar]
- 631.Matthews SG, Challis JR. Regulation of CRH and AVP mRNA in the developing ovine hypothalamus: effects of stress and glucocorticoids. Am J Physiol Endocrinol Metab 268: E1096–E1107, 1995. [DOI] [PubMed] [Google Scholar]
- 632.Matthews SG, Challis JR. Regulation of the hypothalamo-pituitary-adrenocortical axis in fetal sheep. Trends Endocrinol Metab 7: 239–246, 1996. doi: 10.1016/S1043-2760(96)00126-9. [DOI] [PubMed] [Google Scholar]
- 633.Mbaku EM, Zhang L, Pearce WJ, Duckles SP, Buchholz J. Chronic hypoxia alters the function of NOS nerves in cerebral arteries of near-term fetal and adult sheep. J Appl Physiol (1985) 94: 724–732, 2003. doi: 10.1152/japplphysiol.00771.2002. [DOI] [PubMed] [Google Scholar]
- 634.McAuliffe F, Kametas N, Krampl E, Ernsting J, Nicolaides K. Blood gases in pregnancy at sea level and at high altitude. BJOG 108: 980–985, 2001. [DOI] [PubMed] [Google Scholar]
- 635.McCabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol 13: 425–431, 2001. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- 636.McCormick CM, Green MR, Simone JJ. Translational relevance of rodent models of hypothalamic-pituitary-adrenal function and stressors in adolescence. Neurobiol Stress 6: 31–43, 2017. doi: 10.1016/j.ynstr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.McCullough RE, Reeves JT, Liljegren RL. Fetal growth retardation and increased infant mortality at high altitide. Arch Environ Health 32: 36–39, 1977. doi: 10.1080/00039896.1977.10667251. [DOI] [PubMed] [Google Scholar]
- 638.McDonald TJ, Nathanielsz PW. Bilateral destruction of the fetal paraventricular nuclei prolongs gestation in sheep. Am J Obstet Gynecol 165: 764–770, 1991. doi: 10.1016/0002-9378(91)90325-L. [DOI] [PubMed] [Google Scholar]
- 639.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci 1032: 1–7, 2004. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 640.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12: 342–348, 2009. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, Szyf M. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS One 6: e14739, 2011. doi: 10.1371/journal.pone.0014739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35: 901–918, 2003. doi: 10.1016/S1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- 643.McMillen IC, Edwards LJ, Duffield J, Muhlhausler BS. Regulation of leptin synthesis and secretion before birth: implications for the early programming of adult obesity. Reproduction 131: 415–427, 2006. doi: 10.1530/rep.1.00303. [DOI] [PubMed] [Google Scholar]
- 644.McMillen IC, Muhlhausler BS, Duffield JA, Yuen BS. Prenatal programming of postnatal obesity: fetal nutrition and the regulation of leptin synthesis and secretion before birth. Proc Nutr Soc 63: 405–412, 2004. doi: 10.1079/PNS2004370. [DOI] [PubMed] [Google Scholar]
- 645.Mcmillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85: 571–633, 2005. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 646.McNamara PJ, Murthy P, Kantores C, Teixeira L, Engelberts D, van Vliet T, Kavanagh BP, Jankov RP. Acute vasodilator effects of Rho-kinase inhibitors in neonatal rats with pulmonary hypertension unresponsive to nitric oxide. Am J Physiol Lung Cell Mol Physiol 294: L205–L213, 2008. doi: 10.1152/ajplung.00234.2007. [DOI] [PubMed] [Google Scholar]
- 647.Mdaki KS, Larsen TD, Wachal AL, Schimelpfenig MD, Weaver LJ, Dooyema SD, Louwagie EJ, Baack ML. Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction. Am J Physiol Heart Circ Physiol 310: H681–H692, 2016. doi: 10.1152/ajpheart.00795.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev 81: 41–79, 2010. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 649.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24: 1161–1192, 2001. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 650.Meaney MJ, Viau V, Bhatnagar S, Betito K, Iny LJ, O’Donnell D, Mitchell JB. Cellular mechanisms underlying the development and expression of individual differences in the hypothalamic-pituitary-adrenal stress response. J Steroid Biochem Mol Biol 39: 265–274, 1991. doi: 10.1016/0960-0760(91)90072-D. [DOI] [PubMed] [Google Scholar]
- 651.Mehedint MG, Craciunescu CN, Zeisel SH. Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc Natl Acad Sci USA 107: 12834–12839, 2010. doi: 10.1073/pnas.0914328107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Meher S, Duley L. Progesterone for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev (4): CD006175, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Mehta V, Abi-Nader KN, Peebles DM, Benjamin E, Wigley V, Torondel B, Filippi E, Shaw SW, Boyd M, Martin J, Zachary I, David AL. Long-term increase in uterine blood flow is achieved by local overexpression of VEGF-A(165) in the uterine arteries of pregnant sheep. Gene Ther 19: 925–935, 2012. doi: 10.1038/gt.2011.158. [DOI] [PubMed] [Google Scholar]
- 654.Ment LR, Stewart WB, Fronc R, Seashore C, Mahooti S, Scaramuzzino D, Madri JA. Vascular endothelial growth factor mediates reactive angiogenesis in the postnatal developing brain. Brain Res Dev Brain Res 100: 52–61, 1997. doi: 10.1016/S0165-3806(97)00012-6. [DOI] [PubMed] [Google Scholar]
- 655.Mercer JG, Moar KM, Rayner DV, Trayhurn P, Hoggard N. Regulation of leptin receptor and NPY gene expression in hypothalamus of leptin-treated obese (ob/ob) and cold-exposed lean mice. FEBS Lett 402: 185–188, 1997. doi: 10.1016/S0014-5793(96)01525-6. [DOI] [PubMed] [Google Scholar]
- 656.Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCepsilon gene repression in the fetal rat heart. J Mol Cell Cardiol 47: 504–511, 2009. doi: 10.1016/j.yjmcc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Meyer KD, Zhang H, Zhang L. Prenatal cocaine exposure abolished ischemic preconditioning-induced protection in adult male rat hearts: role of PKCepsilon. Am J Physiol Heart Circ Physiol 296: H1566–H1576, 2009. doi: 10.1152/ajpheart.00898.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Michalik KM, You X, Manavski Y, Doddaballapur A, Zörnig M, Braun T, John D, Ponomareva Y, Chen W, Uchida S, Boon RA, Dimmeler S. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res 114: 1389–1397, 2014. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 659.Mihailidou AS, Loan Le TY, Mardini M, Funder JW. Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension 54: 1306–1312, 2009. doi: 10.1161/HYPERTENSIONAHA.109.136242. [DOI] [PubMed] [Google Scholar]
- 660.Mikhailenko VA, Butkevich IP, Bagaeva TR, Makukhina GV, Otellin VA. Short- and long-term influences of hypoxia during early postnatal period of development on behavioral and hormonal responses in rats. Neurosci Lett 464: 214–217, 2009. doi: 10.1016/j.neulet.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 661.Miller S, Tudor C, Nyima, Thorsten VR, Sonam, Droyoung, Craig S, Le P, Wright LL, Varner MW. Maternal and neonatal outcomes of hospital vaginal deliveries in Tibet. Int J Gynaecol Obstet 98: 217–221, 2007. doi: 10.1016/j.ijgo.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Miller S, Tudor C, Thorsten V, Wright L, Varner M. Comparison of maternal and newborn outcomes of Tibetan and Han Chinese delivering in Lhasa, Tibet. J Obstet Gynaecol Res 34: 986–993, 2008. doi: 10.1111/j.1447-0756.2008.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Milner R, Hung S, Erokwu B, Dore-Duffy P, LaManna JC, del Zoppo GJ. Increased expression of fibronectin and the alpha 5 beta 1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol Cell Neurosci 38: 43–52, 2008. doi: 10.1016/j.mcn.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Min JY, Lim SO, Jung G. Downregulation of catalase by reactive oxygen species via hypermethylation of CpG island II on the catalase promoter. FEBS Lett 584: 2427–2432, 2010. doi: 10.1016/j.febslet.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 665.Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardiol 48: 1105–1110, 2010. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 666.Minchenko A, Caro J. Regulation of endothelin-1 gene expression in human microvascular endothelial cells by hypoxia and cobalt: role of hypoxia responsive element. Mol Cell Biochem 208: 53–62, 2000. doi: 10.1023/A:1007042729486. [DOI] [PubMed] [Google Scholar]
- 667.Mishra PK, Givvimani S, Chavali V, Tyagi SC. Cardiac matrix: a clue for future therapy. Biochim Biophys Acta 1832: 2271–2276, 2013. doi: 10.1016/j.bbadis.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol 5: R68, 2004. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Misra C, Chang SW, Basu M, Huang N, Garg V. Disruption of myocardial Gata4 and Tbx5 results in defects in cardiomyocyte proliferation and atrioventricular septation. Hum Mol Genet 23: 5025–5035, 2014. doi: 10.1093/hmg/ddu215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA 102: 9371–9376, 2005. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Mittal M, Roth M, König P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hänze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 672.Mizuuchi M, Cindrova-Davies T, Olovsson M, Charnock-Jones DS, Burton GJ, Yung HW. Placental endoplasmic reticulum stress negatively regulates transcription of placental growth factor via ATF4 and ATF6β: implications for the pathophysiology of human pregnancy complications. J Pathol 238: 550–561, 2016. doi: 10.1002/path.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Mizzen LA, Welch WJ. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol 106: 1105–1116, 1988. doi: 10.1083/jcb.106.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Mody M, Cao Y, Cui Z, Tay KY, Shyong A, Shimizu E, Pham K, Schultz P, Welsh D, Tsien JZ. Genome-wide gene expression profiles of the developing mouse hippocampus. Proc Natl Acad Sci USA 98: 8862–8867, 2001. doi: 10.1073/pnas.141244998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 1: Outcomes. Nat Rev Endocrinol 10: 391–402, 2014. doi: 10.1038/nrendo.2014.73. [DOI] [PubMed] [Google Scholar]
- 676.Molofsky AV, Deneen B. Astrocyte Development: A Guide for the Perplexed. Glia 63: 1320–1329, 2015. doi: 10.1002/glia.22836. [DOI] [PubMed] [Google Scholar]
- 677.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142, 1991. [PubMed] [Google Scholar]
- 678.Moore LG, Charles SM, Julian CG. Humans at high altitude: hypoxia and fetal growth. Respir Physiol Neurobiol 178: 181–190, 2011. doi: 10.1016/j.resp.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Moore LG, Hershey DW, Jahnigen D, Bowes W Jr. The incidence of pregnancy-induced hypertension is increased among Colorado residents at high altitude. Am J Obstet Gynecol 144: 423–429, 1982. doi: 10.1016/0002-9378(82)90248-4. [DOI] [PubMed] [Google Scholar]
- 680.Moore LG, Rounds SS, Jahnigen D, Grover RF, Reeves JT. Infant birth weight is related to maternal arterial oxygenation at high altitude. J Appl Physiol Respir Environ Exerc Physiol 52: 695–699, 1982. [DOI] [PubMed] [Google Scholar]
- 681.Moore LG, Shriver M, Bemis L, Hickler B, Wilson M, Brutsaert T, Parra E, Vargas E. Maternal adaptation to high-altitude pregnancy: an experiment of nature–a review. Placenta 25, Suppl A: S60–S71, 2004. doi: 10.1016/j.placenta.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 682.Moore LG, Young D, McCullough RE, Droma T, Zamudio S. Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am J Hum Biol 13: 635–644, 2001. doi: 10.1002/ajhb.1102. [DOI] [PubMed] [Google Scholar]
- 683.Moore LG, Zamudio S, Zhuang J, Sun S, Droma T. Oxygen transport in tibetan women during pregnancy at 3,658 m. Am J Phys Anthropol 114: 42–53, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 684.Moraga FA, Reyes RV, Herrera EA, Riquelme RA, Ebensperger G, Pulgar VM, Parer JT, Giussani DA, Llanos AJ. Role of the α-adrenergic system in femoral vascular reactivity in neonatal llamas and sheep: a comparative study between highland and lowland species. Am J Physiol Regul Integr Comp Physiol 301: R1153–R1160, 2011. doi: 10.1152/ajpregu.00124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Moresi V, Marroncelli N, Coletti D, Adamo S. Regulation of skeletal muscle development and homeostasis by gene imprinting, histone acetylation and microRNA. Biochim Biophys Acta 1849: 309–316, 2015. doi: 10.1016/j.bbagrm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 686.Mori A, Nishi H, Sasaki T, Nagamitsu Y, Kawaguchi R, Okamoto A, Kuroda M, Isaka K. HLA-G expression is regulated by miR-365 in trophoblasts under hypoxic conditions. Placenta 45: 37–41, 2016. doi: 10.1016/j.placenta.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 687.Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol Regul Integr Comp Physiol 293: R306–R313, 2007. doi: 10.1152/ajpregu.00798.2006. [DOI] [PubMed] [Google Scholar]
- 688.Morrison JL, Zhang S, Tellam RL, Brooks DA, McMillen IC, Porrello ER, Botting KJ. Regulation of microRNA during cardiomyocyte maturation in sheep. BMC Genomics 16: 541, 2015. doi: 10.1186/s12864-015-1693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 443: 289–295, 2006. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 690.Morton JS, Rueda-Clausen CF, Davidge ST. Flow-mediated vasodilation is impaired in adult rat offspring exposed to prenatal hypoxia. J Appl Physiol (1985) 110: 1073–1082, 2011. doi: 10.1152/japplphysiol.01174.2010. [DOI] [PubMed] [Google Scholar]
- 691.Mouillet JF, Chu T, Hubel CA, Nelson DM, Parks WT, Sadovsky Y. The levels of hypoxia-regulated microRNAs in plasma of pregnant women with fetal growth restriction. Placenta 31: 781–784, 2010. doi: 10.1016/j.placenta.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Mouillet JF, Donker RB, Mishima T, Cronqvist T, Chu T, Sadovsky Y. The unique expression and function of miR-424 in human placental trophoblasts. Biol Reprod 89: 25, 2013. doi: 10.1095/biolreprod.113.110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Mounien L, Bizet P, Boutelet I, Vaudry H, Jégou S. Expression of melanocortin MC3 and MC4 receptor mRNAs by neuropeptide Y neurons in the rat arcuate nucleus. Neuroendocrinology 82: 164–170, 2005. doi: 10.1159/000091737. [DOI] [PubMed] [Google Scholar]
- 694.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci 28: 9055–9065, 2008. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Muhlhausler BS, Adam CL, Findlay PA, Duffield JA, McMillen IC. Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J 20: 1257–1259, 2006. doi: 10.1096/fj.05-5241fje. [DOI] [PubMed] [Google Scholar]
- 696.Mühlhäusler BS, McMillen IC, Rouzaud G, Findlay PA, Marrocco EM, Rhind SM, Adam CL. Appetite regulatory neuropeptides are expressed in the sheep hypothalamus before birth. J Neuroendocrinol 16: 502–507, 2004. doi: 10.1111/j.1365-2826.2004.01197.x. [DOI] [PubMed] [Google Scholar]
- 697.Mukhopadhyay A, Ravikumar G, Meraaj H, Dwarkanath P, Thomas A, Crasta J, Thomas T, Kurpad AV, Sridhar TS. Placental expression of DNA methyltransferase 1 (DNMT1): Gender-specific relation with human placental growth. Placenta 48: 119–125, 2016. doi: 10.1016/j.placenta.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 698.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 5: 25–44, 1984. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 699.Muralimanoharan S, Guo C, Myatt L, Maloyan A. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int J Obes 39: 1274–1281, 2015. doi: 10.1038/ijo.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta 33: 816–823, 2012. doi: 10.1016/j.placenta.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Murray TV, Smyrnias I, Schnelle M, Mistry RK, Zhang M, Beretta M, Martin D, Anilkumar N, de Silva SM, Shah AM, Brewer AC. Redox regulation of cardiomyocyte cell cycling via an ERK1/2 and c-Myc-dependent activation of cyclin D2 transcription. J Mol Cell Cardiol 79: 54–68, 2015. doi: 10.1016/j.yjmcc.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Mwaikambo BR, Yang C, Chemtob S, Hardy P. Hypoxia up-regulates CD36 expression and function via hypoxia-inducible factor-1- and phosphatidylinositol 3-kinase-dependent mechanisms. J Biol Chem 284: 26695–26707, 2009. doi: 10.1074/jbc.M109.033480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Myatt L. Placental adaptive responses and fetal programming. J Physiol 572: 25–30, 2006. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Myatt L, Muralimanoharan S, Maloyan A. Effect of preeclampsia on placental function: influence of sexual dimorphism, microRNA’s and mitochondria. Adv Exp Med Biol 814: 133–146, 2014. doi: 10.1007/978-1-4939-1031-1_12. [DOI] [PubMed] [Google Scholar]
- 705.Myers DA, Bell PA, Hyatt K, Mlynarczyk M, Ducsay CA. Long-term hypoxia enhances proopiomelanocortin processing in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol 288: R1178–R1184, 2005. doi: 10.1152/ajpregu.00697.2004. [DOI] [PubMed] [Google Scholar]
- 706.Myers DA, Ducsay CA. Adrenocortical and adipose responses to high-altitude-induced, long-term hypoxia in the ovine fetus. J Pregnancy 2012: 681306, 2012. doi: 10.1155/2012/681306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Myers DA, Ducsay CA. Altitude, attitude and adaptation. Adv Exp Med Biol 814: 147–157, 2014. doi: 10.1007/978-1-4939-1031-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Myers DA, Hanson K, Mlynarczyk M, Kaushal KM, Ducsay CA. Long-term hypoxia modulates expression of key genes regulating adipose function in the late-gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 294: R1312–R1318, 2008. doi: 10.1152/ajpregu.00004.2008. [DOI] [PubMed] [Google Scholar]
- 709.Myers DA, Hyatt K, Mlynarczyk M, Bird IM, Ducsay CA. Long-term hypoxia represses the expression of key genes regulating cortisol biosynthesis in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol 289: R1707–R1714, 2005. doi: 10.1152/ajpregu.00343.2005. [DOI] [PubMed] [Google Scholar]
- 710.Myers DA, Robertshaw D, Nathanielsz PW. Effect of bilateral splanchnic nerve section on adrenal function in the ovine fetus. Endocrinology 127: 2328–2335, 1990. doi: 10.1210/endo-127-5-2328. [DOI] [PubMed] [Google Scholar]
- 711.Myers DA, Singleton K, Kaushal KM, Ducsay CA. Hepatic fibroblast growth factor 21 (FGF21) and perirenal adipose FGF receptor expression in the late gestation ovine fetus: modulation of expression by long term hypoxia. Reprod Sci 21: 271A, 2014. [Google Scholar]
- 712.Myers DA, Singleton K, Kenkel C, Kaushal KM, Ducsay CA. Gestational hypoxia modulates expression of corticotropin-releasing hormone and arginine vasopressin in the paraventricular nucleus in the ovine fetus. Physiol Rep 4: e12643, 2016. doi: 10.14814/phy2.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Naden RP, Rosenfeld CR. Effect of angiotensin II on uterine and systemic vasculature in pregnant sheep. J Clin Invest 68: 468–474, 1981. doi: 10.1172/JCI110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA. Hypoxia induces heart regeneration in adult mice. Nature 541: 222–227, 2017. doi: 10.1038/nature20173. [DOI] [PubMed] [Google Scholar]
- 715.Nakagawa M, Terracio L, Carver W, Birkedal-Hansen H, Borg TK. Expression of collagenase and IL-1 alpha in developing rat hearts. Dev Dyn 195: 87–99, 1992. doi: 10.1002/aja.1001950203. [DOI] [PubMed] [Google Scholar]
- 716.Nakanishi K, Tajima F, Nakamura A, Yagura S, Ookawara T, Yamashita H, Suzuki K, Taniguchi N, Ohno H. Effects of hypobaric hypoxia on antioxidant enzymes in rats. J Physiol 489: 869–876, 1995. doi: 10.1113/jphysiol.1995.sp021099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic Biol Med 64: 20–30, 2013. doi: 10.1016/j.freeradbiomed.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, Khan SA, Zhang X, Kinsman B, Peng YJ, Kumar GK, Fox AP, Godley LA, Semenza GL, Prabhakar NR. Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc Natl Acad Sci USA 109: 2515–2520, 2012. doi: 10.1073/pnas.1120600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Nascimento L, Freitas CM, Silva-Filho R, Leite AC, Silva AB, da Silva AI, Ferreira DS, Pedroza AA, Maia MB, Fernandes MP, Lagranha C. The effect of maternal low-protein diet on the heart of adult offspring: role of mitochondria and oxidative stress. Appl Physiol Nutr Metab 39: 880–887, 2014. doi: 10.1139/apnm-2013-0452. [DOI] [PubMed] [Google Scholar]
- 720.Nauli SM, Williams JM, Gerthoffer WT, Pearce WJ. Chronic hypoxia modulates relations among calcium, myosin light chain phosphorylation, and force differently in fetal and adult ovine basilar arteries. J Appl Physiol (1985) 99: 120–127, 2005. doi: 10.1152/japplphysiol.01131.2004. [DOI] [PubMed] [Google Scholar]
- 721.Negash S, Gao Y, Zhou W, Liu J, Chinta S, Raj JU. Regulation of cGMP-dependent protein kinase-mediated vasodilation by hypoxia-induced reactive species in ovine fetal pulmonary veins. Am J Physiol Lung Cell Mol Physiol 293: L1012–L1020, 2007. doi: 10.1152/ajplung.00061.2007. [DOI] [PubMed] [Google Scholar]
- 722.Neitzke U, Harder T, Plagemann A. Intrauterine growth restriction and developmental programming of the metabolic syndrome: a critical appraisal. Microcirculation 18: 304–311, 2011. doi: 10.1111/j.1549-8719.2011.00089.x. [DOI] [PubMed] [Google Scholar]
- 723.Nemeroff CB. The role of neuropeptides in the pathophysiology of affective disorders. Clin Neuropharmacol 15, Suppl 1 Pt A: 6A–7A, 1992. doi: 10.1097/00002826-199201001-00003. [DOI] [PubMed] [Google Scholar]
- 724.Nestler EJ, Gould E, Manji H. Preclinical models: status of basic research in depression. Biol Psychiatry 52: 503–528, 2002. doi: 10.1016/S0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- 725.Netuka I, Szarszoi O, Maly J, Besik J, Neckar J, Kolar F, Ostadalova I, Pirk J, Ostadal B. Effect of perinatal hypoxia on cardiac tolerance to acute ischaemia in adult male and female rats. Clin Exp Pharmacol Physiol 33: 714–719, 2006. doi: 10.1111/j.1440-1681.2006.04423.x. [DOI] [PubMed] [Google Scholar]
- 726.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S, Caniggia I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol 291: R1085–R1093, 2006. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Newby EA, Myers DA, Ducsay CA. Fetal endocrine and metabolic adaptations to hypoxia: the role of the hypothalamic-pituitary-adrenal axis. Am J Physiol Endocrinol Metab 309: E429–E439, 2015. doi: 10.1152/ajpendo.00126.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Ng SY, Johnson R, Stanton LW. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J 31: 522–533, 2012. doi: 10.1038/emboj.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Nguyen A, Rauch TA, Pfeifer GP, Hu VW. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J 24: 3036–3051, 2010. doi: 10.1096/fj.10-154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. doi: 10.1172/JCI0214276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Nguyen MP, Lee S, Lee YM. Epigenetic regulation of hypoxia inducible factor in diseases and therapeutics. Arch Pharm Res 36: 252–263, 2013. doi: 10.1007/s12272-013-0058-x. [DOI] [PubMed] [Google Scholar]
- 732.Nguyen VB, Probyn ME, Campbell F, Yin KV, Samuel CS, Zimanyi MA, Bertram JF, Black MJ, Moritz KM. Low-dose maternal alcohol consumption: effects in the hearts of offspring in early life and adulthood. Physiol Rep 2: e12087, 2014. doi: 10.14814/phy2.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Ni Y, May V, Braas K, Osol G. Pregnancy augments uteroplacental vascular endothelial growth factor gene expression and vasodilator effects. Am J Physiol Heart Circ Physiol 273: H938–H944, 1997. [DOI] [PubMed] [Google Scholar]
- 734.Nicodemus KK, Marenco S, Batten AJ, Vakkalanka R, Egan MF, Straub RE, Weinberger DR. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Mol Psychiatry 13: 873–877, 2008. doi: 10.1038/sj.mp.4002153. [DOI] [PubMed] [Google Scholar]
- 735.Niermeyer S, Andrade-M MP, Vargas E, Moore LG. Neonatal oxygenation, pulmonary hypertension, and evolutionary adaptation to high altitude (2013 Grover Conference series). Pulm Circ 5: 48–62, 2015. doi: 10.1086/679719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Niermeyer S, Andrade Mollinedo P, Huicho L. Child health and living at high altitude. Arch Dis Child 94: 806–811, 2009. doi: 10.1136/adc.2008.141838. [DOI] [PubMed] [Google Scholar]
- 737.Nilsson C, Larsson BM, Jennische E, Eriksson E, Björntorp P, York DA, Holmäng A. Maternal endotoxemia results in obesity and insulin resistance in adult male offspring. Endocrinology 142: 2622–2630, 2001. doi: 10.1210/endo.142.6.8191. [DOI] [PubMed] [Google Scholar]
- 738.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol 40: 601–609, 2009. doi: 10.1165/2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Noh JS, Sharma RP, Veldic M, Salvacion AA, Jia X, Chen Y, Costa E, Guidotti A, Grayson DR. DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures. Proc Natl Acad Sci USA 102: 1749–1754, 2005. doi: 10.1073/pnas.0409648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Nordentoft M, Lou HC, Hansen D, Nim J, Pryds O, Rubin P, Hemmingsen R. Intrauterine growth retardation and premature delivery: the influence of maternal smoking and psychosocial factors. Am J Public Health 86: 347–354, 1996. doi: 10.2105/AJPH.86.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Novakovic B, Evain-Brion D, Murthi P, Fournier T, Saffery R. VariableDAXXgene methylation is a common feature of placental trophoblast differentiation, preeclampsia, and response to hypoxia. FASEB J 31: 2380–2392, 2017. doi: 10.1096/fj.201601189RR. [DOI] [PubMed] [Google Scholar]
- 742.Nozik-Grayck E, Woods C, Taylor JM, Benninger RK, Johnson RD, Villegas LR, Stenmark KR, Harrison DG, Majka SM, Irwin D, Farrow KN. Selective depletion of vascular EC-SOD augments chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 307: L868–L876, 2014. doi: 10.1152/ajplung.00096.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Numayama-Tsuruta K, Arai Y, Takahashi M, Sasaki-Hoshino M, Funatsu N, Nakamura S, Osumi N. Downstream genes of Pax6 revealed by comprehensive transcriptome profiling in the developing rat hindbrain. BMC Dev Biol 10: 6, 2010. doi: 10.1186/1471-213X-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Oakley RH, Cidlowski JA. Glucocorticoid signaling in the heart: a cardiomyocyte perspective. J Steroid Biochem Mol Biol 153: 27–34, 2015. doi: 10.1016/j.jsbmb.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Oakley RH, Ren R, Cruz-Topete D, Bird GS, Myers PH, Boyle MC, Schneider MD, Willis MS, Cidlowski JA. Essential role of stress hormone signaling in cardiomyocytes for the prevention of heart disease. Proc Natl Acad Sci USA 110: 17035–17040, 2013. doi: 10.1073/pnas.1302546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Oh C, Dong Y, Liu H, Thompson LP. Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts. Am J Obstet Gynecol 199: 78.e1–78.e6, 2008. doi: 10.1016/j.ajog.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 747.Ohnuma Y, Miura T, Miki T, Tanno M, Kuno A, Tsuchida A, Shimamoto K. Opening of mitochondrial K(ATP) channel occurs downstream of PKC-epsilon activation in the mechanism of preconditioning. Am J Physiol Heart Circ Physiol 283: H440–H447, 2002. doi: 10.1152/ajpheart.00434.2001. [DOI] [PubMed] [Google Scholar]
- 748.Oishi PE, Wiseman DA, Sharma S, Kumar S, Hou Y, Datar SA, Azakie A, Johengen MJ, Harmon C, Fratz S, Fineman JR, Black SM. Progressive dysfunction of nitric oxide synthase in a lamb model of chronically increased pulmonary blood flow: a role for oxidative stress. Am J Physiol Lung Cell Mol Physiol 295: L756–L766, 2008. doi: 10.1152/ajplung.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Okamura K, Watanabe T, Tanigawara S, Shintaku Y, Endo H, Iwamoto M, Murotsuki J, Yajima A. Biochemical evaluation of fetus with hypoxia caused by severe preeclampsia using cordocentesis. J Perinat Med 18: 441–447, 1990. doi: 10.1515/jpme.1990.18.6.441. [DOI] [PubMed] [Google Scholar]
- 750.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99: 247–257, 1999. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 751.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol 112: 999–1006, 2008. doi: 10.1097/AOG.0b013e31818a5d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Osol G, Celia G, Gokina N, Barron C, Chien E, Mandala M, Luksha L, Kublickiene K. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am J Physiol Heart Circ Physiol 294: H1381–H1387, 2008. doi: 10.1152/ajpheart.00922.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Osumek JE, Revesz A, Morton JS, Davidge ST, Hardy DB. Enhanced trimethylation of histone h3 mediates impaired expression of hepatic glucose 6-phosphatase expression in offspring from rat dams exposed to hypoxia during pregnancy. Reprod Sci 21: 112–121, 2014. doi: 10.1177/1933719113492212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Otto KB, Lierse W. [The capillaries of various parts of the human brain in the fetal period and during the first years of life]. Acta Anat (Basel) 77: 25–36, 1970. doi: 10.1159/000143525. [DOI] [PubMed] [Google Scholar]
- 756.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 757.Pagida MA, Konstantinidou AE, Malidelis YI, Ganou V, Tsekoura E, Patsouris E, Panayotacopoulou MT. The human neurosecretory neurones under perinatal hypoxia: a quantitative immunohistochemical study of the supraoptic nucleus in autopsy material. J Neuroendocrinol 25: 1255–1263, 2013. doi: 10.1111/jne.12111. [DOI] [PubMed] [Google Scholar]
- 758.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol 180: 1161–1168, 1999. doi: 10.1016/S0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- 759.Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol 80: 1000–1006, 1992. [PubMed] [Google Scholar]
- 760.Pan Z, Sun X, Ren J, Li X, Gao X, Lu C, Zhang Y, Sun H, Wang Y, Wang H, Wang J, Xie L, Lu Y, Yang B. miR-1 exacerbates cardiac ischemia-reperfusion injury in mouse models. PLoS One 7: e50515, 2012. doi: 10.1371/journal.pone.0050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation 16: 265–271, 2009. doi: 10.1159/000216184. [DOI] [PubMed] [Google Scholar]
- 762.Papamatheakis DG, Blood AB, Kim JH, Wilson SM. Antenatal hypoxia and pulmonary vascular function and remodeling. Curr Vasc Pharmacol 11: 616–640, 2013. doi: 10.2174/1570161111311050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Papamatheakis DG, Chundu M, Blood AB, Wilson SM. Prenatal programming of pulmonary hypertension induced by chronic hypoxia or ductal ligation in sheep. Pulm Circ 3: 757–780, 2013. doi: 10.1086/674767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Papamatheakis DG, Patel JJ, Blood Q, Merritt TT, Longo LD, Wilson SM. Depolarization-dependent contraction increase after birth and preservation following long-term hypoxia in sheep pulmonary arteries. Pulm Circ 2: 41–53, 2012. doi: 10.4103/2045-8932.94832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Paradis A, Xiao D, Zhou J, Zhang L. Endothelin-1 promotes cardiomyocyte terminal differentiation in the developing heart via heightened DNA methylation. Int J Med Sci 11: 373–380, 2014. doi: 10.7150/ijms.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Paradis AN, Gay MS, Wilson CG, Zhang L. Newborn hypoxia/anoxia inhibits cardiomyocyte proliferation and decreases cardiomyocyte endowment in the developing heart: role of endothelin-1. PLoS One 10: e0116600, 2015. doi: 10.1371/journal.pone.0116600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Paradis AN, Gay MS, Zhang L. Binucleation of cardiomyocytes: the transition from a proliferative to a terminally differentiated state. Drug Discov Today 19: 602–609, 2014. doi: 10.1016/j.drudis.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Pareja-Galeano H, Sanchis-Gomar F, Mayero S. Autism spectrum disorders: possible implications of BDNF modulation through epigenetics. Acta Psychiatr Scand 128: 97, 2013. doi: 10.1111/acps.12071. [DOI] [PubMed] [Google Scholar]
- 770.Parfenova H, Levine V, Gunther WM, Pourcyrous M, Leffler CW. COX-1 and COX-2 contributions to basal and IL-1 beta-stimulated prostanoid synthesis in human neonatal cerebral microvascular endothelial cells. Pediatr Res 52: 342–348, 2002. doi: 10.1203/00006450-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 771.Parker CR Jr, Everett RB, Quirk JG Jr, Whalley PJ, Gant NF. Hormone production during pregnancy in the primigravid patient. I. Plasma levels of progesterone and 5-alpha-pregnane-3,20-dione throughout pregnancy of normal women and women who developed pregnancy-induced hypertension. Am J Obstet Gynecol 135: 778–782, 1979. doi: 10.1016/0002-9378(79)90391-0. [DOI] [PubMed] [Google Scholar]
- 772.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res 100: 633–644, 2007. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 773.Parraguez VC, Atlagich M, Díaz R, Bruzzone ME, Behn C, Raggi LA. Effect of hypobaric hypoxia on lamb intrauterine growth: comparison between high- and low-altitude native ewes. Reprod Fertil Dev 17: 497–505, 2005. doi: 10.1071/RD04060. [DOI] [PubMed] [Google Scholar]
- 774.Parraguez VH, Atlagich M, Araneda O, García C, Muñoz A, De Los Reyes M, Urquieta B. Effects of antioxidant vitamins on newborn and placental traits in gestations at high altitude: comparative study in high and low altitude native sheep. Reprod Fertil Dev 23: 285–296, 2011. doi: 10.1071/RD10016. [DOI] [PubMed] [Google Scholar]
- 775.Parraguez VH, Atlagich M, Díaz R, Cepeda R, González C, De los Reyes M, Bruzzone ME, Behn C, Raggi LA. Ovine placenta at high altitudes: comparison of animals with different times of adaptation to hypoxic environment. Anim Reprod Sci 95: 151–157, 2006. doi: 10.1016/j.anireprosci.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 776.Parraguez VH, Atlagich MA, Urquieta B, Galleguillos M, De Los Reyes M, Kooyman DL, Araneda S, Raggi LA. Expression of vascular endothelial growth factor and endothelial nitric oxide synthase is increased in the placenta of sheep at high altitude in the Andes. Can J Vet Res 74: 193–199, 2010. [PMC free article] [PubMed] [Google Scholar]
- 777.Parraguez VH, Mamani S, Cofré E, Castellaro G, Urquieta B, De Los Reyes M, Astiz S, Gonzalez-Bulnes A. Disturbances in Maternal Steroidogenesis and Appearance of Intrauterine Growth Retardation at High-Altitude Environments Are Established from Early Pregnancy. Effects of Treatment with Antioxidant Vitamins. PLoS One 10: e0140902, 2015. doi: 10.1371/journal.pone.0140902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Parraguez VH, Urquieta B, De los Reyes M, González-Bulnes A, Astiz S, Muñoz A. Steroidogenesis in sheep pregnancy with intrauterine growth retardation by high-altitude hypoxia: effects of maternal altitudinal status and antioxidant treatment. Reprod Fertil Dev 25: 639–645, 2013. doi: 10.1071/RD12020. [DOI] [PubMed] [Google Scholar]
- 779.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449: 228–232, 2007. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 780.Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol 14: 341–356, 2013. doi: 10.1038/nrm3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Pastore MB, Jobe SO, Ramadoss J, Magness RR. Estrogen receptor-α and estrogen receptor-β in the uterine vascular endothelium during pregnancy: functional implications for regulating uterine blood flow. Semin Reprod Med 30: 46–61, 2012. doi: 10.1055/s-0031-1299597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Patel D, Alhawaj R, Wolin MS. Exposure of mice to chronic hypoxia attenuates pulmonary arterial contractile responses to acute hypoxia by increases in extracellular hydrogen peroxide. Am J Physiol Regul Integr Comp Physiol 307: R426–R433, 2014. doi: 10.1152/ajpregu.00257.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Patt S, Sampaolo S, Théallier-Jankó A, Tschairkin I, Cervós-Navarro J. Cerebral angiogenesis triggered by severe chronic hypoxia displays regional differences. J Cereb Blood Flow Metab 17: 801–806, 1997. doi: 10.1097/00004647-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 784.Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCepsilon gene repression in rat hearts. Circ Res 107: 365–373, 2010. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785.Patterson AJ, Xiao D, Xiong F, Dixon B, Zhang L. Hypoxia-derived oxidative stress mediates epigenetic repression of PKCε gene in foetal rat hearts. Cardiovasc Res 93: 302–310, 2012. doi: 10.1093/cvr/cvr322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Patterson AJ, Zhang L. Hypoxia and fetal heart development. Curr Mol Med 10: 653–666, 2010. doi: 10.2174/156652410792630643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Pearce W. Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol (1985) 100: 731–738, 2006. doi: 10.1152/japplphysiol.00990.2005. [DOI] [PubMed] [Google Scholar]
- 788.Pearce WJ, Williams JM, White CR, Lincoln TM. Effects of chronic hypoxia on soluble guanylate cyclase activity in fetal and adult ovine cerebral arteries. J Appl Physiol (1985) 107: 192–199, 2009. doi: 10.1152/japplphysiol.00233.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Peeters LL, Sparks JW, Grutters G, Girard J, Battaglia FC. Uteroplacental blood flow during pregnancy in chronically catheterized guinea pigs. Pediatr Res 16: 716–720, 1982. doi: 10.1203/00006450-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 790.Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328: 753–756, 2010. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 791.Pena JP, Tomimatsu T, Hatran DP, McGill LL, Longo LD. Cerebral blood flow and oxygenation in ovine fetus: responses to superimposed hypoxia at both low and high altitude. J Physiol 578: 359–370, 2007. doi: 10.1113/jphysiol.2006.119925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Penninga L, Longo LD. Ovine placentome morphology: effect of high altitude, long-term hypoxia. Placenta 19: 187–193, 1998. doi: 10.1016/S0143-4004(98)90008-X. [DOI] [PubMed] [Google Scholar]
- 793.Peters T, Hermans-Beijnsberger S, Beqqali A, Bitsch N, Nakagawa S, Prasanth KV, de Windt LJ, van Oort RJ, Heymans S, Schroen B. Long Non-Coding RNA Malat-1 Is Dispensable during Pressure Overload-Induced Cardiac Remodeling and Failure in Mice. PLoS One 11: e0150236, 2016. doi: 10.1371/journal.pone.0150236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Peyronnet J, Dalmaz Y, Ehrström M, Mamet J, Roux JC, Pequignot JM, Thorén HP, Lagercrantz H. Long-lasting adverse effects of prenatal hypoxia on developing autonomic nervous system and cardiovascular parameters in rats. Pflugers Arch 443: 858–865, 2002. doi: 10.1007/s00424-001-0766-9. [DOI] [PubMed] [Google Scholar]
- 795.Peyronnet J, Roux JC, Geloën A, Tang LQ, Pequignot JM, Lagercrantz H, Dalmaz Y. Prenatal hypoxia impairs the postnatal development of neural and functional chemoafferent pathway in rat. J Physiol 524: 525–537, 2000. doi: 10.1111/j.1469-7793.2000.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Peyronnet J, Roux JC, Mamet J, Perrin D, Lachuer J, Pequignot JM, Dalmaz Y. Developmental plasticity of the carotid chemoafferent pathway in rats that are hypoxic during the prenatal period. Eur J Neurosci 26: 2865–2872, 2007. doi: 10.1111/j.1460-9568.2007.05884.x. [DOI] [PubMed] [Google Scholar]
- 797.Peyter AC, Muehlethaler V, Liaudet L, Marino M, Di Bernardo S, Diaceri G, Tolsa JF. Muscarinic receptor M1 and phosphodiesterase 1 are key determinants in pulmonary vascular dysfunction following perinatal hypoxia in mice. Am J Physiol Lung Cell Mol Physiol 295: L201–L213, 2008. doi: 10.1152/ajplung.00264.2007. [DOI] [PubMed] [Google Scholar]
- 798.Phillips ID, Simonetta G, Owens JA, Robinson JS, Clarke IJ, McMillen IC. Placental restriction alters the functional development of the pituitary-adrenal axis in the sheep fetus during late gestation. Pediatr Res 40: 861–866, 1996. doi: 10.1203/00006450-199612000-00014. [DOI] [PubMed] [Google Scholar]
- 799.Pialoux V, Mounier R. Hypoxia-induced oxidative stress in health disorders. Oxid Med Cell Longev 2012: 940121, 2012. doi: 10.1155/2012/940121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, Draghici S, Espinoza J, Kusanovic JP, Mittal P, Hassan SS, Kim CJ. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol 196: 261.e1–261.e6, 2007. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 801.Pinney SE, Simmons RA. Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol Metab 21: 223–229, 2010. doi: 10.1016/j.tem.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Plomaritas DR, Herbert LM, Yellowhair TR, Resta TC, Gonzalez Bosc LV, Walker BR, Jernigan NL. Chronic hypoxia limits H2O2-induced inhibition of ASIC1-dependent store-operated calcium entry in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 307: L419–L430, 2014. doi: 10.1152/ajplung.00095.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803.Poljaková J, Groh T, Gudino ZO, Hraběta J, Bořek-Dohalská L, Kizek R, Doktorová H, Eckschlager T, Stiborová M. Hypoxia-mediated histone acetylation and expression of N-myc transcription factor dictate aggressiveness of neuroblastoma cells. Oncol Rep 31: 1928–1934, 2014. doi: 10.3892/or.2014.2999. [DOI] [PubMed] [Google Scholar]
- 804.Pollard PJ, Loenarz C, Mole DR, McDonough MA, Gleadle JM, Schofield CJ, Ratcliffe PJ. Regulation of Jumonji-domain-containing histone demethylases by hypoxia-inducible factor (HIF)-1alpha. Biochem J 416: 387–394, 2008. doi: 10.1042/BJ20081238. [DOI] [PubMed] [Google Scholar]
- 805.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O’Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806.Poonyagariyagorn HK, Metzger S, Dikeman D, Mercado AL, Malinina A, Calvi C, McGrath-Morrow S, Neptune ER. Superoxide dismutase 3 dysregulation in a murine model of neonatal lung injury. Am J Respir Cell Mol Biol 51: 380–390, 2014. doi: 10.1165/rcmb.2013-0043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, Dorn GW II, van Rooij E, Olson EN. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res 109: 670–679, 2011. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808.Pralong FP, Roduit R, Waeber G, Castillo E, Mosimann F, Thorens B, Gaillard RC. Leptin inhibits directly glucocorticoid secretion by normal human and rat adrenal gland. Endocrinology 139: 4264–4268, 1998. doi: 10.1210/endo.139.10.6254. [DOI] [PubMed] [Google Scholar]
- 809.Previc FH. Prenatal influences on brain dopamine and their relevance to the rising incidence of autism. Med Hypotheses 68: 46–60, 2007. doi: 10.1016/j.mehy.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 810.Price DJ, Kennedy H, Dehay C, Zhou L, Mercier M, Jossin Y, Goffinet AM, Tissir F, Blakey D, Molnár Z. The development of cortical connections. Eur J Neurosci 23: 910–920, 2006. doi: 10.1111/j.1460-9568.2006.04620.x. [DOI] [PubMed] [Google Scholar]
- 811.Prickaerts P, Adriaens ME, Beucken TVD, Koch E, Dubois L, Dahlmans VEH, Gits C, Evelo CTA, Chan-Seng-Yue M, Wouters BG, Voncken JW. Hypoxia increases genome-wide bivalent epigenetic marking by specific gain of H3K27me3. Epigenetics Chromatin 9: 46, 2016. doi: 10.1186/s13072-016-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812.Prince LR, Maxwell NC, Gill SK, Dockrell DH, Sabroe I, McGreal EP, Kotecha S, Whyte MK. Macrophage phenotype is associated with disease severity in preterm infants with chronic lung disease. PLoS One 9: e103059, 2014. doi: 10.1371/journal.pone.0103059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813.Puckett RE, Lubin FD. Epigenetic mechanisms in experience-driven memory formation and behavior. Epigenomics 3: 649–664, 2011. doi: 10.2217/epi.11.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 814.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157: 565–579, 2014. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR. The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol 308: L229–L252, 2015. doi: 10.1152/ajplung.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816.Qiao J, Chen Y, Yan LY, Yan J, Liu P, Sun QY. Changes in histone methylation during human oocyte maturation and IVF- or ICSI-derived embryo development. Fertil Steril 93: 1628–1636, 2010. doi: 10.1016/j.fertnstert.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 817.Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol 92: 639–646, 1987. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, Elbert T. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry 1: e21, 2011. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.Raff H, Jacobson L, Cullinan WE. Augmented hypothalamic corticotrophin-releasing hormone mRNA and corticosterone responses to stress in adult rats exposed to perinatal hypoxia. J Neuroendocrinol 19: 907–912, 2007. doi: 10.1111/j.1365-2826.2007.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820.Rahnama F, Shafiei F, Gluckman PD, Mitchell MD, Lobie PE. Epigenetic regulation of human trophoblastic cell migration and invasion. Endocrinology 147: 5275–5283, 2006. doi: 10.1210/en.2006-0288. [DOI] [PubMed] [Google Scholar]
- 821.Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 25: 763–769, 2004. doi: 10.1016/j.placenta.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 822.Rakyan V, Whitelaw E. Transgenerational epigenetic inheritance. Curr Biol 13: R6, 2003. doi: 10.1016/S0960-9822(02)01377-5. [DOI] [PubMed] [Google Scholar]
- 823.Ramakrishnan S, Anand V, Roy S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J Neuroimmune Pharmacol 9: 142–160, 2014. doi: 10.1007/s11481-014-9531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824.Ramya S, Shyamasundar S, Bay BH, Dheen ST. Maternal Diabetes Alters Expression of MicroRNAs that Regulate Genes Critical for Neural Tube Development. Front Mol Neurosci 10: 237, 2017. doi: 10.3389/fnmol.2017.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825.Rana S, Rajakumar A, Geahchan C, Salahuddin S, Cerdeira AS, Burke SD, George EM, Granger JP, Karumanchi SA. Ouabain inhibits placental sFlt1 production by repressing HSP27-dependent HIF-1α pathway. FASEB J 28: 4324–4334, 2014. doi: 10.1096/fj.14-252684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Rangasamy S, D’Mello SR, Narayanan V. Epigenetics, autism spectrum, and neurodevelopmental disorders. Neurotherapeutics 10: 742–756, 2013. doi: 10.1007/s13311-013-0227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.Ratajska A, Cleutjens JP. Embryogenesis of the rat heart: the expression of collagenases. Basic Res Cardiol 97: 189–197, 2002. doi: 10.1007/s003950200011. [DOI] [PubMed] [Google Scholar]
- 828.Ream M, Ray AM, Chandra R, Chikaraishi DM. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol 295: R583–R595, 2008. doi: 10.1152/ajpregu.00771.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 829.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science 293: 1089–1093, 2001. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 830.Reini SA, Dutta G, Wood CE, Keller-Wood M. Cardiac corticosteroid receptors mediate the enlargement of the ovine fetal heart induced by chronic increases in maternal cortisol. J Endocrinol 198: 419–427, 2008. doi: 10.1677/JOE-08-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.Ren R, Oakley RH, Cruz-Topete D, Cidlowski JA. Dual role for glucocorticoids in cardiomyocyte hypertrophy and apoptosis. Endocrinology 153: 5346–5360, 2012. doi: 10.1210/en.2012-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J 15: 100–108, 2007. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Reshetnikova OS, Burton GJ, Milovanov AP. Effects of hypobaric hypoxia on the fetoplacental unit: the morphometric diffusing capacity of the villous membrane at high altitude. Am J Obstet Gynecol 171: 1560–1565, 1994. doi: 10.1016/0002-9378(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 834.Resnik ER, Herron JM, Lyu SC, Cornfield DN. Developmental regulation of hypoxia-inducible factor 1 and prolyl-hydroxylases in pulmonary vascular smooth muscle cells. Proc Natl Acad Sci USA 104: 18789–18794, 2007. doi: 10.1073/pnas.0706019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Reul JM, Chandramohan Y. Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology 32, Suppl 1: S21–S25, 2007. doi: 10.1016/j.psyneuen.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 836.Reul JM, Hesketh SA, Collins A, Mecinas MG. Epigenetic mechanisms in the dentate gyrus act as a molecular switch in hippocampus-associated memory formation. Epigenetics 4: 434–439, 2009. doi: 10.4161/epi.4.7.9806. [DOI] [PubMed] [Google Scholar]
- 837.Rexhaj E, Bloch J, Jayet PY, Rimoldi SF, Dessen P, Mathieu C, Tolsa JF, Nicod P, Scherrer U, Sartori C. Fetal programming of pulmonary vascular dysfunction in mice: role of epigenetic mechanisms. Am J Physiol Heart Circ Physiol 301: H247–H252, 2011. doi: 10.1152/ajpheart.01309.2010. [DOI] [PubMed] [Google Scholar]
- 838.Reyes LM, Kirschenman R, Quon A, Morton JS, Shah A, Davidge ST. Aerobic exercise training reduces cardiac function in adult male offspring exposed to prenatal hypoxia. Am J Physiol Regul Integr Comp Physiol 309: R489–R498, 2015. doi: 10.1152/ajpregu.00201.2015. [DOI] [PubMed] [Google Scholar]
- 839.Reyes LM, Morton JS, Kirschenman R, DeLorey DS, Davidge ST. Vascular effects of aerobic exercise training in rat adult offspring exposed to hypoxia-induced intrauterine growth restriction. J Physiol 593: 1913–1929, 2015. doi: 10.1113/jphysiol.2014.288449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, Hafez SA, Grazul-Bilska AT, Redmer DA. Uteroplacental vascular development and placental function: an update. Int J Dev Biol 54: 355–366, 2010. doi: 10.1387/ijdb.082799lr. [DOI] [PubMed] [Google Scholar]
- 841.Rhodes AJ, Hyde JB. Postnatal growth of arterioles in the human cerebral cortex. Growth 29: 173–182, 1965. [PubMed] [Google Scholar]
- 842.Richardson RV, Batchen EJ, Denvir MA, Gray GA, Chapman KE. Cardiac GR and MR: From Development to Pathology. Trends Endocrinol Metab 27: 35–43, 2016. doi: 10.1016/j.tem.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 843.Richter HG, Camm EJ, Modi BN, Naeem F, Cross CM, Cindrova-Davies T, Spasic-Boskovic O, Dunster C, Mudway IS, Kelly FJ, Burton GJ, Poston L, Giussani DA. Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. J Physiol 590: 1377–1387, 2012. doi: 10.1113/jphysiol.2011.226340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem 81: 145–166, 2012. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 845.Risau W, Wolburg H. Development of the blood-brain barrier. Trends Neurosci 13: 174–178, 1990. doi: 10.1016/0166-2236(90)90043-A. [DOI] [PubMed] [Google Scholar]
- 846.Rodríguez-Rodríguez P, López de Pablo AL, García-Prieto CF, Somoza B, Quintana-Villamandos B, Gómez de Diego JJ, Gutierrez-Arzapalo PY, Ramiro-Cortijo D, González MC, Arribas SM. Long term effects of fetal undernutrition on rat heart. Role of hypertension and oxidative stress. PLoS One 12: e0171544, 2017. doi: 10.1371/journal.pone.0171544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Rog-Zielinska EA, Craig MA, Manning JR, Richardson RV, Gowans GJ, Dunbar DR, Gharbi K, Kenyon CJ, Holmes MC, Hardie DG, Smith GL, Chapman KE. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: a role for PGC-1α. Cell Death Differ 22: 1106–1116, 2015. doi: 10.1038/cdd.2014.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 848.Rog-Zielinska EA, Thomson A, Kenyon CJ, Brownstein DG, Moran CM, Szumska D, Michailidou Z, Richardson J, Owen E, Watt A, Morrison H, Forrester LM, Bhattacharya S, Holmes MC, Chapman KE. Glucocorticoid receptor is required for foetal heart maturation. Hum Mol Genet 22: 3269–3282, 2013. doi: 10.1093/hmg/ddt182. [DOI] [PubMed] [Google Scholar]
- 849.Roidl D, Hacker C. Histone methylation during neural development. Cell Tissue Res 356: 539–552, 2014. doi: 10.1007/s00441-014-1842-8. [DOI] [PubMed] [Google Scholar]
- 850.Rondini TA, Baddini SP, Sousa LF, Bittencourt JC, Elias CF. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience 125: 735–748, 2004. doi: 10.1016/j.neuroscience.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 851.Rook W, Johnson CD, Coney AM, Marshall JM. Prenatal hypoxia leads to increased muscle sympathetic nerve activity, sympathetic hyperinnervation, premature blunting of neuropeptide Y signaling, and hypertension in adult life. Hypertension 64: 1321–1327, 2014. doi: 10.1161/HYPERTENSIONAHA.114.04374. [DOI] [PubMed] [Google Scholar]
- 852.Rorabaugh BR, Seeley SL, Bui AD, Sprague L, D’Souza MS. Prenatal methamphetamine differentially alters myocardial sensitivity to ischemic injury in male and female adult hearts. Am J Physiol Heart Circ Physiol 310: H516–H523, 2016. doi: 10.1152/ajpheart.00642.2015. [DOI] [PubMed] [Google Scholar]
- 853.Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol 185: 93–98, 2001. doi: 10.1016/S0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 854.Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol Heart Circ Physiol 232: H231–H235, 1977. [DOI] [PubMed] [Google Scholar]
- 855.Rosenfeld CR, Chen C, Roy T, Liu X. Estrogen selectively up-regulates eNOS and nNOS in reproductive arteries by transcriptional mechanisms. J Soc Gynecol Investig 10: 205–215, 2003. doi: 10.1016/S1071-55760300049-2. [DOI] [PubMed] [Google Scholar]
- 856.Rosenfeld CR, Cornfield DN, Roy T. Ca2+-activated K+ channels modulate basal and E(2)beta-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol Heart Circ Physiol 281: H422–H431, 2001. doi: 10.1152/ajpheart.2001.281.1.H422. [DOI] [PubMed] [Google Scholar]
- 857.Rosenfeld CR, Liu XT, DeSpain K. Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am J Physiol Heart Circ Physiol 296: H1878–H1887, 2009. doi: 10.1152/ajpheart.01185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.Rosenfeld CR, Morriss FH Jr, Makowski EL, Meschia G, Battaglia FC. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol Invest 5: 252–268, 1974. doi: 10.1159/000301658. [DOI] [PubMed] [Google Scholar]
- 859.Ross B, McIntosh M, Rodaros D, Hébert TE, Rohlicek CV. Systemic arterial pressure at maturity in rats following chronic hypoxia in early life. Am J Hypertens 23: 1228–1233, 2010. doi: 10.1038/ajh.2010.160. [DOI] [PubMed] [Google Scholar]
- 860.Rudolph AM, Roman C, Gournay V. Perinatal myocardial DNA and protein changes in the lamb: effect of cortisol in the fetus. Pediatr Res 46: 141–146, 1999. doi: 10.1203/00006450-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 861.Rueda-Clausen CF, Dolinsky VW, Morton JS, Proctor SD, Dyck JR, Davidge ST. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes 60: 507–516, 2011. doi: 10.2337/db10-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862.Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res 81: 713–722, 2009. doi: 10.1093/cvr/cvn341. [DOI] [PubMed] [Google Scholar]
- 863.Rueda-Clausen CF, Morton JS, Lopaschuk GD, Davidge ST. Long-term effects of intrauterine growth restriction on cardiac metabolism and susceptibility to ischaemia/reperfusion. Cardiovasc Res 90: 285–294, 2011. doi: 10.1093/cvr/cvq363. [DOI] [PubMed] [Google Scholar]
- 864.Rueda-Clausen CF, Stanley JL, Thambiraj DF, Poudel R, Davidge ST, Baker PN. Effect of prenatal hypoxia in transgenic mouse models of preeclampsia and fetal growth restriction. Reprod Sci 21: 492–502, 2014. doi: 10.1177/1933719113503401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.Ruijtenbeek K, Kessels LC, De Mey JG, Blanco CE. Chronic moderate hypoxia and protein malnutrition both induce growth retardation, but have distinct effects on arterial endothelium-dependent reactivity in the chicken embryo. Pediatr Res 53: 573–579, 2003. doi: 10.1203/01.PDR.0000055770.07236.98. [DOI] [PubMed] [Google Scholar]
- 866.Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS; ACTS Study Group . Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med 354: 1796–1806, 2006. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 867.Rurak DW. Plasma vasopressin levels during hypoxaemia and the cardiovascular effects of exogenous vasopressin in foetal and adult sheep. J Physiol 277: 341–357, 1978. doi: 10.1113/jphysiol.1978.sp012275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 868.Ryan KJ, Elmes MJ, Langley-Evans SC. The Effects of Prenatal Protein Restriction on β-Adrenergic Signalling of the Adult Rat Heart during Ischaemia Reperfusion. J Nutr Metab 2012: 397389, 2012. doi: 10.1155/2012/397389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869.Sab IM, Ferraz MM, Amaral TA, Resende AC, Ferraz MR, Matsuura C, Brunini TM, Mendes-Ribeiro AC. Prenatal hypoxia, habituation memory and oxidative stress. Pharmacol Biochem Behav 107: 24–28, 2013. doi: 10.1016/j.pbb.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 870.Sabatine MS, Liu E, Morrow DA, Heller E, McCarroll R, Wiegand R, Berriz GF, Roth FP, Gerszten RE. Metabolomic identification of novel biomarkers of myocardial ischemia. Circulation 112: 3868–3875, 2005. doi: 10.1161/CIRCULATIONAHA.105.569137. [DOI] [PubMed] [Google Scholar]
- 871.Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol 24: 225–253, 2003. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 872.Sahu A. Minireview: a hypothalamic role in energy balance with special emphasis on leptin. Endocrinology 145: 2613–2620, 2004. doi: 10.1210/en.2004-0032. [DOI] [PubMed] [Google Scholar]
- 873.Salafia CM, Vogel CA, Bantham KF, Vintzileos AM, Pezzullo J, Silberman L. Preterm delivery: correlations of fetal growth and placental pathology. Am J Perinatol 9: 190–193, 1992. doi: 10.1055/s-2007-999318. [DOI] [PubMed] [Google Scholar]
- 874.Salas SP, Marshall G, Gutiérrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 47: 203–208, 2006. doi: 10.1161/01.HYP.0000200042.64517.19. [DOI] [PubMed] [Google Scholar]
- 875.Salminen A, Kaarniranta K, Hiltunen M, Kauppinen A. Krebs cycle dysfunction shapes epigenetic landscape of chromatin: novel insights into mitochondrial regulation of aging process. Cell Signal 26: 1598–1603, 2014. doi: 10.1016/j.cellsig.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 876.Salzmann C, Otis M, Long H, Roberge C, Gallo-Payet N, Walker CD. Inhibition of steroidogenic response to adrenocorticotropin by leptin: implications for the adrenal response to maternal separation in neonatal rats. Endocrinology 145: 1810–1822, 2004. doi: 10.1210/en.2003-1514. [DOI] [PubMed] [Google Scholar]
- 877.Santos KF, Mazzola TN, Carvalho HF. The prima donna of epigenetics: the regulation of gene expression by DNA methylation. Braz J Med Biol Res 38: 1531–1541, 2005. doi: 10.1590/S0100-879X2005001000010. [DOI] [PubMed] [Google Scholar]
- 878.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res 396: 64–76, 1986. doi: 10.1016/0165-0173(86)90010-X. [DOI] [PubMed] [Google Scholar]
- 879.Sar A, Ponjevic D, Nguyen M, Box AH, Demetrick DJ. Identification and characterization of demethylase JMJD1A as a gene upregulated in the human cellular response to hypoxia. Cell Tissue Res 337: 223–234, 2009. doi: 10.1007/s00441-009-0805-y. [DOI] [PubMed] [Google Scholar]
- 880.Sarada S, Himadri P, Mishra C, Geetali P, Ram MS, Ilavazhagan G. Role of oxidative stress and NFkB in hypoxia-induced pulmonary edema. Exp Biol Med (Maywood) 233: 1088–1098, 2008. doi: 10.3181/0712-RM-337. [DOI] [PubMed] [Google Scholar]
- 881.Sarma K, Reinberg D. Histone variants meet their match. Nat Rev Mol Cell Biol 6: 139–149, 2005. doi: 10.1038/nrm1567. [DOI] [PubMed] [Google Scholar]
- 882.Sartori C, Allemann Y, Trueb L, Delabays A, Nicod P, Scherrer U. Augmented vasoreactivity in adult life associated with perinatal vascular insult. Lancet 353: 2205–2207, 1999. doi: 10.1016/S0140-6736(98)08352-4. [DOI] [PubMed] [Google Scholar]
- 883.Sartori C, Rimoldi SF, Rexhaj E, Allemann Y, Scherrer U. Epigenetics in Cardiovascular Regulation. Adv Exp Med Biol 903: 55–62, 2016. doi: 10.1007/978-1-4899-7678-9_4. [DOI] [PubMed] [Google Scholar]
- 884.Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet 9: 129–140, 2008. doi: 10.1038/nrg2295. [DOI] [PubMed] [Google Scholar]
- 885.Savaskan E. The role of the brain renin-angiotensin system in neurodegenerative disorders. Curr Alzheimer Res 2: 29–35, 2005. doi: 10.2174/1567205052772740. [DOI] [PubMed] [Google Scholar]
- 886.Savel’eva GM, Chekhonin VP, Pavlova TA, Kushch IB, Shalina RI, Rogatkin SO, Morozov SG, Volodin NN. [An immunochemical analysis of the function of the hemato-encephalic barrier in acute fetal hypoxia and asphyxia neonatorum]. Akush Ginekol (Mosk) 2: 43–46, 1991. [PubMed] [Google Scholar]
- 887.Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 296: R493–R500, 2009. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.Schäffler A, Binart N, Schölmerich J, Büchler C. Hypothesis paper Brain talks with fat–evidence for a hypothalamic-pituitary-adipose axis? Neuropeptides 39: 363–367, 2005. doi: 10.1016/j.npep.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 889.Schäffler A, Schölmerich J, Buechler C. The role of ‘adipotropins’ and the clinical importance of a potential hypothalamic-pituitary-adipose axis. Nat Clin Pract Endocrinol Metab 2: 374–383, 2006. doi: 10.1038/ncpendmet0197. [DOI] [PubMed] [Google Scholar]
- 890.Schanen NC. Epigenetics of autism spectrum disorders. Hum Mol Genet 15, Suppl_2: R138–R150, 2006. doi: 10.1093/hmg/ddl213. [DOI] [PubMed] [Google Scholar]
- 891.Schneeberger M, Altirriba J, García A, Esteban Y, Castaño C, García-Lavandeira M, Alvarez CV, Gomis R, Claret M. Deletion of miRNA processing enzyme Dicer in POMC-expressing cells leads to pituitary dysfunction, neurodegeneration and development of obesity. Mol Metab 2: 74–85, 2013. doi: 10.1016/j.molmet.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892.Schröder K, Weissmann N, Brandes RP. Organizers and activators: cytosolic Nox proteins impacting on vascular function. Free Radic Biol Med 109: 22–32, 2017. doi: 10.1016/j.freeradbiomed.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 893.Schumacker PT. Lung cell hypoxia: role of mitochondrial reactive oxygen species signaling in triggering responses. Proc Am Thorac Soc 8: 477–484, 2011. doi: 10.1513/pats.201103-032MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 894.Schumacker PT, Gillespie MN, Nakahira K, Choi AM, Crouser ED, Piantadosi CA, Bhattacharya J. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol 306: L962–L974, 2014. doi: 10.1152/ajplung.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895.Scott J, Hyatt K, Myers DA. Developmental changes in adrenal leptin receptor expression and adrenocortical response to leptin in the ovine fetus. J Soc Gynecol Investig 12: 239A, 2005. [Google Scholar]
- 896.Sdek P, Zhao P, Wang Y, Huang CJ, Ko CY, Butler PC, Weiss JN, Maclellan WR. Rb and p130 control cell cycle gene silencing to maintain the postmitotic phenotype in cardiac myocytes. J Cell Biol 194: 407–423, 2011. doi: 10.1083/jcb.201012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 897.Seckl JR. Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Mol Cell Endocrinol 185: 61–71, 2001. doi: 10.1016/S0303-7207(01)00633-5. [DOI] [PubMed] [Google Scholar]
- 898.Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab 3: 479–488, 2007. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 899.Selemidis S, Seow HJ, Broughton BR, Vinh A, Bozinovski S, Sobey CG, Drummond GR, Vlahos R. Nox1 oxidase suppresses influenza a virus-induced lung inflammation and oxidative stress. PLoS One 8: e60792, 2013. doi: 10.1371/journal.pone.0060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 900.Semenza GL. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol 59: 47–53, 2000. doi: 10.1016/S0006-2952(99)00292-0. [DOI] [PubMed] [Google Scholar]
- 901.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24: 97–106, 2009. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 902.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454, 1992. doi: 10.1128/MCB.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903.Semeralul MO, Boutros PC, Likhodi O, Okey AB, Van Tol HH, Wong AH. Microarray analysis of the developing cortex. J Neurobiol 66: 1646–1658, 2006. doi: 10.1002/neu.20302. [DOI] [PubMed] [Google Scholar]
- 904.Sewer MB, Waterman MR. ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Tech 61: 300–307, 2003. doi: 10.1002/jemt.10339. [DOI] [PubMed] [Google Scholar]
- 905.Sewer MB, Waterman MR. Adrenocorticotropin/cyclic adenosine 3′,5′-monophosphate-mediated transcription of the human CYP17 gene in the adrenal cortex is dependent on phosphatase activity. Endocrinology 143: 1769–1777, 2002. doi: 10.1210/endo.143.5.8820. [DOI] [PubMed] [Google Scholar]
- 906.Sewer MB, Waterman MR. cAMP-dependent protein kinase enhances CYP17 transcription via MKP-1 activation in H295R human adrenocortical cells. J Biol Chem 278: 8106–8111, 2003. doi: 10.1074/jbc.M210264200. [DOI] [PubMed] [Google Scholar]
- 907.Sewer MB, Waterman MR. cAMP-dependent transcription of steroidogenic genes in the human adrenal cortex requires a dual-specificity phosphatase in addition to protein kinase A. J Mol Endocrinol 29: 163–174, 2002. doi: 10.1677/jme.0.0290163. [DOI] [PubMed] [Google Scholar]
- 908.Shah A, Reyes LM, Morton JS, Fung D, Schneider J, Davidge ST. Effect of resveratrol on metabolic and cardiovascular function in male and female adult offspring exposed to prenatal hypoxia and a high-fat diet. J Physiol 594: 1465–1482, 2016. doi: 10.1113/JP271133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 909.Shan Y, Li X, You B, Shi S, Zhang Q, You Y. MicroRNA-338 inhibits migration and proliferation by targeting hypoxia-induced factor 1α in nasopharyngeal carcinoma. Oncol Rep 34: 1943–1952, 2015. doi: 10.3892/or.2015.4195. [DOI] [PubMed] [Google Scholar]
- 910.Sharma S, Grobe AC, Wiseman DA, Kumar S, Englaish M, Najwer I, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Lung antioxidant enzymes are regulated by development and increased pulmonary blood flow. Am J Physiol Lung Cell Mol Physiol 293: L960–L971, 2007. doi: 10.1152/ajplung.00449.2006. [DOI] [PubMed] [Google Scholar]
- 911.Sharma S, Kumar S, Wiseman DA, Kallarackal S, Ponnala S, Elgaish M, Tian J, Fineman JR, Black SM. Perinatal changes in superoxide generation in the ovine lung: Alterations associated with increased pulmonary blood flow. Vascul Pharmacol 53: 38–52, 2010. doi: 10.1016/j.vph.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 912.Sheffer-Mimouni G, Mimouni FB, Dollberg S, Mandel D, Deutsch V, Littner Y. Neonatal nucleated red blood cells in infants of overweight and obese mothers. J Am Coll Nutr 26: 259–263, 2007. doi: 10.1080/07315724.2007.10719609. [DOI] [PubMed] [Google Scholar]
- 913.Shi H, O’Reilly VC, Moreau JL, Bewes TR, Yam MX, Chapman BE, Grieve SM, Stocker R, Graham RM, Chapman G, Sparrow DB, Dunwoodie SL. Gestational stress induces the unfolded protein response, resulting in heart defects. Development 143: 2561–2572, 2016. doi: 10.1242/dev.136820. [DOI] [PubMed] [Google Scholar]
- 914.Shi XF, Wang H, Xiao FJ, Yin Y, Xu QQ, Ge RL, Wang LS. MiRNA-486 regulates angiogenic activity and survival of mesenchymal stem cells under hypoxia through modulating Akt signal. Biochem Biophys Res Commun 470: 670–677, 2016. doi: 10.1016/j.bbrc.2016.01.084. [DOI] [PubMed] [Google Scholar]
- 915.Shimoda LA, Laurie SS. HIF and pulmonary vascular responses to hypoxia. J Appl Physiol (1985) 116: 867–874, 2014. doi: 10.1152/japplphysiol.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916.Shinmura K, Nagai M, Tamaki K, Bolli R. Loss of ischaemic preconditioning in ovariectomized rat hearts: possible involvement of impaired protein kinase C epsilon phosphorylation. Cardiovasc Res 79: 387–394, 2008. doi: 10.1093/cvr/cvn086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 917.Sikarwar AS, Hinton M, Santhosh KT, Chelikani P, Dakshinamurti S. Palmitoylation of Gαq determines its association with the thromboxane receptor in hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol 50: 135–143, 2014. doi: 10.1165/rcmb.2013-0085OC. [DOI] [PubMed] [Google Scholar]
- 918.Silpanisong J, Kim D, Williams JM, Adeoye OO, Thorpe RB, Pearce WJ. Chronic hypoxia alters fetal cerebrovascular responses to endothelin-1. Am J Physiol Cell Physiol 313: C207–C218, 2017. doi: 10.1152/ajpcell.00241.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 919.Silpanisong J, Pearce WJ. Vasotrophic regulation of age-dependent hypoxic cerebrovascular remodeling. Curr Vasc Pharmacol 11: 544–563, 2013. doi: 10.2174/1570161111311050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 920.Sim CB, Ziemann M, Kaspi A, Harikrishnan KN, Ooi J, Khurana I, Chang L, Hudson JE, El-Osta A, Porrello ER. Dynamic changes in the cardiac methylome during postnatal development. FASEB J 29: 1329–1343, 2015. doi: 10.1096/fj.14-264093. [DOI] [PubMed] [Google Scholar]
- 921.Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT, Ge R. Genetic evidence for high-altitude adaptation in Tibet. Science 329: 72–75, 2010. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- 922.Singh RP, Shiue K, Schomberg D, Zhou FC. Cellular epigenetic modifications of neural stem cell differentiation. Cell Transplant 18: 1197–1211, 2009. doi: 10.3727/096368909X12483162197204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923.Skeffington KL, Higgins JS, Mahmoud AD, Evans AM, Sferruzzi-Perri AN, Fowden AL, Yung HW, Burton GJ, Giussani DA, Moore LG. Hypoxia, AMPK activation and uterine artery vasoreactivity. J Physiol 594: 1357–1369, 2016. doi: 10.1113/JP270995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 924.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 469: 336–342, 2011. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 925.Sobrevilla LA, Romero I, Kruger F, Whittembury J. Low estrogen excretion during pregnancy at high altitude. Am J Obstet Gynecol 102: 828–833, 1968. doi: 10.1016/0002-9378(68)90510-3. [DOI] [PubMed] [Google Scholar]
- 926.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab 90: 4299–4308, 2005. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 927.Song MA, Paradis AN, Gay MS, Shin J, Zhang L. Differential expression of microRNAs in ischemic heart disease. Drug Discov Today 20: 223–235, 2015. doi: 10.1016/j.drudis.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928.Song XW, Li Q, Lin L, Wang XC, Li DF, Wang GK, Ren AJ, Wang YR, Qin YW, Yuan WJ, Jing Q. MicroRNAs are dynamically regulated in hypertrophic hearts, and miR-199a is essential for the maintenance of cell size in cardiomyocytes. J Cell Physiol 225: 437–443, 2010. doi: 10.1002/jcp.22217. [DOI] [PubMed] [Google Scholar]
- 929.Soria R, Julian CG, Vargas E, Moore LG, Giussani DA. Graduated effects of high-altitude hypoxia and highland ancestry on birth size. Pediatr Res 74: 633–638, 2013. doi: 10.1038/pr.2013.150. [DOI] [PubMed] [Google Scholar]
- 930.Spence D, Stewart MC, Alderdice FA, Patterson CC, Halliday HL. Intra-uterine growth restriction and increased risk of hypertension in adult life: a follow-up study of 50-year-olds. Public Health 126: 561–565, 2012. doi: 10.1016/j.puhe.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 931.Spencer SJ. Perinatal programming of neuroendocrine mechanisms connecting feeding behavior and stress. Front Neurosci 7: 109, 2013. doi: 10.3389/fnins.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 932.Stanley JL, Andersson IJ, Poudel R, Rueda-Clausen CF, Sibley CP, Davidge ST, Baker PN. Sildenafil citrate rescues fetal growth in the catechol-O-methyl transferase knockout mouse model. Hypertension 59: 1021–1028, 2012. doi: 10.1161/HYPERTENSIONAHA.111.186270. [DOI] [PubMed] [Google Scholar]
- 933.Stanley SA, Small CJ, Murphy KG, Rayes E, Abbott CR, Seal LJ, Morgan DG, Sunter D, Dakin CL, Kim MS, Hunter R, Kuhar M, Ghatei MA, Bloom SR. Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats. Brain Res 893: 186–194, 2001. doi: 10.1016/S0006-8993(00)03312-6. [DOI] [PubMed] [Google Scholar]
- 934.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, Slagboom PE, Heijmans BT. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One 4: e7845, 2009. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322: 1247–1250, 2008. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 936.Stenmark KR, Tuder RM, El Kasmi KC. Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J Appl Physiol (1985) 119: 1164–1172, 2015. doi: 10.1152/japplphysiol.00283.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937.Stewart PA, Hayakawa EM. Interendothelial junctional changes underlie the developmental ‘tightening’ of the blood-brain barrier. Brain Res 32: 271–281, 1987. doi: 10.1016/0165-3806(87)90107-6. [DOI] [PubMed] [Google Scholar]
- 938.Su YM, Lv GR, Xie JX, Wang ZH, Lin HT. Maternal hypoxia increases the susceptibility of adult rat male offspring to high-fat diet-induced nonalcoholic fatty liver disease. Endocrinology 154: 4377–4387, 2013. doi: 10.1210/en.2012-1683. [DOI] [PubMed] [Google Scholar]
- 939.Suganuma T, Workman JL. Signals and combinatorial functions of histone modifications. Annu Rev Biochem 80: 473–499, 2011. doi: 10.1146/annurev-biochem-061809-175347. [DOI] [PubMed] [Google Scholar]
- 940.Sun M, Song MM, Wei B, Gao Q, Li L, Yao B, Chen L, Lin L, Dai Q, Zhou X, Tao J, Chen J, He C, Jin P, Xu Z. 5-Hydroxymethylcytosine-mediated alteration of transposon activity associated with the exposure to adverse in utero environments in human. Hum Mol Genet 25: 2208–2219, 2016. doi: 10.1093/hmg/ddw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 941.Sun X, Sharma S, Fratz S, Kumar S, Rafikov R, Aggarwal S, Rafikova O, Lu Q, Burns T, Dasarathy S, Wright J, Schreiber C, Radman M, Fineman JR, Black SM. Disruption of endothelial cell mitochondrial bioenergetics in lambs with increased pulmonary blood flow. Antioxid Redox Signal 18: 1739–1752, 2013. doi: 10.1089/ars.2012.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 942.Suresh K, Servinsky L, Reyes J, Undem C, Zaldumbide J, Rentsendorj O, Modekurty S, Dodd-O JM, Scott A, Pearse DB, Shimoda LA. CD36 mediates H2O2-induced calcium influx in lung microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 312: L143–L153, 2017. doi: 10.1152/ajplung.00361.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 943.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87: 1171–1180, 1996. doi: 10.1016/S0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 944.Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: The story of the Dutch Famine Study. Am J Epidemiol 147: 213–216, 1998. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- 945.Svitok P, Molcan L, Stebelova K, Vesela A, Sedlackova N, Ujhazy E, Mach M, Zeman M. Prenatal hypoxia in rats increased blood pressure and sympathetic drive of the adult offspring. Hypertens Res 39: 501–505, 2016. doi: 10.1038/hr.2016.21. [DOI] [PubMed] [Google Scholar]
- 946.Swanson AM, David AL. Animal models of fetal growth restriction: considerations for translational medicine. Placenta 36: 623–630, 2015. doi: 10.1016/j.placenta.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 947.Swanson AM, Rossi CA, Ofir K, Mehta V, Boyd M, Barker H, Ledwozyw A, Vaughan O, Martin J, Zachary I, Sebire N, Peebles DM, David AL. Maternal Therapy with Ad.VEGF-A165Increases Fetal Weight at Term in a Guinea-Pig Model of Fetal Growth Restriction. Hum Gene Ther 27: 997–1007, 2016. doi: 10.1089/hum.2016.046. [DOI] [PubMed] [Google Scholar]
- 948.Szarka A, Rigó J Jr, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 11: 59, 2010. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 949.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7: 880–885, 2006. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 950.Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reprod Toxicol 24: 9–19, 2007. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 951.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324: 930–935, 2009. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 952.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 953.Takahashi LK, Kalin NH. Early developmental and temporal characteristics of stress-induced secretion of pituitary-adrenal hormones in prenatally stressed rat pups. Brain Res 558: 75–78, 1991. doi: 10.1016/0006-8993(91)90715-8. [DOI] [PubMed] [Google Scholar]
- 954.Takahashi LK, Turner JG, Kalin NH. Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology 23: 571–581, 1998. doi: 10.1016/S0306-4530(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 955.Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes 58: 1116–1125, 2009. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 956.Tan X, Feng L, Huang X, Yang Y, Yang C, Gao Y. Histone deacetylase inhibitors promote eNOS expression in vascular smooth muscle cells and suppress hypoxia-induced cell growth. J Cell Mol Med 21: 2022–2035, 2017. doi: 10.1111/jcmm.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 957.Tang J, Zhu Z, Xia S, Li N, Chen N, Gao Q, Li L, Zhou X, Li D, Zhu X, Tu Q, Li W, Wu C, Li J, Zhong Y, Li X, Mao C, Xu Z. Chronic hypoxia in pregnancy affected vascular tone of renal interlobar arteries in the offspring. Sci Rep 5: 9723, 2015. doi: 10.1038/srep09723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 958.Tang Y, Zheng J, Sun Y, Wu Z, Liu Z, Huang G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J 50: 377–387, 2009. doi: 10.1536/ihj.50.377. [DOI] [PubMed] [Google Scholar]
- 959.Tanwar V, Gorr MW, Velten M, Eichenseer CM, Long VP III, Bonilla IM, Shettigar V, Ziolo MT, Davis JP, Baine SH, Carnes CA, Wold LE. In Utero Particulate Matter Exposure Produces Heart Failure, Electrical Remodeling, and Epigenetic Changes at Adulthood. J Am Heart Assoc 6: e005796, 2017. doi: 10.1161/JAHA.117.005796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 960.Tarasevičienė V, Grybauskienė R, Mačiulevičienė R. sFlt-1, PlGF, sFlt-1/PlGF ratio and uterine artery Doppler for preeclampsia diagnostics. Medicina (Kaunas) 52: 349–353, 2016. doi: 10.1016/j.medici.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 961.Tartaglia LA. The leptin receptor. J Biol Chem 272: 6093–6096, 1997. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 962.Tashi T, Scott Reading N, Wuren T, Zhang X, Moore LG, Hu H, Tang F, Shestakova A, Lorenzo F, Burjanivova T, Koul P, Guchhait P, Wittwer CT, Julian CG, Shah B, Huff CD, Gordeuk VR, Prchal JT, Ge R. Gain-of-function EGLN1 prolyl hydroxylase (PHD2 D4E:C127S) in combination with EPAS1 (HIF-2α) polymorphism lowers hemoglobin concentration in Tibetan highlanders. J Mol Med (Berl) 95: 665–670, 2017. doi: 10.1007/s00109-017-1519-3. [DOI] [PubMed] [Google Scholar]
- 963.Tashima L, Nakata M, Anno K, Sugino N, Kato H. Prenatal influence of ischemia-hypoxia-induced intrauterine growth retardation on brain development and behavioral activity in rats. Biol Neonate 80: 81–87, 2001. doi: 10.1159/000047125. [DOI] [PubMed] [Google Scholar]
- 964.Taylor DJ. Neurological sequelae of intrauterine deprivation. In: Fetal Monitoring, edited by Spencer JAD. Oxford: Oxford Univ. Press, 1991, p. 20–23. [Google Scholar]
- 965.Tegethoff M, Pryce C, Meinlschmidt G. Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: a systematic review. Endocr Rev 30: 753–789, 2009. doi: 10.1210/er.2008-0014. [DOI] [PubMed] [Google Scholar]
- 966.Teng GQ, Williams J, Zhang L, Purdy R, Pearce WJ. Effects of maturation, artery size, and chronic hypoxia on 5-HT receptor type in ovine cranial arteries. Am J Physiol Regul Integr Comp Physiol 275: R742–R753, 1998. [DOI] [PubMed] [Google Scholar]
- 967.Teng R-J, Eis A, Bakhutashvili I, Arul N, Konduri GG. Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 297: L184–L195, 2009. doi: 10.1152/ajplung.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 968.Teng RJ, Wu TJ, Afolayan AJ, Konduri GG. Nitrotyrosine impairs mitochondrial function in fetal lamb pulmonary artery endothelial cells. Am J Physiol Cell Physiol 310: C80–C88, 2016. doi: 10.1152/ajpcell.00073.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 969.Teran E, Chedraui P, Vivero S, Villena F, Duchicela F, Nacevilla L. Plasma and placental nitric oxide levels in women with and without pre-eclampsia living at different altitudes. Int J Gynaecol Obstet 104: 140–142, 2009. doi: 10.1016/j.ijgo.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 970.Thienpont B, Steinbacher J, Zhao H, D’Anna F, Kuchnio A, Ploumakis A, Ghesquière B, Van Dyck L, Boeckx B, Schoonjans L, Hermans E, Amant F, Kristensen VN, Peng Koh K, Mazzone M, Coleman M, Carell T, Carmeliet P, Lambrechts D. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 537: 63–68, 2016. doi: 10.1038/nature19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971.Thomassin H, Flavin M, Espinás ML, Grange T. Glucocorticoid-induced DNA demethylation and gene memory during development. EMBO J 20: 1974–1983, 2001. doi: 10.1093/emboj/20.8.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 972.Thompson JA, Piorkowska K, Gagnon R, Richardson BS, Regnault TR. Increased collagen deposition in the heart of chronically hypoxic ovine fetuses. J Dev Orig Health Dis 4: 470–478, 2013. doi: 10.1017/S2040174413000299. [DOI] [PubMed] [Google Scholar]
- 973.Thompson LP, Al-Hasan Y. Impact of oxidative stress in fetal programming. J Pregnancy 2012: 582748, 2012. doi: 10.1155/2012/582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 974.Thompson LP, Dong Y. Chronic hypoxia decreases endothelial nitric oxide synthase protein expression in fetal guinea pig hearts. J Soc Gynecol Investig 12: 388–395, 2005. doi: 10.1016/j.jsgi.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 975.Thompson LP, Liu H, Evans L, Mong JA. Prenatal nicotine increases matrix metalloproteinase 2 (MMP-2) expression in fetal guinea pig hearts. Reprod Sci 18: 1103–1110, 2011. doi: 10.1177/1933719111404605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 976.Thompson LP, Pence L, Pinkas G, Song H, Telugu BP. Placental Hypoxia During Early Pregnancy Causes Maternal Hypertension and Placental Insufficiency in the Hypoxic Guinea Pig Model. Biol Reprod 95: 128, 2016. doi: 10.1095/biolreprod.116.142273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 977.Thornburg KL. The programming of cardiovascular disease. J Dev Orig Health Dis 6: 366–376, 2015. doi: 10.1017/S2040174415001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 978.Thornburg KL, Louey S, Giraud GD. The role of growth in heart development. Nestle Nutr Workshop Ser Pediatr Program 61: 39–51, 2008. doi: 10.1159/000113169. [DOI] [PubMed] [Google Scholar]
- 979.Tissot van Patot M, Grilli A, Chapman P, Broad E, Tyson W, Heller DS, Zwerdlinger L, Zamudio S. Remodelling of uteroplacental arteries is decreased in high altitude placentae. Placenta 24: 326–335, 2003. doi: 10.1053/plac.2002.0899. [DOI] [PubMed] [Google Scholar]
- 980.Tissot van Patot MC, Bendrick-Peart J, Beckey VE, Serkova N, Zwerdlinger L. Greater vascularity, lowered HIF-1/DNA binding, and elevated GSH as markers of adaptation to in vivo chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L525–L532, 2004. doi: 10.1152/ajplung.00203.2003. [DOI] [PubMed] [Google Scholar]
- 981.Tissot van Patot MC, Murray AJ, Beckey V, Cindrova-Davies T, Johns J, Zwerdlinger L, Jauniaux E, Burton GJ, Serkova NJ. Human placental metabolic adaptation to chronic hypoxia, high altitude: hypoxic preconditioning. Am J Physiol Regul Integr Comp Physiol 298: R166–R172, 2010. doi: 10.1152/ajpregu.00383.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 982.Tognini P, Napoli D, Pizzorusso T. Dynamic DNA methylation in the brain: a new epigenetic mark for experience-dependent plasticity. Front Cell Neurosci 9: 331, 2015. doi: 10.3389/fncel.2015.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 983.Tokudome S, Sano M, Shinmura K, Matsuhashi T, Morizane S, Moriyama H, Tamaki K, Hayashida K, Nakanishi H, Yoshikawa N, Shimizu N, Endo J, Katayama T, Murata M, Yuasa S, Kaneda R, Tomita K, Eguchi N, Urade Y, Asano K, Utsunomiya Y, Suzuki T, Taguchi R, Tanaka H, Fukuda K. Glucocorticoid protects rodent hearts from ischemia/reperfusion injury by activating lipocalin-type prostaglandin D synthase-derived PGD2 biosynthesis. J Clin Invest 119: 1477–1488, 2009. doi: 10.1172/JCI37413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363, 2002. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 985.Tong W, Xiong F, Li Y, Zhang L. Hypoxia inhibits cardiomyocyte proliferation in fetal rat hearts via upregulating TIMP-4. Am J Physiol Regul Integr Comp Physiol 304: R613–R620, 2013. doi: 10.1152/ajpregu.00515.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986.Tong W, Xue Q, Li Y, Zhang L. Maternal hypoxia alters matrix metalloproteinase expression patterns and causes cardiac remodeling in fetal and neonatal rats. Am J Physiol Heart Circ Physiol 301: H2113–H2121, 2011. doi: 10.1152/ajpheart.00356.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 987.Tong W, Zhang L. Fetal hypoxia and programming of matrix metalloproteinases. Drug Discov Today 17: 124–134, 2012. doi: 10.1016/j.drudis.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 988.Torres A, Belser WW III, Umeda PK, Tucker D. Indicators of delayed maturation of rat heart treated prenatally with dexamethasone. Pediatr Res 42: 139–144, 1997. doi: 10.1203/00006450-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 989.Torres F, González-Candia A, Montt C, Ebensperger G, Chubretovic M, Serón-Ferré M, Reyes RV, Llanos AJ, Herrera EA. Melatonin reduces oxidative stress and improves vascular function in pulmonary hypertensive newborn sheep. J Pineal Res 58: 362–373, 2015. doi: 10.1111/jpi.12222. [DOI] [PubMed] [Google Scholar]
- 990.Tremolizzo L, Doueiri MS, Dong E, Grayson DR, Davis J, Pinna G, Tueting P, Rodriguez-Menendez V, Costa E, Guidotti A. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry 57: 500–509, 2005. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 991.Tsai NP, Lin YL, Wei LN. MicroRNA mir-346 targets the 5′-untranslated region of receptor-interacting protein 140 (RIP140) mRNA and up-regulates its protein expression. Biochem J 424: 411–418, 2009. doi: 10.1042/BJ20090915. [DOI] [PubMed] [Google Scholar]
- 992.Tschöp M, Strasburger CJ, Hartmann G, Biollaz J, Bärtsch P. Raised leptin concentrations at high altitude associated with loss of appetite. Lancet 352: 1119–1120, 1998. doi: 10.1016/S0140-6736(05)79760-9. [DOI] [PubMed] [Google Scholar]
- 993.Tschöp M, Strasburger CJ, Töpfer M, Hautmann H, Riepl R, Fischer R, Hartmann G, Morrison K, Appenzeller M, Hildebrandt W, Biollaz J, Bärtsch P. Influence of hypobaric hypoxia on leptin levels in men. Int J Obes Relat Metab Disord 24, Suppl 2: S151, 2000. doi: 10.1038/sj.ijo.0801309. [DOI] [PubMed] [Google Scholar]
- 994.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature 439: 811–816, 2006. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 995.Turan S, Aberdeen GW, Thompson LP. Chronic hypoxia alters maternal uterine and fetal hemodynamics in the full-term pregnant guinea pig. Am J Physiol Regul Integr Comp Physiol 313: R330–R339, 2017. doi: 10.1152/ajpregu.00056.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 996.Uddin MN, Horvat D, Jones RO, Beeram MR, Zawieja DC, Perger L, Sprague DC, Kuehl TJ. Suppression of aldosterone and progesterone in preeclampsia. J Matern Fetal Neonatal Med 28: 1–6, 2014. [DOI] [PubMed] [Google Scholar]
- 997.Ueno N, Zhao Y, Zhang L, Longo LD. High altitude-induced changes in alpha1-adrenergic receptors and Ins(1,4,5)P3 responses in cerebral arteries. Am J Physiol Regul Integr Comp Physiol 272: R669–R674, 1997. [DOI] [PubMed] [Google Scholar]
- 998.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, 2015. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 999.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409, 2009. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1000.Unger C, Weiser JK, McCullough RE, Keefer S, Moore LG. Altitude, low birth weight, and infant mortality in Colorado. JAMA 259: 3427–3432, 1988. doi: 10.1001/jama.1988.03720230037027. [DOI] [PubMed] [Google Scholar]
- 1001.Unno N, Giussani DA, Hing WK, Ding XY, Collins JH, Nathanielsz PW. Changes in adrenocorticotropin and cortisol responsiveness after repeated partial umbilical cord occlusions in the late gestation ovine fetus. Endocrinology 138: 259–263, 1997. doi: 10.1210/endo.138.1.4880. [DOI] [PubMed] [Google Scholar]
- 1002.Valen G, Kawakami T, Tähepôld P, Dumitrescu A, Löwbeer C, Vaage J. Glucocorticoid pretreatment protects cardiac function and induces cardiac heat shock protein 72. Am J Physiol Heart Circ Physiol 279: H836–H843, 2000. doi: 10.1152/ajpheart.2000.279.2.H836. [DOI] [PubMed] [Google Scholar]
- 1003.Van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic Biol Med 44: 938–955, 2008. doi: 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1004.Van Erp TG, Saleh PA, Rosso IM, Huttunen M, Lönnqvist J, Pirkola T, Salonen O, Valanne L, Poutanen VP, Standertskjöld-Nordenstam CG, Cannon TD. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry 159: 1514–1520, 2002. doi: 10.1176/appi.ajp.159.9.1514. [DOI] [PubMed] [Google Scholar]
- 1005.Van Patot MC, Ebensperger G, Gassmann M, Llanos AJ. The hypoxic placenta. High Alt Med Biol 13: 176–184, 2012. doi: 10.1089/ham.2012.1046. [DOI] [PubMed] [Google Scholar]
- 1006.Van Patot MC, Valdez M, Becky V, Cindrova-Davies T, Johns J, Zwerdling L, Jauniaux E, Burton GJ. Impact of pregnancy at high altitude on placental morphology in non-native women with and without preeclampsia. Placenta 30: 523–528, 2009. doi: 10.1016/j.placenta.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 1007.Vanhoutte PM, Lüscher TF, Gräser T. Endothelium-dependent contractions. Blood Vessels 28: 74–83, 1991. [DOI] [PubMed] [Google Scholar]
- 1008.Varga E, Nagy N, Lazar J, Czifra G, Bak I, Biro T, Tosaki A. Inhibition of ischemia/reperfusion-induced damage by dexamethasone in isolated working rat hearts: the role of cytochrome c release. Life Sci 75: 2411–2423, 2004. doi: 10.1016/j.lfs.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 1009.Vargas VE, Kaushal KM, Monau T, Myers DA, Ducsay CA. Long-term hypoxia enhances cortisol biosynthesis in near-term ovine fetal adrenal cortical cells. Reprod Sci 18: 277–285, 2011. doi: 10.1177/1933719110386242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1010.Vargas VE, Kaushal KM, Monau TR, Myers DA, Ducsay CA. Extracellular signal-regulated kinases (ERK1/2) signaling pathway plays a role in cortisol secretion in the long-term hypoxic ovine fetal adrenal near term. Am J Physiol Regul Integr Comp Physiol 304: R636–R643, 2013. doi: 10.1152/ajpregu.00318.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1011.Veith C, Kraut S, Wilhelm J, Sommer N, Quanz K, Seeger W, Brandes RP, Weissmann N, Schröder K. NADPH oxidase 4 is not involved in hypoxia-induced pulmonary hypertension. Pulm Circ 6: 397–400, 2016. doi: 10.1086/687756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1012.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation 123: 1986–1995, 2011. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1013.Verlohren S, Geusens N, Morton J, Verhaegen I, Hering L, Herse F, Dudenhausen JW, Muller DN, Luft FC, Cartwright JE, Davidge ST, Pijnenborg R, Dechend R. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension 56: 304–310, 2010. doi: 10.1161/HYPERTENSIONAHA.110.153163. [DOI] [PubMed] [Google Scholar]
- 1014.Vij AG, Dutta R, Satija NK. Acclimatization to oxidative stress at high altitude. High Alt Med Biol 6: 301–310, 2005. doi: 10.1089/ham.2005.6.301. [DOI] [PubMed] [Google Scholar]
- 1015.Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res 53: 619–629, 2012. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1016.von Dadelszen P, Dwinnell S, Magee LA, Carleton BC, Gruslin A, Lee B, Lim KI, Liston RM, Miller SP, Rurak D, Sherlock RL, Skoll MA, Wareing MM, Baker PN; Research into Advanced Fetal Diagnosis and Therapy (RAFT) Group . Sildenafil citrate therapy for severe early-onset intrauterine growth restriction. BJOG 118: 624–628, 2011. doi: 10.1111/j.1471-0528.2010.02879.x. [DOI] [PubMed] [Google Scholar]
- 1017.Vuguin PM. Animal models for small for gestational age and fetal programming of adult disease. Horm Res 68: 113–123, 2007. doi: 10.1159/000100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1018.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22: 6810–6818, 2002. 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1019.Walczak-Drzewiecka A, Ratajewski M, Pułaski Ł, Dastych J. DNA methylation-dependent suppression of HIF1A in an immature hematopoietic cell line HMC-1. Biochem Biophys Res Commun 391: 1028–1032, 2010. doi: 10.1016/j.bbrc.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 1020.Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab 302: E19–E31, 2012. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- 1021.Walton SL, Singh RR, Tan T, Paravicini TM, Moritz KM. Late gestational hypoxia and a postnatal high salt diet programs endothelial dysfunction and arterial stiffness in adult mouse offspring. J Physiol 594: 1451–1463, 2016. doi: 10.1113/JP271067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1022.Wang G, Liem DA, Vondriska TM, Honda HM, Korge P, Pantaleon DM, Qiao X, Wang Y, Weiss JN, Ping P. Nitric oxide donors protect murine myocardium against infarction via modulation of mitochondrial permeability transition. Am J Physiol Heart Circ Physiol 288: H1290–H1295, 2005. doi: 10.1152/ajpheart.00796.2004. [DOI] [PubMed] [Google Scholar]
- 1023.Wang KC, Botting KJ, Zhang S, McMillen IC, Brooks DA, Morrison JL. Akt signaling as a mediator of cardiac adaptation to low birth weight. J Endocrinol 233: R81–R94, 2017. doi: 10.1530/JOE-17-0039. [DOI] [PubMed] [Google Scholar]
- 1024.Wang L, Cai R, Lv G, Huang Z, Wang Z. Hypoxia during pregnancy in rats leads to the changes of the cerebral white matter in adult offspring. Biochem Biophys Res Commun 396: 445–450, 2010. doi: 10.1016/j.bbrc.2010.04.114. [DOI] [PubMed] [Google Scholar]
- 1025.Wang L, Ke J, Li Y, Ma Q, Dasgupta C, Huang X, Zhang L, Xiao D. Inhibition of miRNA-210 reverses nicotine-induced brain hypoxic-ischemic injury in neonatal rats. Int J Biol Sci 13: 76–84, 2017. doi: 10.7150/ijbs.17278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1026.Wang L, Li M, Huang Z, Wang Z. The influence of hypoxia during different pregnancy stages on cardiac collagen accumulation in the adult offspring. Biomed Res Int 2014: 419805, 2014. doi: 10.1155/2014/419805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1027.Wang L, Li X, Zhou Y, Shi H, Xu C, He H, Wang S, Xiong X, Zhang Y, Du Z, Zhang R, Lu Y, Yang B, Shan H. Downregulation of miR-133 via MAPK/ERK signaling pathway involved in nicotine-induced cardiomyocyte apoptosis. Naunyn Schmiedebergs Arch Pharmacol 387: 197–206, 2014. doi: 10.1007/s00210-013-0929-1. [DOI] [PubMed] [Google Scholar]
- 1028.Wang L, Sun H, Pan B, Zhu J, Huang G, Huang X, Tian J. Inhibition of histone acetylation by curcumin reduces alcohol-induced expression of heart development-related transcription factors in cardiac progenitor cells. Biochem Biophys Res Commun 424: 593–596, 2012. doi: 10.1016/j.bbrc.2012.06.158. [DOI] [PubMed] [Google Scholar]
- 1029.Wang LW, Berry-Kravis E, Hagerman RJ. Fragile X: leading the way for targeted treatments in autism. Neurotherapeutics 7: 264–274, 2010. doi: 10.1016/j.nurt.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1030.Wang P, Xu J, Hou Z, Wang F, Song Y, Wang J, Zhu H, Jin H. miRNA-34a promotes proliferation of human pulmonary artery smooth muscle cells by targeting PDGFRA. Cell Prolif 49: 484–493, 2016. doi: 10.1111/cpr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1031.Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, Li X. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci USA 105: 2889–2894, 2008. doi: 10.1073/pnas.0800178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1032.Wang X, Abdel-Rahman AA. Estrogen modulation of eNOS activity and its association with caveolin-3 and calmodulin in rat hearts. Am J Physiol Heart Circ Physiol 282: H2309–H2315, 2002. doi: 10.1152/ajpheart.00772.2001. [DOI] [PubMed] [Google Scholar]
- 1033.Wang X, Meng FS, Liu ZY, Fan JM, Hao K, Chen XQ, Du JZ. Gestational hypoxia induces sex-differential methylation of Crhr1 linked to anxiety-like behavior. Mol Neurobiol 48: 544–555, 2013. doi: 10.1007/s12035-013-8444-4. [DOI] [PubMed] [Google Scholar]
- 1034.Wang X, Moazed D. DNA sequence-dependent epigenetic inheritance of gene silencing and histone H3K9 methylation. Science 356: 88–91, 2017. doi: 10.1126/science.aaj2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1035.Wang XC, Sun WT, Yu CM, Pun SH, Underwood MJ, He GW, Yang Q. ER stress mediates homocysteine-induced endothelial dysfunction: Modulation of IKCa and SKCa channels. Atherosclerosis 242: 191–198, 2015. doi: 10.1016/j.atherosclerosis.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 1036.Wang Y, Zhao S. Vascular Biology of the Placenta. San Rafael, CA: Morgan & Claypool Life Sciences, 2010. [PubMed] [Google Scholar]
- 1037.Wang Z, Huang Z, Lu G, Lin L, Ferrari M. Hypoxia during pregnancy in rats leads to early morphological changes of atherosclerosis in adult offspring. Am J Physiol Heart Circ Physiol 296: H1321–H1328, 2009. doi: 10.1152/ajpheart.00440.2008. [DOI] [PubMed] [Google Scholar]
- 1038.Watson CJ, Collier P, Tea I, Neary R, Watson JA, Robinson C, Phelan D, Ledwidge MT, McDonald KM, McCann A, Sharaf O, Baugh JA. Hypoxia-induced epigenetic modifications are associated with cardiac tissue fibrosis and the development of a myofibroblast-like phenotype. Hum Mol Genet 23: 2176–2188, 2014. doi: 10.1093/hmg/ddt614. [DOI] [PubMed] [Google Scholar]
- 1039.Watson JA, Watson CJ, McCann A, Baugh J. Epigenetics, the epicenter of the hypoxic response. Epigenetics 5: 293–296, 2010. doi: 10.4161/epi.5.4.11684. [DOI] [PubMed] [Google Scholar]
- 1040.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res 99: 970–978, 2006. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- 1041.Waypa GB, Schumacker PT. Hypoxia-induced changes in pulmonary and systemic vascular resistance: where is the O2 sensor? Respir Physiol Neurobiol 174: 201–211, 2010. doi: 10.1016/j.resp.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1042.Weaver IC, Korgan AC, Lee K, Wheeler RV, Hundert AS, Goguen D. Stress and the Emerging Roles of Chromatin Remodeling in Signal Integration and Stable Transmission of Reversible Phenotypes. Front Behav Neurosci 11: 41, 2017. doi: 10.3389/fnbeh.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1043.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA 103: 3480–3485, 2006. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1044.Wedgwood S, Lakshminrusimha S, Fukai T, Russell JA, Schumacker PT, Steinhorn RH. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal 15: 1497–1506, 2011. doi: 10.1089/ars.2010.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1045.Wedgwood S, Lakshminrusimha S, Schumacker PT, Steinhorn RH. Hypoxia inducible factor signaling and experimental persistent pulmonary hypertension of the newborn. Front Pharmacol 6: 47, 2015. doi: 10.3389/fphar.2015.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1046.Wedgwood S, Steinhorn RH. Role of reactive oxygen species in neonatal pulmonary vascular disease. Antioxid Redox Signal 21: 1926–1942, 2014. doi: 10.1089/ars.2013.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1047.Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 289: L660–L666, 2005. doi: 10.1152/ajplung.00369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1048.Wehrenberg WB, Chaichareon DP, Dierschke DJ, Rankin JH, Ginther OJ. Vascular dynamics of the reproductive tract in the female rhesus monkey: relative contributions of ovarian and uterine arteries. Biol Reprod 17: 148–153, 1977. doi: 10.1095/biolreprod17.1.148. [DOI] [PubMed] [Google Scholar]
- 1049.Weiner FR, Czaja MJ, Jefferson DM, Giambrone MA, Tur-Kaspa R, Reid LM, Zern MA. The effects of dexamethasone on in vitro collagen gene expression. J Biol Chem 262: 6955–6958, 1987. [PubMed] [Google Scholar]
- 1050.West JB. High Life: A History of High-Altitude Physiology and Medicine. New York: Oxford Univ. Press, 1998. doi: 10.1007/978-1-4614-7573-6 [DOI] [Google Scholar]
- 1051.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 114: e29–e36, 2004. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 1052.White CR, Hamade MW, Siami K, Chang MM, Mangalwadi A, Frangos JA, Pearce WJ. Maturation enhances fluid shear-induced activation of eNOS in perfused ovine carotid arteries. Am J Physiol Heart Circ Physiol 289: H2220–H2227, 2005. doi: 10.1152/ajpheart.01013.2004. [DOI] [PubMed] [Google Scholar]
- 1053.White MM, McCullough RE, Dyckes R, Robertson AD, Moore LG. Chronic hypoxia, pregnancy, and endothelium-mediated relaxation in guinea pig uterine and thoracic arteries. Am J Physiol Heart Circ Physiol 278: H2069–H2075, 2000. doi: 10.1152/ajpheart.2000.278.6.H2069. [DOI] [PubMed] [Google Scholar]
- 1054.Whitehead CL, Teh WT, Walker SP, Leung C, Larmour L, Tong S. Circulating MicroRNAs in maternal blood as potential biomarkers for fetal hypoxia in-utero. PLoS One 8: e78487, 2013. doi: 10.1371/journal.pone.0078487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1055.Wilhelm M, Schlegl J, Hahne H, Gholami AM, Lieberenz M, Savitski MM, Ziegler E, Butzmann L, Gessulat S, Marx H, Mathieson T, Lemeer S, Schnatbaum K, Reimer U, Wenschuh H, Mollenhauer M, Slotta-Huspenina J, Boese JH, Bantscheff M, Gerstmair A, Faerber F, Kuster B. Mass-spectrometry-based draft of the human proteome. Nature 509: 582–587, 2014. doi: 10.1038/nature13319. [DOI] [PubMed] [Google Scholar]
- 1056.Wilkening RB, Meschia G. Fetal oxygen uptake, oxygenation, and acid-base balance as a function of uterine blood flow. Am J Physiol Heart Circ Physiol 244: H749–H755, 1983. [DOI] [PubMed] [Google Scholar]
- 1057.Wilkinson LJ, Neal CS, Singh RR, Sparrow DB, Kurniawan ND, Ju A, Grieve SM, Dunwoodie SL, Moritz KM, Little MH. Renal developmental defects resulting from in utero hypoxia are associated with suppression of ureteric β-catenin signaling. Kidney Int 87: 975–983, 2015. doi: 10.1038/ki.2014.394. [DOI] [PubMed] [Google Scholar]
- 1058.Willard HF, Brown CJ, Carrel L, Hendrich B, Miller AP. Epigenetic and chromosomal control of gene expression: molecular and genetic analysis of X chromosome inactivation. Cold Spring Harb Symp Quant Biol 58: 315–322, 1993. doi: 10.1101/SQB.1993.058.01.037. [DOI] [PubMed] [Google Scholar]
- 1059.Williams JM, Hull AD, Pearce WJ. Maturational modulation of endothelium-dependent vasodilatation in ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol 288: R149–R157, 2005. doi: 10.1152/ajpregu.00427.2004. [DOI] [PubMed] [Google Scholar]
- 1060.Williams JM, Pearce WJ. Age-dependent modulation of endothelium-dependent vasodilatation by chronic hypoxia in ovine cranial arteries. J Appl Physiol (1985) 100: 225–232, 2006. doi: 10.1152/japplphysiol.00221.2005. [DOI] [PubMed] [Google Scholar]
- 1061.Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol 288: R360–R367, 2005. doi: 10.1152/ajpregu.00178.2004. [DOI] [PubMed] [Google Scholar]
- 1062.Williams SJ, Hemmings DG, Mitchell JM, McMillen IC, Davidge ST. Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J Physiol 565: 125–135, 2005. doi: 10.1113/jphysiol.2005.084889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1063.Wilson MJ, Lopez M, Vargas M, Julian C, Tellez W, Rodriguez A, Bigham A, Armaza JF, Niermeyer S, Shriver M, Vargas E, Moore LG. Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am J Physiol Regul Integr Comp Physiol 293: R1313–R1324, 2007. doi: 10.1152/ajpregu.00806.2006. [DOI] [PubMed] [Google Scholar]
- 1064.Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science 286: 481–486, 1999. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 1065.Wood CE. Sinoaortic denervation attenuates the reflex responses to hypotension in fetal sheep. Am J Physiol Regul Integr Comp Physiol 256: R1103–R1110, 1989. [DOI] [PubMed] [Google Scholar]
- 1066.Wood CE, Rabaglino MB, Chang EI, Denslow N, Keller-Wood M, Richards E. Genomics of the fetal hypothalamic cellular response to transient hypoxia: endocrine, immune, and metabolic responses. Physiol Genomics 45: 521–527, 2013. doi: 10.1152/physiolgenomics.00005.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1067.Wood CE, Rabaglino MB, Richards E, Denslow N, Zarate MA, Chang EI, Keller-Wood M. Transcriptomics of the fetal hypothalamic response to brachiocephalic occlusion and estradiol treatment. Physiol Genomics 46: 523–532, 2014. doi: 10.1152/physiolgenomics.00186.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1068.Wood GE, Young LT, Reagan LP, McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav 43: 205–213, 2003. doi: 10.1016/S0018-506X(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 1069.Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry 155: 1661–1670, 1998. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- 1070.Wu H, Sun YE. Reversing DNA methylation: new insights from neuronal activity-induced Gadd45b in adult neurogenesis. Sci Signal 2: pe17, 2009. doi: 10.1126/scisignal.264pe17. [DOI] [PubMed] [Google Scholar]
- 1071.Xia S, Lv J, Gao Q, Li L, Chen N, Wei X, Xiao J, Chen J, Tao J, Sun M, Mao C, Zhang L, Xu Z. Prenatal exposure to hypoxia induced Beclin 1 signaling-mediated renal autophagy and altered renal development in rat fetuses. Reprod Sci 22: 156–164, 2015. doi: 10.1177/1933719114536474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1072.Xiao D, Bird IM, Magness RR, Longo LD, Zhang L. Upregulation of eNOS in pregnant ovine uterine arteries by chronic hypoxia. Am J Physiol Heart Circ Physiol 280: H812–H820, 2001. doi: 10.1152/ajpheart.2001.280.2.H812. [DOI] [PubMed] [Google Scholar]
- 1073.Xiao D, Buchholz JN, Zhang L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: role of PKC/ERK pathway. Am J Physiol Heart Circ Physiol 290: H2337–H2343, 2006. doi: 10.1152/ajpheart.01238.2005. [DOI] [PubMed] [Google Scholar]
- 1074.Xiao D, Dasgupta C, Chen M, Zhang K, Buchholz J, Xu Z, Zhang L. Inhibition of DNA methylation reverses norepinephrine-induced cardiac hypertrophy in rats. Cardiovasc Res 101: 373–382, 2014. doi: 10.1093/cvr/cvt264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1075.Xiao D, Dasgupta C, Li Y, Huang X, Zhang L. Perinatal nicotine exposure increases angiotensin II receptor-mediated vascular contractility in adult offspring. PLoS One 9: e108161, 2014. doi: 10.1371/journal.pone.0108161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1076.Xiao D, Ducsay CA, Zhang L. Chronic hypoxia and developmental regulation of cytochrome c expression in rats. J Soc Gynecol Investig 7: 279–283, 2000. doi: 10.1177/107155760000700502. [DOI] [PubMed] [Google Scholar]
- 1077.Xiao D, Hu XQ, Huang X, Zhou J, Wilson SM, Yang S, Zhang L. Chronic hypoxia during gestation enhances uterine arterial myogenic tone via heightened oxidative stress. PLoS One 8: e73731, 2013. doi: 10.1371/journal.pone.0073731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1078.Xiao D, Huang X, Li Y, Dasgupta C, Wang L, Zhang L. Antenatal Antioxidant Prevents Nicotine-Mediated Hypertensive Response in Rat Adult Offspring. Biol Reprod 93: 66, 2015. doi: 10.1095/biolreprod.115.132381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1079.Xiao D, Huang X, Xu Z, Yang S, Zhang L. Prenatal cocaine exposure differentially causes vascular dysfunction in adult offspring. Hypertension 53: 937–943, 2009. doi: 10.1161/HYPERTENSIONAHA.108.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1080.Xiao D, Huang X, Xue Q, Zhang L. Antenatal hypoxia induces programming of reduced arterial blood pressure response in female rat offspring: role of ovarian function. PLoS One 9: e98743, 2014. doi: 10.1371/journal.pone.0098743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1081.Xiao D, Huang X, Yang S, Zhang L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br J Pharmacol 164: 1400–1409, 2011. doi: 10.1111/j.1476-5381.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1082.Xiao D, Huang X, Yang S, Zhang L. Direct chronic effect of steroid hormones in attenuating uterine arterial myogenic tone: role of protein kinase c/extracellular signal-regulated kinase 1/2. Hypertension 54: 352–358, 2009. doi: 10.1161/HYPERTENSIONAHA.109.130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1083.Xiao D, Huang X, Yang S, Zhang L. Estrogen normalizes perinatal nicotine-induced hypertensive responses in adult female rat offspring. Hypertension 61: 1246–1254, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1084.Xiao D, Huang X, Zhang L. Chronic hypoxia differentially up-regulates protein kinase C-mediated ovine uterine arterial contraction via actin polymerization signaling in pregnancy. Biol Reprod 87: 142, 2012. doi: 10.1095/biolreprod.112.104448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1085.Xiao D, Wang L, Huang X, Li Y, Dasgupta C, Zhang L. Protective Effect of Antenatal Antioxidant on Nicotine-Induced Heart Ischemia-Sensitive Phenotype in Rat Offspring. PLoS One 11: e0150557, 2016. doi: 10.1371/journal.pone.0150557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1086.Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension 51: 1239–1247, 2008. doi: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1087.Xiao D, Yang S, Zhang L. Prenatal cocaine exposure causes sex-dependent impairment in the myogenic reactivity of coronary arteries in adult offspring. Hypertension 54: 1123–1128, 2009. doi: 10.1161/HYPERTENSIONAHA.109.138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1088.Xiao Y, Xiao D, He J, Zhang L. Maternal cocaine administration during pregnancy induces apoptosis in fetal rat heart. J Cardiovasc Pharmacol 37: 639–648, 2001. doi: 10.1097/00005344-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 1089.Xiong F, Lin T, Song M, Ma Q, Martinez SR, Lv J, MataGreenwood E, Xiao D, Xu Z, Zhang L. Antenatal hypoxia induces epigenetic repression of glucocorticoid receptor and promotes ischemic-sensitive phenotype in the developing heart. J Mol Cell Cardiol 91: 160–171, 2016. doi: 10.1016/j.yjmcc.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1090.Xiong F, Xiao D, Zhang L. Norepinephrine causes epigenetic repression of PKCε gene in rodent hearts by activating Nox1-dependent reactive oxygen species production. FASEB J 26: 2753–2763, 2012. doi: 10.1096/fj.11-199422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1091.Xiong F, Zhang L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front Neuroendocrinol 34: 27–46, 2013. doi: 10.1016/j.yfrne.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1092.Xu C, Hu Y, Hou L, Ju J, Li X, Du N, Guan X, Liu Z, Zhang T, Qin W, Shen N, Bilal MU, Lu Y, Zhang Y, Shan H. β-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J Mol Cell Cardiol 75: 111–121, 2014. doi: 10.1016/j.yjmcc.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 1093.Xu D, Guo H, Xu X, Lu Z, Fassett J, Hu X, Xu Y, Tang Q, Hu D, Somani A, Geurts AM, Ostertag E, Bache RJ, Weir EK, Chen Y. Exacerbated pulmonary arterial hypertension and right ventricular hypertrophy in animals with loss of function of extracellular superoxide dismutase. Hypertension 58: 303–309, 2011. doi: 10.1161/HYPERTENSIONAHA.110.166819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1094.Xu EZ, Kantores C, Ivanovska J, Engelberts D, Kavanagh BP, McNamara PJ, Jankov RP. Rescue treatment with a Rho-kinase inhibitor normalizes right ventricular function and reverses remodeling in juvenile rats with chronic pulmonary hypertension. Am J Physiol Heart Circ Physiol 299: H1854–H1864, 2010. doi: 10.1152/ajpheart.00595.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1095.Xu K, Lamanna JC. Chronic hypoxia and the cerebral circulation. J Appl Physiol (1985) 100: 725–730, 2006. doi: 10.1152/japplphysiol.00940.2005. [DOI] [PubMed] [Google Scholar]
- 1096.Xu P, Zhao Y, Liu M, Wang Y, Wang H, Li YX, Zhu X, Yao Y, Wang H, Qiao J, Ji L, Wang YL. Variations of microRNAs in human placentas and plasma from preeclamptic pregnancy. Hypertension 63: 1276–1284, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02647. [DOI] [PubMed] [Google Scholar]
- 1097.Xu X-F, Lv Y, Gu W-Z, Tang L-L, Wei J-K, Zhang L-Y, Du L-Z. Epigenetics of hypoxic pulmonary arterial hypertension following intrauterine growth retardation rat: epigenetics in PAH following IUGR. Respir Res 14: 20, 2013. doi: 10.1186/1465-9921-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1098.Xu XF, Ma XL, Shen Z, Wu XL, Cheng F, Du LZ. Epigenetic regulation of the endothelial nitric oxide synthase gene in persistent pulmonary hypertension of the newborn rat. J Hypertens 28: 2227–2235, 2010. doi: 10.1097/HJH.0b013e32833e08f1. [DOI] [PubMed] [Google Scholar]
- 1099.Xu Y, Arenas IA, Armstrong SJ, Plahta WC, Xu H, Davidge ST. Estrogen improves cardiac recovery after ischemia/reperfusion by decreasing tumor necrosis factor-alpha. Cardiovasc Res 69: 836–844, 2006. doi: 10.1016/j.cardiores.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 1100.Xu Y, Williams SJ, O’Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J 20: 1251–1253, 2006. doi: 10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- 1101.Xu YP, He Q, Shen Z, Shu XL, Wang CH, Zhu JJ, Shi LP, Du LZ. MiR-126a-5p is involved in the hypoxia-induced endothelial-to-mesenchymal transition of neonatal pulmonary hypertension. Hypertens Res 40: 552–561, 2017. doi: 10.1038/hr.2017.2. [DOI] [PubMed] [Google Scholar]
- 1102.Xue J, Nelin LD, Chen B. Hypoxia induces arginase II expression and increases viable human pulmonary artery smooth muscle cell numbers via AMPKα1signaling. Am J Physiol Lung Cell Mol Physiol 312: L568–L578, 2017. doi: 10.1152/ajplung.00117.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1103.Xue Q, Chen P, Li X, Zhang G, Patterson AJ, Luo J. Maternal High-Fat Diet Causes a Sex-Dependent Increase in AGTR2 Expression and Cardiac Dysfunction in Adult Male Rat Offspring. Biol Reprod 93: 49, 2015. doi: 10.1095/biolreprod.115.129916. [DOI] [PubMed] [Google Scholar]
- 1104.Xue Q, Dasgupta C, Chen M, Zhang L. Foetal hypoxia increases cardiac AT(2)R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc Res 89: 300–308, 2011. doi: 10.1093/cvr/cvq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1105.Xue Q, Ducsay CA, Longo LD, Zhang L. Effect of long-term high-altitude hypoxia on fetal pulmonary vascular contractility. J Appl Physiol (1985) 104: 1786–1792, 2008. doi: 10.1152/japplphysiol.01314.2007. [DOI] [PubMed] [Google Scholar]
- 1106.Xue Q, Patterson AJ, Xiao D, Zhang L. Glucocorticoid modulates angiotensin II receptor expression patterns and protects the heart from ischemia and reperfusion injury. PLoS One 9: e106827, 2014. doi: 10.1371/journal.pone.0106827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1107.Xue Q, Xiao D, Zhang L. Estrogen Regulates Angiotensin II Receptor Expression Patterns and Protects the Heart from Ischemic Injury in Female Rats. Biol Reprod 93: 6, 2015. doi: 10.1095/biolreprod.115.129619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1108.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J Pharmacol Exp Ther 330: 624–632, 2009. doi: 10.1124/jpet.109.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1109.Yaka R, Salomon S, Matzner H, Weinstock M. Effect of varied gestational stress on acquisition of spatial memory, hippocampal LTP and synaptic proteins in juvenile male rats. Behav Brain Res 179: 126–132, 2007. doi: 10.1016/j.bbr.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 1110.Yan S, Jiao K. Functions of miRNAs during Mammalian Heart Development. Int J Mol Sci 17: E789, 2016. doi: 10.3390/ijms17050789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1111.Yang D, Gao L, Wang T, Qiao Z, Liang Y, Zhang P. Hypoxia triggers endothelial endoplasmic reticulum stress and apoptosis via induction of VLDL receptor. FEBS Lett 588: 4448–4456, 2014. doi: 10.1016/j.febslet.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 1112.Yang J, Jin Z-B, Chen J, Huang X-F, Li X-M, Liang Y-B, Mao J-Y, Chen X, Zheng Z, Bakshi A, Zheng D-D, Zheng M-Q, Wray NR, Visscher PM, Lu F, Qu J. Genetic signatures of high-altitude adaptation in Tibetans. Proc Natl Acad Sci USA 114: 4189–4194, 2017. doi: 10.1073/pnas.1617042114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1113.Yang Q, Hohimer AR, Giraud GD, Van Winkle DM, Underwood MJ, He GW, Davis LE. Effect of fetal anaemia on myocardial ischaemia-reperfusion injury and coronary vasoreactivity in adult sheep. Acta Physiol (Oxf) 194: 325–334, 2008. doi: 10.1111/j.1748-1716.2008.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1114.Yang Q, Lu Z, Ramchandran R, Longo LD, Raj JU. Pulmonary artery smooth muscle cell proliferation and migration in fetal lambs acclimatized to high-altitude long-term hypoxia: role of histone acetylation. Am J Physiol Lung Cell Mol Physiol 303: L1001–L1010, 2012. doi: 10.1152/ajplung.00092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1115.Yang Q, Sun M, Ramchandran R, Raj JU. IGF-1 signaling in neonatal hypoxia-induced pulmonary hypertension: Role of epigenetic regulation. Vascul Pharmacol 73: 20–31, 2015. doi: 10.1016/j.vph.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1116.Yang S, Banerjee S, Freitas A, Cui H, Xie N, Abraham E, Liu G. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 302: L521–L529, 2012. doi: 10.1152/ajplung.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1117.Yang W, Mao C, Xia F, Zheng J, Wang A, Zhu L, He R, Xu Z. Changed salt appetite and central angiotensin II-induced cellular activation in rat offspring following hypoxia during fetal stages. Peptides 31: 1177–1183, 2010. doi: 10.1016/j.peptides.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1118.Yang Z, Zhuan B, Yan Y, Jiang S, Wang T. Roles of different mitochondrial electron transport chain complexes in hypoxia-induced pulmonary vasoconstriction. Cell Biol Int 40: 188–195, 2016. doi: 10.1002/cbin.10550. [DOI] [PubMed] [Google Scholar]
- 1119.Yaoita H, Ogawa K, Maehara K, Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation 97: 276–281, 1998. doi: 10.1161/01.CIR.97.3.276. [DOI] [PubMed] [Google Scholar]
- 1120.Ye D, Xiong Y. Cancer: Suffocation of gene expression. Nature 537: 42–43, 2016. doi: 10.1038/nature19426. [DOI] [PubMed] [Google Scholar]
- 1121.Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics 43: 534–542, 2011. doi: 10.1152/physiolgenomics.00130.2010. [DOI] [PubMed] [Google Scholar]
- 1122.Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, Zheng H, Liu T, He W, Li K, Luo R, Nie X, Wu H, Zhao M, Cao H, Zou J, Shan Y, Li S, Yang Q, Asan, Ni P, Tian G, Xu J, Liu X, Jiang T, Wu R, Zhou G, Tang M, Qin J, Wang T, Feng S, Li G, Huasang, Luosang J, Wang W, Chen F, Wang Y, Zheng X, Li Z, Bianba Z, Yang G, Wang X, Tang S, Gao G, Chen Y, Luo Z, Gusang L, Cao Z, Zhang Q, Ouyang W, Ren X, Liang H, Zheng H, Huang Y, Li J, Bolund L, Kristiansen K, Li Y, Zhang Y, Zhang X, Li R, Li S, Yang H, Nielsen R, Wang J, Wang J. Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329: 75–78, 2010. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1123.Yin M, Liu S, Yin Y, Li S, Li Z, Wu X, Zhang B, Ang SL, Ding Y, Zhou J. Ventral mesencephalon-enriched genes that regulate the development of dopaminergic neurons in vivo. J Neurosci 29: 5170–5182, 2009. doi: 10.1523/JNEUROSCI.5569-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1124.Yu F, Zheng A, Qian J, Li Y, Wu L, Yang J, Gao X. Prenatal nicotine exposure results in the myocardial fibrosis in the adult male offspring rats. Exp Toxicol Pathol 68: 445–450, 2016. doi: 10.1016/j.etp.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 1125.Yu JY, Chung KH, Deo M, Thompson RC, Turner DL. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res 314: 2618–2633, 2008. doi: 10.1016/j.yexcr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1126.Yuan X, Qi H, Li X, Wu F, Fang J, Bober E, Dobreva G, Zhou Y, Braun T. Disruption of spatiotemporal hypoxic signaling causes congenital heart disease in mice. J Clin Invest 127: 2235–2248, 2017. doi: 10.1172/JCI88725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1127.Yuen BS, McMillen IC, Symonds ME, Owens PC. Abundance of leptin mRNA in fetal adipose tissue is related to fetal body weight. J Endocrinol 163: R11–R14, 1999. doi: 10.1677/joe.0.163R011. [DOI] [PubMed] [Google Scholar]
- 1128.Yuen BS, Owens PC, Symonds ME, Keisler DH, McFarlane JR, Kauter KG, McMillen IC. Effects of leptin on fetal plasma adrenocorticotropic hormone and cortisol concentrations and the timing of parturition in the sheep. Biol Reprod 70: 1650–1657, 2004. doi: 10.1095/biolreprod.103.025254. [DOI] [PubMed] [Google Scholar]
- 1129.Yuen RK, Chen B, Blair JD, Robinson WP, Nelson DM. Hypoxia alters the epigenetic profile in cultured human placental trophoblasts. Epigenetics 8: 192–202, 2013. doi: 10.4161/epi.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1130.Yuen RK, Peñaherrera MS, von Dadelszen P, McFadden DE, Robinson WP. DNA methylation profiling of human placentas reveals promoter hypomethylation of multiple genes in early-onset preeclampsia. Eur J Hum Genet 18: 1006–1012, 2010. doi: 10.1038/ejhg.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1131.Yun C, Mendelson J, Blake T, Mishra L, Mishra B. TGF-beta signaling in neuronal stem cells. Dis Markers 24: 251–255, 2008. doi: 10.1155/2008/747343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1132.Yung HW, Atkinson D, Campion-Smith T, Olovsson M, Charnock-Jones DS, Burton GJ. Differential activation of placental unfolded protein response pathways implies heterogeneity in causation of early- and late-onset pre-eclampsia. J Pathol 234: 262–276, 2014. doi: 10.1002/path.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1133.Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS, Burton GJ. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol 173: 451–462, 2008. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1134.Yung HW, Cox M, Tissot van Patot M, Burton GJ. Evidence of endoplasmic reticulum stress and protein synthesis inhibition in the placenta of non-native women at high altitude. FASEB J 26: 1970–1981, 2012. doi: 10.1096/fj.11-190082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1135.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab 1: 371–378, 2005. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 1136.Zamudio S. High-altitude hypoxia and preeclampsia. Front Biosci 12: 2967–2977, 2007. doi: 10.2741/2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1137.Zamudio S. The placenta at high altitude. High Alt Med Biol 4: 171–191, 2003. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- 1138.Zamudio S, Baumann MU, Illsley NP. Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters. Placenta 27: 49–55, 2006. doi: 10.1016/j.placenta.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1139.Zamudio S, Borges M, Echalar L, Kovalenko O, Vargas E, Torricos T, Khan AA, Alvarez M, Illsley NP. Maternal and fetoplacental hypoxia do not alter circulating angiogenic growth effectors during human pregnancy. Biol Reprod 90: 42, 2014. doi: 10.1095/biolreprod.113.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1140.Zamudio S, Kovalenko O, Echalar L, Torricos T, Al-Khan A, Alvarez M, Illsley NP. Evidence for extraplacental sources of circulating angiogenic growth effectors in human pregnancy. Placenta 34: 1170–1176, 2013. doi: 10.1016/j.placenta.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1141.Zamudio S, Kovalenko O, Vanderlelie J, Illsley NP, Heller D, Belliappa S, Perkins AV. Chronic hypoxia in vivo reduces placental oxidative stress. Placenta 28: 846–853, 2007. doi: 10.1016/j.placenta.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1142.Zamudio S, Leslie KK, White M, Hagerman DD, Moore LG. Low serum estradiol and high serum progesterone concentrations characterize hypertensive pregnancies at high altitude. J Soc Gynecol Investig 1: 197–205, 1994. doi: 10.1177/107155769400100304. [DOI] [PubMed] [Google Scholar]
- 1143.Zamudio S, Palmer SK, Dahms TE, Berman JC, McCullough RG, McCullough RE, Moore LG. Blood volume expansion, preeclampsia, and infant birth weight at high altitude. J Appl Physiol (1985) 75: 1566–1573, 1993. doi: 10.1152/jappl.1993.75.4.1566. [DOI] [PubMed] [Google Scholar]
- 1144.Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol (1985) 79: 15–22, 1995. doi: 10.1152/jappl.1995.79.1.15. [DOI] [PubMed] [Google Scholar]
- 1145.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol (1985) 79: 7–14, 1995. doi: 10.1152/jappl.1995.79.1.7. [DOI] [PubMed] [Google Scholar]
- 1146.Zamudio S, Postigo L, Illsley NP, Rodriguez C, Heredia G, Brimacombe M, Echalar L, Torricos T, Tellez W, Maldonado I, Balanza E, Alvarez T, Ameller J, Vargas E. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol 582: 883–895, 2007. doi: 10.1113/jphysiol.2007.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1147.Zamudio S, Wu Y, Ietta F, Rolfo A, Cross A, Wheeler T, Post M, Illsley NP, Caniggia I. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol 170: 2171–2179, 2007. doi: 10.2353/ajpath.2007.061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1148.Zeisel S. Choline, Other Methyl-Donors and Epigenetics. Nutrients 9: E445, 2017. doi: 10.3390/nu9050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1149.Zhang H, Darwanto A, Linkhart TA, Sowers LC, Zhang L. Maternal cocaine administration causes an epigenetic modification of protein kinase Cepsilon gene expression in fetal rat heart. Mol Pharmacol 71: 1319–1328, 2007. doi: 10.1124/mol.106.032011. [DOI] [PubMed] [Google Scholar]
- 1150.Zhang H, Liu X, Zhang C, Mundo E, Macciardi F, Grayson DR, Guidotti AR, Holden JJ. Reelin gene alleles and susceptibility to autism spectrum disorders. Mol Psychiatry 7: 1012–1017, 2002. doi: 10.1038/sj.mp.4001124. [DOI] [PubMed] [Google Scholar]
- 1151.Zhang H, Meyer KD, Zhang L. Fetal exposure to cocaine causes programming of Prkce gene repression in the left ventricle of adult rat offspring. Biol Reprod 80: 440–448, 2009. doi: 10.1095/biolreprod.108.072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1152.Zhang L, Xiao D, Bouslough DB. Long-term high-altitude hypoxia increases plasma nitrate levels in pregnant ewes and their fetuses. Am J Obstet Gynecol 179: 1594–1598, 1998. doi: 10.1016/S0002-9378(98)70031-6. [DOI] [PubMed] [Google Scholar]
- 1153.Zhang P, Zhu D, Chen X, Li Y, Li N, Gao Q, Li L, Zhou X, Lv J, Sun M, Mao C, Xu Z. Prenatal hypoxia promotes atherosclerosis via vascular inflammation in the offspring rats. Atherosclerosis 245: 28–34, 2016. doi: 10.1016/j.atherosclerosis.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 1154.Zhang S, Morrison JL, Gill A, Rattanatray L, MacLaughlin SM, Kleemann D, Walker SK, McMillen IC. Maternal dietary restriction during the periconceptional period in normal-weight or obese ewes results in adrenocortical hypertrophy, an up-regulation of the JAK/STAT and down-regulation of the IGF1R signaling pathways in the adrenal of the postnatal lamb. Endocrinology 154: 4650–4662, 2013. doi: 10.1210/en.2013-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1155.Zhang TY, Labonté B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology 38: 111–123, 2013. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1156.Zhang W, Cline MA, Gilbert ER. Hypothalamus-adipose tissue crosstalk: neuropeptide Y and the regulation of energy metabolism. Nutr Metab (Lond) 11: 27, 2014. doi: 10.1186/1743-7075-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1157.Zhang X, Li L, Zhang X, Xie W, Li L, Yang D, Heng X, Du Y, Doody RS, Le W. Prenatal hypoxia may aggravate the cognitive impairment and Alzheimer’s disease neuropathology in APPSwe/PS1A246E transgenic mice. Neurobiol Aging 34: 663–678, 2013. doi: 10.1016/j.neurobiolaging.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 1158.Zhang Y, Fei M, Xue G, Zhou Q, Jia Y, Li L, Xin H, Sun S. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: new insights into molecular mechanisms for the disease. J Cell Mol Med 16: 249–259, 2012. doi: 10.1111/j.1582-4934.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1159.Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev 15: 2343–2360, 2001. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 1160.Zhang Y, Shan P, Srivastava A, Jiang G, Zhang X, Lee PJ. An Endothelial Hsp70-TLR4 Axis Limits Nox3 Expression and Protects Against Oxidant Injury in Lungs. Antioxid Redox Signal 24: 991–1012, 2016. doi: 10.1089/ars.2015.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1161.Zhao C, Li T, Han B, Yue W, Shi L, Wang H, Guo Y, Lu Z. DDAH1 deficiency promotes intracellular oxidative stress and cell apoptosis via a miR-21-dependent pathway in mouse embryonic fibroblasts. Free Radic Biol Med 92: 50–60, 2016. doi: 10.1016/j.freeradbiomed.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 1162.Zhao L, Chen CN, Hajji N, Oliver E, Cotroneo E, Wharton J, Wang D, Li M, McKinsey TA, Stenmark KR, Wilkins MR. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation 126: 455–467, 2012. doi: 10.1161/CIRCULATIONAHA.112.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1163.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 436: 214–220, 2005. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 1164.Zhou J, Xiao D, Hu Y, Wang Z, Paradis A, Mata-Greenwood E, Zhang L. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension 62: 599–607, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1165.Zhou L, Zhao Y, Nijland R, Zhang L, Longo LD. Ins(1,4,5)P3 receptors in cerebral arteries: changes with development and high-altitude hypoxia. Am J Physiol Regul Integr Comp Physiol 272: R1954–R1959, 1997. [DOI] [PubMed] [Google Scholar]
- 1166.Zhou W, Ibe BO, Raj JU. Platelet-activating factor induces ovine fetal pulmonary venous smooth muscle cell proliferation: role of epidermal growth factor receptor transactivation. Am J Physiol Heart Circ Physiol 292: H2773–H2781, 2007. doi: 10.1152/ajpheart.01018.2006. [DOI] [PubMed] [Google Scholar]
- 1167.Zhou Y, Zhu Y, Zhang L, Wu T, Wu T, Zhang W, Decker AM, He J, Liu J, Wu Y, Jiang X, Zhang Z, Liang C, Zou D. Human Stem Cells Overexpressing miR-21 Promote Angiogenesis in Critical Limb Ischemia by Targeting CHIP to Enhance HIF-1α Activity. Stem Cells 34: 924–934, 2016. doi: 10.1002/stem.2321. [DOI] [PubMed] [Google Scholar]
- 1168.Zhu R, Hu XQ, Xiao D, Yang S, Wilson SM, Longo LD, Zhang L. Chronic hypoxia inhibits pregnancy-induced upregulation of SKCa channel expression and function in uterine arteries. Hypertension 62: 367–374, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1169.Zhu R, Huang X, Hu XQ, Xiao D, Zhang L. Gestational hypoxia increases reactive oxygen species and inhibits steroid hormone-mediated upregulation of Ca(2+)-activated K(+) channel function in uterine arteries. Hypertension 64: 415–422, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1170.Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol 200: 661.e1–661.e7, 2009. doi: 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 1171.Zubenko SI, Yan L, Zhul’kov MO, Lebed’ko OA, Sazonova EN. Effects of antenatal hypoxia on tissue homeostasis in the myocardium of albino rats: early and delayed consequences. Bull Exp Biol Med 157: 320–323, 2014. doi: 10.1007/s10517-014-2555-4. [DOI] [PubMed] [Google Scholar]
- 1172.Zuena AR, Mairesse J, Casolini P, Cinque C, Alemà GS, Morley-Fletcher S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One 3: e2170, 2008. doi: 10.1371/journal.pone.0002170. [DOI] [PMC free article] [PubMed] [Google Scholar]