Abstract
Skeletal muscle index (SMI) and the controlling nutritional status (CONUT) score are useful for evaluating nutritional status, which is closely associated with cancer prognosis. This study compared the prognostic value of these indicators in patients with gastric cancer (GC) after radical gastrectomy (RG). We retrospectively enrolled 532 patients between 2010 and 2011. SMI was measured via CT images to determine low SMI. The CONUT score was calculated based on serum albumin, total lymphocyte count, and cholesterol. Patients were grouped according to SMI and the CONUT score based on previous research. Spearman's correlation coefficient, the Kaplan‐Meier method, and Cox regression were used. There was no significant correlation between SMI and the CONUT score. Five‐year overall survival (OS) and recurrence‐free survival (RFS) in patients with low SMI were significantly worse than those in patients with high SMI (P < .001). The normal nutrition group had better OS and RFS than did the light and moderate or severe malnutrition groups (P < .05), but the OS and RFS were not significantly different between the light and moderate or severe malnutrition groups (P = .726). Univariate analysis showed that SMI and the CONUT score were associated with OS and RFS, but only SMI remained prognostic in multivariate analysis. Preoperative SMI based on CT images is a more objective predictor than the CONUT score of long‐term survival in GC after RG, but this finding must be confirmed by prospective trials.
Keywords: CONUT score, gastric cancer, long‐term survival, nutritional status, skeletal muscle index
1. INTRODUCTION
Gastric cancer (GC) is the fourth most common malignancy and the second most common cause of cancer‐related deaths worldwide.1, 2 Therefore, accurate evaluation of prognosis in patients with GC may contribute to the development of individualized treatment programs and improve patient prognoses. Recently, nutritional status has been reported as a prognostic factor in patients with cancer.3, 4, 5, 6, 7, 8, 9
Sarcopenia, a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength, results in a decline in function, poor quality of life, and death.10, 11 Although weight can reflect nutritional status, sarcopenia (the loss of muscle mass) is a more accurate and quantitative indicator of frailty (nutritional status),12 and the effectiveness of sarcopenia (low skeletal mass index (SMI)) in predicting prognosis in GC has been widely documented.5, 13, 14, 15, 16 The CONUT score, derived from serum albumin (ALB), total lymphocyte count (TLC), and cholesterol measurements, is an effective tool for assessing the status of immune nutrition.17 The Controlling Nutritional Status (CONUT) score is a prognostic factor for various cancers, including GC.8, 18, 19, 20 Nevertheless, no studies have determined whether SMI or the CONUT score is a better predictor of long‐term prognosis in GC.
Therefore, the aim of this study was to compare the ability of preoperative SMI and the CONUT score to predict long‐term survival in GC after radical gastrectomy (RG).
2. MATERIALS AND METHODS
2.1. Materials
From a prospective database, 864 patients undergoing radical surgery for GC at Fujian Medical University Union Hospital (FMUUH) between 2010 and 2011 were identified. The exclusion criteria for this study were as follows: T4b patients (n = 31), intraoperative evidence of peritoneal tumor dissemination or distant metastasis (n = 9), patients with no available computed tomography (CT) images or with preoperative CT images older than 30 days (n = 235), incomplete clinical and pathologic data (n = 20), gastric stump carcinoma (n = 22), and preoperative neoadjuvant chemotherapy (n = 15). Ultimately, 532 patients were included in this study (Figure S1). Laboratory blood test data were collected within 1 week before surgery, including preoperative ALB and hemoglobin (HB) levels as well as lymphocyte counts and cholesterol concentrations. The type of surgical resection and the extent of lymph node dissection were selected according to the Japanese gastric cancer treatment guidelines,21 and the seventh corresponding edition of the American Joint Committee on Cancer (AJCC) Staging Manual was used to determine the disease stage.22 Patients with stage II‐III GC were advised to undergo adjuvant chemotherapy based on fluorine.23, 24 The study was approved by the FMUUH Institutional Review Board.
2.2. Measurement and grouping of SMI
Abdominal CT images were obtained from the computer center of the hospital, and skeletal muscle parameters were measured under the guidance of a professional radiologist. With Software Osirix version 3.3 (32‐bit; http://www.osirix-viewer.com),25 the third lumbar vertebra (L3) was set as a landmark, and two consecutive slices were selected to measure the cross‐sectional areas of the skeletal muscle. The mean value of two consecutive images was computed for each patient. The muscles in the L3 region include the rectus abdominis, psoas, quadratus lumborum, paraspinal, transverse abdominal, external oblique, internal oblique, and rectus abdominis muscles. Cross‐sectional skeletal muscle area was measured according to attenuation thresholds of −29 to +150 Hounsfield units (HU).26 Muscle areas were normalized for height (m2) to obtain the L3 SMI (cm2/m2).27 According to a previous study conducted by our center,16 32.5 cm2/m2 for men and 28.6 cm2/m2 for women were defined as low SMI. Ultimately, 91 patients (17.1%) with low SMI and 441 patients with high SMI (82.9%) were enrolled in the study.
2.3. Definition and grouping of the CONUT score
The CONUT score was calculated based on serum ALB concentration, peripheral lymphocyte count, and peripheral cholesterol concentration (Table S1). Based on the total scores for the three parameters, nutritional status was categorized as normal nutrition, light malnutrition, moderate malnutrition, or severe malnutrition.17 Because we identified only four patients with severe malnutrition in our study, we integrated moderate and severe malnutrition into a single CONUT group for all subsequent analyses.18 Ultimately, 291 patients (54.7%) were included in the normal nutrition group, 183 patients in the light malnutrition group (34.4%), and 58 patients in the moderate or severe malnutrition group (10.9%).
2.4. Follow‐up
All the patients were followed up by telephone interview, outpatient visits, and letters. All surviving patients were followed up for more than 5 years. All patients were monitored postoperatively by physical examination and laboratory tests, including tests for tumor markers (such as carcinoembryonic antigen (CEA) and carbohydrate antigenic determinant (CA) 19‐9), every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. In addition, examinations, including chest radiography, abdominopelvic CT, and endoscopy, were performed at least once per year. If necessary, further evaluations, such as positron emission tomography or magnetic resonance imaging, were initiated to better identify recurrence.
2.5. Statistical analysis
Statistical analyses were performed with SPSS software, version 18.0 (SPSS Inc., Chicago, IL, USA), and R software, version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria). The significance tests used were Student's t test for continuous variables and the chi‐square test or Fisher's exact test for categorical variables. The relationships among studied parameters were examined using Spearman's correlation coefficient. A correlation was considered weak with coefficient values <0.5 and strong with values >0.8. The Kaplan‐Meier method was used to analyze overall survival (OS) and recurrence‐free survival (RFS), and the differences were assessed with log‐rank tests. A Cox proportional‐hazard model was used to identify variables with significant independent relationships with OS and RFS. Two‐tailed P values <.05 were considered statistically significant.
3. RESULTS
3.1. Clinicopathological data
The clinical and pathological data of the patients are shown in Table 1. Age, female sex, tumor size, tumor‐node‐metastasis (TNM) stage, comorbidities, and American Society of Anesthesiologists (ASA) status were all significantly higher in patients with low SMI than in those with high SMI. BMI, HB, ALB, and lymphocyte count were significantly lower in patients with low SMI than in those with high SMI. Conversely, SMI was not affected by tumor site, histological type, cholesterol concentration, operation method, type of resection, type of reconstruction, surgical duration, intraoperative blood loss, neurovascular invasion, or adjuvant chemotherapy. There were significant differences in age, BMI, tumor size, TNM, HB, ALB, lymphocyte count, cholesterol concentration, ASA, type of resection, and intraoperative blood loss in patients with different nutritional statuses. However, there were no significant differences in tumor site, histological type, comorbidities, operation method, type of reconstruction, neurovascular invasion, or adjuvant chemotherapy.
Table 1.
Clinicopathological characteristics of 532 patients with gastric cancer undergoing radical gastrectomy
| Variable | All | SMI | COUNT score | |||||
|---|---|---|---|---|---|---|---|---|
| Low (n = 91) | High (n = 441) | P value | Normal (n = 291) | Light (n = 183) | Moderate or severe (n = 58) | P value | ||
| Age (y) | 61.1 (11.5) | 68.4 (11.4) | 59.6 (10.9) | <.001 | 58.8 (11.1) | 62.5 (11.4) | 67.7 (10.3) | <.001 |
| Gender | ||||||||
| Female | 129 (24.2) | 41 (45.1) | 88 (20.0) | <.001 | 72 (24.7) | 36 (19.7) | 21 (36.2) | .036 |
| Male | 403 (75.8) | 50 (54.9) | 353 (80.0) | 219 (75.3) | 147 (80.3) | 37 (63.8) | ||
| BMI (kg/m2) | 21.9 (3.5) | 20 (2.7) | 22.3 (3.5) | <.001 | 22.3 (3.5) | 21.3 (2.9) | 21.7 (4.6) | .012 |
| Tumor site | ||||||||
| Upper | 159 (29.9) | 27 (29.7) | 132 (29.9) | .96 | 79 (27.1) | 65 (35.5) | 15 (25.9) | .119 |
| Not upper | 373 (70.1) | 64 (70.3) | 309 (70.1) | 212 (72.9) | 118 (64.5) | 43 (74.1) | ||
| Tumor size (cm) | 4.5 (2.5) | 3.2 (2.0) | 4.8 (2.5) | <.001 | 4.8 (2.5) | 4.1 (2.3) | 4.4 (2.7) | .006 |
| TNM stage | ||||||||
| I | 165 (31) | 16 (17.6) | 149 (33.8) | .001 | 111 (38.1) | 45 (24.6) | 9 (15.5) | .001 |
| II | 123 (23.1) | 17 (18.7) | 106 (24.0) | 63 (26.6) | 46 (25.1) | 14 (24.1) | ||
| III | 244 (45.9) | 58 (63.7) | 186 (42.2) | 117 (40.2) | 92 (50.3) | 35 (60.3) | ||
| Histological type | ||||||||
| Differentiated | 156 (29.3) | 21 (23.1) | 135 (30.6) | .151 | 88 (30.2) | 50 (27.3) | 18 (31) | .758 |
| Undifferentiated | 376 (70.7) | 70 (76.9) | 306 (69.4) | 203 (69.8) | 133 (72.7) | 40 (69) | ||
| Comorbidities | ||||||||
| No | 390 (73.3) | 55 (60.4) | 335 (76) | .002 | 217 (74.6) | 133 (72.7) | 40 (69) | .659 |
| Yes | 142 (26.7) | 36 (39.6) | 106 (24.0) | 74 (25.4) | 50 (27.3) | 18 (31) | ||
| HB (g/dL) | 12.5 (2.1) | 11.4 (2.6) | 12.8 (2.5) | <.001 | 13.5 (2.1) | 12.0 (2.3) | 9.2 (2.5) | <.001 |
| ALB (g/dL) | 3.8 (0.5) | 3.7 (06) | 3.9 (0.5) | .004 | 4.1 (0.3) | 3.7 (0.4) | 2.9 (0.5) | <.001 |
| Lymphocyte (mm3) | 1770 (620) | 1530 (680) | 1820 (600) | <.001 | 2020 (530) | 1540 (610) | 1280 (510) | <.001 |
| Cholesterol (mg/dL) | 189.5 (85.8) | 182.7 (53.4) | 190.9 (91.7) | .405 | 208.1 (37.6) | 178.0 (130.8) | 131.9 (34.9) | <.001 |
| ASA | ||||||||
| I | 202 (38) | 19 (20.9) | 183 (41.5) | .001 | 130 (44.7) | 66 (36.1) | 6 (10.3) | <.001 |
| II | 312 (59.2) | 69 (75.8) | 246 (55.8) | 154 (52.9) | 111 (60.7) | 50 (86.2) | ||
| III | 15 (2.8) | 3 (3.3) | 12 (2.7) | 7 (2.4) | 6 (3.3) | 2 (3.4) | ||
| Operation method | ||||||||
| Open | 76 (14.3) | 15 (16.5) | 61 (13.8) | .51 | 36 (12.4) | 30 (16.4) | 10 (17.2) | .327 |
| Laparoscopic | 456 (85.7) | 76 (83.5) | 380 (86.2) | 255 (87.6) | 153 (83.6) | 48 (82.9) | ||
| Type of resection | ||||||||
| Subtotal gastrectomy | 297 (55.8) | 49 (53.8) | 248 (56.2) | .676 | 176 (60.5) | 88 (48.1) | 33 (56.9) | .03 |
| Total gastrectomy | 235 (44.2) | 42 (46.2) | 193 (43.8) | 115 (39.5) | 95 (51.9) | 25 (43.1) | ||
| Type of reconstruction | ||||||||
| Billroth I | 250 (47) | 41 (45.1) | 209 (47.6) | .776 | 150 (51.5) | 73 (39.9) | 27 (46.6) | .22 |
| Billroth II | 34 (6.4) | 8 (8.8) | 26 (5.9) | 16 (5.5) | 14 (7.7) | 4 (6.9) | ||
| Roux‐en‐Y | 235 (44.2) | 40 (44) | 195 (44.2) | 116 (39.9) | 93 (50.8) | 26 (44.8) | ||
| Other | 13 (2.4) | 2 (2.1) | 11 (2.5) | 9 (3.1) | 3 (1.6) | 1 (1.7) | ||
| Surgical durations | 189.5 (85.8) | 1856.0 (67.2) | 183.4 (58.1) | .706 | 177.5 (56.9) | 191.9 (64.8) | 190.0 (53.8) | .027 |
| Intraoperative blood loss | 96.2 (192.3) | 79.1 (66.5) | 99.7 (208.9) | .353 | 81.6 (110.1) | 98.6 (132.3) | 161.6 (470.5) | .015 |
| Neurovascular invasion | ||||||||
| No | 400 (75.2) | 64 (70.3) | 336 (76.2) | .239 | 229 (78.7) | 129 (70.5) | 42 (72.4) | .115 |
| Yes | 132 (24.8) | 27 (29.7) | 105 (23.8) | 62 (21.3) | 54 (29.5) | 16 (27.6) | ||
| Adjuvant chemotherapy | ||||||||
| No | 280 (52.6) | 46 (50.5) | 234 (53.1) | .662 | 157 (54.0) | 92 (50.3) | 31 (53.4) | .731 |
| Yes | 252 (47.4) | 45 (49.5) | 207 (46.9) | 134 (46.0) | 91 (49.7) | 27 (46.4) | ||
ALB, albumin; ASA, American Society of Anesthesiologists; BMI, body mass index; COUNT, controlling nutritional status; HB, hemoglobin; SMI, skeletal muscle index.
3.2. Correlation analysis
Spearman's correlation analysis showed weak correlations of SMI with ALB, lymphocyte count, and cholesterol (all r s < 0.5) (Table 2).
Table 2.
Correlation between measurements of preoperative SMI and CONUT scores in patients with gastric cancer
| Correlation coefficient (Spearman's p) | SMI |
|---|---|
| ALB | 0.136 |
| Lymphocyte | 0.272 |
| Cholesterol | 0.033 |
ALB, albumin; COUNT, controlling nutritional status; SMI, skeletal muscle index.
3.3. SMI, CONUT score and survival
The median duration of follow‐up was 60 months (range 2‐76 months). The 5‐year OS and RFS after surgery in patients with low SMI were significantly worse than those in patients with high SMI (41.30% vs 68%, P < .001; 42.60%, vs 66.2%, P < .001). Patients with normal nutrition had better 5‐year OS and RFS than did those with light malnutrition (71.40% vs 53.20%, P < .001; 70% vs 51.60%, P < .001), and these metrics were also better than those in patients with moderate or severe malnutrition (71.40% vs 54.50%, P = .006; 70% vs 55.20%; P = .017). However, there were no significant differences in 5‐year OS and RFS between patients with light malnutrition and those with moderate or severe malnutrition (P = .726) (Figure 1).
Figure 1.
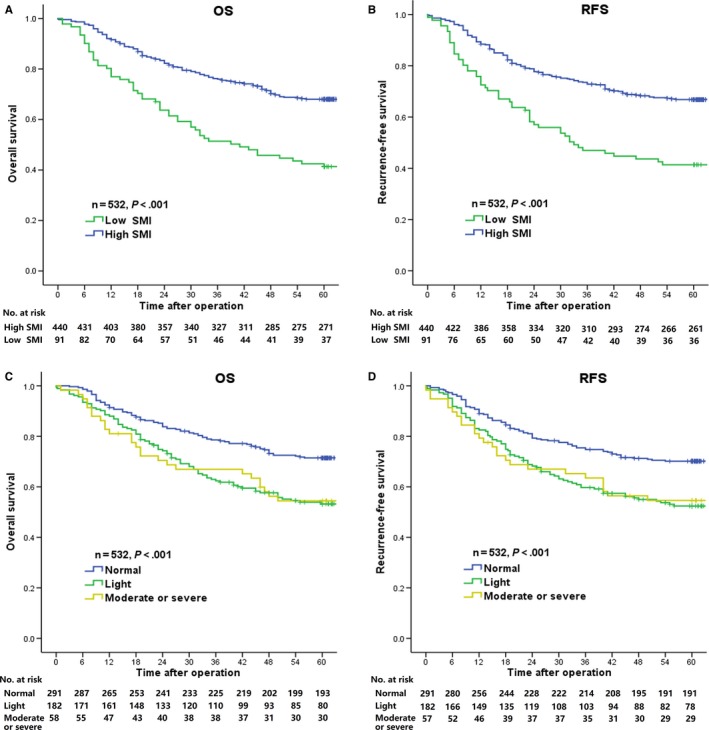
Kaplan‐Meier survival curves for overall survival (OS) according to SMI (A) and the CONUT score (C); Kaplan‐Meier survival curves for recurrence‐free survival (RFS) according to SMI (B) and the CONUT score (D)
In univariate analysis, SMI, the CONUT score, age, tumor site, TNM, HB, ASA, type of resection, type of reconstruction, surgical duration, neurovascular invasion, and adjuvant chemotherapy were associated with 5‐year OS. Regarding 5‐year RFS, univariate analysis showed that SMI, the CONUT score, age, tumor site, TNM, histological type, type of resection, type of reconstruction, surgical duration, neurovascular invasion, and adjuvant chemotherapy were significantly associated (Table 3). In multivariate analysis, only TNM and SMI were independent prognostic factors for 5‐year OS and RFS (Table 3).
Table 3.
Uni‐ and multivariate analyses of factors associated with 5‐year overall survival (OS) and recurrence‐free survival (RFS) rates in patients with gastric cancer
| Variable | Univariate analysis 5‐year OS | Multivariate analysis 5‐year OS | Univariate analysis 5‐year RFS | Multivariate analysis 5‐year RFS | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| SMI | ||||||||
| High | Reference | <.001 | Reference | .002 | Reference | <.001 | Reference | .012 |
| Low | 2.337 (1.702‐2.309) | 1.704 (1.209‐2.403) | 2.125 (1.545‐2.925) | 1.553 (1.101‐2.189) | ||||
| COUNT Score | ||||||||
| Normal | Reference | <.001 | Reference | .173 | Reference | <.001 | Reference | .137 |
| Light | 1.856 (1.367‐2.519) | 1.360 (0.984‐1.879) | 1.837 (1.362‐2.478) | 1.376 (1.005‐1.884) | ||||
| Moderate or severe | 1.839 (1.183‐2.859) | 1.266 (0.753‐2.126) | 1.703 (1.091‐2.659) | 1.154 (0.726‐1.836) | ||||
| Age (y) | ||||||||
| <65 | Reference | .002 | Reference | .766 | Reference | .006 | Reference | .473 |
| ≥65 | 1.563 (1.176‐2.078) | 1.054 (0.733‐1.517) | 1.486 (1.123‐1.968) | 1.124 (0.817‐1.547) | ||||
| Gender | ||||||||
| Female | Reference | .131 | Reference | .051 | ||||
| Male | 0.783 (0.571‐1.075) | 0.736 (0.541‐1.002) | ||||||
| BMI (kg/m2) | ||||||||
| <25 | Reference | .086 | Reference | .156 | ||||
| ≥25 | 0.654 (0.402‐1.063) | 0,716 (0.451‐1.136) | ||||||
| Tumor site | ||||||||
| Upper | Reference | .008 | Reference | .303 | Reference | 0.005 | Reference | .103 |
| Not upper | 0.673 (0.501‐0.903) | 0.824 (0.569‐1.192) | 0.657 (0.491‐0.878) | 0.736 (0.509‐1.064) | ||||
| Tumor size (cm) | ||||||||
| <5.0 | Reference | .076 | Reference | 0.100 | ||||
| ≥5.0 | 0.768 (0.573‐1.028) | 0.786 (0.591‐1.047) | ||||||
| TNM stage | ||||||||
| I | Reference | <.001 | Reference | <.001 | Reference | <.001 | Reference | <.001 |
| II | 2.125 (1.165‐3.876) | 1.931 (1.042‐3.576) | 2.260 (1.267‐4.030) | 2.029 (1.120‐3.678) | ||||
| III | 8.331 (5.009‐13.612) | 7.520 (4.457‐12.690) | 8.178 (5.065‐13.202) | 7.337 (4.365‐12.334) | ||||
| Histological type | ||||||||
| Differentiate | Reference | .063 | Reference | .026 | Reference | .784 | ||
| Undifferentiated | 1.362 (0.984‐1.884) | 1.447 (1.045‐2.004) | 0.953 (0.677‐1.342) | |||||
| Comorbidities | ||||||||
| No | Reference | .352 | Reference | .555 | ||||
| Yes | 1.160 (0.849‐1.583) | 1.098 (0.806‐1.496) | ||||||
| HB (g/L) | ||||||||
| >90 | Reference | .036 | Reference | .702 | Reference | .056 | ||
| ≤90 | 1.535 (1.028‐2.292) | 0.911 (0.564‐1.471) | 1.477 (0.990‐2.204) | |||||
| ASA | ||||||||
| I | Reference | .044 | Reference | .435 | Reference | .102 | ||
| II | 1.483 (1.089‐2.021) | 1.277 (0.880‐1.854) | 1.377 (1.018‐1.863) | |||||
| III | 1.395 (0.602‐3.231) | 1.189 (0.480‐2.945) | 1.501 (0.688‐3.278) | |||||
| Operation method | ||||||||
| Open | Reference | .173 | Reference | .218 | ||||
| Laparoscopic | 0.768 (0.525‐1.123) | 0.788 (0.540‐1.151) | ||||||
| Type of resection | ||||||||
| Subtotal gastrectomy | Reference | <.001 | Reference | .802 | Reference | <.001 | Reference | .944 |
| Total gastrectomy | 1.944 (1.460‐2.591) | 1.089 (0.559‐2.123) | 1.827 (1.379‐2.421) | 1.023 (0.538‐1.945) | ||||
| Type of reconstruction | ||||||||
| Billroth I | Reference | <.001 | Reference | .191 | Reference | <.001 | Reference | .225 |
| Billroth II | 2.582 (1.510‐4.414) | 1.843 (1.044‐3.254) | 2.562 (1.522‐4.313) | 1.745 (1.008‐3.019) | ||||
| Roux‐en‐Y | 2.124 (1.555‐2.901) | 1.158 (0.568‐2.362) | 2.042 (1.505‐2.770) | 1.077 (0.544‐2.132) | ||||
| Other | 1.199 (0.436‐3.294) | 0.926 (0.314‐2.735) | 1.122 (0.409‐3.077) | 0.892 (0.307‐2.590) | ||||
| Surgical durations (min) | ||||||||
| <180 | Reference | .007 | Reference | .237 | Reference | .005 | Reference | .189 |
| ≥180 | 1.502 (1.118‐2.019) | 1.204 (0.885‐1.637) | 1.519 (1.135‐2.032) | 1.224 (0.905‐1.656) | ||||
| Intraoperative blood loss (mL) | ||||||||
| <50 | Reference | 0.380 | Reference | .342 | ||||
| ≥50 | 1.402 (0.659‐2.983) | 1.442 (0.678‐3.066) | ||||||
| Neurovascular invasion | ||||||||
| No | Reference | .008 | Reference | .442 | Reference | .007 | Reference | .406 |
| Yes | 1.523 (1.119‐2.074) | 1.133 (0.824‐1.557) | 1.516 (1.118‐2.057) | 1.141 (0.836‐1.558) | ||||
| Adjuvant chemotherapy | ||||||||
| No | Reference | .036 | Reference | .062 | Reference | .005 | Reference | .281 |
| Yes | 1.357 (1.021‐1.084) | 0.747 (0.552‐1.012) | 1.492 (1126‐1.976) | 0.849 (0.631‐1.143) | ||||
ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidence interval; COUNT, controlling nutritional status; HB, hemoglobin; HR, hazard ratio; OS, overall survival; RFS, recurrence‐free survival; SMI, skeletal muscle index.
4. DISCUSSION
The determinants of cancer progression and prognosis are multifaceted, and increasing attention has been paid to the relationship between cancer and malnutrition.3, 28 Over the past few decades, malnutrition has been associated with a poor response to treatment, decreased quality of life, a higher risk of chemotherapy side effects, and worse prognosis.4, 5, 29, 30
CT imaging to assess body composition has been widely used in the field of cancer treatment and research due to its universality, high accuracy, and low incremental costs. Sarcopenia (low SMI), a multifactorial clinical condition, is closely associated with nutritional deficiencies.5, 31 After analyzing the survival data of 937 GC patients with TNM stage II or III who underwent RG, Zhuang et al13 concluded that sarcopenia (low SMI) was an independent risk factor for OS and RFS. Kensuke's studies suggested that sarcopenia (low SMI) was associated with a negative prognosis in esophagogastric junction cancer or upper GC.14 In addition, previous studies in our center have demonstrated that combining sarcopenia (low SMI) with the cT and cN system could accurately predict long‐term survival after RG for GC.16
The CONUT score has been established as a useful tool to evaluate nutritional status,17 and it is closely related to the prognosis of various cancers.18, 19, 20 The CONUT score not only reflects the nutritional status of patients with GC but also predicts long‐term OS after surgery for GC.8 Takagi et al19 suggested that the CONUT score was a reliable predictor of long‐term prognosis after hepatectomy for hepatocellular carcinoma. In addition, the predictive ability of the CONUT score is better than that of classic indicators, such as the neutrophil to lymphocyte ratio (NLR), prognostic nutritional index (PNI), and modified Glasgow Prognostic Score (mGPS).8, 19 However, whether the predictive power of the CONUT score is superior to that of sarcopenia has not been previously reported.
In this study, the CONUT score and SMI were prognostic factors for OS and RFS after RG according to univariate analysis, but in multivariate analysis, only SMI remained an independent prognostic factor for OS and RFS. Although Kuroda et al8 found that the CONUT score was an independent risk factor for long‐term survival after surgery for GC and was superior to NLR and mGPS, it was not included among the factors for SMI. The present study included both the CONUT score and SMI and revealed that the prognostic ability of SMI was better than that of the CONUT score. The possible reasons for this finding are as follows. The CONUT score is calculated based on plasma ALB concentration, total peripheral lymphocyte count, and total cholesterol concentration. Serum ALB concentration is affected not only by nutritional status but also by changes in body fluid volume, such as dehydration, fluid retention, and chronic disease‐induced inflammatory responses.32, 33 Therefore, the CONUT score is more easily influenced by outside interference. In contrast, SMI is a highly objective measurement based on the use of CT scans to measure body composition, with a reported measurement error of approximately 1.4%.26 Moreover, SMI markers are relatively stable, and rapid fluctuations in skeletal muscle mass are unlikely to occur over a short period of time. This objectivity and stability are conducive to correctly predicting patient prognosis. In contrast to Yoshida et al's study,18 there were no statistically significant differences in 5‐year OS and RFS between patients with light malnutrition and those with moderate or severe malnutrition. This outcome suggests that the ability of the CONUT score to determine the long‐term survival of patients with light and moderate or severe malnutrition remains unproven. This finding might also be associated with the small number of patients with severe malnutrition (n = 58, 10.9%) in our study. These possible explanations require further research to confirm. Nevertheless, SMI is currently a better predictor than the CONUT score of long‐term survival after radical surgery for patients with GC.
We acknowledge several potential limitations of the present study. First, 235 patients were excluded from the study because they had no available abdominal CT data, which might have resulted in selection bias. Second, the design was retrospective, and the cases were obtained from a single center; therefore, the findings must be confirmed in prospective studies. Nevertheless, for the first time, this study compared the prognostic value of preoperative SMI and the CONUT score to predict long‐term outcomes in GC, revealing that SMI was a more stable and objective predictor than the CONUT score.
5. CONCLUSION
Skeletal muscle index based on preoperative CT images is superior to the CONUT score in terms of prognostic value in GC after RG. Therefore, preoperative SMI should be included in preoperative risk assessment, although this conclusion must be confirmed by a large‐scale, prospective validation study.
CONFLICT OF INTEREST
There are no conflict of interests or financial ties to disclose from any authors.
Supporting information
ACKNOWLEDGMENTS
This work was supported by the National Key Clinical Specialty Discipline Construction Program of China (no.[2012] 649), the Scientific and Technological Innovation Joint Capital Projects of Fujian Province (2016Y9031), Construction Project of Fujian Province Minimally Invasive Medical Center (No. [2017]171), the second batch of special support funds for Fujian Province innovation and entrepreneurship talents (2016B013), Youth scientific research subject of Fujian provincial health and family planning commission (No. 2015‐1‐37), and QIHANG funds of Fujian Medical University (No. 2016QH025).
Zheng Z‐F, Lu J, Xie J‐W, et al. Preoperative skeletal muscle index vs the controlling nutritional status score: Which is a better objective predictor of long‐term survival for gastric cancer patients after radical gastrectomy?. Cancer Med. 2018;7:3537–3547. 10.1002/cam4.1548
Z.F.Z and J.L contributed equally to this work and should be considered cofirst authors.
Contributor Information
Chao‐Hui Zheng, Email: wwkzch@163.com.
Chang‐Ming Huang, Email: hcmlr2002@163.com.
Ping Li, Email: 24627878@qq.com.
REFERENCES
- 1. Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;5:277‐300. [DOI] [PubMed] [Google Scholar]
- 2. Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para‐aortic nodal dissection for gastric cancer. N Engl J Med. 2008;5:453‐462. [DOI] [PubMed] [Google Scholar]
- 3. Reisinger KW, van Vugt JL, Tegels JJ, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;2:345‐352. [DOI] [PubMed] [Google Scholar]
- 4. Black D, Mackay C, Ramsay G, et al. Prognostic value of computed tomography: measured parameters of body composition in primary operable gastrointestinal cancers. Ann Surg Oncol. 2017;8:2241‐2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ongaro E, Buoro V, Cinausero M, et al. Sarcopenia in gastric cancer: when the loss costs too much. Gastric Cancer. 2017;4:563‐572. [DOI] [PubMed] [Google Scholar]
- 6. Rey‐Ferro M, Castano R, Orozco O, Serna A, Moreno A. Nutritional and immunologic evaluation of patients with gastric cancer before and after surgery. Nutrition. 1997;10:878‐881. [DOI] [PubMed] [Google Scholar]
- 7. Sun J, Chen X, Gao P, et al. Can the neutrophil to lymphocyte ratio be used to determine gastric cancer treatment outcomes? A systematic review and meta‐analysis. Dis Markers. 2016;2016:7862469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuroda D, Sawayama H, Kurashige J, et al. Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2017;21:204‐212. [DOI] [PubMed] [Google Scholar]
- 9. Sakurai K, Ohira M, Tamura T, et al. Predictive potential of preoperative nutritional status in long‐term outcome projections for patients with gastric cancer. Ann Surg Oncol. 2016;2:525‐533. [DOI] [PubMed] [Google Scholar]
- 10. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;4:412‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;4:249‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wagner D, DeMarco MM, Amini N, et al. Role of frailty and sarcopenia in predicting outcomes among patients undergoing gastrointestinal surgery. World J Gastrointest Surg. 2016;1:27‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhuang CL, Huang DD, Pang WY, et al. Sarcopenia is an independent predictor of severe postoperative complications and long‐term survival after radical gastrectomy for gastric cancer: analysis from a large‐scale cohort. Medicine (Baltimore). 2016;13:e3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kudou K, Saeki H, Nakashima Y, et al. Prognostic significance of sarcopenia in patients with esophagogastric junction cancer or upper gastric cancer. Ann Surg Oncol. 2017;7:1804‐1810. [DOI] [PubMed] [Google Scholar]
- 15. Wang SL, Zhuang CL, Huang DD, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: a prospective study. Ann Surg Oncol. 2016;2:556‐564. [DOI] [PubMed] [Google Scholar]
- 16. Zheng ZF, Lu J, Zheng CH, et al. A novel prognostic scoring system based on preoperative sarcopenia predicts the long‐term outcome for patients after R0 resection for gastric cancer: experiences of a high‐volume center. Ann Surg Oncol. 2017;7:1795‐1803. [DOI] [PubMed] [Google Scholar]
- 17. Ignacio DUJ, Gonzalez‐Madrono A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;1:38‐45. [PubMed] [Google Scholar]
- 18. Yoshida N, Harada K, Baba Y, et al. Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbecks Arch Surg. 2017;2:333‐341. [DOI] [PubMed] [Google Scholar]
- 19. Takagi K, Yagi T, Umeda Y, et al. Preoperative controlling nutritional status (CONUT) score for assessment of prognosis following hepatectomy for hepatocellular carcinoma. World J Surg. 2017;9:2353‐2360. [DOI] [PubMed] [Google Scholar]
- 20. Toyokawa T, Kubo N, Tamura T, et al. The pretreatment controlling nutritional status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016;16:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2010 (Ver. 3). Gastric Cancer. 2011;2:113‐123. [DOI] [PubMed] [Google Scholar]
- 22. Edge SBBDCC. AJCC cancer staging manual, 7th edn New York: Springer; 2009. [Google Scholar]
- 23. Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open‐label, randomised controlled trial. Lancet. 2012;9813:315‐321. [DOI] [PubMed] [Google Scholar]
- 24. Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N Engl J Med. 2007;18:1810‐1820. [DOI] [PubMed] [Google Scholar]
- 25. Dello SA, Lodewick TM, van Dam RM, et al. Sarcopenia negatively affects preoperative total functional liver volume in patients undergoing liver resection. HPB (Oxford). 2013;3:165‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;1:115‐122. [DOI] [PubMed] [Google Scholar]
- 27. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;7:629‐635. [DOI] [PubMed] [Google Scholar]
- 28. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;3:223‐226. [DOI] [PubMed] [Google Scholar]
- 29. Sala A, Rossi E, Antillon F, et al. Nutritional status at diagnosis is related to clinical outcomes in children and adolescents with cancer: a perspective from Central America. Eur J Cancer. 2012;2:243‐252. [DOI] [PubMed] [Google Scholar]
- 30. Zeng Q, Shen LJ, Guo X, Guo XM, Qian CN, Wu PH. Critical weight loss predicts poor prognosis in nasopharyngeal carcinoma. BMC Cancer. 2016;16:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Antoun S, Borget I, Lanoy E. Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr Opin Support Palliat Care. 2013;4:383‐389. [DOI] [PubMed] [Google Scholar]
- 32. de Ulibarri PJ, Fernandez G, Rodriguez SF, Diaz LA. Nutritional screening; control of clinical undernutrition with analytical parameters. Nutr Hosp. 2014;4:797‐811. [DOI] [PubMed] [Google Scholar]
- 33. Cengiz O, Kocer B, Surmeli S, Santicky MJ, Soran A. Are pretreatment serum albumin and cholesterol levels prognostic tools in patients with colorectal carcinoma? Med Sci Monit. 2006;6:R240‐R247. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


