Abstract
Discrete Pavlovian reward cues acquire more potent incentive motivational properties (incentive salience) in some animals (sign-trackers; STs) compared to others (goal-trackers; GTs). Conversely, GTs appear to be better than STs in processing more complex contextual cues, perhaps reflecting their relatively greater bias for goal-directed cue processing. Here, we investigated the activity of two major prefrontal neuromodulatory input systems, dopamine (DA) and acetylcholine (ACh), in response to a discrete Pavlovian cue that was previously paired with cocaine administration in STs and GTs. Rats underwent Pavlovian training in which light cue presentations were either paired or unpaired with an intravenous cocaine infusion. Following a 10-day abstinence period, prefrontal dialysates were collected in STs and GTs during cue presentations in the absence of cocaine. In STs, the cue previously paired with cocaine significantly increased prefrontal DA levels. DA levels remained elevated over baseline across multiple cue presentation blocks, and DA levels and approaches to the cue were significantly correlated. In STs, ACh levels were unaffected by cue presentations. In contrast, in GTs, presentations of the cocaine cue increased prefrontal ACh, but not DA, levels. GTs oriented towards the cue at rates similar to STs, but they did not approach it and elevated ACh levels did not correlate with conditioned orientation. The results indicate a double dissociation between the role of prefrontal DA and ACh in STs and GTs, and suggest that these phenotypes will be useful for studying the role of neuromodulator systems in mediating opponent behavioural-cognitive styles.
Keywords: acetylcholine, addiction, cognition, dopamine, individual differences
Introduction
Rats with a propensity to develop sign-tracking vs. goal-tracking conditioned responses (CRs) evoked by discrete reward cues (STs, GTs) have proved useful in studying psychological traits and underlying neurobiological mechanisms that confer vulnerability for addiction-like behaviour, particularly for characterizing individual variation in the susceptibility to reinstate drug-seeking behaviour in the presence of drug cues (Robinson & Berridge, 2008; Robinson & Flagel, 2009; Flagel & Robinson, 2017). Sign-tracking refers to the propensity of about a third of outbred rats to approach and contact a Pavlovian cue (lever extension) associated with a food reward during a Pavlovian Conditioned Approach (PCA) screening test. This behaviour has been proposed to reflect a rapid and effective attribution of incentive salience to such a cue, rendering it to be ‘attractive and magnetic’ or ‘wanted’ (Berridge & Robinson, 2016). This interpretation is supported by studies showing that food and drug cues are also more effective conditioned reinforcers, and more effective in instigating food and drug-seeking behaviour, in STs than GTs (references above). Furthermore, cue-evoked ventral striatal dopamine signalling is necessary for the attribution of incentive salience to reward cues in STs (Flagel et al., 2011; Saunders et al., 2013; Yager & Robinson, 2013; Yager et al., 2015). In addition, STs exhibit a bias for the stimulus-driven, or ‘bottom-up’ attention, and this bias is mediated via an unresponsive cortical cholinergic input system. These findings indicate that the sign-tracking trait involves broad and complex cognitive-motivational interactions mediated via multiple neuromodulator systems (Paolone et al., 2013; Koshy Cherian et al., 2017).
During PCA testing, GTs exhibit conditioned orienting to the cue, indicating that the cue provides informational or predictive value, but, in contrast to STs, they do not approach it and instead learn to approach the location of pending food delivery. These findings suggest that GTs utilize a more cognitive or top-down analyses of the stimulus situation than STs (Flagel et al., 2009; Clark et al., 2012). This relatively more potent top-down cognitive style of GTs is also indicated by their relatively superior performance of attention tasks (Paolone et al., 2013), and by the relatively greater power of contextual cues to control their behaviour (Saunders et al., 2014). Consistent with the latter, we recently reported that an ‘occasion-setting’ cue was more effective in reinstating cocaine seeking in GTs (Pitchers et al., 2017a). We also found that the relatively superior attentional performance of GTs is associated with a relatively highly responsive cortical cholinergic input system (Paolone et al., 2013). In contrast, in STs, the capacity of the choline transporter is nearly unresponsive to stimulation of cholinergic neurons, which limits their ability to increase cholinergic activity with increasing demand (Koshy Cherian et al., 2017). Confirming the role of acetylcholine (ACh) in the ability of GTs to deploy top-down biases to process discriminative cues, cholinergic cell loss attenuates cocaine seeking evoked by a discriminative stimulus signalling drug availability in GTs but not STs (Pitchers et al., 2017a). Notably, the opponent cognitive-cholinergic biases of STs and GTs are similar to those seen in mice and humans expressing low- and high-capacity choline transporters, respectively (Sarter et al., 2016).
This experiment was designed to measure the effects of presentation of a Pavlovian cue previously associated with IV injections of cocaine on the activity of two major neuromodulatory inputs to the prefrontal cortex, dopamine (DA) and ACh. We hypothesized that STs would express their preference for cue-directed behaviour and that increases in cortical extracellular DA levels would be associated with their bias for processing of the motivational attributes of the cocaine cue, as has been described in cocaine addicts (Milella et al.,2016). Furthermore, in STs, such cue-evoked increases in prefrontal DA levels may stabilize such cue-directed behaviour, even under extinction conditions (Ellwood et al., 2017). Increases in prefrontal DA levels may parallel the well-documented increases in ventral striatal DA levels which, in STs, support the behavioural significance of Pavlovian reward cues (Flagel et al., 2011) and cue-evoked drug-seeking behaviour (Saunders et al., 2013; Fraser & Janak, 2017). Cue-evoked increases in prefrontal and ventral striatal dopaminergic activity together may mediate the cognitive-motivational biases that characterize the behaviour of STs in the presence of Pavlovian reward and drug cues.
In contrast, GTs were expected to exhibit less cue approach behaviour, consistent with the very behaviour that underlies their classification as GTs, and that this behaviour would be associated primarily with increases in prefrontal ACh levels. The results indicate a double dissociation in the influence of a cocaine cue on prefrontal DA and ACh activity STs and GTs, and they suggest that these phenotypes will be useful for studying the role of neuromodulator systems in mediating opponent behavioural-cognitive styles.
Materials and methods
Animals
Sprague-Dawley rats (Envigo, Indianapolis, IN) weighing 250–275 upon arrival were housed in individual cages and kept on a 12-h light/dark cycle (lights on at 0800), with regulated temperature and humidity. STs and GTs were screened from multiple groups of rats, totalling 236 Sprague-Dawley rats. We previously reported that the expression of the ST/GT phenotype in Sprague-Dawley and Heterogeneous Stock rats is not affected by sex, (Pitchers et al., 2015) and thus only one sex (male) was used for this study. After arrival, rats were given 1 week to acclimate to the colony room before experimentation commenced. Food (Rodent Chow, Laboratory Rodent Diet 5001; LabDiet) and water were available ad libitum until the onset of Pavlovian conditioning. Starting 2 days before the first day of Pavlovian conditioning, access to food was limited (20–24 g of regular chow per day) to maintain stable body weight throughout testing and to prevent the unhealthy obesity associated with longterm ad lib feeding (Rowland, 2007). All procedures were approved by the University of Michigan Institutional Animal Care and Use Committee and conducted in Association for Assessment and Accreditation of Laboratory Animal Care-accredited laboratories.
Pavlovian conditioned approach (PCA)
Apparatus
Conditioning test chambers (20.5 × 24.1 cm floor area, 29.2 cm high; Med Associates, St. Albans, VT) were used for PCA training (Meyer et al., 2012). Each chamber was equipped with a food receptacle located 2.5 cm above the floor in the centre of the wall. A catch tray filled with corn-cob bedding was located underneath the floor, which was constructed from stainless steel rods. A red house light was located on the wall opposite the food receptacle and remained on for the duration of training sessions. A retractable lever (Med Associates) was located approximately 2.5 cm to the left or right of the food receptacle, 6 cm above the floor. The side of the lever with respect to the food receptacle was counterbalanced across boxes. A white LED was located inside the lever housing and was used to illuminate the slot through which the lever protruded when it was extended. The lever required a ~15 g force to deflect, such that most contacts with the lever were recorded as a ‘lever press’. The pellet dispenser (Med Associates) delivered one 45-mg banana-flavoured food pellet (Bio-Serv®, #F0059, Frenchtown, NJ) into the food receptacle at a time. Head entry into the food receptacle was recorded each time a rat broke the infrared photobeam located inside the receptacle (1.5 cm above the base of the food cup). Each conditioning chamber was located in a sound-attenuating enclosure, and background noise was supplied by a ventilating fan. Data collection was controlled by med-pc software.
PCA
During the initial 1-week acclimation period, rats were handled regularly. All training sessions were conducted during the 12-h lightson period. The day before the start of training 20 banana-flavoured pellets (Bio-Serv®, #F0059, Frenchtown, NJ) were placed in the rats’ home cages to familiarize them with this food. For pre-training, rats were placed into the test chamber with a red houselight illuminated, and while the lever remained retracted 25 food pellets were delivered on a variable interval (30 s) schedule to assure that rats reliably retrieved pellets from the receptacle. All animals consumed all food pellets by the end of pre-training.
The following day, rats began training on the PCA procedure. During a PCA training session, each individual trial consisted of the insertion of the illuminated lever (conditioned stimulus, CS) into the chamber for 8 s and, immediately following the retraction of the lever, the activation of the pellet dispenser caused the delivery of a single food pellet (unconditioned stimulus, US) into the food receptacle. The intertrial interval (ITI) started immediately following the retraction of the lever. The CS was presented on a variable interval 90-s schedule such that one presentation of the CS occurred on average every 90 s, but the actual time between CS presentations varied randomly between 30 and 150 s. Each Pavlovian training session consisted of 25 trials, yielding 35 to 40-min sessions. PCA training was conducted over 5 consecutive days. For each trial, the measures were taken as follows: (i) number of lever deflections (contacts), (ii) latency to first lever deflection, (iii) number of head entries into the food cup (referred to as food cup entries) during presentation of the CS, (iv) latency to the first cup entry following CS presentation, and (v) number of cup entries during the ITI. All animals included in the final analysis consumed all food pellets during each training session.
At the conclusion of PCA training, the propensity of each individual rat to approach the lever-CS vs. the food cup during the CS period (i.e. sign-tracking or goal-tracking CR) was calculated using a PCA Index score, as previously described (e.g. Meyer et al., 2012). Briefly, the PCA Index score consisted of averaging three measures of conditioned approach: (i) the relative number of contacts with either the lever-CS or food cup during the CS period [(proportion of trials with a lever-CS contact) – (proportion of trials with a food cup contact)]; (ii) the response bias for contacting the lever-CS or the food cup during the CS period [(#lever-CS contacts – #food cup contacts)/(#lever-CS contacts + #food cup contacts)]; and (iii) the latency to contact the lever-CS or the food cup during the CS period [(food cup contact latency – lever-CS contact latency)/8]. If an animal did not make a lever or food cup contact, the latency was recorded as 8 s. Averaging these three measures produces PCA Index scores along a scale ranging from −1.0 to +1.0, where +1 indicates an animal made a sign-tracking CR on every trial and −1 a goal-tracking CR on every trial, and 0 a 50 : 50 distribution of sign- and goal-tracking CRs. For the purpose of classification, rats with an averaged PCA Index score from days 4 and 5 ranging from −1.0 to −0.5 were operationally defined as GTs (i.e. rats more likely to direct behaviour towards food cup than lever), and rats with a PCA Index score between +0.5 and +1.0 were designated as STs (i.e. rats more likely to direct behaviour towards the lever-CS than food cup). Of the rats screened for PCA behaviour, 18 STs and 17 GTs were used in the experiment described below. Rats with scores that fell between −0.49 and +0.49 were classified as intermediates, and their behavioural attention fluctuated between lever-CS and food magazine. Intermediates were not studied in the current experiment.
Surgeries
Intravenous catheter surgery
Following the completion of PCA training and the selection of STs and GTs, chronic indwelling catheters were implanted into the right jugular veins, as described previously (e.g. Pitchers et al., 2017b). Briefly, animals were anesthetized initially with 5% isoflurane in an anaesthetic chamber (Anesco/Surgivet) then maintained with 2% isoflurance via nose cone. Gas was carried via oxygen at a flow rate of 0.6 L/min. Animals’ body temperatures were maintained at 37 °C using Deltaphase isothermal pads (Braintree Scientific) and ophthalmic ointment were provided for lubrication of the eyes. Post-operative pain was managed with carprofen (5 mg/kg, s.c.), given prior to surgery and for 48 h thereafter. The catheter exited through the dorsal skin surface between scapulae. Following surgery, catheters were flushed daily with 0.2 mL of sterile saline containing 5 mg/mL gentamicin sulphate (Vedco) to prevent occlusions and minimize infections. Catheter patency was tested periodically by intravenous (IV) injection of 0.15 mL of methohexital sodium (10 mg/mL in sterile water; JHP Pharmaceuticals). Two animals were removed because they failed to become ataxic after infusion; the data from these animals were discarded from analyses.
Intracranial placement of cannula for microdialysis
Anaesthesia was maintained upon completion of the implantation of the IV catheter surgery, and animals were placed in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA). The microdialysis guide cannula and probes were custom designed. For the construction of the probe, two 75-μm (inner diameter) × 150 μm (outer diameter) fused silica capillary tubings (TSP075150; Polymicro Technologies) were glued together and inserted into a 22-gauge stainless steel tubing, which served as a shaft, with a 2 mm offset at the tip for the inlet capillary. The capillary tip was ensheathed in a 20 kDa molecular weight cutoff polyacrylonitrile membrane (AN69; Hospal). The tip and the base of the membrane were sealed with epoxy resin (Locitite; Henkel). Guide cannulae were implanted directed at the prelimbic region of the medial prefrontal cortex according to the following coordinates, relative to bregma: anteriorposterior (AP) +2.9 mm, medial-lateral (ML) 0.6 mm, and dorsalventral (DV) –1.9 mm (below skull). To prevent clogging, the cannulae were equipped with stainless steel stylets. Cannulae were anchored with three metallic screws and secured to the skull with acrylic dental cement. After surgery, animals were returned to their home cages and monitored for post-surgery injury and infection.
Pavlovian training with cocaine as the unconditioned stimulus (US)
Apparatus
Pavlovian training with cocaine as the US, test day, and conditioned reinforcement test (described below) were conducted in similar behavioural chambers to those used for PCA training (described above) but equipped with a different interface configuration. For Pavlovian cocaine training, instead of a food magazine and adjacent lever, the chambers were equipped with a central cue light (13.5 cm above floor) on the wall opposite to the red house light. A syringe pump connecting to the rats’ catheter back ports delivered cocaine infusions from outside the chamber. The pump tubing was strung through the side of the box enclosing the chamber, and suspended into the chamber through a swivel mechanism, which permitted free movement during sessions.
Pavlovian training
Prior to training, rats were randomly assigned to either paired (CS and US presented together) or unpaired groups (US explicitly not paired with presentation of the CS) ultimately producing four experimental groups: STs paired (n = 10), STs unpaired (n = 8), GTs paired (n = 9) and GTs unpaired (n = 8). Training occurred over 15 days. For the CS-US paired groups, the illumination of the cue light for 20 s coincided with the onset of an I.V. infusion of 0.4 mg/kg/inf (50 μL delivered over 2.8 s) of cocaine hydrochloride (weight of salt; NIDA) dissolved in 0.9% sterile saline. No action was required to initiate the cocaine infusion or the CS illumination. Each session consisted of nine trials (CS-US presentations) occurring on a variable time schedule with a mean of 1200 s (1140–1260 s). This length of time between trials ensured that systemic levels of cocaine were minimal at the time of the next cocaine infusion (Booze et al., 1997). Rats in the CS-US unpaired groups were given nine cocaine infusions of 0.4 mg/kg/inf (50 μL delivered over 2.8 s) explicitly not paired with illumination of the CS. Cocaine infusions were instead delivered on a variable time schedule with a mean of 240 s (210–270 s) after the CS was extinguished.
Video analysis of behaviour
Video was scored offline by two separate observers blind to the experimental condition for two different CRs. (i) Conditioned orientation: an orienting response was scored if the rat made a head and/or body orientation in the direction of the CS during the CS period, regardless of whether the rat approached the CS. (ii) Conditioned approach: an approach response was scored if during the CS period a rat moved towards the CS, bringing its nose to within ~1 cm of the light. To achieve this, the rat had to rear, lifting both front paws off the floor, towards the light. Thus, if an approach response was scored on a given trial then an orienting response would have also been scored, as orientation always preceded approach. However, an orienting response could occur in the absence of an approach response. Conditioned orienting and approach responses were scored for Pavlovian training sessions 1, 3, 5, 10 and 15.
Test day and microdialysis
Following 15 sessions of Pavlovian training with cocaine as the US, animals underwent 10-day cocaine abstinence period (Lu et al., 2004). On days 7–9 of the abstinence period, all animals experienced 2 h of habituation to a tether hooked onto their affixed headstage. The test day occurred on day 10 of the abstinence period. This microdialysis session began with the removal of the stylet and the insertion of a probe. Animals were perfused at rate of 1 μL/min with artificial cerebrospinal fluid (aCSF; pH 7.4) containing the following (in mM): 145.00 NaCl, 2.68 KCl, 1.10 MgSO4, 1.22 CaCl2, 0.50 NaH2PO4, and 1.55 NaHPO4 as well as 25 ascorbic acid. A relatively low concentration of neostigmine (10 nM) was also added to the aCSF perfusate to enhance the recovery of ACh while having minimal effects on basal and activated ACh levels (Himmelheber et al., 1998). Dialysate samples were collected every 4 min, beginning 5 h after inserting the probe.
Collection started with five baseline samples before the first block of Pavlovian cue presentations commenced. Over the next 48 min, four equally spaced 4-min blocks of cue presentation (C1–C4) occurred. Within each cue block, the Pavlovian light cue was presented eight times, each for 5 s every 30 s. Cue presentations during the microdialysis session were shortened to 5 s compared to 20 s during the conditioning phase, for the following reasons. Shorter cue presentations typically result in higher levels of behavioural responding, are associated with less rapid extinction, and allow for a greater number of cue presentations within the 4-min dialysis collection blocks. One dialysate was collected for each C-block. Each C-block was followed by an 8-min period (two 4-min dialysate collections) during which no-cue was presented (NC). At the conclusion of test day, the probe was removed and animals were returned to their home cages. To determine the recovery rate of the probe, the membrane tip of the probe was immersed in recovery solution with known concentrations of DA and ACh, and an additional three dialysate samples were collected. The concentrations acquired from recovery dialysate samples were compared to those of the original recovery solution. Absolute basal neurotransmitter levels were corrected by probe recovery rates (range: 11–34%).
Video analysis of behaviour during microdialysis session
Videos taken during microdialysis sessions were scored offline by two separate observers blind to experimental condition. These analyses determined the types of behaviour associated with altered extracellular levels of DA and ACh, and focused on behaviour during the presentations of the CS (5 s) and during which no drug was administered. Specifically, for all C and NC blocks, we determined whether the animal oriented towards the cue and/or approached the cue (as defined above). Moreover, we divided the behavioural chamber into equal-sized quarters to determine locomotor activity, by measuring number of grid crossings, and we calculated the total time of immobility.
Analysis of ACh and DA levels in dialysates using high performance liquid chromatography coupled with mass spectrometry (HPLC-MS)
Chemicals and reagents
All reagents, drugs and chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. HPLC-grade water was purchased from Thermo-Fisher Scientific (Waltham, MA) and HPLC-grade acetonitrile and methanol were acquired from VWR (Radnor, PA). d4-ACh and d4–choline were obtained from C/D/N isotopes (Pointe-Claire, Canada; Koshy Cherian et al., 2017).
Sample preparation
Benzoyl chloride derivatization of dialysates and preparation of internal standards was performed using a slightly modified version of a previously described method (Song et al., 2011). Calibration curves were generated using standards at 0, 0.1, 0.5, 1, 5, 10, 20 nM for ACh and DA, and 10 or 100-fold higher for other analytes (not reported here). Internal standards were 1 mm glycine, serine and taurine, aspartate, glutamate, and adenosine; 100 μM GABA, histamine, homovanillic acid, 5-hydroxyindoleacetic acid, and 3,4-dihydroxyphenylacetic acid; and 50 μm 5-HT, norepinephrine, and DA. All internal standards were derivatized by adding 100 mm sodium carbonate buffer followed by 2% 13C6 benzoyl chloride in acetonitrile with 0.1% formic acid. The internal standard stock was then diluted 100-fold in 50% acetonitrile in water containing 1% sulphuric acid. d4-ACh and d4-Cho were spiked into the reaction mixture to a final concentration of 50 nm and 5 μm, respectively (Koshy Cherian et al., 2017).
High performance liquid chromatography (HPLC)
Microdialysis samples were analyzed by a Thermo Finnigan (San Jose, CA) Surveyor Plus HPLC system consisting of an Autosampler Plus and MS Pump Plus. Neurochemical separation was achieved with a Phenomenex (Torrance, CA) Kinetex biphenyl LC column (50 × 2.1 mm, 1.7 μm particle size, 100 Å pore size). Mobile phase A was 10 mm ammonium formate with 0.15% (v/v) formic acid in water. Mobile phase B was acetonitrile. The mobile phase gradient for all 16 analytes was as follows: initial, 0% B; 0.1 min, 10% B; 2.3 min, 20% B; 3.7 min, 50% B; 4.0 min, 80% B; 4.5 min, 0% B; 6.5 min, 0% B. The flow rate was 200 μL/min, and sample injection volume was 5 μL. The autosampler and column were maintained at ambient temperature throughout the analysis.
Mass spectrometry (MS)
A Thermo Finnigan TSQ Quantum Ultra triple quadrupole mass spectrometer operating in positive mode was used for detection. Electrospray ionization (ESI) voltage was 3.5 kV, and heated ESI probe (HESI-I) was set at 300 °C. Capillary temperature was 350 °C and sheath gas, aux gas and ion sweep gas were maintained at 25 arb, 15 arb and 0 arb, respectively. Inter-cycle delay was 200 ms. Automated peak integration was performed using Thermo XCalibur QuanBrowser version 2.1. All peaks were visually inspected to ensure proper integration. Calibration curves were constructed based on peak area ratio (Panalyte/PI.S..) vs. concentrations of internal standard by linear regression.
Test for conditioned reinforcement
One day after the microdialysis test day, all animals were placed back in their training/testing chambers for a conditioned reinforcement test. The purpose of this test was to examine whether, in the absence of cocaine delivery, the animals would perform an instrumental task for the drug cue itself to further assess the motivational properties of the cue. Two nose pokes were placed left and right below the Pavlovian cocaine cue light (3 cm above floor). An active nose poke resulted in the 2 s illumination of the cue light, but no cocaine was delivered. A nose poke into the inactive port had no consequence. The active nose poke (left vs. right) was counterbalanced between rats. Each animal completed a 1-h session. Active and inactive nose pokes were recorded to determine a difference score (active minus inactive responses).
Histological analysis
After the conclusion of the experiment, animals were anesthetized using sodium pentobarbital (270 mg/kg; i.p.) and perfused intracardially with 50 mL of 0.9% saline, followed by 500 mL of 4% paraformaldehyde in 0.1 m phosphate buffer (PB). After being perfused, brains were removed and post-fixed for 1 h at 4 °C in the same fixative, then immersed in 20% sucrose and 0.01% sodium azide in 0.1 m PB and stored at 4 °C. Coronal sections (40 μm) were cut with a freezing microtome (SM 2000R; Leica), collected in three parallel series in cryoprotectant solution (30% sucrose and 30% ethylene glycol in 0.1 m PB) and stored at −20 °C. Cresyl violet-stained sections were imaged at 4× magnification using a Leica DM400B digital microscope to verify probe placement.
Experimental design and statistical analysis
The present experiments were designed to investigate the activity of two major prefrontal neuromodulatory input systems, DA and ACh, in response to repeated presentations of a Pavlovian cue that was previously paired with cocaine in STs and GTs. Therefore, the experimental groups consisted of STs and GTs that were selected from outbred rats using an established PCA test and criteria for categorization of the phenotypes. Furthermore, STs and GTs were randomly assigned to either paired (CS and US presented together) or unpaired groups (US explicitly not paired with CS presentation). As indicated in Animals, the expression of the ST/GT phenotype is not affected by sex and thus experiments were conducted using male rats. Final group sizes were n = 18 STs (10 paired, eight unpaired) and n = 17 GTs (nine paired, eight unpaired).
The primary results from this study concern the extracellular levels of mPFC dialysates collected during the test day (Fig. 5), active and inactive nose pokes during the test for conditioned reinforcement (Fig. 6), and the probability to orient or approach the CS during Pavlovian training (Fig. 3) and the microdialysis session (Fig. 4). Linear mixed-models (LMM) analyses were used for all repeated measures data (e.g. session). The covariant structure was explored and modelled for each dependent variable. The best fitting model of repeated measures covariance was determined by the lowest Akaike information criterion score (Verbeke & Molenberghs, 2009). When main effects were found, post hoc comparisons were conducted using the Least Significant Difference (LSD) test. Alpha was set at 0.05, with the exception for mPFC DA or ACh levels. For microdialysis results, alpha was divided by two, and thus set at P < 0.025, because the analyses of transmitter levels were conducted separately for rats with unpaired and paired presentations of the drug cue. In accordance with prior recommendations, exact P values are reported whenever applicable (Greenwald et al., 1996; Sarter & Fritschy, 2008).
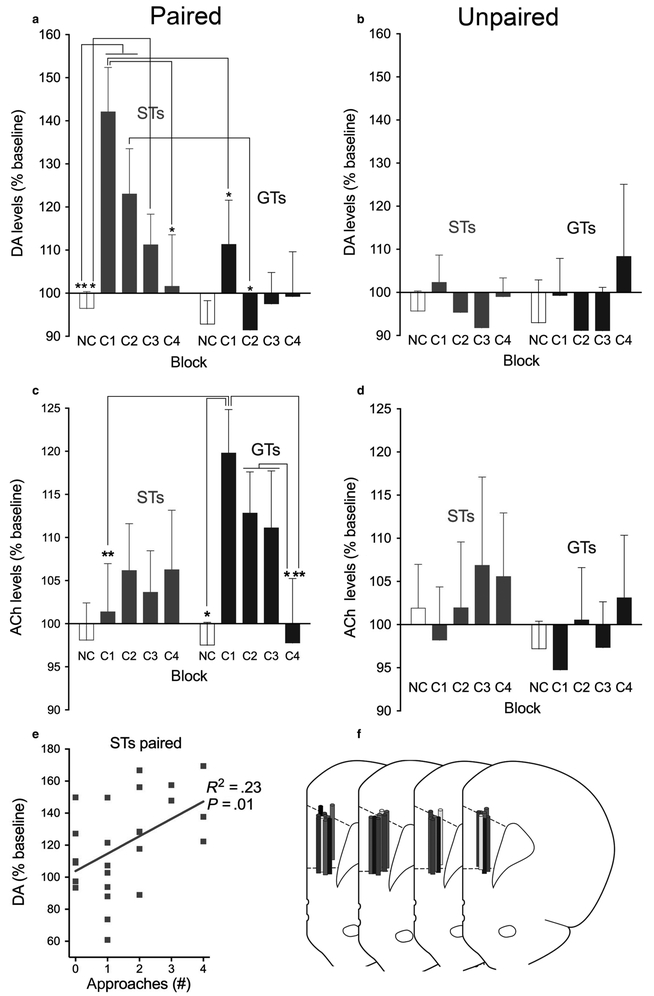
Fig. 5.
Medial prefrontal DA (a,b) and ACh (c,d) levels in paired (a,c) and unpaired (b,d) STs and GTs. One dialysate each was obtained during four 4-min blocks during which the cue was presented (C1–C4), and these blocks were separated by 8-min blocks without cues (NC, data from NC blocks were averaged to yield one data point per condition; note that because of occasional failures to analyse DA or ACh in individual samples, the number of data points per condition varied across neurotransmitters; DA: paired STs, n = 7; paired GTs, n = 5; unpaired STs, n = 5; unpaired GTs, n = 3; ACh: paired STs, n = 10; paired GTs, n = 6; unpaired STs, n = 7; unpaired GTs, n = 6). Cue presentations increased DA levels in STs but not GTs, and ACh levels in GTs, but not STs. In unpaired rats, DA and ACh levels during C1–C4 did not differ from levels seen during NC blocks (M, SEM; multiple comparisons: *P < 0.05; **P < 0.01). (e): In paired STs, individual prefrontal DA levels and approach rates were significantly correlated. No such correlations between approach rates and DA levels were found in paired GTs or unpaired animals, and neither were approach rates correlated with ACh levels in any group of rat (not shown). (f) Placement of the microdialysis probes in the prelimbic cortex (AP level +3.0 mm relative to Bregma) for animals for which dialysate levels were successfully measured. Probe placement is indicated on separate sections for each experimental group for better visualization (left to right: STs paired (n = 10), STs unpaired (n = 7), GTs paired (n = 6), GTs unpaired (n = 7)). [Colour figure can be viewed at https://wileyonlinelibrary.com].
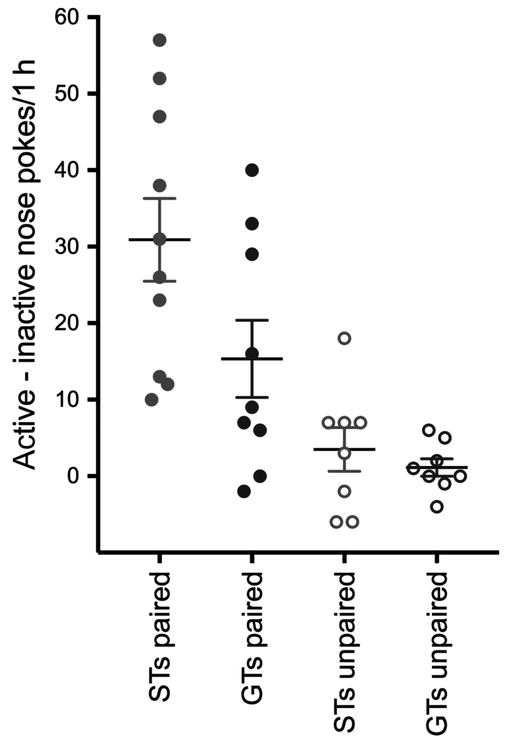
Fig. 6.
Performance during a test of conditioned reinforcement (data points depict the differences in nose pokes into the active minus inactive port; lines depict means and SEM). Paired groups (STs, n = 10; GTs, n = 9) had a significantly greater difference score than unpaired groups (STs, n = 8; GTs, n = 8). Moreover, STs generally scored more active port entries, and thus higher difference scores, than GTs (see Results for anova). [Colour figure can be viewed at https://wileyonlinelibrary.com].
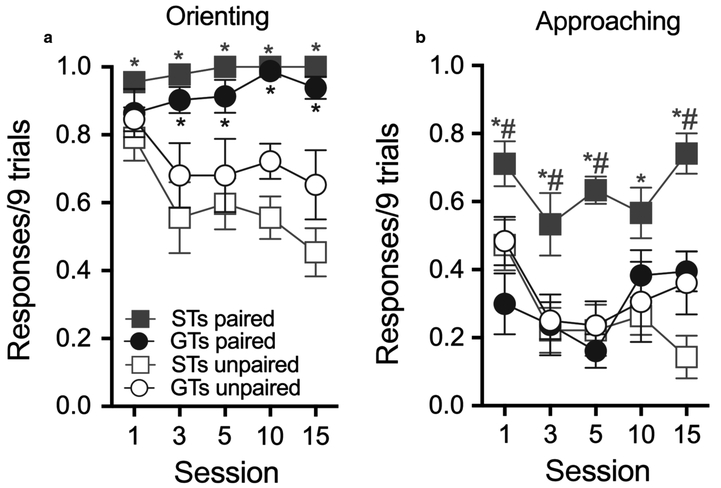
Fig. 3.
CS-directed orientating responses (a) and approach responses (b) in rats trained with paired CS-US presentations and in rats receiving non-contingent cocaine (STs paired: n = 10; GTs paired: n = 9; STs unpaired: n = 8; GTs unpaired, n = 8). (a) Paired STs and GTs reliably oriented to the CS relative to their unpaired controls. (b) Paired STs showed significantly higher rates of approaches to the CS than all other groups, suggesting that only they attributed incentive salience to the CS (M; SEM; see Results for anovas; * indicates post hoc comparisons indicating a significant difference between unpaired and paired rats of the same phenotype; # indicates post hoc comparisons indicating a significant difference between paired STs and paired GTs; *,#P < 0.05). [Colour figure can be viewed at https://wileyonlinelibrary.com].
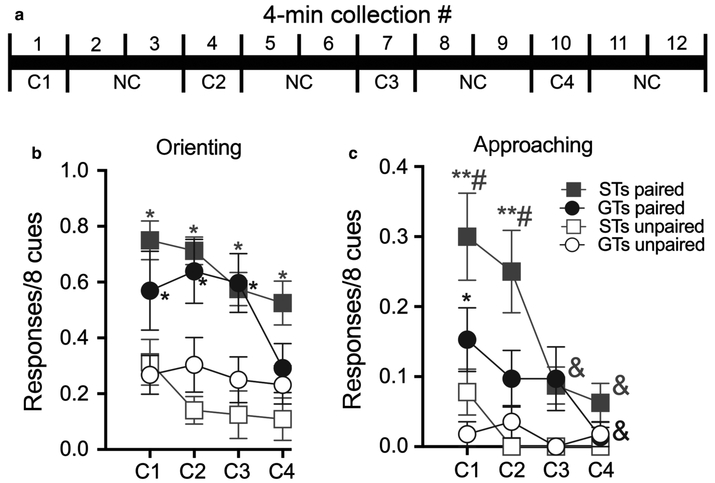
Fig. 4.
The experimental design for the microdialysis test session is shown in (a). Cues were presented during four 4-min blocks of trials (C1–C4; eight cue presentations per block) during which one dialysate was collected. C-blocks were separated by 8 min of no-cue blocks (NC) during which two dialysates were collected. Under extinction conditions (no drug), and while undergoing microdialysis, previously paired STs and GTs continued to orient to the cue (b; post hoc comparisons: *, significantly different from unpaired rats of the same phenotype; *P < 0.05). In contrast, previously paired STs approached the cue more frequently than paired GTs during C1 and C2 (c; #P < 0.05). The rate of approaches in paired STs was significantly higher in C1 and C2 than C3 and C4. Furthermore, the rate of approaches in paired GTs was significantly greater in C1 than in C4 (&P < 0.01). Approach rates in unpaired STs and GTs remained relatively low and significantly lower than approaches counted in paired STs during blocks C1 and C2 (**P < 0.01) and in GTs during C1 (compared with their unpaired counterparts; *P < 0.05. Note that Figs 3 and 4 show different ordinate ranges, reflecting that compared with the rate of cue-directed behaviour in the presence of cocaine the rates of orienting and approach responses were expectedly lower following a period of abstinence and in the absence of cocaine). [Colour figure can be viewed at https://wileyonlinelibrary.com].
Results
Classification of STs and GTs
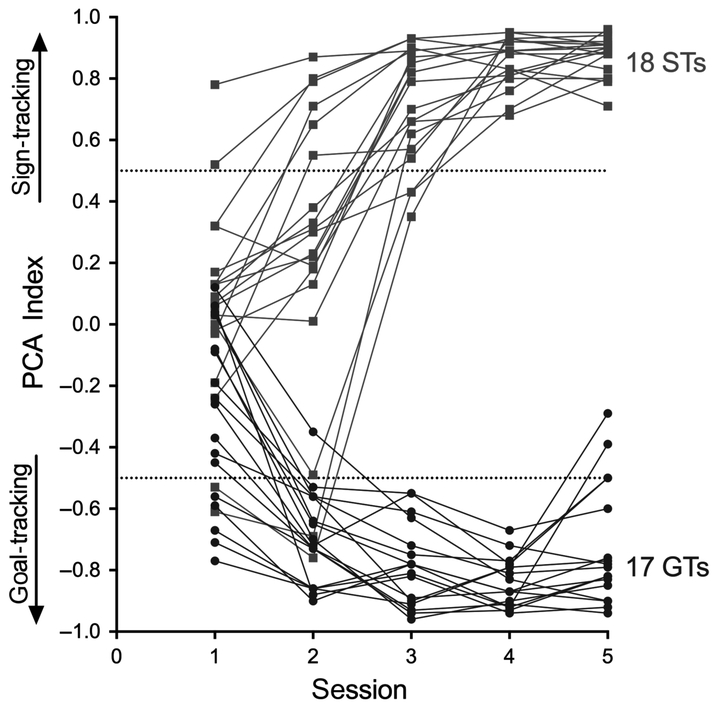
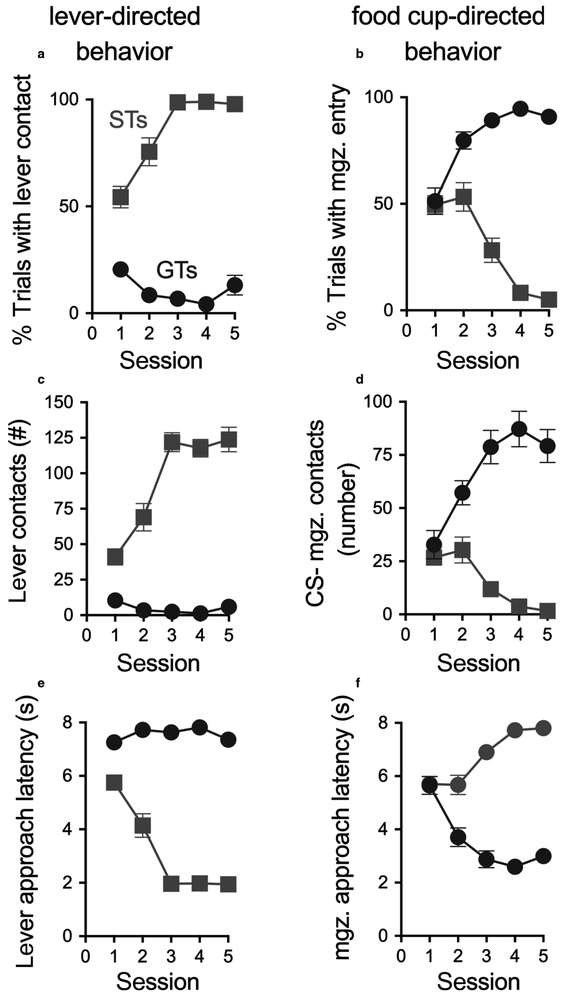
As a result of PCA training, two distinct phenotypes emerged, as previously reported (Flagel et al., 2009; Meyer et al., 2012). Rats were classified as STs or GTs based on the average of PCA Index scores from sessions 4 and 5 (Fig. 1; as rats with intermediate scores were not studied, data from these rats are not shown). For STs, the presentation of the lever-CS over five training sessions evoked conditioned sign-tracking behaviour, which included a greater proportion of contacts with the lever-CS (Fig. 2a), consistent and rapid approach to the lever-CS (Fig. 2e) and vigorous engagement with it (Fig. 2c). In contrast, GTs rarely approached the lever-CS (Fig. 2c). Instead, in GTs, the lever-CS elicited a reliable and rapid approach to the food cup (Fig. 2b,d,f). The lever- or cup-directed responses in STs and GTs, respectively, increased across training days. The resulting STs (n = 18) and GTs (n = 17) used in this study had PCA Index scores that fell between +0.74 to +0.95 (M, SEM: 0.86 ± 0.01) and −0.54 to −0.93 (−0.78 ± 0.03), respectively.
Fig. 1.
Individual PCA index values across five PCA training sessions. The final classification of ST (n = 18) or GT (n = 17) phenotype was based on averaging PCA index values from sessions 4 and 5. The pre-determined PCA index score cutoffs for STs and GTs were 0.5 and −0.5, respectively. [Colour figure can be viewed at https://wileyonlinelibrary.com].
Fig. 2.
Measures of behaviour towards a lever (CS; left column) vs. the location of food delivery (food magazine or cup; right column) over the five PCA training sessions of rats that were eventually classified as STs (n = 18) or GTs (n = 17). Mean ± SEM for (a) the proportion of trials during which the rat approached the lever-CS during the 8 s CS period; (b) the proportion of trials during which the rat approached the food cup during the 8 s CS period; (c) the number of lever contacts; (d) the number of food cup entries during the 8 s CS period; (e) the latency to first lever contact during CS presentation; and (f) the latency to the first food cup entry during CS presentation. [Colour figure can be viewed at https://wileyonlinelibrary.com].
Orienting and approaching a cocaine cue
Conditioned orienting to a CS indicates that the animal acquired the CS-US association and thus that the CS gained informational value. Approaching and contacting a CS, such as the lever during PCA training (above), reflects a CS-evoked motivational state that renders the CS to be attractive and ‘magnetic’ (e.g. Yager & Robinson, 2010; Robinson et al., 2014). Here, we scored a conditioned orientation response if a rat exhibited a head and/or body movement towards the light-CS during the CS period, regardless of whether the rat moved into close proximity to the light. A CS-directed approach response was scored if a rat reared and brought its nose within 1 cm of the light-CS during the CS period. It is worth noting that unlike in previous studies where rats were habituated to the light-CS and infusion procedure to decrease the probability of high levels of responses due to novel stimuli (Yager & Robinson, 2013), the rats in this experiment had no previous experience with the light cue or the infusion procedure. It is probable that the novelty of the stimuli caused high levels of responding in all groups upon the first day of training. However, variations in response between paired/unpaired groups and ST/GT groups emerged across sessions.
Conditioned orientation
As would be expected, paired CS-US presentations, when compared with training of unpaired presentations, generated more reliable orienting towards the CS in both phenotypes (main effect of pairing; F1,33.71 = 84.39, P < 0.001). Multiple comparisons indicate that paired STs exhibited more orienting responses than unpaired STs during all sessions, and that paired GTs oriented more frequently during all but the first test session (Fig. 3a). Orienting responses did not differ by phenotype or session, and the two factors did not interact significantly (all F < 2.31, all P > 0.06). Thus, paired CS-US presentations maintained reliable orienting responses to the CS in both GTs and STs, suggesting that in both phenotypes the CS acquired informational value.
Conditioned approach
Unlike the reliably high rate of orienting responses to the CS by both GTs and STs trained with paired CS-US presentations, only paired STs approached the CS significantly more frequently than their unpaired counterparts. Moreover, paired STs approached the CS more frequently than paired GTs. Indeed, approach responses in paired GTs did not differ from the responses counted in unpaired GTs or unpaired STs (main effect of phenotype: F1,31.32 = 11.28; P = 0.002; pairing; F1,31.32 = 16.47, P < 0.001; phenotype × pairing; F1,31.32 = 23.45, P < 0.001; Fig. 3b). Thus, both STs and GTs trained with paired CS-US presentations learned the cue’s predictive association as indicated by similar conditioned orientation responses, but only STs approached the cue more frequently than unpaired rats. This latter result suggests that the CS evoked a motivational state that rendered the CS to be attractive only in STs.
Microdialysis test session
Following a 10-day abstinence period, including sessions to habituate the animal to microdialysis conditions, animals were presented with the CS, but cocaine was not administered. The light-CS was repeatedly presented (eight times for 5 s every 30 s) during four 4-min blocks (C1–C4). C-blocks were separated by an 8-min block (two dialysate collections) void of cue presentations (no-cue, NC; Fig. 4a).
Conditioned orientation
As was the case during the initial Pavlovian conditioning and when cocaine was the US, following abstinence and now in the absence of cocaine, STs and GTs that were originally presented with the paired CS-US continued to exhibit higher rates of orienting responses to the CS than their unpaired counterparts (pairing: F1,3.06 = 31.62, P < 0.001; phenotype; F1,33.06 = 0.04, P = 0.85; Fig. 4b; pairing × phenotype: F1,33.06 = 2.56, P = 0.12). The number of orienting responses declined in both phenotypes over C1–C4 (block: F3,83.79 = 3.97, P = 0.01; block × phenotype: F3,83.79 = 1.81, P = 0.15), reflecting extinction of the response following repeated CS presentation in the absence of cocaine. Thus, during the microdialysis test session and in the absence of cocaine, STs and GTs continued to indicate that the CS maintained its informational value, at least through the third block of cue presentations (Fig. 4b).
Conditioned approach
STs that previously were presented with light-cocaine pairings approached the CS more frequently than paired GTs (phenotype; F1,46.19 = 5.29, P = 0.026; pairing: (F1,46.19 = 36.77, P < 0.001; pairing × phenotype (F1,46.19 = 1.29, P = 0.03). Furthermore, the rate of approaches decreased in paired STs and GTs over C1–C4 (block; F3,84.76 = 7.61, P < 0.001; block × pairing: F3,84.76 = 3.32, P = 0.02). Multiple comparisons (indicated in Fig. 4c) indicated that paired STs had more approaches than paired GTs and unpaired STs during C1 and C2, and that paired GTs had more approaches than unpaired GTs during block 1. Furthermore, the proportion of trials during which paired STs approached the cue was higher in C1 and C2 than in C3 and C4. In paired GTs, approaches in C1 were higher than in C4.
Thus, during the microdialysis test session, when the CS was presented in the absence of cocaine, both paired STs and GTs continued to orient to the cue at comparable rates. However, paired STs approached the cue more frequently than paired GTs during the first two blocks of cues. Except for the first block C1, paired and unpaired GTs approached the cue at similar and relatively low rates.
Basal extracellular DA and ACh levels
Rats underwent prefrontal microdialysis for DA and ACh while being presented with the CS and during no-cue periods (see Fig. 4a for timeline and dialysate collections taken for analysis).
Absolute basal levels of extracellular DA and ACh, based on five samples taken prior to block C1, did not differ by phenotype and condition (main effects and interactions; all F < 2.57, all P > 0.10; Table 1). Thus, ACh and DA levels obtained during subsequent blocks C1–C4 and during NC blocks (averaged across all NC blocks) were expressed as per cent of basal levels (with basal levels set at 100%; Fig. 5). Note that due to failures of HPLC-MS processing of individual samples, the number of data points indicating DA and ACh levels was not identical across phenotypes and condition (paired/unpaired) and therefore analyzed using LMM. The effects of cue presentation (factor: block (NC, C1–C4)) and phenotype on ACh and DA levels were separately analyzed for paired and unpaired phenotypes therefore alpha was set at 0.05/2.
Table 1.
Absolute basal extracellular levels (nm) of dopamine (DA) and acetylcholine (ACh)
| DA (M ± SEM); n | ACh (M ± SEM); n | |
|---|---|---|
| STs paired | 0.50 ± 0.19; 7 | 28.74 ± 6.79; 10 |
| STs unpaired | 0.67 ± 0.37; 4 | 15.14 ± 4.36; 7 |
| GTs paired | 1.08 ± 0.32; 5 | 25.59 ± 10.88; 6 |
| GTs unpaired | 1.20 ± 0.48; 3 | 16.20 ± 3.88; 6 |
Cue presentation-associated prefrontal DA levels
During the C1–C3 cue presentation blocks, DA levels in paired STs increased significantly over non-cue periods and over DA levels measured during corresponding cue periods in GTs (Fig. 5a). In contrast to DA levels in paired STs, in paired GTs DA levels during cue periods did not differ from non-cue periods (phenotype: F1,9.77 = 6.37, P = 0.03; block: F4,8.2 = 35.45, P < 0.001; phenotype × block: F4,8.2 = 16.66, P = 0.001; see Fig. 5 for results from multiple comparisons). In unpaired STs and GTs, DA levels across cue presentation blocks remained relatively close to baseline levels and did not differ from levels recorded during no-cue presentation blocks (main effects and interaction: all F < 2.64, all P > 0.07; Fig. 5b).
Cue presentation-associated prefrontal ACh levels
In paired STs and GTs, cue presentation elevated ACh levels in GTs but not STs (Fig. 5c), doubly dissociating the effects of phenotype and transmitter. In paired GTs, ACh levels were significantly higher during C1 when compared with ACh levels during their non-cue blocks and the same block in STs (phenotype: F1,61.77 = 2.34, P = 0.075; block: (F4,34.01 = 1.41, P = 0.24; phenotype × block: F4,34.01 = 3.41, P = 0.02). As was the case with DA, ACh levels in unpaired rats did not differ by phenotype or across NC and C1–C4 blocks (all F < 0.89, all P > 0.45; Fig. 5d).
Correlations between DA and ACh release and approach rates
Orienting responses did not differ between paired STs and GTs and remained relatively flat across cue presentation blocks C1–C4 (Fig. 4a), not mirroring the differential increases in DA and ACh, respectively (Fig. 5). Accordingly, orienting responses and neurotransmitter levels were not correlated in any group of rats (DA: all R2 < 0.08, all P > 0.23; ACh: all R2 < 0.15, all P > 0.07). In contrast, in paired STs, the initially higher number of approaches to the cue decreased in the course of cue presentations (Fig. 4b), and this time course was mirrored in these rats’ DA levels (R2 = 0.23, P = 0.01; Fig. 5e; paired GTs: R2 = 0.11, n.s.; unpaired rats: both R2 < 0.008). Correlations between ACh levels and approaches in paired STs (R2 = 0.0005), paired GTs (R2 = 0.08), and in unpaired rats (both R2 < 0.009) were not observed.
To explore the behaviour of GTs in the presence of the cue and while they exhibited relatively low levels of approaches when compared with STs, we scored the animals locomotor activity (grid crossings) and generated a mobility score. When the cue was on (8× for 5 s; total 40 s) during the first block (C1) of cue presentations, both paired STs and GTs were more mobile during cue presentations when compared with unpaired rats (pairing: F1,34 = 9.98, P = 0.004; phenotype: F1,34 = 0.09, P = 0.77; pairing × phenotype: F1,34 = 0.09, P = 0.77; data not shown). Thus, the cue increased mobility in paired rats of both phenotypes, but only STs directed their behaviour towards the cue. While not approaching the cue, GTs did not exhibit overtly distinct behaviours.
Test of conditioned reinforcement
As the effects of cue presentations on DA and ACh were conducted under extinction conditions (no cocaine), the following test day all rats were tested for the ability of the Pavlovian cocaine cue (light-CS) to reinforce instrumental responding. During this test, responses into the active port produced a brief (2 s) presentation of the light-CS (but no drug) previously either paired or unpaired with cocaine infusions. Both paired STs and GTs produced more responses into the active port than those into the inactive port. Difference scores for paired groups were significantly higher than that of unpaired groups (pairing: F1,34 = 23.24, P < 0.001; Fig. 6). Furthermore, the difference score for paired STs was significantly higher than that of paired GTs (phenotype: F1,34 = 4.32, P = 0.04; paired × phenotype: (F1,34 = 2.34, P = 0.14), indicating that a response-dependent presentation of the light cue previously associated with cocaine continued to have greater motivational value in STs than GTs.
Discussion
Both sign- and goal-tracking rats learned a Pavlovian association between the presentation of a light cue (the CS) and an IV injection of cocaine (the US), when the CS and US were paired, but not when they were unpaired. Following a 10-day abstinence period, the effects of presentation of the cocaine cue (under extinction conditions) on extracellular prefrontal DA and ACh levels were determined, using microdialysis, and compared with neurotransmitter levels measured during non-cue periods, in paired and unpaired STs and GTs. Previously paired STs oriented towards and approached the cue and they exhibited increases in DA, but not ACh, levels. In paired STs, the number of approaches and DA levels were significantly correlated. Previously paired GTs also oriented towards the cue but they did not approach it, and they exhibited increases in ACh, but not DA, levels. A subsequent test of conditioned reinforcement confirmed that after the dialysis test day the cue continued to have greater incentive value in STs than GTs.
Reward processing and DA in STs
STs were previously extensively demonstrated to readily perceive reward cues as ‘attractive or magnetic’, reflecting the attribution of incentive salience to such cues. As also reflected by the behaviour of STs during PCA training, STs will approach and interact with such cues. Furthermore, they will work to access such cues, that is, they work for secondary reinforcers, and such cues instigate and energize instrumental action in STs (Flagel et al., 2007, 2009; Saunders & Robinson, 2011; Meyer et al., 2012, 2014; Yager & Robinson, 2013; Flagel & Robinson, 2017). Previous studies also demonstrated that mesolimbic, ventral striatal dopaminergic activity is necessary for the transformation of Pavlovian reward cues in STs to cues that instigate heightened levels of motivation, approach and cue-oriented actions (Flagel et al., 2011; Saunders & Robinson, 2012; Saunders et al., 2013). The present data extend these findings to the prefrontal cortex, suggesting that cortical and subcortical dopaminergic projections act in concert (Carr et al., 1999; Otis et al., 2017) to attribute incentive salience to reward cues in STs (see also Milella et al., 2016). This view is supported by our present finding that the rate of approaches to the cue and prefrontal DA levels were significantly correlated. Increases in prefrontal DA levels may enhance the perceptual ‘magnetism’ of reward cues and the stability of cue-oriented responding (Ellwood et al., 2017), while elevated ventral striatal DA levels may facilitate cue-oriented behaviours, including instrumental behaviours designed to obtain cue access (for more evidence indicating dissociations between the role of prefrontal and ventral striatal regions in cue vs. action processing see Dalley et al., 2002; Brady & O’Donnell, 2004; Goto & Grace, 2005; St Onge et al., 2012; Saddoris et al., 2015).
Although the cocaine cue elevated prefrontal DA in STs, it did not affect prefrontal ACh. The absence of increases in cholinergic modulation may further bias the behaviour of STs towards salient Pavlovian cues, for the following reasons. Our collective evidence indicates that low levels of cholinergic neuromodulation biases humans and rodents towards bottom-up, cue-driven attention, while elevated levels of cholinergic activity favours top-down attentional control and goal-driven analysis of contextual information to optimize performance (Berry et al., 2014, 2015; Sarter et al., 2014, 2016; Lustig & Sarter, 2016). We previously demonstrated that poor attentional control (Paolone et al., 2013) and impulsivity (Lovic et al., 2011) are behavioural components of the trait that is indexed by sign-tracking, and that relatively poor attentional performance is mediated via relatively low levels of cholinergic neuromodulation (Paolone et al., 2013). Moreover, we discovered that the capacity for increases in cholinergic activity is strongly constrained in STs by choline transporters that, upon stimulation of cholinergic neurons, fail to populate the synaptosomal plasma membrane and thus to support elevated levels of ACh synthesis and release (Koshy Cherian et al., 2017). Further proof that a nearly ‘frozen’ cholinergic system in STs contributes to a propensity for attending to a Pavlovian cue, such as seen during PCA training, originated in an experiment that demonstrated that pharmacological inhibition of the choline transporter biases unscreened rats towards exhibiting sign-tracking behaviour (Koshy Cherian et al., 2017). Thus, sign-tracking behaviour, and the associated propensity to attribute incentive salience to a Pavlovian cue, may not only be mediated by elevated levels of mesolimbic DA but also by low or unresponsive levels of cholinergic neuromodulation. The present evidence that, in STs, a cocaine cue elevated prefrontal DA, but not ACh, levels is consistent with a description of STs’ behaviour as biased by dopaminergically and non-cholinergically mediated processing.
Top-down biases and ACh in GTs
In contrast, the behavioural-cognitive style of GTs is biased by cholinergically and non-dopaminergically-mediated processing. We previously demonstrated that GTs exhibit relatively high levels of attentional control that are associated with relatively high increases in prefrontal cholinergic neuromodulation (Paolone et al., 2013). Consistent with this characterization, GTs favour the processing of discriminative stimuli, context, and ‘occasion setters’ will exhibit stronger reinstatement of cocaine seeking than STs if an occasion setter indicates the availability of cocaine. Moreover, such cocaine seeking in GTs requires the basal forebrain cholinergic system (Pitchers et al., 2017a). In other words, GTs are biased towards the selection and processing of relatively complex, discriminative or situational cues, and this bias is hypothesized to be cholinergically mediated. In addition to the present evidence in support of this hypothesis, future research would need to demonstrate whether this bias of GTs remains unaffected by manipulations of frontal and mesolimbic dopaminergic activity.
What behavioural-cognitive processes are favoured by elevated levels of prefrontal ACh in GTs? During PCA training, GTs refrain from approaching the cue, as they did during cue presentations in this experiment. During PCA training, their behaviour is directed towards the food port, reflecting goal-directed attention and behaviour that is attributed to elevated levels of cholinergic neuromodulation (references above). As was previously shown (Pitchers et al., 2017a) and again was the case in this experiment, GTs orient towards the cue at similar rates as STs, indicating that the informational value of the cue was not affected by phenotype. Moreover, in this experiment, the cocaine cue instigated behavioural activity in both phenotypes, yet approaching the cue was not a component of such activation in GTs. Reflecting that the context of cue presentation under extinction conditions did not offer GTs an opportunity to direct cue-evoked activity, we did not observe any particular, post-orientation behaviour in GTs in this study. Thus, elevated levels of ACh in GTs may have yielded the behavioural de-prioritization of the cocaine cue in the absence of cocaine. The finding that, in GTs, orientation rates and ACh levels were nearly significantly correlated (P = 0.07) supports this hypothesis. The behaviour of GTs may also be interpreted in terms of involving ‘model-based’ processes that more readily incorporate higher-order information, such as changes in context, and lead to explicit cognitive expectations of reward (Flagel et al., 2009; Clark et al., 2012).
Interactions between ACh and DA
The neuronal mechanisms underlying the present dissociation between DA and ACh levels in STs and GTs are unclear. Moreover, elevated DA levels may directly attenuate increases in ACh levels, and vice versa, perhaps via local interactions between the terminals of the two cortical input systems and/or long-loop connections between the soma of two neuromodulator systems (Briand et al., 2007). Alternatively, we already know that, in STs, cholinergic neurons have a relatively unresponsive choline transporter that acts as a ‘hard brake’ for cholinergic activation and thus, instead of searching for neuromodulator interactions, the dissociation between cue-evoked effects on DA and ACh levels in STs may simply reflect such cholinergic dysregulation. Conversely, the dissociation seen in GTs may reflect a hitherto unknown fundamental dopaminergic capacity limit. As prior evidence, gained from unscreened rats, also indicated direct interactions between mesolimbic DA and cortical ACh systems (Zmarowski et al., 2005; St Peters et al., 2011), the presence of capacity limits in one of the two systems in STs and GTs would suffice to predict the DA-ACh dissociation seen in this experiment. Clearly, the interactions between the DA and ACh systems, and also with other neuromodulator systems, remain extremely poorly understood. STs and GTs may prove useful in studying such interactions, including dissociations, between neuromodulator systems.
Cognitive styles
STs may have a propensity towards relatively ‘hot’ dopaminergic processing of reward-related information, to attend to salient cues and to attribute incentive value to such cues. GTs, in contrast, may have a propensity towards relatively ‘cold’ cholinergic processing of the utility of cues for goal-directed behaviour. We have suggested that a ST phenotype (a bias towards cue-evoked motivation and poor cognitive control) may confer susceptibility to transition from drug use to addiction. However, depending on the type of cue associated with drug use, individuals with either phenotype may escalate drug use and thus they both may develop addiction-like behaviour (Kawa et al., 2016; Pitchers et al., 2017a). Lastly, in addition to their interest for studying the behavioural and neuronal mechanisms of addiction, STs and GTs may be highly useful for research on individual variation in cognitive style, how this is related to variation in the function of brain neuromodulator systems, and how these variations may contribute to the risk for developing neuropsychiatric disorders.
Acknowledgements
This research was supported by PHS grant DA031656 (T.E.R., M.S.).
Footnotes
Conflict of interest
The authors declare no competing financial interest.
Data accessibility
Raw data generated by these experiments have been stored on a University of Michigan Server. Data and statistical analyses will be made available upon request.
References
- Berridge KC & Robinson TE (2016) Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol, 71, 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Demeter E, Sabhapathy S, English BA, Blakely RD, Sarter M & Lustig C (2014) Disposed to distraction: genetic variation in the cholinergic system influences distractibility but not time-on-task effects. J. Cognitive Neurosci, 26, 1981–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Blakely RD, Sarter M & Lustig C (2015) Cholinergic capacity mediates prefrontal engagement during challenges to attention: evidence from imaging genetics. Neuroimage, 108, 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booze RM, Lehner AF, Wallace DR, Welch MA & Mactutus CF (1997) Dose-response cocaine pharmacokinetics and metabolite profile following intravenous administration and arterial sampling in unanesthetized, freely moving male rats. Neurotoxicol. Teratol, 19, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM & O’Donnell P (2004) Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J. Neurosci, 24, 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Gritton H, Howe WM, Young DA & Sarter M (2007) Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Prog. Neurobiol, 83, 69–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, O’Donnell P, Card JP & Sesack SR (1999) Dopamine terminals in the rat prefrontal cortex synapse on pyramidal cells that project to the nucleus accumbens. J. Neurosci, 19, 11049–11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JJ, Hollon NG & Phillips PE (2012) Pavlovian valuation systems in learning and decision making. Curr. Opin. Neurobiol, 22, 1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Chudasama Y, Theobald DE, Pettifer CL, Fletcher CM & Robbins TW (2002) Nucleus accumbens dopamine and discriminated approach learning: interactive effects of 6-hydroxydopamine lesions and systemic apomorphine administration. Psychopharmacology, 161, 425–433. [DOI] [PubMed] [Google Scholar]
- Ellwood IT, Patel T, Wadia V, Lee AT, Liptak AT, Bender KJ & Sohal VS (2017) Tonic or phasic stimulation of dopaminergic projections to prefrontal cortex causes mice to maintain or deviate from previously learned behavioral strategies. J. Neurosci, 37, 8315–8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB & Robinson TE (2017) Neurobiological basis of individual variation in stimulus-reward learning. Curr. Opin. Behav. Sci, 13, 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE & Akil H (2007) Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology, 191, 599–607. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H & Robinson TE (2009) Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology, 56(Suppl 1), 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM et al. (2011) A selective role for dopamine in stimulus-reward learning. Nature, 469, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser KM & Janak PH (2017) Long-lasting contribution of dopamine in the nucleus accumbens core, but not dorsal lateral striatum, to sign-tracking. Eur. J. Neurosci, 46, 2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y & Grace AA (2005) Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat. Neurosci, 8, 805–812. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Gonzalez R, Harris RJ & Guthrie D (1996) Effect sizes and p values: what should be reported and what should be replicated? Psychophysiology, 33, 175–183. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Fadel J, Sarter M & Bruno JP (1998) Effects of local cholinesterase inhibition on acetylcholine release assessed simultaneously in prefrontal and frontoparietal cortex. Neuroscience, 86, 949–957. [DOI] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS & Robinson TE (2016) Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology, 233, 3587–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy Cherian A, Kucinski A, Pitchers K, Yegla B, Parikh V, Kim Y, Valuskova P, Gurnarni S et al. (2017) Unresponsive choline transporter as a trait neuromarker and a causal mediator of bottom-up attentional biases. J. Neurosci, 37, 2947–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM & Robinson TE (2011) Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav. Brain Res, 223, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT & Shaham Y (2004) Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology, 47(Suppl 1), 214–226. [DOI] [PubMed] [Google Scholar]
- Lustig C & Sarter M (2016) Attention and the cholinergic system: relevance to schizophrenia. Curr. Top. Behav. Neuro, 28, 327–362. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD & Robinson TE (2012) Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One, 7, e38987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Cogan ES & Robinson TE (2014) The form of a conditioned stimulus can influence the degree to which it acquires incentive motivational properties. PLoS One, 9, e98163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milella MS, Fotros A, Gravel P, Casey KF, Larcher K, Verhaeghe JA, Cox SM, Reader AJ et al. (2016) Cocaine cue-induced dopamine release in the human prefrontal cortex. J. Psychiatr. Neurosci, 41, 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Namboodiri VM, Matan AM, Voets ES, Mohorn EP, Kosyk O, McHenry JA, Robinson JE et al. (2017) Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature, 543, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE & Sarter M (2013) Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J. Neurosci, 33, 8321–8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Flagel SB, O’Donnell EG, Woods LC, Sarter M & Robinson TE (2015) Individual variation in the propensity to attribute incentive salience to a food cue: influence of sex. Behav. Brain Res, 278, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Philips KB, Jonte JL, Robinson TE & Sarter M (2017a) Diverse roads to relapse: a discriminative cue signaling cocaine availability is more effective in renewing cocaine-seeking in goal-trackers than sign-trackers, and depends on basal forebrain cholinergic activity. J. Neurosci, 37, 7198–7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Wood TR, Skrzynski CJ, Robinson TE & Sarter M (2017b) The ability for cocaine and cocaine-associated cues to compete for attention. Behav. Brain Res, 320, 302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE & Berridge KC (2008) The incentive sensitization theory of addiction: some current issues. Philos. T. Roy. Soc. B, 363, 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE & Flagel SB (2009) Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol. Psychiat, 65, 869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES & Saunders BT (2014) On the motivational properties of reward cues: individual differences. Neuropharmacology, 76(Pt B), 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE (2007) Food or fluid restriction in common laboratory animals: balancing welfare considerations with scientific inquiry. Comp. Med, 57, 149–160. [PubMed] [Google Scholar]
- Saddoris MP, Cacciapaglia F, Wightman RM & Carelli RM (2015) Differential dopamine release dynamics in the nucleus accumbens core and shell reveal complementary signals for error prediction and incentive motivation. J. Neurosci, 35, 11572–11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M & Fritschy JM (2008) Reporting statistical methods and statistical results in EJN. Eur. J. Neurosci, 28, 2363–2364. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Howe WM, Gritton H & Berry AS (2014) Deterministic functions of cortical acetylcholine. Eur. J. Neurosci, 39, 1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Blakely RD & Koshy Cherian A (2016) Cholinergic genetics of visual attention: human and mouse choline transporter capacity variants influence distractibility. J. Physiology Paris, 110, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT & Robinson TE (2011) Individual variation in the motivational properties of cocaine. Neuropsychopharmacol, 36, 1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT & Robinson TE (2012) The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur. J. Neurosci, 36, 2521–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM & Robinson TE (2013) Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J. Neurosci, 33, 13989–14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, O’Donnell EG, Aurbach EL & Robinson TE (2014) A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacol, 39, 2816–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Mabrouk OS, Hershey ND & Kennedy RT (2011) In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography-mass spectrometry. Anal. Chem, 84, 412–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Ahn S, Phillips AG & Floresco SB (2012) Dynamic fluctuations in dopamine efflux in the prefrontal cortex and nucleus accumbens during risk-based decision making. J. Neurosci, 32, 16880–16891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Peters M, Demeter E, Lustig C, Bruno JP & Sarter M (2011) Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J. Neurosci, 31, 9760–9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke G & Molenberghs G (2009). Linear Mixed Models for Longitudinal Data. Springer, New York. [Google Scholar]
- Yager LM & Robinson TE (2010) Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav. Brain Res, 214, 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM & Robinson TE (2013) A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology, 226, 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Pitchers KK, Flagel SB & Robinson TE (2015) Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacol, 40, 1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmarowski A, Sarter M & Bruno JP (2005) NMDA and dopamine interactions in the nucleus accumbens modulate cortical acetylcholine release. Eur. J. Neurosci, 22, 1731–1740. [DOI] [PubMed] [Google Scholar]