Abstract
Bacteriophages are an attractive alternative to fecal indicator bacteria (FIB), particularly as surrogates of enteric virus fate and transport, due to their closer morphological and biological properties. Based on a review of published data, we summarize densities of coliphages (F+ and somatic), Bacteroides spp. and enterococci bacteriophages (phages) in individual human waste, raw wastewater, ambient fresh and marine waters and removal through wastewater treatment processes utilizing traditional treatments. We also provide comparisons with FIB and enteric viruses whenever possible. Lastly, we examine fate and transport characteristics in the aquatic environment and provide an overview of the environmental factors affecting their survival. In summary, concentrations of bacteriophages in various sources were consistently lower than FIB, but more reflective of infectious enteric virus levels. Overall, our investigation indicates that bacteriophages may be adequate viral surrogates, especially in built systems, such as wastewater treatment plants.
Keywords: bacteriophage, viruses, ambient water, waste, wastewater treatment, fecal indicators
Introduction
Due to intrinsic economic, aesthetic and recreational benefits, an estimated 39% of the United States population, and over 50% of the global population lives in close proximity to rivers, lakes or oceans (Kummu et al. 2011; National Oceanic and Atmospheric Administration 2013). Enteric pathogens can enter aquatic environments through wastewater treatment plants (WWTPs) effluents, industrial waste, concentrated animal feeding operations or by more imperceptible means such as undocumented septic system failures, leaking sewers, as well as from agricultural and wild animals. Human excreta, however, are the primary source of infectious enteric viruses of potential concern to recreators, due to high shedding rates of infected individuals (Osborne et al. 2015; Taniuchi et al. 2015; Sabria et al. 2016) and low dose required for illness (Atmar et al. 2014; Messner et al. 2014). Since enteric viruses are the leading etiologic agents of waterborne disease outbreaks in recreational water settings (Sinclair et al. 2009), exposure to human waste (e.g. untreated sewage, secondary disinfected wastewater effluent, primary wastewater effluent) represents the highest manageable pathogen risk to public health (Soller et al. 2010a; Soller et al. 2010b). While the health risk resulting from exposure to cattle manure may not be substantially different due to the presence of zoonotic pathogens, most other animal sources tend to be of lesser concern (Soller et al. 2010b; Soller et al. 2014).
Due to the difficulties associated with direct detection of pathogens in water, the potential presence of fecal pollution is typically assessed by using fecal indicator bacteria (FIB) which include, fecal coliforms, Escherichia coli and enterococci (Ashbolt 2001). The utility of culture-based assessments of FIB for use in protecting public health, however, has limitations. Since FIB reside in the gastrointestinal tract of many different animals, they cannot be used to distinguish between fecal contamination sources. FIB also display different fate and transport characteristics within waste treatment infrastructure and natural aquatic environments when compared to that of viral and protozoan pathogens (Ashbolt 2001; Boehm et al. 2009a). Bacteriophages are viruses that are dependent on a bacterial host for replication, and those infecting FIB or other commensal intestinal species (e.g. Bacteroides) are subsequently shed by hosts and follow similar routes of diffusion into the environment to that of enteric viral pathogens. Furthermore, bacteriophages have similar morphological characteristics to those of many enteric pathogenic viruses suggesting that they can better mimic their survival (Payment et al. 2001; Payment and Locas 2011). Therefore, bacteriophage have been considered potentially attractive surrogates of viral pathogens because of their similarity in persistence in both built environments (e.g. WWTPs) as well as recreational waters. In this review, we focus on E. coli, Bacteroides spp., and enterococci bacteriophages to explore their potential as fecal indicators.
Bacteriophages infecting E. coli, Bacteroides spp. and enterococci
Coliphages
Coliphages are viruses that have the ability to infect E. coli, and are split into two categories based on the route of bacterial host infection. Somatic coliphages attach through specific protein receptors found on the outer host cell membrane (Kott 1974), while male specific (F+) coliphages (F-RNA & F-DNA) infect via the host’s sex (F) pili (Davis 1961). They are the most extensively studied phages for considerations as possible indicators of fecal pollution and surrogates for viral fate and transport (Skraber et al. 2002; Cole et al. 2003; Duran et al. 2003; Skraber et al. 2004a) and are a part of multiple regulatory frameworks involving monitoring of groundwater (United States Environmental Protection Agency 2006), biosolids (Department of Environment and Conservation 2012), water recycling (Queensland Government Environmental Protection Agency 2005; North Carolina Environmental Quality 2011) and aquaculture practices (Food and Drug Administration and Interstate Shellfish Sanitation Commission 2015). In addition, coliphages are now being considered for monitoring of recreational waters (United States Environmental Protection Agency 2015; 2016). Please see recent reviews regarding coliphages as model organisms (Jofre et al. 2016) and possible indicators of fecal contamination (United States Environmental Protection Agency 2015).
Somatic coliphages are classified into four major taxonomic groups, Myoviridae (linear double-stranded DNA), Siphoviridae (linear double-stranded DNA), Podoviridae (linear double- stranded DNA), Microviridae (circular double-stranded DNA); with the F+ divided into Leviviridae (single-stranded RNA), Inoviridae (single-stranded DNA) and Tectiviridae (double-stranded DNA) (Lute et al. 2004; Mesquita et al. 2010). While somatic coliphages are typically detected using E. coli, they have also been known to also infect Klebsiella and Shigella spp. (Leclerc et al. 2000; Muniesa et al. 2003). Among the F+ coliphage families, F-DNA coliphages belonging to Inoviridae have received less attention as water quality indicators since they are not as abundant as F-RNA coliphages (Leviviridae), and morphologically are less similar to enteric viruses (Furuse 1987; Leclerc et al. 2000). Based on the nucleotide sequence similarities, F-RNA coliphages belonging to the family Leviviridae, are subdivided into two genera Levivirus and Allolevivirus (King et al. 2011). Considering serological properties, and physiochemical measurements, Levivirus is further subdivided into genogroups I and II, and Allolevivirus into III and IV (Vinje et al. 2004; Friedman et al. 2009). Further studies positively link certain genogroups to a specific source of fecal contamination; human excreta largely contain higher proportions of genogroups II and III, while non-human sources (bovine, swine and avian) predominantly, but not exclusively harbor genogroups I and IV (Griffin et al. 2000; Cole et al. 2003; Noble et al. 2003). However, association of F+ genotypes and fecal sources is imperfect as cross-reactivity has been recorded (Hsu et al. 1995; Stewart-Pullaro et al. 2006; Stewart et al. 2006; Wolf et al. 2010; Hartard et al. 2015).
Bacteroides spp. phages
Bacteroides are Gram-negative, obligate anaerobic, pleomorphic rod-shaped bacteria that are commonly found in high concentrations in both human and other animal feces (Harwood et al. 2014). Bacteriophages infecting Bacteroides have been extensively studied to investigate their potential for microbial source tracking (MST) (Gomez-Donate et al. 2011; Nnane et al. 2011; Harwood et al. 2013; Jofre et al. 2014; Diston et al. 2015; Venegas et al. 2015). Some Bacteroides spp. host strains (HSP40, RYC2056 and RYC4023) are useful in discriminating phages from different animal fecal sources (Gomez-Donate et al. 2011), however geographical limitations (Cornax et al. 1990; Grabow et al. 1995; Araujo et al. 1997), cross-reactivity with domestic animal fecal samples (Gomez-Donate et al. 2011), or otherwise low and highly variable detection rates (Tartera and Jofre 1987; Tartera et al. 1989) have prompted research into other more applicable Bacteroides spp. strains. B. thetaiotaomicron (GA17) and B. fragilis (GB124) have been identified as closely associated with human fecal pollution sources for MST purposes (Payan et al. 2005; Ebdon et al. 2012). Distribution of GA17 bacteriophages seems to be largely limited to Europe, although detectable levels have also been reported in Tunisia and Columbia (Blanch et al. 2006; Sirikanchana et al. 2014; Venegas et al. 2015; Yahya et al. 2015), while B. fragilis (GB124) seems to be somewhat more widespread, but suffers from lower concentrations in sewage and environmental waters (Ebdon et al. 2007; Nnane et al. 2011; Ebdon et al. 2012; Harwood et al. 2013; McMinn et al. 2014; Diston and Wicki 2015). Efforts in identifying new Bacteroides spp. bacteriophage hosts continue with the identification of strains that can potentially differentiate between human feces (ARABA 84, CA8) versus animal wastes (RBA 63 and KBA 60) (Wicki et al. 2011; Diston and Wicki 2015; Venegas et al. 2015).
Enterococci phages
Intestinal Enterococcus spp. are facultatively anaerobic, Gram-positive cocci that are abundant in both human and animal feces. Some species (e.g. E. faecalis and E. faecium) have been identified as being frequently found in human intestines, while members of other species (E. casseliflavus, E. mundtii and E. gallinarum) typically reside in non-human hosts (recently reviewed in (Byappanahalli et al. 2012)). This bacterial host strain association suggests that the resulting enterophages could be used for MST purposes (Bonilla et al. 2010; Santiago-Rodriguez et al. 2010; Santiago-Rodriguez et al. 2013). Studies have reported detection of E. faecalis phages in human feces, while absent from non-human sources, so understandably the majority of enterophage MST research has utilized this bacterial species (Bonilla et al. 2010; Santiago-Rodriguez et al. 2010; Purnell et al. 2011; Santiago-Rodriguez et al. 2013). Enterophages have been shown to have survival times similar to human enteric viruses in both fresh and marine waters, however, the majority of this research has been restricted to tropical and subtropical regions of the world (Bonilla et al. 2010; Santiago-Rodriguez et al. 2012; Santiago-Rodriguez et al. 2013). To determine the utility of enterophages as indicators of fecal pollution and enteric virus surrogates, future studies are needed outside this limited geographical area.
Indigenous bacteriophages in wastewater and environmental waters
Our literature search primarily focused on manuscripts (a minimum of two) that reported quantifiable bacteriophage densities rather than presence/absence or percentage data. If available, we also included bacterial indicator (FIB) and viral pathogen measurements (infectious enteric viruses, molecular signal for Adenoviruses and Noroviruses) when reported from the same samples used to generate bacteriophage data. Infectious enteric viruses in this context refers to those organisms that can be grown in continuous mammalian cell lines. The studies included here that reported on the infectious viruses predominantly relied on Buffalo Green Monkey (BGM) kidney cell line, although some also used rhabdo(myo)sarcoma, Human Caucasian Colon Adenocarcinoma (Caco-2) or African Green Monkey kidney (MA-104) lines. Data was collected from text, tables, supplemental materials or estimated from figures when not available otherwise. We collected the mean of each data set from which an overall mean was calculated per organism and/or matrix. In instances where there was no detectable data reported, we substituted it with a zero. Concentrations of all microorganisms were log10 transformed and normalized to 100 mL, irrespective of how the data was originally reported. When necessary log10 reduction values were determined using the following formula: log10 reduction = log10T0−log10TN where T0 is the concentration at the beginning of the wastewater treatment process and TN is the concentration at the end of the treatment stage or the entire treatment train. Lastly, it is important to highlight various assumptions we employed when collecting the data. We assumed that all methods regardless of the protocol used or the intended microbial target perform equally well while also assuming that matrix (human and animal waste, wastewater, freshwater and marine water), geographic location and seasonality did not affect method performance. Because of these limitations we are unable to define any conclusive relationships in the gathered data, but focus instead on describing general trends we observed.
Our review included data from a total of 89 published manuscripts from five continents and 25 countries including Argentina, Austria, Canada, China, Columbia, Cyprus, Finland, France, Germany, Greece, Ireland, Israel, Italy, Japan, Malaysia, Netherlands, Singapore, South African Republic, South Korea, Spain, Sweden, Switzerland, Tunis, United Kingdom and United States. Occurrence data included values from human and non-human excreta, environmental waters as well as WWTP inactivation studies of FIB, coliphages (somatic and F+), B. fragilis bacteriophages (RYC2056, GB124, HSP40), B. thetaiotamicron bacteriophages (GA17), enterophages infecting E. faecalis and viral pathogens (infectious enteric viruses as well as Noroviruses and Adenoviruses quantified via qPCR).
Occurrence in feces and wastewaters
Human fecal sources
The focus of this section is to primarily review the concentrations of indigenous viral indicators, while including bacterial indicators as well as human viral pathogens measured in untreated wastewater. Our emphasis on untreated wastewater rather than human fecal matter and other on-site collection/disposal systems (e.g. septic tanks) was governed by the preponderance of wastewater data in the published literature. When available, studies indicate that concentrations of bacteriophage were typically lower in feces of individual humans and from on-site systems when compared to centralized infrastructure (such as WWTPs) (Calci et al. 1998; Lucena et al. 2003; Harwood et al. 2013; Diston and Wicki 2015). While standard methods are generally used for fecal indicator enumeration, typically little to no data was reported for recoveries from the matrices studied, hence adding uncertainties to reported concentrations.
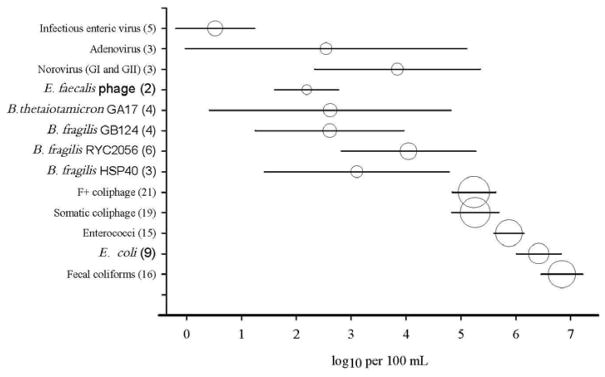
Overall, concentrations of FIB were routinely higher compared to those of bacteriophages, while human viral pathogen levels tended to be the lowest (Figure 1). Of the FIB, mean fecal coliform concentrations (log10 6.84 ± 0.77 CFU/100 mL) were the highest and ranged from log10 4.74 −8.87 per 100 mL, followed by E. coli which ranged from log10 6.34−7.00 per 100 mL (mean log10 6.42 ± 0.63). Mean enterococci concentrations were the lowest of the three (log10 5.88 ± 0.55) and ranged from log10 4.4−6.91 per 100 mL (Figure 1).
Figure 1.
Mean concentrations and 95% confidence intervals of FIB and bacteriophages (expressed as CFU, PFU or MPN), as well as viral pathogens (expressed as MPN for infectious viruses or genomic copies for all others) in untreated wastewater. The size of the symbol is proportionate to the number of studies* (given in parentheses) used to derive the data.
*References: (Tartera et al. 1989; Calci et al. 1998; Chauret et al. 1999; Puig et al. 1999; Baggi et al. 2001; Quinonez-Diaz et al. 2001; Rose et al. 2001; Uemura et al. 2002; Alcaide et al. 2003; Lucena et al. 2003; Queralt et al. 2003; Veschetti et al. 2003; Lucena et al. 2004; Payan et al. 2005; Blanch et al. 2006; Haramoto et al. 2006; Ebdon et al. 2007; Zhang and Farahbakhsh 2007; Reinoso et al. 2008; Petrinca et al. 2009; Boczek et al. 2010; Bonilla et al. 2010; Fu et al. 2010; Santiago-Rodriguez et al. 2010; Vijayavel et al. 2010; Wu et al. 2010; Ebdon et al. 2012; Flannery et al. 2012; Francy et al. 2012; Muniesa et al. 2012a; Harwood et al. 2013; Pradhan et al. 2013; Santiago-Rodriguez et al. 2013; McMinn et al. 2014; Haramoto et al. 2015; Montazeri et al. 2015).
When bacteriophage concentrations were examined, both coliphage types (somatic and F+) were generally higher than any Bacteroides spp. or enterococci phages (Figure 1). The observed mean concentrations for somatic and F+ were log10 5.26 ± 0.96 (range: log10 1.25–8.00) and log10 5.24 ±0.92 (range: none-detected −log10 8.00) PFU per 100 mL, respectively (Figure 1); levels similar to those reported recently in a comprehensive systematic literature review (Eftim 2016). Of the Bacteroides spp. bacteriophages, RYC2056 type was the most numerous and it ranged from none- detected to log10 4.92 with an average of log10 4.05 ± 1.40 PFU per 100 mL. Levels of B. fragilis HSP40 bacteriophage followed with an average of log10 3.10 ± 1.49 (ranging from log10 1.60–4.65) PFU per 100 mL (Figure 1). The average of two remaining Bacteroides spp. phages were lower (log10 2.62 ± 2.25 and log10 2.61 ± 1.38) and ranged from no detection for both groups to log10 5.04 and log10 4.36 PFU per 100 mL for GA17 and GB124, respectively (Figure 1). It should be noted that GA17 and GB124 averages are likely to be affected by an uneven geographic distribution, most notably in the areas where they are not endemic. Bacteriophages infecting E. faecalis exhibited the lowest average concentration (log10 2.19 ± 0.52), ranging from log10 1.15 −2.89 PFU per 100 mL.
Levels of infectious human enteric viruses were clearly the lowest of all microorganisms examined and averaged log10 0.52 ± 0.82, with a range of log10 0.64–2.41 MPN per 100 mL of wastewater (Figure 1). Noting again, that recovery data was generally not provided, yet is known to be highly variable (Petterson et al. 2015). Human viral gene targets measured by qPCR methods were considerably higher compared to culturable infectious virus concentrations, averaging log10 3.84 ± 1.33 for Norovirus (combined average of genogroups I and II) and log10 2.54 ± 2.27 for Adenovirus (Figure 1). Norovirus levels are comparable to those recently reported in a systematic review and meta-analysis detailing Norovirus occurrence in raw sewage (Eftim et al. 2017).
Non-human fecal sources
This section focuses on bacteriophage data measured in animal solid waste as well as animal wastewater and/or fecal slurries. Animal solid waste included samples collected from 21 species belonging to ruminants (cattle, elk, sheep, deer, goat), non-ruminant herbivores (donkey, horse, rabbit), omnivores (baboons, gorillas, orangutans, pigs, vervet monkeys), carnivores (cat, dog) and birds (chicken, duck, goose, pigeon, seagull, turkey). Animal fecal slurries and wastewater represented a mixture of individual samples, in many instances from multiple animal species.
Similar to untreated municipal wastewater, somatic and F+ coliphages were present in the highest concentrations, along with B. fragilis RYC2056 phage. In animal fecal slurries and wastewater, average concentrations were log10 4.67 ± 0.96, 4.39 ± 0.28 and log10 3.61 ±1.89 per 100 mL for F+, RYC2056 and somatic coliphages, respectively (Hill and Sobsey 1998; Puig et al. 1999; Hill and Sobsey 2001; Hill et al. 2002; Payan et al. 2005; Blanch et al. 2006; Ebdon et al. 2007; Muniesa et al. 2012a; Harwood et al. 2013; McMinn et al. 2014).
In animal solid waste, somatic coliphages were higher (log10 2.55 ±2.05 per gram) compared to F+ (log10 1.29 ±1.32 per gram), but there was not enough data to compare for RYC2056 (Grabow et al. 1995; Calci et al. 1998; Miller et al. 1998; Muniesa et al. 1999; Yee et al. 2006; McMinn et al. 2014; Diston and Wicki 2015). The other Bacteroides spp. phages were mainly measured in slurry and wastewater and their concentrations ranged from log10 1.52 ± 1.95, log10 0.69 ± 0.80 and log10 0.23 ± 0.63 per 100 mL for GA17, GB124 and HSP40, respectively (Payan et al. 2005; Ebdon et al. 2007; Muniesa et al. 2012b; Harwood et al. 2013; McMinn et al. 2014; Diston and Wicki 2015). In addition, GB124 phage was not detected in any solids examined (McMinn et al. 2014; Diston and Wicki 2015). Enterophage was also not detected in any animal solid samples, but there was insufficient information for slurry and wastewater (Bonilla et al. 2010; Santiago-Rodriguez et al. 2010; Santiago-Rodriguez et al. 2013).
Removal through conventional municipal wastewater treatment processes
In this subsection, our interest was to describe microbial removal at full-scale wastewater treatment, and therefore we excluded all laboratory and bench scale studies. Due to paucity of the available data we pooled together all Bacteroides spp. phages into one group. Since there was not enough data to describe removals through individual stage(s) of the wastewater treatment train we pooled data and present it as log10 reduction irrespective of any particular unit of operation (e.g. primary treatment, secondary treatment, disinfection). Even though there was uneven number of studies per organism, the distribution of papers describing removal through a particular unit of operation for each microorganism was relatively consistent. When grouped by the absence or presence of disinfection, the percentage of manuscripts belonging to each category was 54/47% (fecal coliforms), 63/37% (E. coli), 57/43% (enterococci), 74/26% (somatic coliphages), 71/29% (F+ coliphages), 68/32% (Bacteroides spp. bacteriophages), 50/50% (Adenoviruses), 44/56% (Nororvirus) and 81/29% (infectious enteric viruses). The pooled data was subjected to Kruskal-Wallis analysis of variance (ANOVA) with Dunn’s post-hoc test performed using Sigma Plot version 13.0 (Systat Software, San Jose, CA) to determine whether statistically significant difference (P > 0.05) exists in log10 reductions among different organisms (Table 1).
Table 1.
Kruskal-Wallis ANOVA with Dunn’s post-hoc tests on log10 reduction values through various wastewater treatment processes for bacterial and viral indicator organisms and pathogens obtained from published literature
| FC (38) | E. coli (32) | Enterococci (36) | SOMPH (111) | F+PH (102) | BSPH (22) | Adenovirus (10) | Norovirus (28) | Inf. virus (26) |
|---|---|---|---|---|---|---|---|---|
| FC | > 0.05 | > 0.05 | < 0.001 | 0.001 | 0.007 | > 0.05 | > 0.05 | < 0.001 |
| E. coli | > 0.05 | 0.010 | 0.019 | 0.040 | > 0.05 | > 0.05 | < 0.001 | |
| Enterococci | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | 0.006 | ||
| SOMPH | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |||
| F+PH | > 0.05 | > 0.05 | > 0.05 | > 0.05 | ||||
| BSPH | > 0.05 | > 0.05 | > 0.05 | |||||
| Adenovirus | > 0.05 | > 0.05 | ||||||
| Norovirus | > 0.05 |
Values in parentheses represent number of observations used for ANOVA, statistically significant values are bolded. FC (fecal coliforms), SOMPH (somatic coliphages), F+PH (F+ coliphages), BSPH (Bacteroides spp. phages).
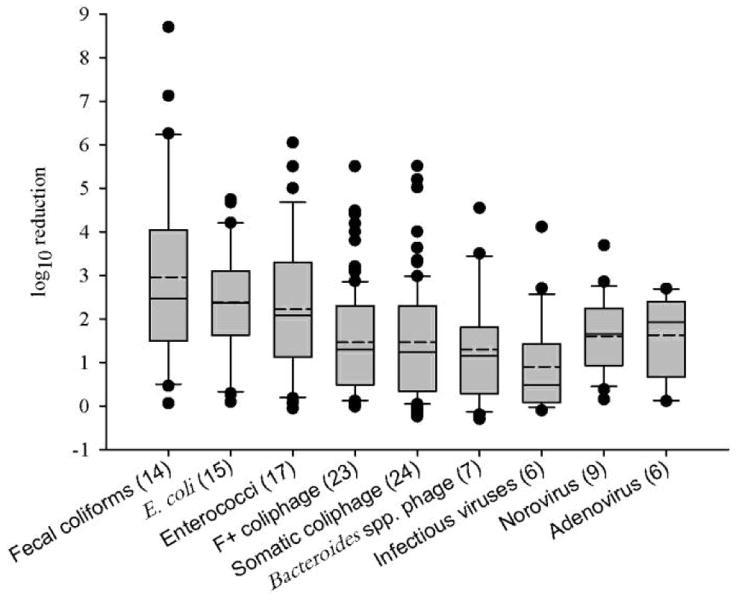
Overall, the greatest removal was achieved for culturable FIB, followed by molecular signal for pathogenic viruses, bacteriophages and finally infectious human enteric viruses (Figure 2). Specifically, reported and/or calculated log10 reductions (± standard deviation) for FIB were as follows: 2.96 ± 2.07, 2.38± 1.26 and 2.22 ± 1.61 for fecal coliforms, E. coli and enterococci, respectively (Figure 2). The average removal of q(RT)-PCR signal of Adenoviruses and Noroviruses was approximately one log10 lower compared to culture-based FIB and measured 1.62 ± 0.93 and 1.60 ± 0.85, respectively (Figure 2). Bacteriophage removal was similar to that for human viruses and averaged 1.46 ±1.24, 1.46 ± 1.18 and 1.29 ± 1.33 for somatic and F+ coliphages and Bacteroides spp. phages, respectively (Figure 2). Infectious human enteric virus removal was the lowest, averaging 0.90 ± 1.01 log10 reduction (Figure 2).
Figure 2.
Reported and/or calculated log10 reduction of FIB, bacteriophages and viral pathogens through wastewater treatment processes. Reduction data per organism type are pooled irrespective of the particular unit of operation (e.g. primary treatment, secondary treatment, disinfection). Box is delimited by 25th and 75th percentiles, solid line within the box represents median and dashed line represents average. Whiskers are 10th and 90th percentile values. Values outside of the range are depicted as filled dots. Number in parenthesis indicate number of studies* used to derive the data.
*References: (Bonadonna et al. 1993; Chauret et al. 1999; Baggi et al. 2001; Bourrouet et al. 2001; Rose et al. 2001; Tanji et al. 2002; Lucena et al. 2004; Harwood et al. 2005; Haramoto et al. 2006; Mandilara et al. 2006; Ottoson et al. 2006; Zhang and Farahbakhsh 2007; Carducci et al. 2008; Costan-Longares et al. 2008; Gomila et al. 2008; Petrinca et al. 2009; Aw and Gin 2010; Boczek et al. 2010; Fu et al. 2010; Ebdon et al. 2012; Flannery et al. 2012; Francy et al. 2012; Muniesa et al. 2012a; Hata et al. 2013; Pradhan et al. 2013; Kitajima et al. 2014; Ulbricht et al. 2014; Dias et al. 2015; Haramoto et al. 2015; Montazeri et al. 2015; Yahya et al. 2015; Mayer et al. 2016).
Comparison of compiled data (Table 1) indicated that fecal coliform and E. coli log10 reductions were significantly higher compared to those of bacteriophages and infectious enteric viruses (P value range 0.019–<0.001), but there was no significant difference when compared to human viral gene targets (i.e. Adenoviruses and Noroviruses) (P>0.05). Enterococci removal was significantly greater compared to infectious enteric viruses (P=0.006), but there were no other statistically significant differences (P>0.05). Furthermore, there were no statistically significant differences when comparing removals of bacteriophages to those of infectious human enteric viruses, including Adenoviruses and Noroviruses (P>0.05). This finding suggests that bacteriophages may be better suited to act as surrogates for human viral pathogen removal through wastewater treatment processes compared to culturable FIB measurements.
Occurrence in the environmental waters
Freshwater
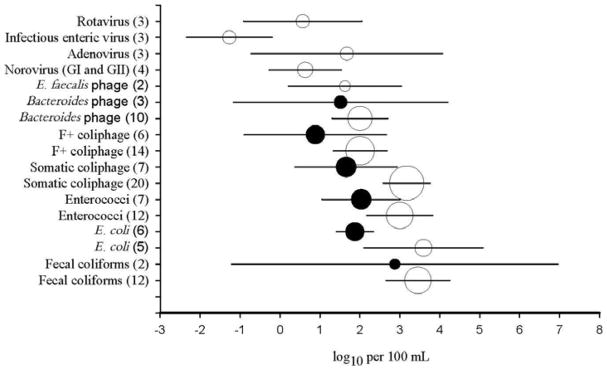
In general, freshwater concentrations of FIB were observed at higher levels than both bacteriophages and human enteric viruses. Results from a literature analysis showed mean concentrations of fecal coliforms, E. coli and enterococci atlog10 3.46 ± 1.41, log10 3.59 ±1.70 and log10 3.00 ± 1.47 CFU/100 mL, respectively (Figure 3). Among bacteriophages, somatic coliphages were detected at the highest concentrations ranging from 0.95 log10 PFU/100 mL to upwards of 5.00 log10 PFU/100 mL in more heavily impacted freshwaters with mean levels of log10 3.18 ±1.35PFU/100 mL typically observed (Figure 3). F+ coliphage levels in surface freshwaters averaged log10 2.00 ±1.29 PFU/100 mL mean levels, approximately log10 1–1.5 lower than somatic coliphages (Figure 3). While detectable levels of Bacteroides phage are found in environmental waters, they were less prevalent than somatic coliphages. In freshwaters, levels of Bacteroides phages (all types combined) were detected at mean concentrations of log10 2.00±1.12 PFU/100 mL, levels similar to that of F+ coliphages, but an order of magnitude lower than mean levels of somatic coliphages (Figure 3). Of the three Bacteroides phages, GB124 and HSP40 were detected at higher mean levels (log10 2.31±1.26 and log10 2.29±1.14 log10 PFU/100 mL, respectively) compared to RYC2056 (log10 0.9±0.7 mean PFU/100 mL). Phages infecting E. faecalis were detected at the lowest levels, averaging log10 1.63±1.02PFU/100 mL, approximately log10 .5 PFU/100 mL lower than Bacteroides phages and F+ coliphages, and ~log10 1.5 PFU/100 mL lower than that of somatic coliphages. Infectious enteric viruses were detected at lower concentrations than both FIB and bacteriophage, with mean levels of log10 −1.27 ± 0.95 PFU/100 mL reported (Figure 3). Levels of Adenovirus (log10 1.67 ± 2.11 genomic copies/100 mL), Norovirus GI and GII (log10 0.63 ± 0.92 genomic copies/100 mL) and Rotavirus (log10 0.57 ±1.30 genomic copies/100 mL) determined by q(RT)-PCR in freshwater were higher than infectious enteric virus concentrations (Figure 3).
Figure 3.
Mean concentrations and 95% confidence intervals of FIB and bacteriophages (expressed as CFU, FU or MPN), as well as viral pathogens (expressed as MPN for infectious viruses or genomic copies for all others) in freshwater (empty symbols) and marine waters (filled symbols). The size of the symbol is proportionate to the number of studies* (given in parentheses) used to derive the data.
*References: (Tartera et al. 1989; Cornax 1991; Lucena 1996; Araujo et al. 1997; Muniesa et al. 1999; Contreras-Coll et al. 2002; Duran et al. 2002; Skraber et al. 2002; Hot et al. 2003; Lucena et al. 2003; Moce-Llivina et al. 2003; Choi and Jiang 2005; Lodder and de Roda Husman 2005; Montemayor et al. 2005; Ebdon et al. 2007; Ibarluzea et al. 2007; Boehm et al. 2009b; Lodder et al. 2010; Vijayavel et al. 2010; Gomez-Donate et al. 2011; Lee et al. 2011; Nnane et al. 2011; Viau et al. 2011; Santiago- Rodriguez et al. 2012; Wyer et al. 2012; Love et al. 2014; Rezaeinejad et al. 2014; Vijayavel 2014; Vergara et al. 2015; Santiago-Rodriguez 2016).
Marine Water
In marine waters, mean concentrations of fecal coliforms, E. coli and enterococci were measured at log10 2.88 ± 2.95, log10 1.87 ± 0.58and log10 2.03 ± 1.33 CFU/100 mL, respectively (Figure 3). Overall, FIB concentrations were one to two orders of magnitude lower compared to freshwaters samples with the largest difference observed for E. coli (Figure 3). Mean somatic coliphages in marine waters (log10 1.66 ± 1.73) were up to log10 1.5 PFU/100 mL lower than the levels in freshwater, and ranged from no detection to log10 6.28 PFU/100 mL (Figure 3). Recorded concentrations of F+ coliphages in marine waters were lower than those of somatic, averaging log10 0.89±2.23 PFU/100 mL (Figure 3), an order of magnitude below levels observed in the freshwater. Bacteroides spp. phage (all types combined) concentrations in marine waters ranged from no detection to log10 4.26 PFU/100 mL, with mean concentrations of log10 1.52 ± 2.37 PFU/100 mL, log10 0.5 PFU/100 mL lower compared to freshwater (Figure 3). There is currently insufficient data detailing the concentrations of Enterococcus phages or human enteric viruses in marine water to be included in these analyses.
Factors influencing fate and transport of bacteriophages
Through various mechanisms such as combined sewer overflows (CSOs), WWTP discharges, failing septic systems, or fecal deposits, bacteriophages can enter environmental waters where they are subject to various selective pressures and environmental insults that govern their fate and transport. Here we briefly examine some biotic and abiotic factors influencing survival of bacteriophages in the environment and provide a comparison with FIB and human enteric viruses when possible. Scarcity of the available data necessitated pooling the information together irrespective of the study design (e.g. laboratory, bench scale or field study) or the coliphage origin (e.g. prototypes such as MS2 and ΦX174 as opposed to indigenous organisms).
Depending on factors such as viral surface charge, pH of the water and levels of suspended solids in water column, bacteriophages and enteric viruses are often attached to particulate matter in aquatic environments. However, the processes of viral dispersion, transport and subsequent attenuation are poorly defined (Ferguson 2003). Attachment to particles is particularly important when considering the movement since it can partition the organisms and therefore prevent them from reaching the surface or ground waters. Attachment of coliphages to particles can be highly variable (ranging from 20% to 60%) and is influenced by environmental factors, as well as heterogeneity of different bacteriophage groups (Characklis et al. 2005). This partitioning behavior seems to be somewhat similar to that of infectious enteric viruses whereby 72 to 78% of samples containing infectious virions are associated with particles as opposed to 14 to 50% found in the overlaying water column (Rao et al. 1984). Studies of coliphage transport indicate that the attachment to particles is the most important process in phage removal, while detachment rates are typically 100 to 1000 times lower (Hijnen et al. 2005), although this process can be considerably influenced by the oxygen content with greater adsorption observed in more oxic environments (Frohnert et al. 2014). By comparison, FIB attachment to particles has been reported to be somewhat more constant (20–55%) (Characklis et al. 2005), a finding that could be attributed to a more uniform isoelectric point of bacteria (Harden and Harris 1953; Jewett et al. 1995; Stevik et al. 1999).
Bacteriophage transport is also dependent on the type of soil/sediment as well as velocity of the surrounding water (Cho et al. 2016). For example, coarse-grained, heterogeneous, gravel aquifers with high velocities have low filtration capacity and to achieve estimated 7 log10 removal of MS2 bacteriophage it would require a setback distance of 3.9 km, but would require only ~129 meters in sandy fine gravel (Pang et al. 2005). The possibility of a “shielding effect” conferred by particle association (Templeton et al. 2005) combined with relatively high concentrations of coliphages (Chung 1993; Paul et al. 1993; Karim et al. 2004; Skraber et al. 2009; Yamahara et al. 2012) and enteric viruses (Smith et al. 1978; LaBelle and Gerba 1979; Rao et al. 1984) typically recorded in sediments and soils (as compared to the overlaying water columns), suggests that they can act as virus reservoirs. In comparison, FIB appear to be similarly influenced by the soil type but less affected by the velocity which is not surprising consider the straining effect of porous media (Ferguson 2003; Hijnen et al. 2005). Similar to coliphages, sediments can also act as reservoirs of FIB (Yamahara et al. 2012). Human activities, such as boating and swimming, along with changes in water currents and activities of wildlife, can resuspended and subsequently physically desorb viruses and bacteria attached to sediment particles therefore potentially increasing the viral loads in the water column (Ferguson 2003).
The effect of temperature on survival of bacteriophages is one of the most studied environmental factors. Numerous experiments demonstrated extended survival at lower temperatures in variety of matrices including sediments/soils (Hurst et al. 1980; Chung 1993; Gantzer et al. 2001; Ottosson and Stenstrom 2003), freshwaters (Long and Sobsey 2004; Skraber et al. 2004b; Yang and Griffiths 2013; Ravva and Sarreal 2016), marine water (Chung 1993), wastewater (Moce-Llivina et al. 2003; McMinn et al. 2014), groundwater (Yates et al. 1985; Gordon and Toze 2003) and tap water (Allwood et al. 2003). In general FIB decay is accelerated compared to bacteriophages and enteric viruses at all of the temperatures examined, while bacteriophages typically survived longer than infectious enteric viruses (Yates et al. 1985; Chung 1993; Gantzer et al. 2001; Allwood et al. 2003; Gordon and Toze 2003; Moce-Llivina et al. 2003; Skraber et al. 2004b; Charles et al. 2009). Somatic coliphages typically persisted longer than the F+ group (Gantzer et al. 2001; Moce-Llivina et al. 2003; McMinn et al. 2014), with different genotypes of the latter exhibiting differential survival characteristics(Long and Sobsey 2004; Muniesa et al. 2009; Yang and Griffiths 2013; Ravva and Sarreal 2016). Survival of Bacteroides spp. phages varied, with some strains behaving more similarly to F+ (Duran et al. 2002; McMinn et al. 2014), while others resembled somatic subgroup more closely (Duran et al. 2002; Moce-Llivina et al. 2003).
Another important factor for bacteriophage survival is sunlight and associated UV radiation. Irrespective of the source of radiation (simulated or ambient sunlight), reported decay of FIB, bacteriophages and enteric viruses were always lower in dark treatments as opposed to sunlight exposed ones (Sinton et al. 1999; Sinton et al. 2002; Noble et al. 2004; Silverman et al. 2013; United States Environmental Protection Agency 2015; Sun et al. 2016). Comparable to the effect of temperature, FIB decay was always greater compared to that of bacteriophages and viruses, but the magnitude of the effect on different microorganisms seems to be at least somewhat dependent on the water type, as differential decay metrics are reported for freshwater compared to marine waters (Sinton et al. 1999; Sinton et al. 2002; Silverman et al. 2013; United States Environmental Protection Agency 2015). Sunlight wavelength (UVA, UVB and visible spectrum) (Sinton et al. 1999; Sinton et al. 2002; Fisher et al. 2011; Silverman et al. 2013; United States Environmental Protection Agency 2015; Sun et al. 2016) as well as the presence of natural organic matter (Silverman et al. 2013; United States Environmental Protection Agency 2015; Sun et al. 2016) are also reported to influence the magnitude and type of bacteriophage inactivation (exogenous, direct and indirect endogenous). Lastly, it is important to note that decay studies characterizing seeded coliphage laboratory strains exhibit different transport and survival characteristics (Brion et al. 2002; Long and Sobsey 2004; Ravva and Sarreal 2016) compared to indigenous organisms. As a result, generalizations combining coliphage survival from spiking experiments and environmental isolates is not prudent.
Summary
Bacteriophages are alternative fecal indicator organisms that are better suited than FIB to act as surrogates for enteric viral pathogens presence and removal in built and natural environments. Our review suggests that removal of bacteriophages through wastewater treatment plants was more similar to that of infectious viral pathogens than FIB to infectious viral pathogens, suggesting the potential advantage of their use as alternative viral indicators in this arena. While bacteriophage densities in fresh and marine waters are lower compared to FIB, but higher than viral pathogens, the accurate assessment of their concentrations is confounded by the lack of recovery data for these matrices. Additionally, there is evidence that bacteriophage fate and transport in the ambient waters may resemble that of viral pathogens more closely than FIB and therefore bacteriophages may be promising surrogates under some environmental conditions.
Footnotes
Conflict of interest: No conflict of interest declared.
Disclaimer
The United States Environmental Protection Agency through its Office of Research and Development funded and managed the research described here. It has been subjected to Agency’s administrative review and approved for publication. The views expressed in this article are those of the author(s) and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- Alcaide L, Oron G, Gillerman L, Salgot M, Manor Y. Removal of fecal coliforms, somatic coliphages and F-specific bacteriophages in a stabilization pond and reservoir system in arid regions. Wa Sci Technol. 2003;3:177–184. [Google Scholar]
- Allwood PB, Malik YS, Hedberg CW, Goyal SM. Survival of F-specific RNA coliphage, feline calicivirus, and Escherichia coli in water: a comparative study. Applied and environmental microbiology. 2003;69:5707–5710. doi: 10.1128/AEM.69.9.5707-5710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo RM, Puig A, Lasobras J, Lucena F, Jofre J. Phages of enteric bacteria in fresh water with different levels of faecal pollution. Journal of applied microbiology. 1997;82:281–286. doi: 10.1046/j.1365-2672.1997.00354.x. [DOI] [PubMed] [Google Scholar]
- Ashbolt NJ, Grabow OK, Snozzi M. Indicators of microbial water quality. In: Fewtrell L, Bartram J, editors. Water quality: guidelines, standards and health. London, United Kingdom: IWA Publishing; 2001. pp. 289–315. [Google Scholar]
- Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Ramani S, Hill H, Ferreira J, Graham DY. Determination of the 50% human infectious dose for Norwalk virus. The Journal of infectious diseases. 2014;209:1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw TG, Gin KY. Environmental surveillance and molecular characterization of human enteric viruses in tropical urban wastewaters. Journal of applied microbiology. 2010;109:716–730. doi: 10.1111/j.1365-2672.2010.04701.x. [DOI] [PubMed] [Google Scholar]
- Baggi F, Demarta A, Peduzzi R. Persistence of viral pathogens and bacteriophages during sewage treatment: lack of correlation with indicator bacteria. Research in microbiology. 2001;152:743–751. doi: 10.1016/s0923-2508(01)01255-4. [DOI] [PubMed] [Google Scholar]
- Blanch AR, Belanche-Munoz L, Bonjoch X, Ebdon J, Gantzer C, Lucena F, Ottoson J, Kourtis C, Iversen A, Kuhn I, Moce L, Muniesa M, Schwartzbrod J, Skraber S, Papageorgiou GT, Taylor H, Wallis J, Jofre J. Integrated analysis of established and novel microbial and chemical methods for microbial source tracking. Applied and environmental microbiology. 2006;72:5915–5926. doi: 10.1128/AEM.02453-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczek LA, Johnson CH, Meckes MC. Chlorine disinfection of blended municipal wastewater effluents. Water environment research : a research publication of the Water Environment Federation. 2010;82:2373–2379. doi: 10.2175/106143010x12681059117175. [DOI] [PubMed] [Google Scholar]
- Boehm AB, Ashbolt NJ, Colford JM, Jr, Dunbar LE, Fleming LE, Gold MA, Hansel JA, Hunter PR, Ichida AM, McGee CD, Soller JA, Weisberg SB. A sea change ahead for recreational water quality criteria. Journal of water and health. 2009a;7:9–20. doi: 10.2166/wh.2009.122. [DOI] [PubMed] [Google Scholar]
- Boehm AB, Yamahara KM, Love DC, Peterson BM, McNeill K, Nelson KL. Covariation and photoinactivation of traditional and novel indicator organisms and human viruses at a sewage-impacted marine beach. Environmental science & technology. 2009b;43:8046–8052. doi: 10.1021/es9015124. [DOI] [PubMed] [Google Scholar]
- Bonadonna L, Liberti R, Volterra L. Distribution of F-specific bacteriophages and coliphages in wastewater. World journal of microbiology & biotechnology. 1993;9:34–36. doi: 10.1007/BF00656512. [DOI] [PubMed] [Google Scholar]
- Bonilla N, Santiago T, Marcos P, Urdaneta M, Domingo JS, Toranzos GA. Enterophages, a group of phages infecting Enterococcus faecalis, and their potential as alternate indicators of human faecal contamination. Water science and technology : a journal of the International Association on Water Pollution Research. 2010;61:293–300. doi: 10.2166/wst.2010.815. [DOI] [PubMed] [Google Scholar]
- Bourrouet A, Garcia J, Mujeriego R, Penuelas G. Faecal bacteria and bacteriophage inactivation in a full-scale UV disinfection system used for wastewater reclamation. Water science and technology : a journal of the International Association on Water Pollution Research. 2001;43:187–194. [PubMed] [Google Scholar]
- Brion GM, Meschke JS, Sobsey MD. F-specific RNA coliphages: occurrence, types, and survival in natural waters. Water research. 2002;36:2419–2425. doi: 10.1016/s0043-1354(01)00547-4. [DOI] [PubMed] [Google Scholar]
- Byappanahalli MN, Nevers MB, Korajkic A, Staley ZR, Harwood VJ. Enterococci in the environment. Microbiology and molecular biology reviews : MMBR. 2012;76:685–706. doi: 10.1128/MMBR.00023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calci KR, Burkhardt W, 3rd, Watkins WD, Rippey SR. Occurrence of male-specific bacteriophage in feral and domestic animal wastes, human feces, and human-associated wastewaters. Applied and environmental microbiology. 1998;64:5027–5029. doi: 10.1128/aem.64.12.5027-5029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci A, Morici P, Pizzi F, Battistini R, Rovini E, Verani M. Study of the viral removal efficiency in a urban wastewater treatment plant. Water science and technology : a journal of the International Association on Water Pollution Research. 2008;58:893–897. doi: 10.2166/wst.2008.437. [DOI] [PubMed] [Google Scholar]
- Characklis GW, Dilts MJ, Simmons OD, 3rd, Likirdopulos CA, Krometis LA, Sobsey MD. Microbial partitioning to settleable particles in stormwater. Water research. 2005;39:1773–1782. doi: 10.1016/j.watres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Charles KJ, Shore J, Sellwood J, Laverick M, Hart A, Pedley S. Assessment of the stability of human viruses and coliphage in groundwater by PCR and infectivity methods. Journal of applied microbiology. 2009;106:1827–1837. doi: 10.1111/j.1365-2672.2009.04150.x. [DOI] [PubMed] [Google Scholar]
- Chauret C, Springthorpe S, Sattar S. Fate of Cryptosporidium oocysts, Giardia cysts, and microbial indicators during wastewater treatment and anaerobic sludge digestion. Canadian journal of microbiology. 1999;45:257–262. [PubMed] [Google Scholar]
- Cho KH, Pachepsky YA, Oliver DM, Muirhead RW, Park Y, Quilliam RS, Shelton DR. Modeling fate and transport of fecally-derived microorganisms at the watershed scale: State of the science and future opportunities. Water research. 2016;100:38–56. doi: 10.1016/j.watres.2016.04.064. [DOI] [PubMed] [Google Scholar]
- Choi S, Jiang SC. Real-time PCR quantification of human adenoviruses in urban rivers indicates genome prevalence but low infectivity. Applied and environmental microbiology. 2005;71:7426–7433. doi: 10.1128/AEM.71.11.7426-7433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Sobsey MD. Comparative survival of indicator viruses and enteric viruses in seawater and sediment. Water science and technology : a journal of the International Association on Water Pollution Research. 1993;27:425–428. [PubMed] [Google Scholar]
- Cole D, Long SC, Sobsey MD. Evaluation of F+ RNA and DNA coliphages as source- specific indicators of fecal contamination in surface waters. Applied and environmental microbiology. 2003;69:6507–6514. doi: 10.1128/AEM.69.11.6507-6514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Coll N, Lucena F, Mooijman K, Havelaar A, Pierz V, Boque M, Gawler A, Holler C, Lambiri M, Mirolo G, Moreno B, Niemi M, Sommer R, Valentin B, Wiedenmann A, Young V, Jofre J. Occurrence and levels of indicator bacteriophages in bathing waters throughout Europe. Water research. 2002;36:4963–4974. doi: 10.1016/s0043-1354(02)00229-4. [DOI] [PubMed] [Google Scholar]
- Cornax R, Morinigo MA, Paez IG, Munoz MA, Borrego JJ. Application of direct plaque assay for detection and enumeration of bacteriophages of Bacteroides fragilis from contaminated-water samples. Applied and environmental microbiology. 1990;56:3170–3173. doi: 10.1128/aem.56.10.3170-3173.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornax R, Morinigo MA, Balebona MC, Castro D, Borrego JJ. Significance of several bacteriophage group as indicators of sewage pollution in marine waters. Water research. 1991;25:673–678. [Google Scholar]
- Costan-Longares A, Montemayor M, Payan A, Mendez J, Jofre J, Mujeriego R, Lucena F. Microbial indicators and pathogens: removal, relationships and predictive capabilities in water reclamation facilities. Water research. 2008;42:4439–4448. doi: 10.1016/j.watres.2008.07.037. [DOI] [PubMed] [Google Scholar]
- Davis JE, Strauss JH, Sinsheimer RL. Bacteriophage MS2: another RNA phage. Science. 1961;134:1427. [Google Scholar]
- Department of Environment and Conservation. Western Australian Guidelines for Biosolids Management. Perth; Australia: 2012. [Google Scholar]
- Dias E, Ebdon J, Taylor H. The application of removal coefficients for viruses in different wastewater treatment processes calculated using stochastic modelling. Water science and technology : a journal of the International Association on Water Pollution Research. 2015;71:1382–1388. doi: 10.2166/wst.2015.086. [DOI] [PubMed] [Google Scholar]
- Diston D, Sinreich M, Zimmermann S, Baumgartner A, Felleisen R. Evaluation of molecular- and culture-dependent MST markers to detect fecal contamination and indicate viral presence in good quality groundwater. Environmental science & technology. 2015;49:7142–7151. doi: 10.1021/acs.est.5b00515. [DOI] [PubMed] [Google Scholar]
- Diston D, Wicki M. Occurrence of bacteriophages infecting Bacteroides host strains (ARABA 84 and GB-124) in fecal samples of human and animal origin. Journal of water and health. 2015;13:654–661. doi: 10.2166/wh.2014.199. [DOI] [PubMed] [Google Scholar]
- Duran AE, Muniesa M, Mendez X, Valero F, Lucena F, Jofre J. Removal and inactivation of indicator bacteriophages in fresh waters. Journal of applied microbiology. 2002;92:338–347. doi: 10.1046/j.1365-2672.2002.01536.x. [DOI] [PubMed] [Google Scholar]
- Duran AE, Muniesa M, Moce-Llivina L, Campos C, Jofre J, Lucena F. Usefulness of different groups of bacteriophages as model micro-organisms for evaluating chlorination. Journal of applied microbiology. 2003;95:29–37. doi: 10.1046/j.1365-2672.2003.t01-1-01948.x. [DOI] [PubMed] [Google Scholar]
- Ebdon J, Muniesa M, Taylor H. The application of a recently isolated strain of Bacteroides (GB-124) to identify human sources of faecal pollution in a temperate river catchment. Water research. 2007;41:3683–3690. doi: 10.1016/j.watres.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Ebdon JE, Sellwood J, Shore J, Taylor HD. Phages of Bacteroides (GB-124): a novel tool for viral waterborne disease control? Environmental science & technology. 2012;46:1163–1169. doi: 10.1021/es202874p. [DOI] [PubMed] [Google Scholar]
- Eftim S. Systematic Literature Reviews and Development of Distributions of Viral Densities in Raw Wastewater. Presented at 2016 UNC Water Microbiology Conference.2016. [Google Scholar]
- Eftim S, Hong T, Soller J, Boehm A, Warren I, Ichihara A, Nappier S. Occurence of Norovirus in Raw Sewage- a systematic literature review and meta-analysis. Water research. 2017;111:366–374. doi: 10.1016/j.watres.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Ferguson C, Husman AM, Altavilla N, Deere D, Ashbolt N. Fate and Transport of Surface Water Pathogens in Watersheds. Critical Reviews in Environmental Science and Technology. 2003;33:299–361. [Google Scholar]
- Fisher MB, Love DC, Schuech R, Nelson KL. Simulated sunlight action spectra for inactivation of MS2 and PRD1 bacteriophages in clear water. Environmental science & technology. 2011;45:9249–9255. doi: 10.1021/es201875x. [DOI] [PubMed] [Google Scholar]
- Flannery J, Keaveney S, Rajko-Nenow P, O’Flaherty V, Dore W. Concentration of norovirus during wastewater treatment and its impact on oyster contamination. Applied and environmental microbiology. 2012;78:3400–3406. doi: 10.1128/AEM.07569-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration and Interstate Shellfish Sanitation Commission. Guide for the Control of Molluscan Shellfish. 2015. National Shellfish Sanitation Program. [Google Scholar]
- Francy DS, Stelzer EA, Bushon RN, Brady AM, Williston AG, Riddell KR, Borchardt MA, Spencer SK, Gellner TM. Comparative effectiveness of membrane bioreactors, conventional secondary treatment, and chlorine and UV disinfection to remove microorganisms from municipal wastewaters. Water research. 2012;46:4164–4178. doi: 10.1016/j.watres.2012.04.044. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Genthner FJ, Gentry J, Sobsey MD, Vinje J. Gene mapping and phylogenetic analysis of the complete genome from 30 single-stranded RNA male-specific coliphages (family Leviviridae) Journal of virology. 2009;83:11233–11243. doi: 10.1128/JVI.01308-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnert A, Apelt S, Klitzke S, Chorus I, Szewzyk R, Selinka HC. Transport and removal of viruses in saturated sand columns under oxic and anoxic conditions--Potential implications for groundwater protection. International journal of hygiene and environmental health. 2014;217:861–870. doi: 10.1016/j.ijheh.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Fu CY, Xie X, Huang JJ, Zhang T, Wu QY, Chen JN, Hu HY. Monitoring and evaluation of removal of pathogens at municipal wastewater treatment plants. Water science and technology : a journal of the International Association on Water Pollution Research. 2010;61:1589–1599. doi: 10.2166/wst.2010.757. [DOI] [PubMed] [Google Scholar]
- Furuse K. Distribution of coliphages in the environment: general considerations. John Wiley & Sons; New York: 1987. [Google Scholar]
- Gantzer C, Gillerman L, Kuznetsov M, Oron G. Adsorption and survival of faecal coliforms, somatic coliphages and F-specific RNA phages in soil irrigated with wastewater. Water science and technology : a journal of the International Association on Water Pollution Research. 2001;43:117–124. [PubMed] [Google Scholar]
- Gomez-Donate M, Payan A, Cortes I, Blanch AR, Lucena F, Jofre J, Muniesa M. Isolation of bacteriophage host strains of Bacteroides species suitable for tracking sources of animal faecal pollution in water. Environmental microbiology. 2011;13:1622–1631. doi: 10.1111/j.1462-2920.2011.02474.x. [DOI] [PubMed] [Google Scholar]
- Gomila M, Solis JJ, David Z, Ramon C, Lalucat J. Comparative reductions of bacterial indicators, bacteriophage-infecting enteric bacteria and enteroviruses in wastewater tertiary treatments by lagooning and UV-radiation. Water science and technology : a journal of the International Association on Water Pollution Research. 2008;58:2223–2233. doi: 10.2166/wst.2008.584. [DOI] [PubMed] [Google Scholar]
- Gordon C, Toze S. Influence of groundwater characteristics on the survival of enteric viruses. Journal of applied microbiology. 2003;95:536–544. doi: 10.1046/j.1365-2672.2003.02010.x. [DOI] [PubMed] [Google Scholar]
- Grabow WOK, Neubrech TE, Holtzhausen CS, Jofre J. Bacteroides fragilis and Escherichia coli Bacteriophages - Excretion by Humans and Animals. Water Science and Technology. 1995;31:223–230. [Google Scholar]
- Griffin DW, Stokes R, Rose JB, Paul JH., 3rd Bacterial Indicator Occurrence and the Use of an F(+) Specific RNA Coliphage Assay to Identify Fecal Sources in Homosassa Springs, Florida. Microbial ecology. 2000;39:56–64. doi: 10.1007/s002489900193. [DOI] [PubMed] [Google Scholar]
- Haramoto E, Fujino S, Otagiri M. Distinct behaviors of infectious F-specific RNA coliphage genogroups at a wastewater treatment plant. The Science of the total environment. 2015;520:32–38. doi: 10.1016/j.scitotenv.2015.03.034. [DOI] [PubMed] [Google Scholar]
- Haramoto E, Katayama H, Oguma K, Yamashita H, Tajima A, Nakajima H, Ohgaki S. Seasonal profiles of human noroviruses and indicator bacteria in a wastewater treatment plant in Tokyo, Japan. Water science and technology : a journal of the International Association on Water Pollution Research. 2006;54:301–308. doi: 10.2166/wst.2006.888. [DOI] [PubMed] [Google Scholar]
- Harden VP, Harris JO. The isoelectric point of bacterial cells. Journal of bacteriology. 1953;65:198–202. doi: 10.1128/jb.65.2.198-202.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartard C, Rivet R, Banas S, Gantzer C. Occurrence of and Sequence Variation among F-Specific RNA Bacteriophage Subgroups in Feces and Wastewater of Urban and Animal Origins. Applied and environmental microbiology. 2015;81:6505–6515. doi: 10.1128/AEM.01905-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood VJ, Boehm AB, Sassoubre LM, Vijayavel K, Stewart JR, Fong TT, Caprais MP, Converse RR, Diston D, Ebdon J, Fuhrman JA, Gourmelon M, Gentry-Shields J, Griffith JF, Kashian DR, Noble RT, Taylor H, Wicki M. Performance of viruses and bacteriophages for fecal source determination in a multi-laboratory, comparative study. Water research. 2013;47:6929–6943. doi: 10.1016/j.watres.2013.04.064. [DOI] [PubMed] [Google Scholar]
- Harwood VJ, Levine AD, Scott TM, Chivukula V, Lukasik J, Farrah SR, Rose JB. Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Applied and environmental microbiology. 2005;71:3163–3170. doi: 10.1128/AEM.71.6.3163-3170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood VJ, Staley C, Badgley BD, Borges K, Korajkic A. Microbial source tracking markers for detection of fecal contamination in environmental waters: relationships between pathogens and human health outcomes. FEMS microbiology reviews. 2014;38:1–40. doi: 10.1111/1574-6976.12031. [DOI] [PubMed] [Google Scholar]
- Hata A, Kitajima M, Katayama H. Occurrence and reduction of human viruses, F- specific RNA coliphage genogroups and microbial indicators at a full-scale wastewater treatment plant in Japan. Journal of applied microbiology. 2013;114:545–554. doi: 10.1111/jam.12051. [DOI] [PubMed] [Google Scholar]
- Hijnen WA, Brouwer-Hanzens AJ, Charles KJ, Medema GJ. Transport of MS2 phage, Escherichia coli, Clostridium perfringens, Cryptosporidium parvum, and Giardia intestinalis in a gravel and a sandy soil. Environmental science & technology. 2005;39:7860–7868. doi: 10.1021/es050427b. [DOI] [PubMed] [Google Scholar]
- Hill VR, Kantardjieff A, Sobsey MD, Westerman PW. Reduction of enteric microbes in flushed swine wastewater treated by a biological aerated filter and UV irradiation. Water Environment Research. 2002;74:91–99. doi: 10.2175/106143002x139785. [DOI] [PubMed] [Google Scholar]
- Hill VR, Sobsey MD. Microbial indicator reductions in alternative treatment systems for swine wastewater. Water Science and Technology. 1998;38:119–122. [Google Scholar]
- Hill VR, Sobsey MD. Removal of Salmonella and microbial indicators in constructed wetlands treating swine wastewater. Water Science and Technology. 2001;44:215–222. [PubMed] [Google Scholar]
- Hot D, Legeay O, Jacques J, Gantzer C, Caudrelier Y, Guyard K, Lange M, Andreoletti L. Detection of somatic phages, infectious enteroviruses and enterovirus genomes as indicators of human enteric viral pollution in surface water. Water research. 2003;37:4703–4710. doi: 10.1016/S0043-1354(03)00439-1. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Shieh YS, van Duin J, Beekwilder MJ, Sobsey MD. Genotyping male- specific RNA coliphages by hybridization with oligonucleotide probes. Applied and environmental microbiology. 1995;61:3960–3966. doi: 10.1128/aem.61.11.3960-3966.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CJ, Gerba CP, Cech I. Effects of environmental variables and soil characteristics on virus survival in soil. Applied and environmental microbiology. 1980;40:1067–1079. doi: 10.1128/aem.40.6.1067-1079.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarluzea JM, Santa Marina L, Moreno B, Serrano E, Larburu K, Maiztegi MJ, Yarzabal A. Somatic coliphages and bacterial indicators of bathing water quality in the beaches of Gipuzkoa, Spain. Journal of water and health. 2007;5:417–426. doi: 10.2166/wh.2007.037. [DOI] [PubMed] [Google Scholar]
- Jewett DG, Hilbert TA, Logan BE, Arnold RG, Bales RC. Bacterial transport in laboratory columns and filters: Influence of ionic strength and pH on collision efficiency. Water research. 1995;29:1673–1680. [Google Scholar]
- Jofre J, Blanch AR, Lucena F, Muniesa M. Bacteriophages infecting Bacteroides as a marker for microbial source tracking. Water research. 2014;55:1–11. doi: 10.1016/j.watres.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Jofre J, Lucena F, Blanch AR, Muniesa M. Coliphages as Model Organisms in the Characterization and Management of Water Resources. Water. 2016;8:1–21. [Google Scholar]
- Karim MR, Manshadi FD, Karpiscak MM, Gerba CP. The persistence and removal of enteric pathogens in constructed wetlands. Water research. 2004;38:1831–1837. doi: 10.1016/j.watres.2003.12.029. [DOI] [PubMed] [Google Scholar]
- King AMQ, Adams MJ, Carstens EB, Lefkowitz WJ. Ninth Report of the International Committee on Taxonomy of Viruses. London, UK: Elsevier Academic Press; 2011. Virus Taxonomy: Classification and Nomenclature of Viruses. [Google Scholar]
- Kitajima M, Iker BC, Pepper IL, Gerba CP. Relative abundance and treatment reduction of viruses during wastewater treatment processes--identification of potential viral indicators. The Science of the total environment. 2014;488–489:290–296. doi: 10.1016/j.scitotenv.2014.04.087. [DOI] [PubMed] [Google Scholar]
- Kott Y, Roze N, Sperber S, Betzer N. Bacteriophages as viral pollution indicators. Water research. 1974;8:165–171. [Google Scholar]
- Kummu M, de Moel H, Ward PJ, Varis O. How close do we live to water? A global analysis of population distance to freshwater bodies. PloS one. 2011;6:e20578. doi: 10.1371/journal.pone.0020578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBelle RL, Gerba CP. Influence of pH, salinity, and organic matter on the adsorption of enteric viruses to estuarine sediment. Applied and environmental microbiology. 1979;38:93–101. doi: 10.1128/aem.38.1.93-101.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc H, Edberg S, Pierzo V, Delattre JM. Bacteriophages as indicators of enteric viruses and public health risk in groundwaters. Journal of applied microbiology. 2000;88:5–21. doi: 10.1046/j.1365-2672.2000.00949.x. [DOI] [PubMed] [Google Scholar]
- Lee JE, Lee H, Cho YH, Hur HG, Ko G. F+ RNA coliphage-based microbial source tracking in water resources of South Korea. The Science of the total environment. 2011;412–413:127–131. doi: 10.1016/j.scitotenv.2011.09.061. [DOI] [PubMed] [Google Scholar]
- Lodder WJ, de Roda Husman AM. Presence of noroviruses and other enteric viruses in sewage and surface waters in The Netherlands. Applied and environmental microbiology. 2005;71:1453–1461. doi: 10.1128/AEM.71.3.1453-1461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder WJ, van den Berg HH, Rutjes SA, de Roda Husman AM. Presence of enteric viruses in source waters for drinking water production in The Netherlands. Applied and environmental microbiology. 2010;76:5965–5971. doi: 10.1128/AEM.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SC, Sobsey MD. A comparison of the survival of F+RNA and F+DNA coliphages in lake water microcosms. Journal of water and health. 2004;2:15–22. [PubMed] [Google Scholar]
- Love DC, Rodriguez RA, Gibbons CD, Griffith JF, Yu Q, Stewart JR, Sobsey MD. Human viruses and viral indicators in marine water at two recreational beaches in Southern California, USA. Journal of water and health. 2014;12:136–150. doi: 10.2166/wh.2013.078. [DOI] [PubMed] [Google Scholar]
- Lucena F, Araujo R, Jofre J. Usefulness of bacteriophages infecting Bacteroides fragilis as index microorganisms of remote faecal pollution. Water research. 1996;30:2812–2816. [Google Scholar]
- Lucena F, Duran AE, Moron A, Calderon E, Campos C, Gantzer C, Skraber S, Jofre J. Reduction of bacterial indicators and bacteriophages infecting faecal bacteria in primary and secondary wastewater treatments. Journal of applied microbiology. 2004;97:1069–1076. doi: 10.1111/j.1365-2672.2004.02397.x. [DOI] [PubMed] [Google Scholar]
- Lucena F, Mendez X, Moron A, Calderon E, Campos C, Guerrero A, Cardenas M, Gantzer C, Shwartzbrood L, Skraber S, Jofre J. Occurrence and densities of bacteriophages proposed as indicators and bacterial indicators in river waters from Europe and South America. Journal of applied microbiology. 2003;94:808–815. doi: 10.1046/j.1365-2672.2003.01812.x. [DOI] [PubMed] [Google Scholar]
- Lute S, Aranha H, Tremblay D, Liang D, Ackermann HW, Chu B, Moineau S, Brorson K. Characterization of coliphage PR772 and evaluation of its use for virus filter performance testing. Applied and environmental microbiology. 2004;70:4864–4871. doi: 10.1128/AEM.70.8.4864-4871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandilara GD, Smeti EM, Mavridou AT, Lambiri MP, Vatopoulos AC, Rigas FP. Correlation between bacterial indicators and bacteriophages in sewage and sludge. FEMS microbiology letters. 2006;263:119–126. doi: 10.1111/j.1574-6968.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- Mayer RE, Bofill-Mas S, Egle L, Reischer GH, Schade M, Fernandez-Cassi X, Fuchs W, Mach RL, Lindner G, Kirschner A, Gaisbauer M, Piringer H, Blaschke AP, Girones R, Zessner M, Sommer R, Farnleitner AH. Occurrence of human-associated Bacteroidetes genetic source tracking markers in raw and treated wastewater of municipal and domestic origin and comparison to standard and alternative indicators of faecal pollution. Water research. 2016;90:265–276. doi: 10.1016/j.watres.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn BR, Korajkic A, Ashbolt NJ. Evaluation of Bacteroides fragilis GB-124 bacteriophages as novel human-associated faecal indicators in the United States. Letters in applied microbiology. 2014;59:115–121. doi: 10.1111/lam.12252. [DOI] [PubMed] [Google Scholar]
- Mesquita MM, Stimson J, Chae GT, Tufenkji N, Ptacek CJ, Blowes DW, Emelko MB. Optimal preparation and purification of PRD1-like bacteriophages for use in environmental fate and transport studies. Water research. 2010;44:1114–1125. doi: 10.1016/j.watres.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Messner MJ, Berger P, Nappier SP. Fractional poisson--a simple dose-response model for human norovirus. Risk analysis : an official publication of the Society for Risk Analysis. 2014;34:1820–1829. doi: 10.1111/risa.12207. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Eblen BS, Oser A, Burkhardt W. Application and evaluation of male- specific bacteriophage as a process integrity or faecal contamination indicator in a pork slaughterhouse environment. Journal of applied microbiology. 1998;85:898–904. doi: 10.1046/j.1365-2672.1998.00599.x. [DOI] [PubMed] [Google Scholar]
- Moce-Llivina L, Muniesa M, Pimenta-Vale H, Lucena F, Jofre J. Survival of bacterial indicator species and bacteriophages after thermal treatment of sludge and sewage. Applied and environmental microbiology. 2003;69:1452–1456. doi: 10.1128/AEM.69.3.1452-1456.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri N, Goettert D, Achberger EC, Johnson CN, Prinyawiwatkul W, Janes ME. Pathogenic Enteric Viruses and Microbial Indicators during Secondary Treatment of Municipal Wastewater. Applied and environmental microbiology. 2015;81:6436–6445. doi: 10.1128/AEM.01218-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montemayor M, Valero F, Jofre J, Lucena F. Occurrence of Cryptosporidium spp. oocysts in raw and treated sewage and river water in north-eastern Spain. Journal of applied microbiology. 2005;99:1455–1462. doi: 10.1111/j.1365-2672.2005.02737.x. [DOI] [PubMed] [Google Scholar]
- Muniesa M, Jofre J, Lucena F. Occurrence and numbers of bacteriophages and bacterial indicators in faeces of yellow-legged seagull (Larus cachinnans) Letters in applied microbiology. 1999;29:421–423. doi: 10.1046/j.1365-2672.1999.00666.x. [DOI] [PubMed] [Google Scholar]
- Muniesa M, Lucena F, Blanch AR, Payan A, Jofre J. Use of abundance ratios of somatic coliphages and bacteriophages of Bacteroides thetaiotaomicron GA17 for microbial source identification. Water research. 2012a;46:6410–6418. doi: 10.1016/j.watres.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Muniesa M, Lucena F, Blanch AR, Payan A, Jofre J. Use of abundance ratios of somatic coliphages and bacteriophages of Bacteroides thetaiotaomicron GA17 for microbial source identification. Water research. 2012b;46:6410–6418. doi: 10.1016/j.watres.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Muniesa M, Moce-Llivina L, Katayama H, Jofre J. Bacterial host strains that support replication of somatic coliphages. Antonie van Leeuwenhoek. 2003;83:305–315. doi: 10.1023/a:1023384714481. [DOI] [PubMed] [Google Scholar]
- Muniesa M, Payan A, Moce-Llivina L, Blanch AR, Jofre J. Differential persistence of F-specific RNA phage subgroups hinders their use as single tracers for faecal source tracking in surface water. Water research. 2009;43:1559–1564. doi: 10.1016/j.watres.2008.12.038. [DOI] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration. Population Trends from 1970 to 2020. 2013. State of the Coast: National Coastal Population Report. [Google Scholar]
- Nnane DE, Ebdon JE, Taylor HD. Integrated analysis of water quality parameters for cost-effective faecal pollution management in river catchments. Water research. 2011;45:2235–2246. doi: 10.1016/j.watres.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Noble RT, Allen SM, Blackwood AD, Chu W, Jiang SC, Lovelace GL, Sobsey MD, Stewart JR, Wait DA. Use of viral pathogens and indicators to differentiate between human and non-human fecal contamination in a microbial source tracking comparison study. Journal of water and health. 2003;1:195–207. [PubMed] [Google Scholar]
- Noble RT, Lee IM, Schiff KC. Inactivation of indicator micro-organisms from various sources of faecal contamination in seawater and freshwater. Journal of applied microbiology. 2004;96:464–472. doi: 10.1111/j.1365-2672.2004.02155.x. [DOI] [PubMed] [Google Scholar]
- North Carolina Environmental Quality. North Carolina Adm. Code 15A NCAC 2U Reclaimed Water. Raleigh, NC: North Carolina Department of Environment and Natural Resources; 2011. [Google Scholar]
- Osborne CM, Montano AC, Robinson CC, Schultz-Cherry S, Dominguez SR. Viral gastroenteritis in children in Colorado 2006–2009. Journal of medical virology. 2015;87:931–939. doi: 10.1002/jmv.24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoson J, Hansen A, Westrell T, Johansen K, Norder H, Stenstrom TA. Removal of noro- and enteroviruses, Giardia cysts, Cryptosporidium oocysts, and fecal indicators at four secondary wastewater treatment plants in Sweden. Water environment research : a research publication of the Water Environment Federation. 2006;78:828–834. doi: 10.2175/106143006x101719. [DOI] [PubMed] [Google Scholar]
- Ottosson J, Stenstrom TA. Growth and reduction of microorganisms in sediments collected from a greywater treatment system. Letters in applied microbiology. 2003;36:168–172. doi: 10.1046/j.1472-765x.2003.01286.x. [DOI] [PubMed] [Google Scholar]
- Pang L, Close M, Goltz M, Noonan M, Sinton L. Filtration and transport of Bacillus subtilis spores and the F-RNA phage MS2 in a coarse alluvial gravel aquifer: implications in the estimation of setback distances. Journal of contaminant hydrology. 2005;77:165–194. doi: 10.1016/j.jconhyd.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Paul JH, Rose JB, Jiang SC, Kellogg CA, Dickson L. Distribution of viral abundance in the reef environment of Key Largo, Florida. Applied and environmental microbiology. 1993;59:718–724. doi: 10.1128/aem.59.3.718-724.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan A, Ebdon J, Taylor H, Gantzer C, Ottoson J, Papageorgiou GT, Blanch AR, Lucena F, Jofre J, Muniesa M. Method for isolation of Bacteroides bacteriophage host strains suitable for tracking sources of fecal pollution in water. Applied and environmental microbiology. 2005;71:5659–5662. doi: 10.1128/AEM.71.9.5659-5662.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payment P, Locas A. Pathogens in water: value and limits of correlation with microbial indicators. Ground water. 2011;49:4–11. doi: 10.1111/j.1745-6584.2010.00710.x. [DOI] [PubMed] [Google Scholar]
- Payment P, Plante R, Cejka P. Removal of indicator bacteria, human enteric viruses, Giardia cysts, and Cryptosporidium oocysts at a large wastewater primary treatment facility. Canadian journal of microbiology. 2001;47:188–193. [PubMed] [Google Scholar]
- Petrinca AR, Donia D, Pierangeli A, Gabrieli R, Degener AM, Bonanni E, Diaco L, Cecchini G, Anastasi P, Divizia M. Presence and environmental circulation of enteric viruses in three different wastewater treatment plants. Journal of applied microbiology. 2009;106:1608–1617. doi: 10.1111/j.1365-2672.2008.04128.x. [DOI] [PubMed] [Google Scholar]
- Petterson S, Grondahl-Rosado R, Nilsen V, Myrmel M, Robertson LJ. Variability in the recovery of a virus concentration procedure in water: Implications for QMRA. Water research. 2015;87:79–86. doi: 10.1016/j.watres.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Pradhan SK, Kauppinen A, Martikainen K, Pitkanen T, Kusnetsov J, Miettinen IT, Pessi M, Poutiainen H, Heinonen-Tanski H. Microbial reduction in wastewater treatment using Fe(3+) and Al(3+) coagulants and PAA disinfectant. Journal of water and health. 2013;11:581–589. doi: 10.2166/wh.2013.241. [DOI] [PubMed] [Google Scholar]
- Puig A, Queralt N, Jofre J, Araujo R. Diversity of Bacteroides fragilis strains in their capacity to recover phages from human and animal wastes and from fecally polluted wastewater. Applied and environmental microbiology. 1999;65:1772–1776. doi: 10.1128/aem.65.4.1772-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell SE, Ebdon JE, Taylor HD. Bacteriophage lysis of Enterococcus host strains: a tool for microbial source tracking? Environmental science & technology. 2011;45:10699–10705. doi: 10.1021/es202141x. [DOI] [PubMed] [Google Scholar]
- Queensland Government Environmental Protection Agency. Queensland Water Recycling Guidelines. Brisbane, Australia: 2005. [Google Scholar]
- Queralt N, Jofre J, Araujo R, Muniesa M. Homogeneity of the morphological groups of bacteriophages infecting Bacteroides fragilis strain HSP40 and strain RYC2056. Current microbiology. 2003;46:163–168. doi: 10.1007/s00284-002-3813-7. [DOI] [PubMed] [Google Scholar]
- Quinonez-Diaz MJ, Karpiscak MM, Ellman ED, Gerba CP. Removal of pathogenic and indicator microorganisms by a constructed wetland receiving untreated domestic wastewater. Journal of environmental science and health Part A, Toxic/hazardous substances & environmental engineering. 2001;36:1311–1120. doi: 10.1081/ese-100104880. [DOI] [PubMed] [Google Scholar]
- Rao VC, Seidel KM, Goyal SM, Metcalf TG, Melnick JL. Isolation of enteroviruses from water, suspended solids, and sediments from Galveston Bay: survival of poliovirus and rotavirus adsorbed to sediments. Applied and environmental microbiology. 1984;48:404–409. doi: 10.1128/aem.48.2.404-409.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravva SV, Sarreal CZ. Persistence of F-Specific RNA Coliphages in Surface Waters from a Produce Production Region along the Central Coast of California. PloS one. 2016;11:e0146623. doi: 10.1371/journal.pone.0146623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinoso R, Torres LA, Becares E. Efficiency of natural systems for removal of bacteria and pathogenic parasites from wastewater. The Science of the total environment. 2008;395:80–86. doi: 10.1016/j.scitotenv.2008.02.039. [DOI] [PubMed] [Google Scholar]
- Rezaeinejad S, Vergara GG, Woo CH, Lim TT, Sobsey MD, Gin KY. Surveillance of enteric viruses and coliphages in a tropical urban catchment. Water research. 2014;58:122–131. doi: 10.1016/j.watres.2014.03.051. [DOI] [PubMed] [Google Scholar]
- Rose JB, Huffman DE, Riley K, Farrah SR, Lukasik JO, Hamann CL. Reduction of enteric microorganisms at the Upper Occoquan Sewage Authority Water Reclamation Plant. Water environment research : a research publication of the Water Environment Federation. 2001;73:711–720. doi: 10.2175/106143001x143457. [DOI] [PubMed] [Google Scholar]
- Sabria A, Pinto RM, Bosch A, Bartolome R, Cornejo T, Torner N, Martinez A, Simon M, Dominguez A, Guix S. Norovirus shedding among food and healthcare workers exposed to the virus in outbreak settings. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2016;82:119–125. doi: 10.1016/j.jcv.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Santiago-Rodriguez TM, Davila C, Gonzalez J, Bonilla N, Marcos P, Urdaneta M, Cadete M, Monteiro S, Santos R, Domingo JS, Toranzos GA. Characterization of Enterococcus faecalis-infecting phages (enterophages) as markers of human fecal pollution in recreational waters. Water research. 2010;44:4716–4725. doi: 10.1016/j.watres.2010.07.078. [DOI] [PubMed] [Google Scholar]
- Santiago-Rodriguez TM, Marcos P, Monteiro S, Urdaneta M, Santos R, Toranzos GA. Evaluation of Enterococcus-infecting phages as indices of fecal pollution. Journal of water and health. 2013;11:51–63. doi: 10.2166/wh.2012.100. [DOI] [PubMed] [Google Scholar]
- Santiago-Rodriguez TM, Toranzos GA, Arce-Nazario JA. Assessing the microbial quality of a tropical watershed with an urbanization gradient using traditional and alternate indicators. Journal of water and health. 2016;14:wh2016041. doi: 10.2166/wh.2016.041. [DOI] [PubMed] [Google Scholar]
- Santiago-Rodriguez TM, Tremblay RL, Toledo-Hernandez C, Gonzalez-Nieves JE, Ryu H, Santo Domingo JW, Toranzos GA. Microbial quality of tropical inland waters and effects of rainfall events. Applied and environmental microbiology. 2012;78:5160–5169. doi: 10.1128/AEM.07773-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AI, Peterson BM, Boehm AB, McNeill K, Nelson KL. Sunlight inactivation of human viruses and bacteriophages in coastal waters containing natural photosensitizers. Environmental science & technology. 2013;47:1870–1878. doi: 10.1021/es3036913. [DOI] [PubMed] [Google Scholar]
- Sinclair RG, Jones EL, Gerba CP. Viruses in recreational water-borne disease outbreaks: a review. Journal of applied microbiology. 2009;107:1769–1780. doi: 10.1111/j.1365-2672.2009.04367.x. [DOI] [PubMed] [Google Scholar]
- Sinton LW, Finlay RK, Lynch PA. Sunlight inactivation of fecal bacteriophages and bacteria in sewage-polluted seawater. Applied and environmental microbiology. 1999;65:3605–3613. doi: 10.1128/aem.65.8.3605-3613.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinton LW, Hall CH, Lynch PA, Davies-Colley RJ. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Applied and environmental microbiology. 2002;68:1122–1131. doi: 10.1128/AEM.68.3.1122-1131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirikanchana K, Wangkahad B, Mongkolsuk S. The capability of non-native strains of Bacteroides bacteria to detect bacteriophages as faecal indicators in a tropical area. Journal of applied microbiology. 2014;117:1820–1829. doi: 10.1111/jam.12646. [DOI] [PubMed] [Google Scholar]
- Skraber S, Gantzer C, Maul A, Schwartzbrod L. Fates of bacteriophages and bacterial indicators in the Moselle river (France) Water research. 2002;36:3629–3237. doi: 10.1016/s0043-1354(02)00063-5. [DOI] [PubMed] [Google Scholar]
- Skraber S, Gassilloud B, Gantzer C. Comparison of coliforms and coliphages as tools for assessment of viral contamination in river water. Applied and environmental microbiology. 2004a;70:3644–3649. doi: 10.1128/AEM.70.6.3644-3649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skraber S, Gassilloud B, Schwartzbrod L, Gantzer C. Survival of infectious Poliovirus-1 in river water compared to the persistence of somatic coliphages, thermotolerant coliforms and Poliovirus-1 genome. Water research. 2004b;38:2927–2933. doi: 10.1016/j.watres.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Skraber S, Schijven J, Italiaander R, de Roda Husman AM. Accumulation of enteric bacteriophage in fresh water sediments. Journal of water and health. 2009;7:372–379. doi: 10.2166/wh.2009.098. [DOI] [PubMed] [Google Scholar]
- Smith EM, Gerba CP, Melnick JL. Role of sediment in the persistence of enteroviruses in the estuarine environment. Applied and environmental microbiology. 1978;35:685–689. doi: 10.1128/aem.35.4.685-689.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller JA, Bartrand T, Ashbolt NJ, Ravenscroft J, Wade TJ. Estimating the primary etiologic agents in recreational freshwaters impacted by human sources of faecal contamination. Water research. 2010a;44:4736–4747. doi: 10.1016/j.watres.2010.07.064. [DOI] [PubMed] [Google Scholar]
- Soller JA, Schoen ME, Bartrand T, Ravenscroft JE, Ashbolt NJ. Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of faecal contamination. Water research. 2010b;44:4674–4691. doi: 10.1016/j.watres.2010.06.049. [DOI] [PubMed] [Google Scholar]
- Soller JA, Schoen ME, Varghese A, Ichida AM, Boehm AB, Eftim S, Ashbolt NJ, Ravenscroft JE. Human health risk implications of multiple sources of faecal indicator bacteria in a recreational waterbody. Water research. 2014;66:254–264. doi: 10.1016/j.watres.2014.08.026. [DOI] [PubMed] [Google Scholar]
- Stevik TK, Ausland G, Hanssen JF, Jenssen PD. The influence of physical and chemical factors on the transport of E. coli through biological filters for wastewater purification. Water research. 1999;33:3701–3706. [Google Scholar]
- Stewart-Pullaro J, Daugomah JW, Chestnut DE, Graves DA, Sobsey MD, Scott GI. F+ RNA coliphage typing for microbial source tracking in surface waters. Journal of applied microbiology. 2006;101:1015–1026. doi: 10.1111/j.1365-2672.2006.03011.x. [DOI] [PubMed] [Google Scholar]
- Stewart JR, Vinje J, Oudejans SJ, Scott GI, Sobsey MD. Sequence variation among group III F-specific RNA coliphages from water samples and swine lagoons. Applied and environmental microbiology. 2006;72:1226–1230. doi: 10.1128/AEM.72.2.1226-1230.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CX, Kitajima M, Gin KY. Sunlight inactivation of somatic coliphage in the presence of natural organic matter. The Science of the total environment. 2016;541:1–7. doi: 10.1016/j.scitotenv.2015.08.136. [DOI] [PubMed] [Google Scholar]
- Taniuchi M, Begum S, Uddin MJ, Platts-Mills JA, Liu J, Kirkpatrick BD, Chowdhury AH, Jamil KM, Haque R, Petri WA, Jr, Houpt ER. Kinetics of poliovirus shedding following oral vaccination as measured by quantitative reverse transcription-PCR versus culture. Journal of clinical microbiology. 2015;53:206–211. doi: 10.1128/JCM.02406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji Y, Mizoguchi K, Akitsu T, Morita M, Hori K, Unno H. Fate of coliphage in waste water treatment process and detection of phages carrying the Shiga toxin type 2 gene. Water science and technology : a journal of the International Association on Water Pollution Research. 2002;46:285–289. [PubMed] [Google Scholar]
- Tartera C, Jofre J. Bacteriophages active against Bacteroides fragilis in sewage- polluted waters. Applied and environmental microbiology. 1987;53:1632–1637. doi: 10.1128/aem.53.7.1632-1637.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartera C, Lucena F, Jofre J. Human origin of Bacteroides fragilis bacteriophages present in the environment. Applied and environmental microbiology. 1989;55:2696–2701. doi: 10.1128/aem.55.10.2696-2701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton MR, Andrews RC, Hofmann R. Inactivation of particle-associated viral surrogates by ultraviolet light. Water research. 2005;39:3487–3500. doi: 10.1016/j.watres.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Uemura S, Takahashi K, Takaishi A, Machdar I, Ohashi A, Harada H. Removal of indigenous coliphages and fecal coliforms by a novel sewage treatment system consisting of UASB and DHS units. Water science and technology : a journal of the International Association on Water Pollution Research. 2002;46:303–309. [PubMed] [Google Scholar]
- Ulbricht K, Selinka HC, Wolter S, Rosenwinkel KH, Nogueira R. A mass balance approach to the fate of viruses in a municipal wastewater treatment plant during summer and winter seasons. Water science and technology : a journal of the International Association on Water Pollution Research. 2014;69:364–370. doi: 10.2166/wst.2013.722. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. Federal Register. 216. Vol. 71. Washington, D.C: 2006. National Primary Drinking Water Regulations: Groundwater rule. Final Rule; 40 CFR Parts 9, 141 and 142. [Google Scholar]
- United States Environmental Protection Agency. Review of coliphages as possible indicators of fecal contamination for ambient water quality No. 820-R-15-098. Washington, D.C: 2015. [Google Scholar]
- United States Environmental Protection Agency. 2016 Coliphage Experts Workshop: Discussion Topics and Findings No. Washington, D.C: 2016. EPA 823-F-16-001. [Google Scholar]
- Venegas C, Diez H, Blanch AR, Jofre J, Campos C. Microbial source markers assessment in the Bogota River basin (Colombia) Journal of water and health. 2015;13:801–810. doi: 10.2166/wh.2015.240. [DOI] [PubMed] [Google Scholar]
- Vergara GG, Goh SG, Rezaeinejad S, Chang SY, Sobsey MD, Gin KY. Evaluation of FRNA coliphages as indicators of human enteric viruses in a tropical urban freshwater catchment. Water research. 2015;79:39–47. doi: 10.1016/j.watres.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Veschetti E, Cutilli D, Bonadonna L, Briancesco R, Martini C, Cecchini G, Anastasi P, Ottaviani M. Pilot-plant comparative study of peracetic acid and sodium hypochlorite wastewater disinfection. Water research. 2003;37:78–94. doi: 10.1016/s0043-1354(02)00248-8. [DOI] [PubMed] [Google Scholar]
- Viau EJ, Goodwin KD, Yamahara KM, Layton BA, Sassoubre LM, Burns SL, Tong HI, Wong SH, Lu Y, Boehm AB. Bacterial pathogens in Hawaiian coastal streams-- associations with fecal indicators, land cover, and water quality. Water research. 2011;45:3279–3290. doi: 10.1016/j.watres.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Vijayavel K, Byappanahalli MN, Ebdon J, Taylor H, Whitman RL, Kashian DR. Enterococcus phages as potential tool for identifying sewage inputs in the Great Lakes region. J Great Lakes Research. 2014;40:989–993. [Google Scholar]
- Vijayavel K, Fujioka R, Ebdon J, Taylor H. Isolation and characterization of Bacteroides host strain HB-73 used to detect sewage specific phages in Hawaii. Water research. 2010;44:3714–3724. doi: 10.1016/j.watres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Vinje J, Oudejans SJ, Stewart JR, Sobsey MD, Long SC. Molecular detection and genotyping of male-specific coliphages by reverse transcription-PCR and reverse line blot hybridization. Applied and environmental microbiology. 2004;70:5996–6004. doi: 10.1128/AEM.70.10.5996-6004.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicki M, Auckenthaler A, Felleisen R, Tanner M, Baumgartner A. Novel Bacteroides host strains for detection of human- and animal-specific bacteriophages in water. Journal of water and health. 2011;9:159–168. doi: 10.2166/wh.2010.165. [DOI] [PubMed] [Google Scholar]
- Wolf S, Hewitt J, Greening GE. Viral multiplex quantitative PCR assays for tracking sources of fecal contamination. Applied and environmental microbiology. 2010;76:1388–1394. doi: 10.1128/AEM.02249-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Li H, Huang X. Indigenous somatic coliphage removal from a real municipal wastewater by a submerged membrane bioreactor. Water research. 2010;44:1853–1862. doi: 10.1016/j.watres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Wyer MD, Wyn-Jones AP, Kay D, Au-Yeung HK, Girones R, Lopez-Pila J, de Roda Husman AM, Rutjes S, Schneider O. Relationships between human adenoviruses and faecal indicator organisms in European recreational waters. Water research. 2012;46:4130–4141. doi: 10.1016/j.watres.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Yahya M, Hmaied F, Jebri S, Jofre J, Hamdi M. Bacteriophages as indicators of human and animal faecal contamination in raw and treated wastewaters from Tunisia. Journal of applied microbiology. 2015;118:1217–1225. doi: 10.1111/jam.12774. [DOI] [PubMed] [Google Scholar]
- Yamahara KM, Sassoubre LM, Goodwin KD, Boehm AB. Occurrence and persistence of bacterial pathogens and indicator organisms in beach sand along the California coast. Applied and environmental microbiology. 2012;78:1733–1745. doi: 10.1128/AEM.06185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Griffiths MW. Comparative persistence of subgroups of F-specific RNA phages in river water. Applied and environmental microbiology. 2013;79:4564–4567. doi: 10.1128/AEM.00612-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MV, Gerba CP, Kelley LM. Virus persistence in groundwater. Applied and environmental microbiology. 1985;49:778–781. doi: 10.1128/aem.49.4.778-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee SYF, Fong NY, Fong GT, Tak OJ, Hui GT, Ming YS. Male-specific RNA coliphages detected by plaque assay and RT-PCR in tropical river waters and animal fecal matter. Int J Environ Heal R. 2006;16:59–68. doi: 10.1080/09603120500398506. [DOI] [PubMed] [Google Scholar]
- Zhang K, Farahbakhsh K. Removal of native coliphages and coliform bacteria from municipal wastewater by various wastewater treatment processes: implications to water reuse. Water research. 2007;41:2816–2824. doi: 10.1016/j.watres.2007.03.010. [DOI] [PubMed] [Google Scholar]