Short abstract
Background
Optic neuritis (ON) is one of the common manifestations both in neuromyelitis-optica spectrum disorders (NMOSD) and in multiple sclerosis (MS).
Objectives
The objective of this paper is to compare clinical presentations, laboratories and imaging findings in ON associated with MS and NMOSD.
Methods
A retrospective chart review was performed in patients presenting with ON in 59 NMOSD patients with 72 eyes’ involvement and 163 ON attacks, and 20 MS patients with 23 eyes’ involvement and 36 ON attacks.
Results
ON-NMOSD patients had recurrent ON more often and tended to have simultaneous bilateral ON involvement at their first ON attack. Individuals with ON-NMOSD revealed worse visual acuity at first ON attacks and also had poorer long-term visual outcome than those with ON-MS, with nearly half of ON-NMOSD patients still having LogMAR visual acuity ≥1 at their last follow-up (p = 0.035). Significant thinner average retinal nerve fiber layer thickness was found in the ON-NMOSD group. We found no significant differences in segmentation location of the optic nerve lesions and the length of involvement between the two groups.
Conclusions
It was difficult to completely differentiate ON-NMOSD from ON-MS. ON-NMOSD patients, however, tended to have simultaneous bilateral ON involvement and poorer long-term visual outcome than individuals with ON-MS.
Keywords: Optic neuritis, neuromyelitis optica, multiple sclerosis, Thai
Introduction
Idiopathic demyelinating optic neuritis (ON) is a demyelinating inflammation of the optic nerve, which accounts for a considerable proportion of unilateral or bilateral, acute-to-subacute visual loss cases.1,2 ON with unilateral involvement is most commonly associated with multiple sclerosis (MS). Atypical ON may, however, be associated with neuromyelitis optica spectrum disorders (NMOSD), acute disseminated encephalomyopathy (ADEM) and other conditions such as systemic autoimmune diseases. Differentiating the etiologies between NMOSD-related ON (ON-NMOSD) and MS-related ON (ON-MS) can be difficult, especially at the first presentation of isolated ON or brainstem encephalitis, for which positive tests for anti-AQP4-antibody (anti-AQP4-Ab)are less frequently found or when no specific manifestations of a particular disease or disease-specific antibodies such as AQP4-Ab (anti-AQP4-Ab)/NMO-immunoglobulin G (IgG) are found.
Different characteristic features of ON-NMOSD and ON-MS by ophthalmologic studies including visual acuity (VA), visual field (VF), imaging and electrophysiologic studies such as visual evoked potentials (VEP) as well as optical coherence tomography (OCT) have been reported in the literature, and these may allow for early discrimination between the two diseases.2–4 Early discrimination between ON-NMOSD and ON-MS is crucial to provide appropriate and timely management because misdiagnosis of ON-NMOSD as ON-MS may lead to inappropriate administration of interferon-beta, natalizumab and fingolimod, which may exacerbate NMOSD relapses.5,6 We, thus, aimed to evaluate the clinical and radiographic findings, including ophthalmologic examinations, of Thai patients presenting with ON related to NMOSD and MS.
Methods
We retrospectively reviewed the medical records of patients attending the MS and Related Disorders Clinic at Siriraj Hospital between January 2009 and December 2015. We included those who (1) were 18 years or older, (2) had at least one attack of ON, (3) had at least one anti-AQP4-Ab test and (4) had an ophthalmologic follow-up after the last ON attack at at least six months. The cell-based assay (CBA) for anti-AQP4-Ab detection in living cells used human embryonic kidney (HEK)-293 cells transfected with the M23 isoform of AQP4, and CBA for myelin oligodendrocyte glycoprotein (MOG) antibodies (anti-MOG-Ab) used live HEK-293 cells transfected with a plasmid containing full-length human MOG complementary DNA as previously described.7 We excluded those who had other causes of visual impairment that were not demyelinating diseases. Diagnosis of either MS or NMOSD was according to the revised McDonald 2010 criteria8 and the NMOSD criteria 2015,9 respectively. Demographic data including sex and age at the onset of the disease along with clinical data including concomitant diseases, disease duration, treatments, blood chemistries, ophthalmologic examinations and magnetic resonance imaging (MRI) findings were collected.
Ophthalmologic examinations
Routine ophthalmologic examinations were recorded including VA measured by ETDRs chart, VF assessed with Humphrey central VF perimetry, VEP and fundoscopic examination. For those who had a VA worse than 6/60, ophthalmologic findings included counting fingers (CF), hand motion (HM), projection of light (PJ) as well as perception of light (PL) or no light perception (no PL). All VA findings were recorded as the logarithm of the minimum angle of resolution (logMAR).10 Findings from OCT performed on a high-definition spectral domain (HD-OCT) (Carl Zeiss Meditec, Dublin, CA, USA) were collected if available. The visual outcomes at one and six months as well as at one year after the onset of ON were collected if available.
MRI protocol and segmentation of the optic images
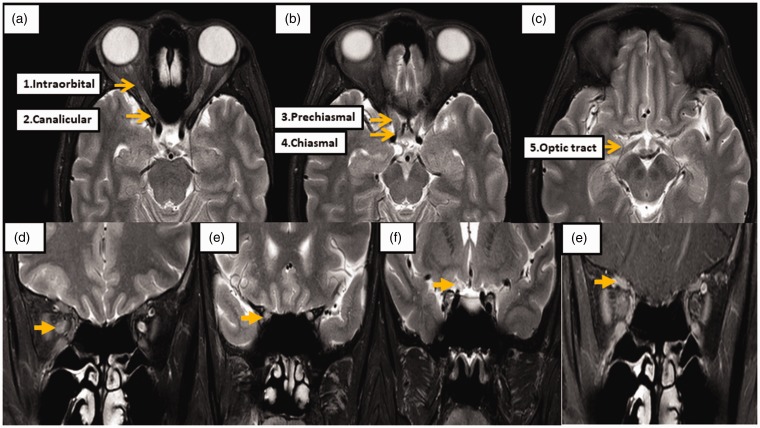
The orbital, brain, and spinal MRI were evaluated using the 3-Tesla MRI Philips Ingenia system (Philips Healthcare). The T2-weighted image with fat-suppression technique (spin-echo, flip angle = 90 degrees, repetition time (TR)/echo time (TE) = 3500/80 and slice thickness = 3 mm) was used to evaluate signal intensity and diameter of the optic nerve. The postcontrast T1-weighted image with fat-suppression sequence (spin-echo, flip angle = 90 degrees, TR/TE = 564/8 and slice thickness = 3 mm) was taken to evaluate optic nerve enhancement. The optic nerve on orbital MRI was divided into five segments: intraorbital (extending from the posterior wall of the optic bulb to the beginning of the optic canal), canalicular (the portion of the nerve that lies within the optic canal), prechiasma (beginning at the exit of the optic canal intracranially and extending to the chiasm), chiasma (across the chiasm) and optic tract (posterior to the chiasm) (Figure 1(a)).11 The optic nerve diameter was evaluated on a coronal view of T2-weighted images and measured at the mid-parts of the following segments: (1) the intraorbital, (2) the canalicular and (3) the prechiasmatic. Optic nerve enhancement was also noted (Figure 1(b)–(e)). The neuroradiologist who interpreted the MRI images was blinded to the clinical information.
Figure 1.
Orbital magnetic resonance imaging (MRI) protocol and segmentations.
The orbital MRI findings were evaluated using 3-Tesla MRI (Philips, Ingenia). (a)–(c) Optic nerves are divided into the following five segments: intraorbital, canalicular, prechiasmal, chiasmal, and optic tract. The T2-weighted image with fat-suppression technique was used for evaluating signal intensity and diameter of the optic nerve. The optic nerves were measured at (d) mid-part of the intraorbital segment, (e) canalicular segment and (f) prechiasmatic segment, and (g) optic nerve enhancement is demonstrated on a T1-weighted image postcontrast study with fat-suppression techniques at the intraorbital part of the right optic nerve.
Statistical analysis
Univariate data are presented as mean (SD) or median (interquartile range (IQR)) as appropriate. Numerical variables between NMOSD and MS patients were analyzed by the two-sample t test and Mann-Whitney U test as appropriate. Categorical variables were analyzed by the Chi-square test or Fisher exact test as appropriate. No adjustment for multiple comparisons was made because this was an exploratory study. All statistical analyses were performed using PASW Statistic SPSS version 18.0 (IBM, Chicago, IL, USA). P values of <0.05 were considered statistically significant.
Results
Demographic data
There were 59 NMOSD patients with involvement of 72 eyes in 163 ON attacks and 20 MS patients with involvement of 23 eyes in 36 ON attacks. Sixty-four percent of NMOSD patients (38 of 59) and 80% of MS patients (16 of 20) experienced ON relapse more than twice during the study period. The mean interval from the onset of ON to visual assessments, including OCT, was not significantly different between the NMOSD and MS groups (4.6 days (IQR 0.0- 17.3) vs 5.9 days (IQR 0.0- 15.6), respectively).
In our practice, we generally treat an acute attack of ON with intravenous methylprednisolone (IVMP) 1 gram per day for five consecutive days then taper off with oral prednisolone within two weeks. If the patient does not respond, plasma exchange on alternate days for five sessions is considered as described in our previous study.12 In the present study, IVMP was given more frequently in ON-NMOSD than ON-MS although this was not significantly different (Table 1). There were also no significant differences in the interval of the treatment of IVMP given and the treatment with plasma exchange or oral prednisolone between the two groups (Table 1).
Table 1.
Demographic data of patients with NMOSD and MS who ever had optic neuritis attacks.
| Results Variables |
Mean ± SD or median (P25, P75) |
p value | |
|---|---|---|---|
| NMOSD (n = 59) | MS (n = 20) | ||
| Number of patients (n) | 59 | 20 | |
| Number of eye involvement with ON (n) | 72 | 32 | |
| Number of ON attacks (n) | 163 | 36 | |
| Number of ON attacks/person (n) | 2 (1, 3) | 1.5 (1, 2.8) | 0.432 |
| Number of ON attacks/eye (n) | 2 (1, 2.5) | 1 (1, 2) | 0.492 |
| Age at onset of disease (years) | 36.5 ± 14.0 | 35.3 ± 13.4 | 0.729 |
| Age at onset of first ON attack (years) | 36.7 ± 14.0 | 34.4 ± 13.5 | 0.524 |
| Disease duration (years) | 8.3 ± 5.1 | 11.7 ± 7.5 | 0.024 |
| Follow up time after first ON attack (years) | 6.7 (3.9, 10.8) | 7.7 (4.8, 10.4) | 0.669 |
| Time from onset to first ON attack (months) | 0 (0, 0) | 5.3 (0, 36.7) | 0.001 |
| Sex: female (n, %) | 55 (93.2) | 19 (95) | 1 |
| Initial manifestation (n, %) | |||
| Optic neuritis | 45 (77.6) | 8 (40) | 0.004 |
| Transverse myelitis | 9 (15.5) | 3 (15) | 1.00 |
| Brainstem syndrome | 2 (3.4) | 8 (40) | <0.001 |
| Cerebellar ataxia | 1 (1.7) | 1 (5) | 0.45 |
| Others | 1 (1.7) | 0 | 1.00 |
| Time to nadir VA (days) | 7 (3, 14) | 7 (5, 14) | 0.284 |
| Simultaneous BON at first ON attack (n, %) | 13/59 (22.4) | 3/20 (15) | 0.749 |
| Simultaneous ever BON attack (n, %) | 31/163 (19.0) | 4/36 (11) | 0.259 |
| Treatment given at ON attacks (n, %) | |||
| IVMP | 49 (92.5) | 14 (77.8) | 0.189 |
| PLEX | 1 (1.9) | 0 | 1.00 |
| Others (oral prednisolone) | 3 (5.7) | 4 (22.2) | 0.064 |
| Time from ON attack to first treatment given (days) | 10 (4, 17) | 7.5 (7, 50) | 0.551 |
| Immunosuppressanta/DMDb given (n, %) | 43a (78.2) | 17b (85) | 0.746 |
| Duration of treatment (years) | 2.4(1.46, 2.91) | 2.62 (1.39, 6.5) | 0.594 |
| CSF findings: n/n tested | 47/59 | 18/59 | |
| Presence of CSF-OCB (n, %) | 6 (12.8) | 13 (72.2) | <0.001 |
| Pleocytosis (n, %) | 18 (38.3) | 7 (43.8) | 0.772 |
| Presence of AQP4-Ab/ (n/n tested, %) | 52/59 (88.1) | 0 | <0.001 |
| Presence of anti-MOG (n/n tested, %) | 1/17 (5.9%) | 0/5 | 1.00 |
| Presence of ANA (n/n-tested, %) | 21/52 (40.4) | 2/13 (15.4) | 0.115 |
IS: immunosuppressant for NMOSD. bDisease-modifying drug for MS.
ANA: antinuclear antibody; anti-MOG: anti-myelin oligodendrocyte; AQP4-Ab: aquaporin-4 antibody; BON: bilateral optic neuritis; CSF: cerebrospinal fluid; IVMP: intravenous methylprednisolone; MS: multiple sclerosis; NMOSD: neuromyelitis optica spectrum disorders; OCB: oligoclonal band; ON: optic neuritis; PLEX: plasma exchange; SD: standard deviation; VA: visual acuity.
There were no significant differences between the NMOSD and MS groups in the proportions of females and males, time to reach nadir VA from the onset of ON attack, age at onset of the disease, age at onset of first ON attack, presence of antinuclear antibody (ANA), proportion of treatment with IVMP and plasma exchange, mean follow-up period after ON attack and time from onset of ON attack to first treatment with IVMP given. The mean disease duration was, however, longer in the MS group than in the NMOSD group (11.7 years (SD 7.5) vs 8.3 years (SD 5.1); p = 0.024). ON was the most common initial manifestation in NMOSD patients and was significantly higher compared to MS patients (77.6% vs 40%, respectively; p = 0.004). Both ON-NMOSD and ON-MS patients presented more frequently with unilateral involvement, and ON-NMOSD patients tended to have a higher incidence of simultaneous bilateral ON (BON) involvement compared to ON-MS patients at their first ON attack and also during the course of the disease although neither was significant (22.4% vs 15%; p = 0.749 and 19% vs 11%; p = 0.259, respectively).
Of the NMOSD patients, 88% (52 of 59) were anti-AQP4-Ab seropositive. Five MS patients and 17 NMOSD patients including all of the seven anti-AQP4-Ab-seronegative NMOSD patients were tested for serum anti-MOG-Ab, and we found only one anti-AQP4-Ab-seronegative NMOSD patient positive for anti-MOG-Ab (Table 1).
Visual presentations and their recovery
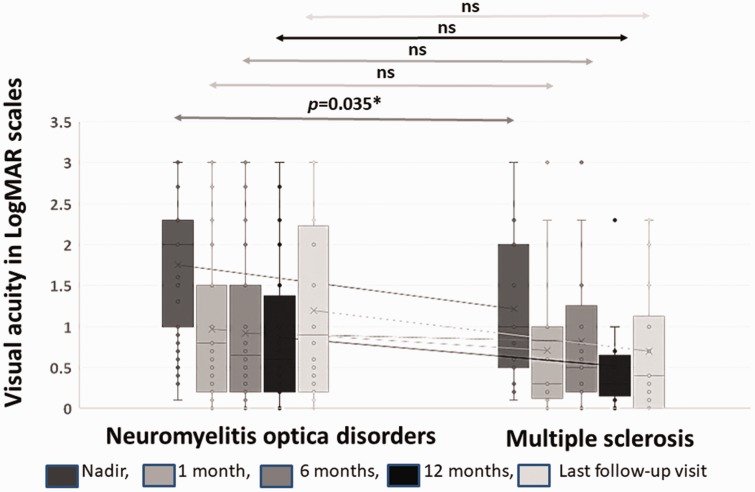
For the first documented ON attack, median VA was significantly worse in the NMOSD group compared to those in the MS group (logMAR 2 vs logMAR 1; p = 0.035) with nearly 80% of the NMOSD patients having a logMAR ≥1.
We found a decreasing prevalence of patients with a VA logMAR ≥1 over time from the time of nadir VA of ON attack to the time of follow-up for VA at one month, six months and 12 months both in the NMOSD and MS groups although no significant difference was seen between the two groups at six- and 12-month follow-up visits (Figure 2).
Figure 2.
Visual acuity (VA) in VA logMAR scales at different times after optic neuritis attack.
logMAR: logarithm of minimum angle of resolution.
At the last follow-up visit, VA with logMAR ≥ 1 was, however, found more frequently in the NMOSD group compared to those in the MS group (49.2% vs 20%, respectively; p = 0.035).
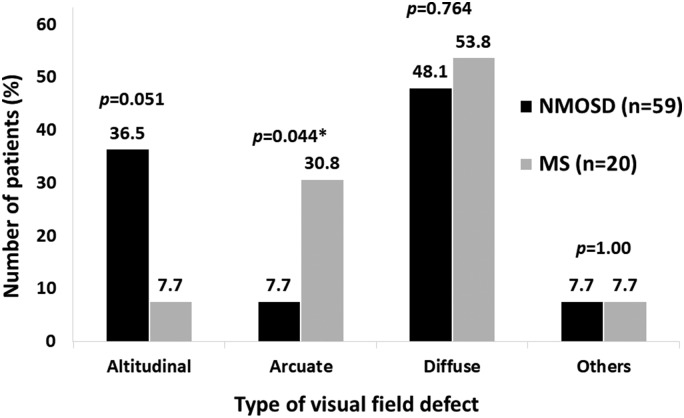
The most common VF defect in the first ON attack was diffuse type in each group. NMOSD patients tended to have an altitudinal type VF defect more often than those in MS group (36.5% vs 7.7%, respectively; p = 0.051) while arcuate type VF defect was significantly more frequently seen in the MS group than the NMOSD group (30.8% vs 7.7%, respectively; p = 0.044) (Figure 3). VEP did not show any difference in the latency or amplitude between the two groups. Compared with the other unaffected eye, we found damping amplitude >75% or completely absent VEP of the affected eyes significantly more often in the NMOSD group than in the MS group (30% vs 11.1%, respectively). The absence of amplitude also affected the interpretation of the latency measure of the VEP.
Figure 3.
Distribution of type of visual field defect in neuromyelitis optica spectrum disorders (NMOSD) and multiple sclerosis (MS).
Half of the patients in each group showed normal disc appearance while the other half revealed disc swelling. At the 12-month follow-up visit, optic disc atrophy was detected more often in the NMOSD group than the MS group although not significantly (83.3% vs 63.6%, respectively; p = 0.213).
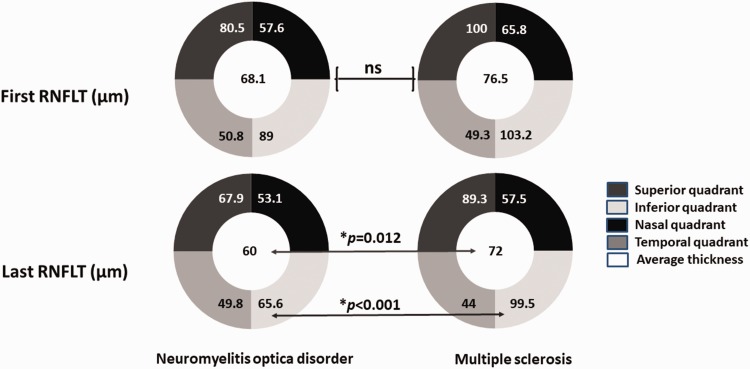
Regarding the available OCT data in the acute phase, the NMOSD group had a trend toward having thinner retinal nerve fiber layer thickness (RNFLT) compared to the MS group, especially at the nasal side (mean thickness 57.6 µm (SD 14.2) vs 65.8 µm (SD 13.2), respectively; p = 0.084). Comparing the NMOSD group to the MS group at the last follow-up visit (median duration of time since first ON attack to last follow-up visit 5.6 years (IQR 1.1- 8.7) vs 6.4 years (IQR 1.5- 7.7), respectively), the last RNFLT measurement, however, showed a significantly lower average of RNFLT (60 µm vs 72 µm, p = 0.012) and a significantly higher average of the inferior quadrant (65.5 µm vs 44 µm, p < 0.001). The thickness of the ganglion cell layer and internal plexiform layer did not show any significant differences between the two groups (Table 2 and Figure 4).
Table 2.
Comparison of visual presentations between NMOSD and MS patients who ever had optic neuritis attacks.
| Results Variables |
Mean ± SD or median (p25, p75) |
p value | |
|---|---|---|---|
| NMOSD (n = 59) | MS (n = 20) | ||
| Nadir LogMAR | 2 (1, 2.3) | 1 (0.5, 2) | 0.035 |
| <0.5 | 5 (6.9) | 3 (17.6) | 0.180 |
| 0.5 – <1 | 10 (13.9) | 3 (17.6) | 0.710 |
| ≥1 | 57 (79.2) | 11 (64.7) | 0.223 |
| VA LogMAR at 1 month | 0.8 (0.2,1.5) | 0.45 (0.2,1) | 0.414 |
| <0.5 | 20 (36.4) | 6 (46.2) | 0.215 |
| 0.5 – <1 | 9 (16.4) | 1 (7.7) | 0.673 |
| ≥1 | 26 (47.3) | 6 (46.2) | 0.529 |
| VA LogMAR at 6 months | 1 ± 0.9 | 0.8 ± 0.9 | 0.442 |
| <0.5 | 22 (39.3) | 7 (50) | 0.467 |
| 0.5 – <1 | 17 (30.4) | 4 (28.6) | 1.000 |
| ≥1 | 17 (30.4) | 3 (21.4) | 0.742 |
| VA LogMAR at 12 months | 0.6 (0.2, 1.4) | 0.3 (0.2, 0.7) | 0.214 |
| <0.5 | 20 (45.5) | 7 (53.8) | 0.594 |
| 0.5 – <1 | 13 (29.5) | 5 (38.5) | 0.735 |
| ≥1 | 11 (25) | 1 (7.7) | 0.261 |
| LOFU LogMAR | 0.8 (0.2, 2.2) | 0.45 (0.1, 1.1) | 0.294 |
| <0.5 | 28 (43.1) | 8 (53.3) | 0.212 |
| 0.5 – <1 | 5 (7.7) | 4 (26.7) | 0.171 |
| ≥1 | 32 (49.2) | 3 (20) | 0.035 |
| Improve ≥2 Snellen lines at 6 mo (n, %) | 33 (50) | 8 (44.4) | 0.792 |
| Improve ≥2 Snellen lines at 12 mo (n, %) | 21 (47.7) | 6 (46.2) | 0.920 |
| Fundoscopic findings (n, %) | |||
| Normal | 34 (50.7) | 9 (50.0) | 1.00 |
| Disc swelling | 33 (49.3) | 9 (50.0) | 1.00 |
| Presence of optic atrophy at 12 mo (n, %) | 30 (83.3) | 7 (63.6) | 0.213 |
| VF defect at first attack (n, %) | 52 | 13 | |
| Altitudinal | 19/52 (36.5) | 1/13 (7.7) | 0.051 |
| Arcuate | 4/52 (7.7) | 4/13 (30.8) | 0.044 |
| Diffuse | 25/52 (48.1) | 7/13 (53.8) | 0.764 |
| Others (Central/peripheral/quadrantanopia) | 4/52 (7.7) | 1/13 (7.7) | 1.00 |
| First RNFL (µm): n tested | 39 | 13 | |
| Average RNFLT | 68.1 ± 18.8 | 76.5 ± 29.0 | 0.235 |
| Superior quadrant | 80.5 (56.3, 99.4) | 100 (59.5, 126.5) | 0.134 |
| Nasal quadrant | 57.6 ± 14.2 | 65.8 ± 13.2 | 0.084 |
| Inferior quadrant | 89.0 ± 36.7 | 103.2 ± 41.7 | 0.268 |
| Temporal quadrant | 50.8 ± 13.2 | 49.3 ± 13.8 | 0.74 |
| Average GCL + IPL thickness (µm) | 56.7 ± 9.1 | 63.6 ± 13.8 | 0.106 |
| Time from first ON attack to first RNFLT tested (years) | 3.0 (0.5, 7.7) | 4.8 (2.5, 9.5) | 0.518 |
| Time from first ON attack to last RNFLT tested (years) | 5.6 (1.1, 8.7) | 6.4 (1.5, 7.7) | 1 |
| Number of ON attacks before last RNFLT (n) | 1 (0, 2) | 0 (0, 0.75) | 0.132 |
| Final RNFLT (µm): n tested | 20 | 4 | |
| Average RNFLT | 60 (54.3, 65.8) | 72 (68.2, 81.75) | 0.012 |
| Superior quadrant | 67.9 ± 14.9 | 89.3 ± 25.3 | 0.19 |
| Nasal quadrant | 53.1 ± 5.4 | 57.5 ± 8.6 | 0.186 |
| Inferior quadrant | 65.6 ± 22.1 | 99.5 ± 6.8 | <0.001 |
| Temporal quadrant | 49.8 ± 7.6 | 44 ± 9.8 | 0.199 |
| Average GCL + IPL thickness (µm) | 53 (50, 60) | 63.5 (59, –) | 0.21 |
| VEP: n tested | 47 | 12 | 0.21 |
| P100 latency (ms) | 132.0 ± 21.8 | 122.9 ± 20.1 | 0.198 |
| P100 amplitude (µV) | 5.4 (4.3, 6.9) | 4.6 (4.2, 6.3) | 0.524 |
µm: micrometer; µV: microvolt; GCL: ganglion cell layer; IPL: inner plexiform layer; LOFU: last of follow-up; mo: months; ms: millisecond; MS: multiple sclerosis; NMOSD: neuromyelitis optica spectrum disorders; ON: optic neuritis; RNFLT: retinal nerve fiber layer thickness; SD: standard deviation; VA: visual acuity; VEP: visual evoked potential; VF: visual field.
Figure 4.
Changes of retinal nerve fiber layer thickness (RNFLT) after the optic neuritis attack in neuromyelitis optica spectrum disorders and multiple sclerosis. ns: not significant.
Paraclinical findings with MRI imaging and cerebrospinal analysis
Brain MRI at the time of the first attack was performed in the 52 NMOSD and 18 MS patients. The most common MRI findings in the NMOSD group were nonspecific findings whereas brain MRI fulfilling the Swanton Criteria was the most common appearance in the MS group (70% vs 15.4%; p ≤ 0.001). For those who had first spinal MRI available to review, spinal involvement with longitudinal extensive transverse myelitis (LETM) was found significantly more frequently in the NMOSD group than the MS group (50% vs 13.3%, respectively; p = 0.016). The two MS patients who had a first spinal MRI available had four and three vertebral segment lengths of spinal lesions with peripheral cross-sectional involvement and typical Swanton brain MRI findings, respectively, and these two MS patients had follow-up times up to seven and eight years, respectively. We found 20.8% of NMOSD patients presented with short myelitis (Table 3).
Table 3.
Comparison of brain and spinal MRI findings between NMOSD and MS patients who ever had optic neuritis attacks.
| Results Variables |
Mean ± SD or median (p25, p75) |
p value | |
|---|---|---|---|
| NMOSD (n = 59) | MS (n = 20) | ||
| MRI brain: n/n tested | 52/59 | 18/20 | |
| Normal (n/n tested, %) | 10/52 (19.2) | 2/18 (11.1) | 0.718 |
| Nonspecific (n/n tested, %) | 20/52 (38.5) | 2/18 (11.1) | 0.040 |
| Swanton criteria (n/n tested, %) | 8/52 (15.4) | 14/18 (70) | <0.001 |
| Other NMOSD lesions (n/n tested, %) | 14/52 (27.0) | 0/18 | 0.015 |
| MRI spine: n/n tested | 48/59 | 15/20 | |
| Normal (n/n tested, %) | 14 (29.2) | 6 (40) | 0.528 |
| Short TM (n/n tested, %) | 10 (20.8) | 7 (46.7) | 0.092 |
| LETM (n/n tested, %) | 24 (50) | 2 (13.3) | 0.016 |
LETM: long extensive transverse myelitis; MRI: magnetic resonance imaging; MS: multiple sclerosis; NMOSD: neuromyelitis optica spectrum disorders; SD: standard deviation; TM: transverse myelitis.
Thirty-seven NMOSD patients and 10 MS patients had orbital MRI available to review, and no significant abnormality was seen in around 40% in each group. The median interval from the onset of ON to MRI assessment of the patients who initially presented with ON was 60 days (0, 214) for NMOSD and 84 days (63, 120) for MS patients (p = 0.311). ON-NMOSD could not be differentiated from ON-MS by orbital MRI by T2/fluid-attenuated inversion recovery/proton techniques, the location of the orbital segment involvement, optic nerve diameter in each segment or having contrast enhancement. Transegmental orbital involvement was commonly found both in ON-NMOSD and ON-MS (66.7% vs 71.4%, respectively; p = 1.0) with an average of 2.52 segments involved vs 2.50 segments involved, respectively (p = 0.968) (Table 4).
Table 4.
Comparison of orbital MRI findings between NMOSD and MS patients who ever had optic neuritis attacks.
| Results Variables |
Mean ± SD or median (p25, p75) |
p value | |
|---|---|---|---|
| NMOSD (n = 59) | MS (n = 20) | ||
| MRI orbit: n/n tested | 37/59 | 10/20 | |
| Normal MRI of the orbit (n/n tested, %) | 14/37 (41.2) | 4/10 (40) | 1 |
| Number of lesions on T2/FLAIR/proton (n/n tested, %) | |||
| Intraorbital | 18/23 (78.3) | 4/6 (66.7) | 0.612 |
| Canalicular | 14/3 (60.9) | 3/6 (50) | 0.669 |
| Prechiasmatic | 15/23 (65.2) | 5/6 (83.3) | 0.633 |
| Optic chiasm | 10/23 (43.5) | 3/6 (50) | 1 |
| Optic tract | 1/23 (4.3) | 0 (0) | 1 |
| Transegmental orbital involvement (n/n tested, %) | 18 (66.7) | 5 (71.4) | 1 |
| Lesion extension in segments/total | 2.52 ± 1.20/5 | 2.50 ± 1.04/5 | 0.968 |
| Optic nerve lesions with contrast enhancement (n/n tested, %) | 6/23 (26.1) | 0 (0) | 0.213 |
| Optic nerve diameter (mm) | |||
| Mid-intraorbital (vertical plane) | 1.98 ± 0.70 | 1.74 ± 0.75 | 0.379 |
| Mid-intraorbital (horizontal plane) | 2.29 ± 0.95 | 2.37 ± 0.22 | 0.841 |
| Mid-canalicular | 1.32 (1.04, 1.86) | 1.67 (1.20, 2.15) | 0.122 |
| Prechiasmatic | 1.44 ± 0.42 | 1.74 ± 0.27 | 0.112 |
MRI: magnetic resonance imaging; MS: multiple sclerosis; NMOSD: neuromyelitis optica spectrum disorders; SD: standard deviation; T2-FLAIR: T2-weighted fluid-attenuated inversion recovery.
The presence of oligoclonal bands in the cerebrospinal fluid (CSF) was significantly higher in the MS group than the NMOSD group (72.2% vs 12.8%, respectively; p ≤ 0.001) while CSF-pleocytosis was not significantly different between the two groups (Table 1).
Discussion
The present study showed the difficulty in completely differentiating ON-NMOSD from ON-MS based only on demographic data, clinical presentation and paraclinical investigations. There were no significant differences between the two groups regarding age at their first ON attack onset, fundoscopic findings, having CSF-pleocytosis, OCT of the RNFL and ganglion cell layer thickness as well as imaging findings. Some characteristic findings might, nevertheless, be suggestive of one condition more than the other. Our study showed ON-NMOSD had recurrent ON more often than ON-MS, and ON-NMOSD tended to manifest with simultaneous BON involvement at the first ON attack. ON-NMOSD was associated with worse VA at the first ON attack and also with a poorer long-term visual outcome than ON-MS. Nearly one-half of the NMOSD group still had a logMAR VA ≥1 at their last follow-up visit even though they had had shorter disease durations compared to the MS group.
While spinal MRI of LETM helps to suggest NMOSD, the presence of CSF-oligoclonal bands and brain MRI fulfilling Swanton criteria is more suggestive of MS. Ten percent to 20% of the patients, nevertheless, did not have typical findings. This borderline demyelinating group may thus need other tools to help to differentiate between the two conditions.
The present study found ON-MS had BON involvement more frequently than previously reported13–15 with incidence of up to 15% at the first ON attack and 11% of the total number of ON attacks. BON involvement may, therefore, not be an appropriate clinical characteristic to predict ON-NMOSD.
Similar to previous studies,16,17 we found worse VA in acute ON attacks in ON-NMOSD than those found in ON-MS with 79.2% of the ON-NMOSD group having VA ≥1 logMAR. Concordant with one previous study,18 we found poorer visual recovery in nearly one-half of the patients with ON-NMOSD who still had a VA of logMAR ≥1 at the last follow-up visit. These findings reaffirm the more severe clinical course of ON-NMOSD.
OCT has been proposed to be a novel tool to differentiate ON-NMOSD from ON-MS and may be used as a predictor of visual recovery outcome and disability progression.19,20 A high index of suspicion for ON-NMOSD is warranted if more severe retinal damage is seen after an ON episode.21–25
Naismith et al. reported that the odds of categorization to the NMOSD group increased by 8% for every 1 µm decrease in RNFLT.21 We did not, however, find significant decreases of RNFLT in ON-NMOSD patients compared to ON-MS patients at the time of ON attacks. We did, however, find a significantly thinner average RNFLT in ON-NMOSD patients than ON-MS patients at the last follow-up visit. Thinning of the RNFLT could result from secondary neurodegeneration after the ON attack, caused by anti-AQP4-Ab targeting the Müller cell and also from other mechanisms that cause retinitis and subsequent dysfunction/damage of the retinal axons and neurons independent of the action of anti-AQP4-Ab.18,26–28
The poorer VA outcome and thinner RNFLT at the last follow-up visit seen in ON-NMOSD compared to ON-MS may suggest the greater spreading of the destruction of astrocytes, myelin and surrounding structure due to pathologic anti-AQP4-Ab resulting in irreversible damage.29
One previous report found altitudinal VF defect to be highly suggestive of ON-NMOSD.30 The authors proposed that an ischemic mechanism mediated by anti-AQP4-Ab in ON-associated NMOSD may explain those findings.31 We also found a trend toward having a higher incidence of altitudinal VF defect in ON-NMOSD than in ON-MS; however, this was not significantly different, perhaps because of small numbers of observations of this variable in the present study. To the best of our knowledge, the present study is the first to report that arcuate VF defect was more commonly found in ON-MS than in ON-NMOSD. This finding, nevertheless, needs to be confirmed by a large sample population.
A higher proportion of normal orbital MRI findings in both groups was found in the present study compared with previous studies.14,32–34 This may be explained by the longer time to MRI assessment at our center (two to three months) compared with that of four weeks in the study by Ramanathan et al.14 and six weeks in the study by Khanna and colleagues.33 The time delay to MRI assessment in the present study could have allowed for sufficient time for the lesion to fade out.
The difference in the radiological findings of orbital MRI in the present study from those reported by Ramanathan et al.14 may be due to several factors. Baseline characteristics were, firstly, not the same. Ramanathan et al. studied a population experiencing their first episode of ON including 19 anti-MOG-Ab, 11 anti-AQP4-Ab, 13 MS and seven others.14 They included pediatric patients (the proportion in each group was not mentioned) and had an age of onset 10 years younger and an onset of ON to MRI assessment of shorter duration than the present study.14 The present study was, however, a cross-sectional study using retrospective data and recruited only adult patients who had experienced at least one attack of ON. The differences between the present study and that of Ramanathan and colleagues in the incidence of ON as an initial manifestation of NMOSD or MS may have been due to the high ratio of patients in the NMOSD group to the MS group in the present study at nearly 5.6:1 (45 NMOSD patients vs eight MS patients). This higher ratio of NMOSD patients to MS patients as well as the small sample sizes in the present study may have caused the reported findings to be imprecise as estimates.
The present study revealed only a trend toward having more simultaneous BON involvement at their first ON attack in the NMOSD group. Our findings were similar to those of Absoud et al.,35 which showed a higher proportion of unilateral ON (40%) than BON involvement (20%), but were different from those of Ramanathan et al., which showed BON predominantly.14
Similar to the present study, Khanna et al.33 showed a trend toward more posterior involvement of the optic nerve in the NMO group with chiasmatic enhancement seen in NMO patients, but this was not significant, while Ramanathan and colleagues14 reported that the majority of anti-AQP4-positive ON patients had involvement of the intracranial portion of the optic nerve and exhibited longitudinally extensive ON. The difference in results may be due to differences in eligibility criteria, time to get orbital MRI assessment (four weeks in the study by Ramanathan et al.,14 six weeks in that by Khanna and colleagues,33 and two to three months in the present study) and small numbers of patients in each stratified group.
The enhancement pattern could not differentiate between the two groups in the present study, which is concordant with two previous studies.14,23 Ramanathan et al.14 reported one anti-AQP4-positive ON patient and about one-half of their anti-MOG-Ab-positive ON patients had optic nerve head swelling radiologically while the present study did not find this in any of the patients. It should, nevertheless, be noted that we found only one anti-MOG-Ab-positive ON patient.
Comparing optic nerve diameter, we did not find significant differences between the ON-NMOSD and ON-MS patients in different dimensions measured by our current MRI protocol. More-advanced MRI techniques focusing on the optic nerve in larger groups of patients may help to clarify whether any true differences exist.
The present study confirmed the findings in the literature that the majority of NMOSD patients had LETM, frequently detected by an association with ANA autoantibodies, and that the majority of MS patients fulfilling Swanton brain MRI criteria had CSF-oligoclonal IgG bands. A proportion of the patients (up to 20%), nevertheless, had borderline presentations or did not manifest with typical presentations. Discrimination between ON-NMOSD and ON-MS in this group could not have been reliable even with clinical presentations, ophthalmologic investigations and orbital MRI findings. In this group, a highly sensitive and specific anti-AQP4-Ab test would have been useful to help to discriminate between these two conditions. In the case of a negative anti-AQP4-Ab test, ON-NMOSD would, however, have still been difficult to discriminate from ON-MS. Other novel biomarkers in the serum and CSF and advanced MRI techniques may help to discriminate between these two entities.7
The present study had several limitations. We, firstly, had small numbers of MS patients with ON; therefore, it may not have been sufficient to declare statistical significance. Our study was, secondly, a retrospective study of real-world practice. Unexpected factors in clinical practice could, thus, have occurred and may have affected the results of the study. The optic nerve lesion after the acute attack may, for example, have been resolved or distorted resulting in changes of the optic nerve length lesion, enhancement pattern and MRI appearances, which may have affected the statistical findings, because orbital MRI was delayed because of the internal referral process between departments. We, finally, did not test anti-MOG-Ab routinely in all of the patients suspected of having demyelinating diseases. Positivity both for anti-AQP4-Ab and anti-MOG-Ab is, however, very rare.36 In the present study, 88% of NMOSD patients were anti-AQP4-Ab positive and all seven anti-AQP4-Ab-negative NMOSD patients were tested for anti-MOG-Ab. We found only one NMOSD patient who was anti-AQP4-Ab seronegative and anti-MOG-Ab seropositive. The presence of very low numbers of unidentified anti-MOG-Ab-positive patients due to lack of testing would, thus, be unlikely to strongly bias the findings.
There is no current consensus on the sequences and protocols for the application of current MRI or advanced imaging for ON, and this lack of standardization may have contributed to the inconsistency of findings in the literature. Standardization of current MRI or advanced imaging techniques to obtain ON lesions would, therefore, be required before these imaging techniques can be considered as reliable measures of outcomes in clinical trials.
A future, well-designed cohort study with larger numbers of patients within specific stratified groups of ON including anti-MOG-Ab-positive-, anti-AQP4-Ab-positive-, anti-AQP4-Ab- negative-associated ON, ON-MS, and isolated-ON is needed to clarify the differences between ON-associated MS and NMOSD as well as other demyelinating diseases.
Conclusion
The present study showed that most of the Thai patients in our study with ON-NMOSD had more relapsing, tended to present initially with simultaneous BON and had poorer visual outcome compared to those with ON-MS.
Acknowledgements
The authors acknowledge Prof Dr Chulaluk Komoltri, who provided data analysis and manuscript preparation.
Author contributions are as follows: Dr Srikajon initiated the project, designed data collection tools, monitored data collection for the whole study, wrote the statistical analysis plan, cleaned and analyzed the data as well as drafted and revised the manuscript. S. Siritho initiated the project, designed data collection tools, monitored data collection for the whole study, drafted and revised the paper as well as approved of the final version of the manuscript for publication. C. Ngamsombat designed data collection tools, monitored data collection for the whole study, interpreted the data and revised the manuscript. N. Prayoonwiwat designed data collection tools, monitored data collection, supervised the study and obtained funding. N. Chirapapaisan designed data collection tools, monitored data collection, interpreted the data and revised the manuscript.
Conflict of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Srikajon, Dr Ngamsombat and Dr Chirapapaisan have nothing to declare. Dr Siritho has received funding for travel and speaker honoraria from Merck Serono, Pacific Healthcare (Thailand), Menarini (Thailand), Biogen Idec, UCB (Thailand) and Novartis. Dr Prayoonwiwat has received funding for travel and received speaker honoraria from Bayer Schering Pharma, Eisai Inc, Pfizer Pharmaceutical Company Limited, Novartis and Sanofi-Aventis.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Siriraj Research Protocol 106/2558 (EC3).
References
- 1.Petzold A, Wattjes MP, Costello F, et al. The investigation of acute optic neuritis: A review and proposed protocol. Nat Rev Neurol 2014; 10: 447–458. [DOI] [PubMed] [Google Scholar]
- 2.Lim YM, Pyun SY, Lim HT, et al. First-ever optic neuritis: Distinguishing subsequent neuromyelitis optica from multiple sclerosis. Neurol Sci 2014; 35: 781–783. [DOI] [PubMed] [Google Scholar]
- 3.Galetta SL, Villoslada P, Levin N, et al. Acute optic neuritis: Unmet clinical needs and model for new therapies. Neurol Neuroimmunol Neuroinflamm 2015; 2: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J 2012; 6: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of neuromyelitis optica: Recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol 2014; 261: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimbrough DJ, Fujihara K, Jacob A, et al. Treatment of neuromyelitis optica: Review and recommendations. Mult Scler Relat Disord 2012; 1: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siritho S, Sato DK, Kaneko K, et al. The clinical spectrum associated with myelin oligodendrocyte glycoprotein antibodies (anti-MOG-Ab) in Thai patients. Mult Scler 2016; 22: 964–968. [DOI] [PubMed] [Google Scholar]
- 8.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holladay JT. Proper method for calculating average visual acuity. J Refract Surg 1997; 13: 388–391. [DOI] [PubMed] [Google Scholar]
- 11.Mealy MA, Whetstone A, Orman G, et al. Longitudinally extensive optic neuritis as an MRI biomarker distinguishes neuromyelitis optica from multiple sclerosis. J Neurol Sci 2015; 355: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olan T, Siritho S, Kittisaresc K, et al. Beneficial effect of plasma exchange in acute attack of neuromyelitis optica spectrum disorders. Mult Scler Relat Disord 2018; 20: 115–121. [DOI] [PubMed] [Google Scholar]
- 13.Burman J, Raininko R, Fagius J. Bilateral and recurrent optic neuritis in multiple sclerosis. Acta Neurol Scand 2011; 123: 207–210. [DOI] [PubMed] [Google Scholar]
- 14.Ramanathan S, Prelog K, Barnes EH, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler 2016; 22: 470–482. [DOI] [PubMed] [Google Scholar]
- 15.Wingerchuk DM, Hogancamp WF, O’Brien PC, et al. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 1999; 53: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 16.Merle H, Olindo S, Bonnan M, et al. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology 2007; 114: 810–815. [DOI] [PubMed] [Google Scholar]
- 17.Masuda H, Mori M, Uzawa A, et al. Recovery from optic neuritis attack in neuromyelitis optica spectrum disorder and multiple sclerosis. J Neurol Sci 2016; 367: 375–379. [DOI] [PubMed] [Google Scholar]
- 18.Silva FR, Vidotti VG, Cremasco F, et al. Sensitivity and specificity of machine learning classifiers for glaucoma diagnosis using spectral domain OCT and standard automated perimetry. Arq Bras Oftalmol 2013; 76: 170–174. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Martin E, Polo V, Larrosa JM, et al. Retinal layer segmentation in patients with multiple sclerosis using spectral domain optical coherence tomography. Ophthalmology 2014; 121: 573–579. [DOI] [PubMed] [Google Scholar]
- 20.Ratchford JN, Quigg ME, Conger A, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology 2009; 73: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naismith RT, Tutlam NT, Xu J, et al. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology 2009; 72: 1077–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett JL, de Seze J, Lana-Peixoto M, et al. Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult Scler 2015; 21: 678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sotirchos ES, Saidha S, Byraiah G, et al. In vivo identification of morphologic retinal abnormalities in neuromyelitis optica. Neurology 2013; 80: 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider E, Zimmermann H, Oberwahrenbrock T, et al. Optical coherence tomography reveals distinct patterns of retinal damage in neuromyelitis optica and multiple sclerosis. PLoS One 2013; 8: e66151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pache F, Zimmermann H, Mikolajczak J, et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation 2016; 13: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeka B, Hastermann M, Kaufmann N, et al. Aquaporin 4-specific T cells and NMO-IgG cause primary retinal damage in experimental NMOSD. Acta Neuropathol Commun 2016; 4: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oertel FC, Kuchling J, Zimmermann H, et al. Microstructural visual system changes in AQP4-antibody-seropositive NMOSD. Neurol Neuroimmunol Neuroinflamm 2017; 4: e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamura T, Nakashima I. Foveal thinning in neuromyelitis optica: A sign of retinal astrocytopathy? Neurol Neuroimmunol Neuroinflamm 2017; 4: e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herwerth M, Kalluri SR, Srivastava R, et al. In vivo imaging reveals rapid astrocyte depletion and axon damage in a model of neuromyelitis optica-related pathology. Ann Neurol 2016; 79: 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakajima H, Hosokawa T, Sugino M, et al. Visual field defects of optic neuritis in neuromyelitis optica compared with multiple sclerosis. BMC Neurol 2010; 10: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merle H, Jeannin S, Hage R, et al. Visual field characteristics in neuromyelitis optica in absence of and after one episode of optic neuritis. Clin Ophthalmol 2013; 7: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akaishi T, Nakashima I, Takeshita T, et al. Lesion length of optic neuritis impacts visual prognosis in neuromyelitis optica. J Neuroimmunol 2016; 293: 28–33. [DOI] [PubMed] [Google Scholar]
- 33.Khanna S, Sharma A, Huecker J, et al. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. J Neuroophthalmol 2012; 32: 216–220. [DOI] [PubMed] [Google Scholar]
- 34.Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: Final optic neuritis treatment trial follow-up. Arch Neurol 2008; 65: 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Absoud M, Lim MJ, Appleton R, et al. Paediatric neuromyelitis optica: Clinical, MRI of the brain and prognostic features. J Neurol Neurosurg Psychiatry 2015; 86: 470–472. [DOI] [PubMed] [Google Scholar]
- 36.Di Pauli F, Höftberger R, Reindl M, et al. Fulminant demyelinating encephalomyelitis: Insights from antibody studies and neuropathology. Neurol Neuroimmunol Neuroinflamm 2015; 2: e175. [DOI] [PMC free article] [PubMed] [Google Scholar]