SUMMARY
Aim:
This retrospective study determined features associated with brain metastasis (BM) in women with breast cancer.
Patients & methods:
A total of 215 initially early breast cancer cases were included. We reviewed files and CT scan images of BM.
Results:
Median age was 47 years and most of our cases were stage III (58.6%), grade III (62.8%), ER negative (62.3%) and nonluminal (59.1%). Median survival after BM was 4 months. Nonluminal, extracranial disease, time to CNS shorter than 15 months, >three brain lesions and poor breast-graded prognostic assessment and recursive partitioning analysis scores were associated with shorter survival. Adding extracranial disease to breast-graded prognostic assessment score also predicted survival after BM. Radiation response was assessed in 57 patients and response tended to be associated with nonluminal phenotype but not with survival.
Conclusion:
Factors associated with both initial tumor and clinical features at BM time are associated with shorter survival in our Latinas population.
KEYWORDS : breast cancer, CNS metastases, phenotype, prognostic factor
Summary points .
Brain metastasis (BM) is associated with a very poor prognosis in breast cancer. Risk factors for developing BM include host factors like race and tumor features of aggressiveness like nonluminal phenotype.
Prognosis after BM can be predicted by features of tumor at diagnosis and at the BM time. Nonluminal phenotype and high number of brain lesions are associated with poor prognosis after BM.
Scores combining clinicopathological features like graded prognostic assessment and recursive partitioning analysis can identify those lesions with shortest survival after BM.
Brain metastasis (BM) is one of the most serious complications of breast cancer (BC) and produces high morbidity and mortality. Approximately 4–5% of early-stage BC and 8% of locally advanced stage develop BM [1,2]. Several host and tumor risk factors have been associated with a higher risk to develop BM. Host factors could be especially important in predisposition and behavior of BM because malignant cells need to overpass host blood–brain barrier (BBB) and move inside the brain parenchyma [3–10]. Host factors age and race can predict BM development and prognosis [4].
By other side some tumor features can predispose to the development of BM as described by Bos et al., who found a gene expression signature associated with BM [1,4–5].
Aggressive features of tumor as high histologic grade (HG), ER-negative and triple-negative (TN) phenotype have been associated with BM. Most BM appears after 2–3 years of the initial diagnosis of BC and only 20% of patients survive after a year [11]. Treatment with whole brain radiotherapy is the standard of care for most BM and increases median survival in around 5 months [12].
Some prognostic scores combining clinical and pathological features have been developed for predicting survival after BM. Recursive partitioning analysis (RPA) and breast graded prognostic assessment (GPA) score have been tested in series of BC patients with BM. BC in the Latina race has been associated with aggressive features like youth, advanced clinical stage (CS), high HG, ER negative and TN phenotype. Additionally, Latina race also appears to have higher BM rates than Caucasians [13,14]. However, these prognostic indicators have not been validated in Latina race and a comprehensive analysis of variables influencing prognosis after BM development including its response to radiation has not been performed [15–17].
We performed a comprehensive analysis of host and tumor factors affecting prognosis of BC cases with BM in a Peruvian population.
Patients & methods
We retrospectively evaluated every new BC cases attended at the Instituto Nacional de Enfermedades Neoplasicas from 2000 to 2011 and identified those 215 early cases who developed BM. Clinicopathological features were obtained from patient files and brain CT scan image was evaluated by a radiologist of the institute.
Evaluated variables at the time of BC diagnosis included: age, CS, surgery technique, adjuvant treatment, HG, hormone receptor (HR), HER2-status and phenotype [18]. Phenotype was classified into luminal A (ER-positive and progesterone receptor [PgR] >20%, HER2-negative and HG1–2), luminal B (ER-positive and any PgR <20 or HER2+++ or HG3), HER2-enriched (ER and PgR-negative and HER2+++) and TN (ER, PgR and HER2-negative) [19].
Evaluated variables at the time of BM included: ECOG 0–4 (functional status), presence and localization of extracranial disease (ECD) evaluated by image test or clinical evaluation, Time to CNS metastases (TTCNS) and response to radiotherapy according to response evaluation criteria in solid tumors (RECIST) 1.1 criteria [20]. TTCNS was measured from the date of primary diagnosis to the date of BM. Survival time after BM was calculated from the date of BM to the date of death or last follow-up (the last time patient came to the hospital). We also evaluated the prognosis impact of recursive partitioning analysis (RPA; based on status performance, age and presence of ECD) and breast-GPA score (based on status performance, age and phenotype) [21,22].
Association among clinicopathological variables including RECIST response was evaluated through Fisher's exact test and Z-test for two proportions of independent samples.
TTCNS and overall survival (OS) after BM were estimated by using the Kaplan–Meier product-limit method. Association between survival after BM and potential prognostic factors (including GPA and class RPA score) were assessed by using the log-rank or Breslow test in univariate analysis.
Statistical significance was accepted if p < 0.05. The data were processed and analyzed using Stata program version 12.0.
Results
We evaluated 215 cases of BM with BC with a median follow-up of 31 months and median survival after CNS metastases of 4 months (0–80 months).
• Features at diagnosis of primary tumor
As Table 1 mentions, we found that median age at BC diagnosis was 47 years. Most cases had ductal histology (97.2%), HG-III (62.8%), ER-negative (62.3%), HER2-negative status (73%), nonluminal (59.1%), TN phenotype (38.6%) and CS-III (58.6%).
Table 1. . Clinical features of included cases.
| Features | Patients (n) | Patients (% of n) |
|---|---|---|
| Clinicopathological features at breast cancer diagnosis | ||
| Patients | 215 | |
| Median age, years (min–max) | 47 (24–80) | |
| Clinical stage | ||
| I–II | 71 | 33.0 |
| III | 126 | 58.6 |
| Unknown | 18 | 8.4 |
| Histology | ||
| Ductal | 209 | 97.2 |
| Lobular | 2 | 0.9 |
| Medullary | 2 | 0.9 |
| Mixto | 2 | 0.9 |
| Histologic grade | ||
| 1–2 | 75 | 34.9 |
| 3 | 135 | 62.8 |
| Unknown | 5 | 2.3 |
| Estrogen receptor | ||
| Negative | 134 | 62.3 |
| Positve | 75 | 34.9 |
| Unknown | 6 | 2.8 |
| HER2 status | ||
| Negative | 157 | 73.0 |
| Positve | 55 | 25.6 |
| Unknown | 3 | 1.4 |
| Phenotype | ||
| Luminal-A | 20 | 9.3 |
| Luminal-B | 59 | 27.4 |
| HER2 enriched | 44 | 20.5 |
| Triple negative | 83 | 38.6 |
| Unknown | 9 | 4.2 |
| Signs and symptoms at brain metastasis | ||
| ECOG at brain metastasis time | ||
| 1 | 121 | 56.3 |
| 2 | 63 | 29.3 |
| 3–4 | 15 | 7.0 |
| Unknown | 16 | 7.4 |
| CT scan image features | ||
| Number of brain lesions | ||
| 1–3 | 118 | 54.9 |
| >3 | 77 | 35.8 |
| Unknown | 1 | 0.5 |
| Carcinomatosis (image or cytology result) | 32 | 14.9 |
| Median diameter of largest lesion, cm (min–max) | 2.25 (0.3–9.0) | |
| Edema | 174 | 80.9 |
| Unknown | 20 | 9.3 |
| Necrosis | 32 | 14.9 |
| Unknown | 21 | 9.8 |
| Hemorrhage | 10 | 4.7 |
| Unknown | 20 | 9.3 |
| Largest lesion shape | ||
| Nodular | 120 | 55.8 |
| Ringed | 34 | 15.8 |
| Lobulated | 11 | 5.1 |
| Irregular | 10 | 4.7 |
| Cystic | 16 | 7.4 |
| Calcifications | 3 | 1.4 |
| Unknown | 21 | 9.8 |
ECOG: Eastern Cooperative Oncology Group.
Median TTCNS was 22 months (0–86 m) and univariate analysis found that it was shorter in patients with CS III (p < 0.001), HG III (p = 0.0011), ER-negative (p < 0.001), HER2-positive status (p = 0.0472), TN (p = 0.0002) and nonluminal phenotype (p < 0.001) (Table 2).
Table 2. . Features related to time to CNS metastases.
| Features | Patients (n) | Brain metastasis at 2 years (%) | p-value |
|---|---|---|---|
| Age (years): | 0.1044 | ||
| – ≤45 | 35 | 40.7 | |
| – >45 | 55 | 50.5 | |
| Clinical stage: | <0.001 | ||
| – I–II | 48 | 72.7 | |
| – III | 38 | 31.4 | |
| Histological grade: | 0.0011 | ||
| – 1–2 | 41 | 56.9 | |
| – 3 | 48 | 38.1 | |
| Estrogen receptor status: | <0.001 | ||
| – Positive | 51 | 70.8 | |
| – Negative | 41 | 32.8 | |
| HER2 status: | 0.0472 | ||
| – Positive | 19 | 38.0 | |
| – Negative | 74 | 49.3 | |
| Molecular subtype: | <0.001 | ||
| – Luminal | 52 | 69.3 | |
| – Nonluminal | 38 | 31.9 | |
| Molecular subtype: | 0.0002 | ||
| – Nontriple negative | 67 | 57.8 | |
| – Triple negative | 23 | 29.5 | |
These features were evaluated in regard to survival after BM, and univariate analysis found that shorter TTCNS (p = 0.0063), ER-negative status (p = 0.0001), nonluminal (p < 0.001) and TN phenotype (p < 0.001) predicted shorter survival (Table 3).
Table 3. . Features related to survival after brain metastases.
| Features | Patients (n) | Survival rate at 1 year (%) | p-value |
|---|---|---|---|
| Age at brain metastases diagnosis (years): | 0.1129 | ||
| – <60 | 165 | 20.6 | |
| – >60 | 27 | 7.4 | |
| Clinical stage: | 0.8690 | ||
| – I–II | 64 | 21.9 | |
| – III | 121 | 17.4 | |
| Histological grade: | 0.2731 | ||
| – I–II | 69 | 20.3 | |
| – III | 126 | 16.7 | |
| Estrogen receptor status: | 0.0001 | ||
| – Positive | 72 | 31.9 | |
| – Negative | 123 | 12.2 | |
| HER2 status: | 0.5627 | ||
| – Positive | 48 | 18.8 | |
| – Negative | 149 | 19.5 | |
| Phenotype luminal: | <0.001 | ||
| – Yes | 75 | 33.3 | |
| – No | 116 | 8.6 | |
| Triple-negative phenotype: | <0.001 | ||
| – No | 114 | 28.1 | |
| – Yes | 77 | 3.9 | |
| ECOG performance status: | 0.1376 | ||
| – 0–1 | 118 | 21.2 | |
| – 2 | 71 | 15.5 | |
| Time to CNS metastases: | 0.0063 | ||
| – ≤15 months | 59 | 21.4 | |
| – >15 months | 140 | 11.9 | |
| Active extracranial disease: | 0.0007 | ||
| – No | 19 | 47.4 | |
| – Yes | 137 | 11.0 | |
| Number of brain lesions: | 0.0275 | ||
| – 1–3 | 108 | 25.0 | |
| – >3 | 73 | 11.0 | |
| Edema: | 0.2687 | ||
| – No | 18 | 38.9 | |
| – Yes | 163 | 17.2 | |
| Brain lesion response to radiation following RECIST: | 0.8633 | ||
| – CR | 12 | 41.7 | |
| – PR | 16 | 43.8 | |
| – SD | 16 | 37.5 | |
| – PD | 13 | 46.2 | |
Time to CNS metastases cut–off of 15 months represents the lower tertile.
CR: Complete response; ECOG: Eastern Cooperative Oncology Group; PD: Progressive disease; PR: Partial response; RECIST: Response evaluation criteria in solid tumors; SD: Stable disease.
Cutoff at 45 years of age was used in Table 2 as we wanted to find out if age could differentiate TTCNs, and a cutoff age to find a significant association was not found. This absence of association is better demonstrated using an age close to the median age of the study series (47.1 years old) – therefore 45 years was decided upon.
Cutoff at 60 years of age was used in Table 3 as we wanted to evaluate those factors that other researchers have found to be associated with BM risk. Breast-GPA score combines the ECOG performance status, tumor phenotype and age with a cutoff of 60 years to give prognosis after BMs.
• Features at the BM event
Performance status ECOG-1 was found in 121 patients (56.3%) and mentally good conscious status at BM event in 164 patients (76.3%). Meningeal symptoms, focalization symptoms and seizures were found in 33.8, 38.6 and 7.1% of the cases, respectively. Most patients had concurrent ECD (68.4%): 50 (23.3%) patients at locoregional area, 41 (19.1%) at bones, 43 (20%) at lungs and 23 (10.7%) at liver. The lungs and the liver are the most frequently compromised places among nonluminal lesions.
Brain image analysis showed one lesion in 41.4% and more than 3 in 35.8%, respectively. Median diameter of largest lesion was 2.25 cm (0.3–9.0) and image of meningeal involvement was found in 14.9%. Important edema, midline shift, necrosis and hemorrhage image were found in 80.9, 38, 14.9 and 4.7% of cases, respectively. Most lesions were nodular (55.8%) and circumscribed (91.8%) (Table 1).
These features were evaluated regarding the survival after BM, and univariate analysis found that the presence of ECD (p = 0.0007) and multiple brain lesions (p = 0.0275) predicted shorter survival. Older age than 60 years (p = 0.1129; age cutoff included in breast-GPA score), poor performance status (p = 0.1376), hemorrhage image (p = 0.3159) and brain edema (p = 0.2687) at the BM presentation showed a trend to shorter survival after BM (Table 3).
• Evaluation of prognostic scores after BM
Breast-GPA score falls between 0–1, 1.5–2, 2.5–3 and 3.5–4 in 6.1, 36.1, 20 and 37.8%, respectively. RPA score falls in class 1, 2 and 3 in 7.5, 84.9 and 7.5%.
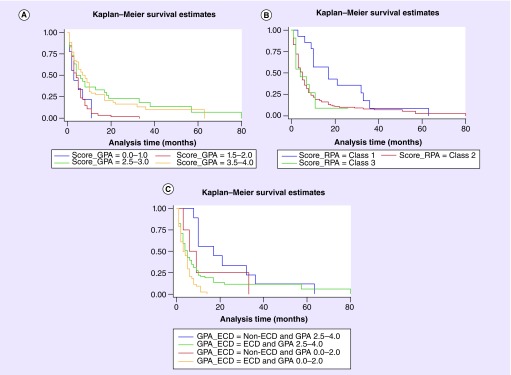
Breast-GPA score (p = 0.0006) and RPA score (p = 0.0315) predicted a shorter survival after BM. Finally, we evaluated the impact of adding ECD to breast-GPA (divided into two groups for obtaining representative population: 0–2 vs 2.5–4) and found that new groups had also different survival after 1 year of BM: 4.4, 22, 19 and 59.9% (p = 0.0007) (Table 4 & Figure 1).
Table 4. . Comparison of prognostic value of graded prognostic assessment and recursive partitioning analysis scores.
| Prognostic score grading | Patients (n) | Survival rate at 1 year (%) | p-value |
|---|---|---|---|
| Breast GPA score: | 0.0006 | ||
| – 0.0–1–0 | 11 | 0.0 | |
| – 1.5–2–0 | 65 | 4.8 | |
| – 2.5–3.0 | 36 | 32.4 | |
| – 3.5–4.0 | 68 | 25.8 | |
| Score RPA: | 0.0315 | ||
| – Class 1 | 15 | 53.3 | |
| – Class 2 | 169 | 16.8 | |
| – Class 3 | 15 | 7.7 | |
| Breast GPA score plus ECD: | 0.0007 | ||
| – Non-ECD and score GPA 2.5–4.0 | 9 | 55.56 | |
| – Non-ECD and score GPA 0.0–2.0 | 5 | 20.0 | |
| – ECD and score GPA 2.5–4.0 | 72 | 17.65 | |
| – ECD and score GPA 0.0–2.0 | 52 | 2.0 | |
ECD: Extracranial disease; GPA: Graded prognostic assessment; RPA: Recursive partitioning analysis.
Figure 1. . Kaplan–Meier survival curves.
Comparison of prognostic value of breast-GPA (A), RPA (B) scores and breast-GPA with addition of ECD (C) by Kaplan–Meier curves. The y-axes indicate probability from 0 to 1.
ECD: Extracranial disease; GPA: Graded prognostic assessment; RPA: Recursive partitioning analysis.
• Features associated with BM response to radiation
Radiation was administered to 197 patients (86.8%) and radiological response according to RECIST 1.1 criteria was evaluated in 57 patients: complete response was observed in 12 patients (21.1%), partial response in 16 (28.1%), stable disease in 16 (28.1%) and Parkinson's disease in 13 (22.8%) (Table 3). No association between RECIST response to radiation and survival was found (p = 0.8633). No association between RECIST response to radiation and luminal phenotype was found (0.722). However, luminal phenotype continued to have better prognosis even in this small subset of 57 cases (p < 0.001) (Table 5).
Table 5. . RECIST response of brain lesions to radiation.
| Response | Luminal A, n (%) | Luminal B, n (%) | HER2, n (%) | TN, n (%) |
|---|---|---|---|---|
| CR | 1 (10.0) | 4 (20.0) | 2 (15.4) | 5 (29.4) |
| PR | 3 (30.0) | 4 (20.0) | 6 (46.2) | 4 (23.5) |
| SD | 2 (20.0) | 7 (35.0) | 4 (30.8) | 5 (29.4) |
| PD | 4 (40.0) | 5 (25.0) | 1 (7.7) | 3 (17.7) |
| Unknown RECIST | 10 | 39 | 31 | 66 |
| Median survival after brain metastasis (months) | 7 ± 13.3 | 6 ± 7.5; p < 0.001 | 4 ± 4 | 3 ± 2.5 |
CR: Complete response; PD: Progressive disease; PR: Partial response; RECIST: Response evaluation criteria in solid tumors; SD: Stable disease; TN: Triple negative.
When we evaluated factors associated with radiation response, we found that most nonluminal (17/30: 56.7%) and few luminal (12/30: 40.0%) cases obtained BM response (p = 0.0939).
Discussion
We initially evaluated the influence of biology from initial breast tumor over BM behavior and similarly to other publications we found that more advanced CS, ER-negative status, nonluminal and TN phenotypes were associated to shorter both TTCNS and survival after BM [1–2,4–10,13]. These factors could predispose cancer cells not only to invade but also to grow inside brain tissue. Remarkably, every mentioned factor has been described as more frequent in Latinas than in Caucasian women as showed in a retrospective analysis we performed in more than 1100 BC Peruvian cases. Higher concentration of these BM-clinical risk factors in Latinas could be responsible for an apparent higher rates of BM we have found in our previous series [13,14].
We also found that HER2-positive status also predisposed to shorter TTCNS but did not predict to shorter survival after BM (despite the fact that patients in our series did not receive anti-HER2 agents at any time), similarly to Anders et al. results [4].
The TTCNS under the lower tertile (15 months) was associated with shorter survival in our series. It could represent the fact that tumors with early ability to invade and grow inside CNS had worst prognosis than those lesions that need for further changes in the tumor biology to invade or progress inside brain [4].
The presence of ECD was associated with poor prognosis in our and other series, and indicates that morbidity and mortality caused by systemic disease appear to be relevant even after BM diagnosis [15,21–22].
Tumor features at BM development have been evaluated through features of brain lesion. More than three brain lesions at CT scan were associated (p = 0.04) and brain edema has a trend to shorter survival (p = 0.08). Similarly, Anders et al. also found an association between the presence of multiple brain lesions and short survival. This finding correlates with the fact that early detection of occult BM appears to improve survival and decrease mortality [4,17].
Host factors at the time of BM diagnosis like older age (>60 years) tended to have shorter survival, but did not reach significance. This absence of relationship has also been previously reported by Anders et al. and Gaspar et al. [4,16,21].
Poor performance status at BM, another host factor, tended to have shorter survival but did not reach significance (p = 0.1376), probably because of the small sample size and because most of our patients were at ECOG 1 (60.9%) and alert (76.8%). Sperduto and Gaspar et al. have demonstrated that poor performance status are strongly associated to shorter survival after BM in BC as well as in other neoplasms [16,21].
Different groups have developed some prognostic-tools with the combination of clinicopathologic factors [15,22]. Breast-GPA score combines the ECOG status, age and tumor phenotype, and identify a subset with poor survival after BM. RPA score combines the first two factors of breast-GPA and adds the ECD factor. We found that both scores achieved significant value in our series. Remarkably, we found also a significant value when we combine breast-GPA score with the presence of ECD.
Our analysis of the subset of patients with postradiotherapy CT scan evaluation found that most nonluminal phenotypes, especially TN BC, achieved BM response and most luminal phenotypes did not; however, it did not achieve significance (we need a larger population to evaluate this trend). However, BM response did not correlate with survival and it appears that even those nonluminal cases with good BM response have short survival. It reminds us the Paradoxical Phenomena previously reported for advanced TN BC that have high response rates to chemotherapy but poor survival [23].
Conclusion & future perspective
In summary, we found that prognosis after BM is affected by features of primary lesion: ER- status, phenotype and TTCNS; as well as features at BM time: the presence of ECD and multiple brain lesions. We found that the addition of the variable ECD to breast-GPA can also identify prognosis after BM. Finally, it appears that BM lesions of most nonluminal BC responded to radiation, but it did not correlate with survival. Further studies are required in the area. I speculate that biological markers in primary lesion that evaluate genes or proteins related to the behavior of brain metastasis as well as functionally image test like PET CT scan with specific markers will be added to these clinico pathological scores in order to improve prognostic value and response prediction accuracy to BM treatment.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Evans AJ, James JJ, Cornford EJ, et al. Brain metastases from breast cancer: identification of a high-risk group. Clin. Oncol. (R. Coll. Radiol.) 2004;16(5):345–349. doi: 10.1016/j.clon.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann. Oncol. 2006;17(6):935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 3.Kleihues P, Cavenee WK International Agency for Research on Cancer. Pathology and Genetics of Tumours of the Nervous System. IARC Press; Lyon, France: 2000. p. 314. [Google Scholar]
- 4.Anders CK, Deal AM, Miller CR, et al. The prognostic contribution of clinical breast cancer subtype, age, and race among patients with breast cancer brain metastases. Cancer. 2011;117(8):1602–1611. doi: 10.1002/cncr.25746. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Retrospective analysis evaluating the influence of race over brain metastasis behavior in breast cancer.
- 5.Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tham YL, Sexton K, Kramer R, Hilsenbeck S, Elledge R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer. 2006;107(4):696–704. doi: 10.1002/cncr.22041. [DOI] [PubMed] [Google Scholar]
- 7.Stemmler HJ, Kahlert S, Siekiera W, Untch M, Heinrich B, Heinemann V. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006;15(2):219–225. doi: 10.1016/j.breast.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J. Clin. Oncol. 2006;24(36):5658–5663. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 9.Hicks DG, Short SM, Prescott NL, et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am. J. Surg. Pathol. 2006;30(9):1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 10.Kwon HC, Oh SY, Kim SH, et al. Clinical outcomes in patients with triple-negative breast cancer and brain metastases. J. Breast Cancer. 2010;13(2):160–166. [Google Scholar]
- 11.Slimane K, Andre F, Delaloge S, et al. Risk factors for brain relapse in patients with metastatic breast cancer. Ann. Oncol. 2004;15(11):1640–1644. doi: 10.1093/annonc/mdh432. [DOI] [PubMed] [Google Scholar]; • Comprehensive analysis of risk factors for developing brain metastasis in a retrospective series.
- 12.Mahmoud-Ahmed AS, Suh JH, Lee SY, Crownover RL, Barnett GH. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int. J. Radiat. Oncol. Biol. Phys. 2002;54(3):810–817. doi: 10.1016/s0360-3016(02)02967-x. [DOI] [PubMed] [Google Scholar]
- 13.Vallejos CS, Gomez HL, Cruz WR, et al. Breast cancer classification according to immunohistochemistry markers: subtypes and association with clinicopathologic variables in a peruvian hospital database. Clin. Breast Cancer. 2010;10(4):294–300. doi: 10.3816/CBC.2010.n.038. [DOI] [PubMed] [Google Scholar]
- 14.Castaneda C, Gomez H, Vallejos C, et al. P2–14–09: comparison between Spanish and Peruvian patients with early breast cancer. Poster Session 2 - epidemiology, risk, and prevention: ethnic/racial aspects. Cancer Res. 2011;71(24Suppl. 3) Abstract P2-14-09. [Google Scholar]
- 15.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997;37(4):745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 16.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Elaboration of a score using tumor phenotype to identify prognosis after brain metastasis: breast-graded prognostic assessment score.
- 17.Niwinska A, Tacikowska M, Murawska M. The effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death. Int. J. Radiat. Oncol. Biol. Phys. 2010;77(4):1134–1139. doi: 10.1016/j.ijrobp.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Simpson JF, Gray R, Dressler LG, et al. Prognostic value of histologic grade and proliferative activity in axillary node-positive breast cancer: results from the Eastern Cooperative Oncology Group Companion Study, EST 4189. J. Clin. Oncol. 2000;18(10):2059–2069. doi: 10.1200/JCO.2000.18.10.2059. [DOI] [PubMed] [Google Scholar]
- 19.Prat A, Cheang MC, Martin M, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J. Clin. Oncol. 2013;31(2):203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalian H, Tore HG, Horowitz JM, Salem R, Miller FH, Yaghmai V. Radiologic assessment of response to therapy: comparison of RECIST Versions 1.1 and 1.0. Radiographics. 2011;31(7):2093–2105. doi: 10.1148/rg.317115050. [DOI] [PubMed] [Google Scholar]
- 21.Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2000;47(4):1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]; •• Elaboration of a score using extra brain metastasis to identify prognosis after brain metastasis: recursive partitioning analysis score.
- 22.Agboola O, Benoit B, Cross P, et al. Prognostic factors derived from recursive partition analysis (RPA) of Radiation Therapy Oncology Group (RTOG) brain metastases trials applied to surgically resected and irradiated brain metastatic cases. Int. J. Radiat. Oncol. Biol. Phys. 1998;42(1):155–159. doi: 10.1016/s0360-3016(98)00198-9. [DOI] [PubMed] [Google Scholar]
- 23.Keam B, Im SA, Kim HJ, et al. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC Cancer. 2007;7:203. doi: 10.1186/1471-2407-7-203. [DOI] [PMC free article] [PubMed] [Google Scholar]