Abstract
The hematogenous metastatic pattern of gastric cancer (GC) was not fully explored. Here we analyzed the frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from GC patients. Data queried for this analysis included GC patients from the Surveillance, Epidemiology, and End Results Program database from 2010 to 2014. All of statistical analyses were performed using the Intercooled Stata 13.0 (Stata Corporation, College Station, TX). All statistical tests were two‐sided. Totally, there were 19 022 eligible patients for analysis. At the time of diagnosis, there were 7792 patients at stage IV, including 3218 (41.30%) patients with liver metastasis, 1126 (14.45%) with lung metastasis, 966 (12.40%) with bone metastasis and 151 (1.94%) with brain metastasis. GC patients with lung or liver metastasis have a higher risk of bone and brain metastasis than those without lung nor liver metastasis. Intestinal subtype had significantly higher rate of liver and lung metastasis, while diffuse type was more likely to have bone metastasis. Proximal stomach had significantly higher risk to develop metastasis than distal stomach. African‐Americans had the highest risk of liver metastasis and Caucasian had the highest prone to develop lung and brain metastasis. The median survival for patients with liver, lung, bone, and brain metastasis was 4 months, 3 months, 4 months and 3 months, respectively. It is important to evaluate the status of bone and brain metastasis in GC patients with lung or liver metastasis. Knowledge of metastatic patterns is helpful for clinicians to design personalized pretreatment imaging evaluation for GC patients.
Keywords: gastric cancer, metastases, SEER
1. INTRODUCTION
Gastric cancer (GC) was a leading cause of cancer‐related deaths in the early 20th century but both incidence and mortality rates have steadily declined over the last century in the United States.1, 2 GC is still the second most common cause of cancer‐related death in developing countries.3, 4, 5, 6, 7, 8, 9, 10 The estimated new GC patients in United States in 2017 are 28 000 and estimated deaths are 10 960.11 Almost one‐third of GC were diagnosed at stage IV.12
From 2010 on, the Surveillance, Epidemiology and End Results (SEER) data started to announce metastatic pattern including liver, lung, bone, and brain. The major feature of recurrence in GC patients is intraabdominal spread.13 Liver is the most common site of hematogenous metastasis of GC.14, 15 Lung metastasis is reported to be around 0.5%‐0.96% in GC patients.16, 17, 18 Bone metastasis by GC is rare, occurring in only 0.9%‐3.8% of gastric cancer patients.19, 20, 21, 22 Brain metastasis from GC is relatively rare and the incidence rate is about 0.16%‐0.69%.23, 24 Most reports on bone and brain metastasis from GC are case reports. Due to the limitation of sample size, the incidence rate of metastasis to above sites may not be estimated sufficiently.
In this study, we used data from the SEER cancer‐registry program of individuals diagnosed with GC from 2010 to 2014 to analyze the metastatic pattern. Knowledge of metastatic distribution may help physicians to design imagine examination, especially in making determinations regarding curative‐intent interventions.
2. METHODS
2.1. Database
The SEER database is the largest publicly available cancer dataset. It is a population‐based cancer registry across several disparate geographic regions. The SEER research data include cancer incidence and prevalence as well as demographic information tabulated by age, sex, race/ethnicity, year of diagnosis, marital status, insurance, Tumor‐Node‐Metastasis (TNM) stage and geographic region. The exact dataset we used for this analysis was SEER Program (http://www.seer.cancer.gov) Research Data (1973‐2014), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, based on the November 2016 submission. The SEER database started to release metastatic information related to liver, lung, bone and brain in 2010. On 7 December 2017, the SEER database just released US Mortality databases including 2015 deaths. Therefore it is possible to analyze the 5 year cause specific survival for patients from 2010 on using the SEER database.
2.2. Outcome variables
Variable definitions information on age at diagnosis, sex, year of diagnosis, race/ethnicity, marital status, primary site, tumor grade and differentiation, histology, lymph node involvement, AJCC 7th TNM stage, insurance status and overall survival were coded and available in SEER database.
The primary site was defined by the following International Classification of Diseases for Oncology (ICD‐O‐2) codes: cardia, (C16.0), fundus (C16.1), body (C16.2), antrum (C16.3), pylorus (C16.4), lesser curvature (C16.5), greater curvature (C16.6), overlapping lesion (C16.7) and stomach, NOS (C16.9).
Grade and differentiated was defined by the following ICD‐O‐2 codes; well differentiated (Code 1), moderate differentiated (Code 2), poorly differentiated (Code 3) and undifferentiated (Code 4).
Histological types were defined by the following ICD‐O‐3 codes: 8140 to 8147, 8210 to 8211, 8220 to 8221, and 8260 to 8263 for adenocarcinoma, 8480 and 8481 for mucinous adenocarcinoma, and 8490 for Signet ring cell carcinoma.
For the Race/Ethnicity, we reclassified patients into five groups: “Caucasian,” “African‐American,” “Asian,” “Others,” and “Unknown.”
For the insurance status, individuals in the “Any Medicaid,” “Insured,” and “Insured/No specifics” groups were clustered together as “Insured group.” Patients were therefore divided into “insured group” and “uninsured group”.
Patients were classified as married and unmarried. Since the group of “Unmarried or domestic partner” is misleading and we removed this group of patients from analysis. Unmarried patients included single, separated/divorced, and widowed.
2.3. Patient population
The study population was based on the SEER cancer registry. We restricted eligibility to patients with gastric adenocarcinoma (including mucinous adenocarcinoma and signet ring cell carcinoma) from 2010 to 2014. We excluded cases without records of follow‐up (survival time code of 0 months) and TNM stage.
2.4. Statistical methods
The patients’ demographic and tumor characteristics were summarized with descriptive statistics. Comparisons of categorical variables were performed using the Chi square test, and continuous variables were compared using Student's t test. The primary endpoint of this study was 5‐year cause specific survival (CSS), which was calculated from the date of diagnosis to the date of cancer specific death. Deaths attributed to gastric cancer were treated as events and deaths from other causes were treated as censored observations. Survival function estimation and comparison among different variables were performed using Kaplan‐Meier estimates and the log‐rank test. The independence of the prognostic factors was adjusted for other known factors including age at diagnosis and tumor stage. All of statistical analyses were performed using the Intercooled Stata 13.0 (Stata Corporation, College Station, TX). Statistical significance was set at two‐sided P < 0.05.
2.5. Informed consent
This study was deemed exempt from institutional review board approval by Sun Yat‐sen University Cancer Center and informed consent was waived.
3. RESULT
3.1. Patient characteristics
The study group consisted of 19 022 patients, including 12 208 men (64.18%) and 6814 women (35.82%). The median age of the whole group was 66 years old. The distribution of AJCC 7th TNM stage from I to IV were 21.63%, 14.92%, 22.48%, and 40.96%, respectively.
3.2. Metastasis pattern
At the time of diagnosis, there were 3218 (16.92%) patients with liver metastasis, 1126 (5.92%) patients with lung metastasis, 966 (5.08%) patients with bone metastasis and 151 (0.79%) patients with brain metastasis. Patients who had metastasis to either one of the four sites accounted for 60.73% (4732/7792) of stage IV diseases. Clinical features of GC patients were presented in Table 1.
Table 1.
Clinical features and metastasis sites for gastric cancer
| Features | Liver (%) | P a | Lung (%) | P b | Bone (%) | P c | Brain (%) | P d | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | |||||
| Sex | ||||||||||||
| Women | 5753 (86.24) | 918 (13.76) | <0.001 | 6276 (94.58) | 360 (5.42) | 0.006 | 6322 (95.18) | 320 (4.82) | 0.083 | 6590 (99.43) | 38 (0.57) | 0.007 |
| Men | 9703 (80.84) | 2300 (19.16) | 11148 (93.57) | 766 (6.43) | 11302 (94.59) | 646 (5.41) | 11816 (99.05) | 113 (0.95) | ||||
| Age at diagnosis | 65.74 ± 14.15 | 65.66 ± 13.20 | 0.785 | 65.82 ± 13.95 | 64.21 ± 14.52 | 0.0002 | 65.99 ± 13.93 | 60.68 ± 13.99 | <0.001 | 65.75 ± 13.98 | 61.34 ± 13.64 | 0.0001 |
| Married status | ||||||||||||
| Married | 8786 (82.61) | 1850 (17.39) | 0.840 | 9925 (94.04) | 629 (5.96) | 0.142 | 10021 (94.71) | 560 (5.29) | 0.874 | 10482 (99.19) | 86 (0.81) | 0.798 |
| Unmarried | 5880 (82.72) | 1228 (17.28) | 6614 (93.50) | 460 (6.50) | 6712 (94.76) | 371 (5.24) | 7003 (99.15) | 60 (0.85) | ||||
| Ethnicity | ||||||||||||
| Caucasian | 10743 (82.21) | 2325 (17.79) | <0.001 | 12128 (93.56) | 835 (6.44) | <0.001 | 12300 (94.53) | 712 (5.47) | 0.151 | 12868 (99.02) | 127 (0.98) | 0.002 |
| African‐American | 1909 (80.18) | 472 (19.82) | 2234 (93.91) | 145 (6.09) | 2265 (95.45) | 108 (4.55) | 2356 (99.58) | 10 (0.42) | ||||
| Asian | 2413 (87.68) | 339 (12.32) | 2623 (95.76) | 116 (4.24) | 2614 (95.47) | 124 (4.53) | 2717 (99.49) | 14 (0.51) | ||||
| Primary tumor sites | ||||||||||||
| Cardia | 5003 (78.77) | 1348 (21.23) | <0.001 | 5806 (92.13) | 496 (7.87) | <0.001 | 5956 (94.20) | 367 (5.80) | <0.001 | 6235 (98.75) | 79 (1.25) | <0.001 |
| Fundus | 520 (76.36) | 161 (23.64) | 616 (91.26) | 59(8.74) | 652 (96.02) | 27(3.98) | 667 (98.23) | 12 (1.77) | ||||
| Body | 1440 (84.51) | 264 (15.49) | 1621 (95.69) | 73 (4.31) | 1603 (94.46) | 94 (5.54) | 1685 (99.59) | 7 (0.41) | ||||
| Antrum | 3112 (87.54) | 443 (12.46) | 3427 (96.81) | 113 (3.19) | 3436 (97.17) | 100 (2.83) | 3526 (99.77) | 8 (0.23) | ||||
| Pylorus | 425 (87.45) | 61 (12.55) | 465 (96.67) | 16(3.33) | 469 (97.10) | 14(2.90) | 480 (99.59) | 2 (0.41) | ||||
| Lesser curvature | 1255 (87.95) | 172 (12.05) | 1367 (96.34) | 52 (3.66) | 1373 (96.55) | 49 (3.45) | 1415 (99.58) | 6 (0.42) | ||||
| Greater curvature | 559 (85.21) | 97 (14.79) | 624 (95.41) | 30(4.59) | 623 (95.70) | 28(4.30) | 650 (99.39) | 4 (0.61) | ||||
| Overlapping | 1248 (85.25) | 216 (14.75) | 1380 (95.11) | 71 (4.89) | 1387 (94.74) | 77 (5.26) | 1457 (99.59) | 6 (0.41) | ||||
| Histology | ||||||||||||
| Adenocarcinoma | 11459 (79.74) | 2912 (20.26) | <0.001 | 13322 (93.5) | 926 (6.5) | <0.001 | 13624 (95.47) | 646 (4.53) | <0.001 | 14134 (99.18) | 117 (0.82) | <0.001 |
| Mucinous | 327 (86.28) | 52 (13.72) | 350 (93.09) | 26(6.91) | 362 (94.52) | 21(5.48) | 376 (99.47) | 2 (0.53) | ||||
| Signet ring cell carcinoma | 3670 (93.53) | 254 (6.47) | 3752 (95.57) | 174 (4.43) | 3638 (92.41) | 299 (7.59) | 3896 (99.19) | 32 (0.81) | ||||
| Grade | ||||||||||||
| Well | 699 (91.25) | 67 (8.75) | <0.001 | 735 (96.2) | 29 (3.8) | <0.001 | 757 (98.95) | 8 (1.05) | <0.001 | 766 (100) | 0 (0) | <0.001 |
| Moderately | 3487 (79.67) | 890 (20.33) | 4075 (93.94) | 263 (6.06) | 4225 (97.28) | 118 (2.72) | 4300 (99.01) | 43 (0.99) | ||||
| Poorly | 8801 (84.78) | 1580 (15.22) | 9753 (94.57) | 560 (5.43) | 9752 (94.32) | 587 (5.68) | 10258 (99.38) | 64 (0.62) | ||||
| Undifferentiated | 243 (90.33) | 26 (9.67) | 257 (95.9) | 11 (4.1) | 262 (97.4) | 7 (2.60) | 266 (99.63) | 1 (0.37) | ||||
| Lauren | ||||||||||||
| Diffuse | 4745 (93.46) | 332 (6.54) | <0.001 | 4857 (95.8) | 213 (4.20) | <0.001 | 4742 (93.25) | 343 (6.75) | <0.001 | 5039 (99.33) | 34 (0.67) | 0.220 |
| Intestinal | 10151 (78.30) | 2814 (21.70) | 11973 (93.15) | 880 (6.85) | 12272 (95.35) | 599 (4.65) | 12741 (99.11) | 114 (0.89) | ||||
| Insurance | ||||||||||||
| Insured | 14472 (82.92) | 2980 (17.08) | 0.017 | 16294 (94.00) | 1040 (6.00) | 0.052 | 16490 (94.91) | 884 (5.09) | 0.001 | 17206 (99.22) | 136 (0.78) | 0.219 |
| Uninsured | 669 (79.17) | 176 (20.83) | 777 (92.06) | 67(7.94) | 776 (92.05) | 67(7.95) | 830 (98.69) | 11 (1.31) | ||||
| Surgery | ||||||||||||
| Yes | 8434 (97.28) | 236 (2.72) | <0.001 | 8611 (99.39) | 53 (0.61) | <0.001 | 8625 (99.65) | 30 (0.35) | <0.001 | 8648 (99.86) | 12 (0.14) | <0.001 |
| No | 6827 (69.70) | 2968 (30.30) | 8608 (88.93) | 1071 (11.07) | 8796 (90.42) | 932 (9.58) | 9552 (98.57) | 139 (1.43) | ||||
NOS, not otherwise specified.
The comparison between patients with and without liver metastasis.
The comparison between patients with and without lung metastasis.
The comparison between patients with and without bone metastasis.
The comparison between patients with and without brain metastasis.
3.3. Liver metastasis
Male patients had higher percentage of liver metastasis than female. For ethnicity, African‐American patients had highest percentage (19.82%) of liver metastasis and Asian patients had lowest (12.32%). Primary tumor at the cardia and fundus had higher percentage of liver metastasis, while antrum, pylorus and lesser curvature had lower liver metastatic rate. Signet ring cell carcinoma had lowest rate of liver metastasis compared with adenocarcinoma and mucinous adenocarcinoma. For the tumor grade, moderately differentiated tumors had highest percentage (20.33%) of liver metastasis and well differentiated tumors had lowest (8.75%). In the Lauren classification, intestinal subtype had significantly higher rate of liver metastasis than diffuse type, 21.70% vs 6.54%, P < 0.001. The metastatic rate in uninsured patients was significantly higher than insured patients, P = 0.017. Age was not significantly different between patients with and without liver metastasis.
3.4. Lung metastasis
Features for patients with lung metastasis were similar to those with liver metastasis, including male predominant and more intestinal subtype. Patients with lung metastasis were significantly younger than those without. Caucasian patients had higher percentage of lung metastasis than African‐American and Asian patients. Mucinous adenocarcinoma patients had higher lung metastatic rate than adenocarcinoma and signet ring cell carcinoma.
3.5. Bone metastasis
There was no significant difference of bone metastasis between male and female. The median age of patients with bone metastasis was 6 years younger than those without. The bone metastatic rate was not significantly different among races. Cardia and body had higher percentages of bone metastasis, while antrum and pyrolus had lowest. Patients with signet ring cell carcinoma had higher percentage of bone metastasis than mucinous and adenocarcinoma. Poorly differentiated tumors had highest bone metastatic rate and well differentiated tumors had the lowest. For Lauren classification, diffuse type had higher bone metastatic rate than intestinal type.
3.6. Brain metastasis
The median age of patients with bone metastasis was 5 years younger than those without. Caucasian patients had higher percentage of brain metastasis than African‐American and Asian patients. Mucinous patients had lower percentage of brain metastasis than adenocarcinoma and signet ring cell carcinoma. Moderately differentiated tumors had higher brain metastatic rate than well, poorly, and undifferentiated tumors. There was no significant difference of brain metastasis between diffuse and intestinal type as well as insured and uninsured type.
3.7. Combination of metastasis patterns
Many patients developed more than one site of metastatic diseases. Table 2 summarized all the possible combinations of these four sites of metastasis. The most common two‐site metastasis combination was liver and lung (2.25%). Only 11 (0.06%) patients had all four sites metastasis.
Table 2.
Frequencies of combination metastasis and 5‐y CSS
| Number (%) | 5‐y CSS (95% CI) | Median OS (mo) | |
|---|---|---|---|
| No metastasis | 11 230 (59.04) | 44.34% (42.47%‐46.19%) | 37 |
| One site | |||
| Only liver | 2247 (11.81) | 5.19% (3.79%‐6.89%) | 5 |
| Only lung | 396 (2.08) | 3.26% (0.09%‐8.11%) | 5 |
| Only bone | 487 (2.56) | 0 | 4 |
| Only brain | 52 (0.27) | 0 | 3 |
| Two sites | |||
| Lung and liver | 428 (2.25) | 2.04% (0.68%‐4.81%) | 3 |
| Lung and bone | 92 (0.48) | 4.71% (1.09%‐12.66%) | 4 |
| Lung and brain | 12 (0.06) | 9.09% (0.54%‐33.29%) | 3 |
| Liver and bone | 172 (0.90) | 1.49% (0.17%‐6.24%) | 4 |
| Liver and brain | 18 (0.09) | 0 | 1 |
| Bone and brain | 15 (0.08) | 4.44% (0.08%‐25.96%) | 2 |
| Three sites | |||
| Lung and liver and bone | 102 (0.54) | 5.08% (1.07%‐14.13%) | 3 |
| Lung and liver and brain | 17 (0.09) | 0 | 2 |
| Liver and bone and brain | 7 (0.04) | 0 | 3 |
| Lung and bone and brain | 6 (0.03) | 16.67% (0.77%‐51.68%) | 1 |
| Four sites | |||
| Liver and lung and bone and brain | 11 (0.06) | 9.09% (0.54%‐33.29%) | 2 |
| Metastasis to other sites | 3060 (16.09) | 2.60% (1.14%‐5.10%) | 7 |
| Metastasis to unknown combination | 670 (3.52) | 2.77% (1.36%‐5.01%) | 4 |
CSS, Cause Specific Survival; CI, confidence interval; OS, Overall survival.
Furthermore, we compared the risk of bone and brain metastasis between patients with and without lung or liver metastasis. We found that patients with lung metastasis had a higher risk of bone (20.24% vs 4.06%, P < 0.001) or brain metastasis (4.27% vs 0.54%, P < 0.001) than patients without (Figure 1). Though a similar phenomenon was noted for liver metastasis, with higher risk of bone (10.20% vs 4.04%, P < 0.001) and brain metastasis (1.75% vs 0.57%, P < 0.001) for liver metastasis patients than those without, GC patients with lung metastasis had a higher incidence rate of bone or brain metastasis than patients with liver metastasis.
Figure 1.
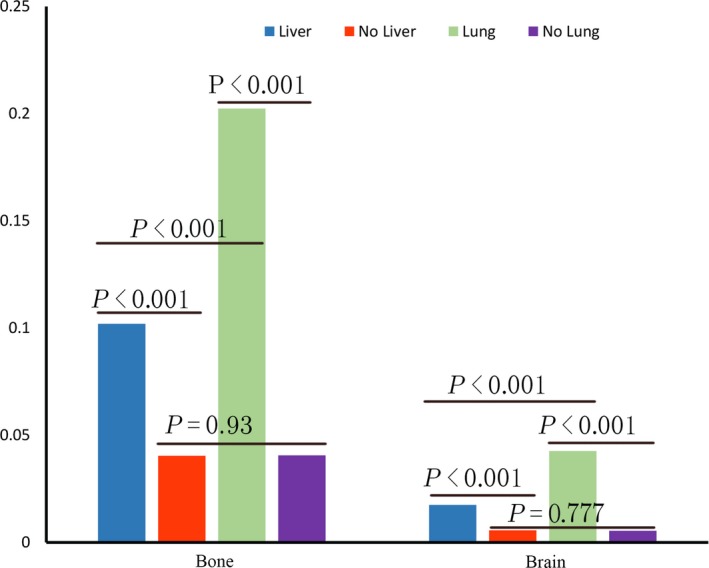
Comparisons of Metastatic Rate to Bone and Brain With and Without Liver or Lung Metastasis
3.8. Survival
In this study, 10 029 deaths (10.56%) were observed. The 5‐year CSS was 27.77% for the whole cohort, with a median OS of 14 months. The 5‐year CSS was 4.28% vs 33.24% for patients with and without liver metastasis (P < 0.001), 3.35% vs 30.01% for patients with and without lung metastasis (P < 0.001), 1.27%% vs 29.86% for patients with and without bone metastasis (P < 0.001) and 2.32% vs 28.68% for patients with and without brain metastasis (P < 0.001) (Figure 2). The median OS for patients with liver, lung, bone, and brain metastasis was 4 months, 3 months, 4 months, and 3 months, respectively. The median OS and 5‐year CSS for patients with different combination of metastasis was showed in Table 2.
Figure 2.
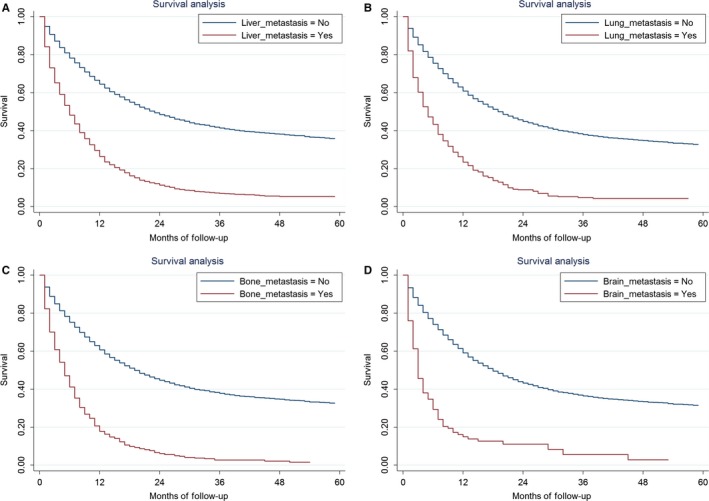
Cause Specific Survival between Patients With and Without Liver Metastasis, P < 0.001 (A), with and without lung metastasis, P < 0.001 (B), with and without bone metastasis, P < 0.001 (C), with and without brain metastasis, P < 0.001 (D)
3.9. Prognostic factors for GC patients with liver metastasis
Since liver metastasis is the most common site of hematogenous metastasis. We analyzed the prognostic factors for liver metastasis from GC patients. Variables showing a trend for association with survival (P < 0.05) in univariated analysis were selected in the cox proportional hazards model. Sex, age, married status, location, Lauren classification, histology, grade, insurance, and surgery were selected in the multivariate analysis. Age, marital status, histologic subtypes, insurance, and surgery were all independent prognostic factors in the multivariable analysis (Table 3).
Table 3.
Survival analysis for GC patients with liver metastasis
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Median OS (mo) | 5‐y CSS (95% CI) | P value | Hazard ratio | 95% CI | P value | |
| Sex | ||||||
| Male | 5 | 4.09% (2.89%‐5.60%) | Reference | |||
| Female | 4 | 4.84% (2.97%‐7.36%) | 0.044 | 1.022 | 0.93‐1.13 | 0.662 |
| Age | ||||||
| <66 | 6 | 4.50% (2.92%‐6.57%) | Reference | |||
| >65 | 3 | 4.00% (2.73%‐5.63%) | <0.001 | 1.276 | 1.17‐1.40 | <0.001 |
| Married status | ||||||
| Married | 5 | 4.28% (3.01%‐5.88%) | Reference | |||
| Unmarried | 3 | 3.72% (2.24%‐5.78%) | <0.001 | 1.239 | 1.13‐1.36 | <0.001 |
| Location | ||||||
| Cardia | 6 | 2.98% (1.66%‐4.92%) | Reference | |||
| Fundus | 3 | 7.02% (2.77%‐14.00%) | 0.932 | 0.75‐1.15 | 0.515 | |
| Body | 4 | 5.50% (2.43%‐10.41%) | 1.042 | 0.87‐1.24 | 0.648 | |
| Antrum | 4 | 8.18% (5.08%‐12.19%) | 1.059 | 0.92‐1.22 | 0.428 | |
| Pylorus | 4 | 7.26% (1.74%‐18.33%) | 0.958 | 0.68‐1.35 | 0.808 | |
| Lesser curvature | 6 | 8.19% (3.18%‐18.06%) | 0.870 | 0.71‐1.07 | 0.193 | |
| Greater curvature | 2 | 1.8% (0.16%‐8.25%) | 1.390 | 1.06‐1.83 | 0.019 | |
| Overlapping | 2 | 0 (0) | 1.481 | 1.23‐1.78 | <0.001 | |
| NOS | 3 | 2.65% (0.9%‐6.09%) | <0.001 | 1.255 | 1.10‐1.44 | 0.001 |
| Race/Ethnicity | ||||||
| Caucasian | 5 | 4.13% (2.94%‐5.62%) | — | |||
| African‐American | 4 | 4.32% (1.88%‐8.35%) | — | |||
| Asian | 5 | 4.53% (2.08%‐8.42%) | — | |||
| Others | 4 | 4.20% (0.44%‐15.65%) | 0.9216 | — | ||
| Lauren | ||||||
| Diffuse | 4 | 2.30% (0.58%‐6.32%) | Reference | |||
| Intestinal | 5 | 4.51% (3.37%‐5.89%) | 0.0144 | 0.87 | 0.66‐1.16 | 0.351 |
| Histology subgroup | ||||||
| Adenocarcinoma | 5 | 4.62% (3.49%‐5.97%) | Reference | |||
| Signet Ring cell | 4 | 1.15% (0.11%‐5.30%) | 1.20 | 1.02‐1.41 | 0.029 | |
| Mucinous adenocarcinoma | 3 | 2.40% (0.19%‐10.79%) | 0.0012 | 1.57 | 1.14‐2.18 | 0.006 |
| Grade | ||||||
| Well differentiated | 4 | 3.59% (0.32%‐14.52%) | Reference | |||
| Moderately differentiated | 6 | 6.43% (4.18%‐9.31%) | 0.81 | 0.59‐1.13 | 0.213 | |
| Poorly differentiated | 4 | 3.26% (2.04%‐4.94%) | 1.05 | 0.76‐1.45 | 0.777 | |
| Undifferentiated | 3 | 0 (0) | <0.001 | 0.83 | 0.46‐1.49 | 0.527 |
| Insurance | ||||||
| Insured | 5 | 4.45% (3.36%‐5.74%) | Reference | |||
| Uninsured | 3 | 2.45% (0.25%‐10.10%) | 0.0262 | 1.31 | 1.07‐1.61 | 0.01 |
| Surgery | ||||||
| Yes | 9 | 17.74% (12.18%‐24.16%) | Reference | |||
| No | 4 | 2.95% (1.97%‐4.23%) | <0.001 | 1.82 | 1.52‐2.18 | <0.001 |
OS, Overall survival; CSS, Cause Specific Survival; CI, Confidence Intervals; NOS, Not otherwise specified.
4. DISCUSSION
In our study, the metastatic rate to liver, lung, bone and brain from GC patients at the time of diagnosis was 16.92%, 5.92%, 5.08%, and 0.79%, respectively. The metastatic rate to above four sites were much higher than previous literature reports, which were 13.5% for liver metastasis,15 0.5%‐0.96% for lung metastasis,16, 17, 18 0.9%‐3.8% for bone metastasis19, 20, 21, 22 and 0.16%‐0.69% for brain metastasis.23, 24 The actual frequency of metastasis to bone and brain originating from GC was underestimated because usually bone and brain imaging was not performed as a routine evaluation and asymptomatic metastasis might be overlooked.25, 26 In our study, we found that the frequency of bone metastasis was similar to lung metastasis. More attention should be paid to evaluate status of metastasis to above sites using appropriate modalities. However it is inappropriate to recommend all the GC patients to screen metastasis to above sites, especially brain metastasis whose metastatic rate was less than 1%. It is feasible to find out the risk factors for metastasis. We found that patients with lung or liver metastasis have a higher risk of bone and brain metastasis than those without lung nor liver metastasis. Our previous study showed similar phenomenon in colorectal cancer.27 The internal mechanism of this distribution remains unknown. Primary lung cancer frequently metastasizes to bone as well as brain.28, 29, 30, 31 Some studies had suggested that there was cross‐talk among lung tumor cells, bone microenvironment, and immune system, which led to bone metastasis formation in primary non‐small cell lung cancer patients.32 This finding is helpful for us to design screen strategy. GC patients with lung or liver metastasis had higher risk of brain and bone metastasis. Therefore it is important to evaluate status of bone and brain metastasis in patients with lung or liver metastasis.
Our analysis found that intestinal subtype had significantly higher rate of liver and lung metastasis while diffuse type was more likely to have bone metastasis. Previous studies showed that liver metastases were more frequent in the intestinal type, while diffuse type had a greater propensity to metastasize to distant organs, including peritoneal dissemination.9, 33 Moderately differentiated tumors were more likely to have liver, lung, and brain metastasis, and poorly differentiated tumors had a prone to develop bone metastasis. The most common histopathological subtype for bone metastasis from GC was adenocarcinoma (79%) with poor differentiation (60.8%).22 Uninsured patients had higher percentages of liver and bone metastasis than insured patients. We did not found related reports in the literatures, while previous reports showed that insured GC patients were more likely to receive surgery and had better prognosis than uninsured.34 We therefore guessed that insured patients might receive more early intervention of GC and have a lower risk to develop metastatic diseases. Moreover, patients with proximal stomach cancer had significantly higher risk to develop metastasis than those with distal stomach cancer. For ethnicity, Asian patients had the lowest possibility to develop metastasis, while African‐Americans had the highest risk of liver metastasis and Caucasian had the highest prone to develop lung and brain metastasis. The relationship between tumor location, ethnicity and metastasis was not clearly described in the literatures. Our findings are helpful for clinicians to design personalized examinations for GC patients.
The outcome for GC patients with metastasis was poor, which were 4 months, 3 months, 4 months, and 3 months for metastasis to liver, lung, bone, and brain, respectively. The 5‐year survival for patients with liver metastasis was 11.4% in Japanese GC patients.35 The median survival times of 3‐4 months after detection of bone metastasis have been reported in some studies36, 37 and the median survival after treatment of brain metastasis was about 3 months.23 The univariated and multivariate analysis showed that younger patient, married, well and moderately differentiated tumor and surgery were related with better prognosis for GC patients with liver metastasis. Our previous studies showed that married GC patients were at lower risk of cancer specific mortality with the possibility that spouse might provide social supports and encourage the patients to seek medical treatments.38
To our knowledge, this is the first SEER‐based study focusing solely on the hematogenous metastatic pattern of GC patients. However, there are obvious limitations due to the retrospective nature of this study, as outlined below. First of all, it is important to note that the database only provide metastatic data to above 4 sites from 2010 on and the follow‐up time is not long enough. Moreover, we only have information on synchronous metastasis to liver, lung, bone, and brain, a relative minority compared to those patients who may develop metachronous metastasis. Furthermore, we don't have information of metastasis to other sites, especially the peritoneal metastasis. Overall, this is the first study to confirm the strong potential for bone and brain metastasis in patients who already have lung or liver metastasis. Based on our finding, we suggest that clinicians take the clinicopathological features into account when designing diagnostic and treatment algorithms.
5. CONCLUSIONS
It is important to evaluate status of bone and brain metastasis in GC patients with lung or liver metastasis. Knowledge of metastatic patterns is helpful for clinicians to design personalized pretreatment imaging evaluation for GC patients.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGMENTS
We would like to thank the staff members of the National Cancer Institute and their colleagues across the United States and at Information Management Services, Inc., who have been involved with the Surveillance, Epidemiology and End Results (SEER) Program.
Qiu M‐Z, Shi S‐M, Chen Z‐H, et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER‐based study. Cancer Med. 2018;7:3662–3672. 10.1002/cam4.1661
Funding Information
This work was supported by National Natural Science Foundation of China (Grant number. 81602066 and 81772587); the Fundamental Research Funds for the Central Universities (Grant number. 16ykpy25); the third outstanding young talents training plan and Medical Scientist program of Sun Yat‐sen University cancer center; Natural Science Foundation of Guangdong Province, China (Grant number. 2014A030312015); Science and Technology Program of Guangdong (2015B020232008); Science and Technology Program of Guangzhou (15570006, 201508020250, 201604020003); Science and Technology Planning Project of Guangzhou, China (Grant number. 201510161726583).
Qiu, Shi and Chen contributed equally to the manuscript.
Contributor Information
Da‐Jun Yang, Email: yangdj@sysucc.org.cn.
Rui‐Hua Xu, Email: xurh@sysucc.org.cn.
REFERENCES
- 1. Wingo PA, Cardinez CJ, Landis SH, et al. Long‐term trends in cancer mortality in the United States, 1930‐1998. Cancer. 2003;97:3133‐3275. [DOI] [PubMed] [Google Scholar]
- 2. Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1‐27. [DOI] [PubMed] [Google Scholar]
- 3. Li YH, Qiu MZ, Xu JM, et al. S‐1 plus cisplatin versus fluorouracil plus cisplatin in advanced gastric or gastro‐esophageal junction adenocarcinoma patients: a pilot study. Oncotarget. 2015;6:35107‐35115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qiu M, Zhou Y, Zhang X, et al. Lauren classification combined with HER2 status is a better prognostic factor in Chinese gastric cancer patients. BMC Cancer. 2014;14:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qiu MZ, Li Q, Wang ZQ, et al. HER2‐positive patients receiving trastuzumab treatment have a comparable prognosis with HER2‐negative advanced gastric cancer patients: a prospective cohort observation. Int J Cancer. 2014;134:2468‐2477. [DOI] [PubMed] [Google Scholar]
- 6. Qiu MZ, Xu RH. The progress of targeted therapy in advanced gastric cancer. Biomark Res. 2013;1:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137‐2150. [DOI] [PubMed] [Google Scholar]
- 8. Qiu MZ, Wang ZQ, Zhang DS, et al. Comparison of 6th and 7th AJCC TNM staging classification for carcinoma of the stomach in China. Ann Surg Oncol. 2011;18:1869‐1876. [DOI] [PubMed] [Google Scholar]
- 9. Marrelli D, Roviello F, de Manzoni G, et al. Different patterns of recurrence in gastric cancer depending on Lauren's histological type: longitudinal study. World J Surg. 2002;26:1160‐1165. [DOI] [PubMed] [Google Scholar]
- 10. Zheng R, Zeng H, Zhang S, Chen W. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer. 2017;36:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7‐30. [DOI] [PubMed] [Google Scholar]
- 12. Jim MA, Pinheiro PS, Carreira H, Espey DK, Wiggins CL, Weir HK. Stomach cancer survival in the United States by race and stage (2001‐2009): findings from the CONCORD‐2 study. Cancer. 2017;123(Suppl 24):4994‐5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236‐242. [DOI] [PubMed] [Google Scholar]
- 14. Li J, Xi H, Cui J, et al. Minimally invasive surgery as a treatment option for gastric cancer with liver metastasis: a comparison with open surgery. Surg Endosc. 2017;32:1422‐1433. [DOI] [PubMed] [Google Scholar]
- 15. Marrelli D, Roviello F, De Stefano A, et al. Risk factors for liver metastases after curative surgical procedures for gastric cancer: a prospective study of 208 patients treated with surgical resection. J Am Coll Surg. 2004;198:51‐58. [DOI] [PubMed] [Google Scholar]
- 16. Koga S, Takebayashi M, Kaibara N, et al. Pathological characteristics of gastric cancer that develop hematogenous recurrence, with special reference to the site of recurrence. J Surg Oncol. 1987;36:239‐242. [DOI] [PubMed] [Google Scholar]
- 17. Kong JH, Lee J, Yi CA, et al. Lung metastases in metastatic gastric cancer: pattern of lung metastases and clinical outcome. Gastric Cancer. 2012;15:292‐298. [DOI] [PubMed] [Google Scholar]
- 18. Kanemitsu Y, Kondo H, Katai H, et al. Surgical resection of pulmonary metastases from gastric cancer. J Surg Oncol. 1998;69:147‐150. [DOI] [PubMed] [Google Scholar]
- 19. Guadagni S, Catarci M, Kinoshita T, Valenti M, De Bernardinis G, Carboni M. Causes of death and recurrence after surgery for early gastric cancer. World J Surg. 1997;21:434‐439. [DOI] [PubMed] [Google Scholar]
- 20. Nakamura K, Tomioku M, Nabeshima K, Yasuda S. Clinicopathologic features and clinical outcomes of gastric cancer patients with bone metastasis. Tokai J Exp Clin Med. 2014;39:193‐198. [PubMed] [Google Scholar]
- 21. Yoshikawa K, Kitaoka H. Bone metastasis of gastric cancer. Jpn J Surg. 1983;13:173‐176. [DOI] [PubMed] [Google Scholar]
- 22. Turkoz FP, Solak M, Kilickap S, et al. Bone metastasis from gastric cancer: the incidence, clinicopathological features, and influence on survival. J Gastric Cancer. 2014;14:164‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Esmaeilzadeh M, Majlesara A, Faridar A, et al. Brain metastasis from gastrointestinal cancers: a systematic review. Int J Clin Pract. 2014;68:890‐899. [DOI] [PubMed] [Google Scholar]
- 24. Kasakura Y, Fujii M, Mochizuki F, Suzuki T, Takahashi T. Clinicopathological study of brain metastasis in gastric cancer patients. Surg Today. 2000;30:485‐490. [DOI] [PubMed] [Google Scholar]
- 25. Lohr F, Pirzkall A, Hof H, Fleckenstein K, Debus J. Adjuvant treatment of brain metastases. Semin Surg Oncol. 2001;20:50‐56. [DOI] [PubMed] [Google Scholar]
- 26. Mikami J, Kimura Y, Makari Y, et al. Clinical outcomes and prognostic factors for gastric cancer patients with bone metastasis. World J Surg Oncol. 2017;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiu M, Hu J, Yang D, Cosgrove DP, Xu R. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget. 2015;6:38658‐38666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coello MC, Luketich JD, Litle VR, Godfrey TE. Prognostic significance of micrometastasis in non‐small‐cell lung cancer. Clin Lung Cancer. 2004;5:214‐225. [DOI] [PubMed] [Google Scholar]
- 29. Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795‐803. [DOI] [PubMed] [Google Scholar]
- 30. Sorensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474‐1480. [DOI] [PubMed] [Google Scholar]
- 31. Rodrigus P, de Brouwer P, Raaymakers E. Brain metastases and non‐small cell lung cancer. Prognostic factors and correlation with survival after irradiation. Lung Cancer. 2001;32:129‐36. [DOI] [PubMed] [Google Scholar]
- 32. Roato I. Bone metastases: When and how lung cancer interacts with bone. World J Clin Oncol. 2014;5:149‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rhomberg W, Gruber U. Liver metastasis in cancer of the stomach and its dependence on the histology of the primary tumor: an autopsy study on 102 cases. Clin Exp Metastasis. 1989;7:585‐590. [DOI] [PubMed] [Google Scholar]
- 34. Sherman KL, Merkow RP, Shah AM, Wang CE, Bilimoria KY, Bentrem DJ. Assessment of advanced gastric cancer management in the United States. Ann Surg Oncol. 2013;20:2124‐2131. [DOI] [PubMed] [Google Scholar]
- 35. Nashimoto A, Akazawa K, Isobe Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahn JB, Ha TK, Kwon SJ. Bone metastasis in gastric cancer patients. J Gastric Cancer. 2011;11:38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee J, Lim T, Uhm JE, et al. Prognostic model to predict survival following first‐line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;18:886‐891. [DOI] [PubMed] [Google Scholar]
- 38. Qiu M, Yang D, Xu R. Impact of marital status on survival of gastric adenocarcinoma patients: results from the Surveillance Epidemiology and End Results (SEER) Database. Sci Rep. 2016;6:21098. [DOI] [PMC free article] [PubMed] [Google Scholar]


