Abstract
Background
Children with a history of maltreatment suffer from altered emotion processing but the neural basis of this phenomenon is unknown. This pioneering functional magnetic resonance imaging (fMRI) study investigated the effects of severe childhood maltreatment on emotion processing while controlling for psychiatric conditions, medication and substance abuse.
Method
Twenty medication-naive, substance abuse-free adolescents with a history of childhood abuse, 20 psychiatric control adolescents matched on psychiatric diagnoses but with no maltreatment and 27 healthy controls underwent a fMRI emotion discrimination task comprising fearful, angry, sad happy and neutral dynamic facial expressions.
Results
Maltreated participants responded faster to fearful expressions and demonstrated hyper-activation compared to healthy controls of classical fear-processing regions of ventromedial prefrontal cortex (vmPFC) and anterior cingulate cortex, which survived at a more lenient threshold relative to psychiatric controls. Functional connectivity analysis, furthermore, demonstrated reduced connectivity between left vmPFC and insula for fear in maltreated participants compared to both healthy and psychiatric controls.
Conclusions
The findings show that people who have experienced childhood maltreatment have enhanced fear perception, both at the behavioural and neurofunctional levels, associated with enhanced fear-related ventromedial fronto-cingulate activation and altered functional connectivity with associated limbic regions. Furthermore, the connectivity adaptations were specific to the maltreatment rather than to the developing psychiatric conditions, whilst the functional changes were only evident at trend level when compared to psychiatric controls, suggesting a continuum. The neurofunctional hypersensitivity of fear-processing networks may be due to childhood over-exposure to fear in people who have been abused.
Key words: Child abuse, childhood maltreatment, fear processing, functional connectivity, insula, limbic, prefrontal
Introduction
Twenty-two per cent of 11- to 17-year-olds in the UK report having experienced physical, emotional, sexual abuse or neglect by a caregiver in their lifetime (Radford et al. 2013) and actual maltreatment rates are likely to be even higher (Brown et al. 1998). Childhood maltreatment is a severe stressor that produces a cascade of physiological, neurochemical, and hormonal changes, which can lead to enduring alterations in brain structure, function and connectivity (Teicher et al. 2003) and is associated with negative outcomes on behavioural, emotional and social functioning (Hart & Rubia, 2012). The human brain is still developing during childhood (Sowell et al. 2003; de Graaf-Peters & Hadders-Algra, 2006). Hence, childhood trauma can disrupt these neurodevelopmental processes.
The ability to categorize facial expressions is generally acquired during childhood and is invaluable for social interaction. Maltreated children are exposed to atypical emotional environments, including less positive (Bugental et al. 1990) and more negative (Herrenkohl et al. 1991) emotion. Altered emotion processing is consistently reported in maltreated children, with neglected children having emotion discrimination deficits (Pollak et al. 2000; Fries & Pollak, 2004; Vorria et al. 2006) and physically abused children displaying response biases for negative emotions such as anger, fear or pain (Pollak et al. 2000; Pollak & Sinha, 2002; Pollak & Tolley-Schell, 2003; Pine et al. 2005).
Physically abused or neglected individuals, have altered event-related potential (ERP) responses when presented with angry or fearful faces, or voices, compared to happy or neutral targets (Pollak et al. 2001; Cicchetti & Curtis, 2005; Parker & Nelson, 2005; Shackman et al. 2007). Functional magnetic resonance imaging (fMRI) studies of emotion processing in neglected/institutionalized children (Maheu et al. 2010; Tottenham et al. 2011), maltreated children (McCrory et al. 2013) and adults with mixed maltreatment histories (Taylor et al. 2006; Dannlowski et al. 2012, 2013; Fonzo et al. 2013) showed enhanced activation of fronto-limbic regions, in particular the amygdala, but also hippocampus, ventromedial prefrontal cortex (vmPFC), anterior cingulate cortex (ACC) and insula, in response to negative emotions, mainly anger and fear.
Previous emotion processing fMRI studies in maltreatment have studied participants with neglect or histories of multiple forms of maltreatment. Neglected and physically maltreated participants differ in their response to emotional stimuli (Pollak et al. 2000) and different maltreatment types may manifest differently. For example, sexually abused and neglected children are most likely to present with social withdrawal, whereas physically abused children are most likely to have problems with aggressive/disruptive behaviour (Trickett & McBride-Chang, 1995). In adults, childhood sexual abuse is particularly associated with sexual problems, emotional abuse with low self-esteem and physical abuse with marital breakdown (Mullen et al. 1996). Ideally, therefore, the effects of each maltreatment type should be studied in isolation. Physical abuse was of particular interest in the current study due to the effect it is thought to have specifically on processing negative emotions (Pollak & Tolley-Schell, 2003). However, although participants were recruited based on their exposure to severe childhood physical abuse, it is unrealistic to separate physical abuse from typically co-occurring emotional abuse and neglect since they are present in almost all cases of physical maltreatment (Edwards et al. 2003; Trickett et al. 2011); hence, our maltreated group had also experienced emotional abuse and neglect. Sexual abuse was excluded due to the known differences in structural, behavioural and psychiatric consequences (Trickett & McBride-Chang, 1995; Ackerman et al. 1998; Heim et al. 2013).
The abovementioned studies have focused on regions of interest (ROIs), thereby limiting hypotheses to a priori regions, primarily the amygdala, providing biased, inappropriately constrained representations (Friston et al. 2006). Another limitation of many maltreatment studies is not controlling for typically co-occurring psychiatric conditions, rendering it impossible to determine whether observed effects are a result of the maltreatment or the associated conditions (McCrory et al. 2011; Hart & Rubia, 2012; Lim et al. 2014). In addition, all emotion processing studies have used traditional static stimuli which do not accurately represent dynamic ‘real world’ emotional expressions which rarely remain static.
Most fMRI studies of emotion processing in maltreatment have concentrated exclusively on functional activation and neglected more sophisticated functional connectivity. Functional communication between brain regions is vital in cognition and emotion, thus examination of altered functional connectivity in childhood maltreatment is vital. One study of women with post-traumatic stress disorder (PTSD) due to intimate partner violence reported that childhood maltreatment severity was correlated with limbic–prefrontal connectivity strength, specifically involving the amygdala, insula, ACC and medial frontal gyrus, while processing fearful or angry faces (Fonzo et al. 2013). It is important that these preliminary findings are further investigated in adolescents without secondary trauma to better understand the effect of maltreatment on brain networks in addition to isolated regions.
The aim of the current study was to investigate for the first time, the effect of child abuse on functional activation and connectivity in adolescents during processing of dynamic emotional facial expressions. The current study aimed to disentangle maltreatment effects from those due to psychiatric conditions by including a psychiatric control group matched with the abused group on psychiatric diagnosis. Furthermore, only medication-naive, drug abuse-free participants were included and whole-brain analyses were used. For this purpose, 20 adolescents who had experienced physical abuse, 20 psychiatric controls matched to the abused group by psychiatric diagnoses but with no abuse and 27 healthy controls were assessed during a dynamic fMRI emotion discrimination task. Based on behavioural findings of a bias towards negative emotions (Pollak et al. 2000; Pollak & Sinha, 2002; Pollak & Tolley-Schell, 2003) and on previous fMRI findings in neglected/institutionalized children (Maheu et al. 2010; Tottenham et al. 2011) and individuals with mixed maltreatment histories (Taylor et al. 2006; Dannlowski et al. 2012, 2013; Fonzo et al. 2013; McCrory et al. 2013), we hypothesized that maltreated individuals would demonstrate an enhanced response and show altered functional activation and connectivity of fronto-limbic networks compared to both healthy and psychiatric controls when processing negative emotions, particularly anger and fear.
Method
Participants
Seventy right-handed adolescents aged 12–20 participated (Table 1). Twenty-three maltreated participants were recruited through Kids Company (http://www.kidsco.org.uk/), Child and Adolescent Mental Health Services (CAMHS) and advertisements. Three were excluded due to motion, resulting in 20 participants. Maltreated participants experienced severe physical abuse prior to age 12, as defined by scores of ⩾13 on the physical abuse subscale of the Childhood Trauma Questionnaire (CTQ; Bernstein & Fink, 1998). Participants undertook the Childhood Experience of Care and Abuse (CECA) Interview (Bifulco et al. 1994) to ascertain detailed maltreatment histories and information was corroborated (with consent) from social services. See Supplementary Table S1A for details of onset and duration of abuse. Physically abused participants frequently had also experienced concurrent emotional abuse, emotional neglect or physical neglect (Table 1). They were diagnosed by an experienced child psychiatrist (K.A.H.M.) using the Development and Well Being Assessment (DAWBA; Goodman et al. 2000).
Table 1.
Demographic, clinical and performance data for 20 maltreated adolescents, 20 psychiatric control adolescents and 27 healthy control adolescents
| Childhood maltreatment | Psychiatric controls | Healthy controls | |
|---|---|---|---|
| Age, years, mean (s.d.) | 17.5 (2.4) | 16.8 (2.6) | 17.5 (1.6) |
| IQ, mean (s.d.) | 89.1 (12.3) | 94.5 (13.2) | 105.4 (10.1) |
| Gender, % female | 30.0 | 50.0 | 29.6 |
| % Caucasian | 50.0 | 15.0 | 48.2 |
| % Afro-Caribbean | 40.0 | 55.0 | 44.4 |
| % Mixed/other ethnicity | 10.0 | 30.0 | 7.4 |
| % No psychiatric condition | 10.0 | 0.0 | 100.0 |
| % PTSD | 35.0 | 35.0 | 0.0 |
| % PTSD with MDD | 10.0 | 10.0 | 0.0 |
| % PTSD with GAD and MDD | 10.0 | 15.0 | 0.0 |
| % PTSD with OCD | 5.0 | 0.0 | 0.0 |
| % PTSD with SP | 0.0 | 5.0 | 0.0 |
| % CD | 10.0 | 10.0 | 0.0 |
| % ODD | 10.0 | 10.0 | 0.0 |
| % Specific phobia with GAD and MDD | 5.0 | 0.0 | 0.0 |
| % PD with agoraphobia | 5.0 | 0.0 | 0.0 |
| % GAD with SP | 0.0 | 5.0 | 0.0 |
| % GAD | 0.0 | 5.0 | 0.0 |
| % PD without agoraphobia | 0.0 | 5.0 | 0.0 |
| CTQ score, mean (s.d.) | |||
| Physical abuse | 21.1 (5.0) | 6.1 (1.6) | 6.2 (3.4) |
| Sexual abuse | 5.2 (0.7) | 5.9 (2.0) | 5.1 (0.4) |
| Emotional abuse | 18.3 (4.2) | 7.1 (1.8) | 6.5 (2.6) |
| Emotional neglect | 18.5 (4.0) | 8.9 (3.8) | 8.2 (3.7) |
| Physical neglect | 14.0 (5.1) | 6.8 (2.2) | 6.0 (2.4) |
| SES score, mean (s.d.) | 2.8 (0.7) | 2.9 (0.7) | 3.2 (0.8) |
| Mean reaction time, ms (s.d.) | |||
| Neutral | 610.9 (106.3) | 570.3 (84.2) | 661.9 (122.5) |
| Happy | 586.3 (89.9) | 578.2 (102.8) | 623.8 (104.2) |
| Sad | 653.1 (111.3) | 623.7 (84.5) | 716.0 (130.0) |
| Anger | 655.5 (108.1) | 632.4 (111.7) | 719.3 (119.1) |
| Fear | 657.1 (133.0) | 684.7 (152.1) | 738.5 (121.6) |
| Mean variability, ms (s.d.) | |||
| Neutral | 281.0 (40.0) | 260.9 (51.2) | 230.2 (32.0) |
| Happy | 229.1 (60.8) | 227.8 (52.0) | 193.7 (63.1) |
| Sad | 304.3 (52.8) | 269.1 (64.7) | 217.9 (60.3) |
| Anger | 283.1 (65.7) | 254.8 (49.9) | 213.2 (46.3) |
| Fear | 277.7 (59.0) | 235.9 (63.3) | 221.6 (48.5) |
| Mean % errors (s.d.) | |||
| Neutral | 16.7 (22.8) | 14.8 (14.2) | 10.4 (12.6) |
| Happy | 7.2 (14.2) | 11.8 (17.8) | 3.1 (5.9) |
| Sad | 18.2 (20.2) | 17.5 (22.0) | 10.5 (11.2) |
| Anger | 26.3 (25.9) | 16.5 (14.7) | 16.8 (22.6) |
| Fear | 19.5 (18.3) | 26.7 (29.4) | 17.5 (24.0) |
CD, Conduct disorder; GAD, generalized anxiety disorder; MDD, major depressive disorder; ODD, oppositional defiant disorder; PD, panic disorder; PTSD, post-traumatic stress disorder; s.d., standard deviation; SP, social phobia.
Twenty psychiatric controls were recruited through Kids Company, CAMHS and advertisements. They had experienced no maltreatment (CTQ subscale scores of ⩽7 for physical abuse, ⩽8 for emotional abuse, ⩽6 for sexual abuse, ⩽9 for emotional neglect and ⩽7 for physical neglect). Diagnoses were again made using the DAWBA and matched as closely as possible one-to-one with maltreated participants (Table 1). Where psychiatric controls had PTSD, causal trauma(s) were unrelated to childhood maltreatment and included bullying, living in war-time Afghanistan, witnessing murder, car accidents and death of a loved one. See Supplementary Table S1B for details of onset and duration of trauma for the 13 participants with PTSD diagnoses.
Twenty seven healthy controls were recruited from advertisements, had experienced no maltreatment and had no psychiatric diagnoses. Both control groups were matched as closely as possible to the maltreated group in ethnicity, though for psychiatric controls matching psychiatric condition was the priority.
In addition to the CTQ and DAWBA, all participants underwent the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) to assess IQ. Socioeconomic status (SES) was measured by two items from the Family Affluence Scale (FAS; Currie et al. 2008). A 10 panel T-cup urine test (http://www.testfield.co.uk) was used to test for substance abuse and participants who tested positive for any of the 10 substances were excluded (resulting in exclusion of three maltreated, one psychiatric control and one healthy control). Other exclusion criteria were left-handedness, IQ < 70, current psychoactive medication, sexual abuse (⩾6 on sexual abuse CTQ subscale), neurological disorder, major head injuries, drug and alcohol abuse, literacy problems, learning disability, psychotic illness, bipolar disorder, schizophrenia, current suicidal behaviour or general MRI contraindications. Participants received £40 as compensation for time and travel. The National Research Ethics Service approved the study and informed consent was obtained from all participants and, if below 18 years old, consent was also obtained from parents or guardians.
fMRI emotion discrimination task
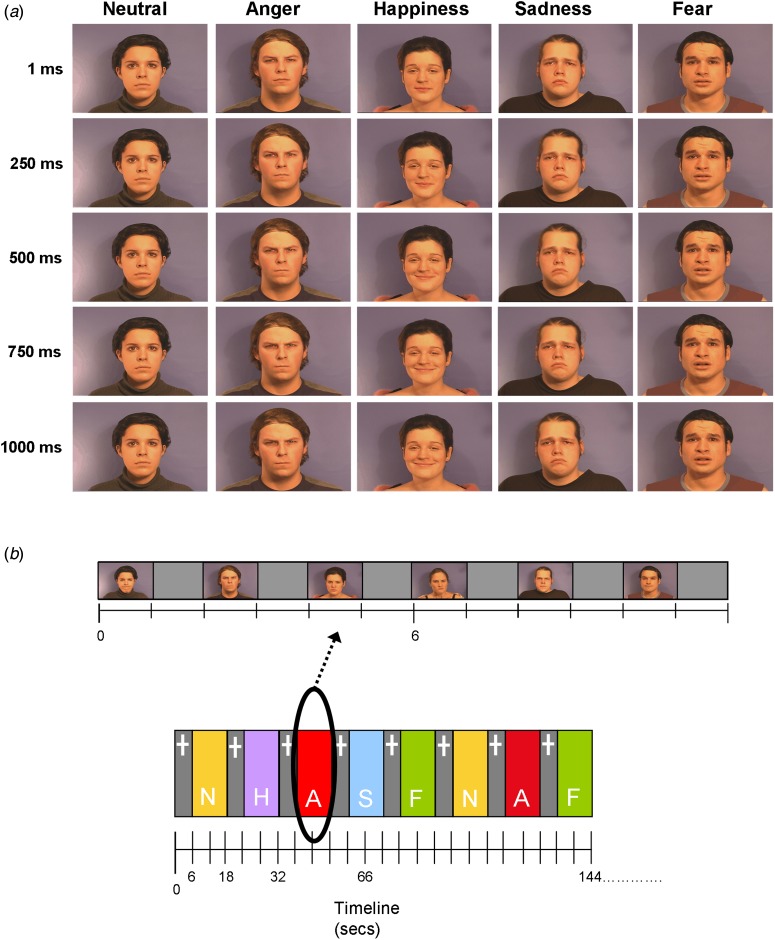
Participants practiced the 8-min block design fMRI Emotion Discrimination task, which measures the ability to categorize dynamic facial expressions of emotions, once prior to scanning. Participants were shown 1-s video clips of six actors (three males) displaying neutral, fearful, angry, sad or happy expressions (Fig. 1). Clips were taken from a validated set of stimuli (Simon et al. 2008) and cut backward from the peak of the expression to avoid different lengths and variability of exposure. Blocks of stimuli (12 s) of each emotion were interspersed with a fixation cross baseline condition (6 s). Each emotion was presented in a block of 6 × 1-s stimuli with each stimuli followed by a 1-s gap. Each emotion block was repeated five times in a pseudo-random order and neutral was repeated six times. Participants were instructed to identify each clip as positive, neutral or negative by immediately pressing one of three buttons with the right index, middle and ring fingers, respectively.
Fig. 1.
(a) Examples of actors expressing the five emotions: neutral, anger, happiness, sadness and fear. Five time points in the clip (1, 250, 500, 750, 1000 ms) are displayed. (b) Showing (top row) an example emotion block (angry) and (bottom row) the block structure of the task comprising 6-s fixation cross blocks (+) interspersed with 12-s emotion blocks (A, angry; F, fear; H, happy; N, neutral; S, sad).
Performance data analysis
To test the hypothesis of enhanced responses to negative emotions in maltreated participants, t tests were carried out to identify group differences between each emotion in percentage errors, reaction times and response time variability. Errors were divided into negative (neutral/happy perceived as negative), positive (neutral/sad/anger/fear perceived as positive) and omission errors (no response) and t tests were carried out. Correlation analyses were carried out between participant age and performance variables, in order to investigate the effect of age on task performance.
fMRI data acquisition and analysis
Data acquisition, pre-processing and first-level analysis are included in the Supplementary material. For second-level, contrast images from the first-level were used to conduct full factorial whole-brain analyses comparing activation across the three participant groups for each emotion contrasted with fixation and all negative emotions contrasted with happy. Age was entered into the analysis as a covariate as, although there were no significant group differences in age, the sample spanned a relatively wide age range. A separate exploratory analysis was also carried out which included percentage error, reaction time and response time variability as covariates. BOLD responses are reported using a cluster threshold of p < 0.05 family-wise error rate (FWER) corrected. Given the limited studies testing brain function differences in maltreated populations, and to control for the false positive rate (using p < 0.05 FWER-corrected cluster statistics) while limiting potential type II errors, we chose an a priori cluster-forming threshold of p < 0.01 for significant between-group differences.
MarsBar (http://marsbar.sourceforge.net/) was used to extract beta values from an 8-mm radius sphere around the peak of activation that differed between groups in order to carry out correlation analyses between neural activation and reaction time, percentage errors, response time variability (Table 1) and participant age for all participants. Additionally correlation analyses were carried out between peak beta values and maltreatment onset, duration (Supplementary Table S1A) and total CTQ scores in the maltreated group and between beta values and trauma onset and duration for psychiatric controls with PTSD (Supplementary Table S2).
Functional connectivity analysis
As effects for fear processing were identified in the main analysis, a post-hoc functional connectivity analysis was carried out for the fear condition only. To assess functional connectivity differences between groups, a generalized psychophysiological interaction (gPPI) analysis was conducted using SPM8. A seed region in the left vmPFC was selected based on the peak of group activation differences for fear. For each participant, an average time-course was extracted from an 8-mm sphere around individual local maximum coordinates closest to the group difference peak (−6, 52, 8). Individual design matrices were computed with three regressors for each emotion, one representing the vmPFC time-course, one representing the time-dependent change as a psychological variable of interest and the third representing the element-by-element product of the previous two (the PPI term). Contrast images were generated for each subject contrasting the fear PPI term with the happy PPI term and entered into a group-level analysis. With this implementation of the gPPI analysis, significant SPM activations of a particular area would reflect changes in functional connectivity for fear processing between the left vmPFC and activated regions. Significant clusters were identified with a threshold of p < 0.001 uncorrected with at least 10 contiguous voxels in the cluster. The combination of height-extent thresholds effectively yields equivalent correction for multiple comparisons (Forman et al. 1995).
Results
Demographic and clinical data
Pearson's χ² tests showed no significant group differences for gender (χ2 = 2.494, p = 0.287) nor ethnicity (χ2 = 11.008, p = 0.088). One-way analyses of variance (ANOVAs) showed no significant group differences for age (F2,64 = 0.936, p = 0.397) nor SES (F2,64 = 2.530, p = 0.091) but for IQ (F2,64 = 11.817, p < 0.001) which is typical in this population (Carrey et al. 1995; De Bellis et al. 2009) (Table 1). Post-hoc t tests revealed that IQ was higher for healthy controls relative to maltreated (p < 0.001) and psychiatric control (p < 0.05) groups, who did not differ from each other (p = 0.735).
Performance data
The maltreated group responded faster than healthy controls for fear (p < 0.05) and psychiatric controls responded faster than healthy controls for neutral (p < 0.01), sad (p < 0.05) and anger (p < 0.05). Maltreated and psychiatric groups had higher response time variability than healthy controls for all emotions (p < 0.05), but did not differ from each other except for fear where maltreated subjects had higher response time variability (p < 0.01). When grouped by psychiatric diagnosis rather than maltreatment and compared with those with no diagnoses, PTSD patients had higher response time variability for neutral (p < 0.05), sad (p < 0.001) and anger (p < 0.05); individuals with disruptive disorder diagnoses (conduct disorder/oppositional defiant disorder) had higher response time variability for neutral (p < 0.001) and sad (p < 0.05) and individuals with other anxiety disorders (generalized anxiety disorder, panic disorder, phobias) demonstrated higher response time variability for neutral (p < 0.001), anger (p < 0.001) and fear (p < 0.05). Individuals with other anxiety disorders had higher response time variability than those with PTSD for neutral and angry (<0.05). There were no significant differences in response time variability between participants with a disruptive disorder and those with PTSD. For neutral errors, t tests showed significant differences between maltreated and psychiatric groups (p < 0.05) and a trend for differences between maltreated and healthy controls (p = 0.071) where maltreated participants made more negative errors (Supplementary Table S2). No significant correlations were found between participant age and reaction time (r = 0.104, p = 0.400), percentage errors (r = 0.228, p = 0.064) nor response time variability (r = 0.083, p = 0.502).
Motion
MANOVAs showed no significant group effects in the extent of 3-dimensional motion as measured by maximum displacement for x, y, and z axes (F6,124 = 1.043, p = 0.401).
Activations and group differences for emotion conditions
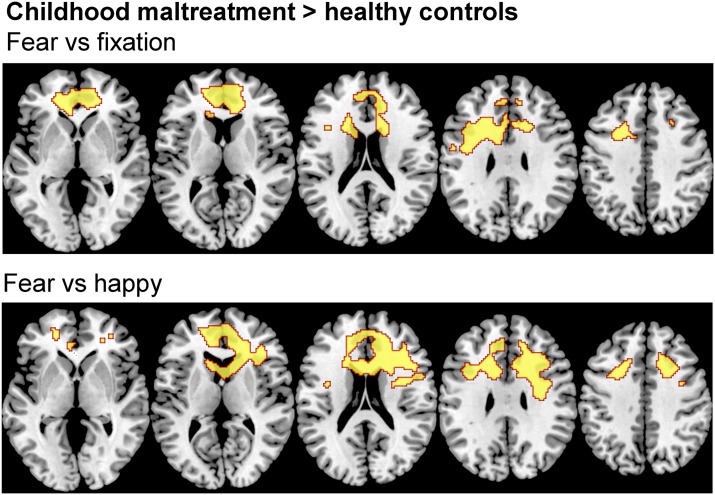
Within-group activations for all emotions v. fixation are shown in Supplementary Fig. S1. Three-group ANOVAs revealed no effect of group for angry, sad or happy v. fixation but revealed significant group effects for fear v. fixation in bilateral vmPFC and ACC (F2,64 = 8.890; p < 0.05 FWER corrected). Post-hoc comparisons showed that maltreated adolescents relative to healthy controls had increased activation in a large bilateral cluster in vmPFC and ACC reaching subcortically into the caudate (Table 2, Fig. 2). Maltreated and psychiatric control groups did not differ at this threshold. To explore potential differences between these two groups, a more lenient threshold of p < 0.005 uncorrected was used, revealing small bilateral clusters of increased activation in occipital cortex, vmPFC and ACC (Supplementary Fig. S2).
Table 2.
Differences in activation between physically maltreated adolescents, psychiatric control adolescents and healthy control adolescents for fear v. fixation and fear v. happy
| Emotion contrast | Subject contrast | Brain regions of activation | Brodmann's area | Cluster level | Peak MNI coordinates | Voxel level Z value |
|
|---|---|---|---|---|---|---|---|
| No. of Voxels | p (corr.) | ||||||
| Fear v. fixation | CM > HC | B MFG, ACC, SFG, IFG, preCG, caudate body | 6/8/9/10/24/32/47 | 4615 | 0.002 | −6, 52, 8 | 3.93 |
| −34, 6, 30 | 3.61 | ||||||
| 12, 52, 8 | 3.6 | ||||||
| Fear v. happy | CM > HC | B ACC, MFG, SFG, IFG, preCG, caudate body | 6/8/9/10/24/32/47 | 7541 | <0.001 | 10, 18, 22 | 3.86 |
| 18, 30, 20 | 3.82 | ||||||
| 32, 24, 22 | 3.78 | ||||||
ACC, Anterior cingulate cortex; B, Bilateral; CM, childhood maltreatment; HC, healthy controls; IFG, inferior frontal gyrus; MFG, medial frontal gyrus; MNI, Montreal Neurological Institute; preCG, precentral gyrus; SFG, superior frontal gyrus.
p value is <0.05 FWER corrected.
Fig. 2.
Between-group differences in brain activation for whole-brain analyses of fear v. happy and fear v. fixation contrasts. Thresholds were p < 0.05 family-wise error rate-corrected. Z coordinates represent distance from the anterior-posterior commissure in millimetres. The right side of the image corresponds to the right side of the brain.
No significant group effects were observed for angry or sad v. happy but there were for fear v. happy in a cluster comprising bilateral ACC and vmPFC reaching into the caudate (F2,64 = 8.821, p < 0.05 FWER corrected). Post-hoc comparisons showed that maltreated adolescents relative to healthy controls had increased activation in this cluster (Table 2, Fig. 2). Maltreated and psychiatric groups did not differ. At an exploratory p < 0.005 uncorrected threshold, maltreated adolescents demonstrated increased activation of bilateral ACC and right vmPFC compared to psychiatric controls (Supplementary Fig. S2).
A boxplot of beta values for each group from an 8-mm sphere around the peak for group activation differences for fear v. fixation in left vmPFC (see Supplementary Fig. S3) shows that, generally, the spread of the psychiatric controls lay somewhere in between that of the maltreated and healthy controls. An exploratory analysis was carried out including performance variables as covariates in the analysis. All main findings remained.
Functional connectivity
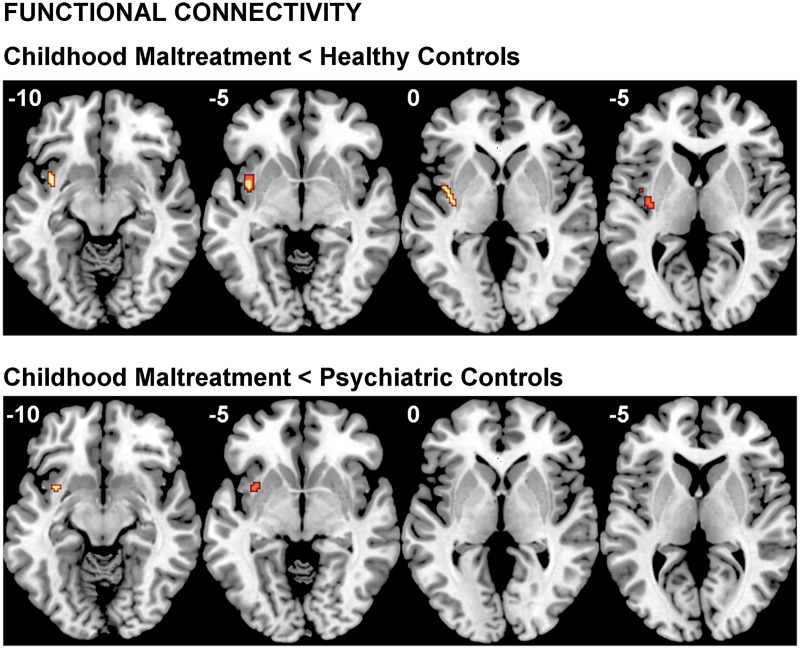
A significant group effect for connectivity was revealed between left vmPFC and left insula during fear v. happy (F2,64 = 8.474; p < 0.001). Post-hoc comparisons showed that maltreated adolescents relative to healthy controls had reduced connectivity between left vmPFC and a cluster in left insula and claustrum and relative to psychiatric controls in a smaller cluster in left insula (see Table 3 and Fig. 3).
Table 3.
Differences in functional connectivity with seed region in left vmPFC between physically maltreated adolescents, psychiatric control adolescents and healthy control adolescents for fear v. happy
| Emotion contrast | Subject contrast | Brain regions of altered connectivity with L vmPFC | No. of voxels | Peak MNI coordinates | p | Z |
|---|---|---|---|---|---|---|
| Fear v. happy | CM < HC | L insula/claustrum | 88 | −38, 4, −8 | <0.001 | 3.67 |
| −36, −10, 2 | <0.001 | 3.41 | ||||
| −40, −2, 2 | 0.001 | 3.18 | ||||
| CM < PC | L insula | 24 | −34, 8, −8 | 0.001 | 3.44 |
vmPFC, Ventromedial prefrontal cortex; MNI, Montreal Neurological Institute; CM, childhood maltreatment; HC, healthy controls; L, left;
Threshold is p < 0.001 uncorrected with a cluster extent of >10.
Fig. 3.
Functional connectivity group differences between the seed region of the left ventromedial prefrontal cortex and the whole brain for the fear v. happy and contrast. The threshold is p < 0.001 uncorrected with an extent threshold of 10 voxels. Z coordinates represent distance from the anterior-posterior commissure in millimetres. The right side of the image corresponds to the right side of the brain.
Correlations
No significant correlations were found between peak beta values and reaction time (r = 0.145, p = 0.242), percentage errors (r = 0.155, p = 0.211), response time variability (r = 0.019, p = 0.878), participant age (r = 0.064, p = 0.604), onset of maltreatment (r = 0.204, p = 0.388) or trauma (r = 0.056, p = 0.849), duration of maltreatment (r = 0.342, p = 0.140) or trauma (r = 0.086, p = 0.769) nor CTQ scores (r = 0.042, p = 0.861).
Discussion
This is the first whole-brain fMRI investigation of the effect of child abuse on functional activation and connectivity during dynamic emotion processing in adolescents. The study demonstrates that maltreated adolescents exhibited altered fear processing at behavioural and neurofunctional levels. Behaviourally, maltreated participants responded faster to fear than healthy controls. Neurofunctionally, relative to healthy controls, maltreated adolescents had increased activation in bilateral vmPFC and ACC for fear, but no differences for other emotions. They also demonstrated, albeit at a more lenient threshold, hyperactivation of ventromedial fronto-cingulate regions relative to psychiatric controls, signifying that the vmPFC/ACC effect may possibly be maltreatment-specific to a degree. Furthermore, maltreated individuals had reduced functional connectivity between left vmPFC and insula relative to both control groups, suggesting that the reduced connectivity is due to maltreatment and not associated psychopathologies Enhanced fear perception in maltreated individuals seems, therefore, to be associated with enhanced fear-related ventromedial fronto-cingulate activation and altered functional connectivity with associated limbic regions.
The behavioural hypersensitivity to fear in maltreated adolescents is consistent with previously reported negative emotion response biases (Pollak et al. 2000; Pollak & Sinha, 2002). The finding of hyperactivity and altered connectivity of fear processing networks is novel and extends previous findings of altered fronto-limbic activity for fear in neglected children (Maheu et al. 2010; Tottenham et al. 2011) and adults with mixed maltreatment (Taylor et al. 2006; Dannlowski et al. 2012; Fonzo et al. 2013) to physically and emotionally abused adolescents. We speculate that maltreated individuals respond faster to dynamic fearful expressions and show increased vmPFC/ACC activation and altered vmPFC-insular connectivity because they have experienced fear more frequently and are therefore better able to recognise fear quickly. Enhanced vmPFC/ACC activation in maltreatment in response to fear is consistent with the concept that these regions play key roles in fear processing, appraising negative emotions and regulating emotional responses via the limbic system (Phelps et al. 2004; Milad et al. 2007; Hansel & von Kanel, 2008; Etkin et al. 2011). The effect of childhood maltreatment on the vmPFC and ACC may reflect the fact that they are late developing, undergoing structural and functional maturation late into childhood and adolescence (Marsh et al. 2008; Velanova et al. 2008; Rubia et al. 2013).
Increased vmPFC/ACC activation may represent a fear regulation deficit. vmPFC and ACC are closely interconnected to limbic structures, particularly the amygdala (Amaral et al. 1992; Ghashghaei et al. 2007) and insula (Morris et al. 1999; Schienle et al. 2002; Sehlmeyer et al. 2009), which are both crucial for fear processing (Davis & Whalen, 2001) and top-down control of emotions (Ochsner & Gross, 2005; Etkin et al. 2011). The effect was much weaker when compared to psychiatric controls, which may be explained by the fact that many individuals in the psychiatric control group (the 65% with PTSD) had also experienced traumatic events, and hence may also have some degree of fear regulation deficit. Indeed, a boxplot of peak beta values for each group shows that, generally, the spread of the psychiatric controls lay between that of the maltreated and healthy controls (Supplementary Fig. S3).
Reduced top-down control over emotions is supported by the finding of diminished functional connectivity between vmPFC and insula, extending findings to the network level suggesting alteration in fear processing networks as well as regions. As mentioned above, the insula is implicated in fear processing and it is thought to convey cortical representations of fear to the amygdala (Phelps et al. 2001). Diminished connectivity between vmPFC and insula in maltreatment may contribute to the observed hyperactivity in ventromedial frontal regions. For example, decreased vmPFC-insula connectivity could result in weakened top-down control of vmPFC over the insula leading to a fear regulation deficit and increased fear sensitivity. Reduced functional connectivity parallels structural connectivity findings in maltreated individuals of reduced white matter tract density in the left uncinate fasciculus, which connects prefrontal with limbic regions, including amygdala and insula (Eluvathingal et al. 2006) and in the cingulum bundle, which connects limbic structures, including the insula, with cortical regions including the cingulate gyrus (Choi et al. 2009). The uncinate fasciculus and cingulum bundle both undergo development changes well into late childhood and adolescence (Lebel et al. 2008) and the insula is known to be involved in regulating glucocorticoids, which play a pivotal role in the stress response (Fornari et al. 2012).
Unexpectedly, we observed no group differences in the amygdala for fear as has been previously reported for neglected and institutionalized children (Maheu et al. 2010; Tottenham et al. 2011) and adults with mixed maltreatment (Taylor et al. 2006; Dannlowski et al. 2012). One reason could be that almost all studies that have reported functional changes in the amygdala specify the amygdala as the main, or only, ROI. Another reason could be maltreatment type differences as the neglected/institutionalized children in Maheu and Tottenham et al.’s studies did not have documented histories of physical maltreatment and the adults in Taylor et al.’s and Dannlowski et al.’s studies only minimal physical maltreatment. In fact, the results of the current study most resemble those from a study of women with intimate partner violence and mixed childhood maltreatment histories, including physical maltreatment (Fonzo et al. 2013). Fonzo and colleagues used whole-brain and limbic ROI approaches and reported that childhood maltreatment severity was correlated with ACC and insula activation and limbic-prefrontal connectivity while processing fear. An alternative explanation could be timing. The hippocampus is fully developed by age 2, amygdala volume peaks at around age 10, but the PFC matures relatively late in life with progressive volumetric changes into adulthood and a sharp growth between 8 and 14 years of age (Lupien et al. 2009). Because of its protracted development, the PFC is conceivably more vulnerable to environmental stressors such as child abuse than are the limbic structures.
Also surprising was lack of correlation between blood oxygen-level dependent (BOLD) beta values and abuse onset, duration and severity (CTQ scores). Previous work has shown relationships between CTQ scores and BOLD signal during face processing (Edmiston & Blackford, 2013). A possible explanation is that the current study only tested potential relationships between BOLD signal and maltreatment measures for the maltreated group who had all experienced severe childhood abuse, whereas Edmiston & Blackford studied a group of young adults with an inhibited temperament with larger variability in degrees of maltreatment, which might be better suited to finding such correlations.
The perception of neutral expressions as negative by maltreated individuals may stem from hyper-vigilance to negative emotions as has been reported in depression (Oliveira et al. 2013; Maniglio et al. 2014) and social anxiety (Cooney et al. 2006). Many of the maltreated group were diagnosed with depression and anxiety so it is unsurprising that they have a tendency to misattribute neutral faces as negative but it is difficult to rationalise the fact that this phenomenon was not observed in the psychiatric controls, who had extremely similar depression and anxiety diagnoses. The current study reported higher response time variability for both maltreated and psychiatric control groups, relative to healthy controls. As this was present for both maltreated and psychiatric groups it is possible that this was related to specific psychiatric condition(s). However, no clear relationship was evident between response time variability and diagnosis.
Among the strengths of this study are that all participants were medication-naive and drug-free, and their abuse experience was carefully assessed and corroborated by social services. Also, we included a psychiatric control group to determine the specificity of maltreatment in our findings. The whole-brain approach ensured that effects outside the expected ROIs were not missed. Finally, the use of dynamic stimuli which more accurately mimic the way in which emotional expressions are observed in everyday life is a novelty of the study.
Limitations include that we cannot categorically state that effects are a result of exclusively physical abuse as many participants also experienced neglect and emotional abuse. However, separating physical from emotional abuse and neglect is unrealistic as the vast majority of maltreated children are subjected to more than one abuse type, with less than 5% occurring in isolation (Ney et al. 1994). Ideally we would like to have investigated specific effects of emotional and physical abuse on neural responses but unfortunately physical and emotional abuse were highly correlated in the sample (r = 0.922, p < 0.001). IQ was not matched between groups which could be considered a limitation. However, since lower IQ is associated with childhood maltreatment (Carrey et al. 1995; De Bellis et al. 2009), artificially matching groups on IQ is inappropriate as it creates unrepresentative groups and it is misguided to covary for a pre-existing group difference as this would lead to potentially spurious results (Miller & Chapman, 2001; Dennis et al. 2009). Another limitation is the inclusion of mixed genders as maltreatment may affect the genders differently (Cooke & Weathington, 2014). Finally, for nine out of 20 participants, maltreatment continued beyond age 12 (Supplementary Table S1A). The broad range of developmental ages at which the maltreatment ended could have affected the neurodevelopmental changes observed.
Conclusion
Childhood abuse is associated with faster fear processing, which was concomitant with elevated activation of vmPFC and ACC and decreased connectivity between vmPFC and insula. Findings suggest that maltreatment leads to behavioural and neurofunctional sensitisation to fearful expressions through altered activation and connectivity of fear processing networks. These alterations could have negative consequences for socio-emotional interaction and this knowledge may help develop new interventions to address social information errors.
Acknowledgements
The research was supported by Kids Company and the Reta Lila Weston Trust for Medical Research. H.H. was supported by Kids Company, the Reta Lila Weston Trust for Medical Research and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. L.L. was supported by the National Medical Research Council (Singapore), Kids Company and the Reta Lila Weston Trust for Medical Research. A.S. and K.R. have received support from the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London.
The authors are grateful to all the individuals and their families who participated in this study. They also thank Dr Kaylita Chantiluke and Ms. Sinead King for their assistance with data collection, staff at Kids Company for assistance with recruitment of participants and Professor Robert Goodman for supervision and advising on DAWBA diagnoses.
Declaration of Interest
K.R. has received speaker's honoraria from Lilly and Shire and received a grant from Lilly for another project. M.M. has acted as a consultant for Cambridge Cognition, Lundbeck and Quintiles and has received fees from Shire for contribution towards education. K.M. has received research and educational grants from GlaxoSmithKline and Shire Pharmaceuticals and has served on the advisory boards of Janssen, Eli Lily and Shire Pharmaceuticals. K.M. has also received honoraria for speaking at conferences organized by Janssen, Eli Lilly and Shire pharmaceuticals.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291716003585.
click here to view supplementary material
References
- Ackerman PT, Newton JE, McPherson WB, Jones JG and Dykman RA (1998) Prevalence of post-traumatic stress disorder and other psychiatric diagnoses in three groups of abused children (sexual, physical, and both). Child Abuse & Neglect 22, 759–774. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A and Carmichael ST (1992) Anatomical organization of the primate amygdaloid complex. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction 1–66. [Google Scholar]
- Bernstein D and Fink L (1998) Childhood Trauma Questionnaire: A Retrospective Self-Report. Manual. San Antonio, TX: The Psychological Cooperation. [Google Scholar]
- Bifulco A, Brown GW and Harris TO (1994) Childhood Experience of Care and Abuse (CECA): a retrospective interview measure. Journal of Child Psychology and Psychiatry 35, 1419–1435. [DOI] [PubMed] [Google Scholar]
- Brown J, Cohen P, Johnson JG and Salzinger S (1998) A longitudinal analysis of risk factors for child maltreatment: findings of a 17-year prospective study of officially recorded and self-reported child abuse and neglect. Child Abuse & Neglect 22, 1065–1078. [DOI] [PubMed] [Google Scholar]
- Bugental DB, Blue J and Lewis J (1990) Caregiver beliefs and dysphoric affect directed to difficult children. Developmental Psychology 26, 631. [Google Scholar]
- Carrey NJ, Butter HJ, Persinger MA and Bialik RJ (1995) Physiological and cognitive correlates of child abuse. Journal of the American Academy of Child & Adolescent Psychiatry 34, 1067–1075. [DOI] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM and Teicher MH (2009) Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry 65, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D and Curtis W (2005) An event-related potential study of the processing of affective facial expressions in young children who experienced maltreatment during the first year of life. Development and Psychopathology 17, 641–677. [DOI] [PubMed] [Google Scholar]
- Cooke BM and Weathington JM (2014) Human and animal research into sex-specific effects of child abuse. Hormones and Behavior 65, 416–426. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugène F and Gotlib IH (2006) Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Research: Neuroimaging 148, 55–59. [DOI] [PubMed] [Google Scholar]
- Currie C, Molcho M, Boyce W, Holstein B, Torsheim T and Richter M (2008) Researching health inequalities in adolescents: the development of the Health Behaviour in School-Aged Children (HBSC) Family Affluence Scale. Social Science & Medicine 66, 1429–1436. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D et al. (2013) Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human Brain Mapping 34, 2899–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D et al. (2012) Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry 71, 286–293. [DOI] [PubMed] [Google Scholar]
- Davis M and Whalen PJ (2001) The amygdala: vigilance and emotion. Molecular Psychiatry 6, 13–34. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hooper SR, Spratt EG and Woolley DP (2009) Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. Journal of the International Neuropsychological Society 15, 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf-Peters VB and Hadders-Algra M (2006) Ontogeny of the human central nervous system: what is happening when? Early Human Development 82, 257–266. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA and Fletcher JM (2009) Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society 15, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston EK and Blackford JU (2013) Childhood maltreatment and response to novel face stimuli presented during functional magnetic resonance imaging in adults. Psychiatry Research: Neuroimaging 212, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards VJ, Holden GW, Felitti VJ and Anda RF (2003) Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. American Journal of Psychiatry 160, 1453–1460. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M et al. (2006) Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics 117, 2093–2100. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T and Kalisch R (2011) Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Flagan TM, Sullivan S, Allard CB, Grimes EM, Simmons AN et al. (2013) Neural functional and structural correlates of childhood maltreatment in women with intimate-partner violence-related posttraumatic stress disorder. Psychiatry Research: Neuroimaging 211, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA and Noll DC (1995) Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster size threshold. Magnetic Resonance in Medicine 33, 636–647. [DOI] [PubMed] [Google Scholar]
- Fornari RV, Wichmann R, Atucha E, Desprez T, Eggens-Meijer E and Roozendaal B (2012) Involvement of the insular cortex in regulating glucocorticoid effects on memory consolidation of inhibitory avoidance training. Memory and Motivational/Emotional Processes 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries AW and Pollak SD (2004) Emotion understanding in postinstitutionalized Eastern European children. Development and Psychopathology 16, 355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Rotshtein P, Geng JJ, Sterzer P and Henson RN (2006) A critique of functional localisers. Neuroimage 30, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H, Hilgetag C and Barbas H (2007) Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34, 905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Ford T, Richards H, Gatward R and Meltzer H (2000) The development and well-being assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. Journal of Child Psychology and Psychiatry 41, 645–655. [PubMed] [Google Scholar]
- Hansel A and von Kanel R (2008) The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? BioPsychoSocial Medicine 2, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H and Rubia K (2012) Neuroimaging of child abuse: a critical review. Frontiers in Human Neuroscience 6, Article 52, pp. 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim CM, Mayberg HS, Mletzko T, Nemeroff CB and Pruessner JC (2013) Decreased cortical representation of genital somatosensory field after childhood sexual abuse. American Journal of Psychiatry 170, 616–623. [DOI] [PubMed] [Google Scholar]
- Herrenkohl RC, Herrenkohl EC, Egolf BP, Wu P (1991). The developmental consequences of child abuse: the lehigh longitudinal study. The Effects of Child Abuse and Neglect: Issues and Research 57, 81. [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L and Beaulieu C (2008) Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40, 1044–1055. [DOI] [PubMed] [Google Scholar]
- Lim L, Radua J and Rubia K (2014) Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. American Journal of Psychiatry 171, 854–863. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR and Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience 10, 434–445. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K et al. (2010) A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective, & Behavioral Neuroscience 10, 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniglio R, Gusciglio F, Lofrese V, Murri MB, Tamburello A and Innamorati M (2014) Biased processing of neutral facial expressions is associated with depressive symptoms and suicide ideation in individuals at risk for major depression due to affective temperaments. Comprehensive Psychiatry 55, 518–525. [DOI] [PubMed] [Google Scholar]
- Marsh R, Gerber AJ and Peterson BS (2008) Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. Journal of the American Academy of Child & Adolescent Psychiatry 47, 1233–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA and Viding E (2011) The impact of childhood maltreatment: a review of neurobiological and genetic factors. Frontiers in Psychiatry 2, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, Samuel S and Viding E (2013) Amygdala activation in maltreated children during pre-attentive emotional processing. British Journal of Psychiatry 202, 269–276. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B and Rauch SL (2007) A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry 62, 1191–1194. [DOI] [PubMed] [Google Scholar]
- Miller G and Chapman J (2001) Misunderstanding analysis of covariance. Journal of Abnormal Psychology 110, 40–48. [DOI] [PubMed] [Google Scholar]
- Morris JS, Scott SK and Dolan RJ (1999) Saying it with feeling: neural responses to emotional vocalizations. Neuropsychologia 37, 1155–1163. [DOI] [PubMed] [Google Scholar]
- Mullen PE, Martin JL, Anderson JC, Romans SE and Herbison GP (1996) The long-term impact of the physical, emotional, and sexual abuse of children: a community study. Child Abuse & Neglect 20, 7–21. [DOI] [PubMed] [Google Scholar]
- Ney PG, Fung T and Wickett AR (1994) The worst combinations of child abuse and neglect. Child Abuse & Neglect 18, 705–714. [DOI] [PubMed] [Google Scholar]
- Ochsner KN and Gross JJ (2005) The cognitive control of emotion. Trends in Cognitive Sciences 9, 242–249. [DOI] [PubMed] [Google Scholar]
- Oliveira L, Ladouceur CD, Phillips ML, Brammer M and Mourao-Miranda J (2013) What does brain response to neutral faces tell us about major depression? Evidence from machine learning and fMRI. PLoS ONE 8, e60121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SW and Nelson CA (2005) The impact of early institutional rearing on the ability to discriminate facial expressions of emotion: an event-related potential study. Child Development 76, 54–72. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI and LeDoux JE (2004) Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43, 897–905. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C and Davis M (2001) Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience 4, 437–441. [DOI] [PubMed] [Google Scholar]
- Pine DS, Mogg K, Bradley BP, Montgomery L, Monk CS, McClure E et al. (2005) Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. American Journal of Psychiatry 162, 291–296. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K and Reed A (2000) Recognizing emotion in faces: developmental effects of child abuse and neglect. Developmental Psychology 36, 679–688. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Klorman R, Thatcher JE and Cicchetti D (2001) P3b reflects maltreated children's reactions to facial displays of emotion. Psychophysiology 38, 267–274. [PubMed] [Google Scholar]
- Pollak SD and Sinha P (2002) Effects of early experience on children's recognition of facial displays of emotion. Developmental Psychology 38, 784–791. [DOI] [PubMed] [Google Scholar]
- Pollak SD and Tolley-Schell SA (2003) Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology 112, 323–338. [DOI] [PubMed] [Google Scholar]
- Radford L, Corral S, Bradley C and Fisher HL (2013) The prevalence and impact of child maltreatment and other types of victimization in the UK: findings from a population survey of caregivers, children and young people and young adults. Child Abuse & Neglect 37, 801–813. [DOI] [PubMed] [Google Scholar]
- Rubia K, Lim L, Ecker C, Halari R, Giampietro V, Simmons A et al. (2013) Effects of age and gender on neural networks of motor response inhibition: from adolescence to mid-adulthood. Neuroimage 83, 690–703. [DOI] [PubMed] [Google Scholar]
- Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P et al. (2002) The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport 13, 2023–2026. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V et al. (2009) Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ONE 4, e5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman JE, Shackman AJ and Pollak SD (2007) Physical abuse amplifies attention to threat and increases anxiety in children. Emotion 7, 838. [DOI] [PubMed] [Google Scholar]
- Simon D, Craig KD, Gosselin F, Belin P and Rainville P (2008) Recognition and discrimination of prototypical dynamic expressions of pain and emotions. Pain 135, 55–64. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL and Toga AW (2003) Mapping cortical change across the human life span. Nature Neuroscience 6, 309–315. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ and Lieberman MD (2006) Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry 60, 296–301. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP and Kim DM (2003) The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews 27, 33–44. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare T, Millner A, Gilhooly T, Zevin J and Casey B (2011) Elevated amygdala response to faces following early deprivation. Developmental Science 14, 190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett PK, Kim K and Prindle J (2011) Variations in emotional abuse experiences among multiply maltreated young adolescents and relations with developmental outcomes. Child Abuse & Neglect 35, 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett PK and McBride-Chang C (1995) The developmental impact of different forms of child abuse and neglect. Developmental Review 15, 311–337. [Google Scholar]
- Velanova K, Wheeler ME and Luna B (2008) Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex, 18, 2505–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorria P, Papaligoura Z, Sarafidou J, Kopakaki M, Dunn J, Van IJzendoorn MH et al. (2006) The development of adopted children after institutional care: a follow-up study. Journal of Child Psychology and Psychiatry 47, 1246–1253. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1999) Wechsler Abbreviated Scale of Intelligence. San Antonio, Texas: The Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291716003585.
click here to view supplementary material