Abstract
Radiotherapy is an important modality for treatment of carcinomas; however, radio‐resistance is still a difficult problem. Aberrant epigenetic alterations play an important role in cancer development. Among epigenetic parameters, DNA methylation has arguably attracted the most attention in the radio‐resistance process. To determine the role of DNA methylation in radiation resistance, several studies were conducted. We summarized previous studies on the role of DNA methylation in radiotherapy. We observed this significant role of DNA methylation in genes related to DNA repair, cell proliferation, cell cycle process, and re‐oxygenation. Furtherly, we also conclude the predictive effect of DNA methylation on tumor radio‐sensitivity and the using of DNA methyltransferase inhibitors in clinical practice. DNA methylation plays a pivotal role in the radio‐sensitivity of tumor radio‐therapy. While hyper‐methylation or hypo‐methylation of genes is related to gene functions.
Keywords: DNA methylation, DNA methyltransferase inhibitors, radio‐resistant, radio‐sensitivity
1. INTRODUCTION
Radiotherapy is a ubiquitous environment factor and 1 of the main therapeutic methods of malignant tumors, and approximately 50% of all cancer patients receive radiotherapy.1 Several tumors are relatively sensitive to radiotherapy, including nasopharyngeal carcinoma, oral cancer, head and neck tumors, breast cancer, and lung cancer. However, parts of patients with above tumors are resistant to irradiation or some initially sensitive patients develop into irradiation resistance. Hence, irradiation resistance is 1 of the major obstacles that leads to loco‐regional recurrence of malignant tumors during treatment.2, 3, 4, 5, 6
To enhance the radiation sensitization, researchers mainly focus on improving the state of hypoxia, increasing the damage of DNA and affecting the cell cycle. Now, commonly used radio‐sensitizers are 5‐fluorouracil, platinum, and gemcitabine in clinical practice. The sensitization mechanism of 5‐fluorouracil is to inhibit the thymidylate synthetase.7 For platinum, its mechanism includes radiation‐induced free radicals prompting the formation of toxic platinum intermediates, inhibition of DNA repair, radiation‐induced enhancement of cell platinum absorption, and cell cycle arrest.8, 9 Via redistributing tumor cells into S phase and causing the depletion of the deoxy ATP, gemcitabine can enhance the radio‐sensitivity of tumor radiotherapy.10 However, these drugs have not only limited sensitization effect, but also overlapped toxicity. Recently, researchers tend to focus the study of sensitizer on epigenetics.
The role of epigenetic modifications, especially DNA methylation, has been extensively explored in the mechanism of radio‐resistance of malignant tumors. During the development of mammalian, CpG islands in DNA are methylated de novo in a tissue‐specific manner, and the patterns are fixed in subsequent cell divisions by methyltransferase activity.11 In general, DNA methylation is established by DNA methyltransferase (DNMTs), including DNMT1 and DNMT3a/b. The DNMTs use S‐adenosylmethionine as a methyl donor to specifically methylate the fifth carbon atom of the cytosine ring. DNA hyper‐methylation of CpG islands in promoter regions plays a major role in the transcriptional silencing of certain genes, especially tumor suppressive genes.12
More recently, increasing evidence supports the suggestion that dysregulated epigenetic control through DNA methylation is important for radio‐resistance of cancer cells.13 The early interest in the effects of DNA methylation dating back to 2002, Kim et al14 found that aberrant methylation of multiple CpG dinucleotides of the ataxia telangiectasia mutated (ATM) gene led to radio‐resistance in a human colorectal tumor cell line. It has been believed that DNA methylation plays a pivotal role in variety of cellular events, including the alterations in apoptosis, cell cycle progression, mitotic checkpoint regulation, and DNA repair.15 The mechanisms of above cellular events have been considered to mediate radiosensitive effects.16
In this systematic review, we describe the effect of DNA methylation on radiation, the mechanisms, and applications. The flowchart of studies selection is shown in Figure 1.
Figure 1.
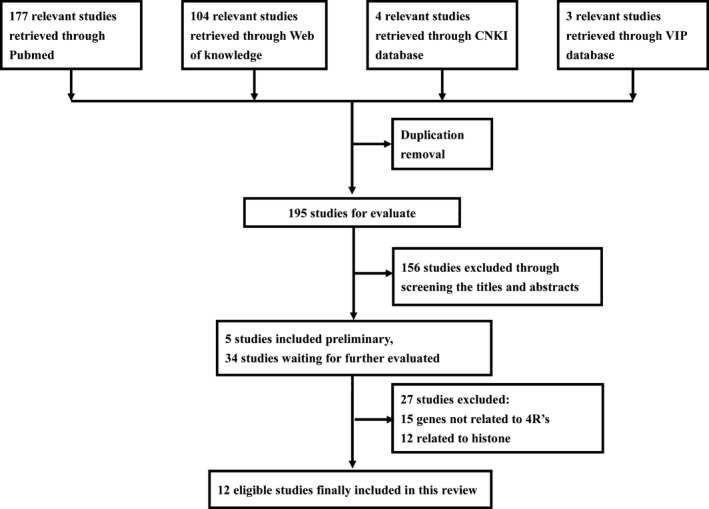
Flow chart of studies selection. 4 R's, repair, repopulation, redistribution, re‐oxygenation. CNKI, China National Knowledge Infrastructure
2. RADIOBIOLOGICAL EFFECT AND THE RESPONSE RATE OF RADIOTHERAPY
Radiotherapy is a mainstay in the treatment of solid tumors, and it is proved to be effective by inducing free radical stress in tumor cells, leading to loss of reproductive integrity. Regarding clinical radiobiological effect, the optimal treatment strategy should consider damage to both tumor and normal cells.17 The clinical radiobiological effect is determined by 5 factors known as the 5 R's of radiobiology including DNA repair, repopulation, re‐oxygenation, redistribution, and radio‐sensitivity, which determine the curative response rate of tumor.18
DNA repair, repopulation, re‐oxygenation, and redistribution were known as 4 R's, which were proposed as the significant factors for the curative effect of radiotherapy of tumors. The 4 R's were discussed from the relationship of cell cycle, proliferation (G1‐S‐G2‐M), and static (G0) period. While radio‐sensitivity is the factor that reflects the response rate of tumor radiotherapy directly. Hence, we hypothesize that the DNA methylation of genes that regulate the process of 4R's plays a pivotal role in tumor radio‐sensitivity.
3. RADIO‐SENSITIVITY AND DNA METHYLATION OF DNA DAMAGE REPAIR‐RELATED GENES
The ataxia telangiectasia mutated (ATM) gene encodes a high‐molecular weight protein kinase which has been proposed to play a pivotal role in triggering appropriate cellular response to genome damage.19 Kim et al14 investigated the radio‐sensitivity of 3 hereditary Non‐Polyposis Colorectal Cancer (HNPCC) cell lines (HCT‐116, LoVo and RKO). Close inspection of the radio‐sensitivity of HNPCC cells showed that HCT‐116 cells were found to have heightened radio‐sensitivity compared to both LoVo and RKO. Further study revealed that the increased sensitivity to radiation in HCT‐116 cells was determined by the aberrant methylation of multiple GpG dinucleotides within the promoter region of ATM gene. Afterwards, Boston scholars Roy et al20 researched the role of methylation of the ATM promoter in the radiation sensitivity in glioma. After examining the ATM methylation status and radio‐sensitivity of 3 glioma cell lines, they observed the same result. For further study, they treated cells with an inhibitor of DNMT (5‐azacytidine) and showed that ATM protein in radiosensitive cells was increased and the radio‐sensitivity was decreased.
For the significant role of nucleotide excision repair (NER) in DNA repair, Chinese researchers studied the relationship between Excision Repair Cross‐complementing rodent repair deficiency 1 (ERCC1) and radio‐sensitivity of glioma.21 Two radiosensitive cells were with the methylated status of ERCC1 gene, while the promoter regions of ERCC1 gene in other 2 radio‐resistant cells were de‐methylated.
Ras Association Domain Family Member 1 (RASSF1A) gene encodes protein similar with the RAS effector proteins. The encoded protein was found to interact with DNA repair protein—Xeroderma pigmentosum complementation group A (XPA). This RASSF1A protein is also shown to inhibit the accumulation of cyclin D1 and thus induces cell cycle arrest.22
Dote et al23 found the enhancement of tumor cell radio‐sensitivity by the DNMT inhibitor (zebularine) in vitro and in vivo. Treating cells with zebularine, the radio‐sensitivity of 3 human tumor cells, including pancreatic carcinoma, glioblastoma, and prostate carcinoma, were more sensitive than control groups.
Due to the gene silencing effect of DNA methylation, we observed that for promoting genes of DNA repair (eg, ATM/ERCC1), DNA methylation could heighten the sensitivity of radiation to tumors, while for the DNA repair suppressive genes (eg, RASSF1A), demethylation could enhance the radio‐sensitivity of human tumor cells. Table 1.
Table 1.
Effect of DNA methylation status of damage repair related genes on radiosensitivity
| Gene | Methylation site | Tumor | Cell line | Methylation status | P‐radiosensitivity | T‐Radiosensitivity |
|---|---|---|---|---|---|---|
| ERCC1 | Promoter | Glioma | MGR1 | Demethylated | Resistant | ‐ |
| MGR2 | Methylated | Sensitive | ‐ | |||
| SF767 | Methylated | Sensitive | ‐ | |||
| T98G | Demethylated | Resistant | ‐ | |||
| ATM | Promoter | Colorectal carcinoma | HCT‐116 | Methylated | Sensitive | Resistant |
| LoVo | Demethylated | Resistant | ‐ | |||
| RKO | Demethylated | Resistant | ‐ | |||
| Glioma | U87 | Methylated | Sensitive | Resistant | ||
| T98G | Demethylated | Resistant | Resistant | |||
| U118 | Demethylated | Sensitive | Sensitive | |||
| RASSF1A | Promoter | Pancreatic carcinoma | MiaPaca | Hypermethylated | Resistant | Sensitive |
| Glioblastoma | U251 | Hypermethylated | Resistant | Sensitive | ||
| Prostate carcinoma | DU145 | Hypermethylated | Resistant | Sensitive | ||
| NPC | CNE2 | Hypermethylated | Resistant | Sensitive | ||
| SUNE2 | Hypermethylated | Resistant | Sensitive |
ATM, Ataxia Telangiectasia mutated; ERCC1, Excision Repair Cross‐Complementing rodent repair deficiency 1; NPC, Nasopharyngeal carcinoma; P‐radiosensitivity, primordial radiosensitivity; RASSF1A, Ras Association Domain Family Member 1A; T‐radiosensitivity, radiosensitivity after treatment with inhibitor of methyltransferase; ‐, undetected.
4. RADIO‐SENSITIVITY AND DNA METHYLATION OF CELL PROLIFERATION‐RELATED GENES
Serine peptidase inhibitor 5 (SERPINB5) gene was originally reported to act as a tumor suppressor gene in epithelial cells, suppressing the ability of cancer cells to invade and metastasize to other tissue.24 Maspin (mammary serine protease inhibitor) is a protein encoded by SERPINB5 gene.25 Several studies have investigated the role of maspin and found its function in cell proliferation.26 Kim et al27 analyzed the global CpG methylation difference between 2 radio‐sensitivity opponent nonsmall cell lung cancer (NSCLC) cell lines. In radio‐resistant NSCLC cell line, CpG islands of SERPINB5 gene were hyper‐methylated which was much higher than that in radiosensitive cells. Reverse transcriptase‐PCR showed higher expression of SERPINB5 gene in radiosensitive cells compared with radio‐resistant cells. Down‐regulation of SERPINB5 gene by small interfering RNA but not methylation inhibitor in radiosensitive cells increased radiation resistance of these cells. Meanwhile, in radio‐resistant cells, they found the hypo‐methylated status of basonuclin‐1 (BNC1) gene encoding finger protein basonuclin‐1 which was considered to regulate the proliferation of keratinocyte.
To investigate the role of DNA methylation inhibitor in tumor radio‐sensitivity, researchers studied 3 human tumor cell line (pancreatic carcinoma, glioblastoma and prostate carcinoma).23 Hyper‐methylated in cancer 1 (HIC1) gene is a growth regulatory and tumor repressor gene. It was detected with methylated status in above 3 radio‐resistant cell lines. And after treating with zebularine, the radiation sensitivity of these 3 radio‐resistant cell lines was enhancement.
Protein encoded by transmembrane 4 L six family member 4 (TM4SF4) gene is a member of the transmembrane 4 family, also known as the tetraspanin family. Most of these members are cell‐surface protein that are characterized by the presence of 4 hydrophobic domains. The TM4SF4 protein is a cell surface glycoprotein that regulates cell proliferation.28 In radio‐resistant lung carcinoma cell lines, TM4SF4 was highly expressed. The detection of CpG methylation status of TM4SF4 gene showed that these cell lines were hypo‐methylated which led to the high expression of TM4SF4.5
Furthermore, scholars also explored the function of microRNA and methylation status in radio‐resistant nasopharyngeal carcinoma (NPC). To identify the role of microRNA 24 (miR24) in NPC radio‐resistance and the mechanism by which miR24 is regulated, Wang et al29 studied 4 NPC cell lines including radio‐sensitive and radio‐resistant cells. Their studies showed that miR24 inhibited NPC cell growth, promoted cell apoptosis, and suppressed the growth of NPC xenografts. Further research found that miR24‐1, 1 of the miR24 precursors, was embedded in a CpG island. Aberrant promoter DNA methylation of miR24‐1 was involved in NPC response to radiotherapy. In radio‐sensitive NPC cells, miR24‐1 was hypo‐methylated while miR24‐1 was hyper‐methylated in radio‐resistant cells.
DNA methylation status of cell proliferation‐related genes affects the radio‐sensitivity differently by their various functions. High expression of tumor proliferation suppressing gene will inhibit the proliferation of tumor cells and then induce radio‐sensitive of radiotherapy. As shown above, SERPINB5 and HIC1 genes were hyper‐methylated in radio‐resistant cells, while TM4SF4 and miR24 were hypo‐methylated in radio‐resistant cells (Table 2).
Table 2.
Effect of DNA methylation status of cell proliferation related genes on radiosensitivity
| Gene | Methylation site | Tumor | Cell line | Methylation status | P‐radiosensitivity | T‐radiosensitivity |
|---|---|---|---|---|---|---|
| SERPINB5 | Promoter | NSCLC | H460 | Methylated | Sensitive | ‐ |
| H1299 | Hypermethylated | Resistant | ‐ | |||
| HIC1 | Promoter | Pancreatic carcinoma | MiaPaca | Hypermethylated | Resistant | Sensitive |
| Glioblastoma | U251 | Hypermethylated | Resistant | Sensitive | ||
| Prostate carcinoma | DU145 | Hypomethylated | Resistant | Sensitive | ||
| Breast carcinoma | MDA‐MB‐231 | Hypermethylated | Resistant | Sensitive | ||
| MDA‐MB‐435 | Hypermethylated | Resistant | Sensitive | |||
| miR24‐1 | Promoter | NPC | CNE‐1 | Hypomethylated | Sensitive | ‐ |
| CNE‐2 | Hypomethylated | Sensitive | ‐ | |||
| CNE‐2R | Hypermethylated | Resistant | ‐ | |||
| HONE‐1 | Hypermethylated | Resistant | ‐ | |||
| TM4SF4 | Promoter + 5′‐UTR | NSCLC | A549 | Hypomethylated | Resistant | ‐ |
| Calu‐3 | Hypomethylated | Resistant | ‐ |
HIC1, hyper‐methylated in cancer 1; miR24‐1, microRNA 24‐1; SERPINB5, serine peptidase inhibitor, clade B, member 5; NPC, nasopharyngeal carcinoma; NSCLC, nonsmall cell lung cancer; 5′‐UTR, 5′ untranslated region; P‐radiosensitivity, primordial radiosensitivity; TM4SF4, transmembrane 4 L six family member 4; T‐radiosensitivity, radiosensitivity after treatment with inhibitor of methyltransferase; ‐, undetected.
5. RADIO‐SENSITIVITY AND DNA METHYLATION OF CELL CYCLE‐RELATED GENES
To explore the mechanism of resistance to head and neck squamous cell cancer (HNSCC) radiotherapy, Chen et al3 analyzed the DNA methylation of cyclin D2 (CCND2) in 2 counterpart HNSCC cell lines. This cyclin forms a complex with cyclin‐dependent kinase 4 (CDK4) or cyclin‐dependent kinase 6 (CDK6) and acts as a regulatory subunit of the complex, whose activity is essential for cell cycle G1/S transition.30 The study showed that CCND2 was hyper‐methylated in radio‐resistant cell line, while in the radiosensitive cells was hypo‐methylated. After treating with 5‐aza‐2′deoxycitidine, a DNMT inhibitor, radio‐resistant cells were more sensitive to radiation.3
Fragile histidine triad (FHIT) gene regulates G2/M checkpoint and is located in a fragile chromosome site (3p13.2), which would likely be damaged by ionizing irradiation, Lin et al6 selected it for study. In oral carcinoma cell lines, the promoter of FHIT gene was hyper‐methylated in radio‐resistant cells. In radio‐sensitive cells, the methylation status of FHIT gene was inverse. Further in vivo study showed that 5‐aza‐2′deoxycitidine significantly re‐sensitized radio‐resistant oral cancer cell xenograft tumors. The S100 calcium binding protein A6 (S100A6) gene encoded protein also involves in the regulation of cell cycle progression. In NSCLC, promoter of this gene was hyper‐methylated in radio‐resistant cell line and hypo‐methylated in radio‐sensitive cell line.27
In NPC, researchers analyzed 2 cell cycle‐related genes including reprimo (RPRM) and cyclin‐dependent kinase inhibitor 2A (CDKN2A). RPRM is a gene located at human chromosome 2q23, whose expression in conjunction with p53, along with other genes induced by p53, is associated with the arrest of cell cycle at the G2 phase.31 CDKN2A is a gene located at chromosome 9, band p21.3. The gene codes for 2 proteins including the INK4 family member p16 and p14arf. Both act as tumor suppressors by regulating the cell cycle.32 In both radio‐resistant NPC cell lines, RPRM gene was hyper‐methylated and CDKN2A gene was hypo‐methylated. Treating with 5‐aza‐2′deoxycitidine also enhanced the radio‐sensitivity of both radio‐resistant cell lines.2
Radio‐sensitivity of different division cycles is not same. Cells in S phase are resistant to irradiation, while cells in M and G2 phases are sensitive to irradiation. Treating with radiotherapy, cells in sensitive phase such as phase M or G2 are selectively killed.20 As shown in studies, in radio‐resistant tumor cell, genes prompting cell cycles to G2/M are hyper‐methylated. Thus, these genes are silenced, and the encoded proteins are with low expression (Table 3).
Table 3.
Effect of DNA methylation status of cell cycle related genes on radio‐sensitivity
| Gene | Methylation site | Tumor | Cell line | Methylation status | P‐radiosensitivity | T‐Radiosensitivity |
|---|---|---|---|---|---|---|
| CCND2 | Promoter | HNSCC | SCC‐61 | Hypomethylated | Sensitive | Sensitive |
| rSCC‐61 | Hypermethylated | Resistant | Sensitive | |||
| FHIT | Promoter | Oral carcinoma | OML1‐P | Hypomethylated | Sensitive | Sensitive |
| OML1‐R | Hypermethylated | Resistant | Sensitive | |||
| S100A6 | Promoter | NSCLC | H460 | Hypomethylated | Sensitive | ‐ |
| H1299 | Hypermethylated | Resistant | ‐ | |||
| RPRM | Promoter | NPC | CNE2 | Hypermethylated | Resistant | Sensitive |
| SUNE1 | Hypermethylated | Resistant | Sensitive | |||
| CDKN2A | Promoter | NPC | CNE2 | Hypomethylated | Resistant | Sensitive |
| SUNE1 | Hypomethylated | Resistant | Sensitive |
CCND2, cyclin D2; FHIT, fragile histidine triad; HNSCC, head and neck squamous cell cancer; P‐radiosensitivity, primordial radiosensitivity; RPRM, reprimo; CDKN2A, cyclin dependent kinase inhibitor 24; NPC, nasopharyngeal carcinoma; NSCLC, nonsmall cell lung cancer; S100A6, S100 calcium binding protein A6; T‐radiosensitivity, radiosensitivity after treatment with inhibitor of methyltransferase; ‐, undetected.
6. RADIO‐SENSITIVITY AND DNA METHYLATION OF RE‐OXYGENATION‐RELATED GENES
Hypoxia Inducible Factor (HIF‐1 α) is a stress responsive transcription factor, which regulates gene expression required for hypoxic adaptation. High expression of HIF‐1 α is significantly associated with radio‐resistance.33 Up to date, there are few studies on the relationship between DNA methylation of oxygenation‐related gene and radio‐sensitivity. Although previous study indicated that hypoxia may be an important but not the main factor leading to the failure of tumor radiotherapy, recent study found that changing the hypoxia status could alter the radio‐sensitivity. The study utilized the prolyl‐hydroxylase inhibitor dimethyl‐oxalylglycine (DMOG) to elevate HIF‐1 α levels in mouse embryonic fibroblasts (MEFs) and demonstrated that DMOG function as radio‐protector by increasing HIF‐1 α protein levels. Further study showed that depletion of Suv39 h1 histone H3 methyltransferase reduced the ability of DMOG to protect cells from radiation damage, implicating increased histone H3 methylation in the radioprotection of cells.34 Thus, we thought that hyper‐methylation may affect tumor radio‐sensitivity by silencing re‐oxygenation‐related genes.
7. THE PREDICTIVE ROLE OF DNA METHYLATION IN TUMOR RADIO‐SENSITIVITY
In tumor cells, abnormal gene expression may result from variant in DNA copy number, sequence mutation or epigenetic changes. Tumor cells always show major disruptions in DNA methylation profiles including aberrant hyper‐methylation and hypo‐methylation of specific genes or global genome.12 Previous studies have shown that DNA methylation of genes may predict the radio‐sensitivity of tumor radiotherapy.
According to previous studies and gene silencing role of DNA hypermethylation of CpG islands at promoter regions, we suppose that DNA methylation affects radio‐sensitivity through gene silencing. For example, DNA damage repair prompting genes are hyper‐methylated in radio‐sensitive tumor cells. Hyper‐methylation of these genes results in the low expression of encoded proteins which are essential to DNA repair. Then damaged tumor cells could not repair effectively and timely, so tumor cells are sensitive to radiotherapy.
In addition, to examine the genome‐wide epigenetic control of radio‐resistance, researchers performed whole‐genome analysis of CpG methylation in normal and tumor lung cells.27 They found 1091 methylated differential genes between radio‐sensitive and radio‐resistant lung cells. Further studies indicated that the differences may be critical to epigenetic regulation of radio‐sensitivity in lung cancer cells. Thus, DNA methylation could be the predictive biomarker for tumor radio‐sensitivity.
8. APPLICATION OF DNA METHYLTRANSFERASE INHIBITORS IN CLINICAL PRACTICE
DNMT inhibitors are drugs that inhibit the DNMT which is function as the initiation and maintenance of DNA methylation.35 Above, we have referred that DNMT inhibitors treating with radiotherapy in cells and animal models can enhance the radio‐sensitivity of original radio‐resistant tumors. Yet, in clinical practice of tumor radiotherapy, the use of DNMT inhibitors is limited. The most commonly studied drugs accompany with irradiation treatment are 5‐azacytiding (5AC), zebularine, and 5‐aza‐2′‐deoxycytidine (decitabine). Among these, 5‐azacytiding and 5‐aza‐2′‐deoxycytidine have been approved by the US Food and Drug Administration (FDA) in 2004 and 2006, respectively, while zebularine is using in preclinical studies. Both drugs of 5‐azacytiding and 5‐aza‐2′‐deoxycytidine are approved for treating myelodysplastic syndromes, acute myeloid leukemia, and other myeloid syndromes.36 But in tumor radiotherapy, they have not been recommended. Considering the toxicity of drugs, 5‐azacytidine with poor selectivity, which can incorporate into both RNA and DNA, has strong toxicity. The rank of toxicity among these drugs is 5‐azacytiding > 5‐aza‐2′‐deoxycytidine > zebularine. Other studied compounds are in their earlier stages, such as RG108 and EGGG.37
9. CONCLUSION
Our knowledge about the role of DNA methylation in radio‐sensitivity is mainly from in vitro and in vivo experimental system. The understanding of this pivotal role in human body is limited. Therefore, these studies should be interpreted with caution. Now, there are few studies on the relationship between hypoxic‐related genes’ methylation and radio‐sensitivity. Maybe, further researches are needed. In addition, 2 FDA approved drugs are limited in clinical practice due to their toxicity. While the low toxicity drug zebularine has not been approved to use in clinic.
According to the summary of these studies, we can conclude as follows. First, the methylation level of DNA damage repair related genes is higher than that of genes negative to DNA repair. Second, except CDKN2A and TN4SF4, cell cycle and cell proliferation‐related genes were hyper‐methylated in radio‐resistant cell lines, while the genes in radio‐sensitive cells were hypo‐methylated. CDKN2A gene which regulates the arrest of cell cycle at the G2 phase is hypo‐methylated in radio‐resistant nasopharyngeal carcinoma cells. The TM4SF4 protein, a cell surface glycoprotein that regulates cell proliferation, is also hypo‐methylated in radio‐resistant nonsmall cell lung cancer cell lines. Thus, the methylation level of genes in tumor cells is related to their functions. From previous studies, the pivotal role of DNA methylation in tumor radio‐sensitivity is obviously clear. And radio‐resistant tumor cells and animal models with hyper‐methylation status can be reversed into radio‐sensitive ones.
Due to the prospective results of tumor cells and animal model experiments, we will validate this pivotal role of DNA methylation in human tumor specimens. Furthermore, with the development of emerging circulating tumor cell (CTC) technology,38 we could detect the methylation status of radio‐sensitivity‐related genes in CTC. Furtherly, comparing the methylation status of human specimen with CTC to analyze the sensitivity and specificity of CTC technology. Supposing that CTC technology could be used to detect DNA methylation in radio‐resistant tumors, and then, it is possible to make clinical practice more convenient and feasible. In addition, studies have shown that the inhibitor of DNMT enhance the radio‐sensitivity of tumor cells. These experimental results can promote the development and application of related drugs in clinical practice of tumor radiotherapy, which will bring gospel to patients.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
We acknowledge financial support from National Key Research and Development Program of China (2016YFC0905500 and 2016YFC0104608).
Zhu X, Wang Y, Tan L, Fu X. The pivotal role of DNA methylation in the radio‐sensitivity of tumor radiotherapy. Cancer Med. 2018;7:3812–3819. 10.1002/cam4.1614
REFERENCES
- 1. Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat Rev Drug Discovery. 2013;12:526‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang W, Li YQ, Liu N, Sun Y, He QM, Jiang N, et al. 5‐Azacytidine enhances the radiosensitivity of CNE2 and SUNE1 cells in vitro and in vivo possibly by altering DNA methylation. PLoS ONE. 2014;9:e93273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen X, Liu L, Mims J, Punska EC, Williams KE, Zhao W, et al. Analysis of DNA methylation and gene expression in radiation‐resistant head and neck tumors. Epigenetics. 2015;10:545‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang L, Zhang Y, Li R, Chen Y, Pan X, Li G, et al. 5‐aza‐2′‐Deoxycytidine enhances the radiosensitivity of breast cancer cells. Cancer Biother Radiopharmaceut. 2013;28:34‐44. [DOI] [PubMed] [Google Scholar]
- 5. Choi SI, Kim SY, Lee J, Cho EW, Kim IG. TM4SF4 overexpression in radiation‐resistant lung carcinoma cells activates IGF1R via elevation of IGF1. Oncotarget. 2014;5:9823‐9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin HY, Hung SK, Lee MS, Chiou WY, Huang TT, Tseng CE, et al. DNA methylome analysis identifies epigenetic silencing of FHIT as a determining factor for radiosensitivity in oral cancer: an outcome‐predicting and treatment‐implicating study. Oncotarget. 2015;6:915‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim YJ, Lee WJ, Woo SM, Kim TH, Han SS, Kim BH, et al. Comparison of capecitabine and 5‐fluorouracil in chemoradiotherapy for locally advanced pancreatic cancer. Radiat Oncol. 2013;8:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta S, Khan H, Barik S, Negi MP. Clinical benefits of concurrent capecitabine and cisplatin versus concurrent cisplatin and 5‐flurouracil in locally advanced squamous cell head and neck cancer. Drug Discov Ther. 2013;7:36‐42. [DOI] [PubMed] [Google Scholar]
- 9. Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor‐risk rectal cancer: N‐SOG 03 Phase II trial. Jpn J Clin Oncol. 2013;43:964‐971. [DOI] [PubMed] [Google Scholar]
- 10. Mattiucci GC, Ippolito E, D'Agostino GR, Alfieri S, Antinori A, Crucitti A, et al. Long‐term analysis of gemcitabine‐based chemoradiation after surgical resection for pancreatic adenocarcinoma. Ann Surg Oncol. 2013;202:423‐429. [DOI] [PubMed] [Google Scholar]
- 11. Kafri T, Ariel M, Brandeis M, Shemer R, Urven L, McCarrey J, et al. Developmental pattern of gene‐specific DNA methylation in the mouse embryo and germ line. Genes Dev. 1992;6:705‐714. [DOI] [PubMed] [Google Scholar]
- 12. McCabe MT, Brandes JC, Vertino PM. Cancer DNA methylation: molecular mechanisms and clinical implications. Clin Cancer Res. 2009;15:3927‐3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyer B, Fabbrizi MR, Raj S, Zobel CL, Hallahan DE, Sharma GG. Histone H3 lysine 9 acetylation obstructs ATM activation and promotes ionizing radiation sensitivity in normal stem cells. Stem Cell Rep. 2016;7:1013‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim WJ, Vo QN, Shrivastav M, Lataxes TA, Brown KD. Aberrant methylation of the ATM promoter correlates with increased radiosensitivity in a human colorectal tumor cell line. Oncogene. 2002;21:3864‐3871. [DOI] [PubMed] [Google Scholar]
- 15. Ren J, Chu Y, Ma H, Zhang Y, Zhang X, Zhao D, et al. Epigenetic interventions increase the radiation sensitivity of cancer cells. Curr Pharm Des. 2014;20:1857‐1865. [DOI] [PubMed] [Google Scholar]
- 16. Kim HJ, Kim JH, Chie EK, Young PD, Kim IA, Kim IH. DNMT (DNA methyltransferase) inhibitors radiosensitize human cancer cells by suppressing DNA repair activity. Radiat Oncol. 2012;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klement RJ. The influence of ketogenic therapy on the 5 R's of radiobiology. Int J Radiat Biol. 2017;93:1‐13. [DOI] [PubMed] [Google Scholar]
- 18. Cui L, Her S, Borst GR, Bristow RG, Jaffray DA, Allen C. Radiosensitization by gold nanoparticles: will they ever make it to the clinic? Radiother Oncol. 2017;124:344‐356. [DOI] [PubMed] [Google Scholar]
- 19. Brown KD, Barlow C, Wynshaw‐Boris A. Multiple ATM‐dependent pathways: an explanation for pleiotropy. Am J Hum Genet. 1999;64:46‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roy K, Wang L, Makrigiorgos GM, Price BD. Methylation of the ATM promoter in glioma cells alters ionizing radiation sensitivity. Biochem Biophys Res Comm. 2006;344:821‐826. [DOI] [PubMed] [Google Scholar]
- 21. Liu Z‐g, Chen H‐y, Xia Y‐f, Kwan AL, Chen Z‐p. Relationship between methylation status of ERCC1 promoter and radio‐sensitivity in glioma cell lines. Chin J Neurooncol. 2007;5:156‐159. [Google Scholar]
- 22. Pabalan N, Kunjantarachot A, Ruangpratheep C, Jarjanazi H, Christofolini DM, Barbosa CP, et al. Potential of RASSF1A promoter methylation as biomarker for endometrial cancer: a systematic review and meta‐analysis. Gynecol Oncol. 2017;146:603‐608. [DOI] [PubMed] [Google Scholar]
- 23. Dote H, Cerna D, Burgan WE, Carter DJ, Cerra MA, Hollingshead MG, et al. Enhancement of in vitro and in vivo tumor cell radiosensitivity by the DNA methylation inhibitor zebularine. Clin Cancer Res. 2005;11:4571‐4579. [DOI] [PubMed] [Google Scholar]
- 24. Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor‐suppressing activity in human mammary epithelial cells. Science (New York, NY). 1994;263:526‐529. [DOI] [PubMed] [Google Scholar]
- 25. Khalkhali‐Ellis Z. Maspin: the new frontier. Clin Cancer Res. 2006;12:7279‐7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, et al. Nuclear cytokine‐activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690‐694. [DOI] [PubMed] [Google Scholar]
- 27. Kim EH, Park AK, Dong SM, Ahn JH, Park WY. Global analysis of CpG methylation reveals epigenetic control of the radiosensitivity in lung cancer cell lines. Oncogene. 2010;29:4725‐4731. [DOI] [PubMed] [Google Scholar]
- 28. Wang L, Feng J, Da L, Li Y, Li Z, Zhao M. Adenovirus‐mediated delivery of siRNA targeting TM4SF4 attenuated liver cancer cell growth in vitro and in vivo. Acta Biochim Biophys Sin. 2013;45:213‐219. [DOI] [PubMed] [Google Scholar]
- 29. Wang S, Zhang R, Claret FX, Yang H. Involvement of microRNA‐24 and DNA methylation in resistance of nasopharyngeal carcinoma to ionizing radiation. Mol Cancer Ther. 2014;13:3163‐3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Misiewicz‐Krzeminska I, Sarasquete ME, Vicente‐Duenas C, Krzeminski P, Wiktorska K, Corchete LA, et al. Post‐transcriptional modifications contribute to the upregulation of Cyclin D2 in multiple myeloma. Clin Cancer Res. 2016;22:207‐217. [DOI] [PubMed] [Google Scholar]
- 31. Xu M, Knox AJ, Michaelis KA, Kiseljak‐Vassiliades K, Kleinschmidt‐DeMasters BK, Lillehei KO, et al. Reprimo (RPRM) is a novel tumor suppressor in pituitary tumors and regulates survival, proliferation, and tumorigenicity. Endocrinology. 2012;153:2963‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor NJ, Mitra N, Goldstein AM, Tucker MA, Avril MF, Azizi E, et al. Germline variation at CDKN2A and associations with nevus phenotypes among members of melanoma families. J Invest Dermatol. 2017;137:2606‐2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilkins SE, Abboud MI, Hancock RL, Schofield CJ. Targeting protein‐protein interactions in the HIF system. ChemMedChem. 2016;11:773‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ayrapetov MK, Xu C, Sun Y, Zhu K, Parmar K, D'Andrea AD, et al. Activation of Hif1alpha by the prolylhydroxylase inhibitor dimethyoxalyglycine decreases radiosensitivity. PLoS ONE. 2011;6:e26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abdelfatah E, Kerner Z, Nanda N, Ahuja N. Epigenetic therapy in gastrointestinal cancer: the right combination. Therap Adv Gastroenterol. 2016;9:560‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zielske SP. Epigenetic DNA methylation in radiation biology: on the field or on the sidelines? J Cell Biochem. 2015;116:212‐217. [DOI] [PubMed] [Google Scholar]
- 37. Zhang P, Pollock RE. Epigenetic regulators: new therapeutic targets for soft tissue sarcoma. Cancer Cell Microenviron. 2014;1:e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ignatiadis M, Lee M, Jeffrey SS. Circulating tumor cells and circulating tumor DNA: challenges and opportunities on the path to clinical utility. Clin Cancer Res. 2015;21:4786‐4800. [DOI] [PubMed] [Google Scholar]


