Abstract
Background:
The aim of this research was to investigate intermediate-term effects of silodosin on lower urinary tract functions and symptoms in patients with lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) according to prostate size, using urodynamics.
Methods:
A total of 70 untreated outpatients with a prostate volume <40 ml [small prostate (SP) group] and 70 with prostate volume ⩾40 ml [large prostate (LP) group] were prospectively enrolled and treated by monotherapy with silodosin for 24 months. Changes in parameters from baseline to 3 months and 24 months after silodosin administration were assessed based on LUTS, voiding and storage function. In addition, withdrawal rates of silodosin due to insufficient effects were compared between the two groups and factors to influence the withdrawal were investigated.
Results:
The International Prostate Symptom Score (IPSS), bladder outlet obstruction index (BOOI), and detrusor overactivity (DO) improved significantly for the 2-year follow up in both groups as compared with the baseline. IPSS, BOOI and DO improved by 40.4%, 41.3%, and 48.1% in the SP group, 32.7%, 35.9%, and 34.4% in the LP group at 3 months, while, 44.3%, 43.5%, and 63.0% in the SP group, 22.6%, 21.1%, and 34.4% in the LP group at 24 months, respectively. Improvement rates in the IPSS and BOOI at 3 months were maintained until 24 months in the SP group, but decreased in the LP group. Storage function improvements continued in both groups for 2 years. Dropout rate due to unsatisfactory effects was significantly higher in the LP group (20% versus 8.6%). Maximum flow rate, BOOI, and intravesical prostatic protrusion had a significant influence on the withdrawal of silodosin treatment in the LP group.
Conclusions:
Silodosin significantly improved lower urinary tract functions for 2 years in patients with LUTS/BPH, regardless of prostate size. However, LUTS and BOO improvements tended to decrease in patients with a large prostate (>40 ml) in the intermediate term.
Keywords: alpha-1 blocker, benign prostatic hyperplasia, bladder outlet obstruction, long-term, urodynamics
Introduction
The standard initial treatment for patients with lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) is pharmacological therapy, and sympathetic α1-adrenoceptor antagonists (α1-blockers) are widely used as the drug of first choice.1–3 α1-blockers have been reported to rapidly improve both voiding and storage symptoms and functions.4–6 However, some reports have stated that the conventional α1-blocker monotherapy is insufficient for long-term improvement of LUTS in patients with a large prostate.7–10 The CombAT study showed that subjective symptoms, as determined by the International Prostate Symptom Score (IPSS), deteriorated 12 months or more after treatment in patients receiving tamsulosin monotherapy, as demonstrated by a worsening of IPSS which was most noteworthy in patients with large prostates (>42 ml).7 Additionally, the same study found that patients with a large prostate at baseline were more likely to have treatment failure with α1-blocker monotherapy.8,9 Given these findings, current guidelines in western countries and Japan recommend a combination therapy of α1-blockers and 5α-reductase inhibitors (5-ARIs) for the treatment of patients with LUTS/BPH with a large prostate.1–3
However, there are few reports to evaluate the effect of α1-blocker monotherapy on voiding and storage functions over a year.4 It has not been clarified whether the therapeutic effects on bladder outlet obstruction (BOO) and storage function decrease if α1-blocker monotherapy is continued for over a year in patients with a large prostate.
Furthermore, little is known about the reasons why patients with a large prostate at baseline tend to have treatment failure with α1-blocker monotherapy. In this study, we prospectively analyzed the intermediate-term effects of α1-blocker monotherapy on the lower urinary tract function as measured by urodynamic studies (UDSs), in addition to the effects on voiding and storage symptoms. The aims of the present study were: (1) to evaluate the intermediate-term effects of α1-blocker monotherapy on storage functions and BOO according to the prostate size, and (2) to determine the factors that have an influence on continuation or withdrawal of α1-blocker monotherapy for patients with a large prostate.
Materials and methods
This was a single-center, prospective study, which was conducted in accordance with the ethical principles of the Declaration of Helsinki. The protocol was approved by the ethics committee of the Nagoya University Graduate School of Medicine, Japan. All participants provided written informed consent before enrolment.
The study included treatment-naïve LUTS/BPH patients who visited our hospital between January 2009 and December 2012. The inclusion criteria were as follows: (1) total IPSS ⩾ 8, (2) IPSS quality of life (QOL) score ⩾ 3, (3) prostate volume ⩾ 25 ml as determined by transabdominal ultrasonography, (4) maximum urinary flow rate (Qmax) < 15 ml/sec at voided a volume of ⩾150 ml, and (5) age ⩾ 50 years. Patients were excluded if they: (1) had received oral or surgical treatment for LUTS, (2) had neurogenic bladder dysfunction, bladder calculi, or active urinary tract infections, or (3) had severe cardiac disease, renal dysfunction, or hepatic dysfunction. Prostate biopsy was performed in all patients who had prostate-specific antigen (PSA) levels >4 ng/ml, and only cancer-free patients were included in the study.
Patients who satisfied all the inclusion and exclusion criteria received monotherapy using silodosin (8 mg/day) for 24 months. To evaluate changes in subjective symptoms, the IPSS, IPSS-QOL, and overactive bladder symptom scores (OABSS)11 were assessed at baseline, 3 months, and 24 months after treatment. The patients also underwent UDS, including uroflowmetry (UFM), cystometrogram (CMG), and pressure flow study (PFS) for the evaluation of objective findings at baseline, and 3 months and 24 months after initiation of treatment. Maximum cystometric capacity (MCC) and detrusor overactivity (DO) were assessed as parameters of storage function, and Qmax, post-void residual urine volume (PVR), detrusor pressure at Qmax (PdetQmax), and bladder outlet obstruction index (BOOI) were evaluated as parameters of voiding function. CMG and PFS were performed according to the standard methods defined by the International Continence Society.12 The data of UDS were de-identified and analyzed independently by our research group members who were not involved in conducting the UDS.
Patients were excluded from the analysis if they discontinued the treatment owing to adverse reactions and urinary retention. As for adherence to therapy, we judged patients, who complain the worsening of subjective symptoms and hope a treatment change, as treatment failure and changed regimen of therapy.
In this study, we defined a small prostate (SP) group as patients with a prostate volume <40 ml, and a large prostate (LP) group as patients with a prostate volume ⩾40 ml, based on previous reports. We compared the subjective and objective effects of silodosin between the SP and the LP groups.3,7,10 Additionally, we divided the LP group into patients who completed the study (LP/completed) and those who dropped out (LP/dropped out). We then compared the backgrounds of these two groups and evaluated factors that may have influenced the continuation or withdrawal of α1-blocker monotherapy.
Sample size calculations were based on a primary outcome of IPSS change, because there were no data on objective findings. The mean IPSS change from baseline to 24 months was 5.0 points in the SP group and 3.8 points in the LP group, based on the CombAT study.7 A standard deviation of this change was calculated as 2.0 points. A total of 45 participants in each group were required for a two-sided significance level of 0.05 and a power of 80%. We expected approximately 35% attrition during the entire study period (after 24 months of study participation), and therefore decided to include 70 participants in each group.
All statistical values are represented as mean ± standard deviation. The Wilcoxon rank-sum test, Student’s t test, and Chi-square test were performed to evaluate changes in subjective symptoms and objective findings obtained by UDS. All tests were two-sided and a p-value <0.05 was considered statistically significant.
Results
Each of 70 patients were enrolled in either the SP or LP group. The characteristics of study participants are shown in Table 1. There were no significant differences in subjective parameters such as IPSS and OABSS between the two groups, although BOOI and the incidence of DO were significantly higher in the LP group. Of the 70 patients in the LP group, 5 (7.1%) discontinued treatment owing to adverse reactions, including nasal congestion (n = 2), postural hypotension (n = 2), and ejaculatory dysfunction disorder (n = 1). Overall, three patients showed urinary retention during the study period. A total of 14 patients (20%) dropped out because of an unsatisfactory effect. On the other hand, in the SP group, 6 patients (8.6%) discontinued treatment because of adverse reactions, including postural hypotension (n = 2), dizziness (n = 1), and ejaculatory dysfunction disorder (n = 3). One patient showed urinary retention and six patients (8.6%) dropped out because of an unsatisfactory effect. Although no significant difference was found between the two groups in the incidence of adverse effects, the rate of withdrawal owing to unsatisfactory effects was significantly higher in the LP group (p = 0.04). As a result, the final analysis included 57 patients in the SP group and 48 patients in the LP group.
Table 1.
Characteristics of the two groups at enrolment.
| SP group |
LP group |
p-value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| n | 70 | 70 | |
| Age (years) | 68.2 ± 9.2 | 70.4± 6.7 | 0.09 |
| Prostate volume (ml) | 31.5 ± 4.5 | 65.0± 17.8 | <0.001 |
| PSA (ng/ml) | 2.2 ± 2.1 | 6.2 ± 4.6 | <0.001 |
| IPSS-total | 18.3 ± 6.2 | 19.3 ± 6.5 | 0.30 |
| IPSS-storage | 7.9 ± 3.0 | 8.1 ± 2.4 | 0.58 |
| IPSS-voiding | 10.4 ± 4.6 | 11.2 ± 4.6 | 0.25 |
| QOL | 4.8 ± 1.0 | 4.7 ± 0.8 | 0.98 |
| OABSS | 5.6 ± 3.0 | 6.6 ±2.4 | 0.11 |
| FDV (ml) | 118 ± 51 | 129 ± 58 | 0.10 |
| MCC (ml) | 248 ± 97 | 244 ± 87 | 0.82 |
| Qmax (ml/s) | 7.9 ± 4.1 | 6.4 ± 2.5 | 0.009 |
| PVR (ml) | 53 ± 53 | 71 ± 61 | 0.07 |
| PdetQmax (cmH20) | 67.8 ± 17.8 | 88.7 ± 23.7 | <0.001 |
| BOOI | 51.9 ± 20.9 | 75.9 ± 25.2 | <0.001 |
| prevalence of DO | 31/70 (44.3%) | 47/70 (67.1%) | 0.01 |
BOOI, bladder outlet obstruction index; DO, detrusor overactivity; FDV, first desire to void; IPSS, International Prostate Symptom Score; LP, large prostate; MCC, maximum cystometric capacity; OABSS, overactive bladder symptom scores; PdetQmax, detrusor pressure at Qmax; PSA, prostate-specific antigen; PVR, post-void residual urine volume; Qmax, maximum urinary flow rate; QOL, quality of life; SD, standard deviation; SP, small prostate.
The changes in subjective symptoms and objective findings are summarized in Tables 2 and 3, and Figures 1–3. At baseline, the mean prostate volume was 31.7 ml in the SP group and 64.2 ml in the LP group. These volumes were nearly unchanged over 24 months in both groups. Significant decreases were observed in the total IPSS, IPSS-storage, IPSS-voiding, IPSS-QOL, and OABSS at 3 months after treatment in both groups. Although further improvements were observed after 24 months in the SP group for all parameters, the improvements in IPSS-total, IPSS-voiding, and IPSS-QOL worsened in the LP group. The improvement rate of IPSS-total at 3 months from baseline was 40.4% in the SP group and 32.7% in the LP group, with no difference between the two groups; however, the improvement rate at 24 months was 44.3% in the SP group and 22.6% in the LP group, which was a significant difference (p = 0.002).
Table 2.
Changes in subjective symptoms between the two groups of patients who completed the study.
| SP group |
p (intra- group) |
LP group | p (intra-group) | p (inter-group) | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| N | 57 | 48 | |||
| Prostate volume (ml) | |||||
| baseline | 31.7 ± 4.3 | 64.2 ± 18.9 | <0.001 | ||
| 3 months | 32.7±9.8 | 0.46 | 65.9 ± 19.7 | 0.93 | <0.001 |
| 24 months | 34.2 ± 9.4 | 0.07 | 66.2 ± 19.9 | 0.86 | <0.001 |
| IPSS | |||||
| baseline | 17.9 ± 5.9 | 19.2 ± 7.1 | 0.31 | ||
| 3 months | 11.0 ± 6.1 | <0.001 | 13.0 ± 6.4 | <0.001 | 0.10 |
| Mean improvement rate from baseline | 40.4 % | 32.7 % | 0.11 | ||
| 24 months | 9.7 ± 6.5 | <0.001 | 14.5 ± 7.5 | 0.002 | <0.001 |
| Mean improvement rate from baseline | 44.3 % | 22.6 % | 0.002 | ||
| IPSS-storage | |||||
| baseline | 7.7 ± 3.0 | 8.3 ± 2.6 | 0.22 | ||
| 3 months | 5.3 ± 3.1 | <0.001 | 5.7 ± 2.5 | <0.001 | 0.37 |
| Mean improvement rate from baseline | 32.5 % | 31.0 % | 0.79 | ||
| 24 months | 4.1 ± 2.8 | <0.001 | 5.8 ± 3.1 | <0.001 | 0.005 |
| Mean improvement rate from baseline | 44.0 % | 31.0 % | 0.05 | ||
| IPSS-voiding | |||||
| baseline | 10.2 ± 4.5 | 10.9 ± 5.1 | 0.43 | ||
| 3 months | 5.7 ± 4.1 | <0.001 | 7.3 ± 4.6 | <0.001 | 0.06 |
| Mean improvement rate from baseline | 47.9 % | 35.0 % | 0.03 | ||
| 24 months | 5.6±4.3 | <0.001 | 8.7 ± 4.6 | 0.02 | <0.001 |
| Mean improvement rate from baseline | 39.6 % | 12.5 % | 0.006 | ||
| IPSS-QOL | |||||
| baseline | 4.8 ± 1.0 | 4.7 ± 0.8 | 0.89 | ||
| 3 months | 3.1 ± 1.3 | <0.001 | 3.1 ± 1.0 | <0.001 | 0.91 |
| Mean improvement rate from baseline | 35.2 % | 33.4 % | 0.66 | ||
| 24 months | 2.9 ± 1.1 | <0.001 | 3.7 ± 1.3* | <0.001 | <0.001 |
| Mean improvement rate from baseline | 39.6 % | 20.6 % | <0.001 | ||
| OABSS | |||||
| baseline | 5.7 ± 3.0 | 7.0 ± 2.6 | 0.09 | ||
| 3 months | 4.0 ± 2.5 | 0.008 | 4.7 ± 2.0 | <0.001 | 0.24 |
| Mean improvement rate from baseline | 26.3 % | 31.4 % | 0.48 | ||
| 24 months | 3.7 ± 2.3 | <0.001 | 4.7 ± 2.2 | <0.001 | 0.02 |
| Mean improvement rate from baseline | 38.2% | 29.6 % | 0.32 | ||
p (intra-group): versus baseline *: p < 0.05 versus 3 months.
IPSS, International Prostate Symptom Score; LP, large prostate; OABSS, overactive bladder symptom scores; QOL, quality of life; SD, standard deviation; SP, small prostate.
Table 3.
Changes in objective findings in the two groups of patients who completed the study.
| SP group |
p
(intra- group) |
LP group |
p (intra-group) | p (inter-group) | |
|---|---|---|---|---|---|
| Mean ± SD (difference in mean change from baseline and 3 months) |
Mean ± SD (difference in mean change from baseline and 3 months) |
||||
| N | |||||
| MCC (ml) |
57 | 48 | |||
| baseline | 250 ± 89 | 233 ± 82 | 0.31 | ||
| 3 months | 288 ± 66 (+38) | 0.04 | 271 ± 72 (+38) | 0.017 | 0.36 |
| 24 months | 296 ± 87 (+46, +8) | 0.007 | 284 ± 80 (+51, +13) | 0.002 | 0.47 |
| Qmax (ml/s) | |||||
| baseline | 8.2 ± 4.1 | 6.7 ± 2.9 | 0.03 | ||
| 3 months | 10.7 ± 4.7 (+2.5) | 0.003 | 9.3 ± 3.9 (+2.6) | <0.001 | 0.11 |
| 24 months | 10.8 ± 4.5 (+2.6, +0.1) | 0.003 | 9.0 ± 6.8 (+2.3, −0.3) | 0.03 | 0.14 |
| PdetQmax (cmH2O) | |||||
| baseline | 67.0 ± 14.3 | 85.3 ± 24.9 | <0.001 | ||
| 3 months | 51.8 ± 12.4 (−15.2) | <0.001 | 66.3 ± 25.1 (−19.0) | <0.001 | <0.001 |
| 24 months | 51.4 ± 14.3 (−15.6, –0.4) | <0.001 | 73.1 ± 25.1 (−12.2, +6.8) | 0.02 | <0.001 |
| PVR (ml) | |||||
| baseline | 49 ± 49 | 64 ± 62 | 0.16 | ||
| 3 months | 25 ± 33 (−24) | 0.003 | 41 ± 43 (−23) | 0.03 | 0.03 |
| 24 months | 30 ± 37 (−19, +5) | 0.03 | 55 ± 48 (−9, +14) | 0.38 | 0.006 |
| BOOI | |||||
| baseline | 50.5 ± 16.9 | 71.8 ± 26.6 | <0.001 | ||
| 3 months | 30.4 ± 15.9 (−20.1) | <0.001 | 47.6 ± 28.1 (−24.2) | <0.001 | <0.001 |
| Mean improvement rate | 41.3 % | 35.9 % | 0.39 | ||
| 24 months | 29.9 ± 18.7 (−20.6, −0.5) | <0.001 | 55.1 ± 33.9 (−16.7, +7.5) | 0.008 |
<0.001 |
| Mean improvement rate | 43.5 % |
21.1% |
<0.001 |
||
| DO case, Disappearing rate (%) | |||||
| baseline | 27/57 | 29/48 | 0.18 | ||
| 3 months | 14/57 (48.1%) | 0.01 | 19/48 (34.4%) | 0.04 | 0.09 |
| 24 months | 10/57 (63.0%) | <0.001 | 19/48 (34.4%) | 0.04 | 0.01 |
BOOI, bladder outlet obstruction index; DO, detrusor overactivity; LP, large prostate; MCC, maximum cystometric capacity; PdetQmax, detrusor pressure at Qmax; Qmax, maximum urinary flow rate; SD, standard deviation; SP, small prostate.
Figure 1.
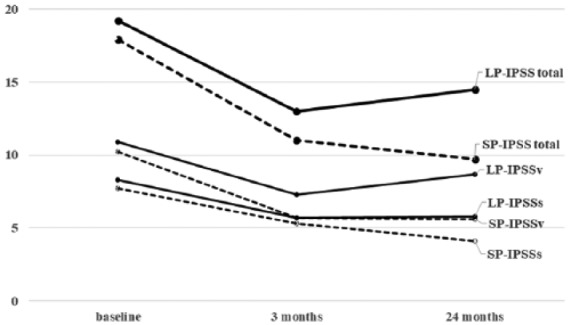
Changes of IPSS according to prostate size between the two groups.
IPSS, International Prostate Symptom Score; IPSSs, IPSS-storage score; IPSSv, IPSS-voiding score; LP, large prostate group; SP, small prostate group.
Figure 2.
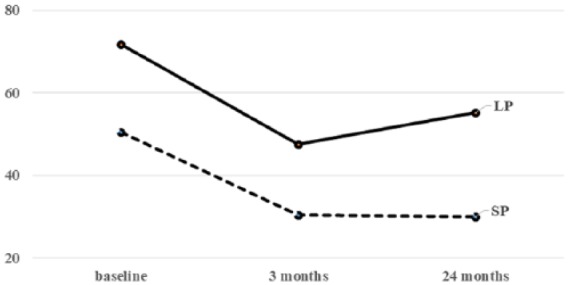
Changes of BOOI according to prostate size between the two groups.
BOOI, bladder outlet obstruction index; LP, large prostate group; SP, small prostate group.
Figure 3.
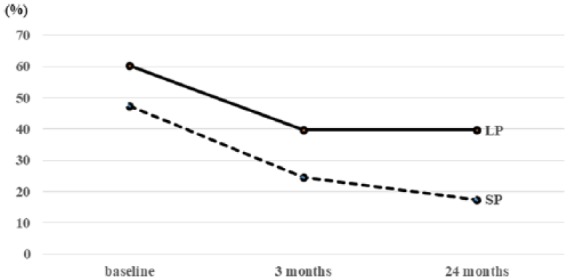
Incidence of DO according to prostate size between the two groups.
DO, detrusor overactivity; LP, large prostate group; SP, small prostate group.
Regarding voiding function, both groups showed significant improvements in Qmax, PdetQmax, and BOOI after both 3 months and 24 months, compared with the baseline measures (Table 3 and Figure 2). In the intermediate-term evaluation, the improvements continued in the SP group, while Qmax and BOOI deteriorated in the LP group. The mean improvement rate of BOOI from baseline was 41.3% in the SP group and 35.9% in the LP group at 3 months, and 43.5% in the SP group and 21.1% in the LP group at 24 months. The efficacy on BOO deteriorated only in the LP group. At baseline, 41 (71.9%) and 44 patients (91.7%) in the SP and LP groups, respectively, showed BOO (BOOI > 40), while the number of the patients with BOO decreased to 13 patients (22.4%) in the SP group and 33 patients (68.8%) in the LP group after 24 months. There was a significant difference in the improvement rate of BOO at 24 months between the two groups (p < 0.001).
Assessment of the storage parameters revealed significant improvements in MCC and the incidence of DO after treatment in both groups. Although the DO relief rate was higher in the SP group at the 24-month evaluation (63.0% in the SP group versus 34.4% in the LP group), the remedial effect on storage functions continued in both groups (Figure 3).
A total of 22 of 70 patients enrolled in the LP group dropped out owing to adverse or unsatisfactory effects. There was no significant difference in age, prostate volume, and the severity of subjective symptoms between the LP/completed and LP/dropped out groups. In contrast, factors were related to BOO, such as Qmax on uroflowmetry (UFM) and BOOI on PFS, had a significant influence on continuation or withdrawal of α1-blocker monotherapy. In particular, intravesical prostatic protrusion (IPP), reported to be a more useful predictor of BPH-induced BOO than either PSA or prostate volume,13,14 was a significant factor in the withdrawal of silodosin (Table 4).
Table 4.
Characteristics of patients in the LP/completed and LP/dropped out groups.
| LP/completed |
LP/dropped out |
p-value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| n | 48 | 22 | |
| Age (years) | 70.2 ± 6.7 | 70.9 ± 6.8 | 0.67 |
| Prostate volume (ml) | 64.2 ± 18.9 | 66.6 ± 15.5 | 0.60 |
| PSA (ng/ml) | 6.0 ± 5.0 | 6.6 ± 3.7 | 0.58 |
| IPSS-total | 19.3 ± 7.1 | 19.7 ± 4.9 | 0.76 |
| IPSS-voiding | 11.0 ± 5.1 | 12.0 ± 3.5 | 0.41 |
| IPSS-storage | 8.3 ± 2.6 | 7.7 ± 2.0 | 0.36 |
| IPSS-QOL | 4.7 ± 0.8 | 5.0 ± 0.6 | 0.12 |
| OABSS | 7.0 ± 2.6 | 6.0 ± 1.9 | 0.16 |
| Qmax (ml/s) on UFM | 8.2 ± 3.5 | 6.5 ± 2.3 | 0.04 |
| PVR (ml) on UFM | 68 ± 59 | 82 ± 50 | 0.35 |
| IPP (mm) | 13.6 ± 6.0 | 17.7 ± 4.6 | 0.006 |
| BOOI | 71.8 ± 26.6 | 84.8 ± 19.6 | 0.04 |
| prevalence of DO | 29/48 (60.4%) | 18/22 (81.8%) | 0.06 |
BOOI, bladder outlet obstruction index; DO, detrusor overactivity; IPP, intravesical prostatic protrusion; IPSS, International Prostate Symptom Score; LP, large prostate; OABSS, overactive bladder symptom scores; PVR, post-void residual urine volume; Qmax, maximum urinary flow rate; QOL, quality of life; PSA, prostate-specific antigen; SD, standard deviation; UFM, uroflowmetry.
Discussion
We evaluated the intermediate-term (2-year) efficacy of α1-blocker monotherapy on voiding and storage functions, according to prostate size. A few studies have evaluated the effects of α1-blocker using PFS, reporting significant improvement of BOO compared with placebo.4,15–17 However, these studies were conducted on patients with a mean prostate size of about 30–40 ml, with no evaluation of patients with larger prostates, or over a year of evaluation. In addition, some papers have reported that the long-term effects of α1-blocker monotherapy deteriorated in patients with a large prostate, based on the evaluation of subjective symptoms.7,8 However, there is a lack of reports investigating changes in objective parameters of lower urinary tract functions such as BOO and DO according to prostate size, following α1-blocker monotherapy. In this study, we showed that the intermediate-term efficacy (24 months) of silodosin gradually decreased in terms of not only subjective symptoms, but also BOO, in patients with a prostate size of >40 ml, although significant improvements were observed in subjective symptoms and BOO compared with baseline evaluation.
Interestingly, the Qmax and BOO improvements at 24 months in the LP group decreased, although the prostatic volume was nearly unchanged. Additionally, the storage functions, such as bladder capacity and DO, showed sustained improvements over 2 years. One question remains to be elucidated, and that is why the storage function benefits observed in the LP group was maintained for 2 years, despite the decreased efficacy of voiding functions. We were unable to determine the precise reason, but can offer some plausible hypotheses. Firstly, it has been reported that structural changes to collagen fibers occur in α1-blocker-treated human prostatic stroma,18 and as a result, the loss of smooth muscle could be one of the factors that influenced the BOO improvements and subjective deterioration in symptoms. In patients with a large prostate, the structural changes caused by α1-blockers are thought to be greater than that in the SP group. Secondly, the increase of bladder blood flow (BBF) caused by α1-blockers has been reported to be responsible for the improvement of storage symptoms and function.19–21 This continual BBF action is thought to contribute to the maintenance of storage function improvements, irrespective of prostate size. In our previous study, we found that the withdrawal of silodosin from combination therapy with silodosin and dutasteride for LUTS/BPH after 12 months led to the worsening of storage functions.22 Further studies are needed in order to understand the long-term efficacy of α1-blockers on storage and voiding functions. However, long-term α1-blocker treatment is thought to be useful with a view to maintaining the improvement of storage symptoms and functions.
Regarding the factors that influenced treatment failure of α1-blockers, prostate volume has been reported as the most powerful factor in some studies.8,9,23 Masumori and colleagues reported that patients with a prostate volume of ⩾35 ml had a 2.14-times higher odds ratio of treatment failure than those with small prostate (<35 ml).8 In our study, the rate of withdrawal owing to unsatisfactory effects was significantly higher in the LP group (p = 0.04). In the comparison between study completed and dropped out, the severity of BOO at baseline was a significant factor to predict the treatment failure of α1-blocker. In particular, IPP was thought to be a more useful and convenient marker than prostate volume and subjective symptoms. We have previously reported that IPP can be a predicting factor for the therapeutic effects of silodosin in LUTS/BPH.24 In the present study, silodosin provided a significant improvement of BOO during 24 months in the LP group; however, about 70% of the LP group still had BOO after 24 months of treatment. This failure to ameliorate BOO in patients with large prostate was thought to influence treatment failure. In patients with a larger prostate and a higher IPP, a combination therapy with 5-ARIs or surgical treatment should be considered at an early stage of treatment.
The present study had some limitations. First, since the present study was not conducted in a placebo-controlled design, a placebo effect could not be eliminated. However, we believe that this effect did not cause a major problem in the evaluation of objective findings by UDS. Secondly, the follow-up period of the present study was only 24 months. Since pharmacotherapy for LUTS should generally be continued for a much longer period, a further long-term efficacy and safety of α1-blocker monotherapy needs to be clarified in future studies.
Conclusion
Silodosin improved not only LUTS but also BOO and storage function in patients with LUTS/BPH for 2 years, regardless of prostate size. However, the improvements in BOO tended to decrease in patients with a large prostate (>40 ml) in the intermediate term and this finding was thought to have an influence on the reduction in subjective symptom improvements and the treatment failure of α1-blocker monotherapy. Given our findings, prostate volume size should be considered when planning treatment for patients with BPH, as α1-blocker monotherapy may be inadequate for patients with larger prostate and higher IPP.
Acknowledgments
We thank all patients for participating and all trial investigators for their contribution to data acquisition and patient care. The study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients had signed written informed consent prior to their inclusion in the study.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
ORCID iD: Yoshihisa Matsukawa  https://orcid.org/0000-0001-7823-2600
https://orcid.org/0000-0001-7823-2600
Contributor Information
Yoshihisa Matsukawa, The Department of Urology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, 466-8550, Japan.
Shun Takai, The Department of Urology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Tsuyoshi Majima, The Department of Urology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Yasuhito Funahashi, The Department of Urology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Masashi Kato, The Department of Urology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Tokunori Yamamoto, The Department of Urology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Momokazu Gotoh, The Department of Urology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
References
- 1. Homma Y, Gotoh M, Yokoyama O, et al. Outline of JUA clinical guidelines for benign prostatic hyperplasia. Int J Urol 2011; 18: 741–756. [DOI] [PubMed] [Google Scholar]
- 2. McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185: 1793–1803. [DOI] [PubMed] [Google Scholar]
- 3. Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2015; 67: 1099–1109. [DOI] [PubMed] [Google Scholar]
- 4. Fusco F, Palmieri A, Ficarra V, et al. α1-blockers improve benign prostatic obstruction in men with lower urinary tract symptoms: a systematic review and meta-analysis of urodynamic studies. Eur Urol 2016; 69: 1091–1101. [DOI] [PubMed] [Google Scholar]
- 5. Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol 1999; 36: 1–13. [DOI] [PubMed] [Google Scholar]
- 6. Matsukawa Y, Gotoh M, Komatsu T, et al. Efficacy of silodosin for relieving benign prostatic obstruction: prospective pressure flow study. J Urol 2009; 182: 2831–2835. [DOI] [PubMed] [Google Scholar]
- 7. Roehrborn CG, Siami P, Barkin J, et al. The influence of baseline parameters on changes in International Prostate Symptom Score with dutasteride, tamsulosin, and combination therapy among men with symptomatic benign prostatic hyperplasia and an enlarged prostate: 2-year data from the CombAT study. Eur Urol 2009; 55: 461–471. [DOI] [PubMed] [Google Scholar]
- 8. Masumori N, Tsukamoto T, Horita H, et al. α1-blocker tamsulosin as initial treatment for patients with benign prostatic hyperplasia: 5-year outcome analysis of a prospective multicenter study. Int J Urol 2013; 20: 421–428. [DOI] [PubMed] [Google Scholar]
- 9. Masumori N, Hashimoto J, Itoh N, et al. Short-term efficacy and long-term compliance/treatment failure of the alpha1 blocker naftopidil for patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Scand J Urol Nephrol 2007; 41: 422–429. [DOI] [PubMed] [Google Scholar]
- 10. Roehrborn CG, Siami P, Barkin J, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57: 123–131. [DOI] [PubMed] [Google Scholar]
- 11. Homma Y, Yoshida M, Seki N, et al. Symptom assessment tool for overactive bladder syndrome–overactive bladder symptom score. Urology 2006; 68: 318–323. [DOI] [PubMed] [Google Scholar]
- 12. Schäfer W, Abrams P, Liao L, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 2002; 21: 261–274. [DOI] [PubMed] [Google Scholar]
- 13. Lim KB, Ho H, Foo KT, et al. Comparison of intravesical prostatic protrusion, prostate volume and serum prostatic-specific antigen in the evaluation of bladder outlet obstruction. Int J Urol 2006; 13: 1509–1513. [DOI] [PubMed] [Google Scholar]
- 14. Franco G, De Nunzio C, Leonardo C, et al. Ultrasound assessment of intravesical prostatic protrusion and detrusor wall thickness–new standards for noninvasive bladder outlet obstruction diagnosis? J Urol 2010; 183: 2270–2274. [DOI] [PubMed] [Google Scholar]
- 15. Martorana G, Giberti C, Di Silverio F, et al. Effects of short-term treatment with the alpha 1-blocker alfuzosin on urodynamic pressure/flow parameters in patients with benign prostatic hyperplasia. Eur Urol 1997; 32: 47. [PubMed] [Google Scholar]
- 16. Abrams P, Speakman M, Stott M, et al. A dose-ranging study of the efficacy and safety of tamsulosin, the first prostate-selective alpha 1A-adrenoceptor antagonist, in patients with benign prostatic obstruction (symptomatic benign prostatic hyperplasia). Br J Urol 1997; 80: 587. [DOI] [PubMed] [Google Scholar]
- 17. Ozbey I, Aksoy Y, Polat O, et al. Effects of doxazosin in men with benign prostatic hyperplasia: urodynamic assessment. Int Urol Nephrol 1999; 31: 471. [DOI] [PubMed] [Google Scholar]
- 18. Imamura T, Ishii K, Kanda H, et al. Structural changes in alpha1-adrenoceptor antagonist-treated human prostatic stroma. Clin Exp Med 2010; 10: 99–106. [DOI] [PubMed] [Google Scholar]
- 19. Okutsu H, Matsumoto S, Hanai T, et al. Effects of tamsulosin on bladder blood flow and bladder function in rats with bladder outlet obstruction. Urology 2010; 75: 235–240. [DOI] [PubMed] [Google Scholar]
- 20. Majima T, Yamamoto T, Funahashi Y, et al. Effect of naftopidil on bladder microcirculation in a rat model of bladder outlet obstruction. LUTS 2017; 9: 111–116. [DOI] [PubMed] [Google Scholar]
- 21. Saito M, Shimizu S, Ohmasa F, et al. Characterization of silodosin and naftopidil in the treatment of bladder dysfunction in the spontaneously hypertensive rat. Neurourol Urodyn 2013; 32: 393–398. [DOI] [PubMed] [Google Scholar]
- 22. Matsukawa Y, Takai S, Funahashi Y, et al. Effects of withdrawing alpha-1 blocker from the combination therapy with alpha-1 blocker and 5-alpha-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol 2017. in press. [DOI] [PubMed] [Google Scholar]
- 23. de la Rosette JJ, Kortmann BB, Rossi C, et al. Long-term risk of re-treatment of patients using alpha-blockers for lower urinary tract symptoms. J Urol 2002; 167: 1734–1739. [DOI] [PubMed] [Google Scholar]
- 24. Matsukawa Y, Ishida S, Majima T, et al. Intravesical prostatic protrusion can predict therapeutic response to silodosin in male patients with lower urinary tract symptoms. Int J Urol 2017; 24: 454–459. [DOI] [PubMed] [Google Scholar]


