The core origins of archaea are comprised of a repeat region and an adjacent gene for an origin recognition complex (ORC) protein, which is homologous to eukaryotic ORC proteins. Haloferax volcanii is exceptional because it contains six replication origins on three chromosomes and an additional 10 orc genes that are not adjacent to an origin. This unique ORC protein repertoire was used to unravel the importance of core origin orc genes and of origin-remote orc genes. Remarkably, all ORC proteins influenced the copy number of at least one chromosome. Some of them influenced those of all three chromosomes, showing that cross-regulation in trans exists in Hfx. volcanii. Furthermore, the evolution of the archaeal ORC protein family was analyzed.
KEYWORDS: Archaea, Haloferax volcanii, ORC proteins, deletion mutants, essentiality, origin recognition complex, phylogenetic analysis, polyploidy, replication origin
ABSTRACT
Replication initiation in archaea involves a protein named ORC, Cdc6, or ORC1/Cdc6, which is homologous to the eukaryotic origin recognition complex (ORC) proteins and to the eukaryotic Cdc6. Archaeal replication origins are comprised of origin repeat regions and adjacent orc genes. Some archaea contain a single replication origin and a single orc gene, while others have more than one of each. Haloferax volcanii is exceptional because it contains, in total, six replication origins on three chromosomes and 16 orc genes. Phylogenetic trees were constructed that showed that orc gene duplications occurred at very different times in evolution. To unravel the influence of the ORC proteins on chromosome copy number and cellular fitness, it was attempted to generate deletion mutants of all 16 genes. A total of 12 single-gene deletion mutants could be generated, and only three orc gene turned out to be essential. For one gene, the deletion analysis failed. Growth analyses revealed that no deletion mutant had a growth defect, but some had a slight growth advantage compared to the wild type. Quantification of the chromosome copy numbers in the deletion mutants showed that all 12 ORC proteins influenced the copy numbers of one, two, or all three chromosomes. The lack of an ORC led to an increase or decrease of chromosome copy number. Therefore, chromosome copy numbers in Hfx. volcanii are regulated by an intricate network of ORC proteins. This is in contrast to other archaea, in which ORC proteins typically bind specifically to the adjacent origin.
IMPORTANCE The core origins of archaea are comprised of a repeat region and an adjacent gene for an origin recognition complex (ORC) protein, which is homologous to eukaryotic ORC proteins. Haloferax volcanii is exceptional because it contains six replication origins on three chromosomes and an additional 10 orc genes that are not adjacent to an origin. This unique ORC protein repertoire was used to unravel the importance of core origin orc genes and of origin-remote orc genes. Remarkably, all ORC proteins influenced the copy number of at least one chromosome. Some of them influenced those of all three chromosomes, showing that cross-regulation in trans exists in Hfx. volcanii. Furthermore, the evolution of the archaeal ORC protein family was analyzed.
INTRODUCTION
The archaeal replication machinery is homologous to that of its eukaryotic counterpart, not to the bacterial replication machinery. However, while eukaryotes contain more replication proteins and/or more paralogs of replication proteins, archaea contain a simpler version of the replication machinery (1). In eukaryotes, replication is initiated by the binding of a heteromeric origin recognition complex (ORC) to replication origins. The ORC is comprised of six subunits, ORC1 to ORC6 (2). With the help of the proteins Cdc6 and Cdt1, it recruits the minichromosome maintenance protein (MCM) complex to the origin, which is the replicative helicase. The MCM complex is also a heteromeric complex comprised of six subunits, MCM2 to MCM7 (2). The eukaryotic ORC has regulatory functions beyond replication initiation, and its malfunction has been connected to the development of various human diseases (3). Very surprisingly, in contrast to the high importance of the ORC, it has recently been found that two of the human orc genes are not essential (4).
Archaea contain a replication initiation protein that is homologous to the ORC proteins and has its highest similarity to ORC1. It is also homologous to Cdc6, and therefore, it is often termed “ORC1/Cdc6.” However, also the names ORC1 and Cdc6 are commonly used. For the sake of simplicity, we will call the archaeal initiation protein ORC in this paper. It was previously shown that in Sulfolobus, the ORC protein fulfills both functions of the eukaryotic ORC and the Cdc6 protein, i.e., binding to the origin and recruitment of the replicative helicase, MCM (5).
Eukaryotes typically contain thousands of replication origins. In stark contrast, bacteria contain only one origin of replication and one replication terminus at opposite sites of a circular chromosome. Some archaea also contain one origin of replication (6). However, it has been shown that various species of archaea contain more than one replication origin, and thus multiorigin replication seems to have evolved in archaea. A recent review gives an excellent overview of the diversity of replication in Archaea (7). The initial observation was that two species of Sulfolobus contain three replication origins (8), but subsequently, 2 to 4 replication origins on the main chromosome were also found in Pyrobaculum calidifontis (9) and in several species of haloarchaea (10, 35). Archaeal replication origins are comprised of (i) a noncoding region containing AT-rich DNA unwinding elements (DUEs), which are important for DNA melting, and origin recognition boxes (ORBs), and (ii) an adjacent orc gene. For Haloarcula hispanica, it has been shown that the origins are specifically dependent on the respective colocalized orc gene (11). Also, for Sulfolobus, it has been shown that each of the three replication origins is addressed by a specialized replication initiation protein, two by an ORC paralog and one by a Cdt1 homolog (12). Typically, the number of replication origins and ORC proteins in archaea is identical, e.g., Pyrococcus has a single replication origin and a single orc gene (13). However, the situation is very different in haloarchaea, which typically contain more orc genes than replication origins. For example, Haloferax volcanii contains six replication origins, three on the major chromosome and one each on three minor chromosomes (14). In the commonly used laboratory strain Hfx. volcanii H26, one of the three minor chromosomes is integrated into the major chromosome, and H26 thus contains three replicons, one with four replication origins and two with one replication origin each (14). In contrast, the Hfx. volcanii genome contains 16 orc genes, and thus the majority of 10 orc genes are not located adjacent to origin repeats (www.halolex.mpg.de) (15). Another feature of Hfx. volcanii is that it is polyploid and contains about 20 copies of the major chromosome (16). The chromosome copy number can vary widely and can range from two to more than 40 (17). The copy number regulation is related to the number of replication initiation events per cell cycle. During steady state, each origin must fire exactly once per cell cycle, irrespective of the chromosome copy number. However, more than one replication event per cell cycle is needed to increase the copy number, and less than one initiation event per cell cycle results in a decrease of copy number. Therefore, several or all of the ORC proteins might be involved in chromosome copy number regulation. To test this hypothesis, all 16 orc genes were chosen to construct single-gene deletion mutants. The effects of the nonessential orc gene deletions on the copy numbers of all three chromosomes and on the fitness of the cells were quantified. Furthermore, phylogenetic trees were constructed to analyze the evolution of the ORC protein family.
RESULTS AND DISCUSSION
Evolution of the Hfx. volcanii ORC proteins.
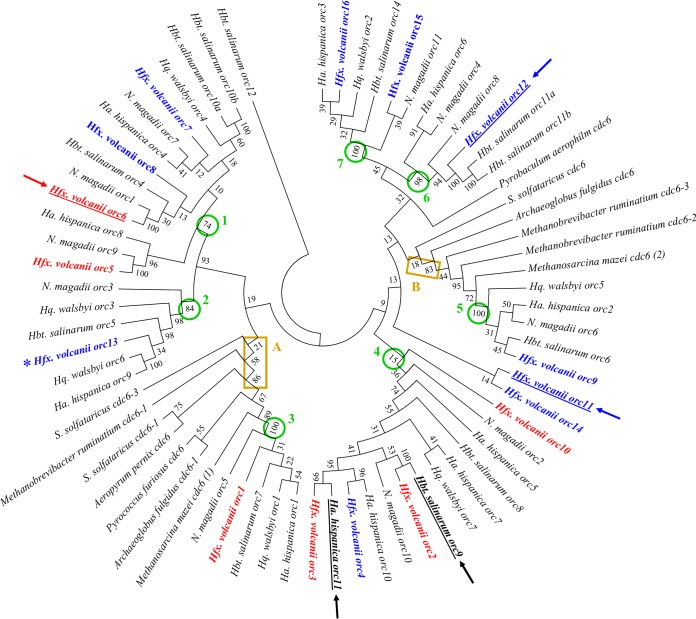
To unravel the evolution of the ORC protein family, the ORC proteins of five haloarchaea (representing five different genera), of two methanogenic archaea, and of five additional euryarchaeota and crenarchaeota were chosen, and phylogenetic trees were generated. Figure 1 shows the consensus tree obtained after 1,000 bootstrap replications using the neighbor-joining approach. A very similar tree was obtained after 1,000 bootstrap replications using the maximal parsimony algorithm (data not shown). The tree revealed that the last common ancestor of Hfx. volcanii, Halobacterium salinarum, Haloarcula hispanica, Haloquadratum walsbyi, and Natrialba magadii already contained seven ORC proteins. The seven nodes are indicated in Fig. 1. In six cases, the monophyletic groups defined by the nodes contain ORC paralogs of all five species; only group six contains paralogs of only four species, and thus it seems that this orc gene has been secondarily lost in Haloquadratum walsbyi. The tree shows that the multiplication and diversification of the orc genes is not a recent event in the genus Haloferax but started early in the evolution of the Halobacteriaceae. Node 3 contains the highly conserved ORC1 protein, which is orthologous to ORC proteins from methanogenic archaea and other euryarchaeota and crenarchaeota (node A in Fig. 1). Thus, the class of node A ORC proteins seems to be the most ancient, because it is widely distributed in many groups of archaea. There is a second family of ORC paralogs that contains proteins from other euryarchaeota, indicating that the presence of two ORC proteins predates the evolution of the Halobacteriaceae (node B in Fig. 1).
FIG 1.
Phylogenetic tree of the ORC protein family. A consensus tree that was generated by neighbor joining after 1,000 bootstrap replications is shown. The bootstrap values (percent) are indicated at the respective nodes. The 12 ORC proteins of this study are colored as follows: the six proteins encoded adjacent to origin repeats are shown in red, and the 12 proteins encoded elsewhere are shown in blue. The essential ORC proteins of Hfx. volcanii, Hbt. salinarum, and Ha. hispanica are underlined and marked with an arrow. The seven nodes that define ORC paralogs that were already present in the common ancestor of Hfx. volcanii, Hbt. salinarum, Har. hispanica, and N. magadii are marked by a circle and are numbered. The nodes that define two ORC paralogs that predated the evolution of the Halobacteriaceae are boxed. The orc gene that could not be analyzed is marked with an asterisk.
The evolution of the seven ORC subfamilies was very diverse; the node 3 and 5 subfamilies contain only a single sequence of the five haloarchaeal species, and thus no further gene duplication occurred. In stark contrast, the node 4 subfamily contains four sequences each of Hfx. volcanii and Har. hispanica, two each of Hbt. salinarum and of N. magadii, and only one of Hqu. walsbyi. The node 7 subfamily contains two sequences of Hfx. volcanii and one sequence each of the four other species and thus gives evidence for a gene duplication event that happened late and only in the evolution of Hfx. volcanii. Taken together, the evolution of the ORC protein family is very complex, and the 16 orc genes of Hfx. volcanii evolved through gene duplications that happened at very different times in its evolution.
Analysis of essentiality of all 16 orc genes.
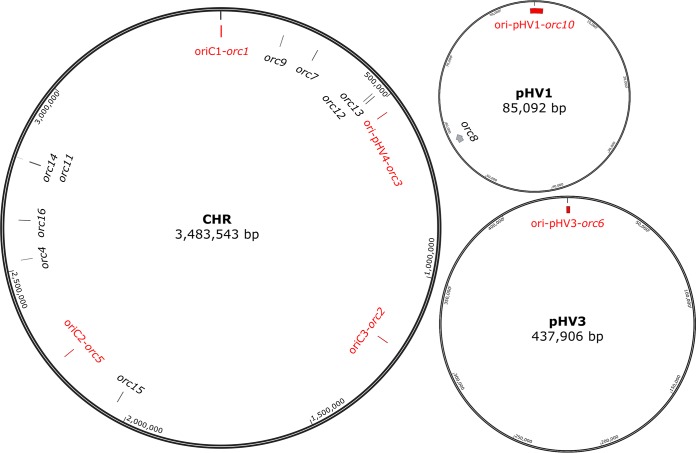
The localization of the 16 orc genes on the three chromosomes is schematically shown in Fig. 2; the origin-adjacent orc genes are shown in red. The minor chromosome pHV3 is essential because it encodes, e.g., the enzymes for nitrate and dimethyl sulfoxide (DMSO) respiration, the degradation pathways for xylose and arabinose, many ABC transporters, translation initiation factor aIF1, and transfer-messenger RNA (tmRNA). It is not clear whether the minor chromosome pHV1 is essential. It contains only 89 protein-coding genes, many of which encode conserved hypothetical proteins. However, it also contains an ABC transporter operon, which might be essential under specific conditions.
FIG 2.
Schematic overview of the three replicons and the localizations of the 16 orc genes of Hfx. volcanii. Names and sizes of the replicons are included; the circles are not drawn to scale.
In other haloarchaea, only a subset of orc genes was found to be essential, e.g., only two of 10 orc genes of Hbt. salinarum are essential (18). Therefore, it was decided to analyze the essentiality of all 16 orc genes of Hfx. volcanii. It was attempted to generate in-frame deletion mutants. This approach involves deletion of a major part of the open reading frame but leaves the very 5′-most and the very 3′-most codons intact. It thereby guarantees that the expression of adjacent genes is not influenced. In two cases, the distance to the downstream gene of the same orientation was only 54 nucleotides (nt) (orc16) and 60 nt (orc9). On the one hand, these distances could include terminators and promoters in the intergenic regions; however, on the other hand, the possibility could not be excluded that these orc genes were cotranscribed into bicistronic transcripts. In three cases, the downstream gene had the opposite orientation, and the distances were so small that overlap of the 3′ untranslated regions (UTRs) were suspected, i.e., 47 nt for orc1, 18 nt for orc12, and −8 nt (= overlap of ORFs) for orc14. The exact regions that were chosen for the deletions are summarized in Table 1. The deletion analysis was performed using the so-called pop-in-pop-out method that is well established for Hfx. volcanii (19–21). In 12 of the 16 cases, the deletion mutant could be generated successfully. The 12 deletion mutants were analyzed by PCR and by Southern blotting analyses and were shown to be homozygous deletion strains (data not shown). The oligonucleotides used for probe generation, and the sizes of the hybridizing restriction fragments are included in Table S2 in the supplemental material.
TABLE 1.
Summary of orc genes in Hfx. volcanii
| Replicon | Gene no. | Gene name | Genomic region/strand deleted regione | Gene length (bp) | Avg coverage (×)f |
|---|---|---|---|---|---|
| Chr | HVO_0001d (ori1)a | orc1d | 258–1952/F | 1,695 | ND |
| 282–1925 | |||||
| Chr | HVO_0194a | orc9 | 174892–176016/F | 1,125 | 1,116 |
| 174909–176000 | |||||
| Chr | HVO_0634d (ori3)a | orc2d | 569203–570429/R | 1,227 | 252 |
| 569215–570384 | |||||
| Chr | HVO_1537a | orc15 | 1403626–1404828/R | 1,203 | 40 |
| 1403674–1404810 | |||||
| Chr | HVO_1725d (ori2)a | orc5d | 1594707–1595912/F | 1206 | 50 |
| 1594719–1595900 | |||||
| Chr | HVO_2042a | orc4 | 1888354–1889583/F | 1,230 | 102 |
| 1888369–1889568 | |||||
| Chr | HVO_2133a | orc16 | 1998477–1999643/R | 1,167 | 10 |
| 1998492–1999634 | |||||
| Chr | HVO_2292a | orc14 | 2160851–2161873/R | 1,023 | ND |
| 2160870–2161857 | |||||
| Chr | HVO_2293b | orc11 | 2162076–2162873/F | 798 | ND |
| pHV4 | HVO_A0001d (ori-pHV4)a | orc3d | 152–1402/F | 1,251 | 62 |
| 167–1387 | |||||
| pHV4 | HVO_A0064c | orc13 | 55398–56897/R | 1,500 | 55 |
| pHV4 | HVO_A0072b | orc12 | 65261–66289/R | 1,029 | ND |
| pHV4 | HVO_A0257a | orc7 | 257391–258668/R | 1,278 | 171 |
| 257415–258656 | |||||
| pHV3 | HVO_B0001d (ori-pHV3)b | orc6d | 201–1433/F | 1,233 | ND |
| 222–1409 | |||||
| pHV1 | HVO_C0001d (ori-pHV1)a | orc10d | 101–1327/F | 1,227 | ND |
| 143–1288 | |||||
| pHV1 | HVO_C0057a | orc8 | 56369–57616/F | 1,248 | 618 |
| 56375–57604 |
Deleted.
Essential.
Pop-in not possible; genome rearrangement occurred.
Part of origin of replication.
F, forward; R, reverse.
ND, nondetectable.
The only genes that could not be deleted were orc11 (HVO_2293), orc12 (HOV_A0072), and orc6 (HVO_B0001). In these three cases, the respective pop-in clones could readily be generated, but after the pop-out selection, none of the more than 100 tested clones turned out to be a deletion mutant, but all had retained the wild-type copy of the gene. Therefore, we concluded that orc11, orc12, and orc6 are essential. The phylogenetic analyses did not give any indication why the three genes should be essential. None of the three genes is closely related to the essential genes of Har. hispanica and Hbt. salinarum. They are also not closely related to one another but are found at different positions in the tree. In addition, orc11 and orc6 are in the same clade as nonessential orc genes, i.e., orc14 for orc11 and orc7 and orc8 for orc6 (compare Fig. 1). Remarkably, orc6 is the sole orc gene of the minor chromosome pHV3, while minor chromosome pHV1 encodes two orc genes, and the major chromosome (with the integrated pHV4) contains 13 orc genes. Thus, in the future, it could be interesting to analyze whether the integration of further orc genes on pHV3 would render orc6 nonessential.
Taken together, five of the six origin-adjacent orc genes were not essential. Therefore, a location adjacent to ORBs or a location far from any origin does not tell anything about the importance of an orc gene in Hfx. volcanii. The essentiality of one of the 16 orc genes, HVO_A0064, could not be analyzed with the same approach as for the other 15 orc genes because attempts to generate the pop-in variant failed. Four independent colonies were analyzed by Southern blotting (for oligonucleotides for probe generation, see Table S2 in the supplemental material), and in all cases, neither the wild-type version nor the deletion version of the gene could be detected, indicating that a genomic rearrangement had occurred. In addition, PCR analyses were performed and did not lead to the amplification of the gene, also suggesting that HVO_A0064 was deleted, together with other genes. The gene is flanked by two nearby transposase genes (HVO_A0062 and HVO_A0066); therefore, it seems possible that integration of the deletion plasmid triggered recombination between these genes, leading to the deletion of the intervening genes, including HVO_A0064. Even if a clear-cut analysis via the pop-in-pop-out method was not possible, the viability of the mutant with the genome rearrangement indicated that also HVO_A0064 is not essential.
Table 1 summarizes all orc genes of Hfx. volcanii; the 12 genes that could be deleted, the three essential orc genes, and the gene that could not be characterized are indicated with footnotes. Table 1 also includes the transcript levels deduced from a recent differential transcriptome sequencing (dRNA-Seq) study (22) in which the transcriptomes of exponentially growing cultures under optimal conditions were analyzed. The transcript levels are given as coverage averages because three biological replicates were analyzed, and thus the raw read numbers had to be normalized prior to the calculation of averages. The transcript levels varied widely, from nondetectable (ND) to more than 1,000. In seven cases, the coverage averages were between 40 and 250. For comparison, the coverage averages of selected transcripts are given, as follows: the transcripts for two enzymes of central metabolism, citrate synthase and isocitrate dehydrogenase, had coverage averages of 202 and 262, and the coverage averages of transcripts for the MCM helicase and DNA topoisomerase I were 126 and 167. The inability to detect the transcripts of six orc genes might be due to their very low levels. Alternatively, the primary transcripts might be processed, and processed transcripts are not detectable by dRNA-Seq.
Phenotypic analysis of 12 orc deletion mutants.
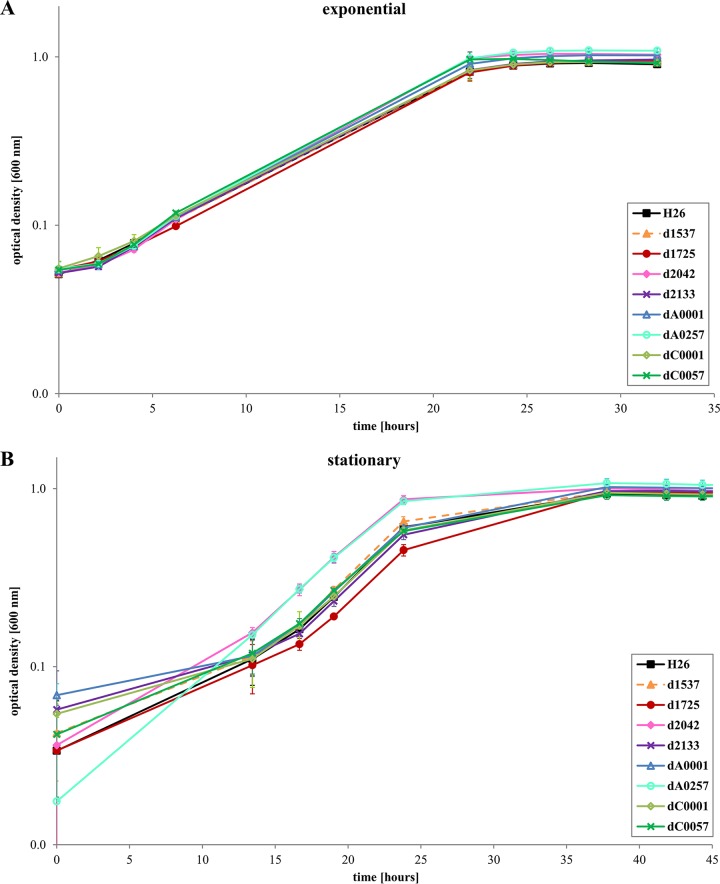
The colony morphologies of the 12 deletion strains and the wild type were compared, and no difference could be detected. The same is true for microscopic comparison of the cell morphologies. To unravel whether the absence of a single ORC protein has an influence on the fitness of Hfx. volcanii cells, growth experiments were performed. The first approach was to inoculate cultures with exponentially growing precultures and grow them in complex medium under optimal conditions. The results are shown in Fig. 3A (eight deletion mutants) and in Fig. S1A in the supplemental material (four deletion mutants). All 12 deletion mutants grew indistinguishably from the wild type.
FIG 3.
Growth of the wild type and eight orc deletion mutants in complex medium. (A) The cultures were inoculated with cells of the mid-exponential growth phase. (B) The cultures were inoculated with stationary-phase cells. Average results from three biological replicates are shown with standard deviations.
Next, cultures were inoculated with 11-day-old stationary-phase precultures. Figure 3B and S1B show that five of the deletion strains had a slight growth advantage compared to the wild type. The growth rates were very similar, so that the main difference was a shorter lag phase of the mutants. One deletion mutant (HVO_1725) had a very slight growth defect, but the difference was very small. The growth yields of all 12 deletion mutants were identical to that of the wild type.
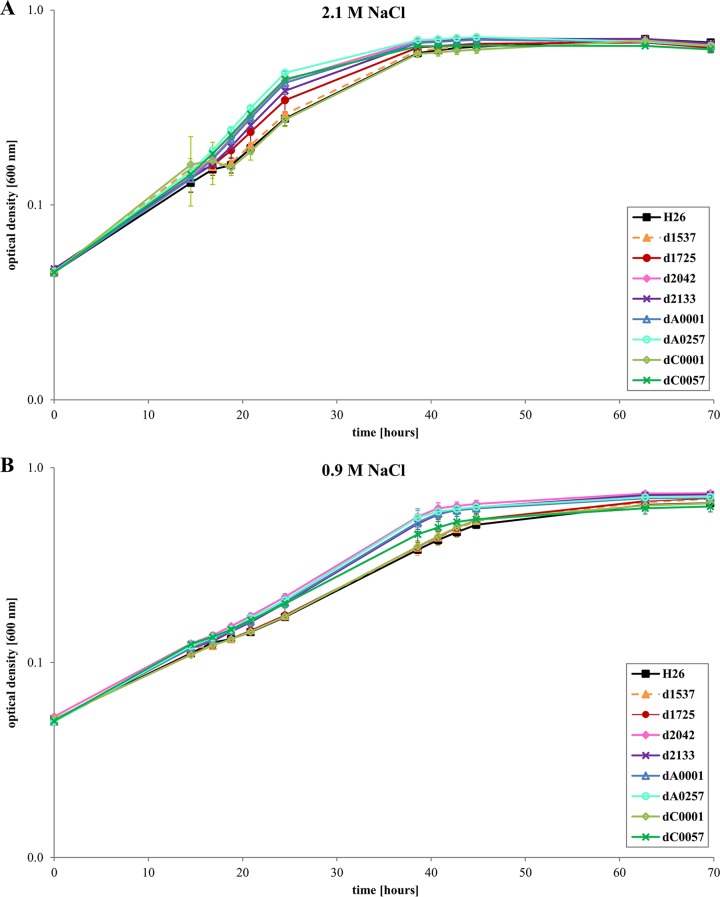
Next, the deletion mutants and the wild type were grown in synthetic medium with glucose as sole carbon and energy source. Figures 4A and S2A show that under this growth condition as well none of the 12 deletion mutants had a growth defect. In contrast, eight deletion mutants had a slight growth advantage. The wild-type and the deletion strains were also grown in glucose synthetic medium with a salt concentration of 0.9 M (Fig. 4B and S2B), which is close to the minimal salt concentration of 0.7 M that Hfx. volcanii can survive (23). Again, none of the deletion strains had a growth defect, and four strains grew somewhat faster than the wild type.
FIG 4.
Growth of the wild-type and eight orc deletion mutants in synthetic medium with glucose as the sole carbon and energy source. (A) The medium had the optimal NaCl concentration of 2.1 M. (B) The medium had an NaCl concentration of 0.9 M, close to the lowest limit for Hfx. volcanii. Average results from three biological replicates are shown with standard deviations.
In summary, the absence of none of the 12 nonessential ORC proteins resulted in a growth defect; in contrast, several deletion strains exhibited a moderate growth advantage. We have shown before that single gene deletion mutants of Hfx. volcanii can grow better than the wild type. Seven of 27 deletion mutants of regulatory small RNA (sRNA) genes had a growth advantage compared to the wild type under at least one of 10 tested conditions (21). Gain-of-function phenotypes have also been reported for deletion mutants of eukaryotic microRNA (miRNA) genes (24, 25). This indicates that evolution does not select for cells that can grow fastest under one specific (laboratory) condition but for cells that have the most flexible regulatory network, which is able to deal with the manifold and dynamic conditions that a cell can experience in nature.
Quantification of the copy numbers of three chromosomes in the wild-type and in 12 orc deletion mutants.
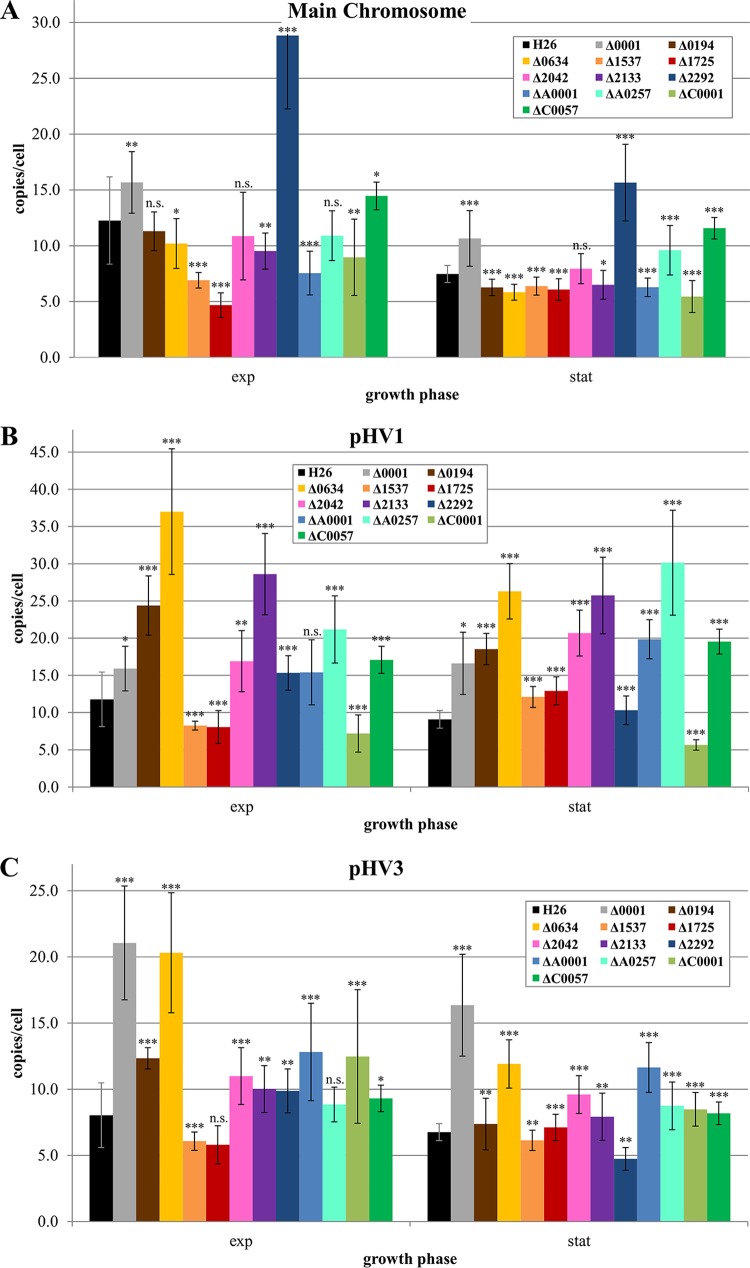
To unravel whether the 12 nonessential ORC proteins might be involved in replicon copy number regulation, the copy numbers of the major chromosome (including the integrated pHV4) and the two minor chromosomes pHV1 and pHV3 were determined in exponentially growing cells as well as in stationary-phase cells of the wild type and the 12 deletion mutants. Figure 5 shows that the absence of each of the 12 ORC proteins has an influence on the copy number of at least one of the three replicons. However, the situation is rather complex. Very unexpectedly, the absence of HVO_0001 led to an increase in the copy number for all three chromosomes under exponential as well as under stationary-phase conditions. The influence of ORC1 on the copy number of the major chromosome and pHV1 is considerable, but the influence on the minor chromosome pHV3 is very high. The absence of HVO_2292 (ORC14) also led to an increase in the copy number for all three chromosomes; however, in this case, the influence on the major chromosome is very large, in contrast to the rather small influence on the other two chromosomes.
FIG 5.
Quantification of the copy numbers of three chromosomes in the wild type and 12 orc deletion mutants. The copy numbers were quantified in cells of the early exponential phase (about 1 × 108 to 3 × 108 cells/ml) and in stationary-phase cells using real-time PCR. The results are based on three biological replicates and 24 technical replicates. Significances of differences in copy number were analyzed with Student's t test. *, P value < 5%, **; P value < 1%; ***, P value < 0.1%; n.s., not significant.
Two other ORC proteins that have an influence on the copy number of all three chromosomes are HVO_1537 and HVO_1725 (ORC15 and ORC5). However, the direction of influence is opposite to that in the above-mentioned cases, i.e., their absence resulted in a decrease in copy number. The effect is considerably greater on the major chromosome than on pHV1 and pHV3, and it is restricted to the exponential growth phase. Another regulatory pattern was observed in the HVO_C0001 deletion mutant. The copy numbers of the major chromosome and pHV1 were decreased in the mutant, whereas, in contrast, the copy number of pHV3 was increased. HVO_A0257 is an example of an ORC protein that influences only two of the three replicons. Its absence led to a decrease in the copy number of the major chromosome and an increase in the copy number of pHV and did not influence the copy number of pHV3. The absence of HVO_0634 led to an increase in the copy numbers of pHV1 and pHV3 in exponential as well as stationary phases, while the copy number of the major chromosome remained unchanged. Taken together, these results clearly show that a specialization of replication initiation proteins for one specific adjacent replication origin, which has been found to exist for Sulfolobus spp. and Har. hispanica (8, 11), does not exist in Hfx. volcanii. Deletion of all 12 nonessential orc genes led to a copy number change of at least two chromosomes in at least one of the two growth phases. In addition, it can be assumed that the three essential ORC proteins also have an influence on the copy number of at least one replicon. Therefore, the copy numbers of the three chromosomes seem to be regulated by a network of many ORC proteins, which can have positive or negative regulatory effects on one or more of the chromosomes. In most cases, the effects were larger in exponential phase than in stationary phase. It has been shown that environmental conditions highly influence the copy number, e.g., the copy number of the major chromosome can be as low as two during phosphate starvation and higher than 40 under phosphate surplus. It is tempting to speculate that individual ORC proteins are specialized in regulating replicon copy numbers in response to specific environmental changes. Further experiments are needed to substantiate this speculation.
Notably, deletion of HVO_0001 revealed that deletion of an orc gene can result in a different phenotype than deletion of the whole replication origin, i.e., the orc gene and the adjacent repeat region. While deletion of HVO_0001 led to an increase in copy number of the major chromosome (Fig. 5), deletion of the whole oriC1 region led to a decrease in copy number (26). The fact that the oriC1 repeat region without the adjacent orc gene has an influence on the chromosome copy number is an additional indication for crosstalk between various ORC proteins and origins. It will be interesting to analyze how many and which ORC proteins can bind to the ORBs of a specific origin.
Conclusions.
The evolution of the ORC protein family is very complex, and duplications happened repeatedly at very different points in time. The attempt to delete the 16 orc genes of Hfx. volcanii revealed that only three of them are essential, while 12 single gene deletion mutants could be generated. None of the 12 deletion mutants had a growth defect, while several deletion mutants grew slightly better than the wild type. The absence of each of the 12 nonessential ORC proteins in the single-gene deletion mutants influenced the copy number of at least one of the three chromosomes. Many ORC proteins influenced the levels of two or all three chromosomes, revealing that the copy number is determined by an intricate regulatory network. Notably, the specialized pairs of autonomous origin repeats with an adjacent orc gene, which have been found in other archaeal species, do not exist in Hfx. volcanii.
MATERIALS AND METHODS
Construction of phylogenetic trees.
Phylogenetic trees were generated using the program MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 (27). Multiple sequence alignments were generated using ClustalW (www.ebi.ac.uk). Low-complexity regions lacking phylogenetic information were removed. Trees were generated using the maximum parsimony and neighbor-joining methods. Bootstrap analyses were performed with 1,000 replications.
Haloarchaeal and bacterial strains.
Haloferax volcanii H26 was obtained from Thorsten Allers (University of Nottingham, UK) and used as wild-type and parent strain for deletion mutants in this study. H26 is a pyrE deletion mutant and is thus auxotrophic for uracil, which can be used as a selection marker. The single-gene deletion strains of 12 orc genes were generated in this study.
Escherichia coli XL1 blue MRF was obtained from Stratagene (Amsterdam, The Netherlands).
Media and culture conditions.
Hfx. volcanii was grown in complex medium (28) and synthetic medium (29) as described. All ingredients were obtained from VWR (Radnor, PA). The complex medium was supplemented with 8 μM FeSO4 (P015.1; Roth, Karlsruhe, Germany), 0.1% (vol/vol) SL-6 trace element solution (30) (all from Roth), 1 ml vitamin solution (B6891; Sigma-Aldrich, St. Louis, MO), and 50 μg/ml uracil (A0667; AppliChem, Darmstadt, Germany). The synthetic medium was supplemented with 10 mM NH4Cl as N source (21236.267; VWR), 1 mM K2HPO4 as P source (6878.2; Roth), 0.5% (wt/vol) glucose as C source (1.08342; Merck, Darmstadt, Germany). and 100 mM morpholinepropanesulfonic acid (MOPS; pH 7.2) (M3183; Sigma-Aldrich). Supplements of the complex medium were also added. For osmotic stress conditions, medium with only 0.9 M NaCl instead of 2.1 M NaCl was used. Cultures were grown in 100-ml Erlenmeyer flasks in a rotary shaker at 42°C and 250 rpm. Phenotypic analyses were performed using growth in microtiter plates (see below).
E. coli XL1 blue MRF was grown under standard conditions (31).
General molecular genetic techniques.
General molecular genetic techniques were performed as described by Green and Sambrook (31). Genomic DNA was isolated from Hfx. volcanii according to Rosenshine et al. (32). Plasmid DNA was isolated using GenElute plasmid midiprep kit (Sigma-Aldrich), and PCR fragments were either purified directly or from preparative agarose gels using a SeqLab PCR-Combi-kit (SeqLab, Göttingen, Germany). Restriction enzymes and T4 DNA ligase were obtained from Thermo Fisher Scientific (Waltham, MA), and, if not otherwise stated, the enzymes were used according to the instructions of the manufacturer. The Pfu and Taq polymerases were isolated in house, and oligonucleotides were synthesized by Sigma-Aldrich or biomers.net (Ulm, Germany). Hfx. volcanii was transformed as described by Cline et al. (28).
Construction of deletion mutants.
The deletion mutants were constructed by the so-called pop-in-pop-out method, as previously described (19, 20). The oligonucleotides used for amplification of the flanking sequences of the respective genes are summarized in Table S1 in the supplemental material. The flanking sequences included a few codons from the beginning and the end of the genes. In each case, the two PCR fragments contained an overlap and could be fused into one fragment by a third PCR. In this way, in-frame deletion variants of the respective orc genes were generated. The genomic localizations of the deleted regions are included in Table 1.
The suicide vector pMH101 was digested with EcoRV, and the fused PCR fragments were ligated into the vector using restriction selection cloning. In the cases of Δ2133 and ΔA0257, the suicide vectors were generated by Gibson assembly (33). The sequences of all plasmids were verified by sequencing (GATC, Constance, Germany). The genomic organizations of deletion mutants were verified by Southern blotting analyses as described below.
Southern blot analysis for verification of deletion mutants.
Southern blot hybridization was performed according to Lange et al. (34). The oligonucleotides, as well as the restriction enzymes used and the expected fragment sizes, are summarized in Table S2.
Phenotypic analysis of ORC deletion mutants.
Phenotypic characterization was generally performed as described by Jantzer et al. (23). Cultures in complex medium were either inoculated with exponentially growing precultures or with cultures that had been in stationary phase for 11 days. Cultures in synthetic medium were inoculated with exponentially growing precultures in the same medium. The cultures were harvested by centrifugation at 8,000 rpm for 5 min, the supernatant was discarded, and the pellet was washed with basal salt solution (medium without a C, N, or P source). After resuspension, the cells were used to inoculate new culture to an optical density at 600 nm (OD600) of 0.05. Only the inner 60 wells of microtiter plates were used for growth; the outmost wells were filled with 200 μl of 1 M NaCl as an evaporation barrier. Growth was monitored with a microtiter plate spectrophotometer. Three biological replicates were performed and average values and standard deviations were calculated.
Quantification chromosome copy numbers using quantitative real-time PCR (qPCR).
For quantification of chromosome copy numbers, a previously established real-time PCR technique was used (16, 17, 34). As a standard fragment for genome copy number determination, a 1-kbp fragment of leuB was amplified by PCR. For pHV1 and pHV3 copy number determination, respectively, a 1-kbp fragment of HVO_C0001 and a 1-kbp fragment of HVO_B0001 were amplified. In the case of ΔC0001, a 1-kbp standard fragment downstream of HVO_C0001 was generated and two different pairs of qPCR oligonucleotides were used to ensure reproducibility of the determined copy number. The standard fragments were purified from a preparative agarose gel as described above and precipitated with ethanol. They were dissolved in Tris-EDTA (TE) buffer, and the DNA concentration was determined by photometric measuring with a NanoDrop ND-1000 spectrophotometer. The concentration of the standards was adjusted to 100 to 200 ng/μl. All oligonucleotides used for standard fragment PCR and real-time PCR are described in Table S3 in the supplemental material.
For determination of chromosome copy numbers, 1 × 108 cells of early exponential (1 × 108 to 3 × 108 cells/ml) and early stationary-phase cultures (1 × 109 to 3 × 109 cells/ml) in complex medium were harvested by centrifugation at 13,000 rpm for 5 min. The supernatant was discarded and the pellet was resuspended in 100 μl of basal salt solution and lysed by addition of 900 μl of distilled water. An aliquot of 40 μl of cell extract was dialyzed for 3 h on membrane filters (Millipore, 13 mm diameter, VSWP01300; Merck) against distilled water. One qPCR mixture contained 0.8 μM of each oligonucleotide, 5.5 μl nuclease-free water, 12.5 μl Maxima SYBR green qPCR master mix (K0223; Thermo Fisher Scientific), and 5 μl of standard or cell extract, to a total volume of 25 μl. Serial dilutions (10−3 to 10−8) of the standard fragment in duplicate and four different dilutions of three biological replicates, also in duplicate, were used for qPCR, so that in total 24 technical replicates were used for copy number determination. A negative control with omission of template DNA was also performed. qPCR conditions were as follows: 10 min at 96°C, 50 cycles with 30 s at 96°C, 30 s at 64°C, and 30 s at 72°C, followed by 5 min at 72°C and a melt curve analysis from 62 to 96°C in 1°C steps.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (German Research Council) through grant SO 264/24.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00161-18.
REFERENCES
- 1.Robinson NP, Bell SD. 2005. Origins of DNA replication in the three domains of life. FEBS J 272:3757–3766. doi: 10.1111/j.1742-4658.2005.04768.x. [DOI] [PubMed] [Google Scholar]
- 2.Duncker BP, Chesnokov IN, McConkey BJ. 2009. The origin recognition complex protein family. Genome Biol 10:214. doi: 10.1186/gb-2009-10-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Z. 2013. The origin recognition complex in human diseases. Biosci Rep 33:e00044. doi: 10.1042/BSR20130036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibata E, Kiran M, Shibata Y, Singh S, Kiran S, Dutta A. 2016. Two subunits of human ORC are dispensable for DNA replication and proliferation. Elife 5:e19084. doi: 10.7554/eLife.19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samson RY, Abeyrathne PD, Bell SD. 2016. Mechanism of archaeal MCM helicase recruitment to DNA replication origins. Mol Cell 61:287–296. doi: 10.1016/j.molcel.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samson RY, Bell SD. 2015. Archaeal chromosome biology. J Mol Microbiol Biotechnol: 24:420–427. doi: 10.1159/000368854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ausiannikava D, Allers T. 2017. Diversity of DNA replication in the Archaea. Genes 8:56. doi: 10.3390/genes8020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundgren M, Andersson A, Chen L, Nilsson Bernander P R. 2004. Three replication origins in Sulfolobus species: synchronous initiation of chromosome replication and asynchronous termination. Proc Natl Acad Sci U S A 101:7046–7051. doi: 10.1073/pnas.0400656101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelve EA, Lindås AC, Knöppel A, Mira A, Bernander R. 2012. Four chromosome replication origins in the archaeon Pyrobaculum calidifontis. Mol Microbiol 85:986–995. doi: 10.1111/j.1365-2958.2012.08155.x. [DOI] [PubMed] [Google Scholar]
- 10.Norais C, Hawkins M, Hartman AL, Eisen JA, Myllykallio H, Allers T. 2007. Genetic and physical mapping of DNA replication origins in Haloferax volcanii. PLoS Genet 3:e77. doi: 10.1371/journal.pgen.0030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, Liu J, Yang H, Liu H, Xiang H. 2014. Multiple replication origins with diverse control mechanisms in Haloarcula hispanica. Nucleic Acids Res 42:2282–2294. doi: 10.1093/nar/gkt1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samson RY, Xu Y, Gadelha C, Stone TA, Faqiri JN, Li D, Qin N, Pu F, Liang YX, She Q, Bell SD. 2013. Specificity and function of archaeal DNA replication initiator proteins. Cell Rep 3:485–496. doi: 10.1016/j.celrep.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsunaga F, Norais C, Forterre P, Myllykallio H. 2003. Identification of short ‘eukaryotic’ Okazaki fragments synthesized from a prokaryotic replication origin. EMBO Rep 4:154–158. doi: 10.1038/sj.embor.embor732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkins M, Malla S, Blythe MJ, Nieduszynski CA, Allers T. 2013. Accelerated growth in the absence of DNA replication origins. Nature 503:544–547. doi: 10.1038/nature12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartman AL, Norais C, Badger JH, Delmas S, Haldenby S, Madupu R, Robinson J, Khouri H, Ren Q, Lowe TM, Maupin-Furlow J, Pohlschroder M, Daniels C, Pfeiffer F, Allers T, Eisen JA. 2010. The complete genome sequence of Haloferax volcanii DS2, a model archaeon. PLoS One 5:e9605. doi: 10.1371/journal.pone.0009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breuert S, Allers T, Spohn G, Soppa J. 2006. Regulated polyploidy in halophilic archaea. PLoS One 1:e92. doi: 10.1371/journal.pone.0000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zerulla K, Chimileski S, Näther D, Gophna U, Papke T, Soppa J. 2014. DNA as a phosphate storage polymer and the alternative advantages of polyploidy for growth or survival. PLoS One 9:e94819. doi: 10.1371/journal.pone.0094819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berquist BR, DasSarma P, DasSarma S. 2007. Essential and non-essential DNA replication genes in the model halophilic archaeon, Halobacterium sp. NRC-1. BMC Genet 8:31. doi: 10.1186/1471-2156-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allers T, Ngo HP, Mevarech M, Lloyd RG. 2004. Development of additional selectable markers for the halophilic archaeon Haloferax volcanii based on the leuB and trpA genes. Appl Environ Microbiol 70:943–953. doi: 10.1128/AEM.70.2.943-953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammelmann M, Soppa J. 2008. Optimized generation of vectors for the construction of Haloferax volcanii deletion mutants. J Microbiol Methods 75:201–204. doi: 10.1016/j.mimet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 21.Jaschinski K, Babski J, Lehr M, Burmester A, Benz J, Heyer R, Dörr M, Marchfelder A, Soppa J. 2014. Generation and phenotyping of a collection of sRNA gene deletion mutants of the haloarchaeon Haloferax volcanii. PLoS One 9:e90763. doi: 10.1371/journal.pone.0090763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babski J, Haas KA, Näther-Schindler D, Pfeiffer F, Förstner KU, Hammelmann M, Hilker R, Becker A, Sharma CM, Marchfelder A, Soppa J. 2016. Genome-wide identification of transcriptional start sites in the haloarchaeon Haloferax volcanii based on differential RNA-Seq (dRNA-Seq). BMC Genomics 17:629. doi: 10.1186/s12864-016-2920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jantzer K, Zerulla K, Soppa J. 2011. Phenotyping in the archaea: optimization of growth parameters and analysis of mutants of Haloferax volcanii. FEMS Microbiol Lett 322:123–130. doi: 10.1111/j.1574-6968.2011.02341.x. [DOI] [PubMed] [Google Scholar]
- 24.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tja M, Urbich C, Zeiher AM, Dimmeler S. 2009. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 25.Boon RA, Dimmeler S. 2015. MicroRNAs in myocardial infarction. Nat Rev Cardiol 12:135–142. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- 26.Maurer S, Ludt K, Soppa J. 2018. Characterization of copy number control of two Haloferax volcanii replication origins using deletion mutants and haloarchaeal artificial chromosomes. J Bacteriol 200:e00517-17. doi: 10.1128/JB.00517-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cline SW, Lam WL, Charelbois RL, Schalkwyk LC. 1989. Transformation methods for halophilic archaebacteria. Can J Microbiol 35:148–152. doi: 10.1139/m89-022. [DOI] [PubMed] [Google Scholar]
- 29.Nieuwlandt DT, Daniels CJ. 1990. An expression vector for the archaebacterium Haloferax volcanii. J Bacteriol 172:7104–7110. doi: 10.1128/jb.172.12.7104-7110.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyall-Smith M. 2008. The halohandbook: protocols for haloarchaeal genetics, version 7. Bathurst, NSW, Australia: http://www.haloarchaea.com/resources/halohandbook/Halohandbook_2008_v7.pdf. [Google Scholar]
- 31.Green MR, Sambrook K. 2012. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 32.Rosenshine I, Zusman T, Werczberger R, Mevarech M. 1987. Amplification of specific DNA sequences correlates with resistance of the archaebacterium Halobacterium volcanii to the dihydrofolate reductase inhibitors trimethoprim and methotrexate. Mol Gen Genet 208:518–522. doi: 10.1007/BF00328149. [DOI] [Google Scholar]
- 33.Gibson DG, Young L, Chuang RY, Venter JC, Huthinson CA, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 34.Lange C, Zerulla K, Breuert S, Sopppa J. 2011. Gene conversion results in the equalization of genome copies in the polyploid haloarchaeon Haloferax volcanii. Mol Microbiol 80:666–677. doi: 10.1111/j.1365-2958.2011.07600.x. [DOI] [PubMed] [Google Scholar]
- 35.Wu Z, Liu J, Yang H, Xiang H. 2014. DNA replication origins in archaea. Front Microbiol 5:179. doi: 10.3389/fmicb.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.