Abstract
Background
Essential tremor (ET) is heterogeneous in nature and cases may be subdivided based on clinical features. ET patients may thus be subdivided by age of onset, family history of tremor, and presence of head tremor. We recently described climbing fiber-Purkinje cell (CF-PC) synaptic abnormalities in ET; however, these CF pathological features have not been studied across different ET subtypes.
Objectives
To explore whether these CF-PC synaptic abnormalities differ across ET subtypes.
Methods
We studied two climbing fiber (CF-PC) synaptic pathologies (CF synaptic density and percentage of CFs in the parallel fiber [PF] territory) in the cerebella of 60 ET cases with a range of clinical presentations and 30 age-matched controls.
Results
Compared to controls, ET cases had lower CF synaptic density and a higher percentage of CFs in the PF territory. ET cases with tremor onset <50 years and tremor onset ≥ 50 years did not differ significantly with respect to CF synaptic density and percentage of CFs in the PF territory. Similar results were found when comparing familial vs. sporadic ET cases, and ET cases with head tremor vs. those without head tremor. Among all ET cases, lower CF synaptic density was associated with lower PC counts and higher torpedo counts. In addition, higher percentage of CFs in the PF territory was associated with lower PC counts and higher torpedo counts.
Conclusions
These findings support the notion that changes in the distribution of CF-PC synapses are broadly part of the neurodegenerative process in the ET cerebellum.
Keywords: Essential tremor, Cerebellum, Climbing fiber, Purkinje cell, Pathology, Neurodegenerative
1. Introduction
Essential tremor (ET) is one of the most prevalent movement disorders among adults [1]. Clinical and neuroimaging evidence both suggest the importance of the cerebellum in tremor generation in ET [2,3]. In recent studies, we observed climbing fiber-Purkinje cell (CF-PC) synaptic abnormalities in the ET cerebellum; compared to controls, ET cases exhibited decreased CF synaptic density and an increased percentage of CFs extending into the parallel fiber (PF) territory [4–7]. These changes coexist with a host of other pathological changes in PCs in ET, such as a decrease in PC counts [8] and an increase in torpedoes and associated PC axonal pathologies [8,9], although PC loss is a finding that is variably reproduced [10–12]. The presence of such pathological changes in the ET cerebellum reinforces the notion that degeneration and reorganization of cerebellar structures may be important for disease progression in ET.
ET is also considered a heterogeneous disorder. Several clinical features may divide ET into subtypes. For example, early onset ET cases might differ in their rates of progression and their tremor-related brain oscillatory circuits when compared to late onset ET cases [13,14]. The presence of family history in ET might indicate an underlying genetic etiology [15,16] as well as possible differences in the ability to metabolize naturally-occurring tremorgenic compounds [17]. In addition, ET cases with head tremor might have different degrees of cerebellar involvement than ET cases without head tremor, based on neuroimaging findings [18]. Collectively, these heterogeneous clinical features of ET could reflect diverse alterations in disease etiology, disease-associated changes in brain circuitry, and disease pathogenesis.
Although we recently described climbing fiber-Purkinje cell (CF-PC) synaptic abnormalities in ET [4–7], we have not explored whether these abnormalities differ across disease subtypes. Therefore, we now investigate CF-PC synaptic abnormalities across disease subtypes in ET: early vs. late onset ET, familial vs. sporadic ET, and ET cases with vs. without head tremor. We also examined the association of these more recently described CF-PC synaptic abnormalities with other described changes in ET (e.g., PC loss and higher torpedo counts), as this has yet to be studied.
2. Materials and methods
2.1. ET brains
ET brains were obtained from the Essential Tremor Centralized Brain Repository (ETCBR), a joint effort between investigators from Yale and Columbia Universities. Three sequential methods were used to carefully assign ET diagnoses, as described at length [9]. Briefly, the clinical diagnoses of ET was initially assigned by treating neurologists and then subsequently confirmed by an ETCBR study neurologist (EDL) using clinical questionnaires, review of medical records, and examination of Archimedes spirals. Third, a detailed, videotaped neurological examination was performed, and published diagnostic criteria were applied as described [19]. A total tremor score (range = 0–36) was assigned to each ET case based on the severity of postural and kinetic tremor (pouring, drinking, using spoon, drawing spirals, finger-nose-finger) on videotaped examination [19]. None of the ET cases had a history of traumatic brain injury, exposure to medications known to cause cerebellar damage, or heavy ethanol use as previously defined [20]. Head tremor was assessed as present or absent using clinical information and the videotaped neurological examination.
2.2. Control brains
Most control brains were obtained from the New York Brain Bank (NYBB, n = 21) and were from individuals followed at the Alzheimer’s Disease (AD) Research Center or the Washington Heights Inwood Columbia Aging Project at Columbia University who were clinically free of Alzheimer’s disease (AD), ET, Parkinson’s disease (PD), Lewy body dementia, and progressive supranuclear palsy (PSP). Seven control brains were from the University of Miami Brain Endowment Bank (University of Miami Hospital, Miami, FL), and two were from the University of Maryland Brain and Tissue Bank (University of Maryland, Baltimore, MD), obtained through the NIH NeuroBioBank. During life, all study subjects signed informed consent approved by their respective University Ethics Boards.
2.3. Sample selection (cases and controls)
We selected ET cases for the current study based on age of onset. The mean age of onset for ET is most commonly reported to be between 45 and 55 years, with a bimodal distribution [21–23]. Of the ET cases in the ETCBR, there were 133 cases with clear documentation of age of tremor onset. This age of tremor onset distribution was bimodal, with a median at 50 years. Therefore, we chose the age of onset of 50 to divide the early vs. late onset ET cases. Using data from our previous publications on the PC pathology in subgroups of ET cases and controls [21,24,25], we determined that with each ET group and control group composed of 30 subjects, we would be powered at 90% to detect differences of the magnitude previously detected [21,24,25]. Accordingly, we randomly selected 30 early onset ET cases, 30 late onset ET cases, and 30 controls in the current study. Of the selected cases for this study, data from 14 of 30 controls and 15 of 60 ET cases were reported in prior publications [21,24,25].
2.4. Standard neuropathological assessment
All ET and control brains had a complete neuropathological assessment. Each brain had a standardized measurement of brain weight (grams), postmortem interval (PMI, hours between death and placement of brain in a cold room or upon ice). No ET cases with Lewy body or PSP pathology were included in this study.
A standard 3 × 20 × 25mm parasagittal, formalin-fixed tissue block was harvested from the neocerebellum, which included white matter and dentate nucleus [4,9]. A senior neuropathologist (PLF), blinded to clinical information, counted torpedoes throughout a single Luxol fast blue Hematoxylin & Eosin (LH&E) stained 7-μm section from this block [8]. PCs were also counted and averaged from 15 microscopic fields at 100 × magnification (LH&E) [8].
2.5. Cerebellar immunohistochemistry
Since CF terminals that form synapses with PCs express vesicular glutamate transporter type 2 (VGlut2, now known as SLC17A6), cerebellar tissues were stained for VGlut2, whose puncta in the molecular layer (ML) represent CF-PC synapses [5]. Seven-micrometer paraffin-embedded cerebellar sections were rehydrated and incubated in 3% hydrogen peroxide, followed by antigen retrieval in Tris-urea buffer (0.1M Tris-base, 5% urea, pH 9.5) for 20 min at 95 °C [5]. A suppressive block of 10% normal goat serum and 0.5% bovine serum albumin was used prior to incubation with polyclonal rabbit anti-VGlut2 antibody (1:250) at 4 °C for 40 h, followed by incubation with biotinylated anti-rabbit IgG (1:100, Vector labs, Burlingame, CA, 15 μg/ml). Signals were amplified via avid in-biotin complex (Vector, Burlingame, CA), and sections were developed with 3,3′-diaminobenzidine chromagen solution (Dako). Images were acquired using bright field microscopy (Leica DFC7000T).
2.6. Assessment of CF synaptic density
We quantified CF synaptic density as previously described [4,5]. Image acquisition and quantification were performed by a trained rater (DL) who was blinded to the diagnoses of each subject. The rater was trained by a neurologist experienced in the study of CF pathology (SHK). The rater and the neurologist had an inter-rater reliability (Pearson’s r) of 0.98, based on the quantification of CF synaptic density in 50 fields (10 fields for each of 5 ET cases).
Using a random digit generator, 10 fields in the cerebellar cortex were randomly selected for each subject, and a 400× image directly above the PC layer was acquired for each field. The total number of visualized VGlut2 puncta on proximal PC dendrites above the PC layer was quantified. In the same 400× field, the total visualized CF length was measured with NeuriteTracer (Neuron J), a plugin of Image J (NIH, Bethesda, MD). CF synaptic density was defined as the total number of visualized VGlut2 puncta, divided by the total CF length. Ten fields were quantified to obtain an average CF synaptic density for each subject.
2.7. Assessment of CFs in the PF territory of the cerebellar cortex
To assess the distribution of CFs abnormally extending into the PF territory, we investigated the percentage of CFs extending into the outer 20% of the ML, as previously described [4,5]. These analyses were similarly performed by DL, who was blinded to the diagnoses of each subject. The neurologist and DL had an inter-rater reliability (Pearson’s r) of 0.89 for the quantification of 50 fields of CFs in the PF territory (10 fields for each of 5 ET cases).
Ten 200 × fields that included the entire thickness of the ML were randomly imaged for each subject. These images were then imported into Image J, where a trained rater (DL) measured the total thickness of the ML, and subsequently drew a line at the border between the outer 20% and inner 80% of the ML in the cerebellar cortex. The number of CFs extending beyond the border was quantified. The percentage of CFs extending into the PF territory was calculated (CFs in the outer 20% of ML/total CFs in field × 100). Ten fields were quantified to obtain an average percentage of CFs extending into the outer 20% of the ML for each subject.
2.8. Statistical analyses
Data were analyzed in SPSS (v24). Clinical and pathological characteristics were first compared between ET cases and controls. We compared four different pathological features across these subjects: PC counts, torpedo counts, CF synaptic density, and CFs in the PF territory. Therefore, we applied a Bonferroni correction, and a p value < 0.0125 (0.05 divided by 4) was considered statistically significant. We divided ET cases into different disease subtypes in a series of 3 parallel analyses: early onset (tremor onset < 50 years) vs. late onset (tremor onset ≥ 50 years) ET, familial vs. sporadic ET, and ET cases with vs. without head tremor. We used two criteria for familial ET: liberal (at least one first- or second-degree relative with reported ET) and conservative (at least one first-degree relative with reported ET) [24].
In secondary analyses, we used different age of onset cutoffs (i.e., 40 years in one analysis and 60 years in a second analysis). In a third analysis, we compared ET cases with childhood onset (≥18 years) vs. adult onset (>18 years) [25].
Kolmogorov-Smirnov test was used to determine the normality of continuous variables. In the above analyses, for normally distributed variables Student’s independent samples t-test was used when comparing two groups (ET cases vs. controls) and comparisons between non-normally distributed variables were performed using Mann-Whitney U tests. For comparisons between three or more groups (e.g., early onset ET, late onset ET, and controls) that involved normally-distributed variables, one-way analysis of variance (ANOVA) was used, followed by Tukey post-hoc analyses between individual groups. Kruskal-Wallis tests were used when dealing with non-normally distributed variables.
We also performed correlation analyses between CF pathological features, PC counts, and torpedo counts. Since all variables in the correlation analysis were non-normally distributed, we used Spearman’s rank correlation coefficients.
3. Results
The 60 ET cases and 30 controls were similar in age at death and gender (Table 1). When compared with controls, ET cases had significantly lower PC counts, more torpedoes, lower CF synaptic density and a higher percentage of CFs extending into the PF territory (Table 1), consistent with previous studies on ET cerebellar pathology [4–9].
Table 1.
Clinical and pathological features of controls and essential tremor cases.
| Variables | Controls | ET cases | p value |
|---|---|---|---|
| N | 30 | 60 | |
| Age at death (years) | 84.2 ± 8.6 | 86.6 ± 5.4 | 0.121a |
| Age of tremor onset (years) | NA | 42.6 ± 21.6 Median = 47.5 | |
| Duration of tremor (years) | NA | 44.0 ± 21.9 Median = 40.5 | |
| Gender | 0.291b | ||
| Male | 15 (50.0%) | 23 (38.3%) | |
| Female | 15 (50.0%) | 37 (61.7%) | |
| PC counts | 10.6 ± 1.9 | 8.9 ± 1.4 | 0.003a |
| Torpedo counts | 5.4 ± 4.0 | 27.4 ± 28.0 Median = 17 | 0.001c |
| CF synaptic density (puncta/100 μm) | 21.9 ± 3.6 | 18.0 ± 2.3 Median = 18 | <0.001c |
| Percentage of CFs extending into the PF territory | 20.2 ± 9.2 | 28.9 ± 6.8 | <0.001a |
Values represent mean ± standard deviation or number (percentage), and for variables with a non-normal distribution, the median is reported as well. Mean number of Purkinje cell counts (PCs) per 100 × microscopic field, among 15 sampled fields. Bolded values are significant (with Bonferroni correction).
NA not applicable.
Abbreviations PC: Purkinje cells, CF: climbing fiber, PF: parallel fiber.
Independent samples t-test.
Chi-square test.
Independent samples Mann-Whitney U test.
There were no significant differences between early and late onset forms of ET in terms of PC counts, torpedo counts, CF synaptic density and percentage of CFs extending into the PF territory (Table 2). Compared to controls, both ET groups had significantly more torpedoes, fewer PCs, lower CF synaptic densities and higher percentages of CFs extending into the PF territory (Table 2 and Fig.1), except for PC counts in early onset ET cases that did not reach statistical significance when comparing to controls (p = 0.068). Additionally, we explored different cut-points to define early and late onset ET cases. Using alternative age cut-points of 40 years and 60 years, as well as comparing childhood onset with adult onset ET cases, we had similar observations (Supplementary Tables 1–3). Specifically, these ET groups did not differ in their pathology, except for a significantly higher CF synaptic density (p = 0.005) in ET cases with tremor onset < 60 years compared to that in ET cases with tremor onset ≥ 60 years, while all other ET subgroups compared to controls had lower CF synaptic density and a higher percentage of CFs in PF territory (Supplementary Tables 1–3).
Table 2.
Clinical and pathological features of controls and essential tremor grouped by age of tremor onset.
| Variables | Controls | ET Cases
|
p value (all three groups) |
p value
|
|||
|---|---|---|---|---|---|---|---|
| Age of tremor onset <50 years | Age of tremor onset ≥50 years | Control vs. ET < 50 years | Control vs. ET ≥ 50 years | ET < 50 years vs. ET ≥ 50 years | |||
| n | 30 | 30 | 30 | ||||
| Age at death (years) | 84.2 ± 8.6 | 86.8 ± 6.1 | 86.3 ± 4.7 | 0.293a | 0.307b | 0.443b | 0.965b |
| Age of tremor onset (years) | NA | 23.7 ± 12.4 | 61.5 ± 7.5 | <0.001c | |||
| Duration of tremor (years) | NA | 63.2 ± 12.3 | 24.8 ± 8.0 | <0.001c | |||
| Gender | 0.421d | 0.605d | 0.1903 | 0.426d | |||
| Male | 15 (50.0%) | 13 (43.3%) | 10 (33.3%) | ||||
| Female | 15 (50.0%) | 17 (56.7%) | 20 (66.7%) | ||||
| PC counts | 10.6 ± 1.9 | 9.3 ± 1.5 | 8.6 ± 1.2 | 0.004a | 0.068b | 0.003b | 0.261b |
| Torpedo counts | 5.4 ± 4.0 | 27.6 ± 28.3 | 27.1 ± 28.3 | 0.006e | 0.003f | 0.002f | 0.990f |
| Median = 4.0 | Median = 18.0 | Median = 17.0 | |||||
| CF synaptic density (puncta/100 μm) | 21.9 ± 3.6 | 18.5 ± 2.6 | 17.4 ± 1.8 | <0.001e | <0.001f | <0.001f | 0.101f |
| Median = 22.5 | Median = 18.0 | Median = 17.0 | |||||
| Percentage of CF extending into the PF territory | 20.2 ± 9.2 | 27.9 ± 7.0 | 29.9 ± 6.6 | <0.001a | 0.001b | <0.001b | 0.583b |
Values represent mean ± standard deviation or number (percentage), and for variables with a non-normal distribution, the median is reported as well. Mean number of PC counts per 100 × microscopic field, among 15 sampled fields. Bolded values are significant (with Bonferroni correction).
NA not applicable.
Abbreviations: PC: Purkinje cells, CF: climbing fiber, PF: parallel fiber.
One-way analysis of variance (ANOVA).
Post hoc multiple comparisons (Tukey HSD) in one-way analysis of variance (ANOVA).
Independent samples t-test.
Chi-square test.
Independent samples Kruskal-Wallis H test.
Independent samples Mann-Whitney U test.
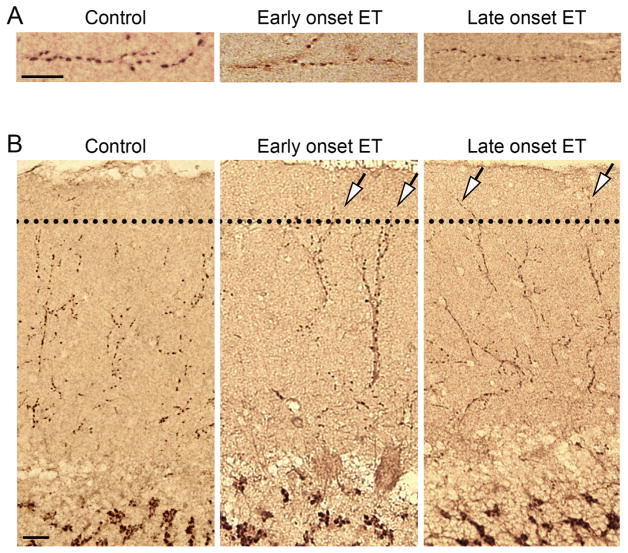
Fig. 1. CF alterations in the ET cerebellar molecular layer.
(A) Decreased CF synaptic density on PC dendritic shafts in the molecular layer of early and late onset ET compared to controls. Representative cerebellar cortical sections labeled with anti-VGlut2 antibody of a control, an early onset ET case, and a late onset ET case are shown, magnified to demonstrate decreased CF synaptic density in ET cases. Scale bar: 10 μm. (B) CFs in the PF territory in the molecular layer of the cerebellar cortex of controls and essential tremor cases. The dotted line indicates the border between the outer 20% and inner 80% of the molecular layer. Representative cerebellar sections of a control, an early onset ET case, and a late onset ET case demonstrate the increased extension of CFs into the PF territory in ET cases. Scale bar: 25 μm.
Between familial and sporadic ET cases, we found no significant differences in terms of CF synaptic density, percentage of CFs extending into the PF territory, PC counts, and torpedo counts, even when both liberal and conservative criteria were used to define ET cases with family history (Table 3). Similar null findings were discovered when comparing controls with ET cases grouped by the presence vs. absence of head tremor (Supplementary Table 4).
Table 3.
Clinical and pathological features of essential tremor cases grouped by family history.
| Variables | Sporadic ET | Familial ET
|
p value
|
||
|---|---|---|---|---|---|
| Liberal criterion | Conservative criterion | Sporadic ET vs. familial ET liberal criterion | Sporadic ET vs. familial ET conservative criterion | ||
| n | 29 | 31 | 26 | ||
| Age at death (years) | 87. ± 4.8 | 85.6 ± 5.9 | 85.7 ± 6.1 | 0.030a | 0.208a |
| Age of tremor onset (years) | 48.8 ± 20.3 | 36.8 ± 21.5 | 33.6 ± 20.6 | 0.024b | 0.007b |
| Median = 55.0 | Median = 40.0 | Median = 33.50 | |||
| Duration of tremor (years) | 38.7 ± 22.1 | 49.0 ± 20.9 | 52.3 ± 20.2 | 0.070a | 0.022a |
| Gender | 0.639c | 0.825c | |||
| Male | 12 (41.4%) | 11 (35.5%) | 10 (38.5%) | ||
| Female | 17 (58.6%) | 20 (64.5%) | 16 (61.5%) | ||
| PC counts | 8.6 ± 1.4 | 9.3 ± 1.3 | 9.4 ± 1.3 | 0.107a | 0.093a |
| Torpedo counts | 23.2 ± 24.9 | 31.4 ± 30.6 | 29.4 ± 32.8 | 0.348b | 0.684b |
| Median = 15.0 | Median = 21.0 | Median = 21 | |||
| CF synaptic density (puncta/100 μm) | 18.0 ± 2.1 | 17.9 ± 2.5 | 17.9 ± 2.6 | 0.748b | 0.689b |
| Median = 18.0 | Median = 18.0 | Median = 17.5 | |||
| Percentage of CFs extending into the PF territory | 28.1 ± 6.8 | 29.7 ± 6.8 | 29.4 ± 7.0 | 0.396a | 0.494a |
Values represent mean ± standard deviation or number (percentage), and for variables with a non-normal distribution, the median is reported as well. Mean number of PC counts per 100 × microscopic field, among 15 sampled fields. Bolded values are significant (with Bonferroni correction).
Abbreviations: PC: Purkinje cells, CF: climbing fiber, PF: parallel fiber.
Independent samples t-test.
Independent samples Mann-Whitney U test.
Chi-square test.
Next, we studied the relationship between CF pathology and ET disease duration or tremor severity and also between CF pathology and PC pathology in our cohort. There were no significant correlations found between disease duration or tremor severity with CF synaptic density or percentage of CFs in the PF territory (Supplementary Table 5). We found that CF synaptic density was correlated with PC counts (r = 0.39, p = 0.005) and inversely correlated with torpedo counts (r= −0.30, p = 0.032). In addition, the percentage of CFs in the PF territory was inversely correlated with PC counts (r= −0.35, p = 0.014) and correlated with torpedo counts (r = 0.37, p = 0.007) (Supplementary Table 5). Finally, CF synaptic density was inversely correlated with the percentage of CFs in the PF territory (r= −0.39, p < 0.001) (Supplementary Table 5). These correlation results confirmed that CF synaptic pathology was inter-related to PC pathological features and could be part of the degenerative process in the ET cerebellum.
4. Discussion
In this study, we found that ET cases and controls differ with respect to CF-PC synaptic pathology, confirming results from previous studies [4–7]. Specifically, ET cases have lower CF synaptic densities and higher percentages of CFs extending into the PF territory compared to controls. We also found that early onset ET cases and late onset ET cases shared similar pathological changes in the cerebellum with regards to CF-PC synaptic pathology, even when considering a range of different age cutoffs. This finding extended to all comparisons involving alternative age cut points, except one. In that one analysis, CF synaptic density was lower in ET cases with an age of onset after 60 years when comparing to ET cases with an age of onset before 60 years, although these two groups of ET cases had similar values for percentage of CFs extending into the PF territory. In sum, differences in age of onset did not seem to drive differences in CF pathologies. We also did not find differences in CF pathologies when comparing familial vs. sporadic ET cases or ET cases with vs. without head tremor.
Although clinical, physiological, and neuroimaging evidence indicates that ET is a heterogeneous disorder [2,3], we found that the ET cases we studied had strikingly homogenous CF pathology. However, as this was a postmortem study, we were only able to investigate the end-point of the disease (i.e., at the time of death). It is possible that the various ET subtypes undergo different disease progressions, yet reach the same end-point pathology. In addition, the homogeneous CF and PC pathology in these ET cases also supports that these pathological features in the cerebellum might be important for the common clinical presentations of these ET cases, i.e. kinetic tremor in the hands, and are the core pathology in ET.
In the correlation analysis between pathological characteristics and CF pathology, we found that lower PC counts and higher torpedo counts correlated with lower CF synaptic densities and higher percentages of CFs in the PF territory. Loss of PCs and increases in torpedoes are hallmarks of the neurodegenerative process in the cerebellum. Spinocerebellar ataxia and multiple system atrophy cases have also been found to exhibit these changes, but to a more severe degree [4]. Interestingly, changes in CF synaptic density might also be the byproducts of a neurodegenerative process, since the degree of decrease in CF synaptic density also correlates with a decrease in PC counts. Although CFs extending into the PF territory also correlated with PC degenerative changes in the current study, this synaptic reorganization of CFs seems to be unique in ET and does not occur in other cerebellar degenerative disorders [4], suggesting a different mechanism. Collectively, these pathological findings suggest that cerebellar degenerative features are seen across all ET subtypes studied and CF pathology is inter-connected with PC degeneration. Future studies in animal models are needed to elucidate these pathological features and the abnormal brain circuitry that generates tremor.
This study has several limitations. First, the pathological features in other relevant brain areas that are known to be connected to the cerebellar cortex, such as the inferior olivary nucleus, were not included. However, we did not find any alterations in the morphology of the inferior olivary neurons in our previous study between ET cases and controls [26], so it is unlikely that changes would have been found among ET subtypes, had we chosen to study this brain region. Second, we did not employ stereological methods for PC counts, although the PC counting method in the current study was previously validated with a random sampling approach [10]. Furthermore, the age of onset is self-reported, and the validity of the self-reported age of onset is unknown [27]. Despite these limitations, our study had several strengths. The ET cohort that we investigated was meticulously diagnosed with strict criteria. Additionally, analysis of CF pathology was performed in a considerable number of ET cases (n = 60). Previously, the largest study on CF pathology in ET cases had a sample size one-third of that in our current study [5].
In conclusion, we did not find any major differences in CF synaptic pathology across ET subtypes studied, although differences between controls and different subtypes of ET cases were confirmed. These data support the notion that the cerebellum is similarly involved across all subgroupings of ET cases and confirms the postulate that changes in the distribution of CF-PC synapses are broadly part of the neurodegenerative process in the ET cerebellum.
Supplementary Material
Acknowledgments
We acknowledge that some of the control brain tissues used in this study were obtained through the NIH NeuroBioBank, with seven brains from the University of Miami Brain Endowment Bank (University of Miami Hospital, Miami, FL) and two from the University of Maryland Brain and Tissue Bank (University of Maryland Medical Center, Baltimore, MD).
Dr. Kuo has received funding from the National Institutes of Health: NINDS #K08 NS083738, the Louis V. Gerstner Jr. Scholar Award, the Parkinson’s Disease Foundation, and the International Essential Tremor Foundation. Dr. Faust has received funding from the National Institutes of Health: NINDS #R21 NS077094, NINDS #R01 NS085136 (principal investigator) and NINDS #RO1 NS088257 (principal investigator). Dr. Louis has received research support from the National Institutes of Health: NINDS #R01 NS094607 (principal investigator), NINDS #R01 NS085136 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator) and NINDS #R01 NS088257 (principal investigator). He has also received support from the Claire O’Neil Essential Tremor Research Fund (Yale University).
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.parkreldis.2018.02.032.
Footnotes
Author contributions
The following is a list of all authors and their contributions in the project and the preparation of the manuscript. These include but are not restricted to: (1) research project: A. conception, B. organization, C. execution; (2) statistical analysis: A. design, B. execution, C. review and critique; and (3) manuscript: A. writing of the first draft, B. review and critique.
Ms. Lee: 1A, 1B, 1C, 2A, 2B, 3A.
Dr. Gan: 1C, 3B.
Dr. Faust: 1A, 2A, 2C, 3B.
Dr. Louis: 1A, 1B, 1C, 2A, 2C, 3B.
Dr. Kuo: 1A, 1B, 2A, 2C, 3B.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 2.Sharifi S, Nederveen AJ, Booij J, van Rootselaar AF. Neuroimaging essentials in essential tremor: a systematic review. Neuroimage Clin. 2014;5:217–231. doi: 10.1016/j.nicl.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dupuis MJM, Evrard FLA, Jacquerye PG, Picard GR, Lermen OG. Disappearance of essential tremor after stroke. Mov Disord. 2010;25:2884–2887. doi: 10.1002/mds.23328. [DOI] [PubMed] [Google Scholar]
- 4.Kuo SH, Lin CY, Wang J, Sims PA, Pan MK, Liou JY, Lee D, Tate WJ, Kelly GC, Louis ED, Faust PL. Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol. 2017;133(1):121–138. doi: 10.1007/s00401-016-1626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137(12):3149–3159. doi: 10.1093/brain/awu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis RJ, Lin CY, Faust PL, Koeppen AH, Kuo SH. Climbing fiber synaptic changes correlate with clinical features in essential tremor. Neurology. 2015;84(22):2284–2286. doi: 10.1212/WNL.0000000000001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo SH, Lin CY, Wang J, Liou JY, Pan MK, Louis RJ, Wu WP, Gutierrez J, Louis ED, Faust PL. Deep brain stimulation and climbing fiber synaptic pathology in essential tremor. Ann Neurol. 2016;80(3):461–465. doi: 10.1002/ana.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(12):3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 9.Babij R, Lee M, Cortes E, Vonsattel JPG, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136(10):3051–3061. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe M, Cortes E, Vonsattel JPG, Kuo SH, Faust PL, Louis ED. Purkinje cell loss in essential tremor: random sampling quantification and nearest neighbor analysis. Mov Disord. 2016;31(3):393–401. doi: 10.1002/mds.26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18(5):626–628. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Symanski C, Shill HA, Dugger B, Hentz JG, Adler CH, Jacobson SA, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. 2014;29:496–500. doi: 10.1002/mds.25845. [DOI] [PubMed] [Google Scholar]
- 13.Deuschl G, Petersen I, Lorenz D, Christensen K. Tremor in the elderly: essential and aging-related tremor. Mov Disord. 2015;30(10):1327–1334. doi: 10.1002/mds.26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muthuraman M, Deuschl G, Anwar AR, Mideksa KG, von Helmolt F, Schneider SA. Essential and aging-related tremor: differences of central control. Mov Disord. 2015t;30(12):1673–1680. doi: 10.1002/mds.26410. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Hernandez N, Kisselev S, Floratos A, et al. Identification of candidate genes for familial early-onset essential tremor. Eur J Hum Genet. 2016;24(7):1009–1015. doi: 10.1038/ejhg.2015.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopfner F, Muller SH, Lorenz D, et al. Mutations in HTRA2 are not a common cause of familial classic ET. Mov Disord. 2015;30(8):1149–1150. doi: 10.1002/mds.26252. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED, Jiang W, Gerbin M, Mullaney MM, Zheng W. Relationship between blood harmane and harmine concentrations in familial essential tremor, sporadic essential tremor and controls. Neurotoxicology. 2010;31(6):674–679. doi: 10.1016/j.neuro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quattrone A, Cerasa A, Messina D, Nicoletti G, et al. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. AJNR Am J Neuroradiol. 2008;29(9):1692–1697. doi: 10.3174/ajnr.A1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis ED, Ottman R, Ford B, Pullman S, et al. The Washington Heights-Inwood genetic study of essential tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16(3):124–133. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 20.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25(2):228–235. [PubMed] [Google Scholar]
- 21.Kuo SH, Wang J, Tate WJ, et al. Cerebellar pathology in early onset and late onset essential tremor. Cerebellum. 2017;16(2):473–482. doi: 10.1007/s12311-016-0826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis ED, Dogu O. Does age of onset in essential tremor have a bimodal distribution? Data from a tertiary referral setting and a population-based study. Neuroepidemiology. 2007;29(3–4):208–212. doi: 10.1159/000111584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology. 1991;41(2):234–238. doi: 10.1212/wnl.41.2_part_1.234. [DOI] [PubMed] [Google Scholar]
- 24.Louis ED, Kuo SH, Wang J, Tate WJ, Pan MK, et al. Cerebellar pathology in familial vs. sporadic essential tremor. Cerebellum. 2017;16(4):786–791. doi: 10.1007/s12311-017-0853-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis ED, Kuo SH, Tate WJ, Kelly GC, Faust PL. Cerebellar pathology in childhood-onset vs. adult-onset essential tremor. Neurosci Lett. 2017;659:69–74. doi: 10.1016/j.neulet.2017.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louis ED, Babij R, Cortes E, Vonsattel JPG, Faust PL. The inferior olivary nucleus: a postmortem study of essential tremor cases versus controls. Mov Disord. 2013;28(6):779–786. doi: 10.1002/mds.25400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louis ED. Age of onset: can we rely on essential tremor patients to report this? Data from a prospective, longitudinal study. Neuroepidemiology. 2013;40(2):93–98. doi: 10.1159/000341903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.