Abstract
Pneumonia is a type of acute lower respiratory infection that is common and severe. The outcome of lower respiratory infection is determined by the degrees to which immunity is protective and inflammation is damaging. Intercellular and interorgan signaling networks coordinate these actions to fight infection and protect the tissue. Cells residing in the lung initiate and steer these responses, with additional immunity effectors recruited from the bloodstream. Responses of extrapulmonary tissues, including the liver, bone marrow, and others, are essential to resistance and resilience. Responses in the lung and extrapulmonary organs can also be counterproductive and drive acute and chronic comorbidities after respiratory infection. This review discusses cell-specific and organ-specific roles in the integrated physiological response to acute lung infection, and the mechanisms by which intercellular and interorgan signaling contribute to host defense and healthy respiratory physiology or to acute lung injury, chronic pulmonary disease, and adverse extrapulmonary sequelae. Pneumonia should no longer be perceived as simply an acute infection of the lung. Pneumonia susceptibility reflects ongoing and poorly understood chronic conditions, and pneumonia results in diverse and often persistent deleterious consequences for multiple physiological systems.
I. INTRODUCTION
Pneumonia is responsible for an extremely large burden of disease across the earth, more than diseases such as cancer, diabetes, HIV/AIDS, malaria, and many other diseases recognized as leading global health problems (330, 335, 336, 350, 351). Burden of disease is calculated from disability-adjusted life years lost, and the appalling global burden of pneumonia results in part from the fact that pneumonia kills more children worldwide than does any other disease (425, 528). In the United States (US), children more commonly survive pneumonia, but even in such advantaged countries pneumonia is the most common reason for children to be hospitalized (566). A fifth of those children need to be in the intensive care unit (ICU), and a third of those require mechanical ventilation (228). After children are released from the hospital, they have increased risk of chronic respiratory diseases including asthma and chronic obstructive pulmonary disease (COPD) (123, 188), which is a further burden of this disease that does not get factored into such calculations.
As dispiriting as those pediatric statistics are, the population most afflicted by pneumonia is older adults, who have incidence and risk of death from pneumonia that are orders of magnitude greater than for children (174, 407). For seniors, pneumonia hospitalization has a higher risk of death than any of the other common causes of hospitalization (147). Pneumonia causes more deaths in the US (and globally) than does any other infectious disease (185). However, most of even the oldest do survive (131). The economic costs are staggering, with estimates ranging from nearly 20 billion dollars to more than 80 billion dollars per year in the US (136, 189, 565). And after all this immediate suffering and cost, additional indirect and longer term consequences include cognitive decline comparable to traumatic brain injury, greater incidence and severity of depression, worsened cardiovascular and cerebrovascular health, physical limitation, and decreased life-span (39, 88, 196, 376, 431, 445). Pneumonia prevention measures like influenza and pneumococcal vaccines are sufficient to decrease risk, thereby demonstrating causal relationships between pneumonia and longer term extrapulmonary outcomes (415, 503).
Pneumonia demands extraordinary attention from the biomedical community, as a direct cause of morbidity and mortality and as a contributor to unhealthy aging and decline. While pneumonia results from microbial infection, the pathogenesis of this disease is driven by the host response. Within the host, pneumonia is by definition within the lungs, but it is a complex disease that involves diverse physiological systems working together. Although pneumonia is an acute event, it is prompted by preexisting chronic conditions, and it has long-term consequences. Thus pneumonia is an acute lower respiratory tract infection that is more than acute, more than lower respiratory tract, and more than infection.
Our goal with this review is to highlight evolving concepts related to pneumonia biology. First, we emphasize the importance of the host response. Pneumonia is an unusual result of infection with commonly encountered microbes; disease is the exception rather than the norm. Knowing “what goes right” to prevent pneumonia during the vast majority of times that these microbes get into our lungs seems key to conceptualizing methods to better prevent and cure this disease. Second, we advocate reenvisioning pneumonia as not just an acute event, but rather as a chronic condition of heightened susceptibility. The mechanisms responsible for susceptibility must be better elucidated so they can be interrupted. Third, we wish to increase attention on pneumonia consequences outside of the lung. Physiological pathways are only beginning to be defined for the extrapulmonary manifestations of pneumonia. And fourth, we highlight that pneumonia has physiological consequences that persist beyond the time course of the pneumonia itself. Pneumonia events lead to prolonged morbidity and earlier mortality, with greater mechanistic insight needed. Improved knowledge in these areas could lead to adjunct therapies that target the wider and longer impacts of pneumonia on its victims.
II. HOST-PATHOGEN INTERACTION
Pneumonia is an infection of the lung causing exudative fluid to accumulate in the pulmonary parenchyma, compromising respiratory function. Diagnosis depends on evidence of pulmonary consolidation (based on auscultation or radiology) in conjunction with evidence of infection (based on microbiology or on general signs such as fever, malaise, leukocyte shifts, etc.) with an acute onset. It does not have a clinical definition that is established by an adjudicating body and uniformly applied by the medical community. Pneumonia is by far the most common cause of sepsis (320, 516), which has been defined by an international task force as organ dysfunction due to a dysregulated host response to infection that is severe enough to be life-threatening (454). Pneumonia is distinguished from other forms of sepsis by the location; when the infection causing dysregulated host response is in the lungs, then it is pneumonia. Pneumonia causes the majority (33, 62) of cases of the acute respiratory distress syndrome (ARDS), which has been defined (486a) as being acute in onset (<1 wk) with diffuse (bilateral) pulmonary edema (not due to elevated hydrostatic pressure) and arterial hypoxemia (the degree of which stratifies severity). Pneumonia is distinguished from other forms of ARDS by etiology; when the lungs contain fluid because of microbes present there, then pneumonia is the cause of ARDS. The fact that patients with pneumonia often become reclassified as patients with sepsis or ARDS when they advance to those stages confounds the discussion and understanding of pneumonia. Sepsis and ARDS are immediately life-threatening forms of severe pneumonia. Characteristics of sepsis and of ARDS, including their pathophysiological mechanisms and poor patient outcomes (19, 315), should be recognized as consequences of severe pneumonia. The definitions of sepsis and ARDS help advance research related to those diseases. The lack of an unambiguous, uniform, and accepted clinical definition for pneumonia may complicate research on this disease.
A. The Microbes Causing Pneumonia
Microbe-targeted approaches have proven useful for pneumonia. The advent of antibiotics was profoundly important, dramatically reducing pneumonia mortality rates in the US during the mid-20th century (336). Vaccines also decrease rates of pneumonia in populations in which they are adopted, modestly but importantly (154, 174, 175). Thus interfering with the microbial side of this host-pathogen interaction driving pneumonia is productive.
Because microbes initiate this disease, changes among microbes infecting the respiratory tract influence pneumonia biology. The mutations and reassortments leading to antigenic drift and shifts of influenza viruses are prominent examples (574). Infamously, a strain of H1N1 influenza virus that emerged in 1918 led to a pandemic with particularly overwhelming increases in morbidity and mortality, largely due to secondary bacterial pneumonias (341, 342). A more recent antigenic shift for influenza virus in 2009 caused a pandemic that included severe pneumonias (574). Sporadic but very severe cases of zoonotic pneumonias caused by animal-tropic influenza viruses demonstrate that highly pathogenic influenza viruses are always nearby (150, 545). Although the influenza viruses presently circulating in animals are not readily transmissible among humans, a few mutations can change that (291). Other zoonoses cause rare pneumonias but get considerable attention because of their bioterrorism potential, including Brucella anthracis, Yersinia pestis, and Francisella tularensis. Some fungi are important causes of pneumonia within restricted geographic regions, such as Coccidioides immitis in the southwestern US or Histoplasma capsulatum in the Missouri, Ohio, and Mississippi River valleys. Microbes that have prominently emerged as pneumonia threats in recent decades include Legionella, Pneumocystis, hantavirus, SARS coronavirus, MERS coronavirus, and more. Some microbes that have recently become recognized as important causes of pneumonia may represent a recent emergence of knowledge more than of microbes, including rhinoviruses C and D, coronaviruses NL63 and HKU1, human metapneumoviruses, and more (230). Among pneumonia-causing bacteria, the emergence and spread of antibiotic resistance is a continuous threat (280). Plasmids containing carbapenem resistance genes are being passed among Klebsiella pneumoniae and other pneumonia agents, including bacteria already resistant to most other antibiotics (133). The recent discovery of plasmid-mediated colistin resistance (294, 402, 527) suggests the frightening prospect of bacterial pneumonias that are resistant to all currently licensed antibiotics. The expanding myriad of microbes combined with future prospects of increasingly ineffective antibiotics mean microbe-targeting such as with antimicrobials and vaccines can achieve successes but not victory. Alternative and supplementary approaches, such as modifications of host responses during pneumonia, are needed.
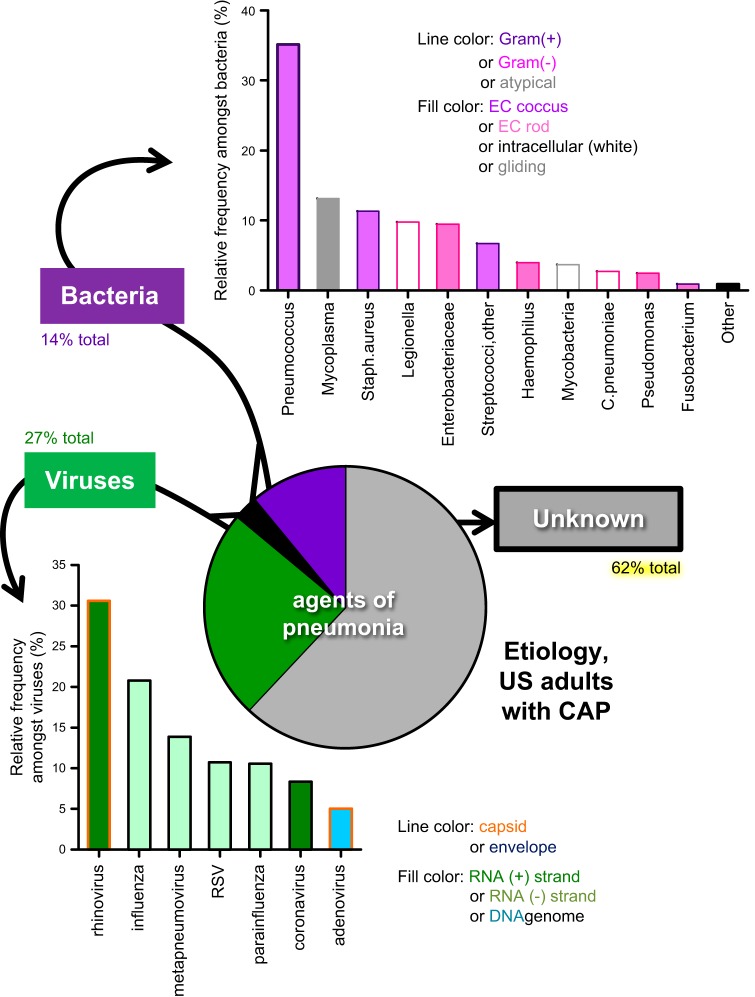
The etiology of pneumonia is complex and poorly understood, because the microbes causing pneumonia are extraordinarily numerous and extremely varied (FIGURE 1). The agents identified include many different viruses and bacteria, and these microbes do not appear to share any particular characteristics (RNA viruses, DNA viruses, enveloped viruses, nonenveloped viruses, Gram-positive bacteria, Gram-negative bacteria, cell wall-free bacteria, extracellular bacteria, intracellular bacteria, etc.). Pneumonia can also be caused by fungal and other infectious agents. Any given microbe accounts for only a small minority each of all pneumonia cases (227), with the most common three for adults hospitalized with community-acquired pneumonia being rhinoviruses (9%), influenza viruses (6%), and pneumococci (5%). Different populations (children, hospitalized patients, nursing home residents, etc.) have different microbes implicated (15, 79), but in each population it is a spectrum of responsible agents rather than a specific microbial type. There are few, if any, unifying principles to the types of microbe that cause pneumonia. These diverse etiologic agents encode a wide variety of microbe-specific virulence pathways that influence the likelihood that respiratory infection will cause pneumonia. While contributing to pneumonia pathogenesis, microbial virulence pathways are beyond the scope of this discussion of host physiology, and readers may wish to consult other reviews specific to relevant microbes (135, 321, 339, 374, 380, 436, 508).
FIGURE 1.
The microbial agents that cause pneumonia are numerous, diverse, and poorly understood. Data represent nonimmunocompromised adult patients hospitalized for community-acquired pneumonia (CAP). Despite intensive efforts, no microbial agent can be identified in the majority of pneumonia cases. Among the viral agents detected, no virus or type of virus was especially prominent. Among bacteria, pneumococcus was most common, but a great many species and types of bacteria were detected. [Data from Jain et al. (227).]
In many cases, a microbial suspect is not identified (FIGURE 1). Even in studies designed for the purpose of identifying etiologic agents, a potentially responsible microbe fails to be detected in about one-fifth of childhood pneumonias (228) and more than half of adult pneumonias (227). When one or more microbes are identified, the degree to which it or they are truly causal is uncertain. In virtually all cases, the agents recognized as responsible are ubiquitous opportunistic microbes. Most people who encounter these microbes do not develop pneumonia and do not get seriously ill. Rather, the interaction usually results in asymptomatic carriage or subclinical infection. The presence of the microbe does not mean an individual gets pneumonia. While the microbe is relevant, whether or not these microbes cause pneumonia depends more on the host and the host response than on the microbe or any specific microbial characteristics.
B. Host Responses to Microbes in the Lung
Whether or not microbes in the lung exceed a host’s capacity to maintain pulmonary homeostasis depends on a complex integration of physiological processes, together which aim to prevent the onset of pneumonia. For these processes to be effective they must provide adequate levels of both immune resistance and tissue resilience (407). Immune resistance refers to the eradication of living pathogens during an infection, whereas tissue resilience involves the prevention of or resolution of injury resulting from the pathogen and/or from the host response to the pathogen. Inappropriate amounts of either disrupt homeostasis, making both equally essential. Despite important advances in our understanding the pathways comprising resistance and resilience, the degree to which certain biological signals under certain circumstances in certain individuals collaborate to dictate pneumonia outcome remains largely unclear.
III. INNATE IMMUNITY AGAINST MICROBES IN THE LUNGS
Innate immunity represents the initial preexisting determinant of resistance against invading pathogens. While innate immunity involves an elaborate network of cells and signals that actively function to eliminate invading organisms and maintain tissue integrity, defense also includes the anatomical barriers that restrict the deposition of microbes within the respiratory tract. Airway architecture not only offers the means to heat, humidify, and distribute air throughout the respiratory tract, but it also provides an efficient physical barrier against microbes and other potentially toxic substances. Examples of anatomic protective measures include nasal hairs, the mucociliary escalator, and the epithelial barrier itself, which ultimately provides the protective interface separating the external and internal environments (429). Even the branching pattern of the respiratory tract represents a critical innate defense. Materials over 3 μm in diameter have extremely limited access to the lower respiratory tract due to filtration and impaction in more proximal airways (21), which has important implications for the dispersal of infectious substances. Yet, this is insufficient to wholly prevent microorganisms from accessing the deeper lung, including the respiratory zone of the alveolocapillary interface. Defense against microbes within the lower respiratory tract then relies on a carefully coordinated immune response that includes both resident and recruited features. Resident defenses such as soluble antimicrobial factors in airway lining fluid and alveolar macrophages (AMs) provide the initial tier of protection against microbes, followed quickly thereafter by recruited elements such as extravasated leukocytes and other immunomodulatory plasma constituents. The resulting inflammation is a hallmark of innate immunity, involving a panoply of intra- and extrapulmonary cells and signals, some of which are highlighted below, that ideally exert an appropriate balance of resistance and resilience in an effort to re-establish lung homeostasis.
A. Lung Innate Immunity
The recognition of lung pathogens elicits robust remodeling of the pulmonary transcriptome (e.g., as described in Refs. 162, 246, 259, 409, 546), resulting in the production and release of mediators that coordinate early protection. These mediators include a plethora of multifactorial cytokines, chemokines, growth factors, antimicrobial substances, opsonins, enzymes, enzyme inhibitors, adhesion molecules, receptors, apoptotic factors, anti-apoptotic factors, and more. This response involves the recruitment and/or activation of numerous cell types, some of which have only recently become appreciated in the setting of lung immunity. Moreover, some of these cells function within the lungs, whereas others do not. All of this must be considered in the context of an integrated physiological response to lung infection. The initial responses are from cells already present within the uninfected lung.
1. Alveolar macrophages
AMs are professional phagocytes that reside on the surface of the lower respiratory tract. They represent an initial line of leukocytic antimicrobial defense. Studies in mouse models indicate that AMs, like other tissue-resident macrophages (387), are yolk sac-derived and extremely long-lived (177, 229, 349). In fact, experiments with GFP-expressing chimeric mice strongly support that the lifespan of AMs approaches the mouse lifespan (229). Examination of human lung transplants mismatched for HLA or for sex chromosomes reveal that alveolar macrophages of the donor lung persist for all of the several years that have been included in analyses (124, 357). This population of resident phagocytes is often maintained over the course of lung injury and infection, remaining after the recruited inflammatory cells have been removed from the airspaces by apoptosis and efferocytosis (229).
Functionally, AMs are extremely diverse, with essential roles in both immune resistance and tissue resilience (4) (FIGURE 2). Under normal homeostatic conditions, AMs suppress inflammation through a variety of mechanisms to be further discussed below. This is critical for limiting immunopathology, as macrophages clear environmental debris, excess surfactant, apoptotic cells, and other innocuous materials (211, 497). In the setting of infection, however, AMs exert significant plasticity, transitioning from an anti-inflammatory housekeeping cell into a central node of immune activity. Macrophages bear an armament of pattern recognition receptors (484), allowing them to respond to a diverse repertoire of pathogens. Upon pathogen recognition, AMs directly contribute to immune resistance through the ingestion and phagocytosis of microbes (244) (FIGURE 2). Transmembrane transport of ions by CFTR, TRPC6, TRPM2, and other channels and pumps coordinately render the phagosome acidic and inhospitable to microbes (103, 104, 418). In addition, synthesis of reactive oxygen and nitrogen intermediates also contributes to alveolar macrophage killing of phagocytized microbes (176, 199). In concert with phagocytosis, apoptosis can contribute to maximal macrophage-mediated killing (111) (FIGURE 2), for example, when triggered by release of cathepsin D into the cytosol to degrade the anti-apoptotic factor Mcl-1 (312). AM apoptosis can also be triggered by extracellular cues such as recognition of the cytokine TRAIL by the DR5 receptor on macrophages (468). Both TRAIL and apoptosis are required for efficient clearance of bacteria in the lungs (35, 468). AM death by pathways other than apoptosis can be stimulated by agents of pneumonia, such as necroptosis mediated by RIP1 and RIP3 kinases and MLKL (85, 165, 255) (FIGURE 2). This does not kill bacteria but instead exacerbates infection (85, 165). Therefore, AM apoptosis is a specialized pathway of immune resistance, while other macrophage death pathways are instead detrimental to the host. The antimicrobial effector functions of AMs can be sufficient to control low pathogen burdens without recruiting additional cells (2, 111).
FIGURE 2.
During pneumonia, macrophages have multiple critical roles in protecting the host against infection (immune resistance) and against injury (tissue resilience). The molecules identified were for illustrative purpose and do not represent an exhaustive presentation.
The direct microbicidal capacity of AMs is complemented by their exceptional ability to coordinate the immune activity of other cells, both neighboring and remote, which is essential when microbes are too virulent or too numerous to be efficiently handled by resident innate immunity. To do so, AMs use RelA from the NF-κB transcription factor family to dispatch numerous cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1α, IL-1β, chemokines, IL-6, and granulocyte colony stimulating factor (G-CSF), all of which are important for eliciting lung innate immunity (61, 171, 173, 194, 239, 240, 392, 408, 413) (FIGURE 2). Isolates of pneumococcus collected from complicated pneumonia patients tend to be lower activators of macrophage NF-κB compared with pneumococci collected from other individuals, and such lower NF-κB activators induce slower cytokine expression and pulmonary defense in mouse models of pneumonia (85), further supporting the concept that the capacity of AMs to elaborate cytokines is key to initiating immune responses in the lung. This capacity may be shaped by prior encounters with invading pathogens. Macrophage NF-κB activation and cytokine elaboration are altered for prolonged periods of time after the resolution of prior respiratory infections (211), suggestive of trained immunity (363). The degree to which such alterations in AM responsiveness may improve or worsen antimicrobial resistance, and mechanisms responsible for altering these macrophage behaviors, demand further attention.
An emerging area of focus related to AMs is the release of cytokines and other immunomodulating agents within membrane-bound vesicles such as exosomes and microparticles (FIGURE 2). IL-36γ, which is essential for efficient resistance against bacterial pneumonia in mouse models, is one such AM product (267). Following bacterial stimulation of human and mouse AMs, this cytokine is released in membrane-bound particles, and it appears within lipid vesicles in the air spaces of human patients with bacterial pneumonia (267). Mechanisms determining which cytoplasmic constituents are encapsulated within the secreted vesicles and how these signal to recipient cells are ongoing areas of research in pulmonary immunity.
Thus macrophages are important as antimicrobial effector cells and as sources of cytokines in the lungs (FIGURE 2), promoting the recruitment and activation of other cells mediating immune resistance, some of which are highlighted below. The profound influence of AMs on pneumonia outcome is further supported by reports of targeted disruption of macrophage function, either pharmacologically or genetically, which impairs innate defense in mouse models of lung infection (55, 111, 187, 198, 389, 392).
2. Epithelial cells
While the accessibility of AMs by bronchoalveolar lavage has provided a wealth of information regarding their functions and regulation in the context of pneumonia and other lung diseases, contributions of other cell types have recently emerged with the advent of more sophisticated isolation and targeting strategies. Among these additional immunomodulatory cell types are those that comprise the lung epithelium, a complex network of epithelial subsets differentially distributed throughout the upper and lower respiratory tract (536). In the alveoli, surfactant proteins (SP) A and D, synthesized by the alveolar epithelial type II cells, have critical roles in immune resistance, by directly inhibiting microbes (184) and also by influencing immune activities (381). In the upper respiratory tract and conducting airways of the lung, a prominent feature is mucociliary clearance. The importance of ciliary action is highlighted by the severe lung disease, particularly recurrent respiratory infections, in patients with primary ciliary dyskinesia (258). Airway ciliated cells are more heterogeneous than previously recognized, and subsets of airway ciliated cells (e.g., those marked by the MIWI2 expression) have immunomodulatory roles that extend beyond the mechanical clearance of mucus (529). Secreted airway mucins, largely synthesized by goblet and club cells, are the primary constituents of the mucus layer, and are independently essential for immune resistance. Genetic targeting of MUC5B but not MUC5AC has been shown to render mice more susceptible to bacterial infection, revealing the former to represent a particularly important mucin in the context of innate immunity (423). CFTR mutations, which compromise the fluidity of mucus in cystic fibrosis patients (191), also underscore the importance of mucus in lung immunity given the prevalence of lung infections in CF patients. In addition to mucus, numerous soluble immunomodulators are constitutively present in epithelial lining fluid throughout the respiratory tract, including but not limited to SP-A, SP-D, lactoferrin, lysozyme, and others (both known and likely unknown), all of which exhibit defense properties (536).
Besides the constitutive defense properties of the epithelial surface, epithelial function is immunologically dynamic following exposure to invading pathogens. Lung epithelial cells can undergo dramatic transcriptional remodeling in response to infection or infectious stimuli (82, 246), and such activity is elicited by both pathogen- and host-derived mediators, as enabled by a wide gamut of receptors for pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), cytokines, and other immunomodulatory agents (181, 455, 536). Epithelial-specific genetic targeting of NF-κB activity, downstream of many of these receptors (408), is necessary and sufficient for the elaboration of innate lung defense (73, 74, 406, 554, 555). With regards to pathogen-elicited responses, several studies in genetic mouse models support Toll-like receptor (TLR) signaling as an important source of immune activation (118, 180, 332, 367, 388, 413). Myeloid differentiation factor 88 (MyD88) is a central adapter protein for much but not all TLR signaling and is essential for pulmonary immune resistance as evidenced by profound susceptibility to lung infections in individuals with genetic MyD88 deficiency, particularly children (518). Mice lacking MyD88 in either hematopoietic and/or nonhematopoietic cells are vulnerable to lung infection (10, 118, 180), and the targeted genetic manipulation of MyD88 in the epithelium specifically yields substantial changes in pulmonary inflammation and defense (118, 332).
Microbial engagement of pathogen recognition receptors (PRRs), while significant, is insufficient for maximal epithelial responses. This is again evidenced by the influence of MyD88 on pulmonary inflammation. MyD88 deficiency appears to have a larger consequence on the development of acute pulmonary inflammation than do combinations of TLR deficiency (456), possibly owing to MyD88’s involvement in signaling downstream of the IL-1 receptor IL-1R1 (332). Along these lines, cytokine stimulation of epithelial cells, particularly that due to IL-1 and TNF-α, is a requirement for maximal epithelial responses in some settings. For instance, direct in vitro stimulation of epithelial cells with Streptococcus pneumoniae fails to elicit an NF-κB response, whereas stimulation with pneumonic airway lining fluid robustly activates this transcription factor in an IL-1- and TNF-α-dependent manner (406). Expression of these cytokines requires macrophage activity (392), which can occur in direct response to pneumococcus (392, 406), suggesting that AMs are a critical relay for initiating epithelial responses to certain microbes (like pneumococcus). The combined importance of IL-1 and TNF-α is consistent with evidence that mice lacking all signaling receptors for these cytokines are exquisitely susceptible to infection (240), and additional studies consistently support the notion that macrophage-derived products such as IL-1 are requisite for epithelial-derived innate immunity (198, 283, 313).
The ability of epithelial cells to respond to AMs does not preclude epithelial activation by other cytokines and cells. IL-22, which has numerous protective properties during pneumonia (23, 71, 220, 272, 371, 395, 498, 510, 549), is produced by multiple cell types, including Th17 cells and innate lymphocytes (71, 272, 371, 373, 510, 549). IL-22-dependent protection includes its capacity to activate epithelial cells via the transcription factor STAT3 to produce the antimicrobial siderophore binding protein lipocalin 2 (LCN2) (23), which is itself required for maximal lung immunity (24, 66, 141). STAT3 activity in lung epithelium also promotes expression of the antimicrobial factor Reg3γ (76). Likewise, the IL-6 family cytokine oncostatin M (OSM) can activate epithelial STAT3 to promote induction of the chemokine CXCL5 (495), which is important for lung neutrophil recruitment (234, 327, 554). Although CXCL5 is induced by STAT3, it is also dependent on NF-κB in epithelial cells of the infected lung (554, 555), and CXCL5 can be stimulated by IL-17 as well (69). CXCL5 is especially interesting because it is derived exclusively from epithelial cells during diverse settings (234, 246, 554, 555). The epithelium can also signal directly to neutrophils by producing granulocyte-macrophage colony stimulating factor (GM-CSF) and secreted and transmembrane 1 (Sectm1) proteins (246, 465, 554). Additional epithelial-specific products induced by lung infection include CCL20 (466, 555), short palate, lung, and nasal epithelial clone 1 (SPLUNC1) (292), thymic stromal lymphopoietin (TSLP) (470), and many others (246) which can confer local immune resistance.
The capacity of epithelial cells to control the immunological tone of the lungs is further exemplified by studies showing that their activation is highly protective against subsequent infectious challenges (130). The intranasal administration of nontypeable Hemophilus influenza lysates confers remarkable protection against subsequent challenges with S. pneumoniae (83). The protective signaling components of this stimulation have been narrowed down to a combination of ligands for TLR2/6 and TLR9 (119). Importantly, this broadly effective inducible resistance conferred by TLR ligand administration appears to solely rely on the activity of epithelial cells (82, 119, 284), which has important implications regarding the functional capacity of this cell type to control pulmonary immune resistance. Pharmacological activation of TLRs is safely tolerated in vivo (11) and confers significant protection against not only S. pneumoniae, but also other bacterial, viral, and fungal pathogens (115, 129). This epithelial stimulation was demonstrated to be sufficiently robust for pneumonia protection in the severely immunocompromised setting of leukemia and its treatment (284). These studies highlight the potential significance of harnessing immune resistance provided by the lung epithelium to prevent pneumonia.
3. Neutrophils
Following exposure to harmful microbes, a major role of AMs, epithelial cells, and other resident cells of the lung is to recruit additional effector cells in the event that initial local defenses are insufficient. This requires the elaboration of cytokines and other intermediates that facilitate the migration of cells into the airspaces of the lungs. Neutrophils, which are sparse or absent in the airspaces of uninfected lungs, are the earliest and most abundantly recruited leukocyte in response to infectious stimuli, representing a hallmark feature of recruited innate immunity in the lungs. Neutrophils have many and diverse roles during pneumonia, as microbe killers and also as important modifiers of the immune milieu (FIGURE 3).
FIGURE 3.
During pneumonia, neutrophils have both effector (antimicrobial) and affector (immunomodulatory) roles. Effector roles include microbial elimination via phagocytosis and degranulation as well as NET formation (top right). Affector roles include activities that enhance antimicrobial activities by other cells, such as macrophage stimulation by neutrophil-derived IL-17 and TRAIL and neutrophil stimulation by IFN-γ. Other immunomodulating activities involve the recruitment of antimicrobial cells (e.g., by CXCL12, CCL17, CXCL10, CXCL2). Signals provided from apoptotic neutrophils are immunoregulatory, enhancing the resolution of inflammation. The molecules identified are for illustrative purpose and do not represent an exhaustive presentation. PhSer, phosphatidylserine.
The known biological mechanisms governing lung neutrophil recruitment are vast, with many more almost certainly remaining to be discovered (90). Indeed, numerous local signals coalesce to drive this response, such as pathogen recognition (by PRRs), transcriptional remodeling of responding resident cells (by transcription factors such as NF-κB and STAT3), production of early-response cytokines and growth factors (which further stimulate neighboring and remote cells), generation of a chemotactic or haptotactic gradient (as accomplished by chemokines, eicosanoids, complement fragments, and other host factors), an appropriate display of adhesion molecules, and the cytoskeletal rearrangements and locomotion of the neutrophils themselves (90, 112). Rapid transmigration is aided by the large marginated pool of neutrophils in the pulmonary vasculature, which means that neutrophil numbers that can exceed the total circulating pool are microns away at the start of infection (113). Neutrophils are relatively short-lived cells (391, 491) compared with other leukocytes, surviving on the order of hours to days, but their survival time can be modified by signals within the inflammatory milieu (86).
Neutrophils are antimicrobial cells (FIGURE 3). Upon activation within the airspaces, neutrophils exert an expansive repertoire of intra- and extracellular antimicrobial activities (260), and their importance in the context of lung infections is evidenced by extreme susceptibility to infection in the absence of functional neutrophils both clinically (during neutropenia and with disorders of neutrophil function such as chronic granulomatous disease) and in animal models (following depletion or targeting of neutrophil-specific factors) (61, 151, 179, 386, 421, 539, 547). The primary means of neutrophil-mediated killing are 1) phagocytosis, during which phagolysosomal fusion exposes ingested organisms to reactive oxygen species (via NADPH-oxidase activity) and acidity; 2) degranulation, during which granules release toxic factors such as myeloperoxidase (MPO), gelatinase B (MMP9), cathepsins, defensins, and other antimicrobial proteins into the phagosome and/or extracellular space; and 3) the formation of neutrophil extracellular traps (NETs), which result from the extrusion of DNA associated with histones and granule-derived antimicrobial proteins. All of these killing mechanisms cooperate to eradicate pathogens. For instance, genetic deletion of neutrophil elastase and cathepsin G in mice increases vulnerability to lung infections with S. pneumoniae (179), whereas S. pneumoniae lacking endonuclease A are less efficient at evading NETs, causing less severe pulmonary infections (32). Thus, while short-lived, the bactericidal capacity of this critical phagocyte population is a consequence of both great numbers and diverse function.
Outside of these effector roles, neutrophils also function in a governing capacity, producing cytokines, chemokines, and other factors that coordinate the ongoing immune functions in the lung (226). Lung neutrophils dispatch a variety of signals that shape acute pulmonary inflammation, providing a second wave of immunomodulatory cargo to expand upon initial responses from resident cells (FIGURE 3). For example, neutrophils recruited in response to lung injury induced by influenza virus or acid aspiration release the chemokine CXCL10, which subsequently enhances both neutrophil activity and recruitment through its receptor CXCR3 (213). Neutrophils produce the neutrophil-attracting and -activating chemokine CXCL2, and neutrophilic production of CXCL2 can drive a self-amplifying feed-forward loop of localized neutrophilia that is important to defense but also a contributor to lethal lung injury (51). Neutrophil production of chemokines like CXCL10 and CXCL2 may contribute to “swarming” behavior of neutrophils (253), in which neutrophil activation amplifies the local recruitment of neutrophils within the air spaces of infected lungs (FIGURE 3). In some cases, neutrophils also can be a source of IL-17 (60, 533), a cytokine driving protective immunity through the induction of CXCL5, CXCL1, G-CSF, and enhanced phagocytic antimicrobial defense (69, 559). Interferon (IFN)-γ, the prototypical driver of type I immunity, is another typically lymphocyte-derived cytokine that can be produced by neutrophils in some settings of lung infection. In mouse models of pneumonia induced by Gram-positive (but not Gram-negative) bacteria, emigrated but not circulating neutrophils represent a prominent source of IFN-γ, which then enhances NET formation to facilitate bacterial killing (163, 553). This finding expands the catalog of neutrophil defense functions while highlighting the need to elucidate the causes and consequences of neutrophil reprogramming as they shift between the extra- and intrapulmonary environments.
While CXCL10, IL-17, and IFN-γ are examples of neutrophil-derived cytokines empowering neutrophil-driven defense, neutrophil products affect other cells as well (FIGURE 3). For instance, neutrophils can enhance macrophage-mediated immunity by serving as a source of TRAIL, which drives antimicrobial apoptotic responses (468). Neutrophils are a requirement for the extravasation of iNKT cells from the pulmonary vasculature, where they are abundant before infection (486). To do so, migrating neutrophils release the chemokine CCL17, which recruits iNKT cells to the interstitium, and this is essential to optimal defense in mice with pneumococcal pneumonia (486). Similarly, neutrophils can recruit lymphocytes for adaptive immunity purposes; after influenza infections, the recruitment of antiviral CD8+ T cells to the lung and optimal influenza elimination requires CXCL12, which is produced exclusively by the migrating neutrophils within the infected lung and deposited in membrane-bound packets along the neutrophil’s path within the interstitium (289). Therefore, neutrophils serve as both a consequence and cause of acute pulmonary inflammation, mediating both effector and affector actions of immune resistance (FIGURE 3).
4. Recruited macrophages
While AMs may be the exclusive resident leukocyte of the airspaces, they do not represent the only macrophage population driving innate immunity. Perhaps this has been somewhat overlooked by the accessibility of AMs through lavage and the overwhelming numbers of recruited neutrophils in the early stages of pneumonia, not to mention the technical challenge of distinguishing resident versus recruited cells of the same type. It is evident that a distinct population of recruited bone marrow-derived macrophages can have an indispensable role in pulmonary innate immunity. In response to inflammatory stimulation, induction of the chemokine CCL2 acts as the primary signal to recruit monocytes into airspaces (72, 317–319), which then become further primed (316), expanding the available macrophage pool. This newly recruited inflammatory monocyte/macrophage population is functionally similar to classically activated (M1) resident macrophages in that they are phagocytes capable of producing inflammatory cytokines such as IL-1, TNF-α, and IL-12, and they can be distinguished from resident cells by a variety of differentially expressed surface markers, the most notably of which is high expression of CD11b (4).
Multiple studies support an essential role for recruited macrophages in maintaining immune resistance in the lungs. CCL2, for example, is both sufficient and necessary for inflammatory monocyte/macrophage recruitment in mice challenged intratracheally with S. pneumoniae, and its expression level is inversely proportional to the number of living bacteria recovered from the lungs (540, 541). Recent studies in mice infected with K. pneumoniae suggest that not only are CCR2+ recruited monocytes critical for lung bacterial clearance, but also that, for a subset of K. pneumoniae isolates, the antibacterial contribution of this cell population exceeds that of neutrophils (547). As discussed with other cell types above, recruited monocytes/macrophages also function to enhance the accumulation of other recruited immune cells. In the setting of sterile inflammation induced by intratracheal lipopolysaccharide (LPS), neutrophil and inflammatory monocyte responses were similarly diminished following interruption of CCR2, suggesting that the neutrophil accumulation requires signaling to monocytes (318). Inflammatory monocytes may also be required for the recruitment of IL-17-producing innate lymphocytes; in mice challenged with K. pneumoniae, recruited monocytes were identified as the prominent source of TNF-α, contributing to the lung recruitment of ILC3s and IL-17-mediated defense (548). Thus the integration of recruited monocytes with other innate defenses has surfaced as a key determinant of immune resistance.
5. Innate lymphocytes
The roles of innate lymphocytes in the context of pneumonia biology are receiving considerable interest. Their study is enabled by increasingly sophisticated tools for characterizing and manipulating lymphocyte subsets. While innate lymphocytes bear functional similarities to the B and T lymphocytes well recognized for their roles in adaptive immune responses, they are innate with regards to pathogen recognition. Natural killer (NK) cells, which were discovered over four decades ago, represent one type of innate lymphocyte enriched in lung tissue, important for defense against both viral and bacterial pathogens (197). Patients with genetic mutations causing NK cell deficiency are especially prone to viral infections (369), and the direct requirement of NK cells for maximal antiviral immunity has been observed in animal models as well (1, 467). While the impact of NK cells on bacterial infections is less delineated, pro-defense roles are beginning to emerge. Mice lacking the NK cell activating receptor NCR1 (NKp46) as well as mice depleted of NK cells (via anti-asialo GM1) exhibit increased lung bacterial burdens and mortality upon infection with S. pneumoniae (125). NK cells also are essential for clearance of K. pneumoniae (549) and S. aureus (457) in murine models of pneumonia, possibly due to their roles in synthesizing IL-22 (549) and IL-15 (457), each of which is independently essential for defense against those respective microbes (23, 457). NK cells may also function at the interface of viral and bacterial pneumonias by limiting the likelihood of superinfection. The number of NK cells and their capacity to produce TNF-α were diminished in mouse lungs with S. aureus pneumonia following influenza infection compared with mice with S. aureus pneumonia and no prior influenza, and adoptive transfer of NK cells (but not TNF-α-deficient or influenza-exposed NK cells) was sufficient to restore antibacterial defense to influenza-infected mice (458).
NK cells were the first recognized subset of the innate lymphocytes now known as innate lymphoid cells (ILCs). These cells have been categorized in three distinct groups: 1) group 1 including ILC1s and NK cells, 2) group 2 including ILC2s, and 3) group 3 including ILC3s and lymphoid tissue-inducer (LTi) cells (461, 462, 519). The categorization of ILCs across three distinct groups is consistent with their capacity to produce cytokines reflective of Th1, Th2, and Th17 adaptive lymphoid cells, yet ILCs are devoid of the known lineage markers associated with adaptive T cells, and they are not antigen specific (461, 519). Research into ILCs and lung infection is in early stages.
ILCs have been identified in the lungs of humans (96) and mice (338), and despite their relatively low abundance, they can play important roles. To our knowledge, ILC1s do not reside or function within the healthy lung. In contrast, ILC2s are present under unchallenged homeostatic conditions (152), although their functional contributions to immune resistance are uncertain. RSV infection promotes accumulation of IL-13-expressing ILC2s in the lung, which associates with increased type 2 inflammatory responses (470), suggesting at least one connection of ILC2s to lower respiratory infection. ILC2s help repair and regenerate injured lung tissue (44, 279, 333), but ILC2-mediated repair after pneumonia specifically is presently speculative. ILC3s are perhaps the most relevant to pneumonia biology and acute pulmonary inflammation, particularly with regards to their influence on IL-17-mediated defense. ILC3s are a prominent source of this cytokine in response to multiple microbial stimuli in the lungs, including P. aeruginosa (31, 347), K. pneumoniae (548), and LPS (347). Similarly, IL-22, often associated with Th17 biology and IL-17-dependent lung immunity (23), has been shown to derive from ILC3 cells during pneumococcal pneumonia (510). In all of these cases, the physiological significance of lung ILC3s is inferred from the already recognized influence of the cytokines they express. In some cases, ILC3-dependent immune effects are more directly supported through depletion strategies (347, 548), although a limitation of these studies is their use of Rag−/− mice lacking adaptive immunity lymphocytes. More precise targeting strategies will be required to definitively distinguish the functional contributions of ILCs during pneumonia.
In addition to ILCs, unconventional T cells (160) are another group of innate lymphocytes promoting lung defense. Invariant natural killer T (iNKT) cells possess an invariant TCR alpha chain and recognize lipid antigens presented by the MHC-like molecule CD1d (53). During pneumococcal pneumonia, Jα18−/− mice lacking iNKT cells exhibit increased mortality in association with impaired bacterial clearance from the lungs (54). Moreover, iNKT cells produce IFN-γ and IL-22 in response to influenza infection (373), although this did not alter immune resistance in this particular setting, it may have defense implications in other infections. γδ-T cells have a limited diversity of TCRs with poorly understood antigen specificity, but they demonstrably function in an innate capacity to modulate pulmonary inflammation (42). During pneumonia, γδ-T cells can be a major source of both TNF-α and IL-17, and mice lacking these cells are more susceptible to lung infections with K. pneumoniae or S. pneumoniae (71, 340, 356). Mucosa-associated invariant T (MAIT) cells recognize riboflavin-related products produced by diverse bacteria when presented by the MHC-related molecule MR1 (145). While MAIT ligands are unexpected in viral infections of any animal, MAIT cell numbers in the peripheral blood during severe avian influenza infections were elevated in human patients who survived, and MAIT activation by the cytokine IL-18 might possibly improve defenses against influenza (297). Although relevant to tuberculosis infection (161), roles of MAIT cells during bacterial pneumonia have yet (to our knowledge) to be demonstrated, but demand further attention.
Beyond ILCs and unconventional T cells, innate-like B cells also can impact early defense in the lungs. B1 cells are a self-renewing B cell population that is a major producer of cross-reactive natural IgM antibodies (30). The innate response activator (IRA) subset of B1a cells has been shown to reside in the pleural space and migrate to the lung parenchyma in response to E. coli pneumonia, where these cells then provide a protective GM-CSF-dependent IgM response (530). Additional studies in the setting of pneumococcal pneumonia also support a requirement for B1a cells to achieve maximal innate defense in the first days of infection (556).
6. Platelets
While best recognized for their roles in coagulation, platelets contribute to innate immunity as well, with multiple potential connections to pneumonia (46, 550). Platelets and their associated platelet GTPases and adhesion molecules enhance LPS-induced neutrophil recruitment in the lung and host defense during Klebsiella pneumonia (98, 99, 375), demonstrating roles in immune resistance. Their myriad effects on coagulation, inflammation, and other aspects of physiology (46, 550) likely shape immune resistance, but also platelets may contribute in some cases through direct antimicrobial activities (269). During severe influenza infections, excess platelet activation amplifies inflammation, lung injury, and mortality (278), consistent with the notion that the challenge to tissue resilience from exuberant inflammation can at times be downstream of platelet activities. The lung is the site for much platelet production by megakaryocytes during homeostasis (282), and platelet production during acute inflammation is increased by maturation of committed progenitors into new megakaryocytes (178). It will be of interest to determine whether and how megakaryocytes in the lung and the differentiation of stemlike precursors into megakaryocytes are influenced by, and in turn influence, pneumonia.
B. Extrapulmonary Innate Immune Physiology
The immunological capacity of the lungs is shaped by a complex and highly dynamic pool of local constituents, some of which are discussed above. Typically, the lungs are remarkably efficient at compartmentalizing both infections and the response that they elicit, with a breach in containment representing insufficient resistance and resilience, potentially resulting in ARDS and/or sepsis. While it is logical, and certainly necessary, to investigate innate immune responses to lung infections from a local intrapulmonary perspective, lung defense does not and cannot occur in a vacuum. Rather, it involves an integrated physiological response in which the lungs selectively send and receive input from extrapulmonary tissues. Elucidating the identity and functional relevance of these signals has been historically challenging, due in large part to limited tools for interrogating tissue-specific contributions and the ever-present complications in distinguishing cause from effect. Yet, advances in gene targeting and other experimental approaches continue to expand our understanding and appreciation for remote processes controlling local immune resistance during pneumonia.
1. Liver
The liver has long been appreciated for its role in mounting the acute phase response (APR). Liver hepatocytes synthesize and secrete significant quantities of circulating acute phase proteins (APPs), whose expression can be dramatically altered in response to virtually any infection or injury (91, 148). The APR was discovered almost 90 yr ago in pneumonia patients, when researchers identified changes a substance (now known as C-reactive protein, CRP) in blood that bound to a polysaccharide-containing fraction of S. pneumoniae (490). There are now dozens of known [and likely many more unknown (403)] APPs, which are functionally diverse, and primarily expressed in the liver (148). The clinical utility of APPs is largely restricted to their application as biomarkers of disease severity, including that of pneumonia (13, 460, 564). The regulation and physiological relevance of APP expression is beginning to be understood.
Lung infections elicit robust hepatic transcriptome remodeling within hours of experimentally induced pneumonia, before the detection of any living organisms into the circulation (403, 404, 531). Regulation of hepatic gene programs guiding APP synthesis is attributed to multiple transcription factors (427), including STAT3, which was originally known as the “acute phase response factor” before it was cloned in the early 1990s (9, 571). In the setting of lung infections, NF-κB and STAT3 are particularly important for hepatic acute phase changes based on studies in mouse models (403). Following a lung infection with S. pneumoniae sufficient to induce over 1,000 gene changes in the liver, targeted simultaneous deletion of NF-κB RelA and STAT3 in hepatocytes virtually eliminated the pneumonia-induced APR (403). This response requires a combination of early-response cytokines (TNF-α, IL-1α, and IL-1β) with IL-6, as mice lacking these cytokine signals exhibit marked defects in hepatic activation of RelA and STAT3, respectively, in association with abrogated APP synthesis (404). These findings confirm the presence of a lung-liver axis, whereby cytokine signals from the lung elicit a rapid hepatic response to remodel the blood proteome. Consequently, APR-null mice lacking hepatocyte RelA and STAT3 not only lack changes in circulating APPs during pneumonia (despite unaffected baseline levels), but they also have diminished amounts of some APPs in alveolar exudate during pneumonia (200, 201). Failure to mount a liver APR is associated with increased mortality and impaired immune resistance both systemically and locally in mice lacking hepatocyte RelA and STAT3 (200, 201, 403), directly demonstrating the physiological significance of lung-liver communication.
While the existence of liver-derived protection during pneumonia is supported by the aforementioned studies, unveiling distinct mechanisms of protection presents a major challenge due to the breadth and diversity of hepatic acute phase changes. Enhancing opsonophagocytosis is one important function. It has been known for over a half century that acute phase serum can enhance opsonophagocytosis (233), and serum obtained from pneumonic mice lacking hepatocyte RelA and STAT3 has a diminished capacity to do so (403), proving that hepatic activity is essential to this blood-borne defense during pneumonia. The reduced opsonophagocytosis after hepatic transcription factor targeting invovlves decreased deposition of complement component 3 (C3) on the surface of pneumococci (403). A role for pneumonia-induced C3 expression is also supported by a recent study showing that IL-22, which is essential for innate defense against intrapulmonary S. pneumoniae, increases hepatic and serum C3 levels to a degree that is sufficient to increase its bacterial deposition as well as pulmonary defense (498). Targeted deletion of the IL-22 receptor on hepatocytes reduces local lung clearance of pneumococcus, indicating that this STAT3-activating cytokine works alongside IL-6 in the context of the lung-liver axis. Short pentraxins such as CRP and serum amyloid P (SAP) are other APPs that bind to bacterial surfaces, where they both activate complement deposition and promote recognition and phagocytosis through FcγRs (299). CRP and SAP are necessary and sufficient to enhance host defense in mouse models of pneumococcal pneumonia (452, 567). Another potential mechanism for liver-derived lung immunity is enhancement of macrophage responsiveness, including increases in the respiratory burst that depend on yet-to-be-identified liver-derived factors (201). Regulation of metal homeostasis may also be an important form of immune resistance provided by the liver. For instance, iron acquisition is essential for bacteria to thrive (414), and host factors that limit iron availability can be protective (235). Hepcidin, which is largely driven by IL-6-dependent STAT3 activity in the liver (361), limits iron availability by controlling its absorption in the intestines (362), and this factor was recently shown to be both sufficient and necessary for promoting defense against K. pneumonia in the lungs (331). Beyond opsonophagocytosis, leukocyte activation, and metal homeostasis, additional relevant APP functions may include protease regulation, hemostasis, toxin inhibition, microbial starvation, and more. In addition to those mentioned above, individual APPs such as lipopolysaccharide binding protein (LBP), serum amyloid A (SAA), and mannose binding lectin (MBL) have been demonstrated significant to pneumonia and acute pulmonary inflammation (52, 149, 164, 416).
Secreted APPs represent only a fraction of the many hepatic gene changes constituting the acute phase response (8, 403), and the importance of liver responses for lung defense demands consideration of non-APP functions. While some “APP-independent” hepatic gene programs, such as those linked to metabolism, protein synthesis, and protein secretion, almost certainly play a support role for APP responses (8), others likely operate to promote defense that is entirely distinct from that afforded by APPs themselves. Cholesterol regulation represents one interesting example of APP-independent liver-derived protection (531). S. pneumoniae lung infections in mice were shown to promote liver gene expression of numerous factors connected to cholesterol biosynthesis (531). In this study, hepatic gene changes were associated with increased plasma cholesterol, and this increase was shown to abrogate pneumolysin-dependent alveolar macrophage necrosis, suggesting that acute phase exudate in the alveolar space directly impairs pneumococcal virulence (531). The functional relevance of the lung-liver axis is now firmly established, and mechanisms governing liver-derived lung immunity are beginning to be resolved.
2. Bone marrow
As outlined above, effective local immune resistance requires both resident and recruited leukocytes in the lungs, the latter of which includes the rapid emigration of neutrophils from the blood to the airspaces. As neutrophils are extracted from the circulation, their supply must be maintained to meet the demand of the infected lung, which is primarily established by the egress of newly formed cells out of the bone marrow. This shift from homeostatic granulopoiesis to “emergency” granulopoiesis in the marrow requires the lung to function in an endocrine capacity, much like it does with the liver to elicit the APR. While multiple cytokines and other immunomodulators have been shown to impact the complex process of granulopoiesis, the most prominent is G-CSF (311), the primary intermediate through which pneumonic lungs trigger bone marrow responses. G-CSF is required for steady-state granulopoiesis and is used therapeutically in patients with neutropenia (379). By inhibiting osteoblast expression of the chemokine CXCL12, G-CSF disrupts the retention of bone marrow neutrophils, which is largely maintained by CXCR4-CXCL12 interactions (121, 441, 442, 480). Diminished CXCL12 content in the bone marrow then enables neutrophils to respond more readily to CXCR2 ligands, such as CXCL1, CXCL2, and CXCL8, promoting neutrophil release from the bone marrow (120, 480).
G-CSF is a pleiotropic neutrophil-targeting cytokine, and a major role of G-CSF during pneumonia is to signal to the marrow from the lung. Lung infections stimulate abundant G-CSF in the blood during pneumonia in both patients and in animal models (382, 410). The intratracheal administration of recombinant G-CSF alone is sufficient to elicit increases in circulating G-CSF, blood neutrophils, and bone marrow granulopoiesis (447), consistent with the notion that lung-derived G-CSF is decompartmentalized to access the bone marrow during pneumonia (410). Consequently, intrapulmonary G-CSF delivery can also significantly amplify alveolar neutrophil recruitment, as demonstrated in multiple settings of acute pulmonary inflammation (26, 137, 360, 570). Genetic targeting of the G-CSF receptor impairs clearance of P. aeruginosa in mouse lungs, and this is associated with reduced survival and dramatic decreases in both circulating and lung-recruited neutrophils (173). Similar consequences are observed in response to pharmacological G-CSF blockade in mice challenged with pneumococcal pneumonia (257). Conditions that alter G-CSF expression also support an important role for this cytokine in immune resistance. For example, impaired G-CSF expression in the setting of alcohol exposure associates with reduced granulopoiesis and enhanced growth of S. pneumoniae in the lungs (451).
3. Spleen
Experimental splenectomy renders pneumonic mice highly vulnerable to systemic infection (449), and this outcome is consistent with increased occurrence and reoccurrence of pneumonia in splenectomized patients (270, 346). Precise contributions of splenic function to local lung defense are presently unknown. Like that of the liver, the anatomy and distribution of phagocytes within the spleen enable reticuloendothelial clearance of circulating pathogens (324), which is essential for controlling systemic defense and inflammation during pneumonia. This is unlikely a direct contributor to immune resistance in the lung, though. As a secondary lymphoid organ, the spleen has essential roles in adaptive immune responses, and these may contribute to local lung defense, particularly the delivery of plasma antibodies in the exudate of an infected lung. The IgM produced by marginal-zone B cells (324) may be particularly important for some respiratory pathogens, like pneumococcus (556). The activation mechanisms and functional roles of splenic B cells involve other leukocytes linked to pneumonia outcome such as neutrophils (78, 400), ILCs (304), and macrophages (263). Beyond the loss of phagocytes, neutropenia may predispose to pneumococcal pneumonia because of defects in T cell-independent antibodies from the spleen; IgM, IgG, and IgA against pneumococcal capsular polysaccharides are produced by splenic B cells requiring neutrophils as helper cells, and all are diminished in the blood of neutropenic patients (400).
4. Gastrointestinal tract
The intestinal mucosa is an immunologically rich environment, containing a microbial landscape shaped by interactions among microbiota, invading pathogens, and host immune functions (438). These interactions also have immunologic consequences in tissue sites outside of the intestines, including the lungs. For instance, meso-diaminopimelic acid-containing peptidoglycan derived from the intestinal microbiota engages the NOD1 receptor of neutrophils in bone marrow, enhancing their in vitro microbicidal capacity against S. pneumoniae and S. aureus (81). Depletion of gut microbiota with antibiotics impairs K. pneumoniae clearance in the lungs in association with reduced pulmonary cytokine responses and impaired ROS synthesis by AMs (80). Oral administration of NOD-like receptor ligands is sufficient to rescue innate immune responses following microbiome depletion, consistent with the hypothesis that gut-derived microbial products promote defense at other tissue sites (80). Similar immunodeficiency is also observed in mice with pneumococcal pneumonia following gut microbiome depletion; broad-spectrum antibiotic treatment ablates intestinal microbiota and significantly increases the number of viable bacteria recovered from the lungs, concurrent with reduced cytokine expression and phagocytosis by AMs (437). Gut-derived pulmonary defense is also evident in the setting of viral pneumonia, in which case microbiome ablation impairs immune responses reliant on inflammasome activity (214). Despite the risk of deleterious effects on immune defenses as well as potentially fostering antibiotic resistance, selective decontamination of the digestive tract has clinical utility; reducing the presence of potentially infectious agents in the gastrointestinal and respiratory systems of vulnerable patients can decrease rates of pneumonia and death in the ICU (43, 97). In addition to microbe-derived substances, host-derived metabolites from the intestines have also been shown to have important immunomodulatory properties. For example, select intestinal short-chain fatty acids have numerous effects on leukocyte recruitment and activation (517). These effects appear to be context specific, and at present their direct influence on immune activity in the respiratory tract is unknown.
5. Fat
Adipose tissue represents another remote contributor to innate pulmonary defense. Leptin is derived primarily from adipocytes, and leptin signaling is essential to optimal immune defense in multiple mouse models of lung infection (308–310, 502). Conversely, excess leptin including hyperleptinemia, a hallmark of obesity, may increase risk of pneumonia (390). Higher leptin levels associate with greater pneumonia risk in nonhospitalized adults, and with greater severity of pneumonia among hospitalized patients, independent of body mass (502). High circulating leptin content in mice, elevated by diverse strategies, can compromise innate immunity in the lungs (502). A specific role for leptin is further supported by the observation of no adverse effects on lung defense in a leptin-independent mouse model of obesity (307). The precise role of leptin is complicated by indirect metabolic consequences of its manipulation and the widespread physiological impacts of obesity (479). Adiponectin is another adipokine with inflammation-regulating properties that may influence pulmonary immune resistance. In the setting of sterile inflammation induced by LPS, adiponectin deficiency exaggerates immune responses (59, 262), but whether or how this factor directly contributes to pneumonia biology is currently unclear. While elements of the obesity phenotype including changes in adipokines may influence pneumonia biology, the evidence does not conclusively demonstrate that obesity per se increases risk of community acquired pneumonia, independent of obesity-associated comorbidities (265, 390).
6. Other extrapulmonary influences on lung innate immunity
The liver, bone marrow, spleen, gut, and adipose tissue are highlighted above for their impact on intrapulmonary immune responses, perhaps all of which involve endocrine activities from these tissues (FIGURE 4). The systemic response to pathogens in the airspaces has other origins, as well. For example, procalcitonin (PCT), which is the thyroid-derived precursor to calcitonin, increases in the blood of critically ill patients and rises in the blood during pneumonia, and it can be used as a biomarker to help distinguish infections of bacterial versus viral origin (22) and as a predictor of pneumonia severity (483). The functional significance of PCT elevation during bacterial pneumonia is unknown. Likewise, febrile-range hyperthermia has numerous consequences on pulmonary inflammation and immunity (186), including elevated neutrophil accumulation and earlier clearance of K. pneumoniae from the lungs (419), suggesting that fever may serve as a systemic mechanism of brain-derived pulmonary defense. Direct biological contributions of fever to pneumonia biology, either good or bad, are largely speculative. Brain function may also influence pneumonia susceptibility, particularly that caused by aspiration, through coordination of airway reflexes such as cough (122). For instance, pneumonia incidence has been shown to correlate with cough reflex sensitivity (355), and among patients with mixed primary neurological disorders the incidence of ARDS is significantly greater in those lacking cough and/or gag reflexes (204). Interestingly, a recent meta-analysis indicated markedly higher pneumonia incidence in subjects with a specific polymorphism in angiotensin converting enzyme that reduces substance P and bradykinin, both of which drive the cough reflex (524), also consistent with the notion that airway reflex sensitivity contributes to pulmonary defense.
FIGURE 4.
Multiple extrapulmonary organs contribute to immune resistance against pulmonary infection. The molecules identified are for illustrative purpose and do not represent an exhaustive presentation. In the few instances where signals have been identified by which pulmonary infection signals to the extrapulmonary organ, this communication is denoted in gray. APPs, acute phase proteins; SCFAs, short-chain fatty acids.
IV. ADAPTIVE IMMUNITY AND PNEUMONIA
The adaptive immune system is well recognized in the fight against pneumonia. One of the earliest effective treatments for pneumonia, used until antibiotics became available, was “serum therapy” in which antibodies collected from horses or rabbits that had previously been serially exposed to pneumococci were administered to pneumonia patients (64, 65). If the antibodies were appropriate to the pneumococcal serotype and were administered soon after pneumonia symptoms developed, “serum therapy” decreased mortality by approximately one-third (64, 65). Along similar lines, but using hybridomas rather than animal sera as an immunoglobulin source and for prevention rather than cure, delivery of a monoclonal antibody against RSV is currently in clinical use for high-risk children (16). The vaccines against influenza, pneumococcus, and Hemophilus influenzae stimulate the host to generate their own circulating antibodies against these microbes, which reduce risk of pneumonia (154, 174, 175). Thus the humoral arm of adaptive immunity can protect the lungs. The emergence of HIV/AIDS in the last half of the 20th century emphasized the importance of cellular immunity in immune defense of the lungs. Patients with low CD4+ T cell counts are highly susceptible to pneumonias caused by diverse organisms including especially Pneumocystis and pneumococcus (169, 440). Although not yet feasible in humans, the adoptive transfer of microbe-specific CD4+ or CD8+ T cells is capable of fighting respiratory infection in inbred animals (182, 232, 558), similar to the transfer of protection achieved with antibodies. Thus cellular immunity also protects the lungs against pneumonia. The regulation and function of specific components of the adaptive immune system in fighting respiratory infection are discussed in several recent reviews (47, 70, 75, 407, 481).
The earliest infections of the youngest children elicit primary adaptive immune responses, and subsequent encounters with those or related microbes trigger secondary or recall responses from memory cells. The immunological memory established by repeated respiratory infections is almost certainly key to the immune defense that helps prevent pneumonia in older children and adults (407). Such “real world” encounters with microbes profoundly rewire immunity, including both innate and adaptive immunity in the lungs (FIGURE 5). Effects of “real world” exposures on immunity were elegantly demonstrated by studies in which the circulating leukocyte transcriptomes of mice and humans were compared (34). In short, laboratory mice have immune systems that reflect those of human infants, whereas mice that were caught in barns or purchased from pet stores have immune systems that more closely match human adults (34). The co-housing of laboratory mice with those purchased from pet stores leads to 1) transcriptional remodeling of blood leukocytes to more closely match that of human adults, 2) circulating antibodies against multiple pathogens of mice, 3) seeding of the lungs (FIGURE 5) and other organs with lymphocytes of the innate and adaptive immune systems, and 4) dramatically improved defenses against experimental infection (34). Here, we overview two important ways in which adaptive immunity is remodeled by prior microbial infections, the establishment of heterotypic immunity and of resident memory, both of which are rapidly advancing areas of research with profound implications for pneumonia defense.
FIGURE 5.
Lungs that have experienced prior infections are different from naive lungs that have not. The T-cell population most notable in lungs from neonatal humans is regulatory T (Treg) cells, whereas the healthy lungs of adults or of laboratory animals that have experienced prior respiratory infections contain abundant resident memory T (TRM) cells. In addition, lungs that have experienced prior respiratory infections exhibit varying degrees of immunological changes including bronchus-associated lymphoid tissue (BALT), innate lymphoid cells (ILCs), and γδ-T cells. In addition, the alveolar macrophages (AM) and epithelial cells of experienced lungs behave differently from their counterparts in naive lungs, likely due to a combination of trained immunity and direction from adaptive immunity.
A. Naturally Acquired Heterotypic Adaptive Immunity
Heterotypic immunity refers to adaptive immunity directed against a microbe that is similar but not identical to the microbe originally establishing immunological memory. Examples include memory to influenza viruses across different seasons (e.g., H1N1 from 2009 and H1N1 from 2010) or across different subtypes (e.g., H3N2 and H1N1), or to multiple of the 94 different serotypes of pneumococcus. Because they are such commonly encountered microbes (FIGURE 1), healthy young adult humans probably have some degree of heterotypic immune memory against all of the most common causes of pneumonia.
Pneumonia defense is strongly influenced by the earliest infections with respiratory pathogens, based on evidence of immunological “imprinting” against influenza viruses (167). Those born before 1968 were likely first infected with influenza viruses containing hemagglutinins (HAs) from phylogenetic group 1 (which includes H1, H2, and H5 HAs), whereas those born after that date were more likely to be first infected by influenza viruses with group 2 HAs (which includes H3 and H7 HAs). For the unfortunate humans who get infected with highly pathogenic zoonotic influenza viruses, the severity of pneumonia correlates strongly with their birth dates; those born before 1968 are more likely to get severe pneumonia from H7N9 rather than H5N1 avian influenza viruses, whereas those born after 1968 are more likely to get severe pneumonia from H5N1 rather than H7N9 (167). These individuals likely did not have neutralizing antibodies against the avian influenza viruses, but their acquired immunity against related viruses (within the same phylogenetic group) provided some level of protection. Such early imprinting of immunity may influence many or all types of respiratory infections.
A combination of humoral and cellular protection is implicated in mediating the naturally acquired heterotypic immunity that protects young adult humans against respiratory infection of the airways. When healthy young adults are experimentally infected with influenza virus or RSV, the amount of heterotypic antibodies in their blood before experimental infection inversely correlates with their viral burden and symptoms after infection (25, 231). In seronegative individuals who have not seen a particular influenza virus before, those with greater numbers of influenza-responsive CD4+ T cells in their blood before infection (FIGURE 6) have less severe infection as measured by viral burden and symptoms during the experimental infection (537). By design necessity, the experimental human infections described above cause mild disease; correlates of protection observed there are inferred to be relevant to pneumonia. Supporting this inference, antibodies with respiratory pathogen specificity found in the blood of seronegative healthy uninfected adults can be sufficient to protect mice against severe viral or bacterial pneumonia (157, 543). Also supporting the association of preexisting heterotypic immunity with more severe infections is an epidemiologic study of a population first experiencing the reassortant H1N1 influenza virus that emerged in 2009 (463). Although all subjects were naive to this particular virus, patients with greater circulating numbers of CD8+ T cells recognizing epitopes conserved in that coming influenza virus (FIGURE 6) demonstrated less symptoms during their naturally acquired infections with that virus (463), suggesting that the preexisting heterotypic immunity provided protection. These results from the epidemiologic study differ in detail from results of experimental infections with influenza or RSV, which showed correlation with elements of preexisting heterotypic immunity but not with blood CD8+ T cells (243, 537). In animal models of influenza infection, both CD4+ T cells and CD8+ T cells are sufficient to transfer heterotypic immunity against pneumonia (182, 232). Together, such studies demonstrate that preexisting heterotypic immunity varies across hosts and pathogens, and variations in the parameters of heterotypic immunity make critical contributions to the outcome of respiratory infection.
FIGURE 6.
T lymphocytes contribute differently to respiratory infection in naive and experienced hosts. A: in naive individuals, who have not previously seen relevant respiratory infections, T lymphocytes responsive to the microbe in the lungs are found in the circulating blood (red) and secondary lymphoid organs such as the spleen (purple) and lymph nodes (LN, blue). It takes several days for them to appear in the lungs and a week for their activities to be manifest. B: in contrast, in experienced hosts who have previously been infected with relevant respiratory pathogens, there are greater numbers of responsive T lymphocytes in the blood and secondary lymphoid organs, and there is a resident memory population of responsive T cells already present in the lungs before infection. These lung T cells are poised to respond more rapidly and to elaborate a broader repertoire of protective cytokines. Thus responsive T cells in experienced hosts are more numerous, localized to the right place, able to respond more quickly, and prone to becoming multifunctional, altogether leading to T cell-mediated defense in the lung that is quicker and more efficacious.
Some heterotypic antibodies in human blood can be neutralizing, preventing viral infection of cells (354, 543). Other heterotypic antibodies direct immune effector activities. Heterotypic antibodies against the conserved stalk region of influenza HA trigger a respiratory burst in phagocytes and provide heterotypic defense against respiratory infection that is Fc receptor (FcR) dependent, whereas homotypic protection provided by antibodies against the more variable head regions of HA do not protect via such pathways (109, 348). This applies uniformly across anti-HA antibodies and to anti-neuraminidase antibodies as well (108), suggesting that FcR-mediated activities may be generally required for protection by heterotypic antibodies. Young adult humans have antibodies recognizing hundreds of protein epitopes from pneumococcus (157), and raising antibodies against some of these pneumococcal antigens by vaccinating mice can improve defense against pneumococcal pneumonia (157, 334), by mechanisms including opsonophagocytosis, decreased adherence, disruption of toxins, and more. Human blood from any of at least three different continents consistently contains IgG antibodies against a common set of diverse pneumococcal proteins, and delivering human IgG (without antigen selection) to mice is sufficient to diminish the severity of infection during pneumococcal pneumonia (538). However, pneumococcus-recognizing antibodies found in the blood after pneumococcal infection are not necessarily capable of mediating heterotypic protection (84, 157). Whether and when the heterotypic antibodies present in human blood can help to prevent pneumonia demands more investigation.
Cellular immunity makes many contributions to heterotypic protection of the lungs (FIGURE 6). Memory CD4+ T cells established by prior respiratory infections are superior to primary effectors in their protection of the respiratory tract, because they are more multifunctional (i.e., producing a greater variety of cytokines) and more prone to yield T follicular helper (TFH) cells in the relevant lung tissue (473). The expansion of such memory CD4+ T cells in the infected lungs requires IL-6 (474). Activities of these virus-specific memory CD4+ T cells include the lysis of infected cells (153, 537) as well as the acceleration and amplification of pathways described above under “innate immunity” but here directed by memory T cell-derived cytokines (472, 475). Similar phenomena apply to bacterial pneumonias. Adult humans have CD4+ T cells that proliferate and produce IL-17 in response to acapsular pneumococcus or pneumococcal proteins, which are heterotypic responses in the sense that they are independent of serotype (301). In humans, experimental pneumococcal infections of the upper airways increase the numbers of cognate multifunctional CD4+ Th17 cells in the blood and lungs (544). IL-17 stimulates host defense against extracellular bacteria and fungi in the lungs (70). Heterotypic protection against pneumococcus in the lungs can be modeled in mice by infecting them with one serotype before challenging them with another, and both IL-17 and CD4+ T cells are necessary for heterotypic protection against pneumonia in this model (526). Furthermore, CD4+ T cells from the spleens of such mice are sufficient to confer defense against respiratory infection, if and only if they have intact genes for IL-17 (526), demonstrating that this systemic cellular immune memory can provide heterotypic protection against bacterial pneumonia.
B. Resident Memory Cells
In addition to the circulating or systemic immune memory described above, which can protect the lungs and all tissues, there are also localized depots of immune memory within the respiratory tract that specifically protect these tissues against respiratory infection. The best recognized such structures are the tertiary lymphoid organs of the upper airways, the tonsils and nasal-associated lymphoid tissue (443), as well as the variable amounts of bronchus-associated lymphoid tissue (BALT) in the lower airways (412). These are sites of local antibody production, as well as sources of memory B cells and plasma cells providing systemic protection. More recently, it has become evident that lung tissue contains numerous memory T cells that reside stably in the interstitium but are not in tertiary lymphoid organs (401, 485, 501). The biology of such resident memory T (TRM) cells has been reviewed (134, 434). Here, we discuss aspects of lung TRM cells that are especially pertinent to pneumonia defense (FIGURE 6).
While identified previously (205), many fundamentals of lung TRM cells in respiratory infection were established with a seminal study from 2011 (485). Mice with a transgenic TCR specific to influenza HA were used to restrict analyses to antigen-specific cells. Memory CD4+ T cells were collected from the lungs or the spleen of mice with a fully resolved influenza virus infection, and then adoptively transferred to normal uninfected mice; the lung-derived memory cells were then found exclusively in the lungs, whereas the spleen-derived cells were found in the spleen and other tissues of the recipient mice. When the bloodstreams of such recipient mice were connected via parabiosis to other uninfected mice who had not received T cell transfers, the lung-derived T cells did not appear in the parabiotic host, whereas the spleen-derived cells did. These data demonstrated that the resolution of lung infection resulted in a population of lung-resident memory cells, retained in the tissue rather than recirculating with a lung-homing propensity. When mice that had received comparable numbers of TCR-transgenic (hence identical antigen specificity to influenza HA) memory T cells from the lungs or from the spleen were challenged with an influenza infection, the lung-derived cells provided better protection as measured by weight loss, survival, and viral burdens, indicating that lung TRM cells have superior abilities to protect against respiratory infection compared with central memory cells. Therefore, the resolution of lower respiratory infection can seed the lungs with TRM cells that remain local and protect that pulmonary tissue against further infections.
Resident memory cells populate the lung tissues after bacterial pneumonia as well (459). When pneumococcus causes a lobar pneumonia, the resulting CD4+ TRM cells concentrate selectively in the previously infected lobe, rather than dispersing throughout the lower respiratory tract (459). Because systemic immunity applies equally to the entirety of the respiratory tract yet TRM cells are restricted to a single lobe, this provides an elegant opportunity to examine the contributions of resident memory above and beyond the combined abilities of all central memory cells, circulating antibodies, and other components of systemic heterotypic adaptive immunity. The lobe with TRM cells demonstrates far superior lung defense against virulent pneumococci compared with the contralateral lobes without TRM cells (FIGURE 7). Trained innate immunity (363), such as improved AM functions, could additionally contribute to the localization of heterotypic immune defense after lobar pneumonia, but the fact that depletion of CD4+ cells compromises such defense (459) highlights a TRM cell role. Thus lung TRM cells protect against diverse types of respiratory pathogens, and the lung protection characteristic of young adults with naturally acquired heterotypic immunological memory cannot be provided by systemic adaptive immunity alone.
FIGURE 7.

The site of respiratory infection determines where protection resides. After pneumococcal lobar pneumonia in mice, CD4+ resident memory T (TRM) cells are found 1–2 mo later in the previously infected lobe rather than contralateral lobes. Polyclonal stimulation of cells collected from the different lobes shows that CD4+ T cells are poised to elaborate Th17 cytokines only in the previously infected lobe. Furthermore, the previously infected lobe is significantly more protected against infection by pneumococci of a different serotype compared with contralateral lobes. [Adapted from Smith et al. (459).]
Human lungs contain TRM cells. A 2011 publication analyzing lobectomy samples from lung cancer patients revealed that the noncancerous regions of lung tissue include an unexpectedly large number of T cells (401), and this has now been reproducibly found in human lung tissues without cancer or any pulmonary disease, from young children through senior citizens (433, 487, 488). These lung lymphocytes include both CD4+ and CD8+ T cells (433, 487, 488). While imperfect, CD69 expression on nonactivated T cells is used as a marker for TRM cells (134, 434), and lung T cells tend to express CD69 (401, 433, 487, 488). Memory T cells begin accumulating in human lungs as early as infancy (487, 488). T cells from human lungs are more likely to proliferate in response to influenza virus antigen presentation than are T cells from the blood or the skin (401), suggesting antigen specificity may be skewed towards respiratory pathogens. While cytokine expression from human lung TRM cells stimulated by presentation of microbial antigens has not (to our knowledge) been reported, pneumococcus presentation to mouse lung TRM cells (459) induces the MHCII-dependent expression of a wide variety of cytokines (including IL-17A, IFN-γ, TNF-α, IL-2, and more), and the antigen-independent polyclonal activation of human lung-derived T cells (401, 433, 487, 488) stimulates the coexpression of many cytokines (e.g., IFN-γ, TNF-α, and IL-2 together), altogether suggesting a predilection for “multifunctional” phenotypes. Thus the human lung contains a preponderance of TRM cells, which likely have specificity for respiratory pathogens and exert multifunctional roles.
The CD8+ TRM cells have been the most extensively studied resident memory cells in the lung, largely by characterizing the cells found in human lungs or by influenza infections of mice. The heterotypic protection against influenza provided by CD8+ TRM cells depends on IFN-γ expression, which is more rapid from TRM cells compared with circulating effector memory T (TEM) cells (323). CD8+ TRM cells from human lungs have distinct transcriptomes compared with CD8+ TEM cells from the blood, including steady-state expression of mRNAs for effector molecules, suggesting that the lung cells are not just better-positioned anatomically but also are better poised molecularly to respond quickly during lower respiratory infection (208) (FIGURE 6). Distinct patterns of expression for chemokine receptors and adhesion molecules (208) may contribute to the prolonged lung localization of CD8+ TRM cells in the lungs. After influenza infection in mice, CD8+ TRM cells reside selectively at sites of prior damage to the tissue (482). The maintenance of CD103+ CD8+ TRM cells in the lung requires signaling from Notch, low levels of T-bet, and the IL-15 receptor (208, 302). Administration of 4–1BB ligand amplifies CD8+ TRM cell accumulation after mucosal vaccination, while 4–1BB-deficient CD8+ T cells fail to seed the lungs after recovery from influenza infection (572), suggesting a requirement for this costimulation pathway. The development of CD103+ CD8+ TRM cells in the lungs after influenza infection also requires IFN-γ from helper CD4+ T cells (276). Whether the pathways described above are broadly applicable, for example, to multiple types of respiratory infection or to CD4+ lung TRM cells, demands more study. The fundamental biology of lung TRM cells, and how lung TRM cells are normally involved in resistance and susceptibility to diverse respiratory infections as well as the roles of alterations in lung TRM cells in diverse populations of susceptible or resistant hosts, remains poorly defined and presents especially great promise for improving our understanding of pneumonia defense.
C. Other Long-Term Changes to Lung Cells After Acute Pneumonia Infection
In addition to changes in adaptive immunity as outlined above, other persistent changes to lung tissue (FIGURE 5) have been noted to result from resolution of respiratory infection, potentially relevant to immune resistance against subsequent pneumonias. Severe respiratory infections can cause BALT formation, and these tertiary lymphoid organs can contribute to local antimicrobial defense (412). Presumably because of the infections experienced, the co-housing of laboratory mice with mice purchased from pet stores is sufficient to thereafter increase the numbers of innate lymphocyte populations in the lungs, including ILC1, ILC2, ILC3, and γδ-T cells (34). Infection with influenza virus is capable of altering the phenotypes of alveolar macrophages and epithelial cells in the mouse lung for months afterwards (107, 247, 394). The degrees to which BALT, innate lymphocyte accumulation, and such remodeling of epithelial cells and macrophages reflects chronic inflammation, trained immunity (363), aberrant repair, or all of the above, and the factors influencing these and other long-term changes in the lung occasionally observed after respiratory infection, are largely unclear.
V. TISSUE RESILIENCE AND PNEUMONIA
Like the local and remote resistance pathways outlined above, biological processes driving tissue resilience also include input from intra- and extrapulmonary sources. The shared goal of these signals is to limit injury resulting from all aspects of infection, which requires countermeasures to damage elicited from the microbes, as well as that from the host response (i.e., immunopathology). Failure to achieve this goal progresses pneumonia to ARDS and sepsis (19, 315). Two major avenues for fortifying tissue resilience in the airspaces are events that 1) provide negative feedback on inflammation, which left unchecked can cause injury; and 2) retain the function and number of viable lung parenchymal cells to ensure an environment supportive of gas exchange. Some of these events will be highlighted below, with consideration to both intra- and extrapulmonary contributions.
A. Resilience in the Lungs
An excess of the signals described above as promoting immune resistance against microbes can be dangerous to the lung tissue itself. Perhaps the most direct and immediate element controlling the magnitude of this response is the size and strength of the initial stimulus, which wanes as a consequence of adequate defense. But the self-limiting nature of infection, or lack thereof, cannot apply a sufficient level of control to ensure tissue protection. This is accomplished, in part, through inducible processes that actively limit innate immunity and acute pulmonary inflammation. For example, classic anti-inflammatory cytokines such as IL-10, transforming growth factor (TGF)-β, and IL-1 receptor antagonist (IL-1RA) are sufficient and necessary to reduce innate immune responses and inflammatory lung injury during pneumonia (94, 170, 195, 505, 507). Of course, this comes with the risk of overly blunting immune resistance and exacerbating infection (507), highlighting the complexity of achieving effective but balanced immune responses to invading pathogens.
Just as AMs are central for establishing pulmonary immune resistance, they are also essential for tissue resilience. The activation state of AMs is tightly regulated by their microenvironment (211). In a state of baseline homeostasis, engagement of CD200R, TGF-βR, and IL-10R by their corresponding ligands at the epithelial surface is an important negative regulator of alveolar macrophage activity (211), desensitizing these cells to innocuous environmental particles common to the airspaces. As the alveolar microenvironment is altered (e.g., during infection), AMs become activated and can exhibit M1 (classical) or M2 (alternative) characteristics, with the former more typically bearing the pro-inflammatory features highlighted in the aforementioned section on immune resistance (4). On the other hand, alternatively activated (M2) AMs generate anti-inflammatory factors such as IL-10 and IL-1RA, and the size of this cell population has been shown to expand during the resolution phase of pneumonia, helping to restore tissues to baseline homeostasis (93, 238, 525).
In addition to fine-tuning inflammation through the release of anti-inflammatory cytokines, an essential role of macrophages in the aftermath of an acute inflammatory event is to remove accumulated debris, a large amount of which includes dead or dying leukocytes. This is largely achieved through phagocytosis of apoptotic cells presenting increased levels of phosphatidylserine and other “eat me” signals on their surface, in a complex and tightly regulated process known as efferocytosis (192, 193). Efferocytosis steers the environment away from an accumulation of necrotic cells, which are inherently pro-inflammatory. Additionally, efferocytosis actively reprograms AMs to release a suite of anti-inflammatory mediators (193), limiting the likelihood of immunopathology. While this includes cytokines like TGF-β (212), it also involves the release of pro-resolving lipid mediators such resolvins, lipoxins, maresins, and protectins (285). These factors elicit resilience responses, including but not limited to inflammatory cytokine regulation, epithelial repair, and efferocytosis itself (28). Pro-resolving lipid mediators limit immunopathology in numerous settings of viral and bacterial pneumonia (28), and recent evidence suggests that inhibition of these pathways may even serve as a virulence determinant for P. aeruginosa and perhaps other important causes of pneumonia (140).
Use of in situ imaging has compellingly revealed a population of nonmotile macrophages in the airspaces, which also appear to have a major influence on tissue resilience (534). These “sessile” macrophages remain adherent to the alveolar surface, where upon stimulation they employ gap junctions to propagate immunosuppressive Ca2+ waves throughout the epithelium. Disruption of these connexin-43-containing gap junctions is sufficient to amplify cytokine expression and alveolar neutrophilia in response to bacteria or bacterial stimuli (534), suggesting that these interactions apply negative feedback to reduce injury in the setting of acute inflammation. Notably, the identification of sessile macrophages challenges existing paradigms of alveolar macrophage biology, produced by decades of studies focused on macrophages lavaged from the airspaces (38). Only a fraction of alveolar macrophages are recovered by bronchoalveolar lavage, which may be a consequence of differential lavageability among several functionally distinct macrophage subsets. The alveolar macrophages that are connected to epithelial cells via gap junctions and not collected by bronchoalveolar lavage may have specialized roles in lung resilience during pulmonary inflammation.
The alveolar-capillary barrier is maintained and restored by the presence of tight junctions and the expression of epithelial Na+ channels (e.g., ENaC and Na+-K+-ATPase) and other membrane transporters (e.g., CFTR and aquaporin 5) which actively limit the airspace liquid accumulation characteristic of pneumonia (37). These surface proteins are dynamically regulated by host factors such as cAMP agonists, glucocorticoids, thyroid hormone, and TRAIL, and disruption of these pathways can enhance acute lung injury (37, 389).
Structural integrity of the epithelial barrier also relies on cellular viability, which is actively supported during pneumonia by tissue-protective signaling networks. STAT3 activity is a critical determinant of epithelial resilience, enabling this cell population to better withstand the barrage of toxic stimuli accrued at the air-liquid interface in an infected lung. Mouse models employing targeted lung-specific epithelial STAT3 deficiency have consistently demonstrated a requirement for this transcription factor to limit cytotoxicity and acute lung injury in response to a variety of stimuli, including virus (314), bacteria (405), LPS (215), hyperoxia (206), and naphthalene (252). Mechanisms of STAT3-mediated epithelial protection likely involve, at least in part, the induction of gene programs that inhibit apoptosis and drive tissue repair (252, 314). Conversely, gain of STAT3 function in lung epithelium can limit acute lung injury, as shown in the setting of hyperoxia (287), but protective STAT3-dependent signals require precise regulation given the potential for the development of epithelial adenocarcinoma (286). Host-derived signals upstream of epithelial STAT3 activation, therefore, represent an important control point for guiding tissue resilience during pneumonia. Leukemia inhibitory factor (LIF) may represent a particularly prominent player in this regard. LIF is induced in lung epithelial cells in response to infection (144, 405, 496), and it is necessary and sufficient to activate lung epithelial STAT3 (409). Pharmacological blockade of LIF causes significant injury in response to bacterial and viral pneumonias (144, 409). Not only does this phenocopy the consequence of epithelial STAT3 inhibition (405), but it is also notable that LIF neutralization does not impact pathogen burdens (144, 409), suggesting that the benefits of LIF are solely attributable to increased tissue resilience. In addition to STAT3, other contributors to epithelial protection and/or repair in the setting of lung infections include β-catenin (568, 569), FOXM1 (293), and p63 (273). The promising and burgeoning area of stem cell-mediated tissue regeneration, while still nascent, is also likely to reveal important mechanisms of epithelial barrier control as the field develops (247, 266).
In addition to its viability and fluid clearance, the epithelium of the lung has significant resilience roles in producing anti-inflammatory signals. SAM Pointed Domain Containing ETS Transcription Factor (SPDEF) is best recognized for driving mucus metaplasia (68), but a nonpathological role of SPDEF is to limit inflammatory gene expression downstream of MyD88 and TRIF (264). The goblet cell promoting factor transcription factor FOXA3 also diminishes antiviral immune resistance gene programs (67). And some mucus proteins exhibit anti-inflammatory roles in the pneumonic lung (254), as highlighted by protective immunosuppressive effects of MUC1 following P. aeruginosa infection in mice (506).
While AMs and lung epithelium represent resident sources of pulmonary resilience, recruited leukocyte populations also have the capability to elicit tissue protective responses. For example, recruited exudate macrophages have been identified as an important source of IL-1RA-dependent prevention of epithelial apoptosis and inflammatory injury in mice with pneumonia caused by LPS or K. pneumoniae (195). Macrophages support the growth of alveolar epithelial cells and of lung epithelial “pneumospheres” in vitro (279), and the CCR2-mediated migration of blood monocytes into lung tissue is essential to maximizing lung regeneration after pneumonectomy (279), together suggesting that the repair of lungs injured by infection may be downstream of monocyte recruitment; although not tested in pneumonia yet, an emerging body of evidence consistently suggests that type 2 immune signals drive macrophage-mediated tissue repair after diverse infections and insults, dependent on macrophage receptors including IL-4Rα and myosin 18A (44, 279, 333). Neutrophils, perhaps the most prototypical “proinflammatory” cell type, cause elastase-dependent activation of a cytoprotective β-catenin response as they migrate across the epithelial barrier (568, 569). Myeloid-derived suppressor cells (MDSCs), which bear resemblances to neutrophils, also accumulate in pneumonic lungs, where they limit immunopathology through synthesis of IL-10 and enhanced efferocytosis of apoptotic neutrophils (396). Recruited cells derived from lymphoid progenitors can also limit inflammatory injury in the lungs. Regulatory T cells (Tregs) accumulate in the lungs of patients and mice with lung injury, and these cells are sufficient and necessary to curb inflammatory cytokine induction and to stimulate resolution in mouse models of acute pulmonary inflammation (5, 94). Antiviral effector T cells, including particularly CD8+ but also CD4+ T cells, can be important additional sources of IL-10-mediated tissue protection during respiratory viral infections (477, 478), thereby exhibiting both pro-resistance and pro-resilience characteristics. Recruited ILC2s can limit immunopathology during pneumonia, as evidenced by their accumulation in the airspaces of mice and humans following influenza infection, combined with their release of the tissue protective factor amphiregulin (338). NK cells can also be tissue protective, producing IL-22 to mediate epithelial repair in response to viral or bacterial lung infections (272, 276, 549). Thus resident and recruited cell types enhance intrapulmonary resilience during pneumonia (FIGURE 8).
FIGURE 8.
Multiple different cell types provide activities to help accomplish tissue resilience and prevent lung injury during pneumonia. Examples shown are not an exhaustive list and pictorialize some of the mechanisms described in text.
B. Resilience From Outside the Lungs
Tissue resilience of the lungs, like immune resistance (see above), can originate from extrapulmonary sources. The brain is one example. As discussed above, brain-dependent processes such as fever and reflex control appear to promote immune defense in the lungs (122, 186), but the central nervous system can also function to limit inflammation potentially reducing immunopathology. For instance, neuroendocrine control of immunity, such as that achieved by autonomics and the hypothalamic-pituitary-adrenal (HPA) axis, is well appreciated (372). Crosstalk between the sympathetic and parasympathetic activity has been attributed to myriad immunological processes, with ACh and catecholamines, particularly norepinephrine, now mechanistically linked to the suppression of cells across the immune system, including but not limited to macrophages, dendritic cells, T cells, and B cells (372). Meanwhile, glucocorticoids derived from the HPA axis are quintessentially anti-inflammatory. The anti-inflammatory effects of glucocorticoids on CXCL5 expression and neutrophilic inflammation in the lungs involve circadian rhythms in airway epithelial cells (156). While the direct impact of endogenously synthesized glucocorticoids such as cortisol on pneumonia is not entirely clear, the HPA axis has long been a target for pharmacological intervention. Many studies have examined the application of corticosteroids for pneumonia patients. While plausible that steroids may pose risk for pneumonia patients (422), results have suggested general trends towards a protective effect, perhaps reducing the risk of ARDS and shortening hospital stays (523). Because it entails risk and only subsets of patients may respond favorably, precision medicine may be needed to apply corticosteroid therapies most effectively for pneumonia. Consistent with this premise, a recent clinical trial showed that severe community-acquired pneumonia patients with the highest levels of inflammation (based on serum CRP) showed significant reductions in treatment failure if they received methylprednisone (494). Investigations in animal models have shed further light on functional connections between neural input and pulmonary immune responses. Vagal denervation reduces acute pulmonary inflammation and mortality in mice with pneumonia induced by E. coli, likely owing to decreased engagement of the anti-inflammatory α7 nicotinic acetylcholine receptor (α7 nAChR) on alveolar macrophages and neutrophils (476). This is further supported by enhanced injury in mice lacking α7 nAChR itself under the same conditions (476). In another example of brain-derived lung resilience, adrenalectomy impairs circadian suppression of airway epithelial cells and exaggerates the lung CXCL5 response, seemingly due to disruption of glucocorticoid receptor occupancy (156), which is consistent with the possibility of an endogenous HPA-dependent reduction of inflammatory injury. Similarly, adrenalectomy promotes inflammatory injury following intrapulmonary challenges with LPS or immune complexes, although this could be more attributable to modulation of catecholamine responses rather than glucocorticoids based on blockade studies of the latter (139). A recently identified anti-inflammatory role of pulmonary neuroendocrine cells also suggests neural regulation of lung immunity (49). These innervated lung epithelial cells release excessive neuropeptides and enhance pulmonary inflammation when their organization is disrupted by targeted mutation of the Robo receptor (49), suggesting that the organization and function of these innervated cells is essential to curbing inflammatory responses to pulmonary challenges such as infection.
Some of the APPs produced in the liver in response to pulmonary infection serve to counter inflammatory injury. Perhaps the most revealing example of liver-dependent tissue protection in the lungs is the pathological consequence of α1-antitrypsin (AAT) deficiency. Patients with this inherited disorder can present with severe liver and lung injury, the latter of which can result in COPD, bronchiectasis, and increased pneumonia incidence (172). Therapeutic treatment with AAT reduces inflammatory injury in patients and animal models (63, 241), and this protective effect may extend beyond AAT’s seminal function of inhibiting neutrophil elastase. Liver-derived APPs contributing to metal homeostasis may also influence pulmonary tissue resilience. For example, in addition to their roles in immune resistance (e.g., bacteriostatic iron sequestration) (235), iron-handling proteins also prevent iron toxicity, oxidative stress, and lung injury (155). Thus it is feasible that APPs including but not limited to antiproteases and those regulating metal homeostasis confer liver-dependent tissue protection in pneumonic lungs. An indirect example of liver-dependent immunoregulation in the lungs relates to MDSC mobilization. Egress of these anti-inflammatory cells from the bone marrow requires gp130-dependent liver activity during sepsis (430), suggesting that the liver may be a requisite intermediate for the lung-protective roles of MDSCs observed in pneumonia (396).
Extrapulmonary organ injury is a common sequela of severe pneumonia, posing concern for sepsis secondary to pulmonary infections (320) and indicating that signals governing extrapulmonary tissue resilience are critical to pneumonia outcome. Liver injury is a prominent feature of sepsis (471). As discussed above, hepatic acute phase changes occur in response to lung infections in mice (403, 404). Pneumonia-induced transcript changes in hepatocytes include hepatoprotective means to limit liver injury, and disturbance of such transcriptome remodeling by interruption of either RelA or STAT3 in hepatocytes can lead to hepatotoxicity (8, 201). The ER stress and antiapoptotic pathways induced by STAT3 and RelA in hepatocytes are examples of inducible tissue resilience outside of the lungs. Increased gut permeability, which is both a cause and consequence of sepsis (256), is also regulated during pneumonia. Results from animal models recapitulate pneumonia-induced intestinal injury and provide evidence for its occurrence being dictated by signals including surfactant proteins A and D (117), PARP (295), EGF (114), Bcl2 (210), and p53 (87). Acute injury to the kidneys and brain is also associated with sepsis, and increased damage to either organ occurs up- and downstream of that of the lung (345, 435, 453). In the heart, pneumococcal pneumonia can lead to bacterial growth and macrophage necroptosis within the mycocardium that leads to acute injury and prolonged scarring (56, 159, 417). The mechanistic basis for most of the crosstalk between these tissues and the lungs is poorly understood, requiring further investigation into biological pathways that promote or limit damaging interactions. Further insights regarding extrapulmonary tissue resilience will be essential for illuminating physiological determinants preventing disseminated injury and sepsis during pneumonia.
VI. LUNG MICROBIOME AND PNEUMONIA
While the lung was previously considered to be a sterile organ, a microbiome in the lung has become appreciated due to culture-independent techniques for detecting, identifying, and quantifying bacteria (105). The bacteria in the lungs of healthy individuals are likely transients that represent a balanced equilibrium of incoming bacteria (from inhalation, aspiration, etc.) with bacterial elimination (by resident innate immunity including the mucociliary escalator, alveolar macrophages, and biochemical components in lung lining fluids, as described above). While healthy lungs are not likely a niche for stable populations of resident bacteria growing there, they are never sterile and those microbes in the lung have physiological consequences (105).
In healthy individuals, the microbiota in the lung are similar to that of the mouth (29, 513). The bacteria are most abundant in the largest airways and diminish with increasing distance into the respiratory tract, suggesting an oral source for the lung microbiome during health (209). Healthy subjects with greater amounts of this microbiota in their air spaces have evidence of low level subclinical inflammation in their BAL fluid (439), implying that recruited immunity may at times need to supplement resident immunity to control lung microbiota and prevent pneumonia. Consistent with the notion that exposure to respiratory pathogens is not sufficient to cause pneumonia, it is not unusual to find bacterial agents that cause pneumonia in the lung microbiome of healthy individuals (127, 202, 337).
One perspective on pneumonia is to frame it as a shift in the lung microbiome (105). During pneumonia, the lung microbiome becomes dominated by the etiologic agent causing infection (105, 221, 385, 492). Thus the pneumonia microbiome is differentiated by low microbial diversity, high microbial biomass, and local bacterial growth (105). A feature that may differentiate the bacteria that cause pneumonia (FIGURE 1) from other bacteria of the normal respiratory tract microbiome is that the causative agents of pneumonia better thrive in a setting of lung inflammation (with differences in nutrients, temperature, biochemical and immune components, etc.). Subsets of organisms within the respiratory microbiome may be better adapted to growth in such environments, and these may be most likely to be causes of pneumonia (209).
In addition to providing agents that cause pneumonia, the microbiome importantly influences pneumonia susceptibility and outcome. Microbes in the respiratory tract microbiome can limit or favor growth of the causative agents of pneumonia (105, 306, 448, 450, 535). Furthermore, the respiratory microbiome shapes the immune system that defends the lungs against pathogens (209, 306). The lung microbiome and lung immunity are both influenced by extrapulmonary microbiomes (58, 81, 106, 190, 209), including that of the gastrointestinal tract as detailed above, highlighting roles of microbiota outside the lungs affecting pneumonia.
Finally, the lung microbiome is altered in chronic pulmonary diseases (105). As discussed below, chronic pulmonary diseases increase the risk of pneumonia, and pneumonia accelerates the course of chronic pulmonary diseases. Changes in the lung microbiome may mediate some of these relationships between chronic pulmonary diseases and pneumonia.
VII. PNEUMONIA SUSCEPTIBILITY
We propose a change in how pneumonia is approached, that the medical community should address pneumonia as a chronic condition of susceptibility rather than merely an acute infection. The argument for such a shift in approach is motivated by the inspiring successes from the cardiovascular community. Deaths from infarction (i.e., heart attacks and strokes) are presently a third of their levels from a half-century ago (353), contrasting sharply with the unchanging mortality rates for pneumonia over this time-frame (20, 147, 290, 336). Both pneumonia and infarction are acute events with disastrous consequences. Infarction is attacked with blood-thinners and anticoagulants that prevent or eliminate clots, analogous to attacking pneumonia using vaccines and antibiotics that prevent or eliminate infections. However, so much more is done to address the underlying chronic disease predisposing to acute infarctions. With so little biological understanding at present, little or nothing can be done to address the underlying chronic disease predisposing to acute pneumonia.
A pivotal event in cardiology was the demonstration that subjects who would later get infarctions already had measurable biological changes: higher blood pressure and serum cholesterol (248). This discovery led to individualized risk factor assessments, behavioral changes (e.g., in diet, exercise, and smoking) designed specifically to alter the relevant risk factors, and the use of drugs such as beta-blockers and statins that target risk factors (rather than acute clots) and thereby lower infarction risk (353). Successful prevention of infarctions results in part from treating the underlying chronic disease process. The approach to pneumonia should move in similar directions. Finding ways to measure and interfere with the chronic processes underlying pneumonia susceptibility should be major goals for the coming years. The ability to approach pneumonia by differentiating those with susceptibility and effectively treating that susceptibility has exceptional promise for diminishing the burden of pneumonia.
So who gets pneumonia? Who are the susceptible? As detailed above, young children and older adults have higher rates of pneumonia then those aged in-between (FIGURE 9A). Multiple clinical and behavioral conditions have been identified as risk factors for pneumonia (discussed further below), and these conditions associate with increased pneumonia risk across the age spectrum (FIGURE 9A) (384). However, while those with recognized risk factors have higher rates of pneumonia, this results from an increased degree of susceptibility rather than a switch from resistant to susceptible. People get pneumonia at every age, even without recognized risk factors (FIGURE 9A). About half of children hospitalized with pneumonia have recognizable underlying conditions, especially premature birth or asthma, while the other half do not (228). Similarly, young adults with pneumonia more often do not have recognized risk factors (FIGURE 9B). In contrast, most older adults hospitalized with pneumonia have underlying conditions that are known risk factors for pneumonia (FIGURE 9B), including chronic respiratory, cardiovascular, neurologic, or metabolic diseases (227). A large fraction of aging adults suffer from one or more of these chronic conditions, tilting the demographics of pneumonia towards the aging group, resulting in a disproportionate number of pneumonia cases in the 60-and-over cohort (FIGURE 9B). Despite this, among adults hospitalized for pneumonia in the US, the majority (around two-thirds) are younger than 65 yr of age (227). This is probably because the chronic conditions that increase pneumonia susceptibility begin in middle age, and there are more middle-aged than elderly people (e.g., over twice as many 55 as 75 yr olds in the US, based on 2016 census data). Finally, only a fraction of the population with any of the recognized risk factors will get pneumonia, but there are presently no means of predicting which subjects within these risk groups are most likely to get pneumonia. Underlying conditions or risk factors are pivotal, but not well understood. We summarize here some of the currently recognized connections between underlying factors and pneumonia susceptibility. However, this is an area that needs more research. Deeper epidemiologic and especially physiological insights are needed before we can recognize, prevent, or reverse pneumonia susceptibility.
FIGURE 9.
Known risk factors for pneumonia are relevant and important, but insufficient to differentiate the susceptible from the resistant. Figure panels were generated from data reported by Pelton et al. (384), representing analyses of 3.4 million individuals in Germany from 2008 to 2012. A: pneumonia rates for individuals stratified by age group and risk factors. For all age groups, the recognized risk factors associate with elevated rates of pneumonia. Rather than susceptible versus resistant, these risk factors associate with an increased pneumonia susceptibility up to severalfold. Even those without recognized risk factors get pneumonia. For all risk groups, pneumonia rates are highest at either end of the age spectrum. B: the distribution of all pneumonia cases among age groups and risk groups. The majority of pneumonias occur in older individuals, most of whom have recognized risk factors. For younger subjects, including children and adults through age 49, more pneumonias are in subjects without recognized risk factors than in those with recognized risk.
A. Age
Although pneumonia can occur across a lifespan, the very young and old are at highest risk. Both the old and young have impaired immune responses to pulmonary pathogens, as well as increased risk of pathogens breaching impaired anatomic barriers to the lower respiratory tract, such as aspiration of oral or gastric contents. The combination of poorer immune clearance and increased alveolar pathogen burden potently increases age-associated risks for developing pneumonia.
Young children have scant prior history with pathogens, leaving them less protected by adaptive immune memory and more dependent on innate immune responses (as detailed above). Their lack of heterotypic immunologic memory against the etiologic agents of pneumonia is likely a predominant predisposing factor for childhood pneumonia. In addition, many specific immune alterations have been noted in the very young, including altered phagocyte function and cytokine production by their AMs (219, 274, 275), a preponderance of Treg cells in their lungs (487), different patterns of cytokine release by their blood leukocytes (261), and altered recruitment and activity of their ILCs during respiratory infection (432). While both are involved, it is unclear whether getting older or gaining experience with microbes (34) is most responsible for “maturing” these and other immune parameters in young children.
Whereas the very young have immature and inexperienced immune systems that predispose to development of pneumonia, excessive activity and chronic inflammation may paradoxically impair immune responses and contribute to pneumonia risks in the aged (45). There is evidence of smoldering inflammation in the aging lung as well. In one study of bronchoalveolar lavage fluid, healthy volunteers older than 65 yr old had increased neutrophil, immunoglobulin, and IL-6 concentrations when compared with younger volunteers (329). The chronic inflammatory milieu of aging induces multiple changes that contribute to increased pneumonia risk. Chronic inflammation increases pathogen adhesion to host cells (92), induces tolerance of TLRs (107), impairs monocyte pathogen clearance (399), and blunts pulmonary innate immune responses to S. pneumoniae (203). In short, the cells of the lung and their inter-communication that is necessary for coordinated immune responses become dysregulated with advancing age (50). In addition, multiple changes in the adaptive immune system associate with aging and reflect a growing state of immunosenescence with diminished efficacy of responses to diverse microbial challenges (166, 322, 557), which may further contribute to the increased susceptibility to pneumonia among the elderly. Modification of compromised immunity resulting from the chronic inflammation and/or immunosenescence of aging may represent targets of future pneumonia risk reduction, once better defined.
B. Comorbidities
At any age, a wide spectrum of comorbidities associates with increasing risk of pneumonia (FIGURE 9). While pneumonia rates are two- to threefold higher in those with comorbidities compared with those without, it is also evident that only a small fraction of those that are young or old and have high risk conditions will get pneumonia in a given year, on the order of 3,000 per 100,000 or 3% per year (FIGURE 9A).
Multiple environmental and behavioral exposures act together to increase pneumonia susceptibility. Tobacco smoke represents the most important modifiable risk factor for pneumonia (368), with nearly one in three pneumonia cases attributable to tobacco smoking (14). Both active and passive tobacco smoke exposures increase nasopharyngeal pathogen (e.g., S. pneumoniae) carriage (281, 366) and alter acute respiratory tract immune responses. Short-term exposure to tobacco smoke disrupts airway mucociliary clearance, alters interferon response to viral pathogens, and attenuates alveolar macrophage, natural killer cell, and dendritic cell responses to pathogens (464). Chronic exposures to cigarette smoke substantially derail antibacterial adaptive immunity, diminishing bacteria-specific antibodies (both IgA and IgG in the BAL fluid and IgG in the blood) and skewing bacterial antigen-induced T-cell responses (whether from lung or spleen) towards IL-17 and away from IFN-γ and IL-4 (300). Such changes worsen host defense while exaggerating inflammation. Mechanisms by which cigarette smoke alters immune cells are multifaceted, involving many diverse particulate materials, toxic chemicals (acrolein, acetone, benzopyrenes, methylcholanthrene, etc.), catalytic agents, noxious gases, and more in cigarette smoke (464). Many of the >4,000 components of cigarette smoke are individually capable of dysregulating immunophysiology, and the habit of cigarette smoking ensures prolonged exposures to large doses of these in combination. High levels of secondhand smoke or of air pollution also associate with increased risk for and severity of pneumonia (7, 364, 538). These exposures contain similar particulate matter and toxic compounds like acrolein, so pneumonia susceptibility due to secondhand smoke and air pollution may be from overlapping mechanisms with tobacco smoking.
Long-term tobacco and poor air quality exposure leads to chronic lung diseases that further exacerbate pneumonia risks. COPD is the most common clinically observed pathological response to long-term exposure to noxious inhaled particles. COPD is characterized by development of fixed airway obstruction, emphysematous remodeling of the alveolar air spaces, and mucus metaplasia with impaired mucociliary clearance in the conducting airways. The AMs of COPD patients adapt to increased oxidative stress by increasing expression of the anti-apoptotic protein Mcl-1, which diminishes antibacterial efficacy of these cells and may be a biological factor contributing to pneumonia susceptibility in these patients (36). Responses of COPD lungs to pathogens are further influenced by alterations in the airway microbiome (305) as well as COPD treatments such as inhaled and systemic immunosuppressive corticosteroid therapies (138, 251). This leads to a three- to fourfold increased risk for pneumonia in COPD patients (444, 493). Smoking cessation reduces the elevated pneumonia risk for COPD patients, but it is not sufficient to eliminate it, and COPD patients maintain an excessive pneumonia susceptibility despite smoking cessation (12). Importantly, patients with COPD who get pneumonia experience acute decrements of lung function that worsen COPD severity and chronic inflammation, both of which increase risk for future pneumonia and perpetuate a cycle of COPD decline (444).
Asthma, defined by reversible airways obstruction, is associated with a 1.5- to 2-fold increased risk for pneumonia, substantially lower than COPD (216). Reasons for lower rates of pneumonia associated with asthma as compared with COPD likely include the younger age, less tobacco smoke exposure, and different anatomical pathologies observed in patients with asthma as compared with COPD. Factors predisposing asthma patients to pneumonia include airways with excessive mucus and immune profiles skewing towards anti-helminthic defense and allergic responses rather than protection against bacteria and viruses (132, 207).
In addition to the increased risk of pneumonia accompanying chronic lung diseases, comorbid conditions involving other organ systems also increase pneumonia risk. Despite the substantial differences in each condition, diabetes mellitus, chronic liver disease, kidney disease, and heart failure all show strong increases (e.g., 1.5- to 4-fold) in pneumonia risks (216, 493). The hyperglycemia of diabetes impairs neutrophil chemotaxis (100) and superoxide-mediated antimicrobial effects (383), although clinical trials of strict control of hyperglycemia fail to demonstrate reductions in pneumonia (6, 486b), implying additional mechanisms contributing to susceptibility. Impaired neutrophil function during liver disease (40) is in part related to tuftsin deficiency (499). Tuftsin modulates activities of phagocytic cells, but requires activation in the spleen; splenic congestion from cirrhotic liver disease is hypothesized to impair tuftsin activation. Uremia during chronic kidney disease impairs neutrophil intracellular killing through unclear mechanisms; however, dialysis partially restores neutrophil function (17). Mechanisms of increased pneumonia risk associated with heart failure have not been experimentally elucidated, although some hypothesize that chronic pulmonary edema may generally impair neutrophil and monocyte chemotaxis and function. Importantly, chronic diseases cluster together (e.g., diabetes is a risk factor for liver and kidney disease as well as heart failure). With the exception of targeted vaccination against influenza and pneumococcus, methods to reduce pneumonia risks among patients with chronic disease are unclear and warrant further study. Understanding the pathophysiological interactions between multiple chronic diseases that lead to pneumonia may identify novel opportunities to interrupt etiological pathways.
Drug and alcohol abuse, dementia, and stroke are also associated with increased risks of pneumonia (168). Increased pneumonia risks associated with these conditions that result in altered mental status occur through immunosuppression, altered host microbiome, and disruption of physical barriers that allow larger burdens of pathogens entry into the lower respiratory tract. Alterations in upper airway reflexes and depressed mental status increase dysphagia risk and the number of pathogens aspirated into lung in patients with either acute or chronic conditions that impair mental status. The risks for pneumonia in conditions disrupting basic anatomical airway protection are further exacerbated by alterations in nasopharyngeal pathogen carriage and local immune suppression, including impairments in alveolar macrophage function with alcohol and drug abuse (57, 288, 325, 326, 424). For example, chronic alcohol ingestion results in oxidative mitochondrial stress leading to dysfunctional alveolar macrophage phagocytosis and increased risks of ARDS during pneumonia; both opiates and opiate withdrawal syndromes have protean immunosuppressive actions involving innate and adaptive immunity (424). Effects of alcohol consumption on redox imbalance and oxidative stress in alveolar macrophages have been attributed, in part, to diminished glutathione availability (57, 95, 288, 560). The inverse relationship between alcohol consumption and glutathione levels has been observed in patients and animal models, and more recent studies have indicated that glutathione supplementation can be used to circumvent alcohol-related macrophage dysfunction (326, 344, 560). Acute neurological insults such as stroke act through vagal α7 nAChR-mediated anti-inflammatory pathways to increase pneumonia risks. Increased vagal tone after stroke activates α7 nAChRs on macrophages and alveolar epithelium to impair the innate immune response and promote pneumonia (126). Compounding risks for patients with substance abuse or dementia, neurological conditions, and impaired physical barrier defenses are comorbid nutritional deficiencies (including vitamin D, zinc, and protein-calorie malnutrition) that exacerbate innate immune dysfunction (27, 183, 296, 411, 420). The aging populace, heralding more strokes and dementia, plus the growing prevalence of opiate abuse suggest increasing burdens of pneumonia over coming years, putting added pressure on elucidating mechanisms by which these major risk factors predispose to pneumonia.
For young children, additional major factors associated with increased risk of pneumonia are factors common in areas of lower socioeconomic status, including malnutrition, vitamin deficiencies, incomplete vaccination, crowded living conditions, indoor air pollution/parental smoking, prematurity or low birth weight, lack of breastfeeding, and HIV infection (142, 225, 277, 365, 514, 542). Breastfeeding is considered one of the most cost-effective interventions to reduce childhood pneumonia, by supplying humoral and cellular adaptive immune components to the neonate with a naive and immature immune system. In addition to being immunocompromised from HIV, childhood comorbid conditions such as gastroesophageal reflux, asthma, and congenital heart diseases also increase pneumonia risk, although diverse mechanisms including increased penetration of pathogens to the lower respiratory tract, recurrent subclinical lung injury, airways disease, pulmonary edema, and chronic inflammation.
C. Acute Illness and Pneumonia
Healthcare-associated pneumonia represents an important and potentially preventable burden to the healthcare system (573). Pneumonia is the most common healthcare-associated infection in the US (303), and ventilator-associated pneumonias alone contributed approximately $3 billion in US healthcare costs in 2009 (573). Healthcare-associated pneumonias represent a ‟perfect stormˮ of pneumonia risk factors. Patients in the healthcare setting often have multiple comorbid conditions that increase pneumonia risks, disruptions to airway barriers from sedative medications and/or endotracheal tubes, sepsis-induced (41) and drug-induced immunosuppression, increased exposures to opportunistic and antibiotic-resistant bacteria, and an altered microbiome from recent antibiotic exposure. It is thus not surprising that hospitalized patients are at high risk for recurrent pneumonias: ~20% of patients hospitalized with pneumonia and 5% of patients hospitalized with sepsis, heart failure, or myocardial infarction are rehospitalized within 30 days with pneumonia (102, 397). Typical of pneumonias occurring during other acute illnesses, ventilator-associated pneumonias represent a pathophysiology driven by simultaneous disruptions to upper airway barrier protection, to lower airway clearance mechanisms, and to immunological responses. Despite efforts such as routine chlorhexidine-based oropharyngeal care and measures to prevent gastroesophageal reflux among patients requiring mechanical ventilation, ~1 in 10 patients requiring mechanical ventilation for more than 2 days develop ventilator-associated pneumonia (328). Endotracheal tubes and sedatives remove epiglottic airway protection and facilitate entry of oropharyngeal flora into the distal lung. Furthermore, preexisting lung injury from prior illness and damage due to mechanical ventilation, combined with the high prevalence of multidrug-resistant pathogens in the nosocomial setting, favors infection with pathogenic bacteria differing from the common causes of community acquired pneumonia, including Staphylococcus aureus, Enterobacteriaceae (especially Klebsiella and E. coli), Pseudomonas, and Acinetobacter (79). Immune suppression due to critical illness, such as impaired leukocyte glycolysis (511), expansion of myeloid-derived suppressor cells (504), and blunted type I interferon signaling (512) may contribute to the inability to mount an effective response to the increased bacterial load faced by the ventilated lung.
Acute viral upper respiratory infections may lead to superinfection with bacterial pathogens in the absence of mechanical ventilation and impaired upper airway protection. Bacterial infections (most commonly S. pneumoniae) complicated nearly all deaths resulting from the 1918 H1N1 influenza pandemic (321, 342) and 25–50% of severe influenza deaths during the 2009 H1N1 pandemic (158). Many mechanisms for bacterial superinfection after viral infection are shared with ventilator- and hospital-acquired pneumonia during critical illness, including epithelial damage increasing susceptibility to bacterial invasion, macrophage depletion and dysfunction, type I interferon dysregulation, and attenuated TH17 responses necessary for bacterial clearance.
The numerous and complex mechanisms by which acute viral infections transiently suppress innate and adaptive immune responses against bacteria in the lung have been excellently summarized by others (101, 242, 249, 321, 428). Most current knowledge relates to interactions between influenza infections and pneumonias caused by pneumococcus or S. aureus, but information may be greatest for these interactions because they have been examined more extensively because of special relationships among these organisms. Many immunological mechanisms for how influenza viruses predispose to secondary infections (101, 242, 249, 321, 428), such as epithelial damage, ineffective macrophages, exuberant interferons, and TH17 cell dysfunction, should also apply to viral infections other than influenza. Supporting such pan-viral generalization, mimicking viral signaling by administering noninfectious polyI:C (as a surrogate for viral double-stranded RNA) is sufficient to render mice susceptible to pneumonia caused by pneumococcus or S. aureus, dependent on signaling from type I IFNs (489) as was previously demonstrated for influenza virus-induced susceptibility (446). Viruses other than influenza, such as RSV and rhinovirus, predispose to secondary bacterial infections in animal studies and associate with secondary bacterial infections in patient studies (101). More than one-tenth of all hospitalized adult pneumonia patients in whom virus could be detected also have evidence of bacterial co-infection, and the fraction of pneumonias in which a copathogen is identified appears roughly similar across all the different viruses (227). Similarly, a plethora of bacterial agents beyond pneumococcus and S. aureus are identified in studies of secondary bacterial pneumonias, including H. influenzae, S. pyogenes, and more (101, 242, 249, 321). Thus, although many virus-specific and bacteria-specific contributions to bacterial superinfections after acute viral infection have been elucidated (249, 321), we suspect that most lower respiratory tract viral infections increase risk for most bacterial pneumonias, through immune pathways triggered by diverse viruses that compromise immune defenses against diverse bacteria.
In summary, aging, tobacco, alcohol or drug abuse, poor air quality, nutritional deficiencies, pulmonary disease, nonpulmonary comorbid conditions, and acute illnesses or exposures interact to increase pneumonia susceptibility. Conditions predisposing to pneumonia enhance susceptibility by increasing upper respiratory colonization, decreasing locally protective barrier mechanisms, and altering local and systemic innate and adaptive immune responses. Importantly, conditions that predispose to pneumonia tend to cluster within individuals and communities, further magnifying pneumonia risks. Because an incident of pneumonia can exacerbate the underlying comorbidity predisposing to pneumonia, this becomes a vicious cycle (FIGURE 10). Increased efforts to interrupt pneumonia susceptibility in those with comorbidities will help break these feed-forward cycles of comorbidity and pneumonia. However, better biological understanding is needed first. At present, we have no biologic metrics that can distinguish those with elevated pneumonia susceptibility from their more resistant peers. Our understanding of the physiological underpinnings of pneumonia susceptibility are too rudimentary and incomplete to guide useful countermeasures that will preserve or restore defects in resistance and resilience that develop due to comorbidities or exposures. In this instance, the epidemiology is leading the way, and biological understanding needs to catch up.
FIGURE 10.
The vicious cycle by which pneumonia drives unhealthy aging. Pneumonia susceptibility is raised by a wide variety of chronic diseases that are recognized as general risk factors for pneumonia (comorbidities). Pneumonia elicits a predisposition to and worsening of those same comorbidities. Thus comorbidities make people more likely to get pneumonia, and pneumonia precipitates and exacerbates comorbidities, which makes individuals yet more likely to get pneumonia, which yet further accelerates comorbidities, and so on. A: the self-amplifying cycle of interactions between pneumonia and comorbidities. B: the decline in physiological function driven by this self-amplifying cycle, schematically rendered to communicate the concept (not actual data).
VIII. PNEUMONIA SEQUELAE
The prior section highlights that pneumonia cannot simply be considered as an acute event, but that conditions preceding infection must be considered as responsible for enabling the pneumonia occurrence. Similarly, the outcomes of pneumonia also should be considered in physiological and temporal context. Death from pneumonia is one potential result, perhaps the most emphasized of the possible outcomes. In addition, pneumonia has other long-term consequences for survivors, which degrade health and hasten mortality after the acute event is over.
An unfortunate aspect of the strong relationship between aging, pneumonia, and death is the prominent notion of pneumonia as the “old man’s friend.” This metaphor is off-base and counterproductive, encouraging some to conclude that pneumonia is inevitable and merciful for the elderly. To our knowledge, this metaphor first appeared in the fifth edition of William Osler’s textbook The Principles and Practice of Medicine, used to imply that pneumonia can provide a quick and painless end for some elderly patients suffering from other slow terminal conditions (370). However, pneumonia is not universally quick, or painless, or in terminal patients. Ironically, Dr. Osler enjoyed good health until he developed pneumonia at age 70, leading to months of distress, decline, and recurrent illness before he eventually succumbed to this disease (3).
Pneumonia is not typically lethal, but rather it is usually survived. The likelihood of survival decreases with age, but even those over 90 yr of age have a 75% survival rate (3). Those elderly pneumonia cases (the vast majority) that end in survival cannot possibly be framed as merciful in any way. They are costly, distressing, and major contributors to morbidity for the aged. It is difficult to precisely quantify the toll pneumonia exacts beyond its (important) impact on mortality, but it is undeniably large. Pneumonia requires hospitalization in 10–20% of cases (with more than 5 days of stay typical), necessitates ICU admission in >20% of hospitalizations, incurs 30-day readmission rates of ~20% after hospitalization, and costs tens of billions of dollars per year in the US (136, 189, 227, 228, 352, 565). For Medicare alone (hence only a subset of the population), pneumonia costs 13 billion dollars in healthcare per year (565). In addition to its immediately attributable burden, pneumonia increases the risk for and worsens the progression of many of the other chronic diseases that also commonly afflict older individuals. For example, as expanded upon below, after matching for initial disease severity and controlling for confounders, survivors of pneumonia consistently experience worse degrees of cognitive decline and dementia, functional disability and limitations, heart attacks, strokes, depression, and risk of death over the ensuing years (39, 88, 196, 376, 431, 445). Implicating causal relationships, vaccination against respiratory pathogens decreases cardiovascular and cerebrovascular disease morbidity and mortality (503, 515). Thus, far from a merciful agent of relief as often and erroneously conceptualized, pneumonia is a major problem for the aged and forms a catastrophic positive feedback loop with other chronic diseases (FIGURE 10), preventing healthy aging and worsening overall decline.
A. Short-Term Consequences of Pneumonia
Severe pneumonia results in direct lung injury from the infectious pathogen, impairing alveolar gas exchange and causing respiratory failure (FIGURE 11). Pneumonia is the leading cause of ARDS, when it causes a bilateral injury that includes the diffuse influx of protein-rich edema and inflammatory cells into the alveolar space, destruction of surfactant, formation of fibrin-rich intra-alveolar hyaline membranes, and loss of gas-exchanging type 1 pneumocytes (315). Treatment of ARDS currently consists of supportive therapy and use of measures to decrease further lung injury caused by mechanical ventilation practices. Patients with ARDS have mortality rates of 30–40%.
FIGURE 11.
Pneumonia sequelae. Pneumonia causes direct injury to the lungs, from a combination of microbial and inflammatory signals. When the degree of injury crosses a physiological threshold defined by blood gases, this is diagnosed as acute respiratory distress syndrome (ARDS). When infection and inflammation disseminate from the lungs and injure other organs, this is diagnosed as sepsis if severe enough to be life-threatening. ARDS and sepsis are well-recognized outcomes of pneumonia. Perhaps less well-recognized are the indirect consequences, including the predisposition to or exacerbation of ongoing chronic diseases such as COPD, atherosclerosis, cognitive decline, and more. The mechanisms driving the sequelae of pneumonia are multifactorial, including systemic inflammation and infection plus localized and diffuse aberrations involving the immune, cardiovascular, microbiome, hematologic, and nervous systems.
Severe pneumonia may also result in injury to organs distant from the lung (FIGURE 11). Prior sections communicated that lung resistance and resilience depended on extrapulmonary tissues, and it is also clear that extrapulmonary tissues can be involved in the acute and severe manifestations of pneumonia. The occurrence of life-threatening organ injury from an infection such as pneumonia is defining for sepsis (454). Extrapulmonary organ damage resulting from pneumonia may have diverse manifestations including encephalopathy, coagulopathy, kidney and liver failure, as well as cardiovascular complications (e.g., shock or arrhythmias). With the exception of antibiotics, intravenous fluid therapy, and organ-supportive treatments (e.g., renal dialysis and mechanical ventilation), no sepsis-specific treatments have been shown to improve patient outcomes. Despite the absence of sepsis-specific therapies, improvements in the processes of critical care delivery have resulted in nearly a 50% reduction in short-term case-fatality rates from sepsis over the past two decades (469), revealing that improvements in healthcare delivery can have substantial impact for this disease. However, this outcome of pneumonia is as grim as ARDS, and severe sepsis patients currently have mortality rates of 33% (469).
Considered in isolation as an acute event, pneumonia is a major cause of morbidity and mortality, often due to ARDS and/or sepsis. But pneumonia is much more than this acute event.
B. Long-Term Consequences of Pneumonia in Adulthood
In the century-plus since Osler depicted pneumonia as friendly to the aged and deemed it “Captain of the Men of Death” (370), pneumonia case-fatality rates in advantaged communities such as the US have dramatically declined (407). ARDS and sepsis have high mortality rates, but they occur in only a fraction of pneumonia cases, and fewer than 10% of all elderly patients hospitalized for pneumonia die from this disease (290). The public health impact of pneumonia in the 21st century is perhaps most driven by its contribution to progressive health decline from long-term complications that often involve extrapulmonary organs (222, 223). Unlike in Dr. Osler’s day, most pneumonia patients today suffer, survive, and deteriorate (FIGURE 11).
Among older adults, survivors of an acute pneumonia hospitalization have higher mortality risks that persist for years following discharge compared with matched patients hospitalized for reasons other than pneumonia (48, 128). Increased mortality risks following pneumonia appear to be less dependent on age or acute severity of pneumonia, but strongly influenced by the nature of the preexisting comorbid conditions (48, 88, 128, 343). However, even after adjusting for comorbid conditions as well as age, patients with pneumonia appear to have worse long-term mortality rates when compared with similar patients who have not suffered pneumonia (398, 561, 563).
Long-term effects of pneumonia are manifest in extrapulmonary organs. Pneumonia accelerates or precipitates declines in cognition (445) and functional status (224), as well as raising risks for cardiovascular complications such as stroke, myocardial infarction, and heart failure (88) and for recurrent infections (397, 398). For example, after accounting for multiple comorbid conditions and prior trajectories of cognitive and functional status, pneumonia (and not other reasons for hospitalization) associates with a 57% increase in risk of developing dementia (445). The risk of myocardial infarction, stroke, or heart failure increases fourfold in patients with pneumonia within 30 days, and remains twofold elevated 1 yr later (88). Risks of cardiovascular events associated with pneumonia exceed what can be accounted for by other cardiovascular risk factors such as diabetes or smoking (88, 89). Finally, nearly one in five pneumonia hospitalizations results in rehospitalization within 30 days, and most of these readmissions are for infection (102). Thus hospital discharge after pneumonia should not imply a full recovery.
The mechanisms through which pneumonia affects long-term risk for cognitive, functional, cardiovascular, and infectious disease are poorly understood. Current hypotheses implicate residual inflammation in the many complications that occur following pneumonia. For example, IL-6 levels measured in the blood at the time of pneumonia hospitalization discharge are associated with increased risk of later death from cardiovascular disease and infection (561). Circulating levels of inflammatory cytokines and acute-phase reactants such as IL-6 and CRP also associate with subsequent cognitive decline (551, 552). Poor cognitive and functional outcomes following pneumonia may additionally result from hypoxia and hypoperfusion during acute illness (445) and from ancillary treatments common to severe pneumonia (e.g., benzodiazepine sedatives and bed rest) that appear to increase inflammation, acute delirium, and long-term cognitive risks (376, 378, 393, 532). Increased risks of recurrent and new infections may be due in part to postinfectious immune paralysis (18, 41). Risks of stroke, myocardial infarction, and heart failure may be increased due to prolonged coagulopathy (562), endothelial injury with accelerated atherosclerosis (250), large volume shifts (116), cardiac injury (218, 245, 426), and arrhythmias (521, 522) that result from pneumonia (FIGURE 10). At least for some types of pneumonia (e.g., pneumococcus), cardiac complications may result from direct infection of cardiac tissue with macrophage necroptosis followed by scarring (56, 159). Use of physical rehabilitation (110, 358, 359, 500), avoidance of benzodiazepines (146, 268), targeted immune modulation (494), and use of cardiovascular risk reduction medications such as statins and aspirin (520) are active areas of investigation seeking to attenuate myriad long-term risks following pneumonia (271). Further studies are required to determine the mechanistic links between pneumonia and these longer term extrapulmonary consequences, as well as the extent to which these complications after pneumonia are modifiable.
C. Long-Term Consequences of Pediatric Pneumonia
Pneumonia affects 50% of children each year in the most economically disadvantaged regions (77) and is the most common reason for childhood hospitalization in regions such as the US that have a more privileged socioeconomic status (566). Studies of childhood pneumonia further refute the notion that pneumonia is an acute illness with limited ramifications for survivors. Rather, children experience high rates of subsequent pulmonary comorbidity following pneumonia, with 10% of children suffering complications following resolution of pneumonia (123). Unlike multisystem disease of adults, the best recognized long-term complications of childhood pneumonias are localized to the affected organ, the lungs. The most common complications following childhood pneumonia are development of restrictive lung disease, asthma, bronchiectasis, and chronic bronchitis (123, 298). Acute effects of pneumonia in children also result in altered lung development that increases the predilection for pneumonia later in life (236, 237). Biological links between early life acute infections and persistent chronic respiratory diseases are not yet well established, to our knowledge.
D. Positive Feedback Loop of Pneumonia and Progressively Declining Health
As shown by the overlap between pneumonia risk factors and pneumonia consequences, iterative positive feedback loops are prominent features of this disease (FIGURE 10). Poor health begets pneumonia, and pneumonia diminishes health. COPD predisposes patients to lung infection, and lung infections accelerate the decline of COPD (444, 493). Cardiovascular diseases raise pneumonia risk, and pneumonia events hasten cardiovascular deterioration (89, 493, 509). Neurological and psychological diseases make pneumonia more likely, and pneumonia compounds the severity of neurological and psychological diseases (39, 376, 445). Such iterative positive feedback loops apply to most of the comorbidities associated with pneumonia. In short, pneumonia is both a symptom of and a cause of unhealthy aging.
IX. CONCLUSIONS AND FUTURE DIRECTIONS
Exposures to the ubiquitous microbes that cause pneumonia are routine and unavoidable. Whether and which subjects get pneumonia upon exposure is dictated by mammalian biology. When the integrated responses of the pulmonary, immune, cardiovascular, neurological, and other systems are appropriate to eliminating microbes while preserving physiological function, respiratory infection is subclinical or mild. Too often, though, this is not the case. We need to develop a better understanding of how the body normally successfully defends itself against respiratory pathogens so that we can recognize and counter decrements in these protective pathways. Comorbidities and exposures render individuals more susceptible to pneumonia, but the biology of this susceptibility is not yet well delineated. The physiology underlying the long-term and extrapulmonary sequelae of pneumonia that accelerate chronic disease and unhealthy aging is only speculative still.
We need new ways to approach pneumonia, to diminish the burden of this disease that has stayed too high for too long. Continued research into fighting microbes will be helpful, hopefully resulting in more and better antibiotics and vaccines, but these directions cannot be enough. The responsible microbes are too numerous, too ubiquitous, and too diverse. We need to reconceptualize pneumonia. Improved understanding of the physiology of the acute infection will lead to new approaches for limiting lung injury and the dissemination of infection, inflammation, and injury beyond the lung. This will reduce ARDS and sepsis. Improved understanding of the biological mechanisms connecting acute pneumonia events to their long-term sequelae will beget new approaches to interrupting the vicious cycle that drives unhealthy aging. This will help people live longer, better. Before people get pneumonia, changes in their physiology are responsible for rendering them susceptible, and these changes are a biological process that is not yet identified but needs to be defined. Pneumonia susceptibility must be considered as a chronic condition so that we can develop medical approaches to combat this chronic condition before pneumonia occurs. Improved understanding of the mechanisms underlying pneumonia susceptibility will direct such development. Just as lowering blood pressure and cholesterol levels help prevent acute infarctions, understanding which biological changes render individuals most susceptible to pneumonia will provide opportunities for interventions that target those biological pathways and reverse or slow the progression of pneumonia susceptibility. Greater physiological insight is needed to more effectively prevent and treat pneumonia.
GRANTS
The authors received research support from the National Instutes of Health that is relevant to this review, including R01 GM120060 and R01 HL111459 (to L. J. Quinton); K01 HL116768 and R01 HL136660 (to A. J. Walkey); and R35 HL135756, R01 AI115053, R61 HL137081, and R56 AI122763 (to J. P. Mizgerd).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: J. P. Mizgerd, Pulmonary Center, Boston University School of Medicine, 72 East Concord St., Boston, MA 02115 (e-mail: jmizgerd@bu.edu).
REFERENCES
- 1.Abboud G, Tahiliani V, Desai P, Varkoly K, Driver J, Hutchinson TE, Salek-Ardakani S. Natural Killer Cells and Innate Interferon Gamma Participate in the Host Defense against Respiratory Vaccinia Virus Infection. J Virol 90: 129–141, 2016. doi: 10.1128/JVI.01894-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberdein JD, Cole J, Bewley MA, Marriott HM, Dockrell DH. Alveolar macrophages in pulmonary host defence the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing. Clin Exp Immunol 174: 193–202, 2013. doi: 10.1111/cei.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adami JG. Last Days of Sir William Osler. Can Med Assoc J 10: 70–77, 1920. [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol 306: L709–L725, 2014. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal NR, Tsushima K, Eto Y, Tripathi A, Mandke P, Mock JR, Garibaldi BT, Singer BD, Sidhaye VK, Horton MR, King LS, D’Alessio FR. Immunological priming requires regulatory T cells and IL-10-producing macrophages to accelerate resolution from severe lung inflammation. J Immunol 192: 4453–4464, 2014. doi: 10.4049/jimmunol.1400146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agus MS, Wypij D, Hirshberg EL, Srinivasan V, Faustino EV, Luckett PM, Alexander JL, Asaro LA, Curley MA, Steil GM, Nadkarni VM; HALF-PINT Study Investigators and the PALISI Network . Tight Glycemic Control in Critically Ill Children. N Engl J Med 376: 729–741, 2017. doi: 10.1056/NEJMoa1612348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn A, Edwards KM, Grijalva CG, Self WH, Zhu Y, Chappell JD, Arnold SR, McCullers JA, Ampofo K, Pavia AT, Bramley AM, Jain S, Williams DJ. Secondhand Smoke Exposure and Illness Severity Among Children Hospitalized With Pneumonia. J Pediatr 167: 869–874.e1, 2015. doi: 10.1016/j.jpeds.2015.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahyi AN, Quinton LJ, Jones MR, Ferrari JD, Pepper-Cunningham ZA, Mella JR, Remick DG, Mizgerd JP. Roles of STAT3 in protein secretion pathways during the acute-phase response. Infect Immun 81: 1644–1653, 2013. doi: 10.1128/IAI.01332-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell 77: 63–71, 1994. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 10.Albiger B, Sandgren A, Katsuragi H, Meyer-Hoffert U, Beiter K, Wartha F, Hornef M, Normark S, Normark BH. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell Microbiol 7: 1603–1615, 2005. doi: 10.1111/j.1462-5822.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 11.Alfaro VY, Goldblatt DL, Valverde GR, Munsell MF, Quinton LJ, Walker AK, Dantzer R, Varadhachary A, Scott BL, Evans SE, Tuvim MJ, Dickey BF. Safety, tolerability, and biomarkers of the treatment of mice with aerosolized Toll-like receptor ligands. Front Pharmacol 5: 8, 2014. doi: 10.3389/fphar.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almirall J, Bolíbar I, Balanzó X, González CA. Risk factors for community-acquired pneumonia in adults: a population-based case-control study. Eur Respir J 13: 349–355, 1999. doi: 10.1183/09031936.99.13234999. [DOI] [PubMed] [Google Scholar]
- 13.Almirall J, Bolíbar I, Toran P, Pera G, Boquet X, Balanzó X, Sauca G; Community-Acquired Pneumonia Maresme Study Group . Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest 125: 1335–1342, 2004. doi: 10.1378/chest.125.4.1335. [DOI] [PubMed] [Google Scholar]
- 14.Almirall J, González CA, Balanzó X, Bolíbar I. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest 116: 375–379, 1999. doi: 10.1378/chest.116.2.375. [DOI] [PubMed] [Google Scholar]
- 15.Anand N, Kollef MH. The alphabet soup of pneumonia: CAP, HAP, HCAP, NHAP, and VAP. Semin Respir Crit Care Med 30: 3–9, 2009. doi: 10.1055/s-0028-1119803. [DOI] [PubMed] [Google Scholar]
- 16.Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev (4): CD006602, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Anding K, Gross P, Rost JM, Allgaier D, Jacobs E. The influence of uraemia and haemodialysis on neutrophil phagocytosis and antimicrobial killing. Nephrol Dial Transplant 18: 2067–2073, 2003. doi: 10.1093/ndt/gfg330. [DOI] [PubMed] [Google Scholar]
- 18.Angus DC, Opal S. Immunosuppression and Secondary Infection in Sepsis: Part, Not All, of the Story. JAMA 315: 1457–1459, 2016. doi: 10.1001/jama.2016.2762. [DOI] [PubMed] [Google Scholar]
- 19.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 369: 840–851, 2013. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA 281: 61–66, 1999. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 21.Asgharian B, Price OT, Oldham M, Chen LC, Saunders EL, Gordon T, Mikheev VB, Minard KR, Teeguarden JG. Computational modeling of nanoscale and microscale particle deposition, retention and dosimetry in the mouse respiratory tract. Inhal Toxicol 26: 829–842, 2014. doi: 10.3109/08958378.2014.935535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341: 515–518, 1993. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14: 275–281, 2008. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachman MA, Lenio S, Schmidt L, Oyler JE, Weiser JN. Interaction of lipocalin 2, transferrin, and siderophores determines the replicative niche of Klebsiella pneumoniae during pneumonia. MBio 3: e00224-11, 2012. doi: 10.1128/mBio.00224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagga B, Cehelsky JE, Vaishnaw A, Wilkinson T, Meyers R, Harrison LM, Roddam PL, Walsh EE, DeVincenzo JP. Effect of Preexisting Serum and Mucosal Antibody on Experimental Respiratory Syncytial Virus (RSV) Challenge and Infection of Adults. J Infect Dis 212: 1719–1725, 2015. doi: 10.1093/infdis/jiv281. [DOI] [PubMed] [Google Scholar]
- 26.Balamayooran G, Batra S, Theivanthiran B, Cai S, Pacher P, Jeyaseelan S. Intrapulmonary G-CSF rescues neutrophil recruitment to the lung and neutrophil release to blood in Gram-negative bacterial infection in MCP-1−/− mice. J Immunol 189: 5849–5859, 2012. doi: 10.4049/jimmunol.1200585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnett JB, Hamer DH, Meydani SN. Low zinc status: a new risk factor for pneumonia in the elderly? Nutr Rev 68: 30–37, 2010. doi: 10.1111/j.1753-4887.2009.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol 16: 51–67, 2016. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB, Beck JM, Curtis JL, Huffnagle GB. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio 6: e00037-15, 2015. doi: 10.1128/mBio.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 11: 34–46, 2011. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 31.Bayes HK, Ritchie ND, Evans TJ. Interleukin-17 Is Required for Control of Chronic Lung Infection Caused by Pseudomonas aeruginosa. Infect Immun 84: 3507–3516, 2016. doi: 10.1128/IAI.00717-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol 16: 401–407, 2006. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 33.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A; LUNG SAFE Investigators; ESICM Trials Group . Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 315: 788–800, 2016. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 34.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, Masopust D. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532: 512–516, 2016. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bewley MA, Marriott HM, Tulone C, Francis SE, Mitchell TJ, Read RC, Chain B, Kroemer G, Whyte MK, Dockrell DH. A cardinal role for cathepsin d in co-ordinating the host-mediated apoptosis of macrophages and killing of pneumococci. PLoS Pathog 7: e1001262, 2011. doi: 10.1371/journal.ppat.1001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bewley MA, Preston JA, Mohasin M, Marriott HM, Budd RC, Swales J, Collini P, Greaves DR, Craig RW, Brightling CE, Donnelly LE, Barnes PJ, Singh D, Shapiro SD, Whyte MKB, Dockrell DH. Impaired Mitochondrial Microbicidal Responses in Chronic Obstructive Pulmonary Disease Macrophages. Am J Respir Crit Care Med 196: 845–855, 2017. doi: 10.1164/rccm.201608-1714OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol 75: 593–615, 2013. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharya J, Westphalen K. Macrophage-epithelial interactions in pulmonary alveoli. Semin Immunopathol 38: 461–469, 2016. doi: 10.1007/s00281-016-0569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N, Dennison CR, Herridge MS, Pronovost PJ, Needham DM. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med 185: 517–524, 2012. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol 9: 727–738, 2011. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD II, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306: 2594–2605, 2011. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Born WK, Lahn M, Takeda K, Kanehiro A, O’Brien RL, Gelfand EW. Role of gammadelta T cells in protecting normal airway function. Respir Res 1: 151–158, 2000. doi: 10.1186/rr26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bos LD, Stips C, Schouten LR, van Vught LA, Wiewel MA, Wieske L, van Hooijdonk RT, Straat M, de Beer FM, Glas GJ, Visser CE, de Jonge E, Juffermans NP, Horn J, Schultz MJ. Selective decontamination of the digestive tract halves the prevalence of ventilator-associated pneumonia compared to selective oral decontamination. Intensive Care Med 43: 1535–1537, 2017. doi: 10.1007/s00134-017-4838-5. [DOI] [PubMed] [Google Scholar]
- 44.Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, Gagliani N, Craft JE, Flavell RA, Ghosh S, Rothlin CV. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356: 1072–1076, 2017. doi: 10.1126/science.aai8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyd AR, Orihuela CJ. Dysregulated inflammation as a risk factor for pneumonia in the elderly. Aging Dis 2: 487–500, 2011. [PMC free article] [PubMed] [Google Scholar]
- 46.Bozza FA, Shah AM, Weyrich AS, Zimmerman GA. Amicus or adversary: platelets in lung biology, acute injury, and inflammation. Am J Respir Cell Mol Biol 40: 123–134, 2009. doi: 10.1165/rcmb.2008-0241TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol 12: 295–305, 2012. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brancati FL, Chow JW, Wagener MM, Vacarello SJ, Yu VL. Is pneumonia really the old man’s friend? Two-year prognosis after community-acquired pneumonia. Lancet 342: 30–33, 1993. doi: 10.1016/0140-6736(93)91887-R. [DOI] [PubMed] [Google Scholar]
- 49.Branchfield K, Nantie L, Verheyden JM, Sui P, Wienhold MD, Sun X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 351: 707–710, 2016. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brandenberger C, Mühlfeld C. Mechanisms of lung aging. Cell Tissue Res 367: 469–480, 2017. doi: 10.1007/s00441-016-2511-x. [DOI] [PubMed] [Google Scholar]
- 51.Brandes M, Klauschen F, Kuchen S, Germain RN. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell 154: 197–212, 2013. doi: 10.1016/j.cell.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Branger J, Florquin S, Knapp S, Leemans JC, Pater JM, Speelman P, Golenbock DT, van der Poll T. LPS-binding protein-deficient mice have an impaired defense against Gram-negative but not Gram-positive pneumonia. Int Immunol 16: 1605–1611, 2004. doi: 10.1093/intimm/dxh161. [DOI] [PubMed] [Google Scholar]
- 53.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 13: 101–117, 2013. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 54.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med 208: 1163–1177, 2011. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R III, Standiford TJ. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun 65: 1139–1146, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown AO, Mann B, Gao G, Hankins JS, Humann J, Giardina J, Faverio P, Restrepo MI, Halade GV, Mortensen EM, Lindsey ML, Hanes M, Happel KI, Nelson S, Bagby GJ, Lorent JA, Cardinal P, Granados R, Esteban A, LeSaux CJ, Tuomanen EI, Orihuela CJ. Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function. PLoS Pathog 10: e1004383, 2014. doi: 10.1371/journal.ppat.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown LA, Ping XD, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol 292: L824–L832, 2007. doi: 10.1152/ajplung.00346.2006. [DOI] [PubMed] [Google Scholar]
- 58.Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol 15: 55–63, 2017. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 59.Cai L, Yi F, Dai Z, Huang X, Zhao YD, Mirza MK, Xu J, Vogel SM, Zhao YY. Loss of caveolin-1 and adiponectin induces severe inflammatory lung injury following LPS challenge through excessive oxidative/nitrative stress. Am J Physiol Lung Cell Mol Physiol 306: L566–L573, 2014. doi: 10.1152/ajplung.00182.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai S, Batra S, Langohr I, Iwakura Y, Jeyaseelan S. IFN-γ induction by neutrophil-derived IL-17A homodimer augments pulmonary antibacterial defense. Mucosal Immunol 9: 718–729, 2016. doi: 10.1038/mi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J Immunol 185: 6214–6225, 2010. doi: 10.4049/jimmunol.0903843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, Ware LB. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 147: 1539–1548, 2015. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cantin AM, Woods DE. Aerosolized prolastin suppresses bacterial proliferation in a model of chronic Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med 160: 1130–1135, 1999. doi: 10.1164/ajrccm.160.4.9807166. [DOI] [PubMed] [Google Scholar]
- 64.Casadevall A, Scharff MD. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother 38: 1695–1702, 1994. doi: 10.1128/AAC.38.8.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cecil RL. Present status of serum therapy in pneumonia. 1939. Bull N Y Acad Med 74: 341–354, 1997. [PMC free article] [PubMed] [Google Scholar]
- 66.Chan YR, Liu JS, Pociask DA, Zheng M, Mietzner TA, Berger T, Mak TW, Clifton MC, Strong RK, Ray P, Kolls JK. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol 182: 4947–4956, 2009. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, Senft AP, Whitsett JA. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med 189: 301–313, 2014. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 119: 2914–2924, 2009. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen K, Eddens T, Trevejo-Nunez G, Way EE, Elsegeiny W, Ricks DM, Garg AV, Erb CJ, Bo M, Wang T, Chen W, Lee JS, Gaffen SL, Kolls JK. IL-17 Receptor Signaling in the Lung Epithelium Is Required for Mucosal Chemokine Gradients and Pulmonary Host Defense against K. pneumoniae. Cell Host Microbe 20: 596–605, 2016. doi: 10.1016/j.chom.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen K, Kolls JK. T cell-mediated host immune defenses in the lung. Annu Rev Immunol 31: 605–633, 2013. doi: 10.1146/annurev-immunol-032712-100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, Alcorn JF, Weaver CT, Kolls JK. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity 35: 997–1009, 2011. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen L, Zhang Z, Barletta KE, Burdick MD, Mehrad B. Heterogeneity of lung mononuclear phagocytes during pneumonia: contribution of chemokine receptors. Am J Physiol Lung Cell Mol Physiol 305: L702–L711, 2013. doi: 10.1152/ajplung.00194.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen SM, Cheng DS, Williams BJ, Sherrill TP, Han W, Chont M, Saint-Jean L, Christman JW, Sadikot RT, Yull FE, Blackwell TS. The nuclear factor kappa-B pathway in airway epithelium regulates neutrophil recruitment and host defence following Pseudomonas aeruginosa infection. Clin Exp Immunol 153: 420–428, 2008. doi: 10.1111/j.1365-2249.2008.03707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, Blackwell TS. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol 178: 6504–6513, 2007. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 75.Chiu C, Openshaw PJ. Antiviral B cell and T cell immunity in the lungs. Nat Immunol 16: 18–26, 2015. doi: 10.1038/ni.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi SM, McAleer JP, Zheng M, Pociask DA, Kaplan MH, Qin S, Reinhart TA, Kolls JK. Innate Stat3-mediated induction of the antimicrobial protein Reg3γ is required for host defense against MRSA pneumonia. J Exp Med 210: 551–561, 2013. doi: 10.1084/jem.20120260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chopra M, Mason E, Borrazzo J, Campbell H, Rudan I, Liu L, Black RE, Bhutta ZA. Ending of preventable deaths from pneumonia and diarrhoea: an achievable goal. Lancet 381: 1499–1506, 2013. doi: 10.1016/S0140-6736(13)60319-0. [DOI] [PubMed] [Google Scholar]
- 78.Chorny A, Casas-Recasens S, Sintes J, Shan M, Polentarutti N, García-Escudero R, Walland AC, Yeiser JR, Cassis L, Carrillo J, Puga I, Cunha C, Bastos H, Rodrigues F, Lacerda JF, Morais A, Dieguez-Gonzalez R, Heeger PS, Salvatori G, Carvalho A, Garcia-Sastre A, Blander JM, Mantovani A, Garlanda C, Cerutti A. The soluble pattern recognition receptor PTX3 links humoral innate and adaptive immune responses by helping marginal zone B cells. J Exp Med 213: 2167–2185, 2016. doi: 10.1084/jem.20150282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cilloniz C, Martin-Loeches I, Garcia-Vidal C, San Jose A, Torres A. Microbial Etiology of Pneumonia: Epidemiology, Diagnosis and Resistance Patterns. Int J Mol Sci 17: E2120, 2016. doi: 10.3390/ijms17122120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immun 82: 4596–4606, 2014. doi: 10.1128/IAI.02212-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 16: 228–231, 2010. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cleaver JO, You D, Michaud DR, Pruneda FA, Juarez MM, Zhang J, Weill PM, Adachi R, Gong L, Moghaddam SJ, Poynter ME, Tuvim MJ, Evans SE. Lung epithelial cells are essential effectors of inducible resistance to pneumonia. Mucosal Immunol 7: 78–88, 2014. doi: 10.1038/mi.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clement CG, Evans SE, Evans CM, Hawke D, Kobayashi R, Reynolds PR, Moghaddam SJ, Scott BL, Melicoff E, Adachi R, Dickey BF, Tuvim MJ. Stimulation of lung innate immunity protects against lethal pneumococcal pneumonia in mice. Am J Respir Crit Care Med 177: 1322–1330, 2008. doi: 10.1164/rccm.200607-1038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cohen JM, Wilson R, Shah P, Baxendale HE, Brown JS. Lack of cross-protection against invasive pneumonia caused by heterologous strains following murine Streptococcus pneumoniae nasopharyngeal colonisation despite whole cell ELISAs showing significant cross-reactive IgG. Vaccine 31: 2328–2332, 2013. doi: 10.1016/j.vaccine.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 85.Coleman FT, Blahna MT, Kamata H, Yamamoto K, Zabinski MC, Kramnik I, Wilson AA, Kotton DN, Quinton LJ, Jones MR, Pelton SI, Mizgerd JP. The capacity of pneumococci to activate macrophage NF-kB determines necroptosis and pneumonia severity. J Infect Dis 216: 425–435, 2017. doi: 10.1093/infdis/jix159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80: 2012–2020, 1992. [PubMed] [Google Scholar]
- 87.Coopersmith CM, Stromberg PE, Davis CG, Dunne WM, Amiot DM II, Karl IE, Hotchkiss RS, Buchman TG. Sepsis from Pseudomonas aeruginosa pneumonia decreases intestinal proliferation and induces gut epithelial cell cycle arrest. Crit Care Med 31: 1630–1637, 2003. doi: 10.1097/01.CCM.0000055385.29232.11. [DOI] [PubMed] [Google Scholar]
- 88.Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, Newman A, Loehr L, Folsom AR, Elkind MS, Lyles MF, Kronmal RA, Yende S. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 313: 264–274, 2015. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet 381: 496–505, 2013. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 90.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun 77: 568–575, 2009. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crispe IN. Hepatocytes as Immunological Agents. J Immunol 196: 17–21, 2016. doi: 10.4049/jimmunol.1501668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cundell DR, Gerard C, Idanpaan-Heikkila I, Tuomanen EI, Gerard NP. PAf receptor anchors Streptococcus pneumoniae to activated human endothelial cells. Adv Exp Med Biol 416: 89–94, 1996. doi: 10.1007/978-1-4899-0179-8_16. [DOI] [PubMed] [Google Scholar]
- 93.D’Alessio FR, Craig JM, Singer BD, Files DC, Mock JR, Garibaldi BT, Fallica J, Tripathi A, Mandke P, Gans JH, Limjunyawong N, Sidhaye VK, Heller NM, Mitzner W, King LS, Aggarwal NR. Enhanced resolution of experimental ARDS through IL-4-mediated lung macrophage reprogramming. Am J Physiol Lung Cell Mol Physiol 310: L733–L746, 2016. doi: 10.1152/ajplung.00419.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 119: 2898–2913, 2009. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.D’Souza NB, Nelson S, Summer WR, Deaciuc IV. Alcohol modulates alveolar macrophage tumor necrosis factor-alpha, superoxide anion, and nitric oxide secretion in the rat. Alcohol Clin Exp Res 20: 156–163, 1996. doi: 10.1111/j.1530-0277.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 96.De Grove KC, Provoost S, Verhamme FM, Bracke KR, Joos GF, Maes T, Brusselle GG. Characterization and Quantification of Innate Lymphoid Cell Subsets in Human Lung. PLoS One 11: e0145961, 2016. doi: 10.1371/journal.pone.0145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJ, Bernards AT, Kuijper EJ, Joore JC, Leverstein-van Hall MA, Bindels AJ, Jansz AR, Wesselink RM, de Jongh BM, Dennesen PJ, van Asselt GJ, te Velde LF, Frenay IH, Kaasjager K, Bosch FH, van Iterson M, Thijsen SF, Kluge GH, Pauw W, de Vries JW, Kaan JA, Arends JP, Aarts LP, Sturm PD, Harinck HI, Voss A, Uijtendaal EV, Blok HE, Thieme Groen ES, Pouw ME, Kalkman CJ, Bonten MJ. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 360: 20–31, 2009. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- 98.De Stoppelaar SF, Van’t Veer C, Roelofs JJ, Claushuis TA, de Boer OJ, Tanck MW, Hoogendijk AJ, van der Poll T. Platelet and endothelial cell P-selectin are required for host defense against Klebsiella pneumoniae-induced pneumosepsis. J Thromb Haemost 13: 1128–1138, 2015. doi: 10.1111/jth.12893. [DOI] [PubMed] [Google Scholar]
- 99.De Stoppelaar SF, van ’t Veer C, Claushuis TA, Albersen BJ, Roelofs JJ, van der Poll T. Thrombocytopenia impairs host defense in gram-negative pneumonia-derived sepsis in mice. Blood 124: 3781–3790, 2014. doi: 10.1182/blood-2014-05-573915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med 14: 29–34, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 101.Deng JC. Viral-bacterial interactions-therapeutic implications. Influenza Other Respi Viruses 7, Suppl 3: 24–35, 2013. doi: 10.1111/irv.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 309: 355–363, 2013. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, Palfrey HC, Nelson DJ. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol 8: 933–944, 2006. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 104.Di A, Kiya T, Gong H, Gao X, Malik AB. Role of the phagosomal redox-sensitive TRP channel TRPM2 in regulating bactericidal activity of macrophages. J Cell Sci 130: 735–744, 2017. doi: 10.1242/jcs.196014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB. The Microbiome and the Respiratory Tract. Annu Rev Physiol 78: 481–504, 2016. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb-Downward JR, Standiford TJ, Huffnagle GB. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol 1: 16113, 2016. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, van Rijt LS, Lambrecht BN, Sirard JC, Hussell T. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med 205: 323–329, 2008. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.DiLillo DJ, Palese P, Wilson PC, Ravetch JV. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest 126: 605–610, 2016. doi: 10.1172/JCI84428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat Med 20: 143–151, 2014. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dinglas VD, Parker AM, Reddy DRS, Colantuoni E, Zanni JM, Turnbull AE, Nelliot A, Ciesla N, Needham DM. A quality improvement project sustainably decreased time to onset of active physical therapy intervention in patients with acute lung injury. Ann Am Thorac Soc 11: 1230–1238, 2014. doi: 10.1513/AnnalsATS.201406-231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, Hellewell PG, Whyte MK. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J Immunol 171: 5380–5388, 2003. doi: 10.4049/jimmunol.171.10.5380. [DOI] [PubMed] [Google Scholar]
- 112.Doerschuk CM. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation 8: 71–88, 2001. doi: 10.1111/j.1549-8719.2001.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 113.Doerschuk CM, Allard MF, Martin BA, MacKenzie A, Autor AP, Hogg JC. Marginated pool of neutrophils in rabbit lungs. J Appl Physiol (1985) 63: 1806–1815, 1987. doi: 10.1152/jappl.1987.63.5.1806. [DOI] [PubMed] [Google Scholar]
- 114.Dominguez JA, Vithayathil PJ, Khailova L, Lawrance CP, Samocha AJ, Jung E, Leathersich AM, Dunne WM, Coopersmith CM. Epidermal growth factor improves survival and prevents intestinal injury in a murine model of Pseudomonas aeruginosa pneumonia. Shock 36: 381–389, 2011. doi: 10.1097/SHK.0b013e31822793c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drake MG, Evans SE, Dickey BF, Fryer AD, Jacoby DB. Toll-like receptor-2/6 and Toll-like receptor-9 agonists suppress viral replication but not airway hyperreactivity in guinea pigs. Am J Respir Cell Mol Biol 48: 790–796, 2013. doi: 10.1165/rcmb.2012-0498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dreyfuss D, Leviel F, Paillard M, Coste F. Resetting of the vasopressin osmostat during infectious pneumonia. Am J Med 90: 407–408, 1991. doi: 10.1016/0002-9343(91)80029-L. [DOI] [PubMed] [Google Scholar]
- 117.Du X, Meng Q, Sharif A, Abdel-Razek OA, Zhang L, Wang G, Cooney RN. Surfactant Proteins SP-A and SP-D Ameliorate Pneumonia Severity and Intestinal Injury in a Murine Model of Staphylococcus aureus Pneumonia. Shock 46: 164–172, 2016. doi: 10.1097/SHK.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 118.Dudek M, Puttur F, Arnold-Schrauf C, Kühl AA, Holzmann B, Henriques-Normark B, Berod L, Sparwasser T. Lung epithelium and myeloid cells cooperate to clear acute pneumococcal infection. Mucosal Immunol 9: 1288–1302, 2016. doi: 10.1038/mi.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duggan JM, You D, Cleaver JO, Larson DT, Garza RJ, Guzmán Pruneda FA, Tuvim MJ, Zhang J, Dickey BF, Evans SE. Synergistic interactions of TLR2/6 and TLR9 induce a high level of resistance to lung infection in mice. J Immunol 186: 5916–5926, 2011. doi: 10.4049/jimmunol.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest 120: 2423–2431, 2010. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 113: 4711–4719, 2009. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ebihara S, Ebihara T, Kohzuki M. Effect of aging on cough and swallowing reflexes: implications for preventing aspiration pneumonia. Lung 190: 29–33, 2012. doi: 10.1007/s00408-011-9334-z. [DOI] [PubMed] [Google Scholar]
- 123.Edmond K, Scott S, Korczak V, Ward C, Sanderson C, Theodoratou E, Clark A, Griffiths U, Rudan I, Campbell H. Long term sequelae from childhood pneumonia; systematic review and meta-analysis. PLoS One 7: e31239, 2012. doi: 10.1371/journal.pone.0031239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eguíluz-Gracia I, Schultz HH, Sikkeland LI, Danilova E, Holm AM, Pronk CJ, Agace WW, Iversen M, Andersen C, Jahnsen FL, Baekkevold ES. Long-term persistence of human donor alveolar macrophages in lung transplant recipients. Thorax 71: 1006–1011, 2016. doi: 10.1136/thoraxjnl-2016-208292. [DOI] [PubMed] [Google Scholar]
- 125.Elhaik-Goldman S, Kafka D, Yossef R, Hadad U, Elkabets M, Vallon-Eberhard A, Hulihel L, Jung S, Ghadially H, Braiman A, Apte RN, Mandelboim O, Dagan R, Mizrachi-Nebenzahl Y, Porgador A. The natural cytotoxicity receptor 1 contribution to early clearance of Streptococcus pneumoniae and to natural killer-macrophage cross talk. PLoS One 6: e23472, 2011. doi: 10.1371/journal.pone.0023472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Engel O, Akyüz L, da Costa Goncalves AC, Winek K, Dames C, Thielke M, Herold S, Böttcher C, Priller J, Volk HD, Dirnagl U, Meisel C, Meisel A. Cholinergic Pathway Suppresses Pulmonary Innate Immunity Facilitating Pneumonia After Stroke. Stroke 46: 3232–3240, 2015. doi: 10.1161/STROKEAHA.115.008989. [DOI] [PubMed] [Google Scholar]
- 127.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, Martinez FJ, Huffnagle GB. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 6: e16384, 2011. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-Year Mortality After Community-Acquired Pneumonia. A Prospective Cohort. Am J Respir Crit Care Med 192: 597–604, 2015. doi: 10.1164/rccm.201501-0140OC. [DOI] [PubMed] [Google Scholar]
- 129.Evans SE, Scott BL, Clement CG, Larson DT, Kontoyiannis D, Lewis RE, Lasala PR, Pawlik J, Peterson JW, Chopra AK, Klimpel G, Bowden G, Höök M, Xu Y, Tuvim MJ, Dickey BF. Stimulated innate resistance of lung epithelium protects mice broadly against bacteria and fungi. Am J Respir Cell Mol Biol 42: 40–50, 2010. doi: 10.1165/rcmb.2008-0260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Evans SE, Xu Y, Tuvim MJ, Dickey BF. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol 72: 413–435, 2010. doi: 10.1146/annurev-physiol-021909-135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ewig S, Birkner N, Strauss R, Schaefer E, Pauletzki J, Bischoff H, Schraeder P, Welte T, Hoeffken G. New perspectives on community-acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax 64: 1062–1069, 2009. doi: 10.1136/thx.2008.109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 363: 2233–2247, 2010. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother 58: 654–663, 2014. doi: 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol 14: 24–35, 2014. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Feldman C, Anderson R. Epidemiology, virulence factors and management of the pneumococcus. F1000 Res 5: 2320, 2016. doi: 10.12688/f1000research.9283.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 122: 130–141, 2010. doi: 10.3810/pgm.2010.03.2130. [DOI] [PubMed] [Google Scholar]
- 137.Fink MP, O’Sullivan BP, Menconi MJ, Wollert SP, Wang H, Youssef ME, Bellelsle JM. Effect of granulocyte colony-stimulating factor on systemic and pulmonary responses to endotoxin in pigs. J Trauma 34: 571–577, 1993. doi: 10.1097/00005373-199304000-00015. [DOI] [PubMed] [Google Scholar]
- 138.Finney L, Berry M, Singanayagam A, Elkin SL, Johnston SL, Mallia P. Inhaled corticosteroids and pneumonia in chronic obstructive pulmonary disease. Lancet Respir Med 2: 919–932, 2014. doi: 10.1016/S2213-2600(14)70169-9. [DOI] [PubMed] [Google Scholar]
- 139.Flierl MA, Rittirsch D, Nadeau BA, Sarma JV, Day DE, Lentsch AB, Huber-Lang MS, Ward PA. Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS One 4: e4414, 2009. doi: 10.1371/journal.pone.0004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Flitter BA, Hvorecny KL, Ono E, Eddens T, Yang J, Kwak DH, Bahl CD, Hampton TH, Morisseau C, Hammock BD, Liu X, Lee JS, Kolls JK, Levy BD, Madden DR, Bomberger JM. Pseudomonas aeruginosa sabotages the generation of host proresolving lipid mediators. Proc Natl Acad Sci USA 114: 136–141, 2017. doi: 10.1073/pnas.1610242114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432: 917–921, 2004. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 142.Fonseca Lima EJ, Mello MJ, Albuquerque MF, Lopes MI, Serra GH, Lima DE, Correia JB. Risk factors for community-acquired pneumonia in children under five years of age in the post-pneumococcal conjugate vaccine era in Brazil: a case control study. BMC Pediatr 16: 157, 2016. doi: 10.1186/s12887-016-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Foronjy RF, Dabo AJ, Cummins N, Geraghty P. Leukemia inhibitory factor protects the lung during respiratory syncytial viral infection. BMC Immunol 15: 41, 2014. doi: 10.1186/s12865-014-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Franciszkiewicz K, Salou M, Legoux F, Zhou Q, Cui Y, Bessoles S, Lantz O. MHC class I-related molecule, MR1, and mucosal-associated invariant T cells. Immunol Rev 272: 120–138, 2016. doi: 10.1111/imr.12423. [DOI] [PubMed] [Google Scholar]
- 146.Fraser GL, Devlin JW, Worby CP, Alhazzani W, Barr J, Dasta JF, Kress JP, Davidson JE, Spencer FA. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and meta-analysis of randomized trials. Crit Care Med 41, Suppl 1: S30–S38, 2013. doi: 10.1097/CCM.0b013e3182a16898. [DOI] [PubMed] [Google Scholar]
- 147.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988-2002. JAMA 294: 2712–2719, 2005. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 148.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340: 448–454, 1999. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 149.Gamble L, Bagby GJ, Quinton LJ, Happel KI, Mizgerd JP, Zhang P, Nelson S. The systemic and pulmonary LPS binding protein response to intratracheal lipopolysaccharide. Shock 31: 212–217, 2009. doi: 10.1097/SHK.0b013e31817c0d7d. [DOI] [PubMed] [Google Scholar]
- 150.Gao HN, Lu HZ, Cao B, Du B, Shang H, Gan JH, Lu SH, Yang YD, Fang Q, Shen YZ, Xi XM, Gu Q, Zhou XM, Qu HP, Yan Z, Li FM, Zhao W, Gao ZC, Wang GF, Ruan LX, Wang WH, Ye J, Cao HF, Li XW, Zhang WH, Fang XC, He J, Liang WF, Xie J, Zeng M, Wu XZ, Li J, Xia Q, Jin ZC, Chen Q, Tang C, Zhang ZY, Hou BM, Feng ZX, Sheng JF, Zhong NS, Li LJ. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 368: 2277–2285, 2013. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 151.Garvy BA, Harmsen AG. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation 20: 499–512, 1996. doi: 10.1007/BF01487042. [DOI] [PubMed] [Google Scholar]
- 152.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350: 981–985, 2015. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ge X, Tan V, Bollyky PL, Standifer NE, James EA, Kwok WW. Assessment of seasonal influenza A virus-specific CD4 T-cell responses to 2009 pandemic H1N1 swine-origin influenza A virus. J Virol 84: 3312–3319, 2010. doi: 10.1128/JVI.02226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gessner BD, Adegbola RA. The impact of vaccines on pneumonia: key lessons from Haemophilus influenzae type b conjugate vaccines. Vaccine 26, Suppl 2: B3–B8, 2008. doi: 10.1016/j.vaccine.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 155.Ghio AJ. Disruption of iron homeostasis and lung disease. Biochim Biophys Acta 1790: 731–739, 2009. doi: 10.1016/j.bbagen.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 156.Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, Farrow S, DeMayo F, Hussell T, Worthen GS, Ray D, Loudon A. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med 20: 919–926, 2014. doi: 10.1038/nm.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Giefing C, Meinke AL, Hanner M, Henics T, Bui MD, Gelbmann D, Lundberg U, Senn BM, Schunn M, Habel A, Henriques-Normark B, Ortqvist A, Kalin M, von Gabain A, Nagy E. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med 205: 117–131, 2008. doi: 10.1084/jem.20071168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gill JR, Sheng ZM, Ely SF, Guinee DG, Beasley MB, Suh J, Deshpande C, Mollura DJ, Morens DM, Bray M, Travis WD, Taubenberger JK. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med 134: 235–243, 2010. doi: 10.1043/1543-2165-134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gilley RP, González-Juarbe N, Shenoy AT, Reyes LF, Dube PH, Restrepo MI, Orihuela CJ. Infiltrated Macrophages Die of Pneumolysin-Mediated Necroptosis following Pneumococcal Myocardial Invasion. Infect Immun 84: 1457–1469, 2016. doi: 10.1128/IAI.00007-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol 16: 1114–1123, 2015. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 161.Gold MC, Napier RJ, Lewinsohn DM. MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol Rev 264: 154–166, 2015. doi: 10.1111/imr.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gomez JC, Dang H, Kanke M, Hagan RS, Mock JR, Kelada SNP, Sethupathy P, Doerschuk CM. Predicted effects of observed changes in the mRNA and microRNA transcriptome of lung neutrophils during S. pneumoniae pneumonia in mice. Sci Rep 7: 11258, 2017. doi: 10.1038/s41598-017-11638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gomez JC, Yamada M, Martin JR, Dang H, Brickey WJ, Bergmeier W, Dinauer MC, Doerschuk CM. Mechanisms of interferon-γ production by neutrophils and its function during Streptococcus pneumoniae pneumonia. Am J Respir Cell Mol Biol 52: 349–364, 2015. doi: 10.1165/rcmb.2013-0316OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gomi K, Tokue Y, Kobayashi T, Takahashi H, Watanabe A, Fujita T, Nukiwa T. Mannose-binding lectin gene polymorphism is a modulating factor in repeated respiratory infections. Chest 126: 95–99, 2004. doi: 10.1378/chest.126.1.95. [DOI] [PubMed] [Google Scholar]
- 165.González-Juarbe N, Gilley RP, Hinojosa CA, Bradley KM, Kamei A, Gao G, Dube PH, Bergman MA, Orihuela CJ. Pore-Forming Toxins Induce Macrophage Necroptosis during Acute Bacterial Pneumonia. PLoS Pathog 11: e1005337, 2015. doi: 10.1371/journal.ppat.1005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goronzy JJ, Weyand CM. Successful and Maladaptive T Cell Aging. Immunity 46: 364–378, 2017. doi: 10.1016/j.immuni.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 354: 722–726, 2016. doi: 10.1126/science.aag1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Grau I, Ardanuy C, Calatayud L, Schulze MH, Liñares J, Pallares R. Smoking and alcohol abuse are the most preventable risk factors for invasive pneumonia and other pneumococcal infections. Int J Infect Dis 25: 59–64, 2014. doi: 10.1016/j.ijid.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 169.Gray DM, Zar HJ. Community-acquired pneumonia in HIV-infected children: a global perspective. Curr Opin Pulm Med 16: 208–216, 2010. [DOI] [PubMed] [Google Scholar]
- 170.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol 155: 722–729, 1995. [PubMed] [Google Scholar]
- 171.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Laichalk LL, McGillicuddy DC, Standiford TJ. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis 173: 159–165, 1996. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 172.Greene CM, Marciniak SJ, Teckman J, Ferrarotti I, Brantly ML, Lomas DA, Stoller JK, McElvaney NG. α1-Antitrypsin deficiency. Nat Rev Dis Primers 2: 16051, 2016. doi: 10.1038/nrdp.2016.51. [DOI] [PubMed] [Google Scholar]
- 173.Gregory AD, Hogue LA, Ferkol TW, Link DC. Regulation of systemic and local neutrophil responses by G-CSF during pulmonary Pseudomonas aeruginosa infection. Blood 109: 3235–3243, 2007. doi: 10.1182/blood-2005-01-015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 369: 155–163, 2013. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Grijalva CG, Zhu Y, Williams DJ, Self WH, Ampofo K, Pavia AT, Stockmann CR, McCullers J, Arnold SR, Wunderink RG, Anderson EJ, Lindstrom S, Fry AM, Foppa IM, Finelli L, Bramley AM, Jain S, Griffin MR, Edwards KM. Association Between Hospitalization With Community-Acquired Laboratory-Confirmed Influenza Pneumonia and Prior Receipt of Influenza Vaccination. JAMA 314: 1488–1497, 2015. doi: 10.1001/jama.2015.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Grimm MJ, Vethanayagam RR, Almyroudis NG, Dennis CG, Khan AN, D’Auria AC, Singel KL, Davidson BA, Knight PR, Blackwell TS, Hohl TM, Mansour MK, Vyas JM, Röhm M, Urban CF, Kelkka T, Holmdahl R, Segal BH. Monocyte- and macrophage-targeted NADPH oxidase mediates antifungal host defense and regulation of acute inflammation in mice. J Immunol 190: 4175–4184, 2013. doi: 10.4049/jimmunol.1202800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210: 1977–1992, 2013. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Haas S, Hansson J, Klimmeck D, Loeffler D, Velten L, Uckelmann H, Wurzer S, Prendergast AM, Schnell A, Hexel K, Santarella-Mellwig R, Blaszkiewicz S, Kuck A, Geiger H, Milsom MD, Steinmetz LM, Schroeder T, Trumpp A, Krijgsveld J, Essers MA. Inflammation-Induced Emergency Megakaryopoiesis Driven by Hematopoietic Stem Cell-like Megakaryocyte Progenitors. Cell Stem Cell 17: 422–434, 2015. doi: 10.1016/j.stem.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 179.Hahn I, Klaus A, Janze AK, Steinwede K, Ding N, Bohling J, Brumshagen C, Serrano H, Gauthier F, Paton JC, Welte T, Maus UA. Cathepsin G and neutrophil elastase play critical and nonredundant roles in lung-protective immunity against Streptococcus pneumoniae in mice. Infect Immun 79: 4893–4901, 2011. doi: 10.1128/IAI.05593-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hajjar AM, Harowicz H, Liggitt HD, Fink PJ, Wilson CB, Skerrett SJ. An essential role for non-bone marrow-derived cells in control of Pseudomonas aeruginosa pneumonia. Am J Respir Cell Mol Biol 33: 470–475, 2005. doi: 10.1165/rcmb.2005-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hallstrand TS, Hackett TL, Altemeier WA, Matute-Bello G, Hansbro PM, Knight DA. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol 151: 1–15, 2014. doi: 10.1016/j.clim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 182.Hamada H, Garcia-Hernandez ML, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol 182: 3469–3481, 2009. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hamer DH, Sempértegui F, Estrella B, Tucker KL, Rodríguez A, Egas J, Dallal GE, Selhub J, Griffiths JK, Meydani SN. Micronutrient deficiencies are associated with impaired immune response and higher burden of respiratory infections in elderly Ecuadorians. J Nutr 139: 113–119, 2009. doi: 10.3945/jn.108.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Han S, Mallampalli RK. The Role of Surfactant in Lung Disease and Host Defense Against Pulmonary Infections. Ann Am Thorac Soc 12: 765–774, 2015. doi: 10.1513/AnnalsATS.201411-507FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hansen V, Oren E, Dennis LK, Brown HE. Infectious Disease Mortality Trends in the United States, 1980-2014. JAMA 316: 2149–2151, 2016. doi: 10.1001/jama.2016.12423. [DOI] [PubMed] [Google Scholar]
- 186.Hasday JD, Shah N, Mackowiak PA, Tulapurkar M, Nagarsekar A, Singh I. Fever, hyperthermia, and the lung: it’s all about context and timing. Trans Am Clin Climatol Assoc 122: 34–47, 2011. [PMC free article] [PubMed] [Google Scholar]
- 187.Hashimoto S, Pittet JF, Hong K, Folkesson H, Bagby G, Kobzik L, Frevert C, Watanabe K, Tsurufuji S, Wiener-Kronish J. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am J Physiol Lung Cell Mol Physiol 270: L819–L828, 1996. [DOI] [PubMed] [Google Scholar]
- 188.Hayden LP, Hobbs BD, Cohen RT, Wise RA, Checkley W, Crapo JD, Hersh CP; COPDGene Investigators . Childhood pneumonia increases risk for chronic obstructive pulmonary disease: the COPDGene study. Respir Res 16: 115, 2015. doi: 10.1186/s12931-015-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hayes BH, Haberling DL, Kennedy JL, Varma JK, Fry AM, Vora NM. Burden of Pneumonia-Associated Hospitalizations: United States, 2001-2014. Chest 153: 427–437, 2018. doi: 10.1016/j.chest.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.He Y, Wen Q, Yao F, Xu D, Huang Y, Wang J. Gut-lung axis: the microbial contributions and clinical implications. Crit Rev Microbiol 43: 81–95, 2017. doi: 10.1080/1040841X.2016.1176988. [DOI] [PubMed] [Google Scholar]
- 191.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, DeMaria GC, Matsui H, Donaldson SH, Davis CW, Sheehan JK, Boucher RC, Kesimer M. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest 124: 3047–3060, 2014. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Henson PM. Cell Removal: Efferocytosis. Annu Rev Cell Dev Biol 33: 127–144, 2017. doi: 10.1146/annurev-cellbio-111315-125315. [DOI] [PubMed] [Google Scholar]
- 193.Henson PM, Tuder RM. Apoptosis in the lung: induction, clearance and detection. Am J Physiol Lung Cell Mol Physiol 294: L601–L611, 2008. doi: 10.1152/ajplung.00320.2007. [DOI] [PubMed] [Google Scholar]
- 194.Herbold W, Maus R, Hahn I, Ding N, Srivastava M, Christman JW, Mack M, Reutershan J, Briles DE, Paton JC, Winter C, Welte T, Maus UA. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect Immun 78: 2620–2630, 2010. doi: 10.1128/IAI.01169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, Albrecht J, Driever F, Vadasz I, Seeger W, Steinmueller M, Lohmeyer J. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med 183: 1380–1390, 2011. doi: 10.1164/rccm.201009-1431OC. [DOI] [PubMed] [Google Scholar]
- 196.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM; Canadian Critical Care Trials Group . Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 364: 1293–1304, 2011. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 197.Hesker PR, Krupnick AS. The role of natural killer cells in pulmonary immunosurveillance. Front Biosci (Schol Ed) 5: 575–587, 2013. doi: 10.2741/S391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hess C, Herr C, Beisswenger C, Zakharkina T, Schmid RM, Bals R. Myeloid RelA regulates pulmonary host defense networks. Eur Respir J 35: 343–352, 2010. doi: 10.1183/09031936.00196408. [DOI] [PubMed] [Google Scholar]
- 199.Hickman-Davis JM, O’Reilly P, Davis IC, Peti-Peterdi J, Davis G, Young KR, Devlin RB, Matalon S. Killing of Klebsiella pneumoniae by human alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 282: L944–L956, 2002. doi: 10.1152/ajplung.00216.2001. [DOI] [PubMed] [Google Scholar]
- 200.Hilliard KL, Allen E, Traber KE, Kim Y, Wasserman GA, Jones MR, Mizgerd JP, Quinton LJ. Activation of Hepatic STAT3 Maintains Pulmonary Defense During Endotoxemia. Infect Immun 83: 4015–4027, 2015. doi: 10.1128/IAI.00464-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hilliard KL, Allen E, Traber KE, Yamamoto K, Stauffer NM, Wasserman GA, Jones MR, Mizgerd JP, Quinton LJ. The Lung-Liver Axis: A Requirement for Maximal Innate Immunity and Hepatoprotection During Pneumonia. Am J Respir Cell Mol Biol 53: 378–390, 2015. doi: 10.1165/rcmb.2014-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, Cookson WO. Disordered microbial communities in asthmatic airways. PLoS One 5: e8578, 2010. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and Toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis 200: 546–554, 2009. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hoesch RE, Lin E, Young M, Gottesman RF, Altaweel L, Nyquist PA, Stevens RD. Acute lung injury in critical neurological illness. Crit Care Med 40: 587–593, 2012. doi: 10.1097/CCM.0b013e3182329617. [DOI] [PubMed] [Google Scholar]
- 205.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med 193: 981–986, 2001. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl AK, Hull WM, Wert SE, Whitsett JA. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Invest 113: 28–37, 2004. doi: 10.1172/JCI19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 18: 673–683, 2012. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 208.Hombrink P, Helbig C, Backer RA, Piet B, Oja AE, Stark R, Brasser G, Jongejan A, Jonkers RE, Nota B, Basak O, Clevers HC, Moerland PD, Amsen D, van Lier RA. Programs for the persistence, vigilance and control of human CD8+lung-resident memory T cells. Nat Immunol 17: 1467–1478, 2016. doi: 10.1038/ni.3589. [DOI] [PubMed] [Google Scholar]
- 209.Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol 10: 299–306, 2017. doi: 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Husain KD, Stromberg PE, Javadi P, Buchman TG, Karl IE, Hotchkiss RS, Coopersmith CM. Bcl-2 inhibits gut epithelial apoptosis induced by acute lung injury in mice but has no effect on survival. Shock 20: 437–443, 2003. doi: 10.1097/01.shk.0000094559.76615.1c. [DOI] [PubMed] [Google Scholar]
- 211.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 14: 81–93, 2014. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 212.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 109: 41–50, 2002. doi: 10.1172/JCI0211638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ichikawa A, Kuba K, Morita M, Chida S, Tezuka H, Hara H, Sasaki T, Ohteki T, Ranieri VM, dos Santos CC, Kawaoka Y, Akira S, Luster AD, Lu B, Penninger JM, Uhlig S, Slutsky AS, Imai Y. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am J Respir Crit Care Med 187: 65–77, 2013. doi: 10.1164/rccm.201203-0508OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA 108: 5354–5359, 2011. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ikegami M, Falcone A, Whitsett JA. STAT-3 regulates surfactant phospholipid homeostasis in normal lung and during endotoxin-mediated lung injury. J Appl Physiol (1985) 104: 1753–1760, 2008. doi: 10.1152/japplphysiol.00875.2007. [DOI] [PubMed] [Google Scholar]
- 216.Inghammar M, Engström G, Kahlmeter G, Ljungberg B, Löfdahl CG, Egesten A. Invasive pneumococcal disease in patients with an underlying pulmonary disorder. Clin Microbiol Infect 19: 1148–1154, 2013. doi: 10.1111/1469-0691.12182. [DOI] [PubMed] [Google Scholar]
- 218.Iribarren C, Chandra M, Rana JS, Hlatky MA, Fortmann SP, Quertermous T, Go AS. High-sensitivity cardiac troponin I and incident coronary heart disease among asymptomatic older adults. Heart 102: 1177–1182, 2016. doi: 10.1136/heartjnl-2015-309136. [DOI] [PubMed] [Google Scholar]
- 219.Islam MA, Uddin MJ, Tholen E, Tesfaye D, Looft C, Schellander K, Cinar MU. Age-related changes in phagocytic activity and production of pro-inflammatory cytokines by lipopolysaccharide stimulated porcine alveolar macrophages. Cytokine 60: 707–717, 2012. doi: 10.1016/j.cyto.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 220.Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, Blanc F, De Trez C, Van Maele L, Dumoutier L, Huerre MR, Eberl G, Si-Tahar M, Gosset P, Renauld JC, Sirard JC, Faveeuw C, Trottein F. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J Virol 87: 6911–6924, 2013. doi: 10.1128/JVI.02943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Iwai S, Huang D, Fong S, Jarlsberg LG, Worodria W, Yoo S, Cattamanchi A, Davis JL, Kaswabuli S, Segal M, Huang L, Lynch SV. The lung microbiome of Ugandan HIV-infected pneumonia patients is compositionally and functionally distinct from that of San Franciscan patients. PLoS One 9: e95726, 2014. doi: 10.1371/journal.pone.0095726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med 153: 204–205, 2010. doi: 10.7326/0003-4819-153-3-201008030-00013. [DOI] [PubMed] [Google Scholar]
- 223.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 60: 1070–1077, 2012. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304: 1787–1794, 2010. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jackson S, Mathews KH, Pulanic D, Falconer R, Rudan I, Campbell H, Nair H. Risk factors for severe acute lower respiratory infections in children: a systematic review and meta-analysis. Croat Med J 54: 110–121, 2013. doi: 10.3325/cmj.2013.54.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jaillon S, Galdiero MR, Del Prete D, Cassatella MA, Garlanda C, Mantovani A. Neutrophils in innate and adaptive immunity. Semin Immunopathol 35: 377–394, 2013. doi: 10.1007/s00281-013-0374-8. [DOI] [PubMed] [Google Scholar]
- 227.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, Chappell JD, Qi C, Hart EM, Carroll F, Trabue C, Donnelly HK, Williams DJ, Zhu Y, Arnold SR, Ampofo K, Waterer GW, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, McCullers JA, Pavia AT, Edwards KM, Finelli L; CDC EPIC Study Team . Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med 373: 415–427, 2015. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, Stockmann C, Anderson EJ, Grijalva CG, Self WH, Zhu Y, Patel A, Hymas W, Chappell JD, Kaufman RA, Kan JH, Dansie D, Lenny N, Hillyard DR, Haynes LM, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, Wunderink RG, Edwards KM, Pavia AT, McCullers JA, Finelli L; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 372: 835–845, 2015. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med 184: 547–560, 2011. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jartti T, Jartti L, Ruuskanen O, Söderlund-Venermo M. New respiratory viral infections. Curr Opin Pulm Med 18: 271–278, 2012. doi: 10.1097/MCP.0b013e328351f8d4. [DOI] [PubMed] [Google Scholar]
- 231.Jegaskanda S, Luke C, Hickman HD, Sangster MY, Wieland-Alter WF, McBride JM, Yewdell JW, Wright PF, Treanor J, Rosenberger CM, Subbarao K. Generation and Protective Ability of Influenza Virus-Specific Antibody-Dependent Cellular Cytotoxicity in Humans Elicited by Vaccination, Natural Infection, and Experimental Challenge. J Infect Dis 214: 945–952, 2016. doi: 10.1093/infdis/jiw262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jelley-Gibbs DM, Dibble JP, Filipson S, Haynes L, Kemp RA, Swain SL. Repeated stimulation of CD4 effector T cells can limit their protective function. J Exp Med 201: 1101–1112, 2005. doi: 10.1084/jem.20041852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jenkin C, Palmer DL. Changes in the titre of serum opsonins and phagocytic properties of mouse peritoneal macrophages following injection of endotoxin. J Exp Med 112: 419–429, 1960. doi: 10.1084/jem.112.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol 32: 531–539, 2005. doi: 10.1165/rcmb.2005-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Johnson EE, Wessling-Resnick M. Iron metabolism and the innate immune response to infection. Microbes Infect 14: 207–216, 2012. doi: 10.1016/j.micinf.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Johnston ID. Effect of pneumonia in childhood on adult lung function. J Pediatr 135: 33–37, 1999. [PubMed] [Google Scholar]
- 237.Johnston ID, Strachan DP, Anderson HR. Effect of pneumonia and whooping cough in childhood on adult lung function. N Engl J Med 338: 581–587, 1998. doi: 10.1056/NEJM199802263380904. [DOI] [PubMed] [Google Scholar]
- 238.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol 47: 417–426, 2012. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jones MR, Quinton LJ, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia. J Infect Dis 193: 360–369, 2006. doi: 10.1086/499312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol 175: 7530–7535, 2005. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jonigk D, Al-Omari M, Maegel L, Müller M, Izykowski N, Hong J, Hong K, Kim SH, Dorsch M, Mahadeva R, Laenger F, Kreipe H, Braun A, Shahaf G, Lewis EC, Welte T, Dinarello CA, Janciauskiene S. Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc Natl Acad Sci USA 110: 15007–15012, 2013. doi: 10.1073/pnas.1309648110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Joseph C, Togawa Y, Shindo N. Bacterial and viral infections associated with influenza. Influenza Other Respi Viruses 7, Suppl 2: 105–113, 2013. doi: 10.1111/irv.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, Almond M, Wong EH, Sykes A, Maybeno M, Del Rosario J, Trujillo-Torralbo MB, Mallia P, Sidney J, Peters B, Kon OM, Sette A, Johnston SL, Openshaw PJ, Chiu C. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun 6: 10224, 2015. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jubrail J, Morris P, Bewley MA, Stoneham S, Johnston SA, Foster SJ, Peden AA, Read RC, Marriott HM, Dockrell DH. Inability to sustain intraphagolysosomal killing of Staphylococcus aureus predisposes to bacterial persistence in macrophages. Cell Microbiol 18: 80–96, 2016. doi: 10.1111/cmi.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kalla C, Raveh D, Algur N, Rudensky B, Yinnon AM, Balkin J. Incidence and significance of a positive troponin test in bacteremic patients without acute coronary syndrome. Am J Med 121: 909–915, 2008. doi: 10.1016/j.amjmed.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 246.Kamata H, Yamamoto K, Wasserman GA, Zabinski MC, Yuen CK, Lung WY, Gower AC, Belkina AC, Ramirez MI, Deng JC, Quinton LJ, Jones MR, Mizgerd JP. Epithelial Cell-Derived Secreted and Transmembrane 1a Signals to Activated Neutrophils During Pneumococcal Pneumonia. Am J Respir Cell Mol Biol 55: 407–418, 2016. doi: 10.1165/rcmb.2015-0261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kanegai CM, Xi Y, Donne ML, Gotts JE, Driver IH, Amidzic G, Lechner AJ, Jones KD, Vaughan AE, Chapman HA, Rock JR. Persistent Pathology in Influenza-Infected Mouse Lungs. Am J Respir Cell Mol Biol 55: 613–615, 2016. doi: 10.1165/rcmb.2015-0387LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J III. Factors of risk in the development of coronary heart disease–six year follow-up experience. The Framingham Study. Ann Intern Med 55: 33–50, 1961. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 249.Kash JC, Taubenberger JK. The role of viral, host, and secondary bacterial factors in influenza pathogenesis. Am J Pathol 185: 1528–1536, 2015. doi: 10.1016/j.ajpath.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kaynar AM, Yende S, Zhu L, Frederick DR, Chambers R, Burton CL, Carter M, Stolz DB, Agostini B, Gregory AD, Nagarajan S, Shapiro SD, Angus DC. Effects of intra-abdominal sepsis on atherosclerosis in mice. Crit Care 18: 469, 2014. doi: 10.1186/s13054-014-0469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kew Kayleigh M, Seniukovich A. Inhaled Steroids and Risk of Pneumonia for Chronic Obstructive Pulmonary Disease. New York: Wiley, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kida H, Mucenski ML, Thitoff AR, Le Cras TD, Park KS, Ikegami M, Müller W, Whitsett JA. GP130-STAT3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am J Pathol 172: 1542–1554, 2008. doi: 10.2353/ajpath.2008.071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kienle K, Lämmermann T. Neutrophil swarming: an essential process of the neutrophil tissue response. Immunol Rev 273: 76–93, 2016. doi: 10.1111/imr.12458. [DOI] [PubMed] [Google Scholar]
- 254.Kim KC. Role of epithelial mucins during airway infection. Pulm Pharmacol Ther 25: 415–419, 2012. doi: 10.1016/j.pupt.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, Wachtel S, Bueno S, Prince A. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog 11: e1004820, 2015. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Klingensmith NJ, Coopersmith CM. The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness. Crit Care Clin 32: 203–212, 2016. doi: 10.1016/j.ccc.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Knapp S, Hareng L, Rijneveld AW, Bresser P, van der Zee JS, Florquin S, Hartung T, van der Poll T. Activation of neutrophils and inhibition of the proinflammatory cytokine response by endogenous granulocyte colony-stimulating factor in murine pneumococcal pneumonia. J Infect Dis 189: 1506–1515, 2004. doi: 10.1086/382962. [DOI] [PubMed] [Google Scholar]
- 258.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med 188: 913–922, 2013. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kobzik L. Searching for a Lifeline: Transcriptome Profiling Studies of Influenza Susceptibility and Resistance. J Innate Immun 9: 232–242, 2017. doi: 10.1159/000457902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13: 159–175, 2013. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 261.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 37: 771–783, 2012. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Konter JM, Parker JL, Baez E, Li SZ, Ranscht B, Denzel M, Little FF, Nakamura K, Ouchi N, Fine A, Walsh K, Summer RS. Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J Immunol 188: 854–863, 2012. doi: 10.4049/jimmunol.1100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Koppel EA, Litjens M, van den Berg VC, van Kooyk Y, Geijtenbeek TB. Interaction of SIGNR1 expressed by marginal zone macrophages with marginal zone B cells is essential to early IgM responses against Streptococcus pneumoniae. Mol Immunol 45: 2881–2887, 2008. doi: 10.1016/j.molimm.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 264.Korfhagen TR, Kitzmiller J, Chen G, Sridharan A, Haitchi HM, Hegde RS, Divanovic S, Karp CL, Whitsett JA. SAM-pointed domain ETS factor mediates epithelial cell-intrinsic innate immune signaling during airway mucous metaplasia. Proc Natl Acad Sci USA 109: 16630–16635, 2012. doi: 10.1073/pnas.1208092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kornum JB, Nørgaard M, Dethlefsen C, Due KM, Thomsen RW, Tjønneland A, Sørensen HT, Overvad K. Obesity and risk of subsequent hospitalisation with pneumonia. Eur Respir J 36: 1330–1336, 2010. doi: 10.1183/09031936.00184209. [DOI] [PubMed] [Google Scholar]
- 266.Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med 20: 822–832, 2014. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kovach MA, Singer B, Martinez-Colon G, Newstead MW, Zeng X, Mancuso P, Moore TA, Kunkel SL, Peters-Golden M, Moore BB, Standiford TJ. IL-36γ is a crucial proximal component of protective type-1-mediated lung mucosal immunity in Gram-positive and -negative bacterial pneumonia. Mucosal Immunol 10: 1320–1334, 2017. doi: 10.1038/mi.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 342: 1471–1477, 2000. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 269.Krijgsveld J, Zaat SA, Meeldijk J, van Veelen PA, Fang G, Poolman B, Brandt E, Ehlert JE, Kuijpers AJ, Engbers GH, Feijen J, Dankert J. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J Biol Chem 275: 20374–20381, 2000. doi: 10.1074/jbc.275.27.20374. [DOI] [PubMed] [Google Scholar]
- 270.Kristinsson SY, Gridley G, Hoover RN, Check D, Landgren O. Long-term risks after splenectomy among 8,149 cancer-free American veterans: a cohort study with up to 27 years follow-up. Haematologica 99: 392–398, 2014. doi: 10.3324/haematol.2013.092460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Krumholz HM. Post-hospital syndrome–an acquired, transient condition of generalized risk. N Engl J Med 368: 100–102, 2013. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol 6: 69–82, 2013. doi: 10.1038/mi.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang Y, Lim B, Chow VT, Crum CP, Xian W, McKeon F. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147: 525–538, 2011. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kurkjian C, Hollifield M, Lines JL, Rogosky A, Empey KM, Qureshi M, Brown SA, Garvy BA. Alveolar macrophages in neonatal mice are inherently unresponsive to Pneumocystis murina infection. Infect Immun 80: 2835–2846, 2012. doi: 10.1128/IAI.05707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kurland G, Cheung AT, Miller ME, Ayin SA, Cho MM, Ford EW. The ontogeny of pulmonary defenses: alveolar macrophage function in neonatal and juvenile rhesus monkeys. Pediatr Res 23: 293–297, 1988. doi: 10.1203/00006450-198803000-00013. [DOI] [PubMed] [Google Scholar]
- 276.Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, Cauley LS, Craft J, Kaech SM. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41: 633–645, 2014. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lamberti LM, Zakarija-Grković I, Fischer Walker CL, Theodoratou E, Nair H, Campbell H, Black RE. Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: a systematic literature review and meta-analysis. BMC Public Health 13, Suppl 3: S18, 2013. doi: 10.1186/1471-2458-13-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lê VB, Schneider JG, Boergeling Y, Berri F, Ducatez M, Guerin JL, Adrian I, Errazuriz-Cerda E, Frasquilho S, Antunes L, Lina B, Bordet JC, Jandrot-Perrus M, Ludwig S, Riteau B. Platelet activation and aggregation promote lung inflammation and influenza virus pathogenesis. Am J Respir Crit Care Med 191: 804–819, 2015. doi: 10.1164/rccm.201406-1031OC. [DOI] [PubMed] [Google Scholar]
- 279.Lechner AJ, Driver IH, Lee J, Conroy CM, Nagle A, Locksley RM, Rock JR. Recruited monocytes and type 2 immunity promote lung regeneration following pneumonectomy. Cell Stem Cell 21: 120–134.e7, 2017. doi: 10.1016/j.stem.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lee B, Boucher HW. Targeting antimicrobial-resistant bacterial respiratory tract pathogens: it is time to ‘get smart’. Curr Opin Pulm Med 21: 293–303, 2015. doi: 10.1097/MCP.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 281.Lee CC, Middaugh NA, Howie SR, Ezzati M. Association of secondhand smoke exposure with pediatric invasive bacterial disease and bacterial carriage: a systematic review and meta-analysis. PLoS Med 7: e1000374, 2010. doi: 10.1371/journal.pmed.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, Krummel MF, Leavitt AD, Passegué E, Looney MR. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544: 105–109, 2017. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.LeibundGut-Landmann S, Weidner K, Hilbi H, Oxenius A. Nonhematopoietic cells are key players in innate control of bacterial airway infection. J Immunol 186: 3130–3137, 2011. doi: 10.4049/jimmunol.1003565. [DOI] [PubMed] [Google Scholar]
- 284.Leiva-Juárez MM, Ware HH, Kulkarni VV, Zweidler-McKay PA, Tuvim MJ, Evans SE. Inducible epithelial resistance protects mice against leukemia-associated pneumonia. Blood 128: 982–992, 2016. doi: 10.1182/blood-2016-03-708511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol 76: 467–492, 2014. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Li Y, Du H, Qin Y, Roberts J, Cummings OW, Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res 67: 8494–8503, 2007. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- 287.Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, Shipley JM, Li T, Senior RM, Du H, Yan C. Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol 174: 7250–7256, 2005. doi: 10.4049/jimmunol.174.11.7250. [DOI] [PubMed] [Google Scholar]
- 288.Liang Y, Harris FL, Brown LA. Alcohol induced mitochondrial oxidative stress and alveolar macrophage dysfunction. BioMed Res Int 2014: 371593, 2014. doi: 10.1155/2014/371593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lim K, Hyun YM, Lambert-Emo K, Capece T, Bae S, Miller R, Topham DJ, Kim M. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science 349: aaa4352, 2015. doi: 10.1126/science.aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lindenauer PK, Lagu T, Shieh M-S, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA 307: 1405–1413, 2012. doi: 10.1001/jama.2012.384. [DOI] [PubMed] [Google Scholar]
- 291.Linster M, van Boheemen S, de Graaf M, Schrauwen EJA, Lexmond P, Mänz B, Bestebroer TM, Baumann J, van Riel D, Rimmelzwaan GF, Osterhaus ADME, Matrosovich M, Fouchier RAM, Herfst S. Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 157: 329–339, 2014. doi: 10.1016/j.cell.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Liu Y, Di ME, Chu HW, Liu X, Wang L, Wenzel S, Di YP. Increased susceptibility to pulmonary Pseudomonas infection in Splunc1 knockout mice. J Immunol 191: 4259–4268, 2013. doi: 10.4049/jimmunol.1202340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Liu Y, Sadikot RT, Adami GR, Kalinichenko VV, Pendyala S, Natarajan V, Zhao YY, Malik AB. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J Exp Med 208: 1473–1484, 2011. doi: 10.1084/jem.20102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16: 161–168, 2016. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 295.Lobo SM, Orrico SR, Queiroz MM, Cunrath GS, Chibeni GS, Contrin LM, Cury PM, Burdmann EA, de Oliveira Machado AM, Togni P, De Backer D, Preiser JC, Szabó C, Vincent JL. Pneumonia-induced sepsis and gut injury: effects of a poly-(ADP-ribose) polymerase inhibitor. J Surg Res 129: 292–297, 2005. doi: 10.1016/j.jss.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 296.Loeb MB. Use of a broader determinants of health model for community-acquired pneumonia in seniors. Clin Infect Dis 38: 1293–1297, 2004. doi: 10.1086/383469. [DOI] [PubMed] [Google Scholar]
- 297.Loh L, Wang Z, Sant S, Koutsakos M, Jegaskanda S, Corbett AJ, Liu L, Fairlie DP, Crowe J, Rossjohn J, Xu J, Doherty PC, McCluskey J, Kedzierska K. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc Natl Acad Sci USA 113: 10133–10138, 2016. doi: 10.1073/pnas.1610750113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lopez Bernal JA, Upton MN, Henderson AJ, Dedman D, McCarthy A, Davey Smith G, Ben-Shlomo Y. Lower respiratory tract infection in the first year of life is associated with worse lung function in adult life: prospective results from the Barry Caerphilly Growth study. Ann Epidemiol 23: 422–427, 2013. doi: 10.1016/j.annepidem.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 299.Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature 456: 989–992, 2008. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lugade AA, Bogner PN, Thatcher TH, Sime PJ, Phipps RP, Thanavala Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J Immunol 192: 5226–5235, 2014. doi: 10.4049/jimmunol.1302584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lundgren A, Bhuiyan TR, Novak D, Kaim J, Reske A, Lu YJ, Qadri F, Malley R. Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine 30: 3897–3907, 2012. doi: 10.1016/j.vaccine.2012.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, Carbone FR. T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 43: 1101–1111, 2015. doi: 10.1016/j.immuni.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 303.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370: 1198–1208, 2014. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Magri G, Miyajima M, Bascones S, Mortha A, Puga I, Cassis L, Barra CM, Comerma L, Chudnovskiy A, Gentile M, Llige D, Cols M, Serrano S, Aróstegui JI, Juan M, Yagüe J, Merad M, Fagarasan S, Cerutti A. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol 15: 354–364, 2014. doi: 10.1038/ni.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Mammen MJ, Sethi S. COPD and the microbiome. Respirology 21: 590–599, 2016. doi: 10.1111/resp.12732. [DOI] [PubMed] [Google Scholar]
- 306.Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 15: 259–270, 2017. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Mancuso P, O Brien E, Prano J, Goel D, Aronoff DM. No Impairment in host defense against Streptococcus pneumoniae in obese CPEfat/fat mice. PLoS One 9: e106420, 2014. doi: 10.1371/journal.pone.0106420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol 168: 4018–4024, 2002. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 309.Mancuso P, Myers MG Jr, Goel D, Serezani CH, O’Brien E, Goldberg J, Aronoff DM, Peters-Golden M. Ablation of leptin receptor-mediated ERK activation impairs host defense against Gram-negative pneumonia. J Immunol 189: 867–875, 2012. doi: 10.4049/jimmunol.1200465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Mancuso P, Peters-Golden M, Goel D, Goldberg J, Brock TG, Greenwald-Yarnell M, Myers MG Jr. Disruption of leptin receptor-STAT3 signaling enhances leukotriene production and pulmonary host defense against pneumococcal pneumonia. J Immunol 186: 1081–1090, 2011. doi: 10.4049/jimmunol.1001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol 14: 302–314, 2014. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 312.Marriott HM, Bingle CD, Read RC, Braley KE, Kroemer G, Hellewell PG, Craig RW, Whyte MK, Dockrell DH. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J Clin Invest 115: 359–368, 2005. doi: 10.1172/JCI200521766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Marriott HM, Gascoyne KA, Gowda R, Geary I, Nicklin MJ, Iannelli F, Pozzi G, Mitchell TJ, Whyte MK, Sabroe I, Dockrell DH. Interleukin-1β regulates CXCL8 release and influences disease outcome in response to Streptococcus pneumoniae, defining intercellular cooperation between pulmonary epithelial cells and macrophages. Infect Immun 80: 1140–1149, 2012. doi: 10.1128/IAI.05697-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Matsuzaki Y, Xu Y, Ikegami M, Besnard V, Park KS, Hull WM, Wert SE, Whitsett JA. Stat3 is required for cytoprotection of the respiratory epithelium during adenoviral infection. J Immunol 177: 527–537, 2006. doi: 10.4049/jimmunol.177.1.527. [DOI] [PubMed] [Google Scholar]
- 315.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 6: 147–163, 2011. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Maus U, Herold S, Muth H, Maus R, Ermert L, Ermert M, Weissmann N, Rosseau S, Seeger W, Grimminger F, Lohmeyer J. Monocytes recruited into the alveolar air space of mice show a monocytic phenotype but upregulate CD14. Am J Physiol Lung Cell Mol Physiol 280: L58–L68, 2001. doi: 10.1152/ajplung.2001.280.1.L58. [DOI] [PubMed] [Google Scholar]
- 317.Maus U, Huwe J, Ermert L, Ermert M, Seeger W, Lohmeyer J. Molecular pathways of monocyte emigration into the alveolar air space of intact mice. Am J Respir Crit Care Med 165: 95–100, 2002. doi: 10.1164/ajrccm.165.1.2106148. [DOI] [PubMed] [Google Scholar]
- 318.Maus U, von Grote K, Kuziel WA, Mack M, Miller EJ, Cihak J, Stangassinger M, Maus R, Schlöndorff D, Seeger W, Lohmeyer J. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med 166: 268–273, 2002. doi: 10.1164/rccm.2112012. [DOI] [PubMed] [Google Scholar]
- 319.Maus UA, Waelsch K, Kuziel WA, Delbeck T, Mack M, Blackwell TS, Christman JW, Schlöndorff D, Seeger W, Lohmeyer J. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2-CCR2 axis. J Immunol 170: 3273–3278, 2003. doi: 10.4049/jimmunol.170.6.3273. [DOI] [PubMed] [Google Scholar]
- 320.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 5: 4–11, 2014. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 12: 252–262, 2014. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 322.McElhaney JE, Kuchel GA, Zhou X, Swain SL, Haynes L. T-Cell Immunity to Influenza in Older Adults: A Pathophysiological Framework for Development of More Effective Vaccines. Front Immunol 7: 41, 2016. doi: 10.3389/fimmu.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-γ Production. J Immunol 195: 203–209, 2015. doi: 10.4049/jimmunol.1402975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol 5: 606–616, 2005. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 325.Mehta AJ, Guidot DM, Weber KT. Alcohol abuse, the alveolar macrophage and pneumonia. Am J Med Sci 343: 244–247, 2012. doi: 10.1097/MAJ.0b013e31823ede77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Mehta AJ, Yeligar SM, Elon L, Brown LA, Guidot DM. Alcoholism causes alveolar macrophage zinc deficiency and immune dysfunction. Am J Respir Crit Care Med 188: 716–723, 2013. doi: 10.1164/rccm.201301-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Mei J, Liu Y, Dai N, Favara M, Greene T, Jeyaseelan S, Poncz M, Lee JS, Worthen GS. CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity 33: 106–117, 2010. doi: 10.1016/j.immuni.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Metersky ML, Wang Y, Klompas M, Eckenrode S, Bakullari A, Eldridge N. Trend in Ventilator-Associated Pneumonia Rates Between 2005 and 2013. JAMA 316: 2427–2429, 2016. doi: 10.1001/jama.2016.16226. [DOI] [PubMed] [Google Scholar]
- 329.Meyer KC, Ershler W, Rosenthal NS, Lu XG, Peterson K. Immune dysregulation in the aging human lung. Am J Respir Crit Care Med 153: 1072–1079, 1996. doi: 10.1164/ajrccm.153.3.8630547. [DOI] [PubMed] [Google Scholar]
- 330.Michaud CM, Murray CJL, Bloom BR. Burden of disease–implications for future research. JAMA 285: 535–539, 2001. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 331.Michels KR, Zhang Z, Bettina AM, Cagnina RE, Stefanova D, Burdick MD, Vaulont S, Nemeth E, Ganz T, Mehrad B. Hepcidin-mediated iron sequestration protects against bacterial dissemination during pneumonia. JCI Insight 2: e92002, 2017. doi: 10.1172/jci.insight.92002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Mijares LA, Wangdi T, Sokol C, Homer R, Medzhitov R, Kazmierczak BI. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. J Immunol 186: 7080–7088, 2011. doi: 10.4049/jimmunol.1003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Minutti CM, Jackson-Jones LH, García-Fojeda B, Knipper JA, Sutherland TE, Logan N, Ringqvist E, Guillamat-Prats R, Ferenbach DA, Artigas A, Stamme C, Chroneos ZC, Zaiss DM, Casals C, Allen JE. Local amplifiers of IL-4Rα-mediated macrophage activation promote repair in lung and liver. Science 356: 1076–1080, 2017. doi: 10.1126/science.aaj2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Miyaji EN, Oliveira ML, Carvalho E, Ho PL. Serotype-independent pneumococcal vaccines. Cell Mol Life Sci 70: 3303–3326, 2013. doi: 10.1007/s00018-012-1234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Mizgerd JP. Lung infection–a public health priority. PLoS Med 3: e76, 2006. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Mizgerd JP. Respiratory infection and the impact of pulmonary immunity on lung health and disease. Am J Respir Crit Care Med 186: 824–829, 2012. doi: 10.1164/rccm.201206-1063PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Molyneaux PL, Mallia P, Cox MJ, Footitt J, Willis-Owen SA, Homola D, Trujillo-Torralbo MB, Elkin S, Kon OM, Cookson WO, Moffatt MF, Johnston SL. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 188: 1224–1231, 2013. doi: 10.1164/rccm.201302-0341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12: 1045–1054, 2011. doi: 10.1038/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Moore ML, Stokes KL, Hartert TV. The impact of viral genotype on pathogenesis and disease severity: respiratory syncytial virus and human rhinoviruses. Curr Opin Immunol 25: 761–768, 2013. doi: 10.1016/j.coi.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Moore TA, Moore BB, Newstead MW, Standiford TJ. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol 165: 2643–2650, 2000. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 341.Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis 195: 1018–1028, 2007. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 342.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 198: 962–970, 2008. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Mortensen EM, Coley CM, Singer DE, Marrie TJ, Obrosky DS, Kapoor WN, Fine MJ. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med 162: 1059–1064, 2002. doi: 10.1001/archinte.162.9.1059. [DOI] [PubMed] [Google Scholar]
- 344.Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med 161: 414–419, 2000. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 345.Mrozek S, Constantin JM, Geeraerts T. Brain-lung crosstalk: implications for neurocritical care patients. World J Crit Care Med 4: 163–178, 2015. doi: 10.5492/wjccm.v4.i3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Mufson MA, Hao JB, Stanek RJ, Norton NB. Clinical features of patients with recurrent invasive Streptococcus pneumoniae disease. Am J Med Sci 343: 303–309, 2012. doi: 10.1097/MAJ.0b013e31822d9860. [DOI] [PubMed] [Google Scholar]
- 347.Muir R, Osbourn M, Dubois AV, Doran E, Small DM, Monahan A, O’Kane CM, McAllister K, Fitzgerald DC, Kissenpfennig A, McAuley DF, Ingram RJ. Innate Lymphoid Cells Are the Predominant Source of IL-17A during the Early Pathogenesis of Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 193: 407–416, 2016. doi: 10.1164/rccm.201410-1782OC. [DOI] [PubMed] [Google Scholar]
- 348.Mullarkey CE, Bailey MJ, Golubeva DA, Tan GS, Nachbagauer R, He W, Novakowski KE, Bowdish DM, Miller MS, Palese P. Broadly Neutralizing Hemagglutinin Stalk-Specific Antibodies Induce Potent Phagocytosis of Immune Complexes by Neutrophils in an Fc-Dependent Manner. MBio 7: e01624-16, 2016. doi: 10.1128/mBio.01624-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Murphy J, Summer R, Wilson AA, Kotton DN, Fine A. The prolonged life-span of alveolar macrophages. Am J Respir Cell Mol Biol 38: 380–385, 2008. doi: 10.1165/rcmb.2007-0224RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Murray CJ, Lopez AD. Evidence-based health policy–lessons from the Global Burden of Disease Study. Science 274: 740–743, 1996. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 351.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197–2223, 2012. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 352.Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 371: 1619–1628, 2014. doi: 10.1056/NEJMra1312885. [DOI] [PubMed] [Google Scholar]
- 353.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med 366: 54–63, 2012. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 354.Nachbagauer R, Choi A, Izikson R, Cox MM, Palese P, Krammer F. Age Dependence and Isotype Specificity of Influenza Virus Hemagglutinin Stalk-Reactive Antibodies in Humans. MBio 7: e01996-15, 2016. doi: 10.1128/mBio.01996-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Nakajoh K, Nakagawa T, Sekizawa K, Matsui T, Arai H, Sasaki H. Relation between incidence of pneumonia and protective reflexes in post-stroke patients with oral or tube feeding. J Intern Med 247: 39–42, 2000. doi: 10.1046/j.1365-2796.2000.00565.x. [DOI] [PubMed] [Google Scholar]
- 356.Nakasone C, Yamamoto N, Nakamatsu M, Kinjo T, Miyagi K, Uezu K, Nakamura K, Higa F, Ishikawa H, O’brien RL, Ikuta K, Kaku M, Fujita J, Kawakami K. Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes Infect 9: 251–258, 2007. doi: 10.1016/j.micinf.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 357.Nayak DK, Zhou F, Xu M, Huang J, Tsuji M, Hachem R, Mohanakumar T. Long-Term Persistence of Donor Alveolar Macrophages in Human Lung Transplant Recipients That Influences Donor-Specific Immune Responses. Am J Transplant 16: 2300–2311, 2016. doi: 10.1111/ajt.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, Brady SL, Brodsky MB, Denehy L, Elliott D, Flatley C, Harabin AL, Jones C, Louis D, Meltzer W, Muldoon SR, Palmer JB, Perme C, Robinson M, Schmidt DM, Scruth E, Spill GR, Storey CP, Render M, Votto J, Harvey MA. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 40: 502–509, 2012. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 359.Needham DM, Kamdar BB, Stevenson JE. Rehabilitation of mind and body after intensive care unit discharge: a step closer to recovery. Crit Care Med 40: 1340–1341, 2012. doi: 10.1097/CCM.0b013e31823b8df7. [DOI] [PubMed] [Google Scholar]
- 360.Nelson S, Summer W, Bagby G, Nakamura C, Stewart L, Lipscomb G, Andresen J. Granulocyte colony-stimulating factor enhances pulmonary host defenses in normal and ethanol-treated rats. J Infect Dis 164: 901–906, 1991. doi: 10.1093/infdis/164.5.901. [DOI] [PubMed] [Google Scholar]
- 361.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276, 2004. doi: 10.1172/JCI200420945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 363.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’Neill LA, Xavier RJ. Trained immunity: a program of innate immune memory in health and disease. Science 352: aaf1098, 2016. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Neupane B, Jerrett M, Burnett RT, Marrie T, Arain A, Loeb M. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am J Respir Crit Care Med 181: 47–53, 2010. doi: 10.1164/rccm.200901-0160OC. [DOI] [PubMed] [Google Scholar]
- 365.Nguyen TK, Tran TH, Roberts CL, Fox GJ, Graham SM, Marais BJ. Risk factors for child pneumonia–focus on the Western Pacific Region. Paediatr Respir Rev 21: 95–101, 2017. doi: 10.1016/j.prrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 366.Nicoletti C, Brandileone MC, Guerra ML, Levin AS. Prevalence, serotypes, and risk factors for pneumococcal carriage among HIV-infected adults. Diagn Microbiol Infect Dis 57: 259–265, 2007. doi: 10.1016/j.diagmicrobio.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 367.Noulin N, Quesniaux VF, Schnyder-Candrian S, Schnyder B, Maillet I, Robert T, Vargaftig BB, Ryffel B, Couillin I. Both hemopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. J Immunol 175: 6861–6869, 2005. doi: 10.4049/jimmunol.175.10.6861. [DOI] [PubMed] [Google Scholar]
- 368.Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, Breiman RF; Active Bacterial Core Surveillance Team . Cigarette smoking and invasive pneumococcal disease. N Engl J Med 342: 681–689, 2000. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 369.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect 4: 1545–1558, 2002. doi: 10.1016/S1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 370.Osler W. The Principles and Practice of Medicine. New York: D. Appleton and Company, 1903. [Google Scholar]
- 371.Paats MS, Bergen IM, Hanselaar WE, van Zoelen EC, Verbrugh HA, Hoogsteden HC, van den Blink B, Hendriks RW, van der Eerden MM. T helper 17 cells are involved in the local and systemic inflammatory response in community-acquired pneumonia. Thorax 68: 468–474, 2013. doi: 10.1136/thoraxjnl-2012-202168. [DOI] [PubMed] [Google Scholar]
- 372.Padro CJ, Sanders VM. Neuroendocrine regulation of inflammation. Semin Immunol 26: 357–368, 2014. doi: 10.1016/j.smim.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, Dumoutier L, Ryffel B, Renauld JC, Gosset P, Gosset P, Si-Tahar M, Faveeuw C, Trottein F. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem 287: 8816–8829, 2012. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Palavecino CE, Cespedes PF, Lay MK, Riedel CA, Kalergis AM, Bueno SM. Understanding Lung Immunopathology Caused by the Human Metapneumovirus: Implications for Rational Vaccine Design. Crit Rev Immunol 35: 185–202, 2015. doi: 10.1615/CritRevImmunol.2015013844. [DOI] [PubMed] [Google Scholar]
- 375.Pan D, Amison RT, Riffo-Vasquez Y, Spina D, Cleary SJ, Wakelam MJ, Page CP, Pitchford SC, Welch HC. P-Rex and Vav Rac-GEFs in platelets control leukocyte recruitment to sites of inflammation. Blood 125: 1146–1158, 2015. doi: 10.1182/blood-2014-07-591040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW; BRAIN-ICU Study Investigators . Long-term cognitive impairment after critical illness. N Engl J Med 369: 1306–1316, 2013. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, Shintani AK, Herr DL, Maze M, Ely EW; MENDS investigators . Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care 14: R38, 2010. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Panopoulos AD, Watowich SS. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 42: 277–288, 2008. doi: 10.1016/j.cyto.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Parker D, Ahn D, Cohen T, Prince A. Innate Immune Signaling Activated by MDR Bacteria in the Airway. Physiol Rev 96: 19–53, 2016. doi: 10.1152/physrev.00009.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc 4: 252–257, 2007. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Pauksen K, Elfman L, Ulfgren AK, Venge P. Serum levels of granulocyte-colony stimulating factor (G-CSF) in bacterial and viral infections, and in atypical pneumonia. Br J Haematol 88: 256–260, 1994. doi: 10.1111/j.1365-2141.1994.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 383.Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev 23: 3–13, 2007. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 384.Pelton SI, Shea KM, Farkouh RA, Strutton DR, Braun S, Jacob C, Klok R, Gruen ES, Weycker D. Rates of pneumonia among children and adults with chronic medical conditions in Germany. BMC Infect Dis 15: 470, 2015. doi: 10.1186/s12879-015-1162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Pendleton KM, Erb-Downward JR, Bao Y, Branton WR, Falkowski NR, Newton DW, Huffnagle GB, Dickson RP. Rapid Pathogen Identification in Bacterial Pneumonia Using Real-Time Metagenomics. Am J Respir Crit Care Med 196: 1610–1612, 2017. doi: 10.1164/rccm.201703-0537LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Pennington JE. Gram-negative bacterial pneumonia in the immunocompromised host. Semin Respir Infect 1: 145–150, 1986. [PubMed] [Google Scholar]
- 387.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol 17: 2–8, 2016. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Perros F, Lambrecht BN, Hammad H. TLR4 signalling in pulmonary stromal cells is critical for inflammation and immunity in the airways. Respir Res 12: 125, 2011. doi: 10.1186/1465-9921-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Peteranderl C, Morales-Nebreda L, Selvakumar B, Lecuona E, Vadász I, Morty RE, Schmoldt C, Bespalowa J, Wolff T, Pleschka S, Mayer K, Gattenloehner S, Fink L, Lohmeyer J, Seeger W, Sznajder JI, Mutlu GM, Budinger GR, Herold S. Macrophage-epithelial paracrine crosstalk inhibits lung edema clearance during influenza infection. J Clin Invest 126: 1566–1580, 2016. doi: 10.1172/JCI83931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Phung DT, Wang Z, Rutherford S, Huang C, Chu C. Body mass index and risk of pneumonia: a systematic review and meta-analysis. Obes Rev 14: 839–857, 2013. doi: 10.1111/obr.12055. [DOI] [PubMed] [Google Scholar]
- 391.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 116: 625–627, 2010. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 392.Pittet LA, Quinton LJ, Yamamoto K, Robson BE, Ferrari JD, Algül H, Schmid RM, Mizgerd JP. Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia. Am J Respir Cell Mol Biol 45: 573–581, 2011. doi: 10.1165/rcmb.2010-0210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Piva S, McCreadie VA, Latronico N. Neuroinflammation in sepsis: sepsis associated delirium. Cardiovasc Hematol Disord Drug Targets 15: 10–18, 2015. doi: 10.2174/1871529X15666150108112452. [DOI] [PubMed] [Google Scholar]
- 394.Pociask DA, Robinson KM, Chen K, McHugh KJ, Clay ME, Huang GT, Benos PV, Janssen-Heininger YMW, Kolls JK, Anathy V, Alcorn JF. Epigenetic and Transcriptomic Regulation of Lung Repair during Recovery from Influenza Infection. Am J Pathol 187: 851–863, 2017. doi: 10.1016/j.ajpath.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, Kolls JK, Alcorn JF. IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol 182: 1286–1296, 2013. doi: 10.1016/j.ajpath.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Poe SL, Arora M, Oriss TB, Yarlagadda M, Isse K, Khare A, Levy DE, Lee JS, Mallampalli RK, Chan YR, Ray A, Ray P. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol 6: 189–199, 2013. doi: 10.1038/mi.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA 313: 1055–1057, 2015. doi: 10.1001/jama.2015.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med 190: 62–69, 2014. doi: 10.1164/rccm.201403-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Puchta A, Naidoo A, Verschoor CP, Loukov D, Thevaranjan N, Mandur TS, Nguyen PS, Jordana M, Loeb M, Xing Z, Kobzik L, Larché MJ, Bowdish DM. TNF Drives Monocyte Dysfunction with Age and Results in Impaired Anti-pneumococcal Immunity. PLoS Pathog 12: e1005368, 2016. doi: 10.1371/journal.ppat.1005368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baró T, de Heredia CD, Torán N, Català A, Torrebadell M, Fortuny C, Cusí V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Aróstegui JI, Juan M, Yagüe J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 13: 170–180, 2011. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One 6: e16245, 2011. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Quan J, Li X, Chen Y, Jiang Y, Zhou Z, Zhang H, Sun L, Ruan Z, Feng Y, Akova M, Yu Y. Prevalence of mcr-1 in Escherichia coli and Klebsiella pneumoniae recovered from bloodstream infections in China: a multicentre longitudinal study. Lancet Infect Dis 17: 400–410, 2017. doi: 10.1016/S1473-3099(16)30528-X. [DOI] [PubMed] [Google Scholar]
- 403.Quinton LJ, Blahna MT, Jones MR, Allen E, Ferrari JD, Hilliard KL, Zhang X, Sabharwal V, Algül H, Akira S, Schmid RM, Pelton SI, Spira A, Mizgerd JP. Hepatocyte-specific mutation of both NF-κB RelA and STAT3 abrogates the acute phase response in mice. J Clin Invest 122: 1758–1763, 2012. doi: 10.1172/JCI59408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Quinton LJ, Jones MR, Robson BE, Mizgerd JP. Mechanisms of the hepatic acute-phase response during bacterial pneumonia. Infect Immun 77: 2417–2426, 2009. doi: 10.1128/IAI.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol 38: 699–706, 2008. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J Immunol 178: 1896–1903, 2007. doi: 10.4049/jimmunol.178.3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Quinton LJ, Mizgerd JP. Dynamics of lung defense in pneumonia: resistance, resilience, and remodeling. Annu Rev Physiol 77: 407–430, 2015. doi: 10.1146/annurev-physiol-021014-071937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Quinton LJ, Mizgerd JP. NF-κB and STAT3 signaling hubs for lung innate immunity. Cell Tissue Res 343: 153–165, 2011. doi: 10.1007/s00441-010-1044-y. [DOI] [PubMed] [Google Scholar]
- 409.Quinton LJ, Mizgerd JP, Hilliard KL, Jones MR, Kwon CY, Allen E. Leukemia inhibitory factor signaling is required for lung protection during pneumonia. J Immunol 188: 6300–6308, 2012. doi: 10.4049/jimmunol.1200256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Quinton LJ, Nelson S, Boé DM, Zhang P, Zhong Q, Kolls JK, Bagby GJ. The granulocyte colony-stimulating factor response after intrapulmonary and systemic bacterial challenges. J Infect Dis 185: 1476–1482, 2002. doi: 10.1086/340504. [DOI] [PubMed] [Google Scholar]
- 411.Quraishi SA, Bittner EA, Christopher KB, Camargo CA Jr. Vitamin D status and community-acquired pneumonia: results from the third National Health and Nutrition Examination Survey. PLoS One 8: e81120, 2013. doi: 10.1371/journal.pone.0081120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Randall TD, Mebius RE. The development and function of mucosal lymphoid tissues: a balancing act with micro-organisms. Mucosal Immunol 7: 455–466, 2014. doi: 10.1038/mi.2014.11. [DOI] [PubMed] [Google Scholar]
- 413.Raoust E, Balloy V, Garcia-Verdugo I, Touqui L, Ramphal R, Chignard M. Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS One 4: e7259, 2009. doi: 10.1371/journal.pone.0007259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol 54: 881–941, 2000. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- 415.Ren S, Newby D, Li SC, Walkom E, Miller P, Hure A, Attia J. Effect of the adult pneumococcal polysaccharide vaccine on cardiovascular disease: a systematic review and meta-analysis. Open Heart 2: e000247, 2015. doi: 10.1136/openhrt-2015-000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Renckens R, Roelofs JJ, Knapp S, de Vos AF, Florquin S, van der Poll T. The acute-phase response and serum amyloid A inhibit the inflammatory response to Acinetobacter baumannii pneumonia. J Infect Dis 193: 187–195, 2006. doi: 10.1086/498876. [DOI] [PubMed] [Google Scholar]
- 417.Reyes LF, Restrepo MI, Hinojosa CA, Soni NJ, Anzueto A, Babu BL, Gonzalez-Juarbe N, Rodriguez AH, Jimenez A, Chalmers JD, Aliberti S, Sibila O, Winter VT, Coalson JJ, Giavedoni LD, Dela Cruz CS, Waterer GW, Witzenrath M, Suttorp N, Dube PH, Orihuela CJ. Severe Pneumococcal Pneumonia Causes Acute Cardiac Toxicity and Subsequent Cardiac Remodeling. Am J Respir Crit Care Med 196: 609–620, 2017. doi: 10.1164/rccm.201701-0104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Riazanski V, Gabdoulkhakova AG, Boynton LS, Eguchi RR, Deriy LV, Hogarth DK, Loaëc N, Oumata N, Galons H, Brown ME, Shevchenko P, Gallan AJ, Yoo SG, Naren AP, Villereal ML, Beacham DW, Bindokas VP, Birnbaumer L, Meijer L, Nelson DJ. TRPC6 channel translocation into phagosomal membrane augments phagosomal function. Proc Natl Acad Sci USA 112: E6486–E6495, 2015. doi: 10.1073/pnas.1518966112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Rice P, Martin E, He JR, Frank M, DeTolla L, Hester L, O’Neill T, Manka C, Benjamin I, Nagarsekar A, Singh I, Hasday JD. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J Immunol 174: 3676–3685, 2005. doi: 10.4049/jimmunol.174.6.3676. [DOI] [PubMed] [Google Scholar]
- 420.Riquelme R, Torres A, el-Ebiary M, Mensa J, Estruch R, Ruiz M, Angrill J, Soler N. Community-acquired pneumonia in the elderly. Clinical and nutritional aspects. Am J Respir Crit Care Med 156: 1908–1914, 1997. doi: 10.1164/ajrccm.156.6.9702005. [DOI] [PubMed] [Google Scholar]
- 421.Robertson CM, Perrone EE, McConnell KW, Dunne WM, Boody B, Brahmbhatt T, Diacovo MJ, Van Rooijen N, Hogue LA, Cannon CL, Buchman TG, Hotchkiss RS, Coopersmith CM. Neutrophil depletion causes a fatal defect in murine pulmonary Staphylococcus aureus clearance. J Surg Res 150: 278–285, 2008. doi: 10.1016/j.jss.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev 3: CD010406, 2016. [DOI] [PubMed] [Google Scholar]
- 423.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defence. Nature 505: 412–416, 2014. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Roy S, Ninkovic J, Banerjee S, Charboneau RG, Das S, Dutta R, Kirchner VA, Koodie L, Ma J, Meng J, Barke RA. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol 6: 442–465, 2011. doi: 10.1007/s11481-011-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 86: 408–416, 2008. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35: 1599–1608, 2007. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- 427.Ruminy P, Gangneux C, Claeyssens S, Scotte M, Daveau M, Salier JP. Gene transcription in hepatocytes during the acute phase of a systemic inflammation: from transcription factors to target genes. Inflamm Res 50: 383–390, 2001. doi: 10.1007/PL00000260. [DOI] [PubMed] [Google Scholar]
- 428.Rynda-Apple A, Robinson KM, Alcorn JF. Influenza and Bacterial Superinfection: Illuminating the Immunologic Mechanisms of Disease. Infect Immun 83: 3764–3770, 2015. doi: 10.1128/IAI.00298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Sahin-Yilmaz A, Naclerio RM. Anatomy and physiology of the upper airway. Proc Am Thorac Soc 8: 31–39, 2011. doi: 10.1513/pats.201007-050RN. [DOI] [PubMed] [Google Scholar]
- 430.Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Müller M, Blander JM, Tacke F, Trautwein C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med 207: 1453–1464, 2010. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Sandvall B, Rueda AM, Musher DM. Long-term survival following pneumococcal pneumonia. Clin Infect Dis 56: 1145–1146, 2013. doi: 10.1093/cid/cis1207. [DOI] [PubMed] [Google Scholar]
- 432.Saravia J, You D, Shrestha B, Jaligama S, Siefker D, Lee GI, Harding JN, Jones TL, Rovnaghi C, Bagga B, DeVincenzo JP, Cormier SA. Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33. PLoS Pathog 11: e1005217, 2015. doi: 10.1371/journal.ppat.1005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38: 187–197, 2013. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity 41: 886–897, 2014. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Schortgen F, Asfar P. Update in sepsis and acute kidney injury 2014. Am J Respir Crit Care Med 191: 1226–1231, 2015. doi: 10.1164/rccm.201502-0307UP. [DOI] [PubMed] [Google Scholar]
- 436.Schrauwen EJ, de Graaf M, Herfst S, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. Determinants of virulence of influenza A virus. Eur J Clin Microbiol Infect Dis 33: 479–490, 2014. doi: 10.1007/s10096-013-1984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, Hoogendijk AJ, de Beer R, de Vos A, Belzer C, de Vos WM, van der Poll T, Wiersinga WJ. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65: 575–583, 2016. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Schuijt TJ, van der Poll T, de Vos WM, Wiersinga WJ. The intestinal microbiota and host immune interactions in the critically ill. Trends Microbiol 21: 221–229, 2013. doi: 10.1016/j.tim.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 439.Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG, Li Y, Shen N, Ghedin E, Morris A, Diaz P, Huang L, Wikoff WR, Ubeda C, Artacho A, Rom WN, Sterman DH, Collman RG, Blaser MJ, Weiden MD. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol 1: 16031, 2016. doi: 10.1038/nmicrobiol.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Segal LN, Methé BA, Nolan A, Hoshino Y, Rom WN, Dawson R, Bateman E, Weiden MD. HIV-1 and bacterial pneumonia in the era of antiretroviral therapy. Proc Am Thorac Soc 8: 282–287, 2011. doi: 10.1513/pats.201006-044WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, Levesque JP, Chappel J, Ross FP, Link DC. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood 106: 3020–3027, 2005. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity 17: 413–423, 2002. doi: 10.1016/S1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 443.Sepahi A, Salinas I. The evolution of nasal immune systems in vertebrates. Mol Immunol 69: 131–138, 2016. doi: 10.1016/j.molimm.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Sethi S. Infection as a comorbidity of COPD. Eur Respir J 35: 1209–1215, 2010. doi: 10.1183/09031936.00081409. [DOI] [PubMed] [Google Scholar]
- 445.Shah FA, Pike F, Alvarez K, Angus D, Newman AB, Lopez O, Tate J, Kapur V, Wilsdon A, Krishnan JA, Hansel N, Au D, Avdalovic M, Fan VS, Barr RG, Yende S. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med 188: 586–592, 2013. doi: 10.1164/rccm.201212-2154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest 119: 1910–1920, 2009. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Shahbazian LM, Quinton LJ, Bagby GJ, Nelson S, Wang G, Zhang P. Escherichia coli pneumonia enhances granulopoiesis and the mobilization of myeloid progenitor cells into the systemic circulation. Crit Care Med 32: 1740–1746, 2004. doi: 10.1097/01.CCM.0000132900.84627.90. [DOI] [PubMed] [Google Scholar]
- 448.Shak JR, Vidal JE, Klugman KP. Influence of bacterial interactions on pneumococcal colonization of the nasopharynx. Trends Microbiol 21: 129–135, 2013. doi: 10.1016/j.tim.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Shinefield HR, Steinberg CR, Kaye D. Effect of splenectomy on the susceptibility of mice inoculated with Diplococcus pneumoniae. J Exp Med 123: 777–794, 1966. doi: 10.1084/jem.123.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Siegel SJ, Weiser JN. Mechanisms of Bacterial Colonization of the Respiratory Tract. Annu Rev Microbiol 69: 425–444, 2015. doi: 10.1146/annurev-micro-091014-104209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Siggins RW, Melvan JN, Welsh DA, Bagby GJ, Nelson S, Zhang P. Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumoniae infection with enhancement of STAT3 signaling. J Immunol 186: 4306–4313, 2011. doi: 10.4049/jimmunol.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Simons JP, Loeffler JM, Al-Shawi R, Ellmerich S, Hutchinson WL, Tennent GA, Petrie A, Raynes JG, de Souza JB, Lawrence RA, Read KD, Pepys MB. C-reactive protein is essential for innate resistance to pneumococcal infection. Immunology 142: 414–420, 2014. doi: 10.1111/imm.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Singbartl K, Bishop JV, Wen X, Murugan R, Chandra S, Filippi MD, Kellum JA. Differential effects of kidney-lung cross-talk during acute kidney injury and bacterial pneumonia. Kidney Int 80: 633–644, 2011. doi: 10.1038/ki.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801–810, 2016. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Sinha M, Lowell CA. Immune Defense Protein Expression in Highly Purified Mouse Lung Epithelial Cells. Am J Respir Cell Mol Biol 54: 802–813, 2016. doi: 10.1165/rcmb.2015-0171OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 292: L312–L322, 2007. doi: 10.1152/ajplung.00250.2006. [DOI] [PubMed] [Google Scholar]
- 457.Small CL, McCormick S, Gill N, Kugathasan K, Santosuosso M, Donaldson N, Heinrichs DE, Ashkar A, Xing Z. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J Immunol 180: 5558–5568, 2008. doi: 10.4049/jimmunol.180.8.5558. [DOI] [PubMed] [Google Scholar]
- 458.Small CL, Shaler CR, McCormick S, Jeyanathan M, Damjanovic D, Brown EG, Arck P, Jordana M, Kaushic C, Ashkar AA, Xing Z. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol 184: 2048–2056, 2010. doi: 10.4049/jimmunol.0902772. [DOI] [PubMed] [Google Scholar]
- 459.Smith NMS, Wasserman GA, Coleman FT, Hilliard KL, Yamamoto K, Lipsitz E, Malley R, Dooms H, Jones MR, Quinton LJ, Mizgerd JP. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol 11: 220–235, 2018. doi: 10.1038/mi.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Smith RP, Lipworth BJ, Cree IA, Spiers EM, Winter JH. C-reactive protein. A clinical marker in community-acquired pneumonia. Chest 108: 1288–1291, 1995. doi: 10.1378/chest.108.5.1288. [DOI] [PubMed] [Google Scholar]
- 461.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med 21: 698–708, 2015. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol 13: 145–149, 2013. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 463.Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 19: 1305–1312, 2013. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 464.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 9: 377–384, 2009. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 465.Standiford LR, Standiford TJ, Newstead MJ, Zeng X, Ballinger MN, Kovach MA, Reka AK, Bhan U. TLR4-dependent GM-CSF protects against lung injury in Gram-negative bacterial pneumonia. Am J Physiol Lung Cell Mol Physiol 302: L447–L454, 2012. doi: 10.1152/ajplung.00415.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Starner TD, Barker CK, Jia HP, Kang Y, McCray PB Jr. CCL20 is an inducible product of human airway epithelia with innate immune properties. Am J Respir Cell Mol Biol 29: 627–633, 2003. doi: 10.1165/rcmb.2002-0272OC. [DOI] [PubMed] [Google Scholar]
- 467.Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol 136: 1435–1441, 1986. [PubMed] [Google Scholar]
- 468.Steinwede K, Henken S, Bohling J, Maus R, Ueberberg B, Brumshagen C, Brincks EL, Griffith TS, Welte T, Maus UA. TNF-related apoptosis-inducing ligand (TRAIL) exerts therapeutic efficacy for the treatment of pneumococcal pneumonia in mice. J Exp Med 209: 1937–1952, 2012. doi: 10.1084/jem.20120983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med 42: 625–631, 2014. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Stier MT, Bloodworth MH, Toki S, Newcomb DC, Goleniewska K, Boyd KL, Quitalig M, Hotard AL, Moore ML, Hartert TV, Zhou B, McKenzie AN, Peebles RS Jr. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol 138: 814–824.e11, 2016. doi: 10.1016/j.jaci.2016.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Strnad P, Tacke F, Koch A, Trautwein C. Liver: guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol 14: 55–66, 2017. doi: 10.1038/nrgastro.2016.168. [DOI] [PubMed] [Google Scholar]
- 472.Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, Huston G, Dutton RW, Swain SL. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med 16: 558–564, 2010. doi: 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Strutt TM, McKinstry KK, Kuang Y, Bradley LM, Swain SL. Memory CD4+ T-cell-mediated protection depends on secondary effectors that are distinct from and superior to primary effectors. Proc Natl Acad Sci USA 109: E2551–E2560, 2012. doi: 10.1073/pnas.1205894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Strutt TM, McKinstry KK, Kuang Y, Finn CM, Hwang JH, Dhume K, Sell S, Swain SL. Direct IL-6 Signals Maximize Protective Secondary CD4 T Cell Responses Against Influenza. J Immunol 197: 3260–3270, 2016. doi: 10.4049/jimmunol.1600033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Strutt TM, McKinstry KK, Marshall NB, Vong AM, Dutton RW, Swain SL. Multipronged CD4(+) T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunol Rev 255: 149–164, 2013. doi: 10.1111/imr.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Su X, Matthay MA, Malik AB. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J Immunol 184: 401–410, 2010. doi: 10.4049/jimmunol.0901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Sun J, Cardani A, Sharma AK, Laubach VE, Jack RS, Müller W, Braciale TJ. Autocrine regulation of pulmonary inflammation by effector T-cell derived IL-10 during infection with respiratory syncytial virus. PLoS Pathog 7: e1002173, 2011. doi: 10.1371/journal.ppat.1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med 15: 277–284, 2009. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Suratt BT. Mouse Modeling of Obese Lung Disease. Insights and Caveats. Am J Respir Cell Mol Biol 55: 153–158, 2016. doi: 10.1165/rcmb.2016-0063PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood 104: 565–571, 2004. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- 481.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol 12: 136–148, 2012. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, Fujisawa M, Chikaishi T, Komeda J, Itoh J, Umemura M, Kyusai A, Tomura M, Nakayama T, Woodland DL, Kohlmeier JE, Miyazawa M. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med 213: 3057–3073, 2016. doi: 10.1084/jem.20160938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Tamura M, Watanabe M, Nakajima A, Kurai D, Ishii H, Takata S, Nakamoto K, Sohara E, Honda K, Nakamura M, Inui T, Wada H, Takizawa H, Goto H. Serial quantification of procalcitonin (PCT) predicts clinical outcome and prognosis in patients with community-acquired pneumonia (CAP). J Infect Chemother 20: 97–103, 2014. doi: 10.1016/j.jiac.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 484.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol 23: 901–944, 2005. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 485.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol 187: 5510–5514, 2011. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Thanabalasuriar A, Neupane AS, Wang J, Krummel MF, Kubes P. iNKT Cell Emigration out of the Lung Vasculature Requires Neutrophils and Monocyte-Derived Dendritic Cells in Inflammation. Cell Reports 16: 3260–3272, 2016. doi: 10.1016/j.celrep.2016.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486a.The ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin Definition. JAMA 307: 2526–2533, 2012. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 486b.The NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360: 1283–1297, 2009. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 487.Thome JJ, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, Granot T, Griesemer A, Lerner H, Kato T, Farber DL. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med 22: 72–77, 2016. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159: 814–828, 2014. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Tian X, Xu F, Lung WY, Meyerson C, Ghaffari AA, Cheng G, Deng JC. Poly I:C enhances susceptibility to secondary pulmonary infections by gram-positive bacteria. PLoS One 7: e41879, 2012. doi: 10.1371/journal.pone.0041879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Tillett WS, Francis T. Serological reactions in pneumonia with non-protein somatic fraction of pneumococcus. J Exp Med 52: 561–571, 1930. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Tofts PS, Chevassut T, Cutajar M, Dowell NG, Peters AM. Doubts concerning the recently reported human neutrophil lifespan of 5.4 days. Blood 117: 6050–6052, 2011. doi: 10.1182/blood-2010-10-310532. [DOI] [PubMed] [Google Scholar]
- 492.Toma I, Siegel MO, Keiser J, Yakovleva A, Kim A, Davenport L, Devaney J, Hoffman EP, Alsubail R, Crandall KA, Castro-Nallar E, Pérez-Losada M, Hilton SK, Chawla LS, McCaffrey TA, Simon GL. Single-molecule long-read 16S sequencing to characterize the lung microbiome from mechanically ventilated patients with suspected pneumonia. J Clin Microbiol 52: 3913–3921, 2014. doi: 10.1128/JCM.01678-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 68: 1057–1065, 2013. doi: 10.1136/thoraxjnl-2013-204282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, Gabarrús A, Sellarés J, Restrepo MI, Anzueto A, Niederman MS, Agustí C. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 313: 677–686, 2015. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 495.Traber KE, Hilliard KL, Allen E, Wasserman GA, Yamamoto K, Jones MR, Mizgerd JP, Quinton LJ. Induction of STAT3-Dependent CXCL5 Expression and Neutrophil Recruitment by Oncostatin-M during Pneumonia. Am J Respir Cell Mol Biol 53: 479–488, 2015. doi: 10.1165/rcmb.2014-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Traber KE, Symer EM, Allen E, Kim Y, Hilliard KL, Wasserman GA, Stewart CL, Jones MR, Mizgerd JP, Quinton LJ. Myeloid-epithelial cross talk coordinates synthesis of the tissue-protective cytokine leukemia inhibitory factor during pneumonia. Am J Physiol Lung Cell Mol Physiol 313: L548–L558, 2017. doi: 10.1152/ajplung.00482.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Trapnell BC, Carey BC, Uchida K, Suzuki T. Pulmonary alveolar proteinosis, a primary immunodeficiency of impaired GM-CSF stimulation of macrophages. Curr Opin Immunol 21: 514–521, 2009. doi: 10.1016/j.coi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Trevejo-Nunez G, Elsegeiny W, Conboy P, Chen K, Kolls JK. Critical Role of IL-22/IL22-RA1 Signaling in Pneumococcal Pneumonia. J Immunol 197: 1877–1883, 2016. doi: 10.4049/jimmunol.1600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Trevisani F, Castelli E, Foschi FG, Parazza M, Loggi E, Bertelli M, Melotti C, Domenicali M, Zoli G, Bernardi M. Impaired tuftsin activity in cirrhosis: relationship with splenic function and clinical outcome. Gut 50: 707–712, 2002. doi: 10.1136/gut.50.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Truong AD, Fan E, Brower RG, Needham DM. Bench-to-bedside review: mobilizing patients in the intensive care unit–from pathophysiology to clinical trials. Crit Care 13: 216, 2009. doi: 10.1186/cc7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 7: 501–510, 2014. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Ubags ND, Stapleton RD, Vernooy JH, Burg E, Bement J, Hayes CM, Ventrone S, Zabeau L, Tavernier J, Poynter ME, Parsons PE, Dixon AE, Wargo MJ, Littenberg B, Wouters EF, Suratt BT. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight 1: e82101, 2016. doi: 10.1172/jci.insight.82101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, Ciszewski A, Vakili H, Hoffman EB, Farkouh ME, Cannon CP. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 310: 1711–1720, 2013. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 504.Uhel F, Azzaoui I, Grégoire M, Pangault C, Dulong J, Tadié JM, Gacouin A, Camus C, Cynober L, Fest T, Le Tulzo Y, Roussel M, Tarte K. Early Expansion of Circulating Granulocytic Myeloid-Derived Suppressor Cells Predicts Development of Nosocomial Infections in Patients with Sepsis. Am J Respir Crit Care Med 196: 315–327, 2017. doi: 10.1164/rccm.201606-1143OC. [DOI] [PubMed] [Google Scholar]
- 505.Ulich TR, Yin S, Guo K, Yi ES, Remick D, del Castillo J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. Am J Pathol 138: 1097–1101, 1991. [PMC free article] [PubMed] [Google Scholar]
- 506.Umehara T, Kato K, Park YS, Lillehoj EP, Kawauchi H, Kim KC. Prevention of lung injury by Muc1 mucin in a mouse model of repetitive Pseudomonas aeruginosa infection. Inflamm Res 61: 1013–1020, 2012. doi: 10.1007/s00011-012-0494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Van der Poll T, Marchant A, Keogh CV, Goldman M, Lowry SF. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis 174: 994–1000, 1996. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- 508.Van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 374: 1543–1556, 2009. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- 509.Van Eeden S, Leipsic J, Paul Man SF, Sin DD. The relationship between lung inflammation and cardiovascular disease. Am J Respir Crit Care Med 186: 11–16, 2012. doi: 10.1164/rccm.201203-0455PP. [DOI] [PubMed] [Google Scholar]
- 510.Van Maele L, Carnoy C, Cayet D, Ivanov S, Porte R, Deruy E, Chabalgoity JA, Renauld JC, Eberl G, Benecke AG, Trottein F, Faveeuw C, Sirard JC. Activation of Type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J Infect Dis 210: 493–503, 2014. doi: 10.1093/infdis/jiu106. [DOI] [PubMed] [Google Scholar]
- 511.Van Vught LA, Klein Klouwenberg PM, Spitoni C, Scicluna BP, Wiewel MA, Horn J, Schultz MJ, Nürnberg P, Bonten MJ, Cremer OL, van der Poll T; MARS Consortium . Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA 315: 1469–1479, 2016. doi: 10.1001/jama.2016.2691. [DOI] [PubMed] [Google Scholar]
- 512.Van Vught LA, Scicluna BP, Wiewel MA, Hoogendijk AJ, Klein Klouwenberg PM, Franitza M, Toliat MR, Nürnberg P, Cremer OL, Horn J, Schultz MJ, Bonten MM, van der Poll T. Comparative Analysis of the Host Response to Community-acquired and Hospital-acquired Pneumonia in Critically Ill Patients. Am J Respir Crit Care Med 194: 1366–1374, 2016. doi: 10.1164/rccm.201602-0368OC. [DOI] [PubMed] [Google Scholar]
- 513.Venkataraman A, Bassis CM, Beck JM, Young VB, Curtis JL, Huffnagle GB, Schmidt TM. Application of a neutral community model to assess structuring of the human lung microbiome. MBio 6: e02284-14, 2015. doi: 10.1128/mBio.02284-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Verani JR, Groome MJ, Zar HJ, Zell ER, Kapongo CN, Nzenze SA, Mulligan C, Moore DP, Whitney CG, Madhi SA. Risk Factors for Presumed Bacterial Pneumonia Among HIV-uninfected Children Hospitalized in Soweto, South Africa. Pediatr Infect Dis J 35: 1169–1174, 2016. doi: 10.1097/INF.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 515.Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Gutierrez-Perez A, Vila-Rovira A, Gomez F, Raga X, de Diego C, Satue E, Salsench E; EPIVAC Study Group . Clinical effectiveness of pneumococcal vaccination against acute myocardial infarction and stroke in people over 60 years: the CAPAMIS study, one-year follow-up. BMC Public Health 12: 222, 2012. doi: 10.1186/1471-2458-12-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K; EPIC II Group of Investigators . International study of the prevalence and outcomes of infection in intensive care units. JAMA 302: 2323–2329, 2009. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 517.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients 3: 858–876, 2011. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Aróstegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yagüe J, Antón J, Pascal M, Chang HH, Janniere L, Rose Y, Garty BZ, Chapel H, Issekutz A, Maródi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova JL. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321: 691–696, 2008. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells–how did we miss them? Nat Rev Immunol 13: 75–87, 2013. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 520.Walkey AJ. Preventing cardiovascular complications of acute infection: a missed opportunity? Circulation 129: 1375–1377, 2014. doi: 10.1161/CIRCULATIONAHA.114.008712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest 146: 1187–1195, 2014. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 306: 2248–2254, 2011. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Wan YD, Sun TW, Liu ZQ, Zhang SG, Wang LX, Kan QC. Efficacy and Safety of Corticosteroids for Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis. Chest 149: 209–219, 2016. doi: 10.1378/chest.15-1733. [DOI] [PubMed] [Google Scholar]
- 524.Wang H, Zhang K, Qin H, Yang L, Zhang L, Cao Y. Genetic Association Between CD143 rs4340 Polymorphism and Pneumonia risk: A Meta Analysis. Medicine (Baltimore) 94: e883, 2015. doi: 10.1097/MD.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Wang J, Li F, Sun R, Gao X, Wei H, Li LJ, Tian Z. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat Commun 4: 2106, 2013. doi: 10.1038/ncomms3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Wang Y, Jiang B, Guo Y, Li W, Tian Y, Sonnenberg GF, Weiser JN, Ni X, Shen H. Cross-protective mucosal immunity mediated by memory Th17 cells against Streptococcus pneumoniae lung infection. Mucosal Immunol 10: 250–259, 2017. doi: 10.1038/mi.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Wang Y, Tian GB, Zhang R, Shen Y, Tyrrell JM, Huang X, Zhou H, Lei L, Li HY, Doi Y, Fang Y, Ren H, Zhong LL, Shen Z, Zeng KJ, Wang S, Liu JH, Wu C, Walsh TR, Shen J. Prevalence, risk factors, outcomes, and molecular epidemiology of mcr-1-positive Enterobacteriaceae in patients and healthy adults from China: an epidemiological and clinical study. Lancet Infect Dis 17: 390–399, 2017. doi: 10.1016/S1473-3099(16)30527-8. [DOI] [PubMed] [Google Scholar]
- 528.Wardlaw TM, Johansson EW, Hodge M; World Health Organization; UNICEF . Pneumonia: the Forgotten Killer of Children. Geneva, Switzerland: WHO Press, 2006. [Google Scholar]
- 529.Wasserman GA, Szymaniak A, Hinds A, Yamamoto K, Kamata H, Smith NMS, Hilliard KL, Carrieri C, Labadorf AT, Quinton LJ, Ai X, Varelas X, Mizgerd JP, Fine A, O’Carroll D, Jones MR. Identification of a discrete population of multiciliated MIWI2-positive epithelial cells that mediate lung inflammation. J Clin Invest 127: 3866–3876, 2017. doi: 10.1172/JCI94639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Weber GF, Chousterman BG, Hilgendorf I, Robbins CS, Theurl I, Gerhardt LM, Iwamoto Y, Quach TD, Ali M, Chen JW, Rothstein TL, Nahrendorf M, Weissleder R, Swirski FK. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J Exp Med 211: 1243–1256, 2014. doi: 10.1084/jem.20131471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Weber M, Lambeck S, Ding N, Henken S, Kohl M, Deigner HP, Enot DP, Igwe EI, Frappart L, Kiehntopf M, Claus RA, Kamradt T, Weih D, Vodovotz Y, Briles DE, Ogunniyi AD, Paton JC, Maus UA, Bauer M. Hepatic induction of cholesterol biosynthesis reflects a remote adaptive response to pneumococcal pneumonia. FASEB J 26: 2424–2436, 2012. doi: 10.1096/fj.11-191957. [DOI] [PubMed] [Google Scholar]
- 532.Weinhouse GL, Schwab RJ, Watson PL, Patil N, Vaccaro B, Pandharipande P, Ely EW. Bench-to-bedside review: delirium in ICU patients–importance of sleep deprivation. Crit Care 13: 234, 2009. doi: 10.1186/cc8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, Brown GD, Steele C. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun 79: 3966–3977, 2011. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 506: 503–506, 2014. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Weyrich LS, Feaga HA, Park J, Muse SJ, Safi CY, Rolin OY, Young SE, Harvill ET. Resident microbiota affect Bordetella pertussis infectious dose and host specificity. J Infect Dis 209: 913–921, 2014. doi: 10.1093/infdis/jit597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol 16: 27–35, 2015. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, Staples KJ, Dong T, Douek DC, McMichael AJ, Xu XN. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 18: 274–280, 2012. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 538.Wilson R, Cohen JM, Reglinski M, Jose RJ, Chan WY, Marshall H, de Vogel C, Gordon S, Goldblatt D, Petersen FC, Baxendale H, Brown JS. Naturally Acquired Human Immunity to Pneumococcus Is Dependent on Antibody to Protein Antigens. PLoS Pathog 13: e1006137, 2017. doi: 10.1371/journal.ppat.1006137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Winkelstein JA, Marino MC, Johnston RB Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 79: 155–169, 2000. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 540.Winter C, Herbold W, Maus R, Länger F, Briles DE, Paton JC, Welte T, Maus UA. Important role for CC chemokine ligand 2-dependent lung mononuclear phagocyte recruitment to inhibit sepsis in mice infected with Streptococcus pneumoniae. J Immunol 182: 4931–4937, 2009. doi: 10.4049/jimmunol.0804096. [DOI] [PubMed] [Google Scholar]
- 541.Winter C, Taut K, Srivastava M, Länger F, Mack M, Briles DE, Paton JC, Maus R, Welte T, Gunn MD, Maus UA. Lung-specific overexpression of CC chemokine ligand (CCL) 2 enhances the host defense to Streptococcus pneumoniae infection in mice: role of the CCL2-CCR2 axis. J Immunol 178: 5828–5838, 2007. doi: 10.4049/jimmunol.178.9.5828. [DOI] [PubMed] [Google Scholar]
- 542.Wonodi CB, Deloria-Knoll M, Feikin DR, DeLuca AN, Driscoll AJ, Moïsi JC, Johnson HL, Murdoch DR, O’Brien KL, Levine OS, Scott JA; Pneumonia Methods Working Group and PERCH Site Investigators . Evaluation of risk factors for severe pneumonia in children: the Pneumonia Etiology Research for Child Health study. Clin Infect Dis 54, Suppl 2: S124–S131, 2012. doi: 10.1093/cid/cir1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O’Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med 208: 181–193, 2011. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Wright AK, Bangert M, Gritzfeld JF, Ferreira DM, Jambo KC, Wright AD, Collins AM, Gordon SB. Experimental human pneumococcal carriage augments IL-17A-dependent T-cell defence of the lung. PLoS Pathog 9: e1003274, 2013. doi: 10.1371/journal.ppat.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 358: 261–273, 2008. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 546.Wu M, Gibbons JG, DeLoid GM, Bedugnis AS, Thimmulappa RK, Biswal S, Kobzik L. Immunomodulators targeting MARCO expression improve resistance to postinfluenza bacterial pneumonia. Am J Physiol Lung Cell Mol Physiol 313: L138–L153, 2017. doi: 10.1152/ajplung.00075.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Xiong H, Carter RA, Leiner IM, Tang YW, Chen L, Kreiswirth BN, Pamer EG. Distinct Contributions of Neutrophils and CCR2+ Monocytes to Pulmonary Clearance of Different Klebsiella pneumoniae Strains. Infect Immun 83: 3418–3427, 2015. doi: 10.1128/IAI.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Xiong H, Keith JW, Samilo DW, Carter RA, Leiner IM, Pamer EG. Innate Lymphocyte/Ly6C(hi) Monocyte Crosstalk Promotes Klebsiella Pneumoniae Clearance. Cell 165: 679–689, 2016. doi: 10.1016/j.cell.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Xu X, Weiss ID, Zhang HH, Singh SP, Wynn TA, Wilson MS, Farber JM. Conventional NK cells can produce IL-22 and promote host defense in Klebsiella pneumoniae pneumonia. J Immunol 192: 1778–1786, 2014. doi: 10.4049/jimmunol.1300039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Yadav H, Kor DJ. Platelets in the pathogenesis of acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 309: L915–L923, 2015. doi: 10.1152/ajplung.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 292: 2237–2242, 2004. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 552.Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 61: 76–80, 2003. doi: 10.1212/01.WNL.0000073620.42047.D7. [DOI] [PubMed] [Google Scholar]
- 553.Yamada M, Gomez JC, Chugh PE, Lowell CA, Dinauer MC, Dittmer DP, Doerschuk CM. Interferon-γ production by neutrophils during bacterial pneumonia in mice. Am J Respir Crit Care Med 183: 1391–1401, 2011. doi: 10.1164/rccm.201004-0592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Yamamoto K, Ahyi AN, Pepper-Cunningham ZA, Ferrari JD, Wilson AA, Jones MR, Quinton LJ, Mizgerd JP. Roles of lung epithelium in neutrophil recruitment during pneumococcal pneumonia. Am J Respir Cell Mol Biol 50: 253–262, 2014. doi: 10.1165/rcmb.2013-0114OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Yamamoto K, Ferrari JD, Cao Y, Ramirez MI, Jones MR, Quinton LJ, Mizgerd JP. Type I alveolar epithelial cells mount innate immune responses during pneumococcal pneumonia. J Immunol 189: 2450–2459, 2012. doi: 10.4049/jimmunol.1200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Yamamoto N, Kerfoot SM, Hutchinson AT, Dela Cruz CS, Nakazawa N, Szczepanik M, Majewska-Szczepanik M, Nazimek K, Ohana N, Bryniarski K, Mori T, Muramatsu M, Kanemitsu K, Askenase PW. Expression of activation-induced cytidine deaminase enhances the clearance of pneumococcal pneumonia: evidence of a subpopulation of protective anti-pneumococcal B1a cells. Immunology 147: 97–113, 2016. doi: 10.1111/imm.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Yanes RE, Gustafson CE, Weyand CM, Goronzy JJ. Lymphocyte generation and population homeostasis throughout life. Semin Hematol 54: 33–38, 2017. doi: 10.1053/j.seminhematol.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Yap KL, Ada GL, McKenzie IF. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature 273: 238–239, 1978. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 559.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194: 519–527, 2001. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Yeligar SM, Harris FL, Hart CM, Brown LA. Glutathione attenuates ethanol-induced alveolar macrophage oxidative stress and dysfunction by downregulating NADPH oxidases. Am J Physiol Lung Cell Mol Physiol 306: L429–L441, 2014. doi: 10.1152/ajplung.00159.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Yende S, D’Angelo G, Kellum JA, Weissfeld L, Fine J, Welch RD, Kong L, Carter M, Angus DC; GenIMS Investigators . Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med 177: 1242–1247, 2008. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Yende S, D’Angelo G, Mayr F, Kellum JA, Weissfeld L, Kaynar AM, Young T, Irani K, Angus DC; GenIMS Investigators . Elevated hemostasis markers after pneumonia increases one-year risk of all-cause and cardiovascular deaths. PLoS One 6: e22847, 2011. doi: 10.1371/journal.pone.0022847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, Reis S, Angus DC. Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med 189: 1065–1074, 2014. doi: 10.1164/rccm.201307-1321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Yip TT, Chan JW, Cho WC, Yip TT, Wang Z, Kwan TL, Law SC, Tsang DN, Chan JK, Lee KC, Cheng WW, Ma VW, Yip C, Lim CK, Ngan RK, Au JS, Chan A, Lim WW; Ciphergen SARS Proteomics Study Group . Protein chip array profiling analysis in patients with severe acute respiratory syndrome identified serum amyloid a protein as a biomarker potentially useful in monitoring the extent of pneumonia. Clin Chem 51: 47–55, 2005. doi: 10.1373/clinchem.2004.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Yu H, Rubin J, Dunning S, Li S, Sato R. Clinical and economic burden of community-acquired pneumonia in the Medicare fee-for-service population. J Am Geriatr Soc 60: 2137–2143, 2012. doi: 10.1111/j.1532-5415.2012.04208.x. [DOI] [PubMed] [Google Scholar]
- 566.Yu H, Wier LM, Elixhauser A. Hospital Stays for Children, 2009. Healthcare Cost and Utilization Project Statistical Brief no. 118. Rockville, MD: Agency for Healthcare Research and Quality, 2011. [PubMed] [Google Scholar]
- 567.Yuste J, Botto M, Bottoms SE, Brown JS. Serum amyloid P aids complement-mediated immunity to Streptococcus pneumoniae. PLoS Pathog 3: 1208–1219, 2007. doi: 10.1371/journal.ppat.0030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, Suzuki T, Yang IV, De Langhe S, Reynolds SD, Mason RJ, Kahn M, Henson PM, Colgan SP, Downey GP. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci USA 108: 15990–15995, 2011. doi: 10.1073/pnas.1110144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Zemans RL, McClendon J, Aschner Y, Briones N, Young SK, Lau LF, Kahn M, Downey GP. Role of β-catenin-regulated CCN matricellular proteins in epithelial repair after inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 304: L415–L427, 2013. doi: 10.1152/ajplung.00180.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Zhang P, Bagby GJ, Kolls JK, Welsh DA, Summer WR, Andresen J, Nelson S. The effects of granulocyte colony-stimulating factor and neutrophil recruitment on the pulmonary chemokine response to intratracheal endotoxin. J Immunol 166: 458–465, 2001. doi: 10.4049/jimmunol.166.1.458. [DOI] [PubMed] [Google Scholar]
- 571.Zhong Z, Wen Z, Darnell JE Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264: 95–98, 1994. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 572.Zhou AC, Wagar LE, Wortzman ME, Watts TH. Intrinsic 4-1BB signals are indispensable for the establishment of an influenza-specific tissue-resident memory CD8 T-cell population in the lung. Mucosal Immunol 10: 1294–1309, 2017. doi: 10.1038/mi.2016.124. [DOI] [PubMed] [Google Scholar]
- 573.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 173: 2039–2046, 2013. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 574.Zimmer SM, Burke DS. Historical perspective–Emergence of influenza A (H1N1) viruses. N Engl J Med 361: 279–285, 2009. doi: 10.1056/NEJMra0904322. [DOI] [PubMed] [Google Scholar]