Methylotrophy, the ability to utilize single-carbon (C1) compounds as the sole carbon and energy sources, is only poorly understood in mycobacteria. Both pathogenic and nonpathogenic mycobacteria, including Mycobacterium tuberculosis, are capable of utilizing C1 compounds to meet their carbon and energy requirements, although the precise pathways are not well studied. Here we present a comprehensive study of methylotrophy in Mycobacterium smegmatis. With several genetic knockouts, we have dissected the entire methanol metabolism pathway in M. smegmatis. We show that while methanol dissimilation in M. smegmatis differs from that in other mycobacterial species, the concluding step of CO2 fixation is similar to that in M. tuberculosis. It is therefore both interesting and important to examine mycobacterial physiology in the presence of alternative carbon sources.
KEYWORDS: methanol dehydrogenase, carbon assimilation pathway, C1 metabolism, methylotrophic metabolism, anaplerotic enzyme
ABSTRACT
The mycobacteria comprise both pathogenic and nonpathogenic bacteria. Although several features related to pathogenicity in various mycobacterial species, such as Mycobacterium tuberculosis, have been studied in great detail, methylotrophy, i.e., the ability of an organism to utilize single-carbon (C1) compounds as the sole source of carbon and energy, has remained largely unexplored in mycobacteria. Reports are available that suggest that mycobacteria, including M. tuberculosis and M. smegmatis, are capable of utilizing alternative C1 compounds to meet their carbon and energy requirements. However, physiological pathways that are functional in mycobacteria to utilize such carbon compounds are only poorly understood. Here we report the identification and characterization of the gene products required for establishing methylotrophy in M. smegmatis. We present N,N-dimethyl-p-nitrosoaniline (NDMA)-dependent methanol oxidase (Mno) as the key enzyme that is essential for the growth of M. smegmatis on methanol. We show that Mno has both methanol and formaldehyde dehydrogenase activities in vitro. Further, M. smegmatis is able to utilize methanol even in the absence of the major formaldehyde dehydrogenase MscR, which suggests that Mno is sufficient to dissimilate methanol and the resulting formaldehyde in vivo. Finally, we show that M. smegmatis devoid of phosphoenolpyruvate carboxykinase, which has been shown to fix CO2 in M. tuberculosis, does not grow on methanol, suggesting that the final step of methanol utilization requires CO2 fixation for biomass generation. Our work here thus forms the first comprehensive report that explores methylotrophy in a mycobacterial species.
IMPORTANCE Methylotrophy, the ability to utilize single-carbon (C1) compounds as the sole carbon and energy sources, is only poorly understood in mycobacteria. Both pathogenic and nonpathogenic mycobacteria, including Mycobacterium tuberculosis, are capable of utilizing C1 compounds to meet their carbon and energy requirements, although the precise pathways are not well studied. Here we present a comprehensive study of methylotrophy in Mycobacterium smegmatis. With several genetic knockouts, we have dissected the entire methanol metabolism pathway in M. smegmatis. We show that while methanol dissimilation in M. smegmatis differs from that in other mycobacterial species, the concluding step of CO2 fixation is similar to that in M. tuberculosis. It is therefore both interesting and important to examine mycobacterial physiology in the presence of alternative carbon sources.
INTRODUCTION
Methylotrophic metabolism allows microorganisms to utilize single-carbon (C1) compounds as the sole sources of carbon and energy (1). Among the various C1 compounds, methylotrophic organisms utilize methanol, a widely available compound that is formed by a mineralization process such as plant cell wall pectin demethylation (2), to carry out the synthesis of biomass and energy production using dedicated biochemical pathways (3). The key enzyme in methanol metabolism is a methanol dehydrogenase, which catalyzes the oxidation of methanol to formaldehyde. Methanol dehydrogenases in different organisms may differ from each other based on their quaternary structure, coenzyme/cofactor requirements, and cellular localization (4, 5). Besides the oxidation of methanol, methanol dehydrogenases in both Gram-negative and Gram-positive methylotrophs have also been shown to have aldehyde dismutase and reductase activities in vitro and thus are likely involved in formaldehyde dissimilation (6–8).
Methanol metabolism initiates with the production of formaldehyde, which then enters the assimilation pathways primarily through a prokaryote-specific ribulose monophosphate (RuMP) or serine cycle, along with a parallel dissimilation pathway leading to CO2 generation. Although the majority of prokaryotes are known to assimilate formaldehyde using RuMP and/or serine cycles, the xylulose monophosphate (XuMP) pathway is the primary functional pathway in yeasts (1, 3). In addition to formaldehyde assimilation by traditional paths, a number of prokaryotic methylotrophs are known to fix the generated CO2 by one or the other pathway (1). Multiple routes for CO2 fixation have now been deciphered, and a majority of them are found in prokaryotes. CO2 fixation thus is a widespread phenomenon and coexists in various domains of life (9–11). Recent findings have also suggested that CO2 fixation is carried out by many methylotrophs for survival on C1 compounds (10, 12–14). The scenario of formaldehyde dissimilation is equally complex. Several formaldehyde oxidation pathways that include cofactors such as tetrahydromethanopterin (H4MPT)-, tetrahydrofolate (H4F)-, bacillithiol (BSH)-, mycothiol (MySH)-, and glutathione (GSH)-dependent formaldehyde dehydrogenases are now known (15, 16). The resultant oxidation of formaldehyde leads to the release of CO2 via formate. In addition to the previously known cofactors, some studied methylotrophs also utilize specific cofactor-dependent formaldehyde dehydrogenases in the course of methanol dissimilation. For example, Bacillus methanolicus is known to use multiple cofactor-dependent dissimilatory pathways operating in parallel (17). Corynebacterium glutamicum and Mycobacterium smegmatis are known to utilize mycothiol-dependent formaldehyde dehydrogenase (18–20).
Methylotrophic metabolism in mycobacteria is only poorly understood and, by far, has been best studied in Mycobacterium sp. strain JC1 (21–24). Interestingly, the most common C1 assimilation pathways (RuMP and serine) have been suggested to be absent in mycobacteria (21), and mycobacterial species (except M. tuberculosis, which does not grow on methanol) have been shown to be capable of utilizing both CO and methanol as the sole carbon and energy sources by utilizing eukaryotic C1 assimilation pathways (3, 21, 23–25). Mycobacterium sp. JC1 utilizes both the XuMP pathway and the ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO)-mediated Calvin cycle (21). Mycobacterium gastri is also known to employ the RuMP pathway for its growth on methanol (26). Thus, the mode for utilization of methanol differs among the different species of mycobacteria. We here present the gene products that constitute the biochemical pathway involved in methanol metabolism in Mycobacterium smegmatis. We show that M. smegmatis produces a dedicated methanol dehydrogenase to support its growth in the presence of methanol as the sole carbon source. We also report that formaldehyde dehydrogenase (MscR), which is known to be essential for the detoxification of excess formaldehyde (18), is dispensable during methanol metabolism, suggesting that methanol dehydrogenase is sufficient to metabolize generated formaldehyde. Our biochemical and genetic evidences allow us to propose that the methanol utilization pathway in M. smegmatis differs significantly from that of any of the currently reported mycobacterial species. Our data also suggest that in M. smegmatis, the phosphoenolpyruvate carboxykinase-catalyzed anaplerotic reaction for the fixation of generated CO2 into oxaloacetate is an essential step during methanol metabolism. M. smegmatis is a soil-dwelling mycobacterial species that demonstrates an admirable adaptive physiology to survive during starvation (27, 28). It is, therefore, important to understand the physiology and metabolism of M. smegmatis during growth on C1 compounds, which have remained largely unexplored. The results presented here form the first comprehensive report on methanol utilization in any mycobacterial species, and the findings can further be extrapolated to other bacteria that utilize methanol as the sole carbon source during methylotrophic metabolism.
RESULTS
Mno from M. smegmatis can oxidize both methanol and formaldehyde in vitro.
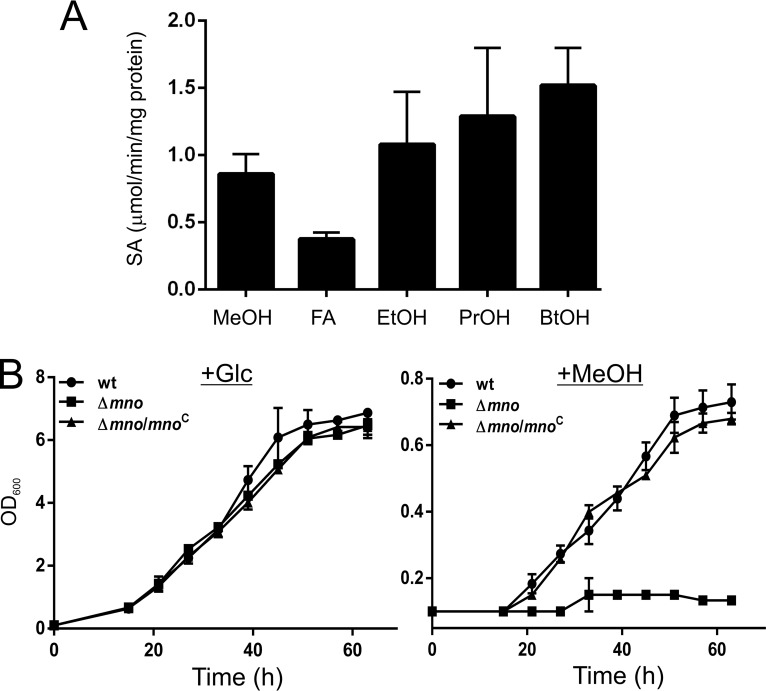
To examine methanol oxidation in M. smegmatis, we selected MSMEG_6242, which shares nearly 99% sequence identity with the previously reported Mdo from Mycobacterium sp. strain JC1 (UniProt accession no. C5MRT8) (22). We proceeded with the cloning and expression of the MSMEG_6242 gene (hereafter referred to as mno) in Escherichia coli and performed protein purification (see Fig. S1 in the supplemental material). The alcohol oxidation activity of the purified Mno was examined for various primary alcohols. In contrast to the previously reported Mdo (22), which is unable to oxidize propanol and butanol, Mno was observed to oxidize methanol along with other primary alcohols (Fig. 1A) in the presence of N,N-dimethyl-p-nitrosoaniline (NDMA). However, the maximum activity of Mno was observed with propanol and butanol, which correlates with the methanol dehydrogenase of Bacillus methanolicus, whose catalytic activity is lower for methanol than for other alcohols (29). Interestingly, Mno is also able to oxidize formaldehyde under the same reaction condition (i.e., in the presence of NDMA) as that for the alcohols (Fig. 1A), suggesting that the enzyme also possesses formaldehyde dehydrogenase activity, which is similar to the Mno isolated from Amycolatopsis methanolicus and M. gastri (8). Our data thus suggest that Mno of M. smegmatis has both methanol oxidase and formaldehyde dehydrogenase activities in vitro.
FIG 1.
Mno can oxidize both primary alcohols and formaldehyde in vitro and is required for bacterial growth on methanol. (A) Specific activities of purified Mno for various alcohols (MeOH, methanol; EtOH, ethanol; PrOH, 1-propanol; BtOH, 1-butanol) and formaldehyde (FA) using NDMA as the electron acceptor. All the assays were carried out at least three times, and the error bars represent standard deviations. (B) The physiological role of mno was confirmed by measuring the OD600 of the culture at specific time intervals and plotting the results. The left and right panels show the bacterial growth in the presence of 2% glucose (+Glc) and 2% methanol (+MeOH), respectively. The data show that M. smegmatis Δmno is unable to utilize methanol as the sole carbon source, whereas M. smegmatis Δmno complemented with pADhpMno (Δmno/mnoC) shows growth on 2% methanol. Both the wild-type M. smegmatis (wt) and the Δmno knockout carry the pSS1 empty vector. The data are an average of results of three independent experiments with standard deviations.
Mno is indispensable for bacterial growth in the presence of methanol as the sole carbon source.
Our in vitro assays suggest that Mno can oxidize single-carbon compounds such as methanol and formaldehyde in vitro. We next asked if M. smegmatis is capable of growing in the presence of methanol as the sole carbon source and if Mno is required to support such growth. Thus, to understand the role of Mno in bacterial growth in the presence of methanol, we first prepared a genetic knockout of Mno in M. smegmatis. The knockout (Δmno) was created by replacing the mno gene with the hygromycin resistance cassette and was further confirmed by PCR and DNA sequencing. Both the wild-type (wt) and knockout (Δmno) strains of M. smegmatis were subjected to growth on solid agar medium containing either 2% glucose or 2% methanol. We observed that while the growth of the two strains is indistinguishable on glucose, the Δmno strain does not grow when methanol is available as the sole carbon source (Fig. 1B; Fig. S2A). Upon complementing the Δmno strain with the wild-type copy of the mno gene on a plasmid under the hsp60 promoter (pMVMno), bacterial growth could be restored in the presence of methanol (Fig. 1B; Fig. S2A). This strongly suggests that Mno is required by M. smegmatis for its growth in the presence of methanol.
Formaldehyde dehydrogenase (MscR) from M. smegmatis can oxidize methanol in vitro but cannot replace Mno in vivo.
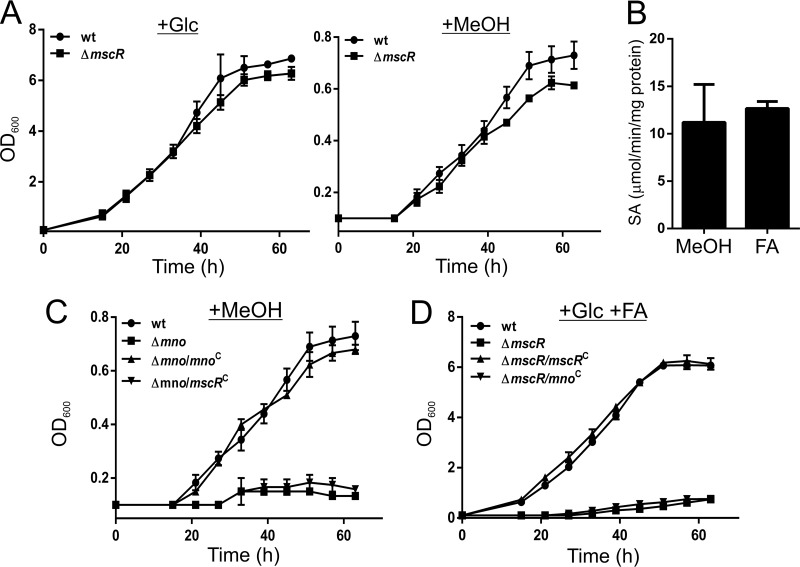
M. smegmatis has been shown to possess a mycothiol-dependent formaldehyde dehydrogenase, MscR, which is overproduced during aldehyde stress (18). We hypothesized that MscR, because of its formaldehyde dehydrogenase activity, is involved in the detoxification of excess formaldehyde generated during methanol oxidation by Mno. Thus, to understand the role of MscR in the methanol dissimilation pathway in M. smegmatis, we constructed an mscR genetic knockout strain. A previous report suggests that the ΔmscR strain cannot grow in the presence of formaldehyde (18). However, we additionally observed that M. smegmatis ΔmscR is able to grow on methanol as the sole carbon source (Fig. 2A; Fig. S2B). Thus, it appears that MscR is dispensable in the dehydrogenation process of formaldehyde generated during methanol metabolism. However, in vitro activity assays of the purified MscR protein (Fig. S1) demonstrate that MscR is capable of oxidizing not only formaldehyde, as reported earlier (30), but also methanol (Fig. 2B). Therefore, the involvement of MscR in the dissimilation of formaldehyde during methanol metabolism cannot be ruled out. Conceivably, MscR should be able to metabolize methanol in vivo and therefore, in principle, should be able to complement the loss of Mno. To explore the latter, we cloned mscR under the hsp60 promoter in an E. coli-mycobacterium shuttle vector and transformed Δmno cells with this plasmid. Growth curve analyses were performed to monitor the growth of the complemented strain in methanol as the sole carbon source. Surprisingly, the bacterium failed to grow on methanol, suggesting that MscR cannot complement the loss of Mno in vivo (Fig. 2C; Fig. S2C). We made a similar observation when we grew the ΔmscR strain by complementing it with mno; although Mno also possesses formaldehyde dehydrogenase activity, it could not complement the loss of MscR to support bacterial growth in the presence of formaldehyde (Fig. 2D; Fig. S2D).
FIG 2.
Both Mno and MscR have methanol and formaldehyde dehydrogenase functions, but one cannot complement the loss of the other in vivo. (A) Growth of M. smegmatis wild-type (wt) and the mscR knockout (ΔmscR) in the presence of either 2% glucose (+Glc) or 2% methanol (+MeOH). Growth was measured by monitoring the OD600 at regular time intervals. Data show that the mscR knockout can utilize methanol as the sole carbon source. (B) Specific activities of purified MscR with methanol and formaldehyde as the substrates, with NAD+ as the electron acceptor. All the assays were carried out at least three times, and the error bars represent standard deviations. (C) Bacterial growth was monitored to assess the complementation of the loss of Mno by MscR, since the latter possesses methanol dehydrogenase activity in vitro. The results show that the wild-type M. smegmatis strain (wt) and the Δmno knockout complemented with pADhpMno (Δmno/mnoC) are able to grow on methanol (MeOH), whereas M. smegmatis Δmno and M. smegmatis Δmno/mscRC (Δmno complemented with pSWhpMscR) cannot. The data show that MscR cannot complement the loss of Mno in vivo. (D) Similarly, Mno, which possesses formaldehyde dehydrogenase activity, is unable to complement the loss of MscR in vivo, when the bacteria are grown in the presence of glucose and formaldehyde (+Glc +FA). Complementation could be successfully accomplished only by using pSWhpMscR in the ΔmscR strain. ΔmscR/mnoC denotes the ΔmscR strain complemented with pADhpMno. In panels C and D, the wt and knockout strains (Δmno and ΔmscR) were transformed with an empty plasmid (pSS1). In panels A, C, and D, the data are an average of results of at least three independent bacterial growth assays with standard deviations.
Multiple factors regulate mno expression.
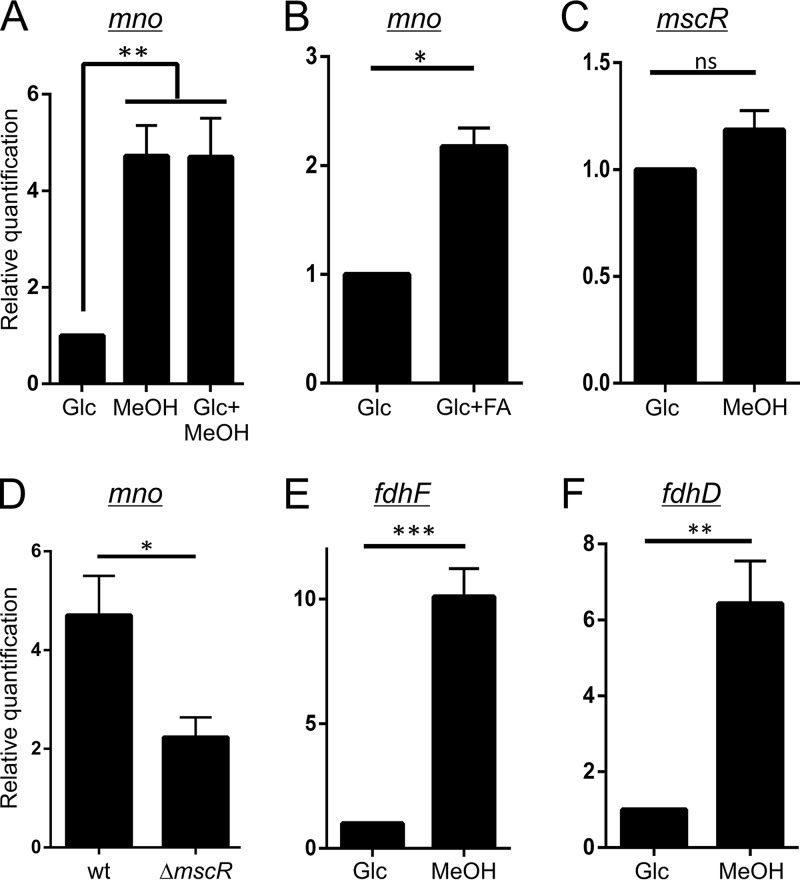
We next assessed the conditions under which Mno is overproduced. Our real-time PCR data suggest that the overexpression of mno occurs in M. smegmatis when the cells are grown in the presence of methanol (Fig. 3A). We also showed that the expression of mno is not affected by the presence of glucose (Fig. 3A), suggesting that mno in not under catabolite repression. Additionally, we observed mno overexpression in the presence of formaldehyde (Fig. 3B). Since Mno is capable of oxidizing formaldehyde in vitro (Fig. 1) and given the fact that mno expression increases in the presence of formaldehyde (Fig. 3B), it appears likely that Mno by itself can carry out the detoxification of formaldehyde generated during methanol oxidation in vivo. Interestingly, cells utilizing methanol as the carbon source do not show overexpression of mscR (Fig. 3C), which has been shown to have higher expression in the presence of formaldehyde (18). It is thus plausible that the formaldehyde generated upon methanol oxidation is insufficient to cause mscR overexpression and is metabolized by Mno itself.
FIG 3.
Expression profiling of various genes involved in methylotrophic metabolism in M. smegmatis RT-qPCR was performed to investigate the differentially expressed genes in M. smegmatis during growth on various carbon sources. The gene in question is shown on the top of each panel. (A and B) mno expression was monitored in cells grown in the presence of only glucose (Glc), only methanol (MeOH), both glucose and methanol (Glc+MeOH), and both glucose and formaldehyde (Glc+FA). (A) Higher mno expression was observed only in the presence of methanol, with or without glucose. (B) Comparatively lower yet significant overexpression of mno was observed when FA was added to the culture medium. (C) No significant overexpression of mscR was observed when the cells were grown in the presence of methanol. (D) Wild-type (wt) M. smegmatis and ΔmscR cells grown in the presence of methanol show differential mno induction. In the latter, comparatively less mno expression is observed, which suggests that there is a loss of methanol-dependent mno overexpression in ΔmscR cells. (E and F) M. smegmatis cells also show a significantly higher expression of fdhF (E) and fdhD (F), which are required for the conversion of formate to CO2. In all panels, gene expression in the presence of only glucose was considered equal to 1 to assess relative quantification. In each plot, error bars represent standard deviations of results from three individual sets of experiments. A P value of <0.05 was considered significant; ns, not significant; *, significant; **, very significant; ***, highly significant.
The presence of multifunctional enzymes (Mno and MscR) for the metabolism of C1 compounds (methanol and formaldehyde) suggests a complex interplay between methanol oxidation and formaldehyde detoxification. MscR is dedicated to formaldehyde detoxification at higher concentrations. However, M. smegmatis ΔmscR grows in the presence of methanol as the sole carbon source and therefore can tolerate the formaldehyde generated during this process. Although the ΔmscR strain can utilize methanol as the sole carbon source, it shows a loss of methanol-dependent induction of mno (Fig. 3D).
The current report suggests a complex regulation of mno expression by either the intracellular formaldehyde levels or the methanol oxidation by-products. It has been shown earlier that during the complete dissimilation process, methanol is converted into CO2 via formaldehyde and formate (1). The M. smegmatis genome harbors genes encoding homologs of formate dehydrogenase accessory protein (FdhD) and formate dehydrogenase H (FdhF) from M. tuberculosis, fdhD and fdhF, respectively. We show that the addition of methanol to the culture medium leads to the overexpression of formate dehydrogenase operon genes fdhD and fdhF, which suggests that the overexpression of these genes is possibly for the conversion of formate into CO2 (Fig. 3E and F). We thus conclude from these experiments that Mno in M. smegmatis metabolizes methanol to formate via formaldehyde, which is then converted into CO2 by the activity of formate dehydrogenase.
Phosphoenolpyruvate carboxykinase-catalyzed carboxylation of phosphoenolpyruvate is essential for methylotrophic metabolism in M. smegmatis.
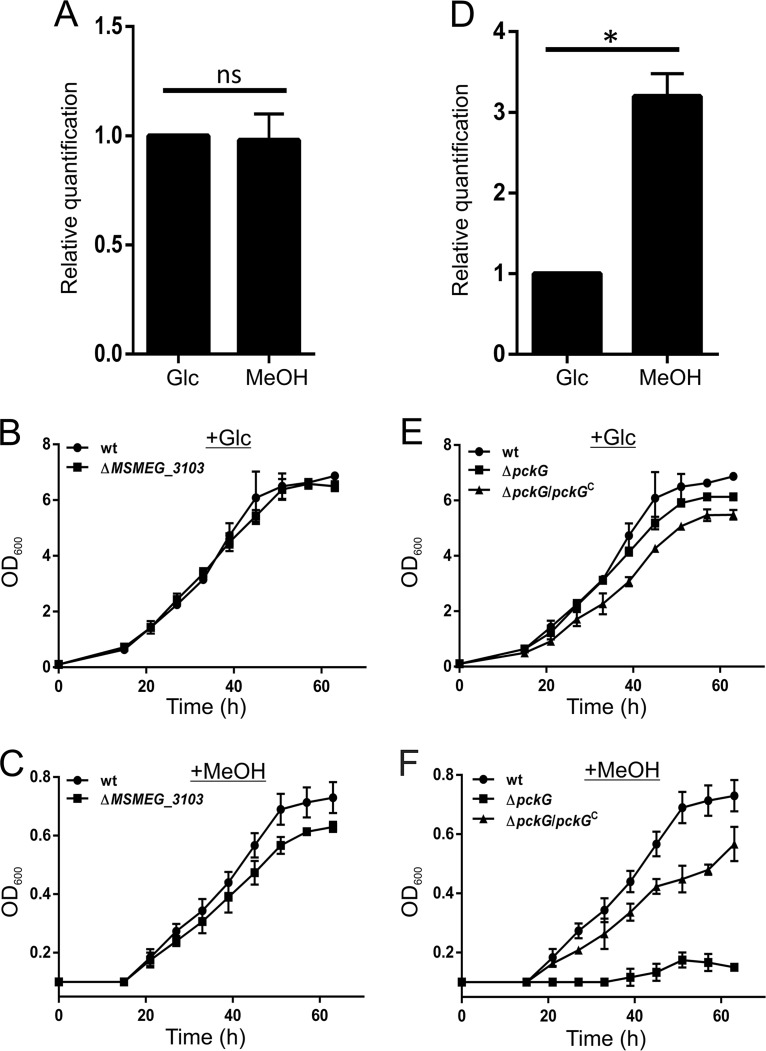
The utilization of methanol as the sole carbon source involves formaldehyde assimilation and/or the fixation of generated CO2 (1, 3). Several mycobacterial species, including M. smegmatis, are known to express dihydroxyacetone synthase (DHAS) and ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) during growth on methanol (21). DHAS, the key enzyme of the XuMP pathway, and RuBisCO have previously been identified and characterized in Mycobacterium sp. JC1 (23–25). Our bioinformatics analysis revealed that MSMEG_3103, which codes for a transketolase, shares 60% sequence identity with the DHAS gene from Mycobacterium sp. JC1. The gene, dasS, coding for DHAS has been shown to overexpress in Mycobacterium sp. JC1 during its growth on methanol (23). However, we failed to observe any overexpression of MSMEG_3103 in M. smegmatis during growth on methanol (Fig. 4A). Nevertheless, to understand the involvement of MSMEG_3103 in methanol utilization in M. smegmatis, we constructed a strain with a genetic knockout of MSMEG_3103 and assessed its methanol utilization ability. Our growth curve data show that M. smegmatis ΔMSMEG_3103 can utilize both glucose (Fig. 4B) and methanol as the sole carbon source (Fig. 4C; Fig. S2E). This strongly indicates that the XuMP pathway is absent in M. smegmatis.
FIG 4.
Phosphoenolpyruvate carboxykinase, and not DHAS-like transketolase, is indispensable for M. smegmatis growth on methanol. (A) M. smegmatis wild-type cells grown in the presence of methanol do not show overexpression of MSMEG_3103 coding for a transketolase, which bears 60% sequence identity with the dihydroxyacetone synthase involved in HCHO fixation via the XuMP pathway of Mycobacterium sp. JC1. The experiment was repeated at least three times, and error bars represent standard deviations. ns, not significant. (B and C) A bacterial growth assay carried out with the M. smegmatis MSMEG_3103 knockout strain (ΔMSMEG_3103) shows that the cells are able to utilize both glucose (B) and methanol (C) as the sole carbon sources. (D) Wild-type M. smegmatis cells grown in the presence of methanol show overexpression of pckG, which codes for GTP-dependent phosphoenolpyruvate carboxykinase. The experiment was repeated at least three times, and error bars represent standard deviations. A P value of <0.05 was considered significant (*). ns, not significant. (E and F) A bacterial growth assay was carried out for the M. smegmatis wild type (wt), the pckG knockout strain (ΔpckG), and the ΔpckG strain harboring pADhpPck (ΔpckG/pckGC) by monitoring the OD600 with time in the presence of either glucose (+Glc) (E) or methanol (+MeOH) (F). Our data suggest that the growth profile of the ΔpckG strain is similar to that of the wild type on glucose, whereas the ΔpckG strain does not show growth on methanol as the sole carbon source. Complementation of the ΔpckG strain with pADhpPck (ΔpckG/pckGC) successfully restores growth on methanol. The growth assays were performed at least three times; averages of OD600 results from three independent experiments were used to plot the curves with standard deviations.
In contrast to DHAS of Mycobacterium sp. JC1, the RuBisCO homolog could not be identified in M. smegmatis (data not shown). However, the overexpression of the M. smegmatis formate dehydrogenase operon in the presence of methanol suggests the conversion of formate to CO2. This immediately is suggestive of the presence of an unknown pathway that M. smegmatis employs for CO2 fixation during methanol metabolism. We therefore attempted to understand how CO2 is utilized by the bacterium. Phosphoenolpyruvate carboxykinase (Pck) and phosphoenolpyruvate carboxylase (Pcl) are the two enzymes that have been reported to catalyze the carboxylation of phosphoenolpyruvate (PEP) to oxaloacetate (OAA) in microorganisms (13, 31). Recent reports have shown the involvement of Pck as an anaplerotic enzyme for this reaction by single-carbon fixation in M. tuberculosis, Mycobacterium bovis, and other organisms, including Bacillus subtilis (31–33). Since M. smegmatis is known to possess a vertebrate-type GTP-dependent Pck, which is also suggested to have similar functions of interconversion between C3 and C4 metabolites (34), we hypothesized that M. smegmatis Pck might be involved in fixing the CO2 with PEP to form OAA by acting as an anaplerotic enzyme during methanol utilization. Thus, to elucidate the role of Pck in methanol metabolism, we monitored the expression of pckG in M. smegmatis cells utilizing methanol as the sole carbon source (Fig. 4D). M. smegmatis cells growing on only methanol show overexpression of pckG. When challenged for growth on only methanol, M. smegmatis with a genetic knockout of pckG (M. smegmatis ΔpckG) shows that it can utilize glucose (Fig. 4E; Fig. S2F) but is unable to utilize methanol as the sole carbon source (Fig. 4F; Fig. S2F). Furthermore, complementation of the loss of pckG in M. smegmatis ΔpckG restores the growth of M. smegmatis ΔpckG on methanol (Fig. 4F; Fig. S2F). Our data thus strongly suggest that Pck is involved in methanol metabolism by carboxylation of PEP with CO2 for the production of OAA during formate dehydrogenation.
DISCUSSION
Methylotrophic metabolism in mycobacteria is not well understood. Here, we have explored the metabolism of methanol by Mycobacterium smegmatis and demonstrate the involvement of various enzymes in the pathway by carrying out both in vivo and in vitro experiments. We present Mno as the one key enzyme required by the bacterium for its growth in the presence of methanol as the sole carbon source.
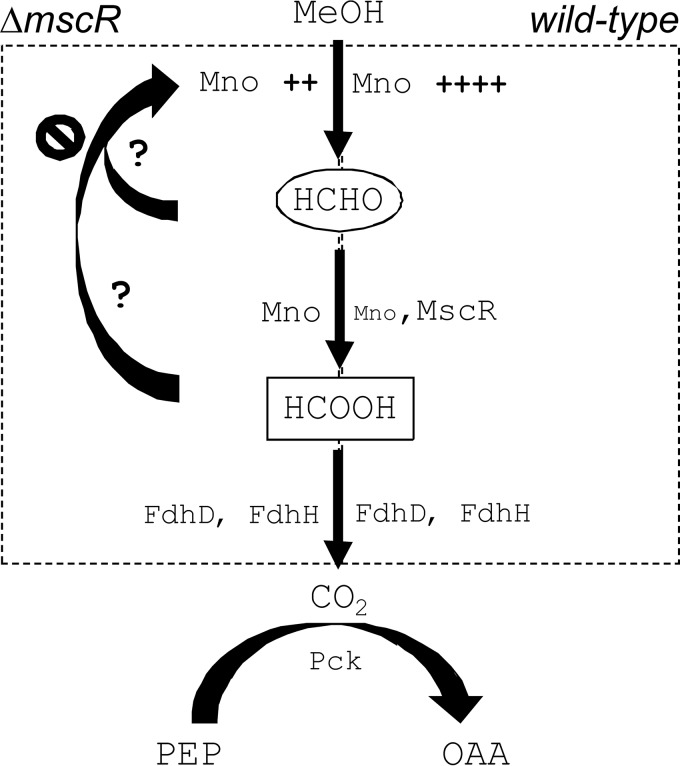
We summarize our findings by a model shown in Fig. 5 that explains methanol metabolism in M. smegmatis. The model shows that the dissimilation of methanol in M. smegmatis is carried out by Mno. While Mno itself can oxidize formaldehyde, thus generating formate, MscR is required when there is an overload of formaldehyde. In the absence of MscR, Mno expression occurs at lower levels. We believe that the expression of Mno is reduced so that methanol oxidation happens at a lower rate and the formaldehyde thus generated can be dissimilated by Mno itself. The addition of methanol to the culture medium results in the overproduction of formate dehydrogenase, suggesting that methanol is, in the end, converted into CO2. The CO2 generated after methanol metabolism in the cell is further used as the substrate for an anaplerotic reaction catalyzed by PEP carboxykinase. A previous report involving 13C labeling of the metabolites clearly demonstrated that mycobacteria can fix CO2 into OAA, leading to biomass generation (31). Our work here thus demonstrates the involvement of various enzymes in methylotrophic metabolism in mycobacteria, which has remained largely unexplored.
FIG 5.
Model summarizing methanol metabolism in M. smegmatis. Methanol metabolism in M. smegmatis initiates with the oxidation of methanol in both the wild-type (right) and mscR knockout (ΔmscR) (left) cells. The presence of methanol in the medium leads to overexpression of mno (Mno ++++) in the wild-type and ΔmscR (Mno ++) cells. However, the level of mno overexpression in ΔmscR cells in the presence of methanol induction is lower than that in the wild type. Further, while formaldehyde produced by methanol oxidation gets converted into formate primarily by MscR and, to a lesser extent, by Mno in wild-type cells, in ΔmscR cells, Mno performs the dual function of methanol and formaldehyde oxidation. The formate thus generated is converted into CO2 by FdhH and FdhD in both wild-type and ΔmscR cells. Eventually, the generated CO2 is fixed by Pck to produced oxaloacetate (OAA) from phosphoenolpyruvate (PEP); this is an essential step during methanol metabolism to produce biomass.
It is both interesting and intriguing that although MscR can oxidize both methanol and formaldehyde in vitro in the presence of NAD+, the mno knockout cells that possess a functional copy of mscR do not grow on methanol. Furthermore, even after such cells are complemented with mscR under the hsp60 promoter, the cells fail to grow when methanol is the only carbon source. Nevertheless, the in vitro activity assay suggests that mscR is likely involved in methylotrophic metabolism. Although both Mno and MscR perform the same function in vitro, it is surprising that they do not complement each other in vivo. We currently do not have sufficient evidence to explain this phenomenon, and it remains open for further exploration.
Our data suggest that the conversion of methanol into biomass in M. smegmatis differs significantly from that in other mycobacterial species. Earlier studies have suggested the involvement of the XuMP pathway and RuBisCO in Mycobacterium sp. JC1 and that of the RuMP pathway in M. gastri (21, 26). However, the involvement of the XuMP pathway in M. smegmatis could be ruled out by monitoring the expression of MSMEG_3103 and by constructing its genetic knockout; the genetic knockout could grow on methanol. Moreover, we also found no homolog of RuBisCO from Mycobacterium sp. JC1 in the M. smegmatis genome. However, our data suggest that methanol is metabolized into CO2, which is further utilized by M. smegmatis to produce biomass by using PEP carboxykinase.
Methylotrophs have found a significant position in ecological, agricultural, and industrial aspects (35–37). We believe that the exploration of methylotrophic metabolism in M. smegmatis, which is already a qualified candidate for industrial applications, will further broaden the range of applications (38, 39). Formaldehyde, which forms the branching point in methanol metabolism, is produced in the cells as an intermediate molecule during cellular metabolism (40). Thus, formaldehyde detoxification has always been of special interest due to its relevance in bacterial physiology and pathogenesis (41). Our work suggests that homologs of the proteins required for methylotrophic metabolism in M. smegmatis are present in pathogenic counterparts such as M. tuberculosis. Thus, an in-depth study of these processes is essential to understand the mechanism of methylotrophic metabolism in this genus, which is represented by both pathogenic and nonpathogenic organisms.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Escherichia coli strains XL1-Blue and BL21(DE3) were used for cloning and protein overexpression, respectively. Both strains were cultured in LB broth (Difco) supplemented with 100 μg/ml ampicillin, 50 μg/ml kanamycin, or 100 μg/ml hygromycin, as required, at 37°C with constant shaking at 200 rpm unless mentioned otherwise. M. smegmatis was cultured in MB7H9 broth (Difco) supplemented with either 2% glucose or 2% methanol, as required, along with 0.05% Tween 80 at 37°C containing kanamycin (25 μg/ml) and/or hygromycin (100 μg/ml), as required. When required, the culture was supplemented with 1 mM formaldehyde, and the tubes were covered with Parafilm. Growth was monitored by recording the optical density at 600 nm (OD600) at regular time intervals in the medium containing the desired carbon sources. Additionally, spot assays were carried out on 1.5% agar plates containing MB7H9 medium, supplemented with either 2% glucose or 2% (vol/vol) methanol; the growth on formaldehyde was monitored on MB7H9 agar plates containing 2% glucose and 1 mM formaldehyde. All the plates had the required antibiotics.
Sequence analysis and cloning of mycobacterial genes.
The sequences of mno (MSMEG_6242), mscR (MSMEG_4340), and pckG (MSMEG_0255) were obtained from the National Center for Biotechnology Information. The genes were PCR amplified from M. smegmatis mc2155 genomic DNA using the primers listed in Table S1 in the supplemental material. The amplicons for mno and mscR were cloned in the quick series pMS-QS-CHS vector as described previously (42), to yield pADt7Mno and pSWt7MscR, respectively. The mycobacterial expression plasmid pMV261 (43) was modified to make pSS1, which can accept blunt-end PCR products at the EcoRV restriction site present downstream to the hsp60 promoter. The vector also provides a hexahistidine tag at the C terminus of the expressed protein. The amplicons mno, mscR, and pckG were cloned in the modified pMV vector to yield pADhpmno, pSWhpmscR, and pADhppckG, respectively. All the clones were screened by colony PCR and were further confirmed by sequencing.
Protein purification and quantification.
Plasmids pADt7Mno and pSWt7MscR were used to transform E. coli BL21(DE3) for Mno and MscR overexpression and purification. The proteins were purified as described previously (44) by Ni-nitrilotriacetic acid (Ni-NTA) column chromatography. The eluted proteins were dialyzed against 40 mM sodium phosphate buffer (pH 7.4), 200 mM NaCl, and 1 mM dithiothreitol (DTT) and quantified by recording their absorbance at 280 nm. The molar extinction coefficients for Mno and MscR were estimated from their protein sequences using the protparam tool available at ExPASy (http://web.expasy.org/protparam/) and found to be 51,590 M−1 cm−1 and 28,585 M−1 cm−1, respectively.
In vitro enzyme activity.
A reaction with Mno possessing N,N-dimethyl-p-nitrosoaniline (NDMA)-dependent activity was carried out as described previously (45) in 20 mM sodium phosphate buffer (pH 7.4), 25 μM NDMA, and 10 mM substrate (methanol, ethanol, 1-propanol, 1-butanol, or formaldehyde). The reaction was initiated by adding 10 μM purified enzyme to the mixture. The methanol-dependent NDMA reduction was monitored by measuring the absorbance at 440 nm at 25°C. The specific activity (SA) of the enzyme was calculated as the amount of reduced NDMA (ε440 = 35,400 M−1 cm−1) in μmol per mg of protein per min. Similarly, MscR activity was measured in the presence of 400 μM NAD+ as the electron acceptor, using the buffer and temperature conditions mentioned above. The SA of MscR was calculated as the amount of reduced NADH (ε340 = 6,220 M−1 cm−1) produced in micromoles per milligram of protein per minute.
RNA isolation and RT-qPCR.
Differential gene expression was monitored by reverse transcription-quantitative PCR (RT-qPCR) for mno, mscR, fdhF, fdhD, the dihydroxyacetone synthase (DHAS)-like transketolase gene (MSMEG_3103), and pckG. M. smegmatis was grown with or without 2% methanol until the log phase (OD600 ∼0.8). RNA was isolated using TRI Reagent (Sigma-Aldrich), and cDNA synthesis was carried out using i-script cDNA synthesis (Bio-Rad) in accordance with the manufacturer's instructions. The generated cDNA was used in qPCR using the oligonucleotides listed in Table S1. The rpoB gene was used as an internal control. qPCR was carried out on a StepOnePlus real-time PCR system (Applied Biosystems) using iTaq universal SYBR green mix (Bio-Rad) in accordance with the manufacturer's instructions. A two-tailed Student t test was performed to calculate the level of significance among three independent sets of experiments.
Protein expression analysis.
To assess Mno, MscR, and Pck production in M. smegmatis, bacterial cells were harvested and resuspended in lysis buffer containing 8 M urea in 1× phosphate-buffered saline (PBS) and lysed by sonication. The lysate was centrifuged at 13,000 rpm for 10 min at room temperature. An equal volume of supernatant was mixed with SDS loading buffer, boiled for 5 min, and loaded on a 12% SDS polyacrylamide gel. The proteins were then transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore), and Western blotting was carried out using anti-His antibody raised in mouse (Sigma-Aldrich), followed by anti-mouse IgG DyLight 680-conjugated secondary antibody (Thermo Scientific). The blots were scanned on an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE) and are presented in Fig. S3.
Construction of genetic knockouts in M. smegmatis.
The genetic knockouts for mno, mscR, MSMEG_3103, and pckG were constructed essentially as described previously (46). In each case, the gene was replaced with a hygromycin cassette (Hygr). The cassette was PCR amplified from the pVV16 plasmid (obtained through BEI Resources, NIAID, NIH; naked plasmid pVV16 for expression in Mycobacterium smegmatis NR-13402) using the oligonucleotides listed in Table S1. DNA segments of ∼500 bp from the upstream and downstream of the genes were also PCR amplified using genomic DNA as the template and the oligonucleotides listed in Table S1. All three fragments were used to generate an allelic-exchange substrate (AES). M. smegmatis mc2155 cells containing pJV53 (kind gift of Graham Hatfull, University of Pittsburgh, Pittsburgh, PA; Addgene plasmid 26904) were induced with 0.2% acetamide at an OD600 of ∼0.6 for 5 h. Cells were then harvested and washed four times with prechilled 10% glycerol to prepare competent cells. These cells were then electroporated with 200 ng of AES for each gene. Gene knockouts were then selected on MB7H9 agar plates containing hygromycin and were confirmed by PCR (Fig. S4) using the oligonucleotides listed in Table S1 and sequencing of the amplified product. Curing of pJV53 was further carried out as described previously (47). Cured cells (Hygr Kans) were used for the complementation experiments.
Supplementary Material
ACKNOWLEDGMENTS
We thank Surya P. Seniya for constructing the pSS1 plasmid. The pVV16 plasmid was obtained through BEI Resources, NIAID, NIH, for expression in Mycobacterium smegmatis NR-13402. pJV53 was a kind gift from Graham Hatfull, University of Pittsburgh.
A.A.D. thanks the Council of Scientific and Industrial Research (CSIR), Government of India, for a senior research fellowship. S.R.W. acknowledges receipt of a junior research fellowship under the DST-INSPIRE scheme from the Department of Science and Technology (DST), Government of India. This work was supported by grants from CSIR [27(0325)/17/EMR-II] and the Department of Biotechnology, Government of India (BT/PR20257/BBE/117/223/2016) and by intramural funds from IISER Bhopal to V.J.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00288-18.
REFERENCES
- 1.Anthony C. 1991. Assimilation of carbon by methylotrophs. Biotechnology 18:79–109. [DOI] [PubMed] [Google Scholar]
- 2.Fall R, Benson AA. 1996. Leaf methanol—the simplest natural product from plants. Trends Plant Sci 1:296–301. doi: 10.1016/S1360-1385(96)88175-0. [DOI] [Google Scholar]
- 3.Anthony C. 1982. The biochemistry of methylotrophs. Academic Press, London, England. [Google Scholar]
- 4.Ghosh R, Quayle JR. 1981. Purification and properties of the methanol dehydrogenase from Methylophilus methylotrophus. Biochem J 199:245–250. doi: 10.1042/bj1990245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bystrykh LV, Vonck J, van Bruggen EF, van Beeumen J, Samyn B, Govorukhina NI, Arfman N, Duine JA, Dijkhuizen L. 1993. Electron microscopic analysis and structural characterization of novel NADP(H)-containing methanol: N,N′-dimethyl-4-nitrosoaniline oxidoreductases from the gram-positive methylotrophic bacteria Amycolatopsis methanolica and Mycobacterium gastri MB19. J Bacteriol 175:1814–1822. doi: 10.1128/jb.175.6.1814-1822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skovran E, Palmer AD, Rountree AM, Good NM, Lidstrom ME. 2011. XoxF is required for expression of methanol dehydrogenase in Methylobacterium extorquens AM1. J Bacteriol 193:6032–6038. doi: 10.1128/JB.05367-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt S, Christen P, Kiefer P, Vorholt JA. 2010. Functional investigation of methanol dehydrogenase-like protein XoxF in Methylobacterium extorquens AM1. Microbiology 156:2575–2586. doi: 10.1099/mic.0.038570-0. [DOI] [PubMed] [Google Scholar]
- 8.Bystrykh LV, Govorukhina NI, Vanophem PW, Hektor HJ, Dijkhuizen L, Duine JA. 1993. Formaldehyde dismutase activities in Gram-positive bacteria oxidizing methanol. J Gen Microbiol 139:1979–1985. doi: 10.1099/00221287-139-9-1979. [DOI] [Google Scholar]
- 9.Berg IA. 2011. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol 77:1925–1936. doi: 10.1128/AEM.02473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabita FR. 2009. The hydroxypropionate pathway of CO2 fixation: fait accompli. Proc Natl Acad Sci U S A 106:21015–21016. doi: 10.1073/pnas.0912486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ragsdale SW. 2008. Enzymology of the Wood-Ljungdahl pathway of acetogenesis. Ann N Y Acad Sci 1125:129–136. doi: 10.1196/annals.1419.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitsui R, Katayama H, Tanaka M. 2015. Requirement of carbon dioxide for initial growth of facultative methylotroph, Acidomonas methanolica MB58. J Biosci Bioeng 120:31–35. doi: 10.1016/j.jbiosc.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Jahn U, Huber H, Eisenreich W, Hugler M, Fuchs G. 2007. Insights into the autotrophic CO2 fixation pathway of the archaeon Ignicoccus hospitalis: comprehensive analysis of the central carbon metabolism. J Bacteriol 189:4108–4119. doi: 10.1128/JB.00047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohhata N, Yoshida N, Egami H, Katsuragi T, Tani Y, Takagi H. 2007. An extremely oligotrophic bacterium, Rhodococcus erythropolis N9T-4, isolated from crude oil. J Bacteriol 189:6824–6831. doi: 10.1128/JB.00872-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vorholt JA. 2002. Cofactor-dependent pathways of formaldehyde oxidation in methylotrophic bacteria. Arch Microbiol 178:239–249. doi: 10.1007/s00203-002-0450-2. [DOI] [PubMed] [Google Scholar]
- 16.Vorholt JA, Marx CJ, Lidstrom ME, Thauer RK. 2000. Novel formaldehyde-activating enzyme in Methylobacterium extorquens AM1 required for growth on methanol. J Bacteriol 182:6645–6650. doi: 10.1128/JB.182.23.6645-6650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller JE, Meyer F, Litsanov B, Kiefer P, Vorholt JA. 2015. Core pathways operating during methylotrophy of Bacillus methanolicus MGA3 and induction of a bacillithiol-dependent detoxification pathway upon formaldehyde stress. Mol Microbiol 98:1089–1100. doi: 10.1111/mmi.13200. [DOI] [PubMed] [Google Scholar]
- 18.Vargas D, Hageman S, Gulati M, Nobile CJ, Rawat M. 2016. S-Nitrosomycothiol reductase and mycothiol are required for survival under aldehyde stress and biofilm formation in Mycobacterium smegmatis. IUBMB Life 68:621–628. doi: 10.1002/iub.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lessmeier L, Hoefener M, Wendisch VF. 2013. Formaldehyde degradation in Corynebacterium glutamicum involves acetaldehyde dehydrogenase and mycothiol-dependent formaldehyde dehydrogenase. Microbiology 159:2651–2662. doi: 10.1099/mic.0.072413-0. [DOI] [PubMed] [Google Scholar]
- 20.Witthoff S, Muhlroth A, Marienhagen J, Bott M. 2013. C1 metabolism in Corynebacterium glutamicum: an endogenous pathway for oxidation of methanol to carbon dioxide. Appl Environ Microbiol 79:6974–6983. doi: 10.1128/AEM.02705-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SW, Hwang EH, Park H, Kim JA, Heo J, Lee KH, Song T, Kim E, Ro YT, Kim SW, Kim YM. 2003. Growth of mycobacteria on carbon monoxide and methanol. J Bacteriol 185:142–147. doi: 10.1128/JB.185.1.142-147.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park H, Lee H, Ro YT, Kim YM. 2010. Identification and functional characterization of a gene for the methanol: N,N′-dimethyl-4-nitrosoaniline oxidoreductase from Mycobacterium sp. strain JC1 (DSM 3803). Microbiology 156:463–471. doi: 10.1099/mic.0.034124-0. [DOI] [PubMed] [Google Scholar]
- 23.Seo JG, Park SW, Park H, Kim SY, Ro YT, Kim E, Cho JW, Kim YM. 2007. Cloning, characterization and expression of a gene encoding dihydroxyacetone synthase in Mycobacterium sp. strain JC1 DSM. 3803. Microbiology 153:4174–4182. doi: 10.1099/mic.0.2007/011965-0. [DOI] [PubMed] [Google Scholar]
- 24.Park SW, Hwang EH, Jang HS, Lee JH, Kang BS, Oh JI, Kim YM. 2009. Presence of duplicate genes encoding a phylogenetically new subgroup of form I ribulose 1,5-bisphosphate carboxylase/oxygenase in Mycobacterium sp. strain JC1 DSM. 3803. Res Microbiol 160:159–165. doi: 10.1016/j.resmic.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Park DO, Park SW, Hwang EH, Oh JI, Kim YM. 2009. Expression and regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase genes in Mycobacterium sp. strain JC1 DSM. 3803. J Microbiol 47:297–307. doi: 10.1007/s12275-008-0210-3. [DOI] [PubMed] [Google Scholar]
- 26.Song Z, Orita I, Yin F, Yurimoto H, Kato N, Sakai Y, Izui K, Li K, Chen L. 2010. Overexpression of an HPS/PHI fusion enzyme from Mycobacterium gastri in chloroplasts of geranium enhances its ability to assimilate and phytoremediate formaldehyde. Biotechnol Lett 32:1541–1548. doi: 10.1007/s10529-010-0324-7. [DOI] [PubMed] [Google Scholar]
- 27.Brown-Elliott BA, Wallace RJ Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev 15:716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smeulders MJ, Keer J, Speight RA, Williams HD. 1999. Adaptation of Mycobacterium smegmatis to stationary phase. J Bacteriol 181:270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krog A, Heggeset TM, Muller JE, Kupper CE, Schneider O, Vorholt JA, Ellingsen TE, Brautaset T. 2013. Methylotrophic Bacillus methanolicus encodes two chromosomal and one plasmid born NAD+ dependent methanol dehydrogenase paralogs with different catalytic and biochemical properties. PLoS One 8:e59188. doi: 10.1371/journal.pone.0059188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogt RN, Steenkamp DJ, Zheng R, Blanchard JS. 2003. The metabolism of nitrosothiols in the mycobacteria: identification and characterization of S-nitrosomycothiol reductase. Biochem J 374:657–666. doi: 10.1042/bj20030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beste DJ, Bonde B, Hawkins N, Ward JL, Beale MH, Noack S, Noh K, Kruger NJ, Ratcliffe RG, McFadden J. 2011. 13C metabolic flux analysis identifies an unusual route for pyruvate dissimilation in mycobacteria which requires isocitrate lyase and carbon dioxide fixation. PLoS Pathog 7:e1002091. doi: 10.1371/journal.ppat.1002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schocke L, Weimer PJ. 1997. Purification and characterization of phosphoenolpyruvate carboxykinase from the anaerobic ruminal bacterium Ruminococcus flavefaciens. Arch Microbiol 167:289–294. doi: 10.1007/s002030050446. [DOI] [PubMed] [Google Scholar]
- 33.Zamboni N, Maaheimo H, Szyperski T, Hohmann HP, Sauer U. 2004. The phosphoenolpyruvate carboxykinase also catalyzes C3 carboxylation at the interface of glycolysis and the TCA cycle of Bacillus subtilis. Metab Eng 6:277–284. doi: 10.1016/j.ymben.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Mukhopadhyay B, Concar EM, Wolfe RS. 2001. A GTP-dependent vertebrate-type phosphoenolpyruvate carboxykinase from Mycobacterium smegmatis. J Biol Chem 276:16137–16145. doi: 10.1074/jbc.M008960200. [DOI] [PubMed] [Google Scholar]
- 35.Chistoserdova L, Kalyuzhnaya MG, Lidstrom ME. 2009. The expanding world of methylotrophic metabolism. Annu Rev Microbiol 63:477–499. doi: 10.1146/annurev.micro.091208.073600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrader J, Schilling M, Holtmann D, Sell D, Filho MV, Marx A, Vorholt JA. 2009. Methanol-based industrial biotechnology: current status and future perspectives of methylotrophic bacteria. Trends Biotechnol 27:107–115. doi: 10.1016/j.tibtech.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Iguchi H, Yurimoto H, Sakai Y. 2015. Interactions of methylotrophs with plants and other heterotrophic bacteria. Microorganisms 3:137–151. doi: 10.3390/microorganisms3020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wendel SO, Perera AS, Pfromm PH, Czermak P, Bossmann SH. 2013. Adaptation of Mycobacterium smegmatis to an industrial scale medium and isolation of the mycobacterial porinMspA. Open Microbiol J 7:92–98. doi: 10.2174/1874285801307010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ofer N, Wishkautzan M, Meijler M, Wang Y, Speer A, Niederweis M, Gur E. 2012. Ectoine biosynthesis in Mycobacterium smegmatis. Appl Environ Microbiol 78:7483–7486. doi: 10.1128/AEM.01318-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teng S, Beard K, Pourahmad J, Moridani M, Easson E, Poon R, O'Brien PJ. 2001. The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes. Chem Biol Interact 130-132:285–296. [DOI] [PubMed] [Google Scholar]
- 41.Chen NH, Djoko KY, Veyrier FJ, McEwan AG. 2016. Formaldehyde stress responses in bacterial pathogens. Front Microbiol 7:257. doi: 10.3389/fmicb.2016.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh MI, Jain V. 2013. Tagging the expressed protein with 6 histidines: rapid cloning of an amplicon with three options. PLoS One 8:e63922. doi: 10.1371/journal.pone.0063922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF, Snapper SB, Barletta RG, Jacobs WR Jr, Bloom BR. 1991. New use of BCG for recombinant vaccines. Nature 351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 44.Pohane AA, Joshi H, Jain V. 2014. Molecular dissection of phage endolysin: an interdomain interaction confers host specificity in lysin A of Mycobacterium phage D29. J Biol Chem 289:12085–12095. doi: 10.1074/jbc.M113.529594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Ophem PW, Van Beeumen J, Duine JA. 1993. Nicotinoprotein [NAD(P)-containing] alcohol/aldehyde oxidoreductases. Purification and characterization of a novel type from Amycolatopsis methanolica. Eur J Biochem 212:819–826. [DOI] [PubMed] [Google Scholar]
- 46.van Kessel JC, Hatfull GF. 2007. Recombineering in Mycobacterium tuberculosis. Nat Methods 4:147–152. doi: 10.1038/nmeth996. [DOI] [PubMed] [Google Scholar]
- 47.Mao XJ, Yan MY, Zhu H, Guo XP, Sun YC. 2016. Efficient and simple generation of multiple unmarked gene deletions in Mycobacterium smegmatis. Sci Rep 6:22922. doi: 10.1038/srep22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.