SUMMARY
Aim:
To report the survival outcomes of patients with multiple brain metastases treated with whole-brain radiotherapy.
Patients & methods:
From 2004 to 2012, patients with brain metastases treated with whole-brain radiotherapy were included. Overall survival (OS) was calculated from the start of radiation treatment. Univariate and multivariate proportional hazard model of OS was conducted. Generalized R2 statistic (ranged from 0 to 1) was calculated to determine the association with the outcome.
Results:
Nine-hundred-ninety-one patients were included. The actuarial median OS time was 2.7 months (95% CI: 2.5–2.9). Patients of older age (>65 years), lower Karnofsky performance status, not postoperative and patients with gastrointestinal, genitourinary or lung as opposed to breast cancer were more likely to have a shorter survival.
Conclusion:
Short median survival of 2.7 months may reflect poorer prognosis of patients referred due to large amount of referrals for radiosurgery. Prognostic factors for survival should be considered at consultation.
KEYWORDS : brain metastases, overall survival, prognostic factors, radiotherapy, whole-brain radiotherapy
Summary points .
Brain metastases occur in 40–60% of advanced cancer patients and are commonly treated with whole-brain radiotherapy (WBRT), radiosurgery, neurosurgery or conservative management with steroids.
Patients with multiple brain metastases are usually treated with WBRT and steroids, however response to radiation can take up to 1 month.
Because 20–30% of patients can die before benefiting from treatment, proper matching of patient to treatment is required.
The purpose of this study was to determine the overall survival of patients with multiple brain metastases treated with WBRT and prognostic factors for survival in this patient population.
In total, 991 patients from 2004 to 2012 referred to Rapid Response Radiotherapy Program receiving WBRT were included: average age was 66 years, median Karnofsky performance status was 60 and the most common primary cancer sites were lung and breast.
The actuarial median survival time was 2.7 months (95% CI: 2.5–2.9 months).
Older age, not postoperative status, lower Karnofsky performance status and gastrointestinal, genitourinary or lung as opposed to breast primary cancer were prognostic factors for shorter survival.
Median survival was lower than other reported series, possibly due to a shift in preference for radiosurgery leaving patients with poorer prognosis being referred for WBRT.
In patients with shorter survival, supportive care should be considered to prevent side effects of WBRT; prognostic factors can be used to determine which patients are likely to have shorter survival.
Brain metastases occur in approximately 40–60% of advanced cancer patients. Certain primary cancer sites such as breast, colorectal, lung, melanoma and kidney are the most common cancers to metastasize to the brain [1]. Patients with brain metastases usually have a limited survival with an observed median survival of 3–6 months following whole-brain radiation treatment (WBRT) [2]. The treatment is usually aimed toward improving health-related quality of life through palliation of symptoms [3].
Due to the presence of the blood–brain barrier, which limits entry or concentration of treatment in the blood of the brain, chemotherapy is not usually considered as a treatment option for patients with brain metastases [4]. Surgical resection can be considered for patients with solitary brain metastasis and good performance status (PS). Radiosurgery may be considered for selected patients with small metastatic disease to brain. Selected patients with single brain metastasis have improved survival when treated with radiosurgery or surgical resection with or without WBRT [5] as compared with WBRT alone. Conversely, in patients with poorer PS, supportive care such as symptom management with medication only may be offered [6]. Patients with multiple brain metastases are typically managed with WBRT and dexamethasone [6]. However, response to WBRT can take up to 1 month during which approximately 20–30% of patients can die due to their cancer before having benefited from the treatment [7].
Due to the limited survival and aims of treatment, proper matching of patients to treatment is of upmost importance; as such the determination of prognostic factors is useful. Other studies investigating prognostic factors in brain metastases patients have identified PS, extracranial disease, age, primary cancer site and extent of metastases as having prognostic significance [8–11]. The purpose of this study was to determine the overall survival (OS) of patients with multiple brain metastases treated with WBRT. Prognostic factors for survival in our patient population were also reported.
Patients & methods
From 2004 to 2012, all patients with multiple brain metastases referred to the Rapid Response Radiotherapy Program at the Odette Cancer Centre in Sunnybrook Health Sciences Centre in Toronto, ON, Canada, receiving WBRT were included in a prospective database. The Rapid Response Radiotherapy Program began in 1996 with the objective of reducing wait times for radiation treatment in patients with reduced life expectancy. These patients had CT and/or MRI documented brain metastases. Common dose fractionations used were 20 Gy in five fractions or 30 Gy in ten fractions at the discretion of the radiation oncologist. General demographic information (age, gender, Karnofsky performance status [KPS], Palliative Performance Scale [PPS], primary cancer site) and radiotherapy information (dose fractionation, date of first treatment, previous radiotherapy, postoperative) were captured. Date of death for patients was collected in January 2014 through searching the Cancer Care Ontario updated database and obituaries.
• Statistics
OS (in months) was calculated from first treatment date to date of death in patients who died, or censored to the last follow-up date in patients who were alive. Kaplan–Meier survival curve was conducted in all patients with 95% CI, and in subgroup of patients with significant covariates. Log-rank test was also performed for subgroup comparisons.
To identify the prognostic factors of OS, univariate and multivariate Cox Proportional Hazard model was conducted using all demographic and clinical details. The OS time (in months) was considered as the outcome variable. Independent variables included age, KPS, PPS, gender, patients coming from hospital or home, by ambulance or not, previous radiotherapy, postoperative, primary cancer site and dose/fractions. Older age at baseline was categorized as age ≥65 years. KPS and PPS were also broken into categories 0–40, 50–70 and 80–100. Poor KPS and PPS were categorized into <70. Patients were either coming from hospital or home, by ambulance or not. Hazard ratio (HR) and its 95% CI were calculated for each factor. The generalized R2 statistic (ranged from 0 to 1) was calculated based on the likelihood ratio statistic (LRT) for testing the global null hypothesis [12] using the formula of R2 = 1 − exp(−LRT/n), where LRT was the difference between the log-likelihood for the null model and the fitted model with covariate, and n was number of observations used. The higher the R2 value is, the stronger the association with the outcome will be. All analyses were conducted using Statistical Analysis Software (SAS version 9.3), p-value <0.05 was considered as statistical significance.
Results
There were 991 patients treated with WBRT from 2004 to 2012 (Table 1). The average age of patients was 66 years of age with a range of 26–93 years. Five hundred and forty nine (55%) patients were female. The median KPS and PPS were 60 with a range of 10–100. The most common primary cancer were lung cancer (594 patients: 60%) and breast cancer (171 patients: 17%). Most patients came from home (64%). Of the included patients, 887 (90%) of patients were treated with 20 Gy in five fractions while 51 (5%) were treated with 30 Gy in 10 fractions; the remaining patients were treated with other varying dosages.
Table 1. . Patient's demographics (n = 991).
| Demographic | Summary |
| Age (years): | |
| – n | 991 |
| – Mean ± SD | 65.6 ± 11.5 |
| – Median (range) | 66 (26–93) |
| Gender: | |
| – Female | 549 (55.40%) |
| – Male | 442 (44.60%) |
| KPS: | |
| – n | 666 |
| – Mean ± SD | 62.6 ± 17.7 |
| – Median (range) | 60 (10–100) |
| KPS categories: | |
| – 0–40 | 113 (16.97%) |
| – 50–70 | 374 (56.16%) |
| – 80–100 | 179 (26.88%) |
| KPS <80: | |
| – No | 179 (26.88%) |
| – Yes | 487 (73.12%) |
| KPS <70: | |
| – No | 318 (47.75%) |
| – Yes | 348 (52.25%) |
| PPS: | |
| – n | 468 |
| – Mean ± SD | 61.7 ± 17.8 |
| – Median (range) | 60 (10–100) |
| PPS categories: | |
| – 0–40 | 87 (18.59%) |
| – 50–70 | 267 (57.05%) |
| – 80–100 | 114 (24.36%) |
| PPS <80: | |
| – No | 114 (24.36%) |
| – Yes | 354 (75.64%) |
| PPS <70: | |
| – No | 216 (46.15%) |
| – Yes | 252 (53.85%) |
| Primary cancer site: | |
| – Lung | 594 (59.94%) |
| – Breast | 171 (17.26%) |
| – GI | 61 (6.16%) |
| – GU | 61 (6.16%) |
| – Other | 54 (5.45%) |
| – Unknown | 50 (5.05%) |
| Patients coming from: | |
| – Home | 623 (63.70%) |
| – Hospital | 355 (36.30%) |
| Dose/fraction: | |
| – 2000 cGy/5 | 887 (89.69%) |
| – 3000 cGy/10 | 51 (5.16%) |
| – Other | 51 (5.16%) |
| Ambulance: | |
| – No | 658 (67.14%) |
| – Yes | 322 (32.86%) |
| Previous radiotherapy: | |
| – No | 772 (78.86%) |
| – Yes | 207 (21.14%) |
| Postoperative: | |
| – No | 954 (96.27%) |
| – Yes | 37 (3.73%) |
| Alive status: | |
| – Alive | 56 (5.65%) |
| – Dead | 935 (94.35%) |
| Duration of follow-up (months): | |
| – n | 991 |
| – Mean ± SD | 5.59 ± 8.64 |
| – Median (range) | 2.6 (0.1–94) |
KPS: Karnofsky performance status; PPS: Palliative performance scale; SD: Standard deviation.
Overall survival
At time of analysis, 935 patients (94%) were confirmed deceased while the remaining was censored at their last follow-up date at the cancer center. The range of OS was between 0.1 and 94 months. The actuarial median survival time was 2.7 months with 95% CI of 2.5–2.9 months (Figure 1). Eighty-two percent of patients were alive at 1 month, 62% of patients were alive at 2 months while only 46% of patients were alive at 3 months follow-up.
Figure 1. . Kaplan–Meier overall survival curve for all patients (n = 991) with 95% CI; median overall survival was 2.7 months (95% CI: 2.5–2.9).
Prognostic factors
From the univariate proportional hazard model of OS (Table 2), all demographic and clinical factors were significantly related to OS except whether the patient had received previous radiotherapy. Through this analysis, patients who were more likely to have a shortened survival prognosis were: older patients (≥65 years old) (p < 0.0001), patients with lower KPS and/or PPS (both <70 and categories) (p < 0.0001), male (p = 0.02) and patients coming from hospital or by ambulance (p < 0.0001). Patients with gastrointestinal (p < 0.0001), genitourinary (p = 0.04) or lung cancer (p = 0.0006) were more likely to have shorter survival prognosis than patients with primary breast cancer.
Table 2. . Univariate cox proportional hazard model of overall survival using collected demographic and medical factors (n = 991).
| Demographic and medical factors | p-value | Hazard ratio | 95% CI of HR | Generalized R2 (%) |
|---|---|---|---|---|
| Age at baseline (years) | <0.0001† | 1.023 | 1.017–1.029 | 5.66 |
| Age ≥65 years | <0.0001† | 1.511 | 1.327–1.722 | 3.83 |
| KPS score | <0.0001† | 0.973 | 0.968–0.978 | 15.89 |
| KPS categories: | <0.0001† | 13.77 | ||
| – 0–40 vs 80–100 | <0.0001 | 3.930 | 3.045–5.073 | |
| – 50–70 vs 80–100 | <0.0001 | 1.576 | 1.307–1.899 | |
| – 0–40 vs 50–70 | <0.0001 | 2.494 | 1.994–3.121 | |
| – KPS <70 | <0.0001† | 1.968 | 1.677–2.311 | 9.71 |
| PPS score | <0.0001† | 0.977 | 0.972–0.983 | 12.44 |
| PPS categories: | <0.0001† | 10.60 | ||
| – 0–40 vs 80–100 | <0.0001 | 3.159 | 2.343–4.258 | |
| – 50–70 vs 80–100 | 0.0007 | 1.493 | 1.185–1.881 | |
| – 0–40 vs 50–70 | <0.0001 | 2.116 | 1.639–2.731 | |
| – PPS <70 | <0.0001† | 1.977 | 1.630–2.398 | 9.75 |
| Gender | 0.0136† | 1.177 | 1.034–1.339 | 0.61 |
| Patients coming from (hospital, home) | <0.0001† | 1.653 | 1.444–1.892 | 5.01 |
| Ambulance | <0.0001† | 1.782 | 1.551–2.047 | 6.13 |
| Previous radiotherapy | 0.4600 | 0.942 | 0.804–1.103 | 0.06 |
| Postoperative | 0.0003† | 0.526 | 0.371–0.746 | 1.59 |
| Primary cancer site: | 0.0006† | 2.22 | ||
| – GI vs breast | <0.0001 | 1.840 | 1.366–2.479 | |
| – GU vs breast | 0.0363 | 1.381 | 1.021–1.869 | |
| – Lung vs breast | 0.0006 | 1.365 | 1.142–1.632 | |
| – Other vs breast | 0.0607 | 1.345 | 0.987–1.834 | |
| – Unknown vs breast | 0.0017 | 1.681 | 1.215–2.326 | |
| Dose/fraction (cGy/fractions): | 0.0319† | 0.78 | ||
| – 2000/5 vs other | 0.9975 | 1.000 | 0.754–1.328 | |
| – 3000/10 vs other | 0.0543 | 0.680 | 0.459–1.007 | |
| – 2000/5 vs 3000/10 | 0.0088 | 1.471 | 1.102–1.963 | |
†p < 0.05.
KPS: Karnofsky performance status; PPS: Palliative performance scale.
Patients treated with 20 Gy in 5 fractions were also found to have a significantly shorter survival prognosis than patients receiving 30 Gy in 10 fractions (p = 0.009). Age ≥65, KPS and PPS categories (0–40, 50–70 and 80–100) were selected to put into the multivariate analysis due to the higher generalized R2 values (3.8% for age ≥65, 13.8% and 10.6% for KPS and PPS categories).
Through the backward stepwise selection procedure in the multivariate analysis to determine the most predictive factors of survival, four demographic and clinical factors remained significant (Table 3). These factors were age, KPS, postoperative and primary cancer site. Patients older than 65 years (p = 0.0002; HR = 1.36), or without postoperative (p = 0.0005; HR = 0.51) were more likely to have a shorter survival prognosis. Median OS for patients <65 years was 3.6 months (95% CI: 3.2–4.3) and 2.2 months (95% CI: 2.0–2.5) in patients ≥65 years. For patients that have undergone neurosurgery, the median OS was 7.4 months (95% CI: 3.2–12.7) and 2.7 months (95% CI: 2.4–2.9) in patients who were not postoperative.
Table 3. . Multivariate cox proportional hazard model of overall survival using demographic and medical factors (n = 666).
| Demographic and medical factors | Coefficient† | p-value | Hazard ratio | 95% CI of hazard ratio |
|---|---|---|---|---|
| Age ≥65 years | 0.309 | 0.0002‡ | 1.362 | 1.155–1.607 |
| KPS categories: | – | <0.0001‡ | – | – |
| – 0–40 vs 80–100 | 1.326 | <0.0001‡ | 3.767 | 2.899–4.894 |
| – 50–70 vs 80–100 | 0.494 | <0.0001‡ | 1.638 | 1.356–1.979 |
| – 0–40 vs 50–70 | – | <0.0001‡ | 2.299 | 1.821–2.887 |
| Postoperative | -0.675 | 0.0005‡ | 0.509 | 0.347–0.747 |
| Primary cancer site: | – | 0.0025‡ | – | – |
| – GI vs breast | 0.728 | 0.0001‡ | 2.070 | 1.426–3.005 |
| – GU vs breast | 0.506 | 0.0081‡ | 1.659 | 1.141–2.413 |
| – Lung vs breast | 0.304 | 0.0087‡ | 1.356 | 1.080–1.702 |
| – Other vs breast | 0.188 | 0.3216 | 1.207 | 0.832–1.751 |
| – Unknown vs breast | 0.483 | 0.0339‡ | 1.620 | 1.037–2.531 |
Generalized R2 = 20.21%
†In the Cox proportional hazard regression analysis, a covariate with a positive coefficient is associated with worse survival prognosis while covariate with a negative coefficient is associated with improved survival prognosis.
‡p < 0.05.
GI: Gastrointestinal; GU: Genitourinary; KPS: Karnofsky performance status.
Patients with 0–40 or 50–70 KPS have higher risk of death comparing to those with 80–100 KPS (HR = 3.77 or 1.64, respectively). Median OS was 1.2 months (95% CI: 0.9–1.5) in patients with KPS 0–40, 2.7 months (95% CI: 2.3–3.0) in patients with KPS 50–70 and 6.2 months (95% CI: 4.6–7.7) in patients with KPS 80–100. Compared to patients with breast cancer, there were shorter survival times in patients with GI, GU or lung (HR = 2.07, 1.66, 1.36, respectively). Median OS was 4.3 months (95% CI: 3.1–5.4) in patients with breast cancer, 2.2 months (95% CI: 1.4–2.8) in patients with GI cancer, 2.1 months (95% CI: 1.6–2.8) in patients with GU cancer, 2.7 months (95% CI: 2.4–3.1) in patients with lung cancer, 2.6 months (95% CI: 1.6–4.8) in patients with other cancer sites and 2.1 months (95% CI: 1.2–2.7) in patients with unknown cancer site. Kaplan–Meier curves for patients in different subgroups for age, KPS, postoperative status and primary cancer sites were displayed as Figures 2–5. Gender, dose/fractions and other factors were not significant any more in the multivariate analysis.
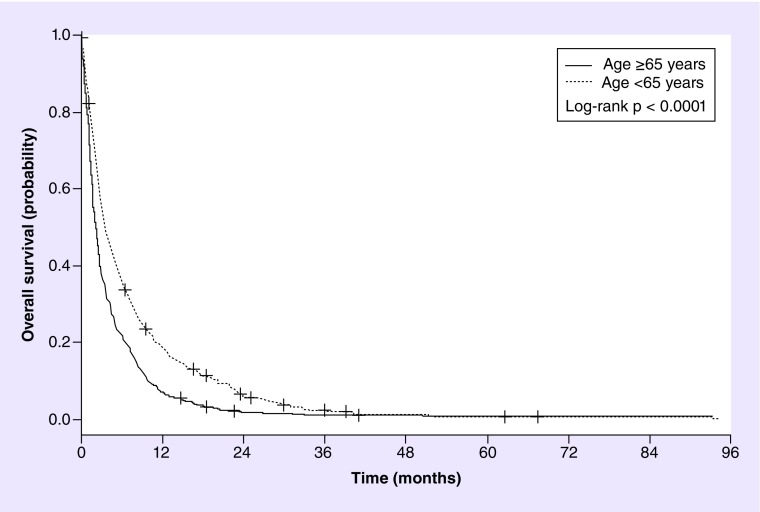
Figure 2. . Kaplan–Meier overall survival curve of patients in different subgroups of age (n = 991); median overall survivals were 3.6 months (95% CI: 3.2–4.3) in patients <65 years, and 2.2 months (95% CI: 2.0–2.5) in patients ≥65 years.
Figure 3. . Kaplan–Meier overall survival curve of patients in different subgroups of Karnofsky performance status (n = 666); median overall survivals were 1.2 months (95% CI: 0.9–1.5) in patients with Karnofsky performance status 0–40, 2.7 months (95% CI: 2.3–3.0) in patients with KPS 50–70, and 6.2 months (95% CI: 4.6–7.7) in patients with Karnofsky performance status 80–100.
Figure 4. . Kaplan–Meier overall survival curve of patients with or without postoperative status (n = 991); median overall survivals were 7.4 months (95% CI: 3.2–12.7) in postoperative patients, and 2.7 months (95% CI: 2.4–2.9) in patients who were not postoperative.
Figure 5. . Kaplan–Meier overall survival curve of patients with different primary cancer sites (n = 991); median overall survivals were 4.3 months (95% CI: 3.1–5.4) in patients with breast cancer, 2.2 months (95% CI: 1.4–2.8) in patients with GI cancer, 2.1 months (95% CI: 1.6–2.8) in patients with GU cancer, 2.7 months (95% CI: 2.4–3.1) in patients with lung cancer, 2.6 months (95% CI: 1.6–4.8) in patients with other cancers and 2.1 months (95% CI: 1.2–2.7) in patients with unknown cancers.
Discussion
In our current study, 60% of our patients had primary lung cancer with the next most common being breast cancer at 17%. Other series have also reported similar proportions of primary cancer sites in their patient population [10–11,13]. This similarity between series in terms of the distribution of primary cancer site is important for comparison due to some evidence which suggests primary cancer site may be an influential factor in the survival of patients with brain metastases [10,14].
Survival in patients with brain metastases has been reported in the literature with different patient cohorts: patients with multiple types of primary cancers or patients with a single primary cancer type. In comparison to our current patient cohort, other reports in patients with multiple primary cancers were chosen. Priestman et al. reported a median OS of 79 days or 2.6 months in patient cohort collected from 1990 to 1993 [10]. Silva et al. reported a median survival time of 4.5 months from 1996 to 2008 [15]. Fleckenstein et al. reported a median survival of 3.8 months in patients from 1997 to 1999 [16]. Lagerwaard et al. reported a median OS of 3.4 months from 1981 to 1990 [13].
Comparatively, our series falls in the lower end of the spectrum, at 2.7 months. In addition, a reported 40% of our patients passed away prior to the 2-month mark. Since median survival was less than 3 months in our reported series and relatively lower than other trials, we suspect patients who are referred to our Rapid Response Radiotherapy Program are those with poor prognostic factors. This may be due to the shift in patients and treatment at our academic center. As there has been an increase use of radiosurgery for patients with brain metastases, we hypothesize more patients with better prognostic factors are directly referred for radiosurgery, thus leaving patients with poorer prognosis to be treated with WBRT.
Our findings highlight the need to more appropriately match patients to treatment, as such prognostic factors were also investigated in our current study. Age, PS (both KPS and PPS), gender, primary cancer sites, postoperative and radiotherapy fractionation were found to be prognostic factors through univariate analysis. Both patient location (home vs hospital) and whether patients were brought by ambulance were also significant upon univariate analysis.
Through multivariate analysis, age, KPS, postoperative and primary cancer sites remained significant. In comparison to other studies investigating prognostic factors, PS [10–11,14–16] and age [10–11,13] were consistently found to be prognostic factors. More specifically, three studies found that KPS ≥70 were prognostic factors, similar to our current study, while other studies used other measurements of PS such as ECOG score [10–11,14–16]. Other studies found that age was a prognostic factor, however age cut-offs were set differently at 60 years, 65 years and 70 years [10–11,13,17]. Only Gaspar et al. used the same age cut-off of 65 years comparable to our current analysis [11]. Despite certain evidence for age being a prognostic factor, studies in literature have also found age to be nonsignificant as a prognostic factor as well [2,15–16].
Other studies have also found prior neurosurgery, or being postoperative to be a prognostic factor while Silva et al. also found that stereotactic radiosurgery is also a prognostic factor [2,15,17–18]. Postoperative status as a prognostic factor is of intuitive sense since other studies have demonstrated that surgery leads to an increased survival in patients with brain metastases [19,20]. Similarly since stereotactic radiosurgery has also been shown to improve survival in patients under the age of 50 years, this may also contribute to it being a prognostic factor [21].
Although we found significance in primary cancer site determining survival, there is mixed evidence in the literature. Similar to our findings, two studies found that breast cancer patients survive longer than lung cancer patients [10,13]. Other studies have found no prognostic significance of histology or no apparent trend [2,14]. However, Broadbent et al. suggest there may be higher survival in patients with adenocarcinoma in comparison to other types of histopathology [14].
In these studies investigating prognostic factors, other factors were found. Most often, significant prognostic factors were presence of extracranial disease [14,16] or control of primary cancer site [11,15–16]. While other prognostic factors include systemic tumor activity [13], dosage of WBRT treatment [14], dose of dexamethasone [10], response to steroid treatment [13] and serum lactate dehydrogenase [13].
Lung and breast primary cancer made up the majority of our patient population. In breast cancer patients with brain metastases, Rades et al. found single brain metastases to be a significant prognostic factor through univariate analysis however, through multivariate analysis, only the presence of extracranial disease continued to be significant (in addition to PS) [22]. Liu et al. in a cohort of nonsmall cell lung cancer patients, found gender, local tumor control and the usage of targeted therapy to be prognostic factors in patients with brain metastases [23].
Similar to our findings through univariate analysis, Lagerwaard et al. have also found that gender may be a prognostic factor [13] however we hypothesized that this was due to the influence of gender-related primary cancer sites. As such we conducted a re-analysis excluding gender-specific primaries (breast, prostate and gynecological primary cancers), resulting in 792 patients and found that gender was not significant in univariate analysis (p = 0.6060; HR = 1.039; 95% CI: 0.899–1.199).
In the quest to determine survival in patients with brain metastases, other groups have developed different systems, which started with the Recursive Partitioning Analysis [11], then to the more prognostic and recent Graded Prognostic Assessment (GPA) [24,25]. GPA was developed as a prognostic index from Radiation Therapy Oncology Group protocols. It utilized age, KPS, number of brain metastases and presence of extracranial metastases [26]. Sperduto et al. summarized the GPA indices in patients treated with various management methods. Prognostic factors were stratified by primary cancer site and were similar to our findings: KPS was common between all tumor types (lung, melanoma, renal cell, breast and gastrointestinal), age was common between lung and breast cancer. Other factors diverged from our findings such as presence of extracranial metastases or number of brain metastases, which may be attributed to the greater variation in patient population in the Sperduto et al. study in comparison to the current study [24]. Furthermore, tumor subtype was also prognostic as demonstrated by a cohort of breast cancer patients [27].
Most physicians remain committed in their belief of the efficacy of WBRT for patients, however whether or not to treat patients with multiple brain metastases using WBRT is a question that still requires a definitive answer [28]. In an effort to determine the appropriateness of WBRT for patients with brain metastases, the QUARTZ trial was conducted. Patients with nonsmall cell lung cancer with brain metastases were randomized to supportive care only or supportive care and WBRT. There were no disadvantages in either group, in terms of survival or quality of life measures [28]. These findings suggest that symptom management and WBRT are worthy of equal consideration.
When considering treatment options for patients, whether individually or at a public health level, other factors also come in consideration. Most palliative patients prefer to spend their remaining time in the comforts of family and friends’ company and not waiting in the cancer centre for clinic visits [29]. Significant amount of resources are required in the process of providing radiation for patients including patient's, family member's and healthcare professionals’ time and cost for the healthcare system to provide radiation such as machine time and ambulance services. These factors, patient's comfort and resources should be taken into consider when deciding how to treat patients with multiple brain metastases.
These patient and healthcare system factors in conjunction with the results of the QUARTZ trial suggests that more patients should be treated with supportive care only [28,29]. Physicians also have a tendency to overestimate survival, lending support to the usage of prognostic factors and indices in the clinical setting [30]. Given the similarities between our prognostic factors and other reported series, this supports the systematic incorporation of age, PS, postoperative status and primary cancer site of the patient into the determination of which patients may be more suited for supportive care or palliative care referrals in clinical practice. Future study should be conducted to determine whether patient outcome improve with these strategies, especially in the Rapid Response Radiotherapy Program.
Conclusion
In our current patient cohort, patients with brain metastases who were treated with WBRT survived a median of 2.7 months following their treatment. Age, PS, postoperative status and primary cancer site were significant prognostic factors for survival. Therefore, such factors should be considered when recommending treatment or considering palliative care referrals for patients. Especially in those with poor survival, they may be better served with supportive care including steroid support only.
Future perspective
Patients with good performance status and limited disease (number of brain metastases) will be treated by stereotactic radio surgery alone reserving whole-brain radiation as salvage treatment. Given the findings of the QUARTZ trial, patients with poorer performance status and multiple brain metastases may be treated with supportive care only such as steroids.
Acknowledgements
The authors thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund and the Ofelia Cancer Research Fund.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J. Neurooncol. 2005;75(1):5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 2.Hall WA, Djalilian HR, Nussbaum ES, Cho KH. Long-term survival with metastatic cancer to the brain. Med. Oncol. 2000;17(4):279–286. doi: 10.1007/BF02782192. [DOI] [PubMed] [Google Scholar]
- 3.Wong J, Hird A, Zhang L, et al. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009;75(4):1125–1131. doi: 10.1016/j.ijrobp.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Mehta MP, Paleologos NA, Mikkelsen T, et al. The role of chemotherapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2010;96(1):71–83. doi: 10.1007/s11060-009-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract. Radiat. Oncol. 2012;2(3):210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bezjak A, Adam J, Barton R, et al. Symptom response after palliative radiotherapy for patients with brain metastases. Eur. J. Cancer. 2002;38(4):487–496. doi: 10.1016/s0959-8049(01)00150-2. [DOI] [PubMed] [Google Scholar]
- 7.Edwards A, Gerrard G. The management of cerebral metastases. Eur. J. Pall. Care. 1998;5:7–11. [Google Scholar]
- 8.Nieder C, Nestle U, Motaref B, et al. Prognostic factors in brain metastases: should patients be selected for aggressive treatment according to recursive partitioning ana-lysis (RPA) classes? Int. J. Radiat. Oncol. Biol. Phys. 2000;46(2):297–302. doi: 10.1016/s0360-3016(99)00416-2. [DOI] [PubMed] [Google Scholar]; • Further confirmed the benefit of utilizing recursive partitioning analysis in determining treatment for patients with brain metastases.
- 9.Auchter RM, Lamond JP, Alexander E, et al. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int. J. Radiat. Oncol. Biol. Phys. 1996;35(1):27–35. doi: 10.1016/s0360-3016(96)85008-5. [DOI] [PubMed] [Google Scholar]
- 10.Priestman TJ, Dunn J, Brada M, et al. Final results of the Royal College of Radiologists’ trial comparing two different radiotherapy schedules in the treatment of cerebral metastases. Clin. Oncol. (R. Coll. Radiol.) 1996;8(5):308–315. doi: 10.1016/s0936-6555(05)80717-4. [DOI] [PubMed] [Google Scholar]
- 11.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997;37(4):745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]; •• Details the recursive partitioning analysis of prognostic factors in patients with brain metastases, suggesting three different classes of patients categorized by prognostic factors.
- 12.Allison PD. Survival Analysis Using the SAS System: A Practical Guide. SAS Institute Inc.; NC, USA: 2010. pp. 282–283. [Google Scholar]
- 13.Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int. J. Radiat. Oncol. Biol. Phys. 1999;43(4):795–803. doi: 10.1016/s0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- 14.Broadbent AM, Hruby G, Tin MM, et al. Survival following whole brain radiation treatment for cerebral metastases: an audit of 474 patients. Radiother. Oncol. 2004;71(3):259–265. doi: 10.1016/j.radonc.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Silva MG, Gomes FC, Saito EY, et al. Whole-brain radiotherapy in the treatment of brain metastases: a 12 year analysis of prognostic factors and survival. Int. J. Rad. Onc. Biol. Phys. 2010;78(3):S584–S585. [Google Scholar]
- 16.Fleckenstein K, Hof H, Lohr F, et al. Prognostic factors for brain metastases after whole brain radiotherapy. Data from a single institution. Strahlenther. Onkol. 2004;180(5):268–273. doi: 10.1007/s00066-004-1234-1. [DOI] [PubMed] [Google Scholar]; • Older study of a similar nature to our present study.
- 17.Stark AM, Tscheslog H, Buhl R, et al. Surgical treatment for brain metastases: prognostic factors and survival in 177 patients. Neurosurg. Rev. 2005;28(2):115–119. doi: 10.1007/s10143-004-0364-3. [DOI] [PubMed] [Google Scholar]
- 18.Yuile PG, Tran MH. Survival with brain metastases following radiation therapy. Australas. Radiol. 2002;46(4):390–395. doi: 10.1046/j.1440-1673.2002.01092.x. [DOI] [PubMed] [Google Scholar]
- 19.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 1990;322(8):494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 20.Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int. J. Radiat. Oncol. Biol. Phys. 1994;29(4):711–717. doi: 10.1016/0360-3016(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 21.Sahgal A, Aoyama H, Kocher M, et al. Individual patient data (IPD) meta-analysis of randomized controlled trials (RCT) comparing stereotactic radiosurgery alone to SRS plus whole brain radiation therapy in patients with brain metastasis. Int. J. Rad. Onc. Biol. Phys. 2013;87(5):1187. [Google Scholar]
- 22.Rades D, Lohynska R, Veninga T, et al. Evaluation of 2 whole-brain radiotherapy schedules and prognostic factors for brain metastases in breast cancer patients. Cancer. 2007;110(11):2587–2592. doi: 10.1002/cncr.23082. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Wu Q, Gong X, et al. Analysis of prognostic factors in NSCLC patients with brain metastases diagnosed by constrast-enhanced MRI after whole brain radiotherapy. Zhongguo Fei Ai Za Zhi. 2011;14(9):719–722. doi: 10.3779/j.issn.1009-3419.2011.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Summary of the Graded Prognostic Assessment, which is a diagnosis-specific prognostic index for patients with brain metastases.
- 25.Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int. J. Radiat. Oncol. Biol. Phys. 2008;70(2):510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 26.Sperduto CM, Watanabe Y, Mullan J, et al. A validation study of a new prognostic index for patients with brain metastases: the Graded Prognostic Assessment. J. Neurosurg. 2008;109(Suppl.):87–89. doi: 10.3171/JNS/2008/109/12/S14. [DOI] [PubMed] [Google Scholar]
- 27.Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012;82(5):2111–2117. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langley RE, Stephens RJ, Nankivell M, et al. Interim data from the Medical Research Council QUARTZ trial: does whole-brain radiotherapy affect the survival and quality of life of patients with brain metastases from non-small cell lung cancer? Clin. Oncol. (R. Coll. Radiol.) 2013;25(3):e23–e30. doi: 10.1016/j.clon.2012.11.002. [DOI] [PubMed] [Google Scholar]; •• Phase III trial comparing whole brain radiotherapy + supportive care with supportive care only, which did not find negative effects of either treatment options on quality of life or survival in patients with brain metastases from nonsmall cell lung cancer.
- 29.Thavarajah N, Wong K, Zhang L, et al. Continued success in providing timely palliative radiation therapy at the Rapid Response Radiotherapy Program: a review of 2008–2012. Curr. Oncol. 2013;20(3):e206–e211. doi: 10.3747/co.20.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes EA, Chow E, Tsao MN, et al. Physician expectations of treatment outcomes for patients with brain metastases referred for whole brain radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010;76(1):187–192. doi: 10.1016/j.ijrobp.2009.01.035. [DOI] [PubMed] [Google Scholar]; • Study of the physician's expectations of whole-brain radiotherapy for patients with brain metastases and a comparison of physician-estimated survival with actual survival.