Abstract
Background
Cardiovascular disease, especially cardiomyopathy, was the major cause of death among owl monkeys (Aotus sp.) at a major colony and threatened colony sustainability. For this study, echocardiography (echo) and electrocardiography (ECG) normal values were established and cardiomyopathy animals identified.
Methods
Forty eight owl monkeys were studied, 30 older than ten years of age (“aged”) and 8 of age five years (“young”). Eight aged owl monkeys had cardiomyopathy.
Results and Conclusions
Aged Aotus had increased left ventricular posterior wall thickness over young animals. Left ventricular diameter, and ejection fraction appeared to be the best identifying measurements for cardiomyopathy. There were no differences in the ECG.
Keywords: AOTUS SP, CARDIOLOGY, CARDIOMYOPATHY, ECHOCARDIOGRAPHY, ELECTROCARDIOGRAPHY, HEART
INTRODUCTION
Cardiovascular disease is the major cause of death among owl monkeys at the University of South Alabama Center for Neotropical Primate Research and Resources (CNPRR). In addition, cardiovascular disease has been identified as the most important problem in maintaining a self-sustaining breeding colony [1].
Owl monkeys are the only nocturnal primates. They are a tree dwelling genus and are found in the Amazon Basin of South America and in Central America. They reach a maximum size of 1.5-2.0 kg. Owl monkeys become sexually mature at three years of age and are full grown at five years[9].
At the Batelle Primate Facility in Richland, Washington, a 1994 report stated that forty percent of owl monkeys that died showed some evidence of cardiovascular disease, such as a large globose heart due to ventricular dilation or concentric left ventricular hypertrophy [22]. At the CNPRR colony, we have also observed left ventricular hypertrophy in some owl monkeys [3]. Spontaneous death of owl monkeys may occur during periods of high stress or physical activity [22]. Many monkeys may display no clinical signs of heart disease before death. Other animals may die suddenly during or after routine manipulations such as weighing or cage changes.
Although observations at the CNPRR have shown that many owl monkeys do not show clinical signs of cardiovascular disease before death, some monkeys may develop clinical signs that can be associated with heart failure. Those signs include ascites, dyspnea, mouth breathing, reduced exercise tolerance, subcutaneous edema [22], and hepatomegaly. Some owl monkeys also have partial or complete paralysis caused by secondary thromboembolic disease [12]. Microscopic lesions such as arteriolosclerosis in the small vessels of the kidney, heart, and small intestine, and microthrombi or emboli in multiple sites have also been identified in owl monkeys post-mortem. Interestingly, these lesions have also been found in humans with chronic severe hypertension [10].
Cardiomyopathy causes the weakening of the heart muscle [22]. Echocardiography (Echo) is effective for diagnosing cardiomyopathy when combined with other diagnostic modalities, such as physical examination, laboratory analysis, radiology and ECG and provides information about other cardiovascular diseases as well. It allows for the study of cardiac function and provides measurements of wall thickness, chamber size, and ejection fraction by using measurements of left ventricular diameter at diastole and systole, interventricular septum at diastole, and left ventricular posterior wall at diastole [5]. This technique is noninvasive and allows the studies to be done rapidly, which is important in small, medically compromised animals. An electrocardiogram (ECG) can also be taken while the animal is anesthetized. Parameters that are measured in the ECG included PR, P, and QRS duration, QT interval, and atrial and ventricular rate.
A study that used both echo and ECG has been performed at the Center for Neotropical Research and Resoources on squirrel monkeys. This study established normal echo and ECG values and compared ECG values of squirrel monkeys and human infants [4]. The 2003 study also identified monkeys that had cardiomyopathy [5]. In 2005, Rishniw and colleagues published echocardiographic data and evaluation for owl monkeys with cardiomyopathy in captivity. They found that the echo parameters for left ventricular end-systolic cross sectional area and left ventricular fractional area change were the two echo parameters that showed the greatest difference between healthy monkeys and cardiomyopathy monkeys [18].
The objectives of the present study were: To establish a set of normal parameters for both echocardiography and electrocardiography for healthy owl monkeys at our center and to identify animals with evidence of cardiomyopathy before the onset of the disease. This study also compares the echocardiographic and electrocardiographic trends of cardiomyopathy in owl monkeys to those found in the previous studies on squirrel monkeys and humans. Specifically, we wished to:
Determine normal parameters of both echocardiography and electrocardiography for healthy owl monkeys (Based on echo, absence of clinical signs and physical examination). Parameters for echocardiography included ejection fraction (EF), left ventricular diameter at end-diastole (LVDd) and end-systole (LVDs), interventricular septal thickness at end-diastole (IVSd), and left ventricular posterior wall thickness at end-diastole (LVPWd). Parameters for electrocardiography included P wave amplitude, P, PR, and QRS duration, QT interval, and atrial and ventricular rate.
Identify owl monkeys with evidence of cardiomyopathy using the above parameters before the onset of the disease, and study the disease progression of two representative cases.
MATERIALS AND METHODS
The University of South Alabama Institutional Animal Care and Use Committee approved this study. All animals were housed at the CNPRR at the University of South Alabama at the time of the study. The Owl Monkey Breeding and Research Resource at the Center for Neotropical Primate Research and Resources consists of approximately 300 animals. Species in the colony include Aotus nancymai, Aotus vociferans, Aotus azarae, and a small number of hybrid animals. Approximately 20% of animals were feral-source. Animals are socially housed whenever possible (either in male-female or same-sex pairs) in stainless steel cages measuring 83 × 83 × 71 cm. Diet consisted of a nutritionally balanced monkey biscuit (Purina 5049 Fiber Plus®, Purina LabDiet, St. Louis, MO, USA) and a canned diet (Zupreem Primate Diet®, Zupreem, Shawnee, KS, USA). Diet was also supplemented with a variety of fresh fruits and vegetables.
Thirty animals, age ten years or older, were the aged animal group in this study. Animals that were feral-source had to be documented as being in captivity longer than ten years. Ten owl monkeys at age five years were selected as a control group. ECGs and echos were recorded with the animal anesthetized with ketamine or isoflurane. The animals were given 30 mg. ketamine by intramuscular injection. Medically fragile animals were anesthetized with isoflurane. Anesthesia was induced with a mixture of oxygen and 4-5% isoflurane and maintained with 1-3% isoflurane. Both the echo and ECG were performed only once for each animal, unless the animal showed signs of cardiomyopathy, such as ascites dyspnea, mouth-breathing. exercise intolerance, subcutaneous edema and hepatomegaly. Nephropathy and severe anemia are also seen in owl monkeys and may show similar clinical signs, but are ruled out by hematology and serum chemistry testing. If signs were seen, the frequency of the echo and ECG examinations was increased based on the progression of the disease. If an owl monkey was identified as healthy initially, and during the course of the study showed signs of cardiomyopathy, then that animal was removed from the study for healthy animals and identified as having cardiomyopathy. Many of the research techniques that were used are similar to published squirrel monkey studies [4,5.]. Diagnosis of cardiomyopathy was based on presence of clinical signs described above and confirmation by ultrasound. Other testing was done as described above to rule out renal disease and severe anemia.
ELECTROCARDIOGRAPHY STUDY
The ECG was recorded after anesthesia induction, but before echo was performed to allow time for muscle relaxation. ECGs were recorded using a Welch Allyn AT-1 electrocardiograph (Welch Allyn Inc., San Diego, CA, USA) and skin contact was made by the application of Welch Allyn disposable ECG Electrodes, which are adhesive gel pads. Lead placement was standard as performed in humans (Leads 1, 2, 3 AVR, AVL, AVF) [4]. All ECGs were recorded at a chart speed of 50 mm sec and the gain was adjusted to one-centimeter deflection per millivolt [4]. A 5 mm × 5 mm patch of fur was clipped from the medial surface of the forearms and ankle for lead placement. The ECG was recorded with the animal positioned in right lateral recumbency. The intervals recorded include PR, P, and QRS duration, QT interval, and the atrial and ventricular rate. These were defined in accordance with standard clinical usage [7, 16].
ECHOCARDIOGRAPHY STUDY
Echo was performed using a Siemens Accuson Aspen® Ultrasound Machine with a V7 transducer (Siemens Accuvue, Mountain View, CA, USA). Chest fur was removed with clippers. The transducer was placed in parasternal, apical, or subcostal areas of the chest and set to a penetration depth of 30 to 40 millimeters and a frequency of 7.0 MHz. The animals were positioned in dorsal recumbency and a foam wedge was used as a positioning aid in most cases. The views that were recorded included: parasternal long axis, parasternal short axis, apical four chamber, apical five chamber, and M-modes of the mitral valve, left ventricle, aortic valve, and left atrium. Placement of the transducer initially began to the right of the sternum and was then moved ventrolaterally to the right pectoralis major muscle. The transducer was then moved deep to the lateral edge of the pectoralis major muscle. The transducer reference mark was oriented craniad. This position allowed for an unobstructed path to and from the free wall of the heart by directing the beam around, instead of through the sternum. This positioning also allowed for the parasternal long axis view. The short-axis view was obtained by keeping the transducer in the same position as the long-axis view, but it was rotated until the view had been obtained. In order to view all four heart chambers, the transducer was positioned subcostally on the left side. These techniques have been used previously in neotropical primates [5]. The parameters recorded include wall thickness, ejection fraction, left ventricular diameter at end-diastole and systole, interventricular septal thickness at end-diastole, and left ventricular posterior wall thickness at end-diastole.
STATISTICAL ANALYSIS
Echocardiography and electrocardiography parameters were analyzed using one way analysis of variance. The groups of monkeys analyzed included healthy owl monkeys, age ten years and older, cardiomyopathy owl monkeys, age ten and over, and the control group, age five years. Comparisons were made between the healthy monkeys (age ten and over) and the control group in order to establish normal parameters. These were then compared to the values found for those owl monkeys found to have cardiomyopathy. Echocardiography provides excellent visualization of the left ventricle and readily distinguishes the three major forms of cardiomyopathy. Dilated cardiomyopathy is characterized by dilated chambers, most prominently the left ventricle, and reduced ejection fraction [17]. All colony animals are necropsied. Necropsy cardiac examination findings are compared with those obtained ante mortem by echocardiographic examination with excellent agreement. For this reason, we consider echocardiographic results to be diagnostic for cardiomyopathy.
The groups compared for statistical analysis are the control versus aged, healthy monkeys, control versus cardiomyopathy monkeys (age ten and over), and healthy (age ten and over) versus cardiomyopathy monkeys (age ten and over). In addition, data was analyzed from aged, healthy owl monkeys in comparison with one of the owl monkeys that had cardiomyopathy. A separate One-Way Analysis of Variance (ANOVA) was conducted for each parameter measured, with each One-Way ANOVA comparing the three groups. A P value of less than 0.05 was considered to be significant. Post-hoc tests were conducted to determine which groups were different. Post-hoc testing was used to check for statistical differences in the ECG and echo between the control group and the healthy group.
In addition, comparisons between the parameters that define cardiomyopathy in owl monkeys were compared to parameters of squirrel monkeys and humans [5]. A statistical analysis software package, Sigma Stat®, was used to analyze the data and graphs were made using Sigma Plot® (Systat Software, Point Richmond, CA, USA).
RESULTS
A total of 48 owl monkeys were studied. There were ten owl monkeys in the control group (age five) and 38 monkeys age ten and over. Thirty were classified as healthy and eight were diagnosed as having cardiomyopathy. The control group had six males and four females. The healthy group had 25 males and five females. The cardiomyopathy group had four males and four females.
Diagnosis of cardiomyopathy was based on physical examination, supplemented by imaging (ultrasound, radiology) and laboratory analysis (serum chemistry, hematology) to rule out other diseases with similar presentations, such as renal and pulmonary disease. Disease other than cardiomyopathy was ruled out for all animals in this study.
The comparison groups had no significant differences in the ECG parameters. Normal parameters for ECG were established for healthy owl monkeys age ten and over (Table 1). In the comparison of healthy aged owl monkeys to the control group, there were no significant differences in ejection fraction, left ventricular diameter at diastole and systole, interventricular septal thickness at end-diastole and data for each parameter is not included. The LVPWd for healthy owl monkeys was 23% higher than the control group (Figure 1). Normal parameters for ECG were established for healthy owl monkeys age ten and over (Table 1).
Table 1.
ECG mean values with standard error for control, healthy, and cardiomyopathy owl monkeys
| Atrial rate (/min) | Ventricular rate (/min) |
P-Wave amp (mm) | P-Wave duration (s) |
PR interval (s) | QRS interval (s) |
QT interval (s) | |
|---|---|---|---|---|---|---|---|
| Control | 237 ± 16.6 | 237 ± 16.6 | 2.3 ± 0.3 | 0.03 ± 0.0 | 0.1 ± 0.0 | 0.04 ± 0.0 | 0.1± 0.0 |
| Healthy 10+ | 225 ± 6.3 | 225 ± 6.3 | 2.7 ± 0.2 | 0.03 ± 0.0 | 0.1 ± 0.0 | 0.04 ± 0.0 | 0.2 ± 0.0 |
| CM | 218 ± 17.3 | 218 ± 17.3 | 2.9 ± 0.4 | 0.04 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
Figure 1.
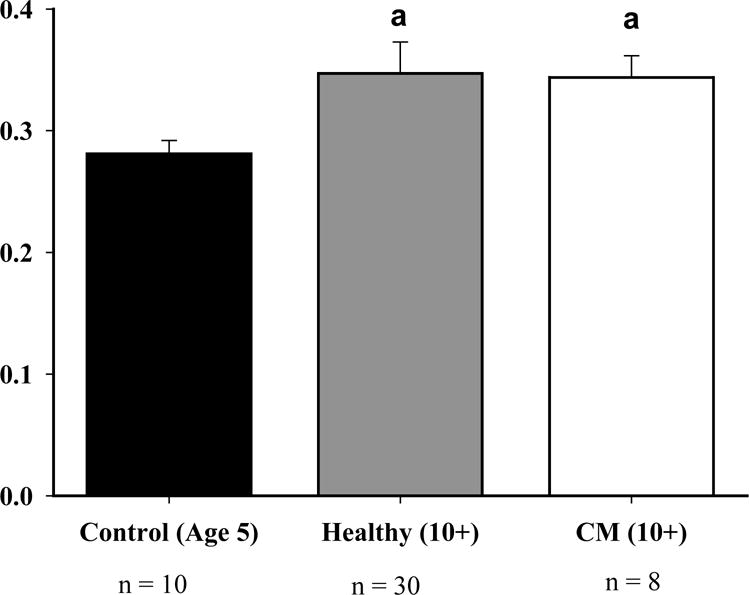
The LVPWd for healthy owl monkeys had a 23% increase when compared to the control group (P<0.05). In addition, there was a 22% increase in cardiomyopathy monkeys when compared to the control group (P<0.05). The a above the graph represents that both healthy and cardiomyopathy were greater than control.
Two cases illustrate the range of clinical progression with owl monkey cardiomyopathy and suggest the potential usefulness of prognostic indicators for the disease:
Case One
Eleven year-old domestically-born male was identified on a research echo as having left ventricular hypertrophy and aortic root dilation. Echo was repeated at six, nine and 34 weeks post-presentation. There was no progression of any of the abnormal measurements during this time. The animal remains alive and asymptomatic at the time of this report.
Case Two
Twelve year old domestically bred male presented for ascites on 9-10-01. The animal was treated with 12 mg furosemide PO twice daily until 3-20-06 when an echocardiogram showed poor contractility, pericardial effusion, an aortic aneurysm and an ejection fraction of 25%. Two mg. enalopril (Enacard®, Merial Ltd, Duluth, GA, USA) and 25 mg spironolactone (both twice daily, Aldactone®, Pharmacia, Chicago, IL, USA) were added to treatment. The animal’s condition stabilized until 4-3-06 when deterioration was again noted and another echo was performed that revealed worsening pericardial effusion and an ejection fraction of 16%. The animal was euthanatized. Necropsy confirmed pericardial effusion, aortic aneurysm, hepatomegaly and revealed pulmonary edema, cardiomegaly, and glomerulonephritis .
When comparing cardiomyopathy owl monkeys to the healthy old and young groups, significant differences were seen in the parameters ejection fraction, left ventricular diameter at end-diastole and end-systole, and left ventricular posterior wall thickness at end-diastole. The interventricular septal thickness at end-diastole had no differences between any of the comparison groups. The ejection fraction was 40% lower when compared to both the healthy (P<0.017) and control (P<0.025) groups (Figure 2). Cardiomyopathy monkeys had a 19% increase when compared to healthy (P<0.017) and a 14% increase when compared to the control group (P<0.025) for the left ventricular diameter at end-diastole (LVDd) (Figure 3). The left ventricular diameter at end-systole (LVDs) had a 36% increase in cardiomyopathy monkeys, when compared to healthy monkeys (P<0.017), and a 25% increase when compared to the control group (P<0.025). The left ventricular posterior wall thickness at end-diastole had a 22% increase in cardiomyopathy monkeys when compared to the control group (P<0.05) (Figure 1).
Figure 2.
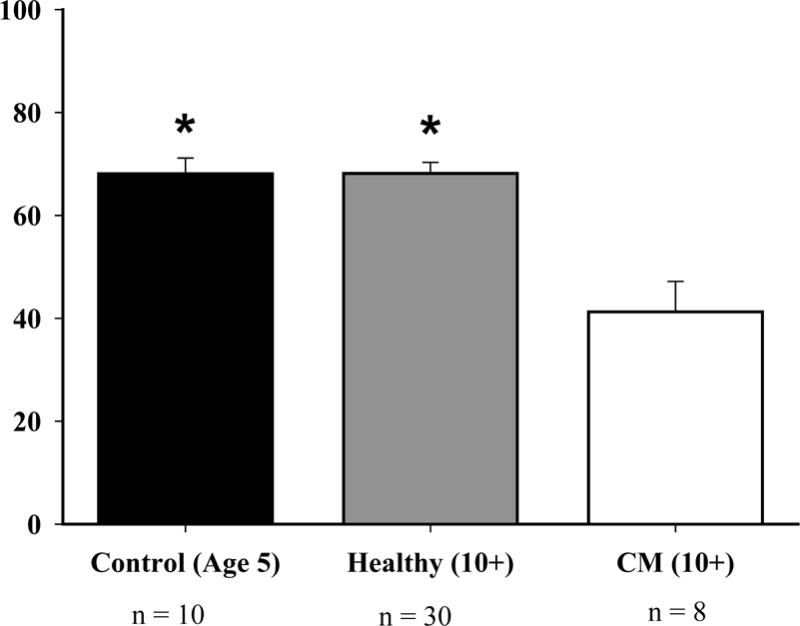
The ejection fraction, which is a measure of pump function, was decreased by 40% in cardiomyopathy owl monkeys when compared to both the healthy owl monkeys (P<0.017) and control owl monkeys (P<0.025). The * above the graph represents that both control and healthy owl monkeys have a greater ejection fraction than cardiomyopathy owl monkeys.
Figure 3.
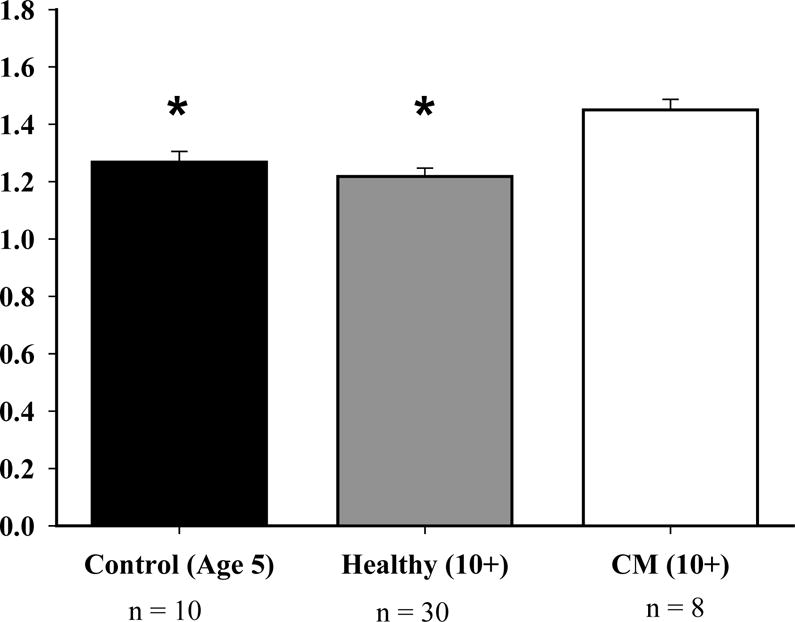
The LVDd for cardiomyopathy owl monkeys increased by 19% when compared to the healthy owl monkeys (P<0.017) and increased by 14% when compared to the control owl monkeys (P<0.025). The * above the graph represents that both control and healthy owl monkeys have a decreased LVDd than cardiomyopathy monkeys.
Owl monkey two was compared to the healthy owl monkeys age ten and over. This monkey had cardiomyopathy based on his echo. Both IVSd and IVSs were 38% thicker when compared to the healthy group. LVPWd was 28% thicker when compared to the healthy group.
DISCUSSION
Our study examined the echocardiographic and electrocardiographic parameters in order to establish normal values for healthy owl monkeys, age ten and over, and to identify owl monkeys that had cardiomyopathy. We found significant changes in parameters for those owl monkeys that were identified as having cardiomyopathy compared with healthy owl monkeys age ten and over and the five-year old control group. There was also a change in parameters for healthy owl monkeys age ten and over when compared to the control group.
In order to determine if there was a change in any of the echo parameters based on increasing age, a comparison was made between healthy owl monkeys, age ten and over, to the control group (age five years). The left ventricular posterior wall thickness at end-diastole was increased in the older healthy group when compared to the control group. In human adults, thickening of the posterior wall is attributed to cardiovascular diseases such as hypertension and coronary disease [6]. We speculate that an increase in left ventricular posterior wall thickness in owl monkeys may also be due to hypertension. From these results, we conclude that aging owl monkeys are more likely to have an increase in LVPWd than younger owl monkeys. Increase in myocardial wall dimensions is associated with aging in humans. Other risk factors for hypertrophy include hypertension, obesity, and valvular heart disease [10]. In our experience, valvular heart disease and obesity are rare in owl monkeys.
Decrease in ejection fraction indicates decreased pumping capacity and is usually associated with systolic heart failure in humans [14]. Cardiomyopathy owl monkeys had an increase in LVPWd. LVPWd was increased in all older owl monkeys, regardless of whether they were diagnosed with cardiomyopathy or not. This increase is indicative of left ventricular hypertrophy [6]. These kind of changes are also seen with advanced age in humans [10]. Although the squirrel monkey study [5] found that LVDd was not identified as clinically important, we believe that it may be clinically important in owl monkeys. A previous owl monkey study documented an increase in LVDd and LVDs, which are possibly associated with systolic heart failure [18]. We conclude that cardiomyopathy owl monkeys may show differences in ejection fraction, left ventricular diameter at end-diastole and end-systole.
The first published echocardiographic values for both normal and cardiomyopathy owl monkeys [18] contain both similarities and differences when compared to our study. The first study found that the most reliable parameters that distinguished between normal and cardiomyopathy owl monkeys were the left ventricular end-systolic cross-sectional area and left ventricular fractional area change. They found that monkeys that developed cardiomyopathy had an increase in left ventricular diameter at systole. Our study did not include these as parameters of study because cross-sectional area is no longer commonly used in primate species due to improvements in echocardiographic imaging. We used instead ejection fraction as a measure of pump function. The ejection fraction is derived from the fractional shortening (end-diastolic diameter minus end systolic diameter divided by end-diastolic diameter, times 1.7) [20], In our study, left ventricular diameter at end-diastole, and ejection fraction appear to be the most important identifying factors of cardiomyopathy in owl monkeys.
Approximately five million patients in the United States have heart failure [14]. Owl monkeys may be useful as a model for heart failure in humans. In our study, three monkeys exhibited the four stages in evolution of heart failure, which was published by the American college of Cardiology and American Heart Association. This classification scheme is inclusive of all types of cardiovascular diseases and not just cardiomyopathy. Stage A identifies a patient, who is at risk of heart failure, but does not have a structural disorder of the heart. In this study, stage A would be represented by healthy aged owl monkeys, because Cardiomyopathy is the most common cause of death in this colony. Stage B identifies patients that have a structural disorder of the heart, but show no symptoms of heart failure. In our study, stage B is represented by healthy owl monkeys that had an increase in wall thickness, demonstrated by an increase in left ventricular posterior wall thickness at end diastole. In humans, this increase is associated with hypertension or infiltrative disease [6]. The third stage, stage C, represents patients, who not only have structural heart disease, but also have had past or current symptoms. Owl monkey One exhibited an increase in interventricular septum thickness at end diastole, which is associated with left ventricular hypertension in humans. The increase in left ventricular posterior wall thickness at end diastole and subjective 2D assessment in the parasternal long axis view demonstrated left ventricular hypertrophy in this animal (Figure 2). Left ventricular hypertrophy may also be seen in humans at stage C. In stage D, patients have clinical signs that are refractory to treatment [14]. Owl monkey Two exhibited this type of end-stage heart failure. Not only did this monkey exhibit poor contractility and both left and right ventricular hypertrophy, but it also had pericardial effusion. Additional findings at necropsy included dilatation of the ascending aorta and a dissecting aortic aneurysm that extended into the renal arteries. In addition, this owl monkey exhibited nephropathy. Most owl monkeys with cardiomyopathy also have nephropathy [13].
An unexpected finding in this study was the large number of animals that were identified with cardiomyopathy. This strengthened our comparison studies for the control, healthy, and cardiomyopathy monkeys.
Studies using non-human primates may have increased variability because some animals may be of feral origin or their genetic backgrounds may not be well-characterized. Most animals in this study were domestically bred and have known pedigrees. This reduces some of this variability. Subject animals were not directly related. The echo imaging may also have variability from one animal to another. This underscores the need to examine echo findings in the broader context of findings from physical examination, laboratory results, echo, and husbandry observations. The variability in imaging technique was reduced by using the same sonographer and using the same methodology previously developed and tested in squirrel monkeys.
Anesthesia may also be a source of variability. In studies with children with congenital heart disease, the use of isoflurane as an anesthetic increased heart rate and lowered vascular resistance, but maintained systemic cardiac output, with little change in contractility [19]. Thus the use of isoflurane versus other halogenated anesthetics, such as halothane, is favored during echo examinations of medically fragile animals. Although the evidence is subjective, we have not observed differences between echocardiograms of owl monkeys anesthetized by the two different methods.
There were no ECG changes observed that were associated with age. This differs from findings in squirrel monkeys, where longer P and QRS durations were found [5] in older animals. A squirrel monkey diagnosed with cardiomyopathy in that report had increased heart rate over its own healthy ECG and age-matched controls. This change was not seen in owl monkeys with cardiomyopathy. The possibility that a larger study might unmask subtle differences cannot be ruled out.
Studies are ongoing to investigate the role of hypertension as a possible cause of cardiomyopathy in owl monkeys. Cardiomyopathy has a decreased prevalence in owl monkeys that have not been in captivity for an extended period of time [13]. A previous study indicated that owl monkeys react to laboratory disturbances with significant, prolonged elevations in blood pressure [21] The study of cardiomyopathy in owl monkeys is not only important in maintaining a self-sustaining breeding colony, but it may be a useful model for human cardiomyopathy.
Figure 4.
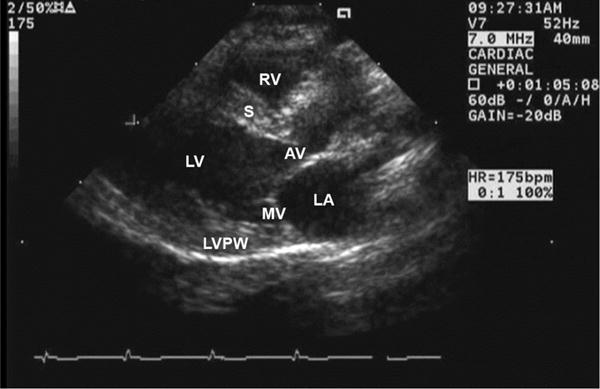
Normal parasternal long axis view for a healthy owl monkey. MV = Mitral Valve; LV = Left Ventricle; LVPW = left ventricular posterior wall; S = Interventricular septum; LA = left atrium; AV = aortic valve; RV = right ventricle
Figure 5.
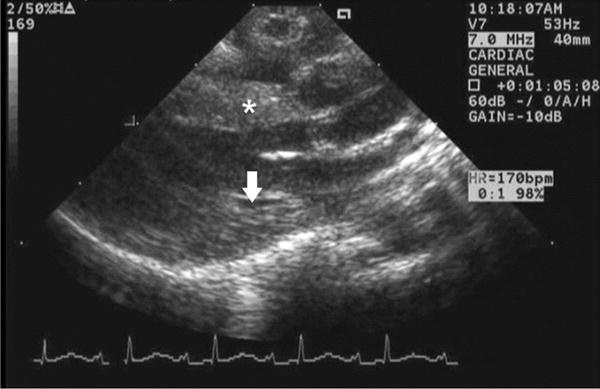
Parasternal long axis view of owl monkey 2, where the arrow indicates thickening of left ventricular posterior wall and the asterisk indicates thickening of the septum.
Table 2.
Echo mean values with standard error for control, healthy, and cardiomyopathy owl monkeys
| Ejection fraction (%) |
LVDd (cm) | LVDs (cm) |
IVSd (cm) |
LVPWd (cm) | Chamber Size (%) | |
|---|---|---|---|---|---|---|
| Control | 68.1 ± 3.0 | 1.3 ± 0.0 | 1.0 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 23.6 ± 1.9 |
| Healthy 10+ | 68.2 ± 2.1 | 1.2 ± 0.0 | 0.9 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 27.1 ± 1.5 |
| CM | 41.3 ± 5.9 | 1.5 ± 0.0 | 1.2 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.0 | 15.9 ± 2.1 |
Acknowledgments
The assistance of Leah Carmichael, George Tustin, Jenne Westberry, and Craig Youngman with this study is gratefully acknowledged. Supported in part by grant 5R24RR020052-03 from the National Center for Research Resources (NCRR). Contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR.
Funding: Supported in part by grant 5R24RR020052-03 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- 1.Akkoc CC. Personal Communication. 2005 [Google Scholar]
- 2.Baer JF, Gibson SV, Weller RE, Buschbom RL, Leathers CW. Naturally occurring aortic aneurysms in owl monkeys (Aotus spp) Lab An Sci. 1992;42:463–466. [PubMed] [Google Scholar]
- 3.Brady AG, Abee CR. Unpublished data. 2003 [Google Scholar]
- 4.Brady AG, Johnson WH, Botchin MB, Williams LE, Scimeca JM, Abee CR. Developmental changes in ECG associated with heart rate are similar in squirrel monkey and human infants. Lab An Sci. 1991;41:596–601. [PubMed] [Google Scholar]
- 5.Brady AG, Watford JW, Massey CV, Rodning KJ, Gibson SV, Williams LE, Abee CR. Studies of heart disease and failure in aged female squirrel monkeys (Saimiri sp) Comp Med. 2003;53:657–662. [PubMed] [Google Scholar]
- 6.Chambers J. Echocardiography in Clinical Practice. New York: The Parthenon Publishing Group; 2002. [Google Scholar]
- 7.Davignon AP, Rautaharju P, Baiselle E, Soumis F, Choquette A. Normal ECG standard for infants and children. Pediatr. Cardiol. 1979/1980;1:123–131. [Google Scholar]
- 8.Feigenbaum H. Echocardiography. Philadelphia: Lea and Febiger; 1986. [Google Scholar]
- 9.Ford S. Taxonomy and distribution of the owl monkey. In: Baer JF, Weller RE, Kakoma I, editors. Aotus: the owl monkey. Philadelphia: Academic Press Inc; 1994. pp. 1–60. [Google Scholar]
- 10.Fuster V, O’Rourke RA, Alexander RW, Nash I, Roberts R, King SB, Prystowski EN. Hurst’s the Heart. New York: The McGraw-Hill Companies, Inc.; 2004. [Google Scholar]
- 11.Gibson SV, Williams LE, Brady AG, Abee CR. Pregnancy associated mortality in squirrel monkeys (Saimiri spp) Am J Primat. 1994;33:211. [Google Scholar]
- 12.Gibson SV, Brady AG, Abee CR. Paresis and paralysis due to thromboembolism in three owl monkeys (Aotus spp) Contemp Topics in Lab Anim Sci. 1995;34:46. [Google Scholar]
- 13.Gozalo A, Dagle GE, Montoya E, Weller RE, Malaga CA. Spontaneous cardiomyopathy and nephropathy in the owl monkey (Aotus sp.) in captivity. J Med Primat. 1992;21:279–284. [PubMed] [Google Scholar]
- 14.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russell RO, Smith SC., Jr C/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2001;38:2101–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 15.Kasper DL, Braunwald E, Fauci A, Hauser S, Longo D, Jameson JL. Harrison’s Principles of Internal Medicine. New York: The McGraw-Hill Companies, Inc; 2005. [Google Scholar]
- 16.Liebman J, Rudy YL. Electrocardiography. In: Liebman J, Moller JH, Neal WA, editors. Fetal, Neonatal, and Infant Cardiac Disease. New York: Appleton and Lange; 1990. pp. 179–245. [Google Scholar]
- 17.Otto C. The Practice of Clinical Echocardiography. Philadelphia: WB Saunders Company; 2002. [Google Scholar]
- 18.Rishniw M, Schiavetta AM, Johnson TO, Erb HN. Cardiomyopathy in captive owl monkeys (Aotus nancymai) Comp Med. 2005;55:162–168. [PubMed] [Google Scholar]
- 19.Rivenes SM, Lewin MB, Stayer SA, Bent ST, Schoenig HM, McKenzie ED, Fraser CD, Andropoulos SB. Cardiovascular effects of sevoflurane, isoflurane, halothane, and fentanyl-midazolam in children with congenital heart disease. Anesthesiology. 2001;94:223–229. doi: 10.1097/00000542-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Rolden C. The Ultimate Echo Guide. Philadelphia: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 21.Smith OA, Astley CA. Naturally occurring hypertension New World nonhuman primates: potential role of the perifornical hypothalamus. Am J Physiol Regul Integr Compu Physiol. 2006;292:R937–R945. doi: 10.1152/ajpregu.00400.2006. [DOI] [PubMed] [Google Scholar]
- 22.Weller RE. Infectious and noninfectious diseases in owl monkeys. In: Baer JF, Kakoma I, Weller RE, editors. Aotus: The Owl Monkey. San Diego: Academic Press Inc.; 1994. pp. 193–196. [Google Scholar]


