Abstract
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute myocardial infarction of emerging significance, which occurs predominantly in young women without coronary artery disease risk factors. Valsalva-like activities such as coughing have been identified as potential triggers for the development of SCAD. We report a case of SCAD in a man in whom the only identifiable predisposing factor was excessive coughing. He presented with atypical chest pain. Troponin I peaked at 29 ng/mL, and ECG showed no evidence of ischaemic changes. He underwent cardiac catheterisation via the radial approach, which revealed a linear second obtuse marginal dissection. He was managed conservatively with medical therapy with a good outcome.
Keywords: cardiovascular medicine, interventional cardiology
Background
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute myocardial infarction (MI), reported to account for 0.1%–4% of all acute coronary syndrome (ACS) pathologies,1 2 with approximately 80% of cases occurring in young women without a history of heart disease or coronary artery disease (CAD) risk factors,1 3 and less than 30% of cases occurring in men.4 Historically, it was first described by Pretty.5
SCAD has emerged as a significant cause of acute MI, necessitating a Scientific Statement by the American Heart Association (AHA) in February, 2018. Misdiagnosis, underdiagnosis and management as atherosclerotic ACS has proven to result in detrimental outcomes, as treatment of SCAD is different from that of atherosclerotic MI.6
Well-recognised predisposing factors for SCAD include fibromuscular dysplasia (FMD), postpartum status (18%), multiparity (≥4 births), connective tissue disorders, systemic inflammatory arteriopathies and hormonal therapy.3 6 7 In a recent study, it was found that more than 50% of cases were associated, possibly on a background of an existing arteriopathy, with a precipitating cardiocirculatory stressor such as intense exercise or emotional stress, labour and delivery, intense Valsalva-type activities, recreational drug use, and aggressive hormonal therapy.7
The majority of cases reported in the literature are of young and middle-aged women. We report a case of SCAD in a middle-aged man who presented with chest pain after prolonged intense coughing episodes.
Case presentation
A 55-year-old Caucasian man with a medical history of hypertension, chronic obstructive pulmonary disease (COPD), presented to the emergency department with a sudden onset of 10/10 substernal chest pain which he developed during violent coughing episodes which had started a week prior, shortly after inhaling engine oil fumes in an enclosed cab of a payloader. He had been treated with an albuterol inhaler and a 5-day course of oral prednisone at a walk-in clinic, due to concern for a COPD exacerbation precipitated by acute bronchitis, but however experienced no improvement in his symptoms. He had no known history of CAD. His medications included lisinopril. His brother had an MI at the age of 40. He denied cocaine use. He had a 40 pack-year smoking history, however had quit 15 years prior.
Physical examination was notable for an oxygen saturation of 79% on room air. He was haemodynamically stable with a blood pressure of 130/90 mm Hg and heart rate of 80 bpm; body mass index was 32 kg/m2. Bilateral rhonchi were noted on lung auscultation; he had a regular heart rate and rhythm with no murmurs, rubs or gallops.
Investigations
Laboratory investigations revealed an initial negative troponin, with ECG showing no evidence of ischaemic changes. Chest CT angiogram ruled out pulmonary embolism and aortic dissection. Troponin I peaked at 29 ng/mL about 24 hours into his admission, subsequent ECG obtained showed non-specific T wave flattening in the lateral leads. Non-ST-elevation MI was diagnosed. Transthoracic echocardiogram reported normal left ventricular wall motion and contractility, ejection fraction 65%.
Differential diagnosis
Our patient was a middle-aged male, with identifiable cardiovascular risk factors including obesity and hypertension. With elevated troponin and non-specific ECG changes, our initial working diagnosis was non-ST-elevation MI in the setting of atherosclerotic CAD, for which reason he was treated with aspirin, carvedilol, atorvastatin, clopidogrel and enoxaparin, prior to cardiac catheterisation.
It was only at coronary angiography that the diagnosis of SCAD was made, which is the case in a vast majority of cases. A high index of suspicion is required to avoid missing a diagnosis of SCAD.
Treatment
Aspirin 325 mg, clopidogrel 300 mg and subcutaneous enoxaparin at 1 mg/kg two times per day were initiated. Cardiac catheterisation with coronary angiography was significant for a filling defect throughout the anterior branch of the second obtuse marginal branch of the left circumflex artery, consistent with a linear dissection with intramural thrombus (figure 1). The right coronary artery (RCA) appeared dilated. Rheumatological screening was negative. The patient was managed conservatively with aspirin 81 mg daily, clopidogrel 75 mg daily, atorvastatin 40 mg daily, lisinopril 20 mg daily and metoprolol tartrate 12.5 mg two times per day.
Figure 1.
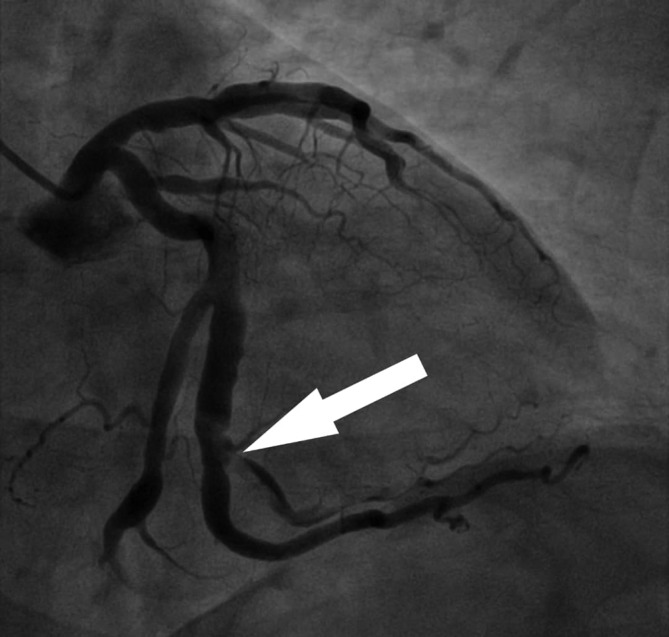
Coronary angiogram: right anterior oblique caudal view showing a linear dissection throughout the anterior branch of the second obtuse marginal artery.
Outcome and follow-up
Our patient was discharged the following day after his cardiac catheterisation, and was seen in follow-up at the outpatient cardiology clinic 2 weeks later, at which time he was doing very well. He did not have repeat coronary angiography to document ‘healing’ of his SCAD lesion. He, however, continues to do well 1 year and 3 months after diagnosis was made and therapy was instituted.
Discussion
Due to the recent heightened awareness of SCAD among healthcare providers and affected patients, use of invasive angiography and advanced intravascular diagnostic imaging techniques, it is suggested that the incidence of SCAD may be higher than previously thought, especially in young women.6 In men, less than 30% of cases have been reported,4 with the principal precipitant in men identified as extreme physical activity.8
The most obvious predisposing factor in our patient was excessive, vigorous coughing. This was also reported by Sivam et al describing a middle-aged woman with intense coughing spasms in the setting of a cystic fibrosis exacerbation.9
We did note, interestingly, on coronary angiography that he had a dilated RCA, raising suspicion for FMD, which has been shown to have a significant association with SCAD.7 10 11 In their study of the angiographic and intravascular manifestations of coronary FMD, Saw et al observed segmental dilatation and ectasia in 56% of patients with SCAD.12 Though CT angiogram of his abdomen and pelvis showed normal-appearing renal and iliac arteries, carotid and cerebral angiograms were not obtained; we wonder whether the finding of a dilated RCA is suggestive of coronary FMD. Also, the course of oral prednisone he received prior to admission could have been an additional predisposing factor. A study reported the occurrence of SCAD as early as the fourth day of initiation of glucocorticoid therapy.13
SCAD is characterised by the spontaneous formation of an intramural haematoma in the wall of a coronary artery, theorised to be either due to an intimal tear resulting in blood from the endoluminal space entering the intimal space, creating a false lumen or a spontaneous rupture of the vasa vasorum within the wall of the coronary arteries, resulting in the creation of a haematoma-filled false lumen.14 The intramural haematoma occupying the dissection compresses the true lumen, leads to coronary insufficiency and MI.6 14 The exact underlying pathophysiology of SCAD is not completely understood. Multiple studies have postulated a complex interplay between an underlying arteriopathy and a potential trigger.7 9 10 15 16 The underlying pathophysiological mechanism by which cough results in SCAD has not been clearly described. Saw et al suggest that the transient significant increase in intrathoracic pressure precipitates SCAD events. We propose that in addition to this, the significant increase in systolic pressure, diastolic pressure and arterial pulse pressure with coughing17 may potentially increase shear stress on the wall of the coronary artery and also predispose to SCAD.
The left anterior descending artery, which is the most commonly affected artery, is involved in 32%–46% of cases. The left circumflex, ramus and obtuse marginal branches are involved in 15%–45% of cases, while the RCA and acute marginal, posterior descending, and posterolateral branches are involved in 10%–39% of cases. The left main artery is the culprit in up to 4% of cases.6 In our patient, the anterior branch of the second obtuse marginal artery was involved.
Percutaneous coronary angiography is the first-line diagnostic imaging technique for SCAD.18 One limitation of this imaging method is its inability to depict the arterial wall, given that it provides two-dimensional images.6 Intravascular ultrasound and optical coherence tomography (OCT) are intracoronary imaging modalities which provide detailed visualisation of the arterial wall, useful in the confirmation of the presence of SCAD.6 18 These techniques, however, are not widely available in all catheterisation laboratories. We did not employ these two novel diagnostic tools in our patient’s case, as we do not have them readily available at our cardiac catheterisation laboratory and diagnosis was accurately made with coronary angiography. Per the Saw angiographic SCAD classification,19 our patient had a type 1 (pathognomonic angiographic appearance with contrast staining of the arterial wall), which is reported to occur in 29.1% of SCAD cases.19
The management of SCAD differs considerably from that of atherosclerotic ACS. While early invasive revascularisation of culprit lesions is advocated for in atherosclerotic ACS,20 evidence from observational studies21–23 has led to the recommendation that haemodynamically stable patients with SCAD with preserved coronary blood flow and no involvement of high-risk anatomy be managed conservatively with antiplatelet therapy, a statin, a beta-blocker, an ACE inhibitor and antianginal therapy, as percutaneous coronary intervention with stenting has been shown to be associated with an increased risk of complications including dissection extension, intramural haematoma propagation resulting in worsening vessel obstruction,24 and subacute and late stent strut mal-apposition resulting from natural resorption of the intramural haematoma with time.25
An observational study of 164 patients with SCAD of which 131 were initially managed conservatively, revealed that only 3 patients required revascularisation for SCAD extension, and all 79 who had repeat angiogram ≥26 days later had spontaneous healing.7
Per the 2018 AHA SCAD Scientific Statement, in haemodynamically unstable patients or in patients with ongoing ischaemia, percutaneous coronary intervention can be considered if feasible, or urgent coronary artery bypass graft (CABG) can be pursued. Clinically stable patients with high-risk anatomy (left main or severe proximal 2-vessel dissection) can be considered for CABG.6 Our patient was conservatively managed with medical therapy given his haemodynamic stability and the location of his culprit lesion, and this resulted in a good outcome.
As stated by the AHA, future large-scale prospective and epidemiological studies are needed to address the considerable gaps in knowledge about the epidemiology, pathogenesis, diagnosis and treatment of SCAD.
Patient’s perspective.
‘I was mostly just scared. I was quite nervous.’
Learning points.
Consider spontaneous coronary artery dissection (SCAD) as a differential in young and middle-aged persons presenting with acute myocardial infarction, including the non-classic male population.
Intense Valsalva-like activities such as coughing or retching are also predisposing factors for SCAD.
In haemodynamically stable patients with SCAD with preserved coronary blood flow, conservative medical therapy has good outcomes.
Footnotes
Contributors: JAY prepared the first draft of this article. HH revised it critically and approved the final version for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mortensen KH, Thuesen L, Kristensen IB, et al. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv 2009;74:710–7. 10.1002/ccd.22115 [DOI] [PubMed] [Google Scholar]
- 2.Nishiguchi T, Tanaka A, Ozaki Y, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2016;5:263–70. 10.1177/2048872613504310 [DOI] [PubMed] [Google Scholar]
- 3.Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579–88. 10.1161/CIRCULATIONAHA.112.105718 [DOI] [PubMed] [Google Scholar]
- 4.Vanzetto G, Berger-Coz E, Barone-Rochette G, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg 2009;35:250–4. 10.1016/j.ejcts.2008.10.023 [DOI] [PubMed] [Google Scholar]
- 5.Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42. BMJ 1931:667.20776438 [Google Scholar]
- 6.Hayes SN, Kim ESH, Saw J, et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation 2018;137:e523–e557. 10.1161/CIR.0000000000000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saw J, Aymong E, Sedlak T, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645–55. 10.1161/CIRCINTERVENTIONS.114.001760 [DOI] [PubMed] [Google Scholar]
- 8.Tweet MS, Eleid MF, Best PJM, et al. Spontaneous Coronary Artery Dissection: Revascularization Versus Conservative Therapy. Circulation 2014;7:777–86. 10.1161/CIRCINTERVENTIONS.114.001659 [DOI] [PubMed] [Google Scholar]
- 9.Sivam S, Yozghatlian V, Dentice R, et al. Spontaneous coronary artery dissection associated with coughing. J Cyst Fibros 2014;13:235–7. 10.1016/j.jcf.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Saw J, Ricci D, Starovoytov A, et al. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013;6:44–52. 10.1016/j.jcin.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 11.Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015;115:1672–7. 10.1016/j.amjcard.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 12.Saw J, Bezerra H, Gornik HL, et al. Angiographic and intracoronary manifestations of coronary fibromuscular dysplasia. Circulation 2016;133:1548–59. 10.1161/CIRCULATIONAHA.115.020282 [DOI] [PubMed] [Google Scholar]
- 13.Keir ML, Dehghani P. Corticosteroids and spontaneous coronary artery dissection: a new predisposing factor? Can J Cardiol 2016;32:395.e7–395.e8. 395 10.1016/j.cjca.2015.06.021 [DOI] [PubMed] [Google Scholar]
- 14.Yip A, Saw J. Spontaneous coronary artery dissection-A review. Cardiovasc Diagn Ther 2015;5:37–48. 10.3978/j.issn.2223-3652.2015.01.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velusamy M, Fisherkeller M, Keenan ME, et al. Spontaneous coronary artery dissection in a young woman precipitated by retching. J Invasive Cardiol 2002;14:198–201. [PubMed] [Google Scholar]
- 16.Lempereur M, Grewal J, Saw J. Spontaneous coronary artery dissection associated with β-HCG injections and fibromuscular dysplasia. Can J Cardiol 2014;30:464.e461–463. 10.1016/j.cjca.2013.11.030 [DOI] [PubMed] [Google Scholar]
- 17.Kern MJ, Gudipati C, Tatineni S, et al. Effect of abruptly increased intrathoracic pressure on coronary blood flow velocity in patients. Am Heart J 1990;119:863–70. 10.1016/S0002-8703(05)80324-2 [DOI] [PubMed] [Google Scholar]
- 18.Yeo I, Feldman DN, Kim LK. Spontaneous coronary artery dissection: diagnosis and management. Curr Treat Options Cardiovasc Med 2018;20:27 10.1007/s11936-018-0622-2 [DOI] [PubMed] [Google Scholar]
- 19.Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2014;84:1115–22. 10.1002/ccd.25293 [DOI] [PubMed] [Google Scholar]
- 20.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol 2016;67:1235–50. 10.1016/j.jacc.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 21.Lettieri C, Zavalloni D, Rossini R, et al. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol 2015;116:66–73. 10.1016/j.amjcard.2015.03.039 [DOI] [PubMed] [Google Scholar]
- 22.Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014;7:777–86. 10.1161/CIRCINTERVENTIONS.114.001659 [DOI] [PubMed] [Google Scholar]
- 23.Rogowski S, Maeder MT, Weilenmann D, et al. Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv 2017;89:59–68. 10.1002/ccd.26383 [DOI] [PubMed] [Google Scholar]
- 24.Alfonso F, Bastante T, García-Guimaraes M, et al. Spontaneous coronary artery dissection: new insights into diagnosis and treatment. Coron Artery Dis 2016;27:696–706. 10.1097/MCA.0000000000000412 [DOI] [PubMed] [Google Scholar]
- 25.Lempereur M, Fung A, Saw J. Stent mal-apposition with resorption of intramural hematoma with spontaneous coronary artery dissection. Cardiovasc Diagn Ther 2015;5:323–9. 10.3978/j.issn.2223-3652.2015.04.05 [DOI] [PMC free article] [PubMed] [Google Scholar]


