Abstract
Objective
To preliminarily explore the effect of electroacupuncture (EA) on bladder and bowel dysfunction in patients with transverse myelitis.
Methods
Sixteen participants were treated with EA at bilateral BL32, BL33, and BL35 once a day, five times a week for the first 4 weeks, and once every other day, three times a week for the following 4 weeks. Patients were then followed up for 6 months. Bladder and bowel function, and the safety of EA, were assessed.
Results
After 8 weeks of treatment, five (5/16, 31%) patients resumed normal voiding, three (6/16, 38%) regained partially normal voiding, and five (5/16, 31%) had no change. After treatment, the residual urine volume decreased by 100 mL (IQR 53–393 mL; P<0.05) in nine patients with bladder voiding dysfunction; in 11 patients with urinary incontinence, the number of weekly urinary incontinence episodes, 24-hour urinary episodes, and nocturia episodes per night diminished by 14 (95% CI 5 to 22), 5 (95% CI 1 to 9), and 4 (95% CI 0 to 7) episodes, respectively (all P<0.05). After 8 weeks of treatment in eight patients with faecal retention, four (4/8, 50%) resumed normal bowel movements, three (3/8, 38%) regained partially normal bowel movements, and one (1/8, 13%) had no change.
Conclusions
EA might be a promising alternative for the management of bladder and bowel dysfunction in patients with transverse myelitis. Randomised controlled trials are needed to confirm the effectiveness and safety of EA for this condition.
Keywords: electroacupuncture, neurology, urology, spine
Introduction
Transverse myelitis (TM) is an inflammatory disorder of the spinal cord, which may be of acute or subacute onset.1 It is a rare condition with an incidence of around one to four new cases per million people per year.2 Patients with TM may experience symptoms including sensory alteration, weakness, paresis, temperature dysregulation and autonomic impairment including bowel and bladder dysfunction below the level of the lesion.3 As the disease progresses, nearly 50% of patients with TM are completely paraplegic, 80–94% have numbness, paraesthesia or band-like dysaesthesia, and virtually all patients have some degree of bladder or bowel dysfunction.2 4 Bladder and bowel dysfunction have been defined as storage or evacuation disorders of urine and stool, respectively, and have variable autonomic manifestations such as urinary urgency, urinary or faecal incontinence, difficulty with voiding or inability to void, incomplete evacuation or complete constipation.5–7 Bladder and bowel dysfunction are common complaints among patients, and are considered an ongoing psychosocial disability, which may have an influence on a patient’s ability to work and his or her quality of life.5 8 Suggested treatments for bladder dysfunction involve pharmacotherapy, pelvic floor training and biofeedback, self-catheterisation and indwelling catheters, sacral neuromodulation, and surgical procedures (such as bladder augmentation, urinary diversion or bladder neck reconstruction).8–10 Methods of management for bowel dysfunction include non-pharmacological therapies (high-fibre diet, abdominal massage, rectal stimulation), pharmacotherapy (suppositories, enemas, laxatives) and surgical interventions (implantation of a sacral nerve stimulator).11 12 However, side effects associated with these therapies, such as urological complications, additional pain from catheterisation, or gerontic cognitive disorder resulting from anticholinergics, cannot be disregarded.13 14 Therefore, an effective, safe and convenient intervention that is likely to be accepted by patients wanting a better quality of life is desirable.8 Systematic reviews and clinical studies have indicated that electroacupuncture (EA) may provide a promising treatment for bladder and bowel dysfunction, with minimal side effects.15–22 After searching Pubmed and China National Knowledge Infrastructure (CNKI), we found that there were no published clinical studies focusing on acupuncture for bladder and bowel dysfunction caused by TM. Therefore, the aim of this preliminary study was to explore the effect of EA on bladder and bowel dysfunction in patients with TM.
Methods
Study design
We conducted a prospective consecutive observational study at the Acupuncture Department of Guang’anmen Hospital affiliated to the China Academy of Chinese Medical Sciences. The project was retrospectively approved by the local ethics review committee on 31 May 2017 (reference no. 2017–050-KY). The duration of the study comprised 8 weeks of treatment and 6 months of follow-up per participant. A senior acupuncturist with more than 20 years’ clinical experience in our department performed the acupuncture procedures. Participant screening was implemented by specialists in our department with a background in neurology. Therapeutic outcome assessment and statistical analyses were conducted by a research assistant and statistician who were both blinded to the treatment procedure.
Participants
In order to be included, participants had to fulfil all the following criteria: (1) have acute or subacute-onset complete or incomplete transverse spinal cord injury; (2) have simultaneous bladder and bowel dysfunction or isolated bladder dysfunction caused by TM that was in the stable stage—that is, after the non-specific inflammatory reaction and tissue oedema was effectively controlled and no longer progressive; (3) have received at least two sessions of acupuncture treatment and had baseline data recorded; (4) have volunteered to participate and provided signed informed consent. Participants with any of the following were omitted: (1) spinal cord compression; (2) severe cardiovascular, hepatic or renal disease; (3) craniocerebral or peripheral nerve injury; (4) cognitive dysfunction, mental disorder or illness that would affect cooperation; (5) regular anticoagulant use or coagulation disorder (antiplatelet treatment using aspirin or clopidogrel was preclusive); or (6) cardiac pacemaker implantation. The study was performed according to the common guidelines for clinical trials in line with the Declaration of Helsinki, and the study protocol was in accordance with the ethical standards of our institution.
Acupuncture protocol
Acupuncture point selection and needle manipulation was based on our prior clinical experience and anatomical relationships according to the pelvic innervation. Bilateral BL32 (Ciliao), BL33 (Zhongliao) and BL35 (Huiyang) were localised according to the WHO’s standard definitions.23 Stainless steel needles (0.45 mm diameter × 100–125 mm length, Hwato, Suzhou Medical Appliance, Suzhou, Jiangsu, China) were inserted inwardly and downwardly at bilateral BL32 and BL33 (overlying the S2 and S3 foramina) at an angle of 45–60° to a depth of 70–90 mm, and shorter and finer needles of the same brand (0.30 mm diameter × 75 mm length, Hwato) were inserted at bilateral BL35 upwardly and laterally to a depth of 50 mm. Paired alligator clips from a GB6805-2 EA apparatus (Medical Supply & Equipment Co, Ltd, Shanghai, China) were attached transversely to the needle holders at bilateral BL32, BL33 and BL35 and a continuous wave with 20 Hz frequency, pulse width 0.5 ms and current intensity 3–10 mA (depending on the individual participant’s tolerance) was applied for 30 min. All participants received EA treatment once a day, five times a week for the first 4 weeks, and once every other day, three times a week for the following 4 weeks. Participants were then followed up for 6 months.
Outcome measurements
Evaluation of bladder function
Normal voiding is achieved by a voluntarily-initiated continuous detrusor contraction that leads to complete bladder emptying within a normal time span, and in the absence of obstruction.24 The proportion of patients with normal voiding, partially normal voiding (able to void without assisted measures, such as intermittent catheterisation or pressing on the bladder, ≥50% of the time) and mostly abnormal voiding (requiring assisted measures >50% of the time) were assessed at baseline, after 8 weeks of treatment, and after a 6-month follow-up period. Only those patients with partially normal voiding underwent voiding cystourethrography to test if there was any reflux after 8 weeks of treatment. For patients with urinary incontinence, the number of weekly urinary incontinence episodes, 24-hour urinary episodes and number of nocturia episodes per night were evaluated at baseline, after the 8 weeks of treatment, and at the 6-month follow-up time point. For patients with voiding dysfunction, the mean post-void residual urine volume (RUV, measured by pelvic ultrasound) was appraised at the same time.
Evaluation of bowel function
The percentage of participants with normal bowel movements (spontaneous defecation without any help from supplementary methods), partially normal bowel movements (spontaneous bowel movements more than half of the time with only occasional use of methods of assistance to aid defecation during the past 48 hours), or complete constipation (requiring methods of assistance for defecation >50% of the time) were assessed at baseline, after the 8 weeks of treatment and after the 6-month follow-up period.
Safety
Acupuncture-related adverse events were documented in detail during the whole study by the acupuncturists. Anticipated adverse events caused by EA were considered to include unbearable pain, haematoma around the site of needling, fainting, nausea or palpitations during the needle manipulation. The time of occurrence, duration, treatment measures required, and time until relief of any adverse events were recorded. Any severe adverse events were required to be reported to the principle investigator and the institutional ethics committee within 24 hours of their occurrence.
Statistical analysis
Normally distributed data were expressed as mean±SD, while skewed data were presented as median (IQR). To analyse the number of weekly urinary incontinence episodes, 24-hour urinary episodes and nocturia episodes, a mixed-effect model with repeated measures (MMRM) was used. The model included baseline, treatment and follow-up as response variables, with time as a fixed-effect factor. The model did not impute missing data points. An unstructured correlation matrix was used to model the within-patient errors. Parameters were estimated using the maximum likelihood method with a Newton-Raphson algorithm. The data generated by the mixed model were presented as least square means with 95% CI. To assess the change from baseline in RUV after 8 weeks of treatment, the Wilcoxon signed rank test was used. We used two-sided tests with a significance level of P<0.05 for all analyses, which were performed using SAS version 9.4 software (SAS Institute, NC, USA).
Results
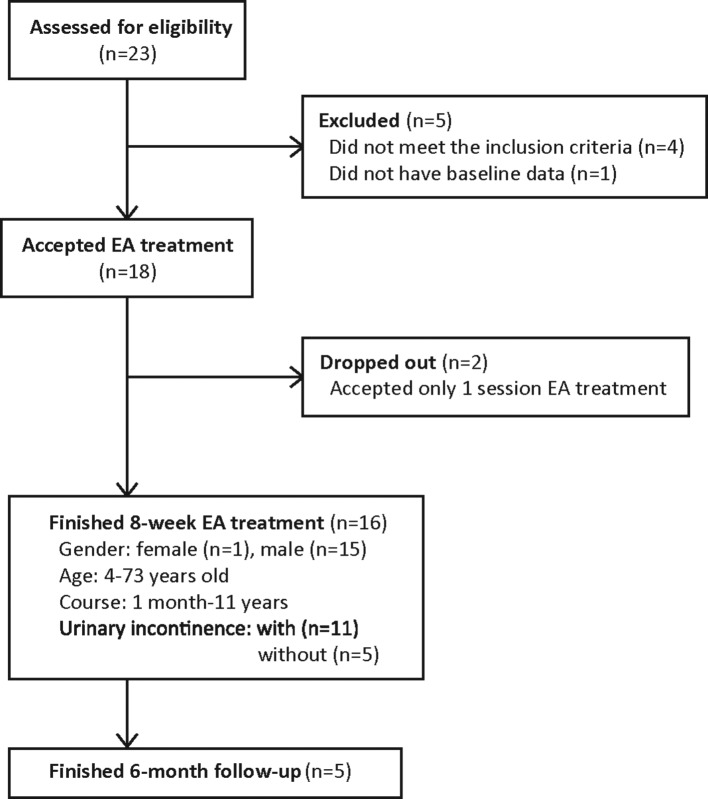
From 1 August 2008 to 31 March 2015, 23 participants were screened (figure 1). Five participants were excluded, leaving 18 participants. Two of these accepted only one session of EA treatment and dropped out; the remaining 16 successfully completed the EA treatment. These participants were 32.6±12.63 years of age with an illness course of 3.5 (2.0–8.5) months. All 16 patients had bladder dysfunction; eight of these had bladder dysfunction together with faecal retention, and one had bladder dysfunction together with faecal incontinence.
Figure 1.
Flow chart of participants. EA, electroacupuncture.
Assessment of bladder function
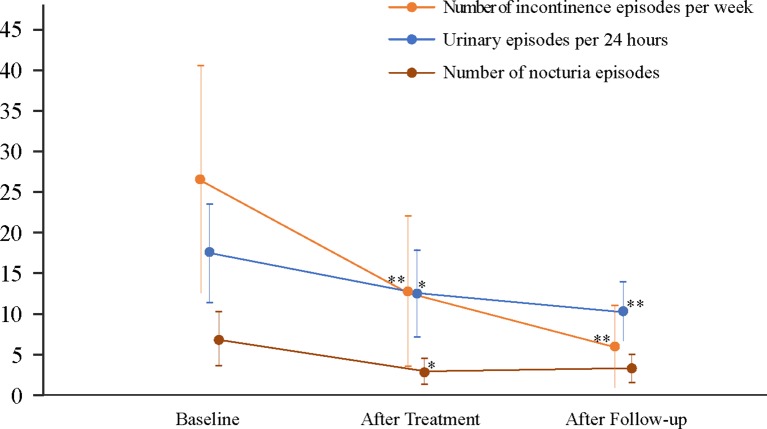
At baseline, all 16 patients had abnormal voiding and required assisted measures >50% of the time to empty the bladder. After the 8-week treatment period, five patients (5/16, 31%) resumed normal voiding, six (6/16, 38%) achieved partially normal voiding assisted by pressing the abdomen to empty the bladder instead of catheterisation with no reflux (tested by voiding cystourethrography), and five (5/16, 31%) had no change. Of the 16 patients with bladder dysfunction, nine provided post-void RUV data at baseline, which ranged from 33–600 mL, with three normal RUV (33% (3/9) ranging from 33–80 mL) and six abnormal RUV (67% (6/9) ranging from 150–600 mL). After the 8 weeks of treatment, the RUV data were all normal (100%, 9/9), and ranged from 12–100 mL (the range of the three normal RUV at baseline decreased to 12–20 mL after treatment, and the range of the six abnormal RUV at baseline declined to 25–100 mL after treatment). Compared with baseline measurements, the median post-void RUV of these nine patients reduced by 100 (IQR 53–393) mL (P<0.05) (table 1). No RUV data were recorded for these nine patients after the 6-month follow-up. With respect to weekly urinary incontinence episodes, 24 hour urinary episodes and nocturia episodes, statistically significant differences were evident between the three points of assessment (figure 2). After 8 weeks of treatment for the 11 patients with urinary incontinence, the number of weekly urinary incontinence episodes, 24 hour urinary episodes and nocturia episodes per night decreased by 14 (95% CI 5 to 22), 5 (95% CI 1 to 9), and 4 (95% CI 0 to 7) episodes, respectively (all P<0.05). After 6 months of follow-up, the number of weekly incontinence episodes and 24-hour urinary episodes declined by 20 (95% CI 10 to 31) and 7 (95% CI 4 to 11) (both P<0.05), while there was no statistically significant reduction in nocturia episodes (4, 95% CI 0 to 7).
Table 1.
Outcome for bladder function assessment
| Outcomes | Baseline (n=11) |
After treatment (n=11) |
After follow-up (n=5) |
P value (time)* | Baseline versus after treatment | Baseline versus after follow-up | ||
| Change | P value | Change | P value | |||||
| Weekly urinary incontinence episodes | 26 (12 to 40) | 13 (4 to 22) | 6 (1 to 11) | 0.005 | 14 (5 to 22) | 0.007 | 20 (10 to 31) | 0.002 |
| 24-hour urinary episodes | 17 (11 to 23) | 12 (7 to 18) | 10 (7 to 14) | 0.004 | 5 (1 to 9) | 0.015 | 7 (4 to 11) | 0.001 |
| Nocturia episodes | 7 (3 to 10) | 3 (1 to 4) | 3 (1 to 5) | 0.015 | 4 (0 to 7) | 0.029 | 4 (0 7) | 0.068 |
| RUV† | 200 (69–420) | 30 (18–52) | – | 100 (53–393) | 0.008 | – | – | |
*P (time) signified the overall difference between each assessment point (baseline, treatment, follow-up).
†For RUV, n=9 at baseline and after 8 weeks of treatment (no data were recorded after follow-up).
Mixed model repeated measures (MMRM) were used to evaluate weekly urinary incontinence episodes, 24-hour urinary episodes and nocturia episodes. Data are presented as least square means (95% CI).
RUV: residual urine volume. Wilcoxon signed ranks test was used to assess RUV; data are presented as median (IQR).
Figure 2.
Weekly incontinence episodes, 24-hour urinary episodes and nocturia episodes before and after 8 weeks electroacupuncture treatment. Data were analysed by mixed model repeated measures (MMRM) and presented as least square means (95% CI). **P<0.01, *P<0.05 compared with baseline.
Assessment of bowel function
At baseline, eight patients had faecal retention (complete constipation) requiring supplementary methods to aid defecation, and one patient had faecal incontinence. After 8 weeks of treatment, of the eight patients with faecal retention, four (50%) resumed normal bowel movements, three (38%) regained partially normal bowel movements with reduced dependence on supplementary methods, and one (13%) had no change. Of the three patients who regained partially normal bowel movements, two exhibited return of sensation of defecation, and one had a return of the anal reflex. The patient who had faecal incontinence at baseline achieved self-controlled ability to defecate after 8 weeks of treatment. Among the patients who resumed normal or partially normal bowel movements, these effects were sustained after 6 months.
Adverse effects
No adverse effects were reported throughout the study except a transient pricking sensation, which was considered consistent with the normal discomfort associated with acupuncture and was well tolerated.
Discussion
Bladder and bowel dysfunction are distressing symptoms that have a significant influence on a patient’s life and work.25 Moreover, bladder dysfunction might be the only sequela of TM,26 which can be troublesome to patients for a long period of time. Our study indicated that EA might be safe and helpful for the recovery of bladder and bowel function. EA may aid the return of patients’ self-voiding and spontaneous bowel movements, with 11 of 16 patients in the present series (69%) regaining normal or partially normal voiding and seven of eight (88%) achieving normal or partially normal bowel movements, together with a reduction in the need for use of supplementary methods throughout the whole study period. Our study suggests that 8 weeks of EA treatment might reduce mean post-void RUV, decrease weekly urinary incontinence episodes, reduce 24-hour urinary episodes, and lower the number of nocturia episodes per night.
An uninhibited bladder is one of the characteristics of bladder dysfunction resulting from TM. Some studies have found that EA or acupuncture could relieve urinary storage disorders.27–29 In our study, the weekly urinary incontinence episodes, 24-hour urinary episodes and nocturia episodes were all decreased after treatment and follow-up. In some randomised controlled trials using medication for bladder dysfunction, the decrease in urinary incontinence per week was about 14.96–16.7,30 31 the change in 24-hour urinary episodes was around 1.8–2.6,31 32 and the decrease in nocturia episodes per night was about 1 (0 to 2).33 Voiding disorder is another characteristic of bladder dysfunction caused by TM. Our previous observational studies on patients with cauda equina injury34 and with traumatic spinal cord injury18 indicated that EA could reduce the postvoid RUV by 303.6±148.8 mL and 190.29±101.87 mL, respectively. The present study also demonstrated a 100 (53–393) mL decrease in RUV after EA treatment. EA may therefore be suitable for patients with urinary retention and incontinence caused by TM, as these two symptoms are commonly associated in patients with TM. Other therapies such as sacral neuromodulation (SNM), that applies electrical stimulation in the pelvic region, and surgical reinnervation with nerve anastomosis, were also effective for bowel and bladder dysfunction.35 Nonetheless, these complicated surgical procedures and their related side effects, as well as the associated costs, may well be unacceptable to some patients.36
The possible therapeutic mechanism underlying the effect of EA at BL32 and BL33 on bowel and bladder dysfunction is likely to involve stimulation of the S2–3 nerve roots, which innervate the urinary bladder, urethra and pelvic floor area. These sacral nerve roots are also the parasympathetic centre of the spinal cord; thus, EA stimulation at S2–S3 may induce stabilisation of the pelvic floor through afferent/efferent neuromodulation37 and help to recover bladder and bowel function.38 39 A previous study reported that stimulation of the sacral nerves can inhibit abnormal micturition through capsaicin-sensitive afferent C-fibres, which further improves bladder function.40 There is also evidence that stimulation of sacral nerves can decrease rectal contractions, enhance anal pressure slow-wave activity, and reduce the number of spontaneous anal relaxation episodes, all of which may help improve bowel function.39
Limitations
Only nine of the 16 participants provided post-void RUV, and no RUV were recorded after the 6-month follow-up, therefore the apparent effect of EA might be not reliable. Moreover, quantitative measures of bowel function (such as bowel movements, spontaneous bowel movements, and faecal incontinence episodes) were lacking, and should be recorded and assessed in future studies. Our study is a non-controlled prospective observational study; therefore, we are unable to distinguish between the natural history of TM, the trends of self-recovery, placebo effects and specific versus non-specific effects of acupuncture without an appropriate control group. Bladder and bowel function may recover spontaneously in patients with TM, which might confound the effect of EA. Therefore, further studies should include patients with a disease course of >1 year. Finally, although we reported acupuncture-related adverse events in this study, the number of participants was insufficient to be able to robustly assess the safety of EA.
Conclusion
EA might represent an effective alternative method for managing bladder and bowel dysfunction in patients with TM. Randomised controlled trials are needed to confirm the effectiveness and safety of EA for this condition.
Acknowledgments
We would like to thank Yan Liu (Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China 100053) for guidance with statistical analysis.
Footnotes
JW and YC contributed equally.
Contributors: ZL conceived the study and took overall control. XL conducted the study and acquired the data. JW and ZQ interpreted and analysed the data. JW and YC drafted the manuscript. ZL, JW and ZQ revised the article. All authors approved the final version of the manuscript accepted for publication.
Funding: This study was supported and funded by the National Natural Science Foundation of China (ref. 81373732).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: IRB of Guang’anmen Hospital, China Academy of Chinese Medical Sciences.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Borchers AT, Gershwin ME. Transverse myelitis. Autoimmun Rev 2012;11:231–48. 10.1016/j.autrev.2011.05.018 [DOI] [PubMed] [Google Scholar]
- 2. Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002;59:499–505. [DOI] [PubMed] [Google Scholar]
- 3. West TW. Transverse myelitis--a review of the presentation, diagnosis, and initial management. Discov Med 2013;16:167–77. [PubMed] [Google Scholar]
- 4. Krishnan C, Kaplin AI, Pardo CA, et al. Demyelinating disorders: update on transverse myelitis. Curr Neurol Neurosci Rep 2006;6:236–43. 10.1007/s11910-006-0011-1 [DOI] [PubMed] [Google Scholar]
- 5. Wiesel PH, Norton C, Glickman S, et al. Pathophysiology and management of bowel dysfunction in multiple sclerosis. Eur J Gastroenterol Hepatol 2001;13:441–8. 10.1097/00042737-200104000-00025 [DOI] [PubMed] [Google Scholar]
- 6. Sakakibara R, Hattori T, Yasuda K, et al. Micturition disturbance in acute transverse myelitis. Spinal Cord 1996;34:481–5. 10.1038/sc.1996.82 [DOI] [PubMed] [Google Scholar]
- 7. Beh SC, Greenberg BM, Frohman T, et al. Transverse myelitis. Neurol Clin 2013;31:79–138. 10.1016/j.ncl.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tawee WA, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Uro 2015;7:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sá MJ. Acute transverse myelitis: a practical reappraisal. Autoimmun Rev 2009;9:128–31. 10.1016/j.autrev.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 10. Engeler DS, Meyer D, Abt D, et al. Sacral neuromodulation for the treatment of neurogenic lower urinary tract dysfunction caused by multiple sclerosis: a single-centre prospective series. BMC Urol 2015;15:105 10.1186/s12894-015-0102-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hughes M. Bowel management in spinal cord injury patients. Clin Colon Rectal Surg 2014;27:113–5. 10.1055/s-0034-1383904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krassioukov A, Eng JJ, Claxton G, et al. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord 2010;48:718–33. 10.1038/sc.2010.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Casey JT, Erickson BA, Navai N, et al. Urethral reconstruction in patients with neurogenic bladder dysfunction. J Urol 2008;180:197–200. 10.1016/j.juro.2008.03.056 [DOI] [PubMed] [Google Scholar]
- 14. Weston AL, Weinstein AM, Barton C, et al. Potentially inappropriate medication use in older adults with mild cognitive impairment. J Gerontol A Biol Sci Med Sci 2010;65:318–21. 10.1093/gerona/glp158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Zhishun L, Peng W, et al. Acupuncture for stress urinary incontinence in adults. Cochrane Database Syst Rev 2013;1:CD009408 10.1002/14651858.CD009408.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang T, Chon TY, Liu B, et al. Efficacy of acupuncture for chronic constipation: a systematic review. Am J Chin Med 2013;41:717–42. 10.1142/S0192415X13500493 [DOI] [PubMed] [Google Scholar]
- 17. Xia LP, Fan F, Tang AL, et al. Effects of electroacupuncture combined with bladder training on the bladder function of patients with neurogenic bladder after spinal cord injury. Int J Clin Exp Med 2014;7:1344–8. [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Z, Wang W, Wu J, et al. Electroacupuncture improves bladder and bowel function in patients with traumatic spinal cord injury: results from a prospective observational study. Evid Based Complement Alternat Med 2013. doi: 10.1155/2013/543174 [Epub ahead of print 7 Dec 2013]. 10.1155/2013/543174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solberg M, Alræk T, Mdala I, et al. A pilot study on the use of acupuncture or pelvic floor muscle training for mixed urinary incontinence. Acupunct Med 2016;34:7–13. 10.1136/acupmed-2015-010828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J, Cheng W, Cai M. Effects of electroacupuncture on overactive bladder refractory to anticholinergics: a single-blind randomised controlled trial. Acupunct Med 2015;33:368–74. 10.1136/acupmed-2015-010770 [DOI] [PubMed] [Google Scholar]
- 21. Zhu X, Liu Z, Qu H, et al. The effect and mechanism of electroacupuncture at LI11 and ST37 on constipation in a rat model. Acupunct Med 2016;34:194–200. 10.1136/acupmed-2015-010897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim KH, Kim DH, Kim HY, et al. Acupuncture for recovery after surgery in patients undergoing colorectal cancer resection: a systematic review and meta-analysis. Acupunct Med 2016;34:248–56. 10.1136/acupmed-2015-010941 [DOI] [PubMed] [Google Scholar]
- 23. WHO Regional Office for the Western Pacific. WHO Standard Acupuncture Point Locations in the Western Pacific Region. Manila, Philippines, 2008. [Google Scholar]
- 24. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003;61:37–49. 10.1016/S0090-4295(02)02243-4 [DOI] [PubMed] [Google Scholar]
- 25. Mutch K, Zhao S, Hamid S, et al. Bladder and bowel dysfunction affect quality of life. A cross sectional study of 60 patients with aquaporin-4 antibody positive neuromyelitis optica spectrum disorder. Mult Scler Relat Disord 2015;4:614–8. 10.1016/j.msard.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 26. Kalita J, Shah S, Kapoor R, et al. Bladder dysfunction in acute transverse myelitis: magnetic resonance imaging and neurophysiological and urodynamic correlations. J Neurol Neurosurg Psychiatry 2002;73:154–9. 10.1136/jnnp.73.2.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu H, Liu B, Wu J, et al. A pilot randomized placebo controlled trial of electroacupuncture for women with pure stress urinary incontinence. PLoS One 2016;11:e0150821 10.1371/journal.pone.0150821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan Z, He C, Yan S, et al. Acupuncture for overactive bladder in female adult: a randomized controlled trial. World J Urol 2015;33:1303–8. 10.1007/s00345-014-1440-0 [DOI] [PubMed] [Google Scholar]
- 29. Emmons SL, Otto L. Acupuncture for overactive bladder: a randomized controlled trial. Obstet Gynecol 2005;106:138–43. 10.1097/01.AOG.0000163258.57895.ec [DOI] [PubMed] [Google Scholar]
- 30. Bödeker RH, Madersbacher H, Neumeister C, et al. Dose escalation improves therapeutic outcome: post hoc analysis of data from a 12-week, multicentre, double-blind, parallel-group trial of trospium chloride in patients with urinary urge incontinence. BMC Urol 2010;10:15 10.1186/1471-2490-10-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gittelman M, Weiss H, Seidman L. A phase 2, randomized, double-blind, efficacy and safety study of oxybutynin vaginal ring for alleviation of overactive bladder symptoms in women. J Urol 2014;191:1014–21. 10.1016/j.juro.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 32. Goldfischer ER, Sand PK, Thomas H, et al. Efficacy and safety of oxybutynin topical gel 3% in patients with urgency and/or mixed urinary incontinence: a randomized, double-blind, placebo-controlled study. Neurourol Urodyn 2015;34:37–43. 10.1002/nau.22504 [DOI] [PubMed] [Google Scholar]
- 33. Gafni-Kane A, Botros SM, Du H, et al. Measuring the success of combined intravesical dimethyl sulfoxide and triamcinolone for treatment of bladder pain syndrome/interstitial cystitis. Int Urogynecol J 2013;24:303–11. 10.1007/s00192-012-1832-x [DOI] [PubMed] [Google Scholar]
- 34. Liu Z, Zhou K, Wang Y, et al. Electroacupuncture improves voiding function in patients with neurogenic urinary retention secondary to cauda equina injury: results from a prospective observational study. Acupunct Med 2011;29:188–92. 10.1136/aim.2010.003913 [DOI] [PubMed] [Google Scholar]
- 35. MaLossi J, Chai TC. Sacral neuromodulation for the treatment of bladder dysfunction. Curr Urol Rep 2002;3:61–6. 10.1007/s11934-002-0012-9 [DOI] [PubMed] [Google Scholar]
- 36. Al Mousa RT, Hassouna MM. Electrical stimulation in the treatment of neurogenic bladder dysfunction. Curr Bladder Dysfunct Rep 2008;3:195–202. 10.1007/s11884-008-0029-0 [DOI] [Google Scholar]
- 37. de Groat WC, Tai C. Impact of bioelectronic medicine on the neural regulation of pelvic visceral function. Bioelectron Med 2015;2015:25–36. [PMC free article] [PubMed] [Google Scholar]
- 38. Hohenfellner M, Humke J, Hampel C, et al. Chronic sacral neuromodulation for treatment of neurogenic bladder dysfunction: long-term results with unilateral implants. Urology 2001;58:887–92. 10.1016/S0090-4295(01)01412-1 [DOI] [PubMed] [Google Scholar]
- 39. Jarrett ME, Mowatt G, Glazener CM, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg 2004;91:1559–69. 10.1002/bjs.4796 [DOI] [PubMed] [Google Scholar]
- 40. Hino K, Honjo H, Nakao M, et al. The effects of sacral acupuncture on acetic acid-induced bladder irritation in conscious rats. Urology 2010;75:730–4. 10.1016/j.urology.2009.04.025 [DOI] [PubMed] [Google Scholar]