Painful sensations induced by capsaicin, the pungent substance in hot peppers, are caused by stimulation of vanilloid receptor 1 (VR1), an ion channel protein expressed by nociceptive primary afferent neurons. VR1 also participates in the detection of at least two additional noxious stimuli, acid (pH < 6) and heat (>43°C). The urinary bladder is rich with capsaicin-sensitive afferent fibers that detect bladder distension or the presence of irritant chemicals and in turn trigger reflex bladder activity. Here, we demonstrate that VR1 is expressed not only by afferent nerves that form close contacts with bladder epithelial (urothelial) cells but also by the urothelial cells themselves. We further show that exogenously applied vanilloids increase intracellular Ca2+ and evoke NO release in urothelial cells and that these responses require VR1. These and other data suggest that urothelial cells work in concert with underlying afferent nerves to detect the presence of irritant stimuli.
Materials and Methods
Reverse Transcription–PCR.
Poly(A)+ RNA was isolated from tissue or cultured cells by homogenization in Trizol (Life Technologies, Grand Island, NY) followed by selection on Oligotex columns (Qiagen, Chatsworth, CA). Twenty nanograms of polyA+ RNA were reverse transcribed with an oligo (dT) primer in 50 mM Tris⋅Cl/75 mM KCl/3 mM MgCl2/0.01 M DTT/20 units of RNase inhibitor/0.5 mM dNTP by using 200 units of Superscript II (Life Technologies), then treated with 1 unit of RNase H. PCR amplification (94°C for 2 min; 35 cycles of 94°C for 30 s, 60°C for 30 s, 72°C for 1 min; 72°C for 2 min) was conducted in 20 mM Tris⋅Cl/50 mM KCl/1.5 mM MgCl2/0.2 mM dNTP/0.4 μM primer pairs, using 1.25 units of platinum Taq (Life Technologies) and the following primers: VR-1, 5′-caaggctgtcttcatcatcc-3′ and 5′-attcccacacacctcccagttc-3′; VRL-1, 5′-gatgaagagggcaatgc-3′, 5′-ccattcagtctccatgaagc-3′. Amplification products were visualized on a 1% agarose gel with ethidium bromide and sequenced to confirm identity.
Cell Culture.
Preparation and characterization of dorsal root ganglia and urothelial cultures has been described in previous reports (1–3). Briefly, bladders excised from anesthetized Sprague–Dawley rats, VR1 null mice (4), or wild-type littermates were cut open and gently stretched (urothelial side down). Muscle was dissected away, and the urothelium was incubated overnight in MEM (Cellgro), penicillin/streptomycin/fungizone and 2.5 mg ml−1 dispase (GIBCO). The urothelium was gently scraped from underlying tissue, treated with 0.25% trypsin, and resuspended in keratinocyte medium (GIBCO). The single cell suspension (0.1 ml, 50,000–150,000 cells per ml) was plated on the surface of collagen-coated dishes. All cells in these cultures were cytokeratin positive (cytokeratins 17 and 20, Dako) and, therefore, were presumably of epithelial origin. All procedures involving rats and mice were conducted in accordance with Institutional Animal Care and Use Committee policies.
Measurement of NO Release and [Ca2+]i.
Urothelial cells were maintained in vitro in Krebs solution and placed in a flow chamber. The tip of a NO-specific sensor (NO detection limit, 1 nM; response time, 1 ms, prepared as described) was placed directly onto the luminal surface of isolated bladder strips or urothelial cells (1, 2). All compounds were applied locally by using a nanoejector. Currents generated by the oxidation of NO to NO+ at the porphyrinic interface were amplified and converted to voltages by using a model 283 Potentiostat (EG & G Princeton Applied Research, Princeton, NJ). Onset of release was rapid (within 1 s), with peak release within 2–3 s. For calcium measurements, cells were loaded with fura-2 AM (1 μM dye, 40 min, 25°C) and alternately illuminated at 340 and 380 nm by using a xenon lamp. Emission was detected at 510 nm and imaged with a Hammamatsu Orca CCD camera with 640 × 480 pixel resolution. A Dage-MTI Gen. II system image intensifier and software package from Compix (Cranberry, PA) were used to collect the data. Data were analyzed by using an unpaired t test.
Immunocytochemistry.
Following perfusion (Krebs; paraformaldehyde fixation) in deeply anesthetized rats or mice (40 mg/kg pentobarbital), portions of urinary bladder whole mounts were postfixed (4% paraformaldehyde fixation), incubated overnight in anti-VR1 (1:20,000), VRL-1 (1:2,000), or cytokeratin 17 (1:1,000) antisera, and localized with cy-3 donkey–anti-rabbit IgG (1:600) (5, 6). Nonspecific staining was assessed without the primary antibody, or by preabsorbing antibody with 10 μg/ml−1 of the VR1 or VRL-1 carboxyl-terminal peptide.
Scanning Laser Confocal Analysis of Bladder Epithelium.
Stained sections were imaged with a Leica DMIRB confocal microscope, using a 100× objective. Z-series of bladder sections taken every 0.5 microns were processed for three-dimensional reconstruction by using METAMORPH imaging software (Universal Imaging, Media, PA).
Results and Discussion
Previous studies have suggested that the expression of VR1 within the bladder is confined to primary afferent neurons (5, 7). Recent functional studies, however, have placed this conclusion in doubt. It was shown, for instance, that capsaicin evoked the release of NO from the luminal surface of bladder strips and that this release was reduced but not completely blocked by denervation (1). Because urothelial cells are known to respond to mechanical and chemical stimuli by releasing neurotransmitters (NO and ATP) (1, 2, 8), it was proposed that capsaicin might activate receptors in the urothelium as well as the underlying afferent nerve plexus to release NO.
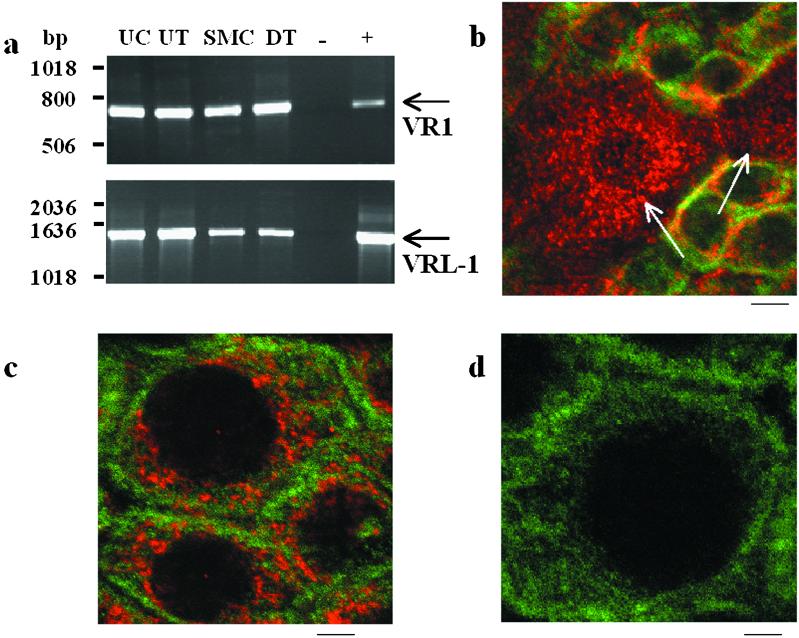
To explore this hypothesis, we initially examined the expression patterns of VR1 and a homologous channel protein, VRL-1, in isolated cell types from rat urinary bladder by using reverse transcription–PCR. In contrast to VR1, VRL-1 can be gated by heat (>53°C) but is insensitive to vanilloid compounds or protons (6). This approach revealed the expression of both VR1 and VRL-1 mRNA in isolated urothelial cells and smooth muscle cells (Fig. 1a). To further evaluate expression within the urothelium at the protein level, sections of rat urinary bladder were processed for either VR1 or VRL-1 immunoreactivity (IR) by using specific polyclonal antisera. The urothelium is a multilayered structure consisting of basal cells, intermediate cells, and large superficial cells (9). Immunostaining revealed VR1-IR in both basal and superficial epithelial cells (Fig. 1 b and c) and VRL-1-IR in superficial epithelial cells (not shown). In both cases, immunoreactivity was absent following incubation of antisera with the corresponding antigenic peptide (Fig. 1d).
Figure 1.
VR1 and VRL-1 expression was detected in urothelial and smooth muscle cells of the urinary bladder from the rat. (a) Reverse transcription–PCR analysis of VR1 and VRL-1 mRNA expression in dissociated bladder urothelial cells (UC), bladder urothelium (UT), dissociated bladder smooth muscle cells (SMC), and de-epithelialized bladder tissue (DT, smooth muscle following removal of the urothelium). Control lanes indicate input of no template (−), dorsal root ganglion cDNA (+ for VR1), or brain cDNA (+ for VRL-1). Arrows indicate predicted product sizes (VR1, 704 bp; VRL-1, 1,436 bp). (b) Confocal image of bladder urothelium in bladder whole mounts stained for VR1 (cy3, red) and cytokeratin 17 (FITC, green), a marker for basal urothelial cells. Diffuse cytoplasmic pattern of VR1 staining can be seen in the apical and underlying urothelial layers (nuclei are unstained). Arrows indicate apical cells within the field from a single plane of focus. (c) Enlarged image of basal cells depicting VR1 (cy3, red) and cytokeratin (FITC, green) immunoreactivity. (d) Elimination of VR1 staining with the antibody preabsorbed with antigenic peptide. (Scale bars: b, 15 μm; c, 5 μm; d, 4 μm.)
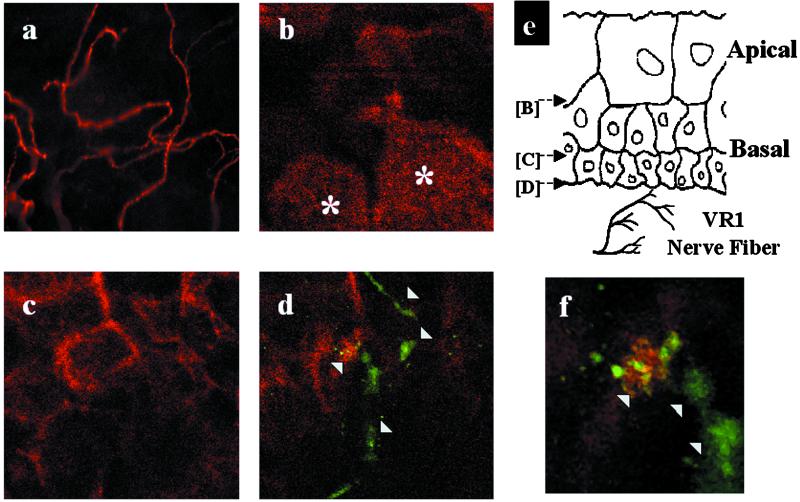
The distribution of VR1- and/or VRL-1-expressing nerve fibers within the bladder urothelium was also examined in frozen and whole mount bladder sections by using immunocytochemical techniques. These studies revealed that VR1-IR nerve fibers were localized throughout the urinary bladder musculature consistent with previous results (5, 10) and in a plexus beneath and extending into the epithelium (Fig. 2a). A more detailed examination of the distribution of VR1-IR nerve fibers in the bladder urothelium using confocal microscopy revealed fibers located mainly in the basal but not in the apical epithelial layer (Fig. 2 b–f). In many cases, VR1-positive nerve fibers were found in close association with basal urothelial cells (Fig. 2 d and f), such that their signals overlapped within 0.5-μm optical sections. Only a few VRL-1-IR fibers were detected in the urinary bladder.
Figure 2.
Confocal imaging of the urothelium reveals VR1-positive nerve fibers located in close proximity to basal cells. (a) Single plane image depicts VR1-IR nerve fibers through the urinary bladder urothelium. (b–d) A series of single plane confocal images taken at distinct z axis positions through a stretched rat bladder preparation stained for VR1 (green, arrowheads) and cytokeratin (red). (e) Schematic illustration of the planes of focus in b–d. Note that VR1-positive nerves are not evident in the same focal plane as the large apical cells indicated by asterisks in b. Punctate VR1 staining in urothelial cells was electronically subtracted to facilitate imaging of the VR1-IR nerve fiber. (f) Enlarged view of d showing close approximation of a VR1-positive nerve fiber (green, arrowheads) with a basal epithelial cell. Colocalization of the green and red fluorescent signals (yellow) suggesting points of cell-to-cell contact.
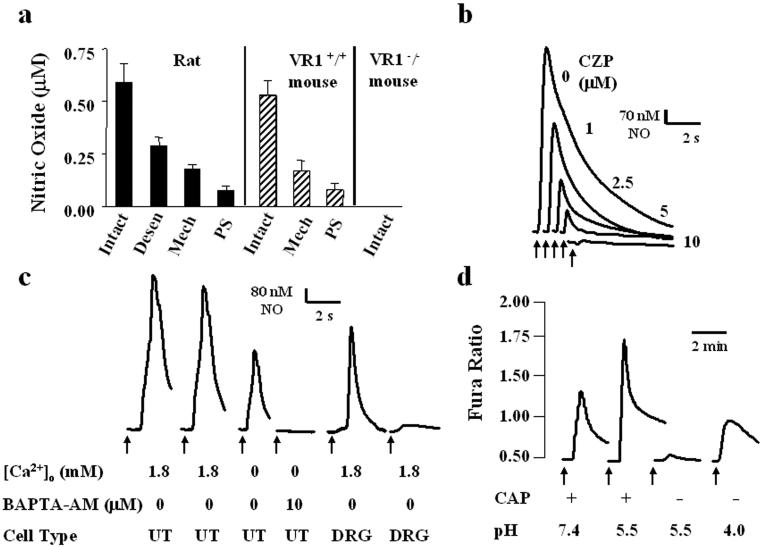
We next evaluated whether VR1 expression in bladder urothelial cells correlates with sensitivity to vanilloid compounds. Previous studies in which a selective porphyrinic microsensor was used to detect NO showed that capsaicin stimulates NO release from isolated dorsal root ganglion neurons (2). Using the same method, we examined the effects of capsaicin (CAP, 500 nM) or its ultrapotent analog resiniferatoxin (RTX, 100 nM) on NO release from the luminal surface of isolated urinary bladder strips from rats. Both CAP (Fig. 3a) and RTX (not shown) induced NO release from rat bladder strips (CAP: mean 582 nM, range 490–670 nM NO; RTX: mean 200 nM, range 150–360 nM NO). Vehicle alone failed to evoke NO release. Vanilloid-evoked release was significantly decreased (72%) following mechanical removal of the urothelium. NO release was similarly diminished (91%) following treatment of the tissue with protamine sulfate (1 mg ml−1), which has been demonstrated to selectively disrupt the epithelium (11). CAP-evoked NO release was also reduced but not ablated following either denervation (bilateral removal of major pelvic ganglia, 4 days before this experiment; 72% mean decrease, not shown) or systemic administration of CAP (100 mg kg−1 s.c., 4 days prior; 52% mean decrease) (Fig. 3a). The latter treatment in rodents has been demonstrated to desensitize CAP receptors and/or destroy sensory nerves (12–15). CAP-induced NO release was blocked following tissue incubation with the competitive vanilloid receptor antagonist, capsazepine (10 μM) (Fig. 3b; see also ref. 16).
Figure 3.
Capsaicin activates VR1 in urothelial cells resulting in NO release. Graphs depict the concentration of CAP-evoked (0.5 μM) NO release from isolated strips of the urinary bladder (UB). (a) CAP-evoked NO release from rat and from VR1+/+ mouse or VR1−/− mouse UB strips. Intact, NO release from the luminal surface of the UB following application of CAP; Desen, CAP-elicited NO release from the luminal surface of the UB in rats previously treated with capsaicin (100 mg kg−1 s.c., 4 days prior); Mech, CAP-evoked NO release from smooth muscle following the mechanical disruption of the bladder urothelium; PS, CAP-evoked NO release following application of protamine sulfate (1 mg ml−1; 20 min) to the UB luminal surface. Protamine damages the mucosal urothelium. Values represent mean ± SEM. (b) Transient NO release from the luminal surface of isolated UB strips in rat was elicited by multiple applications of CAP (0.5 μM, duration 1 s). This release was reduced in a graded manner by increasing concentrations of the CAP antagonist, capsazepine (CZP). Arrows indicate start of drug application. (c) Transient NO release from rat urothelial cells (UT) elicited by successive applications of capsaicin (0.5 μM, arrow, duration 1 s) is diminished in the absence of extracellular calcium (100 μM EGTA) and ablated by 10 μM BAPTA-AM. Transient NO release evoked by CAP (0.5 μM, arrow, duration 1 s) from dissociated dorsal root ganglia cells is significantly reduced following repeated applications of CAP. Tracing is typical of that seen in 10 separate recordings. (d) CAP (0.5 μM, arrow, duration 1 s) elicited an increase in cytosolic calcium in rat urothelial cells. Decreasing the pH of the bath solution (from 7.4 to 5.5) potentiated this response. In the absence of CAP, a reduction in pH to 5.5 did not elicit a significant change in cytosolic calcium; however, a further reduction (pH 4.0) produced a significant increase in cytosolic calcium. Each trace is typical of the data obtained from three to six experiments containing a total of 40–60 cells.
To assess the effect of CAP on urothelial cells in the absence of underlying tissues, we examined the ability of CAP to increase cytosolic calcium and/or evoke NO release in cultured urothelial cells. Both CAP (500 nM) and RTX (100 nM) elicited an increase in both cytosolic-free calcium and NO release from rat cultured urothelial cells (CAP: mean 537 nM, range 481–610 nM NO; RTX: mean 167 nM, range 120–220 nM NO) (Fig. 4). The NO release occurred rapidly (within 1–2 s) and was mediated by a calcium-dependent nitric oxide synthase, as it was diminished by incubation in calcium-free media and ablated following incubation with the rapidly acting, cell-permeant calcium chelator BAPTA-AM (10 μM) (Fig. 3c). The diminished response was not due to desensitization, as repeated administration of CAP or RTX at 10-s intervals in the absence of chelating agent produced no significant tachyphylaxis. Indeed, this lack of vanilloid-evoked desensitization represents one apparent difference between the sensory neuron and urothelial cell preparations. A similar lack of desensitization was also noted for the capsaicin-evoked changes of the intracellular calcium concentration in urothelial cells. The basis for this difference remains unknown, but it may reflect differences in the complement of calcium-dependent kinases and/or phosphatases expressed by these two cell types (17).
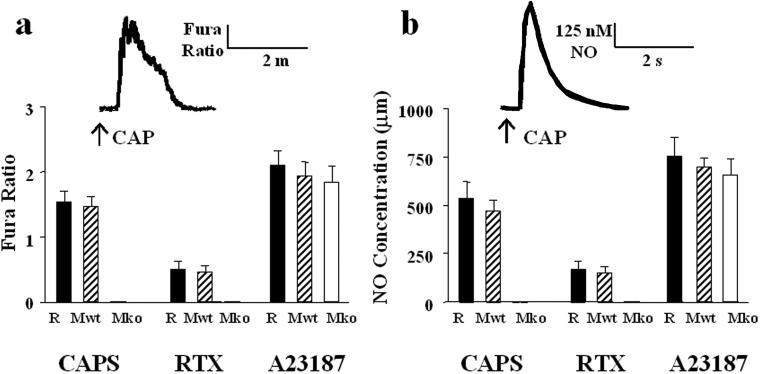
Figure 4.
Vanilloid compounds elicit increased [Ca2+]i and NO release from isolated rat (R) and mouse (wild type, Mwt; null mice, Mko) urothelial cells. (a) Peak increase in fura-2 ratio following local application of CAP (0.5 μM), RTX (0.1 μM), or the calcium ionophore, A23187 (0.5 μM). (b) NO concentration (μM) following local application of CAP, RTX, or A23187. Data are based on calculations from 30–50 cells per treatment, recorded in a minimum of three independent experiments. Values represent mean ± SEM. For CAP and RTX, Mwt vs. Mko, P < 0.0005.
In neurons and heterologous expression systems, protons potentiate VR1 activation by vanilloid compounds or heat and, at higher concentrations, directly activate the channel (7, 18). These two effects are probably distinct because they can be independently attenuated by selective amino acid substitutions in the extracellular domain of VR1 (19). To determine whether vanilloid receptors in urothelial cells exhibit similar properties, we assayed cytosolic Ca2+ in these cells during treatment with low pH bath solutions. A reduction in pH from 7.4 to 5.5 produced little or no change in cytosolic Ca2+ on its own, but potentiated the capsaicin-evoked Ca2+ response by 55 ± 6.2% (Fig. 3d). A further reduction to pH 4.0 produced a significant increase in cytosolic Ca2+, even in the absence of capsaicin. Thus, the functional response of endogenous urothelial cell vanilloid receptors, like that of recombinant VR1, can be modulated by protons.
To determine whether VR1 is responsible for the effect of vanilloid compounds on urothelial cells, we compared vanilloid-evoked NO release and calcium transients in bladder tissues derived from VR1-null mice (4) versus wild-type controls. As with rat-derived bladder strips, CAP (500 nM, Fig. 3a) or RTX (100 nM, not shown) increased cytosolic free calcium and evoked NO release from wild-type mouse bladder strips (CAP: mean 530 nM, range 425–620 nM NO; RTX: mean 170 nM, range 155–210 nM NO). This release could be attenuated following protamine sulfate treatment or mechanical removal of the mucosa (Fig. 3a) and was blocked following application of the competitive antagonist, capsazepine (not shown). At the same concentration, CAP or RTX also increased intracellular calcium and evoked NO release (CAP: mean 472 nM, range 420–580 nM NO; RTX: mean 151 nM, range 118–195 nM NO) from isolated urothelial cells obtained from wild-type mice (Fig. 4). In contrast, vanilloid compounds triggered neither response in urinary bladder strips or urothelial cells isolated from VR1 null mice, even though these responses could be activated by other agents, including ATP (1 μM, not shown), norepinephrine (1 μM, not shown), and the calcium ionophore A23187 (500 nM). VR1 protein expression was observed in the urothelial cells of wild-type mice, but not VR1 null mice, using both immunoblot and immunofluorescence analyses (data not shown). Furthermore, reverse transcription–PCR confirmed the expression of VR1 in the bladders of wild-type mice only (data not shown). These findings strongly suggest that VR1 mediates the responses of urothelial cells to vanilloid compounds.
VR1 is essential for the thermal hypersensitivity associated with inflammation of the hind paw (4, 20). This function most likely stems from the ability of VR1 to simultaneously assess temperature, pH, and the presence of lipid modulators and to be sensitized by G protein-coupled signaling pathways (21). In urothelial cells, VR1 might similarly participate in the detection of irritant stimuli produced by bladder inflammation, infection, or altered urinary composition. Given that the bladder is unlikely to experience the high ambient temperatures required to activate VRL-1, the significance of this latter channel's expression in urothelium is unclear. One possibility is that VRL-1 activity is regulated by growth factors released during bladder or spinal cord injury (22).
Our data, together with previously reported findings, support the idea that urothelial cells exhibit specialized sensory and signaling properties that allow them to respond to their chemical and physical environments and engage in reciprocal communication with neighboring nerves in the bladder wall. These properties include (i) the expression of nicotinic, muscarinic, tachykinin, and adrenergic receptors (1, 23), as well as vanilloid receptors; (ii) responsiveness or sensitivity to transmitters (ATP, Substance P) released from afferent nerves (23); (iii) close physical association with afferent nerves (Fig. 2); and (iv) the ability to release chemical mediators such as ATP and NO that can regulate the activity of underlying nerves and thereby trigger local vascular changes and/or reflex bladder contractions (1, 2, 8, 24). The functional association of urothelial cells with underlying primary afferent nerves would not be unique to the bladder. Taste transduction, for example, involves the activation of specialized epithelial taste cells that are in synaptic contact with afferent fibers of the gustatory pathway (25, 26). A similar relationship exists between hair cells of the inner ear and peripheral auditory fibers (27, 28). Moreover, the concept that the bladder urothelium can participate in nonbarrier functions is supported by the recent observation that in some species, this tissue produces and secretes proteolytic enzymes in a manner regulated by second messengers (29). Ultrastructural characterization of the afferent fiber–urothelial cell association and studies of bladder regulation in mice lacking VR1 may lead to a better understanding of these functions.
Acknowledgments
We thank L. Cysco for technical help with confocal analysis, J. Piccolo for technical help with cell culture, and D. Julius and H. Lee for helpful discussions. This work was supported by National Institutes of Health Grants R01 DK54824 and R01 DK57284 (to L.A.B.) and by grants from the Blaustein Pain Research Fund and the American Cancer Society (to M.J.C.).
Abbreviations
- VR1
vanilloid receptor 1
- IR
immunoreactivity
- CAP
capsaicin
- RTX
resiniferatoxin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Birder L A, Apodaca G, de Groat W C, Kanai A J. Am J Physiol. 1998;275:F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- 2.Birder L A, Kanai A J, de Groat W C. J Urol. 1997;158:1989–1995. doi: 10.1016/s0022-5347(01)64199-5. [DOI] [PubMed] [Google Scholar]
- 3.Truschel S T, Ruiz W G, Shulman T, Pilewski J, Sun T T, Zeidel M L, Apodaca G. J Biol Chem. 1999;274:15020–15029. doi: 10.1074/jbc.274.21.15020. [DOI] [PubMed] [Google Scholar]
- 4.Caterina M J, Leffler A, Malmberg A B, Martin W J, Trafton J, Peterson-Zeitz K R, Koltzenburg M, Basbaum A I, Julius D. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 5.Tominaga M, Caterina M J, Malmberg A B, Rosen T A, Gilbert H, Skinner K, Raumann B E, Basbaum A I, Julius D. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 6.Caterina M J, Rosen T A, Tominaga M, Brake A J, Julius D. Nature (London) 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 7.Caterina M J, Schumacher M A, Tominaga M, Rosen T A, Levine J D, Julius D. Nature (London) 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson D R, Kennedy I, Burton T J. J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis S A. Am J Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 10.Yiangou Y, Facer P, Ford A, Brady C, Wiseman O, Fowler C J, Anand P. Br J Urol Int. 2001;87:774–779. doi: 10.1046/j.1464-410x.2001.02190.x. [DOI] [PubMed] [Google Scholar]
- 11.Lewis S A, Berg J R, Kleine T J. Physiol Rev. 1995;75:561–591. doi: 10.1152/physrev.1995.75.3.561. [DOI] [PubMed] [Google Scholar]
- 12.Jancso G, Kiraly E, Joo F, Such G, Nagy A. Neurosci Lett. 1985;59:209–214. doi: 10.1016/0304-3940(85)90201-0. [DOI] [PubMed] [Google Scholar]
- 13.Buck S H, Burks T F. Pharmacol Rev. 1986;38:179–226. [PubMed] [Google Scholar]
- 14.Maggi C A, Santicioli P, Borsini F, Giulianai S, Meli A. Naunyn Schmiedebergs Arch Pharmacol. 1986;332:276–283. doi: 10.1007/BF00504867. [DOI] [PubMed] [Google Scholar]
- 15.Cheng C L, Ma C P, de Groat W C. Am J Physiol. 1993;265:R132–R138. doi: 10.1152/ajpregu.1993.265.1.R132. [DOI] [PubMed] [Google Scholar]
- 16.Bevan S, Hothi S, Hughes G, James I F, Rang H P. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Docherty R J, Yeats J C, Bevan S, Boddeke H W. Pflugers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- 18.Peterson M, LaMotte R H. Pain. 1993;54:37–42. doi: 10.1016/0304-3959(93)90097-9. [DOI] [PubMed] [Google Scholar]
- 19.Jordt S E, Tominaga M, Julius D. Proc Natl Acad Sci USA. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. . (First Published June 20, 2000; 10.1073/pnas.100129497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis J B, Gray J, Gunthorpe M J, Hatcher J P, Davey P T, Overend P, Harries M H, Latcham J, Clapham C, Atkinson K, et al. Nature (London) 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 21.Premkumar L S, Ahern G P. Nature (London) 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 22.Kanzaki M, Zhang Y Q, Mashima H, Li L, Shibata H, Kojima I. Nat Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 23.Birder, L. A. (2001) Proc. Western Pharmacol. Soc., in press. [PubMed]
- 24.de Groat W C, Booth A M, Yoshimura N. In: The Autonomic Nervous System: Nervous Control of Urogenital System. Maggi C A, editor. Vol. 3. London: Harwood; 1993. pp. 227–290. [Google Scholar]
- 25.Oakley B. Ciba Found Symp. 1991;160:277–287. doi: 10.1002/9780470514122.ch14. [DOI] [PubMed] [Google Scholar]
- 26.Akabas A H. Int Rev Neurobiol. 1990;32:241–279. doi: 10.1016/s0074-7742(08)60585-1. [DOI] [PubMed] [Google Scholar]
- 27.Hudspeth A J, Corey D P. Proc Natl Acad Sci USA. 1977;74:2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell I J, Richardson G P, Cody A R. Nature (London) 1986;321:517–519. doi: 10.1038/321517a0. [DOI] [PubMed] [Google Scholar]
- 29.Deng F M, Ding M, Lavker R M, Sun T T. Proc Natl Acad Sci USA. 2001;98:154–159. doi: 10.1073/pnas.98.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]