Abstract
Adoption of regenerative strategies for heart failure is challenged by mixed outcomes in clinical trials. Ongoing development plans strive to improve biotherapeutic potency, optimize delivery, standardize dosing, and target responsive patient populations. The Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART) program offers advanced experience in clinical development of next generation regenerative therapies.
Keywords: Cardiopoiesis, Clinical Development, Clinical Trial, Disease Severity, Dose, Regenerative Medicine
Patients with cardiac remodelling and ventricular chamber dilation are at risk to progress into pump failure refractory to standard of care. Innovative treatments are needed to alter disease course, avert end-stage organ deterioration, and delay/avoid destination assist-device implantation or transplantation.
Targeting tissue restoration, cell-based therapies are potentially paradigm-shifting interventions. However, clinical trials are confounded by inter-trial and inter-patient variability underscoring the complexity in translating promising biotherapies into viable solutions. Inconsistent efficacy is ascribed to an unpredictable potency of cell products, limited retention following delivery, and disease heterogeneity.1 Calls for transnational cooperation have been issued to ensure that translational and clinical readiness of regenerative therapies is pursued in a systematic manner.2
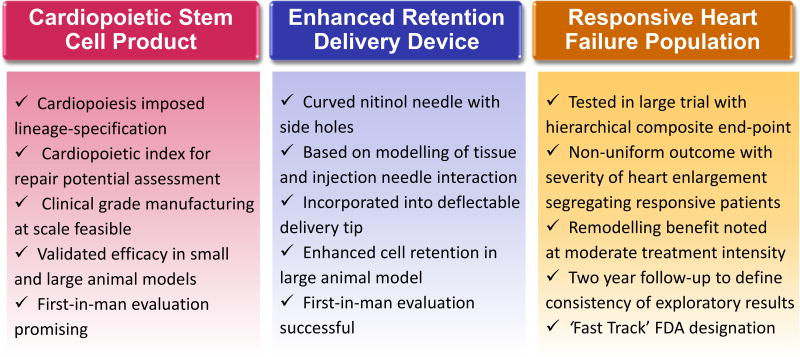
The Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART) development program draws from multinational public-private collaborations. This science-driven discovery-development-delivery pathway leverages an optimizing approach of guided cardiopoiesis to mitigate variability inherent to cell products/patients, and integrates a quality system to certify regenerative proficiency of a biotherapy candidate.3 Cardiopoiesis imposes lineage-specifying instructions on stem cells to promote cardioreparative proclivity (Figure). Accordingly, a ’cardiopoietic index‘ which employs gene-expression profiling was developed to assess the regenerative quotient of patient derived stem cells.4 This quality standard allows pre-assessment of repair potential.
Figure.
The CHART program has provided relevant learnings pertinent to an optimized biotherapeutics, an enhanced delivery system, and scoping of responsive individuals in the setting of the largest cardiopoietic stem cell therapy clinical trial to date.
The cardiopoietic cell phenotype demonstrated promise in proof-of-concept preclinical studies, both in small5 and large6 animal models, providing the foundation for clinical exploration. The first-in-man C-CURE trial (Cardiopoietic stem Cell therapy in heart failURE) evaluated feasibility and safety of the cardiopoiesis technology in 48 randomized patients with chronic ischemic heart failure.7 Cardiopoietic stem cells (dose range: 605 to 1,168 × 106 cells extrapolated from pre-clinical experience) were endomyocardially delivered under electromechanical guidance on average ≈48 months after myocardial infarction. There was no evidence of cardiac or systemic toxicity. The C-CURE trial documented signs of efficacy including improved left ventricular ejection fraction and reduction in left ventricular end-systolic volume. Favorable impact on global parameters, such as the 6-min walk distance test, was noted along with benefit in a composite clinical score encompassing cardiac and general wellness end-points.7
In the ensuing clinical development step, and to address limited cell retention, the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART) program relied on a novel delivery device that yields improved cell retention.8 Based on modelling of tissue and injection needle physical interaction, a curved nitinol needle containing side-holes was incorporated into a deflectable delivery tip (Figure). The CHART program leveraged these advances in the largest cardiovascular regenerative medicine clinical trial to date. The CHART-1 (Congestive Heart Failure CArdiopoietic Regenerative Therapy) trial was conducted across 39 hospitals in 351 randomized ischemic heart failure patients on optimal guidelines-directed standard of care.9 Similar to the C-CURE trial, patients had a history of myocardial infarction yet inclusion did not require documentation of ongoing ischemia. Trial design incorporated a sham-controlled procedure with double-blinded analysis. The single dose of up to 600 × 106 cardiopoietic cells reflected regulatory advice relying on preclinical testing, and was delivered endomyocardially leveraging the new cell retention-enhanced catheter. In the overall study population, the trial was neutral regarding the primary endpoint consisting of a hierarchical composite of mortality, worsening heart failure, Minnesota Living with Heart Failure Questionnaire score, 6-min walk test, left ventricular end-systolic volume, and left ventricular ejection fraction at 9 months follow-up.9 CHART-1 demonstrated a safe profile. While interventionalists-reported unblinded procedural events were higher in the active group, the incidence of overall blinded events - reported by clinicians responsible for patient care - was similar between groups. Notable insights relevant for further evaluation of regenerative interventions were obtained (Figure).
First, the CHART-1 trial set a standard in the design of cardiac regenerative clinical studies. In contrast to conventional clinical criteria-driven randomization, CHART-1 utilized a scheme where randomization followed initial stem cell expansion. This approach aims to mitigate inherent variance in baseline stem cell function. CHART-1 is also the first large randomized cell therapy trial that applied a hierarchical composite end-points.10 In addition, the double-blind design was implemented in the setting of a sham-controlled procedure congruent with stringent protocols. Ethical considerations circumvented potential procedural risk related to placebo injections in a vulnerable population. Rigorous implementation of double-blinded reporting of post-procedural events, in tandem with core laboratory assessments, ensured unbiased evaluation of endpoints. It should be noted that the clinical development path may vary depending on the product or target population. In this regard the methodology of sham controls versus placebo injections differs from the recent ×Cell-DCM trial11 but is comparable to the ongoing DREAM-HF trial.12 CHART-1 likewise differs in cell dosing reflecting the nature of respective biological products and insights from (pre)clinical testing. Similar to CHART-1, modern studies increasingly utilize composite end-points to comprehensively gauge efficacy. We submit that the employed design and methodology are justified as they facilitate timely and adequate readout of clinical status, and as such provide a blueprint for studies that are to follow.
Second, exploration of the CHART-1 trial primary composite endpoint according to heart failure severity at baseline prospectively revealed a clinically relevant patient population that significantly benefited from cardiopoietic cell therapy.9 This target population, representing 60% of the whole study cohort, was identified by the severity of heart enlargement, namely a baseline left ventricular end-diastolic volume (LVEDV) between 200 and 370 mL.9 The effect was sustained at 52 weeks (unpublished data). Patients displaying a lower (<200 mL) or greater (>370 mL) LVEDV did not appear to respond to cell therapy beyond standard of care.9 This observation is consistent with non-uniform responses reported in revascularization, interventions targeting mitral regurgitation or with cardiac resynchronization therapy. The CHART-1 finding of a responsive patient population defined by baseline LVEDV documents for the first time the significance of disease severity in defining outcome of a cell-based therapy.9 This observation is relevant to streamline patient-tailored designs of future trials and/or potential clinical applications of cell therapy targeting individuals with the highest likelihood of response.
Third, besides defining the responsive LVEDV range, pre-defined longitudinal echocardiographic analysis through 52 weeks demonstrated benefit of cardiopoietic cells on LV volumes reduction.13 It is notable that the extent of volume reduction achieved on top of optimal care, was comparable to established medical or device-based interventions associated with improved long-term outcomes.13 These encouraging insights with cardiopoietic cell therapy should be considered in light of the prognostic value of LV reverse remodelling in predicting improved outcomes in patients with advanced heart failure.14
Fourth, LV volumes substantially improved in patients treated with ≤19 injections compared to sham controls or those treated with more injections.13 The inverse relationship between number of injections and improved LV volumes is consistent with a ceiling effect observed in several studies using autologous or allogeneic cell preparations.15 A ceiling-like effect observed in the CHART-1 trial does not appear related to the rise in post-procedural troponin. Troponin T levels, as assessed by the central core laboratory at 6 and 24 h post-procedure, were similar across the range of injections. Rather, it may relate to superior retention of delivered cells due to the optimized needle-myocardial relationship achieved with the employed catheter. Here, factors related to a saturation effect in relation to the extent of eligible segments with 8 mm wall thickness, their distribution or overcrowding during delivery are to be considered. The size of the CHART-1 population and the scheduled 2 year analyses offer a unique opportunity to obtain insights into the relationship between treatment intensity and clinical outcomes, specifically in patients with severely enlarged left ventricles identified as potential high responders. In case of projected benefit detectable already at lower dose regimens, such insights would impact future intervention strategies when using refined delivery methods beyond single-end hole delivery catheters or when using superior delivery platforms. Hence, unlike conventional pharmacological studies, the CHART-1 trial underscores that dose-dependency in interventional cell biotherapy does not follow classical drug regimens established with small chemicals. Absence of traditional dose-dependent relationships in case of optimized cell products with optimized delivery platforms, where “less is better“, implies the need of carefully determining proper posology using tailored approaches that address the integral biotherapy/delivery/patient triad.15
Clinical experience generated by the CHART program informed the decision of the Food and Drug Administration (FDA) to grant ‘Fast Track’ designation to cardiopoietic stem cell therapy for reduction in mortality, hospitalization and improvement in quality of life for patients with chronic heart failure secondary to ischemic cardiomyopathy with baseline LVEDV between 200 and 370 ml. The FDA Fast Track Development Program provision is intended to facilitate development and expedite review of drugs to treat serious and life-threatening conditions so that an approved product can reach patients expeditiously.
Cardiopoietic stem cell therapy has now reached advanced clinical trial testing. Exploratory analyses of treatment intensity on clinical end-points including cardiovascular morbidity and mortality may further refine optimal cardiopoietic cell regimens in target patient populations with cardiac enlargement. The overall clinical experience with the upcoming 2 year-long follow up will be decisive to determine the consistency of early findings and their clinical implications.
Acknowledgments
Sources of Funding
Andre Terzic and Atta Behfar are supported by National Institutes of Health (HL134664), Marriott Family Foundation, Michael S and Mary Sue Shannon Family, Russ and Kathy VanCleve Foundation, Leducq Fondation, Florida Heart Research Institute, and Mayo Clinic Center for Regenerative Medicine. William Wijns is supported by Science Foundation Ireland.
Disclosures
Jozef Bartunek and William Wijns have been members of an institution which co-founded Cardio3Biosciences (now Celyad). Jozef Bartunek reports that all consultancy/speakers fees and research contracts are directed to Cardiac Research Institute, Aalst, Belgium. Andre Terzic and Atta Behfar are co-inventors on patents US 20080019944 and US 20120100533. Mayo Clinic has administered research grants and has rights to future royalties from Celyad. William Wijns reports institutional research grants and speakers fee from device companies (Abbott Vascular, Biotronik, Mi-Cell, MicroPort, Terumo); he is a co-founder of Argonauts Partners, an innovation facilitator.
References
- 1.Terzic A, Behfar A, Filippatos G. Clinical development plan for regenerative therapy in heart failure. Eur J Heart Fail. 2016;18:142–144. doi: 10.1002/ejhf.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández-Avilés F, Sanz-Ruiz R, Climent AM, Badimon L, Bolli R, Charron D, Fuster V, Janssens S, Kastrup J, Kim HS, Lüscher TF, Martin JF, Menasché P, Simari RD, Stone GW, Terzic A, Willerson JT, Wu JC TACTICS (Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes) Writing Group; Authors/Task Force Members. Chairpersons; Basic Research Subcommittee; Translational Research Subcommittee; Challenges of Cardiovascular Regenerative Medicine Subcommittee; Tissue Engineering Subcommittee; Delivery, Navigation, Tracking and Assessment Subcommittee; Clinical Trials Subcommittee; Regulatory and funding strategies subcommittee; Delivery, Navigation, Tracking and Assessment Subcommittee. Global position paper on cardiovascular regenerative medicine. Eur Heart J. 2017;38:2532–2546. doi: 10.1093/eurheartj/ehx248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terzic A, Behfar A. Stem cell therapy for heart failure: Ensuring regenerative proficiency. Trends Cardiovasc Med. 2016;26:395–404. doi: 10.1016/j.tcm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crespo-Diaz R, Yamada S, Bartunek J, Perez-Terzic C, de Waele P, Mauën S, Terzic A, Behfar A. Cardiopoietic index predicts heart repair fitness of patient-derived stem cells. Biomark Med. 2015;9:639–649. doi: 10.2217/bmm.15.31. [DOI] [PubMed] [Google Scholar]
- 5.Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56:721–734. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emmert MY, Wolint P, Jakab A, Sheehy SP, Pasqualini FS, Nguyen TD, Hilbe M, Seifert B, Weber B, Brokopp CE, Macejovska D, Caliskan E, von Eckardstein A, Schwartlander R, Vogel V, Falk V, Parker KK, Gyöngyösi M, Hoerstrup SP. Safety and efficacy of cardiopoietic stem cells in the treatment of post-infarction left-ventricular dysfunction - From cardioprotection to functional repair in a translational pig infarction model. Biomaterials. 2017;122:48–62. doi: 10.1016/j.biomaterials.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 8.Sherman W, Bartunek J, Dolatabadi D, Sanz-Ruiz R, Beleslin B, Wojakowski W, Heyndrickx G, Zefu Kimpalou J, Waldman SA, Laarman GJ, Seron A, Behfar A, Latere JP, Terzic A, Wijns W for the CHART Program. First-in-human use of a retention-enhanced catheter for endomyocardial cell delivery. JACC Cardiovasc Interv. 2018;11:412–414. doi: 10.1016/j.jcin.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 9.Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, Merkely B, Musialek P, Wojakowski W, Andreka P, Horvath IG, Katz A, Dolatabadi D, El Nakadi B, Arandjelovic A, Edes I, Seferovic PM, Obradovic S, Vanderheyden M, Jagic N, Petrov I, Atar S, Halabi M, Gelev VL, Shochat MK, Kasprzak JD, Sanz-Ruiz R, Heyndrickx GR, Nyolczas N, Legrand V, Guédès A, Heyse A, Moccetti T, Fernandez-Aviles F, Jimenez-Quevedo P, Bayes-Genis A, Hernandez-Garcia JM, Ribichini F, Gruchala M, Waldman SA, Teerlink JR, Gersh BJ, Povsic TJ, Henry TD, Metra M, Hajjar RJ, Tendera M, Behfar A, Alexandre B, Seron A, Stough WG, Sherman W, Cotter G, Wijns W. CHART Program. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J. 2017;38:648–660. doi: 10.1093/eurheartj/ehw543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartunek J, Davison B, Sherman W, Povsic T, Henry TD, Gersh B, Metra M, Filippatos G, Hajjar R, Behfar A, Homsy C, Cotter G, Wijns W, Tendera M, Terzic A. Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial design. Eur J Heart Fail. 2016;18:160–168. doi: 10.1002/ejhf.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel AN, Henry TD, Quyyumi AA, Schaer GL, Anderson RD, Toma C, East C, Remmers AE, Goodrich J, Desai AS, Recker D, DeMaria A ixCELL-DCM Investigators. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet. 2016;387:2412–2421. doi: 10.1016/S0140-6736(16)30137-4. [DOI] [PubMed] [Google Scholar]
- 12.Westerdahl DE, Chang DH, Hamilton MA, Nakamura M, Henry TD. Allogeneic mesenchymal precursor cells (MPCs): an innovative approach to treating advanced heart failure. Expert Opin Biol Ther. 2016;16:1163–1169. doi: 10.1080/14712598.2016.1206526. [DOI] [PubMed] [Google Scholar]
- 13.Teerlink JR, Metra M, Filippatos GS, Davison BA, Bartunek J, Terzic A, Gersh BJ, Povsic TJ, Henry TD, Alexandre B, Homsy C, Edwards C, Seron A, Wijns W, Cotter G CHART Investigators. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur J Heart Fail. 2017;19:1520–1529. doi: 10.1002/ejhf.898. [DOI] [PubMed] [Google Scholar]
- 14.Tompkins BA, Rieger AC, Florea V, Banerjee MN, Hare JM. New insights into cell-based therapy for heart failure from the CHART-1 study. Eur J Heart Fail. 2017;19:1530–1533. doi: 10.1002/ejhf.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terzic A, Behfar A. Posology for regenerative therapy. Circ Res. 2017;121:1213–1215. doi: 10.1161/CIRCRESAHA.117.312074. [DOI] [PMC free article] [PubMed] [Google Scholar]