Abstract
Aim:
l-benzyl-3-cetyl-2-methylimidazolium iodide (NH125) can inhibit Staphylococcus aureus growth. We investigated the effects of sub-MIC concentrations of NH125 on S. aureus biofilm and virulence.
Methodology & results:
Three strains of S. aureus were tested. Sub-lethal concentrations of NH125 repressed biofilm formation. At partial sub-MICs, NH125 downregulated the expression of most virulence, while strain-dependent effects were found in the production of α-hemolysin, δ-hemolysin, coagulase and nuclease. In Galleria mellonella model, methicillin-resistant S. aureus pre-exposed to NH125 demonstrated significantly lower killing (p = 0.032 for 1/16 and 1/8 MICs; 0.008 for 1/4 MIC; and 0.001 for 1/2 MIC).
Conclusion:
Sub-MIC concentrations of NH125 inhibited biofilm formation and virulence of S. aureus. These findings provide further support for evaluating the clinical efficacy of NH125 in staphylococcal infection.
Keywords: : NH125, Staphylococcus aureus, sub-MIC, virulence factor
Staphylococcus aureus is a major human pathogen that is associated with serious, deep-seated or systemic infections [1–3]. Treatment of staphylococcal infections is complicated by the development of antibiotic resistance. Moreover, the S. aureus pathogenicity involves multiple virulence factors, such as toxins, enzymes, adhesions, biofilm and gene-expression regulators [4,5]. These factors enable bacteria to adapt to different living environments and are involved in cell surface adhesion, immune evasion and tissue invasion [6,7].
The action of antimicrobial agents on bacterial virulence factor release, especially at suboptimal concentrations, is one of the pivotal aspects to determine their clinical efficacy [8]. A sub-lethal concentration of tigecycline has been demonstrated to reduce the expression of important virulence factors in S. aureus, which may be useful for the treatment of biofilm-mediated infections [7]. Other antibiotics at subinhibitory concentrations, such as tetracycline, erythromycin and quinupristin-dalfopristin, were also found to influence the staphylococcal virulence and biofilm formation [9]. Therefore, the exposure of bacteria to sub-MICs of antimicrobial agents is of great clinical importance as tissue levels of antimicrobial agents vary significantly due to variable blood perfusion of tissues, drug–drug interactions, systemic absorption of topically administered antibiotics, reduction of drug bioavailability or bacterial biofilm development [6].
NH125 (1-Hexadecyl-2-methyl-3-(phenylmethyl)-1H-imidazolium iodide) is an inhibitor of the WalK (a histidine kinase) two-component system (TCS), which is essential in the signal transduction pathway for the cell wall metabolism of notorious pathogens, such as S. aureus [10]. This WalK inhibitor has been demonstrated as a bactericidal agent on methicillin-resistant S. aureus (MRSA) strains [11–14]. Furthermore, we previously reported that NH125 can kill MRSA persisters, a very robust subpopulation within the S. aureus collection of cells that often reside within biofilms or are developed during the biofilm formation process, by inducing membrane permeabilization. Additionally, it can elicit damage to MRSA biofilms [11,15]. Intriguingly, this compound exhibited low toxicity, whereby NH125 did not exhibit significant toxicity to Caenorhabditis elegans at 7.5 μg/ml and did not induce lysis of human red cells at 8 μg/ml [11,15].
TCS may directly or indirectly participate in many bacterial processes, including biofilm formation and virulence [16,17], therefore, making this a plausible target for drug development. Some compounds that inhibit WalK were found to have bactericidal effects on biofilm cells of Staphylococcus epidermidis, indicating that the inhibitors can serve as potential agents against bacterial biofilms [18]. Here, we investigated whether subinhibitory concentrations of TCS inhibitor NH125 could affect biofilm formation and virulence. Also, we gaged the effect of subinhibitory concentrations of NH125 on virulence using Galleria mellonella in vivo model.
Materials & methods
Bacterial strains & antibacterial compound
S. aureus strains MW2 (ATCCBAA-1707), USA300 and Newman were used for this study. The antibacterial compound NH125 was purchased from Sigma-Aldrich (MO, USA) and 5 mg/ml stock solutions of NH125 were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich).
Determination of MICs
MICs of NH125 for S. aureus strains were detected in triplicate using the broth microdilution method, according to standard Clinical and Laboratory Standards Institute (CLSI) protocols [19].
Growth curves
S. aureus strains were cultured at 37°C to an optical density (OD600) of 0.1–0.2 in brain heart infusion broth (BHI, Sigma-Aldrich). Three-hundred microliters of the cultures with different subinhibitory concentrations of NH125 (MIC, 1/2 MIC, 1/4 MIC, 1/8 MIC and 1/16 MIC) were placed into the wells of a 96-well plate (Corning, NY, USA) in three replicates for 24 h [20]. Cultures with 1% DMSO (v/v) served as the control. Bacteria were incubated at 37°C with shaking at 200 r.p.m. A SpectraMax M2 multi-mode microplate reader (Molecular Devices, CA, USA) was used to monitor cell growth at designated time points.
Hemolysis assay
S. aureus strains were grown in BHI broth at 37°C (with shaking at 200 r.p.m.) in the presence of graded subinhibitory concentrations of NH125 (1/2, 1/4, 1/8 and 1/16 MIC) to the postexponential growth phase. Culture supernatants were harvested by centrifugation and filtration. Levels of α-hemolysin (using defibrinated rabbit blood, Rockland Immunochemicals, PA, USA) and δ-hemolysin (using human erythrocytes, Rockland Immunochemicals) were measured in culture supernatants according to the method described by Kim et al. [11]. A 100 μl volume of 4% blood red cells was mixed with 100 μl of supernatant in a 96-well plate, and incubated for 1 h at 37°C. After being centrifuged at 500 × g for 5 min, 100 μl of the clear supernatant was removed to a new microtiter plate, and the OD value was read at 540 nm. Triton-X 100 (0.5%, final concentration) and phosphate-buffered saline (PBS) served as positive and negative controls, respectively. The supernatant of untreated cells (only containing 1% DMSO) was defined as the 100% hemolysis control, and the percent hemolysis was calculated against the control culture. All tests were carried out in triplicate.
Coagulase titer assay
Bacterial culture supernatants were obtained as described above under ‘hemolysis assay’. The supernatants were serially diluted by twofold using BHI broth, and then mixed with iso-volumes (0.5 ml) of BBL lyophilized rabbit plasma (Becton Dickinson, MD, USA) solution. Clotting of the supernatants after incubation at 37°C for 4 h demonstrated the existence of coagulase. The titer was defined as the highest culture supernatant dilution to generate a detectable clot.
Protease activity assay
Proteolytic activity in the supernatants collected as described in the section of hemolysis assay was measured in triplicate [21]. One-hundred microliters of the culture were incubated with 1 ml chromogenic azocasein (1%, Sigma-Aldrich) for 1 h at 37°C. Then, 500 μl of 10% (w/v) trichloroacetic acid (Sigma-Aldrich) was added and incubated for 30 min to cease the reaction. The mixture was centrifuged for 10 min at 10,000 × g, and the OD of the supernatant was measured at 328 nm. The results were reported as the percentage of proteolytic activity in relation to the proteolytic activity of the control.
Nuclease activity assay
Nuclease activity of cultures acquired as depicted above under ‘hemolysis assay’ was detected by using BBL DNase Test Agar with Methyl Green plate (Becton Dickinson). Twenty microliters of supernatants were added to BBL Blank Test Discs (Becton Dickinson). Then, the discs with bacteria supernatants were placed onto DNase Test Agar plates, and incubated at 37°C for 24 h. BHI broth was used as negative control, and the level of nuclease expression was evaluated by a zone diameter around the disc.
Lipase activity assay
Bacteria culture supernatants were prepared and harvested as described above. Lipase activity was measured in triplicate by detecting hydrolysis of 4-nitrophenyl octanoate in the supernatants [22]. Fifty microliters of culture were incubated with 1.2 ml of 4-nitrophenyl octanoate (Sigma-Aldrich) solution at 37°C for 15 min, and the value of OD was measured at 405 nm immediately. The lipase activity was expressed as a percentage of that of the NH125-free culture control.
Biofilm formation assay
Biofilm formation was detected in triplicate using crystal violet (CV) in a 96-well plate format with a slight modification [23]. Bacterial suspension (OD600 = 0.4, 0.5 McFarland standard) was diluted 1:100 in Mueller–Hinton (MH) medium with 2% glucose supplementation (Sigma-Aldrich; MH-G). NH125 dilution was made in MH at graded subinhibitory concentrations (1/2, 1/4, 1/8 and 1/16 MICs). Then, 100 μl of each of bacterial inoculum and the corresponding NH125 dilution were placed into each well of 96-well plates. Inoculated MH-G media with 1% DMSO alone were used as positive control. Plates were cultured statically at 37°C for 48 h. Planktonic bacteria were aspirated and the residual adhesive cells in each well was rinsed three-times with water and stained with 200 μl of 0.3% (w/v) CV (Sigma-Aldrich) for 10 min. The unincorporated CV was washed out and the plates were dried for 3 h at room temperature. The quantity of biofilm in each well was detected by extracting the incorporated dye using 200 μl of 100% ethanol for 30 min and the OD value was determined at 595 nm.
Quantitative reverse transcription polymerase chain reaction
The well-studied S. aureus strain MW2 was selected as a representative strain for assessing the effect of subinhibitory concentrations of NH125 on the expression of virulence genes involved in gene expression regulation, surface protein, polysaccharide intercellular adhesion and hemolysin. In brief, an overnight culture was inoculated into BHI medium at an OD600 = 0.1. Then, bacteria were incubated at 37°C until the culture reached an OD600 of 0.6 and aliquots were subsequently exposed for 3 h to graded subinhibitory concentrations of NH125 [24]. RNA extraction, cDNA synthesis and quantitative reverse transcription (RT)-PCR were carried out as recommended by the manufacturer (Bio-Rad, CA, USA) using the primers listed in Table 1. Cycling conditions were as follows: 95°C for 30 s; 40 cycles at 95°C for 5 s, 55°C for 30 s, and finished with a melt curve analysis from 65 to 95°C. All samples were examined in triplicate, and normalized with respect to the housekeeping gene gyrA expression. Relative gene expression levels were analyzed by the 2-ΔΔCT method described in [24].
Table 1. . Virulence genes and primers used for quantitative RT-PCR detection.
| Gene | Function | Sequence of primer† | Size of product (bp) |
|---|---|---|---|
| hla | α-hemolysin | F: 5′-AATGAATCCTGTCGCTAATGCCGC-3′ | 269 |
| R: 5′-CTGAAGGCCAGGCTAAACCACTTT-3′ | |||
| hld (RNA III) | δ-hemolysin/gene expression regulator RNA III | F: 5′-TAATTAAGGAAGGAGTGATTTCAATG-3′ | 100 |
| R: 5′-TTTTTAGTGAATTTGTTCACTGTGTC-3′ | |||
| agrA | Regulator of gene expression | F: 5′-TGATAATCCTTATGAGGTGCTT-3′ | 164 |
| R: 5′-CACTGTGACTCGTAACGAAAA-3′ | |||
| arlS | Regulator of gene expression | F: 5′-TGGAATACCAATTCCATGATCT-3′ | 103 |
| R: 5′-TGCAATCAAATATGATGTGAAGAA-3′ | |||
| srrA | Regulator of gene expression | F: 5′-AGCATGTGTGGGAGGTATGA-3′ | 118 |
| R: 5′-CCTCTTGGCCATTACTTGCTT-3′ | |||
| saeS | Phosphorylated response regulator | F: 5′-ATCCGAACAACAAGAAAAAACAG-3′ | 110 |
| 5′-TGATTATACCATCACGTAGTCCTTCA-3′ | |||
| yycG | Regulator of gene expression | F: 5′-TACAATCCCTTCATACTAAACTTGTAATTG-3′ | 198 |
| R: 5′-GTGCATTTACGGAGCCCTTTTCGTCATATAC-3′ | |||
| sasG | Surface protein | F: 5′-GGTTTTCAGGTCCTTTTGGAT-3′ | 192 |
| R: 5′-CTGGTGAAGAGCGAGTGAAA-3′ | |||
| spa | Surface protein for bacterial aggregation | F: 5′-GCGCAACACGATGAAGCTCAACAA -3′ | 125 |
| R: 5′-ACGTTAGCACTTTGGCTTGGATCA-3′ | |||
| clfA | Surface protein | F: 5′-CGGTTTTGGACTACTCAGCA-3′ | 151 |
| R: 5′- GCTACTGCCGATAAACTA-3′ | |||
| fnbA | Surface protein | F: 5′-ACTTGATTTTGTGTAGCCTTTTT-3′ | 185 |
| R: 5′-GAAGAAGCACCAAAAGCAGTA-3′ | |||
| fnbB | Surface protein | F: 5′-CGTTATTTGTAGTTGTTTGTGTT-3′ | 118 |
| R: 5′- TGGAATGGGACAAGAAAAAGAA-3′ | |||
| icaA | PIA or PNAG production | F: 5′-AACAGAGGTAAAGCCAACGCACTC-3′ | 85 |
| R: 5′-CGATAGTATCTGCATCCAAGCAC-3′ | |||
| sigB | Regulator of gene expression | F: 5′-TCAGCGGTTAGTTCATCGCTCACT-3′ | 156 |
| R: 5′-GTCCTTTGAACGGAAGTTTGAAGCC-3′ | |||
| codY | Regulator of gene expression | F: 5′-AAAGAAGCGCGCGATAAAGCTG-3′ | 120 |
| R: 5′-TGCGATTAATAGGCCTTCCGTACC-3′ | |||
| sarA | Regulator of gene expression | F: 5′-CCTCGCAACTGATAATCCTTATG-3′ | 173 |
| R: 5′-ACGAATTTCACTGCCTAATTTGA-3′ | |||
| spoVG | Modulator of gene expression | F: 5′-TGTTCGTTGCAATGCCAAGT-3′ | 51 |
| R: 5′-TGTCGCGGAATTCACCATC-3′ | |||
| rot | Transcriptional regulator | F: 5′-AAGAGCGTCCTGTTGACGAT-3′ | 126 |
| R: 5′-TTTGCATTGCTGTTGCTCTA-3′ | |||
| gyrA | DNA gyrase A subunit/internal standard gene | F: 5′-CATTGCCAGATGTTCGTGAC-3′ | 117 |
| R: 5′-CACCAACGATACGTGCTGAT-3′ | |||
†The sequence of primers was designed based on the genome sequence of Staphylococcus aureus strain MW2 (Accession: BA000033.2).
bp: Base pair; PIA: Polysaccharide intercellular adhesion; PNAG: Polymeric N-acetylglucosamine.
The G. mellonella–S. aureus infection model
The inoculum of S. aureus MW2 cells was treated with the graded sub-MICs of NH125 according to the method described above under ‘Quantitative RT-PCR’. Bacteria were collected by centrifugation at 4°C, rinsed two-times with PBS containing the corresponding sub-MIC of compound, then resuspended in the corresponding PBS-sub-MIC NH125 solution at the density of 7.5 × 109 colony-forming unit/ml, respectively. Galleriamellonella larvae were randomly divided into seven groups (ten per group) for the infection experiments. Briefly, four groups of larvae were injected with 10 μl of treated bacteria suspension in PBS containing various sub-MICs of NH125, respectively. The remaining three groups of larvae used as controls were injected with 10 μl sterile PBS, 10 μl of 1 μg/ml of NH125 (1/2 MIC) and 10 μl of untreated bacterial suspension (7.5 × 109 colony-forming unit/ml), respectively. All G. mellonella groups were placed at 37°C and mortality was evaluated daily. Galleria mellonella experiments were carried out twice on separate occasions.
Statistical analysis
Statistical analyses were performed using SPSS 20 software. Data comparisons for the production of hemolysis and exoenzymes, the formation of biofilm and the expression of virulence genes were analyzed by Student’s t-test. Data regarding the influence of sub-MICs NH125 on the bacterial growth were made by one-way analysis of variance (ANOVA). Differences in G. mellonella survival rates were analyzed using the Kaplan–Meier method and survival curves were determined using log-rank test. A p-value <0.05 was considered significant.
Results & discussion
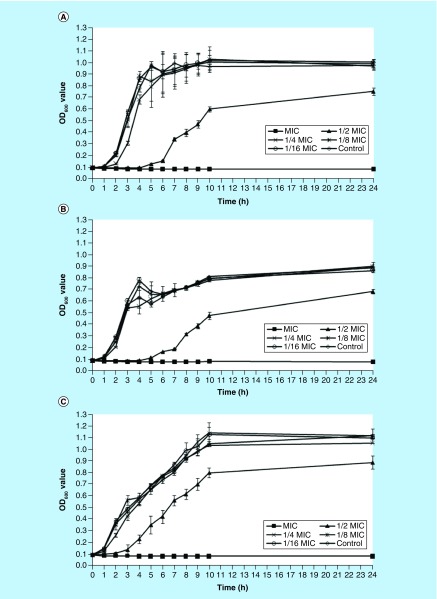
Influence of NH125 on S. aureus growth. The MIC of NH125 against three S. aureus strains (MW2, USA300 and Newman) was found to be 2 μg/ml for each of the tested strains. To assess the influence of sub-lethal concentrations of NH125 on the production of bacterial virulence factors, we initially investigated the effect on bacterial growth. As shown in Figure 1, the bacterial growth rates of all three S. aureus strains studied were significantly reduced by the addition of 1 μg/ml of NH125 in the media, a concentration that represents 1/2 MIC (p < 0.001). Li et al. [25] found a similar growth pattern of S. aureus treated by chlorogenic acid, which could significantly inhibit the growth at concentration of MIC and 1/2 MIC. In addition, the growth of S. aureus was reported to be remarkably attenuated by 1/2 MIC of antimicrobial agents, such as linezolid [8], eugenol [26] and licochalcone A [21]. However, when three S. aureus strains grew in the presence of 1/4, 1/8 or 1/16 MIC of NH125, growth was similar to that of the control. Thus, growth was diminished at 1/2 MIC, but was restored at lower concentrations of NH125.
Figure 1. . Growth curves of Staphylococcus aureus strains in the presence of graded subinhibitory concentrations of NH125.
These curves represent the mean values of three repeat testing results. Error bars represent standard deviation. (A) Strain MW2; (B) strain USA300; (C) strain Newman; control strain treated by 1% DMSO.
OD: Optical density.
NH125 inhibited the biofilm formation. Since sub-lethal concentrations only had modest effects on the overall growth rate of the bacteria, we sought to explore how the introduction of the compound to the bacteria could affect virulence traits that are employed during the infection process. Kim et al. already demonstrated the efficacy of NH125 against persister cells, another part of the sedentary cell population that form a subpopulation harbored within biofilms, where it permeabilized a normally tough membrane [11]. Thus, we speculated that NH125 might be effective against surrounding cells that comprise the biofilm. Our initial investigation focused on the effects to biofilm formation.
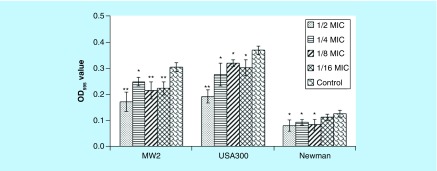
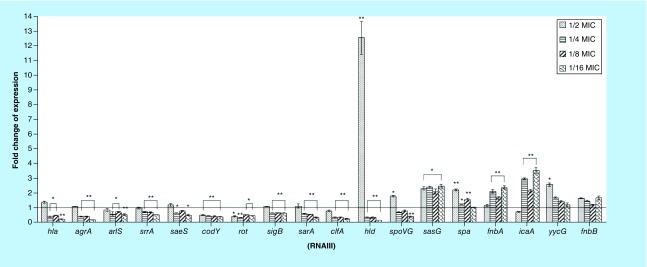
Biofilm formation is extremely important in staphylococcal pathogenesis and it has been associated with seeding chronic infections and establishing recurrent colonization [18]. We found that biofilm formation of the strain MW2 was markedly inhibited by sub-MIC concentrations of NH125 (p = 0.005 at 1/2 MIC, 0.011 at 1/4 MIC and 0.009 at 1/8 and 1/16 MICs). Similar reduction was noted when we examined the S. aureus strain USA300 (p < 0.001 at 1/2 MIC; p = 0.027 at 1/4 MIC, 0.016 at 1/8 MIC and 0.031 at 1/16 MICs; Figure 2). In the case of the Newman strain, NH125 reduced the development of biofilm at 1/8 MIC (p = 0.042), 1/4 MIC (p = 0.031) and 1/2 MIC (p = 0.032). Notably, the obvious decrease in biofilm formation, caused by 1/2 MIC of NH125, might partially be attributed to the inhibition on bacterial growth (Figure 1). More generally, inhibition of biofilm formation is ascribed to repression of proteins necessary for the biofilm, such as cell surface adhesions and exopolysaccharide synthases [27,28], or failure to maintain an adequate supportive structure [29]. Therefore, we further investigated the transcription levels of six biofilm-associated genes using RT-PCR. ClfA (encoding clumping factor A, ClfA) was significantly downregulated by 3.7-, 2.7- and 2.8-fold (p = 0.003 at 1/16 MIC, 0.007 at 1/8 MIC and 0.008 at 1/4 MIC; Figure 3). However, the expression levels of other biofilm-associated genes were increased after exposure to graded sub-MICs NH125. More specifically, sasG (encoding surface protein G) and spa (encoding protein A) were significantly upregulated in S. aureus MW2 cells treated with subinhibitory NH125 concentrations. The transcription levels of icaA (encoding intercellular adhesion A) were enhanced by 3.5-fold (p = 0.001), 2.2-fold (p = 0.004) and 3.0-fold (p = 0.001), when exposed to 1/16, 1/8 and 1/4 MIC of NH125, respectively. The expression levels of fnbA (encoding fibronectin binding protein A) were highly induced at the lower sub-MICs of NH125, while this compound had no prominent influence on fnbB (fibronectin binding protein B) expression. As described above, only clfA among the investigated genes was significantly repressed by all suboptimal concentrations of NH125, indicating that its downregulation could be a major factor to attenuate biofilm formation. Notably, ClfA is a fibrinogen-binding protein anchored to the S. aureus cell wall, and also plays an important role in binding of S. aureus in adhesion to both polyethylene and polyvinyl surfaces [30,31]. Accordingly, we reason that NH125 may interfere with cell wall metabolism and biofilm formation by at least partial inhibiting clfA expression so as to exert its antibiofilm effects.
Figure 2. . Influence of graded subinhibitory concentrations of NH125 on the biofilms formation of Staphylococcus aureus MW2, USA300 and Newman.
The data are presented as mean ± standard deviation (three independent experiments). Control strain treated by 1% DMSO.
*p < 0.05 and **p < 0.01, compared with the results of the corresponding control.
OD: Optical density.
Figure 3. . Influence of graded subinhibitory concentrations of NH125 on the virulence genes expression of Staphylococcus aureus MW2.
The bar graph is a mean of three independent experiments. Error bars represent standard standard deviation. Control strain treated by 1% DMSO.
*p < 0.05 and **p < 0.01, compared with the results of the corresponding control.
Effects of NH125 on hemolysin and hemolysin gene. In S. aureus, TCS is involved in cell viability, biofilm formation and virulence [17]. Hemolysin, one of the main staphylococcal virulence factors, is a pore-forming protein with cytolytic, hemolytic and toxic characteristics that disintegrates erythrocytes and other mammalian cells [32,33]. We evaluated the effects of subinhibitory concentrations of NH125 on the production of α-hemolysin and δ-hemolysin by MRSA strains MW2, USA300 and Newman. The production of hemolysin by S. aureus MW2 cells was reduced by sub-MICs of NH125 in a dose-dependent manner (Table 2). Specifically, NH125 significantly reduced α- and δ-hemolysin within the MW2 strain, when exposed to all the sub-MIC concentrations. On the contrary, NH125 significantly increased δ-hemolysin in the USA300 strain at 1/4 MIC (p = 0.027) and 1/8 MIC (p = 0.035), while the α-hemolysin production kept stable at all tested concentrations of NH125. For the Newman strain, NH125 promoted the δ-hemolytic activities at 1/8 MIC (p = 0.001) and 1/16 (p < 0.001) MIC, and the α-hemolytic activity only at 1/4 MIC (p = 0.004; Table 2). Taken together, these results demonstrated that sub-MICs of NH125 affected the production of hemolysins by S. aureus in a strain-dependent manner.
Table 2. . Hemolytic and enzymatic activities of Staphylococcus aureus culture supernatants dealt with graded subinhibitory concentrations of NH125.
| Toxin/enzyme | Strain MW2 | Strain USA300 | Strain Newman | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/2 MIC | 1/4 MIC | 1/8 MIC | 1/16 MIC | Control | 1/2 MIC | 1/4 MIC | 1/8 MIC | 1/16 MIC | Control | 1/2 MIC | 1/4 MIC | 1/8 MIC | 1/16 MIC | Control | |
| α-hemolysin† | 48.8 ± 0.3# | 55.9 ± 18.4¶ | 72.3 ± 7.3¶ | 64.4 ± 19.8¶ | 100 | 94.4 ± 7.8 | 98.9 ± 2.1 | 104.7 ± 3.9 | 106.2 ± 1.8 | 100 | 98.8 ± 3.2 | 115.9 ± 4.1# | 102.3 ± 3.2 | 101.5 ± 3.7 | 100 |
| δ-hemolysin† | 22.4 ± 5.6# | 39.2 ± 5.7# | 50.7 ± 12.5# | 146.4 ± 32.5 | 100 | 120.7 ± 19.5 | 156.6 ± 27.8¶ | 145.2 ± 24.0¶ | 103.2 ± 55.3 | 100 | 121.1 ± 9.1 | 130.7 ± 18.9 | 293.1 ± 28.1# | 263.7 ± 18.0# | 100 |
| Coagulase titer‡ | 1:1 | 1:1 | 1:1 | 1:2 | 1:4 | 1:16 | 1:16 | 1: 32 | 1: 32 | 1:256 | 1:512 | 1:2048 | 1: 512 | 1: 512 | 1:256 |
| Proteolytic activity† | 96.2 ± 4.7 | 97.7 ± 6.2 | 98.0 ± 5.8 | 95.6 ± 5.8 | 100 | 97.8 ± 3.6 | 98.2 ± 5.8 | 99.7 ± 4.6 | 97.3 ± 3.2 | 100 | 102.0 ± 3.2 | 99.5 ± 4.9 | 101.5 ± 4.3 | 101.0 ± 3.3 | 100 |
| Nuclease activity§ | 10.0 ± 0.9 | 10.0 ± 0.5 | 12.0 ± 0.5# | 12.0 ± 0.9# | 9.0 | 10.0 ± 0.5 | 10.0 ± 0.5 | 10.0 ± 0.9 | 12.0 ± 0.5# | 9.0 | 11.0 ± 0.5 | 11.0 ± 0.9 | 11.0 ± 0.5 | 11.0 ± 0.5 | 10.0 |
| Lipase activity† | 81.9 ± 9.1 | 88.5 ± 4.5 | 86.4 ± 4.2 | 77.2 ± 13.6 | 100 | 93.3 ± 5.5 | 102.2 ± 5.3 | 99.7 ± 3.1 | 114.4 ± 12.0 | 100 | 102.4 ± 0.3 | 97.6 ± 3.4 | 101.0 ± 6.2 | 100.8 ± 2.5 | 100 |
†The data were expressed as percentage. The compound-free culture supernatants were used as the 100% control. Values were the means ± standard deviation of findings of three repeat experiments.
‡The titer is the highest dilution of sample causing coagulation.
§The data indicated the zone size (mm).
¶p < 0.05.
#p < 0.01, compared with the results of the corresponding control. Control strain treated with 1% DMSO.
To determine whether the reduced α-hemolysin and δ-hemolysin production in the presence of various subinhibitory concentrations of NH125 was due to the diminished transcription of hla (α-hemolysin) and hld (δ-hemolysin, encoded by RNA III), we performed real-time RT-PCR to determine the relative expression levels of the investigated genes in S. aureus MW2 after treatment with NH125. We found that the transcription of hla was downregulated by 4.2-fold (p = 0.003), 2.0-fold (p = 0.035) and 2.5-fold (p = 0.016) at 1/16, 1/8 and 1/4 MIC of NH125, respectively, where the transcription of hld was inhibited by 5.8-fold (p < 0.001), 2.9-fold (p = 0.002) and 2.8-fold (p = 0.003) (Figure 3). Notably, treatment with NH125 led to a significant increase by 12.6-fold in the expression of hld. We speculated that NH125 might decrease the hemolysins by controlling other regulatory elements, such as global regulators, which were investigated in the series of experiments detailed below.
NH125 reduced coagulase production. Previous studies demonstrated that the subinhibitory concentrations of compounds with little or no influence on cell growth, could substantially affect the expression of bacterial virulence factors, such as hemolysin, coagulase, protease, nuclease and lipase [24–26,34]. Thus, we also studied the coagulase production and found that the graded subinhibitory concentrations of NH125 altered the production of coagulase in a strain- and dose- dependent manner (Table 2). More specifically, the coagulase titer in S. aureus strain MW2 was reduced by two- and foufolds at 1/8 and 1/16 MIC, whereas the increasing subinhibitory concentrations of NH125 led to a decrease of coagulase production in S. aureus USA300. As for the S. aureus strain Newman, however, the coagulase activities treated with NH125 at 1/2, 1/4, 1/8 and 1/16 MIC were two-, eight-, two- and twofold greater than that of the control, respectively. Interestingly, a strain-dependent difference in the production of coagulase has been previously reported, and Blevins et al. explained that this strain-dependent behavior might be associated with the mutations or deletions of some important regulatory elements [35].
Influence of NH125 on activities of protease, nuclease and lipase. Protease is another crucial virulence factor, which is correlated with the biofilm development in S. aureus [36]. Thus, we detected the activities of S. aureus extracellular enzymes, such as protease, lipase and nuclease, which are the critical virulence factors. As shown on Table 2, NH125 did not significantly affect the total activity of protease in all tested strains. Accordingly, we surmise that the decreased biofilm formation is not related to the protease-mediated destabilization of the biofilm.
In addition, nuclease and lipase are important for bacterial evasion of host response [37]. Our finding showed that sub-MICs of NH125 had no striking influence on the total activity of lipase by the strains studied. The nuclease activity in S. aureus strain Newman was quite stable after treated with grade sub-MIC of NH125, while the increased nuclease activities were observed in S. aureus MW2 at 1/8 MIC (p = 0.002) and 1/16 MIC (p = 0.007), and USA300 at 1/16 MIC (p = 0.007). This change may involve TCS-associated genes and global regulators, which control the expression of many virulence factors [7,25].
Effects of NH125 on TCS-associated gene and global regulator expression. The subinhibitory effects of NH125 are not just restricted to biofilm formation. The compound has more broad scope effects resulting in the altered transcription of other factors that culminate to affect virulence. subinhibitory concentrations of antibiotics may disturb the expression of modulatory determinants in S. aureus [34,38]. As depicted in Figure 3, following exposure NH125 at concentrations ranging from 1/16 to 1/4 MIC, the transcription levels of TCS-associated genes agrA (accessory gene regulator A), arlS and srrA (staphylococcal respiratory response A) in S. aureus MW2 were suppressed by 5.1-, 2.5- and 2.4-fold (p < 0.001 for all three concentrations), 1.8-fold (p = 0.003), 1.4-fold (p = 0.013) and 1.7-fold (p = 0.029), and 1.8-fold (p = 0.001), 1.4-fold (p = 0.004) and 1.4-fold (p = 0.007), respectively. The levels of expression of saeS (encoding S. aureus exoprotein expression S) were decreased by 1.5-fold (p = 0.042) and 1.9-fold (p = 0.014) at 1/4 and 1/16 MIC of NH125, respectively, whereas yycG (also called WalK) showed upregulation by 2.6-folds (p = 0.014) following the addition of 1/2 MIC NH125.
Among the tested global gene regulators, the expression levels of codY and rot (repressor of toxin) were significantly suppressed by all sub-MICs of NH125 (p < 0.05; Figure 3). The levels of transcription of sigB (alternative sigma factor B) and sarA (staphylococcus accessory regulator A) were remarkably decreased by the addition of all subinhibitory concentrations of NH125 but 1/2 MIC, respectively. The expression of spoVG (the stage V sporulation protein G) gene was also impacted by NH125 in a specific manner (promoted by 1.8-fold [p = 0.015] at 1/2 MIC, but reduced by 2.4-fold [p = 0.005] at 1/16 MIC).
Overall, 11 TCS genes and global regulatory genes, which are associated with the regulation of the expression of genes involving bacteria cell density sensing, interconnecting metabolism and virulence, showed dramatic alteration of expression in response to NH125 [14,22,39–45]. The transcription of the genes agrA, saeS, arlS, srrA, sarA, sigB, codY and rot was markedly decreased at different subinhibitory concentrations, while NH125 at different sub-MICs had the opposite impact on the expression of spoVG and hld (RNAIII). The expression of agrA and sarA was obviously downregulated at lower concentrations of NH125 and this finding is in accordance with reports that S. aureus strains expressing agr/sarA at low levels demonstrate increased capacity to form biofilm [46–48]. Also, it should be noted that NH125, as a YycG (WalK) repressor, strikingly enhanced the expression level of yycG at 1/2 MIC, indicating that NH125 inhibits the kinase activity of WalK and regulates kinase expression. Apparently, NH125 may also indirectly modulate the expression of walK by affecting the regulatory factors and NH125 is a nonspecific inhibitor that forms aggregates that bind to multiple proteins [49]. In this context, our finding that NH125 affected the expression of many virulence factors and their regulators may further provide evidence to support that the mechanism might involve multiple regulators or regulatory pathways. Future studies should focus on exploring further the impact of this compound on regulatory pathways that alter the expression of virulence genes.
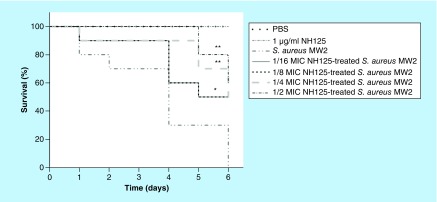
In vivo efficacy of NH125 against S. aureus virulence. The effects of subinhibitory NH125 on virulence traits were ultimately evaluated using the in vivo infection model G. mellonella, a host that has been successfully utilized to assess the virulence of bacterial pathogens [50,51]. To investigate the capacity of NH125 against virulence factors in vivo, the G. mellonella infection model were adopted to evaluate the effect of subinhibitory concentration of NH125 on S. aureus virulence. Herein, each larvae was infected with S. aureus MW2 cells that were pretreated with graded sub-lethal NH125. As shown in Figure 4, NH125 at the concentrations of 1/2 MIC (p = 0.001) and 1/4 MIC (p = 0.008) could remarkably prolong the survival of the infected larvae. Compared with the group infected by untreated bacteria, the survival rates on day 6 were increased by 50% when infected larvae were injected with 1/16 and 1/8 MICs of NH125 (p = 0.032). Therefore, sequential exposure of S. aureus MW2 to sub-MIC of NH125 could attenuate its virulence, corresponding to our conclusion in vitro. The elevated survival rate of infected Galleria further confirms the excellent performance of sub-lethal NH125, which provides important sight into combating the S. aureus.
Figure 4. . Survival curves for Galleria mellonella larvae inoculated with sub-MIC NH125-treated Staphylococcus aureus MW2.
Phosphate-buffered saline and 1 μg/ml (1/2 MIC) of NH125 were injected alone as the control groups. n = 10/group and the experiments were done in duplicate.
*p < 0.05 and **p < 0.01, were considered as with a significant difference, compared with untreated bacteria-infected larvae.
PBS: Phosphate-buffered saline; S. aureus: Staphylococcus aureus.
Conclusion
NH125 could significantly reduce the formation of MRSA biofilms, and this was at least partially due to the reduction of clfA expression. In the presence of graded sub-MICs of NH125, a strain-dependent and concentration-dependent effect was found in the productions of the ubiquitous virulence factors, including α-hemolysin, δ-hemolysin, coagulase and nuclease. Accordingly, some virulence-related genes: agrA, saeS, arlS, srrA, sarA, sigB and codY were found to be repressed by the subinhibitory NH125, whereas the transcription levels of spa, icaA, fnbA, sasG and yycG were increased. Further, the subinhibitory concentrations of NH125 could reduce the virulence of S. aureus MW2 in vivo. Strains of S. aureus secrete a wide list of enzymes and cytotoxins, including hemolysins, nucleases, proteases, lipases and collagenase, while some strains produce one or more additional exoproteins like enterotoxins. Screening for additional virulence factors is beyond the scope of this work. However, future studies should evaluate the effect of NH125 (or other similar compounds) to other virulence factors.
Future perspective
Staphylococcus aureus forms biofilms and produces a wide variety of virulence factors, such as hemolysins, nucleases, proteases and lipases, which contribute to its ability to colonize and cause disease. Moreover, biofilm formation and the expression of these virulence factors hinder our ability to combat staphylococcal infections. Accordingly, the effect of antimicrobial agents on these factors has become the major focus in the study of antimicrobial agents. Herein, we studied the effects of sub-MICs NH125 on the biofilm formation and the ubiquitous and important virulence factors of S. aureus, such as hemolysins, nucleases, proteases, lipases and collagenase. Our results demonstrated that subinhibitory concentrations of NH125 not only inhibited the biofilm formation of S. aureus, but also attenuated the virulence in vitro and in vivo. Future works should evaluate the effect of NH125 (or other similar compounds) to other virulence factors and focus on exploring further the impact of this compound on regulatory pathways to explain why sub-lethal NH125 could alter the expression of certain virulence genes. Additionally, investigation with murine models should be conducted to determine whether NH125 has the therapeutic effects for the treatment of biofilm-mediated infections.
Summary points.
NH125, a WalK two-component system inhibitor, could inhibit biofilm formation of Staphylococcus aureus.
In the presence of graded sub-MICs of NH125, a strain-dependent effect was found in the productions of α-hemolysin, δ-hemolysin, coagulase and nuclease.
Sub-MICs of NH125 had no effect on the total activity of protease and lipase.
At sub-MICs, NH125 repressed the expression of most tested virulence genes, including part of two-component system and global regulatory regulators.
Using the alternative model host Galleria mellonella, we confirmed that NH125 reduces the virulence of S. aureus at sub-MICs in vivo.
Footnotes
Financial & competing interests disclosure
This study was supported by NIH grant P01 AI083214 to E Mylonakis. Q Liu is supported by Shanghai General Hospital Characteristic Discipline Construction Fund and Shanghai Jiao Tong University KC Wong Medical Fellowship Fund. Z Zheng is supported by the China Scholarship Council (CSC) through Chinese Government Graduate Student Overseas Study Program. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Foster TJ. Immune evasion by staphylococci. Nat. Rev. Microbiol. 2005;3(12):948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 2.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015;28(3):603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin. Infect. Dis. 2014;58(Suppl. 1):S10–S19. doi: 10.1093/cid/cit613. [DOI] [PubMed] [Google Scholar]
- 4.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009;7(9):629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus . Clin. Microbiol. Rev. 2000;13(1):16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddadin RN, Saleh S, Al-Adham IS, Buultjens TE, Collier PJ. The effect of subminimal inhibitory concentrations of antibiotics on virulence factors expressed by Staphylococcus aureus biofilms. J. Appl. Microbiol. 2010;108(4):1281–1291. doi: 10.1111/j.1365-2672.2009.04529.x. [DOI] [PubMed] [Google Scholar]; •• Demonstrates that sub-MICs of certain antibiotics interfere with the expression of virulence factors of Staphylococcus aureus biofilms.
- 7.Smith K, Gould KA, Ramage G, Gemmell CG, Hinds J, Lang S. Influence of tigecycline on expression of virulence factors in biofilm-associated cells of methicillin-resistant Staphylococcus aureus . Antimicrob. Agents Chemother. 2010;54(1):380–387. doi: 10.1128/AAC.00155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Provides an evidence that sublethal concentration of tigecycline disturbs the expression of virulence factors in methicillin-resistant S. aureus (MRSA) biofilms.
- 8.Bernardo K, Pakulat N, Fleer S, et al. Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob. Agents Chemother. 2004;48(2):546–555. doi: 10.1128/AAC.48.2.546-555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rachid S, Ohlsen K, Witte W, Hacker J, Ziebuhr W. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis . Antimicrob. Agents Chemother. 2000;44(12):3357–3363. doi: 10.1128/aac.44.12.3357-3363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada A, Igarashi M, Okajima T, et al. Walkmycin B targets WalK (YycG), a histidine kinase essential for bacterial cell growth. J. Antibiot. 2010;63(2):89–94. doi: 10.1038/ja.2009.128. [DOI] [PubMed] [Google Scholar]
- 11.Kim W, Fricke N, Conery AL, et al. NH125 kills methicillin-resistant Staphylococcus aureus persisters by lipid bilayer disruption. Future Med. Chem. 2016;8(3):257–269. doi: 10.4155/fmc.15.189. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates that NH125 kills MRSA persisters at low concentration, and has low toxicity to Caenorhabditis elegans or human cells.
- 12.Yamamoto K, Kitayama T, Ishida N, et al. Identification and characterization of a potent antibacterial agent, NH125 against drug-resistant bacteria. Biosci. Biotechnol. Biochem. 2000;64(4):919–923. doi: 10.1271/bbb.64.919. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto K, Kitayama T, Minagawa S, et al. Antibacterial agents that inhibit histidine protein kinase YycG of Bacillus subtilis . Biosci. Biotechnol. Biochem. 2001;65(10):2306–2310. doi: 10.1271/bbb.65.2306. [DOI] [PubMed] [Google Scholar]
- 14.Dubrac S, Boneca IG, Poupel O, Msadek T. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus . J. Bacteriol. 2007;189(22):8257–8269. doi: 10.1128/JB.00645-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim W, Conery AL, Rajamuthiah R, Fuchs BB, Ausubel FM, Mylonakis E. Identification of an antimicrobial agent effective against methicillin-resistant Staphylococcus aureus persisters using a fluorescence-based screening strategy. PLoS ONE. 2015;10(6):e0127640. doi: 10.1371/journal.pone.0127640. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The authors identify that NH125 is effective against MRSA persisters using a fluorescence-based strategy.
- 16.Krell T, Lacal J, Busch A, Silva-Jimenez H, Guazzaroni ME, Ramos JL. Bacterial sensor kinases: diversity in the recognition of environmental signals. Annu. Rev. Microbiol. 2010;64:539–559. doi: 10.1146/annurev.micro.112408.134054. [DOI] [PubMed] [Google Scholar]
- 17.Gotoh Y, Eguchi Y, Watanabe T, Okamoto S, Doi A, Utsumi R. Two-component signal transduction as potential drug targets in pathogenic bacteria. Current Opin. Microbiol. 2010;13(2):232–239. doi: 10.1016/j.mib.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Qin Z, Zhang J, Xu B, et al. Structure-based discovery of inhibitors of the YycG histidine kinase: new chemical leads to combat Staphylococcus epidermidis infections. BMC Microbiol. 2006;6(1):96. doi: 10.1186/1471-2180-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CLSI. Clinical and Laboratory Standards Institute. PA, USA: 2014. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement, document M100-S24. [Google Scholar]
- 20.Harinen RR, Lampinen J, Raitio M, Kytöniemi V. Turbidometric growth curve measurements and liquid evaporation studies with a microplate photometer. https://tools.thermofisher.com/content/sfs/brochures/MultiskanFC-Turbidometry-Evaporation.pdf
- 21.Qiu J, Jiang Y, Xia L, et al. Subinhibitory concentrations of licochalcone A decrease alpha-toxin production in both methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Lett. Appl. Microbiol. 2010;50(2):223–229. doi: 10.1111/j.1472-765X.2009.02783.x. [DOI] [PubMed] [Google Scholar]
- 22.Schulthess B, Bloes DA, Francois P, et al. The sigmaB-dependent yabJ-spoVG operon is involved in the regulation of extracellular nuclease, lipase, and protease expression in Staphylococcus aureus . J. Bacteriol. 2011;193(18):4954–4962. doi: 10.1128/JB.05362-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazaro-Diez M, Remuzgo-Martinez S, Rodriguez-Mirones C, et al. Effects of subinhibitory concentrations of ceftaroline on methicillin-resistant Staphylococcus aureus (MRSA) biofilms. PLoS ONE. 2016;11(1):e0147569. doi: 10.1371/journal.pone.0147569. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates that ceftaroline affects the expression of virulence factors in MRSA biofilms at sub-MICs.
- 24.Mitchell G, Lafrance M, Boulanger S, et al. Tomatidine acts in synergy with aminoglycoside antibiotics against multiresistant Staphylococcus aureus and prevents virulence gene expression. J. Antimicrob. Chemother. 2012;67(3):559–568. doi: 10.1093/jac/dkr510. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Qiao M, Guo Y, Wang X, Xu Y, Xia X. Effect of subinhibitory concentrations of chlorogenic acid on reducing the virulence factor production by Staphylococcus aureus . Foodborne Pathog. Dis. 2014;11(9):677–683. doi: 10.1089/fpd.2013.1731. [DOI] [PubMed] [Google Scholar]
- 26.Qiu J, Feng H, Lu J, et al. Eugenol reduces the expression of virulence-related exoproteins in Staphylococcus aureus . Appl. Environ. Microbiol. 2010;76(17):5846–5851. doi: 10.1128/AEM.00704-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saising J, Dube L, Ziebandt AK, Voravuthikunchai SP, Nega M, Gotz F. Activity of gallidermin on Staphylococcus aureus and Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2012;56(11):5804–5810. doi: 10.1128/AAC.01296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Gara JP. Ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus . FEMS Microbiol. Lett. 2007;270(2):179–188. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 29.Ren D, Zuo R, Gonzalez Barrios AF, et al. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl. Environ. Microbiol. 2005;71(7):4022–4034. doi: 10.1128/AEM.71.7.4022-4034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 31.Josefsson E, Hartford O, O'Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 2001;184(12):1572–1580. doi: 10.1086/324430. [DOI] [PubMed] [Google Scholar]
- 32.Otto M. Staphylococcus aureus toxins. Curr. Opin. Microbiol. 2014;17:32–37. doi: 10.1016/j.mib.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verdon J, Girardin N, Lacombe C, Berjeaud JM, Hechard Y. Delta-hemolysin an update on a membrane-interacting peptide. Peptides. 2009;30(4):817–823. doi: 10.1016/j.peptides.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Herbert S, Barry P, Novick RP. Subinhibitory clindamycin differentially inhibits transcription of exoprotein genes in Staphylococcus aureus . Infect. Immun. 2001;69(5):2996–3003. doi: 10.1128/IAI.69.5.2996-3003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blevins JS, Beenken KE, Elasri MO, Hurlburt BK, Smeltzer MS. Strain-dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus . Infect. Immun. 2002;70(2):470–480. doi: 10.1128/IAI.70.2.470-480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lister JL, Horswill AR. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front. Cell Infect. Microbiol. 2014;4:178. doi: 10.3389/fcimb.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Böttcher T, Sieber SA. Structurally refined beta-lactones as potent inhibitors of devastating bacterial virulence factors. Chembiochem. 2009;10(4):663–666. doi: 10.1002/cbic.200800743. [DOI] [PubMed] [Google Scholar]
- 38.Kuroda H, Kuroda M, Cui L, Hiramatsu K. Subinhibitory concentrations of beta-lactam induce haemolytic activity in Staphylococcus aureus through the SaeRS two-component system. FEMS Microbiol. Lett. 2007;268(1):98–105. doi: 10.1111/j.1574-6968.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 39.Le KY, Otto M. Quorum-sensing regulation in staphylococci-an overview. Front. Microbiol. 2015;6:1174. doi: 10.3389/fmicb.2015.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discusses the important role of the quorum-sensing system Agr in staphylococcal infection by regulating the expression of a wide variety of virulence genes.
- 40.Chevalier C, Boisset S, Romilly C, et al. Staphylococcus aureus RNAIII binds to two distant regions of coa mRNA to arrest translation and promote mRNA degradation. PLoS Pathog. 2010;6(3):e1000809. doi: 10.1371/journal.ppat.1000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q, Yeo WS, Bae T. The SaeRS two-component system of Staphylococcus aureus . Genes (Basel) 2016;7(10):1–20. [Google Scholar]; • This review explores the major role of two-component system SaeRS in regulating the expression of virulence factors of S. aureus.
- 42.Wu Y, Wang J, Xu T, et al. The two-component signal transduction system ArlRS regulates Staphylococcus epidermidis biofilm formation in an ica-dependent manner. PLoS ONE. 2012;7(7):e40041. doi: 10.1371/journal.pone.0040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oogai Y, Kawada-Matsuo M, Komatsuzawa H. Staphylococcus aureus SrrAB affects susceptibility to hydrogen peroxide and co-existence with Streptococcus sanguinis . PLoS ONE. 2016;11(7):e0159768. doi: 10.1371/journal.pone.0159768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andrey DO, Renzoni A, Monod A, Lew DP, Cheung AL, Kelley WL. Control of the Staphylococcus aureus toxic shock tst promoter by the global regulator SarA. J. Bacteriol. 2010;192(22):6077–6085. doi: 10.1128/JB.00146-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stenz L, Francois P, Whiteson K, Wolz C, Linder P, Schrenzel J. The CodY pleiotropic repressor controls virulence in gram-positive pathogens. FEMS Immunol. Med. Microbiol. 2011;62(2):123–139. doi: 10.1111/j.1574-695X.2011.00812.x. [DOI] [PubMed] [Google Scholar]; • Reviews the regulatory mechanisms of virulence expression in main Gram-positive bacteria by global regulator CodY.
- 46.Beenken KE, Blevins JS, Smeltzer MS. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 2003;71(7):4206–4211. doi: 10.1128/IAI.71.7.4206-4211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beenken KE, Dunman PM, McAleese F, et al. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 2004;186(14):4665–4684. doi: 10.1128/JB.186.14.4665-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vuong C, Saenz HL, Gotz F, Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus . J. Infect. Dis. 2000;182(6):1688–1693. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- 49.Devkota AK, Tavares CD, Warthaka M, et al. Investigating the kinetic mechanism of inhibition of elongation factor 2 kinase by NH125: evidence of a common in vitro artifact. Biochemistry. 2012;51(10):2100–2112. doi: 10.1021/bi201787p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai CJ, Loh JM, Proft T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence. 2016;7(3):214–229. doi: 10.1080/21505594.2015.1135289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Upadhyay A, Venkitanarayanan K. In vivo efficacy of trans-cinnamaldehyde, carvacrol, and thymol in attenuating Listeria monocytogenes infection in a Galleria mellonella model. J. Nat. Med. 2016;70(3):667–672. doi: 10.1007/s11418-016-0990-4. [DOI] [PubMed] [Google Scholar]