Abstract
Owing to advances in modern medicine, life expectancies are lengthening and leading to an increase in the population of older individuals. The aging process leads to significant alterations in many organ systems, with the kidney being particularly susceptible to age-related changes. Within the kidney, aging leads to ultrastructural changes such as glomerular and tubular hypertrophy, glomerulosclerosis, and tubulointerstitial fibrosis, which may compromise renal plasma flow (RPF) and glomerular filtration rate (GFR). These alterations may reduce the functional reserve of the kidneys making them more susceptible to pathological events when challenged or stressed, such as following exposure to nephrotoxicants. An important and prevalent environmental toxicant that induces nephrotoxic effects is mercury (Hg). Since, exposure of normal kidneys to mercuric ions might induce glomerular and tubular injury, aged kidneys, which may not be functioning at full capacity, may be more sensitive to the effects of Hg than normal kidneys. Age-related renal changes and the effects of Hg in the kidney have been characterized separately. However, little is known regarding the influence of nephrotoxicants, such as Hg, on aged kidneys. The purpose of this review was to summarize known findings related to exposure of aged and diseased kidneys to the environmentally relevant nephrotoxicant, Hg.
Keywords: mercury, aging, kidney, glomerulosclerosis, heavy metals, chronic kidney disease
Introduction
In the past century, advances in modern medicine have significantly extended the life-expectancy of humans in developed and developing countries. According to the World Health Organization, the global life expectancy has risen from 64 years in 1990 to 72.1 years in 2013 (WHO 2016). In developed countries such as the United States, the average life expectancy has increased from approximately 47years in 1900 to 79 years in 2011. Similar trends exist for other developed countries such as the United Kingdom, Canada, France, and Germany (WHO 2016). Elevation in life expectancy has led to a rise in the number of older individuals. In the United States alone, the number of individuals over the age of 65 is projected to be 88.5 million by 2050 (USCB 2010). Globally, it is currently estimated that approximately 901 million individuals, i.e., 12% of the population, are over the age of 60. Because of lengthening life expectancies, this figure is expected to rise to 2 billion by 2050 (UN 2016). Older individuals make up a significant fraction of healthcare patients and thus, a thorough understanding of the impact of aging on organ systems, such as the renal/urinary system, is critical when managing the healthcare of these individuals.
Further, aging may enhance individual susceptibility to disease as well as increase one’s risk of being affected adversely by exposure to environmental and/or occupational toxicants. Because of an increased life expectancy and elevated levels of pollution in the environment, it is likely that older individuals may be exposed more frequently and possibly to higher levels of toxic pollutants than individuals were decades ago. In particular, the kidney appears to be a major site of age-related changes and it may also be a target for heavy metals and other toxicants due to its role in filtration and excretion of these compounds. Given this, it is important that normal aging processes and the influence of potential toxicants on kidneys of older individuals are understood more comprehensively.
Aging and the Normal Kidneys
Numerous deleterious structural and physiological changes often occur in the kidneys as individuals age, especially after the seventh decade of life. These changes are likely the consequences of factors such as age-related hemodynamic changes, renal or non-renal disease, and/or life-long exposure to environmental and/or occupational toxicants. Interestingly, aged kidneys are capable of maintaining normal renal function and systemic homeostasis in healthy older individuals even though the normal aging process leads to significant deleterious structural and physiological consequences. The maintenance of normal renal function is at the cost of the renal functional reserve, which is the ability of the kidney to maintain glomerular filtration rate (GFR) when challenged. Once the functional reserve is depleted, kidneys have a reduced capacity to respond to challenges such as those associated with altered hemodynamics. In addition, the ability of the kidneys to eliminate circulating toxicants is most likely lower than normal. Because renal functional reserve may be significantly reduced in older individuals, the kidneys, and possibly other organs, of these individuals may be more susceptible to physiological, pathological, and toxicological challenges.
Molecular Changes within Renal Cells of Aged Kidneys
The aging process results in numerous complex and interrelated changes at the cellular and molecular levels. One such change is an inability to repair or replace injured cells. The mitotic index for renal epithelial cells is normally low, with proliferation occurring in approximately 1% of renal tubular cells (Humphreys et al. 2008). However, this % declines with age and thus, the ability of renal tubules to repair themselves may be reduced significantly in aged kidneys (Schmitt and Cantley 2008). Indeed, evidence from studies in kidneys of aged male C57Bl/6 mice (> 18 months) demonstrated that aging reduces the normal proliferative responses that follow an acute insult to renal tubular cells (Schmitt 2006; Berkenkamp et al. 2014). Analyses of kidneys from older human subjects (> 71 years) showed that renal levels of cytokines such as insulin-like growth factor-1 (IGF-1) and epidermal growth factor (EGF) (Chou et al. 1997; Shurin et al. 2007; Hadem and Sharma 2016), which play roles in cell proliferation, decrease as humans age. The reduction in these cytokines may be an important factor in the inability of aged kidneys to initiate efficient cellular repair mechanisms. In addition, microarray analyses of DNA from kidneys of older humans suggested that certain genes involved in energy metabolism, and nucleotide, amino acid and/or protein turnover are down-regulated in aged kidneys (Melk et al. 2005). Further, a recent study showed that expression of the organic anion transporter 1 (OAT1) and VEGF was decreased in aged animals (Oliveira et al. 2016). In contrast, expression of select genes involved in immune and inflammatory processes was found to be enhanced in aged kidneys (Melk et al. 2005; Brink et al. 2009). For example, tumor necrosis factor (TNF)-α,C-reactive protein (CRP), NF-κB, and interleukin (IL)-6, which are involved in the initiation of an inflammatory response, were expressed at greater levels in older humans (Bruunsgaard et al. 2001; Gerli et al. 2000; Mysliwska et al. 1999; Keller et al. 2007; O’Brown et al. 2015; Wiggins et al. 2010). Elevated expression of NF-κB in kidneys of older individuals appears to be a particularly important regulator of the aging process in that it control cell-cycle exit and mediates age-related transcriptional changes (Helenius et al. 1996; Adler et al. 2007). In addition, it appears that the regulatory control of apoptotic processes is altered by the aging process in that the basal levels of apoptosis were found to be elevated in aged kidneys (Joaquin and Gollapudi 2001).
The aging process also appears to alter regulation of the cell cycle. Ding and colleagues (2001) examined senescent tubular epithelial cells and detected an elevation in the expression of transforming growth factor β−1 (TGF-β1), which plays a role in cell cycle regulation. In addition, p21WAF1/CIP1 and p16INK4A, which are thought to be involved in the regulation of cell growth, appear to be upregulated in senescent renal epithelial cells (Yang and Fogo 2010; Susnik et al. 2015; Berkenkamp et al. 2014). Increased levels of these proteins led to a dysregulation of the cell cycle and cell growth, which consequently resulted in defective cell proliferation and regeneration (Berkenkamp et al. 2014).
Aging has also been associated with alterations in DNA methylation and repair. In most tissues, DNA methylation increases early in life and falls in late adulthood (Jones et al. 2015). A general decrease in the stability and precision of DNA methylation occurs with age and leads to genomic and cellular instability, which contributes to cellular senescence (Pal and Tyler 2016).
Sirtuins have also been implicated as regulators of healthy aging in many organs, including the kidney. These proteins are Class III histone deacetylases that catalyze the deacetylation of proteins in a reaction that consumes NAD+ (Poulose and Raju 2015). Sirtuins are present in renal cells where they appear to protect against development of age-related diseases such renal interstitial fibrosis (Sebastian et al. 2012; Hao and Haase 2010; Ponnusamy et al. 2014). The sirtuin isoform, SIRT1 (silent information regulator-1), appears to promote healthy renal aging by playing a regulatory role in a number of cellular processes, such as gene silencing, lipid homeostasis, mitochondrial biogenesis and cellular respiration (Poulose and Raju 2015; Yuan et al. 2016). Indeed, decreases in the renal levels of SIRT1 protein were detected in aged animals (Lim et al. 2012). In general, deactivation or downregulation of sirtuins appears to lead to dysregulation of a number of cellular processes, which in turn, may promote cell senescence and aging (Poulose and Raju 2015; Yuan et al. 2016; Yang and Fogo 2010).
Another mediator in the aging process is the anti-aging protein, Klotho. Klotho exists as a membrane protein, an intracellular protein, and as a soluble (secretable) protein (Kim et al. 2015a). Klotho is present in a number of organs, but its expression is particularly high in epithelial cells lining the proximal and distal tubules of the kidney (Hu et al. 2010; Li et al. 2004). The mechanisms by which Klotho slows the aging process have not been characterized completely. However, it is thought that the ability of Klotho to suppress reactive oxygen species (ROS)-derived oxidative stress, stimulate nitric oxide (NO) production, and maintain calcium and phosphate homeostasis in renal cells may have important anti-aging effects (Xu and Sun 2015; Saito et al. 1998). Interestingly, inflammation was found to reduce protein levels of Klotho, which may, subsequently, promote the aging process (Ohyama et al. 1998). In addition, hyperperfusion of glomeruli and resulting injury to the glomerular endothelium was also shown to reduce levels of Klotho and accelerate the aging process (Aizawa et al. 1998; Sugiura et al. 2012). Interestingly, studies in Klotho mutant mice demonstrated that development of age-related diseases such as stroke, arteriosclerosis, and osteoporosis was accelerated while overexpression of Klotho in mice appeared to slow the aging process (Masuda et al. 2005).
Injury to mitochondria appears to be an important factor in cellular senescence and aging. The mitochondrial free radical theory of aging, as proposed initially by Harman ( 1972; 1956) as descibed in Eirin et al (2016), suggests that generation of ROS from the mitochondrial respiratory chain increases with age, and consequently leads to oxidative damage within cells. Mitochondrial DNA appears to be a major target for mitochondrial-derived ROS. Injury to mitochondrial DNA impairs the function of the respiratory chain, which results in additional ROS formation and DNA injury. This cycle of events is thought to initiate other oxidative events that may lead to mitochondrial destabilization, cellular injury and cellular aging (Poulose and Raju 2014). These events may promote inflammation and enhanced deposition of extracellular matrix, which results in interstitial and parenchymal fibrosis and acute kidney injury (Guimaraes-Souza et al. 2015; Small et al. 2012; Akcay et al. 2009; Kinsey et al. 2008).
In recent years, a number of investigators started to identify the molecular basis of ROS-induced cellular injury. Data from several studies using aged mice indicate that aged animals display significant reductions in expression of various oxidative stress-related enzymes such as superoxide dismutase (SOD) 1, SOD2, catalase (CAT), and glutathione peroxidase (GPx) (Lim et al. 2012; Gomes et al. 2009; Cand and Verdetti 1989; Wang et al. 2014). These decreases eventually lead to a rise in the level of free radicals, which consequently results in cellular injury. In addition, microRNAs (miRNAs), which are small, non-coding regions of RNA, appear to be involved in the regulation of genes related to cellular metabolism, oxidative stress, and extracellular matrix degradation (Lapierre et al. 2015). Specifically, upregulation of miR-335 and miR-34a appears to diminish the expression of SOD2 and thioredoxin reductase 2 (Txnrd2) (Bai et al. 2011). A reduction in the activities of these enzymes and others may lead to elevated oxidative stress, cellular injury, and cellular aging.
Structural Changes within Glomeruli of Aged Kidneys
Significant ultrastructural changes occur within the glomerulus as the result of the normal aging process. Indeed, several investigators reported that approximately 30–40% of all glomeruli become sclerotic by the eighth decade of life (Kaplan et al. 1975; Epstein 1996; Rodriguez-Puyol 1998; Kappel and Olsen 1980). Typical sclerotic glomeruli are characterized by a thickened glomerular basement membrane, expanded mesangial matrix, and shrinkage and occlusion of the glomerular capillaries (Figure 1) (Rodriguez-Puyol 1998; Zhou 2008; Lerma 2009; Thomas et al. 1998; Bridges et al. 2014; Denic et al. 2016). Although the pathogenesis of glomerulosclerosis is not completely understood, it is postulated to involve multiple factors, including alterations in blood flow, increased susceptibility to inflammatory cytokines, and damage to the glomerular filtration barrier (Figure 2) (Lopez-Novoa 2008; Wiggins et al. 2010; Silva 2005). A reduction in the total number of functioning nephrons appears to be an additional contributing factor in the development of glomerulosclerosis. As nephrons are lost, vascular and glomerular alterations occur in the remaining functional nephrons as an attempt to compensate for diminished total GFR (Lopez-Novoa 2008; Zatz and Fujihara 1994; Denic et al. 2016; Karam and Tuazon 2013).These changes lead to glomerular hypertrophy, hyperperfusion, and hyperfiltration, which increase single nephron GFR (SNGFR) and thus, predispose affected glomeruli to sclerotic changes (Lopez-Novoa 2008; Fogo 2000; Weinstein and Anderson 2010; Anderson and Brenner 1986). Indeed, a positive correlation between glomerular hypertrophy and development of glomerulosclerosis was demonstrated in aging mice (Ferder et al. 1994). In addition, proliferation of mesangial cells and expansion of mesangial matrix, both of which are often associated with glomerular hypertrophy, appears to precede and contribute to the development of glomerulosclerosis (Floege et al. 1992). Alternatively, age-related glomerulosclerosis may occur via immunologic mechanisms whereby formation of circulating or in situ immune complexes leads to glomerulonephritis (Silva 2005).
Figure 1. Age-related glomerulosclerosis and tubular necrosis in rat kidney.
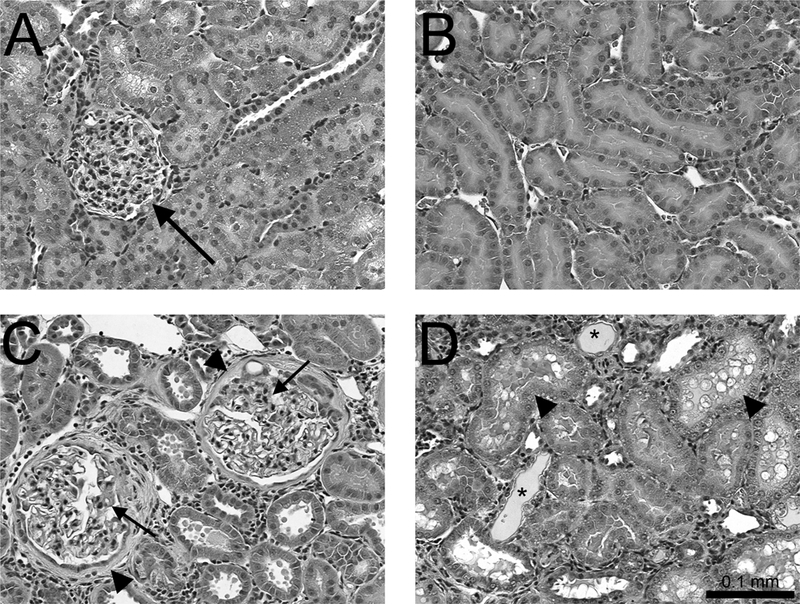
Images shown are representative sections of kidneys from two-month-old (A & B) or 20-month-old (C &D) rats stained with hematoxylin and eosin (H & E). Panel A shows a glomerulus (arrow) within the renal cortex of young rats that displays normal glomerular morphology. Panel B shows normal proximal tubules (PT) within the outer stripe of the outer medulla (OSOM) of young rats. Panel C shows the renal cortex of older rats wherein glomeruli were swollen with thickened basement membranes (arrows) and capsules (arrowheads). Panel D shows proximal tubules (arrowheads) within the OSOM of older rats. Tubules were swollen with numerous cytoplasmic vacuoles and protein deposits (*) (200x).
Figure 2. Possible pathogenesis of age-related glomerulosclerosis.
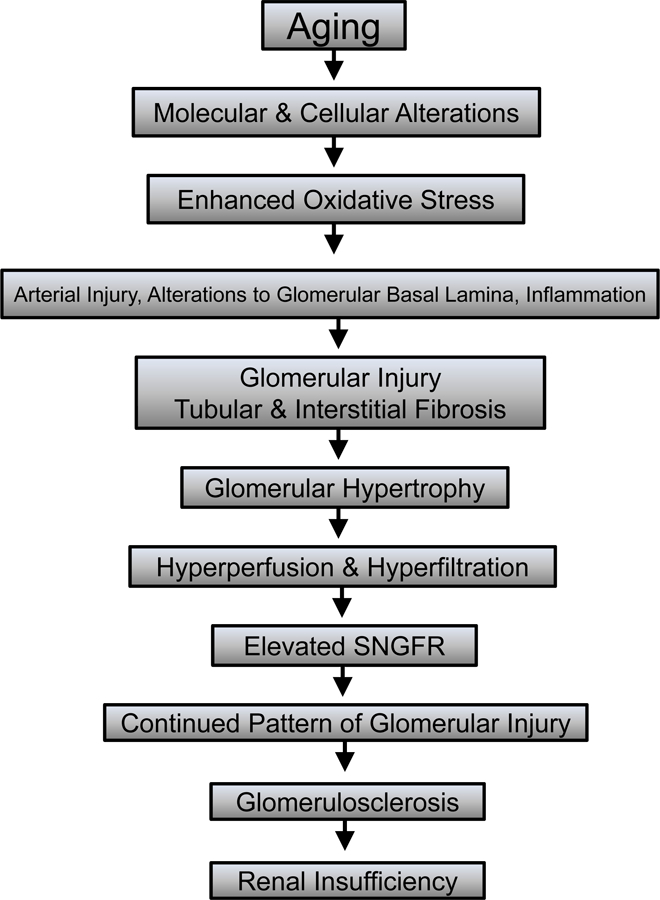
Numerous factors, such as molecular and cellular changes and alterations in the hemodynamics and structure of the glomerulus may contribute to age-related glomerulosclerosis. These alterations may lead to glomerular hypertrophy followed by hyperperfusion and hyperfiltration of affected glomeruli. As a result of these hypertrophic changes, the single nephron glomerular filtration rate often increases, leading to glomerular injury, glomerulosclerosis, and renal insufficiency.
Interestingly, glomeruli in the outer cortex appear to be affected earlier and more severely by sclerotic changes than those in the juxtamedullary region (Rodriguez-Puyol 1998; Ljungqvist and Lagergren 1962; Choudhury 2004). As cortical glomeruli degenerate, glomerular capillaries atrophy, which leads to sclerosis. Interestingly, in juxtamedullary glomeruli, a direct channel is formed between afferent and efferent arterioles resulting in arterioles that do not communicate hemodynamically with a glomerulus (Lopez-Novoa 2008). In general, these aberrant glomeruli tend to be larger than cortical glomeruli; therefore, a significant reduction in the functionality of this population of glomeruli likely leads to significant decrease in whole-body GFR. An important marker of glomerular damage is proteinuria, which results from a disruption of the glomerular filtration barrier. Indeed, several studies suggested that injury to and dysfunction of podocytes, which are key components of the glomerular filtration barrier, may play a role in the pathogenesis of age-related glomerulosclerosis (Wiggins 2009; Camici et al. 2011; Wharram et al. 2005).
Structural Changes in Renal Tubules and Interstitium of Aged Kidneys
Age-related changes also occur in renal tubules and interstitium. These alterations include atrophy and degeneration, formation of diverticula, irregular thickening of the tubular basement membrane and tubulointerstitial fibrosis, which is associated with interstitial inflammation, fibroblast activation, and enhanced deposition of collagen (Thomas et al. 1998; Ding et al. 2001; Abrass et al. 1995; Zhou et al. 2008; Denic et al. 2016; Karam and Tuazon 2013). Macrophages and myofibroblasts, which are involved in collagen deposition, were identified in interstitial areas of fibrosis (Nakatsuji et al. 1998). The presence of myofibroblasts in these areas correlates with greater deposition of collagens (types I and III) (Nakatsuji et al. 1998) and non-collagenous proteins (Abrass et al. 1995) found in the interstitial space of fibrotic nephrons. This deposition is probably a primary cause of fibrosis and expansion of the interstitium. In addition, apoptosis of tubular and interstitial cells appears to be enhanced in fibrotic areas (Thomas et al. 1998). Apoptosis of these cell populations may be one reason for the observed decrease in volume, length, and number of tubular segments from fibrotic nephrons (Lindeman and Goldman 1986; Silva 2005; Zhou et al. 2008). As a consequence of these structural changes, alterations in tubular function may also occur. These alterations may include a diminished ability to concentrate/dilute urine (Rowe et al. 1976), maintain acid/base balance (Adler et al. 1968), and filter solutes (Macias-Nunez and Lopez-Novoa 2008; Zhou et al. 2008).
Physiologic Changes in the Aged Kidney
Since the aging kidney is subjected to considerable alterations in structure, it is not surprising to find that GFR also changes significantly as the kidney ages (Choudhury 2004; Zhou et al. 2008; Musso and Oreopoulos 2011; Rodriguez-Puyol 1998; Davies and Shock 1950; Lerma 2009). Beginning around 40 years of age, it is estimated that total GFR decreases by approximately 10% per decade of life (Baylis and Schmidt 1996; Davies and Shock 1950; Lerma 2009; Silva 2005; Weinstein and Anderson 2010). The rate of decline was found to rise after the age of 65 (Macias-Nunez and Lopez-Novoa 2008; Shock 1946; Davies and Shock 1950). The Baltimore Longitudinal Study of Aging, which collected data from patients aged 17 to 96, over a 23-year period, found that creatinine clearance (i.e., GFR) declined by 0.75 ml/min per year (Lindeman et al. 1985; Shock 1984). This decrease appears to be due to multiple factors including glomerulosclerosis, injury to and inadequate regeneration of podocytes, and an eventual reduction in the total number of functioning nephrons (Rule et al. 2011; Wharram et al. 2005; Hoshi et al. 2002). The overall findings of the Baltimore Longitudinal Study of Aging indicated that GFR fell steadily over time. However, of the 254 patients evaluated, 92 (36%) showed no significant reduction in GFR (Lindeman et al. 1985). Interestingly, individual variations in the expression of certain genes, such as those related to cell cycle regulation, may be linked to the tendency to develop age-related glomerular changes that lead to diminished GFR (Zheng et al. 2003; Melk et al. 2004; 2005; Zha et al. 2008). Thus, individual variations in gene expression may account for the lack of change in GFR observed in some patients. Despite the mixed findings of the Baltimore Longitudinal Study, there is overwhelming evidence to suggest that a decline in GFR is a normal consequence of aging (Glassock and Winearls 2009; Davies and Shock 1950; Epstein 1996; Silva 2005; Glassock and Rule 2016).
In addition to changes in GFR, aging also appears to affect renal blood flow (RBF) to the kidney (Choudhury 2004; Epstein 1996; Lerma 2009). Maximal RBF is reached around the third decade following which, RBF was found to decrease by approximately 10% per decade of life (Rodriguez-Puyol 1998). This fall appears to be related to alterations in vascular resistance in afferent and efferent arterioles rather than to changes in cardiac output (Naeije et al. 1993) or renal perfusion (Hollenberg et al. 1974). Because the decrease in total GFR is generally less than reduction in RBF, filtration fraction (FF) rises in most patients (Silva 2005; Epstein 1996; Choudhury 2004; Baylis and Schmidt 1996). It should be pointed out that although overall RBF falls, variable changes in blood flow occur at the level of the individual nephron. Within a single, hypertrophied nephron, RBF increases as a result of the hypertrophic changes that occur within that nephron (Fine 1992; Hayslett 1979). Elevation in RBF may lead to increased intraglomerular pressure, which may consequently result in glomerular injury.
Renal functional reserve (RFR) may also be altered in older individuals. Renal functional reserve has been defined as the ability of the kidney to increase its basal RBF and GFR by 20% or more after a stimulus, such as a protein load (Musso and Oreopoulos 2011; Bosch et al. 1986). Studies of RFR in older individuals yielded mixed results. While some investigations found that RFR is preserved to some extent in healthy individuals, aged 61–82 (Bohler et al. 1993; Fliser et al. 1993), Esposito and colleauges (2007) demonstrated that RFR is reduced or depleted in older individuals in order to accommodate for the age-related decline in renal function and as an attempt to preserve normal renal function. Musso et al. (2011) also found that RFR of older individuals remained intact, but its magnitude was lower than that in younger individuals. Similarly, in vivo studies using aged Sprague-Dawley rats provided evidence for an age-related decline in RFR (Uriu et al. 2000). In addition, the functional reserve of the kidneys appears to be decreased or absent in patients with diseases such as diabetes and hypertension (Raes et al. 2007;Zaletel et al. 2004;Gabbai 1995; Pecly et al. 2006).
Aging and Kidney Disease
There is an apparent association between aging and development of kidney disease. One can speculate that as the kidneys age, these organs lose their capacity to cope with certain challenges. Therefore, when an aged kidney is challenged, physiologically, pathologically or toxicologically, renal function may be affected. Indeed, an epidemiological study analyzing 437 cases of acute renal failure in Spain demonstrated that acute renal failure was 3.5-fold more prevalent in adults over the age of 70 than in younger adults (Pascual et al. 1990). Similarly, an analysis of data obtained from the Third National Health and Nutrition Examination Study (NHANES) in the United States noted that the incidence of chronic kidney disease (CKD) increases with age (Coresh et al. 2003; USRDS 2015). Coresh et al. (2003) noted that, even in the absence of other diseases such as hypertension and diabetes, approximately 11% of individuals over the age of 65 were diagnosed with moderate to severe renal failure. The progression of renal failure also appeared to occur more rapidly in aged than younger patients (Coresh et al. 2003; O’Hare et al. 2007). Ali and colleagues (2007) examined elderly patients with acute or chronic renal failure and suggested that older individuals are at greater risk of morbidity associated with their disease.
Comorbidities such as hypertension, cardiovascular disease, diabetes, and obesity are common in the elderly population (Table 1) and are considered to be significant risk-factors for the development of CKD (Abdelhafiz et al. 2010; Silva 2005; Anderson et al. 2009). In the United States, approximately 30% of the adult population is affected by hypertension (Yoon SS 2010; Keenan and Rosendorf 2011; Nwankwo et al. 2013), the incidence of which has been shown to increase with age (Nwankwo et al. 2013). Indeed, approximately 65% of adults over the age of 60 were diagnosed with hypertension (Keenan and Rosendorf 2011; Nwankwo et al. 2013), which has been associated with more rapid progression of CKD (Perry et al. 1995). A similar trend exists for diabetes, which affects approximately 29 million individuals in the United States (CDC 2014). Of individuals aged 65 and older, nearly 26% were diagnosed with diabetes (CDC 2014) and approximately 30% of these patients may develop CKD later in life (NKF 2016). The incidence of cardiovascular disease and obesity, which are also risk factors for CKD, is also greater in the older population (> 60 years) (Ogden et al. 2015; CDC 2014) The presence of diseases such as hypertension and/or diabetes in aging patients may enhance the normal age-related decline in renal function (Abdel-Kader and Palevsky 2009). Therefore, it is reasonable to suggest that older patients with superimposed diseases are more susceptible to development of CKD. It is also important to consider the rise in prescription drug use in older individuals. Not only does aging lead to a reduction in renal function, but it may also lead to a decrease in drug metabolism and elimination of drug metabolites (Jerkic et al. 2001; Rawlins et al. 1987; Kinirons and O’Mahony 2004; Hilmer et al. 2005). For example, non-steroidal anti-inflammatory drugs (NSAIDs) may be used frequently by older individuals. In fact, the use of these drugs by the aged and elderly population is estimated to be 3-fold higher than that of the younger population (Jerkic et al. 2001). In addition, the time required to metabolize and eliminate drugs was demonstrated to be greater in the elderly than in younger individuals (Cuny et al. 1979; Hilmer et al. 2005; Ruscin 2016). The altered handling of drugs may be due, in part, to age-related loss of renal function. Considering the elevated use and reduced elimination of certain drugs in older individuals, it is possible that utilization of these drugs may predispose individuals to renal injury and/or insufficiency.
Table 1.
Estimated number of older adults in the United States affected by chronic kidney disease (CKD) and its comorbidities.
| Hypertension > 60 years |
Type II Diabetes > 65 years |
Obesity > 60 years |
Cardiovascular Disease | CKD >60 years |
||
|---|---|---|---|---|---|---|
| 65–74 years | > 75 years | |||||
| U. S. Adults |
65.0 %1 | 25.9 %2 | 37.0 %3 | 24.6 %4 | 35.0 %4 | 33.2 %5 |
Aging and Exposure to Mercury
Mercury is a Major Environmental Nephrotoxicant
Since toxic metals are abundant in the environment as well as in many occupational settings, human exposure to these metals is inadvertent and inevitable. Exposure of older individuals to toxic metals that are also nephrotoxicants may promote or enhance the progression of renal disease (Jarup et al. 1995; 1998; Kim et al. 2015b; Hellstrom et al. 2001; Moriguchi et al. 2005). Mercury (Hg) is a toxic metal that is of particular concern since certain forms are highly nephrotoxic while exposure to other forms may lead to significant neurotoxic effects. Mercury can exist in elemental (metallic), inorganic, and/or organic forms. Elemental mercury (Hg0), which exists as a liquid at room temperature, has a low vapor pressure and thus, vaporizes readily to become Hg vapor. Inorganic Hg may be found as mercurous (Hg1+) or mercuric (Hg2+) ions, which are usually bound to chlorine, sulfur, or oxygen to form mercurous or mercuric salts. In the environment, inorganic Hg is usually found in the mercuric form. Organic forms of Hg include phenylmercury, dimethylmercury, and monomethylmercury. Of these forms, methylmercury (MeHg) is the most frequently encountered in the environment. MeHg is formed primarily when inorganic mercuric ions are methylated by microorganisms present in soil and water (ATSDR 2008; Clarkson and Magos 2006; Rooney 2007; Zalups 2000).
Humans may be exposed to various forms of Hg through occupational and environmental settings, although exposure may also be due to the presence of dental amalgams or the use of certain medicinal products (ATSDR 2008; Clarkson and Magos 2006; Risher and De Rosa 2007; Zalups 2000). The most common route of human exposure, however, is via the ingestion of food, primarily fish, contaminated with MeHg (Nunes et al. 2014; 2014; Wolff et al, 2016; Lopez-Barrera and Barragan-Gonzalez, 2016). The flesh of large predatory fish, such as northern pike, swordfish, shark, and some species of tuna may contain high levels of CH3Hg+ and represents a major source of Hg exposure (Siedlikowski et al. 2016; Burger and Gochfeld 2011). Upon ingestion, MeHg is absorbed readily by the gastrointestinal tract (Vazquez et al. 2013; 2014) following which, mercuric ions enter systemic circulation and are delivered to target organs.
Renal Handling of Mercury
Inorganic and organic forms of Hg accumulate readily in the kidney. Although the kidney is the primary site of accumulation and toxicity of inorganic forms of Hg, exposure to organic Hg (e.g., MeHg) may also lead to serious adverse effects in the kidney (Murphy et al. 1979; Rowens et al. 1991; Samuels et al. 1982; Yasutake et al. 1989). Within the kidney, the proximal tubule is the primary site of uptake and accumulation of mercuric species (Zalups 2000). It is important to note that following exposure to MeHg, a fraction of the absorbed CH3Hg+ is oxidized within tissues and cells to form Hg2+ (Gage 1964; Norseth and Clarkson 1970a; 1970b; Omata et al. 1980). It should also be noted that within biological systems, mercurous, mercuric, or methylmercuric ions do not exist as inorganic salts, or in an unbound, “free” ionic state (Hughes 1957). Alternatively, mercuric ions are bound to one or more thiol-containing biomolecules, such as GSH, cysteine (Cys), homocysteine (Hcy), N-acetylcysteine (NAC), or albumin. Hg2+ binds to thiol-containing molecules in a linear II, coordinate covalent manner whereas MeHg forms linear I, coordinate covalent complexes with thiol-containing molecules (Figure 3) (Fuhr and Rabenstein 1973; Rubino et al. 2004). In addition, Hg may also bind to selenium (Se). Several studies in humans showed that administration of Se to individuals exposed to Hg reduces the overall intoxication induced by Hg (Bjorklund 2015; Chen et al. 2006). It is not clear if Hg-Se complexes are transportable forms of Hg. It is interesting to note that the binding affinity of Hg is greater for Se than for thiols (Sugiura et al. 1978); however, the concentration of Se in blood is approximately 11 µmol/L (Kucharzewski et al. 2002) while the concentration of Cys and GSH in blood is approximately 275 and 850 µmol/L, respectively (Michelet et al. 1995; El-Khairy et al. 2001). Since the normal blood concentrations of Cys and GSH are significantly greater than that of Se, it appears that circulating Hg is more likely to be bound to Cys and/or GSH than to Se.
Figure 3. Similarities between mercuric species and amino acids.
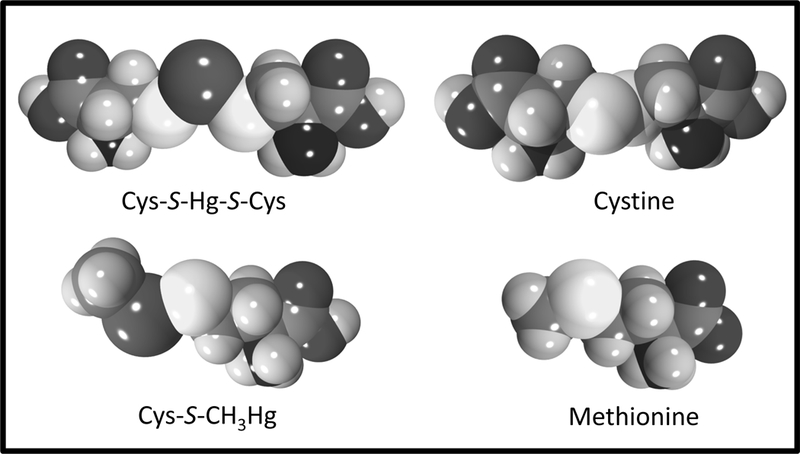
The binding of cysteine (Cys) to inorganic mercury (Hg2+) forms a linear II coordinate covalent complex (Cys-S-Hg-S-Cys) while binding of Cys to methylmercury (CH3Hg+) forms a linear I coordinate covalent complex (Cys-S-CH3Hg). Cys-S-Hg-S-Cys is similar in shape and size to the amino acid, cystine while Cys-S-CH3Hg is similar to the amino acid, methionine.
Inorganic Hg accumulates rapidly in renal tissue with as much as 50% of a nontoxic dose present in the kidneys within a few hr after exposure (Zalups 1993). The majority of mercuric ions accumulate in the epithelial cells lining the proximal tubule (Zalups 2000). Mercuric ions appear to be taken into proximal tubular cells via transport mechanisms present on the luminal and basolateral membranes (Cannon et al. 2000; 2001; Zalups 1998; Zalups and Lash 1997; Zalups and Minor 1995). The primary species of Hg2+ that is transported into cells at the luminal membrane appears to be a Cys S-conjugate (Cys-S-Hg-S-Cys) of Hg2+ (Zalups and Barfuss 1995;1996;1998). Subsequent studies in isolated perfused tubules implicated amino acid transporters in the luminal uptake of thiol-S-conjugates of Hg+ by proximal tubular cells (Cannon et al. 2000; 2001). Cys-S-Hg-S-Cys is similar in size and shape to the amino acid cystine (Figure 3), and thus, it was suggested that this mercuric conjugate may utilize a cystine transporter to gain access to the intracellular compartment of proximal tubular cells. Studies using Madin-Darby Canine Kidney (MDCK) cells stably transfected with the sodium-independent cystine transporter, System b0,+, provide evidence implicating this carrier in the uptake of Cys-S-Hg-S-Cys from the tubular lumen into proximal tubular cells (Figure 4) (Bridges et al. 2004). Similar studies reported that the Hcy S-conjugate of Hg2+ (Hcy-S-Hg-S-Hcy) is also transported by System b0,+ (Bridges and Zalups 2004). As of yet, a sodium-dependent transport mechanism has not been identified for the luminal uptake of Cys-S-Hg-S-Cys and/or Hcy-S-Hg-S-Hcy into proximal tubular cells. In contrast, evidence from studies using Xenopus laevis oocytes indicates that Cys-S-conjugates of MeHg (Cys-S-MeHg), which are similar in shape to methionine (Figure 3) are substrates of the sodium-dependent amino acid carrier, System B0,+ (Bridges and Zalups 2006). Currently, there are no apparent data supporting a role for System B0,+ in the uptake of Cys-S-Hg-S-Cys or Hcy-S-Hg-S-Hcy (Bridges and Zalups 2006).
Figure 4. Schematic representation of the transport and toxicity of mercury in proximal tubular cells.
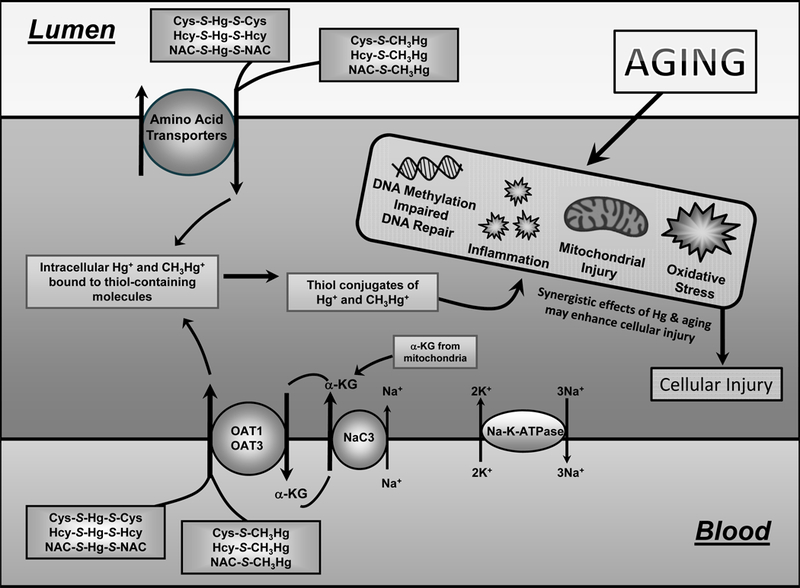
Inorganic (Hg2+) and organic (CH3Hg+) forms of mercury enter proximal tubular cells as conjugates of thiols (cysteine, Cys; homocysteine, Hcy; N-acetylcysteine, NAC) at the luminal membrane via amino acid transporters and at the basolateral membrane via organic anion transporters (OAT) 1 and 3. OAT1 and 3 are driven by the circular movement of α-ketoglutarate (α-kg), which enters the cell via the Na+-dicarboxylate transporter (NaC3). Once inside the cell, mercuric conjugates may lead to changes in DNA methylation and repair, inflammation, mitochondrial injury, and oxidative stress. These cellular changes may also be caused by the aging process. Exposure of aged individuals to mercuric compounds may enhance the susceptibility of cells to injury because of the synergistic effects of age and mercury on the cells.
Approximately 40–60% of the Hg2+ that accumulates in proximal tubular cells appears to be taken up at the basolateral plasma membrane (Zalups and Barfuss 1995; 1998a; 1998b; Zalups 1995; 1998a; 1998b; Zalups and Minor 1995). The organic anion transporters (OAT) 1 and 3 are localized in the basolateral plasma membrane of proximal tubular epithelial cells (Kojima et al. 2002; Motohashi et al. 2002) and appear to be involved in the uptake of Cys-S-Hg-S-Cys, NAC-S-Hg-S-NAC, and Hcy-S-Hg-S-Hcy at the basolateral membrane of proximal tubular cells (Figure 4) (Zalups 1995; 1998a; 1998b; Zalups and Barfuss 1995; 1998a; 1998b; Zalups and Lash 1997; Aslamkhan et al. 2003; Zalups and Ahmad 2004; Zalups et al. 2004). Studies using cultured MDCK cells stably transfected with OAT1 indicate that mercuric conjugates of Cys, Hcy, and NAC (NAC-S-Hg-S-NAC) are all substrates of this carrier (Aslamkhan et al. 2003; Koh et al. 2002; Zalups and Ahmad 2004; Zalups et al. 2004). In addition, Cys-S-Hg-S-Cys also appears to be a transportable substrate of OAT3 (Aslamkhan et al. 2003). OAT1 was also found to mediate the basolateral uptake of MeHg, as a conjugate of Cys, NAC, or Hcy (Zalups and Ahmad 2005a; 2005b; 2005c). Interestingly, single nucleotide polymorphisms (SNP) in OAT1 and OAT3 have been associated with enhanced urinary excretion of Hg in certain populations (Engstrom et al. 2013). It should be noted that other studies suggest that SNP in OAT1 and OAT3 may not occur frequently enough in a population to significantly alter the renal handling of substrates by a large fraction of the population (Fujita et al. 2005; Xu et al. 2005).
Once mercuric ions enter the intracellular compartment of cells, they form strong bonds with protein and non-protein thiol-containing biomolecules. In particular, mercuric ions have a strong affinity for intracellular metallothionein (MT) (Cherian and Clarkson 1976; Zalups and Koropatnick 2000). Exposure of animals to various forms of Hg increases intracellular levels of MT (Wisniewska et al. 1970; Piotrowski et al. 1974; Planas-Bohne et al. 1985). MT binds to mercuric ions within the cells and forms a stable complex that appears to reduce the intoxication produced by mercuric ions (Satoh et al. 1997). This complex is also less likely to be transported out of cells leading to retention of mercuric ions within cells (Planas-Bohne et al. 1985;Zalups et al. 1993;1995).
Mercuric ions also have a strong affinity for GSH (Fuhr and Rabenstein 1973) and may bind to GSH, both intracellularly and extracellularly. The concentration of GSH in renal tubular cells is in the mM range (Brehe et al. 1976), which makes GSH well-suited for binding to and neutralizing intracellular mercuric ions. Indeed, exposure of mice to HgCl2 lowered renal levels of intracellular GSH (Bridges et al. 2013), suggest that GSH is utilized to counteract the oxidative effects of Hg2+. In addition, the mRNA expression of glutamate-cysteine ligase (GCL), the rate-limiting enzyme in the synthesis of GSH, was found to be elevated following exposure to HgCl2 (Bridges et al. 2013; Oliveira et al. 2016). This increased expression is likely an attempt to synthesize additional GSH in order to counteract the oxidative stress induced by exposure to Hg2+. Although the binding of mercuric ions to intracellular biomolecules, such as GSH, is important to diminish the possibility of Hg-induced intoxication within the cell, this binding may also contribute to retention of mercuric ions within cells in that these GSH S-conjugates of Hg2+ may not be transported easily and/or readily out of cells.
In order to efficiently remove mercuric ions from within renal epithelial cells, metal complexing agents, such as 2,3-bis(sulfanyl)propane-1-sulfonic acid (formally known as 2,3-dimercaptopropane-1-sulfonic acid; DMPS) (Ruprecht 2008) or 2,3-dimercaptosuccinic acid (DMSA) (Aposhian 1983; Aposhian et al. 1992; 1995; Planas-Bohne 1981; Ruprecht 2008; Zalups 1993) are required. Several investigators demonstrated that DMPS and DMSA gain access to proximal tubular cells via OAT1, OAT3 and/or the sodium-dependent dicarboxylate transporter (NaC2) in the basolateral plasma membrane (Rodiger et al. 2010; Bahn et al. 2002; Burckhardt et al. 2002; Islinger et al. 2001). Once inside the cell, it is postulated that DMPS and DMSA (in their reduced forms) bind to mercuric ions to form DMPS or DMSA S-conjugates of Hg2+ or CH3Hg+. These complexes appear to be secreted into the tubular lumen for eventual excretion in the urine (Aposhian 1983; Aposhian et al. 1992). Early studies examining the tubular secretion of thiol S-conjugates of CH3Hg+ implicated the multidrug resistance-associated protein 2 (Mrp2) in the export of NAC-S-conjugates of MeHg from proximal tubular cells (Aremu et al. 2008; Madejczyk et al. 2007). Similarly, studies utilizing TR— (Mrp2-deficient) rats showed that Mrp2 is involved in the DMPS- and DMSA-mediated extraction of Hg2+ from proximal tubular cells (Bridges et al. 2008a; 2008b). In addition, in vitro studies utilizing inside-out, brush-border membrane vesicles prepared from Sf9 cells transfected with human MRP2 provided direct evidence indicating that DMPS- and DMSA-S-conjugates of Hg2+ are transportable substrates of MRP2 (Bridges et al. 2008a; 2008b). MRP2 polymorphisms were also identified in certain populations and appear to enhance the urinary excretion of Hg in these populations (Engstrom et al. 2013). Ballatori (2002) proposed that GSH-S-conjugates of Hg2+ or CH3Hg+ may be transportable substrates of MRP2; however, at present, there are no apparent data to support this theory.
In addition to MRP2, the breast cancer resistance protein (BCRP) may play a role in proximal tubular export of mercuric ions. Studies using Bcrp knockout rats (Bcrp−/−) noted that mercuric ions are retained within target organs such as kidney and liver in the absence of Bcrp (Bridges et al. 2015). These data suggest that Bcrp may play a role in the export of mercuric species from within renal tubular cells. Additional studies using inside-out membrane vesicles from Sf9 cells overexpressing human BCRP provide direct evidence showing that DMPS S-conjugates of Hg+2 are transportable substrates of BCRP (Bridges et al. 2015). Further in vivo studies are needed to fully characterize the role of BCRP in the transport of various thiol S-conjugates of Hg2+.
Renal Effects of Mercury Exposure
Exposure to all forms of Hg might lead to nephrotoxic effects (Murphy et al. 1979; Rowens et al. 1991; Samuels et al. 1982; Yasutake et al. 1989). In fact, numerous epidemiological studies reported evidence of renal injury following acute and chronic exposure to various forms of Hg (Ha et al. 2016; Pollack et al, 2015). However, the most severe nephropathy is induced following exposure to Hg2+. In vitro studies indicate that this nephropathy initially affects the pars recta of the proximal tubule, located in the inner cortex and outer stripe of the outer medulla, suggesting that this section of the nephron is the most sensitive to the toxic effects of Hg (Zalups 2000; Bridges et al. 2013; Zalups et al. 2014; Zalups and Diamond 1987). Exposure to moderate or high doses of HgCl2 results in cellular necrosis along the pars recta, pars convoluta and distal segments of the nephron (McDowell et al. 1976; Zalme et al. 1976; Zalups et al. 2014). Under these conditions, cellular injury along the pars recta is rapid and signs of injury such as swelling of mitochondrial matrices and the presence of pyknotic nuclei may be detected via electron microscopy in as little as 3 hr (Rodin and Crowson 1962; Gritzka and Trump 1968). After 6 hr, cells begin to lose microvilli, mitochondrial swelling worsens and the endoplasmic reticulum becomes dilated (McDowell et al. 1976; Gritzka and Trump 1968). The activities of selected enzymes including alkaline phosphatase (AP), succinic dehydrogenase, arginase II in proximal tubular cells also appear to be diminished (Zalme et al. 1976; Kanda et al. 2008). Twelve hr after exposure to HgCl2, electron microscopic analyses of pars recta segments revealed rupture of the plasma membrane, loss of microvilli, decreased contact with the basement membrane, and loss of cell shape (Gritzka and Trump 1968). After 24 hr, cellular fragments may be identified in the tubular lumen, junctional complexes between cells are absent, and nuclear structure is compromised (Gritzka and Trump 1968; Rodin and Crowson 1962; Zalme et al. 1976). When the plasma membrane of tubular epithelial cells is compromised, numerous brush-border and intracellular enzymes, such as kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), AP, γ-glutamyltransferase (γ-GT), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and glutathione peroxidase (GPx) were detected in urine (Zalups 1988a; 2000; Gotelli et al. 1985; Price 1982; Planas-Bohne 1981; Zalups and Diamond 1987;Prozialeck and Edwards 2010; Roels et al. 1999; Yasutake et al. 1997). Exposure of rats to nephrotoxic doses of Hg2+ was found to enhance renal expression of Kim-1 and Ngal (Bridges et al. 2014). As Hg-induced renal injury progresses, there is also a simultaneous rise in urinary excretion of mercuric ions (Clarkson and Magos 1967; Magos and Stoytchev 1969; Trojanowska et al. 1971).
Accumulation of Hg within proximal tubular cells induces oxidative stress and exerts significant effects on enzymes that are responsible for reducing oxidative stress and managing detoxification within cells (Joshi et al. 2014a; 2014b; Stacchiotti et al. 2009). In a study of healthy male industrial workers in Saudi Arabia, chronic exposure to Hg was found to decrease renal expression of proteins involved in detoxification and the reduction of oxidative stress such as NAD(P)H:quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), alpha-glutathione S-transferase (GSTA1), MT-1, and heat shock protein 70 (HSP70) (Al Bakheet et al. 2013). Reductions in these protective mechanisms may further enhance the susceptibility of cells to intoxication and injury by toxicants such as Hg. Similarly, when healthy adult rats were exposed chronically to HgCl2, renal levels of thiobarbituric acid reactive substances (TBARS) and GPx were found to be elevated, suggesting that Hg induces intracellular oxidative stress in renal epithelial cells (Agrawal et al. 2014). In the same study, levels of SOD, CAT, GSH, and glutathione disulfide (GSSG) were reported to be lowered suggesting that these enzymes were depleted in the process of counteracting the oxidative effects of Hg (Agrawal et al. 2014). In contrast, acute exposure of healthy rats to HgCl2 led to an rise in enzymes such as AP and LDH (Joshi et al. 2014b), suggesting that the initial exposure to Hg leads to an early increase in enzyme levels, which may become depleted eventually if exposure to Hg becomes chronic. Exposure of renal cells to various species of Hg and subsequent induction of intracellular oxidative stress may also initiate mitochondrial injury and dysfunction, which results in a reduction in overall cell viability (Lund et al. 1993; Shenker et al. 1998; Carranza-Rosales et al. 2005; Carocci et al. 2014). Since many of the injurious cellular effects of Hg exposure are similar to those induced by aging, one should consider that exposure of aged kidneys to Hg may lead to effects that are synergistic, producing enhanced intoxication of proximal tubular cells.
Exposure to mercuric species may also result in pathological changes in glomeruli, such as membranous glomerulonephritis, which is characterized by a thickened glomerular basement membrane and proliferation of mesangial cells. Tubulointerstitial injury and fibrosis, granular degeneration of epithelial cells and infiltration of immune cells also occurs (Li et al. 2010; Zalups et al. 2014). Rats exposed to a non-nephrotoxic dose of HgCl2 for 21 weeks developed significant tubular, interstitial and glomerular lesions (Hall et al. 1986), even though there were no significant differences in the measurable parameters of renal function such as plasma urea nitrogen, plasma creatinine, and protein excretion. Similarly, exposure of rats to MeHg for two years led to fibrotic changes in a fraction of glomeruli (Eto et al. 1997). In addition, deposits of IgG, IgM and C3 were detected along the glomerular basal lamina (Eto et al. 1997), which is characteristic of an immunological glomerulopathy (Druet et al. 1978; Bigazzi 1992; 1988; Zalups 2000). Glomerular changes such as fibrosis and thickening of the glomerular basal lamina often result in reductions in GFR. Therefore, it is not surprising that following exposure to Hg2+, decrease in GFR was observed (McDowell et al. 1976; Vanholder et al. 1982).
Based on these studies, it is apparent that acute and chronic exposure to mercuric compounds produces numerous, deleterious effects in the kidneys. Given that older individuals may have reduced functional renal mass due to glomerulosclerosis, tubular atrophy and interstitial fibrosis and GFR, it is possible that exposure of these individuals to mercuric compounds may be even more detrimental to the health of the patient. Unfortunately, few studies examined the link between age, exposure to mercuric compounds, and patient health.
Aging and the Effects of Mercury Exposure on the Kidney
Older individuals make up a significant percentage of the population, and exposure to mercuric compounds appears to be increasing, yet little is known regarding the relationship between aging kidneys and effects of Hg exposure on kidneys. Recent epidemiological studies in human populations indicate that the renal burden of Hg increases with age (Habiba et al. 2016; Song et al. 2016; Carneiro et al. 2014). Similarly, studies measuring bioaccumulation in wild roe deer from Spain and free-range European hares from Serbia also showed that the renal burden of heavy metals such as Hg increases as animals age (Petrovic et al. 2014; Hermoso de Mendoza Garcia et al. 2011) Recent in vivo studies utilizing two- and 20-month-old Wistar rats, demonstrated that the overall accumulation of Hg2+ was greater in kidneys of older than younger rats (Bridges et al. 2014; Oliveira et al. 2016). Similarly, Kostial et al (1978) compared the distribution of Hg2+ in 2-week-old to 21-week-old rats and found that renal accumulation of Hg2+ was higher in kidneys of older rats (Kostial et al. 1978). Interestingly, chronic exposure to MeHg was found to correlate with development of Type II diabetes (Schumacher and Abbott 2016) and hypertension (Houston 2011; Rajaee et al. 2015), which are considered to be significant risk factors of CKD (Ginsberg et al, 2014). Therefore, exposure to Hg may contribute to the development of CKD or it may enhance the progression of the disease. Indeed, a recent study of residents living near the Chatian Hg mine in southwestern China reported that older (> 60 years) individuals had higher blood Hg and serum creatinine levels (indicative of lower GFR) than adults (18 – 60 years) and children (< 18 years) in the same area (Li et al. 2013). Data suggest that older individuals who are exposed to Hg are more likely to experience renal insufficiency or CKD than older individuals who are not exposed to Hg. However, it is not clear if the increased blood Hg and creatinine levels are due simply to the age of the individuals or if there is a valid correlation between renal function and exposure to Hg. Alternatively, exposure to Hg may enhance progression or exacerbate symptoms of CKD in current patients. Because many patients in the early stages of CKD are asymptomatic (CDC 2012), a diagnosis is often not made until renal function has been compromised significantly and GFR is well below the normal range. During this period, patients may continue to be exposed to nephrotoxicants including Hg. Such exposure may enhance morbidity and mortality as these patients may be more sensitive to the adverse effects of Hg. While an association between age, exposure to Hg, and CKD has not been established with certainty, there are a number of studies showing that exposure to nephrotoxic metals, such as lead (Pb), cadmium(Cd) and cisplatin, exacerbate renal insufficiency in older individuals (Ferraro et al. 2010; Noonan et al. 2002; Roels et al. 1991; Sommar et al. 2013; Kim et al. 2015b; Wen et al. 2015). Since exposure to Cd or Pb were found to exert adverse effects on renal function of older individuals, it may be postulated that exposure of these individuals to Hg may also exacerbate renal disease.
Although, there are few human studies linking exposure of Hg to an enhanced risk of renal injury in older individuals, there are several reports using rat models that link exposure to Hg with reduced renal function. In aged kidneys, the total number of functioning nephrons has decreased significantly, which is similar to the renal mass of a uninephrectomized rat. Although the uninephrectomized rat model does not precisely mimic changes that occur in an aging kidney, there are similarities between the two systems. In each, a significant number of nephrons are lost and remaining nephrons need to undergo a compensatory, hypertrophic phase in order to maintain normal fluid and electrolyte homeostasis (Fine and Norman 1989; Fine 1992; Hayslett 1979). Hyperperfusion and hyperfiltration appear to occur in hypertrophied nephrons and may result in these nephrons being exposed to higher levels of nephrotoxicants such as Hg. This increased exposure may enhance susceptibility of these nephrons to the deleterious effects of Hg or other nephrotoxicants (Houser and Berndt 1986; 1988; Ramos-Frendo et al. 1979; Zalups 1991, 1997; Zalups et al. 1987;1992; Zalups and Diamond 1987) .
Several studies utilizing uninephrectomized rats support the idea that hypertrophied nephrons are more sensitive to the adverse effects of Hg. Ramos-Frendo and colleagues (1979) exposed uninephrectomized and sham rats to a nephrotoxic dose of HgCl2 and found that development of Hg-induced renal failure was more pronounced in uninephrectomized animals than in shams. Similarly, Houser and Berndt (1986) exposed uninephrectomized and sham rats to a nephrotoxic dose of HgCl2, following which they assessed renal susceptibility to mercuric ions. Exposure to HgCl2 induced glomerular and tubular dysfunction, which appeared to be more severe in uninephrectomized rats than in sham rats (Houser and Berndt 1986) . In a separate, more detailed study using uninephrectomized and sham Sprague-Dawley rats exposed to a nephrotoxic dose of HgCl2, it was discovered that mercuric ions were redistributed within the kidney following uninephrectomy (Houser and Berndt 1988). The concentration of mercuric ions was greater in renal cortex and outer stripe of the outer medulla in uninephrectomized rats than in shams. Not surprisingly, the urinary excretion of mercuric ions (per kidney) was greater in uninephrectomized rats than in shams (Houser and Berndt 1986; 1988).Similarly, Zalups and Diamond (1987) treated uninephrectomized and sham Long Evans hooded rats to nephrotoxic doses of HgCl2 and noted that Hg-induced proximal tubular necrosis was more extensive in uninephrectomized than sham animals. In addition, urinary excretion of cellular enzymes and plasma proteins, including LDH, γ-glutamyltransferase and albumin, was greater in uninephrectomized than sham animals (Zalups 1997; Zalups and Diamond 1987). Collectively, the results of these studies indicate that kidneys of animals with reduced renal mass are more susceptible to adverse effects of Hg2+. Similarly, it might be postulated that elderly and aged individuals who display reduced renal function due to normal aging processes and/or superimposed disease processes may be more susceptible to renal injury following exposure to a nephrotoxicant such as Hg.
Summary
The aging process in the kidneys was studied and characterized comprehensively. It is well-known that glomerulosclerosis leads to decreases in GFR and RBF. However, there is little information regarding the response of aged kidneys to environmental toxicants such as Hg. Because of the prevalence of Hg in the environment, human exposure is inadvertent and nearly unavoidable. Further, it is well-known that acute and chronic exposures to toxic metals may be detrimental to kidneys of normal adults, thus it may be postulated that exposure of individuals with CKD to these metals may result in additional reductions in renal function. Individuals with compromised renal function, either from aging, disease or a combination of aging and disease, may be particularly susceptible to nephrotoxicants. The potential association between aging and susceptibility to nephrotoxicants is supported by only a few studies. However, the available data appear to show an association between exposure to Hg and an increase in incidence and severity of renal disease. It is important to note that early signs of renal dysfunction often go unnoticed (CDC 2012), thus, individuals with reduced renal function are often unaware that they may be at risk during the early stages of disease. If exposure to nephrotoxicants occurs during this early period, this may be especially detrimental to these individuals. Therefore, a thorough and complete understanding of the manner in which nephrotoxicants are handled by aging kidneys is of utmost importance. Because of the paucity of data available on this topic, additional studies are clearly necessary.
Acknowledgments
Funding Details
This work was supported by the National Institutes of Health under grant ES019991.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
Contributor Information
Christy C. Bridges, Mercer University School of Medicine, Division of Basic Medical Sciences, Macon, Georgia 31207
Rudolfs K. Zalups, Mercer University School of Medicine, Division of Basic Medical Sciences, Macon, Georgia 31207
References
- Abdel-Kader K, and Palevsky PM 2009. Acute kidney injury in the elderly. Clin. Geriatr Med. 25: 331–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhafiz AH, Brown SH, Bello A, and El Nahas M 2010. Chronic kidney disease in older people: physiology, pathology or both? Nephron Clin. Pract 116: c19–c24. [DOI] [PubMed] [Google Scholar]
- Abrass CK, Adcox MJ, and Raugi GJ 1995. Aging-associated changes in renal extracellular matrix. Am. J. Pathol 146:742–752. [PMC free article] [PubMed] [Google Scholar]
- Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, and Chang HY 2007. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev 21: 3244–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler S, Lindeman RD, Yiengst MJ, Beard E, and Shock NW 1968. Effect of acute acid loading on urinary acid excretion by the aging human kidney. J. Lab. Clin. Med 72: 278–289. [PubMed] [Google Scholar]
- Agrawal S, Flora G, Bhatnagar P, and Flora SJ 2014. Comparative oxidative stress, metallothionein induction and organ toxicity following chronic exposure to arsenic, lead and mercury in rats. Cell Mol. Biol 60:13–21. [PubMed] [Google Scholar]
- Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, Kimura K, Hasegawa A, Kurabayashi M, Kuro-o M, Nabeshima Y, and Nagai R 1998. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem. Biophys. Res. Commun 249: 865–871. [DOI] [PubMed] [Google Scholar]
- Akcay A, Nguyen Q, and Edelstein CL 2009. Mediators of inflammation in acute kidney injury. Mediators Inflamm 2009:137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Bakheet SA, Attafi IM, Maayah ZH, Abd-Allah AR, Asiri YA, and Korashy HM 2013. Effect of long-term human exposure to environmental heavy metals on the expression of detoxification and DNA repair genes. Environ. Pollut 181: 226–232. [DOI] [PubMed] [Google Scholar]
- Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, and Macleod A 2007. Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J. Am. Soc. Nephrol 18:1292–1298. [DOI] [PubMed] [Google Scholar]
- Anderson S, and Brenner BM 1986. Effects of aging on the renal glomerulus. Am. J. Med 80: 435–442. [DOI] [PubMed] [Google Scholar]
- Anderson S, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kaysen GA, Kusek JW, Nayfield SG, Schmader K, Tian Y, Ashworth JR, Clayton CP, Parker RP, Tarver ED, Woolard NF, and High KP 2009. Prediction, progression, and outcomes of chronic kidney disease in older adults. J. Am. Soc. Nephrol 20:1199–1209. [DOI] [PubMed] [Google Scholar]
- Aposhian HV 1983. DMSA and DMPS--water soluble antidotes for heavy metal poisoning. Annu. Rev. Pharmacol. Toxicol 23: 193–215. [DOI] [PubMed] [Google Scholar]
- Aposhian HV, Maiorino RM, Gonzalez-Ramirez D, Zuniga-Charles M, Xu Z, Hurlbut KM, Junco-Munoz P, Dart RC, and Aposhian MM 1995. Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology 97: 23–38. [DOI] [PubMed] [Google Scholar]
- Aposhian HV, Maiorino RM, Rivera M, Bruce DC, Dart RC, Hurlbut KM, Levine DJ, Zheng W, Fernando Q, and Carter D 1992. Human studies with the chelating agents, DMPS and DMSA. J. Toxicol. Clin. Toxicol 30: 505–528. [DOI] [PubMed] [Google Scholar]
- Aremu DA, Madejczyk MS, and Ballatori N 2008. N-acetylcysteine as a potential antidote and biomonitoring agent of methylmercury exposure. Environ. Health Persp 116: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslamkhan AG, Han YH, Yang XP, Zalups RK, and Pritchard JB 2003. Human renal organic anion transporter 1-dependent uptake and toxicity of mercuric-thiol conjugates in Madin-Darby canine kidney cells. Mol. Pharmacol 63: 590–596. [DOI] [PubMed] [Google Scholar]
- ATSDR, Agency for Toxicological Sciences and Disease Registry. 2008. Toxicological Profile for Mercury, edited by United States Public Heath Services, Department of Health and Human Services Atlanta, GA: Centers for Disease Control. [Google Scholar]
- Bahn A, Knabe M, Hagos Y, Rodiger M, Godehardt S, Graber-Neufeld DS, Evans KK, Burckhardt G, and Wright SH 2002. Interaction of the metal chelator 2,3-dimercapto-1-propanesulfonate with the rabbit multispecific organic anion transporter 1 (rbOAT1). Mol. Pharmacol 62: 1128–1136. [DOI] [PubMed] [Google Scholar]
- Bai XY, Ma Y, Ding R, Fu B, Shi S, and Chen XM 2011. miR-335 and miR-34a Promote renal senescence by suppressing mitochondrial antioxidative enzymes. J. Am. Soc. Nephrol 22: 1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatori N 2002. Transport of toxic metals by molecular mimicry. Environ. Health. Persp 110 Suppl. 5: 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis C, and Schmidt R 1996. The aging glomerulus. Semin. Nephrol 16: 265–276. [PubMed] [Google Scholar]
- Berkenkamp B, Susnik N, Baisantry A, Kuznetsova I, Jacobi C, Sorensen-Zender I, Broecker V, Haller H, Melk A, and Schmitt R 2014. In vivo and in vitro analysis of age-associated changes and somatic cellular senescence in renal epithelial cells. PLoS One 9: e88071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigazzi PE 1988. Autoimmunity induced by chemicals. J. Toxicol. Clin. Toxicol 26: 125–156. [DOI] [PubMed] [Google Scholar]
- Bigazzi PE 1992. Lessons from animal models: The scope of mercury-induced autoimmunity. Clin. Immunol. Immunopathol 65: 81–84. [DOI] [PubMed] [Google Scholar]
- Bjorklund G 2015. Selenium as an antidote in the treatment of mercury intoxication. Biometals 28: 605–614. [DOI] [PubMed] [Google Scholar]
- Bohler J, Gloer D, Reetze-Bonorden P, Keller E, and Schollmeyer PJ 1993. Renal functional reserve in elderly patients. Clin. Nephrol 39:145–150. [PubMed] [Google Scholar]
- Bosch JP, Lew S, Glabman S, and Lauer A 1986. Renal hemodynamic changes in humans. Response to protein loading in normal and diseased kidneys. Am. J. Med 81: 809–815. [DOI] [PubMed] [Google Scholar]
- Brehe JE, Chan AW, Alvey TR, and Burch HB 1976. Effect of methionine sulfoximine on glutathione and amino acid levels in the nephron. Am. J. Physiol 231:1536–1540. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Bauch C, Verrey F, and Zalups RK 2004. Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0,+): implications of molecular mimicry. J. Am. Soc. Nephrol 15: 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, van den Heuvel JJ, Russel FG, and Zalups RK 2013. Glutathione status and the renal elimination of inorganic mercury in the Mrp2(−/−) mouse. PLoS One 8: e73559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, and Zalups RK 2008a. MRP2 and the DMPS- and DMSA-mediated elimination of mercury in TR(−) and control rats exposed to thiol S-conjugates of inorganic mercury. Toxicol. Sci 105: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, and Zalups RK 2008b. Multidrug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J. Pharmacol. Exp. Ther 324: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, and Zalups RK 2014. Aging and the disposition and toxicity of mercury in rats. Exp. Gerontol 53: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, and Zalups RK 2004. Homocysteine, system b0,+ and the renal epithelial transport and toxicity of inorganic mercury. Am. J. Pathol 165:1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, and Zalups RK 2006. System B0,+ and the transport of thiol-s-conjugates of methylmercury. J. Pharmacol. Exp. Ther 319: 948–956. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK, and Joshee L 2015. Toxicological significance of renal Bcrp: Another potential transporter in the elimination of mercuric ions from proximal tubular cells. Toxicol. Appl. Pharmacol 285:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink TC, Regenbrecht C, Demetrius L, Lehrach H, and Adjaye J 2009. Activation of the immune response is a key feature of aging in mice. Biogerontology 10: 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, and Pedersen BK 2001. Aging and proinflammatory cytokines. Curr. Opin. Hematol 8:131–136. [DOI] [PubMed] [Google Scholar]
- Burckhardt BC, Drinkuth B, Menzel C, Konig A, Steffgen J, Wright SH, and Burckhardt G 2002. The renal Na(+)-dependent dicarboxylate transporter, NaDC-3, translocates dimethyl- and disulfhydryl-compounds and contributes to renal heavy metal detoxification. J. Am. Soc. Nephrol 13: 2628–2638. [DOI] [PubMed] [Google Scholar]
- Burger J, and Gochfeld M 2011. Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci. Total Environ 409: 1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camici M, Carpi A, Cini G, Galetta F, and Abraham N 2011. Podocyte dysfunction in aging--related glomerulosclerosis. Front Biosci 3: 995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cand F, and Verdetti J 1989. Superoxide dismutase, glutathione peroxidase, catalase, and lipid peroxidation in the major organs of the aging rats. Free Radic. Biol. Med 7: 59–63. [DOI] [PubMed] [Google Scholar]
- Cannon VT, Barfuss DW, and Zalups RK 2000. Molecular homology and the luminal transport of Hg2+ in the renal proximal tubule. J. Am. Soc. Nephrol 11: 394–402. [DOI] [PubMed] [Google Scholar]
- Cannon VT, Zalups RK, and Barfuss DW 2001. Amino acid transporters involved in luminal transport of mercuric conjugates of cysteine in rabbit proximal tubule. J. Pharmacol. Exp. Ther 298: 780–789. [PubMed] [Google Scholar]
- Carneiro MF, Grotto D, and Barbosa F Jr. 2014. Inorganic and methylmercury levels in plasma are differentially associated with age, gender, and oxidative stress markers in a population exposed to mercury through fish consumption. J. Toxicol. Environ. Health A 77: 69–79. [DOI] [PubMed] [Google Scholar]
- Carocci A, Rovito N, Sinicropi MS, and Genchi G 2014. Mercury toxicity and neurodegenerative effects. Rev. Environ. Contam. Toxicol 229: 1–18. [DOI] [PubMed] [Google Scholar]
- Carranza-Rosales P, Said-Fernandez S, Sepulveda-Saavedra J, Cruz-Vega DE, and Gandolfi AJ 2005. Morphologic and functional alterations induced by low doses of mercuric chloride in the kidney OK cell line: Ultrastructural evidence for an apoptotic mechanism of damage. Toxicology 210: 111–121. [DOI] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance System - United States 2012 [cited 09–16-16 2016] 2016 Available from www.cdc.gov/ckd.
- CDC, Centers for Disease Control and Prevention. 2014. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, edited by United States Department of Heath and Human Services Atlanta, GA. [Google Scholar]
- CDC, Centers for Disease Control and Prevention. 2014. Summary Health Statistics: National Health Interview Survey, 2014, edited by United States Deparment of Heath and Human Services Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Chen C, Yu H, Zhao J, Li B, Qu L, Liu S, Zhang P, and Chai Z 2006. The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure. Environ. Health Persp 114: 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian MG, and Clarkson TW 1976. Biochemical changes in rat kidney on exposure to elemental mercury vapor: effect on biosynthesis of metallothionein. Chem. Biol. Interact 12: 109–120. [DOI] [PubMed] [Google Scholar]
- Chou JS, Reiser IW, and Porush JG 1997. Aging and urinary excretion of epidermal growth factor. Ann. Clin. Lab. Sci 27: 116–122. [PubMed] [Google Scholar]
- Choudhury D, Raj DSC, Levi M 2004. Effect of aging on renal function and disease. In The Kidney, edited by Brenner BM. Philadelphia: Elsevier; pp.2306–2341. [Google Scholar]
- Clarkson TW, and Magos L 1967. The effect of sodium maleate on the renal deposition and excretion of mercury. Br. J. Pharmacol. Chemother 31: 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, and Magos L 2006. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol 36: 609–662. [DOI] [PubMed] [Google Scholar]
- Coresh J, Astor BC, Greene T, Eknoyan G, and Levey AS 2003. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney. Dis 41:1–12. [DOI] [PubMed] [Google Scholar]
- Cuny G, Royer RJ, Mur JM, Serot JM, Faure G, Netter P, Maillard A, and Penin F 1979. Pharmacokinetics of salicylates in elderly. Gerontology 25: 49–55. [DOI] [PubMed] [Google Scholar]
- Davies DF, and Shock NW 1950. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J. Clin. Invest 29: 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denic A, Glassock RJ, and Rule AD 2016. Structural and functional changes within the aging kidney. Adv. Chronic Kidney Dis 23:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Franki N, Kapasi AA, Reddy K, Gibbons N, and Singhal PC 2001. Tubular cell senescence and expression of TGF-beta1 and p21(WAF1/CIP1) in tubulointerstitial fibrosis of aging rats. Exp. Mol. Pathol 70: 43–53. [DOI] [PubMed] [Google Scholar]
- Druet P, Druet E, Potdevin F, and Sapin C 1978. Immune type glomerulonephritis induced by HgCl2 in the Brown Norway rat. Ann. Immunol 129C: 777–792. [PubMed] [Google Scholar]
- Eirin A, Lerman A, and Lerman LO 2016. The emerging role of mitochondrial targeting in kidney disease. Handbook Exp. Pharmacol epub ahead of print: 10.1007/164_2016_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khairy L, Ueland PM, Refsum H, Graham IM, and Vollset SE 2001. Plasma total cysteine as a risk factor for vascular disease: The European Concerted Action Project. Circulation 103: 2544–2549. [DOI] [PubMed] [Google Scholar]
- Engstrom K, Ameer S, Bernaudat L, Drasch G, Baeuml J, Skerfving S, Bose-O’Reilly S, and Broberg K 2013. Polymorphisms in genes encoding potential mercury transporters and urine mercury concentrations in populations exposed to mercury vapor from gold mining. Environ. Health Persp 121: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M 1996. Aging and the kidney. J. Am. Soc. Nephrol 7: 1106–1122. [DOI] [PubMed] [Google Scholar]
- Esposito C, Plati A, Mazzullo T, Fasoli G, De Mauri A, Grosjean F, Mangione F, Castoldi F, Serpieri N, Cornacchia F, and Dal Canton A 2007. Renal function and functional reserve in healthy elderly individuals. J. Nephrol 20: 617–625. [PubMed] [Google Scholar]
- Eto K, Yasutake A, Miyamoto K, Tokunaga H, and Otsuka Y 1997. Chronic effects of methylmercury in rats. II. Pathological aspects. Tohoku J. Exp. Med 182:197–205. [DOI] [PubMed] [Google Scholar]
- Ferder L, Inserra F, Romano L, Ercole L, and Pszenny V 1994. Decreased glomerulosclerosis in aging by angiotensin-converting enzyme inhibitors. J. Am. Soc. Nephrol 5:1147–1152. [DOI] [PubMed] [Google Scholar]
- Ferraro PM, Costanzi S, Naticchia A, Sturniolo A, and Gambaro G 2010. Low level exposure to cadmium increases the risk of chronic kidney disease: Analysis of the NHANES 1999–2006. BMC Public Health 10: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine LG, and Norman J 1989. Cellular events in renal hypertrophy. Annu. Rev. Physiol 51: 19–32. [DOI] [PubMed] [Google Scholar]
- Fine LG, Norman JT, Kujubu DA, Knecht A 1992. Renal hypertrophy. In The Kidney: Physiology and Pathophysiology, edited by Giebisch G, Seldin DW New York: Raven Press; pp.3113–3133. [Google Scholar]
- Fliser D, Zeier M, Nowack R, and Ritz E 1993. Renal functional reserve in healthy elderly subjects. J. Am. Soc. Nephrol 3: 1371–1377. [DOI] [PubMed] [Google Scholar]
- Floege J, Johnson RJ, and Couser WG 1992. Mesangial cells in the pathogenesis of progressive glomerular disease in animal models. Clin. Invest 70: 857–864. [DOI] [PubMed] [Google Scholar]
- Fogo AB 2000. Glomerular hypertension, abnormal glomerular growth, and progression of renal diseases. Kidney Int. Suppl 75: S15–S21. [PubMed] [Google Scholar]
- Fuhr BJ, and Rabenstein DL 1973. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX. The binding of cadmium, zinc, lead, and mercury by glutathione. J. Am. Chem. Soc 95: 6944–6950. [DOI] [PubMed] [Google Scholar]
- Fujita T, Brown C, Carlson EJ, Taylor T, de la Cruz M, Johns SJ, Stryke D, Kawamoto M, Fujita K, Castro R, Chen CW, Lin ET, Brett CM, Burchard EG, Ferrin TE, Huang CC, Leabman MK, and Giacomini KM 2005. Functional analysis of polymorphisms in the organic anion transporter, SLC22A6 (OAT1). Pharmacogenet. Genom 15: 201–209. [DOI] [PubMed] [Google Scholar]
- Gabbai FB 1995. Renal reserve in patients with high blood pressure. Semin. Nephrol 15: 482–487. [PubMed] [Google Scholar]
- Gage JC 1964. Distribution and excretion of methyl and phenyl mercury salts. Br. J. Ind. Med 21:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerli R, Monti D, Bistoni O, Mazzone AM, Peri G, Cossarizza A, Di Gioacchino M, Cesarotti ME, Doni A, Mantovani A, Franceschi C, and Paganelli R 2000. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech. Ageing Dev 121: 37–46. [DOI] [PubMed] [Google Scholar]
- Ginsberg G, Sonawane B, Nath R and Lewandowski P 2014. Methylmercury-induced inhibition of paraoxonase-1 (PON-1) -Implications for cardiovascular risk. J. Toxicol. Environ. Health A. 77: 1004–1023. [DOI] [PubMed] [Google Scholar]
- Glassock RJ, and Rule AD 2016. Aging and the kidneys: Anatomy, physiology and consequences for defining chronic kidney disease. Nephron 132: 24–29. [DOI] [PubMed] [Google Scholar]
- Glassock RJ, and Winearls C 2009. Ageing and the glomerular filtration rate: truths and consequences. Trans. Am. Clin. Climatol. Assoc 120: 419–428. [PMC free article] [PubMed] [Google Scholar]
- Gomes P, Simao S, Silva E, Pinto V, Amaral JS, Afonso J, Serrao MP, Pinho MJ, and Soares-da-Silva P 2009. Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure. Oxid. Med. Cell. Longev 2: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotelli CA, Astolfi E, Cox C, Cernichiari E, and Clarkson TW 1985. Early biochemical effects of an organic mercury fungicide on infants: “dose makes the poison”. Science 227: 638–640. [DOI] [PubMed] [Google Scholar]
- Gritzka TL, and Trump BF 1968. Renal tubular lesions caused by mercuric chloride. Electron microscopic observations: degeneration of the pars recta. Am. J. Pathol 52: 1225–1277. [PMC free article] [PubMed] [Google Scholar]
- Guimaraes-Souza NK, Yamaleyeva LM, Lu B, Ramos AC, Bishop CE, and Andersson KE 2015. Superoxide overproduction and kidney fibrosis: A new animal model. Einstein (Sao Paulo) 13: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha E, Basu N, Bose-O’Reilly S, Dorea JG, McSorley E, Sakamoto M, and Chan HM 2016. Current progress on understanding the impact of mercury on human health. Environ Res epub ahead of print: 10.1016/j.envres.2016.06.042 [DOI] [PubMed] [Google Scholar]
- Habiba G, Abebe G, Bravo AG, Ermias D, Staffan A, and Bishop K 2016. Mercury human exposure in populations living around Lake Tana (Ethiopia). Biol. Trace Elem. Res epub ahead of print: 10.1007/s12011-016-0745-9. [DOI] [PubMed] [Google Scholar]
- Hadem IK, and Sharma R 2016. Age- and tissue-dependent modulation of IGF-1/PI3K/Akt protein expression by dietary restriction in mice. Horm. Metab. Res 48: 201–206. [DOI] [PubMed] [Google Scholar]
- Hall RL, Wilke WL, and Fettman MJ 1986. Renal resistance to mercuric chloride toxicity during prolonged exposure in rats. Vet. HumanToxicol 28: 305–307. [PubMed] [Google Scholar]
- Hao CM, and Haase VH 2010. Sirtuins and their relevance to the kidney. J. Am. Soc. Nephrol 21: 1620–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D 1956. Aging: a theory based on free radical and radiation chemistry. J. Gerontol 11: 298–300. [DOI] [PubMed] [Google Scholar]
- Harman D 1972. The biologic clock: the mitochondria? J. Am. Geriatr. Soc 20: 145–147. [DOI] [PubMed] [Google Scholar]
- Hayslett JP 1979. Functional adaptation to reduction in renal mass. Physiol. Rev 59:137–164. [DOI] [PubMed] [Google Scholar]
- Helenius M, Hanninen M, Lehtinen SK, and Salminen A 1996. Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem. J 318: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom L, Elinder CG, Dahlberg B, Lundberg M, Jarup L, Persson B, and Axelson O 2001. Cadmium exposure and end-stage renal disease. Am. J. Kidney Dis 38:1001–1008. [DOI] [PubMed] [Google Scholar]
- Hermoso de Mendoza Garcia M, Hernandez Moreno D, Soler Rodriguez F, Lopez Beceiro A, Fidalgo Alvarez LE, and Perez Lopez M 2011. Sex- and age-dependent accumulation of heavy metals (Cd, Pb and Zn) in liver, kidney and muscle of roe deer (Capreolus capreolus) from NW Spain. J. Environ. Sci. Health. Part A: Toxicol Hazard Subst Environ Eng 46: 109–116. [DOI] [PubMed] [Google Scholar]
- Hilmer SN, Shenfield GM, and Le Couteur DG 2005. Clinical implications of changes in hepatic drug metabolism in older people. Ther. Clin. Risk. Manage 1: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg NK, Adams DF, Solomon HS, Rashid A, Abrams HL, and Merrill JP 1974. Senescence and the renal vasculature in normal man. Circ. Res 34: 309–316. [DOI] [PubMed] [Google Scholar]
- Hoshi S, Shu Y, Yoshida F, Inagaki T, Sonoda J, Watanabe T, Nomoto K, and Nagata M 2002. Podocyte injury promotes progressive nephropathy in zucker diabetic fatty rats. Lab. Invest 82: 25–35. [DOI] [PubMed] [Google Scholar]
- Houser MT, and Berndt WO 1986. The effect of unilateral nephrectomy on the nephrotoxicity of mercuric chloride in the rat. Toxicol. Appl. Pharmacol 83: 506–515. [DOI] [PubMed] [Google Scholar]
- Houser MT, and Berndt WO 1988. Unilateral nephrectomy in the rat: Effects on mercury handling and renal cortical subcellular distribution. Toxicol. Appl. Pharmacol 93: 187–194. [DOI] [PubMed] [Google Scholar]
- Houston MC 2011. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J. Clin. Hypertens 13: 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, and Moe OW 2010. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J .24: 3438–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes WL 1957. A physicochemical rationale for the biological activity of mercury and its compounds. Ann. N. Y. Acad. Sci 65: 454–460. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, and Bonventre JV 2008. Intrinsic epithelial cells repair the kidney after injury. Cell. Stem. Cell 2: 284–291. [DOI] [PubMed] [Google Scholar]
- Islinger F, Gekle M, and Wright SH 2001. Interaction of 2,3-dimercapto-1-propane sulfonate with the human organic anion transporter hOAT1. J. Pharmacol. Exp. Ther 299: 741–747. [PubMed] [Google Scholar]
- Jarup L, Berglund M, Elinder CG, Nordberg G, and Vahter M 1998. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scand. J. Work. Environ. Health 24 Suppl 1: 1–51. [PubMed] [Google Scholar]
- Jarup L, Persson B, and Elinder CG 1995. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup. Environ. Med 52: 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerkic M, Vojvodic S, and Lopez-Novoa JM 2001. The mechanism of increased renal susceptibility to toxic substances in the elderly. Part I. The role of increased vasoconstriction. Int. Urol. Nephrol 32: 539–547. [DOI] [PubMed] [Google Scholar]
- Joaquin AM, and Gollapudi S 2001. Functional decline in aging and disease: a role for apoptosis. J. Am. Geriatr. Soc 49: 1234–1240. [DOI] [PubMed] [Google Scholar]
- Jones MJ, Goodman SJ, and Kobor MS 2015. DNA methylation and healthy human aging. Aging Cell 14: 924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D, Kumar MD, Kumar SA, and Sangeeta S 2014a. Reversal of methylmercury-induced oxidative stress, lipid peroxidation, and DNA damage by the treatment of N-acetyl cysteine: a protective approach. J. Environ. Pathol. Toxicol. Oncol 33:167–182. [DOI] [PubMed] [Google Scholar]
- Joshi D, Mittal DK, Shukla S, Srivastav AK, and Srivastav SK 2014b. N-acetyl cysteine and selenium protects mercuric chloride-induced oxidative stress and antioxidant defense system in liver and kidney of rats: A histopathological approach. J. Trace Elem. Med. Biol 28: 218–226. [DOI] [PubMed] [Google Scholar]
- Kanda H, Kikushima M, Homma-Takeda S, Sumi D, Endo A, Toyama T, Miura N, Naganuma A, and Kumagai Y 2008. Downregulation of arginase II and renal apoptosis by inorganic mercury: Overexpression of arginase II reduces its apoptosis. Arch. Toxicol 82: 67–73. [DOI] [PubMed] [Google Scholar]
- Kaplan C, Pasternack B, Shah H, and Gallo G 1975. Age-related incidence of sclerotic glomeruli in human kidneys. Am. J. Pathol 80: 227–234. [PMC free article] [PubMed] [Google Scholar]
- Kappel B, and Olsen S 1980. Cortical interstitial tissue and sclerosed glomeruli in the normal human kidney, related to age and sex. A quantitative study. Virchows Arch. A. Pathol. Anat. Histol 387: 271–277. [DOI] [PubMed] [Google Scholar]
- Karam Z, and Tuazon J 2013. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med 29: 555–564. [DOI] [PubMed] [Google Scholar]
- Keenan NL, and Rosendorf KA 2011. Prevalence of hypertension and controlled hypertension - United States, 2005–2008. MMWR Suppl 60: 94–97. [PubMed] [Google Scholar]
- Keller CR, Odden MC, Fried LF, Newman AB, Angleman S, Green CA, Cummings SR, Harris TB, and Shlipak MG 2007. Kidney function and markers of inflammation in elderly persons without chronic kidney disease: the health, aging, and body composition study. Kidney Int 71: 239–244. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hwang KH, Park KS, Kong ID, and Cha SK 2015a. Biological role of anti-aging protein Klotho. J. Lifestyle Med 5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NH, Hyun YY, Lee KB, Chang Y, Ryu S, Oh KH, and Ahn C 2015b. Environmental heavy metal exposure and chronic kidney disease in the general population. J. Korean Med. Sci 30: 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinirons MT, and O’Mahony MS 2004. Drug metabolism and ageing. Br. J. Clin. Pharmacol 57: 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey GR, Li L, and Okusa MD 2008. Inflammation in acute kidney injury. Nephron Exp. Nephrol 109: e102–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh AS, Simmons-Willis TA, Pritchard JB, Grassl SM, and Ballatori N 2002. Identification of a mechanism by which the methylmercury antidotes N-acetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: transport by the renal organic anion transporter-1. Mol. Pharmacol 62:921–926. [DOI] [PubMed] [Google Scholar]
- Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, and Endou H 2002. Immunolocalization of multispecific organic anion transporters, OAT1, OAT2, and OAT3, in rat kidney. J. Am. Soc. Nephrol 13: 848–857. [DOI] [PubMed] [Google Scholar]
- Kostial K, Kello D, Jugo S, Rabar I, and Maljkovic T 1978. Influence of age on metal metabolism and toxicity. Environ. Health Persp 25: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzewski M, Braziewicz J, Majewska U, and Gozdz S 2002. Concentration of selenium in the whole blood and the thyroid tissue of patients with various thyroid diseases. Biol. Trace Elem. Res 88: 25–30. [DOI] [PubMed] [Google Scholar]
- Lapierre LR, Kumsta C, Sandri M, Ballabio A, and Hansen M 2015. Transcriptional and epigenetic regulation of autophagy in aging. Autophagy 11: 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma EV 2009. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med 25: 325–329. [DOI] [PubMed] [Google Scholar]
- Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, and Takei K 2004. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell. Struct. Funct 29: 91–99. [DOI] [PubMed] [Google Scholar]
- Li SJ, Zhang SH, Chen HP, Zeng CH, Zheng CX, Li LS, and Liu ZH 2010. Mercury-induced membranous nephropathy: Clinical and pathological features. Clin. J. Am. Soc. Nephrol 5: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang B, Yang L, and Li H 2013. Blood mercury concentration among residents of a historic mercury mine and possible effects on renal function: A cross-sectional study in southwestern China. Environ. Monit. Assess 185: 3049–3055. [DOI] [PubMed] [Google Scholar]
- Lim JH, Kim EN, Kim MY, Chung S, Shin SJ, Kim HW, Yang CW, Kim YS, Chang YS, Park CW, and Choi BS 2012. Age-associated molecular changes in the kidney in aged mice. Oxid. Med. Cell. Longev 2012: 171383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman RD, and Goldman R 1986. Anatomic and physiologic age changes in the kidney. Exp. Gerontol 21: 379–406. [DOI] [PubMed] [Google Scholar]
- Lindeman RD, Tobin J, and Shock NW 1985. Longitudinal studies on the rate of decline in renal function with age. J. Am. Geriatr. Soc 33: 278–285. [DOI] [PubMed] [Google Scholar]
- Ljungqvist A, and Lagergren C 1962. Normal intrarenal arterial pattern in adult and ageing human kidney. A microangiographical and histological study. J. Anat 96: 285–300. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barrera BA and Barragan-Gonzalez RG 2016. Metals and metalloid in eight fish species consumed by citizens of Bogota, DC , Columbia and potential risk to human. J Toxicol Environ Health A 79: 232–243. [DOI] [PubMed] [Google Scholar]
- Lopez-Novoa JM 2008. The mechanisms of age-associated glomerular sclerosis. In The Aging Kidney in Health and Disease, edited by Macias Nunez JF, Cameron JS, Oreopoulos DG New York, NY: Springer; pp.113–126. [Google Scholar]
- Lund BO, Miller DM, and Woods JS 1993. Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem. Pharmacol 45: 2017–2024. [DOI] [PubMed] [Google Scholar]
- Macias-Nunez JF, Lopez-Novoa JM 2008. Physiology of the healthy aging kidney. In The Aging Kidney in Health and Disease, edited by Macias Nunez JF, Cameron JS, Oreopoulos DG New York, NY: Springer; pp. 93–112. [Google Scholar]
- Madejczyk MS, Aremu DA, Simmons-Willis TA, Clarkson TW, and Ballatori N 2007. Accelerated urinary excretion of methylmercury following administration of its antidote N-acetylcysteine requires Mrp2/Abcc2, the apical multidrug resistance-associated protein. J. Pharmacol. Exp. Ther 322: 378–384. [DOI] [PubMed] [Google Scholar]
- Magos L, and Stoytchev T 1969. Combined effect of sodium maleate and some thiol compounds on mercury excretion and redistribution in rats. Br. J. Pharmacol 35: 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Chikuda H, Suga T, Kawaguchi H, and Kuro-o M 2005. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in Kotho mutant mice. Mech. Ageing Dev 126: 1274–1283. [DOI] [PubMed] [Google Scholar]
- McDowell EM, Nagle RB, Zalme RC, McNeil JS, Flamenbaum W, and Trump BF 1976. Studies on the pathophysiology of acute renal failure. I. Correlation of ultrastructure and function in the proximal tubule of the rat following administration of mercuric chloride. Virchows Arch. B. Cell. Pathol 22: 173–196. [DOI] [PubMed] [Google Scholar]
- Melk A, Mansfield ES, Hsieh SC, Hernandez-Boussard T, Grimm P, Rayner DC, Halloran PF, and Sarwal MM 2005. Transcriptional analysis of the molecular basis of human kidney aging using cDNA microarray profiling. Kidney Int 68: 2667–2679. [DOI] [PubMed] [Google Scholar]
- Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, and Halloran PF 2004. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int 65: 510–520. [DOI] [PubMed] [Google Scholar]
- Michelet F, Gueguen R, Leroy P, Wellman M, Nicolas A, and Siest G 1995. Blood and plasma glutathione measured in healthy subjects by HPLC: Relation to sex, aging, biological variables, and life habits. Clin. Chem 41: 1509–1517. [PubMed] [Google Scholar]
- Moriguchi J, Ezaki T, Tsukahara T, Fukui Y, Ukai H, Okamoto S, Shimbo S, Sakurai H, and Ikeda M 2005. Effects of aging on cadmium and tubular dysfunction markers in urine from adult women in non-polluted areas. Int. Arch. Occup. Environ. Health 78: 446–451. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, Fukatsu A, Ogawa O, and Inui K 2002. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol 13: 866–874. [DOI] [PubMed] [Google Scholar]
- Murphy MJ, Culliford EJ, and Parsons V 1979. A case of poisoning with mercuric chloride. Resuscitation 7: 35–44. [DOI] [PubMed] [Google Scholar]
- Musso CG, and Oreopoulos DG 2011. Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 119 Suppl 1: 1–5. [DOI] [PubMed] [Google Scholar]
- Musso CG, Reynaldi J, Martinez B, Pierangelo A, Vilas M, and Algranati L 2011. Renal reserve in the oldest old. Int. Urol. Nephrol 43: 253–256. [DOI] [PubMed] [Google Scholar]
- Mysliwska J, Bryl E, Foerster J, and Mysliwski A 1999. The upregulation of TNF alpha production is not a generalised phenomenon in the elderly between their sixth and seventh decades of life. Mech. Ageing Dev 107:1–14. [DOI] [PubMed] [Google Scholar]
- Naeije R, Fiasse A, Carlier E, Opsomer M, and Leeman M 1993. Systemic and renal haemodynamic effects of angiotensin converting enzyme inhibition by zabicipril in young and in old normal men. Eur. J. Clin. Pharmacol 44: 35–39. [DOI] [PubMed] [Google Scholar]
- Nakatsuji S, Yamate J, and Sakuma S 1998. Macrophages, myofibroblasts, and extracellular matrix accumulation in interstitial fibrosis of chronic progressive nephropathy in aged rats. Vet. Pathol 35: 352–360. [DOI] [PubMed] [Google Scholar]
- NKF, National Kidney Foundation. 2016. Diabetes - A major risk factor for kidney disease [accessed September 23, 2016]. Available from https://www.kidney.org/atoz/content/diabetes.
- Noonan CW, Sarasua SM, Campagna D, Kathman SJ, Lybarger JA, and Mueller PW 2002. Effects of exposure to low levels of environmental cadmium on renal biomarkers. Environ. Health Persp 110: 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norseth T, and Clarkson TW 1970a. Biotransformation of methylmercury salts in the rat studied by specific determination of inorganic mercury. Biochem. Pharmacol 19: 2775–2783. [DOI] [PubMed] [Google Scholar]
- Norseth T, and Clarkson TW 1970b. Studies on the biotransformation of 203Hg-labeled methyl mercury chloride in rats. Arch. Environ. Health 21: 717–727. [DOI] [PubMed] [Google Scholar]
- Nunes E, Cavaco A, and Carvalho C 2014a. Children’s health risk and benefits of fish consumption: Risk indices based on a diet diary follow-up of two weeks. J. Toxicol. Environ. Health. A 77: 103–114. [DOI] [PubMed] [Google Scholar]
- Nunes E, Cavaco A, and Carvalho C 2014b. Exposure assessment of pregnant Portuguese women to methylmercury through the ingestion of fish: Cross-sectional survey and biomarker validation. J. Toxicol. Environ. Health. A 77: 133–142. [DOI] [PubMed] [Google Scholar]
- Nwankwo T, Yoon SS, Burt V, and Gu Q 2013. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief: pp. 1–8. [PubMed] [Google Scholar]
- O’Brown ZK, Van Nostrand EL, Higgins JP, and Kim SK 2015. The inflammatory transcription factors NFkappaB, STAT1 and STAT3 drive age-associated transcriptional changes in the human kidney. PLoS Genet 11: e1005734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, and Landefeld CS 2007. Age affects outcomes in chronic kidney disease. J. Am. Soc. Nephrol 18: 2758–2765. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Fryar CD, and Flegal KM 2015. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief: pp. 1–8. [PubMed] [Google Scholar]
- Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura Y, Kuro-o M, Nabeshima Y, and Nagail R 1998. Molecular cloning of rat klotho cDNA: Markedly decreased expression of Klotho by acute inflammatory stress. Biochem. Biophys. Res. Commun 251: 920–925. [DOI] [PubMed] [Google Scholar]
- Oliveira CS, Joshee L, Zalups RK, and Bridges CC 2016. Compensatory renal hypertrophy and the handling of an acute nephrotoxicant in a model of aging. Exp. Gerontol 75: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata S, Sato M, Sakimura K, and Sugano H 1980. Time-dependent accumulation of inorganic mercury in subcellular fractions of kidney, liver, and brain of rats exposed to methylmercury. Arch. Toxicol 44: 231–241. [DOI] [PubMed] [Google Scholar]
- Pal S, and Tyler JK 2016. Epigenetics and aging. Sci. Adv 2: e1600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual J, Orofino L, Liano F, Marcen R, Naya MT, Orte L, and Ortuno J 1990. Incidence and prognosis of acute renal failure in older patients. J. Am. Geriatr. Soc 38: 25–30. [DOI] [PubMed] [Google Scholar]
- Pecly IM, Genelhu V, and Francischetti EA 2006. Renal functional reserve in obesity hypertension. Int. J. Clin. Pract 60:1198–1203. [DOI] [PubMed] [Google Scholar]
- Perry HM Jr., Miller JP, Fornoff JR, Baty JD, Sambhi MP, Rutan G, Moskowitz DW, and Carmody SE 1995. Early predictors of 15-year end-stage renal disease in hypertensive patients. Hypertension 25: 587–594. [DOI] [PubMed] [Google Scholar]
- Petrovic Z, Teodorovic V, Djuric S, Milicevic D, Vranic D, and Lukic M 2014. Cadmium and mercury accumulation in European hare (Lepus europaeus): Age-dependent relationships in renal and hepatic tissue. Environ. Sci. Pollut. Res. Int 21:14058–14068. [DOI] [PubMed] [Google Scholar]
- Piotrowski JK, Trojanowska B, Wisniewska-Knypl JM, and Bolanowska W 1974. Mercury binding in the kidney and liver of rats repeatedly exposed to mercuric chloride: induction of metallothionein by mercury and cadmium. Toxicol. Appl. Pharmacol 27: 11–19. [DOI] [PubMed] [Google Scholar]
- Planas-Bohne F 1981. The effect of 2,3-dimercaptorpropane-1-sulfonate and dimercaptosuccinic acid on the distribution and excretion of mercuric chloride in rats. Toxicology 19: 275–278. [DOI] [PubMed] [Google Scholar]
- Planas-Bohne F, Taylor DM, and Walser R 1985. The influence of administered mass on the subcellular distribution and binding of mercury in rat liver and kidney. Arch. Toxicol 56: 242–246. [DOI] [PubMed] [Google Scholar]
- Pollack AZ, Mumford SL, Mendola P, Perkins NJ, Rotman Y, Wactawski-Wende J, and Schisterman EF 2015. Kidney biomarkers associated with blood lead, mercury, and cadmium in premenopausal women: A prospective cohort study. J Toxicol Environ Health A 78: 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy M, Zhou X, Yan Y, Tang J, Tolbert E, Zhao TC, Gong R, and Zhuang S 2014. Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. J. Pharmacol. Exp. Ther 350: 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose N, and Raju R 2014. Aging and injury: alterations in cellular energetics and organ function. Aging Dis 5: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose N, and Raju R 2015. Sirtuin regulation in aging and injury. Biochim. Biophys. Acta 1852: 2442–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RG 1982. Urinary enzymes, nephrotoxicity and renal disease. Toxicology 23:99–134. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, and Edwards JR 2010. Early biomarkers of cadmium exposure and nephrotoxicity. Biometals 23: 793–809. [DOI] [PubMed] [Google Scholar]
- Raes A, Donckerwolcke R, Craen M, Hussein MC, and Vande Walle J 2007. Renal hemodynamic changes and renal functional reserve in children with type I diabetes mellitus. Pediatr. Nephrol 22: 1903–1909. [DOI] [PubMed] [Google Scholar]
- Rajaee M, Sanchez BN, Renne EP, and Basu N 2015. An investigation of organic and inorganic mercury exposure and blood pressure in a small-scale gold mining community in Ghana. Int. J. Environ. Res. Public Health 12: 10020–10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Frendo B, Perez-Garcia R, Lopez-Novoa JM, and Hernando-Avendano L 1979. Increased severity of the acute renal failure induced by HgCl2 on rats with reduced renal mass. Biomedicine 31:167–170. [PubMed] [Google Scholar]
- Rawlins MD, James OF, Williams FM, Wynne H, and Woodhouse KW 1987. Age and the metabolism of drugs. Quart. J. Med 64: 545–547. [PubMed] [Google Scholar]
- Risher JF, and De Rosa CT 2007. Inorganic: the other mercury. J. Environ. Health 70: 9–16. [PubMed] [Google Scholar]
- Rodiger M, Zhang X, Ugele B, Gersdorff N, Wright SH, Burckhardt G, and Bahn A 2010. Organic anion transporter 3 (OAT3) and renal transport of the metal chelator 2,3-dimercapto-1-propanesulfonic acid (DMPS). Can. J. Physiol. Pharmacol 88: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin AE, and Crowson CN 1962. Mercury nephrotoxicity in the rat. 2. Investigation of the intracellular site of mercury nephrotoxicity by correlated serial time histologic and histoenzymatic studies. Am. J. Pathol 41: 485–499. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Puyol D 1998. The aging kidney. Kidney Int 54: 2247–2265. [DOI] [PubMed] [Google Scholar]
- Roels HA, Hoet P, and Lison D 1999. Usefulness of biomarkers of exposure to inorganic mercury, lead, or cadmium in controlling occupational and environmental risks of nephrotoxicity. Renal Fail 21: 251–262. [DOI] [PubMed] [Google Scholar]
- Roels HA, Lauwerys RR, Bernard AM, Buchet JP, Vos A, and Oversteyns M 1991. Assessment of the filtration reserve capacity of the kidney in workers exposed to cadmium. Br. J. Ind. Med 48: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney JP 2007. The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology 234: 145–156. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Shock NW, and DeFronzo RA 1976. The influence of age on the renal response to water deprivation in man. Nephron 17: 270–278. [DOI] [PubMed] [Google Scholar]
- Rowens B, Guerrero-Betancourt D, Gottlieb CA, Boyes RJ, and Eichenhorn MS 1991. Respiratory failure and death following acute inhalation of mercury vapor. A clinical and histologic perspective. Chest 99: 185–190. [DOI] [PubMed] [Google Scholar]
- Rubino FM, Verduci C, Giampiccolo R, Pulvirenti S, Brambilla G, and Colombi A 2004. Molecular characterization of homo- and heterodimeric mercury(II)-bis-thiolates of some biologically relevant thiols by electrospray ionization and triple quadrupole tandem mass spectrometry. J. Am. Soc. Mass. Spectrom 15: 288–300. [DOI] [PubMed] [Google Scholar]
- Rule AD, Cornell LD, and Poggio ED 2011. Senile nephrosclerosis - does it explain the decline in glomerular filtration rate with aging? Nephron Physiol. 119 Suppl 1: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht J 2008. Scientific Monograph for Dimaval Houston, TX. [Google Scholar]
- Ruscin, J. M. L., S.A. Pharmacokinetics in the Elderly. Merck & Co., 2016 2016 [cited 4–21-16 2016] 2016 Available from http://www.merckmanuals.com/professional/geriatrics/drug-therapy-in-the-elderly/pharmacokinetics-in-the-elderly.
- Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, and Nagai R 1998. Klotho protein protects against endothelial dysfunction. Biochem. Biophys. Res. Commun 248: 324–329. [DOI] [PubMed] [Google Scholar]
- Samuels ER, Heick HM, McLaine PN, and Farant JP 1982. A case of accidental inorganic mercury poisoning. J. Anal. Toxicol 6: 120–122. [DOI] [PubMed] [Google Scholar]
- Satoh M, Nishimura N, Kanayama Y, Naganuma A, Suzuki T, and Tohyama C 1997. Enhanced renal toxicity by inorganic mercury in metallothionein-null mice. J. Pharmacol. Exp. Ther 283: 1529–1533. [PubMed] [Google Scholar]
- Schmitt R, and Cantley LG 2008. The impact of aging on kidney repair. Am. J. Physiol. Renal. Physiol 294: F1265–F1272. [DOI] [PubMed] [Google Scholar]
- Schmitt R, Zhu X, Cantley LG 2006. Tubular epithelial cells contribute directly to the age-related increase in susceptibility to ischemic acute kidney injury. J. Am. Soc. Nephrol 17: 530A. [Google Scholar]
- Schumacher L, and Abbott LC 2016. Effects of methyl mercury exposure on pancreatic beta cell development and function. J. Appl. Toxicol [DOI] [PubMed] [Google Scholar]
- Sebastian C, Satterstrom FK, Haigis MC, and Mostoslavsky R 2012. From sirtuin biology to human diseases: An update. J. Biol. Chem 287: 42444–42452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, Guo TL, and Shapiro IM 1998. Low-level methylmercury exposure causes human T-cells to undergo apoptosis: Evidence of mitochondrial dysfunction. Environ. Res 77: 149–159. [DOI] [PubMed] [Google Scholar]
- Shock NW 1946. Kidney function tests in aged males. Geriatrics 1: 232–239. [PubMed] [Google Scholar]
- Shock NW, Greulick RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin JD 1984. Normal Human Aging: the Baltimore Longitudinal Study of Aging, edited by Public Health Service U.S. Department of Health and Human Services. [Google Scholar]
- Shurin GV, Yurkovetsky ZR, Chatta GS, Tourkova IL, Shurin MR, and Lokshin AE 2007. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine 39: 123–129. [DOI] [PubMed] [Google Scholar]
- Siedlikowski M, Bradley M, Kubow S, Goodrich JM, Franzblau A, and Basu N 2016. Bioaccessibility and bioavailability of methylmercury from seafood commonly consumed in North America: In vitro and epidemiological studies. Environ. Res 149: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FG 2005. The aging kidney: a review -- Part I. Int. Urol. Nephrol 37: 185–205. [DOI] [PubMed] [Google Scholar]
- Silva FG 2005. The aging kidney: a review--Part II. Int. Urol. Nephrol 37: 419–432. [DOI] [PubMed] [Google Scholar]
- Small DM, Bennett NC, Roy S, Gabrielli BG, Johnson DW, and Gobe GC 2012. Oxidative stress and cell senescence combine to cause maximal renal tubular epithelial cell dysfunction and loss in an in vitro model of kidney disease. Nephron Exp. Nephrol 122: 123–130. [DOI] [PubMed] [Google Scholar]
- Sommar JN, Svensson MK, Bjor BM, Elmstahl SI, Hallmans G, Lundh T, Schon SM, Skerfving S, and Bergdahl IA 2013. End-stage renal disease and low level exposure to lead, cadmium and mercury: A population-based, prospective nested case-referent study in Sweden. Environ. Health 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Lee CK, Kim KH, Lee JT, Suh C, Kim SY, Kim JH, Son BC, Kim DH, and Lee S 2016. Factors associated with total mercury concentrations in maternal blood, cord blood, and breast milk among pregnant women in Busan, Korea. Asia. Pac. J. Clin. Nutr 25: 340–349. [DOI] [PubMed] [Google Scholar]
- Stacchiotti A, Morandini F, Bettoni F, Schena I, Lavazza A, Grigolato PG, Apostoli P, Rezzani R, and Aleo MF 2009. Stress proteins and oxidative damage in a renal derived cell line exposed to inorganic mercury and lead. Toxicology 264: 215–224. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Yoshida T, Shiohira S, Kohei J, Mitobe M, Kurosu H, Kuro-o M, Nitta K, and Tsuchiya K 2012. Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am. J. Physiol . Renal. Physiol 302: F1252–F1264. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Tamai Y, and Tanaka H 1978. Selenium protection against mercury toxicity: high binding affinity of methylmercury by selenium-containing ligands in comparison with sulfur-containing ligands. Bioinorg. Chem 9:167–180. [DOI] [PubMed] [Google Scholar]
- Susnik N, Melk A, and Schmitt R 2015. Cell aging and kidney repair. Cell Cycle 14: 3521–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Anderson S, Gordon KL, Oyama TT, Shankland SJ, and Johnson RJ 1998. Tubulointerstitial disease in aging: Evidence for underlying peritubular capillary damage, a potential role for renal ischemia. J. Am. Soc. Nephrol 9: 231–242. [DOI] [PubMed] [Google Scholar]
- Trojanowska B, Piotrowski JK, and Szendzikowski S 1971. The influence of thioacetamide on the excretion of mercury in rats. Toxicol. Appl. Pharmacol 18: 374–386. [DOI] [PubMed] [Google Scholar]
- UN, United Nations 2016. World Population Ageing 1950–2050. II. Magnitude and Speed of Population Ageing, United Nations, Geneva, Switzerland. [Google Scholar]
- Uriu K, Kaizu K, Qie YL, Ito A, Takagi I, Suzuka K, Inada Y, Hashimoto O, and Eto S 2000. Long-term oral intake of low-dose cadmium exacerbates age-related impairment of renal functional reserve in rats. Toxicol. Appl. Pharmacol 169:151–158. [DOI] [PubMed] [Google Scholar]
- USCB, United States Census Bureau. 2010. The Next Four Decades. The Older Population in the US: 2010–2050 US Census Bureau. [Google Scholar]
- USRDS. 2015. 2015 USRDS Annual Data Report, edited by United States Renal Disease System Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- Vanholder RC, Praet MM, Pattyn PA, Leusen IR, and Lameire NH 1982. Dissociation of glomerular filtration and renal blood flow in HgCl2-induced acute renal failure. Kidney Int 22: 162–170. [DOI] [PubMed] [Google Scholar]
- Vazquez M, Calatayud M, Velez D, and Devesa V 2013. Intestinal transport of methylmercury and inorganic mercury in various models of Caco-2 and HT29-MTX cells. Toxicology 311: 147–153. [DOI] [PubMed] [Google Scholar]
- Vazquez M, Velez D, and Devesa V 2014. In vitro characterization of the intestinal absorption of methylmercury using a Caco-2 cell model. Chem. Res. Toxicol 27: 254–264. [DOI] [PubMed] [Google Scholar]
- Wang X, Bonventre JV, and Parrish AR 2014. The aging kidney: increased susceptibility to nephrotoxicity. Int. J. Mol. Sci 15:15358–15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JR, and Anderson S 2010. The aging kidney: physiological changes. Adv. Chronic Kidney Dis 17: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Zeng M, Shu Y, Guo D, Sun Y, Guo Z, Wang Y, Liu Z, Zhou H, and Zhang W 2015. Aging increases the susceptibility of cisplatin-induced nephrotoxicity. Age (Dordr) 37: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, and Wiggins RC 2005. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol 16: 2941–2952. [DOI] [PubMed] [Google Scholar]
- WHO, World Health Organization. Global Health Observatory Data Repository. 2016.
- Wiggins J 2009. Podocytes and glomerular function with aging. Semin. Nephrol 29: 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JE, Patel SR, Shedden KA, Goyal M, Wharram BL, Martini S, Kretzler M, and Wiggins RC 2010. NFkappaB promotes inflammation, coagulation, and fibrosis in the aging glomerulus. J. Am. Soc. Nephrol 21: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska JM, Trojanowska B, Piotrowski J, and Jakubowski M 1970. Binding of mercury in the rat kidney by metallothionein. Toxicol. Appl. Pharmacol 16: 754–763. [DOI] [PubMed] [Google Scholar]
- Wolff S, Brown G, Chen J, Meals K, Thorton C, Brewer S, Cizdziel JV and Willett KL 2016. mercury concentrations in fish from three major lakes in north Mississippi: Spatial and temporal differences and human health risk assessment. J Toxicol Environ Health A 79: 894–904. [DOI] [PubMed] [Google Scholar]
- Xu G, Bhatnagar V, Wen G, Hamilton BA, Eraly SA, and Nigam SK 2005. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)]. Kidney Int 68:1491–1499. [DOI] [PubMed] [Google Scholar]
- Xu Y, and Sun Z 2015. Molecular basis of Klotho: from gene to function in aging. Endocr. Rev 36: 174–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, and Fogo AB 2010. Cell senescence in the aging kidney. J. Am. Soc. Nephrol 21:1436–1439. [DOI] [PubMed] [Google Scholar]
- Yasutake A, Hirayama K, and Inoue M 1989. Mechanism of urinary excretion of methylmercury in mice. Arch. Toxicol 63: 479–483. [DOI] [PubMed] [Google Scholar]
- Yasutake A, Nakano A, Miyamoto K, and Eto K 1997. Chronic effects of methylmercury in rats. I. Biochemical aspects. Tohoku J. Exp. Med 182:185–196. [DOI] [PubMed] [Google Scholar]
- Yoon SS OY, Louis T 2010. Recent trends in the prevalence of high blood pressure and its treatment and control, 1999−−2008, edited by Department of Health and Human Services, National Center for Health Statistics. Hyattsville, MD. [Google Scholar]
- Yuan Y, Cruzat VF, Newsholme P, Cheng J, Chen Y, and Lu Y 2016. Regulation of SIRT1 in aging: Roles in mitochondrial function and biogenesis. Mech. Ageing Dev 155:10–21. [DOI] [PubMed] [Google Scholar]
- Zaletel J, Cerne D, Lenart K, Zitta S, Jurgens G, Estelberger W, and Kocijancic A 2004. Renal functional reserve in patients with Type 1 diabetes mellitus. Wien. Klin. Wochenschr 116:246–251. [DOI] [PubMed] [Google Scholar]
- Zalme RC, McDowell EM, Nagle RB, McNeil JS, Flamenbaum W, and Trump BF 1976. Studies on the pathophysiology of acute renal failure. II. A histochemical study of the proximal tubule of the rat following administration of mercuric chloride. Virchows Arch. B. Cell. Pathol 22: 197–216. [DOI] [PubMed] [Google Scholar]
- Zalups RK 1991. Autometallographic localization of inorganic mercury in the kidneys of rats: effect of unilateral nephrectomy and compensatory renal growth. Exp. Mol. Pathol 54: 10–21. [DOI] [PubMed] [Google Scholar]
- Zalups RK 1993a. Early aspects of the intrarenal distribution of mercury after the intravenous administration of mercuric chloride. Toxicology 79: 215–228. [DOI] [PubMed] [Google Scholar]
- Zalups RK 1993b. Influence of 2,3-dimercaptopropane-1-sulfonate (DMPS) and meso-2,3-dimercaptosuccinic acid (DMSA) on the renal disposition of mercury in normal and uninephrectomized rats exposed to inorganic mercury. J. Pharmacol. Exp. Ther 267: 791–800. [PubMed] [Google Scholar]
- Zalups RK 1995. Organic anion transport and action of gamma-glutamyl transpeptidase in kidney linked mechanistically to renal tubular uptake of inorganic mercury. Toxicol. Appl. Pharmacol 132: 289–298. [DOI] [PubMed] [Google Scholar]
- Zalups RK 1997a. Enhanced renal outer medullary uptake of mercury associated with uninephrectomy: Implication of a luminal mechanism. J. Toxicol. Environ. Health 50: 173–194. [DOI] [PubMed] [Google Scholar]
- Zalups RK 1997b. Reductions in renal mass and the nephropathy induced by mercury. Toxicol. Appl. Pharmacol 143: 366–379. [DOI] [PubMed] [Google Scholar]
- Zalups RK 1998a. Basolateral uptake of inorganic mercury in the kidney. Toxicol. Appl. Pharmacol 151:192–199. [DOI] [PubMed] [Google Scholar]
- Zalups RK 1998b. Basolateral uptake of mercuric conjugates of N-acetylcysteine and cysteine in the kidney involves the organic anion transport system. J. Toxicol. Environ. Health A 55: 13–29. [DOI] [PubMed] [Google Scholar]
- Zalups RK 2000a. Evidence for basolateral uptake of cadmium in the kidneys of rats. Toxicol. Appl. Pharmacol 164:15–23. [DOI] [PubMed] [Google Scholar]
- Zalups RK 2000b. Molecular interactions with mercury in the kidney. Pharmacol. Rev 52:113–143. [PubMed] [Google Scholar]
- Zalups RK, and Ahmad S 2004. Homocysteine and the renal epithelial transport and toxicity of inorganic mercury: Role of basolateral transporter organic anion transporter 1. J. Am. Soc. Nephrol 15: 2023–2031. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Ahmad S 2005a. Handling of cysteine S-conjugates of methylmercury in MDCK cells expressing human OAT1. Kidney Int 68:1684–1699. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Ahmad S 2005b. Handling of the homocysteine S-conjugate of methylmercury by renal epithelial cells: Role of organic anion transporter 1 and amino acid transporters. J. Pharmacol. Exp. Ther 315: 896–904. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Ahmad S 2005c. Transport of N-acetylcysteine s-conjugates of methylmercury in Madin-Darby canine kidney cells stably transfected with human isoform of organic anion transporter 1. J. Pharmacol. Exp. Ther 314:1158–1168. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Aslamkhan AG, and Ahmad S 2004. Human organic anion transporter 1 mediates cellular uptake of cysteine-S conjugates of inorganic mercury. Kidney Int 66: 251–261. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Barfuss DW 1995a. Accumulation and handling of inorganic mercury in the kidney after coadministration with glutathione. J. Toxicol. Environ. Health 44: 385–399. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Barfuss DW 1995b. Pretreatment with p-aminohippurate inhibits the renal uptake and accumulation of injected inorganic mercury in the rat. Toxicology 103: 23–35. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Barfuss DW 1996. Nephrotoxicity of inorganic mercury co-administrated with L-cysteine. Toxicology 109:15–29. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Barfuss DW 1998a. Participation of mercuric conjugates of cysteine, homocysteine, and N-acetylcysteine in mechanisms involved in the renal tubular uptake of inorganic mercury. J. Am. Soc. Nephrol 9: 551–561. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Barfuss DW 1998b. Small aliphatic dicarboxylic acids inhibit renal uptake of administered mercury. Toxicol. Appl. Pharmacol 148:183–193. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Barfuss DW, and Kostyniak PJ 1992. Altered intrarenal accumulation of mercury in uninephrectomized rats treated with methylmercury chloride. Toxicol. Appl. Pharmacol 115: 174–182. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Cherian MG, and Barfuss DW 1993. Mercury-metallothionein and the renal accumulation and handling of mercury. Toxicology 83: 61–78. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Cherian MG, and Barfuss DW 1995. Lack of luminal or basolateral uptake and transepithelial transport of mercury in isolated perfused proximal tubules exposed to mercury-metallothionein. J. Toxicol. Environ. Health 44:101–113. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Cox C, Diamond GL 1988. Histological and urinalysis assessment of nephrotoxicity induced by mercuric chloride in normal and uninephrectomized rats. In Biological Monitoring of Toxic Metals, edited by Clarkson TW,Friberg L, Nordberg GF, Sager PR New York: Plenum Publishing Corporation; pp. 531–535. [Google Scholar]
- Zalups RK, and Diamond GL 1987a. Intrarenal distribution of mercury in the rat: effect of administered dose of mercuric chloride. Bull. Environ. Contam. Toxicol 38: 67–72. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Diamond GL 1987b. Mercuric chloride-induced nephrotoxicity in the rat following unilateral nephrectomy and compensatory renal growth. Virchows Arch. B. Cell. Pathol. Incl. Mol. Pathol 53: 336–346. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Joshee L, and Bridges CC 2014. Novel Hg2+-induced nephropathy in rats and mice lacking mrp2: Evidence of axial heterogeneity in the handling of Hg2+ along the proximal tubule. Toxicol. Sci 142: 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalups RK, Klotzbach JM, and Diamond GL 1987. Enhanced accumulation of injected inorganic mercury in renal outer medulla after unilateral nephrectomy. Toxicol. Appl. Pharmacol 89: 226–236. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Koropatnick J 2000. Temporal changes in metallothionein gene transcription in rat kidney and liver: relationship to content of mercury and metallothionein protein. J. Pharmacol. Exp. Ther 295: 74–82. [PubMed] [Google Scholar]
- Zalups RK, and Lash LH 1997. Binding of mercury in renal brush-border and basolateral membrane-vesicles. Biochem. Pharmacol 53: 1889–1900. [DOI] [PubMed] [Google Scholar]
- Zalups RK, and Minor KH 1995. Luminal and basolateral mechanisms involved in the renal tubular uptake of inorganic mercury. J. Toxicol. Environ. Health 46: 73–100. [DOI] [PubMed] [Google Scholar]
- Zatz R, and Fujihara CK 1994. Glomerular hypertrophy and progressive glomerulopathy. Is there a definite pathogenetic correlation? Kidney Int. Suppl 45:S27–S29; discussion S30-S21. [PubMed] [Google Scholar]
- Zha Y, Taguchi T, Nazneen A, Shimokawa I, Higami Y, and Razzaque MS 2008. Genetic suppression of GH-IGF-1 activity, combined with lifelong caloric restriction, prevents age-related renal damage and prolongs the life span in rats. Am. J. Nephrol 28: 755–764. [DOI] [PubMed] [Google Scholar]
- Zheng F, Plati AR, Banerjee A, Elliot S, Striker LJ, and Striker GE 2003. The molecular basis of age-related kidney disease. Sci. Aging Knowledge Environ 2003: PE20. [DOI] [PubMed] [Google Scholar]
- Zhou XJ, Laszik ZG, Silva FG 2008. Anatomical Changes in the Aging Kidney. In The Aging Kidney in Health and Disease, edited by Nunez JFM, Cameron JS, Oreopoulos DG New York, NY: Springer; pp. 39–54. [Google Scholar]
- Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND, and Silva FG 2008. The aging kidney. Kidney Int 74: 710–720. [DOI] [PubMed] [Google Scholar]


