Abstract
Introduction
Population‐based data on the incidence and prognosis of bone metastases at diagnosis of breast cancer are currently limited. Hence, we conducted this study to analyze the incidence proportions and prognostic factors of patients with breast cancer and bone metastases at the time of cancer diagnosis.
Materials and methods
Patients with primary invasive breast cancer and bone metastases at initial diagnosis between 2010 and 2014 were identified using the Surveillance, Epidemiology, and End Results (SEER) dataset and Fudan University Shanghai Cancer Center (FUSCC) cohort. Multivariable logistic regression was performed to identify predictors of the presence of bone metastases at diagnosis. Univariate and multivariate analyses were performed to determine the effects of each variable on survival.
Results
Of 229, 195 patients from SEER database included in the analysis, 8295 patients had bone metastases at initial diagnosis, reflecting 3.6% of the entire study population, and 65.1% of the subset with metastatic disease to any distant site. Patients with hormone receptor (HR)‐positive human epidermal growth factor receptor 2 (HER2)‐negative represented the highest incidence proportions among patients with metastatic disease (73.9%). Among entire cohort, multivariable logistic regression identified eight factors as predictors of the presence of bone metastases at diagnosis. Median OS for the patients with bone metastases in SEER and FUSCC cohorts was 30.0 and 68.2 months, respectively. Patients with HR‐positive HER2‐positive subtype had the longest median OS, and patients with triple‐negative subtype showed the shortest median OS. Multivariable Cox model in SEER cohort confirmed age, histology, grade, tumor subtype, extraosseous metastatic sites, history of primary surgery, insurance status, marital status, and income as independent prognostic factors for both OS and BCSS.
Conclusions
The findings of this study provide population‐based estimates of the incidence and prognosis for patients with bone metastases at initial diagnosis of breast cancer.
Keywords: bone metastases, breast cancer, incidence, prognosis
1. INTRODUCTION
Breast cancer is the most common cancer in women and is the leading causes of cancer‐related death in women. Globally, it accounted for approximately 1.67 million cases in 2012 and 0.52 million deaths, and both numbers have continued to increase.1 Approximately 5% of patients present with distant metastases at their initial diagnosis of breast cancer, with bone being the most common site.2, 3 Of those patients who die of breast cancer, approximately 70% will have evidence of bone metastases.4
Bone metastases are associated with lower survival in patients with advanced breast cancer and an increased risk of serious complications during the patients’ disease course.5, 6, 7 The sites and extent of metastases determine the complications, which are called skeletal‐related events (SREs); these include pathological fractures, severe bone pain, bone marrow infiltration, spinal cord compression, and hypercalcemia.8, 9 The median time from bone metastases diagnosis to first SRE among breast cancer patients with bone metastases is only 1.8 months, and the 1‐year incidence of SREs is as high as 40%.2 Studies have demonstrated that nearly 50% of breast cancer patients with bone metastases experience at least one of these SREs, resulting in the reduced quality of life.2, 7, 10
There is growing evidence indicating that patterns of metastases vary between different hormone receptor (HR) and human epithelial growth factor receptor 2 (HER2) statuses of breast tumors.11, 12, 13, 14, 15, 16, 17, 18 Patients with HR‐positive breast cancer are at increased risk for the development of bone metastases, whereas bone metastases are less frequent in cases of triple‐negative tumors.15, 16, 17, 18, 19, 20, 21 Previous studies also indicated that tumor subtype is an important prognostic factor for the survival of patients with bone metastases, where worse survival was seen in patients with triple‐negative breast cancer.22, 23
An early diagnosis of bone metastases is necessary to start intervention early and reduce complications. However, current guidelines do not recommend routine screening of bone metastases in patients with localized breast cancer only if directed by signs or symptoms.24 Among patients with bone metastases at initial diagnosis of breast cancer, there is a lack of data regarding patient characteristics and the clinical and sociodemographic predictors of outcome at a population level, which needs to be supplemented and studied.
In this study, we used the Surveillance, Epidemiology, and End Results (SEER) database to survey the incidence of bone metastases when breast cancer was initially diagnosed. We also attempted to investigate the influence of tumor subtype and other prognostic factors on the survival of patients with bone metastases at the time of cancer diagnosis using both SEER cohort and another independent cohort from Fudan University Shanghai Cancer Center (FUSCC).
2. MATERIALS AND METHODS
2.1. Study population
We obtained data from the current SEER database, which consists of 18 population‐based cancer registries. This database collects and publishes cancer incidence and survival data covering approximately 28% of the total population in the United States. SEER*Stat Version 8.3.4 (http://www.seer.cancer.gov/seerstat) from the National Cancer Institute was used to identify eligible patients in this study.25
Because the SEER database began collecting information on the presence or absence of bone metastases at the time of diagnosis in 2010, we included adult patients (≥18 years of age) diagnosed with microscopically confirmed invasive breast cancer between 1 January 2010 and 31 December 2014. We selected patients with only one primary malignancy in their lifetime. In total, 229 195 patients were eligible for inclusion in the incidence analyses. Among whom 8295 were diagnosed with bone metastases. We subsequently removed patients with an unknown follow‐up, leaving 7482 patients eligible for survival analyses.
To validate the preliminary findings obtained from the SEER database, we used data from 198 breast cancer patients with bone metastases at first diagnosis who were treated between January 2004 and December 2017 at FUSCC. All patients included in the analysis were histopathologically reconfirmed independently by two experienced pathologists according to the ASCO/CAP 2010 criteria. The cutoff for estrogen receptor or progesterone receptor positivity was ≥1% of tumor cells with nuclear staining.26 Cytoplasmic staining was ignored.27 Pathologic HER2 status was defined according to the ASCO/CAP guidelines.28
This study was conducted with approval from the Ethical Committee Review Board of Fudan University Shanghai Cancer Center and all patients provided written informed consent.
2.2. Statistical analysis
Descriptive statistics were used to examine the baseline characteristics of the patient population. These variables were stratified by breast cancer subtype: HR‐positive HER2‐negative, HR‐positive HER2‐positive, HR‐negative HER2‐positive, and triple‐negative (HR‐negative HER2‐negative). Residence type, median household income, and education level (percentage of adults ≥25 years with a high school education) were estimated with county attributes from the US Census 2010‐2014 American Community Survey 5‐year data files, which were provided through the SEER*Stat software. Patient characteristics were compared among the subgroups with the chi‐square test and Fisher's exact test for categorical variables and with the Kruskal‐Wallis test for continuous variables. Within each variable, patients with unknown data were excluded from the comparative analysis.
Absolute numbers and incidence proportions were calculated for patients with bone metastases at the time of their breast cancer diagnosis. Patients were also stratified by breast cancer subtype. Incidence proportion was defined as the percentage of breast cancer patients diagnosed with bone metastases among either the entire study cohort or the patients with metastatic disease to any distant site.
Multivariable logistic regression was used to determine predictors of the presence of bone metastases at diagnosis. Information regarding the presence of brain, lung, and liver metastases at diagnosis is available in the SEER database and FUSCC cohort and was used to calculate the number of extraosseous metastatic sites in this study.
Overall survival (OS) and breast cancer‐specific survival (BCSS) were the primary study outcomes. OS was defined as the date of diagnosis to the date of death due to any cause or the date of last follow‐up. BCSS was calculated as the time from the date of diagnosis to the date of death attributed to breast cancer or the date of last follow‐up. We used the Kaplan‐Meier method to obtain survival probabilities and analyzed the differences between groups using the log‐rank test. Univariate and multivariate Cox proportional hazard models were applied to assess the independent association of several variables with BCSS and OS, which were reported as hazard ratios and their 95% confidence intervals (95% CIs).
All statistical analyses were performed using R software, version 3.4.3 (http://www.r-project.org) and SPSS software, version 22.0 (SPSS, Chicago, IL, USA). All P values reported were two‐sided, and P values <0.05 were considered statistically significant.
3. RESULTS
3.1. Patient characteristics
A total of 229 195 patients in the United States were diagnosed with breast cancer between 2010 and 2014 and were included in the incidence analysis. Table S1 shows the distribution of patient characteristics according to tumor subtype. Most patients had HR‐positive HER2‐negative tumors (66.7%) while the fewest patients had HR‐negative HER2‐positive tumors (4.4%). Within this cohort, 7482 patients had bone metastases at their initial diagnosis and complete survival data; thus, they were included in the survival analysis, and their demographic and clinical characteristics are shown in Table 1. Among the cohort for the survival analysis, 59.5%, 5.9%, 14.9%, 8.2%, and 11.4% of patients had HR‐positive HER2‐negative, HR‐negative HER2‐positive, HR‐positive HER2‐positive, triple‐negative, and unknown subtypes, respectively. At the time of diagnosis, bone was the only site of metastases in 4078 patients (54.5%). Compared with other patients, patients with bone metastases from HR‐positive/HER2‐negative breast cancer were older (P < 0.001), were more likely to be white (P < 0.001), had a higher rate of lobular histology (P < 0.001), had a lower tumor grade (P < 0.001), and had a higher household income (P < 0.001). In contrast, patients with HR‐negative/HER2‐positive patients were younger (P < 0.001), were less likely to be white (P < 0.001), had fewer extraosseous metastatic sites to the lung, liver, and brain (P < 0.001), and were more likely to live in urban area (P = 0.006). The basic characteristics of the patients in the FUSCC cohort are presented in Table S2.
Table 1.
Demographic characteristics of patients in the SEER cohort included in the survival analysis according to tumor subtypes
| Patient characteristics | Tumor subtype | Total | P‐value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR+/HER2‐ | HR‐/HER2+ | HR+/HER2+ | Triple‐negative | Unknowna | |||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||
| All Patients | 4454 | 59.5 | 445 | 5.9 | 1112 | 14.9 | 616 | 8.2 | 855 | 11.4 | 7482 | 100.0 | |
| Age at diagnose | |||||||||||||
| 18‐49 | 895 | 20.1 | 133 | 29.9 | 322 | 29.0 | 150 | 24.4 | 115 | 13.5 | 1615 | 21.6 | <0.001 |
| 50‐64 | 1775 | 39.9 | 201 | 45.2 | 491 | 44.2 | 247 | 40.1 | 309 | 36.1 | 3023 | 40.4 | |
| ≥65 | 1784 | 40.1 | 111 | 24.9 | 299 | 26.9 | 219 | 35.6 | 431 | 504 | 2844 | 38.0 | |
| Sex | |||||||||||||
| Female | 4400 | 98.8 | 443 | 99.6 | 1095 | 98.5 | 610 | 99.0 | 840 | 98.2 | 7388 | 98.7 | 0.332 |
| Male | 54 | 1.2 | 2 | 0.4 | 17 | 1.5 | 6 | 1.0 | 15 | 1.8 | 94 | 1.3 | |
| Race | |||||||||||||
| White | 3051 | 68.5 | 267 | 60.0 | 695 | 62.5 | 376 | 61.0 | 592 | 69.2 | 4981 | 66.6 | <0.001 |
| Black | 630 | 14.1 | 72 | 16.2 | 195 | 17.5 | 144 | 23.4 | 126 | 14.7 | 1167 | 15.6 | |
| Hispanic | 439 | 9.9 | 61 | 13.7 | 138 | 12.4 | 64 | 10.4 | 84 | 9.8 | 786 | 10.5 | |
| Asian | 292 | 6.6 | 40 | 9.0 | 75 | 6.7 | 26 | 4.2 | 44 | 5.1 | 477 | 6.4 | |
| Othersb | 26 | 0.6 | 2 | 0.4 | 6 | 0.5 | 5 | 0.8 | 4 | 0.5 | 43 | 0.6 | |
| Unknowna | 16 | 0.4 | 3 | 0.7 | 3 | 0.3 | 1 | 0.2 | 5 | 0.6 | 28 | 0.4 | |
| Laterality | |||||||||||||
| Left | 2154 | 48.4 | 221 | 49.7 | 573 | 51.5 | 307 | 49.8 | 375 | 43.9 | 3630 | 48.5 | 0.486 |
| Right | 2145 | 48.2 | 215 | 48.3 | 512 | 46.0 | 285 | 46.3 | 322 | 37.7 | 3479 | 46.5 | |
| Bilateral, single primary | 21 | 0.5 | 5 | 1.1 | 7 | 0.6 | 3 | 0.5 | 16 | 1.9 | 52 | 0.7 | |
| Unknowna | 134 | 3.0 | 4 | 0.9 | 20 | 1.8 | 21 | 3.4 | 142 | 16.6 | 321 | 4.3 | |
| Histology | |||||||||||||
| IDC | 2882 | 64.7 | 350 | 78.7 | 881 | 79.2 | 444 | 72.1 | 377 | 44.1 | 4934 | 65.9 | <0.001 |
| ILC | 703 | 15.8 | 16 | 3.6 | 45 | 4.0 | 31 | 5.0 | 1204 | 6.7 | 883 | 11.8 | |
| Othersc | 869 | 19.5 | 79 | 17.8 | 186 | 16.7 | 141 | 22.9 | 5041 | 28.0 | 1665 | 22.3 | |
| Grade | |||||||||||||
| I | 406 | 9.1 | 3 | 0.7 | 21 | 1.9 | 12 | 1.9 | 36 | 4.2 | 478 | 6.4 | <0.001 |
| II | 1908 | 42.8 | 105 | 23.6 | 398 | 35.8 | 105 | 17.0 | 165 | 19.3 | 2681 | 35.8 | |
| III/IV | 1227 | 27.5 | 260 | 58.4 | 514 | 46.2 | 402 | 65.3 | 193 | 22.6 | 2596 | 34.7 | |
| Unknowna | 913 | 20.5 | 77 | 17.3 | 179 | 16.1 | 97 | 15.7 | 461 | 53.9 | 1727 | 23.1 | |
| Surgery | |||||||||||||
| No surgery | 3212 | 72.1 | 302 | 67.9 | 785 | 70.6 | 398 | 64.6 | 724 | 84.7 | 5421 | 72.5 | 0.002 |
| BCS | 369 | 8.3 | 31 | 7.0 | 101 | 9.1 | 63 | 10.2 | 44 | 5.1 | 608 | 8.1 | |
| Mastectomy | 844 | 18.9 | 108 | 24.3 | 217 | 19.5 | 149 | 24.2 | 74 | 8.7 | 1392 | 18.6 | |
| Unknowna | 29 | 0.7 | 4 | 0.9 | 9 | 0.8 | 6 | 1.0 | 13 | 1.5 | 61 | 0.8 | |
| Extraosseous metastatic sites to lung, liver, and brain, No. | |||||||||||||
| 0 | 2653 | 59.6 | 159 | 91.5 | 522 | 46.9 | 282 | 45.8 | 462 | 54.0 | 4078 | 54.5 | <0.001 |
| 1 | 1174 | 26.4 | 163 | 4.9 | 360 | 32.4 | 207 | 33.6 | 240 | 28.1 | 2144 | 28.7 | |
| 2 | 339 | 7.6 | 76 | 1.5 | 150 | 13.5 | 77 | 12.5 | 77 | 9.0 | 719 | 9.6 | |
| All 3 | 45 | 1.0 | 22 | 0.3 | 19 | 1.7 | 24 | 3.9 | 9 | 1.1 | 119 | 1.6 | |
| Unknowna | 243 | 5.5 | 25 | 1.9 | 61 | 5.5 | 26 | 4.2 | 67 | 7.8 | 422 | 5.6 | |
| Marital status | |||||||||||||
| Married | 1935 | 43.4 | 199 | 44.7 | 497 | 44.7 | 277 | 45.0 | 326 | 38.1 | 3234 | 43.2 | 0.769 |
| Unmarriedd | 2270 | 51.0 | 218 | 49.0 | 548 | 49.3 | 315 | 51.1 | 491 | 57.4 | 3842 | 51.3 | |
| Unknowna | 249 | 5.6 | 28 | 6.3 | 67 | 6.0 | 24 | 3.9 | 38 | 4.4 | 406 | 5.4 | |
| Insurance | |||||||||||||
| Insured | 4178 | 93.8 | 422 | 94.8 | 1024 | 92.1 | 576 | 93.5 | 799 | 93.5 | 6999 | 93.5 | 0.272 |
| Uninsured | 184 | 4.1 | 15 | 3.4 | 58 | 5.2 | 29 | 4.7 | 39 | 4.6 | 325 | 4.3 | |
| Unknowna | 92 | 2.1 | 8 | 1.8 | 30 | 2.7 | 11 | 1.8 | 17 | 2.0 | 158 | 2.1 | |
| Residence type | |||||||||||||
| Urban | 4003 | 89.9 | 403 | 90.6 | 976 | 87.8 | 529 | 85.9 | 742 | 86.8 | 6653 | 88.9 | 0.006 |
| Rural | 451 | 10.1 | 42 | 9.4 | 136 | 12.2 | 87 | 14.1 | 113 | 13.2 | 829 | 11.1 | |
| Median household income | 56 640 | 56 590 | 56 490 | 55 870 | 56 590 | 56 590 | <0.001 | ||||||
| High school education, % | 86.7 | 86.2 | 85.8 | 86.1 | 86.2 | 86.6 | 0.083 | ||||||
BCS, breast conserving surgery; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IDC, infiltrating ductal carcinoma; ILC, infiltrating lobular carcinoma.
Unknown patients are excluded from the comparative analysis.
Including American Indian/Alaskan native and Pacific Islander.
Including other histology of invasive breast cancer except IDC and ILC.
Including divorced, separated, single (never married), and widowed.
3.2. Incidence analysis
Figure 1 shows the number and incidence proportions of patients with breast cancer and bone metastases at diagnosis according to tumor subtype in the SEER cohort. A total of 8295 patients presented with bone metastases, reflecting 3.6% of the entire cohort and 65.1% of the subset with metastatic breast cancer. Patients with the HR‐positive HER2‐negative (3.2% of the entire cohort, 73.9% of the metastatic subset) and HR‐positive HER2‐positive (5.2% of the entire cohort, 64.1% of the metastatic subset) subtypes had the highest incidence proportions.
Figure 1.
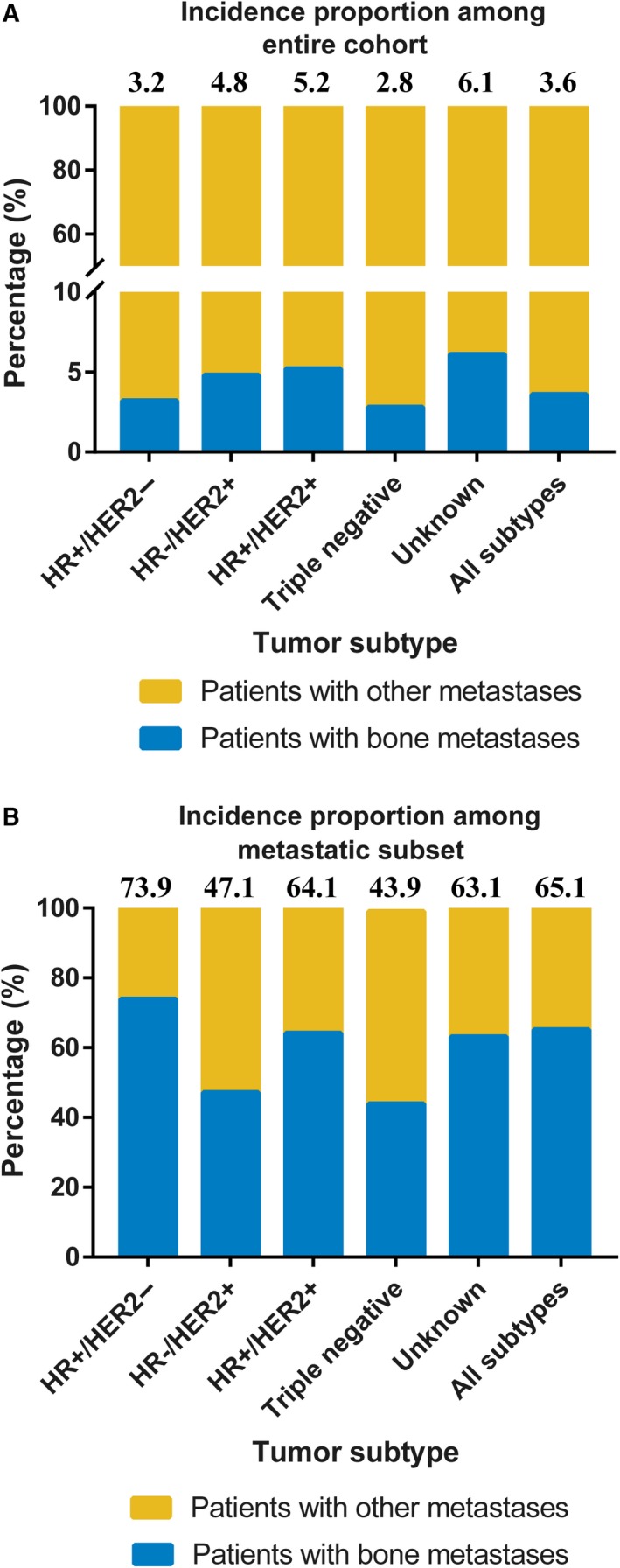
The Incidence Proportion of Patients with Breast Cancer and Bone Metastases at the Time of Initial Diagnosis According to Tumor Subtype in the SEER Cohort. A, The incidence proportion of patients among entire cohort. B, The incidence proportion of patients among subset with metastatic disease. The number above the histogram denotes the incidence proportion of patients with bone metastases. HER2, human epidermal growth factor receptor 2; HR, hormone receptor
Multivariable logistic regression was performed among the entire cohort and the subset with metastatic disease in the SEER cohort (Table 2). Among the entire cohort, age 50‐64 years (P = 0.003), male sex (P = 0.003), infiltrating lobular carcinoma (P < 0.001) and other histology (P < 0.001), grade II (P < 0.001) and III/IV (P < 0.001), metastatic disease to 1 extraosseous site (P < 0.001), 2 extraosseous sites (P < 0.001) and 3 extraosseous sites (P < 0.001), uninsured status (P < 0.001), unmarried status (P < 0.001), and higher education level (P < 0.001) were associated with significantly increased risk of bone metastases at diagnosis. Hispanic (P = 0.001) and Asian (P < 0.001) race, HR‐negative HER2‐positive (P < 0.001) and triple‐negative subtypes (P < 0.001), higher median household income (P < 0.001) were associated with significantly reduced risk of bone metastases at diagnosis. Residence type was not associated with a risk of bone metastases at diagnosis in the multivariable model. Among patients with metastatic cancer, age, race, histology, grade, tumor subtype, extraosseous metastatic sites, and education level were identified as predictors of the presence of bone metastases at diagnosis.
Table 2.
Multivariable logistic regression for the presence of bone metastases at diagnosis of breast cancer in the SEER cohort
| Patient characteristics | Among entire cohort | Among subset with metastatic disease | ||
|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Age at diagnosis | ||||
| 18‐49 | Reference | Reference | Reference | Reference |
| 50‐64 | 1.109 (1.036‐1.188) | 0.003 | 1.000 (0.899‐1.112) | 0.996 |
| ≥65 | 1.000 (0.931‐1.073) | 0.993 | 0.826 (0.740‐0.922) | 0.001 |
| Sex | ||||
| Female | Reference | Reference | Reference | Reference |
| Male | 1.450 (1.136‐1.850) | 0.003 | 1.207 (0.835‐1.744) | 0.317 |
| Race | ||||
| White | Reference | Reference | Reference | Reference |
| Black | 0.989 (0.915‐1.068) | 0.773 | 0.808 (0.725‐0.901) | <0.001 |
| Hispanic | 0.864 (0.792‐0.942) | 0.001 | 0.893 (0.783‐1.019) | 0.093 |
| Asian | 0.775 (0.699‐0.859) | <0.001 | 0.874 (0.747‐1.021) | 0.090 |
| Othersa | 0.798 (0.564‐1.130) | 0.203 | 0.897 (0.549‐1.466) | 0.666 |
| Histology | ||||
| IDC | Reference | Reference | Reference | Reference |
| ILC | 1.996 (1.840‐2.165) | <0.001 | 1.243 (1.065‐1.451) | 0.006 |
| Othersb | 1.238 (1.157‐1.325) | <0.001 | 0.927 (0.837‐1.026) | 0.143 |
| Grade | ||||
| I | Reference | Reference | Reference | Reference |
| II | 2.449 (2.219‐2.702) | <0.001 | 1.125 (0.928‐1.365) | 0.229 |
| III/IV | 2.872 (2.587‐3.187) | <0.001 | 0.712 (0.587‐0.863) | 0.001 |
| Unknown | 7.576 (6.770‐8.478) | <0.001 | 0.992 (0.813‐1.212) | 0.940 |
| Tumor subtype | ||||
| HR+/HER‐ | Reference | Reference | Reference | Reference |
| HR‐/HER2+ | 0.618 (0.548‐0.698) | <0.001 | 0.390 (0.338‐0.450) | <0.001 |
| HR+/HER2+ | 1.063 (0.981‐1.151) | 0.136 | 0.737 (0.656‐0.828) | <0.001 |
| Triple‐negative | 0.470 (0.426‐0.519) | <0.001 | 0.340 (0.301‐0.384) | <0.001 |
| Unknown | 0.780 (0.624‐0.788) | <0.001 | 0.669 (0.591‐0.756) | <0.001 |
| Extraosseous metastatic sites, No. | ||||
| 0 | Reference | Reference | Reference | Reference |
| 1 | 44.896 (41.868‐48.143) | <0.001 | 0.391 (0.358‐0.426) | <0.001 |
| 2 | 89.690 (78.928‐101.919) | <0.001 | 0.814 (0.709‐0.934) | 0.003 |
| All 3 | 192.634 (130.771‐283.765) | <0.001 | 1.895 (1.286‐2.792) | 0.001 |
| Unknown | 4.906 (4.396‐5.475) | <0.001 | 0.494 (0.423‐0.577) | <0.001 |
| Marital status | ||||
| Married | Reference | Reference | Reference | Reference |
| Unmarriedc | 1.367 (1.296‐1.443) | <0.001 | 1.049 (0.966‐1.139) | 0.258 |
| Unknown | 0.959 (0.852‐1.079) | 0.483 | 0.894 (0.755‐1.060) | 0.197 |
| Insurance | ||||
| Insured | Reference | Reference | Reference | Reference |
| Uninsured | 1.592 (1.387‐1.827) | <0.001 | 1.069 (0.887‐1.289) | 0.483 |
| Unknown | 0.571 (0.476‐0.683) | <0.001 | 0.743 (0.577‐0.955) | 0.021 |
| Residence type | ||||
| Urban | Reference | Reference | Reference | Reference |
| Rural | 0.992 (0.906‐1.086) | 0.863 | 0.908 (0.792‐1.042) | 0.169 |
| Median household income (per $10 000 annual increase) | 0.951 (0.931‐0.972) | <0.001 | 0.977 (0.946‐1.010) | 0.173 |
| High school education (per 10% increase) | 1.066 (1.024‐1.111) | 0.002 | 1.081 (1.016‐1.151) | 0.015 |
CI, confidence interval; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IDC, infiltrating ductal carcinoma; ILC, infiltrating lobular carcinoma.
Unknown age and unknown race removed from model owing to nonconvergence (n = 1798).
Including American Indian/Alaskan native and Pacific Islander.
Including other histology of invasive breast cancer except IDC and ILC.
Including divorced, separated, single (never married), and widowed.
3.3. Survival analysis
The median OS of the SEER cohort and FUSCC cohort included in the survival analysis was 30 and 68.2 months, respectively (Figure 2A,B). Significant differences were observed from the OS analysis according to tumor subtype (Figure 2C,D; log‐rank P < 0.001). Patients with the HR‐positive HER2‐positive subtype experienced the longest median survival in both SEER cohort and FUSCC cohort (41.0 months and not reached, respectively), whereas patients with the triple‐negative subtype experienced the shortest median survival (11.0 and 15.1 months, respectively). Survival estimates stratified by extraosseous metastatic sites are displayed in Figure 2E,F; and patients with more extraosseous metastatic sites showed worse OS (log‐rank P < 0.001 in the SEER cohort and log‐rank P = 0.002 in the FUSCC cohort).
Figure 2.
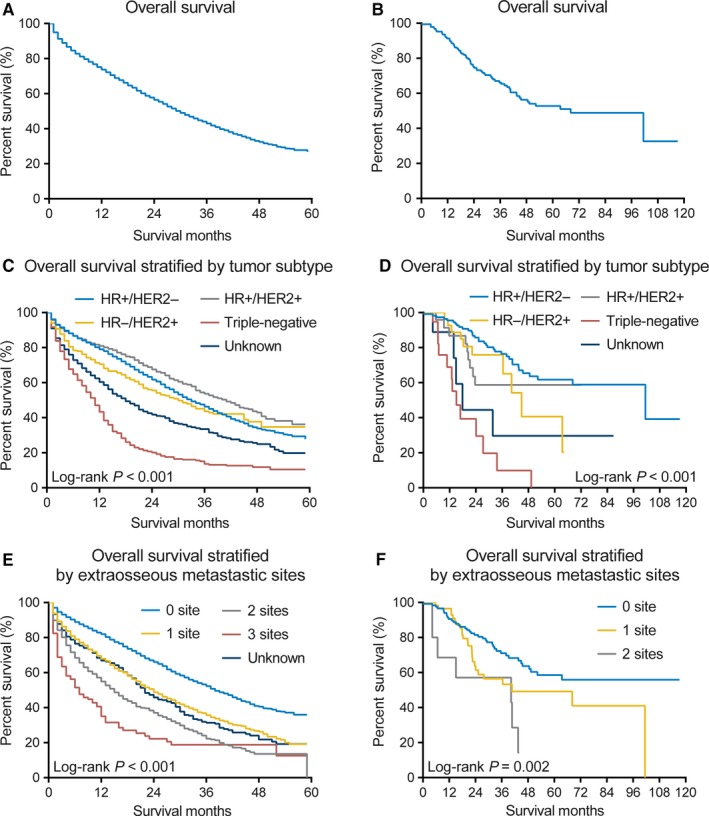
Kaplan‐Meier curves for Overall Survival Among Patients with Breast Cancer and Bone Metastases at the Time of Initial Diagnosis in both SEER and FUSCC Cohorts. A‐B, The whole population included in the survival analysis in the (A) SEER and (B) FUSCC cohort, respectively. C‐D, According to the tumor subtype in the (C) SEER and (D) FUSCC cohort, respectively. E‐F, According to the number of extraosseous metastatic sites to lung, liver, and brain in the (E) SEER and (F) FUSCC cohort, respectively. HER2, human epidermal growth factor receptor 2; HR, hormone receptor
The impact of the presence of extraosseous metastases on OS in the SEER cohort is shown in Figure 3. Patients with metastases to the bone and other sites had significantly shorter survival (median OS: 21.0 months) than those with metastases to the bone only (median OS: 38.0 months, log‐rank P < 0.001). There were also significant differences in OS between patients with liver metastases vs those without liver metastases (median OS: 18.0 vs 35.0 months, log‐rank P < 0.001), patients with lung metastases vs those without lung metastases (median OS: 21.0 vs 34.0 months, log‐rank P < 0.001), and patients with brain metastases vs those without brain metastases (median OS: 12.0 vs 32.0 months, log‐rank P < 0.001).
Figure 3.
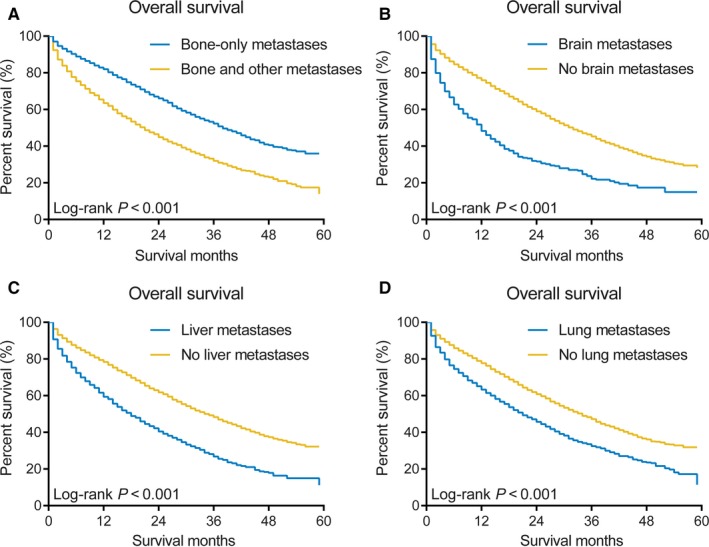
Kaplan‐Meier curves for Overall Survival According to Individual Metastases in the SEER Cohort. A, Patients with bone‐only metastases vs those with bone and other metastases. B, Patients with brain metastases vs those without brain metastases. C, Patients with liver metastases vs those without liver metastases. D, Patients with lung metastases vs those without lung metastases
We used univariate and multivariate Cox proportional hazard models to determine the prognostic factors of breast cancer patients with bone metastases at diagnosis in the SEER cohort, the results of which are shown in Table 3. In the univariate analysis, age, tumor grade, tumor subtype, extraosseous metastatic sites, history of surgery, insurance status, marital status, residence type, median household income, and high school education level were significantly associated with OS and BCSS (P < 0.05). Multivariable Cox analysis confirmed that age, histology, tumor grade, tumor subtype, extraosseous metastatic sites, history of primary surgery, insurance status, marital status, and median household income are independent prognostic factors for both OS and BCSS (P < 0.05). Sex, race, residence type, and education level did not reach significance in the multivariable test. We also found that there is no significant difference of survival time between patients with de novo osseous and extraosseous metastatic disease, except for patients with liver metastases at initial diagnosis (Table S3).
Table 3.
Cox regression analysis of overall survival and breast cancer‐specific survival among patients with bone metastases in the SEER cohort
| Patient characteristics | Overall survival | Breast cancer‐specific survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| Age at diagnosis | ||||||||
| 18‐49 | Reference | Reference | Reference | Reference | ||||
| 50‐64 | 1.398 (1.268‐1.542) | <0.001 | 1.334 (1.208‐1.472) | <0.001 | 1.398 (1.262‐1.547) | <0.001 | 1.331 (1.201‐1.475) | <0.001 |
| ≥65 | 1.861 (1.690‐2.049) | <0.001 | 1.782 (1.611‐1.970) | <0.001 | 1.776 (1.606‐1.965) | <0.001 | 1.702 (1.532‐1.891) | <0.001 |
| Sex | ||||||||
| Female | Reference | Reference | Reference | |||||
| Male | 1.061 (0.783‐1.439) | 0.702 | 1.063 (0.782‐1.444) | 0.697 | 0.970 (0.695‐1.354) | 0.858 | 0.979 (0.700‐1.369) | 0.901 |
| Race | ||||||||
| White | Reference | Reference | Reference | Reference | ||||
| Black | 1.258 (1.148‐1.378) | <0.001 | 1.095 (0.995‐1.204) | 0.062 | 1.233 (1.120‐1.357) | <0.001 | 1.061 (0.960‐1.173) | 0.243 |
| Hispanic | 0.902 (0.804‐1.012) | 0.079 | 0.961 (0.853‐1.083) | 0.512 | 0.889 (0.788‐1.003) | 0.056 | 0.938 (0.827‐1.063) | 0.314 |
| Asian | 0.900 (0.778‐1.041) | 0.154 | 0.976 (0.842‐1.132) | 0.747 | 0.883 (0.758‐1.030) | 0.114 | 0.954 (0.816‐1.115) | 0.555 |
| Othersa | 0.893 (0.554‐1.439) | 0.641 | 0.962 (0.595‐1.555) | 0.875 | 0.977 (0.606‐1.575) | 0.924 | 1.049 (0.648‐1.696) | 0.846 |
| Histology | ||||||||
| IDC | Reference | Reference | Reference | Reference | ||||
| ILC | 1.007 (0.904‐1.121) | 0.900 | 1.189 (1.061‐1.333) | 0.003 | 1.008 (0.900‐1.128) | 0.892 | 1.213 (1.077‐1.368) | 0.002 |
| Othersb | 1.291 (1.193‐1.398) | <0.001 | 1.161 (1.063‐1.269) | 0.001 | 1.284 (1.182‐1.396) | <0.001 | 1.152 (1.049‐1.264) | 0.003 |
| Grade | ||||||||
| I | Reference | Reference | Reference | Reference | ||||
| II | 1.279 (1.085‐1.507) | 0.003 | 1.293 (1.095‐1.527) | 0.002 | 1.344 (1.126‐1.604) | 0.001 | 1.360 (1.137‐1.626) | 0.001 |
| III/IV | 1.861 (1.582‐2.189) | <0.001 | 1.892 (1.598‐2.241) | <0.001 | 1.984 (1.666‐2.362) | <0.001 | 1.998 (1.666‐2.395) | <0.001 |
| Tumor subtype | ||||||||
| HR+/HER‐ | Reference | Reference | Reference | Reference | ||||
| HR‐/HER2+ | 1.165 (1.003‐1.353) | 0.045 | 1.022 (0.876‐1.191) | 0.786 | 1.220 (1.046‐1.423) | 0.012 | 1.049 (0.895‐1.230) | 0.553 |
| HR+/HER2+ | 0.829 (0.743‐0.925) | 0.001 | 0.749 (0.669‐0.838) | <0.001 | 0.835 (0.744‐0.937) | 0.002 | 0.747 (0.664‐0.841) | <0.001 |
| Triple‐negative | 2.868 (2.582‐3.185) | <0.001 | 2.530 (2.265‐2.826) | <0.001 | 2.959 (2.652‐3.301) | <0.001 | 2.584 (2.303‐2.900) | <0.001 |
| Unknown | 1.673 (1.515‐1.848) | <0.001 | 1.407 (1.268‐1.560) | <0.001 | 1.718 (1.549‐1.905) | <0.001 | 1.450 (1.302‐1.616) | <0.001 |
| Extraosseous metastatic sites, No. | ||||||||
| 0 | Reference | Reference | Reference | Reference | ||||
| 1 | 1.662 (1.538‐1.797) | <0.001 | 1.546 (1.428‐1.675) | <0.001 | 1.705 (1.571‐1.850) | <0.001 | 1.589 (1.461‐1.727) | <0.001 |
| 2 | 2.457 (2.210‐2.732) | <0.001 | 2.275 (2.040‐2.538) | <0.001 | 2.556 (2.289‐2.854) | <0.001 | 2.365 (2.111‐2.650) | <0.001 |
| All 3 | 3.674 (2.948‐4.579) | <0.001 | 3.184 (2.544‐3.984) | <0.001 | 3.960 (3.163‐4.958) | <0.001 | 3.404 (2.707‐4.281) | <0.001 |
| Surgery | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| BCS | 0.485 (0.420‐0.559) | <0.001 | 0.527 (0.455‐0.609) | <0.001 | 0.495 (0.427‐0.574) | <0.001 | 0.542 (0.465‐0.631) | <0.001 |
| Mastectomy | 0.528 (0.480‐0.581) | <0.001 | 0.565 (0.511‐0.625) | <0.001 | 0.531 (0.480‐0.587) | <0.001 | 0.569 (0.512‐0.633) | <0.001 |
| Insurance | ||||||||
| Insured | Reference | Reference | Reference | Reference | ||||
| Uninsured | 1.252 (1.068‐1.466) | 0.006 | 1.186 (1.009‐1.395) | 0.039 | 1.263 (1.071‐1.490) | 0.006 | 1.186 (1.002‐1.405) | 0.048 |
| Marital status | ||||||||
| Married | Reference | Reference | ||||||
| Unmarriedc | 1.425 (1.327‐1.529) | <0.001 | 1.304 (1.213‐1.402) | <0.001 | 1.400 (1.300‐1.508) | <0.001 | 1.290 (1.196‐1.392) | <0.001 |
| Residence type | ||||||||
| Urban | Reference | Reference | Reference | Reference | ||||
| Rural | 1.118 (1.007‐1.241) | 0.036 | 1.015 (0.902‐1.141) | 0.808 | 1.119 (1.003‐1.248) | 0.044 | 0.984 (0.870‐1.113) | 0.800 |
| Median household income (per $10 000 annual increase) | 0.940 (0.919‐0.962) | <0.001 | 0.948 (0.920‐0.976) | <0.001 | 0.933 (0.910‐0.955) | <0.001 | 0.937 (0.909‐0.966) | <0.001 |
| High school education (per 10% increase) | 0.931 (0.889‐0.975) | 0.002 | 0.968 (0.917‐1.022) | 0.241 | 0.925 (0.882‐0.970) | 0.001 | 0.969 (0.916‐1.025) | 0.271 |
CI, confidence interval; HER2, human epidermal growth factor receptor 2; HR, hormone receptor IDC, infiltrating ductal carcinoma; ILC, infiltrating lobular carcinoma.
We hid the unknown data in order to avoid confusion.
Including American Indian/Alaskan native and Pacific Islander.
Including other histology of invasive breast cancer except IDC and ILC.
Including divorced, separated, single (never married), and widowed.
4. DISCUSSION
This study analyzed recently available data from the SEER database on the incidence proportion and survival of patients with metastatic breast cancer who had bone metastases at their initial diagnosis. We also validated the results of survival analysis in another independent cohort from FUSCC. Some studies have evaluated the epidemiology and prognosis of breast cancer patients with bone metastases at the population level, but most of the patients in these studies presented bone metastases after a diagnosis of early‐stage breast cancer.2, 20, 29, 30, 31, 32, 33 However, limited data have been reported in the specific group of patients with bone metastases upon their initial diagnosis of breast cancer.34 Because early detection and systemic treatment of bone metastases in patients with breast cancer may modify the natural progression of bone metastases, reduce the probability of SREs, and improve progression‐free survival, quality of life and cost‐effective care, it is important for us to study patients who present with de novo bone metastases in a large independent cohort.
In this large retrospective study, we found that 3.6% of patients with invasive breast cancer had bone metastases at diagnosis, and 65.1% of those with any metastases at diagnosis had bone metastases. This result is slightly different from that of a previously published meta‐analysis.35 Body et al included and analyzed observational studies and clinical trials published between 1999 and 2013. The median (range) proportion of patients who had bone metastases was 14.7% (1.4%‐61.8%) in the breast cancer cohort and 58.3% (18.2%‐91.7%) in the metastatic cohort. This difference is possibly since consensus guidelines for patients with early‐stage breast cancer do not recommend a bone scan unless signs or symptoms have developed. As a result, the incidence proportion of bone metastases in patients with breast cancer in our study is likely underestimated.
We also observed significant differences in demographic and clinical characteristics of patients according to tumor subtype. Some of the relative high‐risk features in patients with triple‐negative breast cancer included black race, higher tumor grade, more extraosseous metastatic sites, and lower rate of primary tumor surgery. In addition, our study demonstrated that different tumor subtypes showed a different tendency to bone metastases. The incidence proportion of bone metastases was highest among patients with the HR‐positive HER2‐negative and HR‐positive HER2‐positive subtypes, which are in accordance with other publications describing the patterns of metastatic breast cancer.11, 12, 36 The multivariable logistic regression indicated that patients with the HR‐positive HER2‐negative subtype had significantly greater odds of having bone metastases at diagnosis than patients with other subtypes, whether among the entire cohort or within the subset with metastatic disease. Previous findings have confirmed that patients with an HR‐positive status are more likely to have bone metastases than those with HR‐negative status. The absence of WNT/β‐catenin signaling and the involvement of transforming growth factor β and fibroblast growth factor signaling have been found to promote HR‐positive breast cancer metastases to bone.12, 37
We identified predictors of the presence of bone metastases at diagnosis with the use of multivariate logistic regression to distinguish patients at increased risk of bone metastases. Our results could serve as a basis for future studies to evaluate the utility of a bone scan among these high‐risk patients. Among the entire cohort, we found that patients who were uninsured (vs insured, OR, 1.592; 95% CI, 1.387‐1.827; P < 0.001), were unmarried (vs married, OR, 1.367; 95% CI, 1.296‐1.443; P < 0.001) and had a higher school education (per 10% increase, OR, 1.066; 95% CI, 1.024‐1.111; P = 0.002) had a significantly greater likelihood of presenting bone metastases at diagnosis, but these associations were not observed among the cohort with metastatic breast cancer at diagnosis (except for the education level). Patients with uninsured status and unmarried status are more likely to ignore symptoms or signs and be diagnosed at late stage. However, there is no research studying this association and further study for this finding is warranted.
The median OS was 30 and 68.2 months from initial diagnosis in the SEER cohort and FUSCC cohort, respectively, which is similar with the survival reported by previous authors.20, 29, 30, 33 We assume that the longer survival in the FUSCC cohort was because the patients in this cohort were much younger and had less extraosseous metastatic sites. Notably, we found that the median survival for patients with breast cancer and bone metastases varied significantly by tumor subtypes. Compared with HR‐positive HER2‐negative patients, HR‐positive HER2‐positive patients experienced a 25.1% reduction in the hazards of overall mortality while triple‐negative patients experienced a 153.0% increase in the hazards of overall mortality. Our data are consistent with some previous retrospective studies.22, 23, 38, 39 We found that HR status in patients who are HER2‐positive is an important prognostic factor. Patients with bone metastases could be treated with chemotherapy, endocrine therapy or HER2‐targeted therapy according to the tumor subtypes. HR‐positive HER2‐positive have the option to undergo endocrine therapy, which may improve OS; HR‐negative HER2‐positive patients do not have this option.40
The presence of extraosseous disease in patients with bone metastases had a worse impact on survival. A recent study showed that compared with patients with visceral metastases with or without bone metastases, patients with bone‐only metastases at start of first‐line therapy had an improved median OS (54 months vs 28 months).29 Other studies also found that the median survival of patients with bone‐only metastases was approximately twofold to threefold that of patients with additional visceral metastases.30, 41, 42, 43 The results of our cohort study reached the same conclusion that patients with metastases to the bone only had better survival than those with metastases to both the bone and extraosseous sites. In addition, we found that patients with more extraosseous sites had worse survival, even after adjusting for other prognostic factors in the multivariate model. When we analyzed specific extraosseous metastatic sites by the log‐rank test, we identified that the presence of metastases at another sites such as the brain, liver, or lung had a significant negative impact on OS. We speculate that the improved survival in patients with bone‐only metastases patients is because bone is not a vital organ, and patients with bone‐only metastases have a slower onset of vital organ dysfunction. In general, our findings underscore that tumor subtypes and properties may be associated with the correlation between the clinical aggressiveness of the tumor and the metastases to specific sites.
Some limitations of our study should be acknowledged. First, information relating to recurrence or metastases after a diagnosis of breast cancer is not available in the SEER database. Therefore, we were unable to obtain and analyze the data on patients who showed progression in their disease course. Second, for patients with early‐stage breast cancer, routine screening for bone metastases is recommended only if directed by signs or symptoms. However, a subset of patients with bone metastases do not present symptoms, resulting in an underestimation of the actual rate of patients with de novo bone metastases. Third, we do not have information about systemic treatment, such as endocrine therapy, HER2‐targeted therapy, or chemotherapy, which may contribute to some bias in the survival analysis. Fourth, the SEER database only provides information about four sites of metastases at diagnosis: bone, brain, lung, and liver. Information on other sites of metastases such as the pleura and skin is lacking, which may influence the prognostic assessment of the extraosseous metastases group. Fifth, the finding of our analysis is limited to the United States, as some socioeconomic factors such as insurance, income, and education level are different in other parts of the world. Finally, residence type, median household income, and education level were defined at the county level, not the patient level. This may cause deviations in the analysis.
To the best our knowledge, our study is the first population‐based analysis of patients with bone metastases upon initial diagnosis of breast cancer. It provides important information for clinicians to consider conducting studies that evaluate the utility of a bone scan among patients at higher risk for bone metastases. Our study does not suffer from confounding effects that systemic therapies might cause on the timing of development and potential drug resistance of bone metastases, so the findings about the prognostic impact of tumor subtypes and extraosseous metastatic sites could be used for prognostic assessments and risk stratification of breast cancer patients with bone metastases at initial diagnosis. However, whether an earlier diagnosis of bone metastases may impact outcomes warrants further investigation.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Our study was approved by Shanghai Cancer Center Ethical Committee. Cancer a reportable disease in every state in the United States; informed patient consent is not required for the data released by the SEER database.
AVAILABILITY OF DATA AND MATERIAL
The datasets generated and analyzed during this study are available from Surveillance, Epidemiology, and End Results (SEER) Program (http://www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (1973‐2014 varying), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We would like to thank SEER for providing open access to the database.
Gong Y, Zhang J, Ji P, Ling H,Hu X, Shao Z‐M. Incidence proportions and prognosis of breast cancer patients with bone metastases at initial diagnosis. Cancer Med. 2018;7:4156–4169. 10.1002/cam4.1668
Funding information
This study was supported by grants from the Ministry of Science and Technology of China (National Key R&D Program of China, MOST2016YFC0900300), the National Natural Science Foundation of China (81572583, 81672601, 81602311), and the Shanghai Committee of Science and Technology Funds (15410724000). The funders had no role in the study design, collection, and analysis of the data, decision to publish, or manuscript preparation.
Both Yue Gong and Jing Zhang contributed equally to this work.
Contributor Information
Xin Hu, Email: xinhu@fudan.edu.cn.
Zhi‐Ming Shao, Email: zhi_ming_shao@163.com.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Jensen AO, Jacobsen JB, Norgaard M, Yong M, Fryzek JP, Sorensen HT. Incidence of bone metastases and skeletal‐related events in breast cancer patients: a population‐based cohort study in Denmark. BMC Cancer. 2011;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s‐6249s. [DOI] [PubMed] [Google Scholar]
- 5. Martin M, Bell R, Bourgeois H, et al. Bone‐related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res. 2012;18(17):4841‐4849. [DOI] [PubMed] [Google Scholar]
- 6. Saad F, Lipton A, Cook R, Chen Y‐M, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110(8):1860‐1867. [DOI] [PubMed] [Google Scholar]
- 7. Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal‐related events among women with breast cancer: a population‐based analysis of U.S. Medicare beneficiaries, 1999‐2006. Breast Cancer Res Treat. 2012;131(1):231‐238. [DOI] [PubMed] [Google Scholar]
- 8. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J, ESMO Guidelines Working Group . Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25(Suppl 3):iii124‐37. [DOI] [PubMed] [Google Scholar]
- 9. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165‐176. [DOI] [PubMed] [Google Scholar]
- 10. Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335(24):1785‐1791. [DOI] [PubMed] [Google Scholar]
- 11. Metzger‐Filho O, Sun Z, Viale G, et al. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node‐negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013;31(25):3083‐3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108‐3114. [DOI] [PubMed] [Google Scholar]
- 13. Koenders PG, Beex LV, Langens R, Kloppenborg PW, Smals AG, Benraad TJ. Steroid hormone receptor activity of primary human breast cancer and pattern of first metastasis. Breast Cancer Res Treat. 1991;18(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 14. Leone JP, Leone J, Zwenger AO, Iturbe J, Leone BA, Vallejo CT. Prognostic factors and survival according to tumour subtype in women presenting with breast cancer brain metastases at initial diagnosis. Eur J Cancer. 2017;74:17‐25. [DOI] [PubMed] [Google Scholar]
- 15. Wu SG, Li H, Tang LY, et al. The effect of distant metastases sites on survival in de novo stage‐IV breast cancer: A SEER database analysis. Tumour Biol. 2017;39(6):1010428317705082. [DOI] [PubMed] [Google Scholar]
- 16. Wu Q, Li J, Zhu S, et al. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget. 2017;8(17):27990‐27996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang H, Zhang C, Zhang J, Kong L, Zhu H, Yu J. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: a SEER based study. Oncotarget. 2017;8(16):26368‐26379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leone BA, Vallejo CT, Romero AO, et al. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Cancer Res Treat. 2017;161(3):537‐548. [DOI] [PubMed] [Google Scholar]
- 19. Gong Y, Liu YR, Ji P, Hu X, Shao ZM. Impact of molecular subtypes on metastatic breast cancer patients: a SEER population‐based study. Sci Rep. 2017;7:45411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liede A, Jerzak KJ, Hernandez RK, Wade SW, Sun P, Narod SA. The incidence of bone metastasis after early‐stage breast cancer in Canada. Breast Cancer Res Treat. 2016;156(3):587‐595. [DOI] [PubMed] [Google Scholar]
- 21. Wei B, Wang J, Bourne P, et al. Bone metastasis is strongly associated with estrogen receptor‐positive/progesterone receptor‐negative breast carcinomas. Hum Pathol. 2008;39(12):1809‐1815. [DOI] [PubMed] [Google Scholar]
- 22. Diessner J, Wischnewsky M, Stuber T, et al. Evaluation of clinical parameters influencing the development of bone metastasis in breast cancer. BMC Cancer. 2016;16:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Press DJ, Miller ME, Liederbach E, Yao K, Huo D. De novo metastasis in breast cancer: occurrence and overall survival stratified by molecular subtype. Clin Exp Metastasis. 2017;34(8):457‐465. [DOI] [PubMed] [Google Scholar]
- 24. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology. Breast cancer (version 4.2017) [requires login]. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed February 7, 2018. [DOI] [PubMed]
- 25. National Cancer Institute . Surveillance, Epidemiology, and End Results (SEER) Program (http://www.seer.cancer.gov) SEER*Stat Database, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission.
- 26. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784‐2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rody A, Diallo R, Poremba C, et al. Estrogen receptor alpha and beta, progesterone receptor, pS2 and HER‐2/neu expression delineate different subgroups in ductal carcinoma in situ of the breast. Oncol Rep. 2004;12:695‐699. [PubMed] [Google Scholar]
- 28. Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118‐145. [DOI] [PubMed] [Google Scholar]
- 29. Schroder J, Fietz T, Kohler A, et al. Treatment and pattern of bone metastases in 1094 patients with advanced breast cancer ‐ Results from the prospective German Tumour Registry Breast Cancer cohort study. Eur J Cancer. 2017;79:139‐148. [DOI] [PubMed] [Google Scholar]
- 30. Harries M, Taylor A, Holmberg L, et al. Incidence of bone metastases and survival after a diagnosis of bone metastases in breast cancer patients. Cancer Epidemiol. 2014;38(4):427‐434. [DOI] [PubMed] [Google Scholar]
- 31. Kuchuk I, Hutton B, Moretto P, Ng T, Addison CL, Clemons M. Incidence, consequences and treatment of bone metastases in breast cancer patients‐Experience from a single cancer centre. J Bone Oncol. 2013;2(4):137‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hagberg KW, Taylor A, Hernandez RK, Jick S. Incidence of bone metastases in breast cancer patients in the United Kingdom: results of a multi‐database linkage study using the general practice research database. Cancer Epidemiol. 2013;37(3):240‐246. [DOI] [PubMed] [Google Scholar]
- 33. Cetin K, Christiansen CF, Sværke C, Jacobsen JB, Sørensen HT. Survival in patients with breast cancer with bone metastasis: a Danish population‐based cohort study on the prognostic impact of initial stage of disease at breast cancer diagnosis and length of the bone metastasis‐free interval. BMJ Open. 2015;5(4):e007702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shen T, Siegal GP, Wei S. Clinicopathologic factors associated with de novo metastatic breast cancer. Pathol Res Pract. 2016;212(12):1167‐1173. [DOI] [PubMed] [Google Scholar]
- 35. Body JJ, Quinn G, Talbot S, et al. Systematic review and meta‐analysis on the proportion of patients with breast cancer who develop bone metastases. Crit Rev Oncol Hematol. 2017;115:67‐80. [DOI] [PubMed] [Google Scholar]
- 36. Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271‐3277. [DOI] [PubMed] [Google Scholar]
- 37. Smid M, Wang Y, Klijn JG, et al. Genes associated with breast cancer metastatic to bone. J Clin Oncol. 2006;24(15):2261‐2267. [DOI] [PubMed] [Google Scholar]
- 38. Lobbezoo DJ, van Kampen RJ, Voogd AC, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2‐positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141(3):507‐514. [DOI] [PubMed] [Google Scholar]
- 39. Yamashiro H, Takada M, Nakatani E, et al. Prevalence and risk factors of bone metastasis and skeletal related events in patients with primary breast cancer in Japan. Int J Clin Oncol. 2014;19(5):852‐862. [DOI] [PubMed] [Google Scholar]
- 40. Fiteni F, Villanueva C, Bazan F, et al. Long‐term follow‐up of patients with metastatic breast cancer treated by trastuzumab: impact of institutions. Breast. 2014;23(2):165‐169. [DOI] [PubMed] [Google Scholar]
- 41. Jacobson AF, Shapiro CL, Van den Abbeele AD, Kaplan WD. Prognostic significance of the number of bone scan abnormalities at the time of initial bone metastatic recurrence in breast carcinoma. Cancer. 2001;91(1):17‐24. [DOI] [PubMed] [Google Scholar]
- 42. Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer. implications for management. Eur J Cancer. 2000;36(4):476‐482. [DOI] [PubMed] [Google Scholar]
- 43. Solomayer EF, Diel IJ, Meyberg GC, Gollan C, Bastert G. Metastatic breast cancer clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat. 2000;59(3):271‐278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


