Abstract
Introduction:
Prostate cancer is a highly heterogeneous disease, with remarkably different prognosis across all stages. Increased circulating tumor cell (CTC) count (≥ 5) using the CellSearch assay has been identified as one of the markers that can be used to predict surv<ival, with added value beyond currently available prognostic factors. Recently, androgen receptor splice variant 7 (AR-V7) detection has been associated with worse outcomes for patients with castration-resistant prostate cancer (CRPC) treated with novel androgen receptor-signaling (ARS) inhibitors such as abiraterone and enzalutamide but not taxane chemotherapies.
Areas covered:
In this manuscript, the authors review the available biomarkers in CRPC and discuss emerging data on the value of CTC-derived AR-V7 status to assess prognosis and its potential role to guide treatment selection for patients with advanced prostate cancer.
Expert Commentary:
Current evidence supports AR-V7 status as a prognostic biomarker and also as a potential predictive biomarker for patients with mCRPC. The authors expect that the incorporation of AR-V7 status and other biomarkers (e.g. AR mutations) in the sequential assessment of patients with advanced prostate cancer will lead to a more rational use of available and future therapies, with significant improvements in outcomes for our patients.
Keywords: AR-V7, prognostic, predictive, biomarker, castration-resistant prostate cancer
1. INTRODUCTION
Prostate cancer remains one of the leading causes of cancer death worldwide1,2 despite many recent advances in the understanding of the disease biology and increasing treatment options for patients with advanced prostate cancer.
For many decades androgen deprivation therapy (ADT) has been the mainstay of therapy for patients with metastatic prostate cancer, with high rates of PSA declines, symptom improvement and disease control. Despite this initial favorable response, most patients eventually develop resistance to ADT and present with disease progression. Over the past decade we have learned that there are several different mechanisms of progression in the setting of castrate levels of testosterone, but most patients present with a rising PSA, reflecting continuous androgen receptor (AR) signaling activation3.
Better understanding of the mechanisms of disease progression has led to the development and approval of several active systemic therapies for castration-resistant prostate cancer (CRPC) over the last several years (Table 1), including novel AR-signaling inhibitors (abiraterone4,5 and enzalutamide6,7), taxane chemotherapy with docetaxel8 and cabazitaxel9, and agents with alternative mechanisms of action such as radium-22310 and sipuleucel-T11. Recently, clinical trials evaluating the use of docetaxel and abiraterone in castration-sensitive prostate cancer (CSPC) have demonstrated further improvements in outcomes and increased survival compared to ADT alone12–15, particularly in those with metastatic castration-sensitive disease, suggesting that earlier use of active drugs can lead to even more profound clinical benefits.
Table 1.
Current systemic treatment options for metastatic prostate cancer.
| CASTRATION-SENSITIVE PROSTATE CANCER | |
|---|---|
|
ADT •LHRH agonists vs. antagonists •Continuous vs. intermittent |
•Mainstay of therapy for mCSPC. •No clear superiority of LHRH agonists or antagonists. •Intermittent ADT not proven to be non-inferior to continuous47. •Should be used continuously in the setting of mCRPC. |
| ADT plus Docetaxel | • Two RCTs and meta-analysis demonstrating improved outcomes for de novo mCSPC, including OS12,13,48. |
| ADT plus Abiraterone and prednisone | • Two RCTs demonstrating significant improvements in outcomes for de novo mCSPC, including OS14,15. |
| CASTRATION-RESISTANT PROSTATE CANCER | |
|
AR-signaling inhibitors •Abiraterone / prednisone •Enzalutamide |
•Both with RCTs demonstrating significant improvements in OS and QoL in pre- and post-chemo settings4–7. •Sequential use not proven to improve clinically meaningful outcomes49. |
|
Taxane-based chemotherapy •Docetaxel •Cabazitaxel |
•RTCs demonstrating improvements in clinically meaningful outcomes8,9. •Cabazitaxel approved for patients previously treated with docetaxel9. •Both taxanes retain activity in patients with AR-V733,34. |
|
Radiopharmaceuticals • Radium-223 |
•1st radiopharmaceutical to demonstrate OS benefit10. •Can be used in pre- and post-chemo settings, and in combination with osteoclast-inhibitory agents. |
|
Immunotherapy • Sipuleucel-T |
• Despite OS improvement demonstrated in one RCT, no benefit in terms of PSA decline, objective response or PFS11. |
Abbreviations: ADT: androgen deprivation therapy; aPC: advanced prostate cancer; CRPC: castration-resistant prostate cancer; RCT: randomized clinical trials; OS: overall survival; QoL: quality of life; PFS: progression-free survival; chemo: chemotherapy; PSA: prostatic specific antigen.
Despite these recent achievements, many patients derive short-term benefits from available therapies and even those who have a prolonged disease control will still develop eventual progressive disease at some point. It is widely known that prostate cancer is a highly heterogeneous disease, with some patients presenting with very indolent disease and others with highly aggressive clinical course and refractory disease. Therefore, it is of paramount importance to establish prognosis in this setting but also to identify predictive biomarkers of benefit to specific therapies (i.e. treatment-selection markers) to help us select the best drug for the appropriate patient at the appropriate time and with the appropriate toxicities (i.e., “precision medicine”). In this article, we will review the available prognostic markers for advanced prostate cancer, with a focus on CTC-detected AR splice variant 7 (AR-V7), and also to describe potential predictive biomarkers in this setting.
2. MECHANISMS OF ESCAPE TO ANDROGEN RECEPTOR-SIGNALING INHIBITORS
The mechanisms of resistance to ADT and other AR-signaling (ARS) inhibitors for metastatic prostate cancer can be broadly divided into androgen-dependent and androgen-independent groups16–18 (Figure 1). Despite not being the main subject of this review, it is important to recognize that many of these resistance mechanisms have prognostic implications since they usually lead to less responsiveness to other available therapies and consequently to a worse overall prognosis. One of the most important examples of a prognostic and predictive marker is the detection of AR-V7 in circulating tumor cells (CTCs), which has been demonstrated in several single-center studies to be associated with a poor prognosis, as discussed in detail over the next several sections of this paper.
Figure 1. Androgen receptor signaling axis and potential androgen-dependent and independent mechanisms of disease progression.
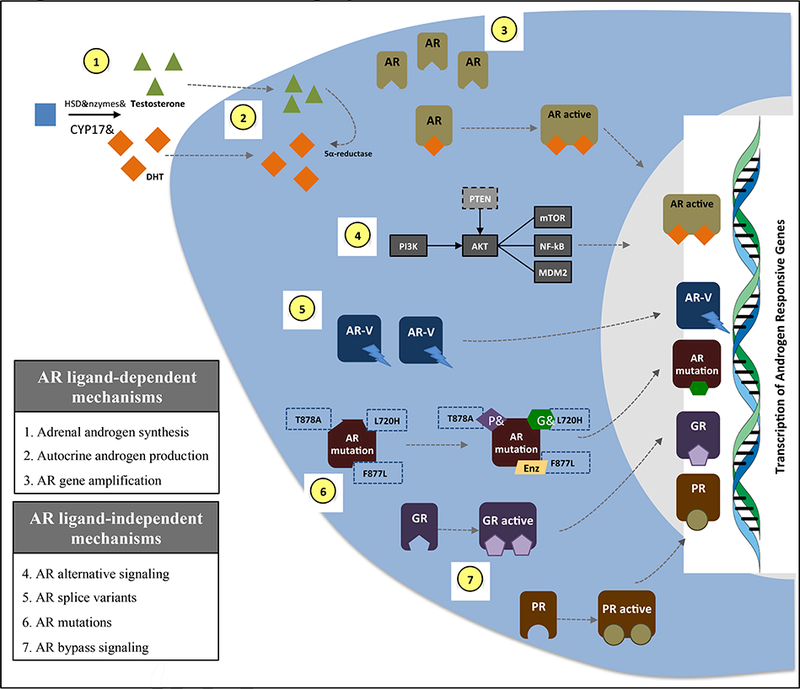
Figure 1 highlights the androgen receptor (AR)-signaling axis, with conversion of testosterone to dihydrotestosterone (DHT) by the 5α-reductase enzyme, and subsequent AR activation, dimerization, nuclear translocation and activation of transcriptional activation of target genes. The figure summarizes potential mechanisms of resistance to AR-signaling inhibitors by using a schematic representation of a prostate cancer cell. Not shown are multiple additional androgen/AR-independent mechanisms of escape including activated Wnt pathway signaling, loss of the RB1 and/or TP53 genes, overexpression of DNA repair pathways proteins including PARP1 and DNA-PK, epigenetic dysregulation (e.g. via EZH2 overexpression), and neuroendocrine/small cell transformation.
Abbreviations: CYP17: cytochrome P450 17alpha-hydroxylase; PI3K: phosphoinositide 3-kinase; AKT: protein kinase B; PTEN: phosphatase and tensin homolog; mTOR: mammalian target of rapamycin; NF-kB: nuclear factor kappa B; MDM2: mouse double minute 2 homolog; AR-V: AR splice variant; P: progesterone; PR: progesterone receptor; G: glucocorticoid; GR: glucocorticoid receptor.
3. BIOMARKERS IN METASTATIC PROSTATE CANCER
3.1. Biomarker definitions
The term biomarker refers to clinical or molecular characteristics that can be objectively and reproducibly measured to indicate a biologic condition, including normal or pathogenic processes, and also a response to a specific therapeutic intervention19,20. Therefore, biomarkers can be clinical features (such as performance status), laboratory analytes (such as hemoglobin, PSA, etc.), imaging studies, or molecular alterations (such as gene mutations). These biomarkers are classified based on their contexts of use (Table 2). Importantly, each biomarker under development must undergo the prerequisite analytical and clinical validation steps before it can be reliably used to inform a medical decision. Analytical validation comprises several steps to guarantee accuracy and reproducibility of the biomarker assay and the clinical validation is established when clinical trials demonstrate that the specific biomarker provides useful information to guide management19,20.
Table 2.
Biomarker category definitions19 and examples in advanced prostate cancer.
| Biomarker Category | Definition | Examples |
|---|---|---|
| Diagnostic | Used to confirm presence of disease or identify disease subtype. | Pathology (prostate biopsy). |
| Monitoring | Used serially to assess status or extent of a disease. | PSA, bone scan, CTC count. |
| Response | Used to show a biologic response to an agent or product. | PSA, Bone scan, CTC conversion. |
| Prognostic | Identifies at baseline the likelihood of a clinical event (e.g., progression, death), irrespective of treatment. | Performance status, LDH, visceral disease, presence of pain, etc. |
| Predictive | Identifies individuals at baseline who are more likely to experience a favorable or unfavorable effect from one agent compared to another. | None validated so far. Potential candidates are AR-V7, CTC heterogeneity, and DDR gene alterations. |
| Safety | Indicates the likelihood or extent of toxicity as an adverse event of an agent. | Performance status, hepatic function for some therapies, renal function for other therapies. |
| Susceptibility | Indicates the potential risk for developing a medical condition. | Family history, germline BRCA2 mutation, African American race. |
Abbreviations: PSA: prostatic specific antigen; LDH: lactate dehydrogenase; AR-V7: androgen receptor splice variant 7; MSI: microsatellite instability; DDR: DNA damage repair.
Several biomarkers have been identified as prognostic factors for patients with advanced prostate cancer21. Many of these markers that are associated with clinically meaningful outcomes, such as survival, can be identified at baseline and others can be identified after treatment over the course of the disease (Table 3). A useful prognostic model for predicting OS in patients with mCRPC treated with first-line chemotherapy has been developed using several of these markers22.
Table 3.
Key prognostic biomarkers in metastatic prostate cancer.
| Laboratory biomarkers22 | Clinical biomarkers22 |
|---|---|
| - PSA (baseline and kinetics) -LDH - Hemoglobin level - Alkaline phosphatase - Serum albumin - CTC count ≥ 5 vs. <5 at baseline and CTC count conversion (<5) after 12 weeks of therapy23–26 |
- Performance status - Presence of pain / use of opioids |
| Imaging biomarkers | |
| - Sites of metastasis50 - Extent of disease12,51 | |
| Pathologic / Molecular biomarkers | |
| - Aggressive histologic variants (e.g. small cell) - Rb1, TP53 and/or PTEN alterations52. AR-V7+ detection 32,40 DDR gene alterations |
Abbreviations: PSA: prostatic specific antigen; LDH: lactate dehydrogenase; CTC: circulating tumor cell; AR-V7 androgen receptor splice variant 7.
3.2. Circulating Tumor Cell Count and Prognosis
Circulating tumor cell enumeration in patients with mCRPC has been correlated with survival in several studies, both retrospective and prospectively23–25. It has been demonstrated that patients with a CTC count ≥ 5 at baseline have worse survival and also that patients with a CTC count decline (from ≥ 5 to < 5) after 12 weeks of therapy have better outcomes than patients with no CTC declines26. Two recent studies have demonstrated that incorporating the CTC count with other known prognostic markers significantly improves the prognostic assessment and may serve as a surrogate for survival in mCRPC27,28. In the COU-AA-301 phase III randomized trial comparing abiraterone plus prednisone versus placebo plus prednisone for patients with mCRPC previously treated with docetaxel, the analysis of CTC count and other known prognostic biomarkers (PSA, LDH, hemoglobin [Hb], albumin [Alb] and alkaline phosphatase levels [AlkPhos]) as a surrogate for survival was prospectively assessed as a secondary endpoint. The final analysis included 711 patients and demonstrated that a panel of CTC count plus LDH level was able to stratify patients according to three different groups in terms of prognosis: low (CTC <5; any LDH), intermediate (CTC≥ 5 and LDH ≤ 250), and high-risk (CTC ≥ 5 and LDH ≥ 250), with median OS being 8.7, 12.0 and 22.2 months, respectively (P<0.001), satisfying Prentice surrogacy criteria for survival27. Another recent study examined the added prognostic value of CTC count to a model containing LDH, PSA, Hb, Alb and AlkPhos in mCRPC patients enrolled in two prospective randomized trials (COU-AA-301 and ELM-PC4)28. This study also demonstrated that including the CTC count in current prognostic models increased its discriminating power for predicting survival in mCRPC patients.
It should be noted that most of these studies on CTC enumeration as a prognostic biomarker were performed using the CellSearch assay, which relies on capturing CTCs of epithelial origin from role blood, and is the only FDA cleared method for CTC detection. There are some limitations of the CellSearch assay, including its limited sensitivity since it can only detect EPCAM+ cells (would miss cells undergoing mesenchymal transition), and the uncertainty of whether detected cells are truly viable cancer cells rather than necrotic cells. Thus, many other assays are under development to improve CTC detection sensitivity by including other CTC detection methods, such as the use of multiple antibodies (CK, HER-2< CD44, CD24, ALDH1) and also DNA- or RNA-based RT-PCR assays. A detailed review of CTC detection methods has been reported elsewhere29.
3.3. AR-V7 as a Prognostic and Predictive Biomarker in CRPC
Androgen receptor splice variants are constitutively active forms of the AR that lack the ligand-binding domain and have been implicated as one of the mechanisms of resistance to the currently available ARS inhibitors. Most of these variants arise from abnormal splicing (and retention) of intronic sequences containing cryptic exons which (when translated) result in frameshift events leading to truncated protein products30. To date, several AR splice variants have been described, and AR-V7 is the most prevalent and biologically relevant17. The first pilot study to demonstrate a potential correlation between AR-V7 and clinical outcomes included 62 patients with mCRPC treated with abiraterone or enzalutamide and assessed the AR-V7 status by a CTC-based RT-PCR assay detecting the AR-V7 messenger RNA31. Although preliminary, results demonstrated no PSA declines ≥ 50% from baseline (PSA50) in patients with AR-V7 positive (+) CTCs and also worse PFS and OS compared to AR-V7 negative (−) patients. These results suggested that AR-V7 could potentially serve as a prognostic tool but also possibly a predictive biomarker for response to abiraterone and enzalutamide. Recently, an update of this study with a larger sample size was published, including 202 patients treated with abiraterone or enzalutamide32. Importantly, the authors correlated clinical outcomes with 3 different biomarker groups based on CTC detection and AR-V7 detection: CTC(−) vs. CTC(+)/AR-V7(−) vs. CTC(+)/AR-V7(+). Overall, PSA50 response rates were 75.5% in CTC(−) patients, 52.2% in CTC(+)/AR-V7(−) patients and 13.9% (5 of 36) CTC(+)/AR-V7(+) patients (p<0.001). CTC(+)/AR-V7(+) patients were more likely to have higher Gleason scores (≥ 8), prior ARS inhibitor and taxane use, and worse performance status (ECOG ≥ 1). On multivariable analysis, PFS and OS outcomes were more favorable for CTC(−) patients, intermediate for CTC(+)/AR-V7(−) patients and worse for CTC(+)/AR-V7(+) patients32.
In another study conducted in 37 patients with mCRPC treated with taxane-based chemotherapy, no statistical difference in terms of PSA50, PFS or OS was detected between AR-V7 (+) vs. AR-V7(−) patients, suggesting that patients may still respond to taxanes regardless of the AR-V7 status33. Although a numeric superiority was detected in AR-V7(−) patients in terms of PFS (6.9 vs. 5.1 months, P=0.02) and OS (14.7 vs. 9.2 months, P=0.11), no statistically significant difference was observed. Important to note is that the OS analysis was exploratory and the low number of patients enrolled limits the evaluation of AR-V7 as a prognostic marker in this study. Therefore, it is certainly possible that CTC-based AR-V7 detection using this mRNA assay may be associated with inferior clinical outcomes to chemotherapy if more patients are studied, although the effect is not likely to be as great as in the context of ARS inhibitor therapy.
In a larger study with 161 men with mCRPC treated with ARS inhibitors (abiraterone, enzalutamide or apalutamide) or taxanes (docetaxel, cabazitaxel or paclitaxel), AR-V7 status was determined using a separate AR-V7 protein immunofluorescent assay performed on a non-EpCAM-based CTC detection platform34. This study also demonstrated that patients with AR-V7(+) CTCs treated with ARS inhibitors had worse outcomes by all event measures, including radiographic PFS (2.3 vs. 14.5 months; P<0.001), time on therapy (2.1 vs. 6.8 months; P<0.001) and OS (4.6 vs. not reached; P<0.001). Among patients treated with taxanes, no difference was observed in terms of PFS or time on therapy, but patients with AR-V7 positive CTCs demonstrated inferior OS than AR-V7 negative (8.9 vs. 19.8 months; P<0.001), suggesting that AR-V7 status may be prognostic even in taxane-treated patients. Very importantly, in the AR-V7(+) subset, patients treated with taxanes had superior outcomes, including OS, compared to men treated with ARS inhibitors (positive statistical interaction between biomarker status and therapy type), supporting the preliminary evidence of a predictive role of protein-based AR-V7 detection in this setting34. In addition, a subsequent study from these same investigators demonstrated that the predictive ability of the biomarker with respect to discriminating the two different types of therapies was greatest when nuclear-localized AR-V7 presence was required to define a biomarker-positive test, rather than any (nuclear or cytoplasmic) presence of AR-V7 protein35. For this reason, this assay is now been defined as positive only if AR-V7 is nuclear-localized; this definition is now being used in multiple prospective validation studies.
One important limitation of some of the CTC-based AR-V7 detection tests is that a subset of patients may present with AR-low or AR-negative CTCs, often associated with an aggressive phenotype and lack of response to ARS inhibitors, and therefore may be classified as AR-V7 negative. Although this may be a limitation of EPCAM-based CTC assays, CTCs have been identified in AR-low prostate cancer cells using non-EPCAM based tests), including neuroendocrine prostate carcinomas36. Also, AR-V7 has been identified in AR-low prostate cancer such as neuroendocrine carcinomas37.
In addition to CTC-derived AR-V7 detection, there are other ways to interrogate AR-V7 using liquid biopsies. For example, two recent studies correlated AR-V7 mRNA detection in the whole blood with clinical outcomes in patients with mCRPC treated with abiraterone or enzalutamide38,39. The first study, using semi-quantitative mRNA analysis of AR-V7 using analog PCR in peripheral whole blood of 85 men demonstrated worse outcome in patients with high-AR-V7 expression levels, including no PSA50 responses, shorter PFS and OS38. The second study assessed AR-V7 (and PSA) mRNA levels in the whole blood of mCRPC patients using a highly-quantitative digital droplet PCR (ddPCR) methodology, and observed potential prognostic value of both tests on survival of patients treated with ASR inhibitors39. A summary of all methods currently used to detect AR-V7 in tumor and liquid biopsies is provided in Table 4.
Table 4.
Currently used methods to detect AR-V7 in tumor and liquid biopsies
| Tissue-based AR-V7 detection methods |
| - Western blot for protein detection53 - IHC for protein detection54 - RNA in situ hybridization (RISH) for mRNA55–57 - RT PCR for mRNA detection58 - RNA sequencing for mRNA detection59 |
| Blood-based AR-V7 detection methods |
| - CTC based RT PCR for mRNA detection31 - CTC based ddPCR for mRNA detection60 - CTC based RNA-seq61 - CTC based protein (immunofluorescence] detection34 - Whole blood analog RT PCR for mRNA detection38 - Whole blood ddPCR for mRNA detection39 - Cell free RNA detection by RT-PCR62 - Exosome derived PCR detection of mRNA63 |
Abbreviations: AR-V7: androgen receptor splice variant 7; IHC: immunohistochemistry; RT PCR: reverse transcription polymerase chain reaction; mRNA: messenger ribonucleic acid.
To add further evidence of the prognostic impact of AR-V7 in patients with mCRPC treated with ARS inhibitors, a meta-analysis on this subject was recently published40. In the PFS and OS analysis, a total of eight trials encompassing 490 patients were included and demonstrated that both PFS and OS were better in AR-V7(−) than AR-V7(+) patients. Prospective validation of AR-V7 is now urgently needed.
The first large prospective randomized trial to demonstrate the prognostic significance of AR-V7 status in mCRPC was the ARMOR3-SV trial, which compared galeterone vs. enzalutamide in patients with CTC-based AR-V7(+) disease who had not previously received ARS inhibitors or taxane agents41. Galeterone is a selective multi-targeted molecule that acts as a CYP17 inhibitor, AR antagonist and degrader (including AR-V7 degrader), with potential activity in AR-V7(+) patients based on initial preclinical and initial clinical studies42. The AR-V7 biomarker test used for eligibility assessment was a derivation of the mRNA-based RT-PCR assay first reported by the Johns Hopkins group31. Of the 953 patients screened using this assay, only 8% (73) were AR-V7(+) and 38 patients were randomized to enzalutamide or galeterone. In this trial, AR-V7 detection was associated with worse prognostic features, including higher baseline PSA levels, more bone lesions, higher ECOG PS status, prior anti-androgen and docetaxel use compared to patients with no CTC detected or AR-V7(−) CTCs41. Because 7 of the first 12 treated AR-V7(+) patients developed rapid disease progression (early censoring rate of 58%), the data and safety monitoring committee (DSMC) recommended premature termination of the study since it was unlikely to meet its primary endpoint. As a result of this early trial closure, galeterone is no longer being developed as a prostate cancer therapeutic.
Despite all of available data, we must emphasize that further prospective validation studies are important to confirm the role of AR-V7 as a prognostic biomarker in patients with mCRPC. One such study is the PROPHECY trial (NCT02269982). In this trial, 120 mCRPC patients who are beginning next-generation ARS inhibitor therapy will be prospectively sampled at baseline using 3 different AR-V7 liquid biopsies (two are PCR-based mRNA assays, and one is a immunofluorescence-based protein assay), and then patients will be monitored using a prospectively-defined follow-up schema until clinical or radiographic progression of their disease. At the time of progression, patients will be re-sampled using the same 3 liquid biopsy platforms, and will then be offered a taxane chemotherapy. At the time of progression on the taxane agent, a third set of liquid biopsy samples for AR-V7 analysis will be collected. At the time of writing, this study has completed enrolment of all 120 men, and preliminary data on the primary endpoint of PFS are eagerly awaited. One of the unique advantages of this study is its ability to assess the prognostic value of each of the 3 AR-V7 assays, and to indirectly compare the analytical and clinical characteristics of each test against the others. A preliminary communication of the top-line results from the PROPHECY trial is expected by Q2 2018.
4. POTENTIAL TOOLS FOR TREATMENT SELECTION
An important unmet need for patients with metastatic CRPC is the identification of biomarkers that can be predictive for response to available therapies, to guide treatment selection at each specific time point in the course of disease, with the ultimate goal of maximizing outcomes and limiting toxicity. Although there are no currently available validated predictive biomarkers in this setting, many are under development and hopefully in the near future will help us to select the right therapy for the right patient at the right time.
AR-V7 is one of these candidates as a putative predictor of benefit to the newer ARS inhibitors, as demonstrated in several single-center trials and a recent meta-analysis, as discussed above31,32,34,38,40. These studies demonstrate in aggregate that the benefit of ARS inhibitors is limited to AR-V7(−) patients and very few patients with AR-V7(+) disease demonstrate benefit with abiraterone or enzalutamide. On the other hand, it appears that AR-V7 status does not interfere as dramatically with responsiveness to taxane-based chemotherapy33,34. Therefore, AR-V7 status may represent a marker for therapy selection in mCRPC if larger studies confirm its predictive role in this scenario. Recently, two studies presented data suggesting that some patients with AR-V7(+) may achieve PSA declines with enzalutamide or abiraterone, although a transient PSA reduction does not always equate with a meaningful clinical benefit. The disappointing results of the ARMOR3-SV study comparing galeterone vs. enzalutamide in AR-V7(+) patients were recently reported as discussed above, also showing some PSA50 responses to enzalutamide41. Another recent study described that 6 of 21 AR-V7(+) patients demonstrated PSA reductions under treatment with abiraterone or enzalutamide, but again this does not necessarily equate with clinical benefit43. Despite several discussion points on the interpretation of these data44,45, specially related to benefit demonstrated only by PSA decline and no other outcome endpoints, the bottom of line is that large prospective validation studies are critical to define the real impact of AR-V7 status on treatment selection between ARS inhibitors and taxane chemotherapy. Interestingly however, a recent study from the Johns Hopkins group evaluating the real-world clinical utility of AR-V7 testing using a commercial CLIA-certified assay suggested that physicians used the AR-V7 results more often than not to inform their clinical decisions, and that the physicians who used the AR-V7 test results to direct the next line of systemic therapy observed greater rates of PSA50 declines with the subsequent systemic therapy compared to physicians who did not use the results to change treatment46. These provocative but preliminary findings suggest that AR-V7 testing is already beginning to demonstrate broader clinical utility outside of specialized academic centers.
Other potential predictive biomarkers under investigation for mCRPC therapy selection are: (1) AR mutations detected by cell-free DNA; (2) DNA damage repair gene alterations as predictive for response to PARP inhibitors; (3) gene alterations related to aggressive prostate cancer phenotype and response to platinum agents; and (4) microsatellite instability (MSI) as a predictor for benefit to pembrolizumab, an anti-PD inhibitor. In the near future, these and possibly others markers may help us to select the best treatments and avoid futile therapies for patients with metastatic castration-resistant prostate cancer. A list of these and other putative predictive markers under investigation is included in Table 5.
Table 5.
Potential predictive biomarkers under investigation for mCRPC therapy-selection.
| Potential Predictive Biomarker | Therapy Selection |
|---|---|
| AR-V7 31,32,34,64 | Not detected: ARS inhibitors Detected: taxane chemotherapy |
| Activating AR-LBD mutations and/or AR gene amplification65,66 | Not detected: ARS inhibitors Detected: taxane chemotherapy |
| CTC heterogeneity67 (Shannon index) | Low (Shannon <1.5): TARS inhibitors High (Shannon ≥1.5): taxane chemotherapy |
| DDR gene alterations | PARP inhibitors68 or platinum agents69 |
| Microsatellite instability (MSI-high) | Immunotherapy with anti-PD-1 (pembrolizumab)70 |
Abbreviations: ARS: androgen receptor signaling; AR-V7: androgen receptor splice variant 7; AR-LBD: androgen receptor ligand-binding domain; CTC: circulating tumor cell; DDR: DNA damage repair; PARP: poly ADP (adenosine diphosphate)-ribose polymerase; PD-1 programmed death.
5. CONCLUSIONS
There is a growing body of evidence supporting blood-based AR-V7 status as a prognostic biomarker in metastatic castration-resistant prostate cancer. Patients with AR-V7(+) disease tend to present with more aggressive features, evidenced by higher PSA levels, higher disease burden and worse performance status. The predictive role of the AR-V7 biomarker to aid in treatment selection between ARS inhibitors and taxane agents requires further validation, but preliminary evidence to date suggests limited efficacy of abiraterone or enzalutamide in AR-V7(+) patients while taxanes appear to retain some level of sensitivity despite AR-V7 detection. While definitive prospective trials (such as PROPHECY) are awaited, existing data point to some evidence of clinical utility of the AR-V7 biomarker in real-world oncology practices. The era of precision medicine for prostate cancer is very near!
6. Expert commentary
Recent advances in the understanding of prostate cancer biology and mechanisms of progression to androgen deprivation therapy have led to the development of several biomarkers that can be used to assess prognostic and also potentially predictive biomarkers which could serve as treatment-selection tools. To date, there are many prognostic biomarkers identified in prospective studies, including lactate dehydrogenase (LDH) levels, ECOG performance status, PSA level, sites of disease, hemoglobin, baseline CTC count (≥ 5) and decline (to < 5) with therapy, among others. Recently, AR-V7 status has been correlated with clinical outcomes and patients with AR-V7 detected in liquid biopsies tend to present with more aggressive disease and shorter progression-free and overall survival.
As discussed herein, an important unmet need for patients with metastatic castration-resistant prostate cancer (mCRPC) is the identification of predictive biomarkers that could be used by oncologists to recommend therapies with higher likelihood of response and to avoid ineffective drugs, maximizing clinical benefits to patients while minimizing toxicities. To date, there is a growing body of evidence supporting AR-V7 status as a potential tool in this setting, since studies have demonstrated a lack of significant benefit with novel AR signaling inhibitors in AR-V7 positive patients, but partial sensitivity to taxane-based chemotherapy that may potentially occur in the context of AR-V7 conversions from positive to negative. Although these findings still require validation in larger studies, recent data indicate that AR-V7 status may have an important role to guide treatment decisions in clinical practice. Another promising biomarker under investigation that could be integrated together with the assessment of the AR-V7 status is the presence of specific AR mutations, which also can help to guide the best choice of systemic therapies. For example, a patient with mCRPC receiving abiraterone with a rising PSA who is found using a liquid biopsy to harbor the AR T878A mutation (associated with abiraterone resistance) could be switched to enzalutamide early before developing clinical progression on abiraterone. If that same patient presented with AR-V7 positive CTCs as well, it is unlikely that he would respond to enzalutamide and would probably be best served with alternative agents including docetaxel, cabazitaxel, or even radium-223.
There are many other promising biomarkers under investigation beyond AR-V7 and AR mutations, including AR-independent pathways such as the presence of DNA damage response (DDR) gene alterations, which may predict benefit to PARP inhibitors and platinum chemotherapies, the presence of microsatellite instability which might predict benefit to immunotherapy (anti-PD1 agents, i.e. pembrolizumab) and other gene alterations such as Rb loss and TP53 mutations, which have been associated with an aggressive phenotype and poor response to AR-directed therapies. We expect that the incorporation of these and other biomarkers in the sequential assessment of patients with advanced prostate cancer - i.e. precision medicine - will lead to a more rational use of available and future therapies, with significant improvements in outcomes for our patients.
7. Five-year view
We believe that in the near future therapy selection for patients with metastatic castration-resistant prostate cancer will be guided by predictive biomarkers identified in liquid biopsies and/or tumor tissue. It is likely that many of the potential biomarkers under investigation will be integrated into routine clinical practice, including assessment of AR-V7 status, AR mutations, CTC heterogeneity, presence of DNA repair gene alterations, microsatellite instability, and potentially others. Moreover, a better understanding of the disease biology in an individual patient at a particular moment in time will help to integrate other strategies, such as potentially local therapy to the prostate gland and also rational drug combination strategies. This will likely result in increased efficacy and value of the available therapies, ultimately leading to improvements in important endpoints such as survival and quality of life.
Key issues.
Currently, there are several life-prolonging therapies available for patients with mCRPC, but treatment selection is largely based on clinical factors and no validated predictive biomarkers are yet available to guide therapeutic choices.
The mechanisms of escape to novel androgen-receptor signaling (ARS) inhibitors are now better understood and include ligand-dependent and -independent mechanisms.
mCRPC remains a highly heterogeneous disease and many prognostic biomarkers have been identified to help estimate survival in this setting, such as lactate dehydrogenase (LDH), hemoglobin, ECOG performance status, PSA, albumin, and others.
Recently, CTC count and AR-V7 status have been described as prognostic markers. The presence of a baseline CTC count ≥5 (and post-treatment CTC conversion to <5) and/or AR-V7 detection are associated with overall worse survival.
There have been significant advances to identify biomarkers for therapy selection, such as AR-V7 status, AR mutations, CTC heterogeneity, the presence of DNA damage repair gene alterations. Prospective validation of these biomarkers is still pending prior to incorporation in routine clinical use.
AR-V7 is a constitutionally active form of the AR and has been implicated in primary and acquired resistance to ARS inhibitors such abiraterone and enzalutamide, but is compatible with sensitivity to taxane chemotherapies.
Patients with AR-V7-positive CTCs may still respond to taxane-based chemotherapy, including some cases that are associated with AR-V7 conversions from positive to negative. Therefore, if these findings are confirmed in validation studies, AR-V7 status may serve as a treatment-selection tool for patients with mCRPC.
Acknowledgments
Funding
This article was funded by NIH Grants R01 CA185297 and P30 CA006973, the Patrick C. Walsh fund and the Prostate Cancer Foundation.
Declaration of Interest
DA Bastos has served as a paid consultant/advisor for Janssen, Astellas, Sanofi, Merck, and Roche; and has received research funding to his institution from Janssen. ES Antonarakis has served as a paid consultant/advisor for Janssen, Astellas, Sanofi, Dendreon, Merck, Essa, and Medivation; has received research funding to his institution from Janssen, Johnson & Johnson, Medivation, Sanofi, Dendreon, Bristol Myers Squibb, Genentech, Novartis, Bayer and Tokai; and is a co-inventor of a biomarker technology that has been licensed to Tokai and Qiagen. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. March 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. January 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. September 20 2011;29(27):3651–3658. [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. May 26 2011;364(21):1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. The New England journal of medicine. December 10 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. The New England journal of medicine. September 27 2012;367(13):1187–1197. [DOI] [PubMed] [Google Scholar]
- 7.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. The New England journal of medicine. June 1 2014. [DOI] [PubMed] [Google Scholar]
- 8.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. The New England journal of medicine. October 7 2004;351(15):1502–1512. [DOI] [PubMed] [Google Scholar]
- 9.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. October 2 2010;376(9747):1147–1154. [DOI] [PubMed] [Google Scholar]
- 10.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. The New England journal of medicine. July 18 2013;369(3):213–223. [DOI] [PubMed] [Google Scholar]
- 11.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine. July 29 2010;363(5):411–422. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. The New England journal of medicine. August 20 2015;373(8): 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. March 19 2016;387(10024):1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. The New England journal of medicine. July 27 2017;377(4):338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. The New England journal of medicine. July 27 2017;377(4): 352–360. [DOI] [PubMed] [Google Scholar]
- 16.Tsao CK, Small AC, Galsky MD, Oh WK. Overcoming castration resistance in prostate cancer. Current opinion in urology. May 2012;22(3):167–174. [DOI] [PubMed] [Google Scholar]
- 17.Nakazawa M, Antonarakis ES, Luo J. Androgen receptor splice variants in the era of enzalutamide and abiraterone. Hormones & cancer. October 2014;5(5):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakazawa M, Paller C, Kyprianou N. Mechanisms of Therapeutic Resistance in Prostate Cancer. Current oncology reports. February 2017;19(2):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FDA-NIH Biomarker Working Group. BEST (Biomarkers, Endpoints, and other Tools) Resource. Silver Spring (MD): Food and Drug Administration (US): Co-published by National Institutes of Health (US) and Bethesda (MD); 2016. [PubMed] [Google Scholar]
- 20.Scher HI, Morris MJ, Larson S, Heller G. Validation and clinical utility of prostate cancer biomarkers. Nature reviews. Clinical oncology. April 2013;10(4):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong AJ, Eisenberger MA, Halabi S, et al. Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. European urology. March 2012;61(3):549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. March 01 2014;32(7):671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. December 01 2007;13(23):7053–7058 [DOI] [PubMed] [Google Scholar]
- 24.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. October 01 2008;14(19):6302–6309. [DOI] [PubMed] [Google Scholar]
- 25.Goldkorn A, Ely B, Quinn DI, et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. April 10 2014;32(11):1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorente D, Olmos D, Mateo J, et al. Decline in Circulating Tumor Cell Count and Treatment Outcome in Advanced Prostate Cancer. European urology. December 2016;70(60):985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. *.Scher HI, Heller G, Molina A, et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. April 20 2015;33(12):1348–1355. Study including 711 patients with CRPC demonstrated that a panel of CTC count plus LDH level was able to stratify patients according to three different groups in terms of prognosis, satisfying surrogacy criteria for survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heller G, Fizazi K, McCormack R, et al. The Added Value of Circulating Tumor Cell Enumeration to Standard Markers in Assessing Prognosis in a Metastatic Castration-Resistant Prostate Cancer Population. Clinical cancer research : an official journal of the American Association for Cancer Research. April 15 2017;23(8):1967–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toss A, Mu Z, Fernandez S, Cristofanilli M. CTC enumeration and characterization: moving toward personalized medicine. Annals of translational medicine. November 2014;2(11):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonarakis ES, Armstrong AJ, Dehm SM, Luo J. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate cancer and prostatic diseases. September 2016;19(3):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. The New England journal of medicine. September 11 2014;3 71 (11): 102 8–103 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. **.Antonarakis ES, Lu C, Luber B, et al. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First-and Second-Line Abiraterone and Enzalutamide. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. July 01 2017;35(19):2149–2156. Study with 202 patients with mCRPC treated with abiraterone or enzalutamide that correlated clinical outcomes with 3 different biomarker groups based on CTC detection and AR-V7 detection, demonstrating that clinical outcomes were more favorable for CTC(−) patients, intermediate for CTC(+)/AR-V7(−) patients and worse for CTC(+)/AR-V7(+) patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. **.Antonarakis ES, Lu C, Luber B, et al. Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer. JAMA oncology. August 2015;1(5):582–591. Initial preliminary evidence that patients with mCRPC may respond to taxane-based chemotherapy regardless of the AR-V7 status. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. **.Scher HI, Lu D, Schreiber NA, et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA oncology. November 01 2016;2(11):1441–1449. Study with 161 men with mCRPC treated with ARS inhibitors or taxanes that added further evidence of AR-V7 status as a prognostic and predictive biomarker for patients treated with abiraterone or enzalutamide. This study also demonstrated that patients might respond to taxanes regardless of the AR-V7 status. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scher HI, Graf RP, Schreiber NA, et al. Nuclear-specific AR-V7 Protein Localization is Necessary to Guide Treatment Selection in Metastatic Castration-resistant Prostate Cancer. European urology. June 2017;71(6):874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beltran H, Jendrisak A, Landers M, et al. The Initial Detection and Partial Characterization of Circulating Tumor Cells in Neuroendocrine Prostate Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. March 15 2016;22(6):1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nature medicine. March 2016;22(3):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seitz AK, Thoene S, Bietenbeck A, et al. AR-V7 in Peripheral Whole Blood of Patients with Castration-resistant Prostate Cancer: Association with Treatment-specific Outcome Under Abiraterone and Enzalutamide. European urology. November 2017;72(5):828–834. [DOI] [PubMed] [Google Scholar]
- 39.Qu F, Xie W, Nakabayashi M, et al. Association of AR-V7 and Prostate-Specific Antigen RNA Levels in Blood with Efficacy of Abiraterone Acetate and Enzalutamide Treatment in Men with Prostate Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. February 01 2017;23(3):726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. *.Li H, Wang Z, Tang K, et al. Prognostic Value of Androgen Receptor Splice Variant 7 in the Treatment of Castration-resistant Prostate Cancer with Next generation Androgen Receptor Signal Inhibition: A Systematic Review and Meta-analysis. European urology focus. January 23 2017. Meta-analysis of eight trials encompassing 490 patients that added further evidence of the prognostic impact of AR-V7 in patients with mCRPC treated with ARS inhibitors, which demonstrated that both PFS and OS were better in AR-V7(−) than AR-V7(+) patients. [DOI] [PubMed] [Google Scholar]
- 41.Taplin ME, Antonarakis ES, Ferrante KJ, et al. Clinical factors associated with AR-V7 detection in ARMOR3-SV, a randomized trial of galeterone (Gal) vs enzalutamide (Enz) in men with AR-V7+ metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 35, 2017. (suppl; abstr 5005). [Google Scholar]
- 42.Bastos DA, Antonarakis ES. Galeterone for the treatment of advanced prostate cancer: the evidence to date. Drug design, development and therapy. 2016;10:2289–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernemann C, Schnoeller TJ, Luedeke M, et al. Expression of AR-V7 in Circulating Tumour Cells Does Not Preclude Response to Next Generation Androgen Deprivation Therapy in Patients with Castration Resistant Prostate Cancer. European urology. January 2017;71(1):1–3. [DOI] [PubMed] [Google Scholar]
- 44.Antonarakis ES, Scher HI. Do Patients With AR-V7-Positive Prostate Cancer Benefit from Novel Hormonal Therapies? It All Depends on Definitions. European urology. January 2017;71(1):4–6. [DOI] [PubMed] [Google Scholar]
- 45.Steinestel J, Bernemann C, Schrader AJ, Lennerz JK. Reply from Authors re: Emmanuel S. Antonarakis, Howard I. Scher. Do Patients With AR-V7-Positive Prostate Cancer Benefit from Novel Hormonal Therapies? It All Depends on Definitions. Eur Urol 2017;71:4–6: Unsplicing a Conflict. European urology. January 2017;71(1):6–7. [DOI] [PubMed] [Google Scholar]
- 46. *.Markowski MC, Silberstein JL, Eshleman JR, Eisenberger MA, Luo J, Antonarakis ES. Clinical utility of CLIA-grade AR-V7 testing in patients with metastatic castration-resistant prostate cancer. JCO Precision Oncology 2017; epub ahead of print. Study evaluating the real-world clinical utility of AR-V7 testing suggesting that physicians who used the AR-V7 test results to direct the next line of systemic therapy observed greater rates of PSA50 declines with the subsequent systemic therapy compared to physicians who did not use the results to change treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussain CMT M, Higano CS, Crawford ED, Liu G, Wilding G, Prescott S, Akdas A, Small EJ, Dawson NA, Donnelly BJ, Venner P, Vaishampayan UN, Schellhammer PF, Quinn DI, Raghavan D, Vogelzang NJ, Thompson IM Intermittent versus continuous androgen deprivation in hormone sensitive metastatic prostate cancer patients: Results of S9346 (INT-0162), an international phase III trial. Paper presented at: J Clin Oncol 30 (suppl; abstr 4) 2012. [Google Scholar]
- 48.Vale CL, Burdett S, Rydzewska LHM, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. The lancet oncology. February 2016;17(2):243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Handy CE, Antonarakis ES. Sequencing Treatment for Castration-Resistar Cancer. Current treatment options in oncology. December 2016;17(12):64. [DOI] [PubMed] [Google Scholar]
- 50.Halabi S, Kelly WK, Ma H, et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men With Castration-Resistant Prostate Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. May 10 2016;34(14):1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. The New England journal of medicine. April 04 2013;368(14): 1314–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aparicio AM, Shen L, Tapia EL, et al. Combined Tumor Suppressor Defects Characterize Clinically Defined Aggressive Variant Prostate Cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. March 15 2016;22(6):1520–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hornberg E, Ylitalo EB, Crnalic S, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PloS one. April 28 2011;6(4):el9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welti J, Rodrigues DN, Sharp A, et al. Analytical Validation and Clinical Qualification of a New Immunohistochemical Assay for Androgen Receptor Splice Variant-7 Protein Expression in Metastatic Castration-resistant Prostate Cancer. European urology. October 2016;70(4):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saylor PJ, Lee RJ, Arora KS, et al. Branched Chain RNA In Situ Hybridization for Androgen Receptor Splice Variant AR-V7 as a Prognostic Biomarker for Metastatic Castration-Sensitive Prostate Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. January 15 2017;23(2):363–369. [DOI] [PubMed] [Google Scholar]
- 56.Guedes LB, Morais CL, Almutairi F, et al. Analytic Validation of RNA In Situ Hybridization (RISH) for AR and AR-V7 Expression in Human Prostate Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. September 15 2016;22(18):4651–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Y, Sharp A, Anderson CM, et al. Novel Junction-specific and Quantifiable In Situ Detection of AR-V7 and its Clinical Correlates in Metastatic Castration-resistant Prostate Cancer. European urology. August 30 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer research. January 01 2009;69(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. May 21 2015;161(5):1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Y, Luk A, Young FP, et al. Droplet Digital PCR Based Androgen Receptor Variant 7 (AR-V7) Detection from Prostate Cancer Patient Blood Biopsies. International journal of molecular sciences. August 04 2016;17(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyamoto DT, Zheng Y, Wittner BS, et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. September 18 2015;349(6254):1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Usher JL, Athreya K, Ishiba T, et al. Detection of AR-V7 using cell-free RNA (cfRNA) in prostate cancer.J Clin Oncol 34, 2016. (suppl; abstr el6613). [Google Scholar]
- 63.Del Re M, Biasco E, Crucitta S, et al. The Detection of Androgen Receptor Splice Variant 7 in Plasma-derived Exosomal RNA Strongly Predicts Resistance to Hormonal Therapy in Metastatic Prostate Cancer Patients. European urology. April 2017;71(4):680–687. [DOI] [PubMed] [Google Scholar]
- 64.Antonarakis ES, Lu C, Chen Y, et al. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC).J Clin Oncol 33, 2015. (suppl 7; abstr 138). [Google Scholar]
- 65.Romanel A, Gasi Tandefelt D, Conteduca V, et al. Plasma AR and abiraterone-resistant prostate cancer. Science translational medicine. November 04 2015;7(312):312re310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Azad AA, Volik SV, Wyatt AW, et al. Androgen Receptor Gene Aberrations in Circulating Cell-Free DNA: Biomarkers of Therapeutic Resistance in Castration-Resistant Prostate Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. May 15 2015;21(10):2315–2324. [DOI] [PubMed] [Google Scholar]
- 67. *.Scher HI, Graf RP, Schreiber NA, et al. Phenotypic Heterogeneity of Circulating Tumor Cells Informs Clinical Decisions between AR Signaling Inhibitors and Taxanes in Metastatic Prostate Cancer. Cancer research. October 15 2017;77(20):5687–5698. Study evaluating CTC heterogeneity as a treatment selection tool, suggesting that patients withmCRPC and high CTC heterogeneity do not benefit from ARS inhibitors and may benefit from taxane-base chemotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. The New England journal of medicine. October 29 2015;373(18):1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pomerantz MM, Spisak S, Jia L, et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer. September 15 2017;123(18):3532–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine. June 25 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]


