Abstract
Familial and sporadic essential tremor (ET) cases differ in several respects. Whether they differ with respect to cerebellar pathologic changes has yet to be studied. We quantified a broad range of postmortem features (Purkinje cell (PC) counts, PC axonal torpedoes, a host of associated axonal changes, heterotopic PCs, and hairy basket ratings) in 60 ET cases and 30 controls. Familial ET was defined using both liberal criteria (n = 27) and conservative criteria (n = 20). When compared with controls, ETcases had lower PC counts, more torpedoes, more heterotopic PCs, a higher hairy basket rating, an increase in PC axonal collaterals, an increase in PC thickened axonal profiles, and an increase in PC axonal branching. Familial and sporadic ET had similar postmortem changes, with few exceptions, regardless of the definition criteria. The PC counts were marginally lower in familial than sporadic ET (respective p values = 0.059 [using liberal criteria] and 0.047 [using conservative criteria]). The PC thickened axonal profile count was marginally lower in familial ET than sporadic ET (respective p values = 0.037 [using liberal criteria] and 0.17 [using conservative criteria]), and the PC axonal branching count was marginally lower in familial than sporadic ET (respective p values = 0.045 [using liberal criteria] and 0.079 [using conservative criteria]). After correction for multiple comparisons, however, there were no significant differences. Overall, familial and sporadic ET cases share very similar cerebellar postmortem features. These data indicate that pathological changes in the cerebellum are a part of the pathophysiological cascade of events in both forms of ET.
Keywords: Essential tremor, Cerebellum, Neurodegenerative, Purkinje cell, Pathology, Family history
Introduction
Essential tremor (ET) is among the most prevalent movement disorders [1], although disease mechanisms remain obscure. Both clinical and neuroimaging evidence suggests that the cerebellum plays an important role in the generation of tremor in ET [2–6]. In prior studies, we observed a constellation of pathological changes in the ET cerebellum, present primarily in the cerebellar cortex and involving the Purkinje cell (PC) and its neighboring neuronal populations. These include an increase in torpedoes and associated PC axonal pathologies [7, 8], an increase in heterotopic PCs [9], and abnormal basket cell axons with a dense and tangled appearance (“hairiness”) surrounding the PC soma and elongated processes extending past the PC axon initial segment [10, 11]. In addition to these changes, we have reported PC loss [7, 12], a finding that has been variably reproduced [13–15]. These pathological changes reinforce the notion that the cerebellum is of mechanistic importance in ET [16].
Both familial and sporadic forms of ET exist [17, 18]. Some familial ET cases can have an earlier age of disease onset; in fact, the onset of ET during childhood is generally familial [19]. The two forms of ET differ with respect to underlying etiologies, with familial forms being the result of underlying susceptibility genes [20–24]. They have also been shown to differ with respect to blood concentrations of neurotoxin harmane, being increased in both familial and sporadic ET cases versus controls but to a greater degree in familial ET cases [25–27]. The higher concentration in familial ET cases suggests that the mechanism for this elevated harmane concentration may be at least partly genetic and/or metabolic (i.e., possibly a combination of an inherited tendency for decreased metabolism in the setting of increased exposure) [26]. Given their etiological differences, it is possible that familial and sporadic ET could be pathophysiologically distinct as well. We capitalized on a large, prospectively assembled collection of ET brains, including both familial and sporadic forms, to investigate whether the postmortem changes in the cerebellum differ across these two disease groups.
Methods
Brain Repository, Study Subjects, and Neuropathological Assessment
ET brains were from the Essential Tremor Centralized Brain Repository (ETCBR), a joint effort between investigators at Yale and Columbia Universities [28]. All ET diagnoses were carefully assigned using three sequential methods, as described in detail [8]. In brief, the clinical diagnosis of ET was initially assigned by treating neurologists and then confirmed by an ETCBR study neurologist (EDL) using questionnaires, review of medical records, and review of Archimedes spirals. Third, a detailed, videotaped, neurological examination was performed and published diagnostic criteria applied, as described [29]. Total tremor scores (range = 0–36) were assigned based on the severity of postural and kinetic tremor (pouring, drinking, using spoon, drawing spirals, finger-nose-finger) on examination [28]. None of the ET cases had a history of traumatic brain injury, a history of exposure to medications known to cause cerebellar damage or heavy ethanol use, as previously defined [8, 30].
Familial ET was defined using both conservative and liberal criteria. Using conservative criteria, familial ET was the presence, by the case’s report, of at least one first-degree relative with reported ET (n = 20). Using liberal criteria, familial ET was the presence, by the case’s report, of at least one first-or second-degree relative with reported ET (n = 27). Sporadic ET was defined as the absence of any first- or second-degree relatives with reported ET. There were 33 cases.
The majority of the control brains were obtained from the New York Brain Bank (NYBB) (n = 21) and were from individuals followed at the Alzheimer disease (AD) Research Center or the Washington Heights Inwood Columbia Aging Project at Columbia University [28]. They were followed prospectively with serial neurological examinations and were clinically free of AD, ET, Parkinson’s disease (PD), Lewy body dementia, or progressive supranuclear palsy (PSP). Nine control brains were from Harvard Brain Tissue Resource Center (McLean Hospital, Belmont, MA) [28]. During life, all study subjects signed informed consent approved by these University Ethics Boards.
These analyses were performed on a sample of 90 brains comprising a 2:1 age match of 60 ETcases (approximately one-half familial and one-half sporadic) and 30 controls [28]. We performed a power analysis that utilized data from our previous publications on PC counts [7], torpedo counts [7], and heterotopic PCs [9] in ET cases and controls. With a sample size of 14–26 in each comparison group, we would be powered at 90% to detect differences of the magnitude previously detected.
All ET and control brains had a complete neuropathological assessment at the NYBB and Harvard Brain Bank. Brains had standardized measurements of brain weight (grams), postmortem interval (PMI, hours between death and placement of brain in a cold room or upon ice), Braak and Braak AD staging for neurofibrillary tangles [31, 32], and Consortium to Establish a Registry for AD (CERAD) ratings for neuritic plaques [33]. We did not include ET cases with Lewy body pathology (α-synuclein staining) [7] or PSP pathology [34].
Characterization of Cerebellar Pathology
A standard 3 × 20 × 25 mm parasagittal, formalin-fixed, tissue block was harvested from the neocerebellum; the block included the cerebellar cortex, white matter, and dentate nucleus [8]. A senior neuropathologist (P.L.F.) who was blinded to all clinical information counted torpedoes and heterotopic PCs (PCs whose cell bodies were completely surrounded by the molecular layer and that did not contact the granule layer) throughout one entire LH&E 7-μm-thick section [9]. As described, PCs were counted and averaged from 15 microscopic fields at ×100 magnification (LH&E) [8, 28].
In addition, 7-μm-thick paraffin sections were stained by modified Bielschowsky silver technique and a semi-quantitative basket rating scale was applied in each section: 0 (few, or no discernible processes); 1 (sparse number of processes); 2 (moderate number of processes); and 3 (dense tangle of processes). In some instances, as described, the rater used intermediate values (0.5, 1.5, and 2.5) [10, 28].
CalbindinD28k immunohistochemistry was performed in free-floating 100-μm-thick, formalin-fixed vibratome sections of cerebellar cortex to visualize PC axonal morphology. The sections were heated at 37 °C for 10 min in 20 μg/mL Proteinase K (Roche Applied Science) in 10 mM Tris, 0.1 mM EDTA, pH 8, followed by 1% hydrogen peroxide in PBS for 30 min and serum blocking solution (10% normal goat serum, 1% IgG-free bovine serum albumin [Jackson Immunoresearch], 1% Triton™ X-100, in PBS) for 1 h. Rabbit polyclonal anti-calbindin D28k (1:1000, Swant) was applied overnight at 4 °C in antibody diluent (1% IgG-free bovine serum albumin, 1% Triton™ X-100 in PBS). Secondary antibody (1:200, 2 h, biotin-SP goat-anti-rabbit [Fisher Scientific]), followed by streptavidin-horseradish per-oxidase (1:200, 1 h, AbD Serotec, for biotinlyated antibodies) was developed with 3,3′ diaminobenzidene chromogen solution (Dako). As described, PC axonal morphology in 10 randomly selected 100X images was quantified: axon recurrent collaterals (with at least a 90° turn back towards the PC layer from their initial trajectory), thickened PC axonal profiles (axons at least double the width of other apparently normal axons), and PC axonal branching (any PC axon with at least one branch point; multiple bifurcations on the same axon were not separately counted) [8, 28].
Statistical Analyses
We first compared clinical and pathological characteristics between ET cases and controls (Table 1), and then further compared these features in sporadic ET cases vs. familial ET cases (defined using both liberal and conservative methods) (Table 2). Clinical characteristics such as gender were compared using chi-square tests. Age at death and PC counts were normally distributed; thus, we compared groups using Student’s t tests. Torpedoes, heterotopic PCs, hairy basket rating, PC axonal recurrent collateral counts, PC thickened axon counts, and PC axonal branching counts were not normally distributed (Kolmogorov-Smirnov test). Therefore, we used Mann-Whitney tests. We compared postmortem features in 14 comparisons (Table 2); to correct for multiple comparisons, Bonferroni p value was set at 0.0036. Data were analyzed in SPSS (v23).
Table 1.
Clinical and pathological features of controls and ET cases
| Variables | Controls | ET cases | p value |
|---|---|---|---|
| n | 30 | 60 | |
| Age at death (years) | 85.2 ± 5.7 | 86.0 ± 6.6 | 0.57b |
| Age of tremor onset (years) | NA | 44.0 ± 21.6 | NA |
| Median = 47.5 | |||
| Duration of symptoms (years) | NA | 42.0 ± 21.5 | NA |
| Median = 39.0 | |||
| Gender | 0.23c | ||
| Male | 15 (50.0%) | 22 (36.7%) | |
| Female | 15 (50.0%) | 38 (63.3%) | |
| Total tremor scores | NA | 12.45 ± 3.19 | NA |
| Purkinje cell countsa | 10.52 ± 1.49 | 8.82 ± 1.48 | <0.001b |
| Torpedo counts | 3.90 ± 3.28 | 15.38 ± 15.02 | <0.001d |
| Median = 3.00 | Median = 12.00 | ||
| Heterotopic Purkinje cell counts | 6.23 ± 11.45 | 7.65 ± 9.14 | 0.020d |
| Median = 2.00 | Median = 4.50 | ||
| Hairy basket ratings | 1.57 ± 0.61 | 2.00 ± 0.78 | 0.004d |
| Median = 1.50 | Median = 2.00 | ||
| Purkinje cell axonal recurrent collateral counts | 1.01 ± 1.16 | 1.88 ± 1.53 | 0.006d |
| Median = 0.60 | Median = 1.42 | ||
| Purkinje cell thickened axonal profile counts | 0.96 ± 1.60 | 1.76 ± 2.32 | 0.017d |
| Median = 0.55 | Median = 1.09 | ||
| Purkinje cell axonal branching counts | 0.09 ± 0.10 | 0.37 ± 0.40 | <0.001d |
| Median = 0.07 | Median = 0.27 |
Values represent mean ± standard deviation or number (percentage), and for variables with non-normal distribution, the median is reported as well
NA not applicable
Mean number of Purkinje cell counts (PCs) per ×100 microscopic field, among 15 sampled fields
Independent samples t test
Chi-square test
Independent samples Mann-Whitney U test
The italicized entries are statistically significant
Table 2.
Clinical and pathological features of ET cases grouped by family history
| Variables | Sporadic ETa | Familial ET
|
|||
|---|---|---|---|---|---|
| Liberal criterionb | Conservative criterionc | Sporadic ET vs. familial ET liberal criterion | Sporadic ET vs. familial ET conservative criterion | ||
| n | 33 | 27 | 20 | ||
| Age at death (years) | 85.6 ± 7.3 | 86.6 ± 5.8 | 87.5 ± 6.1 | 0.53d | 0.33d |
| Age of tremor onset (years) | 42.7 ± 23.4 | 45.6 ± 19.5 | 45.2 ± 20.0 | 0.61d | 0.70d |
| Duration of symptoms (years) | 42.8 ± 22.0 | 41.0 ± 21.3 | 42.3 ± 21.7 | 0.75d | 0.93d |
| Gender | |||||
| Male | 12 (36.4%) | 10 (37.0%) | 9 (45.0%) | 0.96e | 0.55e |
| Female | 21 (63.6%) | 17 (63.0%) | 11 (55.0%) | ||
| Total tremor scores | 25.3 ± 7.1 | 24.5 ± 5.7 | 22.9 ± 5.8 | 0.65d | 0.27d |
| Purkinje cell counts | 9.09 ± 1.48 | 8.18 ± 2.17 | 8.24 ± 1.50 | 0.059d | 0.047d |
| Torpedo counts | 18.21 ± 18.12 | 11.93 ± 9.24 | 13.15 ± 9.86 | 0.26f | 0.53f |
| Median = 15.0 | Median = 9.00 | Median = 9.00 | |||
| Heterotopic Purkinje cell counts | 9.58 ± 11.21 | 5.30 ± 4.94 | 6.45 ± 5.24 | 0.14f | 0.62f |
| Median = 6.00 | Median = 4.00 | Median = 6.00 | |||
| Hairy basket ratings | 1.98 ± 0.80 | 2.02 ± 0.78 | 2.08 ± 0.69 | 0.84f | 0.70f |
| Median = 2.00 | Median = 2.00 | Median = 2.00 | |||
| Purkinje cell axonal recurrent collateral counts | 2.00 ± 1.69 | 1.76 ± 1.39 | 1.94 ± 1.61 | 0.77f | 0.91f |
| Median = 1.66 | Median = 1.11 | Median = 1.29 | |||
| Purkinje cell thickened axonal profile counts | 2.14 ± 2.54 | 1.37 ± 2.06 | 1.70 ± 2.42 | 0.037f | 0.17f |
| Median = 1.57 | Median = 0.78 | Median = 0.92 | |||
| Purkinje cell axonal branching counts | 0.51 ± 0.50 | 0.25 ± 0.22 | 0.25 ± 0.23 | 0.045c | 0.079c |
| Median = 0.49 | Median = 0.20 | Median = 0.16 | |||
Values represent mean ± standard deviation or number (percentage), and for variables with non-normal distribution, the median is reported as well Mean number of Purkinje cell counts (PCs) per ×100 microscopic field, among 15 sampled fields
Sporadic ET requires absence of either first- or second-degree relative with reported ET
Liberal criteria requires at least one first- or second-degree relative with reported ET
Conservative criteria requires at least one first-degree relative with reported ET
Student’s t test
Chi-square test
Independent samples Mann-Whitney U test
The italicized entries are statistically significant
Results
The 60 ET cases and 30 controls were similar in age at death and gender (Table 1). When compared with controls, ET cases had lower PC counts, more torpedoes, more heterotopic PCs, a higher hairy basket rating, an increase in PC axonal collaterals, an increase in PC thickened axonal profiles, and an increase in PC axonal branching (Table 1).
We compared the clinical characteristics of cases with familial ET to cases with sporadic ET; familial ET was defined using both liberal and conservative criteria (Table 2). There were no differences in age at death, age of tremor onset, duration of symptoms, gender, or total tremor score (Table 2). Familial and sporadic ET cases were similar with respect to the large majority of postmortem features, with few exceptions (Fig. 1). The PC counts were marginally lower in familial ET (liberal criterion = 8.18 ± 2.17, conservative criterion = 8.24 ± 1.50) than sporadic ET (9.09 ± 1.48), respective p values = 0.059 and 0.047, Table 2. The PC thickened axonal profile count was significantly or marginally significantly lower in familial ET (liberal criterion = 1.37 ± 2.06 [median = 0.78], conservative criterion = 1.70 ± 2.42 [median = 0.92]) than sporadic ET (2.14 ± 2.54 [median = 1.57]), respective p values = 0.037 and 0.17, Table 2. Similarly, the PC axonal branching count was significantly or marginally significantly lower infamilialET (liberalcriterion = 0.25±0.22 [median = 0.20], conservative criterion = 0.25 ± 0.23 [median = 0.16]) than sporadic ET (0.51 ± 0.50 [median = 0.49]), respective p values = 0.045 and 0.079, Table 2. There were no pathological changes that differed to a significant degree between sporadic ET and both definitions of familial ET (liberal definition and conservative definition). We made 14 comparisons; after correction for multiple comparisons, there were no group differences.
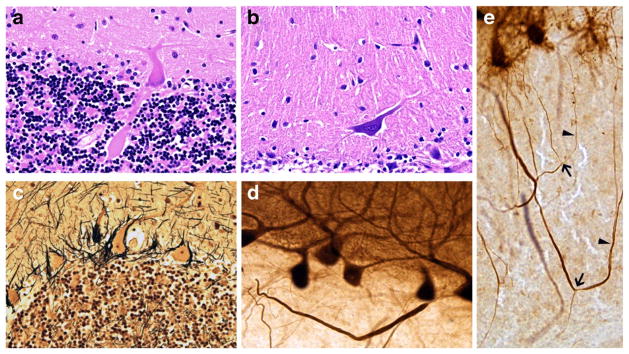
Fig. 1.
a, b Seven micrometer LH&E stained cerebellar cortical sections. a PC axonal torpedo, ×400. b Heterotopic PC mis-localized in the molecular layer, ×200. c Hairy Basket Plexus in 7-um Bielschowsky stained cerebellar cortical section, ×200. d, e Calbindin immunohistochemistry on 100-um cerebellar cortical sections. d Thickened PC axon profile, ×200. e PC axonal branching (arrows) and recurrent collaterals (carets), ×200
Discussion
We capitalized on a large, prospectively assembled collection of ET brains, including both familial and sporadic forms, to investigate whether the postmortem changes in the cerebellum differ across these two disease groups. To our knowledge, this specific issue has not been the focus of prior in-depth analysis. We found that familial ET and sporadic ET cases shared similar pathological changes in cerebellum. We defined familial ET using alternative sets of criteria, and this did not influence the results either. These data indicate that pathological changes in the cerebellum are a part of the pathophysiological cascade of events in both forms of ET. The data further suggest that both groups reach the same pathological endpoints at a similar age of death.
While the two groups of patients, familial and sporadic ET, obviously differ with respect to their presumed underlying etiologies, prior studies have not been able to detect substantive differences between familial ET and sporadic ET in terms of a wide range of clinical variables, including rate of progression [35, 36]. Hence, it is possible that postmortem features share certain similarities as well. It may be that the differences between familial and sporadic ET will eventually become apparent on a molecular level, once the molecular bases for ET are better explored.
A limitation of the current study is that we did not study pathological features in other relevant brain areas that are known to be connected to cerebellar cortex and have been postulated as part of oscillatory loops, such as the inferior olivary nucleus. Nonetheless, we did not observe any morphological alterations in the inferior olivary nucleus in our previous study between ET cases and controls [37]; therefore, it is unlikely that we will discover the differences in this brain region in familial vs. sporadic ET. Another limitation is that we did not employ stereological methods for PC counts; nonetheless, we have previously validated PC counting with a random sampling approach [13]. Finally, familial ET was defined based on reported presence or absence of tremor among relatives of cases (i.e., by history); the relatives were not examined directly to validate their diagnoses nor were issues of false paternity explored. Our study had several strengths. First, we investigated a carefully diagnosed ET cohort. Second, we studied a broad range of pathological changes. Third, our sample size, of 90 brains, including approximately 30 in each group, was considerable.
In conclusion, we did not find major pathological differences in the cerebellar cortex of familial and sporadic ET cases. These data do not support the notion that these forms of ET represent distinct clinical-pathological entities. These data suggest that pathological changes in the cerebellum are a part of the pathophysiological cascade of events in both forms of ET and that both groups reach the same pathological endpoints at a similar age of death.
Acknowledgments
Dr. Louis has received research support from the National Institutes of Health: NINDS #R01 NS094607 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R01 NS086736 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator), and NINDS #R01 NS088257 (principal investigator). He has also received support from the Claire O’Neil Essential Tremor Research Fund (Yale University). Dr. Kuo has received funding from the National Institutes of Health: NINDS #K08 NS083738 (principal investigator), and the Louis V. Gerstner Jr. Scholar Award, Parkinson’s Disease Foundation, and International Essential Tremor Foundation. Dr. Wang has received funding from the Jiangsu Government Scholarship for Overseas studies and Qing Lan Project supported by Jiangsu Provincial Department of Education, China. Dr. Vonsattel has received funding from the National Institutes of Health: NINDS #R01 NS088257 (coinvestigator) and NINDS #R01 NS086736 (coinvestigator). Dr. Faust has received funding from the National Institutes of Health: NINDS #R01 NS088257 (principal investigator) and NINDS #R01 NS085136 (principal investigator).
Footnotes
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–41. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 2.Bares M, Lungu OV, Husárová I, Gescheidt T. Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson’s disease. Cerebellum. 2010;9:124–35. doi: 10.1007/s12311-009-0133-5. [DOI] [PubMed] [Google Scholar]
- 3.Benito-Leon J, Labiano-Fontcuberta A. Linking essential tremor to the cerebellum: clinical evidence. Cerebellum. 2016;15:253–62. doi: 10.1007/s12311-015-0741-1. [DOI] [PubMed] [Google Scholar]
- 4.Cerasa A, Quattrone A. Linking essential tremor to the cerebellum-neuroimaging evidence. Cerebellum. 2016;15:263–75. doi: 10.1007/s12311-015-0739-8. [DOI] [PubMed] [Google Scholar]
- 5.Gitchel GT, Wetzel PA, Baron MS. Slowed saccades and increased square wave jerks in essential tremor. Tremor Other Hyperkinet Mov (N Y) 2013:3. doi: 10.7916/D8251GXN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quattrone A, Cerasa A, Messina D, et al. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. Am J Neuroradiol. 2008;29:1692–27. doi: 10.3174/ajnr.A1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 8.Babij R, Lee M, Cortes E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–61. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo SH, Erickson-Davis C, Gillman A, Faust PL, Vonsattel JP, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. J Neurol Neurosurg Psychiatry. 2011;82:1038–40. doi: 10.1136/jnnp.2010.213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson-Davis CR, Faust PL, Vonsattel JP, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–71. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo SH, Tang G, Louis ED, et al. Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta Neuropathol. 2013;125:879–89. doi: 10.1007/s00401-013-1108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis ED, Babij R, Lee M, Cortes E, Vonsattel JP. Quantification of cerebellar hemispheric purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord. 2013;28:1854–9. doi: 10.1002/mds.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe M, Cortes E, Vonsattel JG, Kuo SH, Faust PL, Louis ED. Purkinje cell loss in essential tremor: random sampling quantification and nearest neighbor analysis. Mov Disord. 2016;31:393–401. doi: 10.1002/mds.26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Symanski C, Shill HA, Dugger B, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. 2014;29:496–500. doi: 10.1002/mds.25845. [DOI] [PubMed] [Google Scholar]
- 15.Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18:626–8. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED. Linking essential tremor to the cerebellum: neuropathological evidence. Cerebellum. 2016;15:235–42. doi: 10.1007/s12311-015-0692-6. [DOI] [PubMed] [Google Scholar]
- 17.Bain PG, Findley LJ, Thompson PD, et al. A study of hereditary essential tremor. Brain. 1994;117:805–24. doi: 10.1093/brain/117.4.805. [DOI] [PubMed] [Google Scholar]
- 18.Louis ED, Ford B, Frucht S, Barnes LF, Tang M, Ottman R. Risk of tremor and impairment from tremor in relatives of patients with essential tremor: a community-based family study. Ann Neurol. 2001;49:761–9. doi: 10.1002/ana.1022. [DOI] [PubMed] [Google Scholar]
- 19.Louis ED, Clark LN, Ottman R. Familial versus sporadic essential tremor: what patterns can one decipher in age of onset? Neuroepidemiology. 2015;44:166–72. doi: 10.1159/000381807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YW, Rong TY, Li HH, et al. Analysis of Lingo1 variant in sporadic and familial essential tremor among Asians. Acta Neurol Scand. 2011;124:264–8. doi: 10.1111/j.1600-0404.2010.01466.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Hernandez N, Kisselev S, et al. Identification of candidate genes for familial early-onset essential tremor. Eur J Hum Genet. 2016;24:1009–15. doi: 10.1038/ejhg.2015.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopfner F, Muller SH, Lorenz D, Appenzeller S, Klebe S, Deuschl G, Kuhlenbaumer G. Mutations in HTRA2 are not a common cause of familial classic ET. Mov Disord. 2015;30:1149–50. doi: 10.1002/mds.26252. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Martin E, Martinez C, Alonso-Navarro H, et al. H1-MAPT and the risk for familial essential tremor. PLoS One. 2012;7:e41581. doi: 10.1371/journal.pone.0041581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan EK, Teo YY, Prakash KM, et al. LINGO1 variant increases risk of familial essential tremor. Neurology. 2009;73:1161–2. doi: 10.1212/WNL.0b013e3181bacfc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis ED, Factor-Litvak P, Liu X, et al. Elevated brain harmane (1-methyl-9H-pyrido[3,4-b]indole) in essential tremor cases vs. controls. Neurotoxicology. 2013;38:131–5. doi: 10.1016/j.neuro.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louis ED, Benito-Leon J, Moreno-Garcia S, et al. Blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentration in essential tremor cases in Spain. Neurotoxicology. 2013;34:264–8. doi: 10.1016/j.neuro.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louis ED, Jiang W, Gerbin M, Mullaney MM, Zheng W. Relationship between blood harmane and harmine concentrations in familial essential tremor, sporadic essential tremor and controls. Neurotoxicology. 2010;31:674–9. doi: 10.1016/j.neuro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo SH, Wang J, Tate WJ, et al. Cerebellar pathology in early onset and late onset essential tremor. Cerebellum. 2016 doi: 10.1007/s12311-016-0826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood genetic study of essential tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16:124–33. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 30.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–35. [PubMed] [Google Scholar]
- 31.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18:S85–8. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging. 1997;18:S91–4. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 34.Louis ED, Babij R, Ma K, Cortes E, Vonsattel JP. Essential tremor followed by progressive supranuclear palsy: postmortem reports of 11 patients. J Neuropathol Exp Neurol. 2013;72:8–17. doi: 10.1097/NEN.0b013e31827ae56e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology. 1991;41:234–8. doi: 10.1212/wnl.41.2_part_1.234. [DOI] [PubMed] [Google Scholar]
- 36.Putzke JD, Whaley NR, Baba Y, Wszolek ZK, Uitti RJ. Essential tremor: predictors of disease progression in a clinical cohort. J Neurol Neurosurg Psychiatry. 2006;77:1235–7. doi: 10.1136/jnnp.2006.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Louis ED, Babij R, Cortes E, Vonsattel JP, Faust PL. The inferior olivary nucleus: a postmortem study of essential tremor cases versus controls. Mov Disord. 2013;28:779–86. doi: 10.1002/mds.25400. [DOI] [PMC free article] [PubMed] [Google Scholar]