Abstract
Stroke is a leading cause of mortality and chronic neurologic disability. Yet, the successful treatment remains limited. In this study, we investigated the efficacy and the mechanism of a novel treatment, microRNA-210 (miR-210) inhibition, in protecting acute ischemic brain injury in adult mice. Focal cerebral ischemia was induced by middle cerebral artery occlusion (MCAO) in adult male C57BL/6 mice. MiR-210-LNA (miR-210 inhibitor) or the negative control was administered via intracerebroventricular injection 24 h prior or 4 h after MCAO. Cerebral infarction volume and behavioral deficits were determined 48 h after MCAO. The expression of inflammation-related genes and infiltration/activation of various immune cells in the brain were assessed by RT-qPCR, flow cytometry, and immunohistochemistry. Acute ischemic stroke significantly increased miR-210 levels in the brain, which was abolished by miR-210-LNA administered prior to MCAO. Pre- and post-MCAO treatments with miR-210-LNA significantly decreased cerebral infarction and ameliorated behavioral deficits induced by MCAO. Long-term behavioral recovery was also improved by miR-210-LNA post-treatment. At the same time, inhibition of miR-210 significantly reduced the expression of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and chemokines (CCL2 and CCL3), but had no significant effect on anti-inflammatory factors (TGF-β and IL-10). In addition, MCAO-induced macrophage infiltration and microglial activation in the brain were inhibited by the miR-210-LNA treatment. In summary, inhibition of miR-210 suppresses pro-inflammatory response and reduces brain damage in the acute phase of ischemic stroke, providing new insight in molecular basis of a novel therapeutic strategy of miR-210 inhibition in the treatment of acute ischemic stroke.
Keywords: miR-210, microRNA, Ischemic stroke, Infarction, Inflammation, MRI
1. Introduction
Stroke is the leading cause of adult chronic disability and the fifth leading cause of death in the United States (Benjamin et al., 2017). About $36.5 billion are spent due to stroke every year (Benjamin et al., 2017), and this cost will be steadily rising over the next 40 years (Howard and Goff, 2012). Unfortunately, our ability to treat stroke remains extremely limited. Thus, it has been urgent to explore novel and effective treatment strategies for stroke.
Growing evidence has been reported that recently discovered microRNAs (miRNAs) are essential mediators in neural development and diverse neurological diseases (Fiore et al., 2008), including ischemic stroke (Jeyaseelan et al., 2008; Rink and Khanna, 2011). MiRNAs are a class of non-coding RNAs, participating in post-transcriptional gene regulation. Mature miRNAs are single-stranded nucleotides of ~21–22 in length and can bind to 3′-untranslated region (3′UTR) of target mRNAs, leading to translation repression and mRNA degradation (Boyd, 2008; Filipowicz et al., 2008). A group of miRNAs are upregulated by hypoxia, termed ‘hypoxamirs’ (Chan and Loscalzo, 2010). It is possible that hypoxamirs are of importance in stroke, as hypoxia is a crucial pathogenetic element of ischemic stroke that caused by occlusion of cerebral blood supply. Among hypoxamirs, miR-210 is a ‘master hypoaxmir’ that is robustly induced by hypoxia in all species including humans and all cell types investigated up to date (Kulshreshtha et al., 2007; Pulkkinen et al., 2008).
Few studies reported the role of miR-210 in ischemic stroke and the results were somewhat controversial. Brain miR-210 levels were shown to be upregulated at 24 h and downregulated at 48 h in transient ischemic stroke rats (Jeyaseelan et al., 2008). Another study, using the same stroke model, reported that post-stroke brain cortex miR-210 levels continually increased to 7 days (Lou et al., 2012). In addition, it was demonstrated that upregulation of miR-210 induced pro-apoptotic gene caspase 3 expression and increased endothelial cell apoptosis (Chan et al., 2009). In contrast, miR-210 overexpression appeared antiapoptotic in PC12 cells and neural progenitor cells under oxygen-glucose deprivation (Chio et al., 2013) or hypoxia alone (Wang et al., 2013). These studies imply that the role of miR-210 may vary in different cell types and treatments. Nonetheless, the cause and effect role of miR-210 in brain injury in vivo in the setting of acute ischemic stroke remains undetermined.
Our previous study indicated that inhibition of miR-210 provided a neuroprotective effect in a neonatal rat hypoxic-ischemic encephalopathy (HIE) model (Ma et al., 2016). However, the effect of miR-210 inhibition on the outcome of adult ischemic stroke, especially in the acute phase, remains unclear. Herein, we provide evidence that inhibition of miR-210 suppresses pro-inflammatory response and reduces ischemic brain injury in the acute phase of stroke in adult mice, suggesting a novel therapeutic strategy of the miR-210 blockade in the treatment of acute ischemic stroke.
2. Materials and methods
2.1. Experimental animals and group allocation
Total 85 eight-week-old male C57BL/6 mice were purchased from Charles River Laboratories (Portage, MI). Animals were maintained at 20 ± 2 °C and housed in a 12-h light-dark cycle with access to food and water ad libitum. Mice were randomly divided into 2 major groups and 7 subgroups using a sequence of computer-generated random numbers. Two major groups include 1) negative control (n = 41); and 2) miR-210-LNA treatment (n = 44). Experimental subgroups were listed as followed: Group 1: infarction and behavioral function assays after pre-treatment (n = 5 each group); Group 2: quantitative real-time PCR of cytokines and chemokines (n = 4 and 5 in negative control group and treatment group respectively at 6, 12 and 24 h post-MCAO); Group 3: Enzyme-Linked Immunosorbent Assay (ELISA) of chemokines (n = 4 each group); Group 4: Flow cytometry analysis of immune cells (n = 5 each group); Group 5: Immunofluorescence analysis of reactive microglia (n = 4 each group); Group 6: infarction and behavioral function assays for post-treatment (n = 5 each group); Group 7: long-term investigation of functional recovery after post-treatment (n = 6 each group). All the experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Animal model of focal cerebral ischemia
Focal cerebral ischemia was induced by intraluminal middle cerebral artery occlusion (MCAO) as described previously (Huang et al., 2014). Briefly, mice were anesthetized with 1.5% isoflurane using a face mask. Under a microscope, the left common carotid artery and external carotid artery were exposed through a midline neck incision. A 6–0 nylon monofilament coated with silicon rubber (Doccol) was introduced into the left internal carotid artery through the external carotid stump to occlude the origin of the MCA and block blood flow to the striatum and cortex. After 60 min of occlusion, reperfusion was introduced by filament withdrawal, and the incision was sutured. Throughout the whole procedure, body temperature was maintained at 37 ± 0.5 °C. The surgeon was blinded to the animal grouping. No animal died during MCAO procedure. The mortality of follow-up study was 15% in negative control group and 12% in miR-210-LNA treatment group. To compensate the dropouts, eleven additional mice (six for the negative control group and five for the treatment group) were recruited to the study population. Overall 96 mice were used in our study.
2.3. Intracerebroventricular injection (i.c.v)
Adult C57BL/6 mice were anesthetized with isoflurane and placed in a stereotaxic frame (Stoelting Co., Wood Dale, IL, USA). The ocular ointment was applied to the eyes to prevent drying, animal heads were wiped with 70% ethanol, and the skin on the head was cut open at the midline. A small burr hole (0.5 mm diameter, F.S.T.) was drilled into the skull (0.3 mm posterior to bregma; 1.0 mm lateral to sagittal suture). MiR-210-LNA (Exiqon) and the negative control - LNA scrambled oligonucleotides (miRCURY LNA Power Inhibitor control, #199006–111, Exiqon), were prepared according to manufacturer’s instructions. The miR-210-LNA sequence was AGCCGCTGT CACACGCACA. The LNA scrambled control sequence was TAACACGTCTATACGCCCA, which has no known microRNA targets in miRase (www.mirbase.org). 100 pmol miR-210-LNA or the negative control with a total volume of 4 μl were injected over a 5-minute period into the left lateral ventricle (depth: 3.0 mm dorsal), 24 h prior to (Pretreatment study) or 4 h after (Post-treatment study) MCAO. The wound was closed with suture, and mice were waiting to wake up on a heating pad.
2.4. MRI
MRI was performed on a Bruker 11.7-T BioSpec (Billerica, MA, USA). Body temperature was monitored and maintained at 37.0 ± 1.0 °C during MRI scan, using a thermostatically controlled water flow system. T2-weighted image was acquired on 2 days after MCAO, with following parameters: TR/TE = 4600/90 ms, Field of view (FOV) = 3 cm × 3 cm, slice thickness = 1 mm, interslice distance = 1 mm, number of slices = 20, matrix = 128 × 128, number of average = 2. Infarct volume was quantified by blinded observer, based on the previously reported method (Huang et al., 2017), using Mango and ImageJ. The threshold of signal intensity was determined from contralateral hemisphere in each slice and used for identifying normal brain region in the stroke-affected hemisphere. Irregular regions of interest (ROIs) were drawn to encircle the infarction exhibited as a hyperintense signal within abnormal brain region in each slice. The whole infarct volume was equal to the summed infarct areas multiplied by the slice thickness. Edema correction was also performed, and infarction was expressed as a percentage of infarct volume to the volume of the contralateral hemisphere. The equations used to calculate uncorrected (%HLVu) and edema-corrected lesion volume (%HLVe) were as follows: %HLVu = [2·(infarct volume)/(ipsilateral hemisphere volume + contralateral hemisphere volume)]·100; %HLVe = [(contralateral hemisphere volume – ipsilateral hemisphere volume + infarct volume)/contralateral hemisphere volume]·100. The space-occupying effect due to brain edema (%HSE) was also calculated as previously described (Gerriets et al., 2004): %HSE = %HLVu − % HLVe.
2.5. Behavioral tests
Behavioral tests were performed with the neurological score test and foot-fault test. Each mouse was subjected to the tests on 1 day before and on 2 days after MCAO. The neurological score test was based on the 18-points graded scoring system (Garcia et al., 1995). This scoring system consisted six trials, including spontaneous activity, symmetry in the movement of limbs, forepaw outstretching, climbing, body proprioception and response to vibrissae touch. The lower the score is, the more severe the injury is. The test was videotaped, and the scores were analyzed by an observer blinded to experimental groups. The foot-fault test measures the forelimb misplacement on a grid during locomotion. The performance of mouse was videotaped for 5 min or until 50 steps were taken with one forelimb. The total number of step and number of times each forelimb fell below the grid were counted by an observer blinded o experimental groups. The percentage of footfaults for right forelimb (affected by the stroke) to total steps was calculated and presented, as previous reported (Huang et al., 2017). The foot fault test was performed 10, 20 and 30 days after MCAO for long-term behavioral evaluation.
2.6. Measurement of miR-210
Total RNA was extracted from ipsilateral and contralateral mouse brain tissues 24 h after MCAO. MiR-210 abundance was determined using miScript II RT kit (Qiagen) and miScript SYBR Green PCR kit with miScript Primer Assay kit (Qiagen), as previously described (Ma et al., 2016). Primers included miR-210 miScript Primer Assay (Mm_miR-210_2; MS00032564; Qiagen) and SNORD61 miScript Primer Assay (Hs_SNORD61_11; MS00033705; Qiagen). PCR was performed in triplicate, and threshold cycle numbers were averaged for each sample.
2.7. Real-time PCR for expression of inflammation-related genes
Total RNA was extracted from ipsilateral and contralateral mouse brain tissues 6, 12, and 24 h after MCAO, using TRIzol reagent (Invitrogen). cDNA was made using Superscript III First-Strand Synthesis System (Invitrogen), following the manufacturer’s instructions. Target gene mRNA abundance was determined by real-time PCR using SYBR Green Supermix (Biomake). PCR reaction conditions were a 5-minute hold at 95 °C, followed by 40 cycles of activation for 15 s at 95 °C and annealing/extending for 60 s at 60 °C. Primers used were listed in Supplement Table 1. PCR reactions were carried out in triplicate, and threshold cycle numbers were averaged for each sample. Ct values were normalized relative to GAPDH. The Livak (2−ΔΔCt) method was used to calculate changes in target gene expression relative to the contralateral hemisphere of the negative control group.
2.8. Enzyme-linked immunosorbent assay (ELISA) for chemokine quantification
Ipsilateral and contralateral mouse brain tissues 24 h after MCAO were homogenized in lysis buffer (150 mmol/L NaCl, 50 mmol/L Tris HCl, 10 mmol/L EDTA, 0.1% Tween-20, 1% Triton, 0.1% β-mercaptoethanol, 0.1 mmol/L phenylmethylsulfonyl fluoride, 5 μg/mL leupeptin, and 5 μg/mL aprotinin, pH 7.4). Protein was extracted by centrifuging with 14,000 g at 4 °C for 30 min. Total protein concentration was determined by bicinchoinic acid (BCA) protein assay (#23227, Thermo Scientific). Levels of chemokine CCL2 and CCL3 were quantified using ELISA Kit (CCL2: #MJE00, R & D systems, Inc.; CCL3: #EMCCL3, Thermo Scientific), according to the manufacturer’s instructions. All samples were analyzed in duplicates and the data were normalized to the concentration of chemokine in 1 mg/ml of total protein of lysates.
2.9. Cell isolation and flow cytometry
Twenty-four hours after stroke, the mouse brain was isolated and divided into contralateral and ipsilateral hemispheres after removing the cerebellum. The mononuclear cells were separated through the 70/30% percoll gradients, as previously described (Pino and Cardona, 2011). Briefly, the hemisphere was mechanically dissociated in RPMI buffer and passed through 40-μm nylon cell strainer. The cell pellet was resuspended in 7 mL 30% percoll and layered it on top of 70% percoll. The solution was then centrifuged for 30 min at 500 g at room temperature in the absence of a brake. The cells were collected from the interphase of two density gradients. After been washed twice, the isolated cells were incubated with anti-mouse CD16/CD32 (Biolegend) to block Fc fragment receptor (FcR), and stained with a mix of fluorochrome-conjugated antibodies for 20mins at 4 °C. The antibodies were used as follows: Fixable viability dye eF506 (eBioscience), CD45-Alexa Fluor®647(#103123, Biolegend), CD11b-Alexa Fluor®488(#101219, Biolegend), CD11c-Pacific Blue (#117321, Biolegend), CD3-PE (#100205, Biolegend), and CD8a-APC (#100765, Biolegend). After washes, cells were resuspended in 2% paraformaldehyde and analyzed in an MACSQuant Analyzer (Miltenyi Biotec). The multi-color compensation was performed using MACS Comp bead kit (Miltenyi Biotec). Flowmagic and FlowJo software were used for analysis. Cell gating was performed to identify immune cells, as the previous report (Gelderblom et al., 2009). Dead cells were excluded based on viability dye staining. Cells were then gated for CD45 high and CD45 intermediate populations. CD11b+/CD11c− macrophages and CD3+/CD8+ T-cells were separated from the former population; CD45intermediate+/CD11b+ population were separated from the latter population and considered as microglia cells (Ford et al., 1995; Gelderblom et al., 2009).
2.10. Immunohistochemistry
Twenty-four hours after stroke, mice were deeply anesthetized and transcardially perfused, first with normal saline, then with a solution of phosphate-buffered 4% paraformaldehyde (PFA) solution. Brain samples were post-fixed in 4% PFA for 24 h and dehydrated; coronal sections (20 μm) were prepared using a Leica cryostat. Nonspecific binding was blocked by incubation in 10% goat serum in PBS, pH 7.4, containing 0.1% Tween 20, 0.3% Triton X-100. Sections were then incubated with primary antibody (rabbit Anti-Iba-1, 1:300, Wako) at 4 °C overnight. After washed for three times with PBS, the slices were incubated with Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (Invitrogen, 1:500) for 1 h at room temperature. Brain slices were mounted and coverslipped, using mounting media (Vector Labs). All slices were scanned with a Zeiss LSM710 confocal microscopy (Zeiss). For quantification of active microglia cells, 4 randomly selected fields (0.07 mm2) from the ipsilateral and contralateral cortex of stroke were analyzed in 3 nonadjacent sections (100 μm apart) by the investigator who blinded to the experimental groups. Images were captured at 20× magnification. Average body size of rest microglia in the contralateral cortex was quantified. ImageJ was used to exclude rest microglia (Iba-1+, ramified shape and body size < 25 μm2) and calculate the average number of active microglia (Iba-1 staining positive, amoeboid shape and body size over 25 μm2) per field.
2.11. Statistical analysis
Data were expressed as mean ± SEM. The Infarction and edema caused space-occupying effect (%HSE) were compared between groups using Student’s unpaired t-tests. One-way ANOVA with post-hoc Tukey’s test was used for comparing cytokine expression time course. Two-way ANOVA with post-hoc Sidak’s multiple comparisons test was used for analyzing the data of miR-210 abundance, functional evaluation, expression of cytokines and chemokines, protein level of chemokines, immune cell infiltration and microglial activation. P < 0.05 was considered significant.
3. Results
3.1. MiR-210-LNA pretreatment reduced brain infarct volume and brain edema in mice with MCAO
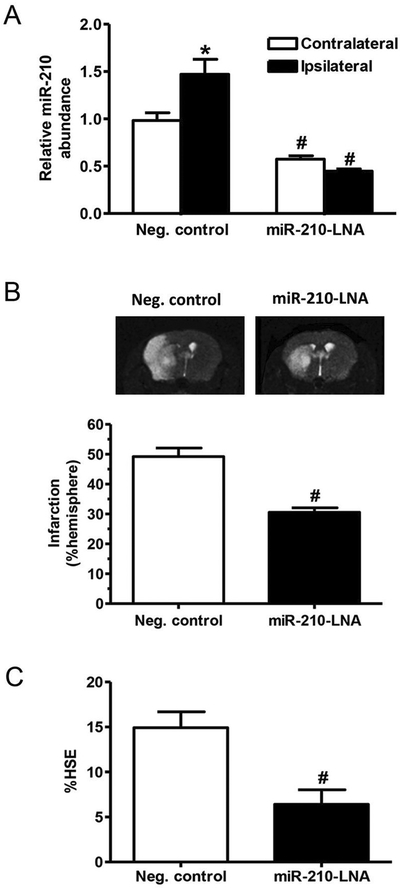
We first measured brain miR-210 levels after MCAO in both negative control and miR-210-LNA pretreated groups. As shown in Fig. 1A, compared to the contralateral hemisphere, MCAO significantly increased miR-210 levels in the ipsilateral hemisphere in the control group, which was blocked by the miR-210-LNA treatment. Brain infarction and correlated edema were evaluated by MRI T2 weighted image 48 h after stroke. Compared to the negative control group, the infarct volume was significantly reduced in the miR-210-LNA pretreated group (30.6% ± 1.5% vs. 49.2% ± 2.9%, P < 0.01, Fig. 1B). In addition, miR-210-LNA significantly decreased MCAO-induced brain edema, as compared to the negative control (6.4% ± 1.6% vs. 14.9% ± 1.8%, P < 0.05, Fig. 1C).
Fig. 1.
MiR-210-LNA decreased MCAO-induced brain infarction and edema. Adult male mice were administered with miR-210-LNA (100 pmol) or the negative control via intracerebroventricular injection (i.c.v) 24 h prior to MCAO. (A) Forty-eight hours after MCAO, miR-210 abundance was measured in the ipsilateral and contralateral hemisphere. Data are presented as mean ± SEM, n = 5. *P < 0.05, ipsilateral vs. contralateral hemisphere; #P < 0.05, miR-210-LNA vs. Neg. control, by two-way ANOVA with post-hoc Sidak’s test; (B) Brain infarction volume was determined by MRI (T2 weighted image) and expressed as a percentage of the volume of contralateral hemisphere. Data are presented as mean ± SEM, n = 5. #P < 0.05, miR-210-LNA vs. Neg. control, by Student’s t-test; (C) Brain edema caused space-occupying effect was calculated based on MRI data (%HSE, % of hemisphere). Data are presented as mean ± SEM, n = 5. #P < 0.05, miR-210-LNA vs. Neg. control, by Student’s t-test.
3.2. MiR-210-LNA pretreatment ameliorated the behavioral deficits after stroke
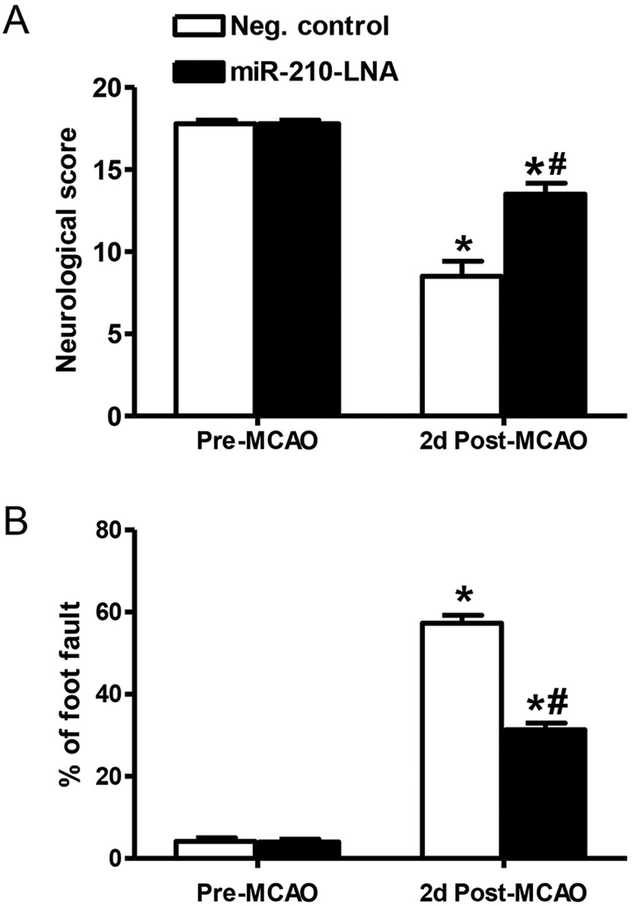
Neurological score and foot fault test were performed 48 h after stroke. The neurological score was decreased in both groups after stroke, compared to the pre-MCAO score (Fig. 2A). However, the score was significantly improved in the miR-210-LNA pre-treated group, as compared to the control group (13.5 ± 0.7 vs. 8.5 ± 0.9, P < 0.01, Fig. 2A). For the foot fault test, the percentage of foot fault dramatically increased after stroke in both groups (Fig. 2B). However, this was significantly reduced in the miR-210-LNA pre-treated group, as compared with the control group (31.35% ± 1.6% vs. 57.3% ± 1.9%, P < 0.001, Fig. 2B).
Fig. 2.
MiR-210-LNA reduced MCAO-induced behavioral deficits. Adult male mice were administered with miR-210-LNA (100 pmol) or the negative control via intracerebroventricular injection (i.c.v) 24 h prior to MCAO. Neurological score test (A) and foot fault test (B) were conducted 24 h before and 48 h after MCAO. Data are presented as mean ± SEM, n = 5. *P < 0.05, post-MCAO vs. pre-MCAO; #P < 0.05, miR-210-LNA vs. Neg. control, by two-way ANOVA with post-hoc Sidak’s test.
3.3. MiR-210-LNA pretreatment suppressed pro-inflammatory cytokines
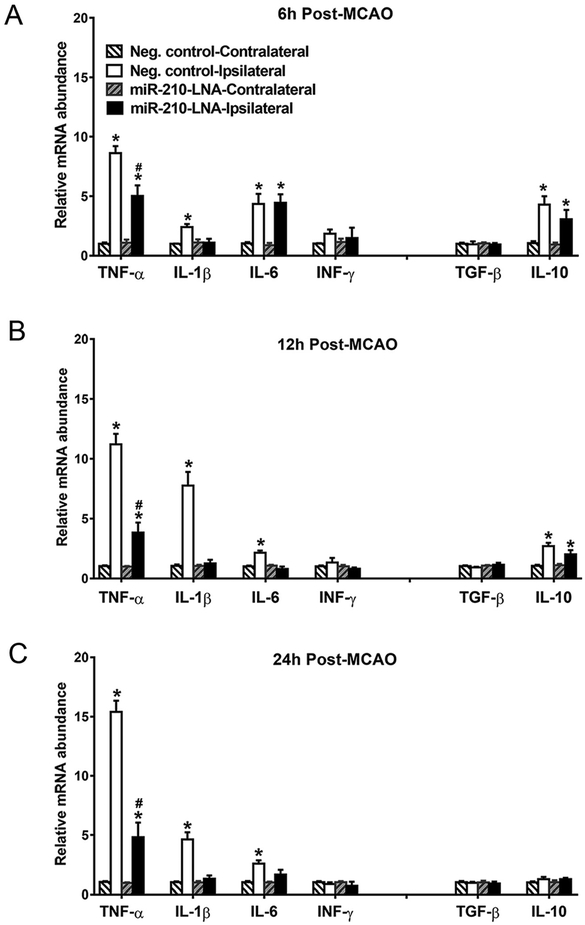
The mRNA levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IFN-γ) and anti-inflammatory cytokines (TGF-β, and IL-10) were determined in contralateral and ipsilateral hemispheres at 6, 12 and 24 h after stroke. As shown in Fig. 3, MCAO induced differential time courses of pro-inflammatory cytokine productions in the ipsilateral hemisphere, as compared to the contralateral side. In the control group, TNF-α dramatically increased at 6 h and continued increasing at 12 and 24 h (8.6 ± 0.6 folds at 6 h; 11.2 ± 0.9 folds at 12 h; 15.4 ± 0.9 folds at 24 h; 12 h vs. 6 h, P < 0.05, 24 h vs. 12 h, P < 0.05, one-way ANOVA). IL-1β significantly increased at 6 h and peaked at 12 h (2.4 ± 0.3 folds at 6 h; 7.7 ± 1.2 folds at 12 h; 4.6 ± 0.6 folds at 24 h; 12 h vs. 6 h, P < 0.01, 24 h vs. 12 h, P < 0.05, one-way ANOVA). In contrast, IL-6 peaked at 6 h and decreased but remained elevated at 12 and 24 h (4.4 ± 0.8 folds at 6 h; 2.2 ± 0.2 folds at 12 h; 2.6 ± 0.3 folds at 24 h; 12 h vs. 6 h, P < 0.05, 24 h vs. 12 h, P > 0.05, one-way ANOVA). IFN-γ was not affected. Inhibition of miR-210 by the miR-210-LNA treatment prior to MCAO significantly inhibited TNF-α productions and completely blocked IL-1β productions at all time points. MiR-210-LNA had no effect on IL-6 at 6 h but blocked IL-6 productions in the later phase of 12 and 24 h. Similar to the time course of IL-6 productions, MCAO induced a significant increase in an anti-inflammatory cytokine IL-10 production at 6 h, which were progressively reduced at 12 and 24 h. In contrast to its inhibitory effect on pro-inflammatory cytokines, miR-210-LNA had no significant effect on MCAO-induced IL-10 productions in the brain. TGF-β was not affected by either MCAO or miR-210-LNA treatments.
Fig. 3.
MiR-210-LNA suppressed MCAO-induced pro-inflammatory cytokine expression in the brain. Adult male mice were administered with miR-210-LNA (100 pmol) or the negative control via intracerebroventricular injection (i.c.v) 24 h prior to MCAO. mRNA abundance of inflammatory cytokines were determined 6 (A), 12 (B) or 24 h (C) after MCAO. Data are presented as mean ± SEM, n = 4 to 5. *P < 0.05, ipsilateral vs. contralateral hemisphere; #P < 0.05, miR-210-LNA vs. Neg. control, by two-way ANOVA with post-hoc Sidak’s test.
3.4. MiR-210-LNA pretreatment inhibited MCAO-induced chemokine expression
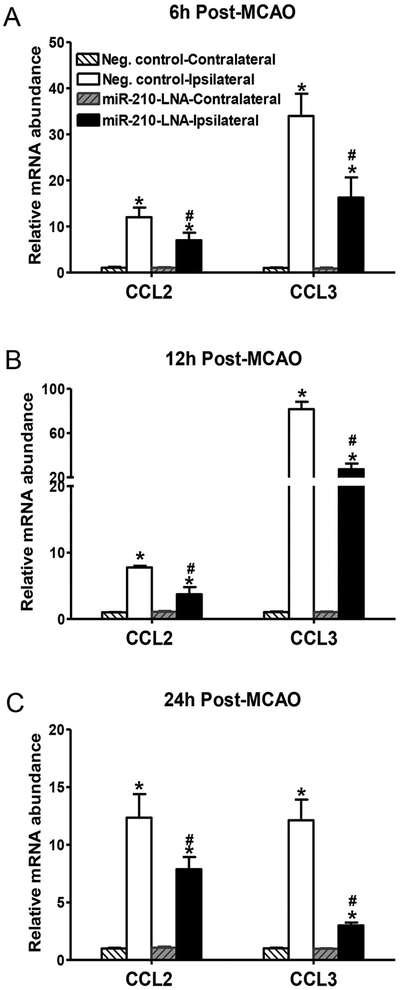
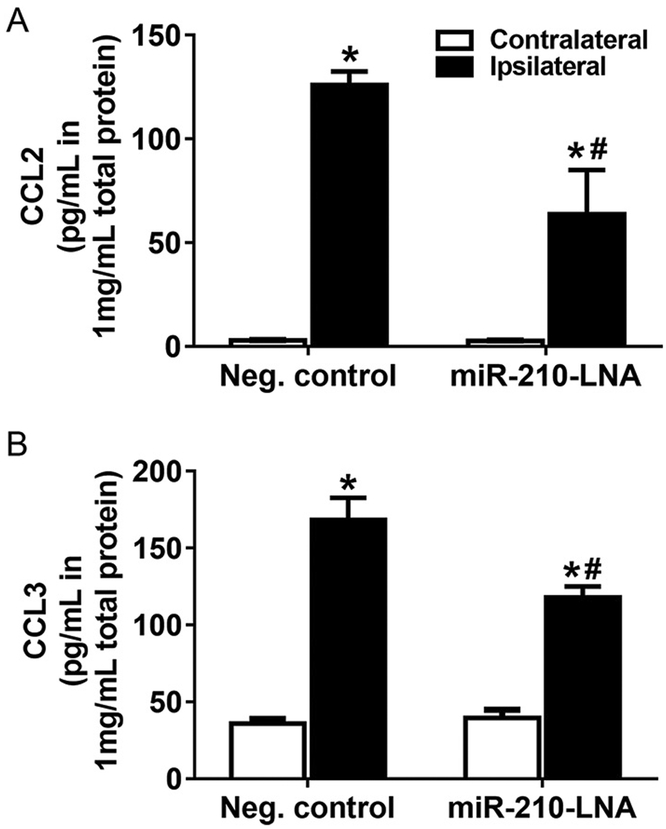
Chemokines are critical factors to recruit peripheral monocytes/macrophages and neutrophils into the brain parenchyma during neuroinflammation. As shown in Fig. 4, MCAO significantly increased mRNA abundance of chemokines CCL2 and CCL3 in the ipsilateral hemisphere. CCL2 was increased at 6 h after MCAO and maintained elevated at 12 and 24 h. The increase of CCL3 was much greater and reached the peak of ~80-fold increase at 12 h. MiR-210-LNA pretreatment prior to MCAO significantly inhibited MCAO-induced CCL2 and CCL3 expression (Fig. 4). Protein levels of CCL2 and CCL3 were also quantified by ELISA. The levels of CCL2 and CCL3 in the ipsilateral hemisphere were significantly increased 24 h after MCAO (Fig. 5). The miR-210-LNA treatment significantly decreased both CCL2 and CCL3 levels in the ipsilateral hemisphere, compared to the negative control (CCL2: 63.5 ± 21.5 vs. 126.0 ± 6.6, P < 0.05; CCL3: 118.1 ± 6.9 vs. 168.2 ± 14.5, P < 0.05; Fig. 5).
Fig. 4.
MiR-210-LNA inhibited MCAO-induced chemokine expression in the brain. Adult male mice were administered with miR-210-LNA (100 pmol) or the negative control via intracerebroventricular injection (i.c.v) 24 h prior to MCAO. mRNA abundance of chemokines, CCL2 and CCL3 were determined 6 (A), 12 (B) or 24 h (C) after MCAO. Data are presented as mean ± SEM, n = 4 to 5. *P < 0.05, ipsilateral vs. contralateral hemisphere; #P < 0.05, miR-210-LNA vs. Neg. control, by two-way ANOVA with post-hoc Sidak’s test.
Fig. 5.
MiR-210-LNA reduced protein level of chemokine after stroke. Adult male mice were administered with miR-210-LNA (100 pmol) or the negative control via intracerebroventricular injection (i.c.v) 24 h prior to MCAO. Chemokines, CCL2 (A) and CCL3 (B) level of ipsilateral and contralateral hemisphere were quantified by ELISA 24 h after MCAO. Data are presented as mean ± SEM, n = 4. *P < 0.05, ipsilateral vs. contralateral hemisphere; #P < 0.05, miR-210-LNA vs. Neg. control, by two-way ANOVA with post-hoc Sidak’s test.
3.5. Effect of miR-210-LNA pretreatment on immune cell infiltration and microglial activation
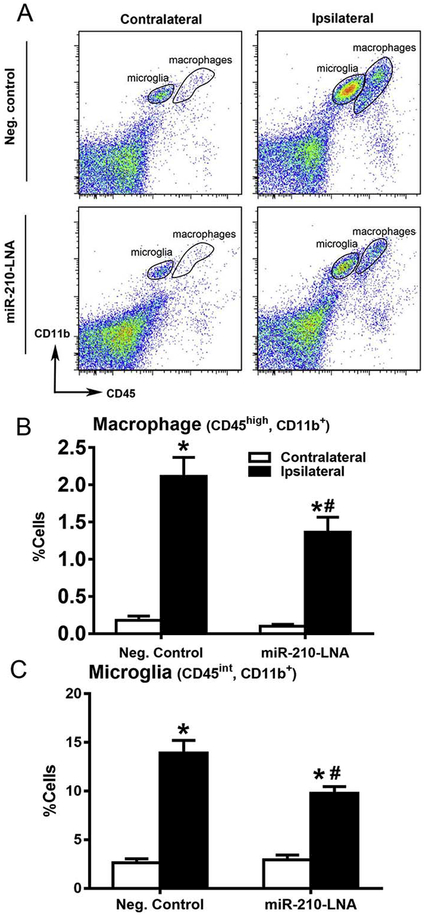
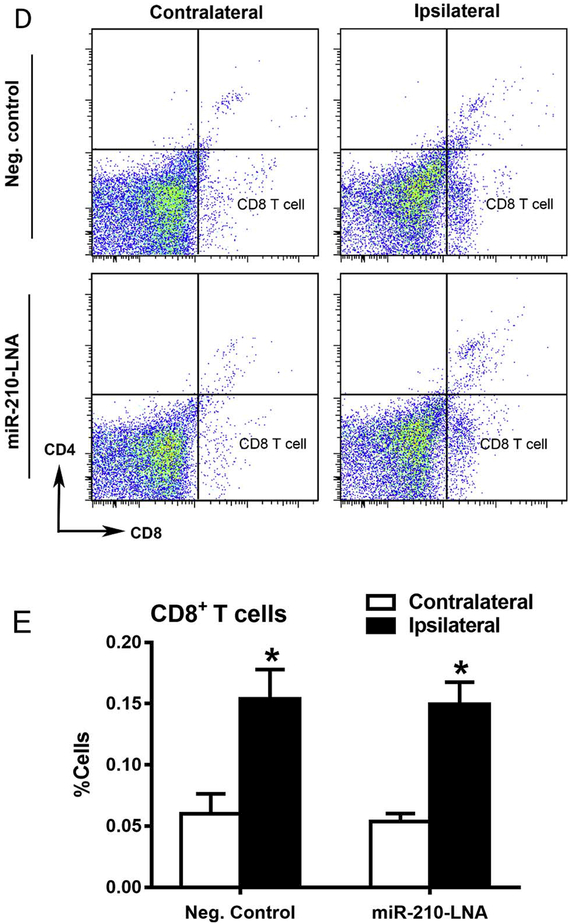
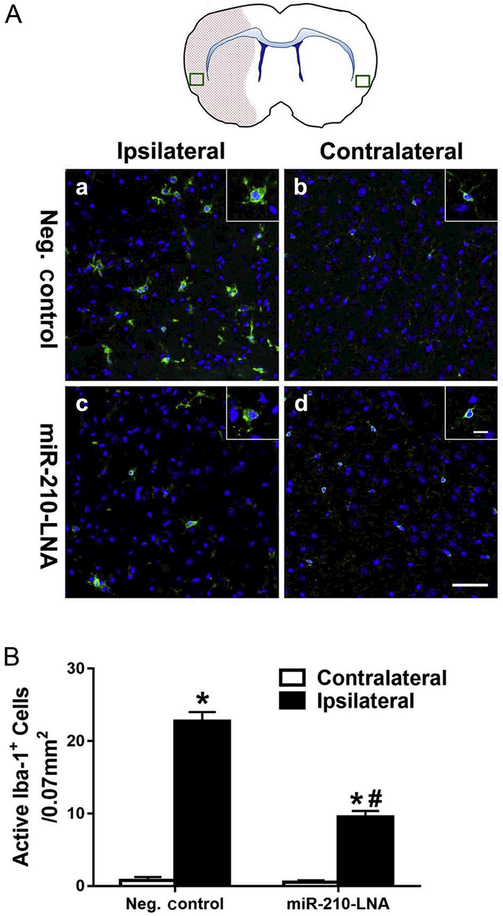
Flow cytometry was performed to quantify cerebral immune cells 24 h after stroke. As shown in Fig. 6, compared to the contralateral hemisphere, there were significant increases in macrophages (Fig. 6B), microglia (Fig. 6C) and CD8+ T cells (Fig. 6E) in the ipsilateral hemisphere after MCAO. MiR-210-LNA pretreatment prior to MCAO significantly decreased MCAO-induced macrophages (Fig. 6B), microglia (Fig. 6C) in the ipsilateral hemisphere. In contrast, miR-210-LNA had no significant effect on MCAO-induced infiltration of CD8+ T cells in the brain (Fig. 6E). In addition, the activation of microglia after MCAO was demonstrated by immunostaining with the microglial marker, Iba-1. As shown in Fig. 7A, immunostaining of microglial marker Iba-1 was increased in the ischemic, ipsilateral core, as compared with the nonischemic, contralateral hemisphere, and a greater number of microglial cells exhibited reactive amoeboid morphology in the ipsilateral cortex as compared with the typical ramified shape of resting microglia in the contralateral hemisphere. Of importance, miR-210-LNA pretreatment prior to MCAO significantly reduced the microglial activation (10 ± 1 vs. 23 ± 1, P < 0.01, Fig. 7B).
Fig. 6.
MiR-210-LNA decreased MCAO-induced immune cell infiltration and activation in the brain. Adult male mice were administered with miR-210-LNA (100 pmol) or the negative control via intracerebroventricular injection (i.c.v) 24 h prior to MCAO. Representative flow-cytometry plots are shown to illustrate immune cells after stroke (A, D). Macrophages (B), microglia (C) and CD8+ T cells (E) were measured by flow cytometry 24 h after MCAO. Data are presented as mean ± SEM, n = 5. *P < 0.05, ipsilateral vs. contralateral hemisphere; #P < 0.05, miR-210-LNA vs. Neg. control, by twoway ANOVA with post-hoc Sidak’s test.
Fig. 7.
MiR-210-LNA decreased MCAO-induced microglial activation. Anti-Iba-1 immunostaining of microglia 24 h after MCAO is shown in Panel A. The box in the brain diagram indicates the region of imaging. Red area highlights the lesion area in the ipsilateral hemisphere. Inset shows a higher magnification view of microglia. Scale bar = 50 μm and 10 μm (inset). Quantification of active microglia is presented in Panel B. Data are presented as mean ± SEM, n = 4. *P < 0.01, ipsilateral vs. contralateral hemisphere; #P < 0.01, miR-210-LNA vs. Neg. control, by two-way ANOVA with post-hoc Sidak’s test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
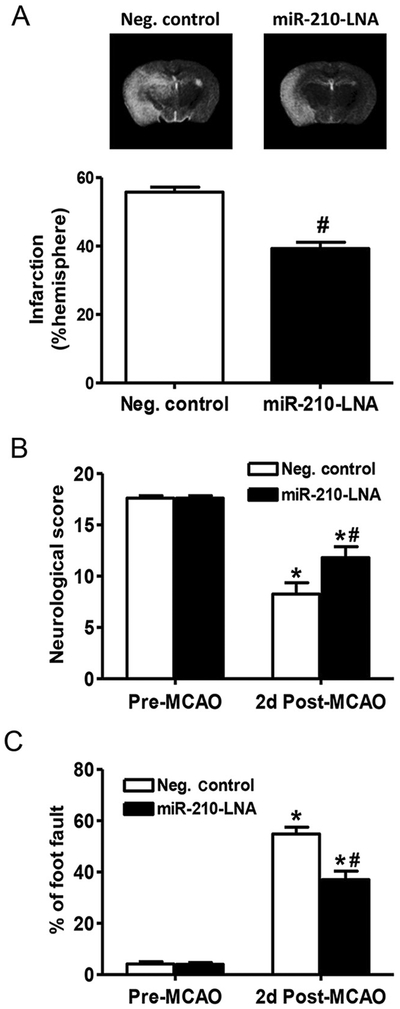
3.6. MiR-210-LNA posttreatment conferred protection in mice with MCAO
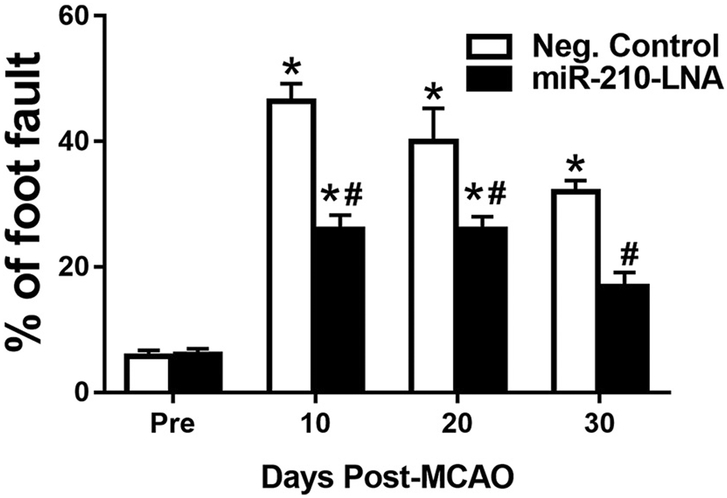
To further evaluate the therapeutic potential of miR-210 inhibition in clinical application, miR-210-LNA was administered 4 h after the MCAO treatment. Brain infarction and behavioral function were determined 48 h after MCAO. Consistent with the findings of miR-210-LNA pretreatment, the infarct volume was significantly reduced in the miR-210-LNA treated group, as compared to the negative control group (39.3% ± 1.9% vs. 55.8% ± 1.4%, P < 0.01, Fig. 8A). Moreover, the neurological score was significantly higher in the miR-210-LNA treated group than that in the control group (11.8 ± 1.1 vs. 8.3 ± 1.1, P < 0.05, Fig. 8B). In addition, the percentage of foot fault was significantly reduced in the miR-210-LNA treated group, as compared to the negative control group (37.0% ± 3.4% vs. 54.8% ± 2.6%, P < 0.01, Fig. 8C). We also evaluated the long-term effect of miR-210-LNA post-treatment on behavioral deficits. Compared to the negative control group, miR-210-LNA significantly reduced foot fault 10 (26.0% ± 2.3% vs. 46.4% ± 2.8%. P < 0.01), 20 (26.2% ± 2.7% vs. 40.0% ± 5.3%, P < 0.01) and 30 (16.8% ± 2.3% vs. 32.0% ± 1.8%, P < 0.01) days after MCAO (Fig. 9).
Fig. 8.
MiR-210-LNA post-treatment reduced MCAO-induced brain infarction and behavioral deficits. Adult male mice were administered with miR-210-LNA (100 pmol) or the negative control via intracerebroventricular injection (i.c.v) 4 h after MCAO. (A) Brain infarction volume was determined 48 h after MCAO by MRI and expressed as a percentage of the volume of contralateral hemisphere. Data are presented as mean ± SEM, n = 5. #P < 0.05, miR-210-LNA vs. Neg. control, by Student’s t-test. For behavioral tests, neurological score test (B) and foot fault test (C) were conducted 24 h before and 48 h after MCAO. Data are presented as mean ± SEM, n = 5. *P < 0.05, post-MCAO vs. pre-MCAO; #P < 0.05, miR-210-LNA vs. Neg. control, by two-way ANOVA with post-hoc Sidak’s test.
Fig. 9.
MiR-210-LNA post-treatment improved long-term behavioral recovery. Adult male mice were administered with miR-210-LNA (100 pmol) or the negative control via intracerebroventricular injection (i.c.v) 4 h after MCAO. Foot fault test was conducted 24 h before, 10 days, 20 days and 30 days after MCAO. Data are presented as mean ± SEM, n = 6. *P < 0.05, post-MCAO vs. pre-MCAO; #P < 0.05, miR-210-LNA vs. Neg. control, by two-way ANOVA with post-hoc Sidak’s test.
4. Discussion
The present study demonstrated that inhibition of miR-210 by miR-210-LNA significantly reduces brain damage and ameliorates behavioral deficits in the acute phase of ischemic stroke. In addition, miR-210 inhibition decreased post-ischemic inflammation response. Of importance, the finding that miR-210-LNA posttreatment 4 h after stroke confers neuroprotection similar to that seen in the pretreatment suggests that silencing miR-210 may present as a novel and potential therapeutic intervention in the setting of acute ischemic stroke.
The finding that inhibition of miR-210 reduced brain infarction caused by stroke provides a cause-and-effect link and suggests that endogenous miR-210 plays a detrimental role in the stroke development. Although ischemic stroke and hypoxia cause a dramatic increase in miR-210 levels in the brain or cultured neural cells, the biological function of miR-210 in hypoxic-ischemic brain injury or neuronal cell death is still elusive, even controversial. Chio et al. reported that knocking down miR-210 expression reduced OGD-induced apoptosis in mouse neuroblastoma neuro-2a cells, through alleviation of antiapoptotic Bcl-2 expression (Chio et al., 2013). In contrast, Qiu et al. demonstrated that OGD-induced cell apoptosis is decreased by overexpression of miR-210 in rat pheochromocytoma (PC12) cells (Qiu et al., 2013a). The similar controversial pattern has also been observed in in-vivo studies. Reducing miR-210, through miR-210 inhibitor intraventricular injection, has shown a decrease of neuronal apoptosis in rat HIE model (Qiu et al., 2013b). However, our previous study, using the same animal model, demonstrated that inhibition of miR-210 reduced neuronal death and brain infarction through directly increasing the abundance of glucocorticoid receptor (GR) in neonatal rats (Ma et al., 2016). The present study for the first time provides the evidence that miR-210 inhibition has the neuroprotective effect in an adult stroke model. Consistent with our findings, a recent study has demonstrated that downregulation of miR-210 decreases bupivacaine-induced neurotoxicity in dorsal root ganglion (Wang et al., 2016). It is possible that the distinctive effects of miR-210 are related to the variety of its targets. More than twenty direct targets of miR-210 have been reported (Devlin et al., 2011), including both beneficial and detrimental factors in hypoxic/ischemic brain injury. For example, luciferase activity assay confirmed that pro-apoptosis gene caspase-8-associated protein-2 (Casp8ap2) is a direct target of miR-210, and abrogation of miR-210 significantly increases the expression of Casp8ap2, thereby leading to apoptosis in ischemic preconditioned mesenchymal stem cell (MSC) (Kim et al., 2009). On the other hand, the iron‑sulfur cluster assembly protein (ISCU1/2), a critical protein for mitochondrial electron transport, is another direct target of miR-210 (Chan et al., 2009). Increasing miR-210 levels disrupt mitochondrial metabolism, which leads to apoptosis of human endothelial cells (Chan et al., 2009). Thus, the final consequence of manipulating miR-210 likely depends on the balance and integration of multiple target genes actions. High-throughput methodology to detect the expression of miR-210 target genes should be considered in the future study in order to better understand the mechanisms underlying the neuroprotection of miR-210 inhibition seen in vivo in the setting of acute ischemic stroke.
Neuroinflammation is a major causative event for neuronal death in stroke. After stroke, brain-resident immune cells, microglia, are activated, and peripheral immune cells including neutrophils, monocytes/macrophages, and T cells, are recruited and infiltrated into the brain parenchyma through the disrupted blood-brain-barrier (BBB) (Tobin et al., 2014). Vast amounts of toxic inflammatory factors are secreted by immune cells, thereby causing irreversible cell death. The present study demonstrated that the miR-210-LNA treatment had a lasting effect in inhibiting miR-210 and blocked MCAO-increased miR-210 in the ipsilateral hemisphere up to 48 h after MCAO. This is in agreement with the previous finding that the effect of miR-16 antagomir on inhibiting miR-16 expression lasted for at least three days after intracerebral injection (Krutzfeldt et al., 2007). Of importance, the present study demonstrated that inhibition of miR-210 significantly reduced acute inflammatory reaction and decreased the expression of pro-inflammatory cytokines and chemokines up to 24 h after MCAO, which may account for the neuroprotective effect of miR-210 blockade. Yet, inhibition of miR-210 had no effects on the expression of anti-inflammatory factors, such as IL-10 and TGF-β. These implicate that the neuroprotective effect of miR-210 inhibition mainly relies on the reduction of pro-inflammatory factors. Of interest, miR-210-LNA produced differential inhibitory effects among pro-inflammatory cytokines. For example, miR-210-LNA decreased TNF-α expression but completely blocked IL-1β expression at all time points examined. Although it had no effect on IL-6 expression at the early time point of 6 h after MCAO, it blocked IL-6 at later phases of 12 and 24 h. At present, it is not clear whether these pro-inflammatory cytokines are the direct targets of miR-210. However, miR-210 may regulate the expression of upstream signaling proteins to these factors, like ten eleven translocation 2 (TET2). Recent studies have demonstrated that TET2 is required to resolve inflammation by suppressing IL-1β, as well as IL-6 at the later phase of inflammatory responses (Fuster et al., 2017; Zhang et al., 2015). TET2 mRNA 3′UTR has a perfect binding sequence to miR-210, which is highly homologous and is identical among human and rodents. It is possible that ischemia-induced increase in brain miR-210 may downregulate TET2 protein abundance and its effect on suppression of inflammatory mediators, including IL-6 and IL-1β.
In addition, we found that stroke-induced expression of chemokines CCL2 and CCL3 in the brain were suppressed by miR-210 inhibition. CCL2 and CCL3, which are released by activated microglia and injury endothelial cells after the stroke, are the critical mediators to recruit peripheral immune cells, including monocytes/macrophage, neutrophils, and lymphocytes into the brain. Thus, the finding suggests that the infiltration of immune cells in the brain after stroke could be decreased by the treatment of miR-210 inhibitor. Indeed, our flow cytometry data indicated that the number of macrophages and activated microglia were significantly reduced by the treatment with miR-210-LNA. Furthermore, immunostaining of Iba-1, a microglia marker, demonstrated that post-stroke activation of microglia was suppressed by inhibition of miR-210. To our surprise, the number of CD8+ T cells was not decreased by the miR-210-LNA treatment. This is consistent with the finding that the expression of IFN-γ was not affected by miR-210-LNA. IFN-γ has been reported to recruit CD8+ T cells after the stroke, and its expression peaked at 10–14 days after stroke (Yasuda et al., 2011). Thus, the impact of miR-210 inhibition on the activity of specific immune cells in the brain for a prolonged period of days warrants further investigation.
Of importance, the finding that a single dose of miR-210-LNA administration 4 h after MCAO significantly reduced brain injury and improved short-term and long-term neurological function is of high interest and suggests a new therapeutic strategy for the treatment of stroke. Growing evidence indicates that the application of miRNA-related agent has a high translational potential for the treatment of neurological diseases. Firstly, single intracerebral injection of miR-16 antagomir significantly decreased miR-16 expression in the mouse brain three days later (Krutzfeldt et al., 2007), which implies the efficiency of miR inhibition with a single dose injection. Secondly, a recent phase I/II clinical trial has reported that no long-term safety issues were observed along with miR inhibitor administration for treating hepatitis (van der Ree et al., 2014), which alleviates the clinical concern for the safety of such agents. Lastly but not the least, the chemical modifications make the miR-related products more stable and membrane-penetrable (Lam et al., 2015; Mook et al., 2007). The present study showed that intracerebroventricular administration of miR-210 inhibitor effectively reduced brain infarction and behavioral deficits after acute stroke, which sheds light on its therapeutic potential. Similarly, our previous study demonstrated the equal efficacy of intranasal administration of miR-210 inhibitor as the intracerebroventricular injection for protecting brain injury in a neonatal HIE model (Ma et al., 2016). Substantial evidence demonstrates that nucleotides can pass the blood-brain-barrier (BBB) through this non-invasive noise-to-brain delivery route (Han et al., 2007). In line with these findings, further investigation of the effects of miR-210 inhibitor through intranasal administration in stroke is needed for clinical translation. In the present study, although the treatment of miR-210-LNA 4 h after stroke showed the neuroprotective effect, future studies are needed to further evaluate the optimized treatment windows.
5. Conclusion
The present investigation identifies a novel cause-and-effect mechanism of miR-210 in regulating pro-inflammatory response and brain injury in the acute phase of ischemic stroke in a mouse model. Although it may be difficult to translate directly these findings into humans, several lines of evidence support the significance of the present study in clinical implications. Thus, mature miR-210 is identical between humans and mice, and hypoxia increases miR-210 in cells and tissues of all species that have been studied, including humans and mice. Given that neuroinflammatory response to acute cerebral hypoxia-ischemia is a major contributor to the pathophysiology of brain injury, the findings that inhibition of miR-210 suppresses pro-inflammatory cytokines and ameliorates brain injury and neurobehavioral deficits suggest the potential of miR-210 inhibition as an effective therapeutic strategy for treating hypoxic-ischemic brain injury.
Supplementary Material
Acknowledgements
A portion of this research used the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core, a facility is supported in part by the National Science Foundation through the Major Research Instrumentation program of the Division of Biological Infrastructure Grant No. 0923559 and the Loma Linda University School of Medicine.
Sources of funding
This work was supported in part by the National Institutes of Health grants HL118861 (LZ) and NS103017 (LZ).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.expneurol.2017.10.024.
Disclosures
None.
References
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, 2017. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135, e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd SD, 2008. Everything you wanted to know about small RNA but were afraid to ask. Lab. Investig 88, 569–578. [DOI] [PubMed] [Google Scholar]
- Chan SY, Loscalzo J, 2010. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle 9, 1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J, 2009. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 10, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio CC, Lin JW, Cheng HA, Chiu WT, Wang YH, Wang JJ, Hsing CH, Chen RM, 2013. MicroRNA-210 targets antiapoptotic Bcl-2 expression and mediates hypoxia-induced apoptosis of neuroblastoma cells. Arch. Toxicol 87, 459–468. [DOI] [PubMed] [Google Scholar]
- Devlin C, Greco S, Martelli F, Ivan M, 2011. miR-210: more than a silent player in hypoxia. IUBMB Life 63, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N, 2008. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet 9, 102–114. [DOI] [PubMed] [Google Scholar]
- Fiore R, Siegel G, Schratt G, 2008. MicroRNA function in neuronal development, plasticity and disease. Biochim. Biophys. Acta 1779, 471–478. [DOI] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD, 1995. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol 154, 4309–4321. [PubMed] [Google Scholar]
- Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, Walsh K, 2017. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ, 1995. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 26, 627–634 (discussion 635). [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T, 2009. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40, 1849–1857. [DOI] [PubMed] [Google Scholar]
- Gerriets T, Stolz E, Walberer M, Muller C, Kluge A, Bachmann A, Fisher M, Kaps M, Bachmann G, 2004. Noninvasive quantification of brain edema and the space-occupying effect in rat stroke models using magnetic resonance imaging. Stroke 35, 566–571. [DOI] [PubMed] [Google Scholar]
- Han IK, Kim MY, Byun HM, Hwang TS, Kim JM, Hwang KW, Park TG, Jung WW, Chun T, Jeong GJ, Oh YK, 2007. Enhanced brain targeting efficiency of intranasally administered plasmid DNA: an alternative route for brain gene therapy. J. Mol. Med. (Berl) 85, 75–83. [DOI] [PubMed] [Google Scholar]
- Howard G, Goff DC, 2012. Population shifts and the future of stroke: forecasts of the future burden of stroke. Ann. N. Y. Acad. Sci 1268, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Wong S, Snyder EY, Hamblin MH, Lee JP, 2014. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res Ther 5, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Liu Y, Lu J, Cerqueira B, Misra V, Duong TQ, 2017. Intraarterial transplantation of human umbilical cord blood mononuclear cells in hyperacute stroke improves vascular function. Stem Cell Res Ther 8, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaseelan K, Lim KY, Armugam A, 2008. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 39, 959–966. [DOI] [PubMed] [Google Scholar]
- Kim HW, Haider HK, Jiang S, Ashraf M, 2009. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J. Biol. Chem 284, 33161–33168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzfeldt J, Kuwajima S, Braich R, Rajeev KG, Pena J, Tuschl T, Manoharan M, Stoffel M, 2007. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 35, 2885–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M, 2007. A microRNA signature of hypoxia. Mol. Cell. Biol 27, 1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JK, Chow MY, Zhang Y, Leung SW, 2015. siRNA versus miRNA as therapeutics for gene silencing. Mol. Ther.–Nucleic Acids 4, e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou YL, Guo F, Liu F, Gao FL, Zhang PQ, Niu X, Guo SC, Yin JH, Wang Y, Deng ZF, 2012. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol. Cell. Biochem 370, 45–51. [DOI] [PubMed] [Google Scholar]
- Ma Q, Dasgupta C, Li Y, Bajwa NM, Xiong F, Harding B, Hartman R, Zhang L, 2016. Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol. Dis 89, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook OR, Baas F, de Wissel MB, Fluiter K, 2007. Evaluation of locked nucleic acidmodified small interfering RNA in vitro and in vivo. Mol. Cancer Ther 6, 833–843. [DOI] [PubMed] [Google Scholar]
- Pino PA, Cardona AE, 2011. Isolation of brain and spinal cord mononuclear cells using percoll gradients. J. Vis. Exp 48 10.3791/2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen K, Malm T, Turunen M, Koistinaho J, Yla-Herttuala S, 2008. Hypoxia induces microRNA miR-210 in vitro and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett. 582, 2397–2401. [DOI] [PubMed] [Google Scholar]
- Qiu J, Zhou XY, Zhou XG, Cheng R, Liu HY, Li Y, 2013a. Neuroprotective effects of microRNA-210 against oxygen-glucose deprivation through inhibition of apoptosis in PC12 cells. Mol. Med. Rep 7, 1955–1959. [DOI] [PubMed] [Google Scholar]
- Qiu J, Zhou XY, Zhou XG, Cheng R, Liu HY, Li Y, 2013b. Neuroprotective effects of microRNA-210 on hypoxic-ischemic encephalopathy. Biomed. Res. Int 2013, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ree MH, van der Meer AJ, de Bruijne J, Maan R, van Vliet A, Welzel TM, Zeuzem S, Lawitz EJ, Rodriguez-Torres M, Kupcova V, Wiercinska-Drapalo A, Hodges MR, Janssen HL, Reesink HW, 2014. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antivir. Res 111, 53–59. [DOI] [PubMed] [Google Scholar]
- Rink C, Khanna S, 2011. MicroRNA in ischemic stroke etiology and pathology. Physiol. Genomics 43, 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O, 2014. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J. Cereb. Blood Flow Metab 34, 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Xiong L, Huang X, Zhao T, Wu LY, Liu ZH, Ding X, Liu S, Wu Y, Zhao Y, Wu K, Zhu LL, Fan M, 2013. miR-210 suppresses BNIP3 to protect against the apoptosis of neural progenitor cells. Stem Cell Res 11, 657–667. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ni H, Zhang W, Wang X, Zhang H, 2016. Downregulation of miR-210 protected bupivacaine-induced neurotoxicity in dorsal root ganglion. Exp. Brain Res 234, 1057–1065. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Shimoda T, Uno K, Tateishi N, Furuya S, Tsuchihashi Y, Kawai Y, Naruse S, Fujita S, 2011. Temporal and sequential changes of glial cells and cytokine expression during neuronal degeneration after transient global ischemia in rats. J. Neuroinflammation 8, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, Zhao D, Liu Y, Wang C, Zhang X, Su X, Liu J, Ge W, Levine RL, Li N, Cao X, 2015. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.