Abstract
Aim
This study aimed to investigate circumferential resection margin (CRM) as a prognostic factor for long‐term oncologic survival after rectal cancer surgery.
Methods
Patients diagnosed with malignant rectal cancer between 1 January 2010 and 31 December 2014, from the Surveillance, Epidemiology, and End Results (SEER) program were identified for this study. The patients were divided into five CRM groups to compare the baseline characteristics and assess cancer‐specific survival (CSS): 0‐1 mm, 1.1‐2.0 mm, 2.1‐5.0 mm, 5.1‐10.0 mm, and >10 mm. The main endpoint was CSS.
Results
Circumferential resection margin ≤1 mm was independently associated with 99% increased risk of cancer‐specific mortality in rectal cancer [hazard ratio (HR) = 1.990, 95% confidence interval (CI) = 1.613‐2.454, P < 0.001, using CRM (1.1‐2.0 mm) as a reference]. CRM (5.1‐10.0 mm) was independently associated with 29.2% decreased risk of cancer‐specific mortality [HR = 0.708, 95% CI = 0.525‐0.954, P = 0.152, using group (2.1‐5.0 mm) as reference]. CRM ≤2 mm or ≤0.4 mm was not obviously associated with CSS.
Conclusions
circumferential resection margin is an independent prognostic factor in rectal cancer. Surgeons should try to maximize the CRM. Rectal cancer patients with CRM ≤1 mm should receive more postoperative attention depending on individual situation. Also, CRM should be accurately measured in millimeters in a preoperative magnetic resonance imaging or pathological report, rather than simply described as “involved” or “clear.”
Keywords: circumferential resection margin, prognostic, rectal cancer, SEER
1. INTRODUCTION
Circumferential resection margin (CRM) is the closest distance between the radial resection margin and the tumor tissue by either direct tumor spread, areas of neural or vascular invasion, or the nearest involved lymph node.1 Despite the routine use of preoperative chemoradiotherapy (CT) followed by total mesorectal excision (TME) for locally advanced rectal cancer, the local recurrence and mortality remain high, and the search for potential prognostic factors has become increasingly important.2, 3
While several studies showed that CRM should not be used as a prognostic factor in rectal cancer,4, 5 other studies demonstrated the importance of CRM as an independent prognostic factor of local recurrence and long‐term survival,6, 7, 8, 9 including the first report by Quirke et al10 suggesting that CRM might be a strong predictor of long‐term oncologic outcomes. According to the European Society for Medical Oncology (ESMO) Clinical Practice Guidelines for rectal cancer, CRM is defined as involved if it is ≤1 mm from the tumor‐free margin, leading to an increased risk of local recurrence, distant metastases, and poorer survival.11
Many studies considered CRM as positive when it was ≤1 mm (R1) and associated with obviously poor prognosis as compared to CRM >1 mm (R0), which was in accordance with the ESMO guidelines.6, 8, 12, 13, 14, 15, 16, 17 The criterion to define a positive CRM remains unclear. However, some researchers believed that CRM within 2 mm was associated with a negative prognosis.18, 19, 20, 21, 22 In addition, Kelly et al1 argued that a CRM clearance >5 mm should be achieved to optimize curative treatment. Recently, Beaufrère et al23 found that the prognosis after rectal cancer surgery was worse with a CRM ≤0.4 mm.
Given that the aforementioned studies had a relatively small sample size, the Surveillance, Epidemiology, and End Results (SEER) program conducted a large population‐based study to analyze the prognostic ability of CRM distance in rectal cancer.
2. PATIENTS AND METHODS
2.1. CRM in SEER database
The SEER database is an authoritative source of information on the most recent cancer incidence, mortality, prevalence, and lifetime risk statistics in the United States. It is a comprehensive source of population‐based information including all newly diagnosed cancer cases among people residing in SEER‐participating areas and covering approximately 28% of the US population.
The CRMs in SEER database are, expressed as the nearest tenth in millimeters (mm), the distance between the leading edge of the tumor and the nearest edge of surgically dissected margin, as recorded in the pathology report according to The American Joint Committee on Cancer (AJCC) Seventh Edition Cancer Staging Manual: the CRM is the surgically dissected nonperitonealized surface of the specimen.
2.2. Study design
Using the SEER‐Stat software (SEER*Stat 8.3.4), patients diagnosed with malignant rectal cancer between 1 January 2010 and 31 December 2014, from the SEER Program of the National Cancer Institute were identified (Figure 1). Among these patients, patients with known CRM (eg, CS Site‐Specific Factor 6, 2004, varying by Schema 000, 032, and 981) were included in the analysis. Patients with rectal cancer whose CS Site‐Specific Factor 6 was 996 (means “>5 mm”; cannot be grouped in this study), with unknown seventh AJCC stage or unknown race record, were excluded. Patients were then divided into five CRM groups: 0‐1 mm, 1.1‐2.0 mm, 2.1‐5.0 mm, 5.1‐10.0 mm, and >10 mm to compare the baseline characteristics and assess cancer‐specific survival (CSS). Next, patients whose CS Site‐Specific Factor 6 was 000 (CRM <1 mm and <0.4 mm; unknown) were also excluded. Thus, the target population for further analysis was obtained. These patients were divided into six CRM groups: ≤0.4 mm, 0.5‐1.0 mm, 1.1‐2.0 mm, 2.1‐5.0 mm, 5.1‐10.0 mm, and >10 mm.
Figure 1.
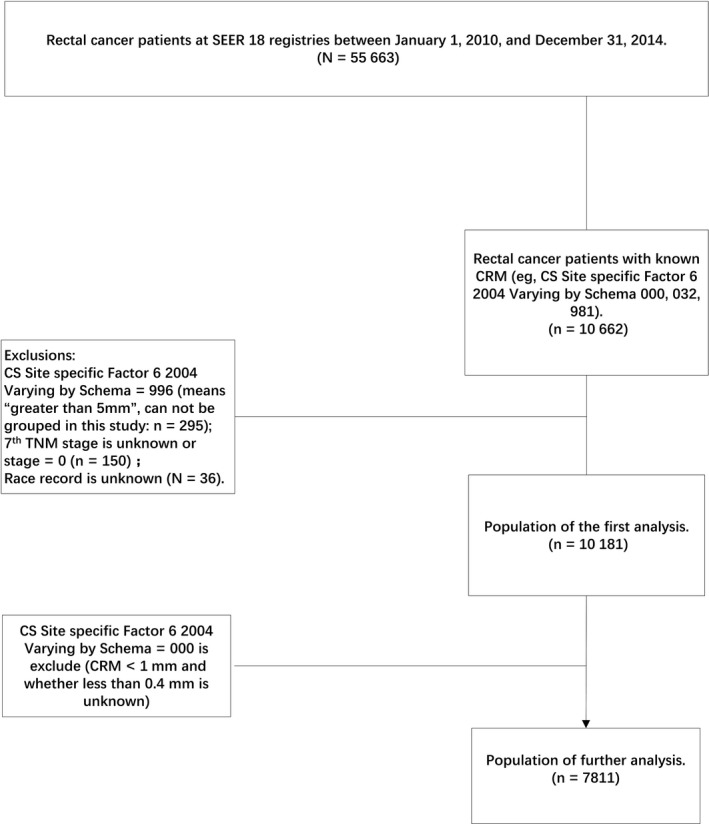
Flow diagram of patient population selection from the SEER database
2.3. Statistical analyses
Several Cox proportional hazards models were built to identify independent prognostic variables at a median survival time of 22 months (range 0‐59 months). All hazard ratios (HR) were shown with 95% confidence intervals (CI). A multivariate survival analysis was performed using a Cox proportional hazards model, including all variables associated with a P value <0.2 in univariate analysis. Variables including AJCC stage, tumor size, age at diagnosis, race, gender, year of diagnosis, and grade were included in the Cox multivariate survival analysis. The TNM staging used in the present study was the seventh edition of the AJCC cancer staging system, the newest TNM staging that could be obtained from the SEER database. The primary outcome of interest was CSS. The Kaplan‐Meier survival curves were used to evaluate the prognostic prediction of different factors. The log‐rank tests were used to assess statistical significance. All tests were two sided, and P values <0.05 were considered statistically significant. Statistical analysis was performed using Statistic Package for Social Science (SPSS) version 22 (SPSS Inc., IL, USA).
3. RESULTS
3.1. Patient characteristics of the overall cohort
A total of 10 181 patients with rectal cancer after surgery were identified from the SEER database. The baseline demographic characteristics of the patients are summarized in Table 1. A total of 4232 (41.6%) patients whose CRM was between 0 and 1 mm were included in the analyses. The overall cohort showed that higher AJCC stages (P < 0.001), larger tumor size (P < 0.001), black people (P < 0.001), earlier year of diagnosis (P < 0.001), and higher grades (P < 0.001) were associated with a CRM between 0 and 1 mm. Differences in other characteristics were not significant.
Table 1.
Comparison of baseline characteristics by various circumferential resection margins
| Variable | Distance to circumferential resection margin | P value | ||||
|---|---|---|---|---|---|---|
| 0‐1.0 mm | 1.1‐2.0 mm | 2.1‐5.0 mm | 5.1‐10.0 mm | >10.0 mm | ||
| AJCC stage | ||||||
| Stage I | 620 (32.9%) | 214 (11.3%) | 355 (18.8%) | 166 (8.8%) | 531 (28.2%) | <0.001 |
| Stage II | 1071 (39.3%) | 320 (11.8%) | 428 (15.7%) | 274 (10.1%) | 630 (23.1%) | |
| Stage III | 1772 (41.1%) | 482 (11.2%) | 671 (15.6%) | 426 (9.9%) | 957 (22.2%) | |
| Stage IV | 769 (60.8%) | 110 (8.7%) | 127 (10.0%) | 84 (6.6%) | 174 (13.8%) | |
| Tumor size | ||||||
| ≤5 cm | 2379 (37.9%) | 704 (11.2%) | 1039 (16.6%) | 602 (9.6%) | 1549 (24.7%) | <0.001 |
| >5 cm | 1499 (47.7%) | 315 (10.0%) | 444 (14.1%) | 283 (9.0%) | 601 (19.1%) | |
| Unknown | 354 (46.2%) | 107 (14.0%) | 88 (12.8%) | 65 (8.5%) | 142 (18.5%) | |
| Age at diagnosis (y) | ||||||
| ≤60 | 1903 (41.4%) | 504 (11.0%) | 700 (15.2%) | 433 (9.4%) | 1061 (23.1%) | 0.757 |
| >60 | 2329 (41.7%) | 622 (11.1%) | 881 (15.8%) | 517 (9.3%) | 1231 (22.1%) | |
| Race | ||||||
| White | 3388 (40.9%) | 932 (11.2%) | 1313 (15.8%) | 779 (9.4%) | 1876 (22.6%) | <0.001 |
| Black | 408 (51.3%) | 84 (10.6%) | 104 (13.1%) | 54 (6.8%) | 145 (18.2%) | |
| Other | 436 (39.7%) | 110 (10.0%) | 164 (14.9%) | 117 (10.7%) | 271 (24.7%) | |
| Gender | ||||||
| Male | 2543 (42.0%) | 688 (11.4%) | 907 (15.0%) | 562 (9.3%) | 1354 (22.4%) | 0.287 |
| Female | 1689 (40.9%) | 438 (10.6%) | 674 (16.3%) | 388 (9.4%) | 938 (22.7%) | |
| Year of diagnosis | ||||||
| 2010 | 895 (48.5%) | 225 (12.2%) | 290 (15.7%) | 157 (8.5%) | 280 (15.2%) | <0.001 |
| 2011 | 874 (42.9%) | 229 (11.3%) | 317 (15.6%) | 172 (8.5%) | 443 (21.8%) | |
| 2012 | 873 (41.3%) | 216 (10.2%) | 325 (15.4%) | 208 (9.8%) | 491 (23.2%) | |
| 2013 | 803 (38.8%) | 220 (10.6%) | 317 (15.3%) | 213 (10.3%) | 516 (24.9%) | |
| 2014 | 787 (37.2%) | 236 (11.1%) | 332 (15.7%) | 200 (9.4%) | 562 (26.5%) | |
| Grade | ||||||
| Grade I | 279 (43.7%) | 75 (11.8%) | 110 (17.2%) | 61 (9.6%) | 113 (17.7%) | <0.001 |
| Grade II | 2835 (38.1%) | 859 (11.5%) | 1217 (16.3%) | 730 (9.8%) | 1806 (24.3%) | |
| Grade III | 725 (55.6%) | 116 (8.9%) | 165 (12.7%) | 88 (6.7%) | 210 (16.1%) | |
| Grade IV | 170 (60.1%) | 25 (8.8%) | 27 (9.5%) | 18 (6.4%) | 43 (15.2%) | |
| Unknown | 223 (43.8%) | 51 (10.0%) | 62 (12.2%) | 53 (10.4%) | 120 (23.6%) | |
3.2. R1 CRM was strongly associated with poor survival in rectal cancer
The median follow‐up duration for the overall cohort was 22 months (range 0‐59 months). At the end of the follow‐up, 1262 (12.4%) patients died of rectal cancer.
A multivariate analysis was conducted to identify the variables independently associated with CSS in the overall cohort. The results of multivariate analyses by Cox regression are detailed in Table 2. R1 CRM was found to be independently associated with CSS of 10 181 patients with rectal cancer and had a 99.0% increased risk of cancer‐specific mortality [HR = 1.990, 95% CI = 1.613‐2.454, P < 0.001, using group (1.1‐2.0 mm) as a reference]. In addition, Table 2 shows that lower AJCC stages, younger age, and lower grades were independent protective factors.
Table 2.
Multivariate Cox regression analyses of CSS to study CRM ≤1 mm
| Variable | Reference | Characteristic | Cancer‐specific survival | ||
|---|---|---|---|---|---|
| HR (95%CI) | SE | P value | |||
| CRM | 1.1‐2.0 mm | 0‐1.0 mm | 1.990 (1.613‐2.454) | 0.107 | <0.001 |
| 2.1‐5.0 mm | 1.106 (0.855‐1.429) | 0.131 | 0.443 | ||
| 5.1‐10.0 mm | 0.783 (0.569‐1.075) | 0.162 | 0.131 | ||
| >10.0 mm | 0.825 (0.637‐1.070) | 0.132 | 0.147 | ||
| AJCC stage | Stage I | Stage II | 2.426 (1.769‐3.327) | 0.161 | <0.001 |
| Stage III | 4.759 (3.538‐6.400) | 0.151 | <0.001 | ||
| Stage IV | 15.909 (11.773‐21.499) | 0.154 | <0.001 | ||
| Tumor size | Unknown | ≤5 cm | 0.803 (0.645‐1.000) | 0.112 | 0.050 |
| >5 cm | 1.129 (0.904‐1.411) | 0.114 | 0.284 | ||
| Age at diagnosis (y) | ≤60 | >60 | 1.777 (1.584‐1.993) | 0.058 | <0.001 |
| Year of diagnosis | 2010 | 2011 | 1.124 (0.973‐1.299) | 0.074 | 0.111 |
| 2012 | 0.884 (0.747‐1.046) | 0.086 | 0.152 | ||
| 2013 | 1.014 (0.832‐1.237) | 0.101 | 0.887 | ||
| 2014 | 0.830 (0.611‐1.128) | 0.156 | 0.234 | ||
| Grade | Grade I | Grade II | 1.286 (0.942‐1.757) | 0.159 | 0.113 |
| Grade III | 2.195 (1.584‐3.041) | 0.166 | <0.001 | ||
| Grade IV | 2.793 (1.914‐4.074) | 0.193 | <0.001 | ||
| Unknown | 1.439 (0.963‐2.150) | 0.205 | 0.076 | ||
Kaplan‐Meier CSS curves were used to analyze the prognosis of different CRMs (Figure 2). Group (0‐1.0 mm) was associated with poorer CSS (90.0% for 1‐year CSS and 73.8% for 3‐year CSS). The 3‐year CSS of group (1.1‐2.0 mm), group (2.1‐5.0 mm), group (5.1‐10.0 mm), and group (>10.0 mm) was 88.2%, 87.3%, 91.4%, and 90.8%, respectively. However, the differences between group (1.1‐2.0 mm) and group (2.1‐5.0 mm), group (1.1‐2.0 mm) and group (5.1‐10.0 mm), and group (5.1‐10.0 mm) and group (>10.0 mm) were not statistically significant. The Cox multivariate CSS analysis also showed no significant difference between group (2.1‐5.0 mm) and group (1.1‐2.0 mm) [HR = 0.905, 95% CI = 0.700‐1.169, P = 0.443, using group (2.1‐5.0 mm) as reference] (Table S1). However, group (5.1‐10.0 mm) had more favorable prognosis as compared to group (2.1‐5.0 mm) [HR = 0.708, 95% CI = 0.525‐0.954, P = 0.152, using group (2.1‐5.0 mm) as reference].
Figure 2.
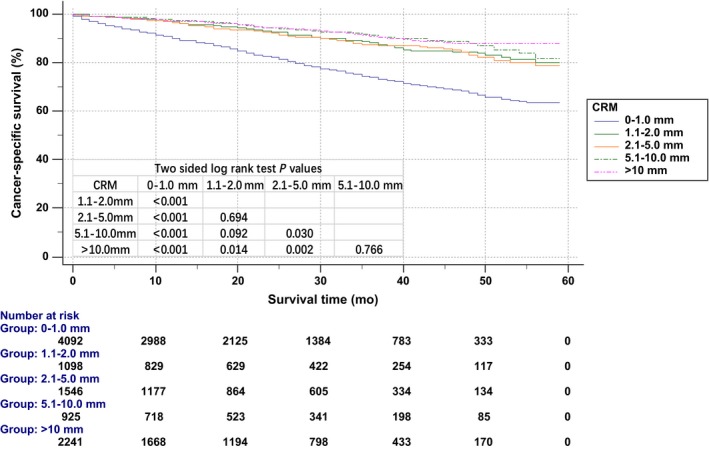
Kaplan‐Meier cancer‐specific survival curve according to circumferential resection margin (CRM)
Kaplan‐Meier OS curves were also used to analyze the prognosis of different CRMs (Figure 3). Group (0‐1.0 mm) was associated with poorer OS (84.9% for 1‐year OS and 62.4% for 3‐year OS). The 3‐year OS of group (1.1‐2.0 mm), group (2.1‐5.0 mm), group (5.1‐10.0 mm), and group (>10.0 mm) was 78.5%, 79.0%, 81.3%, and 83.8%, respectively. However, the differences between group (1.1‐2.0 mm) and group (2.1‐5.0 mm), group (1.1‐2.0 mm) and group (5.1‐10.0 mm), group (2.1‐5.0 mm) and group (5.1‐10.0 mm), and group (5.1‐10.0 mm) and group (>10.0 mm) were not statistically significant.
Figure 3.
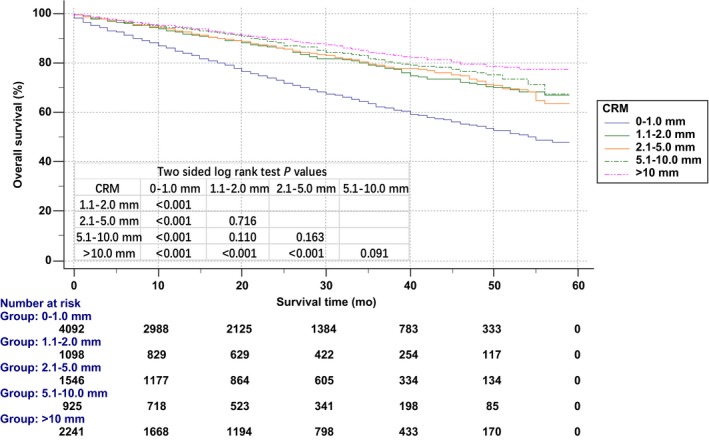
Kaplan‐Meier overall survival curve according to circumferential resection margin (CRM)
3.3. Further analysis of R1 CRM
Patients whose CRM was not known to be <0.4 mm were excluded. Hence, 7811 patients with rectal cancer were identified for further analysis of R1 CRM. R1 CRM was further divided into group (≤0.4 mm) and group (0.5‐1.0 mm).
A multivariate analysis was conducted to identify whether the CSS was different between group (≤0.4 mm) and group (0.5‐1.0 mm) in this target population. The results of multivariate analyses by Cox regression are detailed in Table 3. The difference between group (≤0.4 mm, n = 741) and group (0.5‐1.0 mm, n = 576) was HR = 0.834, 95% CI 0.645‐1.078, using group (≤0.4 mm) as reference, but it was not statistically significant (log‐rank test, P = 0.166).
Table 3.
Multivariate Cox regression analyses of CSS to study CRM ≤0.4 mm
| Variable | Reference | Characteristic | Cancer‐specific survival | ||
|---|---|---|---|---|---|
| HR (95%CI) | SE | P value | |||
| CRM | ≤0.4 mm | 0.5‐1.0 mm | 0.834 (0.645‐1.078) | 0.131 | 0.166 |
| 1.1‐2.0 mm | 0.704 (0.536‐0.924) | 0.139 | 0.012 | ||
| 2.1‐5.0 mm | 0.773 (0.602‐0.992) | 0.127 | 0.043 | ||
| 5.1‐10.0 mm | 0.543 (0.397‐0.741) | 0.159 | <0.001 | ||
| >10.0 mm | 0.577 (0.448‐0.742) | 0.129 | <0.001 | ||
| AJCC stage | Stage I | Stage II | 2.041 (1.388‐3.001) | 0.197 | <0.001 |
| Stage III | 4.361 (3.060‐6.216) | 0.181 | <0.001 | ||
| Stage IV | 15.773 (10.949‐22.722) | 0.186 | <0.001 | ||
| Tumor size | Unknown | ≤5 cm | 0.941 (0.685‐1.292) | 0.162 | 0.707 |
| >5 cm | 1.283 (0.927‐1.775) | 0.166 | 0.133 | ||
| Age at diagnosis (y) | ≤60 | >60 | 1.962 (1.674‐2.298) | 0.081 | <0.001 |
| Grade | Grade I | Grade II | 1.125 (0.738‐1.714) | 0.215 | 0.583 |
| Grade III | 2.077 (1.331‐3.241) | 0.227 | 0.001 | ||
| Grade IV | 2.899 (1.685‐4.987) | 0.277 | <0.001 | ||
| Unknown | 1.027 (0.584‐1.805) | 0.288 | 0.926 | ||
4. DISCUSSION
While CRM is widely accepted as a strong independent prognostic factor of long‐term oncologic survival, the criterion used to define a positive CRM remains controversial.16 Therefore, this retrospective study was conducted to assess the influence of CRM on prognosis after rectal cancer surgery. The present study included more than 10 000 patients with rectal cancer, which greatly exceeded the number of cases in previous studies and hence the results of this study are persuasive and depict real‐world scenario.
R1 CRM was found to be an independent factor for poor prognosis and had 99.0% increased risk of cancer‐specific mortality as compared to group (1.1‐2.0 mm). This is consistent with most studies on the prognostic prediction of CRM.6, 7, 8, 12, 13, 14, 15, 16
However, some researchers argued that CRM <2 mm was associated with a negative prognosis.18, 19, 20, 21, 22 A CRM clearance of >5 mm and 0.4 mm was proposed by Kelly et al1 in 2009 and Beaufrère et al23 in 2017, respectively. Relevant analyses were also conducted in this study to assess previous study results. The Cox multivariate analysis showed that the differences in CSS between group (1.1‐2.0 mm) and group (2.1‐5.0 mm) were not statistically significant. Yet, group (5.1‐10.0 mm) had 29.2% decreased cancer‐specific mortality as compared to group (2.1‐5.0 mm). After excluding the patients whose CRM was <0.4 mm or unknown, the Cox multivariate analysis showed no statistically significant difference between group (≤0.4 mm) and group (0.5‐1 mm). Given the large sample size in the present study, it was believed that 2 and 0.4 mm were not optimal cutoff values, in partial agreement with Kelly et al. In the study by Kelly et al, the multivariate analysis showed 32.4% increased cancer‐specific mortality in group (>1 and ≤5 mm) as compared to group (>5 and ≤10 mm), which was similar to the result of the present study. However, there was no obvious difference in CSS between group (0‐1.0 mm) and group (1.1‐2.0 mm) in the present study.
The treatment modalities have dramatically changed in the recent years. The introduction of newer surgical techniques (TME and laparoscopy) and neoadjuvant chemoradiotherapy have reduced the incidence of positive CRMs.9 Well‐performed TMEs with a resection margin on the mesorectal plane showed <10% of margin positivity.24, 25 The European Organization for Research and Treatment of Cancer trial showed that neoadjuvant radiochemotherapy had 9% decreased margin positivity as compared to short‐course radiotherapy.26
MRI is the most accurate method for preoperative diagnosis of rectal cancer and can detect tumor invasion.27 The results of the present study suggested that neoadjuvant radiochemotherapy should be considered if the distance of tumor and the mesorectal fascia is predicted to be <1 mm by preoperative MRI in rectal cancer. While some recent studies have reported that postoperative treatment did not improve outcomes in this situation,28, 29, 30 we hypothesize that CRM could guide postoperative treatment in combination with preoperative MRI assessment and neoadjuvant chemotherapy.31, 32, 33 Also, the prognosis is typically better when the distance of the tumor is larger from the radial resection margin. Therefore, surgeons should try to maximize the CRM and at least 1 mm of CRM should be reached. Given the 32.4% increased cancer‐specific mortality in group (>1, ≤5 mm) as compared to group (>5, ≤10 mm), 5 mm of CRM should also be considered. Whether patients with the distance between tumor and the mesorectal fascia predicted as less than 5 mm by preoperative MRI need neoadjuvant chemoradiotherapy or with CRM ≤5 mm need more intensive postoperative attention should depend on the individual situations.
The present study also found that higher AJCC stages, larger tumor size, black people, earlier year of diagnosis, and higher grades were associated with a CRM between 0 and 1 mm, resulting in poor prognosis. AJCC stage, tumor size, and tumor grade are known prognostic factors in rectal cancer, adding to the evidence that CRM is strongly associated with the prognosis in rectal cancer.34, 35 The incidence of R1 CRM is reducing every year due to the improvements in treatment. Black people are more likely to achieve R1 CRM and should receive adequate attention. This is attributed to the financial conditions and biological differences between races.
This study was the largest till date and included more than 10 000 patients for the analyses of the prognostic prediction of CRM and was the first to simultaneously analyze postoperative R1 CRM and R0 CRM in depth.
This study had several limitations. First, the SEER database lacked the data on local recurrence, which is an important factor that influences the survival of rectal cancer. However, patients with an R1 CRM often die from metastatic disease before local recurrence.7, 36 In addition, definitions of local recurrence were different in previous studies, making it difficult to examine the prognosis of different CRMs. Therefore, CSS is thought to be a more robust endpoint to assess the prognostic prediction of CRM.37 Second, the lack of factors influencing the treatment might have affected the results to some extent. However, the large sample size could offset this influence. Further, the lack of preoperative treatment had minimal effect since CRM in the present study was measured post‐operation and would have improved after preoperative treatment. The longest follow‐up duration was only 59 months, not exceeding 5 years. Besides, the present analysis was solely based on retrospective data. Hence, prospective clinical studies on CRM are needed.
In summary, CRM is an independent prognostic factor in rectal cancer, and surgeons should try to maximize the CRM. R1 CRM indicates a poor prognosis. Patients with rectal cancer having R1 CRM should receive more postoperative attention. Also, 5 mm of CRM should be adequately monitored and further investigated. The closest distance between the radial resection margin and the tumor tissue should be accurately measured in millimeters in preoperative MRI or pathological report, rather than simply described as “involved” or “clear.” This may provide better treatment guidelines for clinicians.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
Supporting information
Liu Q, Luo D, Cai S, Li Q, Li X. Circumferential resection margin as a prognostic factor after rectal cancer surgery: A large population‐based retrospective study. Cancer Med. 2018;7:3673–3681. 10.1002/cam4.1662
Qi Liu and Dakui Luo contributed equally to this work.
Contributor Information
Qingguo Li, Email: oncosurgeonli@sohu.com.
Xinxiang Li, Email: 1149lxx@sina.com.
REFERENCES
- 1. Kelly SB, Mills SJ, Bradburn DM, et al. Effect of the circumferential resection margin on survival following rectal cancer surgery. Br J Surg. 2011;98(4):573‐581. [DOI] [PubMed] [Google Scholar]
- 2. Fokas E, Ströbel P, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy as a prognostic factor and individual‐level surrogate for disease‐free survival in rectal cancer. J Natl Cancer Inst. 2017;109(12): 10.1093/jnci/djx095 [DOI] [PubMed] [Google Scholar]
- 3. Ikoma N, You YN, Bednarski BK, et al. Impact of recurrence and salvage surgery on survival after multidisciplinary treatment of rectal cancer. J Clin Oncol. 2017;35(23):JCO2016721464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engelen SM, Maas M, Lahaye MJ, et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur J Cancer. 2013;49(10):2311‐2320. [DOI] [PubMed] [Google Scholar]
- 5. Nikberg M, Kindler C, Chabok A, et al. Circumferential resection margin as a prognostic marker in the modern multidisciplinary management of rectal cancer. Dis Colon Rectum. 2015;58(3):275. [DOI] [PubMed] [Google Scholar]
- 6. Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235(4):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cawthorn SJ, Parums DV, Gibbs NM, et al. Extent of mesorectal spread and involvement of lateral resection margin as prognostic factors after surgery for rectal cancer. Lancet. 1990;335(8697):1055. [DOI] [PubMed] [Google Scholar]
- 8. Wibe DA, Rendedal PR, Svensson E, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer †. Br J Surg. 2002;89(3):327‐334. [DOI] [PubMed] [Google Scholar]
- 9. Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26(2):303. [DOI] [PubMed] [Google Scholar]
- 10. Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2(8514):996. [DOI] [PubMed] [Google Scholar]
- 11. Schmoll HJ, Van CE, Stein A, et al. Esmo consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23(10):2479. [DOI] [PubMed] [Google Scholar]
- 12. Adam IJ, Martin IG, Finan P, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344(8924):707. [DOI] [PubMed] [Google Scholar]
- 13. de Haas‐Kock DF, Baeten CG, Jager JJ, et al. Prognostic significance of radial margins of clearance in rectal cancer. Br J Surg. 1996;83(12):781‐785. [DOI] [PubMed] [Google Scholar]
- 14. Glynne‐Jones R, Mawdsley S, Novell JR. The clinical significance of the circumferential resection margin following preoperative pelvic chemo‐radiotherapy in rectal cancer: Why we need a common language. Colorectal Dis. 2006;8(9):800. [DOI] [PubMed] [Google Scholar]
- 15. Lin HH, Lin JK, Lin CC, et al. Circumferential margin plays an independent impact on the outcome of rectal cancer patients receiving curative total mesorectal excision. Am J Surg. 2013;206(5):771‐777. [DOI] [PubMed] [Google Scholar]
- 16. Park JS, Huh JW, Park YA, et al. A circumferential resection margin of 1 mm is a negative prognostic factor in rectal cancer patients with and without neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2014;57(8):933‐940. [DOI] [PubMed] [Google Scholar]
- 17. Trakarnsanga A, Gonen M, Shia J, et al. What is the significance of the circumferential margin in locally advanced rectal cancer after neoadjuvant chemoradiotherapy? Ann Surg Oncol. 2013;20(4):1179‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernstein TE, Endreseth BH, Romundstad P, et al. Circumferential resection margin as a prognostic factor in rectal cancer. Br J Surg. 2009;96(11):1348‐1357. [DOI] [PubMed] [Google Scholar]
- 19. Bouzourene H, Bosman FT, Matter M, et al. Predictive factors in locally advanced rectal cancer treated with preoperative hyperfractionated and accelerated radiotherapy. Hum Pathol. 2003;34(6):541‐548. [DOI] [PubMed] [Google Scholar]
- 20. Luna‐Pérez P, Bustos‐Cholico E, Alvarado I, et al. Prognostic significance of circumferential margin involvement in rectal adenocarcinoma treated with preoperative chemoradiotherapy and low anterior resection. J Surg Oncol. 2005;90(1):20. [DOI] [PubMed] [Google Scholar]
- 21. Nagtegaal ID, Marijnen CA, Kranenbarg EK, et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26(3):350‐357. [DOI] [PubMed] [Google Scholar]
- 22. Tilney HS, Rasheed S, Northover JM, et al. The influence of circumferential resection margins on long‐term outcomes following rectal cancer surgery. Dis Colon Rectum. 2009;52(10):1723‐1729. [DOI] [PubMed] [Google Scholar]
- 23. Beaufrère A, Guedj N, Maggiori L, et al. Circumferential margin involvement after total mesorectal excision for mid or low rectal cancer: are all r1 resections equal? Colorectal Dis. 2017;19(11):O377. [DOI] [PubMed] [Google Scholar]
- 24. Nagtegaal ID, Velde CJHVD, Worp EVD, et al. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20(7):1729. [DOI] [PubMed] [Google Scholar]
- 25. Quirke P, Sebagmontefiore D, Steele R, et al. Local recurrence after rectal cancer resection is strongly related to the plane of surgical dissection and is further reduced by pre‐operative short course radiotherapy. Preliminary results of the medical research council (mrc) cr07 trial . 2006.
- 26. Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results–eortc 22921. J Clin Oncol. 2005;23(24):5620. [DOI] [PubMed] [Google Scholar]
- 27. Brown G, Daniels IR. Preoperative staging of rectal cancer: the mercury research project [M]. Berlin Heidelberg: Springer; 2005. [DOI] [PubMed] [Google Scholar]
- 28. Breugom AJ, Gijn WV, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a dutch colorectal cancer group (dccg) randomized phase iii trial. Ann Oncol. 2015;26(4):696‐701. [DOI] [PubMed] [Google Scholar]
- 29. Breugom AJ, Swets M, Bosset JF, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta‐analysis of individual patient data. Lancet Oncol. 2015;16(2):200‐207. [DOI] [PubMed] [Google Scholar]
- 30. Loree JM, Kennecke HF, Renouf DJ, et al. Effect of adjuvant chemotherapy on stage ii rectal cancer outcomes after preoperative short‐course radiotherapy. Clin Colorectal Cancer. 2016;15(4):352‐359. e1. [DOI] [PubMed] [Google Scholar]
- 31. Bouzourene H, Bosman FT, Seelentag W, et al. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94(4):1121. [PubMed] [Google Scholar]
- 32. Chie EK, Lee J, Kim K, et al. The influence of treatment response on the impact of resection margin status after preoperative chemoradiotherapy in rectal cancer. J Clin Oncol. 2013;31(4 suppl):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taylor FG, Quirke P, Heald RJ, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease‐free survival and local recurrence: 5‐year follow‐up results of the mercury study. J Clin Oncol. 2014;32(1):34. [DOI] [PubMed] [Google Scholar]
- 34. Kwon KA, Kim SH, Oh SY, et al. Clinical significance of preoperative serum vascular endothelial growth factor, interleukin‐6, and c‐reactive protein level in colorectal cancer. BMC Cancer. 2010;10(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song JH, Kim SH, Lee JH, et al. Significance of histologic tumor grade in rectal cancer treated with preoperative chemoradiotherapy followed by curative surgery: a multi‐institutional retrospective study. Radiother Oncol. 2016;118(2):387. [DOI] [PubMed] [Google Scholar]
- 36. Hall NR, Finan PJ, Al‐Jaberi T, et al. Circumferential margin involvement after mesorectal excision of rectal cancer with curative intent. Predictor of survival but not local recurrence? Dis Colon Rectum. 1998;41(8):979‐983. [DOI] [PubMed] [Google Scholar]
- 37. Dent OF, Haboubi N, Chapuis PH, et al. Assessing the evidence for an association between circumferential tumour clearance and local recurrence after resection of rectal cancer. Colorectal Dis. 2007;9(2):112‐121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


