Abstract
The jury on transmission of HIV through breast-feeding is still on. Data from a clinical trial in children born to HIV-positive mothers were evaluated with respect to their relationship to mother-to-child transmission. A total of 1629 infants who were not infected at age 6 weeks, had HIV results available at 12 months and who were breast-fed were included in this study. Exclusive breast feeding (EBF) rates declined from 85% at 2 months to < 30% by 4 months. EBF was associated with a sustained and significant reduction in HIV infection. With every incremental month of EBF, HIV infection was reduced by 16% [multivariable (risk ratio) RR: 0.84, CI: 0.72–0.98, p = 0.03] from enrollment to 6 months of age and by 18% (multivariable RR: 0.82, CI: 0.72–0.94, p = 0.005) from enrollment to 12 months of age. EBF significantly reduces the risk of vertical HIV transmission through 12 months of age.
Keywords: Exclusive Breast Feeding, HIV and PMTCT
INTRODUCTION
The most common mode of acquisition of HIV infection in infants is through breast-feeding by the HIV-infected mother.
Various studies in relation to reducing mother-to-child transmission (MTCT) of HIV among breast-feeding infants of HIV-infected mothers have been done, and some are still ongoing. (1–3)
In the context of MTCT, the United Nations Children's Emergency Fund/United Nations guidelines state: ‘When replacement feeding is Acceptable, Feasible, Affordable, Sustainable and safe, avoidance of breast feeding by HIV-infected mothers is recommended’. Cessation of breast-feeding in the absence of replacement feeds that are acceptable, feasible, affordable, sustainable and safe is associated with malnutrition, more frequent and severe gastrointestinal and upper respiratory infections and increased mortality (1–4)
This study describes the role of exclusive breast-feeding (EBF) and the transmission of HIV infection among offspring’s of HIV-infected mothers in Tanzania.
METHODS
Study design and population
This study was part of the randomized, double-blind, placebo-controlled trial of micronutrients (clinicaltrials.gov identifier NCT00197730) done in 2004–08 (5). Women aged ≥18 years, presenting for prenatal care at the 32nd week of gestation or earlier in one of eight antenatal clinics in Dar es Salaam were offered HIV screening with pre- and post-eligibility, including the intention to reside in Dar es Salaam for the duration of follow-up. Maternal HIV-1 aerostats was determined by two sequential enzyme-linked immunosorbent assays (ELISAs) using Murex HIV antigen/antibody (Abbott Murex, UK), followed by the Enzygnost anti-HIV laboratories. Written informed consent was obtained from women for participation in the trial while still pregnant. Eligibility for infant participation in the trial included singleton birth and age between 5 and 7 weeks at randomization. Infants born of multiple gestations or those with serious congenital anomalies or other conditions that would interfere with study procedures, including the ability to consume a daily micronutrient supplement, were excluded. Detailed feeding of the child was captured in the questionnaires administered by a registered nurse. Institutional approval was granted by the Harvard T.H. Chan School of Public Health Human Subjects Committee, the Muhimbili University of Health and Allied Sciences Senate Research and Publications Committee, the Tanzanian National Institute of Medical Research and the Tanzanian Food and Drugs Authority.
Mothers were counseled on the risks and benefits of breast-feeding. Women who chose to breast-feed were instructed not to give any additional foods or fluids while they were exclusively breast-feeding, in keeping with the World Health Organization (WHO) and the Tanzanian Ministry of Health and Social Welfare guidelines supporting EBF. (6–8)
When the study was proposed, routine medical care for pregnant women with HIV infection included malaria prophylaxis, iron and folate supplementation, diagnosis and treatment of sexually transmitted infections and prophylaxis and diagnosis and treatment of opportunistic infections. ARV medication was limited to Nevirapine (NVP) prophylaxis for MTCT (one dose given to the mother at the onset of labor and one dose given to the infant within 72 h of birth) (9). As the study progressed, the availability of ARVs increased substantially through programs such as the US President’s Emergency Plan for AIDS Relief and Tanzanian governmental and nongovernmental programs. Beginning in July 2005, women and children in the study were screened for Anti-Retro Viral drugs (ARV) eligibility and treated according to the Tanzanian Ministry of Health guidelines. For adults, eligibility was based on WHO stage IV HIV disease, or CD4 cell count <200 cells/µl or WHO stage III and CD4 cell count <350/µl.
Therefore, some mothers did not receive ARVs because of the eligibility criteria at that time, but all those who fulfilled the criteria received ARVs as recommended.
All children were tested for HIV infection at 6 weeks of age using the Amplicor HIV-1 DNA polymerase chain reaction (PCR) assay version 1.5 (Roche Molecular Systems Inc, NJ, USA). Blood samples from children at 3 months, 6 months, 9 months and 12 months were stored for further tests later. Tests at 18 months of age were performed sequentially using Murex HIV antigen/antibody (Abbott Murex), followed by the Enzygnost anti-HIV-1/2 Plus (Dade Behring) ELISAs; discordant results were resolved by using a Western blot test. Samples from children who tested positive at 18 months were then back tested via a PCR to estimate the time of transmission. Time of transmission was estimated as the date halfway between the last negative and first positive HIV test result. Only those who had results available up to 12 months were analyzed; moreover, few continued to breast-feed beyond 12 months. Maternal body mass index (BMI), CD4 counts and hemoglobin were tested from pregnancy and throughout the study period, and were analyzed as time-varying covariants. Details of the baseline characteristics included maternal and child characteristics and confirmed that measures of age, socioeconomic characteristics, WHO disease stage of mother, rates of low birth weight and rates of EBF between the placebo and intervention group were similar and are described in the primary outcomes publication in full details. (5)
Only those infants who were breast-feeding after 6 weeks of age and had an HIV test available at 12 months were analyzed. Those who were HIV infected at 6 weeks were excluded from the analysis because of the difficulty in teasing out the intrapartum HIV infection, and those who may have suffered from early postpartum infection through breast-feeding were also excluded.
Statistical analysis
Cox proportional hazards (Cox-PH) regression models were used to examine the relationship between EBF and time to HIV infection (10). A counting process data structure with one record per child visit was used to facilitate the handling of time-varying covariates (11, 12). EBF was entered into the model as a time-varying continuous variable (duration of EBF in months) at the visits when a child was exclusively breast-fed, and as the final EBF duration (months) at the visits after which complementary foods were introduced to the child. Analyses were run for 0–12 months of follow-up because few infants were breast-fed after 12 months. For the analysis of time to HIV infection, HIV-uninfected children were censored at the minimum of the time that breast-feeding was stopped and the last HIV-negative test. For inclusion in the model, we considered potential confounders from a list of known and suspected risk factors for the outcome. These variables were categorized as follows: maternal age (<28 years, ≥28 years); education level (none, 1–7 years, 8+ years); employment (housewife without income, housewife with income, others); daily food expenditure(≤500, >500 T shillings); marital status (single, married/living with partner); number of prior pregnancies (0, 1–3, 4+); maternal WHO stages (I/II, III/IV); CD8 counts (<767, ≥767 cells/mm3); lymphocyte counts (<2000, ≥2000 cells/mm3); ARV treatment during pregnancy (yes/no); maternal time-varying BMI (<18.5, 18.5–<25.0, 25.0–<30, 30+ kg/m2); time-varying hemoglobin level (<11, ≥11 g/dl); time-varying maternal CD4 counts (<350, ≥350 cells/mm3); child gender (male/female); birth weight(<2500, ≥2500 g); preterm birth (<37, ≥37 weeks); and child time-varying HIV status (yes/no). Risk ratios (RRs) and their corresponding 95% confidence intervals (CIs) are reported. Kaplan–Meier survival curves were generated for EBF (13).
We performed secondary analyses to examine whether the associations of EBF with HIV infection were modified by the child’s time-varying HIV status, maternal ARV therapy initiated during pregnancy, maternal baseline CD4+ cell counts, CD8+ cell counts and lymphocyte counts. Likelihood ratio tests were used to assess the statistical significance of the interactions examined. All statistical analyses were performed with the statistical software package SAS, 9.2 (SAS Institute Inc, Cary, North Carolina, USA). The significance tests were two-sided, and differences were considered significant at p < 0.05.
EBF is defined as the receipt of only breast milk since birth; only oral rehydration solution, drops and syrups (vitamins, minerals or medicines) are permitted. (14, 15)
RESULTS
Details of the baseline characteristics and the recruitment are available elsewhere. (5) There were 262 deaths in children, and they are not part of this analysis; there were only 20% of women who were on ARVs at the time of the study.
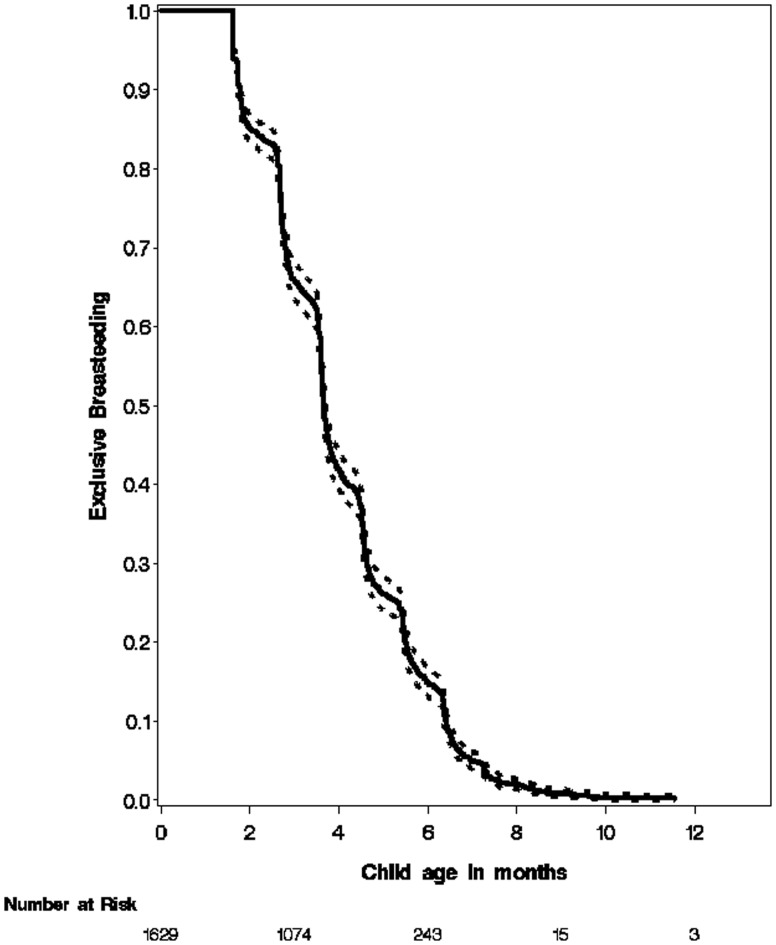
Two thousand eighty-six children with completed data collection forms were analyzed, and among them, 2349 were breast-fed (98.5%), and 2088 (88.8%) were exclusively breast-fed (EBF) for various periods of time. HIV test results at 12 months were available in 1629 infants. The mean duration of BF duration was 4.0 months, ranging from 0 to 21.7 months; and the mean duration of EBF duration was 3 months, ranging from 0 to 13.3 months. Fig. 1 describes the rates of breast-feeding. With few continuing breast-feeding after 12 months, the analysis for HIV acquisition was limited to 12 months.
Fig. 1.
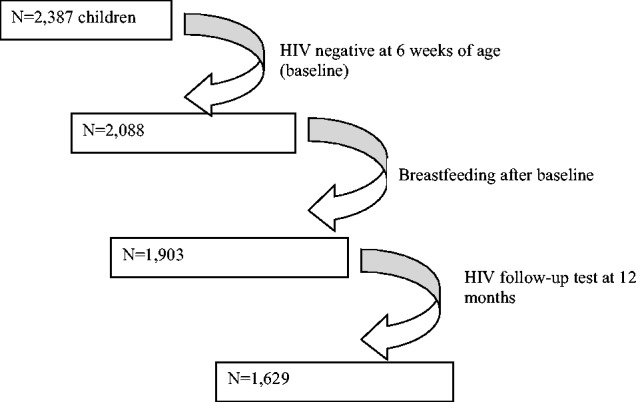
Subjects enrolled in analysis of breast-feeding and HIV infection in Tanzania.
Fig. 2.
The breast-feeding rates and duration over time among the 1629 selected infants.
As indicated in Table 1, 72 infants became infected by 12 months, of whom 65 were infected by the age of 6 months. During the first 6 months of life, for every 1 month longer for EBF, there was an additional 16% protection to acquisition of HIV (adjusted model). This benefit continued up to 12 months, over which the additional protection was 18%.
Table 1.
Duration of EBF in relation to HIV-infection, the 12-month follow-up period among infants born to HIV-infected mothersa
| Outcome | HIV+ve | Univariate |
Multivariateb |
|||
|---|---|---|---|---|---|---|
| n | RR (95% CI) | p-value | RR (95%CI) | p-value | ||
| HIV infection | ||||||
| 0–6 months | 65 | 1629 | 0.82 (0.71–0.95) | 0.008 | 0.84(0.72–0.98) | 0.03 |
| 7–12 months | 7 | 132 | 0.83 (0.63–1.09) | 0.18 | –c | |
| 0–12 months | 72 | 1629 | 0.83 (0.73–0.94) | 0.003 | 0.82(0.72–0.94) | 0.005 |
aTime-varying Cox-PH regression models were used to estimate the RR and its 95% CIs.
bAdjusted for maternal age, education level, employment, daily food expenditure, marital status, number of prior pregnancy, maternal WHO stages, CD8 T-cell counts, lymphocyte counts, ARV treatment during pregnancy, maternal time-varying BMI, hemoglobin level, maternal CD4 T-cell counts, child gender, birth weight and preterm birth.
cRR could not be estimated in the multivariate model because of the small number of events in this period and because EBF rates were lower after 4 months.
Potential modifiers included maternal ARV use during pregnancy and maternal CD4 T-cell counts. Table 2 shows that there were no significant effect modifiers for the association between EBF and reduction of acquisition of HIV.
Table 2.
The association of EBF with HIV infection, within strata of potential modifiers during the 12-month follow-up
| Potential modifier | HIV +ve | N | RR (95% CI)a | P a | Pintb |
|---|---|---|---|---|---|
| Maternal ARV therapy | |||||
| No | 53 | 1287 | 0.86 (0.73–1.00) | 0.05 | 0.37 |
| Yes | 18 | 308 | 0.74 (0.51–1.08) | 0.11 | |
| Maternal baseline CD4+ cell count (/mm3) | |||||
| <350 | 29 | 379 | 0.83 (0.63–1.10) | 0.2 | 0.2 |
| ≥350 | 35 | 1088 | 0.83 (0.69–0.99) | 0.04 | |
| Maternal baseline CD8+ cell count (/mm3) | |||||
| <767 | 4 | 357 | –c | 0.62 | |
| ≥767 | 57 | 1079 | 0.82 (0.71–0.94) | 0.006 | |
| Maternal baseline lymphocyte count (/mm3) | |||||
| <2000 | 7 | 324 | 0.84 (0.35–2.02) | 0.75 | 0.69 |
| ≥2000 | 54 | 1116 | 0.85 (0.73–0.99) | 0.04 | |
aRR, 95% CI and p-values are from the adjusted Cox-PH regression models. Covariates adjusted for were the same as in Table 1.
b p-value, tests for interaction between EBF and potential modifiers were obtained from likelihood ratio tests.
cRR could not be estimated in the multivariate model because of the small number of events.
DISCUSSION
EBF rates in this study indicate a low uptake and are still lower than expected. Despite a universal initiation of breast-feeding in Tanzania, as seen from our data, where 98.5% initiated breast-feeding, pre-lacteal feeds and mixed feeding were equally prevalent, and the rates of EBF rapidly declined to 88% within a few days, to just over 75% by 2 months and <40% by 4 months. This practice exposes the offspring of an HIV-infected mother to a higher risk of HIV transmission as seen in this cohort. It has been reported that EBF is not widely practiced in Tanzania (14). The median duration of breast-feeding in mainland Tanzania was reported to be 21.1 months in 2004; 20.8 months was the median in urban populations and 21.2 months in rural area. (13, 14). In the context of Prevention of Mother to Child Transmission of HIV (PMTCT), early rapid cessation of breast-feeding had been previously recommended in Tanzania as per the WHO recommendations(15) but was revised in late 2007 because of adverse health consequences of early and rapid cessation of breast-feeding for both mothers (mastitis) and infants (growth failure, HIV transmission during rapid weaning and mortality). The revised national guidelines follow the new WHO 2013 recommendations on HIV and EBF (7, 15–19).
Our findings are similar to that reported in another trial, whereby 80.1%, 34.2% and 13.3% of women reported EBF up to 2, 4 and 6 months, respectively. The median duration of EBF among women who ever breast-fed was 3 months (20).
Two major strategies for further reduction of MTCT are provision of ARV prophylaxis to the breast-feeding infant and the provision of triple therapy to the lactating mother. Various studies are looking into these, and several studies have reported variable outcomes, with a trend toward the extended use of oral ARV for the infant and use of triple therapy in the mother. (15, 16–23). ARV use during pregnancy for reduction of MTCT has indicated that these are safe, and their effect is augmented when coupled with EBF. (25, 26)
In this study, the role of EBF in MTCT was evaluated, and for every additional month of EBF, a reduction of transmission of 18% was noted (p = 0.005), confirming further that EBF still holds good, although the effect in subgroups of ARV use and CD4 count was not statistically significant, despite evidence from other studies. (7, 26, 27)
Therefore, promoting EBF is crucial and urgent to save children from acquiring HIV infection and reducing growth failure. Recent findings have suggested that breast-feeding transmission of HIV can be reduced further by the provision of a single dose of NVP orally to the baby for up to 6 months and beyond (23). HIV-infected pregnant women, who require triple therapy for her own health, shall continue using them even after cessation of breast-feeding, and thus reduce transmission even during the weaning period. (28–33). In a study done in Tanzania during the era where ARVs were not available, EBF was protective for HIV acquisition and death, especially during the first 5 months of life. (34).
In conclusion: EBF significantly reduces the risk of vertical HIV transmission through 12 months of age. More data are needed to evaluate the risk of MTCT, as new antiretroviral therapy is rolled out to HIV-positive pregnant women regardless of their disease stage as per WHO option B+.
There is a need to revive emphatically campaigns to promote EBF in the community. HIV-infected mothers should be counseled and EBF should be promoted now more ever before.
FUNDING
This trial was registered at clinicaltrials.gov as NCT00197730. It was funded by (NICHD R01 HD043688-01 and K24HD058795).
ACKNOWLEDGMENTS
We thank the mothers and children and field teams, including physicians, nurses, midwives, supervisors, laboratory staff and administrative staff, who made this study possible; Muhimbili University of Health and Allied Sciences, for their institutional support; the field and study staff for their tireless efforts (Rehema Mtonga, Frank Killa, Edgar Basheka, Susie Welty, Rachel Steinfeld, Anne Marie Darling, Angela Jardin, Sibtain Moledina, Faheem Sheriff, Mohamed Manji, Frank Lazaro, Mohamed Aloo and Elizabeth Long); and members of the DSMB (Marcello Pagano – Chair, Nicholas Horton, Andrew Kitua and Charles Mgone).
The authors’ responsibilities were as follows—KPM, CD, WWF and RJB designed the research; KPM, R Kupka, Kisenge and SA conducted the research; JO and EL analyzed the data; and KPM wrote the manuscript and had primary responsibility for its final content. All authors contributed to and approved the final manuscript.
REFERENCES
- 1.Vanamail P, Sehgal R, Kriplani A. Status and awareness of HIV/AIDS among antenatal Indian women. Indian Journal of Health and Wellbeing. 2015;5(3).
- 2. De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA 2000;283:1175–82. 1; [DOI] [PubMed] [Google Scholar]
- 3. Mofenson LM, McIntyre JA. Advances and research directions in the prevention of mother-to-child HIV-1 transmission. Lancet 2000;355:2237–44. 24; [DOI] [PubMed] [Google Scholar]
- 4. Coovadia H, Bland R. Preserving breastfeeding practice through the HIV pandemic. Trop Med Int Health 2007;12:1116–33. [DOI] [PubMed] [Google Scholar]
- 5. Duggan C, Manji KP, Kupka R, et al. Multiple micronutrient supplementation in Tanzanian infants born to HIV-infected mothers: a randomized, double-blind, placebo-controlled clinical trial. Am J ClinNutr 2012;96:1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. USAID, AED, University of California Davis, et al. Indicators for Assessing Infant and Young Child Feeding Practices. Geneva: WHO, 2008. [Google Scholar]
- 7. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a public health approach. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 8. Ministry of Health and Social Welfare of the United Republic of Tanzania Prevention of Mother-to-Child Transmission of HIV (PMTCT): National Guidelines. Dar es Salaam: Ministry of Health and Social Welfare, 2007. [Google Scholar]
- 9. Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with Zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomized trial. Lancet 1999;354:795–802. [DOI] [PubMed] [Google Scholar]
- 10. Cox DR. Regression models and life tables. J R Stat Soc Ser B 1972;34:187–220. [Google Scholar]
- 11. Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 12. World Health Organization, UNICEF, UNAIDS. HIV and Infant Feeding Counseling: A Training Course. Geneva: WHO, 2000. [Google Scholar]
- 13. Therneau TM, Grambach PM. The counting process form of a Cox model In: Modeling Survival Data. Springer: New York, NY, 2000, 68–77 [Google Scholar]
- 14. Young SL, Israel-Ballard KA, Dantzer EA, et al. Infant feeding practices among HIV-positive women in Dar es Salaam, Tanzania, indicate a need for more intensive infant feeding counseling. Public Health Nutr 2010;13:2027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bland RM, Little KE, Coovadia HM, et al. Intervention to promote exclusive breastfeeding for the first 6 months of life in a high HIV prevalence area. Aids 2008;22:883–91. [DOI] [PubMed] [Google Scholar]
- 16. Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet 2007;369:1107–16. [DOI] [PubMed] [Google Scholar]
- 17. Kuhn L, Reitz C, Abrams EJ. Breastfeeding and AIDS in the developing world. Curr Opin Pediatr 2009;21:83–93. [DOI] [PubMed] [Google Scholar]
- 18. Kesho Bora Study Group . Safety and effectiveness of antiretroviral drugs during pregnancy, delivery and breastfeeding for prevention of mother-to-child transmission of HIV-1: the Kesho Bora Multicentre Collaborative Study rationale, design, and implementation challenges. Contemp Clin Trials 2011;32:74–85. [DOI] [PubMed] [Google Scholar]
- 19. Thior I, Lockman S, Smeaton LM, et al. Breastfeeding plus infant Zidovudine prophylaxis for 6 months vs. formula feeding plus infant Zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashie Study. JAMA 2006;296:794–805. [DOI] [PubMed] [Google Scholar]
- 20. Kilewo C, Karlsson K, Massawe A, et al. Prevention of mother-to child transmission of HIV-1 through breastfeeding by treating infants prophylactic ally with lamivudine in Dar es Salaam, Tanzania: the MITRA Study. Jaids 2008;48:315–23. [DOI] [PubMed] [Google Scholar]
- 21. Vyankandondera J, Luchters S, Hassink E, et al. SIMBA—Stopping Infection from Mother to Child via Breastfeeding in Africa. In: Program and abstracts of the 3rd International AIDS Society Conference on HIV Pathogenesis, Paris, France, 2003. Abstract LB07.
- 22. Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended dose Nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomized controlled trials. Lancet 2008;372:300–13. [DOI] [PubMed] [Google Scholar]
- 23. Coovadia HM, Brown ER, Fowler MG, et al. and HPTN 046 protocol team. Efficacy and safety of an extended Nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomized, double-blind, placebo-controlled trial. Lancet 2012;379:221–8. doi: 10.1016/S0140-6736(11)61653-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Vincenzi and Kesho Bora study Group. Triple antiretroviral compared with Zidovudine and single-dose Nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomized controlled trial. Lancet Infect Dis 2011;11:171–80. [DOI] [PubMed] [Google Scholar]
- 25.UNAIDS J. Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva: UNAIDS. 2013. [Google Scholar]
- 26. Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related millennium development goals: time for a public health approach. Lancet 2011;378:282–4. Jul 16 [DOI] [PubMed] [Google Scholar]
- 27. Arpadi S, Fawzy A, Aldrovandi GM, et al. Growth faltering due to breastfeeding cessation in uninfected children born to HIV-infected mothers in Zambia. Am J ClinNutr 2009;90:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuhn L, Aldrovandi GM, Sinkala M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med 2008;359:130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas TK, Masaba R, Borkowf CB, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding–the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med 2011;8:e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Homsy J, Moore D, Barasa A, et al. Breastfeeding, mother-to-child HIV transmission, and mortality among infants born to HIV-infected women on highly active antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr 2010;53:28–35. [DOI] [PubMed] [Google Scholar]
- 32. The United Republic of Tanzania. Comprehensive guidelines for Prevention of mother to child transmission of HIV; National PMTCT guidelines. In: Ministry of health and social welfare, Vol 2, 2011 [Google Scholar]
- 32. Mofenson LM. Prevention in neglected subpopulations: prevention of mother-to-child transmission of HIV infection. Clin Infect Dis 2010;50:S130–48. [DOI] [PubMed] [Google Scholar]
- 33. Natchu UCM, Liu E, Duggan C, et al. Exclusive breastfeeding reduces risk of mortality in infants up to 6 months of age born to HIV-positive Tanzanian women. Am J ClinNutr 2012;96:1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]