Abstract
Background:
Although the 6-min walk test (6MWT) is the gold standard for assessing exercise-induced impairment of gas exchange, it cannot easily be performed in a clinical office environment. The aim of this study was to compare the 1-min sit-to-stand test (1STST) with the 6MWT for the ability to assess exercise-induced oxygen desaturation in patients with interstitial lung diseases (ILDs).
Methods:
A total of 107 patients were enrolled and classified into three groups: sarcoidosis, fibrotic idiopathic interstitial pneumonia (f-IIP), and other forms of ILD. The 6MWT and 1STST were performed on the same day, and pulmonary function tests, pulse oxygen saturation (SpO2), and dyspnea and fatigue (modified Borg scale) were assessed. SpO2 desaturation was evaluated by intraclass correlation coefficient (ICC), Bland–Altman analysis, and kappa (κ) coefficient in the whole population and the patient subgroups.
Results:
The SpO2 nadir during the 1STST and 6MWT showed good consistency [mean ± standard deviation: 92.5% ± 5% and 90% ± 7%, respectively; ICC 0.77, 95% confidence interval (CI) 0.71–0.83] and correlated strongly (r = 0.9, p < 0.0001). The frequency of patients with oxygen desaturation ⩾4% was also consistent for the two exercise tests (κ = 0.68, 95% CI 0.54–0.82). The number of repetitions in the 1STST correlated with the 6MWT distance (r = 0.5, p < 0.0001), but the dyspnea scores were higher during the 1STST than the 6MWT (p < 0.0001). These findings did not differ for the three patient subgroups.
Conclusion:
The 1STST can measure exercise-induced desaturation in ILD patients and could be used as an alternative test to the 6MWT in office practice.
Keywords: 1-min sit-to-stand test, 6-min walk test, exercise, functional tests, interstitial lung disease
Introduction
Interstitial lung diseases (ILDs) are a heterogeneous group of conditions characterized by interstitial inflammation or fibrosis. ILDs are associated with progressive loss of lung function marked by lung restriction and impaired gas exchange.1 Resting gas exchange, as evaluated by arterial blood gas analysis, remains normal for an extended time, and resting hypoxemia only occurs when the disease is advanced.2 Gas exchange impairment can be detected at early stages of ILD as a reduction in the diffusing capacity of carbon monoxide (DLco) or a decrease in SpO2 or PaO2 during exercise tests.3,4 However, resting DLco is an insensitive predictor of abnormal gas exchange during exercise.5,6 Changes in oxygenation can be measured during both maximal and submaximal exercise tests in patients with normal arterial blood gas, as shown for idiopathic pulmonary fibrosis (IPF) patients. The recent guidelines for the management of IPF7 include assessments of exercise-induced gas exchange impairment. This is routinely measured in expert centers because it correlates with the extent of ILD measured by biopsy and imaging7,8 as well as the severity9 and prognosis10–12 of the disease. However, such tests are not easily performed in primary care settings or office practice and there is a need to identify a simple and inexpensive test that can estimate exercise-induced desaturation and can be performed in office practice.
The 6-min walk test (6MWT) is the gold standard exercise test and has been validated for most chronic lung diseases. In patients with ILDs, the 6MWT is commonly used to evaluate exercise tolerance (distance walked) and alterations in gas exchange [lowest oxygen saturation (SpO2 nadir)].13,14 The 6MWT test is sensitive, reproducible, easy to perform, and does not use any specialized equipment.15 However, it does require a 30-m corridor, which is uncommon in health centers and office practices. Shorter corridors require more turns, which is likely to distort the test results, especially the total distance traveled. To overcome these technical and spatial limitations, several additional exercise tests, such as the 1-min sit-to-stand test (1STST), are currently being evaluated.16 The 1STST requires only a chair and is easy to perform, making it feasible for use in the physician’s office.17 Studies to date have shown the 1STST to be well tolerated, sensitive, and reproducible in chronic obstructive pulmonary disease (COPD)16,18–20 and cystic fibrosis21 patients. However, its validity has never been investigated in ILD patients.
With this in mind, we compared the ability of the 1STST and the 6MWT to detect oxygen desaturation in a large cohort of patients with various ILDs.
Methods
Patients
We enrolled all ILD patients (n = 107) admitted for assessment at the Rare Lung Disease Competence Center in Lille, France, between May and December 2015, regardless of the etiology or severity of ILD. Exclusion criteria were an inability to perform either the 6MWT or the 1STST, unstable respiratory status, or recent respiratory infection. Hypoxemic patients on long-term oxygen therapy were also excluded. Clinical characteristics were recorded on the same day as the pulmonary function tests and both exercise tests. All data were retrospectively collected. Patients were classified into three groups according to ILD etiology: (i) sarcoidosis (n = 31); (ii) fibrotic idiopathic interstitial pneumonia (f-IIP), including IPF, fibrotic nonspecific idiopathic pneumonia (f-NSIP), and chronic hypersensitivity pneumonitis (n = 51); and (iii) other ILD (nonsarcoidosis, non-f-IIP ILD; n = 25). Approval for the use of these data was provided by the Institutional Review Board of the French Learned Society for Pulmonology (CEPRO 2011-039) and informed consent was obtained from all patients.
Study design
Patients underwent plethysmography and then performed the 6MWT followed by the 1STST, or vice versa, on the same day. A resting period of at least 30 min was allowed between the exercise tests to ensure complete recovery. Each patient performed both tests with the same tester.
1STST
The 1STST was performed as previously described16 with a chair of standard height (46 cm) without arm rests positioned against a wall. The patient was seated upright on the chair with knees and hips flexed at 90°, feet placed flat on the floor a hip-width apart, and arms held stationary by placing their hands on their hips. Patients were asked to perform repetitions of standing upright and then sitting down in the same position at a self-paced speed (safe and comfortable) as many times as possible for 1 min. They were instructed not to use their arms for support while rising or sitting. Patients were permitted to rest during the 1-min period. The number of repetitions was recorded. The modified Borg scale (0–10) was used to assess dyspnea and fatigue immediately before and after each test. A finger oxymeter (Nellcor OxiMax N-65; Covidien, Minneapolis, MN, USA) was connected throughout the test for continuous recording of SpO2 and heart rate (HR). A desaturation level ⩾4% was considered clinically significant for this study.22,23
6MWT
The 6MWT was performed in accordance with international recommendations.15 Patients were asked to walk as far as possible in a 30-m indoor corridor at our center. They were allowed to stop during the test if necessary. Dyspnea was assessed before and after the test, and SpO2 and HR were monitored continuously, as described for the 1STST. Desaturation ⩾4% was considered clinically significant.
Pulmonary function tests
Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and total lung capacity were measured by spirometry and plethysmography using a Jaeger–Masterlab cabin. Single-breath DLco was measured and adjusted for hemoglobin using Cotes’ equation.24 Predicted values for lung volumes and DLco were based on the European Respiratory Society guidelines.25,26
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation (SD). Normality of the distributions was checked graphically and using the Shapiro–Wilk test. Comparisons between the three patient groups were performed as follows: continuous variables at baseline were compared using analysis of variance; the frequency and percentage of steroid and immunosuppressive drug treatment were compared using a Chi-square test; and variables assessed at the end of the exercise tests were compared using a covariance analysis (ANCOVA) adjusted to the baseline value. For significant results (p < 0.05), post-hoc analyses with Bonferroni’s correction were performed. The relationship between the number of repetitions or SpO2 nadir during the 1STST and the DLco or FVC was evaluated by Pearson’s correlation coefficient.
Agreement between the values obtained in the 6MWT and 1STST for SpO2, peak HR, and Borg scale scores was evaluated with the intraclass correlation coefficient (ICC), with p-values <0.5, 0.5–0.75, 0.75–0.9, and >0.9 indicating poor, moderate, good, and excellent agreement, respectively. A paired Student’s t test was used to compare differences in the SpO2, peak HR, and Borg scale scores between the 6MWT and 1STST in the three patient subgroups. A Bland–Altman analysis was conducted to graphically represent the limits of agreement between the SpO2 in the 6MWT and 1STST for the whole population. Agreement between the ability of the two exercise tests to detect desaturation ⩾4% was assessed using Cohen’s kappa coefficient (κ) with 95% confidence intervals (CIs). κ values of <0, 0–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1 were considered to indicate no, slight, fair, moderate, substantial, and almost perfect agreement, respectively.27 The relationship between the distance walked in the 6MWT and the number of repetitions in the 1STST was evaluated by Pearson’s correlation coefficient. Data were analyzed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) and all statistical tests were performed with a two-tailed alpha risk of 0.05.
Results
Patients
A total of 107 patients (62 men, 45 women) aged 57 ± 14 years (mean ± SD) were included and completed the study. Within the three patient groups, 31 were diagnosed with sarcoidosis, 51 with f-IIP (including 21 with IPF, 25 with f-NSIP, and 5 with chronic hypersensitivity pneumonitis), and 25 with miscellaneous other ILDs. Patient sociodemographic, clinical, and functional characteristics are summarized in Table 1. There were no significant inter-group differences in FVC or FEV1. Sarcoidosis patients had a lower FEV1/FVC than the other two groups, but this was statistically significant only for the comparison with f-IIP patients. The f-IIP group showed significantly lower DLco than the sarcoidosis group (Table 1). A number of patients in each group received long-term drug therapy for ILD, including antifibrotic drugs (f-IIP patients only), steroids (all three groups), and immunosuppressive drugs (primarily f-NSIP and chronic hypersensitivity pneumonitis patients).
Table 1.
Patient clinical and functional characteristics.
| Variable | All patients (n = 107) |
Sarcoidosis (n = 31) |
f-IIP (n = 51) |
Other ILD (n = 25) |
p-value |
|---|---|---|---|---|---|
| Age (years) | 57 ± 14 | 50 ± 11a | 63 ± 13 | 57 ± 15 | <0.001 |
| BMI (kg/m²) | 28 ± 5 | 29 ± 5 | 29 ± 5 | 28 ± 6 | 0.74 |
| Steroids, n (%) | 54 (50) | 16 (52) | 28 (55) | 10 (40) | 0.47 |
| Immunosuppressive drugs, n (%) | 26 (24) | 2 (6)a,b | 17 (33) | 7 (28) | 0.020 |
| Antifibrotic drugs, n (%) | 17 (16) | 0 | 17 (33) | 0 | – |
| FEV1 (% predicted) | 75 ± 22 | 73 ± 23 | 79 ± 17 | 73 ± 25 | 0.13 |
| FVC (% predicted) | 85 ± 19 | 86 ± 19 | 78 ± 17 | 86 ± 22 | 0.41 |
| FEV1/FVC (%) | 73 ± 13 | 68 ± 13a | 80 ± 7 | 69 ± 17a | <0.001 |
| DLco (% predicted) | 51 ± 19 | 64 ± 20a,b | 42 ± 13 | 50 ± 18 | <0.001 |
Significantly different from f-IIP.
Significantly different from other ILD.
BMI, body mass index; DLco, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in 1 s; f-IIP, fibrotic idiopathic interstitial pneumonia; FVC, forced vital capacity; ILD, interstitial lung disease.
1STST and 6MWT
The average (± SD) number of repetitions performed by the whole population during the 1STST was 21 ± 6. No significant differences were observed between the three patient groups (Table 2). The number of repetitions did not correlate with FVC (r = 0.17, p = 0.07) and correlated only weakly with DLco (r = 0.24, p = 0.01). The mean SpO2 was 97% ± 1% at baseline and was markedly reduced during exercise (SpO2 nadir 92% ± 5%). This value also did not differ significantly between the groups. There were moderate but statistically significant correlations between the SpO2 nadir during the 1STST and both FVC (r = 0.35, p < 0.001) and DLco (r = 0.56, p < 0.001). A total of 54 of the 107 patients (50%) had oxygen desaturation of ⩾4% (23%, 69%, and 48% in the sarcoidosis, f-IIP, and other ILD groups, respectively). Sarcoidosis patients experienced less severe dyspnea at the end of the test compared with the other two groups, although the differences did not reach the level of statistical significance (Table 2).
Table 2.
1-min sit-to-stand test and 6-min walk test results.
| Variable | All patients (n = 107) |
Sarcoidosis (n = 31) |
f-IIP (n = 51) |
Other ILD (n = 25) |
p-value |
|---|---|---|---|---|---|
| 1STST | |||||
| Number of repetitions | 21 ± 6 | 23 ± 7 | 20 ± 6 | 22 ± 5 | 0.058 |
| Baseline SpO2 (%) | 97 ± 1 | 97.1 ± 0.8 | 96.3 ± 1.1 | 96.5 ± 1.5 | 0.23 |
| SpO2 nadir (%) | 92 ± 5 | 93 ± 3 | 91 ± 4 | 92 ± 6 | 0.19c |
| Baseline heart rate (bpm) | 81 ± 14 | 83 ± 14 | 80 ± 14 | 82 ± 14 | 0.59 |
| Heart rate at 1 min (bpm) | 112 ± 17 | 117 ± 18b | 109 ± 17 | 109 ± 17 | 0.047c |
| Baseline Borg score | 0.8 ± 1.2 | 0.6 ± 1.0 | 1.1 ± 1.4 | 0.7 ± 1.2 | 0.16 |
| Borg score at 1 min | 4.9 ± 2.2 | 4.0 ± 2.4 | 5.3 ± 2.0 | 5.1 ± 2.0 | 0.068c |
| 6MWT | |||||
| Distance walked (m) | 436 ± 87 | 476 ± 86a,b | 417 ± 83 | 422 ± 83 | 0.008 |
| Baseline SpO2 (%) | 97 ± 1 | 97 ± 1 | 97 ± 1 | 97 ± 1 | 0.82 |
| SpO2 nadir (%) | 90 ± 7 | 94 ± 4a,b | 89 ± 7 | 89 ± 7 | 0.002c |
| Baseline heart rate (bpm) | 80 ± 12 | 83 ± 11 | 78 ± 13 | 79 ± 12 | 0.27 |
| Heart rate at 6 min (bpm) | 112 ± 16 | 116 ± 14 | 112 ± 17 | 108 ± 14 | 0.14c |
| Baseline Borg score | 0.4 ± 0.8 | 0.4 ± 0.8 | 0.5 ± 0.7 | 0.2 ± 0.7 | 0.38 |
| Borg score at 6 min | 3.7 ± 2.1 | 3.0 ± 2.0a,b | 4.0 ± 2.1 | 4.1 ± 2.1 | 0.034c |
Values are the mean ± SD.
Significantly different from f-IIP.
Significantly different from other ILD.
Adjusted to baseline value.
1STST, 1-min sit-to-stand test; 6MWT, 6-min walk test; bpm, beats per minute; f-IIP, fibrotic idiopathic interstitial pneumonia; ILD, interstitial lung disease; SpO2, oxygen saturation.
The average 6MWT distance for the whole population was 436 ± 87 m (Table 2). The sarcoidosis group walked a significantly longer distance (476 ± 86 m) than the f-IIP group (417 ± 83 m) and the other ILD group (422 ± 83 m). The mean baseline SpO2 for the whole population was 97% ± 1%, and this did not differ significantly between the patient groups. The SpO2 nadir was 90% ± 7%, with the sarcoidosis group showing significantly less desaturation than the other two groups. A total of 59 patients (55%) showed oxygen desaturation of ⩾4% (23%, 76%, and 60% in the sarcoidosis, f-IIP, and other ILD groups, respectively). The sarcoidosis patients experienced significantly less severe dyspnea at the end of the test than either the f-IIP or other ILD group (Table 2).
Comparison of SpO2 between the 1STST and 6MWT
There was a strong correlation between the SpO2 nadir value in the 6MWT and the 1STST (r = 0.9, p < 0.0001; Figure 1). The 6MWT and 1STST SpO2 nadir values showed good agreement when evaluated for the whole population [ICC 0.77, 95% CI (0.71–0.83); Table 3], the other ILD group [0.81 (0.67–0.90)], and the sarcoidosis group [0.73 (0.48–0.85)] and moderate agreement for the f-IIP group [0.68 (0.56–0.76)]. SpO2 nadir values in the 1STST and the 6MWT were significantly lower for patients with the poorest functional status (Table 4). The SpO2 nadir recorded in the 1STST was 2% higher than that in the 6MWT (Table 3, Figure 2). According to a linear regression model, the SpO2 nadir in the 6MWT can be estimated from that obtained in the 1STST according to the formula: 6MWT SpO2 nadir = (1.16 × 1STST SpO2 nadir) − 16.4.
Figure 1.
Correlation between aspects of the 6MWT and 1STST.
Left: SpO2 nadir values during the 6MWT and 1STST. Right: Distance walked in the 6MWT and number of repetitions in the 1STST.
1STST, 1-min sit-to-stand test; 6MWT, 6-min walk test; SpO2, oxygen saturation.
Table 3.
Agreement between the 6-min walk test and 1-min sit-to-stand test results.
| Variable | 6MWT (n = 107) |
1STST (n = 107) |
ICC (95% CI) |
p-value |
|---|---|---|---|---|
| SpO2 nadir (%) | 90 ± 7 | 92 ± 5 | 0.77 (0.71–0.83) | <0.001 |
| End of test Borg dyspnea score | 3.7 ± 2.1 | 4.9 ± 2.2 | 0.45 (0.29–0.60) | <0.001 |
| Peak HR (bpm) | 112 ± 16 | 112 ± 17 | 0.71 (0.59–0.79) | 0.90 |
Values are presented as the mean ± SD.
1STST, 1-min sit-to-stand test; 6MWT, 6-min walk test; bpm, beats per minute; CI, confidence interval; HR, heart rate; ICC, intraclass coefficient correlation; SD, standard deviation; SpO2, oxygen saturation.
Table 4.
Mean nadir SpO2 during the 1STST and the 6MWT.
| FVC > 70% (n = 82) |
FVC < 70% n = 25 |
p-value | DLco > 50% n = 51 |
DLco < 50% n = 56 |
p-value | |
|---|---|---|---|---|---|---|
| Nadir SpO2 1STST | 92.2 ± 4.8 | 89.8 ± 4.3 | 0.008 | 94.7 ± 2.5 | 89.6 ± 5.1 | <0.0001 |
| Nadir SpO2 6MWT | 91.3 ± 6.2 | 86.5 ± 6.5 | 0.001 | 94.2 ± 3.2 | 86.5 ± 6.5 | <0.0001 |
Values are presented as the mean ± SD.
1STST, 1-min sit-to-stand test; 6MWT, 6-min walk test; DLco, diffusing capacity of carbon monoxide; FVC, forced vital capacity; SpO2, oxygen saturation.
Figure 2.
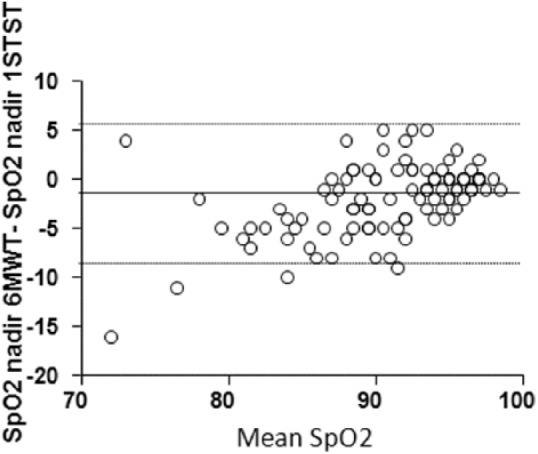
Bland–Altman plot of the difference in SpO2 nadir between the 6MWT and 1STST as a function of the mean SpO2 nadir in both tests.
Solid black line represents the mean difference and the dotted lines define the limits of the 95% confidence interval.
1STST, 1-min sit-to-stand test; 6MWT, 6-min walk test; SpO2, oxygen saturation.
The 6MWT and 1STST showed good agreement in their ability to detect oxygen desaturation ⩾4%, with a κ coefficient of 0.68 (95% CI 0.54–0.82). The number of patients showing desaturation levels ⩾4% during only one of the two tests was not significantly different between the 1STST (n = 11) and the 6MWT (n = 6; Table 5). Strong correlations were found between the SpO2 nadir during the 1STST and 6MWT (r = 0.76, p < 0.0001) for patients with oxygen desaturation below 88% during the 6MWT.
Table 5.
Agreement between exercise-induced oxygen desaturation during 1STST and 6MWT.
| ∆SpO2 ⩾ 4% in the 1STST |
∆SpO2 ⩾ 4% in the 6MWT |
|
|---|---|---|
| Yes | No | |
| Yes | 42a | 11 |
| No | 6 | 48 |
Number of patients.
1STST, 1-min sit-to-stand test; 6MWT, 6-min walk test; SpO2, oxygen saturation.
Comparison of exercise capacity between the 1STST and 6MWT
The number of repetitions in the 1STST correlated moderately with the distance traveled during the 6MWT (r = 0.5, p < 0.0001; Figure 1). Similar results were obtained when the sarcoidosis (r = 0.4, p = 0.02), f-IIP (r = 0.5, p = 0.002), and other ILD (r = 0.5, p = 0.003) groups were analyzed separately.
The whole patient population and all three patient subgroups experienced more severe dyspnea (Borg score) at the end of the 1STST than the 6MWT: 4.9 ± 2.2 versus 3.7 ± 2.1, 107 (p < 0.0001) for the whole cohort (Table 3), 4.0 ± 2.4 versus 3.0 ± 2.0 (p = 0.004) for the sarcoidosis group, 5.3 ± 2.0 versus 4.0 ± 2.1 (p < 0.0001) for the f-IIP group, and 5.1 ± 2.0 versus 4.1 ± 2.1 (p = 0.006) for the other ILD group. Borg values at the end of the two tests showed poor agreement in the whole population [ICC 0.45, 95% CI (0.29–0.60)] and in all patient subgroups: 0.44 (0.08–0.70), 0.45 (0.22–0.66), and 0.40 (0.14–0.59) for the sarcoidosis, f-IIP, and other ILD patient groups, respectively.
We observed no significant difference in the increase in HR between the 6MWT and 1STST in the total population (Table 3) or any of the patient subgroups (data not shown). The two exercise tests showed moderate to good agreement in peak HR, with ICC (95% CI) values of 0.71 (0.59–0.79) for the whole population (Table 3), and 0.58 (0.25–0.80), 0.78 (0.67–0.86), and 0.69 (0.48–0.81) for the sarcoidosis, f-IIP, and other ILD patient groups, respectively.
Discussion
Our study establishes that the 1STST is a reliable test to estimate exercise-induced oxygen desaturation in patients with ILDs, as shown by good correlation (r = 0.9) and agreement [ICC 0.77, 95% CI (0.71–0.83)] between the SpO2 nadir values in the 1STST and the 6MWT. Moreover, this finding holds for the groups with ILDs of various etiologies, including f-IIP, sarcoidosis, and other ILDs.
Exercise-induced gas exchange impairment can be detected early in the course of ILD, before changes in resting DLco and PaO2. Resting DLco is a specific but insensitive predictor of abnormal gas exchange during exercise, as assessed by the 6MWT (SpO2 nadir) or cardiopulmonary exercise tests (peak alveolar-arterial oxygen difference).5,6,28 Gas exchange impairment is associated with exertional dyspnea,29,30 exercise limitation,30,31 and altered quality of life.31 In IPF patients, desaturation during a 6MWT is an independent predictor of mortality.10 For these reasons, assessment of exercise-induced gas exchange impairment, in addition to indicators such as FVC and DLco, should be factored into decision-making when initiating, changing, or interrupting the treatment of ILD patients.32
Although the 6MWT is acknowledged to be the simplest test to evaluate gas exchange impairment during exercise and is widely used to evaluate patients with various chronic respiratory disorders, it is not feasible to perform it in the majority of office-based practices. In Europe, 14.5% of IPF patients are followed up in such practices,29 which generally do not have the flat, straight 30-m corridor necessary for the 6MWT. This may contribute to the underuse of exercise tests to evaluate IPF patients in clinical practice.33
In contrast, the 1STST is easily performed in office practice34 but it had not previously been evaluated for ILD patients. Our results show that patients with moderate-to-severe ILD (FVC 85% ± 19%, DLco 51% ± 19%) performed a mean of 21 ± 6 repetitions during the 1STST, which is similar to the number of repetitions by age-matched COPD populations.18–20 The number of repetitions performed during the 1STST in our study correlated poorly with the 6MWT distance. Previous work has shown that exercise capacity in the 6MWT and 1STST is affected by various anthropometric characteristics, pulmonary rehabilitation, and, potentially, by steroid-induced muscle dysfunction. However, we found that oxygen desaturation did not differ significantly between the two tests. Indeed, the SpO2 nadir was concordant in both tests and correlated strongly. This concordance was particularly robust for patients with nonfibrotic idiopathic interstitial pneumonitis (sarcoidosis and other ILDs). In our study, the SpO2 nadir was higher during the 1STST than the 6MWT, which is consistent with previous studies of COPD patients showing that oxygen desaturation was more pronounced during walking than cycling,21,35 and during the 6MWT compared with the 1STST.18 Similar observations were made when a 6-min stepper test and the 6MWT were compared in ILD patients.36 Further, we observed that the 1STST and the 6MWT were both able to detect a desaturation ⩾4%, which is the threshold of the variability of SpO2 measurement.37
Several sit-to-stand tests have been described that measure the number of repetitions performed over a defined time (30 s,38 1 min, and 3 min39) or the time to perform a certain number of repetitions.40 The availability of multiple tests has undoubtedly slowed the development of a standardized sit-to-stand test. We chose to evaluate the 1STST because it seemed well-suited for our ILD population: it is sufficiently short to be practical for testing on a daily basis and sufficiently long to detect desaturation in patients who exhibit desaturation in the 6MWT.17,34 In the future, it will be interesting to perform the 1STST sequentially to evaluate the prognosis, survival, or progression-free survival of patients with ILD, especially IPF, as has previously been performed with the 6MWT. Indeed, Lama and colleagues reported that desaturation during the 6MWT has important prognostic value for patients with IPF and fibrotic NSIP.10 Such data could enhance our understanding of the course of the disease and help to assess the efficacy of therapeutic interventions. In this regard, Vaidya and coworkers have reported that the 1STST test is a simple and sensitive test and performs comparably to the 6MWT in detecting a change in exercise tolerance in COPD patients after pulmonary rehabilitation.41
Study limitations
The main limitation of our study was the absence of a control group of healthy subjects for comparison with the 1STST performance of ILD patients. Strassman and colleagues recently reported reference values for the 1STST in a healthy adult population, but they did not report oxygen saturation values.42 Although the validity and reproducibility of the 1STST was recently demonstrated in COPD patients,18 further studies will be needed to confirm our findings in ILD.
Conclusion
Our study highlights the ability of the 1STST to detect exercise-induced gas exchange impairment as effectively as the 6MWT in patients with ILD of various etiologies. The 1STST is fast and well tolerated, easy to perform, understand, and interpret; and does not require specialized hardware. These attributes could make it an ideal test for use in office settings and during routine consultations.
Acknowledgments
The authors wish to thank Anne M. O’Rourke for editing the manuscript. The authors made the following contributions to this manuscript: Conception and design: JB, BW, and LSW; analysis and interpretation: JB, BW, CC, and LSW; statistical analysis: HB; drafting the manuscript for important intellectual content: JB, HB, BW, CC, and LSW
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Benoit Wallaert  https://orcid.org/0000-0002-2075-4315
https://orcid.org/0000-0002-2075-4315
Contributor Information
Justine Briand, CHU Lille, Service de Pneumologie et Immuno-Allergologie, Centre de Compétence des Maladies Pulmonaires Rares, F-59000 Lille, France University of Lille, F-59000 Lille, France.
Hélène Behal, University of Lille, F-59000 Lille, France CHU Lille, EA 2694, Santé Publique: Epidémiologie et Qualité des Soins, Unité de Biostatistiques, F-59000 Lille, France.
Cécile Chenivesse, CHU Lille, Service de Pneumologie et Immuno-Allergologie, Centre de Compétence des Maladies Pulmonaires Rares, F-59000 Lille, France.
Lidwine Wémeau-Stervinou, CHU Lille, Service de Pneumologie et Immuno-Allergologie, Centre de Compétence des Maladies Pulmonaires Rares, F-59000 Lille, France.
Benoit Wallaert, CHU Lille, Service de Pneumologie et Immuno-Allergologie, Centre de Compétence des Maladies Pulmonaires Rares, Lille F-59000, France.
References
- 1. Plantier L, Cazes A, Dinh-Xuan A-T, et al. Physiology of the lung in idiopathic pulmonary fibrosis. Eur Respir Rev. Epub ahead of print 31 March 2018. DOI: 10.1183/16000617.0062-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennett D, Fossi A, Bargagli E, et al. Mortality on the waiting list for lung transplantation in patients with idiopathic pulmonary fibrosis: a single-centre experience. Lung 2015; 193: 677–681. [DOI] [PubMed] [Google Scholar]
- 3. Chetta A, Marangio E, Olivieri D. Pulmonary function testing in interstitial lung diseases. Respir Int Rev Thorac Dis 2004; 71: 209–213. [DOI] [PubMed] [Google Scholar]
- 4. Wallaert B, Wemeau-Stervinou L, Salleron J, et al. Do we need exercise tests to detect gas exchange impairment in fibrotic idiopathic interstitial pneumonias? Pulm Med 2012; 2012: 657180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sue DY, Oren A, Hansen JE, et al. Diffusing capacity for carbon monoxide as a predictor of gas exchange during exercise. N Engl J Med 1987; 316: 1301–1306. [DOI] [PubMed] [Google Scholar]
- 6. Kaminsky DA, Whitman T, Callas PW. DLCO versus DLCO/VA as predictors of pulmonary gas exchange. Respir Med 2007; 101: 989–994. [DOI] [PubMed] [Google Scholar]
- 7. Cottin V, Crestani B, Cadranel J, et al. [French practical guidelines for the diagnosis and management of idiopathic pulmonary fibrosis. 2017 update. Full-length update]. Rev Mal Respir. Epub ahead of print 21 September 2017. DOI: 10.1016/j.rmr.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 8. Fulmer JD, Roberts WC, von Gal ER, et al. Morphologic-physiologic correlates of the severity of fibrosis and degree of cellularity in idiopathic pulmonary fibrosis. J Clin Invest 1979; 63: 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lettieri CJ, Nathan SD, Browning RF, et al. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med 2006; 100: 1734–1741. [DOI] [PubMed] [Google Scholar]
- 10. Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003; 168: 1084–1090. [DOI] [PubMed] [Google Scholar]
- 11. du Bois RM, Albera C, Bradford WZ, et al. 6-Minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J 2014; 43: 1421–1429. [DOI] [PubMed] [Google Scholar]
- 12. du Bois RM. 6-minute walk distance as a predictor of outcome in idiopathic pulmonary fibrosis. Eur Respir J 2014; 43: 1823–1824. [DOI] [PubMed] [Google Scholar]
- 13. du Bois RM, Weycker D, Albera C, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med 2011; 183: 1231–1237. [DOI] [PubMed] [Google Scholar]
- 14. Holland AE, Dowman L, Fiore J, et al. Cardiorespiratory responses to 6-minute walk test in interstitial lung disease: not always a submaximal test. BMC Pulm Med 2014; 14: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 16. Ozalevli S, Ozden A, Itil O, et al. Comparison of the Sit-to-Stand Test with 6 min walk test in patients with chronic obstructive pulmonary disease. Respir Med 2007; 101: 286–293. [DOI] [PubMed] [Google Scholar]
- 17. Bui K-L, Nyberg A, Maltais F, et al. Functional tests in chronic obstructive pulmonary disease, part 1: clinical relevance and links to the international classification of functioning, disability, and health. Ann Am Thorac Soc 2017; 14: 778–784. [DOI] [PubMed] [Google Scholar]
- 18. Crook S, Büsching G, Schultz K, et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur Respir J. Epub ahead of print March 2017. DOI: 10.1183/13993003.01871-2016. [DOI] [PubMed] [Google Scholar]
- 19. Meriem M, Cherif J, Toujani S, et al. Sit-to-stand test and 6-min walking test correlation in patients with chronic obstructive pulmonary disease. Ann Thorac Med 2015; 10: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reychler G, Boucard E, Peran L, et al. One minute sit-to-stand test is an alternative to 6MWT to measure functional exercise performance in COPD patients. Clin Respir J. Epub ahead of print 8 June 2017. DOI: 10.1111/crj.12658. [DOI] [PubMed] [Google Scholar]
- 21. Gruet M, Peyré-Tartaruga LA, Mely L, et al. The 1-Minute sit-to-stand test in adults with cystic fibrosis: correlations with cardiopulmonary exercise test, 6-minute walk test, and quadriceps strength. Respir Care 2016; 61: 1620–1628. [DOI] [PubMed] [Google Scholar]
- 22. Poulain M, Durand F, Palomba B, et al. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest 2003; 123: 1401–1407. [DOI] [PubMed] [Google Scholar]
- 23. Hadeli KO, Siegel EM, Sherrill DL, et al. Predictors of oxygen desaturation during submaximal exercise in 8,000 patients. Chest 2001; 120: 88–92. [DOI] [PubMed] [Google Scholar]
- 24. Cotes JE, Dabbs JM, Elwood PC, et al. Iron-deficiency anaemia: its effect on transfer factor for the lung (diffusiong capacity) and ventilation and cardiac frequency during sub-maximal exercise. Clin Sci 1972; 42: 325–335. [DOI] [PubMed] [Google Scholar]
- 25. Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16: 1–100. [PubMed] [Google Scholar]
- 26. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968. [DOI] [PubMed] [Google Scholar]
- 27. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]
- 28. Chenivesse C, Boulanger S, Langlois C, et al. Oxygen desaturation during a 6-minute walk test as a predictor of maximal exercise-induced gas exchange abnormalities in sarcoidosis. J Thorac Dis 2016; 8: 1995–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kreuter M, Swigris J, Pittrow D, et al. Health related quality of life in patients with idiopathic pulmonary fibrosis in clinical practice: insights-IPF registry. Respir Res 2017; 18: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahler DA, Harver A, Rosiello R, et al. Measurement of respiratory sensation in interstitial lung disease. Evaluation of clinical dyspnea ratings and magnitude scaling. Chest 1989; 96: 767–771. [DOI] [PubMed] [Google Scholar]
- 31. Swigris JJ, Streiner DL, Brown KK, et al. Assessing exertional dyspnea in patients with idiopathic pulmonary fibrosis. Respir Med 2014; 108: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maher TM, Molina-Molina M, Russell A-M, et al. Unmet needs in the treatment of idiopathic pulmonary fibrosis-insights from patient chart review in five European countries. BMC Pulm Med 2017; 17: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mapel DW, Samet JM, Coultas DB. Corticosteroids and the treatment of idiopathic pulmonary fibrosis. Past, present, and future. Chest 1996; 110: 1058–1067. [DOI] [PubMed] [Google Scholar]
- 34. Bui K-L, Nyberg A, Maltais F, et al. Functional tests in chronic obstructive pulmonary disease, part 2: measurement properties. Ann Am Thorac Soc 2017; 14: 785–794. [DOI] [PubMed] [Google Scholar]
- 35. Palange P, Forte S, Onorati P, et al. Ventilatory and metabolic adaptations to walking and cycling in patients with COPD. J Appl Physiol 1985. 2000; 88: 1715–1720. [DOI] [PubMed] [Google Scholar]
- 36. Chéhère B, Bougault V, Gicquello A, et al. Cardiorespiratory response to different exercise tests in interstitial lung disease. Med Sci Sports Exerc 2016; 48: 2345–2352. [DOI] [PubMed] [Google Scholar]
- 37. Jubran A. Pulse oxymetry. In: Tobin MJ. (ed.) Principles and practice of intensive care monitoring. New York: McGraw-Hill, 1998, pp. 261–287. [Google Scholar]
- 38. Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999; 70: 113–119. [DOI] [PubMed] [Google Scholar]
- 39. Aguilaniu B, Roth H, Gonzalez-Bermejo J, et al. A simple semipaced 3-minute chair rise test for routine exercise tolerance testing in COPD. Int J Chron Obstruct Pulmon Dis 2014; 9: 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jones SE, Kon SSC, Canavan JL, et al. The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax 2013; 68: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 41. Vaidya T, de Bisschop C, Beaumont M, et al. Is the 1-minute sit-to-stand test a good tool for the evaluation of the impact of pulmonary rehabilitation? Determination of the minimal important difference in COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 2609–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Strassmann A, Steurer-Stey C, Lana KD, et al. Population-based reference values for the 1-min sit-to-stand test. Int J Public Health 2013; 58: 949–953. [DOI] [PubMed] [Google Scholar]



