Abstract
Background:
Cardiac arrest in the Catheterization Lab is a rare and unique scenario that is often logistically challenging. It often has dire prognosis especially in patients suffering from severe pre-existing illnesses (high risk patient) such as acute myocardial infarction with cardiogenic shock, or patients undergoing high risk procedures. As the number of complex interventional procedures increases, cardiac arrest in the cath lab will become more common and optimal management of this scenario is critical for both the patient and operator.
Conclusion:
In this review, we will discuss the special challenges during the resuscitation efforts in cath lab, especially with tradition chest compression. We will discuss the alternative options including mechanical compression devices and Invasive Percutaneous Mechanical Circulatory Support Devices. Finally, we will offer management suggestions on selecting the appropriate circulatory support device based on clinical and anatomic risks.
Keywords: Cardiac arrest, catheterization lab, mechanical compression devices, PCI, invasive percutaneous mechanical circulatory support devices, LUCAS, LifeStat, AutoPulse, impella, ECMO
1. INTRODUCTION
Cardiac arrest occurring in the catheterization laboratory (cath lab) is uncommon but many times results in poor outcomes. This often occurs in patients suffering from severe pre-existing illnesses (high-risk patients) such as acute massive myocardial infarction with cardiogenic shock, or patients undergoing high-risk procedures. The incidence increases substantially when a complication occurs such as acute vessel closure or coronary perforation.
Historically, the mortality risk for cardiac catheterization procedures was low (0.1% for all comers) [1]. With rapid advancements in percutaneous coronary techniques coupled with hemodynamic support devices, many very high-risk patients nowadays are offered PCI (percutaneous coronary intervention) and they have higher mortality rate (>30% in those with sustained cardiogenic shock) [2]. In recent years, there has been proliferation of structural heart disease interventions including percutaneous transaortic valve replacement (TAVR), mitral valve repair, and left appendage closure which substantially add to the high-risk procedural volume in the cath lab. In the PARTNER trial, 8% of patients undergoing TAVR required emergent mechanical circulatory support for hemodynamical instability and in a recent TAVR series, 4.3% of patients suffered cardiac arrest [3].
The increasing number of high-risk patients, as well as high-risk procedures, will likely make cardiac arrest in the cath lab more common and optimizing the management of this scenario is critical for both the patient and operator.
2. THE CATHETERIZATION LABORATORY ENVIRONMENT AND CARDIAC ARREST
Cardiac arrest in the cath lab is a unique scenario. Even though the patient may have predispositions for the event due to underlying illnesses, the precipitating factor is usually iatrogenic; or considered to be so by default. Thus, while a failed resuscitation effort for out-of-hospital or in-hospital cardiac arrest is generally well accepted, cardiac arrest during a cath lab procedure is considered a serious complication. This puts enormous pressure on the treatment team and so heroic resuscitation effort is often the norm. The main objectives of such event are to maintain vital organ perfusion and reversing the precipitating cause.
There are specific advantages when cardiac arrest occurs in the cath lab. The patient has continuous monitoring of arterial pressure and ECG so the event is rapidly recognized. Defibrillation pads are also usually pre-connected so time to defibrillation can be minimized. Continuous arterial pressure also gives immediate feedback if chest compression quality is poor. There is also a wide range of equipment available to support the patient including hemodynamic support devices, cardiac medications, pacing wire, and pericardiocentesis kit etc. The treatment team including cardiologists and cath lab staff are also well trained to respond to these emergencies.
The cath lab environment also presents special challenges during the resuscitation efforts, particularly with the traditional manual chest compression method. A major problem is the difficulty in delivering high-quality chest compressions to support cardio-cerebral perfusion. The rescuer is usually limited by physical space, must wear a lead apron, and stretch the hands at an angle due to the C-arm and other cath lab equipment leading to ineffective compressions and easy fatigability. There are also frequent interruptions (“hold compression”) for defibrillation or requested by the operator who is working to reverse the underlying problem (e.g. balloon the occluded vessel). The rescuer also faces health risks due to extensive radiation (being next to the X-ray source and having the hands directly in the beam) as well as potential orthopedic injuries from poor ergonomic and panning of the cath table. The operator also encounters procedural difficulties such as stent positioning due to poor visualization from the hands in the X-ray field as well as excessive movements of the heart during chest compression.
3. ALTERNATIVES TO MANUAL CHEST COMPRESSION IN THE CATH LAB
Given the limitations of manual chest compressions to provide circulatory support during cardiac arrest in the cath lab, alternative methods have been explored to overcome these obstacles. The ideal circulatory support strategy would be one that provides adequate vital organ perfusion (most importantly, the brain and coronaries), readily available and can be commenced quickly, has low complication rates and cost-effective.
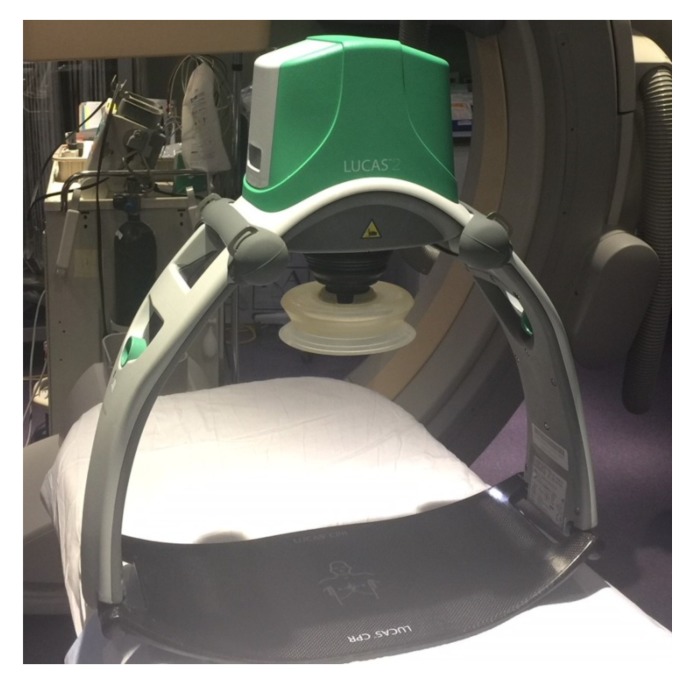
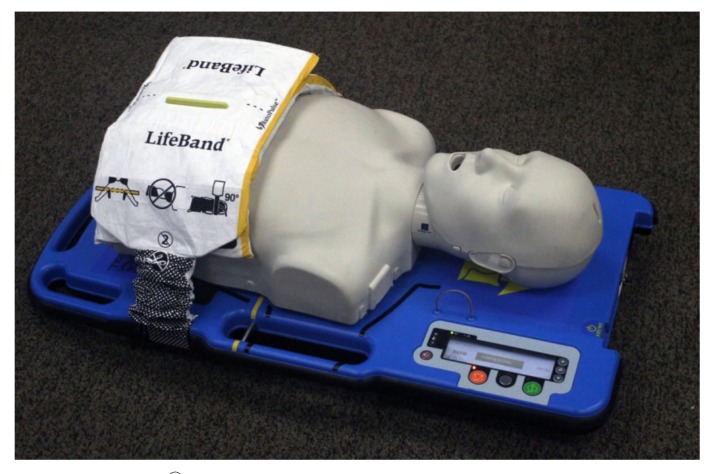
Currently, the circulatory support options can be broadly divided into the mechanical chest compression devices (MCD) and the invasive percutaneous mechanical circulatory support devices (Table 1). The three commercially available mechanical chest compression devices are: LUCASTM (Lund University Cardiopulmonary Assist System), LifeStatTM, and AutoPulse® (Figs. 1-3). The two main percutaneous mechanical circulatory support devices (pMCS) that can be used during cardiac arrest are V-A ECMO (veno-arterial extracorporeal membrane oxygenation) and Impella® as they can be inserted rapidly.
Table 1.
Mechanical circulatory support options in the cardiac cath laboratory.
| Mechanic Chest Compression Devices (MCD) LUCASTM LifeStatTM AutoPulse® |
| Invasive Percutaneous Mechanical Circulatory Support Devices (pMCS) Impella® Veno-arterial ECMO (extracorporal membrane oxygenation) Intra-aortic Balloon Pump TandemHeart® |
Fig. (1).
Lund university cardiopulmonary assistance system (LUCASTM).
Fig. (3).
AutoPulse®
When a patient has elevated risks of cardiac arrest undergoing high-risk PCI, the “hemodynamic support” strategy can be employed where a pMCS is inserted prior to the actual intervention. This approach is based on the notions that pMCS may lessen the risk of cardiac arrest during the ischemic insult; or if the patient does have hemodynamic collapse, circulatory support is already in place to provide organ perfusion and the operator can focus all the efforts on correcting the precipitating cause. In addition to the two pMCS above, TandemHeart® left atrial-to-aorta extracorporeal device may be used for this purpose. Intra-aortic balloon pump can potentially reduce ischemia by improving coronary flow but is ineffective if cardiac arrest occurs. The main drawbacks of this approach are the additional risks of the pMCS insertion which typically require multiple large vascular cannulations and the high cost of the pMCS.
4. MECHANICAL CHEST COMPRESSION DEVICES
Mechanical chest compression devices address several limitations of manual compression. After placement, the rescuer is no longer susceptible to the health hazards noted above and is also available to perform other tasks to treat the patient. There is essentially no interruption in compressions because rotating of rescuers is not necessary and the device can continue during defibrillation. MCDs also deliver consistent high-quality compressions including rate, depth, and release without fatigue. Since these devices are placed over the front of the chest, they do obscure visualization of the X-ray images in the PA projections. However, coronary interventions are typically performed in oblique projections making this a minor inconvenience. The main disadvantage of MCD compared to manual chest compression is the delay of circulatory support to apply the device at which time the patient has no circulation. With training, Levy et al. showed that the time to first mechanical compression can be reduced to a median of 7 seconds -- which is significantly briefer than the typical interruption periods during manual compression [4].
Mechanical chest compression devices can be divided into two categories: piston-driven and load-distribution. The piston-driven technology produces antero-posterior sternal compressions with energy and frequency specifications following International Liaison Committee on Resuscitation guidelines while the load-distribution band is applied around the chest to distribute the force more evenly.
The most studied piston-driven chest compression device is the LUCASTM. In pre-clinical ventricular fibrillation swine model, this device produces superior cardiac output, carotid artery blood flow, end-tidal CO2, and the return of spontaneous circulation (ROSC) compared to manual compression [5]. The first major trial (n=2589) comparing the LUCAS to manual compression in patients suffering from out-of-hospital cardiac arrest demonstrated no significant difference in short (4 hour) or long-term (6 months) survival between the two groups [6]. A subsequent larger trial (n=4471) also showed no improvement in 30-day survival [7]. The other available piston-driven LifeStatTM device (based on the former Thumper® CPR system) does not have robust clinical data.
The AutoPulseTM uses the load-distribution technology with the potential advantage of minimizing chest trauma by distributing the energy over a larger surface area. In pre-clinical studies, it was shown to improve coronary perfusion pressure and cerebral blood flow, and minimizing rib fracture and lung injuries [8, 9]. Cohort studies suggested that this device was associated with improved survival to discharge [10, 11]. However, both major randomized controlled trials (ASPIRE n=1071 and CIRC n=4753) demonstrated no improvement in survival to hospital discharge for patients with out-of-hospital cardiac arrest [12, 13]. In fact, ASPIRE showed that the AutoPulseTM was associated with worse neurologic outcomes.
Overall, randomized data do not show superior outcomes with MCDs for out-of-hospital cardiac arrest and the 2015 AHA guidelines give a IIb recommendation for the use of these devices as a reasonable alternative to manual compression [14]. The evidence for the use of these devices in the cath lab is scarce. A recent retrospective study (n=53) showed that mechanical chest compression was associated with higher rates of ROSC [15]. Another small prospective study (n=42) suggested that mechanical compression was associated with improved survival with good neurologic recovery [16]. Since these events are uncommon, it’s unlikely that a large randomized study is feasible. Despite limited beneficial data for the patient, mechanical chest compression devices will at least spare the rescuer from potential harm in the cath lab. Its use in this setting is supported by the AHA (Class IIb) and “strongly recommended” by the European Guidelines [16, 17].
5. INVASIVE PERCUTANEOUS MECHANICAL CIRCULATORY SUPPORT DEVICES
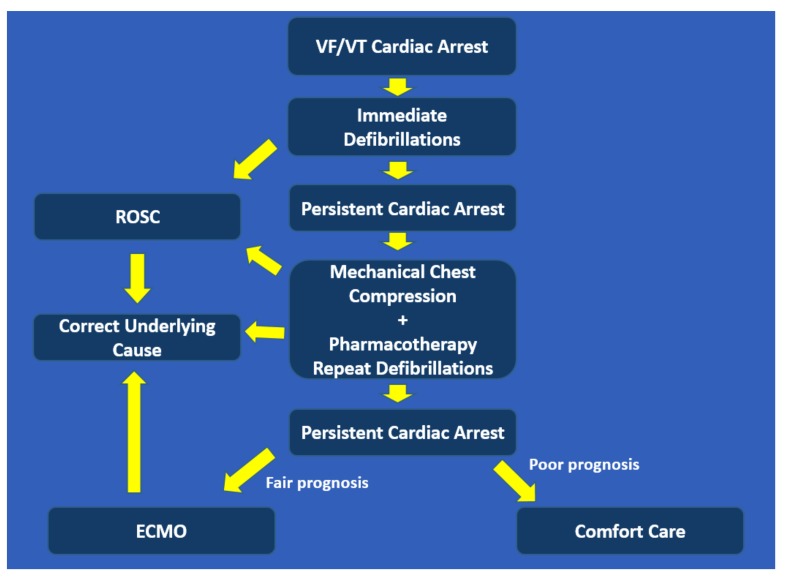
The percutaneous mechanical circulatory support devices aim to provide circulation to vital organs when the heart is ineffective during cardiac arrest. The device historically used for this purpose is V-A ECMO, also referred to as extracorporeal CPR (eCPR). Recently, the Impella device has also been used in this context. The advantages of these devices are that they provide moderate-to-high level of circulatory support and do not cause significant cardiac motions as compared to manual or mechanical chest compression. However, they entail significant expertise for insertion and post-insertion management, require large vascular access which often leads to vascular complications, and are costly. Intra-aortic balloon counter-pulsation is typically not used in cardiac arrest as it needs intrinsic cardiac function to augment. The TandemHeart® with left atrium-to-aorta circuit is a viable support device but requires left atrial access which is impractical during cardiac arrest. Our protocol for managing cardiac arrest in the cath lab is outlined in Fig. (4).
Fig. (4).
Suggested protocol for managing cardiac arrest in the Cath Lab.
Veno-arterial ECMO removes blood from a 18-21 French venous cannula, circulates through an oxygenator and returns blood via a 15-22 French arterial cannula, effectively bypassing the heart and lungs. While providing essentially full circulatory support, there are some hemodynamic disadvantages of V-A ECMO. The elevated left atrial pressure can cause pulmonary edema and left ventricular pressure overload increases wall stress and myocardial oxygen consumption [18]. In a ventricular fibrillation swine model comparing pMCS, V-A ECMO achieved the highest mean arterial pressure, followed by TandemHeart, and the least being Impella (although the addition of norepinephrine brought the arterial pressure in the Impella group to the same level as the TandemHeart group) [19]. For out-of-hospital cardiac arrest, large observational studies demonstrated that V-A ECMO is associated with improved survival and better neurologic outcomes compared to conventional CPR [20, 21]. In a study where patients with refractory VF/VT cardiac arrest being brought directly to the cath lab for V-A ECMO and revascularization, 42% survived to hospital discharge with favorable neurologic outcomes compared to 15% in historical control [22]. For cardiac arrest occurring in the cath lab during PCI and TAVR, Arlt et al demonstrated in their 14 patients case series that using ECMO allowed ROSC on all patients and 50% those survived to discharge [23]. Due to limited evidence, both the AHA and European Resuscitation Council give weak recommendations for the use of V-A ECMO during cardiac arrest in the cath lab [16, 17].
A newer pMCS is the Impella which is inserted via a 13-14 French arterial sheath. It can provide up to 4 L/min of cardiac output using axial, non-pulsatile technology to pump blood from the left ventricle into the ascending aorta. Compared to V-A ECMO, the Impella device provides lower level of circulatory support, does not assist the right ventricle (unless a right sided Impella is also placed), and lacks the ability to oxygenate blood. However, it has the advantages of using smaller vascular access, and unloads the left ventricle. Several experimental reports suggest that in a porcine model of cardiac arrest, the Impella 2.5 device can support the systemic circulation without concurrent chest compressions and improved the rate of return of spontaneous circulation [24, 25]. An early clinical experience of 8 refractory cardiac arrest patients showed that using the Impella plus conventional treatment was associated with 50% survival to discharge [26]. In short, Impella is potentially a good circulatory support option for cardiac arrest but clinical data is lacking.
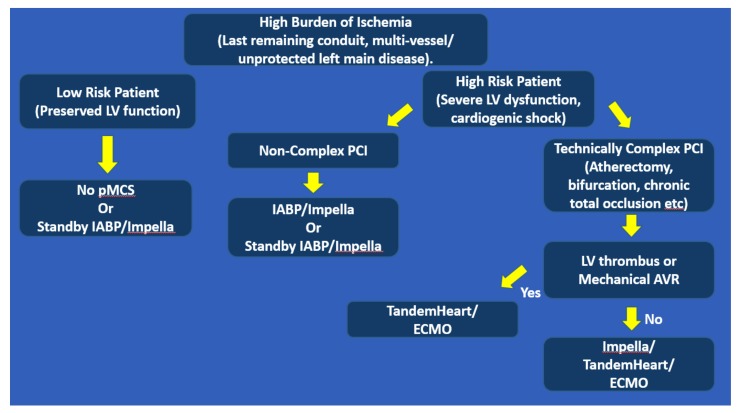
The hemodynamic support devices to support high-risk PCI include Intra-aortic balloon pump, Impella, TandemHeart, and V-A ECMO. The IABP continues to be a popular choice given its ease of insertion, availability, low complications, and low cost. This provides modest augmentation of cardiac output (~0.5 L/min) with conflicting evidence of improvement in coronary blood flow [27]. In the Balloon Pump–Assisted Coronary Intervention Study, elective IABP in high-risk PCI failed to reduce major adverse cardiac and cerebrovascular events (MACCE) compared to control at hospital discharge [28]. The Impella is increasingly used for high-risk PCI indication. The PROTECT II trial which compared Impella 2.5 to IABP demonstrated no difference in 30 days major adverse events despite the superior hemodynamic support of the Impella. The rate of CPR or ventricular dysrhythmia requiring cardioversion was 3.2% in the IABP group and 2.2% in the Impella group but the event rates were too few to determine a statistical significance [29]. The TandemHeart provides up to 4 L/min of flow from left atrium-to-descending aorta with an advantage over V-A ECMO in that it reduces left ventricular filling pressure. It can also be used in the scenario of the mechanical aortic valve or left ventricular thrombus; a limitation of Impella. However, device insertion requires trans-septal access which necessitates longer time and additional operator expertise. TandemHeart enhanced cardiac output, lower PCWP and PA pressures but did not improve 30-day mortality, and was associated with higher vascular complications when compared to IABP [30]. Veno-arterial ECMO provides oxygenation in addition to perfusion making it a good option for those with severe hypoxemia or right ventricular dysfunction. Its provisional use in high-risk PCI is limited to small studies likely due to the availability of other devices with less rigorous requirements (perfusionist, surgeon etc). Our suggested algorithm for choosing the appropriate pMCS in patients at higher risk for cardiac arrest with PCI is depicted in Fig. (5).
Fig. (5).
Suggested algorithm for choosing pMCS in patients with elevated risks of cardiac arrest undergoing high-risk PCI.
CONCLUSION
Although a historically very rare event in the cath lab, cardiac arrest will become more frequent due to increasing high-risk patients and procedures being performed. The general goals of treatment are to perfuse vital organs and to correct the underlying cause. Conventional manual chest compression is suboptimal for the cath lab setting due to poor quality circulatory support as well as being a health hazard to the rescuers. Alternative options are available and each has its own advantages and disadvantages (Table 2). While there is robust clinical data for mechanical chest compression in out-of-hospital cardiac arrest, clinical evidence for cath lab specific cardiac arrest is sparse. Clinicians must take into consideration numerous factors when making decisions. These include local expertise and ancillary support, patient overall prognosis, complication risks, and cost.
Table 2.
Comparison of different circulatory support options in the catheterization laboratory.
| Manual Compression | Mechanical Compression | IABP | Impella | TandemHeart | ECMO | |
|---|---|---|---|---|---|---|
| Risks to rescuer | ++ | None | None | None | None | None |
| Level of support | + | ++ | * | ++ | +++ | ++++ |
| Time to initiation | Immediate | Short | Longer | Longer | Very long | Longer |
| Readily available (Institution dependent) | +++ | +++ | +++ | ++ | + | + |
| Post-placement Management | Simple | Simple | Moderate | Moderate | Complex | Complex |
| Vascular complication | 0 | 0 | + | ++ | +++ | +++ |
| Cost | 0 | $ | $ | $$$ | $$$ | $$$ |
| Other | L sided support only | Increases LVEDP, also oxygenate |
*IABP is ineffective during cardiac arrest.
Fig. (2).
LifeStatTM.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.William P., Rao P., Kanakadandi U.B., Asencio A., Kern K.B. Mechanical cardiopulmonary resuscitation in and on the way to the cardiac catheterization laboratory. Circ. J. 2016;80(6):1292–1299. doi: 10.1253/circj.CJ-16-0330. [DOI] [PubMed] [Google Scholar]
- 2.Brennan J.M., Curtis J.P., Dai D., et al. National Cardiovascular Data Registry. Enhanced mortality risk prediction with a focus on high-risk percutaneous coronary intervention: Results from 1,208,137 procedures in the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc. Interv. 2013;6(8):790–799. doi: 10.1016/j.jcin.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Banjac I., Petrovic M., Akay M.H., et al. Extracorporeal membrane oxygenation as a procedural rescue strategy for transcatheter aortic valve replacement cardiac complications. ASAIO J. 2016;62(1):e1–e4. doi: 10.1097/MAT.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 4.Levy M., Yost D., Walker R.G., Scheunemann E., Mendive S.R. A quality improvement initiative to optimize use of a mechanical chest compression device within a high-performance CPR approach to out-of-hospital cardiac arrest resuscitation. Resuscitation. 2015;92:32–37. doi: 10.1016/j.resuscitation.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Steen S., Liao Q., Pierre L., Paskevicius A., Sjöberg T. Evaluation of LUCAS, a new device for automatic mechanical compression and active decompression resuscitation. Resuscitation. 2002;55(3):285–299. doi: 10.1016/s0300-9572(02)00271-x. [DOI] [PubMed] [Google Scholar]
- 6.Rubertsson S., Lindgren E., Smekal D., et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of hospital cardiac arrest. The LINC Randomized Trial. JAMA. 2014;311(1):53–61. doi: 10.1001/jama.2013.282538. [DOI] [PubMed] [Google Scholar]
- 7.Perkins G.D., Lall R., Quinn T., et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): A pragmatic, cluster randomized controlled trial. Lancet. 2015;385:947–955. doi: 10.1016/S0140-6736(14)61886-9. [DOI] [PubMed] [Google Scholar]
- 8.Ikeno F., Kaneda H., Hongo Y., et al. Augmentation of tissue perfusion by a novel compression device increases neurologically intact survival in a porcine model of prolonged cardiac arrest. Resuscitation. 2006;68(1):109–118. doi: 10.1016/j.resuscitation.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Halperin H.R., Paradis N., Ornato J.P., et al. Cardiopulmonary resuscitation with a novel chest compression device in a porcine model of cardiac arrest: Improved hemodynamics and mechanisms. J. Am. Coll. Cardiol. 2004;44:2214–2220. doi: 10.1016/j.jacc.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 10.Ong M.E., Ornato J.P., Edwards D.P., et al. Use of an automated, load-distributing band chest compression device for out-of-hospital cardiac arrest resuscitation. JAMA. 2006;295(22):2629–2637. doi: 10.1001/jama.295.22.2629. [DOI] [PubMed] [Google Scholar]
- 11.Hock Ong M.E., Fook-Chong S., Annathurai A., et al. Improved neurologically intact survival with the use of an automated, load-distributing band chest compression device for cardiac arrest presenting to the emergency department. Crit. Care. 2012;16(4):R144. doi: 10.1186/cc11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallstrom A., Rea T.D., Sayre M.R., et al. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: A randomized trial. JAMA. 2006;295(22):2620–2628. doi: 10.1001/jama.295.22.2620. [DOI] [PubMed] [Google Scholar]
- 13.Wik L., Olsen J.A., Persse D., et al. Manual vs integrated automatic load-distributing band CPR with equal survival after out of hospital cardiac arrest. Resuscitation. 2014;85:741–748. doi: 10.1016/j.resuscitation.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Venturini J.M., Retzer E., Estrada J.R., et al. Mechanical chest compressions improve rate of return of spontaneous circulation and allow for initiation of percutaneous circulatory support during cardiac arrest in the cardiac catheterization laboratory. Resuscitation. 2017;115:56–60. doi: 10.1016/j.resuscitation.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 15.Wagner H., Hardig B.M., Rundgren M., et al. Mechanical chest compressions in the coronary catheterization laboratory to facilitate coronary intervention and survival in patients requiring prolonged resuscitation efforts. Scand. J. Trauma Resusc. Emerg. Med. 2016;24:4. doi: 10.1186/s13049-016-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavonas E.J., Drennan I.R., Gabrielli A., et al. Part 10: Special circumstances of resuscitation: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18) Suppl. 2:S501–S518. doi: 10.1161/CIR.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 17.Truhlář A., Deakin C.D., Soar J., et al. Cardiac arrest in special circumstances section Collaborators. European resuscitation council guidelines for resuscitation 2015: Section 4. Cardiac arrest in special circumstances. Resuscitation. 2015;95:148–201. doi: 10.1016/j.resuscitation.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Meani P., Gelsomino S., Natour E., et al. Modalities and effects of left ventricle unloading on extracorporeal life support: A review of the current literature. Eur. J. Heart Fail. 2017;19(Suppl. 2):84–91. doi: 10.1002/ejhf.850. [DOI] [PubMed] [Google Scholar]
- 19.Ostadal P., Mlcek M., Holy F., et al. Direct comparison of percutaneous circulatory support systems in specific hemodynamic conditions in a porcine model. Circ Arrhythm Electrophysiol. 2012;5(6):1202–1206. doi: 10.1161/CIRCEP.112.973123. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y.S., Lin J.W., Yu H.Y., et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto T., Morimura N., Nagao K., et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: A prospective observational study. Resuscitation. 2014;85:762–768. doi: 10.1016/j.resuscitation.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Yannopoulos D., Bartos J.A., Raveendran G., et al. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J. Am. Coll. Cardiol. 2017;70(9):1109–1117. doi: 10.1016/j.jacc.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 23.Arlt M., Philipp A., Voelkel S., et al. Early experiences with miniaturized extracorporeal life-support in the catheterization laboratory. Eur. J. Cardiothorac. Surg. 2012;42(5):858–863. doi: 10.1093/ejcts/ezs176. [DOI] [PubMed] [Google Scholar]
- 24.Tuseth V., Salem M., Pettersen R., et al. Percutaneous left ventricular assist in ischemic cardiac arrest. Crit. Care Med. 2009;37:1366–1372. doi: 10.1097/CCM.0b013e31819c0642. [DOI] [PubMed] [Google Scholar]
- 25.Derwall M., Brucken A., Ebeling A., et al. Doubling survival and improving neurological outcomes using a left ventricular assist device instead of chest compressions for resuscitation after prolonged cardiac arrest. Crit. Care. 2015;19:123–134. doi: 10.1186/s13054-015-0864-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vase H., Christensen S., Christiansen A., et al. The Impella CP device for acute mechanical circulatory support in refractory cardiac arrest. Resuscitation. 2017;112:70–74. doi: 10.1016/j.resuscitation.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Myat A., Patel N., Tehrani S., Banning A.P., Redwood S.R., Bhatt D.L. Percutaneous circulatory assist devices for high-risk coronary intervention. JACC Cardiovasc. Interv. 2015;8(2):229–24. doi: 10.1016/j.jcin.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Perera D., Stables R., Thomas M., et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: A randomized controlled trial. JAMA. 2010;304:867–874. doi: 10.1001/jama.2010.1190. [DOI] [PubMed] [Google Scholar]
- 29.O’Neill W.W., Kleiman N.S., Moses J., et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126(14):1717–1727. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- 30.Thiele H., Sick P., Boudriot E., et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 2005;360:1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]