Abstract
The availability of genome-wide genetic data on hundreds of thousands of people has led to an equally rapid growth in methodologies available to analyse these data. While the motivation for undertaking genome-wide association studies (GWAS) is identification of genetic markers associated with complex traits, once generated these data can be used for many other analyses. GWAS have demonstrated that complex traits exhibit a highly polygenic genetic architecture, often with shared genetic risk factors across traits. New methods to analyse data from GWAS are increasingly being used to address a diverse set of questions about the aetiology of complex traits and diseases, including psychiatric disorders. Here, we give an overview of some of these methods and present examples of how they have contributed to our understanding of psychiatric disorders. We consider: (i) estimation of the extent of genetic influence on traits, (ii) uncovering of shared genetic control between traits, (iii) predictions of genetic risk for individuals, (iv) uncovering of causal relationships between traits, (v) identifying causal single-nucleotide polymorphisms and genes or (vi) the detection of genetic heterogeneity. This classification helps organise the large number of recently developed methods, although some could be placed in more than one category. While some methods require GWAS data on individual people, others simply use GWAS summary statistics data, allowing novel well-powered analyses to be conducted at a low computational burden.
Key words: Genetics, methods, polygenic, review
Introduction
The reduction in costs of genotyping technologies in recent years has led to an explosion of genetic and phenotypic information collected on large numbers of people. The primary aim of these studies is to identify genetic polymorphisms associated with a quantitative trait or with an increased risk of disease. These genome-wide association studies (GWAS) have led to an important increase in understanding of the underpinnings of psychiatric and other disorders (Sullivan et al. 2012; Visscher et al. 2012; Gratten, 2016). They have provided empirical data demonstrating that common disorders are polygenic and have allowed interrogation of the genetic architecture, in terms of number, frequency, effect size and interactions of genetic risk factors. However, the potential use of GWAS data goes far beyond identification of trait associated loci.
Here, we present an overview of some recently developed methods utilising genome-wide genotype and phenotype data on large numbers of individuals and show how they can be applied to the research of psychiatric disorders. These new methods serve at least one of the following purposes: (i) estimation of the extent of genetic influence on traits (Estimation of proportion of variance attributable to genome-wide single-nucleotide polymorphisms (SNPs), SNP-heritability or h2SNP), (ii) uncovering of shared genetic control between traits (Estimation of genetic correlation from using genome-wide SNPs), (iii) predictions of genetic risk for individuals (Polygenic risk prediction), (iv) uncovering of causal relationships between traits [Mendelian randomisation (MR)], (v) identifying causal SNPs and genes (Fine-mapping and gene prioritisation), or (vi) Detection of genetic heterogeneity. There is often an overlap between these applications: some methods can be applied for more than one purpose, and some of the available software implements more than one method. In this overview, it is not possible to be exhaustive, and we apologise to authors whose methods have not been included.
Most of the methods presented here require genetic data in either of two formats. One is that of full individual level genotype data and phenotypic measurements on each person, where the genetic data can be represented as a matrix with allele counts for each genetic marker for each person. While this offers the largest range of analytic options, file sizes can be very large, which can become prohibitive as computational burden is usually non-linear with increasing numbers of individuals and markers. Moreover, privacy concerns can prevent this type of data from being shared across research groups. Summary statistics of genome-wide association analysis represent the second data format, for which data sharing has fewer privacy concerns (Pasaniuc & Price, 2016). GWAS summary statistics comprise the association test statistic (including direction of effect for a reference allele), standard error, p value of association and allele frequency of each SNP. While it has been shown that it is possible to infer whether an individual was part of a cohort using summary statistics, the power to do so is limited (Homer et al. 2008; Sankararaman et al. 2009; Visscher & Hill, 2009), and in any case requires the genome-wide genotype data of the individual to be identified. To guard against any privacy concerns GWAS summary statistics can be provided using allele frequencies estimated in large independent samples of the same ethnicity. Methods which require only summary statistic data benefit from shorter analytical run-times, much reduced computer memory requirements, and applicability to a larger number of traits. These benefits usually come at the cost of lower precision (larger standard errors), when compared with methods that utilise individual level genotype data.
While genetic data in one of these two formats are required by all methods presented here, some methods additionally make use of other information, such as genomic annotation or expression quantitative trait locus (eQTL) data. Genomic annotation can give clues about the functional importance of a region in which a SNP resides, whereas eQTL data are the result of an association test where the phenotype analysed is expression of a gene.
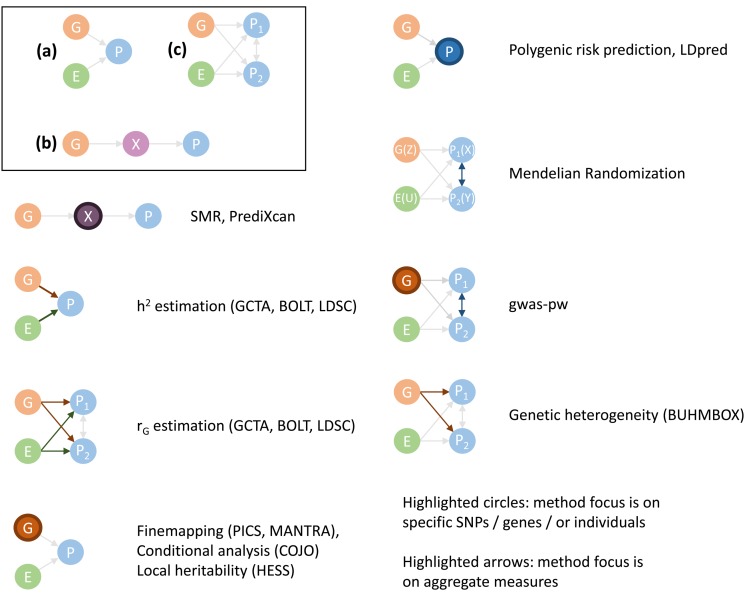
Here we review a range of different polygenic methods and highlight their aims and the input data they require (Fig. 1). For each method, we provide some examples of applications relevant to psychiatric genetics research (Table 1).
Fig. 1.
Schematic representation of the basic models underlying the polygenic methods reviewed. All models assume that a phenotype (P) is influenced by genetic (G) and environmental (E) factors (with environmental defined loosely as anything not captured by G including stochastic variation and measurement error). (a) Model which considers only one phenotype and no gene expression. (b) Model which considers only one phenotype and gene expression (X). (c) Model which considers two or more phenotypes and no gene expression. Methods can be grouped into those where the focus lies on individual SNPs, genes or people (nodes highlighted), and those where the focus lies on aggregate measures affecting the relationship between genetic and environmental factors and a phenotype (edges highlighted).
Table 1.
Overview of selected polygenic methods
| Program/method | Data needed | URL |
|---|---|---|
| Estimation of h2, rG | ||
| GCTA (GREML) | Individual-level genotype data | http://cnsgenomics.com/software/gcta/ |
| BOLT-REML/BOLT-LMM | Individual-level genotype data | https://data.broadinstitute.org/alkesgroup/BOLT-LMM/ |
| LD score regression | Summary statistics + LD scores | https://github.com/bulik/ldsc; http://ldsc.broadinstitute.org/ |
| HESS (local heritability) | Summary statistics + LD reference panel | http://bogdan.bioinformatics.ucla.edu/software/hess |
| Polygenic risk prediction | ||
| PLINK | Summary statistics + individual-level data | https://www.cog-genomics.org/plink2 |
| PRSice | Summary statistics + individual-level data | http://prsice.info/ |
| GCTA, MTG2 (GBLUP, MTGBLUP) | Individual-level genotype data | http://cnsgenomics.com/software/gcta/; https://sites.google.com/site/honglee0707/mtg2 |
| BayesR | Individual-level genotype data | https://github.com/syntheke/bayesR |
| LDpred | Summary statistics + individual-level data | https://github.com/bvilhjal/ldpred |
| Causality of phenotypes | ||
| Mendelian randomisation | Individual-level genotype data or summary statistics | http://www.mrbase.org/ |
| gwas-pw | Summary statistics | https://github.com/joepickrell/gwas-pw |
| Causality of genes (gene prioritisation) | ||
| gwas-pw | Summary statistics | https://github.com/joepickrell/gwas-pw |
| SMR | Summary statistics + eQTL | http://cnsgenomics.com/software/smr/ |
| PrediXcan | Individual-level genotype data + eQTL | https://github.com/hakyimlab/PrediXcan |
| metaXcan | Summary statistics + eQTL | https://github.com/hakyimlab/MetaXcan |
| TWAS/FUSION | Summary statistics + eQTL | http://gusevlab.org/projects/fusion/ |
| DEPICT | Summary statistics | https://data.broadinstitute.org/mpg/depict/ |
| Causality of SNPs (fine-mapping) | ||
| PICS (Fine-mapping) | Summary statistics | http://pubs.broadinstitute.org/pubs/finemapping/ |
| GCTA (COJO) | Summary statistics | http://cnsgenomics.com/software/gcta/ |
| MANTRA | Summary statistics | Available on request from the author |
| Detection of genetic heterogeneity | ||
| BUHMBOX | Summary statistics + individual-level data | http://software.broadinstitute.org/mpg/buhmbox/ |
| Subtest | Summary statistics | https://github.com/jamesliley/subtest |
Estimation of proportion of variance attributable to genome-wide SNPs
Heritability is the proportion of phenotypic variance that can be attributed to genetic factors. It is a key quantity in genetics research as it summarises the role of causal inherited variation. Trait heritability can be estimated by comparing the phenotypic resemblance among family members to their coefficients of relationships. However, estimating heritability from relatives can result in upwards-biased estimates (Vinkhuyzen et al. 2013) if non-genetic factors shared by relatives cannot be disentangled from the shared genetic relationships. This can be circumvented by estimating heritability from genome-wide markers in unrelated (that is, very distantly related) individuals. Genomic-restricted maximum-likelihood analysis (GREML) can be used to estimate the proportion of phenotypic variance, which is captured by genotyped SNPs (SNP-heritability), by using genetic data of unrelated individuals (Yang et al. 2010). SNP-heritability estimates are typically lower than those from twin or family-based heritability estimates, because genotyped SNPs account only for a subset of all genetic effects (the remainder includes other types of polymorphisms and SNPs that are not tagged by genotyped SNPs). Hence, the parameter estimated by SNP-heritability analysis depends on the genotype data available in the data set analysed, and can only converge to the traditional parameter being estimated from family data, when the genotypes available are fully representative of the variation in the genome.
Estimation of SNP-heritability has been of particular importance for disease traits, especially those of low lifetime risk (<1% is typical of most common diseases) for which it is difficult to collect the large samples needed to calculate heritability from estimates of increased risk in relatives of those affected. Both traditional-heritability and SNP-heritability estimates are presented on the liability scale (and depend on lifetime risk of disease in the population), and empirical data of the GWAS era (Lee et al. 2012a; Stahl et al. 2012) demonstrates that the polygenic model implied in these estimates is justified. GWAS case–control samples for disease traits are usually heavily oversampled for cases compared with a population sample and so SNP-heritability estimates are made on this binary case–control scale and transformed to the liability scale accounting for this ascertainment (Lee et al. 2011). The SNP-heritability estimates are relatively robust to choice of lifetime risk for most common diseases (lifetime risk <1%). However, for the very common diseases such as major depressive disorder (MDD) (lifetime risk 15%), SNP-heritability estimates are more sensitive to the choice of lifetime risk estimate, and to screening v. non-screening of controls (Peyrot et al. 2016).
The GREML method for estimation of SNP-heritability is based on a linear mixed model (Meuwissen et al. 2001), where a central component is the genetic relationship (or similarity) matrix, which captures the genetic relatedness of all pairs of individuals (Hayes et al. 2009). For application to human data, the algorithm is implemented in GCTA (Yang et al. 2011a). Several independent studies have confirmed that GREML results in unbiased estimates of SNP heritability when the model assumptions are met, or have investigated how departures from these assumptions can influence the results (Speed et al. 2012; Zhou & Stephens, 2014). Potential biases that might influence heritability estimation are an association between minor allele frequency (MAF) and SNP effect size, which does not fit the assumptions of the model, or an overrepresentation of causal SNPs in regions of high or low linkage disequilibrium (LD) (Lee et al. 2011; Speed et al. 2012), both of which can be overcome in GCTA through the use of the GREML–LDMS approach (Yang et al. 2015a). The runtime of GCTA–GREML is a function of both the number of markers, M, and the number of individuals, N. Compute time is O(N3 + MN2), which includes a component for the construction of the genetic relationship matrix and a component for the actual REML algorithm. The steep increase in runtime with larger N makes it impractical for data sets with very large numbers of individuals. The software BOLT-LMM can estimate variance components through a stochastic approximation algorithm, which circumvents the costly calculation of a genetic relationship matrix and thus reduces the runtime to only O(MN1.5) (Loh et al. 2015). Note that both GCTA and BOLT-LMM have other features such as linear mixed model association analysis (Yang et al. 2014) and genotype × environment (G × E) analysis, which are not the topic of this review.
LD score regression (LDSC) (Bulik-Sullivan et al. 2015b) is a method, which requires only summary statistics to estimate SNP-heritability and has therefore shorter runtime than the methods discussed so far. Under polygenic genetic architecture, SNPs which are highly correlated with many other SNPs (have a high LD score) are more likely to tag a causal SNP and are therefore expected, on average, to have a higher association test statistic than SNPs which are not highly correlated with many other SNPs. The regression coefficient of the association test statistics of all SNPs on their LD score is a function of SNP-heritability (Yang et al. 2011b; Bulik-Sullivan et al. 2015b). Any factor that increases the association statistic of a SNP independently of its LD score (as might be found in population stratification, which induces correlations in test statistics across chromosomes) will increase the intercept term of this regression (Bulik-Sullivan et al. 2015b). LDSC estimates SNP-heritability with vastly reduced computational speed compared with GREML, but with higher standard error (s.e.) of estimates. The s.e. of GREML SNP-heritability estimates is accurately approximated as 316/N, where N is the total sample size (Visscher et al. 2014). For LDSC the standard errors of the variance component estimates are typically larger (usually by 50% or more) than those of a GREML analysis for the same sample size (Yang et al. 2015b). However, it is typical that LDSC can be applied to larger data sets (which generate smaller s.e.) since only summary statistics are needed. Comparisons of estimates from GREML and LDSC show that the accuracy of estimates from LDSC are dependent on LD scores calculated from a population representative of the population used to estimate GWAS summary statistics (Yang et al. 2015a; Brown et al. 2016).
GREML, LDSC and similar methods can also be used to estimate the SNP-heritability based on genomic partitioning, for example by chromosome, minor allele frequency, genomic annotations or single loci (Shi et al. 2016). Analyses partitioning SNP-heritability by cell type-specific genomic annotations have shown enrichment of GWAS discoveries in trait relevant cells (Finucane et al. 2015).
Examples of applications to psychiatric disorders
GREML SNP-heritability estimated for psychiatric disorders usually ranges from 15% to 30%, depending on the disease, and reported estimates are at most half of the estimates derived from family studies (Lee et al. 2012a, 2013; Anttila et al. 2016). SNP-heritability estimates of quantitative mental traits tend to be relatively low (<0.15), for example, a meta-analysis of the Big Five personality traits reported significant SNP-heritability estimates below 0.2 for all five traits (Lo et al. 2016). This is in line with another study of up to 300 000 people finding SNP-heritability estimates for subjective well-being, depressive symptoms and neuroticism in the same range (Okbay et al. 2016). Partitioning heritability by tissue-specific genomic annotations has identified the central nervous system as the most relevant tissue in the aetiology of schizophrenia and bipolar disorder (Finucane et al. 2015).
Estimation of genetic correlation using genome-wide SNPs
Two traits are genetically correlated, if there is a correlation between the true effect sizes of SNPs affecting the two traits, or in other words, when, on-average, SNPs have directionally similar effects on two traits. Genetic correlation can reflect pleiotropy, which is defined as a genetic variant affecting both traits. However, while a non-null genetic correlation can imply pleiotropy is present at many SNPs, a genetic correlation of zero could arise when pleiotropy is common, but the direction of effects are uncorrelated across SNPs. Genetic correlations (rG) are of interest because they suggest a shared aetiology. However, misdiagnosis between two genetically uncorrelated diseases can generate significant estimates of rG (Kendler, 1987; Wray et al. 2012). The availability of genetic marker data on disease case–control samples has allowed the interrogation of the genetic relationship between diseases, often for the first time, since traditional methods to estimate genetic correlation based on increased risk of a disease in relatives of those with another disease requires often unattainably large samples (Visscher et al. 2014).
Formally, genetic correlation is defined as genetic covariance between two traits, scaled by the genetic standard deviations of the two contributing traits. Methods used to estimate SNP heritability can be extended into a bivariate form to estimate rG. In order to estimate genetic correlation using GREML, individual-level genetic data and measurements on two phenotypes are required (from the same or from different individuals). The power of bivariate GREML analyses to detect rG departing from 0 or from 1 depends on the population value of rG, the SNP-heritability of both traits, on the sample sizes, on whether the same or different samples are used for the two traits, and for disease traits, on the proportion of cases in the sample (Visscher et al. 2014). For example, for two diseases with lifetime prevalence of 1%, SNP-h2 of 0.2 and genetic correlation of 0.5, 5000 cases and 5000 controls for each disease are sufficient to have 89% power at type 1 error rate of 5% to detect a genetic correlation greater than 0, corresponding to a standard error of 0.06. The BOLT-LMM software is also capable of calculating rG in a bivariate GREML analysis with shorter runtime (Loh et al. 2015). In contrast to heritability estimates, rG estimates are scale independent (approximately) and hence scale transformation is not needed (Lee et al. 2012b).
If summary statistics on two or more traits are available, LDSC can be used to estimate rG between them, albeit with higher standard errors than GREML. To make the application of LDSC for SNP-heritability and rG estimation even more user-friendly, the LD Hub resource has been developed, which provides access to summary statistics from more than 200 different traits. LD Hub then calculates genetic correlation estimates between each of these traits and any additional traits for which the user provides summary statistics data (Zheng et al. 2017).
Estimation of rG is most commonly applied to uncover genetic relationships between two different traits, but it can also be applied to detect heterogeneity in genetic effects between two different groups, for example a trait might be under different genetic control in men and in women or in old people and young people (i.e. G × E). It has also been applied to two data sets of the same disease, where rG is expected to be one (Lee et al. 2013), to infer between-sample heterogeneity or two data sets of the same disease but of different ethnicity (De Candia et al. 2013). Calculating rG across populations using LDSC is not straightforward, however, due to between population differences in both allele frequencies and LD structure. The program Popcorn addresses this problem and allows one to estimate the transethnic genetic correlation based on summary statistics and LD matrices from two populations (Brown et al. 2016).
Examples of applications to psychiatric disorders
One of the first applications of the bivariate GREML method to disease traits was to estimate genetic correlations between psychiatric disorders, presenting evidence that most pairs of disorders result in estimates that are significantly different from zero (PGC cross disorder group (Lee et al. 2013)). From those initial estimates the high correlations between schizophrenia and bipolar disorder (~0.6) and between bipolar and MDD (0.5) were considered plausible given evidence from family studies; however, the high genetic correlation between schizophrenia and MDD was more surprising for the clinical community. In fact, close study of the literature from family studies (PGC Cross disorder group, 2013) showed that all three genetic correlation estimates were consistent with published increased risk to relatives (RR) of one disorder for individuals diagnosed with another. However, for the same genetic correlation the size of RR involving a very common disorder (~15% lifetime risk) such as MDD is much smaller than when both disorders are less common (<1% lifetime risk for both schizophrenia and bipolar disorder).
Genetic correlation can arise through misdiagnosis between two diseases. For example, those first presenting with clinical features consistent with a diagnosis of bipolar disorder can in the long-term receive a diagnosis of schizophrenia (and vice versa) (Joyce, 1984; Meyer & Meyer, 2009). However, it can be shown analytically that very high misclassification rate of 20% would be needed under no shared aetiology to result in rG of ~0.6 estimated between schizophrenia and bipolar disorder (Wray et al. 2012). In contrast, a high genetic correlation between disorders would be consistent with some clinical presentations being difficult to classify.
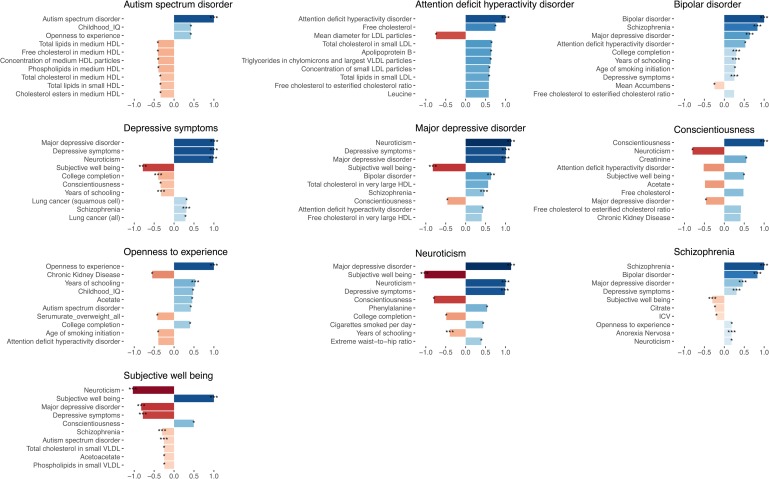
LDSC has been applied to a full battery of GWAS summary statistics and report higher estimates of genetic correlations estimates between pairs of psychiatric disorders than between psychiatric- and non-psychiatric disorders (Bulik-Sullivan et al. 2015a). Some notable examples include a positive genetic correlation between schizophrenia and anorexia nervosa, and a positive genetic correlation between bipolar disorder and years of education. A study investigating genetic sharing between neurological and psychiatric traits found that among neurological disorders significant genetic correlations are rare, but that there is some overlap in genetic risk between migraine and MDD, and ADHD and Tourette syndrome (Anttila et al. 2016). On the other hand, genetic correlations between psychiatric traits and personality traits are more common. Figure 2 shows the top genetic correlations for psychiatric disorders and traits. Data obtained from LD Hub.
Fig. 2.
Genetic correlations between psychiatric disorders and traits, and almost 200 other traits. For each trait, the 10 traits with the highest absolute genetic correlations are shown. Colours indicate whether genetic correlations are positive or negative. One star indicates a genetic correlation p value <0.05. Three starts indicate a p value below the Bonferroni threshold of 2.81 × 10−6 for 17 766 tested trait pairs. Data obtained from LD Hub (Zheng et al. 2017).
Polygenic risk prediction
Estimates of SNP effects can be used to predict the genetic risk of individuals. Simple risk scores for each individual are calculated as the sum over all per SNP effects, where the per SNP effect is the allele count of the SNP for the individual multiplied by the effect size of the SNP (Wray et al. 2007; Purcell et al. 2009). Here the SNP effects come from a typically large and well-powered discovery (sometimes called training) data set. While the ultimate goal of genetic risk prediction is in applications where the phenotype has not yet been observed, in research applications the risk predictor is evaluated for individuals in a target (sometimes called validation or testing) data set where the phenotype has already been recorded, so that the efficacy of the predictor can be evaluated.
Screening of high-risk individuals for early intervention or prevention programs is a potential clinical application of polygenic risk prediction that at present is not widely used because of the low accuracy of genetic risk predictors (Chatterjee et al. 2016). However, there are applications of polygenic risk prediction in research where low prediction accuracy is less limiting; for example, it could be a cost-effective strategy to conduct follow-up studies in samples ascertained to be low or high for polygenic risk. The genetic predictor is evaluated against a measured phenotype in the target sample, which may or may not be the same phenotype from which the predictor was constructed. Generally, if the predicted and the measured trait are genetically correlated, then there should be positive prediction accuracy, given enough power in both data sets (Dudbridge, 2013).
In the usual implementation of polygenic risk scores, SNP effect sizes have been estimated from the standard, one SNP at a time GWAS analysis. The construction of polygenic risk scores is based on some decision about the proportion of SNPs to include in the predictor. As the discovery sample p value threshold becomes more lenient, the increased predictive power of including estimated effect sizes from more true positive associated SNPs is balanced by the inclusion of more false positives. The optimum proportion of SNPs to include depends on the (unknown) genetic architecture and size of the discovery sample. In the latest schizophrenia GWAS, the optimum p value threshold was identified as 0.05 (based on variance explained in out-of-sample prediction across many samples), although inclusion of all SNPs did not lower accuracy drastically (Ripke et al. 2014). Often a range of different p value thresholds are used to determine the best predictor, although this approach is prone to overfitting. Software such as PLINK (Purcell et al. 2007; Chang et al. 2015) implements basic polygenic risk scoring, while PRSice compares polygenic risk predictors using a large number of p value cutoffs to find the optimum threshold given the data (Euesden et al. 2015). In polygenic risk scoring, LD among SNPs is usually accounted for by applying LD-clumping, i.e. pruning of SNPs based on LD but with higher preference to SNPs with lower p values (usually simply termed ‘clumping’). Prediction accuracy can be improved by using methods that provide estimates of SNP effects that are conditional on all other SNPs, thereby directly taking SNP LD correlations into account (de Los Campos et al. 2013), such as Genomic Best Linear Unbiased Prediction (GBLUP) (Meuwissen et al. 2001), which is widely used in animal breeding. In GWAS, there are many more SNPs compared with individuals so effects sizes of each SNP are not estimable in a multiple regression model. In GBLUP, it is assumed that SNP effect sizes are drawn from a normal distribution and a shrinkage term, or penalty term, proportional to the trait heritability is introduced in the model. More complex models are BayesR (Moser et al. 2015) or LASSO (least absolute shrinkage and selection operator) (Abraham et al. 2013). These methods shrink the SNP effect estimates (i.e. the effect attributable to SNPs in LD with each other is shared between the correlated SNPs) and will ensure the predicted phenotypes are more accurate, and in LASSO and BayesR shrink a proportion of SNPs to zero. The gains in prediction accuracy are dependent on underlying genetic architecture (Moser et al. 2015).
If individual-level genotype data are not available, it is still possible to transform marginal SNP effects (standard GWAS summary statistics) into penalised, conditional SNP effects, by making use of an LD reference data set (Yang et al. 2012; Robinson et al. 2017). This is implemented in GCTA (Yang et al. 2011a) and in LDpred (Vilhjálmsson et al. 2015), which not only accounts for LD between SNPs, but also uses a Bayesian framework to adjust the SNP effects for traits where an infinitesimal model (all SNPs have some effect) is not the best fit to the data (e.g. autoimmune disorders). This is analogous to the selecting SNPs based on p value in standard polygenic risk prediction and can further improve accuracy.
Finally, GBLUP approaches can be extended to a multi-trait model, which can further improve prediction accuracy when phenotypes are genetically correlated, because measurements on each trait provide information on the genetic values of the other correlated traits. This approach has been implemented in the program MTG2 and has been shown to improve prediction accuracy for schizophrenia and bipolar disorder (Maier et al. 2015).
Examples of applications to psychiatric disorders
Polygenic risk prediction is widely used in psychiatric genetics, not to infer an individual's case control status, but to gain a better understanding of disease aetiology. Polygenic risk prediction in applications relevant to psychiatry has been reviewed previously (Wray et al. 2014). Some more recent examples include schizophrenia polygenic risk scores calculated for community samples of individuals, which explain variation in creativity (Power et al. 2015) and cannabis use (Power et al. 2014). An association between schizophrenia polygenic risk scores and negative symptoms and anxiety disorder in adolescents gives reason to hope that these kind of studies can not only lead to a better understanding of disease aetiology, but may someday also contribute to early intervention programmes (Jones et al. 2016; Kendler, 2016). Polygenic risk prediction additionally provides a novel approach to studying gene – environment interactions (G × E). Traditional G × E studies, which test for an interaction effect between single genetic variants and an environmental exposure on disease risk, often suffered from low power caused by the small amount of variance explained by individual genetic loci. In contrast, interactions between a polygenic risk score and environmental exposure can be detected more easily, because polygenic risk scores explain more of the variance in disease risk than individual loci. This type of G × E study has been applied to investigate a potential interaction between a polygenic risk score for MDD and childhood trauma on the risk for MDD. Two independent studies found that both the polygenic risk score and exposure to childhood trauma increase the risk for MDD, but came to different conclusions about the nature of the interaction between the two: One study found a positive interaction, meaning that those with both exposure to childhood trauma and high polygenic risk scores are at the greatest risk of developing MDD (Peyrot et al. 2014), while another study found a negative interaction, where people with exposure to childhood trauma and low polygenic risk scores are at the highest risk (Mullins et al. 2016).
Mendelian randomisation
MR analysis investigates the causal relationships between traits. It is a specific form of an instrumental variable analysis (Evans & Davey Smith, 2015), where the goal is to test the causal effect of an explanatory variable (exposure to a risk factor) on a dependent outcome variable (such as disease risk). In MR, genetic markers are used as the instrumental variables. The MR poster child is the application to blood lipid levels and myocardial infarction (Voight et al. 2012). Together with a number of randomised controlled trials (Keene et al. 2014), this application of MR has had major impact on drug development by providing evidence against a causal role of HDL and thus helped in the search for effective ways of preventing myocardial infarction, and demonstrates how evidence from an MR analysis could be used to circumvent costly randomised controlled trials.
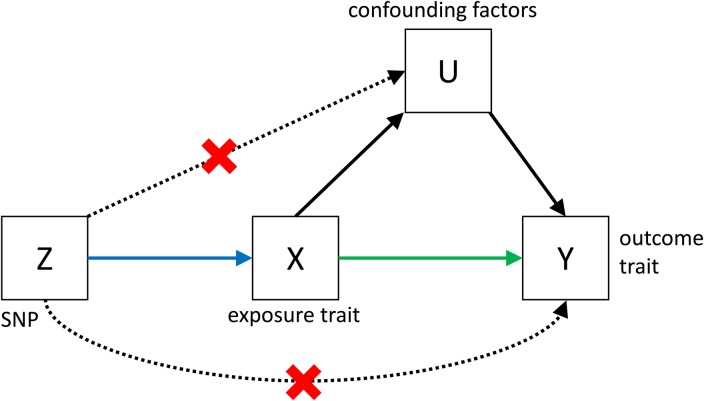
Despite its great potential, MR is often limited by low power, and by the fact that it is very difficult to show that all the assumptions (see Fig. 3) that are necessary to infer causality are met. However, if MR is applied bidirectionally for trait pairs of approximately comparable power, and evidence for significant causality is detected in only one direction, then this can help to infer causality over pleiotropy. The power in MR studies is a function of the true causal association between exposure and outcome and of the variance explained by the instrumental variables (Brion et al. 2013). Since statistically significantly associated SNPs often only explain a small proportion of the genetic variance, for many pairs of traits, very large sample sizes are needed to achieve sufficient power to detect causal associations [see online calculator (Brion et al. 2013)].
Fig. 3.
Causal pathways in an MR experiment. A causal effect of exposure (X) on outcome (Y) can be inferred if three core assumptions are met. These assumptions concern the genetic instrumental variable, Z, and state that: (i) Z has to be robustly associated with the exposure variable, (ii) Z cannot be related to any common causal factors of X and the outcome Y (these are labelled U), and (iii) Z may only be related to Y through X (Solovieff et al. 2013). The latter two assumptions can be summarised as the absence of pleiotropy for the instrumental variable.
In recent years, many improvements to the MR method have been developed. Of particular importance for polygenic traits is the extension from single-SNP instrumental variables to instrumental variables that comprise multiple SNPs (Evans et al. 2013). This may increase power since in polygenic traits individual SNPs are likely to be weak instruments. Two-sample MR is an extension that makes it possible to combine independent data for genotype – exposure and genotype – outcome in an MR experiment, thus allowing to investigate causal associations among all phenotypes for which well-powered GWAS data are available (Burgess et al. 2013). Further developments of MR include better ways to test some of the assumptions, modifications which allow the relaxation of the no-pleiotropy assumption, and improvements which increase the power to detect causal effects (Evans & Davey Smith, 2015). The web-based resource MR BASE has been developed to simplify the application of MR to test causality between a large number of traits and to compare different variations of the method (Hemani et al. 2016). Summary data for more than a thousand traits have been collected and can be tested for causal associations with data provided by the user.
Examples of applications to psychiatric disorders
Several MR studies have investigated a potential causal influence of variables, which are known to be associated with psychiatric traits and diseases from observational studies. One study which looked at BMI as a potential risk factor concluded that there is no evidence of BMI being a causal influence on schizophrenia and bipolar disorder, but weak evidence of BMI conferring a higher risk of MDD. It was noted, however, that the BMI association suffers from low power caused by small sample sizes for MDD (Hartwig et al. 2016). C-Reactive protein (CRP) is a potential risk factor for psychiatric disorders with undisputed correlational association, but unclear causality. A study from 2016 surprisingly found a protective role of genetically elevated CRP levels on the risk for schizophrenia, as well as weak (nominally significant) evidence for a risk increasing effect on bipolar disorder as well as a range of other somatic traits (Prins et al. 2016). This is in contradiction with another 2016 study, which identified elevated CRP levels to confer an increased risk of schizophrenia (Inoshita et al. 2016). There is much debate on whether cannabis is a risk factor for psychosis or schizophrenia, or whether the association is due to reverse causation or due to a confounding factor (McGrath et al. 2010; Gage et al. 2016). Recently MR studies have provided evidence for a causal role of cannabis in the development of schizophrenia, but also for a reverse causation (Gage et al. 2017; Vaucher et al. 2017). Several other risk factors for schizophrenia, anxiety and depression have been investigated through MR with negative results (Bjørngaard et al. 2013; Gage et al. 2013; Taylor et al. 2016).
In summary, MR studies investigating risk factors for psychiatric disorders could in a few cases provide evidence for a risk increasing effect. As the power of GWAS with increasing sample sizes, there will be more robust SNP associations which can be used as instrumental variables. This will provide more evidence for whether the many negative results were just caused by low power or by the absence of a true causal association, but interpretation of causality needs to carefully consider confounding factors.
Fine-mapping and gene prioritisation
LD between SNPs is both a blessing and a curse for GWAS. On one hand, it makes it possible to probe only a subset of all genetic variants yet still detect associations for a much larger set, either through tagging of non-genotyped SNPs by genotyped SNPs or through LD-based imputation to sequenced reference samples. On the other hand, it means that a detected association does not necessarily imply a causal role for the associated SNPs. Fine-mapping attempts to identify which out of a number of associated SNPs in a LD region have a causal role, and which are merely associated because they are in LD with causal SNPs (Sekar et al. 2016).
To better understand disease aetiology, it may be of interest to identify causal genes, rather than causal SNPs. In many cases the causal gene may simply be the gene closest to the most strongly associated SNP in a region, but this is not always so (Claussnitzer et al. 2015).
Identifying causal SNPs
A range of different approaches have been developed for the fine-mapping of SNPs. Most of them use information on functional annotation of the genome and LD between SNPs, in addition to SNP association statistics on one or more diseases. An algorithm (PICS) utilising all these kinds of information has recently been applied to 21 autoimmune disorders and identified many putative causal variants by integrating information from different types of functional annotations, including epigenetic marks and gene expression information (Farh et al. 2015).
Fine-mapping methods can use LD information to either identify causal SNPs within a region that may not have the strongest association signal, but are located in a functional genomic element like an enhancer, or they can use LD information to identify multiple independently associated SNPs, by calculating the association signal conditionally on the association signal of neighbouring SNPs. Traditionally, this would require full genotype data on the trait of interest. However, it has been demonstrated that it is possible to borrow LD information from a reference genotype data set for a conditional analysis, making it possible to apply this approach to traits for which only summary statistics are available (Yang et al. 2012; GCTA-cojo). For this to work well, the LD structure in the reference genotype population should be a good approximation of the LD structure in the population on which the GWAS has been performed. Cross-ethnic genetic studies can aid fine mapping of disease loci, exploiting differences in allele frequency and LD (Morris, 2011), but application to psychiatric disorders has been limited by the dearth of non-European data sets.
Fine-mapping can benefit from data on multiple traits. When two traits share regions of significant genetic associations it can be investigated if they share causal loci at those shared regions, or if different loci drive the regional association in each trait. This has been investigated in a Bayesian framework using only summary statistics and resulted in the identification of 341 loci associated with more than one trait across 42 different phenotypes (Pickrell et al. 2016). SNPs associated with two traits form the basis of the previously discussed MR methods.
Identifying causal genes
Genome-wide association studies suffer from a massive multiple-testing burden, owing to the large number of association tests between SNPs and phenotype. To minimise the number of false positive results, associations are usually required to be significant at a p value of 5 × 10−8 (Bonferroni correction of a million independent tests). Gene-based tests have a reduced multiple-testing burden (~20 000 independent tests) and give biological meaning to association results. In gene-based tests SNPs are aggregated into larger groups (Neale & Sham, 2004) assuming that SNPs exert their effect through nearby genes, which is not always true. The PrediXcan method refines this aggregation step by including external tissue specific eQTL data to predict gene expression levels based on SNP data (Gamazon et al. 2015). This has several advantages over conventional gene-based tests as it limits the multiple-testing burden by only using SNPs, which are known to affect gene expression, and the direction of effect of a SNP on expression levels is not lost when aggregating multiple SNPs. By using tissue-specific eQTL data, associations can be tested between a phenotype and expression changes in tissues relevant to the phenotype. PrediXcan has been used to identify genes which may play causal roles in amyloid deposition and cognitive changes in Alzheimer's disease (Hohman et al. 2017) and genes associated with Asthma (Ferreira et al. 2017). While PrediXcan requires individual-level genotype data, the extension MetaXcan requires only summary statistics and promises similar accuracy, if the right reference population is used for LD estimation (Barbeira et al. 2016). Transcriptome-wide association study (TWAS) is a summary statistics based method similar to MetaXcan, which differs in the algorithm used to predict expression (Gusev et al. 2016a). More recently, TWAS has been applied to detect pairs of traits with genetic correlations at the level of predicted expression (Mancuso et al. 2017). A current limiting factor in this and other expression-based methods is the quality of tissue-specific eQTL data. The previously mentioned methods prioritise genes based on predicted effects of SNPs on expression. This is in contrast to other methods, such as DEPICT, which use gene expression data to predict gene function and prioritise genes at specific loci based on the predicted function (Pers et al. 2015).
While an association of predicted gene expression and a phenotype is suggestive of a causal role for that gene, pleiotropy is an alternative explanation for this association. That is, the same SNPs could independently lead to expression changes in one gene and via a different route have an effect on the phenotype. The summary statistics-based MR (SMR) method (Zhu et al. 2016) attempts to distinguish between these two scenarios using eQTL SNPs as instrumental variables and gene expression as exposure variable. This method also applies a follow-up test (HEIDI), which identifies and excludes regions where multiple linked SNPs are independently associated with gene expression and phenotype, as a strategy to prioritise regions with evidence of a simple causal mechanism for functional follow-up. The method was applied to several complex human traits and has identified 126 putatively causal genes, of which 77 were not the closest gene to their respective top associated GWAS hit.
MetaXcan, TWAS and SMR use the same type of data to identify genes of interest. However, there are many subtle differences between the methods, which will likely lead to unique results for each method. To date, no systematic comparison of their relative performance has been published.
Examples of applications to psychiatric disorders
PrediXcan has been applied to bipolar disorder, resulting in the identification of two genes, PTPRE and BBX, for which predicted increased expression in whole blood and the anterior cingulate cortex, respectively, was associated with increased risk of bipolar disorder (Shah et al. 2016). SMR has been applied to schizophrenia, highlighting two genes, SNX19 and NMRAL1, with a potentially causal influence (Zhu et al. 2016). The previous two examples have highlighted genes by using eQTL data, but the concept can be extended to account for the fact that chromatin modifications may mediate the association between genetic variants and eQTLs, and that splice-QTLs, rather than eQTLs, may underlie the genetic effect of a SNP. A TWAS study on schizophrenia has incorporated these ideas and has highlighted 157 genes, many of which were identified due to brain specific splice-QTLs (Gusev et al. 2016b).
Detection of genetic heterogeneity
Most genetic studies are based on the assumption that individuals who exhibit similar symptoms or who have been diagnosed with the same disease are representatives of the same underlying biology defined by a common genetic architecture. Under a polygenic disease architecture, each individual is likely to have a unique combination of risk loci, but with each combination drawn from the pool of risk loci. Genetic heterogeneity occurs when individuals with the same clinical presentation have risk alleles drawn from independent (or perhaps correlated) sets of risk loci, and the genetic risk profile of one or more subgroups of cases departs from that of the rest. This may arise through misclassification of some cases, or through distinct aetiological pathways leading to the same diagnosis (Flint & Kendler, 2014; Jeste & Geschwind, 2014). The inherent phenotypic heterogeneity within psychiatry makes detection of genetic heterogeneity an appealing goal and identification of distinct pathways holds the promise of shedding light on disease aetiology. Furthermore, a biological basis for disease stratification could lead to more personalised treatments (Kapur et al. 2012).
Although the concept of identifying genetic sub-groups is intuitively appealing simulations suggest it is very difficult to find meaningful genetic groupings if each sub-group has a genetic architecture of a large number of loci with small effects. The large number of combinations of risk loci, their small effect sizes, the uncertainty about the size or even about the presence of genetically heterogeneous groups, and the challenging disentanglement from population stratification all contribute to the difficulty of this problem. As a result, even data sets comprising hundreds of thousands of individuals may not provide sufficient power for naïve approaches to detecting even the simplest scenarios of genetic heterogeneity (Han et al. 2016; Maier et al. unpublished results).
The BUHMBOX method (Han et al. 2016) frames the question of disease heterogeneity in a different way and sets out to test if two diseases that share a genetic basis (rG > 0) are correlated because the shared genetic risk factors are present in the whole sample (pleiotropy) or are confined to only a subgroup of individuals (identifiable genetic sub-type) (Han et al. 2016). The BUHMBOX method investigates LD-independent risk loci for disease B in individuals diagnosed with disease A. If only a subgroup of individuals has a higher genetic risk for disease B, this will induce a correlation among the disease B risk loci, which would support the presence of genetic heterogeneity and not pleiotropy. This approach can demonstrate presence of a genetic sub-group without identifying which specific individuals diagnosed with disease A are genetically more similar to those with disease B. The method found evidence for heterogeneity among seronegative rheumatoid arthritis cases, suggesting that they may contain a significant proportion of seropositive cases. The power of the BUHMBOX method to detect heterogeneity depends on the number of cases, number of markers, risk allele frequency, odds ratio and proportion of disease A cases that are genetically disease B cases (heterogeneity proportion). For example, with 2000 cases and 2000 controls, a heterogeneity proportion of 0.2 and 50 risk loci, the power to detect heterogeneity at a significance threshold of 0.05 is 92% (Han et al. 2016).
Alternatively, genetic heterogeneity can be studied by first grouping individuals (for example, disease cases) based on non-genetic data and then testing for genetic heterogeneity between these disease subtypes. A recent method follows this approach by jointly modelling the probability for each SNP of whether its frequency differentiates cases and controls and/or differentiates disease subgroups. Applied to type 1 diabetes, this method suggests that cases with and without autoantibodies exhibit a different genetic architecture for type 1 diabetes disease risk (Liley et al. 2016).
Examples of applications to psychiatric disorders
The BUHMBOX method was used to investigate the shared genetic basis between MDD and schizophrenia, and found no evidence that suggested that a subset of MDD cases was genetically more similar to schizophrenia cases, implying that the genetic correlation estimated between the disorders reflect pleiotropy (Han et al. 2016). Application of this method will become more interesting as sample sizes increase.
Conclusions
For many psychiatric disorders, genetic factors explain more variation in disease risk in the population than any other known risk factors (Sullivan et al. 2012), but only recently has it become possible to resolve the overall familial genetic risk into individual risk factors at the DNA level. The evidence is now conclusive that psychiatric disorders, like many other common disease and disorders are highly polygenic underpinned by thousands of genetic loci, each of which contributes a small amount to the overall genetic risk. After a period in which many candidate gene studies have reported association results, which failed to replicate (Chanock et al. 2007), the hypothesis-free GWAS approach has established itself as the dominating paradigm to find associated genetic loci. With ever growing sample sizes, more and more SNPs surpass the stringent p value threshold for almost all investigated traits. However, it is also becoming clear that the bulk of genetic risk factors remains hidden among those loci that do not achieve genome-wide significance. Many of the methods presented in this review leverage the large amount of information that is harboured by genetic variants, regardless of whether or not they achieve significance. While the focus of some methods is on individual SNPs or genes, other methods aggregate over a potentially large number of loci to answer questions such as ‘What is the combined genetic effect of all measurable SNPs on phenotypic variance?’, ‘Do these traits have a shared genetic aetiology?’ or ‘Do these traits causally influence one another?’. One thing that all of these methods have in common is that their utility crucially depends on the power to detect an association, which in turn depends on sample size. Larger sample sizes lead to a higher computational burden, but for most analytical questions, which have been presented here, there are methods which can utilise summary statistics and thus drastically reduce runtime and memory requirements.
The literature on new methods is ever-growing and while we have tried to present an overview of key methods to help navigation of this complex field, it is difficult to be fully exhaustive. In particular, pathway analyses are important application of GWAS summary statistics, but these methods have been reviewed recently (Wang et al. 2010) and so were not considered here. We have illustrated the methods with some example applications; however, we expect the full potential of the data will only be revealed in coming years when studies with half a million or more people will be widely available. A key issue for the field is to develop cost-effective strategies to capture larger sample sizes with both DNA samples and phenotypic data as these are needed to evaluate the extent to which genetic data can explain phenotypic heterogeneity and to fulfil the potential of more personalised medicine.
Acknowledgements
This research is funded by the Australian National Health and Medical Research Council (N.R.W., grant numbers 1078901, 1087889), (P.M.V., grant number 1078037).
Declaration of Interest
None.
Ethical Standards
Not applicable.
References
- Abraham G, Kowalczyk A, Zobel J and Inouye M (2013) Performance and robustness of penalized and unpenalized methods for genetic prediction of complex human disease. Genetic Epidemiology 37, 184–195. [DOI] [PubMed] [Google Scholar]
- Anttila AV, Finucane H, Bras J, Duncan L, Falcone G, Gormley P et al. (2016) Analysis of shared heritability in common disorders of the brain Brainstorm consortium. bioRxiv 48991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeira A, Shah KP, Torres JM, Wheeler HE, Torstenson ES, Edwards T et al. (2016) Metaxcan: summary statistics based gene-level association method infers accurate PrediXcan results. bioRxiv 45260. [Google Scholar]
- Bjørngaard JH, Gunnell D, Elvestad MB, Davey Smith G, Skorpen F, Krokan H et al. (2013) The causal role of smoking in anxiety and depression: a Mendelian randomization analysis of the HUNT study. Psychological Medicine 43, 711–719. [DOI] [PubMed] [Google Scholar]
- Brion MJA, Shakhbazov K and Visscher PM (2013) Calculating statistical power in Mendelian randomization studies. International Journal of Epidemiology 42, 1497–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BC, Ye CJ, Price AL and Zaitlen N (2016) Transethnic genetic-correlation estimates from summary statistics. American Journal of Human Genetics 99, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R et al. (2015a) An atlas of genetic correlations across human diseases and traits. Nature Genetics 47, 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N et al. (2015b) LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nature Genetics 47, 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Butterworth A and Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genetic Epidemiology 37, 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Candia TR, Lee SH, Yang J, Browning BL, Gejman PV, Levinson DF et al. (2013) Additive genetic variation in schizophrenia risk is shared by populations of African and European descent. American Journal of Human Genetics 93, 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM and Lee JJ (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G et al. (2007) Replicating genotype-phenotype associations. Nature 447, 655–660. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Shi J and García-Closas M (2016) Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nature Reviews Genetics 14210, 14205–14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussnitzer M, Dankel SN, Kim K-H, Quon G, Meuleman W, Haugen C et al. (2015) FTO obesity variant circuitry and adipocyte browning in humans. The New England Journal of Medicine 373, 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Los Campos G, Vazquez AI, Fernando R, Klimentidis YC and Sorensen D (2013) Prediction of complex human traits using the genomic best linear unbiased predictor. PLoS Genetics 9, e1003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudbridge F (2013) Power and predictive accuracy of polygenic risk scores. PLoS Genetics 9, e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euesden J, Lewis CM and O'Reilly PF (2015) PRSice: polygenic risk score software. Bioinformatics 31, 1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DM, Brion MJA, Paternoster L, Kemp JP, McMahon G, Munafò M et al. (2013) Mining the human phenome using allelic scores that index biological intermediates. PLoS Genetics 9, e1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DM and Davey Smith G (2015) Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annual Review of Genomics and Human Genetics 16, 327–350. [DOI] [PubMed] [Google Scholar]
- Farh KK-H, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S et al. (2015) Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MAR, Jansen R, Willemsen G, Penninx B, Bain LM, Vicente CT et al. (2017) Gene-based analysis of regulatory variants identifies 4 putative novel asthma risk genes related to nucleotide synthesis and signaling. Journal of Allergy and Clinical Immunology 139, 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane HK, Bulik-Sullivan BK, Gusev A, Trynka G, Reshef Y, Loh P-R et al. (2015) Partitioning heritability by functional annotation using genome-wide association summary statistics. Nature Genetics 47, 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J and Kendler KS (2014) The genetics of major depression. Neuron 81, 484–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage SH, Hickman M and Zammit S (2016) Association between cannabis and psychosis: epidemiologic evidence. Biological Psychiatry 79, 549–556. [DOI] [PubMed] [Google Scholar]
- Gage SH, Jones HJ, Burgess S, Bowden J, Davey Smith G, Zammit S et al. (2017) Assessing causality in associations between cannabis use and schizophrenia risk: a two-sample Mendelian randomization study. Psychological Medicine 47, 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage SH, Smith GD, Zammit S, Hickman M and Munafò MR (2013) Using Mendelian randomisation to infer causality in depression and anxiety research. Depression and Anxiety 30, 1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamazon ER, Wheeler HE, Shah KP, Mozaffari SV, Aquino-Michaels K, Carroll RJ et al. (2015) A gene-based association method for mapping traits using reference transcriptome data. Nature Genetics 47, 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratten J (2016) Rare variants are common in schizophrenia. Nature Neuroscience 19, 1426–1428. [DOI] [PubMed] [Google Scholar]
- Gusev A, Ko A, Shi H, Bhatia G, Chung W, Penninx BWJH et al. (2016a) Integrative approaches for large-scale transcriptome-wide association studies. Nature Genetics 48, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusev A, Mancuso N, Finucane HK, Reshef Y, Song L, Safi A et al. (2016b) Transcriptome-wide association study of schizophrenia and chromatin activity yields mechanistic disease insights. bioRxiv 67355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Pouget JG, Slowikowski K, Stahl E, Lee CH, Diogo D et al. (2016) A method to decipher pleiotropy by detecting underlying heterogeneity driven by hidden subgroups applied to autoimmune and neuropsychiatric diseases. Nat Genet 48, 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig FP, Bowden J, Loret de Mola C, Tovo-Rodrigues L, Davey Smith G and Horta BL (2016) Body mass index and psychiatric disorders: a Mendelian randomization study. Scientific Reports 6, 32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes BJ, Visscher PM and Goddard ME (2009) Increased accuracy of artificial selection by using the realized relationship matrix. Genetics Research 91, 47–60. [DOI] [PubMed] [Google Scholar]
- Hemani G, Zheng J, Wade KH, Laurin C, Elsworth B, Burgess S et al. (2016) MR-Base: a platform for systematic causal inference across the phenome using billions of genetic associations. bioRxiv 078972. [Google Scholar]
- Hohman TJ, Dumitrescu L, Cox NJ, Jefferson AL and For The Alzheimer's Neuroimaging Initiative (2017) Genetic resilience to amyloid related cognitive decline. Brain Imaging and Behavior 11, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer N, Szelinger S, Redman M, Duggan D, Tembe W, Muehling J et al. (2008) Resolving individuals contributing trace amounts of DNA to highly complex mixtures using high-density SNP genotyping microarrays. PLoS Genetics 4, e1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshita M, Numata S, Tajima A, Kinoshita M, Umehara H, Nakataki M et al. (2016) A significant causal association between C-reactive protein levels and schizophrenia. Scientific Reports 6, 26105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jeste SS and Geschwind DH (2014) Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nature Reviews Neurology 10, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HJ, Stergiakouli E, Tansey KE, Hubbard L, Heron J, Cannon M et al. (2016) Phenotypic manifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry 73, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce PR (1984) Age of onset in bipolar affective disorder and misdiagnosis as schizophrenia. Psychological Medicine 14, 145–149. [DOI] [PubMed] [Google Scholar]
- Kapur S, Phillips AG and Insel TR (2012) Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Molecular Psychiatry 17, 1174–1179. [DOI] [PubMed] [Google Scholar]
- Keene D, Price C, Shun-Shin MJ and Francis DP (2014) Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ (Clinical research ed.) 349, g4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS (1987) The impact of diagnostic misclassification on the pattern of familial aggregation and coaggregation of psychiatric illness. Journal of Psychiatric Research 21, 55–91. [DOI] [PubMed] [Google Scholar]
- Kendler KS (2016) The schizophrenia polygenic risk score. JAMA Psychiatry 73, 193. [DOI] [PubMed] [Google Scholar]
- Lee SH, DeCandia TR, Ripke S, Yang J, Sullivan PF, Goddard ME et al. (2012a) Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nature Genetics 44, 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH et al. (2013) Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature Genetics 45, 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Wray NR, Goddard ME and Visscher PM (2011) Estimating missing heritability for disease from genome-wide association studies. American Journal of Human Genetics 88, 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Yang J, Goddard ME, Visscher PM and Wray NR (2012b) Estimation of pleiotropy between complex diseases using single-nucleotide polymorphism-derived genomic relationships and restricted maximum likelihood. Bioinformatics (Oxford, England) 28, 2540–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley J, Todd JA and Wallace C (2016) A method for identifying genetic heterogeneity within phenotypically defined disease subgroups. Nature Genetics 49, 310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M-T, Hinds DA, Tung JY, Franz C, Fan C-C, Wang Y et al. (2016) Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nature Genetics 49, 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh P-R, Tucker G, Bulik-Sullivan BK, Vilhjálmsson BJ, Finucane HK, Salem RM et al. (2015) Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nature Genetics 47, 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R, Moser G, Chen G-B, Ripke S, Coryell W, Potash JB et al. (2015) Joint analysis of psychiatric disorders increases accuracy of risk prediction for schizophrenia, bipolar disorder, and major depressive disorder. The American Journal of Human Genetics 96, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso N, Shi H, Goddard P, Kichaev G, Gusev A and Pasaniuc B (2017) Integrating gene expression with summary association statistics to identify genes associated with 30 complex traits. The American Journal of Human Genetics 100, 473–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Welham J, Scott J, Varghese D, Degenhardt L, Hayatbakhsh MR et al. (2010) Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Archives of General Psychiatry 67, 440–447. [DOI] [PubMed] [Google Scholar]
- Meuwissen THE, Hayes BJ and Goddard ME (2001) Prediction of total genetic value using genome-wide dense marker maps. Genetics 157, 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F and Meyer TD (2009) The misdiagnosis of bipolar disorder as a psychotic disorder: some of its causes and their influence on therapy. Journal of Affective Disorders 112, 174–183. [DOI] [PubMed] [Google Scholar]
- Morris AP (2011) Transethnic meta-analysis of genomewide association studies. Genetic Epidemiology 35, 809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser G, Lee SH, Hayes BJ, Goddard ME, Wray NR and Visscher PM (2015) Simultaneous discovery, estimation and prediction analysis of complex traits using a Bayesian mixture model. PLoS Genetics 11, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins N, Power RA, Fisher HL, Hanscombe KB, Euesden J, Iniesta R et al. (2016) Polygenic interactions with environmental adversity in the aetiology of major depressive disorder. Psychological Medicine 46, 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM and Sham PC (2004) The future of association studies: gene-based analysis and replication. American Journal of Human Genetics 75, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Baselmans BML, De Neve J-E, Turley P, Nivard MG, Fontana MA et al. (2016) Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nature Genetics 48, 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasaniuc B and Price AL (2016) Dissecting the genetics of complex traits using summary association statistics. Nature Reviews Genetics 18, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pers TH, Karjalainen JM, Chan Y, Westra H-J, Wood AR, Yang J et al. (2015) Biological interpretation of genome-wide association studies using predicted gene functions. Nature Communications 6, 5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrot WJ, Boomsma DI, Penninx BWJH and Wray NR (2016) Disease and polygenic architecture: avoid trio design and appropriately account for unscreened control subjects for common disease. American Journal of Human Genetics 98, 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrot WJ, Milaneschi Y, Abdellaoui A, Sullivan PF, Hottenga JJ, Boomsma DI et al. (2014) Effect of polygenic risk scores on depression in childhood trauma. The British Journal of Psychiatry: The Journal of Mental Science 205, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell JK, Berisa T, Liu JZ, Ségurel L, Tung JY, Hinds DA et al. (2016) Detection and interpretation of shared genetic influences on 42 human traits. Nature Genetics 48, 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power RA, Steinberg S, Bjornsdottir G, Rietveld CA, Abdellaoui A, Nivard MM et al. (2015) Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nature Neuroscience 18, 953–955. [DOI] [PubMed] [Google Scholar]
- Power RA, Verweij KJH, Zuhair M, Montgomery GW, Henders AK, Heath AC et al. (2014) Genetic predisposition to schizophrenia associated with increased use of cannabis. Molecular Psychiatry 19, 1201–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins BP, Abbasi A, Wong A, Vaez A, Nolte I, Franceschini N et al. (2016) Investigating the causal relationship of C-reactive protein with 32 complex somatic and psychiatric outcomes: a large-scale cross-consortium Mendelian randomization study. PLoS Medicine 13, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics 81, 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF et al. (2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans PA et al. (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MR, Kleinman A, Graff M, Vinkhuyzen AAE, Couper D, Miller MB et al. (2017) Genetic evidence of assortative mating in humans. Nature Publishing Group. Nature Human Behaviour 1, 16. [Google Scholar]
- Sankararaman S, Obozinski G, Jordan MI and Halperin E (2009) Genomic privacy and limits of individual detection in a pool. Nature Genetics 41, 965–967. [DOI] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N et al. (2016) Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Wheeler HE, Gamazon ER, Nicolae DL, Cox NJ and Im HK (2016) Genetic predictors of gene expression associated with risk of bipolar disorder. bioRxiv. [Google Scholar]
- Shi H, Kichaev G and Pasaniuc B (2016) Contrasting the genetic architecture of 30 complex traits from summary association data. The American Journal of Human Genetics 99, 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovieff N, Cotsapas C, Lee PH, Purcell SM and Smoller JW (2013) Pleiotropy in complex traits: challenges and strategies. Nature Reviews Genetics 14, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed D, Hemani G, Johnson MR and Balding DJ (2012) Improved heritability estimation from genome-wide SNPs. The American Journal of Human Genetics 91, 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl EA, Wegmann D, Trynka G, Gutierrez-Achury J, Do R, Voight BF et al. (2012) Bayesian inference analyses of the polygenic architecture of rheumatoid arthritis. Nature Genetics 44, 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Daly MJ and O'Donovan M (2012) Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nature Reviews Genetics 13, 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AE, Burgess S, Ware JJ, Gage SH, Richards JB, Davey Smith G et al. (2016) Investigating causality in the association between 25(OH)D and schizophrenia. Scientific Reports 6, 26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucher J, Keating BJ, Lasserre AM, Gan W, Lyall DM, Ward J et al. (2017) Cannabis use and risk of schizophrenia: a Mendelian randomization study. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilhjálmsson BJ, Yang J, Finucane HK, Gusev A, Lindström S, Ripke S et al. (2015) Modeling linkage disequilibrium increases accuracy of polygenic risk scores. The American Journal of Human Genetics 97, 576–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkhuyzen AAE, Wray NR, Yang J, Goddard ME and Visscher PM (2013) Estimation and partition of heritability in human populations using whole-genome analysis methods. Annual Review of Genetics 47, 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher PM, Brown MA, McCarthy MI and Yang J (2012) Five years of GWAS discovery. American Journal of Human Genetics 90, 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher PM, Hemani G, Vinkhuyzen AAE, Chen G-B, Lee SH, Wray NR et al. (2014) Statistical power to detect genetic (co)variance of complex traits using SNP data in unrelated samples. PLoS Genetics 10, e1004269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher PM and Hill WG (2009) The limits of individual identification from sample allele frequencies: theory and statistical analysis. PLoS Genetics 5, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK et al. (2012) Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. The Lancet 380, 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M and Hakonarson H (2010) Analysing biological pathways in genome-wide association studies. Nature Reviews Genetics 11, 843–854. [DOI] [PubMed] [Google Scholar]
- Wray N, Goddard M and Visscher P (2007) Prediction of individual genetic risk to disease from genome-wide association studies. Genome Research 17, 1520–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Lee SH and Kendler KS (2012) Impact of diagnostic misclassification on estimation of genetic correlations using genome-wide genotypes. European Journal of Human Genetics 20, 668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Lee SH, Mehta D, Vinkhuyzen AAE, Dudbridge F and Middeldorp CM (2014) Research review: polygenic methods and their application to psychiatric traits. Journal of Child Psychology and Psychiatry 55, 1068–1087. [DOI] [PubMed] [Google Scholar]
- Yang J, Bakshi A, Zhu Z, Hemani G, Vinkhuyzen AAE, Lee SH et al. (2015a) Genetic variance estimation with imputed variants finds negligible missing heritability for human height and body mass index. Nature Genetics 47, 1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bakshi A, Zhu Z, Hemani G, Vinkhuyzen AAE, Nolte IM et al. (2015b) Genome-wide genetic homogeneity between sexes and populations for human height and body mass index. Human Molecular Genetics 24, 7445–7449. [DOI] [PubMed] [Google Scholar]
- Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR et al. (2010) Common SNPs explain a large proportion of the heritability for human height. Nature Genetics 42, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ferreira T, Morris AP, Medland SE, Madden PAF, Heath AC et al. (2012) Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nature Genetics 44, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME and Visscher PM (2011a) GCTA: a tool for genome-wide complex trait analysis. American Journal of Human Genetics 88, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weedon MN, Purcell S, Lettre G, Estrada K, Willer CJ et al. (2011b) Genomic inflation factors under polygenic inheritance. European Journal of Human Genetics 19, 807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zaitlen NA, Goddard ME, Visscher PM and Price AL (2014) Advantages and pitfalls in the application of mixed-model association methods. Nature Genetics 46, 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC et al. (2017) LD hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics (Oxford, England) 33, 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X and Stephens M (2014) Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nature Methods 11, 407–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE et al. (2016) Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nature Genetics 48, 481–487. [DOI] [PubMed] [Google Scholar]