Abstract
Background:
Natalizumab significantly reduces the disease activity in patients with relapsing-remitting multiple sclerosis but due to the risk of progressive multifocal leukoencephalopathy it is often discontinued. Fingolimod is seen as an alternative, but there are no long-term analyses of the efficacy of fingolimod in this setting using the no evidence of disease activity (NEDA)-3 criteria. We provide an assessment of patients who discontinued natalizumab and switched to fingolimod or other treatments by evaluating the proportion of patients who fulfil NEDA-3 criteria after prolonged follow-up periods.
Methods:
We conducted a retrospective observational study of multiple sclerosis patients, who were treated with continuous natalizumab or who had switched to fingolimod or other treatments after natalizumab discontinuation. We assessed NEDA-3 status, annual relapse rate and determined the odds ratio between disease course after treatment switch and other patient and treatment characteristics.
Results:
A total of 61 patients on continuous natalizumab treatment and 53 patients who switched from natalizumab to fingolimod or other treatments were accompanied for up to 5 years. While the proportion of natalizumab patients fulfilling NEDA-3 criteria remained stable at 90% during the entire follow-up period, the proportion of patients switching to fingolimod or other therapies dropped to 76.7% in the first year after discontinuation, and to 50% in the years thereafter. While the median Expanded Disability Status Scale remained stable and the percentage of relapsing patients did not change significantly, recurring magnetic resonance imaging activity was found in up to 42% of the patients after switching from natalizumab to other treatments. New disease activity was significantly correlated with extended treatment gap between natalizumab discontinuation and the start of a new therapy.
Discussion:
Patients remain clinically stable after discontinuing natalizumab and switching to other therapies. However, when considering NEDA-3 criteria, a considerable proportion of patients show disease reactivation. Careful monitoring and early evaluation of alternatives is necessary after switching from natalizumab to other treatments.
Keywords: fingolimod, multiple sclerosis, natalizumab, NEDA, progressive multifocal leukoencephalopathy, treatment switch
Introduction
Natalizumab (NTZ) is an alpha-4-integrin antagonist used for the treatment of patients with relapsing-remitting multiple sclerosis (RRMS).1 Compared with interferons (IFNs) or glatiramer acetate (GA), NTZ significantly reduces the relapse rate, the disability progression and the radiological progression in RRMS patients.1,2 In Switzerland, NTZ is licensed for second-line treatment of RRMS patients who did not respond to other disease-modifying treatments (DMTs) or as a first-line therapy option for highly active RRMS. However, the use of NTZ is associated with progressive multifocal leukoencephalopathy (PML) caused by John-Cunningham virus (JCV) in approximately 4.2 per 1000 patients, with 731 confirmed PML cases worldwide (as of June 2017).3,4 Seropositivity for anti-JCV antibodies is highly prevalent in the general population. Primary exposure typically occurs early in life,5 and approximately 55% of the multiple sclerosis (MS) population are seropositive for anti-JCV antibodies.3,6–8 As the risk of developing PML increases with anti-JCV positivity and increasing duration of NTZ treatment, NTZ treatment discontinuation should be considered for JCV seropositive patients who had been treated with NTZ for prolonged periods.4 Other factors that may lead to NTZ discontinuation are NTZ hypersensitivity, NTZ antibody seropositivity, suboptimal treatment efficacy, or a preference for oral therapy.3 Although, there is evidence of reappearing disease activity after NTZ cessation, there are no widely accepted guidelines on how to best avoid disease reactivation after NTZ withdrawal. Therefore, alternative treatments to control disease activity must be evaluated.9–12
Most commonly, immunomodulatory agents are considered after NTZ withdrawal,13 with the functional antagonist of the sphingosine-1-phosphate receptor fingolimod (FTY) being regarded the best alternative.14–17 FTY shows a superior efficacy compared with IFNs, and there is evidence of FTY benefits after discontinuation of NTZ.2,14,16,18 Nevertheless, other studies revealed clinically relevant disease activity during the first weeks of FTY treatment in patients with severe MS or after extended NTZ washout periods prior to initiating FTY treatment.19–22 However, none of these studies used ‘no evidence of disease activity’ (NEDA) criteria to assess disease activity/reactivation.
To the best of our knowledge, the majority of the available studies analyzing the disease course after switching from NTZ to FTY focus on disease activity within the first six months after changing the treatment. In addition, only certain aspects of the disease activity status are assessed (e.g. relapse frequency or radiological disease progression), and outcome measures other than NEDA criteria are used.14–16 Thus far, there are no long-term data assessing the efficacy of FTY after NTZ treatment discontinuation using the NEDA-3 criteria that take both clinical and radiological parameters into account in order to stringently assess the disease activity and predict mid- to long-term prognosis.23 Therefore, we aimed to assess the long-term efficacy of FTY in patients who had discontinued the treatment with NTZ by evaluating the proportion of patients who fulfil NEDA-3 criteria after prolonged follow-up periods. Furthermore, we assessed which parameters might predict a higher risk of recurrence of disease activity after switching from NTZ to FTY.
Methods
A retrospective observational study at the MS outpatient clinic at the Cantonal Hospital Aarau, Switzerland, was conducted. The MS outpatient clinic at the Cantonal Hospital Aarau has a catchment area of around 600,000 people, and provides diagnostic services and comprehensive care for MS patients at all disease stages with about 1500 patient contacts/visits per year. The study was approved by the local ethical committee (ethical committee for northwest and central Switzerland, permit number 2016-02233).
Patient records of all MS patients treated at our clinic between 2007 and 2016 were searched for patients treated with NTZ or for patients who had previously received NTZ treatment, but who had changed the treatment regimen. Three categories were defined: Patients receiving continuous NTZ treatment until the end of the observational period (NTZ group) formed the control group. The other two groups consisted of patients who had discontinued the treatment with NTZ and started a treatment with FTY during the observational period (FTY group), and of patients who had switched from NTZ to a different disease-modifying treatment, (dimethyl fumarate, teriflunomide; DMT group). We analyzed patients switching to FTY separately, as it is the most often used treatment after NTZ withdrawal and because it is also used as an escalation therapy. In addition to demographic data, the NEDA-3 status was assessed. NEDA-3 is a composite measure of disease activity based on the absence of relapses, no evidence of disability progression by the Expanded Disability Status Scale (EDSS), and no new T2 lesions or gadolinium-enhancing magnetic resonance imaging (MRI) lesions. Disability was measured by EDSS. The EDSS including the functional system scores was determined by an experienced neurologist. Furthermore, we assessed the annualized relapse rate (ARR). Relapses were diagnosed by an experienced neurologist when new or previous symptoms appeared which were reported subjectively or could be objectified by a clinical examination. The symptoms had to exist for at least 24 h, the time interval to the previous relapse had to be at least 30 days, and the symptoms could not be explained by a change in body temperature or by infection.
The data were analyzed using descriptive statistics. Group comparisons were made between NTZ patients, FTY patients and DMT patients, and were analyzed for demographic and clinical variables by using the Kruskal–Wallis test and Mann–Whitney U test. A Chi-square test was used to calculate the distribution of sex, reasons for beginning with NTZ and treatment before NTZ initiation within groups. We used a multiple logistic regression model to estimate the association [odds ratio (OR)] between disease course after treatment switch and sex, age, disease duration, previous disease course as measured by ARR or treatment gap between NTZ and the subsequent treatment. The significance threshold was set to a level of <0.05. Statistical analysis was performed using SPSS 24 (IBM Corp., Armonk, NY, USA).
Results
Patient demographics and clinical characteristics
From 2007 to 2016 we took care of 549 MS patients. During this period 152 RRMS patients were treated with NTZ [mean age 42 years, female n = 100 (65.8%)]. All patients received NTZ within the treatment criteria valid for Switzerland as a second-line treatment in patients who did not respond to other DMTs or as a first-line treatment in patients with highly active disease. Overall, 26 patients were lost to follow up because they had transferred to other treatment centers. Complete follow-up data were available for 126 patients [mean age 42.4 years (standard deviation (SD) ± 12.3), female n = 87 (69%), male n = 39 (31%)]. The median EDSS at NTZ initiation was 2.5 (SD ± 1.1) and the mean follow-up time was 5.9 years (SD ± 1.8).
A total of 61 of the 126 patients received continuous NTZ treatment, while 65 patients discontinued NTZ. Overall, 12 patients in the discontinuation group were excluded from the analysis for the following reasons: Pregnancy was the reason for NTZ discontinuation in one patient, whereas PML led to treatment cessation in two patients. These patients were excluded from the analysis because they had experienced very long treatment gaps. Five patients discontinued NTZ without subsequent treatment and four patients suffered anaphylactic reaction within the first 3 months of treatment with NTZ and were excluded as well. The courses of 61 patients on continuous NTZ treatment and 53 patients who discontinued NTZ therapy were included in the data analysis. Out of these 53 patients, 43 started treatment with FTY (FTY group), while 10 patients began treatment with another DMT (DMT group). As part of our clinical routine all patients were examined according to the same schedule with clinical visits at least twice a year and MRI scans at least once a year. The mean number of yearly clinical visits was 2.5 (SD ± 0.32) and 3.5 (SD ± 1.9) in the FTY group and the DMT group respectively. In the NTZ group patients had 2.1 (SD ± 0.4) visits with complete EDSS assessment and in addition a short monthly clinical visit with every infusion. The mean annual number of MRIs was 1.5 (SD ± 0.6) in the NTZ group, 1.1 (SD ± 0.4) in the FTY group and 1.2 (SD ± 0.8) in the DMT group. Patients who ceased NTZ treatment were older and had longer disease duration than patients who continued treatment with NTZ. However, after applying a multiple logistic regression model these factors had no predictive value. Demographic and clinical data of all groups are shown in Table 1.
Table 1.
Demographic and baseline characteristics of all patients who completed follow up. Stated p-values refer to comparisons between patients on continual NTZ treatment with patients switching to alternative therapies (FTY and other DMTs).
| Patients treated with NTZ | Patients switching from NTZ to FTY | Patients switching from NTZ to other DMT | p-value | |
|---|---|---|---|---|
| N (%) | 61 (53.5) | 43 (37.7) | 10 (8.8) | |
| Female, N (%) | 41 (67.2) | 31 (72.1) | 7 (70.0) | p = 0.867 |
| Mean age in years (SD) | 39.6 (±12.3) | 46.19 (±11.3) | 46.0 (±13.3) | p = 0.009 |
| Mean time since first symptoms in years (SD) | 11.4 (±7.8) | 15.8 (±9.2) | 11.1 (±4.7) | p = 0.022 |
| Mean time since MS diagnosis in years (SD) | 9.2 (±5.3) | 13.3 (±7.5) | 9.5 (±3.9) | p = 0.013 |
| Number of relapses before NTZ mean (SD) | 3.7 (±2.2) | 4.5 (±2.9) | 2.9 (±2.1) | p = 0.148 |
| Median EDSS before NTZ | 2 | 2.5 | 2.5 | p = 0.783 |
| MS treatment prior to natalizumab, N (%) | ||||
| Interferon-beta | 27 (44.3) | 29 (67.4) | 5 (50.0) | p = 0.064 |
| Glatiramer acetate | 8 (13) | 9 (20.9) | 2 (20.0) | p = 0.550 |
| Azathioprine | 0 (0) | 2 (4.7) | 0 (0) | p = 0.186 |
| Teriflunomide | 1 (1.6) | 0 (0) | 0 (0) | p = 0.645 |
| Fingolimod | 4 (6.5) | 0 (0) | 0 (0) | p = 0.165 |
| Mitoxantron | 0 (0) | 3 (6.9) | 0 (0) | p = 0.079 |
| Treatment-naïve | 27 (44.3) | 11 (25.6) | 4 (40.0) | p = 0.349 |
| Primary reasons for beginning NTZ treatment, % (n) | ||||
| Disease progression despite previous treatment | 34 (55.7) | 26 (60.5) | 6 (60.0) | p = 0.882 |
| Lack of tolerance of previous treatment | 0 (0) | 6 (13.9) | 0 (0) | p = 0.005 |
| First treatment, highly active disease | 27 (44.3) | 11 (25.6) | 4 (40.0) | p = 0.349 |
DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale; FTY, fingolimod; MS, multiple sclerosis; NTZ, natalizumab; SD, standard deviation.
Safety considerations in patients with JCV antibody seropositivity were the main reason for NTZ discontinuation and switch to FTY or other DMTs. A total of two patients developed malignancy while on NTZ treatment and switched to interferon or dimethyl fumarate. All reasons are shown in Table 2. No significant differences in terms of reasons for withdrawal were found between the groups.
Table 2.
Reasons for natalizumab discontinuation.
| Primary reasons for natalizumab discontinuation | Patients switching from NTZ to FTY, n (%) | Patients switching from NTZ to DMT n (%) | p-value |
|---|---|---|---|
| Positive JCV status | 39 (90.8) | 8 (80.0) | p = 0.336 |
| Presence of anti-natalizumab antibodies | 1 (2.3) | 0 (0) | |
| Patient choice | 2 (4.6) | 0 (0) | |
| Disease progression | 1 (2.3) | 0 (0) | |
| Malignancy | 0 (0) | 2 (20.0) |
DMT, disease-modifying treatment; FTY, fingolimod; JCV, John-Cunningham virus; NTZ, natalizumab; SD, standard deviation.
Disease activity based on NEDA
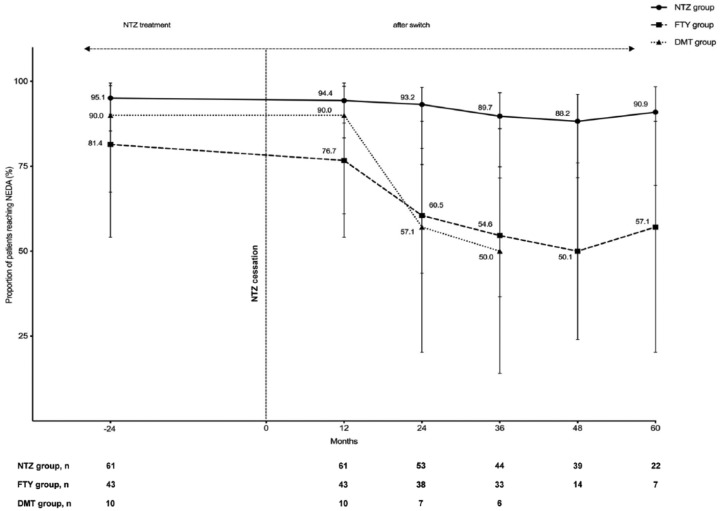
While on NTZ treatment, the proportion of patients in the total study population fulfilling NEDA-3 criteria was 90%. This proportion remained stable in the patients who continued treatment with NTZ with 90.9% of patients fulfilling NEDA-3 after 5 years of follow up. In patients switching from NTZ to FTY, the proportion of patients fulfilling NEDA-3 dropped from 81.4% in the last year of NTZ treatment to 76.7% in the first year after discontinuation, and to 50% in the following 3 years thereafter. In patients switching to another DMT, the proportion of patients fulfilling NEDA-3 also dropped to 50%. In this group, the longest follow up was 36 months. Figure 1 shows the detailed NEDA-3 course by treatment group.
Figure 1.
Detailed course of the proportion of patients reaching NEDA-3 presented by the treatment groups. Data were calculated using the 61 patients on continuous NTZ treatment and 53 patients who discontinued NTZ. Maximum follow-up time in the DMT group was 3 years.
DMT, other disease-modifying treatment; FTY, fingolimod; NEDA, no evidence of disease activity; NTZ, natalizumab.
ARR and proportion of patients with relapses
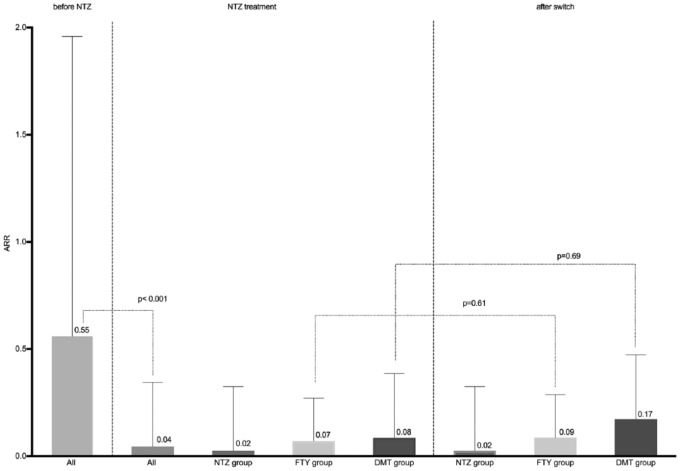
After NTZ initiation, the ARR in the entire study population dropped markedly from 0.55 to 0.04 (p < 0.001) and remained stable in patients on continuous NTZ treatment (ARR = 0.02). Patients who switched from NTZ to FTY also exhibited a stable ARR at low levels with an ARR of 0.09 after the switch compared with 0.07 while being treated with NTZ. A slight, however not statistically significant increase in ARR from 0.08 to 0.17 was observed in the patients who had switched to other DMTs (Figure 2).
Figure 2.
In all patients, a highly significant drop in ARR from 0.55 to 0.04 was noted after initiation of NTZ treatment. In the patients continuously treated with NTZ the ARR remained stable throughout the entire study period. Nonsignificant changes in the ARR were noted in both patient cohorts after discontinuation of NTZ and switching to either FTY therapy or treatment with other DMTs. Data were calculated using the 61 patients on continuous NTZ treatment and 53 patients who discontinued NTZ.
ARR, annualized relapse rate; DMT, other disease-modifying treatment; FTY, fingolimod; NTZ, natalizumab.
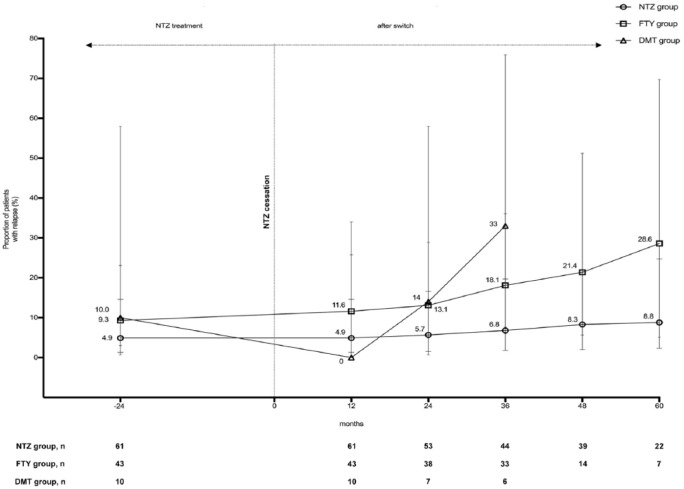
The proportion of relapsing patients did not change significantly in patients continuing with NTZ treatment. A nonsignificant increase in the percentage of relapsing patients was seen after switching from NTZ to FTY (11.6% 1 year after the switch from NTZ to 28.6% after 60 months) or another DMT (to 33% after 36 months; Figure 3). None of the relapsing patients developed severe rebound activity with severe clinical worsening or fulminant MRI activity. All relapses were treated with 500 mg methylprednisolone for 5 days.
Figure 3.
Proportion of patients receiving relapses presented by the treatment groups. A nonsignificant increase in the percentage of relapsing patients was seen after switching from NTZ to FTY or another DMT. Data were calculated using the 61 patients on continuous NTZ treatment and 53 patients who discontinued NTZ. Maximum follow-up time in the DMT group was 3 years.
DMT, other disease-modifying treatment; FTY, fingolimod; NTZ, natalizumab.
MRI activity
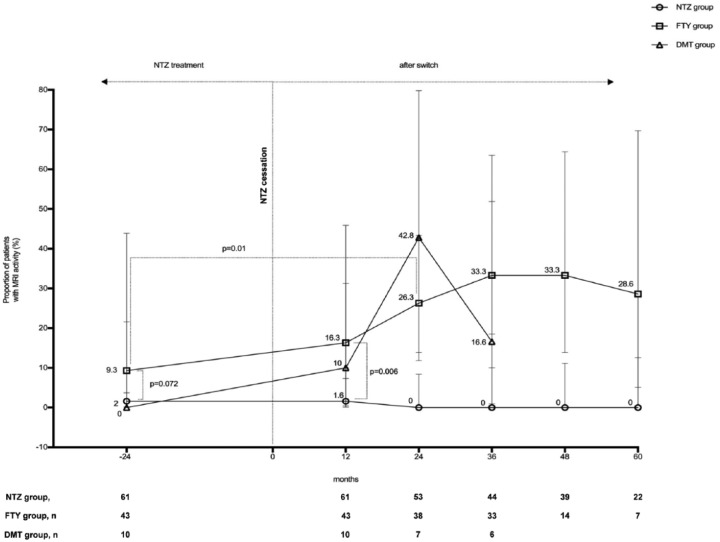
The proportion of patients with new or enlarging lesions on MRI activity within the entire patient population was lower than 10% during NTZ treatment. No change of MRI activity was observed throughout the entire follow-up period in patients remaining on treatment with NTZ. By contrast, the proportion of patients with MRI activity increased after a switch from NTZ to FTY, from 9.3% during NTZ treatment to 16.3% after 12 months post switching. The percentage plateaued and reached significance at approximately 30% during longer follow-up periods. The patient percentage experiencing new or enhancing MRI activity in the DMT group reached a peak at 42.8% 24 months after 24 months post switching (Figure 4).
Figure 4.
Proportion of patients with new or enlarging lesions on MRI activity presented by the treatment groups. No change of MRI activity in patients remaining on treatment with NTZ but an increased proportion of patients with MRI activity after a switch from NTZ to FTY or another DMT. Maximum follow-up time in the DMT group was 3 years.
DMT, other disease-modifying treatment; FTY, fingolimod; MRI, magnetic resonance imaging; NTZ, natalizumab.
EDSS after switching
During the observational period, the median EDSS remained stable at levels of minimal or mild disability (i.e. EDSS of 2.0 or 2.5) after switching from NTZ to FTY or another DMT (Table 3).
Table 3.
Number of relapses and median EDSS stratified by treatment group. Stated p-values refer to comparisons between patients on continual NTZ treatment with patients switching to alternative therapies (FTY and other DMTs).
| Patients treated with NTZ | Patients switching from NTZ to FTY | Patients switching from NTZ to other | p-value | |
|---|---|---|---|---|
| Number of relapses before NTZ mean (SD) | 3.7 (±2.2) | 4.5 (±2.9) | 2.9 (±2.1) | p = 0.148 |
| Median EDSS before NTZ | 2 | 2.5 | 2.5 | p = 0.783 |
| Number of relapses during NTZ mean (SD) | 0.1 (±0.4) | 0.3 (±0.6) | 0.4 (±0.9) | p = 0.607 |
| Median EDSS during NTZ | 2 | 2 | 2 | p = 0.296 |
| Mean NTZ exposure in weeks (±SD) | 267.2 (±148.5) | 190.2 (±92.1) | 246.3 (±131.2) | p = 0.017 |
| Positive JCV status, N (%) | 16 (26.2) | 40 (93.0) | 8 (80.0) | |
| Number of relapses during FTY mean (± SD) | 0.28 (±0.62) | |||
| Median EDSS during FTY | 2.5 | |||
| Mean FTY exposure in weeks (SD) | 166.73 (±63.92) | |||
| Mean washout period (SD) (weeks) | 14 (±14) | 17.9 (±21.1) | ||
| Number of relapses during other medication mean (SD) | 0.30 (±0.48) | |||
| Median EDSS during other medication | 2.0 | |||
| Mean medication exposure in weeks (SD) | 91.86 (±43.14) | |||
| MS treatment after NTZ, n (%) | ||||
| Dimethyl fumarate | 6 (60.0) | |||
| Teriflunomide | 4 (40.0) |
DMT, other disease-modifying treatment; EDSS, Expanded Disability Status Scale; FTY, fingolimod; JCV, John-Cunningham virus; NTZ, natalizumab; SD, standard deviation.
Factors correlated with new disease activity
We performed a multinomial logistic regression analysis to assess factors related to reappearing disease activity. A longer washout period after cessation of NTZ and before starting a new treatment was the only factor correlated with new disease activity (p = 0.023). Patients who remained NEDA had a mean washout period of 11.9 weeks (SD ± 8.7). Patients who were no longer NEDA after the switch had a mean washout period of 22.01 weeks (SD ± 24.7). No association was found between disease reactivation and age, sex, disease duration, or EDSS. Furthermore, disease activity prior to NTZ treatment as measured by the relapse rate or duration of NTZ treatment showed no correlation with disease reactivation after changing the treatment.
Discussion
The question of which subsequent therapy option to choose after NTZ therapy is of high importance as NTZ is highly effective, but has been shown to lead to PML in a subset of patients.3,4 Recent studies revealed that discontinuation of NTZ may lead to reappearance of MRI activity and disease rebound after the clearance of NTZ 3–6 months after discontinuation, despite subsequent treatment.21,24–26 Only limited data are available about the long-term course in these patients, and therapeutic strategies for anti-JCV antibody positive MS patients who need highly effective treatment remain to be optimized. To the best of our knowledge, this is the first study analyzing the long-term course of RRMS patients switching from NTZ to FTY or other DMT and using the NEDA-3 concept.
Existing data about ARR after switching from NTZ are contradictory. In prior reports a marked and aggressive increase in relapse activity has been reported.14,27 In contrast, no increase of ARR has been reported in a recently published work of a cohort of 21 patients followed up for 12 months.13 Consistently with the latter, in our patient cohort of 53 patients no significant increases in ARR were observed in patients switching to other therapeutics (0.09 versus 0.07 in the FTY and 0.08 versus 0.17 in the DMT group respectively), even in patients followed for up to 5 years. Furthermore, the ARR of all treatment groups remained substantially below the pre-NTZ ARR of 0.78 (Figure 2) and no increased disability as measured by the EDSS was notable during the observation period. In those patients who perceived relapses, no severe clinical worsening or fulminant MRI activity correspondent to severe rebound activity was noted and standard relapse treatment with methylprednisolone was sufficient.
The duration of the washout period between NTZ cessation and the start of the following therapy as a predictor of relapses was investigated in several studies.14,20 Data from the literature show that disease reactivation occurred most frequently after 3–6 months after the last NTZ infusion.21,24–26 In our cohort the duration of the washout period was not the same during the observation period but shortened over time, as more recent data showed that shorter washout periods are also safe and may reduce the risk of new disease activity.20 However, in line with previous data, we found a correlation between duration of the washout period and new disease activity as measured by MRI, although the relapse rate was not increased after cessation of NTZ.21,24–26
In the past, it was suggested that radiologic disease reactivation after NTZ treatment discontinuation was influenced by the duration of NTZ exposure or the relapse activity prior to NTZ start.22 In our study, the disease activity after cessation of NTZ was not influenced by relapse rate before NTZ start, the duration of NTZ treatment or the disease duration.
Taken together, these data suggest that the patients remain stable when considering clinical parameters after discontinuing NTZ treatment and switching to different treatment regimens. However, when taking MRI data into account for the assessment of disease activity, by considering the concept of NEDA-3, a considerable proportion of patients show signs of disease reactivation in the FTY and DMT groups after extended follow-up periods.
After 1 year from cessation of NTZ, 76.7% of the FTY treated patients and 90% of the other DMT-treated patients fulfilled the NEDA-3 criteria. This proportion of patients who are free of clinical relapse, worsening of disability, and radiological activity decreases further to approximately 50% after longer follow-up periods; up to 36 months for the patients in the DMT group and 60 months for patients receiving FTY. The majority of patients who did not meet the NEDA-3 criteria after switching showed radiological progression. At the same time, the rate of patients who fulfil the NEDA-3 criteria remains stable throughout the entire observation period in the NTZ-treated group. One could assume that the reported increase of MRI activity is due to a closer MRI monitoring after changing the treatment. In our cohort patients under NTZ therapy had a higher mean annual number of MRIs than patients who switched to another treatment. However, we cannot exclude the possibility that this higher number was partially driven by a few JCV-positive patients with closer MRI schedule.
Our novel long-term findings support the assumption of recent short-term observational studies, suggesting that FTY and other DMTs are less efficient than NTZ in controlling disease activity.28–30 The novelty of our study is that our data show that aggressive rebounds of disease activity are rare in a real-life cohort of RRMS patients ceasing NTZ treatment and switching to another therapy. However, disease activity reappears after several months of clinical stability.31 Our findings suggest that radiological disease activity should be assessed on a regular basis to monitor subclinical radiological changes in patients without clinical disease activity as subclinical MRI activity has been associated with a worse prognosis even in patients on treatment.32,33
The consequent use of NEDA-3 criteria may prove crucial in enabling early treatment of subclinical disease activity and, hence, in helping to improve the long-term prognosis of RRMS patients. Nevertheless, in the absence of treatment guidelines, our study provides additional guidance on the treatment after NTZ discontinuation. Generally, the treatment gap should not exceed 12 weeks.20 Furthermore, close MRI monitoring even in clinically stable patients should be performed to capture recurring disease activity and to establish treatments with higher efficacy. For these patients, highly efficient novel treatment options with improved risk–benefit profiles like alemtuzumab or ocrelizumab are needed.
When interpreting our data, the limitations of a retrospective and observational study with different follow-up times resulting from different time points of treatment switch must be considered. A further shortcoming of our study could be that patient selection is heterogeneous, as there were no clear criteria for the change in therapy. However, in our clinical routine we have strictly adhered to the Swiss criteria for the start of NTZ therapy and the switch to another drug, so that there is finally a certain standardization that represents the clinical practice accepted in Switzerland and abroad with data from a real cohort of treated MS patients.4,34–36 Furthermore, one point that could be criticized is the fact that among the changing NTZ patients there were also a few patients who were positive for NTZ antibodies, showed MRI activity or developed malignancies in the year before the change of treatment. This may be a weakness from the perspective of a randomized trial but this is a retrospective observational study under real-life conditions. Under these conditions, and although NTZ is one of the most effective MS drugs, it can be expected that a small proportion of patients under NTZ therapy will develop NTZ antibodies or show radiological or clinical signs of disease activity. In these patients or in patients with contraindications for NTZ therapy, such as malignancies, similar to JCV-positive patients, the unanswered question arises as to which alternative therapy should be chosen? We therefore believe that it is reasonable to include these patients in our evaluation. Finally, the generalizability of the results of the other-DMT group is limited, as the number of patients in this group is small.
In conclusion, our data show that FTY and other DMTs are valid and efficient treatment options after NTZ discontinuation, but close monitoring is necessary to catch recurring disease activity in some patients. In patients who show signs of disease activity under a new therapy, the use of highly effective drugs such as rituximab must be considered at an early stage. Unfortunately, rituximab was not approved for MS treatment in Switzerland during the observation period and data on the safety and efficacy of rituximab only appeared around the end of the observation period.37 Ideally, the duration of any treatment gap after NTZ discontinuation should be restricted to less than 3 months to minimize the risk of disease reactivation.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Lara Diem, Department of Neurology, Cantonal Hospital Aarau, Aarau, Switzerland.
Krassen Nedeltchev, Department of Neurology, Cantonal Hospital Aarau, Aarau, Switzerland; Department of Neurology, Inselspital, University Hospital Bern and University of Bern, Bern, Switzerland.
Timo Kahles, Department of Neurology, Cantonal Hospital Aarau, Aarau, Switzerland.
Lutz Achtnichts, Department of Neurology, Cantonal Hospital Aarau, Aarau, Switzerland.
Oliver Findling, Department of Neurology, Cantonal Hospital Aarau, Tellstrasse, Aarau 5000, Switzerland.
References
- 1. Hutchinson M, Kappos L, Calabresi PA, et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol 2009; 256: 405–415. [DOI] [PubMed] [Google Scholar]
- 2. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 3. Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012; 366: 1870–1880. [DOI] [PubMed] [Google Scholar]
- 4. Tysabri® (natalizumab) safety update, https://medinfo.biogen.com (2017, accessed 8 August 2017). [Google Scholar]
- 5. Ferenczy MW, Marshall LJ, Nelson CD, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 2012; 25: 471–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol 2010; 68: 295–303. [DOI] [PubMed] [Google Scholar]
- 7. Tysabri(R) (natalizumab) - United States full prescribing information [Internet], https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125104s0576lbl.pdf (2012, accessed 8 Augst 2017).
- 8. Tysabri (natalizumab) – Summary of product characteristics [Internet], http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000603/WC500044686.pdf (2016, accessed 8 August 2017).
- 9. O’Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 2011; 76: 1858–1865. [DOI] [PubMed] [Google Scholar]
- 10. Sorensen PS, Koch-Henriksen N, Petersen T, et al. Recurrence or rebound of clinical relapses after discontinuation of natalizumab therapy in highly active MS patients. J Neurol 2014; 261: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 11. Killestein J, Vennegoor A, Strijbis EM, et al. Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol 2010; 68: 392–395. [DOI] [PubMed] [Google Scholar]
- 12. Prosperini L, Annovazzi P, Capobianco M, et al. Natalizumab discontinuation in patients with multiple sclerosis: profiling risk and benefits at therapeutic crossroads. Mult Scler 2015; 21: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 13. Villaverde-Gonzalez R, Gracia Gil J, Perez Sempere A, et al. Observational study of switching from natalizumab to immunomodulatory drugs. Eur Neurol 2017; 77: 130–136. [DOI] [PubMed] [Google Scholar]
- 14. Comi G, Gold R, Dahlke F, et al. Relapses in patients treated with fingolimod after previous exposure to natalizumab. Mult Scler 2015; 21: 786–790. [DOI] [PubMed] [Google Scholar]
- 15. Cohen JA, Barkhof F, Comi G, et al. Fingolimod versus intramuscular interferon in patient subgroups from TRANSFORMS. J Neurol 2013; 260: 2023–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Havla J, Tackenberg B, Hellwig K, et al. Fingolimod reduces recurrence of disease activity after natalizumab withdrawal in multiple sclerosis. J Neurol 2013; 260: 1382–1387. [DOI] [PubMed] [Google Scholar]
- 17. Laroni A, Brogi D, Milesi V, et al. Early switch to fingolimod may decrease the risk of disease recurrence after natalizumab interruption. Mult Scler 2013; 19: 1236–1237. [DOI] [PubMed] [Google Scholar]
- 18. Rasia S, Cordioli C, De Rossi N, et al. Natalizumab to fingolimod switching in multiple sclerosis: results from a “real word” retrospective analysis. J Mult Scler (Foster City) 2015; 2: 142–147. [Google Scholar]
- 19. Cohen M, Maillart E, Tourbah A, et al. Switching from natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol 2014; 71: 436–441. [DOI] [PubMed] [Google Scholar]
- 20. Kappos L, Radue EW, Comi G, et al. Switching from natalizumab to fingolimod: a randomized, placebo-controlled study in RRMS. Neurology 2015; 85: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centonze D, Rossi S, Rinaldi F, et al. Severe relapses under fingolimod treatment prescribed after natalizumab. Neurology 2012; 79: 2004–2005. [DOI] [PubMed] [Google Scholar]
- 22. Vellinga MM, Castelijns JA, Barkhof F, et al. Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology 2008; 70: 1150–1151. [DOI] [PubMed] [Google Scholar]
- 23. Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol. 2015; 72: 152–158. [DOI] [PubMed] [Google Scholar]
- 24. Jander S, Turowski B, Kieseier BC, et al. Emerging tumefactive multiple sclerosis after switching therapy from natalizumab to fingolimod. Mult Scler 2012; 18: 1650–1652. [DOI] [PubMed] [Google Scholar]
- 25. Rinaldi F, Seppi D, Calabrese M, et al. Switching therapy from natalizumab to fingolimod in relapsing-remitting multiple sclerosis: clinical and magnetic resonance imaging findings. Mult Scler 2012; 18: 1640–1643. [DOI] [PubMed] [Google Scholar]
- 26. Gueguen A, Roux P, Deschamps R, et al. Abnormal inflammatory activity returns after natalizumab cessation in multiple sclerosis. J Neurol Neurosurg Psychiatry 2014; 85: 1038–1040. [DOI] [PubMed] [Google Scholar]
- 27. Sempere AP, Martin-Medina P, Berenguer-Ruiz L, et al. Switching from natalizumab to fingolimod: an observational study. Acta Neurol Scand 2013; 128: e6–e10. [DOI] [PubMed] [Google Scholar]
- 28. Barbin L, Rousseau C, Jousset N, et al. Comparative efficacy of fingolimod vs natalizumab: a French multicenter observational study. Neurology 2016; 86: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carruthers RL, Rotstein DL, Healy BC, et al. An observational comparison of natalizumab vs. fingolimod using JCV serology to determine therapy. Mult Scler 2014; 20: 1381–1390. [DOI] [PubMed] [Google Scholar]
- 30. Kalincik T, Horakova D, Spelman T, et al. Switch to natalizumab versus fingolimod in active relapsing-remitting multiple sclerosis. Ann Neurol 2015; 77: 425–435. [DOI] [PubMed] [Google Scholar]
- 31. Braune S, Lang M, Bergmann A; NTC Study Group. Second line use of Fingolimod is as effective as Natalizumab in a German out-patient RRMS-cohort. J Neurol 2013; 260: 2981–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bermel RA, You X, Foulds P, et al. Predictors of long-term outcome in multiple sclerosis patients treated with interferon beta. Ann Neurol 2013; 73: 95–103. [DOI] [PubMed] [Google Scholar]
- 33. Prosperini L, Gallo V, Petsas N, et al. One-year MRI scan predicts clinical response to interferon beta in multiple sclerosis. Eur J Neurol 2009; 16: 1202–1209. [DOI] [PubMed] [Google Scholar]
- 34. NHS. Clinical commissioning policy: disease-modifying therapies for patients with Multiple Sclerosis (MS), https://www.england.nhs.uk/wp-content/uploads/2013/10/d04-p-b.pdf2014 (2014, accessed April 2018).
- 35. Qualitätshandbuch MS / NMOSD [Internet]. Kompetenznetz Multiple Sklerose, http://www.kompetenznetz-multiplesklerose.de/wp-content/uploads/2017/09/KKNMS_Qualit%C3%A4tshandbuch_2017_webfrei.pdf (2017, accessed 16 March 2018).
- 36. Professional Information Tysabri (Natalizumab) [Internet], https://compendium.ch/mpro/mnr/15730/html/en#7100 (2017, accessed 16 March 2018).
- 37. Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol 2016; 79: 950–958. [DOI] [PubMed] [Google Scholar]