SUMMARY
Gliosarcoma (GS) is a malignant, uncommon variant of high-grade glioma comprised of infiltrative glial and atypical sarcomatous cells, identified in adult and pediatric populations. GS has been subcategorized into primary (de novo) and secondary tumors, with the latter typically arising in the setting of prior glioblastoma. Due to its rarity, the pathogenesis, epidemiology and optimal therapy of GS have been based on small retrospective cohort studies, with treatment presently utilizing regimens established for other high-grade gliomas, including combination of resection, radiotherapy and temozolomide-based chemotherapy. As more information is gathered about GS molecular profiles, novel treatment strategies may be developed to improve outcomes of GS patients. Here we summarize results of GS management with focus on the temozolomide era.
KEYWORDS : adult, gliosarcoma, high-grade glioma, management, pediatric, radiotherapy, surgery, temozolomide
Practice points .
Gliosarcoma (GS) is a rare, aggressive tumor affecting both adult and pediatric populations, which is considered a variant of high-grade glioma, histopathologically comprised of both infiltrative glial and atypical sarcomatous, mesenchymal cells.
Primary GS is diagnosed in patients with no prior history of brain tumor, whereas secondary GS is identified in patients with prior glioblastoma (GBM) that was treated with radiotherapy.
On imaging, GS may appear as a well-circumscribed lesion (‘meningioma-like’) or as in infiltrated high-grade glioma (‘GBM like’), although radiographic characteristics and surgical impression do not necessarily correlate with pathologic findings.
Current treatment strategy for GS utilizes trimodality therapy of maximal safe resection followed by radiotherapy and temozolomide-based chemotherapy, using the European Organisation for Research and Treatment of Cancer derived regimen for GBM.
As the molecular signature of GS is further characterized, novel treatment strategies utilizing targeted therapies, likely in conjunction with standard local control therapies, are expected to emerge and improve outcomes of these patients.
Gliosarcoma overview
• Histopathologic features
WHO has classified gliosarcoma (GS), first described in 1895 by Stroeb, as a variant of the high-grade primary brain tumor, glioblastoma (GBM) [1]. Morphologically, GS has components of both classic GBM, which includes infiltrative, pleomorphic glial cells with areas of focal necrosis, microvascular proliferation and mitotic figures, and atypical sarcomatous, mesenchymal differentiation (Figure 1) [2].
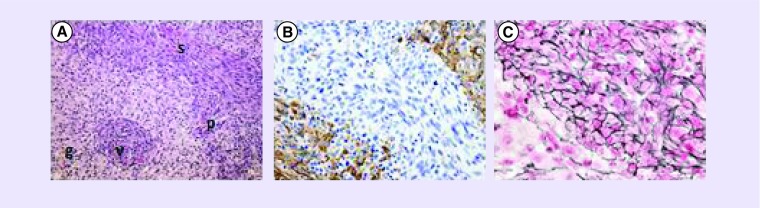
Figure 1. . Gliosarcoma histopathology.
Representative images demonstrating biphasic glial and sarcomatous elements of gliosarcoma. (A) Hematoxylin and eosin staining, demonstrating features of classic glioblastoma (g; pleomorphic, hyperchromatic cells with glassy astrocytic cytoplasm, pseudopallisading [p] and microvascular proliferation [v]) and of atypical mesenchymal, sarcomatous cells (s) at 100× magnification. (B) Glial component highlighted by positive glial fibrillary acidic protein immunohistochamical staining at 200× magnification. (C) Sarcomatous component highlighted by positive reticulin staining at 400× magnification.
Images are courtesy of G Fuller, The University of Texas, MD Anderson Cancer Center, TX, USA.
Historically, GS was thought to derive from separate malignant clones, one from glial origin and the other sarcomatous part from malignant transformation of adjacent endothelial cells [3]. Although the exact pathogenesis of GS is unknown, one current theory proposes that GS develops from a single abnormal cell with specific genetic alterations producing the biphasic tumor [2]. In support of this monoclonal origin, similar genetic changes have been seen in both glial and sarcomatous components. Molecular alterations in GS include those seen in both primary and secondary GBMs, such as TP53 and PTEN mutations, and p16 deletion, but incidence of these changes has been found to fall somewhere in between that of de novo GBM and dedifferentiated lower-grade astrocytomas [3]. A notable difference between GS and primary GBM is the very low frequency of EGFR amplification in the former compared with the latter [3]. In addition, mutation of IDH-1 and IDH-2 genes, identified in the majority of low-grade gliomas and secondary GBM, associated with good prognosis, has only been rarely observed in GS [3]. Several molecular features unique to GS include altered c-kit and B-RAF signaling and expression of genes associated with epithelial–mesenchymal transition (EMT) and metastatic potential, including MMP-2 and MMP-9, Twist and Slug [2].
GS has been subdivided into primary and secondary tumors. The term secondary GS has been used to describe GS identified in patients with a prior history of GBM. There is a smaller subset of patients who develop GS following external beam radiotherapy for nonglial intracranial tumors, such as ependymoma, leukemia, and pituitary adenoma. These tumors have been called radiation-induced GS [4,5]. Distinction between secondary and radiation-induced GSs is subtle, since most patients with GBM receive radiotherapy as part of initial management, and hence can be considered radiation-induced. The length of time following radiotherapy to the development of GS in setting of prior GBM, however, is shorter (<12 months) than that observed in cases of nonglial radiation-induced GS (15+ months) [4–7]. The exact mechanism leading to the development of secondary/radiation-induced GS, as for primary GS, is unknown. Histologically, the sarcomatous component of primary and secondary GSs has been shown to differ, with the former demonstrating malignant fibrous histiocytoma, osteosarcoma or fibrosarcoma characteristics and the latter predominantly having only fibrosarcoma features [2]. Survival of patients with secondary GS has been noted to exceed 1 year, which is twofold higher than radiation-induced GS patients in one review [4,5]; however, another report suggested that median survival of both groups is very poor, being only several months [6]. Since occurrence of all GSs, collectively, is rare, and cases of secondary and radiation-induced GSs are even more uncommon, with <60 instances having been documented in the literature [6,7], it is difficult to draw meaningful conclusions from these analyses.
• Epidemiology & clinical features
GS is a rare tumor comprising only 1–5% of all grade IV glioma cases [2]. While most cases of GS are diagnosed in patients in their sixth to eighth decade, GS has also been identified in the pediatric population at a median age of 11 years but with a high incidence reported in children <3 years old [2,8]. GS can occur either as a primary (de novo) malignancy or secondary tumor from dedifferentiation of a pre-existing intracranial neoplasm (e.g., GBM) and/or following cranial radiotherapy for such tumors. Cases of primary and secondary GS have been described in both adult and pediatric cohorts [2,4–5,8–9]. The incidence of GS is higher in males than females, regardless of age, with reported male-to-female ratio of 1.4–1.8:1 in adults and 1.2:1 in children [8,10]. While there are published reports that attempt to define the specific epidemiology of GS (see, e.g., [8] for pediatric cases and [11] for the adult population), due to the rarity of this tumor, no distinct profile has been identified to date.
As with GBM, the most common presenting signs of GS in both adult and pediatric populations are attributable to increased intracranial pressure [8,10]. Seizure at presentation has been less frequently reported [8]. More specific symptoms, such as aphasia or hemiparesis, are dependent upon tumor location. GS occurs predominantly in the supratentorial brain. In the pediatric cohort, these tumors are observed most frequently in the frontal lobes, followed by equal occurrence in parietal and temporal lobes [8]. GS in adult subjects is also most commonly detected in the frontal and temporal regions of the brain [10].
Initial work-up of a patient with evidence of increased intracranial pressure standardly includes brain imaging. There are two distinct imaging profiles of GS: one has heterogeneous contrast enhancement with areas of central necrosis and irregular, infiltrative borders; the other has uniform contrast uptake and clearly defined borders (Figure 2) [10]. The former group is best characterized as being similar to GBM on CT and MRI scans, whereas the latter has features associated with meningioma. A unifying imaging finding is significant peritumoral edema [10]. There are also variable descriptions of GS gross specimens at time of resection, ranging from a poorly demarcated, diffusely infiltrative tumor to a well-defined, solid mass, often peripheral, in close proximity to the dura mater. Although these features appear to parallel radiographic findings of the two subsets of GS detailed above, in a series of 15 GS patients with gross pathologic analysis following craniotomy with tumor resection, 14 of whom had preoperative CT scans, there was no cross-correlation of imaging and anatomic findings [12]. Use of CT-based radiography in this study may account for this discrepancy. Nonconcordance of imaging and surgical findings was also reported in a more contemporary retrospective study of 20 GS patients from the University of California, San Francisco (UCSF, CA, USA), 19 of whom had MRI imaging prior to resection [9].
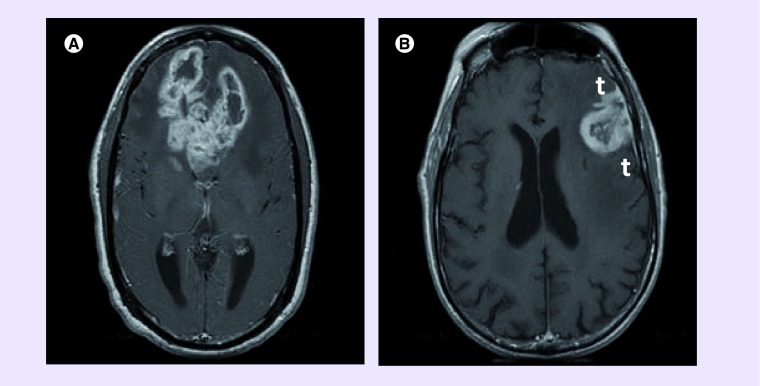
Figure 2. . Gliosarcoma imaging.
Representative T1-weighted, postcontrast MRI of (A) infiltrative, heterogeneously enhancing, centrally necrotic, glioblastoma-like and (B) more uniformly enhancing, well-defined, meningioma-like gliosarcoma tumors. Note the dural-tail (t) in the meningioma-like tumor.
Despite lack of agreement between radiographic and gross tumor characteristics, clinical outcomes of GS patients appear to correspond to the macroscopic tumor features. Well-circumscribed, solid, meningioma-like GS tumors are more readily resected than necrotic, hypervascular, infiltrative, GBM-like ones. In the UCSF cohort with primary GS, a survival benefit was seen for those possessing meningioma features. Median survival was 16 months in the meningioma-type GS patients, significantly better when compared with 9.5 months in those with GBM features [9]. It is unclear if the survival advantage in the former group derives from the relative ease of, and thus, more complete surgical resection of the well-demarcated masses versus a unique genetic profile associated with the distinct pathologic entities, or perhaps a combination of the two. Identification of a radiographic signature to distinguish between these types of GS currently remains elusive.
In contradistinction to patients with GBM in whom occurrence of extracranial metastases is exceedingly uncommon, the presence of metastatic foci, most often involving lungs and liver, has been well documented in adult patients with GS (as reviewed in [10]). Despite the small patient numbers in the pediatric subgroup, there is a report of one 11-year-old GS patient from the German HIT-GBM trial who developed thoracic metastases [8]. While analysis of the metastatic lesions often revealed both glial and sarcomatous components, others were comprised of only sarcoma tissue (see, e.g., [13]). It is hypothesized that the predilection of GS to metastasize outside the CNS is attributable to the nature of sarcomas to spread hematogenously.
Mean survival of the entire adult GS cohort ranges from 4 to 11.5 months [2], whereas median survival in pediatric patients just exceeds 1 year. Of note, infants with GS have been found to have an even greater overall survival (OS) rate, with 50% surviving 2 years after diagnosis [8]. Although histopathologic features of GS in adults and pediatric patients are reportedly similar, difference in survival, particularly in the youngest patients, suggests that there may be distinctions, as yet undefined, between tumors in these populations.
Historic treatment
Since identification of GS as a variant of GBM, therapeutic management of GS, as with other high-grade gliomas (HGGs), has utilized a multimodality approach. Surgical resection has been combined with adjuvant radiation therapy and/or systemic therapy to treat both adult and pediatric GS patients.
• Surgery
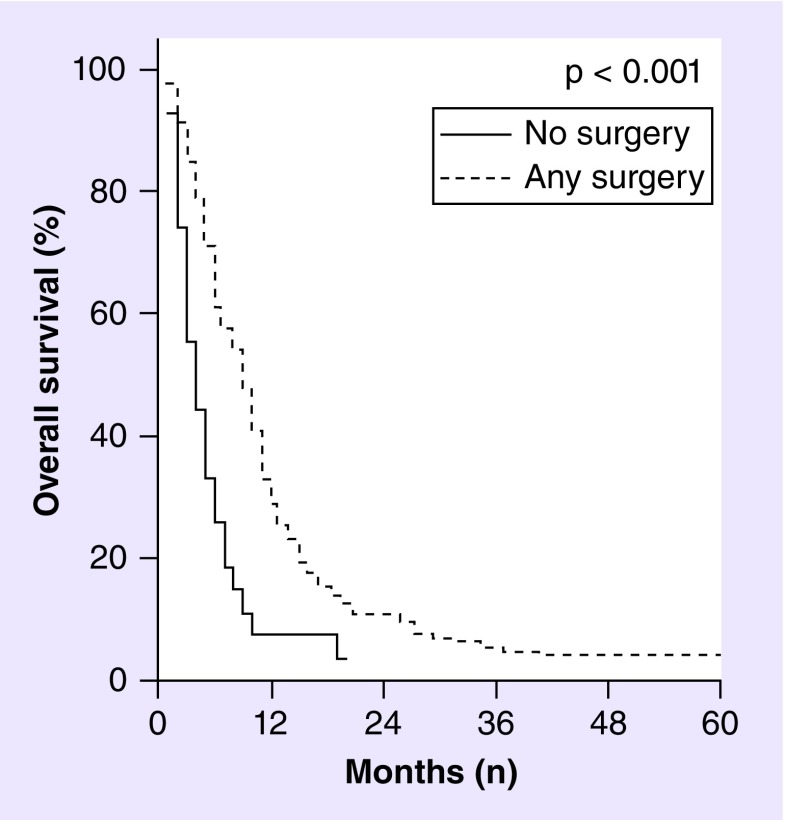
The cornerstone of GS treatment, similar to other HGGs, has been and remains maximal safe resection. On multivariate analysis of 353 adult GS patients identified from 1988 to 2004 in the database of the Surveillance, Epidemiology, and End Results (SEER) program that gathers information on incidence and survival of cancer patients in the USA, extent of surgical resection was shown to improve survival, with any surgical intervention having a hazard ratio (HR) of 0.34 (95% CI: 0.11–0.98; p = 0.004) compared with no surgical intervention (see Figure 3).
Figure 3. . Overall survival of gliosarcoma as a function of surgical resection.
Kaplan–Meier curves showing overall survival of gliosarcoma patients who received biopsy only (solid line) versus any cancer-directed surgical excision of tumor (dashed line) based on analysis of 353 adult gliosarcoma patients identified from the 1988–2004 Surveillance, Epidemiology, and End Results database. Median survival of gliosarcoma patients was significantly improved from 4 months following biopsy only to 7–11 months following any resection.
Adapted with permission from [11].
Extent of GS resection has been evaluated vis-à-vis intraoperative impression and postoperative pathologic findings. In one report of 11 subjects from Italy [14], there was nearly 100% concordance of firm, meningioma-like tumors, as determined both by preoperative imaging and the surgeon's impression at time of tumor resection, to have predominantly sarcomatous features and diffuse, infiltrative lesions to be mostly glial. Extent of resection was reported as total (defined as ≤10% residual tumor based on postoperative MRI in five cases and surgical impression for the others) for all of the meningioma-like lesions, whereas only two of the six glial-prevalent tumors could be completely resected. The authors of this study noted that survival was significantly improved in the sarcoma-rich tumors, with median survival of 71 weeks versus 63 weeks in the glial group. Of note, mean survival of the two totally resected glial tumors in this review was 65.5 weeks versus 52 weeks in the subtotally resected ones, supporting the SEER finding that extent of resection may be as critical to survival in GS patients as proportion of sarcomatous to glial component of the tumor.
Not all reports have corroborated intraoperative and pathologic findings in GS patients, however, Singh et al. [15] in their cohort of GS patients from India observed that seven of 15 tumors deemed GBM-like by the surgeon's report at time of resection were sarcoma-prevalent on histologic analysis, and one-third of the six meningioma-like tumors were predominantly glial or mixed. Despite this discrepancy, there was a significant association between final pathology and extent of resection, with 75% of sarcoma-prevalent tumors being gross totally resected (as defined for the Italian cohort above), whereas 100% of the glial-predominant tumors had ≤50% residual tumor. The authors of this study did not report how extent of residual disease in this series was determined. The survival of 14 evaluable patients in the entire group was 18.5 months, but the survival of patients with the more glial tumors was only 8.5 months. While these authors were unable to demonstrate a statistically significant correlation between extent of resection and survival in this cohort, there is a trend supporting the SEER findings.
• Radiotherapy
Radiotherapy has been reported to improve survival following surgical resection of GS dating back to one of the earliest reviews of outcomes in a cohort of 24 GS patients by Morantz et al. [16]. A planned 50–60 Gy was delivered to 13 of 18 patients in this study, and mean survival of patients receiving radiotherapy was 33 weeks from the date of surgery compared with 21 weeks in patients undergoing resection alone. Similar median OS of 8.3 months was also observed in an analysis of 26 subjects with GS treated with various radiotherapy regimens on five consecutive Phase II–III Radiation Therapy Oncology Group trials between 1974 and 1983 [17]. In two more contemporary single-institution, retrospective reviews having at least 20 GS patients, >90% of the patients received postoperative radiation [6,9]. The details of radiation treatment were provided for only one of these two studies in which 21 of 43 (48.8%) irradiated patients were treated using intensity modulated radiotherapy or three-dimensional conformal radiotherapy to a median dose of 60 Gy (range: 30–63 Gy) [6]. OS of these modern cohorts was greater than that reported for the earlier historical groups, with median survival of 12.5 months in the patients from MD Anderson Cancer Center (MDACC, TX, USA) [6] and ranging from 10.4 to 13.9 months in the UCSF patients [9]. The reason for improved survival, although likely multifactorial, may be due, at least in part, to utilization of more advanced radiotherapeutic techniques in the more modern cohorts.
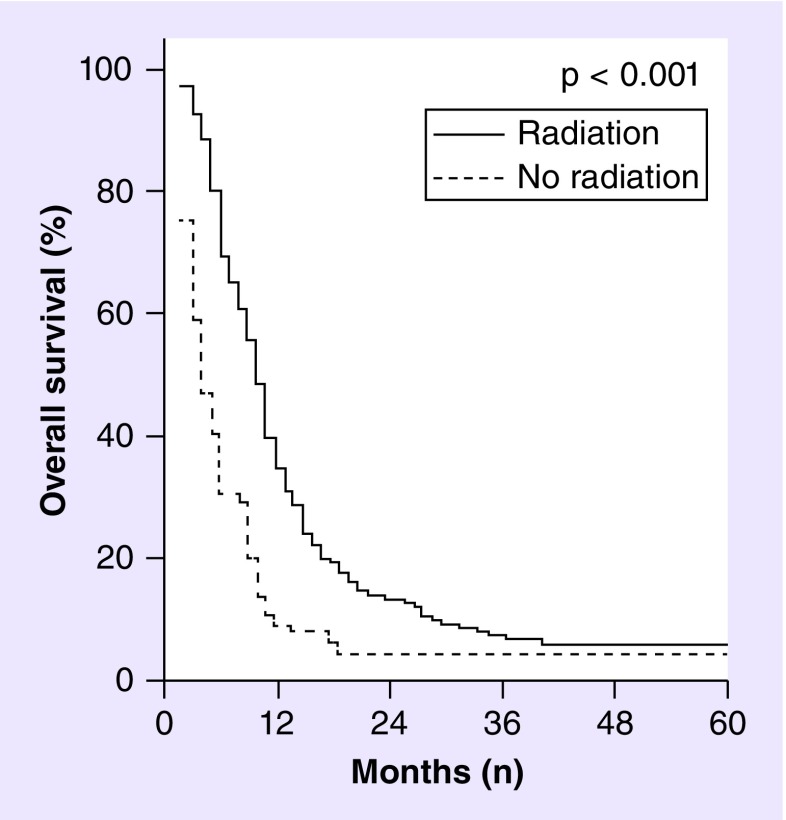
Adjuvant use of radiotherapy following surgery has been identified on multivariate analysis of the adult GS patients identified in the SEER database as a factor associated with improved OS (Figure 4). Specifically, the HR of death for adult GS patients from 1988 to 2004 not receiving adjuvant radiotherapy was increased by 2.75 (95% CI: 2.06–3.67) over those treated with radiation [11]. This finding was supported in a recent update of outcomes of adult GS subjects using the SEER database from 1973 to 2008 [6]. Despite the evidence, albeit retrospective, for improved survival of patients with GS receiving adjuvant radiotherapy after surgical resection, only about one-third of those identified in the SEER database from 2000 to 2003 and 2006 to 2008 were identified as having received this treatment [6].
Figure 4. . Overall survival of gliosarcoma as a function of adjuvant radiotherapy.
Kaplan–Meier curves showing overall survival of gliosarcoma patients who received adjuvant radiotherapy (solid line) vs no adjuvant radiotherapy (dashed line) based on analysis of 353 adult gliosarcoma patients identified from the 1988–2004 Surveillance, Epidemiology, and End Results database, excluding those patients who did not survive 2 months from diagnosis. Median survival of gliosarcoma patients included in this analysis was significantly improved from 4 months with no radiation to 10 months with radiation treatment.
Adapted with permission from [11].
Radiotherapy use in pediatric patients as a general rule has been discouraged due to concerns of late sequelae, which include neurocognitive and neuroendocrine deficits when radiotherapy is employed to treat brain tumors. Case reports of pediatric GS patients treated adjuvantly with radiotherapy, however, support prolonged survival when this modality was utilized, with up to 2-year survival following resection reported not only in adolescent patients [18] but also in a 3-month-old infant [19]. The radiation dose used to treat older pediatric patients has ranged from 40 to in excess of 60 Gy, whereas the case of infantile GS treated with radiotherapy noted above received only 30 Gy, with equivalent prolonged survival, underscoring again the possibility of a unique pathogenesis of this tumor type in the very young patient.
While there appears to be a benefit of radiotherapy use in all GS patients, this finding may simply reflect selection bias of patients 'healthy' enough, postoperatively, to receive and complete a prescribed course of radiation. Due to the lack of prospective data and the small patient numbers in both adult and pediatric GS cohorts, determining the role as well as dose and target of radiotherapy in these patients remains speculative, at best.
• Chemotherapy
As for other HGGs, chemotherapy has been used to treat GS in the adjuvant setting, most often with and/or following postoperative radiotherapy, to augment treatment outcomes of surgery and radiotherapy. Prior to the introduction of temozolomide into clinical use in the USA in 1999 and elsewhere in 2000, various agents were employed to treat GS following resection. In the early review of GS management by Morantz et al. [16], only nine of 24 patients received chemotherapy, and five of these received mithramycin, an RNA-synthesis inhibitor. In this analysis, the patients receiving chemotherapy in addition to radiotherapy postoperatively had on average 3 weeks longer survival compared with those receiving adjuvant radiation alone and 15 weeks longer survival compared with all patients. In an analysis of four consecutive Phase III trials conducted by the North Central Cancer Treatment group from 1979 to 1996, 18 adult GS patients were identified who were treated adjuvantly with 60–65 Gy external beam radiotherapy and nitrosourea chemotherapeutic agents including carmustine (BCNU) and two lesser known nitrosoureas [20]. The median survival time of this group of GS patients was 9 months, which is comparable to the 36 weeks survival reported by Morantz et al. two decades earlier. Other Phase III trials in the 1990s investigating adjuvant BCNU in combination with other systemic agents including diaziquone, mitomycin C, 6-mercaptopurine and cisplatin plus radiotherapy (see, e.g., [21–23]) enrolled only a minority of subjects with GS, but no combination resulted in superior outcomes. It was not until the introduction of temozolomide into the armamentarium of agents to treat HGGs at the turn of the millennium and the results of a pivotal Phase III clinical trial for patients with GBM conducted by the European Organisation for Research and Treatment of Cancer (EORTC) and National Cancer Institute of Canada (NCIC) Clinical Trials Group were published by Stupp et al. in 2005 [24] that hope for further improvements in outcome in patients with GS were reported.
GS treatment in the temozolomide era
The EORTC–NCIC trial examined OS of GBM patients treated adjuvantly with 60 Gy standard fractionated radiotherapy with concurrent daily temozolomide at 75 mg/m2 followed, after a 4-week break, by temozolomide at 150–200 mg/m2 daily for 5 days on an every 28-day cycle for six cycles versus radiotherapy alone. Addition of temozolomide resulted in improved OS to 14.6 months, increased from 12.1 months in the radiotherapy alone arm (unadjusted HR: 0.63; 95% CI: 0.52–0.75; log rank test p < 0.001) [24]. The survival benefit of the combined therapy in this patient population was upheld in the 5-year analysis, with 27.2% and 10.9% of patients treated with the chemoradiotherapy regimen still alive compared with only 10.9% and 1.9% of those treated with radiotherapy alone at 2 and 5 years, respectively [25]. Only 85% of the 573 subjects randomized had central pathology review, and 7% of these had non-GBM histologies [25].
Although no patients in this trial were reported to have GS, the promise of potential improved outcomes in this GBM variant prompted adjuvant use of temozolomide with radiotherapy for GS patients. In more modern series published after the clinical introduction of temozolomide, use of the Stupp-like regimen was found to have mixed results in GS patients. In the Italian series of 11 GS patients, the median survival was 69.5 weeks (˜16 months) in four patients receiving 60 Gy radiotherapy plus temozolomide compared with 63 weeks (˜14.5 months) in five others receiving radiotherapy alone [14]. Similarly, 2-year OS of the 17 of 46 GS patients at MDACC treated with Stupp-like therapy was 20.0%, compared with 10.2% in the others [6]. While statistical significance was not reached in the MDACC analysis and was not reported in the Italian series, these trends mirror the results of the EORTC–NCIC study. The other large contemporary series of 20 GS patients from UCSF found the median survival time to be 10.4 months in the 10 who received Stupp-like therapy versus 13.9 months in the others [9]. The median time to disease progression was 2 months longer in the Stupp-like treated group (5 vs 3 months in the non-Stupp-like group). These data also failed to meet statistical significance, likely due to the small patient numbers.
An important epigenetic factor associated with improved outcomes observed in the EORTC–NCIC trial GBM patients is methylation of the MGMT promoter that silences expression of a DNA repair protein and thus augmenting the antineoplastic effect of alkylating agents like temozolomide [24,25]. In a recent analysis of 26 cases of GS, less than 12% of cases were identified as having the methylated MGMT promoter [3]. In a separate series of 12 GS patients, methylation-specific polymerase chain reaction confirmed methylated MGMT promoter in six of these tumors. Median OS and progression-free survival in GS cases having methylated MGMT promoters were 15 and 10.5 months, respectively, exceeding survival times of 11.3 and 7.3 months in the corresponding unmethylated group [26]. Similar methylation-specific polymerase chain reaction analysis of another 16 GS patients identified methylated MGMT promoter in five cases [15]. While no statistically significant improvement in survival was noted in the methylated group in this analysis, after a mean follow-up of 12.8 months, all GS patients with methylated MGMT promoters were still alive, in comparison to six of 11 patients with unmethylated tumors. Lack of statistical significance in this study is, as with all GS studies, likely attributable to small patient numbers.
Conclusion & future perspective
GS is a rare, highly malignant, variant of GBM, comprised of glial and sarcomatous components, affecting both pediatric and adult populations. Given small patient numbers, data guiding optimal management have been derived from retrospective cohort studies. Maximal safe resection, as with other HGGs, is the cornerstone of GS therapy; although improved OS and time to disease progression have been observed in GS patients by addition of adjuvant radiotherapy in conjunction with temozolomide. As more detailed molecular analyses establish a molecular signature for GS distinct from classic GBM, novel GS-specific therapeutic targets, such as the MMPs, B-RAF and/or c-kit, may be identified to augment current trimodality treatment for this still devastating disease.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Louis D, Ohgaki H, Wiestler O, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karsy M, Gelbaum M, Shah P, Balumbu O, Moy F, Arslan E. Established and emerging variants of glioblastoma multiforme: review of morphological and molecular features. Folia Neuropathol. 2012;50(4):301–321. doi: 10.5114/fn.2012.32361. [DOI] [PubMed] [Google Scholar]; •• Details the evolving molecular and morphological variants of glioblastoma, including gliosarcoma, since the release of WHO 2007 classification guidelines.
- 3.Lee D, Kang S, Suh Y-L, Jeong J, Lee J-I, Nam D-H. Clinicopathologic and genomic features of gliosarcomas. J. Neurooncol. 2012;107(3):643–650. doi: 10.1007/s11060-011-0790-3. [DOI] [PubMed] [Google Scholar]
- 4.Han SJ, Yang I, Otero JJ, et al. Secondary gliosarcoma after diagnosis of glioblastoma: clinical experience with 30 consecutive patients. J. Neurosurg. 2010;112(5):990–996. doi: 10.3171/2009.9.JNS09931. [DOI] [PubMed] [Google Scholar]; •• Describes the largest modern series of secondary gliosarcoma from a single institution.
- 5.Han SJ, Yang I, Tihan T, Chang SM, Parsa AT. Secondary gliosarcoma: a review of clinical features and pathological diagnosis. J. Neurosurg. 2010;112(1):26–32. doi: 10.3171/2009.3.JNS081081. [DOI] [PubMed] [Google Scholar]
- 6.Walker G, Gilbert M, Prabhu S, Brown P, McAleer MF. Temozolomide use in adult patients with gliosarcoma: an evolving clinical practice. J. Neurooncol. 2013;112(1):83–89. doi: 10.1007/s11060-012-1029-7. [DOI] [PubMed] [Google Scholar]; •• Describes the largest modern series of adult gliosarcoma patients treated at a single institution in the temozolomide era.
- 7.Andaloussi-Saghir K, Oukabli M, El Marjany M, Sifat H, Hadadi K, Mansouri H. Secondary gliosarcoma after the treatment of primary glioblastoma multiforme. N. Am. J. Med. Sci. 2011;3(11):527–530. doi: 10.4297/najms.2011.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karremann M, Rausche U, Fleischhack G, et al. Clinical and epidemiological characteristics of pediatric gliosarcomas. J. Neurooncol. 2010;97(2):257–265. doi: 10.1007/s11060-009-0021-3. [DOI] [PubMed] [Google Scholar]; • Describes the largest series of gliosarcoma in pediatric patients.
- 9.Han S, Yang I, Tihan T, Prados M, Parsa A. Primary gliosarcoma: key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity. J. Neurooncol. 2010;96(3):313–320. doi: 10.1007/s11060-009-9973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han SJ, Yang I, Ahn BJ, et al. Clinical characteristics and outcomes for a modern series of primary gliosarcoma patients. Cancer. 2010;116(5):1358–1366. doi: 10.1002/cncr.24857. [DOI] [PubMed] [Google Scholar]
- 11.Kozak KR, Mahadevan A, Moody JS. Adult gliosarcoma: epidemiology, natural history, and factors associated with outcome. Neuro Oncol. 2009;11(2):183–191. doi: 10.1215/15228517-2008-076. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Compares outcomes of adult glioblastoma and gliosarcoma patients treated between 1988 and 2004 identified in the Surveillance, Epidemiology, and End Results-17 Registry database.
- 12.Parekh HC, O'Donovan DG, Sharma RR, Keogh AJ. Primary cerebral gliosarcoma: report of 17 cases. Br. J. Neurosurg. 1995;9(2):171–178. doi: 10.1080/02688699550041511. [DOI] [PubMed] [Google Scholar]
- 13.Smith DR, Hardman JM, Earle KM. Contiguous glioblastoma multiforme and fibrosarcoma with extracranial metastasis. Cancer. 1969;24:270–276. doi: 10.1002/1097-0142(196908)24:2<270::aid-cncr2820240210>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Salvati M, Caroli E, Raco A, Giangaspero F, Delfini R, Ferrante L. Gliosarcomas: analysis of 11 cases do two subtypes exist? J. Neurooncol. 2005;74(1):59–63. doi: 10.1007/s11060-004-5949-8. [DOI] [PubMed] [Google Scholar]
- 15.Singh G, Mallick S, Sharma V, et al. A study of clinico-pathological parameters and O6–methylguanine DNA methyltransferase (MGMT) promoter methylation status in the prognostication of gliosarcoma. Neuropathology. 2012;32(5):534–542. doi: 10.1111/j.1440-1789.2012.01297.x. [DOI] [PubMed] [Google Scholar]; • Highlights the benefit of temozolomide therapy for gliosarcoma patients regardless of O6-methylguanine DNA methyltransferase promoter methylation status (contrasts with [26]).
- 16.Morantz RA, Feigin I, Ransohoff J., III Clinical and pathological study of 24 cases of gliosarcoma. J. Neurosurg. 1976;45:398–408. doi: 10.3171/jns.1976.45.4.0398. [DOI] [PubMed] [Google Scholar]
- 17.Meis JM, Martz KL, Nelson JS. Mixed glioblastoma multiforme and sarcoma. A clinicopathologic study of 26 radiation therapy oncology group cases. Cancer. 1991;67(9):2342–2349. doi: 10.1002/1097-0142(19910501)67:9<2342::aid-cncr2820670922>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Salvati M, Lenzi J, Brogna C, et al. Childhood's gliosarcomas: pathological and therapeutical considerations on three cases and critical review of the literature. Childs Nerv. Syst. 2006;22(10):1301–1306. doi: 10.1007/s000381-006-0057-z. [DOI] [PubMed] [Google Scholar]
- 19.Ono N, Nakamura M, Inoue HK, Tamura M, Murata M. Congenital gliosarcoma; so-called sarcoglioma. Childs Nerv. Syst. 1990;6(7):416–420. doi: 10.1007/BF00302231. [DOI] [PubMed] [Google Scholar]
- 20.Galanis E, Buckner JC, Dinapoli RP, et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. J. Neurosurg. 1998;89(3):425–430. doi: 10.3171/jns.1998.89.3.0425. [DOI] [PubMed] [Google Scholar]
- 21.Schold SC, Herndon JE, Burger PC, et al. Randomized comparison of diaziquone and carmustine in the treatment of adults with anaplastic glioma. J. Clin. Oncol. 1993;11(1):77–83. doi: 10.1200/JCO.1993.11.1.77. [DOI] [PubMed] [Google Scholar]
- 22.Halperin EC, Herndon J, Schold, et al. A Phase III randomized prospective trial of external beam radiotherapy, mitomycin C, carmustine, and 6-mercaptopurine for the treatment of adults with anaplastic glioma of the brain. Int. J. Radiat. Oncol. Biol. Phys. 1996;34(4):793–802. doi: 10.1016/0360-3016(95)02025-x. [DOI] [PubMed] [Google Scholar]
- 23.Buckner JC, Ballman KV, Michalak JC, et al. Phase III Trial of carmustine and cisplatin compared with carmustine alone and standard radiation therapy or accelerated radiation therapy in patients with glioblastoma multiforme: North Central Cancer Treatment Group 93–72–52 and Southwest Oncology Group 9503 trials. J. Clin. Oncol. 2006;24(24):3871–3879. doi: 10.1200/JCO.2005.04.6979. [DOI] [PubMed] [Google Scholar]
- 24.Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 25.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised Phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 26.Kang S-H, Park K-J, Kim C-Y, et al. O6-methylguanine DNA methyltransferase status determined by promoter methylation and immunohistochemistry in gliosarcoma and their clinical implications. J. Neurooncol. 2011;101(3):477–486. doi: 10.1007/s11060-010-0267-9. [DOI] [PubMed] [Google Scholar]; • Describes improved outcomes of gliosarcoma patients with methylated O6-methylguanine DNA methyltransferase promoter (contrasts with [15]).