Abstract
Sphingosine‐1‐phosphate (S1P) is a bioactive lipid that exerts various pathophysiological functions through binding to its receptor family (S1PRs). Since first report of the breast cancer (BCA) promoting function by S1P production (through the function of sphingosine kinases) and S1P/S1PR signaling, their antagonists have never been successfully progress to clinics after three decades. Taking advantage of bioinformatics linking to gene expression to disease prognosis, we examined the impact of associated genes in BCA patients. We found high gene expressions involved in S1P anabolism suppressed disease progression of patients who are basal cell type BCA or receiving adjuvant therapy. In addition, S1PRs expression also suppressed disease progress of multiple categories of BCA patient progression. This result is contradictory to tumor promoter role of S1P/S1PRs which revealed in the literature. Further examination by directly adding S1P in BCA cells found a cell growth suppression function, which act via the expression of S1PR1. In conclusion, our study is the first evidence claiming a survival benefit function of S1P/S1PR signaling in BCA patients, which might explain the obstacle of relative antagonist apply in clinics.
Keywords: Breast cancer (BCA), Sphingosine‐1‐Phosphate (S1P), S1PR
1. INTRODUCTION
Sphingosine‐1‐phosphate (S1P) is a bioactive sphingolipid metabolite involved in many pathophysiological processes.1, 2, 3, 4 The intracellular level of S1P as a secondary messenger 5 is regulated by several enzymes including anabolism and catabolism. For anabolism, such as sphingosine kinases 1 and 2 (SPHK1, SPHK2) that are responsible for S1P synthesis. And S1P lyase1 (SGPL1) and S1P phosphatase 1 and 2 (SGPP1, SGPP2) that mediated S1P catabolism.5, 6, 7 Besides, lipid phosphate phosphatases (LPP1/2/3) regulates lipid dephosphorylation including hydrolyzing S1P. In addition, S1P could be exported to extracellular environment by the ATP‐binding cassette transporters (ABCA1, ABCC1).5, 8 Recent studies indicated spinster homolog 2 (Spns2) expressed on endothelial cells (ECs) function as S1P transporter.9, 10 In addition, studies showed that red blood cells and platelets requires the major facilitator superfamily transporter 2b (Mfsd2b) to export S1P 11, 12 and maintained S1P level in blood stream. The function of S1P is go through its receptors S1PRs (S1PR1‐5) which belongs to the G‐protein coupled receptor (GPCR) family 2 and each receptor, respectively, involved in cell growth, apoptosis, proliferation, angiogenesis, chemo resistance, or vascular stability.5, 13, 14, 15 It is also a chemotactic factor for immune cell trafficking 5, 16, 17 and a microenvironmental regulator for cancer development.18 The growth rate of breast cancer cells was found to be decreased in SPHK1 or SPHK2 deficient mice.19 Based on these observations, S1P promote breast cancer (BCA) development was hypothesized. The utilization of online cDNA microarray databases meta‐analysis that predicts the outcome with appropriately powered cohorts and provides feasible, unbiased approach to analyze genes in cancer progression. There are online databases to validate the importance of gene expressions in BCA patient survival (http://kmplot.com/analysis/index.php?p=service&cancer=breast). Beneficiary from the advances of bioinformatics, using the web‐based genome‐scale gene expression survival analyzer followed with pathway weighting algorithm, we are able to evaluate gene clusters/pathways‐of‐genes in cancer patient prognosis in a hypothesis‐driven basis.20 To test our hypothesis, we performed Kaplan Meier plotter to investigate the role of S1P in BCA patient survival (http://kmplot.com/analysis/index.php?p=service&cancer=breast) and the association between S1P metabolism and BCA progression. The role of S1P receptors (S1PRs) in BCA development was also investigated.
2. MATERIALS AND METHODS
2.1. Kaplan‐Meier plot for evaluating the impact of genes in BCA patient survival
Data mining was performed using Kaplan‐Meier Plotter (http://kmplot.com/analysis/index.php?p=service&cancer=breast).21 The following genes that may play a role in breast cancer 10‐year relapse free survival (RFS) were assessed SPHK1 (219257_s_at), SPHK2 (209857_s_at), SGPP1 (208381_s_at), SGPP2 (226560_at), LPP1 (210946_at), LPP2 (209529_at), LPP3 (212230_at), SGPL1 (223391_at), S1PR1 (204642_at), S1PR2 (208537_at), S1PR3 (228176_at), S1PR4 (206437_at), and S1PR5 (221417_x_at). Databases of the following cohorts were analyzed: nonclassified BCA (n = 1660); ER +/− (n = 695/313); PR +/− (n = 489/372); HER2 +/− (n = 150/635); lymph node metastasis positive and negative (n = 724/496); grade 1, grade 2, and 3 (n = 108/227/444); and intrinsic subtypes including basal (n = 339), luminal A (n = 841), and luminal B (n = 407): Tp53 mutant or wild type (n = 132/82); with systemically treatment (n = 751); include or exclude endocrine therapy or Tamoxifen only (n = 335/275/161); and with or without chemotherapy (n = 255/243). According to the gene expression we use median to divide the patients into two groups, low and high expression.
2.2. Determination of Hazard Ratios (HR score)
The following formula was used to calculate HR scores20:
2.3. Cell culture, reagents, and chemicals
The human breast cancer cell line MCF‐7 and human triple negative breast cancer (TNBC) cell lines MDA‐MB‐231 were purchased from the Food Industry Research and Development Institute in Taiwan. Cells were grown in Dulbecco's Modified Eagle Medium (GIBCO, USA) with 10% fetal bovine serum (GIBCO, CA,USA) and 1% penicillin‐streptomycin solution (CORNING, NY, USA) at 37°C and 5% CO2. Sphingosine‐1‐phosphate was obtained commercially (Sigma‐Aldrich, MO, USA) and dissolved in methanol.
2.4. Cell colony formation assay
Effects of S1P on the growth of various types of BCA cells were assessed by colony formation assay. 500 each of MCF‐7 and MDA‐MB 231 cells were seeded separately in wells of a 6‐well plate. S1P was added to the cultures at 0, 2, 4, 6, 8, 10 μmol/L 24 hours after seeding. After 2 weeks of incubation, culture media were removed, and cells were washed with PBS once. The cells in each well were then fixed with 1 mL of 4% formaldehyde for 30 minutes. After removal of the formaldehyde solution, cells in each well were stained with 1.5 mL of 2% crystal violet for 30‐60 minutes, and colony number of each cell type was counted.
2.5. Lentiviral production and infection
To investigate the role of S1P in the growth of BCA cells, miRNAs targeting the genes encoding its receptors S1PR1 and S1PR2 were introduced into cells using lentiviral vectors as described previously. Lentiviral plasmid vectors containing shRNA genes targeting S1PR1 (TRCN0000221119; shS1PR1 sequence: CCGGCCCATGTGAAAGCGTCTCTTTCTCGAGAAAGAGACGCTTTCACATGGGTTTTT) and S1PR2 (TRCN0000356882; shS1PR2 sequence: CCGGCAAGGTCCAGGAACACTATAACTCGAGTTATAGTGTTCCTGGACCTTGTTTTTG) and the control plasmid (pLKO.1‐shLuciferase) were obtained from the National RNAi Core Facility, Academia Sinica, Taipei, Taiwan. To produce lentiviral vectors, HEK293T cells were transfected with each of these plasmid together with packaging plasmid (psPAX2) and envelope plasmid (pMD2.G) (Addgene, MA, USA) at a ratio of 2:1:1 by liposome in 3 mL of serum free DMEM (GIBCO, CA, USA). The culture medium was then replaced by 10 mL of fresh DMEM with 10% FBS 4‐6 hours after transfection. The culture supernatants containing recombinant lentiviruses were harvested 48 hours after incubation and filtered through a 0.45 μm filter to remove dead cells and debris. BCA cells at 40‐50% confluence were washed with PBS once and then incubated with 5 mL culture medium containing lentiviral vectors and 8 μg/mL of polybrene (Sigma‐Aldrich, MO, USA) for 24‐48 hours. Cells that were then incubated in fresh DMEM containing puromycin (1 μg/mL) to eliminate those that were not infected with lentivirus.
2.6. RT‐PCR
To evaluate S1PR1 and S1PR2 knockdown, RT‐PCR was performed as described. Briefly, RNAs were isolated from lentivirus infected cells (80‐90% confluence) using the Trizol reagent (Invitrogen, CA, USA) and reverse transcribed to cDNAs using the PrimeScript TM RT reagent kit (TAKARA Bio Inc., Kyoto, Japan). The real‐time PCR was performed using the KAPA TM SYBR FAST One‐Step qRT‐PCR Kit (KAPABIOSYSTEMS, MA, USA) with the following primers: S1PR1 (forward 5′‐CGAGAGCAC TATGCAGTCAG‐3′, reverse 5′‐CGATGAGTGATCCAGGCTTT‐3′), S1PR2 (forward 5′‐AGGTCGTCTCCTGCGTTTC, reverse 5′‐GCCGGCCTAGCCAGTTCT‐3′). The KAPA TM SYBR FAST One‐Step qRT‐PCR Kit (KAPABIOSYSTEMS, MA, USA) and temperature condition were used according to the manufacturers’ instructions.
2.7. Western blot
To measure S1PR1 and S1PR2 knockdown effect in protein level, western blot was performed. Briefly, protein separation through SDS‐PAGE by gel electrophoresis. Gel percentage was 8% and then transferred to a PVDF membrane (Millipore, MA, USA). The membrane was then incubated with the target antibodies, S1PR1/2 (R12‐3478/R12‐2725, AssaybioTech, CA, USA). After incubation, ECL reagent (Millipore, MA, USA) was applied to the membranes, and used Chemidoc XRS+ (BioRad) with a charge‐coupled device (CCD) camera to capture the signals.
2.8. Statistics
Significant difference between the experimental group and the control group was determined using student's t‐test. Results were expressed as mean ± SEM.
3. RESULT
3.1. S1P producing enzyme expression attenuates breast cancer development
The effects of S1P anabolic enzymes (SPHK 1 and SPHK 2) (Figure 1A) on BCA progression were first investigated. Analyses of databases of various BCA cohorts using KM Plotter with a defined prognosis threshold as 10‐years recurrence risks (relapse‐free survival; RFS) 22 for evaluating various patient classifications. The results exhibited that little influence on most classifications of BCA patients (Figure 1B). SPHK1 had no significant effect on 10‐year relapse free survival (data not shown). However, SPHK2 expression was found to have a positive impact in patients with nonclassified (Figure 1C, HR = 0.67, P‐value = 2.1e‐11) or basal cell type BCA (Figure 1D, HR = 0.62, P‐value = 4.1e‐4) and in those received adjuvant therapy (Figure 1E, HR = 0.45, P‐value <1e‐16). Those data indicating SPHK2 is the determining enzyme to suppress adjuvant treatment BCA patient progression.
Figure 1.
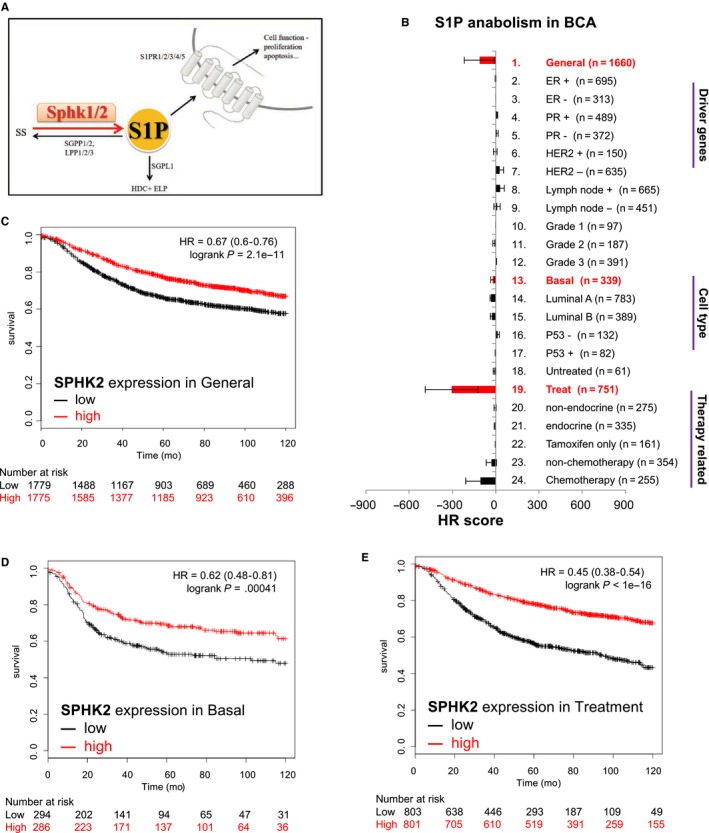
S1P anabolism attenuate prognosis for BCA patients. A, The illustration of S1P production and the involved enzyme (Sphk1 and Sphk2). SS: sphingosine; SPHK 1/2: sphingosine kinase 1/2; SGPP1/2: Sphingosine‐1‐phosphate phosphatase 1/2; S1P: Sphingosine‐1‐phosphate; SGPL1: Sphingosine‐1‐phosphate lyase1; LPP 1/2/3: lipid phosphate phosphatases 1/2/3; HDC: Hexadecenal; ELP: Ethanolamine phosphate; S1PR1/2/3/4/5: Sphingosine‐1‐phosphate receptor 12/3/4/5. B, HR score to evaluate the impact of S1P anabolism in BCA prognosis. The items on the right are the patient classification for KM plotter analyses. C‐E, Kaplan‐Meier plot survival analysis of SPHK2 in overall patients (C), basal cell type (D), and patients with adjuvant therapy (E). In X‐axis is survival of observe patients, Y‐axis is observed time for 10 years. The words low and high means the target gene expression was low or high in patients. Number at risk means the observe number of patients
3.2. S1P catabolism plays redundant roles in breast cancer progression
As the dynamic homeostasis of S1P is also related to its catabolism (Figure 2A), the roles of enzymes (SGPP1/2, SGPL1, and LPP1/2/3) responsible for its degradation in BCA progression were examined. Results showed that SGPL1 expression significantly negative‐correlated with BCA progression in patients of the nonclassified (Figure 2C, HR = 1.32, P‐value = 2.5e‐06). However, LPP3 showed positive correlation with BCA progression in general condition (Figure 2D, HR = 0.69, P‐value = 6.5e‐11). In brief, S1P degradation may not be one of the factors affecting the progression of breast cancer.
Figure 2.
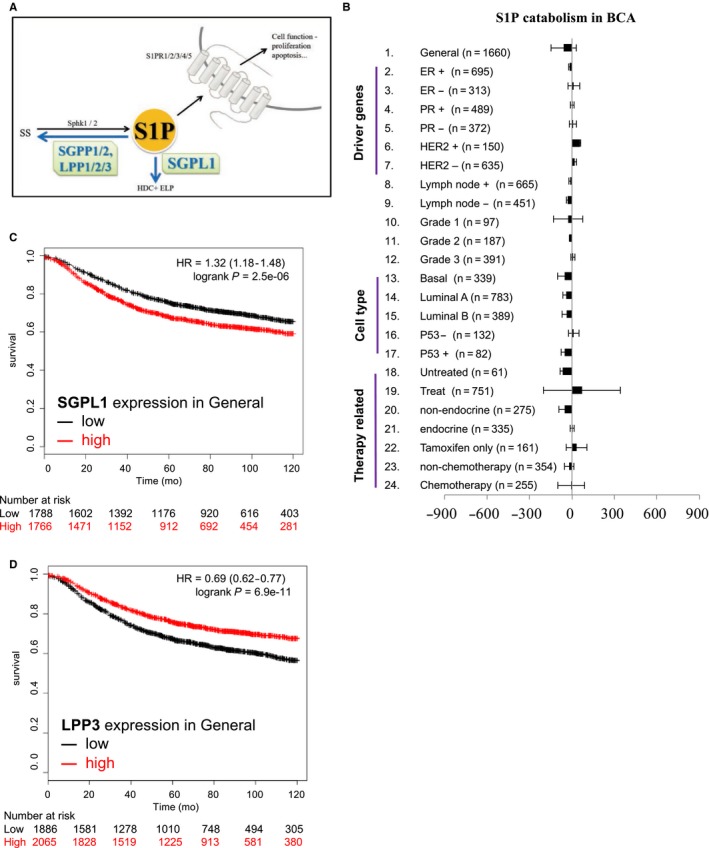
S1P catabolism did not affect prognosis for BCA patients. A, The illustration of S1P catabolism and the involved enzyme (SGPP1/2, SGPL1 and LPP 1/2/3). B, HR score to evaluate the impact of S1P catabolism in BCA prognosis. The items on the left is the patient classification for KM plotter analyses. C and D, Kaplan‐Meier plot survival analysis of SGPL1 in overall patients (C) and LPP3 in general parameter (D)
3.3. S1P receptors expression attenuates breast cancer progression
As S1P exerts its biological functions through S1P receptors (S1PRs), the association of S1PR expression and BCA progression was investigated (Figure 3A; S1PR1‐5) Results showed that the expression of S1PRs had a positive impact on BCA patients (Figure 3B). Individual dissection of S1PRs revealed that S1PR1 expression levels were found to suppressed the progression of tumors in patients with nonclassified BCA (Figure 3C; HR = 0.62 with P‐value = 1.1e‐15) and in those received treatments (Figure 3G; HR = 0.55 with P‐value = 1.2e‐11). However, S1PR1 expression had no significant effects on patients with basal cell type BCA (Figure 3E; HR‐0.8 with P‐value = .093). S1PR2 expression was found to have a positive impact on patients with nonclassified (Figure 3D, HR = 0.72, P‐value = 4.7e‐08) or basal cell type (Figure 3F, HR = 0.59, P‐value = 1.1e‐04) BCA and on those received treatments (Figure 3H; HR = 0.49 with P‐value = 1.1e‐16).
Figure 3.
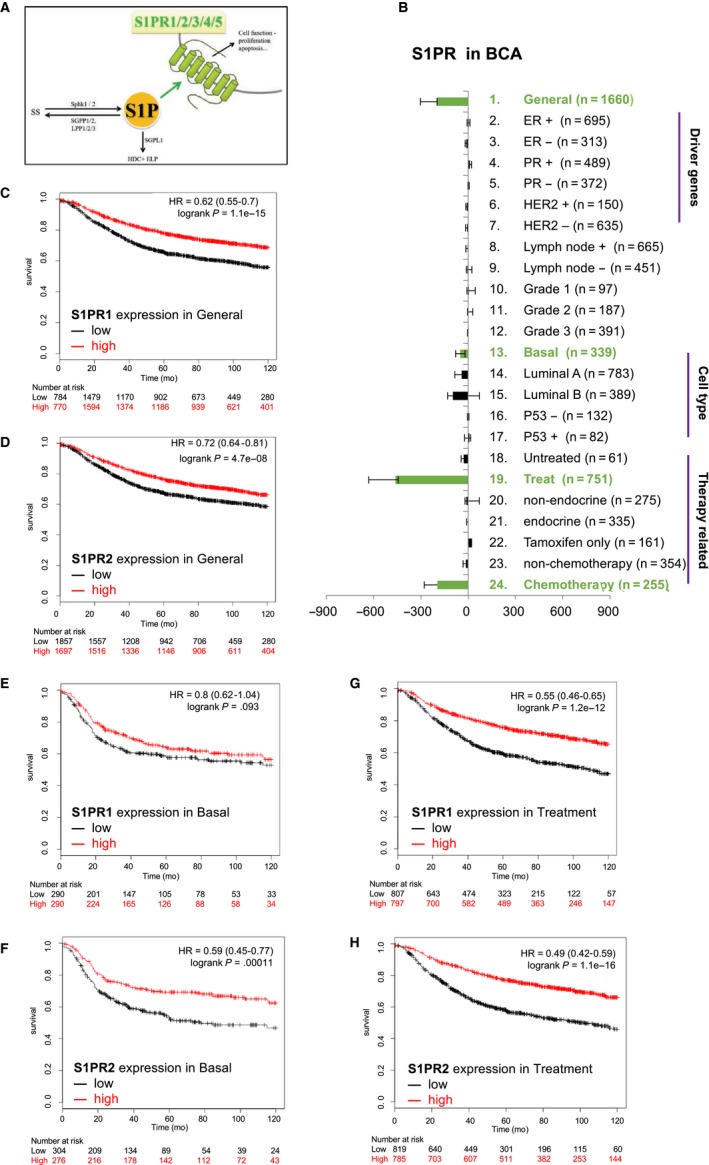
S1P receptors attenuate prognosis for BCA patients. A, The illustration of S1P effective receptors (S1PR1,2,3,4,5). B, HR score to evaluate the impact of S1P catabolism in BCA prognosis. The items listed on the right‐hand side is the patient classification for KM plotter analyses. C,E,G, Kaplan‐Meier plot survival analysis of S1PR1 in overall patients (C), basal cell type (E), and patients with adjuvant therapies (G). D,F,H, Kaplan‐Meier plot survival analysis of S1PR2 in overall patients (D), basal cell type (F), and patients with adjuvant therapy (H)
3.4. S1P suppresses breast cancer cell growth through S1PR1 in vitro
To confirm the inhibitory effect of S1P on BCA progression, the effect of S1P on the growth of BCA cells was investigated in vitro. To achieve the goal, MCF‐7 (hormone sensitive) and MDA‐MB‐231 (triple negative breast cancer, TNBC) cells were treated with 0‐10 μmol/L of S1P for 2 weeks and then evaluated for growth by colony formation assay. Results showed that S1P inhibited the proliferation of these cells in a dose‐dependent manner (Figure 4A), suggesting that SIP can inhibit the growth of both hormone sensitive and hormone insensitive BCA cells.
Figure 4.
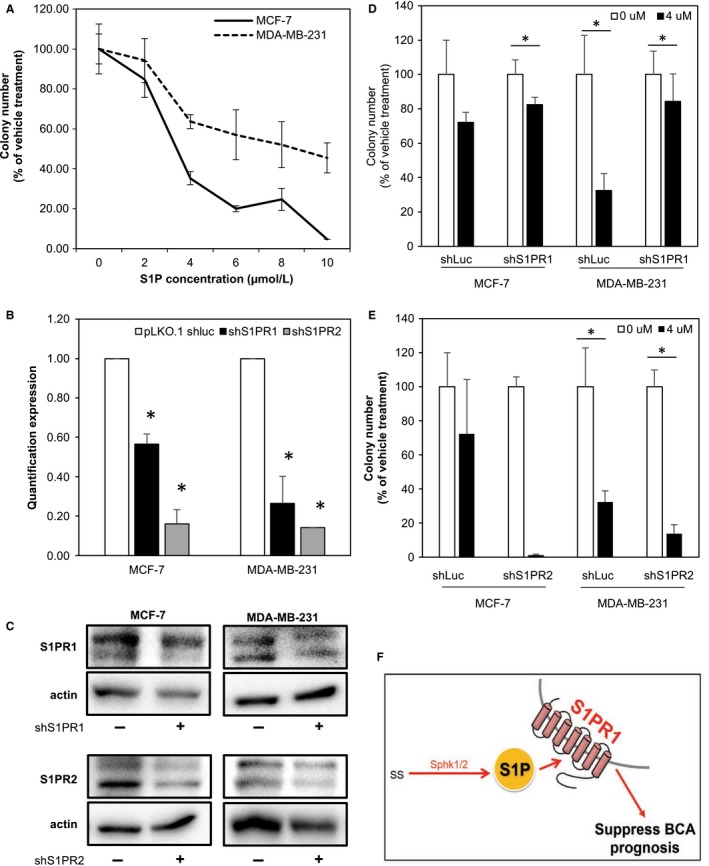
S1P inhibit cell colony through S1PR1. (A) S1P suppress BCA cells colony formatting ability. MCF7 was estrogen sensitive cells, MDA‐MB‐231 was triple negative breast cancer cell. The shRNA efficiency of S1PR1 and S1PR2 expression in quantification RCR and western blot (B, C). (D) Colony formation of knockdown S1P receptor 1 in MCF‐7 and MDA‐MB‐231 cells. S1P inhibitory effect was abolished when knockdown S1PR1 in MDA‐MB‐231 cell. E. Colony formation of knockdown S1P receptor 2 in MCF‐7 and MDA‐MB‐231 cells. The * indicating P‐value <.05. The data shown were the mean value of reproducible three biological repeats. (F) The carton shows S1P suppressed breast cancer prognosis through S1PR1. High abundance of S1P suppresses breast cancer prognosis (red arrows); on the contrary, low level of S1P promotes breast cancer prognosis (blue arrows)
To confirm the suppressive effect of S1P on the growth of BCA cells, the expression of S1PR1 or S1PR2 in MCF‐7 and MDA‐MB‐231 cells was knocked down by introducing lentivirus carrying S1PR1 or S1PR2 shRNA gene. Results showed that the expression of both S1PR1 and S1PR2 was successfully knocked down by more than 85% (Figure 4B,C) These S1PR1 and S1PR2 knockdown cells were then treated with 0 or 4 μmol/L of S1P for 2 weeks and then assayed for colony formation. As shown in Figure 4D, S1PR1 knockdown MDA‐MB‐231 yielded poor inhibition than vehicle cell while treat with S1P; yet, we did not observe S1PR1 knockdown reverse S1P effect in MCF‐7 cells. In Figure 4E, knockdown S1PR2 did not influence the S1P inhibition in MDA‐MB‐231 cell, moreover, MCF‐7 showed more dramatic suppression in S1P treatment. These data suggest that S1P inhibit BCA cells partially go through S1PR1 and irrelevant to S1PR2 cells.
4. DISCUSSION
4.1. SPHK/S1P/S1PR signaling in BCA cells
Our data showed that S1P can suppress BCA prognosis and this effect was partially through S1PR1 expression (Figure 4E). This result is contradictory with those of previous studies.6, 19, 23, 24 We discuss this inconsistency in the following.
Ceramide and sphingosine are the precursors of S1P. Literatures indicated that ceramide and sphingosine involved in cell death, apoptosis, and anti‐proliferation.25, 26, 27 SGPP1 is the enzyme revert S1P to ceramide/sphingosine, add‐in S1P might increase ceramide/sphingosine level from SGPP1. However, the results from Le Stunff et al28 showed that increase in S1P did not upregulate ceramide level unless overexpressed SGPP1 expression. Since that, ceramide and sphingosine from S1P catabolism might not contributor to the S1P‐mediated cancer inhibitory effect.
SPHK1 is an enzyme responsible for S1P production and is involved in many cellular processes, such as promoting cellular proliferation and stimulating cell survival and metastasis.2, 3, 4 SPHK1 mainly express in the cytosol and needed to be translocated to the membrane while be activated. SPHK1 increases S1P levels in the local environment and induced proliferation signaling.29 On the other hand, SPHK2 presented and form S1P in the nucleus.30 In our study, we added S1P in vitro which increases extracellular S1P concentration to mimic Sphk1 pathway to induce proliferation signals. Since we discovered the S1P cancer growth inhibition effect, we excluded the growth promoting mechanisms as the signaling route. According to the literatures, Insulin‐like growth factors (IGFs) stimulated cell proliferation by activated MAPK, Akt 31 and S1P have been found to inhibit keratinocyte proliferation through inhibit Akt/PKB pathway induced by Insulin‐like growth factor I (IGF‐I).32 In the cancer condition, triple negative breast cancer was reported to express high level of IGF‐I.31, 33 Therefore, S1P/S1PR1 signaling might downregulate IGF‐1/Akt/PKB axis to suppress cell proliferation.
Results of previous study indicated that overexpression of SPHK1 in MDA‐MB‐231 resulted in a decrease in sphingosine concentration and knockdown of SPHK1 with shRNA increased sphingosine level.23 The function of SPHK1 was suggested to upregulate sphingosine levels and influence cell proliferation. Besides, the study in 2008 also showed SPHK1 correlated with poor prognosis in ER‐negative breast cancer.34 Moreover, in 2010 Watson et al35 found that high expression of SPHK1 and ERK‐1/2 is associated with shorter recurrence time in ER positive patients. Besides, high level of S1PR1 and ERK1/2 or high expression of S1PR3 and ERK1/2 also indicated the association with shorter recurrence times. These studies illustrated that overexpression of SPHK1 has been shown to increase cancer progression. Ohotski et al36 also indicated that nuclear localization of sphingosine kinase 1 and S1PR2 played an important role in breast cancer prognosis. Yet it is still unknown whether this effect is directly related to S1P level in these studies.
Previous study has been shown that S1P stimulates OVCA invasion at low concentration (0.5 μmol/L), but inhibits its invasion at high concentration (20 μmol/L).37 In our study, the minimum dosage required to inhibit colony formation of BCA cells was 4 μmol/L (Figure 4A). Literatures showed that the levels of S1P in the ascitic fluids ranged from 0.42‐2.17 μmol/L,38 and the serum S1P concentration in healthy individual is less than 1 μmol/L.5 Referencing with the S1P concentration and our observations, we speculated that local S1P concentration may suppresses BCA cell proliferation. Recently, Nagahashi et al39 used the breast cancer patients tissue to measured sphingolipid level and found S1P level was higher in tumor tissue than normal tissue. However, they did not establish the relationship with S1P level, disease progression and patient survival.
S1P signal transduction is through binding to a family of five G protein‐coupled receptors (GPCRs). S1P receptors are divided into five subtypes: S1PR1, S1PR2, S1PR3, S1PR4, and S1PR5, and S1PRs are differentially coupled to heterotrimeric G proteins and Rac or Rho to regulate various effectors, such as MAPKs.1, 14, 40, 41 Previous studies have shown that S1P‐SIPRs signaling can enhance cell proliferation and migration. The high S1PR1 expression in glioblastoma patients was positively associated with favorable survival.42 However, S1PR2 signaling negatively regulates tumor angiogenesis and tumor growth in vivo.43 S1PR2 is also involved in the reduction of platelet‐derived growth factor mediated‐cell motility and proliferation.44 A knockdown in the expression of S1PR2 by shRNA in satellite cells resulted in a twofold increase in cell migration.45 As mentioned above, we observed that S1P can enhance BCA cancer cell growth when it binds to S1PR1 and in the KM plotter analysis, S1PR2 expression was found to have a better survival benefit than S1PR1 expression. There also a study investigated other receptor, such as S1PR4, and the authors suggested S1PR4 related to ER‐negative breast cancer progression.46 Since our KM plotter did not showed the significant result in S1PR4, we do not rule out the possibility that different S1PR subtype will have diverse function in different type of breast cancer.
Another study to analyze sphingolipid level in tumor tissue of breast patients and demonstrated high expression of pSPHK1 is associated with higher levels of S1P, which in term is associated with lymphatic metastasis in breast cancer.47 Some references providing clinical evidence to show the S1P axis involved in the therapies resistant of tumors. In Katsuta et al48 study, it suggested that the expression of sphingosine kinase 1 tended to be higher in doxorubicin‐resistant human cancer and cell lines. In Nagahashi et al49 recent study, they used FTY720 to target SPHK1/S1P/S1PR1 axis and suggested that this pathway is associated with obesity‐related inflammation and breast cancer metastasis. Since our KM plotter results did not show the relationship with metastasis and we did not investigate the drug resistant effect, we were optimistic about the above studies that S1P might played different role in different tumor progression.
4.2. S1PR as a target for BCA treatment
S1P axis was initially found to play important roles in lymphocyte egress from lymphoid tissue. It means that targeting S1P may have immunosuppressive function. This possibility has been investigated in organs transplantation and autoimmune diseases. Although the effects of targeting S1P axis for cancer treatment are still preliminary, a few clinical trials have explored the anti‐tumor effects of S1P related modulators. The currently known drug targeting S1P is FTY720 (Fingolimod), which is an analog of sphingosine and functions as S1PR agonist. However, while FTY720 phosphorylated by SPHK2 to form FTY720‐phosphate (FTY720‐p) was suggested to a functional antagonist of S1PR1 through binding to S1PR1 and induces internalization and degradation, leading to sequestration of thymocytes and lymphocytes from secondary lymphoid organs thereby reducing inflammation.2, 5, 50, 51 FTY720 demonstrates anti‐cancer properties and may have a potential in cancer treatment.52, 53 There are many in vitro and in vivo studies demonstrated the growth arrest and apoptosis‐inducing ability of FTY720. The anti‐cancer ability of FTY720 may be through the inhibition of sphingosine kinase 1. For example, the cisplatin‐resistant melanoma cells treated with FTY720 might activated p53‐independent caspase and caused SPHK1 degradation and might inhibit the Akt/mTOR pathway.54 In another way, not all relevant features of FTY720 are helpful for cancer treatment. Nagaoka et al52 showed that FTY720‐P did not decrease the viability of BCA cells, suggesting that the anticancer effect of FTY720 was not mediated by its phosphorylate form. Noticeably, they also found that S1P can suppress the growth of BCA as we did in this study. KRP‐203 is a kind of S1PR1 selective agonists. It has been demonstrated to regulate both T and B cells and improve the outcome of atherosclerosis disease.55 SEW2871 is also a selective S1PR1 agonist and has been used to treat acute kidney injury and ischemia‐reperfusion injury in mice.56, 57 SEW2871 binds to S1PR1 causing its internalization and recycling. Those S1PR1 agonists could be candidates for the further investigation of the role of S1P/S1PR in cancer progression. Although we did not observe effect of S1P‐S1PR2 binding in the inhibition of colony formation of BCA cells, results of our bioinformatic analyses showed a positive correlation between S1PR2 expression and BCA progression. There is a compound designated CYM‐5520 58 has been shown to more selective for activating S1PR2 and may be suitable for BCA treatment. Although these compounds are currently available only for research, they may become effective regimens for BCA treatment, based on our findings in this study.
5. CONCLUSIONS
In this study, we found a cancer suppressive function of S1P through S1PR1. As this finding is contradictory with those of previous studies, further investigations are needed to develop S1PR agonists for cancer therapy.
CONFLICT OF INTEREST
All authors in this study claim no interest of conflict.
ACKNOWLEDGMENTS
This work was supported by grants from Taiwan Ministry of Science and Technology (MOST104‐2314‐B‐039‐046; 106‐2320‐B‐039‐008, 106‐2221‐E‐039‐011‐MY3), Taiwan National Health Research Institute (NHRI‐EX107‐10705BI), Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW106‐TDU‐B‐212‐133019; MOHW106‐TDU‐B‐212‐113006), China Medical University/Hospital (CMU106‐S‐30; CMU106‐N‐05; DMR‐107‐075), and Chia‐Yi Chang‐Gong Memorial Hospital (CMRPG6G0111; CMRPG8D0743; CMRPG6E0122).
Lei F‐J, Cheng B‐H, Liao P‐Y, et al. Survival benefit of sphingosin‐1‐phosphate and receptors expressions in breast cancer patients. Cancer Med. 2018;7:3743–3754. 10.1002/cam4.1609
Fu‐Ju Lei and Bi‐Hua Cheng are equal contribution as first authors.
Contributor Information
Li‐Ching Chu, Email: 8705025@cgmh.org.tw.
Wen‐Lung Ma, Email: maverick@mail.cmu.edu.tw.
REFERENCES
- 1. Tabasinezhad M, Samadi N, Ghanbari P, et al. Sphingosin 1‐phosphate contributes in tumor progression. J Cancer Res Ther. 2013;9:556‐563. [DOI] [PubMed] [Google Scholar]
- 2. Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine‐1‐phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim ES, Kim JS, Kim SG, Hwang S, Lee CH, Moon A. Sphingosine 1‐phosphate regulates matrix metalloproteinase‐9 expression and breast cell invasion through S1P3‐Galphaq coupling. J Cell Sci. 2011;124:2220‐2230. [DOI] [PubMed] [Google Scholar]
- 4. Salas A, Ponnusamy S, Senkal CE, et al. Sphingosine kinase‐1 and sphingosine 1‐phosphate receptor 2 mediate Bcr‐Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 2011;117:5941‐5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside‐out” signaling of sphingosine‐1‐phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohotski J, Rosen H, Bittman R, Pyne S, Pyne NJ. Sphingosine kinase 2 prevents the nuclear translocation of sphingosine 1‐phosphate receptor‐2 and tyrosine 416 phosphorylated c‐Src and increases estrogen receptor negative MDA‐MB‐231 breast cancer cell growth: the role of sphingosine 1‐phosphate receptor‐4. Cell Signal. 2014;26:1040‐1047. [DOI] [PubMed] [Google Scholar]
- 7. Pyne S, Pyne NJ. Sphingosine 1‐phosphate signalling in mammalian cells. Biochem J. 2000;349:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi N, Kobayashi N, Yamaguchi A, Nishi T. Characterization of the ATP‐dependent sphingosine 1‐phosphate transporter in rat erythrocytes. J Biol Chem. 2009;284:21192‐21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukuhara S, Simmons S, Kawamura S, et al. The sphingosine‐1‐phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Investig. 2012;122:1416‐1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine‐1‐phosphate transporter in vascular endothelial cells. PLoS ONE. 2012;7:e38941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vu TM, Ishizu AN, Foo JC, et al. Mfsd2b is essential for the sphingosine‐1‐phosphate export in erythrocytes and platelets. Nature. 2017;550:524‐528. [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi N, Kawasaki‐Nishi S, Otsuka M, Hisano Y, Yamaguchi A, Nishi T. MFSD2B is a sphingosine 1‐phosphate transporter in erythroid cells. Sci Rep. 2018;8:4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y, Wada R, Yamashita T, et al. Edg‐1, the G protein‐coupled receptor for sphingosine‐1‐phosphate, is essential for vascular maturation. J Clin Investig. 2000;106:951‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee MJ, Thangada S, Claffey KP, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine‐1‐phosphate. Cell. 1999;99:301‐312. [DOI] [PubMed] [Google Scholar]
- 15. Wang F, Van Brocklyn JR, Hobson JP, et al. Sphingosine 1‐phosphate stimulates cell migration through a G(i)‐coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343‐35350. [DOI] [PubMed] [Google Scholar]
- 16. Cyster JG. Chemokines, sphingosine‐1‐phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127‐159. [DOI] [PubMed] [Google Scholar]
- 17. Spiegel S, Milstien S. Sphingosine‐1‐phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397‐407. [DOI] [PubMed] [Google Scholar]
- 18. Mukhopadhyay P, Ramanathan R, Takabe K. S1P promotes breast cancer progression by angiogenesis and lymphangiogenesis. Breast Cancer Manag. 2015;4:241‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weigert A, Schiffmann S, Sekar D, et al. Sphingosine kinase 2 deficient tumor xenografts show impaired growth and fail to polarize macrophages towards an anti‐inflammatory phenotype. Int J Cancer. 2009;125:2114‐2121. [DOI] [PubMed] [Google Scholar]
- 20. Chang WC, Huang SF, Lee YM, et al. Cholesterol import and steroidogenesis are biosignatures for gastric cancer patient survival. Oncotarget. 2017;8:692‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gyorffy B, Lanczky A, Eklund AC, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725‐731. [DOI] [PubMed] [Google Scholar]
- 22. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738‐2746. [DOI] [PubMed] [Google Scholar]
- 23. Kotelevets N, Fabbro D, Huwiler A, Zangemeister‐Wittke U. Targeting sphingosine kinase 1 in carcinoma cells decreases proliferation and survival by compromising PKC activity and cytokinesis. PLoS ONE. 2012;7:e39209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagahashi M, Ramachandran S, Kim EY, et al. Sphingosine‐1‐phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72:726‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woodcock J. Sphingosine and ceramide signalling in apoptosis. IUBMB Life. 2006;58:462‐466. [DOI] [PubMed] [Google Scholar]
- 26. Cuvillier O. Sphingosine in apoptosis signaling. Biochim Biophys Acta. 2002;1585:153‐162. [DOI] [PubMed] [Google Scholar]
- 27. Ahn EH, Schroeder JJ. Induction of apoptosis by sphingosine, sphinganine, and C(2)‐ceramide in human colon cancer cells, but not by C(2)‐dihydroceramide. Anticancer Res. 2010;30:2881‐2884. [PubMed] [Google Scholar]
- 28. Le Stunff H, Galve‐Roperh I, Peterson C, Milstien S, Spiegel S. Sphingosine‐1‐phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J Cell Biol. 2002;158:1039‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bao Y, Guo Y, Zhang C, Fan F, Yang W. Sphingosine Kinase 1 and Sphingosine‐1‐Phosphate Signaling in Colorectal Cancer. Int J Mol Sci. 2017;18:pii: E2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K. Sphingosine‐1‐phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediators Inflamm. 2016;2016:8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chandran S, Harmey JH, Toomey S. Inhibition of IGF signalling pathway in MDA‐MB‐231 triple negative breast cancer cells. BMC Proc. 2012;6:O14. [Google Scholar]
- 32. Kim DS, Kim SY, Kleuser B, Schafer‐Korting M, Kim KH, Park KC. Sphingosine‐1‐phosphate inhibits human keratinocyte proliferation via Akt/protein kinase B inactivation. Cell Signal. 2004;16:89‐95. [DOI] [PubMed] [Google Scholar]
- 33. Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin‐like growth factor‐1 system in breast cancer. Mol Cancer. 2015;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruckhaberle E, Rody A, Engels K, et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112:41‐52. [DOI] [PubMed] [Google Scholar]
- 35. Watson C, Long JS, Orange C, et al. High expression of sphingosine 1‐phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal‐regulated kinase‐1/2 is associated with development of tamoxifen resistance in estrogen receptor‐positive breast cancer patients. Am J Pathol. 2010;177:2205‐2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ohotski J, Edwards J, Elsberger B, et al. Identification of novel functional and spatial associations between sphingosine kinase 1, sphingosine 1‐phosphate receptors and other signaling proteins that affect prognostic outcome in estrogen receptor‐positive breast cancer. Int J Cancer. 2013;132:605‐616. [DOI] [PubMed] [Google Scholar]
- 37. Devine KM, Smicun Y, Hope JM, Fishman DA. S1P induced changes in epithelial ovarian cancer proteolysis, invasion, and attachment are mediated by Gi and Rac. Gynecol Oncol. 2008;110:237‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hong G, Baudhuin LM, Xu Y. Sphingosine‐1‐phosphate modulates growth and adhesion of ovarian cancer cells. FEBS Lett. 1999;460:513‐518. [DOI] [PubMed] [Google Scholar]
- 39. Nagahashi M, Tsuchida J, Moro K, et al. High levels of sphingolipids in human breast cancer. J Surg Res. 2016;204:435‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spiegel S, Milstien S. Sphingosine 1‐phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851‐25854. [DOI] [PubMed] [Google Scholar]
- 41. Shimizu YFH, Tamashiro PM, Kawamori T. Involvement of sphingosine kinases/sphingosine‐1‐phosphate (S1P)/S1P receptors in breast cancer subtypes. J Oncobiomarkers. 2013;1:1‐6. [Google Scholar]
- 42. Yoshida Y, Nakada M, Harada T, et al. The expression level of sphingosine‐1‐phosphate receptor type 1 is related to MIB‐1 labeling index and predicts survival of glioblastoma patients. J Neurooncol. 2010;98:41‐47. [DOI] [PubMed] [Google Scholar]
- 43. Du W, Takuwa N, Yoshioka K, et al. S1P(2), the G protein‐coupled receptor for sphingosine‐1‐phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res. 2010;70:772‐781. [DOI] [PubMed] [Google Scholar]
- 44. Goparaju SK, Jolly PS, Watterson KR, et al. The S1P2 receptor negatively regulates platelet‐derived growth factor‐induced motility and proliferation. Mol Cell Biol. 2005;25:4237‐4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Calise S, Blescia S, Cencetti F, Bernacchioni C, Donati C, Bruni P. Sphingosine 1‐phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochim Biophys Acta. 2012;1823:439‐450. [DOI] [PubMed] [Google Scholar]
- 46. Ohotski J, Long JS, Orange C, et al. Expression of sphingosine 1‐phosphate receptor 4 and sphingosine kinase 1 is associated with outcome in oestrogen receptor‐negative breast cancer. Br J Cancer. 2012;106:1453‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsuchida J, Nagahashi M, Nakajima M, et al. Breast cancer sphingosine‐1‐phosphate is associated with phospho‐sphingosine kinase 1 and lymphatic metastasis. J Surg Res. 2016;205:85‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Katsuta E, Yan L, Nagahashi M, et al. Doxorubicin effect is enhanced by sphingosine‐1‐phosphate signaling antagonist in breast cancer. J Surg Res. 2017;219:202‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nagahashi M, Yamada A, Katsuta E, et al. Targeting the SphK1/S1P/S1PR1 Axis That Links Obesity, Chronic Inflammation, and Breast Cancer Metastasis. Cancer Res. 2018;78:1713‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen B, Roy SG, McMonigle RJ, et al. Azacyclic FTY720 analogues that limit nutrient transporter expression but lack S1P receptor activity and negative chronotropic effects offer a novel and effective strategy to kill cancer cells in vivo. ACS Chem Biol. 2016;11:409‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pitman MR, Woodcock JM, Lopez AF, Pitson SM. Molecular targets of FTY720 (fingolimod). Curr Mol Med. 2012;12:1207‐1219. [DOI] [PubMed] [Google Scholar]
- 52. Nagaoka Y, Otsuki K, Fujita T, Uesato S. Effects of phosphorylation of immunomodulatory agent FTY720 (Fingolimod) on antiproliferative activity against breast and colon cancer cells. Biol Pharm Bull. 2008;31:1177‐1181. [DOI] [PubMed] [Google Scholar]
- 53. Azuma H, Takahara S, Ichimaru N, et al. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002;62:1410‐1419. [PubMed] [Google Scholar]
- 54. White C, Alshaker H, Cooper C, Winkler M, Pchejetski D. The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget. 2016;7:23106‐23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Poti F, Gualtieri F, Sacchi S, et al. KRP‐203, sphingosine 1‐phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL‐R‐/- mice. Arterioscler Thromb Vasc Biol. 2013;33:1505‐1512. [DOI] [PubMed] [Google Scholar]
- 56. Jo S, Bajwa A, Awad A, Lynch K, Okusa M. Sphingosine‐1‐phosphate receptors: biology and therapeutic potential in kidney disease. Kidney Int. 2008;73:1220‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dong J, Wang H, Wu G, et al. Oral treatment with SEW2871, a sphingosine‐1‐phosphate type 1 receptor agonist, ameliorates experimental colitis in interleukin‐10 gene deficient mice. Clin Exp Immunol. 2014;177:94‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Satsu H, Schaeffer MT, Guerrero M, et al. A sphingosine 1‐phosphate receptor 2 selective allosteric agonist. Bioorg Med Chem. 2013;21:5373‐5382. [DOI] [PMC free article] [PubMed] [Google Scholar]


