Abstract
Background:
Out of Hospital Cardiac Arrest (OHCA) remains not an uncommon occurrence in USA and the rest of the world. However, the survival to discharge following an episode of OHCA in adults is still very disappointing at around 10%. Several areas of improvement including education of general public in early Cardio Pulmonary Resuscitation (CPR) by bystander, chest compression first, and improvement of Emergency Medical response time have had a positive effect on the outcomes and survival but still much needs to be done. Recently, new data has emerged with regards to post resuscitation care and mild induced hypothermia (now preferably called; Targeted Temperature Management {TTM}) and several advances have been made.
Conclusion:
The purpose of this review is to summarize and compare the most recent guidelines and also provide a practical approach to TTM especially with regards to the field of cardiology.
Keywords: Out of hospital cardiac arrest, cardio pulmonary resuscitation, emergency medical response, targeted temperature management, post resuscitation care
1. INTRODUCTION
Out of Hospital Cardiac Arrest (OHCA) is a not an uncommon occurrence in the general United States population with an estimated annual incidence of 7307 events per year in pediatric population and 110.8 events per 100,000 adult population [1]. Which roughly translates into 374,000 episodes of OHCA in the US adult population [1]. Despite improvement in delivery of health care, the survival to hospital discharge post OHCA remains abysmal at 10.5% in adults >18 years, 23.5% for children 13 to 18 years, 16.6% for children >1 to 12 years, and 6.2% for infants [1]. However, there has been a concerted effort by several organizations and also at at community level to improve the efficiency and response time by Emergency Medical Services. In addition, there have been recent trials which have specifically focused on post resuscitative care including targeted temperature management and new data have emerged. The International Liaison Committee on Resuscitation (ILCOR) issued an advisory statement on temperature management in 2015 and this was followed by American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care [2, 3]. This review summarizes and compares these recommendations along with the European guidelines and also provides a practical overview of the process of targeted temperature management especially with regards to the cardiologists. In this review, the term Targeted Temperature Management (TTM) will be used to refer to mild induced therapeutic hypothermia as suggested by the most recent guidelines.
2. CURRENT GUIDELINES
The 2015 ILCOR Advisory statement gave specific recommendations about the following 3 questions: should induced mild hypothermia be used in post cardiac arrest syndrome, when should it be instituted and for how long?
With regards to the first question whether TTM be used in the following 3 scenarios were described [2].
For patients with OHCA and initial shockable rhythm (i.e. Ventricular fibrillation or pulseless ventricular tachycardia), when compared to no TTM, the pooled Relative risk (RR) reduction was 0.75 (95% Confidence Interval {CI} 0.61-0.92) and 0.73 (95% CI, 0.60-0.88) for mortality and for poor neurological or functional outcome at 6 months or at hospital discharge respectively [4, 5].
While for OHCA survivors with initial non-shockable rhythm, the adjusted pooled Odds Ratio (OR) was, 0.90; (95% CI, 0.45-1.82) [6-8].
On the other hand, for in hospital cardiac arrest (IHCA), the evidence to support use of TTM was less impressive with an adjusted OR for mortality of 1.11; (95% CI, 0.81-1.54) and for poor neurological outcome of (adjusted OR, 1.08; 95% CI, 0.76-1.5) [9].
Based on the above review of evidence they gave a strong recommendation for OHCA and shockable rhythm, a weak recommendation for OHCA and non-shockable rhythm and weak recommendation for IHCA of any rhythm.
They however broadened the range for TTM from 32-34 degree Celsius to 32-36 degree Celsius in keeping with a large randomized controlled trial comparing 33 vs 36 degree temperature targets which found no difference between the two temperature groups [10].
The 2015 American Heart Association (AHA) Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care, however, gave a Class I, Level of evidence (LOE) B-R (randomized) for shockable rhythm OHCA; a Class I, LOE C-Expert Opinion (EO) for non-shockable and in-hospital cardiac arrests [3]. They agreed with ILCOR Advisory statement and also broadened the temperature range to 32°C to 36°C during TTM (Class I, LOE B-R).
While the European guidelines simply endorsed the ILCOR Advisory statement [11].
With regards to the 2nd question of how soon after cardiac arrest TTM be initiated the following recommendations were made:
Based on the review of randomized control trials pre-hospital cooling with rapid infusion of normal saline compared to no cooling was not associated with improved neurologic outcomes outcome (RR, 1.00; 95% CI, 0.95–1.06) [2]. Nor was there a mortality benefit (RR, 0.98; 95% CI, 0.92–1.04). However, the rearrests and occurrence of pulmonary edema were found to be significantly increased in the pre-hospital cooling arm (RR, 1.22; 95% CI, 1.01–1.46), and (RR, 1.34; 95% CI, 1.15-1.57) respectively [2, 12]. Based on these data, all three guidelines recommended against the routine use of pre-hospital cooling with rapid infusion of intravenous fluids. However, the effects of cooling in pre-hospital setting using methods other than rapid infusion of intravenous fluids are yet to be investigated.
2.1. Definition of Comatose
OHCA patients after Return of Spontaneous Circulation (ROSC) who remain comatose i.e. who are unable to fully follow all verbal commands, should be considered for TTM [13]. However, one usually encountered clinical dilemma is, that patient may not be appropriately following verbal commands within few minutes of ROSC, but after a certain interval, most patients do show some improvement in neurologic status even prior to institution of TTM. The question then becomes whether to institute TTM or not. The Guidelines however, don’t specifically address this question but the 2 most important trials excluded patients if they could follow verbal commands, thus, common sense would dictate that the patient’s most recent neurologic status rather than immediate post ROSC neurologic status should dictate whether TTM should be instituted or not [4, 5].
2.2. Contraindications to TTM
Although there are very few true contraindications to TTM, they include intracranial bleeding, severe sepsis, excessive bleeding to refractory hypotension and pregnancy [13].
2.3. Duration of Hypothermia
The ILCOR Advisory statement recommended TTM for at least 24 hours post arrest as was the trial design of the two largest trials [5, 10].
2.3.1. Temperature Management Post Rewarming
For Patients who are managed with TTM post arrest fever will usually not occur until after 48-72 hours [3]. However, post rewarming, fever can be observed in 41-52% of patients [14, 15] and there is observational data that suggests that fever post arrest can be associated with poor outcomes [16, 17]. Hence, the AHA guidelines gave a Class IIB, LOE C-LD (limited data) for actively preventing fever in post rewarming period in the unconscious patients and the most practical method may be to leave the equipment used for TTM in place longer than the duration of TTM [3].
2.3.2. Technique of TTM
The process of TTM can broadly be divided into three distinct phases: induction, maintenance and rewarming [18].
2.3.3. Induction
In this phase the goal is to drop the mean core temperature to the target temperature using various external or internal cooling methods.
2.3.4. Choosing the Target Temperature
Previously AHA Guidelines and European Resuscitation Council (ERC) guidelines had suggested a target temperature of 32◦ to 34◦ C [19]. However, a large randomized controlled trial showed no difference in outcomes when using 33◦C versus 36◦C targets [9]. Therefore, the ILCOR advisory committee, AHA and ERC have accordingly broadened the range for TTM to 32◦ to 36◦ C. However, choosing the target temperature should include clinical information like presence of high risk for bleeding, like a post Percutaneous Coronary Intervention (PCI) patient, who could be cooled to a higher target temperature to minimize risk of bleeding. On the other hand, patients with evidence of seizures or cerebral edema etc. could be cooled to a lower temperature.
2.4. How to Cool
Different methods have used and shown to be effective. The most commonly used ones with their pros and cons are enumerated below:
Cooling by ice packs or wet towels: cheap and effective in decreasing the core temperature but maintenance of hypothermia is difficult and requires excessive diligence on part of the nursing staff [20-23]. Also rewarming is unreliable.
External air or water circulating cooling pads or blankets: easy to apply reliable temperature control [24].
External water circulating cooling pads with gel coating [25, 26].
Intravascular cooling by way of a large bore central venous access which circulates cool saline through different sized balloons in the venous circulation which removes heat from blood by conduction and brings down the core temperature [27-30].
Evaporative trans nasal cooling: this can be started before the return of ROSC [8-10].
Extra Corporeal Membrane Oxygenation (ECMO): rarely used for just this purpose but if patient is maintained on ECMO for clinically required hemodynamic support then it can also be used to monitor and regulate core temperature [31, 32].
Of the above mentioned methods cooling by gel coated pads with circulating water and invasive methods like cooling with intravascular cooling catheters are more commonly employed and will be described below in detail.
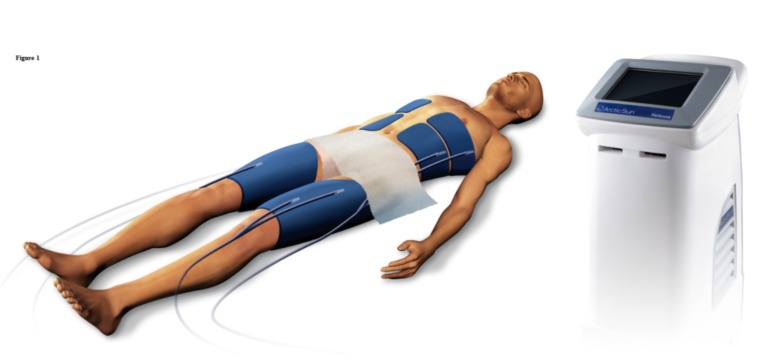
Cooling by gel coated water circulating adhesive pads as depicted in the Fig. (1), offers special advantages. Studies have shown that it is as effective as conventional methods in reducing the core temperature [33]. One randomized controlled study actually showed that the number of patients who reached core temperatures below 34 degrees was higher for Artic Sun® than conventional cooling methods although not statistically significant [25]. It also reliably maintains the core temperature and also provides more reliably rewarming [18]. The cons include additional cost associated with system. The prototype for this in US is Artic Sun®5000 (Brad medical, Denver, Colorado, USA) and ZOLL’S STx™ (Asahi Kasei/Zoll medical corporation, San Jose, California, USA) Surface Pad System. Another potential concern for these external cooling pads is a patient with skin injury or burns or very obese patients.
Fig. (1).
Artic Sun gel coated water circulating cooling pads with the typical conformation of pads.
Cooling by invasive catheters requires placement of a large bore central venous line either in internal jugular, subclavian or femoral position. The most common brands include Coolgard TM 3000/Alsius Icy heat Exchange catheter Thermal Regulating system (Alsius Corporation, Irvine, California, USA) and ZOLL Thermogard XP® Temperature Management System (Asahi Kasei/Zoll medical corporation, San Jose, California, USA). The catheters contain balloons through which cool saline goes in and cools the venous blood and the warm saline is removed. It is however a closed system so that the coolant does not enter the venous circulation of the patient. Most catheters in the market in addition to inlet and out let ports for the cooling fluid also have ports which can be used for central venous access Fig. (2). Cons include placing a large bore central venous catheter as well as small risk of catheter associated infection and thrombosis which is seen with any central venous catheter [18].
Fig. (2).
Coolgard TM 3000/Alsius Icy heat Exchange catheter Thermal regulating system with the inlet and out ports along with a third port for intravenous infusion.
2.4.1. Maintenance Phase
During this phase the target core temperature is maintained for least 24 hours.
2.4.2. Rewarming Phase
During this phase the core temperature is gradually raised roughly 0.25-0.5◦C/hour [34]. There can be significant electrolyte derangements and fluid shifts during the maintenance and rewarming phases. It has been shown that rebound hyperthermia in the post TTM phase is associated with poor outcomes [16, 17]. Therefore, it may be prudent to leave the equipment used for TTM in place a bit longer to potentially prevent this phenomenon. In addition use of prophylactic methods like scheduled acetaminophen or other anti-pyretics can be instituted but, such interventions have not be studied in randomized trials. The AHA 2015 guidelines give a Class IIb, (LOE C-LD; Limited data) recommendation for actively preventing fever in post TTM patients [3].
2.5. The Post Resuscitation Syndrome
It describes a condition following global body ischemia/reperfusion and it resembles systemic inflammatory syndrome in several ways including release of inflammatory mediators, cytokines and endothelial dysfunction [35-38]. There are several metabolic and electrolyte derangements which are quite frequently observed and which can get exaggerated with TTM like elevated lactic acid, hypokalemia, hypocalcemia and hypomagnesemia [10, 18, 39]. In addition there are also coagulation abnormalities which become more exaggerated with the institution of TTM and may increase susceptibility to bleeding [40, 41]. This becomes especially relevant in the setting of recent coronary intervention. So for a cardiologist taking care of patients with OHCA, it is quite essential to be familiar with these derangements as it may require adjustments of the medicine dosages like heparin infusion etc. Also the key branching point with regards to management of OHCA is the post Return of Spontaneous Circulation (ROSC) Electrocardiogram (ECG). If the ECG shows ST segment elevation or new Left Bundle Branch Block (LBBB) then there is at least observational data that immediate coronary angiogram and possibly primary Percutaneous Coronary Angioplasty (PTCA) could result in improved survival [42-44]. The guidelines agree on immediate coronary angiography in this particular situation. The AHA guidelines give a (Class I, LOE B-NR) recommendation while the European guidelines give a strong recommendation with limited low quality data [11]. On the other hand, if the post ROSC ECG does not show clear STEMI or LBBB or then proceeding with coronary angiography is advocated by some but randomized data is lacking. The PEARL Study which is actively recruiting patients to answer this specific question: whether immediate coronary angiography and if feasible PCI improves outcomes in non-STEMI OHCA patients when compared to no angiography [45].
CONCLUSION
In summary the OHCA is a not an uncommon occurrence and in some instances the precipitating factor or at least a contributing factor may be myocardial ischemia/ infarction in addition to several other non-cardiac causes. The ILCOR statement and American and ESC guidelines are consistent with regards to institution of TTM. However, they differ in the strength of recommendation. In any case, there seems to be a growing body of data which supports TTM in all cases of OHCA patients but the minimum target temperature to be achieved has now been changed to 36◦ in view of recent data.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
All authors have contributed substantially to the design, performance, analysis, or reporting of the work as well as the article’s drafting the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Benjamin E.J., Blaha M.J., Chiuve S.E., et al. Heart disease and stroke statistics’2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnino M.W., Andersen L.W., Berg K.M., et al. Temperature management after cardiac arrest. Circulation. 2015;132(25):2448–2456. doi: 10.1161/CIR.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 3.Callaway C.W., Donnino M.W., Fink E.L., et al. Part 8: Post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18):S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard S.A., Gray T.W., Buist M.D., et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 5.England T.N. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 6.Vaahersalo J., Hiltunen P., Tiainen M., et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in Finnish intensive care units: The FINNRESUSCI study. Intensive Care Med. 2013;39(5):826–837. doi: 10.1007/s00134-013-2868-1. [DOI] [PubMed] [Google Scholar]
- 7.Dumas F., Grimaldi D., Zuber B., et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients?: Insights from a large registry. Circulation. 2011;123(8):877–886. doi: 10.1161/CIRCULATIONAHA.110.987347. [DOI] [PubMed] [Google Scholar]
- 8.Testori C., Sterz F., Behringer W., et al. Mild therapeutic hypothermia is associated with favourable outcome in patients after cardiac arrest with non-shockable rhythms. Resuscitation. 2011;82(9):1162–1167. doi: 10.1016/j.resuscitation.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Nichol G., Huszti E., Kim F., et al. Does induction of hypothermia improve outcomes after in-hospital cardiac arrest? Resuscitation. 2013;84(5):620–625. doi: 10.1016/j.resuscitation.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen N., Wetterslev J., Cronberg T., et al. Targeted temperature management at 33degreeC versus 36degreeC after cardiac arrest. N. Engl. J. Med. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 11.Nolan J.P., Soar J., Cariou A., et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015. Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Kim F., Nichol G., Maynard C., et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest. JAMA. 2014;311(1):45. doi: 10.1001/jama.2013.282173. [DOI] [PubMed] [Google Scholar]
- 13.Scirica B.M. Therapeutic hypothermia after cardiac arrest. Circulation. 2013;127(2):244–250. doi: 10.1161/CIRCULATIONAHA.111.076851. [DOI] [PubMed] [Google Scholar]
- 14.Leary M., Grossestreuer A.V., Iannacone S., et al. Pyrexia and neurologic outcomes after therapeutic hypothermia for cardiac arrest. Resuscitation. 2013;84(8):1056–1061. doi: 10.1016/j.resuscitation.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Cocchi M.N., Boone M.D., Giberson B., et al. Fever after rewarming: Incidence of pyrexia in postcardiac arrest patients who have undergone mild therapeutic hypothermia. J. Intensive Care Med. 2014;29(6):365–369. doi: 10.1177/0885066613491932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bro-Jeppesen J., Hassager C., Wanscher M., et al. Post-hypothermia fever is associated with increased mortality after out-of-hospital cardiac arrest. Resuscitation. 2013;84(12):1734–1740. doi: 10.1016/j.resuscitation.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Winters S.A., Wolf K.H., Kettinger S.A., Seif E.K., Jones J.S., Bacon-Baguley T. Assessment of risk factors for post-rewarming “rebound hyperthermia” in cardiac arrest patients undergoing therapeutic hypothermia. Resuscitation. 2013;84(9):1245–1249. doi: 10.1016/j.resuscitation.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Polderman K.H., Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: Practical considerations, side effects, and cooling methods*. Crit. Care Med. 2009;37(3):1101–1120. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- 19.Peberdy M.A., Callaway C.W., Neumar R.W., et al. Part 9: Post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18) Suppl. 3:S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 20.Larsson I.M., Wallin E., Rubertsson S. Cold saline infusion and ice packs alone are effective in inducing and maintaining therapeutic hypothermia after cardiac arrest. Resuscitation. 2010;81(1):15–19. doi: 10.1016/j.resuscitation.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Bernard S.A., Gray T.W., Buist M.D., et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 22.Knafelj R., Radsel P., Ploj T., Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation. 2007;74(2):227–234. doi: 10.1016/j.resuscitation.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Hovdenes J., Laake J.H., Aaberge L., Haugaa H., Bugge J.F. Therapeutic hypothermia after out-of-hospital cardiac arrest: Experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol. Scand. 2007;51(2):137–142. doi: 10.1111/j.1399-6576.2006.01209.x. [DOI] [PubMed] [Google Scholar]
- 24.Bro-Jeppesen J., Kjaergaard J., Horsted T.I., et al. The impact of therapeutic hypothermia on neurological function and quality of life after cardiac arrest. Resuscitation. 2009;80(2):171–176. doi: 10.1016/j.resuscitation.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Heard K.J., Peberdy M.A., Sayre M.R., et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation. 2010;81(1):9–14. doi: 10.1016/j.resuscitation.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haugk M., Sterz F., Grassberger M., et al. Feasibility and efficacy of a new non-invasive surface cooling device in post-resuscitation intensive care medicine. Resuscitation. 2007;75(1):76–81. doi: 10.1016/j.resuscitation.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Gaieski D.F., Band R.A., Abella B.S., et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–424. doi: 10.1016/j.resuscitation.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Sunde K., Pytte M., Jacobsen D., et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Aberle J., Kluge S., Prohl J., Rother J., De Heer G., Kreymann G. Hypothermia after CPR through conduction and convection - Initial experience on an ICU. Intensivmed. Notfallmed. 2006;43(1):37–43. [Google Scholar]
- 30.Feuchtl A., Gockel B., Lawrenz T., Bartelsmeier M., Stellbrink C. Endovascular cooling improves neurological short-term outcome after prehospital cardiac arrest. Intensivmed. 2007;44(1):37–42. [Google Scholar]
- 31.Stub D., Bernard S., Pellegrino V., et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation. 2015;86:88–94. doi: 10.1016/j.resuscitation.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Nagao K., Kikushima K., Watanabe K., et al. Early induction of hypothermia during cardiac arrest improves neurological outcomes in patients with out-of-hospital cardiac arrest who undergo emergency cardiopulmonary bypass and percutaneous coronary intervention. Circ. J. 2010;74(1):77–85. doi: 10.1253/circj.cj-09-0502. [DOI] [PubMed] [Google Scholar]
- 33.Shinada T., Hata N., Yokoyama S., et al. Usefulness of a surface cooling device (Arctic Sun®) for therapeutic hypothermia following cardiac arrest. J. Cardiol. 2014;63(1):46–52. doi: 10.1016/j.jjcc.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Arrich J. Clinical application of mild therapeutic hypothermia after cardiac arrest*. Crit. Care Med. 2007;35(4):1041–1047. doi: 10.1097/01.CCM.0000259383.48324.35. [DOI] [PubMed] [Google Scholar]
- 35.Cerchiari E.L., Safar P., Klein E., Diven W. Visceral, hematologic and bacteriologic changes and neurologic outcome after cardiac arrest in dogs. The visceral post-resuscitation syndrome. Resuscitation. 1993;25(2):119–136. doi: 10.1016/0300-9572(93)90090-d. [DOI] [PubMed] [Google Scholar]
- 36.Adrie C., Adib-Conquy M., Laurent I., et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106(5):562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 37.Adrie C., Laurent I., Monchi M., Cariou A., Dhainaou J-F., Spaulding C. Postresuscitation disease after cardiac arrest: A sepsis-like syndrome? Curr. Opin. Crit. Care. 2004;10(3):208–212. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- 38.Bro-Jeppesen J., Kjaergaard J., Wanscher M., et al. Systemic inflammatory response and potential prognostic implications after out-of-hospital cardiac arrest: A substudy of the target temperature management trial*. Crit. Care Med. 2015;43(6):1223–1232. doi: 10.1097/CCM.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 39.Polderman K.H., Peerdeman S.M., Girbes A.R.J. Hypophosphatemia and hypomagnesemia induced by cooling in patients with severe head injury. J. Neurosurg. 2001;94(5):697–705. doi: 10.3171/jns.2001.94.5.0697. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen N., Hovdenes J., Nilsson F., et al. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol. Scand. 2009;53(7):926–934. doi: 10.1111/j.1399-6576.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 41.Brinkman A.C.M., ten Tusscher B.L., de Waard M.C., de Man F.R., Girbes A.R.J., Beishuizen A. Minimal effects on ex vivo coagulation during mild therapeutic hypothermia in post cardiac arrest patients. Resuscitation. 2014;85(10):1359–1363. doi: 10.1016/j.resuscitation.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Gräsner J., Meybohm P., Caliebe A., et al. Postresuscitation care with mild therapeutic hypothermia and coronary intervention after out-of-hospital cardiopulmonary resuscitation: A prospective registry analysis. Crit. Care. 2011;15(1):R61. doi: 10.1186/cc10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumas F., White L., Stubbs B.A., Cariou A., Rea T.D. Long-term prognosis following resuscitation from out of hospital cardiac arrest: Role of percutaneous coronary intervention and therapeutic hypothermia. J. Am. Coll. Cardiol. 2012;60:21–27. doi: 10.1016/j.jacc.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 44.Callaway C.W., Schmicker R.H., Brown S.P., et al. Early coronary angiography and induced hypothermia are associated with survival and functional recovery after out-of-hospital cardiac arrest. Resuscitation. 2014;85(5):657–663. doi: 10.1016/j.resuscitation.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. https://clinicaltrials.gov/ct2/show/ https://clinicaltrials.gov/ct2/show/