Abstract
Background
Pro-resolving mediators (PRMs) are considered as emerging analgesics for chronic pain. Maresin 1 (MaR1) is a newly identified member of PRMs, and recent studies implicate its potential role in some pain conditions. As the function of MaR1 in neuropathic pain remains unclear, we investigated the effects of MaR1 on pain hypersensitivity and the underlying mechanism using a rat spinal nerve ligation (SNL) model of neuropathic pain.
Materials and methods
MaR1 (100 ng/10 μL) or commensurable artificial cerebrospinal fluid was delivered via intrathecal catheter from days 3 to 5 post-SNL followed by assessment of mechanical allodynia and thermal hyperalgesia. Ipsilateral L4–L5 spinal cord tissue was collected on day 7 post-SNL and assessed by Western blotting, enzyme-linked immunosorbent assay or immunohistochemistry.
Results
Intrathecal MaR1 significantly attenuated mechanical allodynia and thermal hyperalgesia from day 5 to day 7 post-SNL, which was associated with decreased spinal levels of glial markers, GFAP and IBA1. It was also found that intrathecal MaR1 downregulated phosphorylation levels of NF-κB p65 and its nuclear translocation, as well as decreased protein levels of pro-inflammatory cytokines, TNF-α, IL-1β and IL-6. Further, MaR1 treatment restored PSD95 and synapsin II levels, suggesting that MarR1 also protected synaptic integrity.
Conclusion
Our results indicate that MaR1 ameliorates the SNL-induced neuropathic pain by regulating glial activities and pro-inflammatory cytokines release. The present study offers insight into the potential of MaR1 as a novel intervention to ameliorate neuropathic pain.
Keywords: maresin 1, neuropathic pain, spinal nerve ligation, inflammation, NF-κB p65
Introduction
Neuropathic pain, which often follows injury to the somatosensory system, is a refractory disease of extremely complicated nature.1 In addition, the serious side effects of conventional therapeutic strategies limit their range and long-term application.2,3 Therefore, it is necessary to explore a novel treatment with potent therapeutic effects and lesser side effects for neuropathic pain. A large number of studies have demonstrated that neuro-inflammation is involved in the occurrence and maintenance of neuropathic pain, and the inflammatory cytokines- and chemokines-induced activation of glia accelerate the progress of neuropathic pain and thus result in peripheral and central sensitization.4–8 Moreover, the activated glia cells and the neuroglia integrations reciprocally exacerbate the inflammatory response.9,10 Therefore, inhibiting the inflammatory cascades from different aspects, especially the suppression of activated glial cells, has been implicated to ameliorate neuropathic pain.
Recent studies reported a kind of endogenously biosynthesized pro-resolving mediators (PRMs) that play a pivotal role in the maintenance of homeostasis and restrict the development of inflammation.10 One of these important protective mediators, maresin 1 (MaR1), has been identified.11 It is synthesized in macrophages and derived from fatty acid docosahexaenoic acid (DHA), inducing the biosynthesis of protective mediators such as MaR1 (7R, 14S-dihydroxy-4Z, 8E, 10E, 12Z, 16Z, 19Z-DHA).11,12 Previous studies have shown that MaR1 protects the central nervous system from ischemic injuries by its pro-resolution effects, and reduces the production and release of inflammatory cytokines and chemokines in acute lung injury and lung fibrosis.13,14 Furthermore, it has been demonstrated that MaR1 can suppress the activation of astrocytes and microglia in rats cerebral ischemic/reperfusion model and ameliorate the injuries.15 However, there is little evidence showing the therapeutic effects of MaR1 on neuropathic pain with no explicit mechanisms.16 Therefore, this study was designed to examine the effects of MaR1 on neuropathic pain and to elucidate the underlying mechanisms.
Materials and methods
Animals
Adult male Sprague Dawley rats weighting 250–350 g used in this study were purchased from the Experimental Animal Center of the Tongji Medical College (Wuhan, China). Rats were housed at 25 ± 1°C in a 12 h light/dark cycle. Experimental protocols were approved by the Institutional Animal Care and Use Committee in Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China) and were performed in accordance with the guide for the Care and Use of Laboratory Animals of Tongji Medical College.
Intrathecal catheter implantation
The intrathecal catheter was implanted 7 days before spinal nerve ligation (SNL) using a modification of the Malkmus and Yaksh protocol.17 Briefly, rats were anesthetized with sodium pentobarbital (50 mg/kg). A 2 cm incision was made along the midline of the ear line, and the superficial muscles were bluntly separated to expose the atlanto-occipital membrane. A 1–2 mm longitudinal slit was made along the midline of the membrane, and a polyethylene catheter (0.6 mm outer diameter, 0.28 mm inter diameter; Smiths Medical, Minneapolis, MN, USA) marked at 3.3 cm was carefully inserted into the spinal subarachnoid space. Successful catheterization was accompanied by a slight resistance and rising cerebrospinal fluid surface in the catheter. Then, the catheter was firmly fixed in the muscle, with 3 cm of the catheter left externally (sealed using heat) above the skull for bolus drug injections. Rats showing neurological dysfunction after catheter placement were euthanized.
Neuropathic pain model induced by SNL
Rat model of neuropathic pain was induced by SNL as described previously.18 Briefly, the rats were anesthetized with 2% sodium pentobarbital intraperitoneally (50 mg/kg body weight), and a midline incision was made along the skin of L4–L6 spinal dorsal horn. After separating the left paraspinal muscles and removing the L6 transverse process, the left L5 spinal nerve was exposed. Subsequently, the L5 spinal nerve was separated carefully and ligated tightly with a 4-0 silk thread. The rats in the sham group underwent the same operation except for nerve ligation.
Treatment and groups
The day of surgery was marked as day 0, and the behavioral tests were performed daily for 7 days after surgery. The rats were randomly divided into five groups: 1) control; 2) sham; 3) SNL; 4) SNL + artificial cerebrospinal fluid (ACSF); and 5) SNL + MaR1. MaR1 (Cayman Chemical, Ann Arbor, MI, USA; 100 ng/10 μL) and commensurable ACSF (20 mM NaHCO3, 126 mM NaCl, 0.09 mM Na2HPO4, 6 mM KCl, 1.4 mM CaCl2, 0.09 mM MgSO4 and 5 mM glucose 1 L) were intrathecally injected on days 3, 4 and 5 after SNL in SNL + MaR1 and SNL + ACSF groups, respectively. There was no effect on motor coordination and balance after injection.
Behavioral tests
To measure the mechanical withdrawal threshold (MWT) and paw withdrawal latency (PWL), the rats were habituated to plastic cages with metal mesh floor (for MWT test) or glass floor (for PWL test) for at least 30 min.19 In MWT test, an electronic Von Frey device (IITC Life Science Inc., Woodland Hills, CA, USA) was used to stimulate the mid-plantar surface of the hind paw and the corresponding readout was recorded until a withdrawal reflex was observed. In PWL test (Type 7370, Planter Test Instrument; Ugo Basile, Gemonio, Italy), a beam of radiant heat was employed to stimulate the mid-plantar surface of the hind paw and its withdrawal latency was recorded. Twenty seconds was set as the cut-off time to prevent damage on the paws. Measurements were repeated three times on the same paw with a 5-min interval. In addition, after the behavior tests, we found that there were no differences between the sham mice and the control mice and thus we included only the sham mice in the results.
Immunofluorescence assay
After measurements for behavioral tests on day 7, L4–L5 spinal cord tissue was collected and paraffin-embedded. The spinal cord was sliced into 5 mm sections that were permeabilized with PBS containing 1% Triton X-100 for 10 min, blocked with 10% normal goat serum for 1 h and then incubated overnight at 4°C with rabbit-anti-IBA1 (1:100; Abcam, Cambridge, UK) or rabbit-anti-GFAP (1:100; Abcam) primary antibodies. After three washes with PBS, the sections were incubated with donkey anti-rabbit immunoglobulin G (1:200; Abbkine, Wuhan, China) for 1 h at room temperature followed by DAPI (Sigma, St. Louis, MO, USA). Images were captured using an Olympus fluorescence microscope (Olympus, Tokyo, Japan).
Western blot analysis
Ipsilateral L4–L5 spinal cord tissue was collected on day 7 after SNL and homogenized with radio-immunoprecipitation assay (RIPA) buffer for total proteins or the NE-PER Nuclear and Cytoplasmic Extraction Reagent kit (Pierce Biotechnology, Waltham, MA, USA) for nuclear proteins supplemented with a protease inhibitor cocktail and phosphatase inhibitors (Roche Molecular Biochemicals, Inc., Mannheim, Germany). Proteins were separated by 10% SDS-PAGE and electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% nonfat milk for 2 h at room temperature, and then incubated with primary antibodies against NF-κB p65, p-p65 (1:1,000; EMD Millipore, Billerica, MA, USA), β-actin or lamin B (1:1000; Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C. Then, the membranes were incubated with a corresponding secondary antibody for 1 h at room temperature. The bands were analyzed using ImageJ Software (version 1.45s; NIH, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay (ELISA)
For ELISA detection, the ipsilateral L4–L5 spinal dorsal horn was collected after the measurement of MWT and PWL on day 7. Levels of TNF-α, IL-1β and IL-6 were quantified using ELISA kits (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions. Samples were measured in duplicate, and readings from each sample were normalized to the protein concentration.
Statistical analysis
Data are presented as mean ± SD. GraphPad Prism 5.0 (San Diego, CA, USA) software was used for all analyses. The differences in the behavioral test, and the results of ELISA and immunofluorescence were analyzed by one-way or two-way analysis of variance (ANOVA), followed by Tukey post hoc test. Statistical significance was defined as P < 0.05.
Results
MaR1 ameliorated SNL-induced mechanical allodynia and thermal hyperalgesia
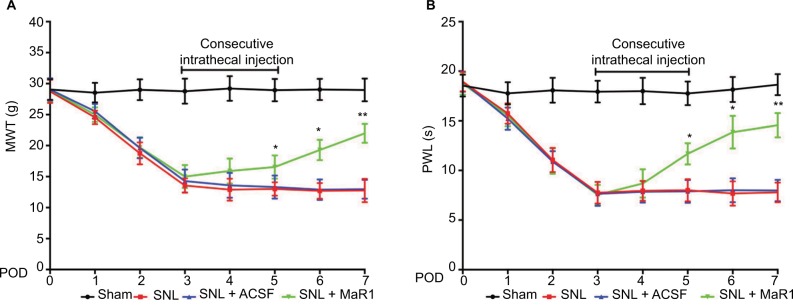
First, we examined whether MaR1 could attenuate mechanical allodynia and thermal hyperalgesia induced by SNL. There was a dramatic decrease of ipsilateral MWT and PWL from day 1, and the measurements reached the lowest value on day 3 after surgery (MWT: from 28.76 ± 1.86 g to 13.55 ± 1.56 g, P < 0.05; PWL: from 18.25 ± 2.01 s to 8.06 ± 1.78 s, P < 0.05; SNL vs. sham group, two-way ANOVA), which were maintained till day 7 after SNL (time duration of the study). After intrathecal injection of MaR1 from day 3 to day 5, there was a significant increase of MWT and PWL from day 5 (Figure 1A, MWT: 12.89 ± 1.05 g to 16.54 ± 1.27 g, P < 0.05; Figure 1B, PWL: 10.65 ± 1.02 s to 12.57 ± 1.78 s, P < 0.05; SNL + MaR1 vs. SNL group, two-way ANOVA) till day 7 (Figure 1A, MWT: 12.76 ± 1.75 g to 21.98 ± 1.53 g, P < 0.05; Figure 1B, PWL: 11.25 ± 1.72 s to 15.66 ± 1.58 s, P < 0.05 vs. SNL group, two-way ANOVA). No significant differences in MWT and PWL were detected between ACSF and SNL groups. The results indicated that MaR1 ameliorated neuropathic pain.
Figure 1.
Intrathecal injection of MaR1 attenuated the mechanical allodynia and thermal hyperalgesia induced by SNL in ipsilateral spinal cord. MWT (A) and PWL (B) showed a sharp decrease after SNL in rats, whereas intrathecal injection of MaR1 alleviated the mechanical allodynia and thermal hyperalgesia induced by SNL. *P < 0.05 and **P < 0.01 vs SNL. Data are expressed as mean ± SD (n = 10).
Abbreviations: MaR1, maresin 1; SNL, spinal nerve ligation; MWT, mechanical withdrawal threshold; PWL, paw withdrawal latency; ACSF, artificial cerebrospinal fluid; POD, postoperative day.
MaR1 inhibited the activation of microglia and astrocytes induced by SNL in spinal dorsal horn
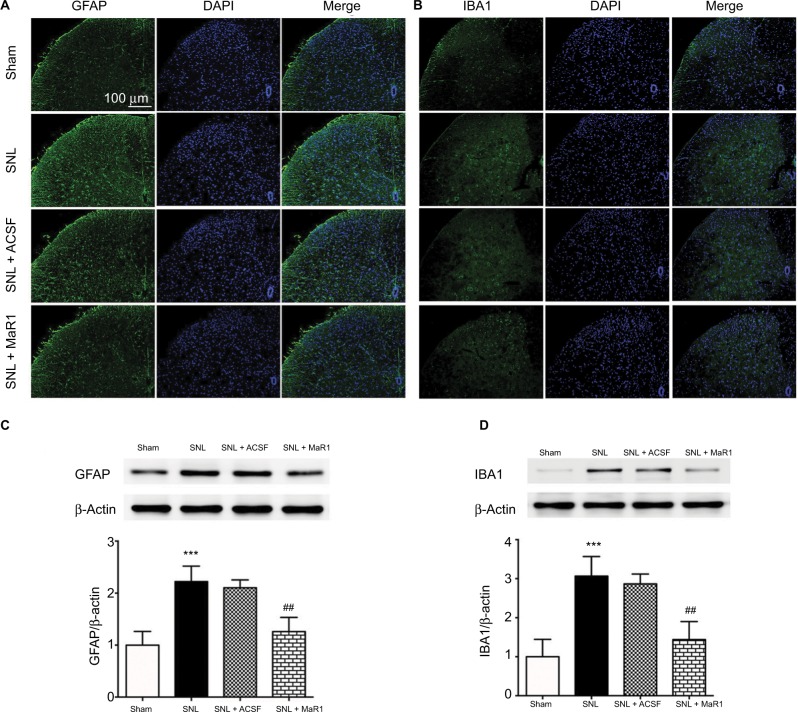
Maintaining the resting state of glial cells plays a crucial role in the homeostasis of the central nervous system.4,8 Noxious stimulus and inflammation can facilitate the activation of glial cells and result in further exacerbation of inflammatory response and intensified neuropathic pain.5,6 Therefore, we checked the number of microglia and astrocytes using cell markers IBA1 and GFAP, respectively. In the sham group, only a few positive cells could be detected in the ipsilateral L4–L5 spinal dorsal horn (Figure 2A and B). Conversely, the levels of IBA1- and GFAP-positive cells which increased significantly following the SNL reduced markedly after intrathecal injection of MaR1 (Figure 2A and B). Western blotting showed that the level of GFAP protein was increased significantly in SNL rats compared with that in sham rats (Figure 2C, P < 0.001; SNL vs. sham group, one-way ANOVA), and MaR1 decreased the expression of GFAP after SNL (Figure 2C, P < 0.01; MaR1 + SNL vs. SNL group, one-way ANOVA). Results of IBA1 protein expression paralleled that of GFAP (Figure 2D). These results validated the role of microglia and astrocytes in the occurrence of neuropathic pain and suggested that intrathecal injection of MaR1 could significantly inhibit the activation of microglia and astrocytes.
Figure 2.
MaR1 suppressed the activation of microglia and astrocytes. Representative immunofluorescent staining of GFAP (A, marker for astrocytes) and IBA1 (B, marker for microglia) to detect activated microglia and astrocytes in the ipsilateral L4–L5 spinal dorsal horn. Western blotting indicated that administration of MaR1 significantly inhibited the expression of GFAP and IBA1 (C and D). ***P < 0.001 vs sham and ##P < 0.01 vs SNL. Data are expressed as mean ± SD (n = 10).
Abbreviations: GFAP, glial fibrillary acidic protein; MaR1, maresin 1; SNL, spinal nerve ligation; ACSF, artificial cerebrospinal fluid.
MaR1 suppressed the activation of NF-κB
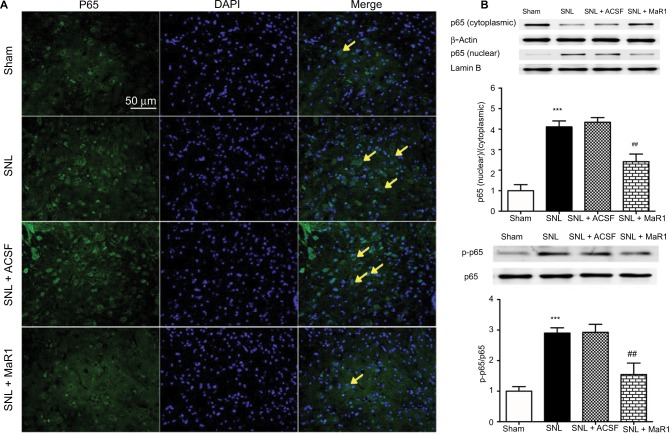
NF-κB has long been identified in the development of inflammatory response and neuropathic pain.5,6,8 To determine the effects of MaR1 on the activation of NF-κB in ipsilateral L4–L5 spinal cord following SNL, we examined the activity of NF-κB on day 7 after surgery using immunofluorescence and Western blot. Immunofluorescence staining indicated that SNL facilitated the translocation of NF-κB p65 from cytoplasm to nuclei, which was reduced by the administration of MaR1 after SNL (Figure 3A). Western blot showed that the nuclear–cytoplasmic ratio of p65 was significantly increased after SNL compared with that in the sham rats (Figure 3B, P < 0.001; SNL vs. sham group, one-way ANOVA), while the nuclear–cytoplasmic ratio of p65 was largely decreased after treatment with MaR1 (Figure 3B, P < 0.01; MaR1 + SNL vs. SNL group, one-way ANOVA). p65 phosphorylation at Ser536 increased sharply following SNL (Figure 3B, P < 0.001; SNL vs. sham group, one-way ANOVA) but was significantly inhibited by intrathecal injection of MaR1 (Figure 3B, P < 0.01; MaR1 + SNL vs. SNL group, one-way ANOVA). These results confirmed the correlation between neuropathic pain and activation of NF-κB and indicated the deactivation of NF-κB by MaR1.
Figure 3.
MaR1 inhibited SNL-induced NF-κB activation and nuclear translocation. (A) Representative immunofluorescent staining of p65 nuclear translocation (arrows) in ipsilateral L4–L5 spinal cord dorsal horn. (B) Western blotting was used to analyze nuclear and cytoplasmic p65 and p65 phosphorylation. Treatment with MaR1 significantly decreased p65 nuclear translocation and phosphorylation. ***P < 0.001 vs sham and ##P < 0.01 vs SNL. Data are expressed as mean ± SD (n = 10).
Abbreviations: MaR1, maresin 1; SNL, spinal nerve ligation; ACSF, artificial cerebrospinal fluid.
Intrathecal injection of MaR1 reduced the production and release of spinal TNF-α, IL-1β and IL-6
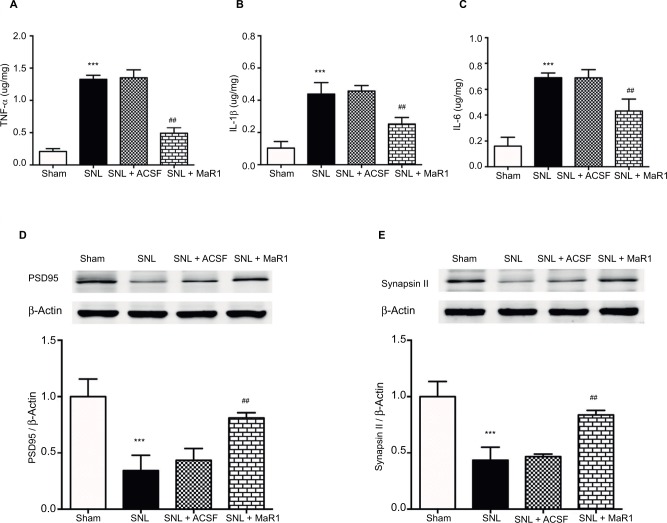
Previous studies have suggested that pro-inflammatory cytokines contribute to the initiation and maintenance of neuropathic pain due to their role in inflammatory propagation and neutrophil recruitment.20 We detected a significant increase of TNF-α, IL-1β and IL-6 in the ipsilateral spinal cord by ELISA on day 7 after SNL (Figure 4A–C, P < 0.001; MaR1 + SNL vs. sham group, one-way ANOVA), while their levels were suppressed by intrathecal injection of MaR1 for 3 continuous days (Figure 4A–C, P < 0.01; MaR1 + SNL vs. SNL group, one-way ANOVA). We also assessed the expression of synapsin II and PSD95, which are crucial synaptic proteins, in the ipsilateral L4–L5 spinal cord. The results revealed that treatment with intrathecal injection of MaR1 significantly recovered the expression of PSD95 and synapsin II (Figure 4D and E, P < 0.01), implying that MaR1 may attenuate neuropathic pain by restoring the synaptic integrity.
Figure 4.
MaR1 inhibited SNL-induced production of cytokines and preserved the synaptic-related proteins from destruction. After the behavioral test on day 7 after surgery, ipsilateral L4–L5 spinal cord tissue was collected for ELISA detection of TNF-α, IL-1β and IL-6 (A–C). MaR1 significantly downregulated the production of TNF-α, IL-1β and IL-6 after 3 days’ continuous injection. Western blotting was used to examine the expression of PSD95 and synapsin II (D and E). MaR1 treatment protected the PSD95 and synapsin II from degradation. ***P < 0.01 vs. sham and ##P < 0.05 vs. SNL. Data are expressed as mean ± SD (n = 10).
Abbreviations: MaR1, maresin 1; SNL, spinal nerve ligation; ELISA, enzyme-linked immunosorbent assay; ACSF, artificial cerebrospinal fluid.
Discussion
In the present study, we demonstrated that MaR1, a PRM, ameliorates neuropathic pain induced by SNL. By suppressing the activation of glia, production and release of pro-inflammatory factors and activation of NF-κB, and restoring synaptic stability, MaR1 exerts anti-inflammatory and pro-resolution effects in combating neuropathic pain of peripheral origin.
Some studies have demonstrated that MaR1 promotes the resolution of inflammation and restores the neurological function in spinal cord injury and peripheral nerve injury in animal models.16,21 Combined with our previous study on the protective effect of MaR1 on ischemic/reperfusion injury in cortex, the present study confirmed the role of MaR1 in neuroprotection and pain relief.
Activation of microglia and astrocytes has been well documented in the exacerbation of chronic neuropathic pain and pain hypersensitivity.22,23 The activation of microglia and astrocytes has been found to facilitate the production and release of inflammatory cytokines and chemokines such as TNF-α, IL-1β and IL-6; reciprocally, these cytokines and chemokines intensify the activation of glial cells.22,23 It is of note that upregulation of these cytokines and activated microglia can also induce NF-κB, which in turn promotes the activation of microglia.24 It has been found that spinal nerve injuries can promote the phosphorylation of NF-κB at p65, which is then translocated to the nucleus, resulting in the upregulation of its downstream target genes such as IL-1β, IL-6 and TNF-α.25 Although the present study could not identify the cellular localization of p65, our results indicated that SNL accelerated the nuclear translocation of p65 and MaR1 inhibited the translocation, which confirmed its therapeutic effect on neuropathic pain and inflammation. Since p65 was reported to be expressed in astrocytes, microglia and neurons in spinal dorsal horn, the cellular localization of p65 needs further study.
Indeed, in the present study, we observed prominent mechanical allodynia and thermal hyperalgesia along with significantly increased number of microglia and astrocytes after nerve injury. In particular, our results indicated that SNL injury activated the NF-κB and elevated inflammatory markers TNF-α, IL-1β and IL-6. This is consistent with previous studies,26 which demonstrated that the production and release of various inflammatory cytokines and chemo-kines, such as TNF-α, IL-1β and IL-6, are rapidly increased within 1–3 days after spinal nerve injury.27–30 In our study, the symptoms of mechanical allodynia and thermal hyperalgesia appeared to be most prominent,19 as well as the expression of inflammatory cytokines and chemokines on day 3 following nerve injury. All the studied cytokines play a major role in pain processing and regional, intrathecal or intracerebro-ventricular injection of IL-1β or/and IL-6 has been found to induce dramatic pain hypersensitivity in rats and mice.27,28 Interventions aimed at reducing these targets were found to produce effective pain relief; for example, TNF-α neutralizing antibody can ameliorate mechanical allodynia and thermal hyperalgesia in different types of animal models of neuropathic pain.31
Heightened inflammatory cascades play a prominent role in neuropathic pain as shown in previous studies;8,32 therefore, an anti-inflammatory or pro-resolving therapy would be an effective option for the treatment of neuropathic pain. Maresins, synthesized in macrophages, are a newly discovered class of lipid mediators. Since MaR1 is an endogenous anti-inflammatory and pro-resolution effector, it has been widely studied in various types of animal models related to inflammatory injury, such as acute lung injury, acute peritonitis and cerebral ischemia/reperfusion injury.13,14,16 Here, intrathecal injection of MaR1, starting on day 3 following nerve injury, significantly reduced the number of active microglia and astrocytes. Further, MaR1 also decreased the translocation and phosphorylation of NF-κB and subsequently reduced the pro-inflammatory cytokines TNF-α, IL-1β and IL-6, suggesting that the potent anti-inflammatory capability of MaR1 can be a therapeutic strategy in combating neuropathic pain.
Further, we examined the synaptic proteins, PSD95 and synapsin II, as activated microglia contribute to synaptic dysfunction and degradation of synaptic proteins.33 Levels of these proteins have been found to significantly decrease after nerve injury.34,35 Here treatment with MaR1 after SNL significantly normalized the expression of PSD95 and synpasin II, suggesting the restoration of synaptic integrity by MaR1. However, the exact mechanisms of these effects need further study.
Limitations
There are some limitations in the present study that should be discussed. First, we only tested the effects of MaR1 on neuropathic pain in the acute phase; long-term evaluation should be investigated in a future study. Second, other models of neuropathic pain must be examined, since SNL is only one type of a neuropathic pain model. Third, we only focused on the effects of MaR1 on neuro-inflammation and NF-κB pathway; other aspects and pathways relating to neuropathic pain also need further research.
Conclusion
In conclusion, MaR1 treatment ameliorated mechanical allodynia and thermal hyperalgesia, inhibiting the activation of microglia and astrocytes as well as the pro-inflammatory response in SNL-induced neuropathic pain. The therapeutic effects of MaR1 on pain hypersensitivity are correlated with decreased activation of microglia and astrocytes and NF-κB signaling pathway, suppressing the cellular inflammatory cascades.
Acknowledgments
The authors are grateful for the enthusiastic support of Mr Kai Zhang (Anhui Film and Television Media Co., Ltd. Wuhu, Anhui, China) in creating the figures. This study was supported by the National Natural Science Foundation of China (Grant No. 80171075 and 81171274), and the Fundamental Research Funds for the Central Universities to Chaoliang Tang (Grant No. WK9110000044).
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Jensen TS, Baron R, Haanpaa M, et al. A new definition of neuropathic pain. Pain. 2011;152(10):2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Buckley DA, Jennings EM, Burke NN, et al. The development of translational biomarkers as a tool for improving the understanding, diagnosis and treatment of chronic neuropathic pain. Mol Neurobiol. 2018;55(3):2420–2430. doi: 10.1007/s12035-017-0492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torigoe K, Nakahara K, Rahmadi M, et al. Usefulness of olanzapine as an adjunct to opioid treatment and for the treatment of neuropathic pain. Anesthesiology. 2012;116(1):159–169. doi: 10.1097/ALN.0b013e31823c7e56. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Couture R, Hong Y. Activated microglia in the spinal cord underlies diabetic neuropathic pain. Eur J Pharmacol. 2014;728:59–66. doi: 10.1016/j.ejphar.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 5.Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153(2):319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333(6048):1462–1466. doi: 10.1126/science.1206243. [DOI] [PubMed] [Google Scholar]
- 7.Sacerdote P, Franchi S, Moretti S, et al. Cytokine modulation is necessary for efficacious treatment of experimental neuropathic pain. J Neuroimmune Pharmacol. 2013;8(1):202–211. doi: 10.1007/s11481-012-9428-2. [DOI] [PubMed] [Google Scholar]
- 8.Jha MK, Jeon S, Suk K. Glia as a link between neuroinflammation and neuropathic pain. Immune Netw. 2012;12(2):41–47. doi: 10.4110/in.2012.12.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354(6312):572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan CN, Yang R, Martinod K, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206(1):15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalli J, Zhu M, Vlasenko NA, et al. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013;27(7):2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Li R, Chen L, et al. Maresin 1 inhibits epithelial-to-mesenchymal transition in vitro and attenuates bleomycin induced lung fibrosis in vivo. Shock. 2015;44(5):496–502. doi: 10.1097/SHK.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 14.Gong J, Wu ZY, Qi H, et al. Maresin 1 mitigates LPS-induced acute lung injury in mice. Br J Pharmacol. 2014;171(14):3539–3550. doi: 10.1111/bph.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xian W, Wu Y, Xiong W, et al. The pro-resolving lipid mediator maresin 1 protects against cerebral ischemia/reperfusion injury by attenuating the pro-inflammatory response. Biochem Biophys Res Commun. 2016;472(1):175–181. doi: 10.1016/j.bbrc.2016.02.090. [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN, Dalli J, Karamnov S, et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26(4):1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malkmus SA, Yaksh TL. Intrathecal catheterization and drug delivery in the rat. Methods Mol Med. 2004;99:109–121. doi: 10.1385/1-59259-770-X:011. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Liu H, Xu S, et al. Spinal translocator protein alleviates chronic neuropathic pain behavior and modulates spinal astrocyte-neuronal function in rats with L5 spinal nerve ligation model. Pain. 2016;157(1):103–116. doi: 10.1097/j.pain.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 19.Yao CY, Weng ZL, Zhang JC, Feng T, Lin Y, Yao S. Interleukin-17A acts to maintain neuropathic pain through activation of CaMKII/CREB signaling in spinal neurons. Mol Neurobiol. 2016;53(6):3914–3926. doi: 10.1007/s12035-015-9322-z. [DOI] [PubMed] [Google Scholar]
- 20.Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun. 2010;24(5):708–723. doi: 10.1016/j.bbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Francos-Quijorna I, Santos-Nogueira E, Gronert K, et al. Maresin 1 promotes inflammatory resolution, neuroprotection, and functional neurological recovery after spinal cord injury. J Neurosci. 2017;37(48):11731–11743. doi: 10.1523/JNEUROSCI.1395-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao YJ, Zhang L, Samad OA, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neu-ropathic pain. J Neurosci. 2009;29(13):4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milligan ED, Twining C, Chacur M, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23(3):1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Xie R, Hu S, et al. Upregulation of cystathionine beta-synthetase expression by nuclear factor-kappa B activation contributes to visceral hypersensitivity in adult rats with neonatal maternal deprivation. Mol Pain. 2012;8:89. doi: 10.1186/1744-8069-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KM, Kang BS, Lee HL, et al. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci. 2004;19(12):3375–3381. doi: 10.1111/j.0953-816X.2004.03441.x. [DOI] [PubMed] [Google Scholar]
- 26.Sun T, Song WG, Fu ZJ, Liu ZH, Liu YM, Yao SL. Alleviation of neuropathic pain by intrathecal injection of antisense oligonucleotides to p65 subunit of NF-kappaB. Br J Anaesth. 2006;97(4):553–558. doi: 10.1093/bja/ael209. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Cao DL, Jiang BC, Yang T, Gao YJ. MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord. Brain Behav Immun. 2015;49:119–129. doi: 10.1016/j.bbi.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, Jiang BC, Cao DL, et al. TRAF6 upregulation in spinal astrocytes maintains neuropathic pain by integrating TNF-alpha and IL-1beta signaling. Pain. 2014;155(12):2618–2629. doi: 10.1016/j.pain.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zang Y, Chen SX, Liao GJ, et al. Calpain-2 contributes to neuropathic pain following motor nerve injury via up-regulating interleukin-6 in DRG neurons. Brain Behav Immun. 2015;44:37–47. doi: 10.1016/j.bbi.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Yang D, Yang Q, Wei X, et al. The role of miR-190a-5p contributes to diabetic neuropathic pain via targeting SLC17A6. J Pain Res. 2017;10:2395–2403. doi: 10.2147/JPR.S133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin TB, Hsieh MC, Lai CY, et al. Fbxo3-dependent Fbxl2 ubiquitination mediates neuropathic allodynia through the TRAF2/TNIK/GluR1 cascade. J Neurosci. 2015;35(50):16545–16560. doi: 10.1523/JNEUROSCI.2301-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazur C, Fitzsimmons B, Kamme F, Nichols B, Powers B, Wancewicz E. Development of a simple, rapid, and robust intrathecal catheterization method in the rat. J Neurosci Methods. 2017;280:36–46. doi: 10.1016/j.jneumeth.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Zimmermann K, Hioki H, Pfeifer A, Baader SL. Efficient and graded gene expression in glia and neurons of primary cerebellar cultures transduced by lentiviral vectors. Histochem Cell Biol. 2015;143(1):109–121. doi: 10.1007/s00418-014-1260-8. [DOI] [PubMed] [Google Scholar]
- 34.Schmidtko A, Luo C, Gao W, Geisslinger G, Kuner R, Tegeder I. Genetic deletion of synapsin II reduces neuropathic pain due to reduced glutamate but increased GABA in the spinal cord dorsal horn. Pain. 2008;139(3):632–643. doi: 10.1016/j.pain.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Peng HY, Chen GD, Lai CY, Hsieh MC, Lin TB. Spinal SIRPalpha1-SHP2 interaction regulates spinal nerve ligation-induced neuropathic pain via PSD-95-dependent NR2B activation in rats. Pain. 2012;153(5):1042–1053. doi: 10.1016/j.pain.2012.02.006. [DOI] [PubMed] [Google Scholar]