Abstract
Vacuolar ATPase (V‐ATPase) is an ATP‐dependent H+‐transporter that pumps protons across intracellular and plasma membranes. It consists of a large multi‐subunit protein complex and influences a wide range of cellular processes. This review focuses on emerging evidence for the roles for V‐ATPase in cancer. This includes how V‐ATPase dysregulation contributes to cancer growth, metastasis, invasion and proliferation, and the potential link between V‐ATPase and the development of drug resistance.
Keywords: cancer, drug resistance, invasion, metastasis, novel therapy, V‐ATPase
1. INTRODUCTION
V‐ATPase is a multi‐protein complex that catalyzes the ATP‐dependent transport of protons across intracellular and plasma membranes. The resulting acidification of organelle lumens and the extracellular space influences a diverse range of cellular processes, many of which are dysregulated in cancers. Here, we review current evidence that V‐ATPase function promotes multiple cancer‐associated hallmarks, especially invasion and metastasis, and that V‐ATPase inhibition may have utility as a novel anti‐cancer therapeutic strategy.
2. V‐ATPASE STRUCTURE AND FUNCTION IN NORMAL PHYSIOLOGY
Mammalian V‐ATPase comprises 13 distinct subunits that are part of either the cytosolic V1 (A, B, C, D, E, F, G and H) or membrane‐integral Vo (a, c, c″, d and e) domains. ATP hydrolysis occurs within the core A3B3 hexamer of the V1‐domain and drives rotation of the central stalk resulting in H+‐transport via the Vo‐domain. The complex is orientated such that protons are transported from the cytoplasm to the lumen of organelles or, for plasma membrane‐located V‐ATPase, to the extracellular space. The key structural and functional features of V‐ATPase are summarized in Figure 1, and readers are referred to recent reviews for more detailed information.1, 2 To clarify, the nomenclature of the genes encoding the subunits A in the V1 and a in Vo domains are ATP6V1A and ATP6V0A, respectively.
Figure 1.
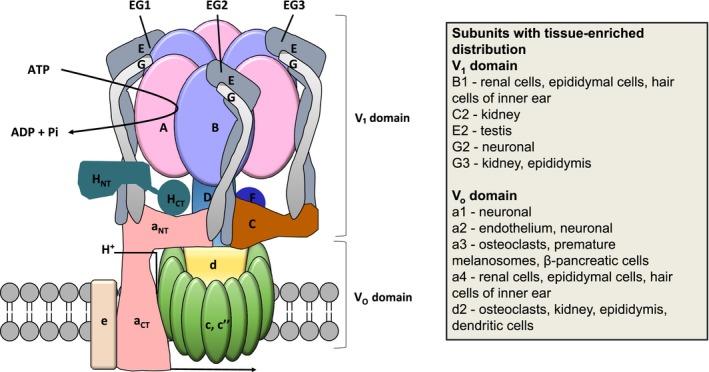
V‐ATPase structure. The V1 domain contains the A3B3 catalytic hexamer, the peripheral stalk made up of subunits E, G, C, and H, and D and F of the central rotor. ATP hydrolysis occurs in the A3B3 catalytic hexamer and the energy generated is used to drive the rotary mechanism. The Vo domain is integrated into the membrane and is responsible for proton translocation; it consists of subunits a, d, e and the proteolipid ring made up of c and c″. Many of the subunits are expressed in the form of multiple isoforms; the tissue enriched localization of some of the important isoforms is shown
The peripheral stalks in the V1 domain are composed of 3 V1E and V1G subunits, which form dimers that are connected by the V1C and V1H subunits and the N‐terminus of Voa in the Vo domain (Figure 1). The peripheral stator has an important role in tethering the A3B3 hexamer to the N‐terminus of subunit Voa, thus resisting the torque generated by the rotation of the central stalk. Protons enter the cytoplasmic hemi‐channel in the Vo domain via the transmembrane Voa subunit and bind to a conserved glutamic acid residue in the Voc or Voc″ subunit. The clockwise rotation of the central stalk sequentially rotates the proteolipid ring, allowing the protonated glutamic acid residues to come into contact with an arginine residue within a hemi‐channel in the Voa subunit. This results in the deprotonation of the glutamic acid residue and the release of the proton into the lumen.
Intracellular membrane‐associated V‐ATPase is ubiquitous in mammalian cells where it acidifies lysosomes, endosomes, and secretory vesicles, and thereby influences many processes associated with these organelles, including vesicular trafficking, endocytosis, autophagy, receptor recycling, and protein degradation.1 V‐ATPase‐dependent lysosome acidification also drives the coupled transport of small molecules/ions into the cytoplasm, especially of amino acids and calcium ions. A summary of some important V‐ATPase functions is shown in Figure 2.
Figure 2.
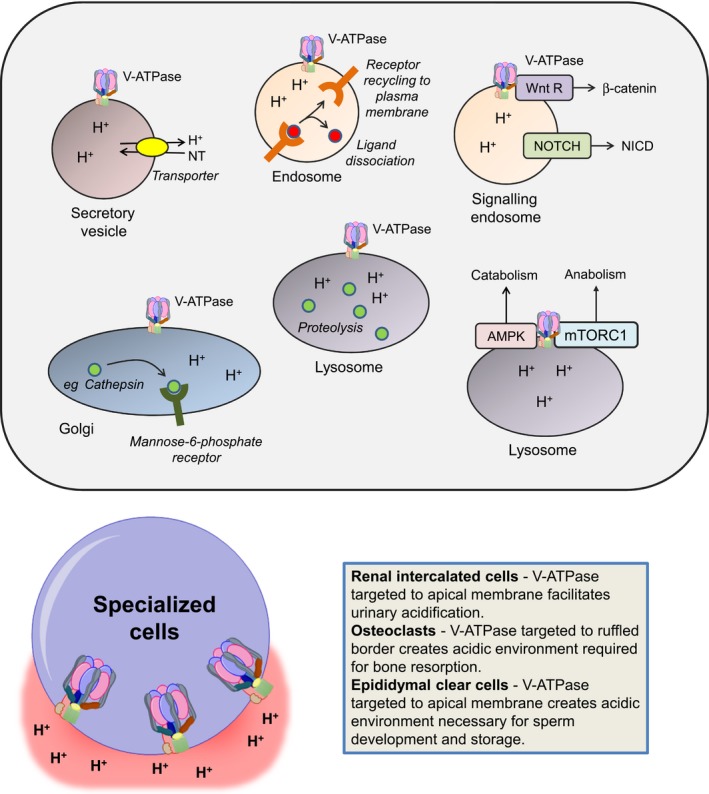
V‐ATPase function. A, Intracellular V‐ATPase regulates multiple intracellular processes. In secretory vesicles, V‐ATPase generates a proton gradient that is used to drive the H+‐dependent uptake of neurotransmitters. In endosomes, low pH promotes the dissociation of ligands, such as low density lipoproteins, from their receptors, facilitating receptor recycling to the plasma membrane. In signaling endosomes, V‐ATPase promotes signaling to β‐catenin downstream of Wnt receptors, and can promote NOTCH signaling via enhanced cleavage to generate NICD. In the Golgi, V‐ATPase promotes binding of hydrolases to the mannose‐6‐phosphate receptor which is important for their delivery to the lysosome. In lysosomes, low pH is required for optimal activity of acid‐dependent proteases, such as cathepsins. Lysosomal V‐ATPase also acts to coordinate activity of AMPK and mTORC1 to regulated cellular catabolism vs anabolism in response to shifting microenvironmental cues. Note the figure is designed to illustrate some key functions of V‐ATPase and is not intended to portray all of the diverse functions that have been ascribed to V‐ATPase. B, Plasma membrane functions of V‐ATPase in specialized cell types including renal intercalated cells, osteoclasts, and clear epididymal cells
Intracellular V‐ATPase plays important roles in several key cell signaling pathways.2, 3 For example, in some systems, V‐ATPase is required for optimal signaling downstream of NOTCH receptors as V‐ATPase‐mediated acidification of endosomes enhances γ‐secretase‐mediated proteolysis of NOTCH to generate the active NICD cleavage product.4, 5 However, in other cell systems (ie, triple negative breast cancer cells) V‐ATPase appears to negatively regulate NOTCH signaling as chemical inhibition of V‐ATPase activity or RNAi‐mediated knockdown of the Voa2 subunit, results in increased NOTCH signaling associated with increased NICD expression.6 Consistent with this inhibitory effect, conditional deletion of Voa2 in mouse mammary glands results in reduced mammary gland development, associated with increased NOTCH (and TGFβ) signaling.7 V‐ATPase is also required for Wnt signaling, including in developing Xenopus embryos, in which the V‐ATPase accessory protein, ATP6AP2 (also known as prorenin receptor), acts as an adaptor between Wnt receptor complexes and V‐ATPase.8
V‐ATPase acts as common activator for both mTORC1 signaling, which is activated under conditions of nutrient abundance and growth factor stimulation to promote cell growth, and AMPK (AMP‐activated protein kinase) which leads to enhanced catabolic activity.9 Thus, V‐ATPase coordinates a molecular switch between anabolism and catabolism in response to shifting metabolic cues. Although AMPK is classically activated in response to rising AMP/ATP ratios, recent studies have demonstrated that V‐ATPase plays a role in AMP‐independent AMPK activation. Thus, binding of aldolase to V‐ATPase in the absence of the glycolytic intermediate fructose‐1,6‐bisphosphate results in AMPK activation.10, 11
In contrast to the ubiquitous role of V‐ATPase at intracellular membranes, function of plasma membrane V‐ATPase is enriched in specific cell types, including osteoclasts and epididymal clear cells (where it is important for extracellular acidification) and renal epithelial intercalated cells (where it is important for acid secretion). In these cells, trafficking of V‐ATPase to the plasma membrane is regulated via intracellular signaling. For example, enhanced “delivery” from V‐ATPase‐rich intracellular vesicles increases the density of the complex in the apical plasma membrane of renal intercalated cells in response to reduced plasma pH.12 Here, PKA‐mediated phosphorylation of V‐ATPase controls fusion and endocytosis of V‐ATPase‐containing vesicles.13, 14 Interestingly, the PKA‐mediated increase in apical V‐ATPase in epididymal clear cells and renal intercalated cells is antagonized by AMPK and the V1A subunit appears to be a shared target for modulation by PKA and AMPK‐mediated phosphorylation.14, 15 Additionally, the Voa subunits are important for targeting V‐ATPases to the plasma membrane of specialized acidifying cells. For example, plasma membrane localization of V‐ATPase in osteoclasts and epididymal cells is associated with expression of tissue‐enriched Voa3/a4 subunits.16, 17 Key tissue‐enriched subunit isoforms are shown in Figure 1.
In addition to regulated trafficking, reversible association of V1 and Vo domains plays an important role in controlling V‐ATPase function. Disassociation of V1/Vo domains is accompanied by release of “free” V1C subunit, which plays a central role in the reassembly process. Multiple stimuli promote V‐ATPase assembly in mammalian cells such as the presence of growth factors, amino acid starvation, and increased glucose concentration.1, 18 For example, it has been proposed that in the presence of growth factors, such as EGF, increased V‐ATPase assembly is crucial to generate sufficient levels of amino acids from lysosomal protein degradation for mTORC1 stimulation.19 Increased V‐ATPase assembly due to amino acid starvation may be due to increased intracellular levels of free amino acids as a result of increased protein degradation in the lysosome.20 PI3‐kinase‐dependent signaling appears to play a central role in inducing V‐ATPase assembly in response to increased glucose, but not amino‐acid deprivation.20, 21 V‐ATPase assembly is also promoted by interaction with aldolase or phosphofructokinase22, 23 and this may be important for AMPK activation.10
V‐ATPase function can also be modulated by incorporation of specific subunit isoforms, many of which are expressed in a tissue‐enriched pattern (eg, Voa3/a4) or by binding of auxiliary subunits such as ATP6AP1 (Ac45) and ATP6AP2.24 V‐ATPase function has also been demonstrated to be influenced by the transmembrane protein TM9SF4 and ceramide synthase (LASS2/TMSG1) although the molecular basis for these functional links remain only partially understood.25, 26
3. DYSREGULATION OF V‐ATPASE IN CANCER
The bulk of the evidence linking dysregulation of V‐ATPase to cancer derives from studies showing altered expression or subcellular localization of specific V‐ATPase subunits in malignant cells versus normal counterparts and/or correlations between variable subunit expression and clinical features of disease.
For example, analysis of the TCGA database revealed that amplification/overexpression of ATP6VC1 was observed in 34% of human breast cancers and was associated with a relatively poor outcome.27 In a separate study, expression of the V1C1 subunit, which bridges between the V1 and Vo domains, was detectable in all examined stages of Barrett's esophagus (a precursor lesion for ESCC associated with an ~0.3% annual risk of progression to adenocarcinoma) using immunohistochemistry (IHC), indicating that its increased expression may play a role early in the transformation in these tissues.28 Interestingly, immunofluorescence staining revealed that at least a proportion of V‐ATPase was localized to the plasma membrane in ESCC cells.29 Furthermore, a recent detailed study using IHC showed that the V1E1 subunit was overexpressed in esophageal squamous cell carcinoma (ESCC) compared to normal esophagus. Unusually high V1E1 subunit protein level in cancer cells was associated with tumor invasiveness and lymph node (LN) metastasis, and with reduced disease‐free and overall survival, including in early stage disease. Importantly, V1E1 expression was an independent predictor of outcome30 and potentially used as a marker of disease. A similar study has investigated the significance of the V1A subunit in gastric cancer using IHC. Again, increased V‐ATPase subunit expression was observed in cancer, compared to normal, and high tumor expression was associated with poor differentiation, lymph node metastasis, advanced stage and was an independent predictor of poor outcome.31
Other studies have described expression of V‐ATPase/specific subunits in pancreatic cancer,32, 33 nonsmall cell lung cancer,34 oral squamous cell carcinoma,35, 36 ovarian cancer,37 cervical cancer,38 breast cancer,39, 40 gliomas,41 and hepatocellular carcinoma.42 A summary of studies investigating clinical significance of V‐ATPase in cancer is provided in Table 1. It is important to note that most of these studies focused on individual subunits and lack information on localization, expression of other subunits, and/or analysis of the ATP hydrolysis and proton transport activities of V‐ATPase. Further investigation into whether altered gene expression and/or protein accumulation of one of the subunits of V‐ATPase lead to malfunction of V‐ATPase function in the primary human tumors remains an important area for future investigation.
Table 1.
Evidence for V‐ATPase subunit dysregulation in patient tumor tissues
| Cancer type | Subunits investigated | Techniques | Key evidence | References |
|---|---|---|---|---|
| Breast | Voa | RT‐qPCR, IHC | Voa3 mRNA expression was upregulated in all breast cancer tissues tested compared to normal tissue and a3 mRNA expression correlated with tumor grade. Invasive breast cancer tissue had greater a3 staining than ductal carcinoma in situ, suggesting a3 expression increases with invasive potential. | Cotter et al39 |
| Breast and melanoma | Voa2 | IHC, IF | Highly positive staining of Voa2 in breast and skin tumors compared to their respective normal tissues. | Katara et al40 |
| Cervical | V1C1 | IHC | V‐ATPase expression significantly increased in patients with cervical adenocarcinoma. In patients with bulky cervical tumor expression was correlated with poor disease‐free survival | Song et al38 |
| Gastric | V1A | IHC | V1A overexpressed in gastric cancer tissue compared to normal tissue. V1A expression correlated with advanced tumor grade, vascular invasion, lymph node metastasis and was associated with worse survival than patients with negative V1A staining. | Liu et al31 |
| Glioma | Voa | RT‐qPCR | Voa4 isoform increased compared to brain biopsies of epileptic patients. | Gleize et al41 |
| Human hepatocellular carcinoma | Voc (ATP6L) | RT‐qPCR, WB, IHC | Greater ATP6L mRNA and protein expression in HCC tissues compared to normal liver tissue. Baf‐A1 inhibition retarded growth of HCC in liver of mice. | Xu et al19, 42 |
| Lung | V‐ATPase complex | IHC | V‐ATPase complex overexpressed in NSCLC. Expression was significantly lower in grade II adenocarcinoma and squamous cell lung cancer than grade III. V‐ATPase expression was found to be positively correlated to drug resistance in NSCLC samples and a significant positive correlation was obtained for V‐ATPase expression and common cancer chemotherapeutic agents. | Lu et al34 |
| Esophageal | Voa and V1C1 | Confocal microscopy, IHC | V‐ATPase expressed in all stages of neoplastic progression in Barrett's esophagus. | Chueca et al28 |
| V‐ATPase complex | IHC/IF | Complex highly expressed in esophageal squamous cancer cells. | Huang et al29 | |
| V1E1 | IHC | V1E1 expression increased in esophageal cancer tissues than normal esophageal tissue and was directly correlated with tumor invasiveness and poor prognosis. Can act as independent prognostic factor. | Son et al30 | |
| Oral squamous cell carcinoma | V1C1 | RT‐qPCR | V1C1 overexpressed in OSCC tissues compared to healthy oral mucosa samples. | Perez‐Sayans et al36 |
| V1C1 | IHC | V1C1 overexpressed in OSCC tissues compared to healthy oral mucosa tissue. | Garcia‐Garcia et al35 | |
| Ovarian | Voa | IF, IHC | Voa2 expression increased and a3 subunits had greater staining in ovarian cancer tissues compared to normal ovarian tissues. | Kulshrestha et al37 |
| Pancreatic | Voc | RT‐qPCR, IHC, IP | Voc overexpressed in pancreatic carcinoma tissues compared to normal pancreatic tissue. Expression was linked to invasive capabilities as the overexpression of Voc was characteristic of invasive ductal adenocarcinomas. | Ohta et al32 |
| V1E | IHC | V‐ATPase staining was significantly increased from low‐grade pancreatic intraepithelial neoplasia (PanIN) to invasive PDAC. | Chung et al33 |
IHC, Immunohistochemistry; IF, Immunofluorescence; WB, Western blot; IP, Immunoprecipitation.
An important new advance that moves beyond differences in subunit expression in cancer is the recent identification of V‐ATPase subunit mutations. Somatically acquired mutations of ATP6V1B1 or the accessory subunit ATP6AP1 are detected in ~20% of follicular lymphoma (FL).43, 44 These V‐ATPase mutations frequently co‐occur with mutations in the RRAGC gene encoding the Rag‐GTPase family member RagC which, with V‐ATPase and the V‐ATPase interaction partner Ragulator, form an amino acid “sensing” supercomplex which is required for mTORC1 activation.45 FL RRAGC mutations appear to be gain‐of‐function and promote inappropriate mTORC1 activity following amino‐acid depletion.44 Functional data are lacking, but V‐ATPase mutations may also promote inappropriate mTORC1 activation in FL. A relatively large number of V‐ATPase subunit sequence variants have been reported in the COSMIC database in a wide spectrum of cancer types46 suggesting that the occurrence and importance of V‐ATPase mutations may extend beyond lymphoma. However, validating studies will be required to confirm which sequence variants are truly cancer specific and have important functional consequences.
4. V‐ATPASE FUNCTION IN CANCER CELLS
Functional characterization of V‐ATPase in human cancer cells has focused on its role in potentiation of migration and invasion, consistent with the common association between elevated subunit expression and advanced stage/metastasis observed in several cancer types (Table 1). Increased cancer cell invasive activity is frequently associated with aberrant V‐ATPase plasma membrane localization suggesting that increased acidification of the extracellular space plays an important role.29, 33, 34, 37, 47, 48, 49, 50, 51, 52
Various cell lines have been used to study the functional links between V‐ATPase and invasive activity. For example, using 2 closely related human breast cancer cell lines, MCF10a and MCF10CA1a, Capecci et al found that the levels of mRNA encoding Voa1 and Voa3 were highest in the invasive MCF10CA1a cells.48 Furthermore, the Voa3 isoform was also expressed in lung and bone metastasis of B16‐F10 cells, suggesting this isoform might be relevant to the increased metastatic potential of melanoma cells.51 As previously discussed, Voa isoforms can be expressed in a tissue‐enriched pattern and are associated with plasma membrane localization. Therefore overexpression of these isoforms might be an example of a cancer cells hijacking a normal cellular process to increase H+ extrusion.
V‐ATPase in the invasive breast cancer MDA‐MB‐231 cell line was shown to be localized to the plasma membrane and V‐ATPase activity was significantly higher than less invasive cell lines. To investigate whether plasma membrane or intracellular V‐ATPases were responsible for the invasive phenotype of breast cancer cells, Cotter et al inhibited plasma membrane V‐ATPase activity in MDA‐MB‐231 cells with a monoclonal antibody to the V5 epitope of a V5 tagged Voc subunit construct (which, in contrast to pharmacological inhibitors or RNAi‐mediated subunit ablation, would be expected to specifically target plasma membrane localized V‐ATPase) and found that specific inhibition significantly decreased in vitro invasion.49 Other studies of breast cancer cells have also linked V‐ATPase subunit expression to invasion. For example, siRNA knockdown of Voa3 (but not a1, a2 or a4) significantly decreased invasion of MCF10CA1a cells. Interestingly, in this system, knockdown of Voa4 led to an increase in Voa3 expression and a combined knockdown of both Voa3 and a4 led to the greatest reduction in MCF10CA1a cell invasion.48
The precise mechanisms by which V‐ATPase promotes invasion/migration require further investigation, but appear multifactorial. V‐ATPase can increase the activity of extracellular proteases, such as cathepsins and matrix metalloproteinases, via both increased protease secretion and decreased extracellular pH which enhances activity of these enzymes.53, 54, 55 Once activated, these proteases increase extracellular matrix degradation and facilitate migration of cancer cells. For example, in ovarian cancer cells, Voa2 was found to be localized in endosomes and inhibition showed a decrease in MMP‐9 and ‐2 activity.37 A study in hepatocellular carcinoma (HCC) found that inhibiting the Voc subunit led to decreased MMP‐2 expression and extracellular MMP‐2 activity.56
Other potential mechanisms may involve modulation of intracellular organelles. Intracellular V‐ATPase may play a role in tumor cell invasion by either assisting in the activation of lysosomal proteases or by activating cytosolic proteins which aid in trafficking these proteases to the surface of the cell.57, 58 V‐ATPase localizes with vesicles containing the small GTPase Rab27B, which promotes secretion of proinvasive growth factors and is associated with poor prognosis in breast cancer. V‐ATPase inhibition alters distribution and size of Rab27B vesicles, and reduces collagen type I invasion, cell cycle, and invasive growth in the chorioallantoic membrane assay. Importantly, V‐ATPase inhibition reduces the Rab27B dependent extracellular secretion of Hsp90α, which is a molecular chaperone required for MMP‐2 activation.57
The role of V‐ATPase in tumor migration has also been linked to tumor cell stiffness. Recent evidence suggests that a loss of cell stiffness is correlated with an increase in invasion and proliferation.59 For example, in HCC V‐ATPase inhibition was shown to increase cell stiffness due to depletion of cholesterol in the plasma membrane and decreased Ras signaling.60 However, due to the complexity of the tumor microenvironment, the impact of V‐ATPase on tumor stiffness is not clear. In breast cancer, in vivo deletion of the ATP6V0A2 gene encoding Voa2 subunit caused a reduction in breast tissue stiffness due to defective extracellular matrix glycosylation and altered Golgi morphology, resulting in an inflammatory and metastatic phenotype. Furthermore, normal breast tissue from patients who had reported lymph node metastasis (LNM) was analyzed using IHC and was found to have significantly lower Voa2 expression than those without LNM, indicating that low Voa2 expression is associated with metastatic disease.61
V‐ATPases may also promote increased cancer cell migration via interaction with the cytoskeleton. In breast cancer 4T1 cells, following lentiviral depletion of V1C1 subunit expression, the actin cytoskeletons lost their regular orientation resulting in decreased migratory and invasive activity, suggesting that V1C1 is able to regulate actin cytoskeleton rearrangements.62 Moreover, V‐ATPase inhibition is associated with F‐actin reorganization in PC‐3 prostate cancer cells.63 In addition, inhibition of V‐ATPase using archazolid A or Voc subunit siRNA in SKBR3 breast cancer cells altered the distribution and localization of EGFR and Rac‐1, which are associated with cellular migration.64
In addition to effects on migration/invasion linked to extracellular acidification, it has also been proposed that H+‐extrusion by V‐ATPase can counter intracellular acidification associated with altered metabolism.1 Thus, cancer cell growth is often associated with a reprogramming of metabolism towards aerobic glycolysis. This is termed the Warburg effect and is associated with tumor hypoxia as well as activation of oncogenes, such as MYC and RAS, and inactivation of tumor suppressors, such as p53.65, 66, 67 This results in production of lactic acid and H+, and plasma membrane V‐ATPase may be key under these conditions for maintaining intracellular pH homeostasis.68 In addition, via its action as a coordinator of regulators of opposing anabolic versus catabolic pathways (eg, mediated via mTORC1 or AMPK, respectively).10 V‐ATPase may support “metabolic plasticity” allowing tumor cells to adapt to distinct and shifting tumor microenvironments (eg, nutrient/O2 replete versus limited).
In prostate cancer cells, V‐ATPase co‐localized with prostate specific antigen (PSA; a secreted tumor marker that is regulated by the androgen receptor [AR]) in the Golgi and V‐ATPase inhibition resulted in a relocalization of PSA to lysosome‐like intracellular vesicles.50 Interestingly, in androgen‐sensitive LNCaP prostate cancer cells, V‐ATPase inhibition also reduced PSA mRNA expression, suggesting upstream inhibition of AR's transcription activating activity.50
Finally, V‐ATPase expression in cancer cells may influence immune cells to promote tumorigenesis indirectly. For example, a soluble cleavage fragment of Voa2 (a2NTD) has been shown to polarize macrophages toward a “tumor‐associated” or M2 macrophage phenotype.40, 69
5. V‐ATPASE AS A POTENTIAL CANCER THERAPEUTIC TARGET
V‐ATPase function is intimately involved in the control of multiple normal cellular processes. However, genetic manipulation experiments suggest the potential to target V‐ATPase function in cancer cells, with relatively little effect in normal cells, both in vitro and in vivo. This suggests that cancer cells may become particularly dependent on V‐ATPase complex function, or of specific subunits, to support cancer‐associated hallmarks, including growth and survival.
In the breast cancer cell lines MDA‐MB‐231, MCF‐7, and MDA‐MB‐435, shRNA‐mediated knockdown of the V1C1 subunit significantly inhibited cell proliferation, whereas in the untransformed C3H10T1/2 cell line, there was no effect on proliferation.27 In addition, 4T1 cells with 90% V1C1 depletion had significantly less metastatic potential and reduced osteolytic lesions.27 Alternatively, siRNA knockdown of the ATP6L (Voc) subunit in HCCLM3 hepatocellular carcinoma cells resulted in significantly reduced invasion, decreased MMP‐2 expression, reduced average xenograft size, and a dramatic reduction in intrahepatic metastases.56 In support of this, siRNA‐mediated knockdown of the Voc subunit led to a reduction in both SK‐N‐MC and A‐673 Ewing sarcoma cell number.47
In addition to these genetic experiments, chemical inhibitors of V‐ATPase, plecomacrolide bafilomycin‐A1 (baf‐A1), concanamycin‐A (con‐A), benzolactone enamides, and archazolid, have been used to understand consequence of V‐ATPase inhibition in vitro and in vivo. Both baf‐A1 and con‐A bind to the Voc subunit responsible for proton translocation and are highly specific V‐ATPase inhibitors with IC50 values in the nanomolar range. Benzolactone enamides have been extracted from marine organisms such as the sponge Haliclona sp. and the tunicate Aplidium lobatum as well as the gram negative bacterium Psudomonas sp. and the myxobacterium Chondromyces sp. Archazolid is one of the novel V‐ATPase inhibitors originally isolated from Archangium gephyra, which competes with con‐A for binding to the Voc subunit and also has IC50 values in the low nanomolar range.70
V‐ATPase inhibition has been shown to reduce cancer cell growth and induce apoptosis in a number of cell lines across a range of cancer types. Importantly, chemical inhibition appears to show selectivity for cancer cells compared to normal cells. For example, baf‐A1 inhibition resulted in significant reductions in hepatoblastoma cell growth compared to normal human hepatocytes.71 One of the benzolactone enamides and a derivative of salicylihalamide was shown to have a significantly synergistic effect on the viability of NCI‐H1155 lung cancer cells when applied with paclitaxel increasing the sensitivity of the latter 1000‐fold.72, 73 Additionally, archazolid had a significantly increased toxic effect in SKBR3 breast carcinoma cells compared to nontumor MCF10A cells.74
The biological mechanisms in which V‐ATPase inhibition induces cancer cell death are diverse and complex. V‐ATPase inhibition has been shown to result in increased reactive oxygen species (ROS) in cancer cells75, 76, 77 and HIF1α upregulation.74 Furthermore, V‐ATPase inhibition induces caspase‐dependent apoptosis in invasive tumor cells via the mitochondrial pathways.74, 78 Archazolid was also shown to induce cell cycle arrest in MDA‐MB‐231 cells and double‐strand breaks in all cell lines investigated.79 This evidence indicates that V‐ATPase inhibition can induce a cellular stress response, autophagy, and eventually apoptosis in tumor cells. However, it should be noted that the relationship between V‐ATPase and autophagy is complex particularly as the V‐ATPase has a well‐established role in activation of lysosomal acid hydrolases that mediate proteolysis during autophagy.80, 81, 82 Moreover, although V‐ATPase inhibition can result in increased autophagic markers in some settings,74 V‐ATPase is required for activation of noncanonical autophagy.83 In a very recent study, the autophagy‐related protein ATG5 was demonstrated to displace ATP6V1E1 from V‐ATPase, causing it to accumulate in exosomes.84 The function of autophagy in cancer is also complex; both pro‐survival and pro‐death rolls have been described, dependent on cellular context.85
There is also evidence for the stimulation of signaling pathways as MAPKs such as ERK, p38, and JNK were upregulated in response inhibition which might potentially provide a stimulus to apoptosis.78 This is in agreement with findings in breast cancer as ERK activation was shown to be increased as a pro‐survival mechanism in response to baf‐A1. Interestingly, inhibition of ERK using sorafenib augmented bafilomycin mediated cell death in tumor cells under hypoxic conditions.86
Furthermore, iron chelators deferoxamine and 3‐AP, showed a synergistic cytotoxic effect when used in combination with archazolid. It was therefore suggested that V‐ATPase induced cytotoxicity may primarily be due to disturbed iron receptor recycling, impairing the iron metabolism of tumor cells.79 Consistent with this, the expression of genes responsive to a decrease in iron was greatly increased in response to V‐ATPase inhibition. Iron was also inversely related to the cytotoxic hypersensitivity of cancer cells and therefore may act as a key determinant of cancer cell sensitivity to V‐ATPase inhibition.87
In addition to these in vitro studies, chemical V‐ATPase inhibition has recently been shown to be effective using in vivo models. For example, in a 4T1‐Luc mouse breast cancer xenograft model, archazolid treatment inhibited lung metastasis at a concentration (1 mg/kg i.v.) which did not cause signs of obvious toxicity.64 Similar results were obtained using baf‐A1, in which treatment led to the reduction of average tumor volume by 50% in MCF‐7 and MDA‐MB‐231 xenograft mouse models. Again, no toxic side effects were observed using baf‐A1 (1 mg/kg). Additionally, when combined with sorafenib, it was shown that V‐ATPase treatment could result in tumor regression in MDA‐MD‐231 xenograft mice.86 Another study demonstrated that growth of a HepG2 orthotopic HCC xenograft model in nude mice was retarded by baf‐A1.42
Finally, the potential “repurposing” of protein pump inhibitors (PPI), which are widely used for the treatment of gastroesophageal reflux and gastric ulcers, has garnered considerable interest.88 Although deployed as H+/K+‐ATPase inhibitors, PPI such as omeprazole and esomeprazole, also inhibit V‐ATPase and can increase sensitivity to chemotherapeutics in vitro and in vivo.89, 90 However, it should be noted that the concentration of PPI's required to inhibit V‐ATPase is considerably higher than for H+/K+‐ATPase.91
6. V‐ATPASE AS A MEDIATOR OF RESISTANCE FOR CONVENTIONAL CANCER THERAPIES
In addition to a role in cancer cell growth, survival, migration, and invasion, V‐ATPase dysregulation is also linked to therapy resistance. This may be explained, in part, to studies linking drug resistance in cancer cells, to reversal of the normal pH gradient between the cytoplasm and extracellular environment.1 Mechanistically, in some cases, lowering of extracellular pH protonates drugs leading to impaired cellular entry and/or vesicular trapping. Indeed, it remains unclear whether correlations between V‐ATPase (subunit) dysregulation and poor clinical outcome (Table 1) reflect an impact of V‐ATPase on cancer cell behavior per se vs a response to treatment.
In breast cancer, V‐ATPase inhibition was able to induce apoptosis in the trastuzumab resistant JIMT‐1 cells, impair HER2 signaling, and decrease HER2 surface expression. Moreover, treatment with archazolid significantly reduced the proportion of strongly HER2 positive cells and tumor growth in a JIMT‐1 cell xenograft.92 Chemical V‐ATPase inhibition was also shown to overcome Bcl‐xL‐ and Bcl‐2 mediated resistance in Ms‐1 cells and induce apoptosis. Baf‐A1 was able to suppress the mitochondrial protective function of Bcl‐xL and allow the taxol to decrease MMP levels.93 V‐ATPase expression was found to be higher in cisplatin resistant cells than other drug‐resistant cell lines. It has been shown that baf‐A1 and cisplatin had a synergistic effect on cell cytotoxicity, which was higher in cisplatin resistant cells than cisplatin sensitive.94
Again, a potential role for V‐ATPase in drug resistance has been demonstrated using siRNA ablation of specific subunits. You et al used siRNA targeting the ATP6L (Voc) subunit in human drug resistant MCF‐7/ADR breast cancer cells. The knockdown cells were more sensitive to chemotherapeutic agents such as doxorubicin and 5‐FU than control MCF‐7/ADR cells. ATP6L knockdown was associated with an increase of lysosomal pH (ie alkalinization) and increase caspase‐3/7 activity and PARP expression.95
7. ROLE OF V‐ATPASE MODULATORY PROTEINS IN CANCER
In addition to the core V‐ATPase subunits (Figure 1), proteins which directly modulate V‐ATPase activity have been shown to contribute to cancer progression. LASS2/TMSG1 is a negative V‐ATPase regulatory protein which directly binds to the Voc subunit and was found to be inversely related to the metastatic potential of tumor cells. It was shown to be expressed at low levels in the highly metastatic prostate cancer cell line PC‐3M‐1E8 and highly expressed in the low metastatic PC‐3M‐2B4. Furthermore, MMP‐9 and MMP‐2 activity were found to be significantly increased in LASS2/TMSG1 shRNA PC‐3M‐2B4 cells. Moreover, mouse xenografts of PC‐3M‐2B4 cells transfected with LASS/TMSG1 shRNA exhibited significantly increased average tumor size and weight compared to controls. Those with LASS/TMSG1 shRNA also had more LNM, suggesting loss of LASS2/TMSG1 induced tumor cell growth, proliferation, invasion, and metastasis likely as a result of loss of control in V‐ATPase activity.96
Furthermore, these modulatory proteins also may have a role in cancer therapy resistance. It has been demonstrated that the expression of LASS2/TMSG1 was significantly lower in doxorubicin resistant MCF‐7/ADR breast cancer cells than sensitive MCF‐7 cells. LASS2/TMSG1‐positive tumors had a positive correlation with disease‐free and overall survival. It was shown that overexpression of LASS2/TMSG1 increased chemosensitivity to a number of chemotherapeutic agents in drug resistant MCF‐7/ADR cells. Overexpression of LASS2/TMSG1 inhibited pHi recovery and significantly decreased MCF‐7/ADR cell migration due to the suppression of V‐ATPase function via LASS2/TMSG1 binding to subunit Voc.26 Furthermore, the downregulation of a positive regulator of V‐ATPase activity, TM9SF4, significantly inhibited tumor cell invasiveness and increased the cytotoxic effect of 5‐FU in colon cancer cells. This group hypothesized that in malignant cancer cells, TM9SF4 binds to the V1H subunit, leading to stabilization of the V‐ATPase complex and permanent activation of the enzyme.25
8. CONCLUSION
There is emerging evidence to support a role for V‐ATPase in cancer biology through impact on processes including cancer cell invasion, metastasis, and proliferation. Evidence also supports a potential link between V‐ATPase and conventional cancer therapy and that V‐ATPase may represent a direct anti‐cancer target. Future work is still required to understand whether the expression of individual subunits represents a casual or causal relationship with cancer progression. It is also important to uncover whether there are other functional consequences of V‐ATPase dysregulation in cancer and if more specific agents can be developed with more acceptable toxicity profiles.
CONFLICT OF INTERESTS
None declared.
Whitton B, Okamoto H, Packham G, Crabb SJ. Vacuolar ATPase as a potential therapeutic target and mediator of treatment resistance in cancer. Cancer Med. 2018;7:3800–3811. 10.1002/cam4.1594
REFERENCES
- 1. Stransky L, Cotter K, Forgac M. The function of V‐ATPases in cancer. Physiol Rev. 2016;96:1071‐1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun‐Wada G‐H, Wada Y. Role of vacuolar‐type proton ATPase in signal transduction. Biochim Biophys Acta. 2015;1847:1166‐1172. [DOI] [PubMed] [Google Scholar]
- 3. Pamarthy S, Kulshrestha A, Katara GK, Beaman KD. The curious case of vacuolar ATPase: regulation of signaling pathways. Mol Cancer. 2018;17:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vaccari T, Duchi S, Cortese K, Tacchetti C, Bilder D. The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development. 2010;137:1825‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yan Y, Denef N, Schüpbach T. The vacuolar proton pump (V‐ATPase) is required for Notch signaling and endosomal trafficking in Drosophila. Dev Cell. 2009;17:387‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pamarthy S, Jaiswal MK, Kulshreshtha A, Katara GK, Gilman‐Sachs A, Beaman KD. The vacuolar ATPase a2‐subunit regulates Notch signaling in triple‐negative breast cancer cells. Oncotarget. 2015;6:34206‐34220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pamarthy S, Mao L, Katara GK, et al. The V‐ATPase a2 isoform controls mammary gland development through Notch and TGF‐β signaling. Cell Death Dis. 2016;7:e2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cruciat CM, Ohkawara B, Acebron SP, et al. Requirement of prorenin receptor and vacuolar H+‐ATPase‐mediated acidification for Wnt signaling. Science. 2010;327:459‐463. [DOI] [PubMed] [Google Scholar]
- 9. Zhang CS, Jiang B, Li M, et al. The lysosomal v‐ATPase‐Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab. 2014;20:526‐540. [DOI] [PubMed] [Google Scholar]
- 10. Zhang CS, Hawley SA, Zong Y, et al. Fructose‐1,6‐bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. 2017;548:112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin S‐C, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27:299‐313. [DOI] [PubMed] [Google Scholar]
- 12. Breton S, Brown D. New insights into the regulation of V‐ATPase‐dependent proton secretion. Am J Physiol Renal Physiol. 2007;292:F1‐F10. [DOI] [PubMed] [Google Scholar]
- 13. Al‐bataineh MM, Gong F, Marciszyn AL, Myerburg MM, Pastor‐Soler NM. Regulation of proximal tubule vacuolar H(+)‐ATPase by PKA and AMP‐activated protein kinase. Am J Physiol Renal Physiol. 2014;306:F981‐F995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alzamora R, Thali RF, Gong F, et al. PKA regulates vacuolar H+‐ATPase localization and activity via direct phosphorylation of the A subunit in kidney cells. J Biol Chem. 2010;285:24676‐24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hallows KR, Alzamora R, Li H, et al. AMP‐activated protein kinase inhibits alkaline pH‐ and PKA‐induced apical vacuolar H+‐ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol. 2009;296:C672‐C681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toei M, Saum R, Forgac M. Regulation and isoform function of the V‐ATPases. Biochemistry. 2010;49:4715‐4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toyomura T, Murata Y, Yamamoto A, et al. From lysosomes to the plasma membrane: localization of vacuolar type H+‐ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem. 2003;278:22023‐22030. [DOI] [PubMed] [Google Scholar]
- 18. Sautin YY, Lu M, Gaugler A, Zhang L, Gluck SL. Phosphatidylinositol 3‐Kinase‐mediated effects of glucose on vacuolar H+‐ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells. Mol Cell Biol. 2005;25:575‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu Y, Parmar A, Roux E, et al. Epidermal growth factor‐induced vacuolar (H+)‐ATPase assembly: a role in signaling via mTORC1 activation. J Biol Chem. 2012;287:26409‐26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stransky LA, Forgac M. Amino acid availability modulates vacuolar H+‐ATPase assembly. J Biol Chem. 2015;290:27360‐27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGuire CM, Forgac M. Glucose starvation increases V‐ATPase assembly and activity in mammalian cells through AMP kinase and phosphatidylinositide 3‐Kinase/Akt signaling. J Biol Chem. 2018; 10.1074/jbc.RA117.001327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu M, Ammar D, Ives H, Albrecht F, Gluck SL. Physical Interaction between Aldolase and vacuolar H+‐ATPase is essential for the assembly and activity of the proton pump. J Biol Chem. 2007;282:24495‐24503. [DOI] [PubMed] [Google Scholar]
- 23. Chan CY, Parra KJ. Yeast phosphofructokinase‐1 subunit Pfk2p is necessary for pH homeostasis and glucose‐dependent vacuolar ATPase reassembly. J Biol Chem. 2014;289:19448‐19457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jansen EJ, Martens GJ. Novel insights into V‐ATPase functioning: distinct roles for its accessory subunits ATP6AP1/Ac45 and ATP6AP2/(pro) renin receptor. Curr Protein Pept Sci. 2012;13:124‐133. [DOI] [PubMed] [Google Scholar]
- 25. Lozupone F, Borghi M, Marzoli F, et al. TM9SF4 is a novel V‐ATPase‐interacting protein that modulates tumor pH alterations associated with drug resistance and invasiveness of colon cancer cells. Oncogene. 2015;34:5163‐5174. [DOI] [PubMed] [Google Scholar]
- 26. Fan S, Niu Y, Tan N, et al. LASS2 enhances chemosensitivity of breast cancer by counteracting acidic tumor microenvironment through inhibiting activity of V‐ATPase proton pump. Oncogene. 2013;32:1682‐1690. [DOI] [PubMed] [Google Scholar]
- 27. McConnell M, Feng S, Chen W, et al. Osteoclast proton pump regulator Atp6v1c1 enhances breast cancer growth by activating the mTORC1 pathway and bone metastasis by increasing V‐ATPase activity. Oncotarget. 2017;8:47675‐47690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chueca E, Apostolova N, Esplugues JV, García‐González MA, Lanas Á, Piazuelo E. Proton pump inhibitors display antitumor effects in Barrett's adenocarcinoma cells. Front Pharmacol. 2016;7:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang L, Lu Q, Han Y, Li Z, Zhang Z, Li X. ABCG2/V‐ATPase was associated with the drug resistance and tumor metastasis of esophageal squamous cancer cells. Diagn Pathol. 2012;7:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Son SW, Kim S‐H, Moon E‐Y, Kim D‐H, Pyo S, Um SH. Prognostic significance and function of the vacuolar H(+)‐ATPase subunit V1E1 in esophageal squamous cell carcinoma. Oncotarget. 2016;7:49334‐49348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu P, Chen H, Han L, Zou X, Shen W. Expression and role of V1A subunit of V‐ATPases in gastric cancer cells. Int J Clin Oncol. 2015;20:725‐735. [DOI] [PubMed] [Google Scholar]
- 32. Ohta T, Numata M, Yagishita H, et al. Expression of 16 kDa proteolipid of vacuolar‐type H(+)‐ATPase in human pancreatic cancer. Br J Cancer. 1996;73:1511‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chung C, Mader CC, Schmitz JC, et al. The vacuolar‐ATPase modulates matrix metalloproteinase isoforms in human pancreatic cancer. Lab Invest. 2011;91:732‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu Q, Lu S, Huang L, et al. The expression of V‐ATPase is associated with drug resistance and pathology of non‐small‐cell lung cancer. Diagn Pathol. 2013;8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia‐Garcia A, Perez‐Sayans Garcia M, Rodriguez MJ, et al. Immunohistochemical localization of C1 subunit of V‐ATPase (ATPase C1) in oral squamous cell cancer and normal oral mucosa. Biotech Histochem. 2012;87:133‐139. [DOI] [PubMed] [Google Scholar]
- 36. Perez‐Sayans M, Reboiras‐Lopez MD, Somoza‐Martin JM, et al. Measurement of ATP6V1C1 expression in brush cytology samples as a diagnostic and prognostic marker in oral squamous cell carcinoma. Cancer Biol Ther. 2010;9:1057‐1064. [DOI] [PubMed] [Google Scholar]
- 37. Kulshrestha A, Katara GK, Ibrahim S, et al. Vacuolar ATPase ‘a2’ isoform exhibits distinct cell surface accumulation and modulates matrix metalloproteinase activity in ovarian cancer. Oncotarget. 2015;6:3797‐3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song T, Jeon HK, Hong JE, et al. Proton pump inhibition enhances the cytotoxicity of paclitaxel in cervical cancer. Cancer Res Treat. 2017;49:595‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cotter K, Liberman R, Sun‐Wada G, et al. The a3 isoform of subunit a of the vacuolar ATPase localizes to the plasma membrane of invasive breast tumor cells and is overexpressed in human breast cancer. Oncotarget. 2016;7:46142‐46157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katara GK, Jaiswal MK, Kulshrestha A, Kolli B, Gilman‐Sachs A, Beaman KD. Tumor‐associated vacuolar ATPase subunit promotes tumorigenic characteristics in macrophages. Oncogene. 2014;33:5649‐5654. [DOI] [PubMed] [Google Scholar]
- 41. Gleize V, Boisselier B, Marie Y, Poea‐Guyon S, Sanson M, Morel N. The renal v‐ATPase a4 subunit is expressed in specific subtypes of human gliomas. Glia. 2012;60:1004‐1012. [DOI] [PubMed] [Google Scholar]
- 42. Xu J, Xie R, Liu X, et al. Expression and functional role of vacuolar H(+)‐ATPase in human hepatocellular carcinoma. Carcinogenesis. 2012;33:2432‐2440. [DOI] [PubMed] [Google Scholar]
- 43. Green MR, Kihira S, Liu CL, et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc Natl Acad Sci USA. 2015;112:E1116‐E1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Okosun J, Wolfson RL, Wang J, et al. Recurrent mTORC1‐activating RRAGC mutations in follicular lymphoma. Nat Genet. 2016;48:183‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zoncu R, Bar‐Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside‐out mechanism that requires the vacuolar H(+)‐ATPase. Science. 2011;334:678‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Forbes SA, Beare D, Boutselakis H, et al. COSMIC: somatic cancer genetics at high‐resolution. Nucleic Acids Res. 2017;45:D777‐D783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Avnet S, Di Pompo G, Lemma S, et al. V‐ATPase is a candidate therapeutic target for Ewing sarcoma. Biochim Biophys Acta. 2013;1832:1105‐1116. [DOI] [PubMed] [Google Scholar]
- 48. Capecci J, Forgac M. The function of vacuolar ATPase (V‐ATPase) a subunit isoforms in invasiveness of MCF10a and MCF10CA1a human breast cancer cells. J Biol Chem. 2013;288:32731‐32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cotter K, Capecci J, Sennoune S, et al. Activity of plasma membrane V‐ATPases is critical for the invasion of MDA‐MB231 breast cancer cells. J Biol Chem. 2015;290:3680‐3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Michel V, Licon‐Munoz Y, Trujillo K, Bisoffi M, Parra KJ. Inhibitors of vacuolar ATPase proton pumps inhibit human prostate cancer cell invasion and prostate‐specific antigen expression and secretion. Int J Cancer. 2013;132:E1‐E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nishisho T, Hata K, Nakanishi M, et al. The a3 isoform vacuolar type H+‐ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res. 2011;9:845‐855. [DOI] [PubMed] [Google Scholar]
- 52. Hinton A, Sennoune SR, Bond S, et al. Function of a subunit isoforms of the V‐ATPase in pH homeostasis and in vitro invasion of MDA‐MB231 human breast cancer cells. J Biol Chem. 2009;284:16400‐16408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zou P, Yang Y, Xu X, et al. Silencing of vacuolar ATPase c subunit ATP6V0C inhibits the invasion of prostate cancer cells through a LASS2/TMSG1‐independent manner. Oncol Rep. 2018;39:298‐306. [DOI] [PubMed] [Google Scholar]
- 54. Uhlman A, Folkers K, Liston J, Pancholi H, Hinton A. Effects of vacuolar H(+)‐ATPase inhibition on activation of Cathepsin B and Cathepsin L secreted from MDA‐MB231 breast cancer cells. Cancer Microenviron. 2017;10:49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle. 2007;6:60‐64. [DOI] [PubMed] [Google Scholar]
- 56. Lu X, Qin W, Li J, et al. The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res. 2005;65:6843‐6849. [DOI] [PubMed] [Google Scholar]
- 57. Hendrix A, Sormunen R, Westbroek W, et al. Vacuolar H+ ATPase expression and activity is required for Rab27B‐dependent invasive growth and metastasis of breast cancer. Int J Cancer. 2013;133:843‐854. [DOI] [PubMed] [Google Scholar]
- 58. Kubisch R, Fröhlich T, Arnold GJ, et al. V‐ATPase inhibition by archazolid leads to lysosomal dysfunction resulting in impaired cathepsin B activation in vivo. Int J Cancer. 2014;134:2478‐2488. [DOI] [PubMed] [Google Scholar]
- 59. Lin H‐H, Lin H‐K, Lin IH, et al. Mechanical phenotype of cancer cells: cell softening and loss of stiffness sensing. Oncotarget. 2015;6:20946‐20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bartel K, Winzi M, Ulrich M, et al. V‐ATPase inhibition increases cancer cell stiffness and blocks membrane related Ras signaling ‐ a new option for HCC therapy. Oncotarget. 2017;8:9476‐9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Katara GK, Kulshrestha A, Mao L, et al. Mammary epithelium‐specific inactivation of V‐ATPase reduces stiffness of extracellular matrix and enhances metastasis of breast cancer. Mol Oncol. 2018;12:208‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Feng S, Cai M, Liu P, et al. Atp6v1c1 may regulate filament actin arrangement in breast cancer cells. PLoS ONE. 2014;9:e84833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Licon‐Munoz Y, Michel V, Fordyce CA, Parra KJ. F‐actin reorganization by V‐ATPase inhibition in prostate cancer. Biol Open. 2017;6:1734‐1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wiedmann RM, von Schwarzenberg K, Palamidessi A, et al. The V‐ATPase‐inhibitor archazolid abrogates tumor metastasis via inhibition of endocytic activation of the Rho‐GTPase Rac1. Cancer Res. 2012;72:5976‐5987. [DOI] [PubMed] [Google Scholar]
- 65. Tarrado‐Castellarnau M, de Atauri P, Cascante M. Oncogenic regulation of tumor metabolic reprogramming. Oncotarget. 2016;7:62726‐62753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85. [DOI] [PubMed] [Google Scholar]
- 67. Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fogarty FM, O'Keeffe J, Zhadanov A, Papkovsky D, Ayllon V, O'Connor R. HRG‐1 enhances cancer cell invasive potential and couples glucose metabolism to cytosolic/extracellular pH gradient regulation by the vacuolar‐H+ ATPase. Oncogene. 2013;33:4653. [DOI] [PubMed] [Google Scholar]
- 69. Katara GK, Kulshrestha A, Jaiswal MK, Pamarthy S, Gilman‐Sachs A, Beaman KD. Inhibition of vacuolar ATPase subunit in tumor cells delays tumor growth by decreasing the essential macrophage population in the tumor microenvironment. Oncogene. 2015;35:1058. [DOI] [PubMed] [Google Scholar]
- 70. Huss M, Wieczorek H. Inhibitors of V‐ATPases: old and new players. J Exp Biol. 2009;212:341‐346. [DOI] [PubMed] [Google Scholar]
- 71. Morimura T, Fujita K, Akita M, Nagashima M, Satomi A. The proton pump inhibitor inhibits cell growth and induces apoptosis in human hepatoblastoma. Pediatr Surg Int. 2008;24:1087‐1094. [DOI] [PubMed] [Google Scholar]
- 72. Lebreton S, Jaunbergs J, Roth MG, Ferguson DA, De Brabander JK. Evaluating the potential of vacuolar ATPase inhibitors as anticancer agents and multigram synthesis of the potent salicylihalamide analog saliphenylhalamide. Bioorg Med Chem Lett. 2008;18:5879‐5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Whitehurst AW, Bodemann BO, Cardenas J, et al. Synthetic lethal screen identification of chemosensitizer loci in cancer cells. Nature. 2007;446:815‐819. [DOI] [PubMed] [Google Scholar]
- 74. von Schwarzenberg K, Wiedmann RM, Oak P, et al. Mode of cell death induction by pharmacological vacuolar H(+)‐ATPase (V‐ATPase) inhibition. J Biol Chem. 2013;288:1385‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hong J, Nakano Y, Yokomakura A, et al. Nitric oxide production by the vacuolar‐type (H+)‐ATPase inhibitors Bafilomycin A1 and Concanamycin A and its possible role in apoptosis in RAW 264.7 cells. J Pharmacol Exp Ther. 2006;319:672‐681. [DOI] [PubMed] [Google Scholar]
- 76. Schempp CM, von Schwarzenberg K, Schreiner L, et al. V‐ATPase inhibition regulates Anoikis resistance and metastasis of cancer cells. Mol Cancer Ther. 2014;13:926‐937. [DOI] [PubMed] [Google Scholar]
- 77. McHenry P, Wang WLW, Devitt E, et al. Iejimalides A and B inhibit lysosomal vacuolar H+‐ATPase (V‐ATPase) activity and induce S‐phase arrest and apoptosis in MCF‐7 cells. J Cell Biochem. 2010;109:634‐642. [DOI] [PubMed] [Google Scholar]
- 78. Wu YC, Wu WK, Li Y, et al. Inhibition of macroautophagy by bafilomycin A1 lowers proliferation and induces apoptosis in colon cancer cells. Biochem Biophys Res Commun. 2009;382:451‐456. [DOI] [PubMed] [Google Scholar]
- 79. Schneider LS, von Schwarzenberg K, Lehr T, et al. Vacuolar‐ATPase inhibition blocks iron metabolism to mediate therapeutic effects in breast cancer. Cancer Res. 2015;75:2863‐2874. [DOI] [PubMed] [Google Scholar]
- 80. McGuire C, Cotter K, Stransky L, Forgac M. Regulation of V‐ATPase assembly and function of V‐ATPases in tumor cell invasiveness. Biochim Biophys Acta. 2016;1857:1213‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mauvezin C, Nagy P, Juhász G, Neufeld TP. Autophagosome–lysosome fusion is independent of V‐ATPase‐mediated acidification. Nat Commun. 2015;6:7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mijaljica D, Prescott M, Devenish RJ. V‐ATPase engagement in autophagic processes. Autophagy. 2011;7:666‐668. [DOI] [PubMed] [Google Scholar]
- 83. Gao Y, Liu Y, Hong L, et al. Golgi‐associated LC3 lipidation requires V‐ATPase in noncanonical autophagy. Cell Death Dis. 2016;7:e2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guo H, Chitiprolu M, Roncevic L, et al. Atg5 disassociates the V1V0‐ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev Cell. 2017;43:716‐730.e7. [DOI] [PubMed] [Google Scholar]
- 85. Santana‐Codina N, Mancias JD, Kimmelman AC. The role of autophagy in cancer. Annu Rev Cancer Biol. 2017;1:19‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Graham RM, Thompson JW, Webster KA. Inhibition of the vacuolar ATPase induces Bnip3‐dependent death of cancer cells and a reduction in tumor burden and metastasis. Oncotarget. 2014;5:1162‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Straud S, Zubovych I, De Brabander JK, Roth MG. Inhibition of iron uptake is responsible for differential sensitivity to V‐ATPase inhibitors in several cancer cell lines. PLoS ONE. 2010;5:e11629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ikemura K, Hiramatsu S, Okuda M. Drug repositioning of proton pump inhibitors for enhanced efficacy and safety of cancer chemotherapy. Front Pharmacol. 2017;8:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Luciani F, Spada M, De Milito A, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96:1702‐1713. [DOI] [PubMed] [Google Scholar]
- 90. De Milito A, Canese R, Marino ML, et al. pH‐dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer. 2010;127:207‐219. [DOI] [PubMed] [Google Scholar]
- 91. Sabolic I, Brown D, Verbavatz JM, Kleinman J. H(+)‐ATPases of renal cortical and medullary endosomes are differentially sensitive to Sch‐28080 and omeprazole. Am J Physiol Renal Physiol. 1994;266:F868‐F877. [DOI] [PubMed] [Google Scholar]
- 92. von Schwarzenberg K, Lajtos T, Simon L, Müller R, Vereb G, Vollmar AM. V‐ATPase inhibition overcomes trastuzumab resistance in breast cancer. Mol Oncol. 2014;8:9‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sasazawa Y, Futamura Y, Tashiro E, Imoto M. Vacuolar H+‐ATPase inhibitors overcome Bcl‐xL‐mediated chemoresistance through restoration of a caspase‐independent apoptotic pathway. Cancer Sci. 2009;100:1460‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Murakami T, Shibuya I, Ise T, et al. Elevated expression of vacuolar proton pump genes and cellular PH in cisplatin resistance. Int J Cancer. 2001;93:869‐874. [DOI] [PubMed] [Google Scholar]
- 95. You H, Jin J, Shu H, et al. Small interfering RNA targeting the subunit ATP6L of proton pump V‐ATPase overcomes chemoresistance of breast cancer cells. Cancer Lett. 2009;280:110‐119. [DOI] [PubMed] [Google Scholar]
- 96. Xu X, Liu B, Zou P, Zhang Y, You J, Pei F. Silencing of LASS2/TMSG1 enhances invasion and metastasis capacity of prostate cancer cell. J Cell Biochem. 2014;115:731‐743. [DOI] [PubMed] [Google Scholar]


