Abstract
Aim
The aim of the work was to catch potential errors with daily EPID measurements of repeatability of the dose distribution during irradiation of IMRT patients.
Materials and methods
In the first stage, measurements were made using an anthropomorphic phantom in which the method of collecting data with an EPID device and the possibility of detecting errors in positioning were developed. Next, for 23 patients, the pelvis (P) and head and neck (H&N) regions, images were collected with an EPID device for each IMRT subfield daily and compared to reference images using the gamma method (DTA 3 mm, DD 3%). Finally, the dependencies between treatment plan parameters, pre-verification results and repeatability of collected images were evaluated.
Results
The anthropomorphic phantom study has shown what kind of effects we can expect with EPID measured at potential shifts during radiotherapy. For the clinical case, score results were obtained for individual tumor regions as below: (P) 0.786 ± 1.046, (H&N) 0.720 ± 1.552. For most evaluated cases, score values were below 1%: (P) 75.5% and (H&N) 83.9% of analyzed fields. 95% of all evaluated data was with the score below: (P) 2.86% and (H&N) 3.40%. The relationship between the results of the analysis of daily collected images and the results of pre-verification, field size and irradiation time was shown.
Conclusions
The EPID-based daily verification can provide extra information about day-to-day repeatability of treatment, without additional dose.
Keywords: EPID, Fluency map, Treatment repeatability, IMRT
1. Introduction
The dynamic techniques, such as IMRT (intensity modulated radiotherapy) and VMAT, have become very popular in the clinical routine of radiotherapy in recent years. This can offer substantial benefits to the patient, both in terms of better dose distribution in the target volume and improved sparing of the surrounding normal tissues and critical organs.7, 8, 9 Since these techniques are much more complex than conventional open-field 3DCRT (3D conformal radiotherapy), more advanced and precise quality control of treatment should be implemented.1, 2, 3
In present clinical procedures, the pre-treatment dosimetry verification of IMRT plan is used at the hospital as a standard for dynamic techniques before treatment and patient's geometric verification (MV, OBI: kV, CBCT) is also performed.4 QA of the linac is performed periodically according to the national and international reports and the government recommendations, but still not daily, for each fraction and each patient. In the pre-treatment dosimetry verification, it is checked that the linac is capable of achieving the planned dose distribution prior to initiation of therapy, after which no information is available on the correct dose distribution during irradiation. Patient's geometric verification gives information about patient position, but, usually, it is not performed daily and it relates to additional doses from imaging. Machine QA (especially MLC tests are important for IMRT/VMAT techniques.) are conducted weekly, monthly or even less often, so, in general, unexpected errors in beam delivery are hard to catch with conventional QA. It is more often said that QA for more sophisticated techniques should be performed daily or for every patient and each fraction.2, 5, 6
During the whole treatment, there are many things which can change: patient's anatomy, patient position, linac settings: MLC, output or geometry. To ensure that the patient is adequately irradiated, dosimetry verification should be performed daily, throughout the entire therapy.
There are some dosimetric tools for daily verification of dynamic techniques, such as Dolphin®, IBA Dosimetry), PerFraction® (Sun Nuclear), DAVID® (PTW), or recently very popular the transit dosimetry method.1, 2, 5, 6 These tools give some information about daily delivery of the treatment. However, they are quite expensive or there is limited availability (for example one tool at the hospital for many linacs), so it is important (especially for developing countries) to find a more available method or use a simple method for additional verification of patient treatment.
This is possible through the use of an electronic portal imaging device (EPID). This device is an integral part of most available treatment machines in radiotherapy, so there is no additional cost. In recent years, EPID has gained new importance and usefulness in radiotherapy: it can be used not only for geometric verification of patient position or for pre-verification dosimetry but also as a useful tool for QA of linac: verification of output, geometry or MLCs.3, 4 There is also interest in transit dosimetry and patient dose reconstruction based on measurement fluency map collected during treatment.2, 6 It is easy to use and can be placed under treatment couch with patients lying on it. This device can be used to collect daily fluency for patients during IMRT. The EPID-based method is cost-effective, integrated and involves no additional doses to the patient.
The aim of the work was to catch potential errors with daily EPID measurements of repeatability of the dose distribution during irradiation of IMRT patients, to verify the developed method on an anthropomorphic phantom with simple test plan and to determine the impact of the treatment plan parameters on the reproducibility of irradiation.
2. Material and methods
In our hospital, for dynamic techniques (IMRT/VMAT) quality control is carried out according to established procedures. For this study, a therapeutic line vendor by Varian was used: TPS Eclipse, Clinac (6 and 20 MV), imaging system: MV, OBI (kV, CBCT). For this study, to assess repeatability of irradiation, the fluency maps were collected with EPID aS500, which was placed under treatment couch (SDD 140 cm) with a phantom/patient placed on it (Fig. 1). All measured images were related to a reference map using the gamma evaluation method with definition created by Low at al. with considerate criteria: DTA 3 mm, DD 3% of maximum dose. Score value was defined as the ratio of points that do not meet the gamma criteria to all analyzed points:
Finally, the data was analyzed statistically using the Spearman correlation (p < 0.05 is statistical significance).
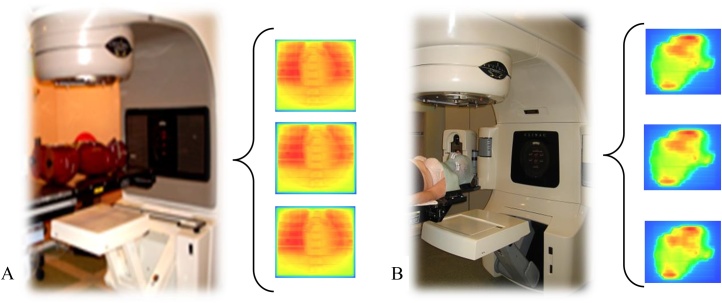
Fig. 1.
The picture of principle of (A) phantom measurement (B) daily measurement and examples of collected fluency map using an EPID.
In the first part of the study, anthropomorphic phantom (phantom case) was used, in the second, repeatability of 23 patient (clinical case) was assessed.
2.1. Phantom case
In the first stage, measurements were done for an anthropomorphic phantom (Alderson Radiation Therapy Phantom) irradiated with a homogeneous static field. The method of collecting data using an EPID device and the possibility of detecting errors in positioning were developed. Images were collected for two gantry angles: 0 and 90 degrees and the phantom was shifted within range from 0 mm to 12 mm in the lateral, longitudinal and vertical directions. In position 0 mm, the reference image was measured. To estimate differences between images and compare it, the gamma method was used.
2.2. Clinical case
Next, for 23 patients, the pelvis (P) and head and neck (H&N) regions, images were collected with an EPID device for each IMRT subfield daily and compared to reference images using the gamma method (DTA 3 mm, DD 3%). Patients were positioned using the kV/MV/CBCT system before each treatment session, according to hospital procedures or weekly on average. Images collected during the first treatment sessions for each subfield were established as a reference fluency map. All measured fluency maps were associated with proper reference maps and analyzed using the gamma evaluation method. Table 1 presents the number of patients, mean number of fields, mean number of sessions during which images were successfully collected and total number of images to investigate. Additionally, the correlation between median score and gamma coefficient values for each subfield and parameters of plan (field size, time of treatment) or pre-treatment verification results were assessed.
Table 1.
Number of patient, mean number of fields, mean number session during which images were successfully collected and total number of images to analyzed.
| Region | Imaging method | Number of patients | Mean number of fields per patients | Mean number of fractions per patients for collected data | Total data to analyze |
|---|---|---|---|---|---|
| P | kV, CBCT | 5 | 11 | 18 | 999 |
| MV | 11 | 9 | 20 | 2040 | |
| H&N | MV | 7 | 11 | 21 | 1588 |
3. Results and discussion
3.1. Phantom case
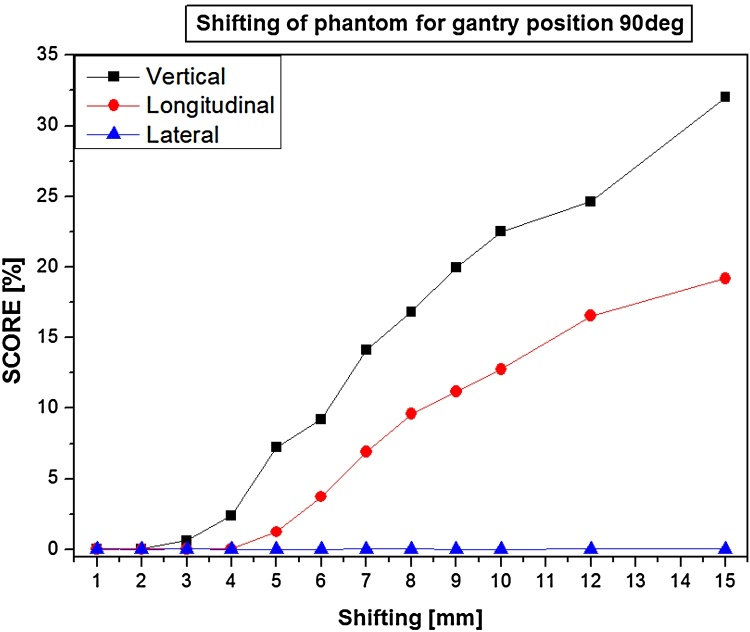
Results show a correlation between score value and phantom position (shifted from reference), depending on gradient of tissue density in irradiated regions and gantry angles, for example (Fig. 2): there is a strong correlation for the vertical and longitudinal position but not for the lateral direction for 90 degrees (Spearman's R range: 90 degrees: Vrt: 0.999–1.000, Lng: 0.998–1.000, Lat: 0.091–0.591; 0 degree: Vrt: 0.480–0.521, Lng: 0.999–1.000, Lat: 0.967–0.998). When the phantom was shifted along the axis of the gantry-EPID, the differences are almost imperceptible. If there was a shift in one direction (Vrt or Lat), the differences could only be seen for the subfields with a respective gantry angle. The relationship between the score value and phantom displacement to the reference position depends on the complexity of the irradiated area. Areas with a higher density gradient (soft tissue – bone) will give rise to greater score value differences than those that only pass through soft tissue. The anthropomorphic phantom study has shown in a simple way, what kind of effects and results of score values we can expect with EPID measured at potential shifts during radiotherapy.
Fig. 2.
Correlation between score value (%) and position of the phantom (mm) (shifted from reference in vrt, lng and lat direction) for 90-degree gantry position.
3.2. Clinical case
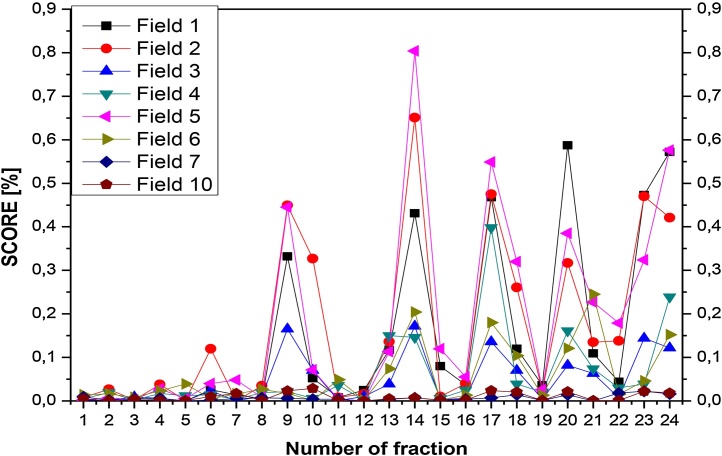
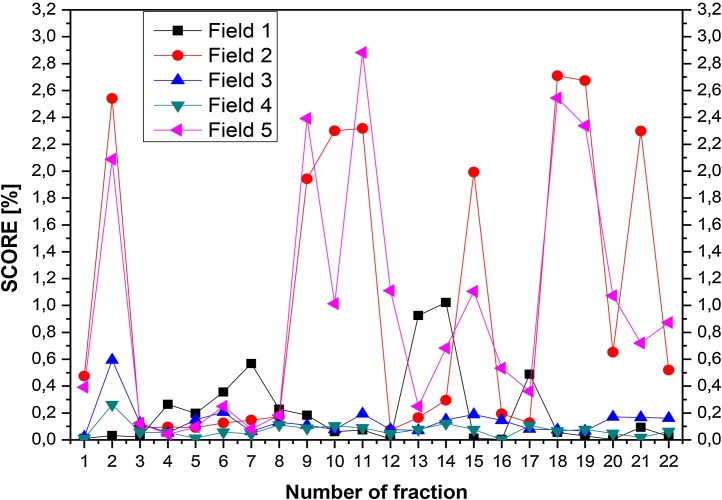
Results of the score value were studied for each patient in respect of variability of results of individual subfields and repeatability of irradiation of patients (example of reproducibility of patient with H&N (Fig. 3) and with P (Fig. 4) are presented. The results of repeatability depend on many parameters, so each patient was scrutinized individually. For patient study, the reproducibility of irradiation is affected by: the linac output (especially, the precision of the MLC), the internal movements or changes in anatomy of the patient and above mentioned (phantom case) geometrical position of the patient.
Fig. 3.
Results for head and neck: example of repeatability of score for each field for consecutive patient's fractions.
Fig. 4.
Results for pelivis: example of repeatability of score for each field for consecutive patient's fractions.
The example of a patient with H&N was presented in Fig. 3. The plan consists of 8 fields, patient's irradiation was observed to be repeatable for the first 8 registered fractions (less than 0.1% of score value), then it was getting worse. Generally, with longer time from computed tomography, which was used for creating treatment plan and calculations, the results were worse, but none them exceeded 1% of score value. Moreover, it is obvious that some fields are more repeatable and we could predict before treatment to which we should pay more attention.
For the pelvis region (example on Fig. 4), the plan consists of 5 fields, and the results were a bit worse than for H&N, probably because of worse immobilization, but still the 2.5% score was not exceeded. What is interesting, in the case of two symmetric fields: 2 (50 degrees of gantry angle) and 5 (310 degrees of gantry angle) vary considerably. Possibly, because these fields partly cover the regions with the femoral heads and better fixation should be used.
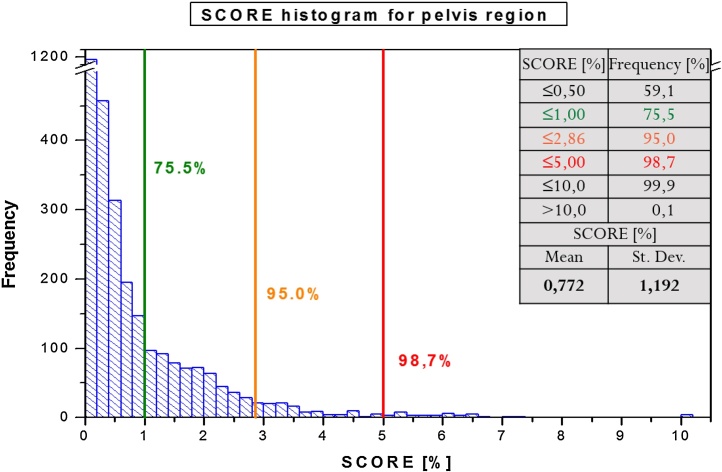
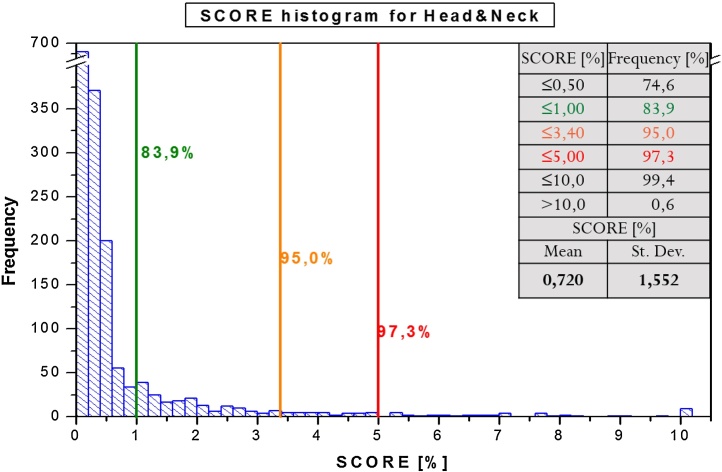
For the clinical case, score results (mean and standard deviation) were obtained for individual tumor regions as below: (P) 0.786 ± 1.046, (H&N) 0.720 ± 1.552. For most evaluated cases, score values were below 1%: (P) 75.5% (Fig. 6) and (H&N) 83.9% (Fig. 5) of analyzed fields. 95% of all evaluated data reached the score below: (P) 2.86% and (H&N) 3.40%.
Fig. 6.
Score value histogram for all analyzed patients with pelvis region.
Fig. 5.
Score value histogram for all analyzed patients with H&N region.
The relationship between the results of the analysis of daily collected images and the results of pre-verification, field size and irradiation time is shown. Table 2 presents the correlation between: a median score values and results of the pre-treatment IMRT verification for each analyzed subfield, mean gamma for H&N (p = 0.00), P (p = 0.00), score value H&N (p = 0.00); P (p = 0.10). There is also a correlation between median score values for each field and its size (for both H&N and P: p = 0.00) and time of radiation H&N (p = 0.01), P: (p = 0.00).
Table 2.
Results: median gamma and score values versus chosen parameters. Spearman correlation (p < 0.05 is statistically significant values and denoted in bold).
| Region | Parameters | Field size X × Y (cm2) | Time of treatment (MU) | Pre-treatment gamma result | Pre-treatment score result |
|---|---|---|---|---|---|
| Pelvis | Median gamma | 0.6083 | 0.0034 | 0.6530 | 0.0106 |
| Median score | 0.0000 | 0.0000 | 0.0013 | 0.1004 | |
| H&N | Median gamma | 0.0000 | 0.3567 | 0.0001 | 0.0015 |
| Median score | 0.0000 | 0.0069 | 0.0000 | 0.0000 | |
Because of the low invasiveness of the method (no additional dose), it can be used as extra information demonstrating the correctness of the radiotherapy procedure. However, the result of the analysis may have the EPID response, so extra care should be taken for the device and additional calibration procedures should be considered to minimize the effect of fluctuations of the device response on collected data. In addition, it is important to consider the time-consuming nature of the method and the absence of dose distribution reconstructed in the patient CT scans, but only the two-dimensional distribution composed after the radiation pass through the patient's body, treatment couch and accessories. Nevertheless, it can give an indication of the need of use of: additional patient immobilization, the use of more frequent or advanced imaging devices, or facilitate the decision to do new CT scan and changed treatment plans. EPID-based verification method is forward-looking, especially after the introduction of automated data analysis and could improve the quality control of radiotherapy process for dynamic techniques. In the future, more restricted criteria for gamma analysis should be considered for a larger group of patients and a detailed response threshold for each tumor site should be set.
4. Conclusions
The results confirm that for good repeatability of patients’ irradiation both positioning (phantom case) and physiological motion (patient case) are very important and can have a significant influence on variability of dose distributions. There were better reproducibility results for the irradiated H&N region than for the pelvic region, mainly due to more accurate immobilizing of patients. There is a correlation between the results of the analysis of daily repeatable of patient irradiation based on EPID and the results of pre-verification, field size and irradiation time. Because of the low invasiveness of the method (no additional dose), it can be used as extra information demonstrating the correctness of the radiotherapy procedure. EPID-based verification is forward-looking, especially after the introduction of automated data analysis.
Conflict of interest
None declared.
Financial disclosure
The study was granted from Greater Poland Cancer Centre (Contract No. 1 of 12.05.2009).
References
- 1.Rozendaal R.A., Mijnheer B.J., Hamming-Vrieze O., Mans A., van Herk M. Impact of daily anatomical changes on EPID-based in vivo dosimetry of VMAT treatments of head-and-neck cancer. Radiother Oncol. 2015;116(July (1)):70–74. doi: 10.1016/j.radonc.2015.05.020. Epub 2015 June 30. [DOI] [PubMed] [Google Scholar]
- 2.Mijnheer B.J., González P., Olaciregui-Ruiz I., Rozendaal R.A., van Herk M., Mans A. Overview of 3-year experience with large-scale electronic portal imaging device-based 3-dimensional transit dosimetry. Pract Radiat Oncol. 2015;5(November–December (6)):e679–e687. doi: 10.1016/j.prro.2015.07.001. Epub 2015 July 9. [DOI] [PubMed] [Google Scholar]
- 3.Sumida I., Yamaguchi H., Das I.J. Intensity-modulated radiation therapy dose verification using fluence and portal imaging device. J Appl Clin Med Phys. 2016;17(January (1)):259–271. doi: 10.1120/jacmp.v17i1.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh E.S., Hansen K.S., Kent M.S., Saini S., Dieterich S. Can a commercially available EPID dosimetry system detect small daily patient setup errors for cranial IMRT/SRS? Pract Radiat Oncol. 2017;7(July–August (4)):e283–e290. doi: 10.1016/j.prro.2016.12.005. Epub 2016 December 24. [DOI] [PubMed] [Google Scholar]
- 5.Peca S., Brown D.W., Smith W.L. A simple method for 2-D in vivo dosimetry by portal imaging. Technol Cancer Res Treat. 2017;16(6):944–955. doi: 10.1177/1533034617711354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olaciregui-Ruiz I., Rozendaal R., van Oers R.F.M., Mijnheer B., Mans A. Virtual patient 3D dose reconstruction using in air EPID measurements and a back-projection algorithm for IMRT and VMAT treatments. Phys Med. 2017;37(May):49–57. doi: 10.1016/j.ejmp.2017.04.016. Epub 2017 April 21. [DOI] [PubMed] [Google Scholar]
- 7.Low D.A., Dempsey J.F. Evaluation of the gamma dose distribution comparison method. Med Phys. 2003;30(September (9)):2455–2464. doi: 10.1118/1.1598711. [DOI] [PubMed] [Google Scholar]
- 8.Slosarek K., Szlag M., Bekman B., Grzadziel A1. EPID in vivo dosimetry in RapidArc technique. Rep Pract Oncol Radiother. 2010;15(February (1)):8–14. doi: 10.1016/j.rpor.2010.01.003. eCollection 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piermattei A., Fidanzio A., Stimato G. In vivo dosimetry by an aSi-based EPID. Med Phys. 2006;33(November (11)):4414–4422. doi: 10.1118/1.2360014. [DOI] [PubMed] [Google Scholar]