Abstract
Background:
Copper is an essential element that is used widely in agriculture as fungicides and insecticides; for example, it is used to control schistosomiasis and as an antiseptic and germicide. Copper sulfate (CuSO4) induces multiorgan dysfunction through the stimulation of reactive oxygen species and oxidative stress. Despite the numerous pharmacological effects of curcumin (CUR), its pharmacokinetic properties are less promising. Hence, there is an urgent need for novel, effective strategies to attenuate heavy metal toxicity and consequently improve the treatment efficiency. Liposomal curcumin (L-CUR) improves the dissolution, stability, and bioavailability of treatment agents. This study compared the efficacy of CUR and L-CUR with that of desferrioxamine (DES), which is a heavy metal chelator against CuSO4 hepatotoxicity.
Methods:
All treatments with the aforementioned antioxidants were administered for 7 days along with CuSO4. Serum levels of alanine aminotransferase, aspartate transaminase, lactate dehydrogenase, and C-reactive protein, hepatic nitric oxide (NO), and lipid peroxides (malondialdehyde) were measured; protein expression of cyclooxygenase 2 and DNA fragmentation were evaluated. Histopathological examinations were also conducted.
Results:
A toxic dose of CuSO4 induced elevations in the previously measured parameters; these increases were reduced by the tested antioxidants, whereas glutathione (GSH) and superoxide dismutase (SOD) levels were decreased. Treatment with the antioxidants in question modulated these levels. Liposomal CUR has more hepatoprotective efficiency than CUR, and its efficacy was similar to that of DES. The histopathological examinations confirmed these results.
Conclusions:
Liposomal CUR may be useful for the prevention of CuSO4-induced liver injury. Cyclooxygenase 2 protein expression and DNA fragmentation were involved in CuSO4 toxicity and treatment.
Keywords: liposomal curcumin, COX-2, DNA, desferrioxamine
Introduction
Copper sulfate (CuSO4) is used widely in agriculture as fungicides and insecticide.1 In medicine, it is used to control schistosomiasis infections in tropical countries and as an antiseptic and germicide.2 Copper enters systemic circulation through different routes, including the gastrointestinal tract, lungs, and skin,3 and is transported to various organs in the body, leading to multiorgan dysfunction through the stimulation of reactive oxygen species (ROS) formation and, subsequently, the induction of oxidative stress.3 The medical signs of CuSO4 poisoning include erosive gastropathy involved in gastrointestinal manifestations; intravascular hemolysis and methemoglobinemia; and acute liver failure, as the liver is one of the first organs that can be affected by copper deposition, acute kidney injury, arrhythmia, and seizures.4
Desferrioxamine (DES) has the formula N-{5-[3-[[(5-aminopentyl) hydroxycarbamoyl]-propionamido] pentyl}-3-[5-(N-hydroxyacetamido)-pentyl] carbamoyl]-propionhydroxamic acid. It is a chelating agent with a specific ability to form a complex with iron for the treatment and prevention of iron overload.5 It is a naturally occurring compound produced by Streptomyces pilosus;6 despite poor oral absorption characteristics, it can be administered through both intravenous and intramuscular routes.5 Desferrioxamine forms complexes with iron to form a stable chelate ferrioxamine, which prevents the participation of iron in further chemical reactions.7 Desferrioxamine is used to reduce tissue iron stores, prevent iron-induced organ damage, and facilitate the removal of metals in the treatment of metal overload.8
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a naturally occurring compound found in Curcuma longa.9 Curcumin has numerous biological and pharmacological effects, such as antioxidant, anti-inflammatory, and anti-carcinogenic activity.10 Owing to its potent antioxidant activity, it can increase hepatic glutathione, leading to a decrease in the level of lipid hydroperoxides, and also has the ability to decrease the serum level of ROS.11 However, as a result of its low aqueous solubility, the bioavailability of CUR is poor.12
In order to overcome the poor bioavailability of CUR, nanoparticle-based drug delivery systems were developed.12 Scientists have synthesized nanoparticle-encapsulated CUR, which increased solubility with no effects on its activity.12 Nanoparticles can also offer several other advantages, such as prolonged half-life of injected drugs, drug delivery targeted to specific tissues, and increased metabolic stability. Furthermore, it can increase membrane permeability through its specific physiochemical properties, such as size, surface, charge, and hydrophobicity. These advantages have enabled improved clinical outcomes and reduced the side effects of CUR treatment.13 The aims of this study were to compare the hepatoprotective effect of L-CUR against CuSO4 toxicity with that of native CUR or DES and to discover the new mechanisms underlying their actions.
Materials and Methods
Chemicals
Copper sulfate and CUR were obtained as powders from Sigma Chemical Co. Liposomal CUR was obtained from Lipolife (Saint Louis Co, United Kingdom). Desferrioxamine was purchased from Novartis Pharma AG, Basel, Switzerland.
Experimental Design
In this study, the use of animals was under strict compliance with the local ethics committee in King Saud University. Forty adult male albino rats, weighing 150 to 200 g, were obtained from the Experimental Animal Center of King Saud University. The animals were allowed a 1-week acclimatization period in the laboratory under standard environmental conditions of 25°C and a natural light/dark cycle. A standard rat pellet diet and ad libitum access to distilled water were provided.
Rats were divided into 5 groups of 8t rats each and fasted overnight before treatment. Group I, the negative control rats, received carboxy methyl cellulose (CMC). In group II, rats were orally administered 100 mg/kg/d CuSO4.1 In group III, the positive control rats, were intraperitoneally administered 23 mg/kg/d DES.14 In group IV, rats were orally administered 80 mg/kg/d CUR.3 Group V rats were orally administered 80 mg/kg/d L-CUR. All treatments with the agents used in the study were administered for 7 days along with CuSO4 treatment. On the eighth day, all rats were killed. The blood samples were collected for serum separation by centrifugation at 3000 × g for 10 minutes. Samples of the liver were collected, weighed, and then homogenized in normal saline solution to yield 20% homogenates. The homogenates were centrifuged at 3000 rpm at 4°C for 30 minutes, and the supernatants were collected for further analysis. Sections of the liver were stored under nitrogen for Western blot analysis; other parts of the liver were stored in 4% formalin for histopathological study.
Biochemical Studies
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) levels were measured in accordance with the manufacturer’s recommendations (Randox Laboratories Ltd., United Kingdom). The determination of malondialdehyde (MDA), reduced glutathione (GSH), and superoxide dismutase (SOD) activity was performed by using colorimetric assay kits in accordance with the manufacturer’s instructions (Biodiagnostic, Egypt). Nitric oxide was assayed using a colorimetric assay kit in accordance with the manufacturer’s instructions (Cayman Chemical Co. USA). C-reactive protein (CRP) was determined by enzyme-linked immunosorbent assay by using a rat CRP immunoassay kit in accordance with the manufacturer’s recommendations (R&D Systems).
Western Blot Analysis for Cyclooxygenase 2
Western blot was performed to determine the protein expression of cyclooxygenase 2 (COX-2). Lung sections were homogenated in lysis buffer (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) HEPES, 2 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol (DTT), 0.1% sodium dodecyl sulphate (SDS), 1 mM phenyl methane sulphonyl floride (PMSF), pH 7.4) on ice. The supernatants were harvested by centrifugation at 12 000 × g at 4°C for 10 minutes. Protein concentrations were determined by a Bradford assay.15
Protein (20 mg) was separated on a 12% SDS-PAGE gel and transferred to polyvinylidiene floride (PVDF) membranes. Protein bands were visualized using the ECL-Plus detection system (Amersham Life Sciences, Buckinghamshire, United Kingdom) in accordance with the manufacturer’s instructions.
DNA Fragmentation
In brief, liver tissues were homogenized, washed overnight at 37°C in phosphate-buffered saline, and lysed in 0.5 mL of DNA extraction buffer (50 mM Tris-HCl, 10 mM EDTA, 0.5% triton, and 100 μg/mL proteinase K, pH 8.0). The lysate was then incubated with 100 μg/mL DNase-free RNase for 2 hours at 37°C followed by 3 extractions of an equal volume of phenol/chloroform (1:1 vol/vol) and a subsequent re-extraction with chloroform by centrifuging at 21 913 × g for 5 minutes at 4°C. The extracted DNA was precipitated in 2 volumes of ice-cold 100% ethanol with 1/10 volume of 3 M sodium acetate, pH 5.2 at −20°C for 1 hour, followed by centrifuging at 21 913 × g for 15 minutes at 4°C. After washing with 70% ethanol, the DNA pellet was air-dried and dissolved in 10 mM Tris-HCl/1 mM EDTA, pH 8.0. The DNA was then electrophoresed on 1.5% agarose gel and stained with ethidium bromide in Tris/acetate/EDTA buffer (pH 8.5, 2 mM EDTA, and 40 mM Tris-acetate). A 100-bp DNA ladder (Invitrogen) was included as a molecular size marker and DNA fragments were visualized and photographed by exposing the gels to ultraviolet transillumination using a Covaris S220 (Covaris, Woburn, Massachusetts, United Kingdom).8,16
Histopathological Examination
Sections of the liver were cut, stained with hematoxylin and eosin, and used for histopathological examination.
Statistical Analysis
All values were expressed as the mean ± SEM. The results were analyzed by 1-way analysis of variance (ANOVA), followed by the Tukey’s test for multiple comparisons. Analyses were computed using SPSS for Windows (version 11); P < .05 was considered to represent a significant difference.
Results
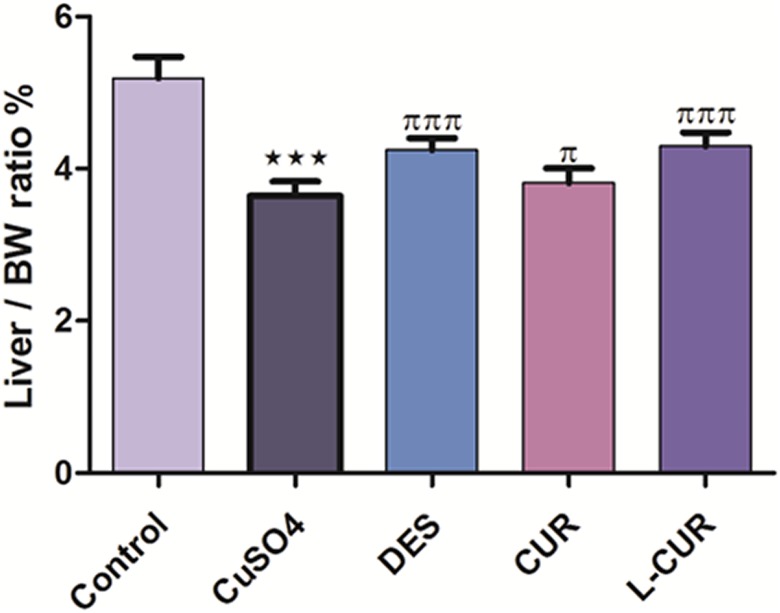
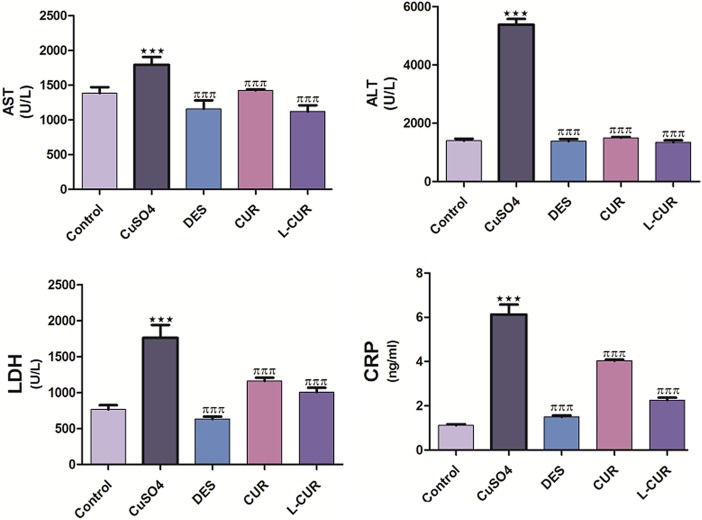
Copper sulfate caused a reduction in the liver weight to body weight ratio, whereas treatment with the antioxidants resulted in a significant increase in this ratio compared to CuSO4 treatment (Figure 1). Treatment with CuSO4 caused an elevation in serum AST, ALT, LDH, and CRP levels compared to the negative control group, while treatment with DES, CUR, and L-CUR decreased their levels relative to the CuSO4-treated group. Liposomal CUR treatment resulted in greater downregulation of these levels compared to the CUR treated group (Figure 2).
Figure 1.
The liver weight to body weight ratio in the control and treatment groups. The data are presented as the mean ± standard error mean (SEM) (n = 8). ***P ≤ .001 versus control, and πππ P ≤ .001 versus CuSO4-treated group.
Figure 2.
Serum AST, ALT, LDH, and CRP levels in the control and treatment groups. The data are presented as the mean ± SEM (n = 8). ***P ≤ .001 versus control, and πππ P ≤ .001 versus CuSO4-treated group. ALT indicates alanine aminotransferase; AST aspartate aminotransferase; CRP, C-reactive protein; LDH, lactate dehydrogenase.
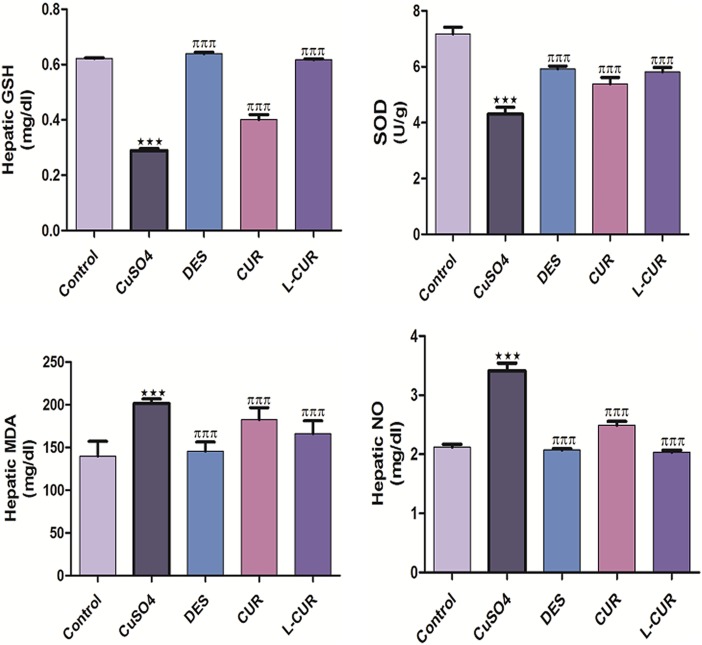
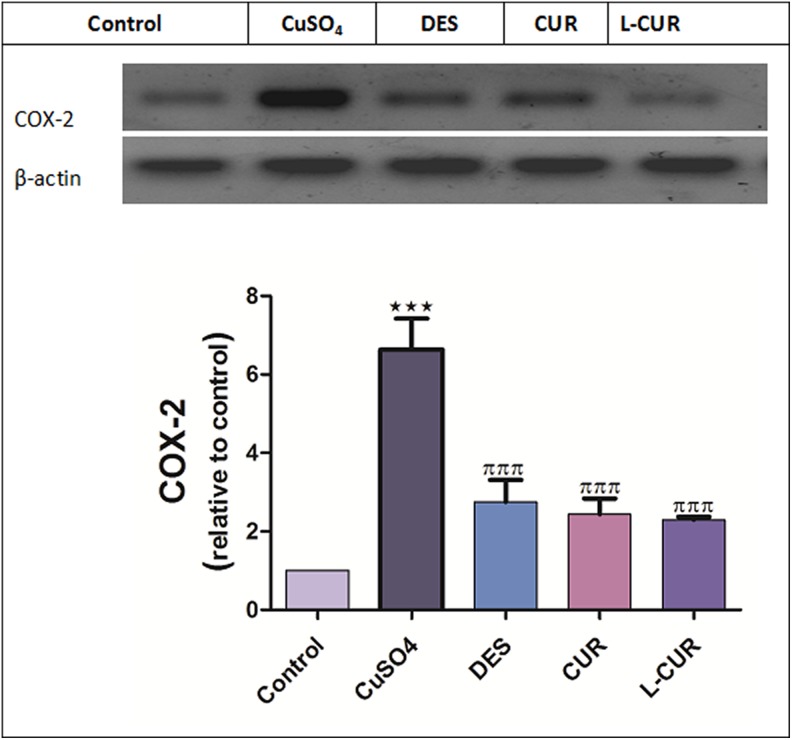
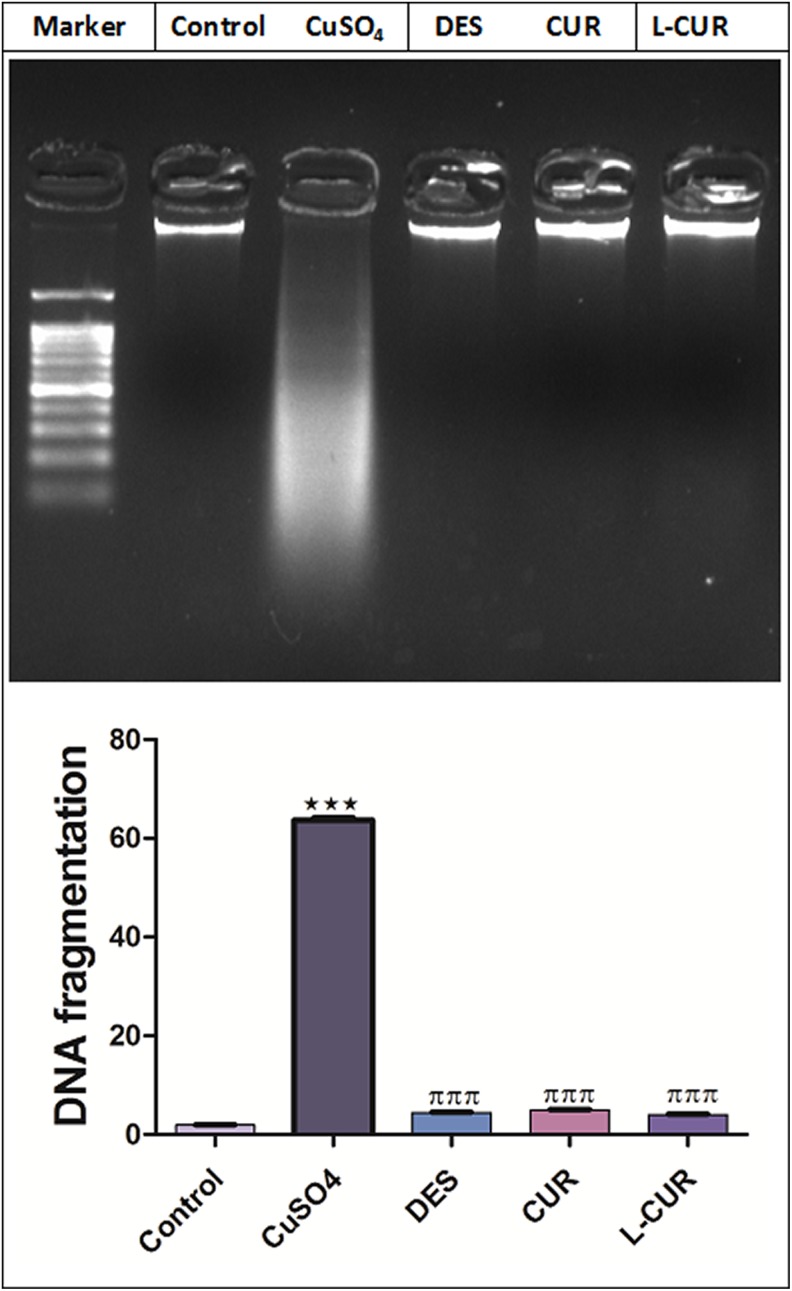
The levels of hepatic MDA, NO, and protein expression of COX-2 were increased, while GSH and SOD levels were decreased by CuSO4 treatment. The administration of the antioxidants in question markedly ameliorated all the previously measured parameters (Figures 3 and 4). Liposomal CUR administration resulted in the most pronounced improvements in GSH, MDA, COX-2 levels, and NO compared to CUR-administered group; therefore, L-CUR was more hepatoprotective than native CUR. A significant increase in DNA fragmentation was caused by CuSO4 treatment (Figure 5) while treatment with antioxidants markedly prohibited this fragmentation.
Figure 3.
Hepatic levels of GSH, SOD, MDA, and NO in the control and treatment groups. GSH glutathione; SOD superoxide dismutase; NO, nitric oxide; MDA, malondialdehyde. The data are presented as the mean ± SEM (n = 8). ***P ≤ .001 versus control, and πππ P ≤ .001 versus CuSO4-treated group. MDA, malondialdehyde.
Figure 4.
Cyclooxygenase 2 protein expression in the control and treatment groups. The data are presented as the mean ± SEM (n = 8). ***P ≤ .001 versus control, and πππ P ≤ .001 versus CuSO4-treated group.
Figure 5.
DNA fragmentation in the control and treatment groups. The data are presented as the mean ± SEM (n = 8). ***P ≤ .001 versus control, and πππ P ≤ .001 versus CuSO4-treated group.
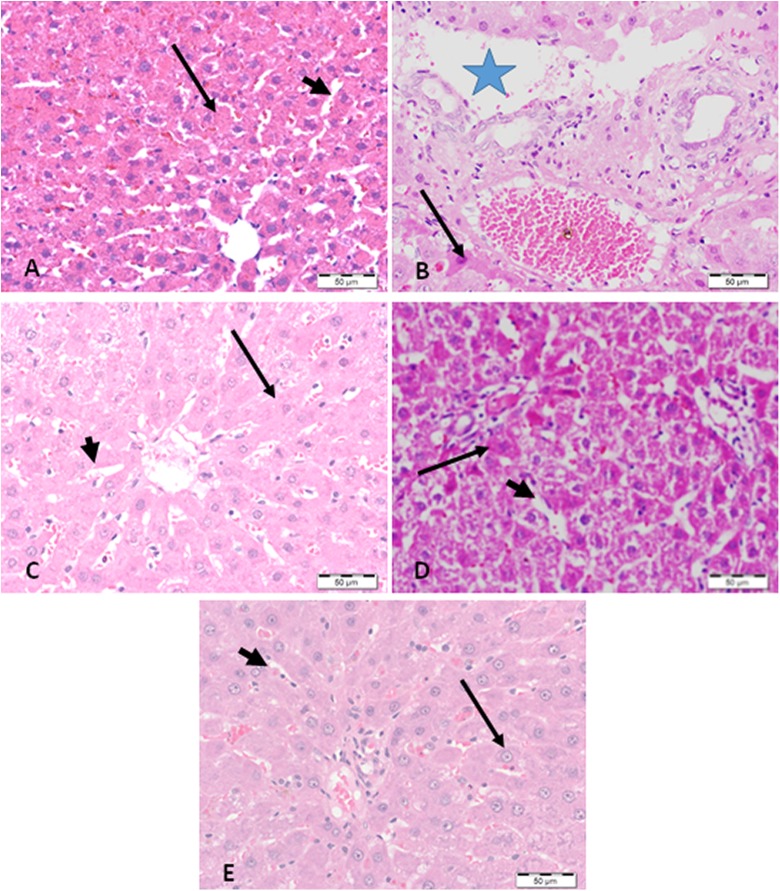
The histopathological study demonstrated massive cellular degeneration and necrosis of hepatocytes induced by the treatment with CuSO4. Treatment with DES, CUR, and L-CUR resulted in cellular improvement and healing of the necrosis induced by CuSO4 (Figure 6). Liposomal CUR-treated group demonstrated the most obvious improvement in cellular damage compared to CUR-treated group and displayed the strongest hepatoprotective effect.
Figure 6.
Light photomicrographs of liver sections stained with hematoxylin and eosin. A, The liver section from a negative control-treated rat show normal hepatocytes (arrow) with normal blood sinusoids (arrowhead). B, The liver section from a rat administered CuSO4 shows foci of massive cellular degeneration and necrosis (star) near the portal area surrounded by degenerated hepatocytes (arrow). C, The liver section from a rat administered desferrioxamine with CuSO4 shows a marked decrease in the degenerative changes, with appearance of normal hepatocytes (arrow) and sinusoids (arrow head). D, The liver section from a rat administered CUR with CuSO4 shows moderate healing of the necrosis (arrow) and a slight decrease of the dilated sinusoids (arrow head). E, The liver section from a rat administered Liposomal CUR with CuSO4 shows a marked improvement in hepatocyte degeneration (arrow), with normal sinusoids (arrow head).
Discussion
Copper (Cu) is an essential element, dispersed extensively throughout different animal tissues. It participates in the formation of a number of metalloenzymes, including catalase, cytochrome oxidase, and peroxidase.17 An increase of Cu concentration in drinking water is a prospective ecological risk. Copper is a catalyst for oxidative stress via the creation of ROS and peroxidative damage of membrane lipids.18 Copper ingested from the gastrointestinal tract enters into systemic circulation and binds to plasma amino acids and albumin, from where it is transported to the liver and involved into ceruloplasmin formation. The main target organs of CuSO4 are liver and kidney leading to hepatotoxicity and renal failure.19,20 Therefore, Cu toxicity is of particular concern.
Catalase and GSH catalyze the exchange of hydrogen peroxide to water. SOD helps to ameliorate the damage caused by O and defends different organs from lipoperoxidation and the damage induced by ROS. A decrease in SOD and GSH activities or an increase in O may cause an imbalance of the oxidant/antioxidant system.3 Previous results indicated that Cu triggers the transcriptional induction of the inducible form of NO synthase (NOS-II). Cyclooxygenase 2 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-dependent genes involved in the inflammatory response were transcriptionally induced by copper. These results suggest that a physiopathological state, characterized by hypercupremic situations, may lead to the onset of inflammation through the production of ROS and activation of NF-κB.21
In the present work, the ingestion of a toxic dose of CuSO4 induced the elevation of serum biomarkers, including AST, LDH, the inflammatory marker CRP, hepatic NO, lipid peroxide, protein expression of COX-2, and DNA fragmentation. In contrast, GSH and SOD levels were suppressed.
Curcumin, a bioactive phenolic compound has numerous biological functions, including anti-inflammatory, antimutagenic, and antioxidant activities.22 It is used to treat many disorders, such as a cough, anorexia, sinusitis, and hepatic and renal diseases.23 It is reported to be involved in several aspects of health and disease conditions. Despite the beneficial therapeutic effects of CUR, its pharmacokinetic properties are less promising owing to poor bioavailability, low aqueous solubility, and rapid metabolism. An encouraging strategy is the development of nanocarriers (liposomes) that can transfer drugs and discharge them at a precise specific target, improve dissolution, protect the chief bioactive compound from degradation, enhance the physical stability of the compound, and improve its oral bioavailability.12
Although the use of CUR as a protective and therapeutic agent against Cu toxicity has been investigated, the pharmacological mechanisms responsible for the expression of different types of inflammatory proteins that cause liver tissue damage during CuSO4 toxicity, as well as the hepatoprotective power of L-CUR, remain unclear.
The current study demonstrated that CUR and L-CUR had hepatoprotective potential against CuSO4 toxicity in rats, as documented by the decline in serum AST, AST, LDH, CRP, hepatic NO, and lipid peroxides. Likewise, decreased protein expression of COX-2 and DNA fragmentation was observed; in contrast, GSH and SOD levels were increased. Liposomal CUR was more hepatoprotective than CUR. These results were supported by histopathological analyses, which revealed that CUR and L-CUR improved liver architecture and necrosis induced by a toxic dose of CuSO4.
Conclusion
Liposomal CUR has a more hepatoprotective effect than CUR, and its efficacy was similar to that of DES. The present study has established that CUR is a useful tool for the prevention of liver injury induced by CuSO4. Cyclooxygenase 2 protein expression and DNA fragmentation were shown to be involved in both CuSO4 toxicity and treatment.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research project was supported by a grant from the Research Center of the Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University, Saudi Arabia.
References
- 1. Rufus OA, Olaoluwa SO, Chris EI, et al. A study of two weeks administration of copper sulphate on markers of renal function and feeding pattern of Wistar rats. African J Biochem Res. 2014;8(9):158–165. [Google Scholar]
- 2. King CH, Bertsch D. Historical perspective: snail control to prevent schistosomiasis. Plos Negl Trop Dis. 2015;9(4):e0003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hashish EA, Elgaml SA. Hepatoprotective and nephroprotective effect of curcumin against copper toxicity in rats. Indian J Clin Biochem. 2015;31(3):270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gamakaranage CS, Rodrigo C, Weerasinghe S, Gnanathasan A, Puvanaraj V, Fernando H. Complications and management of acute copper sulphate poisoning; a case discussion. J Occup Med Toxicol. 2011;6(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kontoghiorghe CN, Kontoghiorghes GJ. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des Devel Ther. 2016;10:465–481. doi:10.2147/DDDT.S79458. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aronson J, Meyler L. Meyler’s Side Effects of Drugs. 16th ed USA: Elsevier, 2016:846–860. [Google Scholar]
- 7. Flora SJ, Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010;7(7):2745–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gumienna-Kontecka E, Pyrkosz-Bulska M, Szebesczyk A, Ostrowska M. Iron chelating strategies in systemic metal overload, neurodegeneration and cancer. Curr Med Chem. 2014;21(33):3741–3767. [DOI] [PubMed] [Google Scholar]
- 9. Hewlings SJ, Kalman DS. Curcumin: a review of its’ effects on human health. Foods. 2017;6(10):E92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nalli M, Ortar G, SchianoMoriello A, Di Marzo V, De Petrocellis L. Effects of curcumin and curcumin analogues on TRP channels. Fitoterapia. 2017;122:126–131. [DOI] [PubMed] [Google Scholar]
- 11. Arablou T, Kolahdouz-Mohammadi R. Curcumin and endometriosis: review on potential roles and molecular mechanisms. Biomed Pharmacother. 2018;97:91–97. [DOI] [PubMed] [Google Scholar]
- 12. Bisht S, Feldmann G, Soni S, et al. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Onoue S, Yamada S, Chan HK. Nanodrugs: pharmacokinetics and safety. Int J Nanomedicine. 2014;9:1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deferoxamine mesylate (Desferal mesylate). A specific iron-chelating agent for treating acute iron intoxication. Clin Pharmacol Ther. 1969;10(4):595–596. [DOI] [PubMed] [Google Scholar]
- 15. Jackson LM, Wu KC, Mahida YR, Jenkins D, Hawkey CJ. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut. 2000;47(6):762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sedlackova T, Repiska G, Celec P, Szemes T, Minarik G. Fragmentation of DNA affects the accuracy of the DNA quantitation by the commonly used methods. Biol Proced Online. 2013;5(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101(3):294–301. [DOI] [PubMed] [Google Scholar]
- 18. Abuja PM, Albertini R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin Chim Acta. 2001;306(1-2):1–17. [DOI] [PubMed] [Google Scholar]
- 19. Galhardi CM, Diniz YS, Faine LA, et al. Toxicity of copper intake: lipid profile, oxidative stress and susceptibility to renal dysfunction. Food Chem Toxicol. 2004;42(12):2053–2060. [DOI] [PubMed] [Google Scholar]
- 20. Hassan S, Shaikh MU, Ali N, Riaz M. Copper sulphate toxicity in a young male complicated by methemoglobinemia, rhabdomyolysis and renal failure. J Coll Physicians Surg Pak. 2010;20(7):490–491. [PubMed] [Google Scholar]
- 21. Persichini T, Percario Z, Mazzon E, Colasanti M, Cuzzocrea S, Musci G. Copper activates the NF-kappaB pathway in vivo. Antioxid Redox Signal. 2006;8(9-10):1897–1904. [DOI] [PubMed] [Google Scholar]
- 22. Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 23. Eigner D, Scholz D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J Ethnopharmacol. 1999;67(1):1–6. [DOI] [PubMed] [Google Scholar]