Abstract
Purpose: ST-162 and ST-168 are small-molecule bifunctional inhibitors of MEK and PI3K signaling pathways that are being developed as novel antitumor agents. Previous small-molecule and biologic MEK inhibitors demonstrated ocular toxicity events that were dose limiting in clinical studies. We evaluated in vitro and in vivo ocular toxicity profiles of ST-162 and ST-168.
Methods: Photoreceptor cell line 661W and adult retinal pigment epithelium cell line ARPE-19 were treated with increasing concentrations of bifunctional inhibitors. Western blots, cell viability, and caspase activity assays were performed to evaluate MEK and PI3K inhibition and dose-dependent in vitro toxicity, and compared with monotherapy. In vivo toxicity profile was assessed by intravitreal injection of ST-162 and ST-168 in Dutch-Belted rabbits, followed by ocular examination and histological analysis of enucleated eyes.
Results: Retinal cell lines treated with ST-162 or ST-168 exhibited dose-dependent inhibition of MEK and PI3K signaling. Compared with inhibition by monotherapies and their combinations, bifunctional inhibitors demonstrated reduced cell death and caspase activity. In vivo, both bifunctional inhibitors exhibited a more favorable toxicity profile when compared with MEK inhibitor PD0325901.
Conclusions: Novel MEK and PI3K bifunctional inhibitors ST-162 and ST-168 demonstrate favorable in vitro and in vivo ocular toxicity profiles, supporting their further development as potential therapeutic agents targeting multiple aggressive tumors.
Keywords: : toxicity, photoreceptors, retina, MEKAR, PD0325901, efficacy
Introduction
Tumorigenesis requires aberrant activation of key cellular processes. Activation of MAP kinase (Ras/MEK/ERK) and PI3K/AKT/mTOR signaling pathways are critical for K-Ras-mediated transformation.1,2 A wide range of tumors demonstrate mutations in the RAS family and increased PI3K/AKT signaling is observed frequently in leukemia, melanoma, breast, ovarian, brain, lung, and prostate cancers. Concomitant activation of both Ras/MEK/ERK and PI3K/AKT/mTOR underlies the crosstalk across these signaling pathways. This crosstalk has been shown to facilitate drug resistance during tumor monotherapy designed to target PI3K or MEK signaling pathways individually.3–8 In contrast, simultaneous inhibition of PI3K and MEK signaling synergistically enhances tumor cell death in vitro and in vivo.9–11 Since Ras/MEK/ERK and PI3K/AKT/mTOR pathways are regulated by different mechanisms, concomitant targeting of these key complementary signaling pathways could provide improved response to treatment for many tumor types.12–14
Several inhibitors of the PI3K/AKT/mTOR or the Ras/MEK/ERK signaling pathways have been developed and evaluated for tumor targeting, including the monotherapeutic agents ZSTK474 and PD0325901. The PI3K inhibitor ZSTK474 is a highly selective and Adenosine triphosphate (ATP)-competitive antagonist with excellent antitumor activity.15–19 Previous studies have shown that this inhibitor is effective against all 4 of the class I PI3K isoforms, and it has demonstrated relatively low cytotoxicity levels in animal models.16,18 ZSTK474 is very potent, displaying IC50 values between 3.9 and 20.8 nM for the 4 PI3K isoforms.20 PD0325901 is a second-generation inhibitor of both MEK1 and MEK2 isoforms that has been evaluated in phase 1 clinical trials for the treatment of several different solid tumors.21 This early phase clinical study of PD0325901, which enrolled 66 patients with various late-stage cancers, revealed numerous ocular adverse events, including transient blurred vision (7 patients), optic neuropathy (1 patient), and retinal vein occlusion (2 patients), which developed after 4 months of treatment.21 Further development of PD0325901 was abandoned following the phase 1 trial due to neurologic and ophthalmic dose-limiting toxicity.21 The toxicity profile of this particular MEK inhibitor increases in a dose-dependent fashion in vitro, and can lead to adverse events such as retinal vein occlusion when administered at higher concentrations in a preclinical animal model.22–24 Ophthalmic adverse events appear to be a class effect not specific to PD0325901, as 2 other MEK inhibitors approved by the FDA (trametinib and cobimetinib) as well as those in late-stage clinical testing, including selumetinib and binimetinib, demonstrate a variety of ocular findings.23–25 These adverse events include cystoid macular edema, serous retinal detachments, and uveitis, collectively described as MEK inhibitor-associated retinopathy (MEKAR).25–27 Ocular toxicity, including MEKAR, has been reported after systemic exposure to multiple classes of MEK inhibitors.23–29 Additionally, MEKAR was seen in preclinical studies with MEK inhibitors. Huang et al. found that 1 week after intravitreal injection of PD0325901 into rabbit eyes, the retinal findings progressed to retinal detachment and edema, both hallmarks of MEKAR.22 MEK inhibitors with improved ocular toxicity profile would provide a larger therapeutic window for the treatment of aggressive tumors.
While monotherapeutic agents, including ZSTK474 and PD0325901, have shown clinical promise in targeting tumorigenesis, concurrent inhibition of PI3K and MEK using 2 monotherapeutic drugs has emerged as a means to counter the redundant and reciprocal roles that are exhibited by these 2 signaling pathways in cancer.8–10,30–35 In recent years, there has been increasing interest toward developing multifunctional, single-agent compounds for modulating multiple biological targets.36–39 As part of this strategy, we recently reported the development of prototype small-molecule MEK/PI3K bifunctional inhibitors for simultaneous targeting of these critical regulators of K-Ras-mediated transformation.40,41 Bifunctional inhibitors were designed by covalent linking of structural analogs of the ATP-competitive pan-PI3K inhibitor ZSTK474 with analogs of the allosteric MEK inhibitors RO5126766 or PD0325901. One such bifunctional inhibitor, designated as ST-162, was formed by linkage of analogs of the PI3K inhibitor ZSTK474 and the MEK inhibitor PD0325901. Both in vitro and in vivo studies have demonstrated the effectiveness of ST-162 against MEK and PI3K enzyme activity.41,42 A structural analog of ST-162, ST-168, demonstrates higher inhibitory activity toward MEK1 and all 4 class I PI3K isoforms.20 The side effect profile of both ST-168 and ST-162 is much less significant as compared with single-pathway inhibition (PD0325901 or ZSTK474) or cocktail inhibition.20,42
In this study, we characterized the in vitro and in vivo toxicity profiles of bifunctional MEK/PI3K inhibitors, ST-162 and ST-168, in retinal cells and in a rabbit preclinical model. Our results show that these novel inhibitors demonstrate favorable in vitro and in vivo ocular toxicity profiles compared with PD0325901, supporting their further development as potential therapies for diverse tumors.
Methods
Ethics statement
All experiments were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol was approved by the University Committee on Use and Care of Animals of the University of Michigan (Protocol No.: PRO00006215).
Cell culture
The immortalized 661W cell line was supplied by Dr. David Zacks (Department of Ophthalmology and Visual Sciences, University of Michigan, Ann Arbor, MI). The ARPE-19 cells (ATCC® CRL2302™) were acquired from American Type Culture Collection (Manassas, VA). Both cell types were grown to 90% confluency before experimental treatment in an atmosphere regulated at 37°C consisting of 5% CO2 and 95% air. The 661W culture was maintained in Dulbecco's modified Eagle's medium (DMEM) (Catalog No.: 11995065; Thermo Fisher Scientific), which consists of 10% fetal bovine serum (FBS, Catalog No.: 10082147; Thermo Fisher Scientific), 40 μg L−1 of both hydrocortisone 21-hemisuccinate and progesterone, 40 μL L−1 β-mercaptoethanol and 32 mg L−1 Putrescine dihydrochloride. This medium was supplemented with streptomycin (0.09 mg mL−1) and penicillin (90 units mL−1). The ARPE-19 cells were maintained in DMEM:F12 (Catalog No.: 11320033; Thermo Fisher Scientific) supplemented with 10% FBS, streptomycin (0.09 mg mL−1), and penicillin (90 units mL−1). Penicillin–Streptomycin (5,000 U/mL) was purchased from Thermo Fisher Scientific (Catalog No.: 15070063). The 661W and ARPE-19 cells were passaged, and then plated at 300,000 cells/well in 6-well culture dishes in growth media. The cells were treated with PD0325901, ZSTK474, PD0325901, and ZSTK474 cocktail (equimolar concentrations of PD0325901 and ZSTK474) and bifunctional MEK/PI3K inhibitors, ST-162 and ST-168, at 1, 5, 10, 25, 50, and 100 μM for 24 h. Both untreated cells and DMSO (0 μM) that was used as a vehicle were taken as controls. Cells were harvested after 24 h and prepared as described for western analysis.
Cell viability and caspase activity
The 661W and ARPE-19 cells were seeded in a white-walled 96-well plate (Catalog No.: 07-200-587; Fisher Scientific) at 5,000 cells/well for 24 h before treatment. The cells were then treated with single agents PD0325901, ZSTK474, PD0325901, and ZSTK474 cocktail and bifunctional MEK/PI3K inhibitors ST-162 and ST-168 at 1, 5, 10, 25, 50, and 100 μM for 24 h. Both untreated cells and DMSO (0 μM) that was used as a vehicle were taken as controls. The number of viable cells was measured based on quantitation of ATP using the CellTiter-Glo Luminescent Cell Viability Assay Kit (Catalog No.: G7570; Promega, Madison, WI) following the manufacturer's recommendations. Caspase activities were measured according to the manufacturer's instructions using the Caspase-Glo 8 (Catalog No.: G8200) and Caspase-Glo 3/7 Assay Kits (Catalog No.: G8090). Luminescence was measured using a Veritas Microplate Luminometer (Turner Biosystems, Sunnyvale, CA). The assay kits were purchased from Promega. Statistical analysis was performed using Prism 6.0 (GraphPad Software, San Diego, CA).
Western blot analysis
Cells were lysed in RIPA Lysis and Extraction Buffer (Catalog No.: 89900; Life Technologies Corporation, Grand Island, NY). One tablet of protease inhibitor (Complete Mini; Roche Diagnostics, Indianapolis, IN) and 1 tablet of phosphatase inhibitor (PhosSTOP; Roche Diagnostics) per 10 mL were added to the lysis buffer before use to prevent proteolysis and maintain protein phosphorylation. The cellular debris was removed by centrifugation and protein concentrations of supernatants were determined by the Pierce BCA Protein Assay Kit (Life Technologies Corporation) and quantified with the FLUOstar Omega multi-mode microplate reader (BMG Labtech, Offenburg, Germany). Protein samples were separated by SDS NuPAGE Novex 10% gels (Invitrogen, Carlsbad, CA), transferred onto Polyvinylidene difluoride membranes that were blocked in blocking buffer (5% nonfat dry milk in phosphate-buffered saline and 0.1% Tween 20) for 1 h, incubated with primary antibody, washed, and incubated with horseradish peroxidase-conjugated secondary antibody, and developed using SuperSignal West Dura Extended Duration Substrate. Images were captured digitally on an Azure c500 (Azure Biosystems, Dublin, CA).
In vivo toxicity analysis
Male Dutch-Belted rabbits with an approximate weight of 1.5 to 2.5 kg were anesthetized with sevoflurane. An external heat source was used to prevent hypothermia. Immediately before the intravitreal injections, each animal underwent a clinical examination to rule out any preexistent ocular disease. Eyes were anesthetized with proparacaine, and pupils were dilated with 1% Tropicamide and 2.5% Phenylephrine ophthalmic drops. A Flynn pediatric lid speculum was placed in the eye and betadine was applied to the injection site. The temporal sclera was marked with calipers 2 mm from the corneal border. A 30-gauge needle was inserted into the mid-vitreous ∼62° from the horizontal axis (28° from the vertical axis). Fifty microliters of DMSO (vehicle), PD0325901 (0.5 or 1 mg), ST-162 (1, 2, or 4 mg), or ST-168 (4 mg) was administered intravitreally and delivery of the test agent into the eye was confirmed with inspection. Animals underwent ocular examination daily for 1 week after intravitreal injection, performed by the senior author who is a retina specialist. Retina was visualized using a video indirect ophthalmoscope (Heine Optotechnik, Herrsching, Germany). Retinal images in still frames were captured from fundus videos.
Histopathology
Rabbits were euthanatized, and the eyes were enucleated 7 days after intravitreal injections for histopathology. Whole eyes were fixed overnight at 4°C in phosphate-buffered saline with 4% paraformaldehyde (pH 7.4). The specimens were embedded in paraffin and were then placed in a tissue processor (Tissue-Tek II; Sakura, Tokyo, Japan) for standard paraffin embedding. Eyes were then sectioned at a width of 6 μm on a standard paraffin microtome. The sections were deparaffinized and rehydrated in a graded ethanol series. Hematoxylin–Eosin staining was performed and images were captured on a Leica DM6000 microscope (Leica Microsystems, Wetzlar, Germany).
Results
Bifunctional PI3K/MEK inhibitors, ST-162 and ST-168, show dose-dependent inhibition of PI3K and MEK activities in 661W and ARPE-19 cells
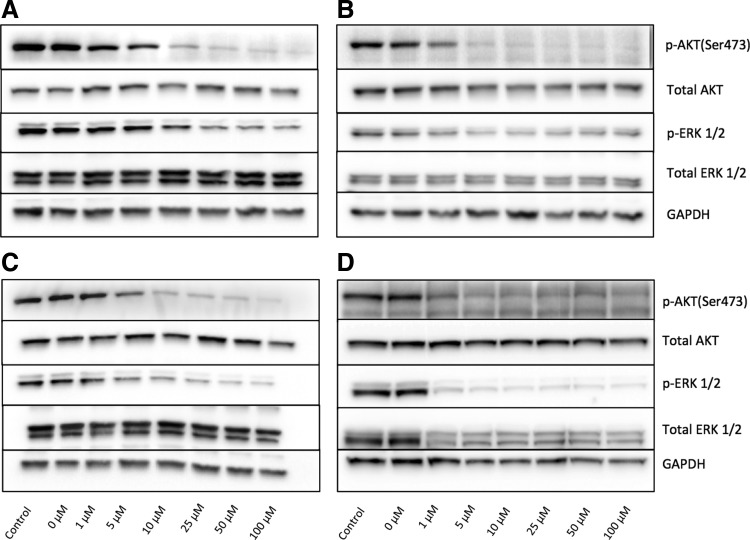
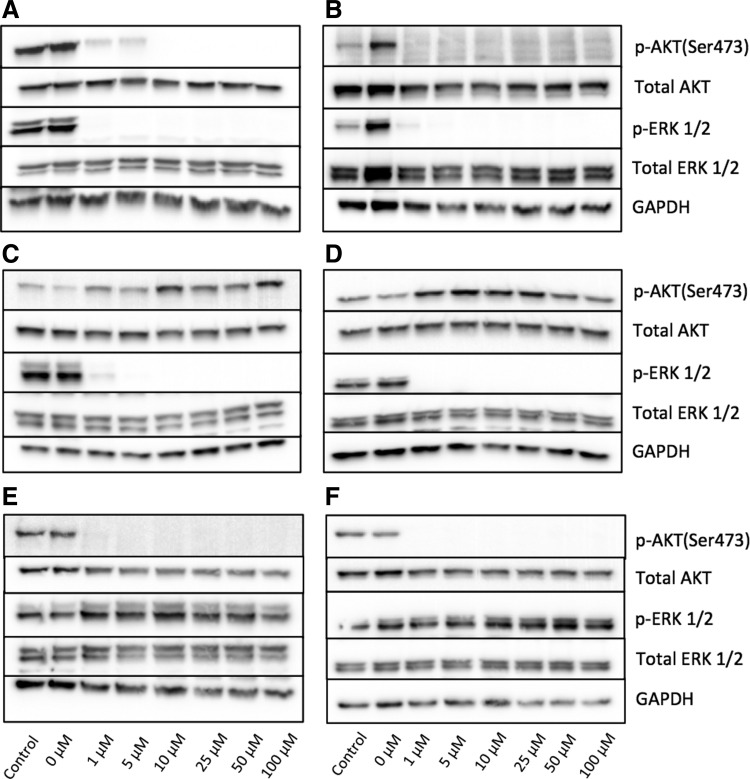
To measure the inhibitory effect of bifunctional MEK/PI3K inhibitors, we treated 661W photoreceptor cells and ARPE-19 cells with various concentrations of ST-162 and ST-168. Both retinal cell types display high levels of PI3K and MEK activity under normal cell culture conditions, as measured by AKT and ERK1/2 phosphorylation (Fig. 1A, B). The bifunctional inhibitor ST-162 showed dose-dependent inhibition of both PI3K/AKT and MAPK/ERK pathways (Fig. 1A, B). Similarly, ST-168, exhibited a dose-dependent inhibition of AKT and ERK phosphorylation at 24 h in both 661W and ARPE-19 cells (Fig. 1C, D), with an improved activity against MEK in both cell types compared with ST-162. The effects of bifunctional inhibitors on MEK and PI3K activities in vitro were similar to those seen with the combined inhibition using PD0325901 and ZSTK474 cocktail (Fig. 2A). PD0325901 and ZSTK474 were selective against MEK and PI3K, respectively, in retinal cells in vitro (Fig. 2C–F).
FIG. 1.
Dual PI3K/MEK inhibitors, ST-162 and ST-168, effectively target AKT and ERK phosphorylation in a dose-dependent fashion. (A, B) Western blot analysis of 661W cells (A) and ARPE-19 cells (B) treated for 24 h with ST-162 analyzed with antibodies against MEK and PI3K signaling proteins. (C, D) Western blot analysis of 661W cells (C) and ARPE-19 cells (D) treated for 24 h with ST-168 analyzed with antibodies against MEK and PI3K signaling proteins. GADPH is shown as the loading control.
FIG. 2.
(A, B): Dual PIK3 and MEK inhibition with PD0325901+ZSTK474 cocktail effectively prevents AKT and ERK phosphorylation. Western blot analysis of 661W cells (A) and ARPE-19 cells (B) treated for 24 h with PD0325901+ZSTK474 and analyzed with antibodies against MEK and PI3K signaling proteins. (C, D) MEK inhibitor PD0325901 prevents baseline ERK phosphorylation and PI3K inhibitor ZSTK474 prevents baseline AKT phosphorylation in retinal cells. Western blot analysis of 661W cells (C) and ARPE-19 cells (D) treated for 24 h with PD0325901 or ZSTK474 (E, F) and analyzed with antibodies against PI3K and MEK signaling proteins. GADPH is shown as the loading control.
Bifunctional MEK/PI3K inhibitors ST-162 and ST-168 demonstrate reduced in vitro toxicity compared with monotherapeutic agents PD0325901 and ZSTK474
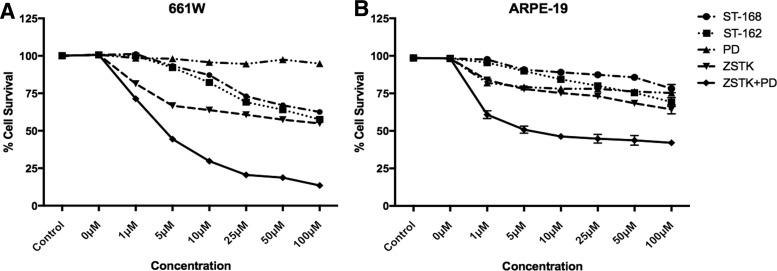
We next determined the viability of 661W and ARPE-19 cells exposed to MEK and PI3K inhibitors. The 661W cells were sensitive to PI3K inhibition as demonstrated by close to 50% cell death at 24 h with ZSTK474 concentrations above 5 μM (Fig. 3A). Although MEK inhibition alone had little effect on 661W cell survival, addition of PD0325901 to ZSTK474 significantly increased cell death with >75% cell death by 24 h (Fig. 3A). In contrast, 661W cells were more resistant to bifunctional inhibitors ST-162 and ST-168 with increased survival at all concentration levels, demonstrating over 50% survival compared with near total cell death induced by PD0325901 and ZSTK474 cocktail at 24 h (Fig. 3A).
FIG. 3.
In vitro toxicity profiles of single and dual PI3K/MEK inhibitors. Cell viability of 661W cells (A) and ARPE-19 cells (B) was measured in the presence of ZSTK474, PD0325901, ST-162, ST-168, and PD0325901+ZSTK474 cocktail. The % cell survival was determined 24 h after treatment. Increased cell death observed for PD0325901+ZSTK474 cocktail compared with ST-162 or ST-168 was statistically significant. Mean ± SEM; P < 0.05, n = 15.
Unlike 661W cells, ARPE-19 cells were sensitive to both MEK and PI3K inhibition alone, although the cell death was much less robust even with the highest concentrations of PD0325901 and ZSTK474 (Fig. 3B). However, dual MEK and PI3K inhibition by PD0325901 and ZSTK474 nearly doubled the amount of cell death. In contrast, ST-168, and to a lesser extent ST-162, showed much less toxicity in ARPE-19 cells (Fig. 3B).
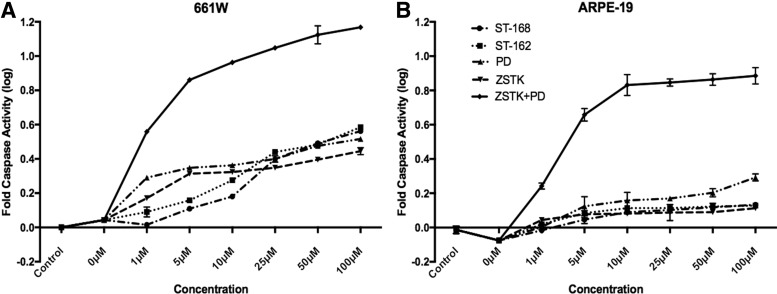
We next evaluated the activation of apoptotic effectors in retinal cells exposed to PI3K and MEK inhibitors. In the 661 W cells, inhibition of PI3K and MEK with monofunctional and bifunctional inhibitors mildly increased caspase 3/7 activity, consistent with low amounts of cell death observed in cell viability experiments (Fig. 4A). In contrast, simultaneous PI3K and MEK inhibition with PD0325901/ZSTK474 cocktail treatment led to robust Caspase 3/7 activity, likely accounting for significantly more death seen in the viability experiments (Fig. 4A). At lower drug concentrations, both ST-162 and ST-168 showed less caspase activation compared with monofunctional inhibitors. Similar to 661W cells, ARPE-19 cells demonstrated little caspase activity when treated with monofunctional or bifunctional inhibitors alone (Fig. 4B). In contrast, PD0325901/ZSTK474 cocktail showed significant caspase 3/7 activation, demonstrating the synergistic effect of PI3K and MEK inhibition on apoptotic signaling and cell viability (Fig. 4B). These experiments demonstrated that inhibition of PI3K and MEK pathways together lead to apoptotic cell death in retinal cells. In contrast, bifunctional inhibitors, ST-162 and ST-168, displayed reduced caspase activation, demonstrating their increased tolerability for retinal cells.
FIG. 4.
Activation of caspases with single and dual PI3K/MEK inhibitors. Caspase 3/7 activation in 661W cells (A) and ARPE-19 cells (B) was measured in the presence of ZSTK474, PD0325901, ST-162, ST-168, and PD0325901+ZSTK474 cocktail for 24 h. PD0325901+ZSTK474 cocktail increased caspase activation significantly more than ST-162 or ST-168. Mean ± SEM; P < 0.05, n = 15.
Bifunctional MEK/PI3K inhibitors demonstrate a favorable in vivo toxicity profile
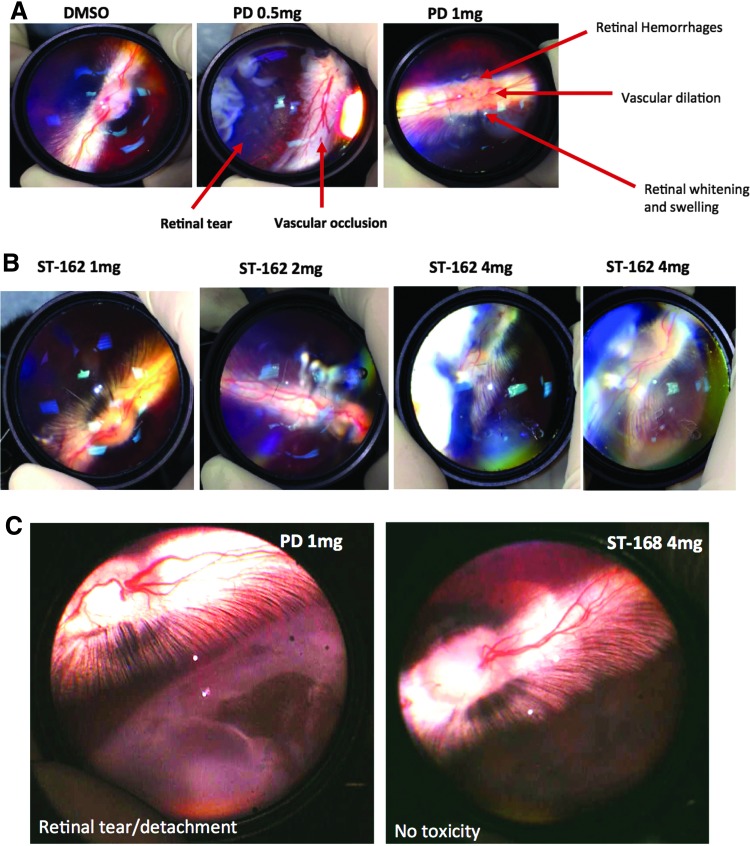
Clinical trials of PD0325901 in patients with solid tumors were complicated by central retinal vein occlusion, and Huang et al. have replicated similar findings in a rabbit model through intravitreal injection of PD0325901.22 Similar to what was discovered in the study conducted by Huang et al., we found that PD0325901 induced ocular changes simulating retinal vein occlusion, including focal vascular occlusions with proximal vascular dilation and retinal hemorrhages (Fig. 5A). In addition, intravitreal treatment with PD0325901 caused retinal whitening, retinal swelling, and retinal tears with associated retinal detachment (Fig. 5A and C). In contrast, intravitreal administration of bifunctional inhibitors, ST-162 and ST-168, were well tolerated at all tested doses and did not demonstrate any clinically observable adverse effects (Fig. 5B, C).
FIG. 5.
In vivo toxicity profiles of PD0325901 and bifunctional inhibitors. (A) Both 0.5 and 1 mg doses of PD0325901 led to adverse events following intravitreal injection compared to control injection with DMSO (vehicle). (B) No adverse events in the retina or retinal vasculature were observed with increasing doses of ST-162 1 week after injection (C) Pronounced toxicity difference between eyes treated with PD0325901 and ST-168 is demonstrated by retinal tear and detachment in PD0325901-treated eye only after 3 days.
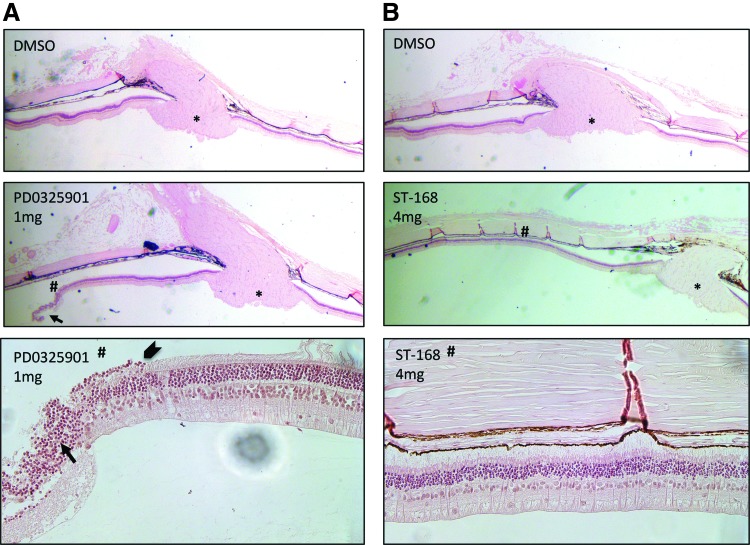
Histopathology of enucleated rabbit eyes treated with MEK inhibitor PD0325901 revealed retinal toxicity as demonstrated by loss of photoreceptors in the outer nuclear layer, RPE cell death, disorganization of retinal layers, and retinal tear (Fig. 6A). In contrast, rabbit retinas exposed to bifunctional inhibitor ST-168 displayed normal anatomy with no evidence of inner or outer retinal toxicity (Fig. 6B).
FIG. 6.
Ocular histopathology of rabbit eyes treated with PD0325901 and bifunctional inhibitor ST-168. Representative retinal sections are shown. (A) Intravitreal treatment with 1 mg of PD0325901 caused retinal thinning and tear compared with control injection with DMSO (vehicle). Magnified image (#) shows disorganization of retinal layers (arrow). Transition from normal to abnormal retina is marked by an arrowhead. (B) No histological changes were observed with increasing doses of ST-168 or DMSO vehicle. Magnified inset image (#) shows preservation of retinal layers. *optic nerve.
Discussion
The primary purpose of our study was to determine the ocular toxicity profiles of 2 novel bifunctional MEK/PI3K inhibitors, ST-162 and ST-168, which are being developed for the treatment of tumors with K-Ras-mediated transformation. In our previous studies, we investigated the systemic toxicity of the bifunctional inhibitors and found that the side effect profiles of ST-162 and ST-168 are much less significant as compared with single-pathway inhibition (PD0325901 or ZSTK474) or inhibition of both signaling pathways with PD0325901 and ZSTK474 cocktail.20,42 Moreover, treatment with ST-162 in colorectal and melanoma mouse xenograft models has demonstrated no significant systemic toxicity (assessed as more than 10% of body weight loss posttreatment).42 Since earlier preclinical and clinical studies of MEK inhibitors focused on pathological changes largely in the outer retina, we used outer retinal cell line models of photoreceptors and RPE for in vitro toxicity analysis. For our in vivo analysis, we chose a previously described rabbit model. Our in vitro and in vivo studies showed that these 2 bifunctional inhibitors are effective in reducing PI3K and MEK signaling in retinal cells while demonstrating improved retinal toxicity profiles, thus increasing their potential to become successful lead compounds for tumor targeting.
In our in vitro models for photoreceptors and RPE cells, we found that PI3K or MEK inhibition alone induced mild caspase activation and apoptotic cell death. Photoreceptor cells were sensitive to PI3K inhibition and showed little cell death after MEK inhibition. This is consistent with the critical role of active PI3K signaling in inhibiting apoptotic pathways in cells with neuronal lineage.43 In contrast, non-neuronal RPE cells were susceptible to both PI3K and MEK inhibition, although with only mild amount of cell death. However, both cell types showed much more robust caspase activation and significantly more cell death when both pathways were inhibited together using a cocktail combination of PI3K and MEK inhibitors, demonstrating the more potent effect of combination therapy.
Compared with the PI3K/MEK inhibitor cocktail, bifunctional PI3K/MEK inhibitors showed improved toxicity profiles in 661W and ARPE-19 cells. Similarly, both bifunctional PI3K/MEK inhibitors showed favorable in vivo toxicity profiles compared with MEK inhibitor in a rabbit model. There are several possibilities for this improved toxicity profile that is associated with ST-162 and ST-168. First, bifunctional inhibitors may have reduced off-target kinase effects compared with parent compound combinations. This is a subject of ongoing research, but previous studies have demonstrated that one of the bifunctional inhibitors, ST-162, has generated unique modulation of specific genes, which were revealed using transcriptome analysis of melanoma cells.42 The unique gene set was shown to be different than the 1 found in cells exposed to combination therapy using the individual MEK and PI3K inhibitors. These findings revealed specific and unique cellular signaling properties of these compounds, which may be, in part, contributing to the observed reduction in off-target kinase effects.42 Another possibility for this lowered toxicity profile is a decreased tissue distribution. Furthermore, it should be noted that bifunctional inhibitors, ST-162 and ST-168, are less potent (higher IC50 enzymatic inhibitory values) versus parent MEK and PI3K compounds, thus complicating a direct comparison of activity and toxicity across drug concentrations. In fact, data presented in Fig. 1 reveal that significant cell-based inhibition of p-ERK 1/2 and p-AKT occurs at ∼5–10 μM for exposure of ARPE-19 cells to ST-162 and ST-168, whereas complete inhibition of p-ERK 1/2 and p-AKT occurred when these cells were treated with 1 μM of PD0325901 or ZSTK474, respectively, as shown in Fig. 2. However, cell survival toxicity data showed that ZSTK474+PD0325901 had IC50 values of ∼1–5 μM for 661W and ARPE-19 cell lines versus ST-162 and ST-168, which was ∼100 μM or not reached for 661W and ARPE-19 cells, respectively.
Using the same rabbit model as Huang et al., we found similar adverse ocular effects of PD0325901, including retinal vein occlusion, retinal tears, and retinal whitening. In the same preclinical model, we showed that bifunctional inhibitors, ST-162 and ST-168, were better tolerated versus PD0325901 at the doses tested with minimal effect on retina or retinal vasculature. Our findings demonstrate the potential for decreased ocular toxicity and support further preclinical development of bifunctional PI3K/MEK inhibitors for multiple types of aggressive tumors systemically as well as potential intraocular targets, including ocular melanoma and retinoblastoma. In vivo functional and anatomic assessment with electroretinogram (ERG) and optical coherence tomography (OCT) is expected to provide additional information on potential electrophysiological and microstructural changes associated with bifunctional inhibitors, a limitation of the current study. We plan to examine the effects of the bifunctional inhibitors with ERG and OCT in our future studies. Although more research is needed, it is important to note that the development of ST-162 and ST-168 and further analogs open up the potential for intraocular administration of PI3K/MEK inhibitors rather than systemic administration. For example, chemical modifications of the linker can be accomplished to improve aqueous solubility for intraocular formulation and dosing.
The etiology of MEKAR is not fully understood. One previously proposed mechanism includes dysfunctional transmembrane transport of water or chloride ions across the RPE in the presence of MEK inhibition.25 Electrophysiological studies have demonstrated that even a slight alteration in the localization of RPE ion channels can considerably influence fluid movement across the RPE. MEK signaling pathway may regulate the function and localization of volume-regulated chloride channels in RPE.25,44 Similarly, the expression levels of aquaporins, transmembrane water channels found in the RPE, have been shown to exhibit sensitivity to the inhibition of MEK.45 If normal water and ion transport across the RPE is contingent on MEK, inhibition of MEK signaling in the RPE may be one of the underlying mechanisms of MEKAR. Our in vitro and in vivo data suggest that bifunctional inhibitors, ST-162 and ST-168, are better tolerated by the RPE and may show reduced rates of ocular toxicity, including MEKAR, in future clinical trials. Some of the adverse effects of kinase inhibitors may not be seen in in vitro studies or in the preclinical models. Although data presented in this study support the improved safety of ST-162 and ST-168, these bifunctional inhibitors could potentially lead to ocular changes similar to MEKAR, which may only be seen in human trials.
In conclusion, we describe a favorable in vitro and in vivo toxicity profile of 2 novel bifunctional inhibitors of oncogenic PI3K and MEK signaling pathways. The continued development of these lead compounds will identify candidates for preclinical and clinical studies for the treatment of K-Ras-mediated tumors.
Acknowledgments
NIH Grants 5K08EY023982 (CGB), P01CA085878 (BDR), R35CA197701 (BDR) and support were received from the University of Michigan MCubed program (CGB, BDR, GMT).
Author Disclosure Statement
B.D.R. and M.V.D. hold patents on the underlying therapeutic compounds, which were exclusively licensed from the University of Michigan to Sarisa Therapeutics, LLC, a company in which B.D.R has an ownership interest.
References
- 1.Engelman J.A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer. 9:550–562, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Montagut C., and Settleman J. Targeting the RAF–MEK–ERK pathway in cancer therapy. Cancer Lett. 283:125–134, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Castellano E., and Downward J. RAS interaction with PI3K: more than just another effector pathway. Genes Cancer. 2:261–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serra V., Scaltriti M., Prudkin L., et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 30:2547–2557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aksamitiene E., Kiyatkin A., and Kholodenko B.N. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem. Soc. Trans. 40:139–146, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Chandarlapaty S. Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer Discov. 2:311–319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turke A.B., Song Y., Costa C., et al. MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Res. 72:3228–3237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guenther M.K., Graab U., and Fulda S. Synthetic lethal interaction between PI3K/Akt/mTOR and Ras/MEK/ERK pathway inhibition in rhabdomyosarcoma. Cancer Lett. 337:200–209, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Hoeflich K.P., Merchant M., Orr C., et al. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 72:210–219, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Williams T.M., Flecha A.R., Keller P., et al. Co-targeting MAPK and PI3K signaling with concurrent radiotherapy as a strategy for the treatment of pancreatic cancer. Mol. Cancer Ther. 11:1193–1202, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renshaw J., Taylor K.R., Bishop R., et al. Dual blockade of the PI3K/AKT/mTOR (AZD8055) and RAS/MEK/ERK (AZD6244) pathways synergistically inhibits rhabdomyosarcoma cell growth in vitro and in vivo. Clin. Cancer Res. 19:5940–5951, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britten C.D. PI3K and MEK inhibitor combinations: examining the evidence in selected tumor types. Cancer Chemother. Pharmacol. 71:1395–1409, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Stewart A., Thavasu P., de Bono J.S., et al. Titration of signalling output: insights into clinical combinations of MEK and AKT inhibitors. Ann. Oncol. 26:1504–1510, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temraz S., Mukherji D., and Shamseddine A. Dual inhibition of MEK and PI3K pathway in KRAS and BRAF mutated colorectal cancers. Int. J. Mol. Sci. 16:22976–22988, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong D., Okamura M., Yoshimi H., et al. Antiangiogenic effect of ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor. Eur. J. Cancer. 45:857–865, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Kong D., and Yamori T. ZSTK474 is an ATP-competitive inhibitor of class I phosphatidylinositol 3 kinase isoforms. Cancer Sci. 98:1638–1642, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rewcastle G.W., Gamage S.A., Flanagan J.U., et al. Synthesis and biological evaluation of novel analogues of the pan class I phosphatidylinositol 3-kinase (PI3K) inhibitor 2-(difluoromethyl)-1-[4, 6-di (4-morpholinyl)-1, 3, 5-triazin-2-yl]-1 H-benzimidazole (ZSTK474). J. Med. Chem. 54:7105–7126, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Yaguchi S., Fukui Y., Koshimizu I., et al. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J. Natl. Cancer Inst. 98:545–556, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Zhao W., Guo W., Zhou Q., et al. In vitro antimetastatic effect of phosphatidylinositol 3-kinase inhibitor ZSTK474 on prostate cancer PC3 cells. Int. J. Mol. Sci. 14:13577–13591, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Dort M.E., Galbán S., Nino C.A., et al. Structure-guided design and initial studies of a bifunctional MEK/PI3K inhibitor (ST-168). ACS. Med. Chem. Lett. 8:808–813, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LoRusso P.M., Krishnamurthi S.S., Rinehart J.J., et al. Phase I pharmacokinetic and pharmacodynamic study of the oral MAPK/ERK kinase inhibitor PD-0325901 in patients with advanced cancers. Clin. Cancer Res. 16:1924–1937, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Huang W., Yang A.H, Matsumoto D., et al. PD0325901, a mitogen-activated protein kinase kinase inhibitor, produces ocular toxicity in a rabbit animal model of retinal vein occlusion. J. Ocul. Pharmacol. Ther. 25:519–530, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Duncan K.E., Chang L.Y., and Patronas M. MEK inhibitors: a new class of chemotherapeutic agents with ocular toxicity. Eye. 29:1003–1012, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stjepanovic N., Velazquez-Martin J.P., and Bedard P.L. Ocular toxicities of MEK inhibitors and other targeted therapies. Ann. Oncol. 27:998–1005, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Montana C.L., and Apte R.S. MEKanisms of a serous complication. JAMA. Ophthalmol. 135:413–414, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Urner-Bloch U., Urner M., Jaberg-Bentele N., et al. MEK inhibitor-associated retinopathy (MEKAR) in metastatic melanoma: long-term ophthalmic effects. Eur. J. Cancer. 65:130–138, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Weber M.L., Liang M.C., Flaherty K.T., et al. Subretinal fluid associated with MEK inhibitor use in the treatment of systemic cancer. JAMA. Ophthalmol. 134:855–862, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Messersmith W.A., Hidalgo M., Carducci M., et al. Novel targets in solid tumors: MEK inhibitors. Clin. Adv. Hematol. Oncol. 4:831–836, 2006 [PubMed] [Google Scholar]
- 29.Al-Tweigeri T., Nabholtz J.M., and Mackey J.R. Ocular toxicity and cancer chemotherapy: a review. Cancer. 78:1359–1373, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Weinstein I.B., and Joe A.K. Mechanisms of disease: oncogene addiction—a rationale for molecular targeting in cancer therapy. Nat. Clin. Pract. Oncol. 3:448–457, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Grant S. Cotargeting survival signaling pathways in cancer. J. Clin. Invest. 118:3003–3006, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valentino J.D., Li J., Zaytseva Y.Y., et al. Cotargeting the PI3K and RAS pathways for the treatment of neuroendocrine tumors. Clin. Cancer Res. 20:1212–1222, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson A.L., Anderson L.K., Greeley A.D., et al. Co-targeting the MAPK and PI3K/AKT/mTOR pathways in two genetically engineered mouse models of schwann cell tumors reduces tumor grade and multiplicity. Oncotarget. 5:1502–1514, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong H., Sanchez C., Spitzer D., et al. Synergistic effects of concurrent blockade of PI3K and MEK pathways in pancreatic cancer preclinical models. PLoS One. 8:e77243, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heavey S., O'Byrne K.J., and Gately K. Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer Treat. Rev. 40:445–456, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Li Q., Wu J., Zheng H., et al. Discovery of 3-(2-aminoethyl)-5-(3-phenyl-propylidene)- thiazolidine-2, 4-dione as a dual inhibitor of the Raf/MEK/ERK and the PI3K/Akt signaling pathways. Bioorg. Med. Chem. Lett. 20:4526–4530, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Guerrant W., Patil V., Canzoneri J.C., et al. Dual targeting of histone deacetylase and topoisomerase I with novel bifunctional inhibitors. J. Med. Chem. 55:1465–1477, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heffron T.P., Ndubaku C.O., Salphati L., et al. Discovery of clinical development candidate GDC- 0084, a brain penetrant inhibitor of PI3K and mTOR. ACS. Med. Chem. Lett. 7:351–356, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodrik-Outmezguine V.S., Okaniwa M., Yao Z., et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. 534:272–276, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Dort M.E., Galbán S., Wang H., et al. Dual inhibition of allosteric mitogen-activated protein kinase (MEK) and phosphatidylinositol 3-kinase (PI3K) oncogenic targets with a bifunctional inhibitor. Bioorg. Med. Chem. 23:1386–1394, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Dort M.E., Hong H., Wang H., et al. Discovery of bifunctional oncogenic target inhibitors against allosteric mitogen-activated protein kinase (MEK1) and phosphatidylinositol 3- kinase (PI3K). J. Med. Chem. 59:2512–2522, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galbán S., Apfelbaum A.A., Espinoza C., et al. A bifunctional MAPK/PI3K antagonist for inhibition of tumor growth and metastasis. Mol. Cancer Ther. 16:2340–2350, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsui-Pierchala B.A., Encinas M., Milbrandt J., et al. Lipid rafts in neuronal signaling and function. Trends Neurosci. 25:412–417, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Van Dijk E.H.C., Van Herpen C.M.L., Marinkovic M., et al. Serous retinopathy associated with mitogen-activated protein kinase kinase inhibition (binimetinib) for metastatic cutaneous and uveal melanoma. Ophthalmology. 122:1907–1916, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Jiang Q., Cao C., Lu S., et al. MEK/ERK pathway mediates UVB-induced AQP1 downregulation and water permeability impairment in human retinal pigment epithelial cells. Int. J. Mol. Med. 23:771–777, 2009 [DOI] [PubMed] [Google Scholar]