Abstract
Purpose
To investigate the factors affecting microvascular responses in the bulbar conjunctiva of habitual contact lens (HCL) wearers.
Methods
A functional slit-lamp biomicroscope (FSLB) was used to image the temporal bulbar conjunctiva of habitual contact lens (HCL) wearers and non–contact lens (NCL) wearers. The vessel diameters and blood flow velocities (BFVs) were measured. Fractal analysis using Dbox as vessel density and D0 as vessel complexity were used to quantitatively analyze the microvascular network. One eye each of 91 NCL wearers and 75 HCL wearers was imaged.
Results
The BFV of NCL wearers was 0.50 ± 0.14 mm/s, which was negatively correlated with age (r = −0.22, P < 0.05). The BFV, vessel diameter, Dbox, and D0 of HCL wearers was significantly higher than NCL wearers (P < 0.05). In these HCL wearers, BFVs were positively correlated with contact lens (CL) hours of wear per day and CL days of wear per week. BFV, Dbox, and D0 were not related to CL years of wear, CL power, CL base curve, and CL diameter (P > 0.05).
Conclusions
Vascular responses on the bulbar conjunctiva occurred in HCL wearers and appeared to be unrelated to sex or age, CL years of wear, and lens parameters, indicating that wearing a CL itself may be the predominant factor inducing these responses.
Keywords: bulbar conjunctiva, blood flow velocity, microvascular network, functional slit-lamp biomicroscopy (FSLB), hemodynamics
Contact lenses (CLs) have become popular for both medical and cosmetic use. It has been estimated that more than 140 million people wear contact lenses worldwide, including 38 million in the United States.1–3 With the increasing uptake of CL wear, more and more people are potentially at risk for CL-related issues.4 Common CL-related complications include corneal infiltration, sterile corneal ulcers, and CL-related ocular discomfort (CLD).2,5–7 These complications are often accompanied by a common clinical sign, bulbar conjunctival redness (conjunctival hyperemia), which is also a principal clinical sign of a conjunctival vascular response to CL wear and underlying ocular inflammation. Our previous studies using advanced ophthalmic imaging on conjunctival microvasculature and microcirculation have indicated that vascular responses on the ocular surface, especially on the bulbar conjunctiva, occur in neophyte CL wearers8 and habitual CL wearers.9 Chen et al.8 studied the microvascular responses to short-term contact lens wear in neophyte contact lens wearers and found that blood flow velocity of the bulbar conjunctiva was increased after 6 hours of CL wear. Shi et al.9 studied the blood flow velocity in the bulbar conjunctiva in a small group of habitual CL wearers and found that conjunctival blood flow velocity was elevated in daily CL wearers when they did not wear their lenses during imaging. However, these previous studies did not characterize the vasculature in the healthy population, nor the factors affecting the vascular responses to long-term lens wear.
Despite the alteration of the vasculature in the ocular surface, most CL wearers successfully wear their lenses long-term and rarely develop severe complications, such as infection,10 sterile keratitis, or corneal infiltrative events.11,12 It is critical to first understand the ocular vasculature in the healthy population, then determine the factors that affect the vascular responses in long-term successful CL wearers. Clinical observation of a conjunctival vascular response is subjective, with poor reliability and repeatibility.13–15 With the introduction of an advanced modality for imaging conjunctival microvasculature and microcirculation, the functional slit-lamp biomicroscope (FSLB), quantification of subclinical vascular responses can be readily performed noninvasively.16 The goal of the research was to study the vasculature of the conjunctiva in a healthy population and determine the factors affecting microvascular responses in the bulbar conjunctiva in habitual CL wearers.
Materials and Methods
Microcirculation and Microvasculature Measurement by FSLB
The FSLB imaging system has been described in detail in our previous studies4,5 and the imaging protocol was the same as used in our previous studies.8,9 FSLB devices in Miami, FL, USA, and Wenzhou, China, have similar configurations and both devices were carefully calibrated. Briefly, a traditional slit-lamp was adapted with a digital camera that can measure the blood flow velocity and vessel diameter. Both FSLB devices were attached with the Canon digital camera (Canon 60D; Canon Inc, Melville, NY, USA). The inherent Movie Crop Function (MCF) in the camera generates the equivalent of approximately ×7 magnification, and it combines with the built-in slit-lamp optical magnification of up to ×30, which resulted in total magnification of up to approximately ×210. In the present study, the FSLB based on the Nikon slit-lamp (Nikon FS-2; Nikon, Inc., Melville, NY, USA) in Miami has a field of view of 0.9 × 0.7 mm2 with the MCF and slit-lamp magnification setting of ×30. The FSLB based on the Kanghua slit-lamp (SLM-4ER; Kanghua, Inc., Chongqing, China) in Wenzhou has a field of view of 1.1 × 0.9 mm2 with the MCF and slit-lamp magnification setting of ×25. Image size of 640 × 480 pixels was used in the video recording mode (ISO 400, shutter speed 1/60). Six different locations approximately 1 mm away from the limbus on the temporal bulbar conjunctiva were imaged for the measurements of blood flow velocity, vessel diameter, and flow rate. To obtain the bulbar conjunctiva's microvascular network, the camera was set to a still photo shot model with an ISO of 500 and a shutter speed of 1/15. The magnification was approximately ×22 optical magnification with an image size of 5184 × 3456 pixels. A green filter was used to capture a field of 15.74 × 10.50 mm2 of the temporal conjunctiva with the Miami FSLB. A green filter was used to capture a field of 14.63 × 9.75 mm2 with the Wenzhou FSLB.
Custom software has been developed and used for the quantification of blood flow velocity, vessel diameter, blood flow rate, and vessel density as described in our previous studies.8,9 With input of camera settings and fields of view of each of the FSLB devices, vessel diameters, BFVs, and flow rates were measured through a series of image-processing procedures from the video recording.16 Blood flow velocity measurements were performed using the automatic space-time image technique to track motion of the red blood cell cluster.16 The vessel diameter was defined as the full width at the half-maximum (FWHM) of the intensity profile, which was perpendicular to the center line of the vessel. The flow rate was calculated based on blood flow velocity and vessel diameter using the question previously published.17 Using custom developed software, the microvascular network was automatically segmented with a series of image-rocessing procedures,16 and fractal analysis was performed using a commercially available software program (Benoit; TruSoft Inc., St. Petersburg, FL, USA).16 The monofractal and multifractal values were obtained to evaluate the vessel density (Dbox) and complexity (D0).
Subjects and Image Procedure
This study was conducted at two research sites: Bascom Palmer Eye Institute, University of Miami, Miami, FL, USA, and the Eye Hospital of Wenzhou Medical University, Wenzhou, China. This study was approved by the Institutional Review Board of Human Research at the University of Miami and the Ethics Committee of the Eye Hospital of Wenzhou Medical University. Informed consent was obtained from each subject at both sites, and all subjects were treated in accordance with the tenets of the Declaration of Helsinki.
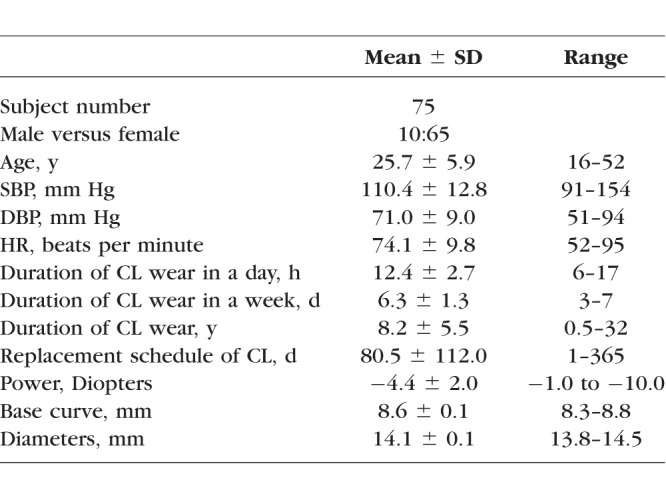
Two groups of subjects were recruited. Non-CL (NCL) wearers included self-reported healthy subjects who never wore CLs. Habitual CL (HCL) wearers included those who had worn soft CLs daily for more than 6 months. The exclusion criteria included a history of extended wear (overnight), laser treatment, trauma, eye surgery, systemic diseases, tobacco or alcohol use, and the use of medication. Excluded compounding factors included hypertension, diabetes, sickle cell anemia, cerebral small vessel disease, stroke, cardiovascular diseases, and other vascular diseases. The average age of the 91 NCL wearers (36 males and 55 females, ranged from 17 to 56 years old) was 34.2 ± 8.8 years, the average systolic blood pressure (SBP) of the NCL wearers was 113.9 ± 11.3 (ranged from 93 to 145) mm Hg, the average diastolic blood pressure (DBP) was 74.4 ± 8.8 (ranged from 55 to 91) mm Hg, the average heart rate (HR) was 72.0 ± 9.9 (ranged from 52 to 95) beats per minute. The characteristics of the HCL wearers are shown in Table 1. Of 75 HCL wearers, 19 (25%) wore daily disposable CLs, 16 (21%) wore 1- to 4-weekly disposable CLs, and 40 (53%) wore 1 month or more disposable CLs. All HCL wearers wore silicone hydrogel CLs. Seventeen (23%) HCL wearers wore Johnson & Johnson Vision Care Inc. (JJVC; Jacksonville, FL, USA) brand CLs, 16 (21%) wore Bausch & Lomb Inc. (B&L; Rochester, NY, USA) brand CLs, and 42 (56%) wore other brands of CLs.
Table 1.
Characteristics of the HCL Wearers Group
Imaging sessions were conducted from 9 AM to 5 PM for both NCL wearers and HCL wearers during a study visit. The previous study suggested the time of day is not a factor of BFV in NCL wearers.18 One eye of each subject was imaged using FSLB.
Statistical Analysis
All data management and statistical analyses were performed in Excel (version 2010; Microsoft, Redmond, WA, USA) and SAS (version 9.4; SAS Institute, Cary NC, USA). The sample size was calculated by a software program (Gpower, version 3.1.9) recommended by Faul et al.19 and Bonett and Wright.20 According to our previous study,9 a sample size of 58 subjects in each group of the NCL and HCL groups would be enough to detect the true difference of the blood flow velocity in the bulbar conjunctiva with a detection power of 0.9. In the present study, 75 HCL wearers and 91 NCL subjects were recruited, which would ensure enough power to detect the true difference between groups. All parameters were compared between HCL wearers and NCL wearers through analysis of covariance (ANCOVA) with adjusted age and sex. Pearson correlation coefficients were used to determine the relationships between the microvascular parameters and other parameters. All data were presented as the mean ± SD and a P value less than 0.05 was considered statistically significant.
Results
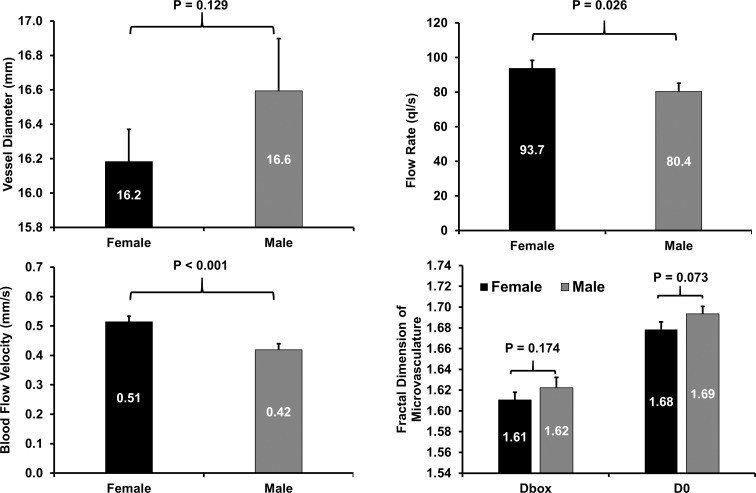
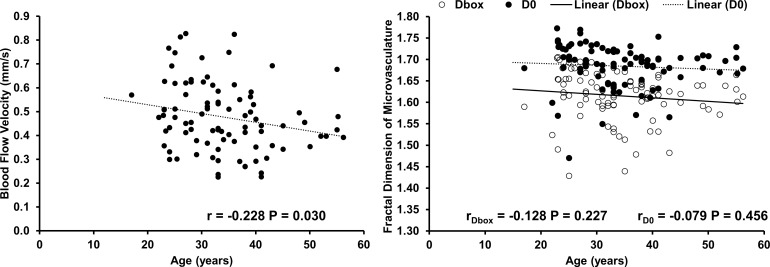
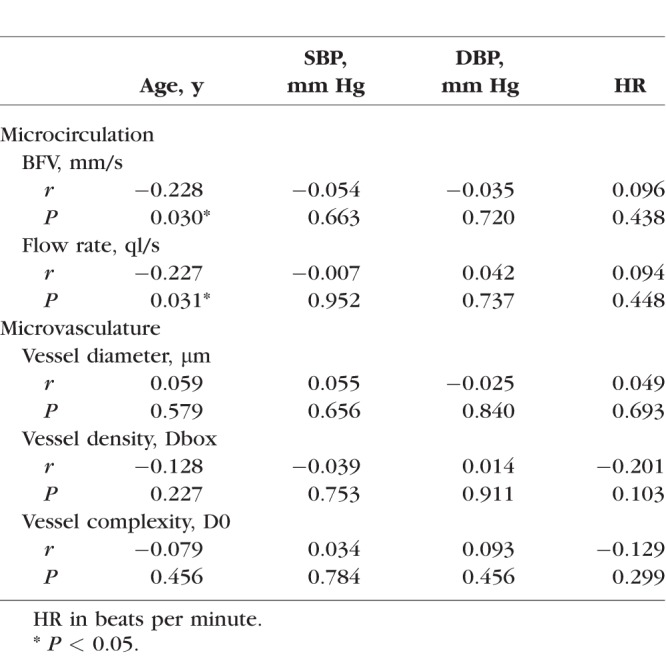
In NCL wearers, the BFV was 0.51 ± 0.14 mm/s in female NCL wearers, which was significantly higher than that of male NCL wearers (0.42 ± 0.13 mm/s, P < 0.001, Fig. 1). The flow rate was significantly higher in female NCL wearers than that in male NCL wearers (P = 0.026, Fig. 1). No significant differences were observed in vessel diameter, microvascular density, and complexity between females and males (Fig. 1). The BFV and flow rate were significantly and negatively correlated with age (r = −0.228, P = 0.030 and r = −0.227, P = 0.031, separately, Fig. 2; Table 2). No significant correlations were found between microvascular network density and complexity with age, SBP, DBP, and HR (Table 2; Fig. 2).
Figure 1.
Conjunctival vascular measurements in female NCL wearers and in male NCL wearers. BFV (bottom left) and flow rate (top right) were significantly higher in females compared with males (P < 0.05). Vessel diameter (top left) and fractal dimension (bottom right) showed that microvascular network density (Dbox) and complexity (D0) were not significantly different between males and females (P > 0.05). Bars: standard error.
Figure 2.
Correlations of BFVs, and microvascular network density and complexity with age in 91 NCLs. A significant negative correlation was found between age and BFV (P = 0.030, left). No significant correlation was found between age and microvascular measurements (density and complexity) (right).
Table 2.
Correlations Between the Characteristics and the Microcirculation and Microvasculature in NCL Wearers
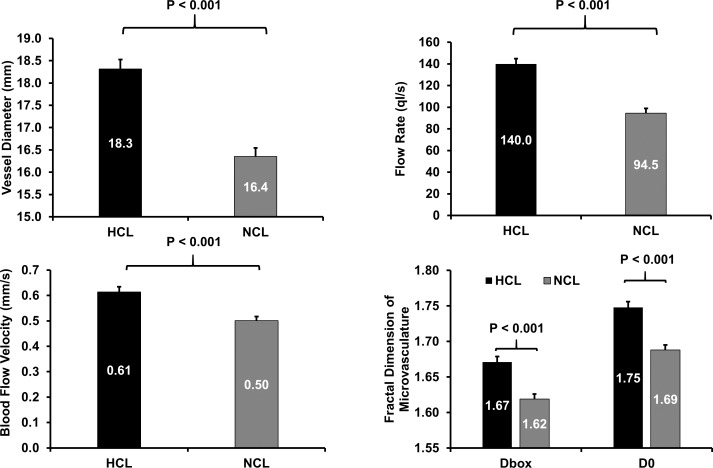
The BFV of HCL wearers was 0.61 ± 0.15 mm/s, which was significantly higher than that in in NCL wearers (0.50 ± 0.14 mm/s, P < 0.001, Fig. 3), after the age and sex were adjusted. The flow rate was significantly higher in HCL wearers than that in the NCL wearers (P < 0.001; Fig. 3). The vessel diameter was significantly larger in HCL wearers than that in NCL wearers (P < 0.001; Fig. 3). Similarly, microvascular network density and complexity in HCL wearers were both significantly higher than those in NCL wearers (both P < 0.001; Fig. 3).
Figure 3.
Conjunctival vascular measurements in HCL wearers and NCL wearers. No significant difference in vessel diameter (top left) was found. The flow rate (top right) and BFV (bottom left) showed that the microcirculation was significantly greater in HCL wearers compared with NCL wearers after age and sex adjustment. The fractal dimension of microvasculature (bottom right) showed that microvascular network density (Dbox) and complexity (D0) were significantly higher in HCL wearers compared with NCL wearers. Bars: standard error.
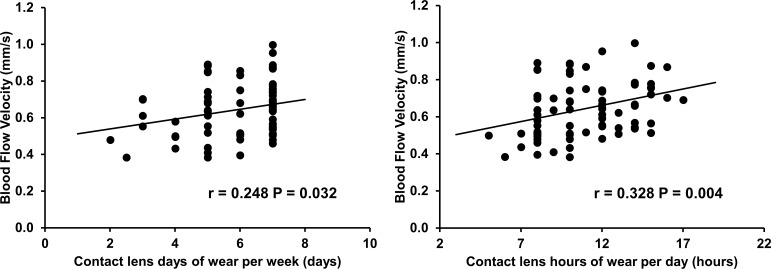
In HCL wearers, no significant difference in microvascular parameters between male and female HCL wearers was found. BFV was significantly and positively correlated with CL hours of wear per day and CL days of wear per week (r = 0.328, r = 0.248, separately, both P < 0.05; Fig. 4). The vessel diameter was significantly and positively correlated with CL replacement schedule (r = 0.298, P = 0.009), but negatively correlated with CL hours of wear per day (r = −0.240, P = 0.038). BFV, flow rate, vessel diameter, and microvascular network density and complexity were not correlated with the time of day (o'clock), CL years of wear, CL power, CL base curve, and CL diameter; the microvascular network density and complexity also were not correlated with CL hours of wear per day, CL days of wear per week, and CL replacement schedule.
Figure 4.
Correlations between BFV and CL days of wear per week and hours of wear per day. In HCLs wearers (n = 75), BFV was positively related to CL days of wear per week (P = 0.032, left) and positively related to CL hours of wear per day (P = 0.004, right).
Discussion
This study characterized microcirculation and the microvascular network in the bulbar conjunctiva in a relatively large population of healthy subjects and HCL wearers. The bulbar conjunctival vasculature is regarded as the terminal vascular bed of the human internal carotid artery. Studies of the conjunctival microvasculature have shown that systemic diseases (i.e., diabetes, stroke, and sickle cell retinopathy) affect the microcirculation of the conjunctival microvasculature.21–23 Studying changes in microstructure and microcirculation in healthy subjects can better explain changes caused by disease in the human conjunctival microvasculature and the factors of microvascular responses in HCL wearers. The results of the present study showed that bulbar conjunctival BFV declined with age, but neither the density nor complexity of the microvascular network changed.
Age was identified as a factor affecting conjunctival BFV in the present study. The observation of BFV during aging mirrors the findings in the retina. Burgansky-Eliash et al.24 reported a reduction in retinal venular flow velocity of 9.7% per decade above 40 years of age. Wei et al.25 reported a decrease of 3.5% per decade of age in retinal venular flow velocity. BFV and retinal vascular network density both decrease with aging.25 In contrast to the findings in the retina,25 the density of the bulbar conjunctival vasculature does not appear to change during aging, as found in the present study, possibly due to the terminal vessel bed and its location, which may be influenced by the external environment and ocular conditions in old age. For example, ocular dryness is a common condition in the elderly, which may cause the vessel density to increase slightly to offset the possible changes due to aging.26
Sex is another crucial factor in the vascular system of the human body. The difference in BFV in the bulbar conjunctiva found between females and males may be explained by the sex difference in the carotid artery, which supplies the conjunctival vascular system. A higher BFV in the carotid artery is reported in females compared with males.27 Although this difference in the carotid artery should be considered at clinically relevant thresholds for intervention, notable differences in conjunctival BFV may not be clinically significant. The BFV in females was approximately 0.5 mm/s, which was much lower than that of HCL wearers, and the difference between sexes appeared to be suppressed in response to CL wear.
Characterization of the microvasculature and microcirculation in healthy subjects and factors that affect these measurements (i.e., age and sex) could serve as a foundation to better understand changes in response to CL wear. Cheung et al.28 reported that HCL wearers displayed microvascular abnormalities in morphology representing conjunctival vasculopathies. Elevated BFV and changes in microvascular network in HCL wearers have been documented previously,8,9 but no relationships between vascular responses and other recorded parameters in HCL wearers have been identified. Elevated BFV and hyperemia in the bulbar conjunctiva was also found in neophyte CL wearers, but BFV and hyperemia were not related to base curves of the lenses during 2-week daily wear.29 Compared with healthy subjects, the microcirculation was elevated in HCL wearers, but they were unrelated to CL years of wear, CL power, CL base curve, and CL replacement schedule. The relationship between BFV and CL hours of wear per day and CL days of wear per week was also identified, but the relationship was not strong, indicating that all CL wearers fall under a general category based on the perspective that a CL is a foreign object attached to the ocular surface that engages the vasculature to elicit a response, which has been regarded as possible subclinical chronic inflammation.30
This subclinical chronic inflammation can be characterized as the middle ground between the basal state and infected or damaged states. This phenomenon is due to elevated immune activity, creating a steady state of alertness. It could be speculated that wearing a lens itself may be the predominant factor eliciting vascular responses, surpassing other factors such as CL power and CL base curve. In other words, wearing CLs triggers or modulates vascular responses, which are maintained at a low level in the middle ground between basal and extreme states (Figs. 5, 6). This may explain the elevated vascular response to CL wear alone rather than in combination with other affecting factors, except for CL hours of wear per day and CL days of wear per week in the present study. Although increased BFV and microvascular network density can be explained by an underlying inflammatory response, the relationship between BFV and inflammatory markers has not been established, warranting further studies.
Figure 5.
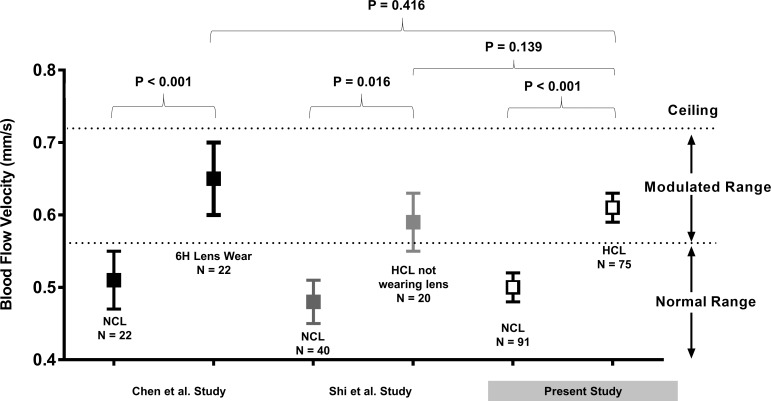
Comparison of BFV among studies using the same imaging protocol. The present study and two previously published studies used the same imaging protocol and measured BFV. Chen et al.8 examined the BFVs of 22 NCL wearers after 6 hours of CL wear and found a significant increase in BFVs. Shi et al.9 examined 20 HCL wearers who were imaged when they were not wearing their CLs after one night of sleep and found that BFV remained higher compared with that of 40 NCL wearers. In the present study, a significantly higher BFV was observed in 75 HCL wearers than that in 91 NCL wearers. Comparing BFVs of HCL wearers in the present study with that of NCLs after 6 hours of CL wear and HCLs when they were not wearing their CLs, no significant differences were found. Overall, it appeared that wearing lenses caused an elevated BFV and a possible ceiling effect limited the evaluation within a modulated range.
Figure 6.
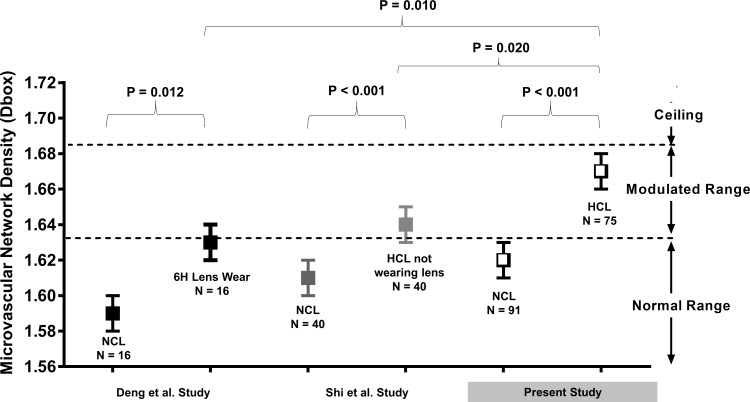
Comparison of microvascular network density (Dbox) among studies using the same imaging protocol. The present study and two previously published studies used the same imaging protocol to measure microvascular network density. Deng et al.31 examined 16 NCL wearers and found a significantly elevated Dbox after 6 hours of CL wear. Shi et al.9 examined 20 HCL wearers who removed their CLs for one night of sleep and found an elevated Dbox compared with 40 NCL wearers. In the present study, a significantly higher Dbox was identified in 75 HCL wearers compared with 91 NCL wearers. The Dbox of HCL wearers while wearing CLs was significantly different from that when they were not wearing CLs and NCL wearers after 6 hours of CL wear. Overall, it appeared that wearing lenses caused elevated vessel density and a possible ceiling effect limited the evaluation within the modulated range.
As with most studies, our findings should be considered in the context of our study limitations. First, we did not study microvascular changes at the end of the day right before the CL wearers removed their lenses, which may have helped determine the possible ceiling point, and we did not evaluate these NCL wearers after a night of rest. Second, we did not study recovery after the CL wearers stopped wearing their lenses for days or weeks. Third, we did not measure inflammatory mediators and ocular discomfort or explore the relationship among BFV inflammatory mediators and ocular discomfort; further studies are needed.
In summary, vascular responses on the bulbar conjunctiva occurred in HCL wearers and appeared to be unrelated to demographic characteristics, CL years of wear, and CL parameters, indicating that wearing a CL itself may be the predominant factor inducing these responses.
Acknowledgments
Supported by National Institutes of Health Center Grant P30 EY014801, and a grant from Research to Prevent Blindness.
Disclosure: L. Hu, None; C. Shi, None; H. Jiang, None; Y. Shi, None; Z. Sethi, None; J. Wang, None
References
- 1.Nichols JJ, Green-Church KB. Mass spectrometry-based proteomic analyses in contact lens-related dry eye. Cornea. 2009;28:1109–1117. doi: 10.1097/ICO.0b013e3181a2ad81. [DOI] [PubMed] [Google Scholar]
- 2.Dumbleton K, Caffery B, Dogru M, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on epidemiology. Invest Ophthalmol Vis Sci. 2013;54:TFOS20–TFOS36. doi: 10.1167/iovs.13-13125. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton F, Keay L, Jalbert I, Cole N. The epidemiology of contact lens related infiltrates. Optom Vis Sci. 2007;84:257–272. doi: 10.1097/OPX.0b013e3180485d5f. [DOI] [PubMed] [Google Scholar]
- 4.Forister JF, Forister EF, Yeung KK, et al. Prevalence of contact lens-related complications: UCLA contact lens study. Eye Contact Lens. 2009;35:176–180. doi: 10.1097/ICL.0b013e3181a7bda1. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Wang J, Shen M, et al. Tear menisci and ocular discomfort during daily contact lens wear in symptomatic wearers. Invest Ophthalmol Vis Sci. 2011;52:2175–2180. doi: 10.1167/iovs.10-5780. [DOI] [PubMed] [Google Scholar]
- 6.Masoudi S, Stapleton FJ, Willcox MD. Contact lens-induced discomfort and protein changes in tears. Optom Vis Sci. 2016;93:955–962. doi: 10.1097/OPX.0000000000000888. [DOI] [PubMed] [Google Scholar]
- 7.Nichols JJ, Willcox MD, Bron AJ, et al. The TFOS International Workshop on Contact Lens Discomfort: executive summary. Invest Ophthalmol Vis Sci. 2013;54:TFOS7–TFOS13. doi: 10.1167/iovs.13-13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Xu Z, Jiang H, Zhou J, Wang L, Wang J. Altered bulbar conjunctival microcirculation in response to contact lens wear. Eye Contact Lens. 2017;43:95–99. doi: 10.1097/ICL.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Hu L, Chen W, Qu D, Jiang H, Wang J. Evaluated conjunctival blood flow velocity in daily contact lens wearers. Eye Contact Lens. doi: 10.1097/ICL.0000000000000389. [published online ahead of print April 13, 2017] http://doi: 10.1097/ICL.0000000000000389. [DOI] [PMC free article] [PubMed]
- 10.Willcox MD, Holden BA. Contact lens related corneal infections. Biosci Rep. 2001;21:445–461. doi: 10.1023/a:1017991709846. [DOI] [PubMed] [Google Scholar]
- 11.Morgan PB, Efron N, Brennan NA, Hill EA, Raynor MK, Tullo AB. Risk factors for the development of corneal infiltrative events associated with contact lens wear. Invest Ophthalmol Vis Sci. 2005;46:3136–3143. doi: 10.1167/iovs.05-0133. [DOI] [PubMed] [Google Scholar]
- 12.Radford CF, Minassian D, Dart JK, Stapleton F, Verma S. Risk factors for nonulcerative contact lens complications in an ophthalmic accident and emergency department: a case-control study. Ophthalmology. 2009;116:385–392. doi: 10.1016/j.ophtha.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 13.Schulze MM, Hutchings N, Simpson TL. The perceived bulbar redness of clinical grading scales. Optom Vis Sci. 2009;86:E1250–E1258. doi: 10.1097/OPX.0b013e3181bb4225. [DOI] [PubMed] [Google Scholar]
- 14.Efron N, Morgan PB, Katsara SS. Validation of grading scales for contact lens complications. Ophthalmic Physiol Opt. 2001;21:17–29. [PubMed] [Google Scholar]
- 15.McMonnies CW, Chapman-Davies A. Assessment of conjunctival hyperemia in contact lens wearers. Part I. Am J Optom Physiol Opt. 1987;64:246–250. doi: 10.1097/00006324-198704000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Zhong J, DeBuc DC, et al. Functional slit lamp biomicroscopy for imaging bulbar conjunctival microvasculature in contact lens wearers. Microvasc Res. 2014;92:62–71. doi: 10.1016/j.mvr.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koutsiaris AG, Tachmitzi SV, Batis N, et al. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44:375–386. [PubMed] [Google Scholar]
- 18.Xu Z, Jiang H, Tao A, et al. Measurement variability of the bulbar conjunctival microvasculature in healthy subjects using functional slit lamp biomicroscopy (FSLB) Microvasc Res. 2015;101:15–19. doi: 10.1016/j.mvr.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 20.Bonett DG, Wright TA. Sample size requirements for estimating Pearson, Kendall and Spearman correlations. Psychometrika. 2000;65:23–28. [Google Scholar]
- 21.Khansari MM, Wanek J, Tan M, et al. Assessment of conjunctival microvascular hemodynamics in stages of diabetic microvasculopathy. Sci Rep. 2017;7:45916. doi: 10.1038/srep45916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kord VA, Wanek J, Mukarram F, Zelkha R, Testai FD, Shahidi M. Feasibility of assessment of conjunctival microvascular hemodynamics in unilateral ischemic stroke. Microvasc Res. 2015;100:4–8. doi: 10.1016/j.mvr.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kord VA, Wanek J, Zelkha R, et al. Conjunctival microvascular haemodynamics in sickle cell retinopathy. Acta Ophthalmol. 2015;93:e275–e280. doi: 10.1111/aos.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgansky-Eliash Z, Lowenstein A, Neuderfer M, et al. The correlation between retinal blood flow velocity measured by the retinal function imager and various physiological parameters. Ophthalmic Surg Lasers Imaging Retina. 2013;44:51–58. doi: 10.3928/23258160-20121221-13. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, Jiang H, Shi Y, et al. Age-related alterations in the retinal microvasculature, microcirculation, and microstructure. Invest Ophthalmol Vis Sci. 2017;58:3804–3817. doi: 10.1167/iovs.17-21460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Batawi HI, Alava JR, et al. Bulbar conjunctival microvascular responses in dry eye. Ocul Surf. 2017;15:193–201. doi: 10.1016/j.jtos.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comerota AJ, Salles-Cunha SX, Daoud Y, Jones L, Beebe HG. Gender differences in blood velocities across carotid stenoses. J Vasc Surg. 2004;40:939–944. doi: 10.1016/j.jvs.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Cheung AT, Hu BS, Wong SA, et al. Microvascular abnormalities in the bulbar conjunctiva of contact lens users. Clin Hemorheol Microcirc. 2012;51:77–86. doi: 10.3233/CH-2011-1513. [DOI] [PubMed] [Google Scholar]
- 29.Sorbara L, Maram J, Simpson T, Hutchings N. Corneal, conjunctival effects and blood flow changes related to silicone hydrogel lens wear and their correlations with end of day comfort. Cont Lens Anterior Eye. 2018;41:193–200. doi: 10.1016/j.clae.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Efron N. Contact lens wear is intrinsically inflammatory. Clin Exp Optom. 2017;100:3–19. doi: 10.1111/cxo.12487. [DOI] [PubMed] [Google Scholar]
- 31.Deng Z, Wang J, Jiang H, et al. Lid wiper microvascular responses as an indicator of contact lens discomfort. Am J Ophthalmol. 2016;170:197–205. doi: 10.1016/j.ajo.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]