Abstract
Riociguat is the treatment of choice for inoperable patients with chronic thromboembolic pulmonary hypertension (CTEPH). We addressed here whether additional balloon pulmonary angioplasty (BPA) provides further benefits. A prospective series of 36 consecutive patients with inoperable CTEPH were treated with riociguat at least three months before BPA. All patients underwent diagnostic workup at baseline, before BPA treatments, and six months after final intervention. The main outcome measures were pulmonary hemodynamic parameters and World Health Organization (WHO) functional class (FC). Significant improvements in pulmonary hemodynamics and physical capacity were observed for riociguat treatment, and subsequent BPA interventions yielded further benefits. With targeted medication, WHO FC improved by at least one class in 13 (36.1%) patients (P = 0.01). Hemodynamic assessment showed significant improvements in mean pulmonary arterial pressure (mPAP) (49 ± 12 mmHg vs. 43 ± 12 mmHg; P = 0.003) and PVR (956 ± 501 dyn·s·cm–5 vs. 517 ± 279 dyn·s·cm–5; P = 0.0001). Treatment with a combination of targeted medication and BPA resulted in WHO FC improvement in 34 (94.4%) patients. Hemodynamic assessment showed significant improvement in mPAP (43 ± 12 mmHg vs. 34 ± 14 mmHg; P = 0.0001) and PVR (517 ± 279 dyn·s·cm–5 vs. 360 ± 175 dyn·s·cm–5; P = 0.0001). These findings provide, for the first time, support for the therapeutic strategy recommended by current guidelines.
Keywords: chronic thromboembolic pulmonary hypertension, balloon pulmonary angioplasty, targeted medication, riociguat
Approximately one-third of all patients with diagnosed chronic thromboembolic pulmonary hypertension (CTEPH) are not amenable to surgical pulmonary endarterectomy (PEA), mainly due to peripheral localization of pulmonary vascular obstructions.1 Several pulmonary arterial hypertension (PAH) therapies have been considered for use in CTEPH patients: bosentan, an endothelial receptor antagonist,2 and sildenafil, an inhibitor of phosphodiesterase-5,3 were found to improve pulmonary hemodynamics, but there was no significant change in patients’ physical capacity, and randomized controlled trials (RCT) failed to meet their primary endpoint. Riociguat, a stimulator of soluble guanylate cyclase, is the first drug that improves not only pulmonary hemodynamics but also the physical capacity of inoperable CTEPH patients, and it is the first drug that has been approved for this indication.4–6
An increasing number of inoperable CTEPH patients are currently being treated with balloon pulmonary angioplasty (BPA), but the use of targeted medical treatment differs among various centers.7–13 As the evidence for BPA is still scarce, with a lack of long-term data and/or controlled clinical trials, this interventional therapy is not clearly recommended in current guidelines. Moreover, guidelines describe BPA as a further, additional treatment option for inoperable CTEPH patients (IIb C) after initiating riociguat without any evidence to support this recommendation.14
Therefore, the aim of the present study was to determine the effects of riociguat treatment in inoperable CTEPH patients with BPA-feasible target lesions determined by angiography. Furthermore, additional effects of BPA on top of medical treatment were investigated.
Methods
Patient selection
Patients admitted to the Kerckhoff Clinic, Bad Nauheim, Germany, who, after evaluation in a multidisciplinary CTEPH conference, were scheduled for BPA between March 2014 and July 2017 were considered eligible for inclusion in the present prospective, observational cohort study. The Kerckhoff Clinic serves as an international reference center for CTEPH, with >150 PEA and >200 BPA procedures performed per year. CTEPH was diagnosed in symptomatic patients who presented in at least World Health Organization (WHO) functional class (FC) II with a mean pulmonary arterial pressure (mPAP) of at least 25 mmHg at rest and with pulmonary vascular lesions on computed tomography and conventional biplanar pulmonary artery angiography. Patients were deemed technically inoperable based on a comprehensive assessment of imaging findings; they were included in the study if they were considered to be amenable to BPA. Patients were treated with riociguat and BPA was offered after a period of at least three months of targeted medication if WHO FC was still ≥II.
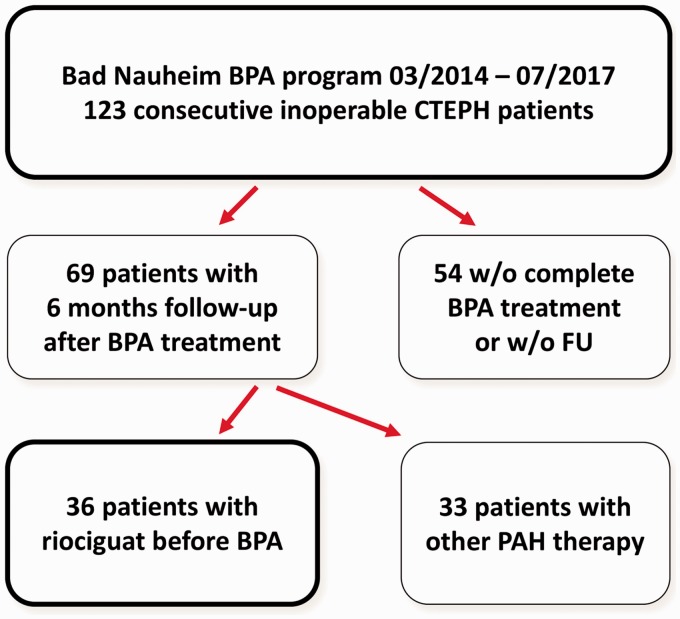
A total of 123 consecutive patients underwent BPA treatment. Follow-up after six months was completed in 69 patients. Of these, 36 patients were without targeted medication at the time of referral (Fig. 1); these patients served as the study cohort.
Fig. 1.
Flow chart describing the balloon pulmonary angioplasty program in Bad Nauheim showing patient selection.
All patients were informed in detail about the investigational nature of the study, including potential risks and benefits, and gave written informed consent to participate. The local ethics committee approved this prospective observational study (AZ 43/14, Giessen University Ethics Committee). All patients included were also enrolled in the New International CTEPH Database of the International CTEPH Association (NCT02656238).
Clinical assessment
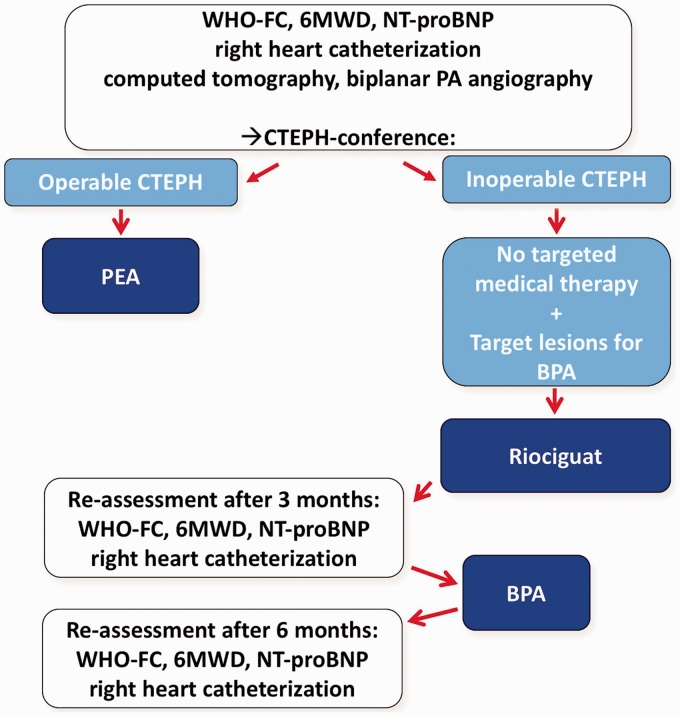
All patients underwent standardized assessment: (1) before initiation of medical treatment; (2) before the first BPA; and (3) six months after the last intervention. Assessment included WHO FC, 6-min walking distance (6MWD), serum levels of creatinine (with calculation of the estimated creatinine clearance) and of the N-terminal fragment of pro-brain natriuretic peptide (NT-proBNP), and right heart catheterization to determine right atrial pressure (RAP), pulmonary arterial pressures (PAP), pulmonary artery wedge pressure (PAWP), cardiac output (CO), cardiac index (CI), and pulmonary vascular resistance (PVR) (Fig. 2).
Fig. 2.
Study flow chart for the 36 patients included in the study.
Targeted medical therapy
Targeted medical therapy with riociguat was initiated in an outpatient setting. The initial dose was 1 mg three times daily, which was increased to the maximally tolerated dose (up to 2.5 mg three times daily) within 4–6 weeks.
Balloon pulmonary angioplasty
BPA was performed as a staged procedure, with a limited number of pulmonary segments being treated during each session. All procedures were performed in conscious patients under local anesthesia (and light sedation, if required). The standard procedure has been described previously.13 Briefly, using femoral or jugular access, a sheath was placed in the pulmonary artery and a guiding catheter was inserted into the target segmental arteries. The guide wire was then advanced into the target subsegmental branches, which were subsequently dilated by multiple balloon inflations. Final pulmonary angiography documented the post-procedural morphological result.
Statistical analysis
All data for continuous variables are expressed as mean ± SD or as median and interquartile range (IQR), as appropriate. Categorical variables are reported as number and percentage. Continuous variables were compared using the Wilcoxon signed-rank test. Longitudinal comparisons were made across repeated observations without correction for multiple comparisons. The cohort data were distributed parametrically, as determined by the Kolmogorov–Smirnov test. All statistical tests were performed with SPSS software, version 22.0. A two-tailed P value <0.05 was considered to indicate statistical significance.
Results
Baseline characteristics and procedures
A total of 36 consecutive patients underwent BPA after initiation of targeted medication with riociguat. The demographics and baseline characteristics of these patients before riociguat administration are given in Table 1. The hemodynamics and functional capacity before riociguat are shown in Table 2. The follow-up was concluded in July 2017.
Table 1.
Baseline characteristics of patients at time of inclusion.
| Last measurement before riociguat treatment | |
|---|---|
| Patients (n (%)) | 36 (100) |
| Age (years) (median (IQR)) | 62 (50–71) |
| Female (n (%)) | 14 (38.9) |
| Body mass index (kg/m2) (median (IQR)) | 24 (23–27) |
| History of VTE (n (%)) | 10 (27.8) |
| Interval between first symptoms to CTEPH diagnosis (months) (median (IQR)) | 16 (6–44) |
| Pulmonary function | |
| TLC (% pred) | 97 ± 25 |
| FVC (% pred) | 84 ± 20 |
| FEV1 (% pred) | 83 ± 22 |
| Anticoagulation | |
| Vitamin K antagonist (n (%)) | 6 (16.7) |
| FXa inhibitor (n (%)) | 30 (83.3) |
Values are given as mean ± SD unless otherwise indicated.
IQR, interquartile range; VTE, venous thromboembolism; CTEPH, chronic thromboembolic pulmonary hypertension; BPA, balloon pulmonary angioplasty; TLC, total lung capacity; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s.
Table 2.
Baseline functional capacity and hemodynamics of patients at time of inclusion.
| n | Last measurement before riociguat treatment | |
|---|---|---|
| Exercise capacity | ||
| WHO FC (n (%)) | 36 | |
| I | 0 (0) | |
| II | 0 (0) | |
| III | 19 (52.8) | |
| IV | 17 (47.2) | |
| 6MWD (m) | 26 | 389 ± 108 |
| Hemodynamics and NT-proBNP | ||
| mPAP (mmHg) | 36 | 49 ± 12 |
| PAWP (mmHg) | 36 | 9 ± 4 |
| CO (L/min) | 36 | 4.3 ± 1.3 |
| CI (L/min/m2) | 36 | 2.2 ± 0.6 |
| PVR (dyn·s·cm–5) | 36 | 956 ± 501 |
| NT-proBNP (ng/L) (median (IQR)) | 31 | 1137 (283–2142) |
Values are given as mean ± SD unless otherwise indicated.
WHO, World Health Organization; FC, functional class; 6MWD, 6-min walking distance; mPAP, mean pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; CO, cardiac output; CI, cardiac index; PVR, pulmonary vascular resistance; NT-proBNP, N-terminal fragment of pro-brain natriuretic peptide.
All patients were treated with riociguat and medical therapy was continued even after the six-month follow-up. In eight patients, the full dose could not be administered due to side effects (e.g. arterial hypotension, gastrointestinal disorders). The interval between initiation of targeted medication to first BPA was five months (range = 2–18 months). A total number of 195 interventions were performed. The median number of sessions per patient was five (range = 5–6). The median number of pulmonary segments targeted in all interventions was 11 (range = 8–13). The median duration from first BPA to the six-month follow-up assessment was 14 months (IQR = 12–16 months).
Response to treatment with targeted medication
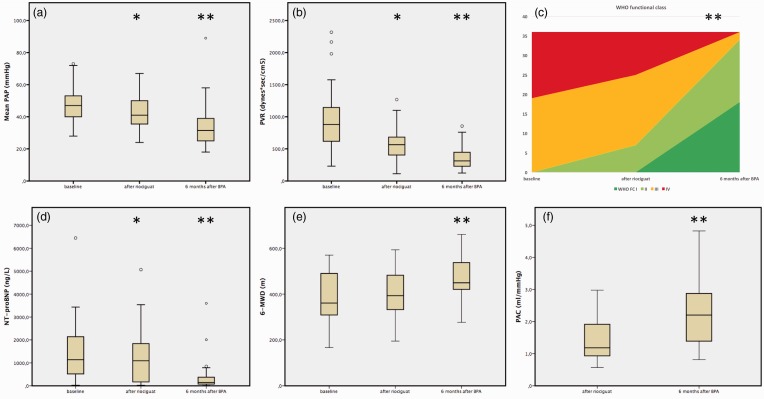
The effects of targeted medical treatment with riociguat on hemodynamics, serum NT-proBNP, and exercise capacity (WHO FC, 6MWD) in comparison with baseline are presented in Table 3 and Fig. 3. The WHO FC improved by at least one class in 13 (36.1%) patients and remained unchanged in 23 (63.9%) patients (P = 0.01). The 6MWD improved by an average of 20 m (5% from baseline; range = 1–17%), but this was not a significant change (P = 0.88). Hemodynamic assessment showed significant improvements in mPAP (49 ± 12 mmHg vs. 43 ± 12 mmHg; P = 0.003) and PVR (956 ± 501 dyn·s·cm–5 vs. 517 ± 279 dyn·s·cm–5; P = 0.0001). NT-proBNP levels were significantly decreased (baseline = 1137 ng/L [IQR = 283–2142] vs. under riociguat = 1010 ng/L [IQR = 128–1887]; P = 0.02).
Table 3.
Functional capacity and hemodynamics with riociguat and 6 months after BPA.
| n | Under riociguat | n | 6 months after BPA | P value | |
|---|---|---|---|---|---|
| Exercise capacity | |||||
| WHO FC (n (%)) | 36 | 36 | 0.0001 | ||
| I | 0 (0) | 18 (50.0) | |||
| II | 7 (19.4) | 16 (44.4) | |||
| III | 18 (50.0) | 2 (5.6) | |||
| IV | 11 (30.6) | 0 (0) | |||
| 6MWD (m) | 32 | 409 ± 102 | 30 | 467 ± 95 | 0.0001 |
| Hemodynamics | |||||
| Right atrial pressure (mmHg) | 36 | 7 ± 4 | 36 | 6 ± 3 | 0.02 |
| mPAP (mmHg) | 36 | 43 ± 12 | 36 | 34 ± 14 | 0.0001 |
| sPAP (mmHg) | 36 | 74 ± 21 | 36 | 59 ± 25 | 0.0001 |
| dPAP (mmHg) | 36 | 25 ± 7 | 36 | 18 ± 8 | 0.0001 |
| PAWP (mmHg) | 36 | 10 ± 3 | 36 | 10 ± 3 | 0.92 |
| DPG (mmHg) | 36 | 15 ± 7 | 36 | 8 ± 8 | 0.0001 |
| TPG (mmHg) | 36 | 33 ± 11 | 36 | 24 ± 13 | 0.0001 |
| CO (L/min) | 36 | 5.0 ± 1.5 | 36 | 5.5 ± 1.3 | 0.0001 |
| CI (L/min/m2) | 36 | 2.6 ± 0.7 | 36 | 2.9 ± 0.6 | 0.02 |
| PVR (dyn·s·cm–5) | 36 | 517 ± 279 | 36 | 360 ± 175 | 0.0001 |
| PAC (mL/mmHg) | 36 | 1.4 ± 0.6 | 36 | 2.3 ± 1.0 | 0.0001 |
| HR (bpm) | 36 | 78 ± 12 | 36 | 70 ± 11 | 0.001 |
| Laboratory findings | |||||
| NT-proBNP (ng/L) (median (IQR)) | 29 | 1,010 (128–1,887) | 35 | 150 (75–385) | 0.0001 |
| Creatinine* (mg/dL) | 28 | 0.98 ± 0.31 | 36 | 0.91 ± 0.28 | 0.02 |
| eGFR (mL/min) | 28 | 82 ± 28 | 36 | 94 ± 59 | 0.05 |
Values are given as mean ± SD unless otherwise indicated.
To convert mg/dL to mmol/L divide by 11.3.
WHO, World Health Organization; mPAP, mean pulmonary artery pressure; sPAP, systolic pulmonary artery pressure; dPAP, diastolic pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; DPG, diastolic pressure gradient; TPG, transpulmonary gradient; CO, cardiac output; CI, cardiac index; PVR, pulmonary vascular resistance; PAC, pulmonary arterial compliance; HR, heart rate; NT-proBNP, N-terminal fragment of pro-brain natriuretic peptide; eGFR, estimated glomerular filtration rate.
Fig. 3.
Effects of riociguat and BPA on (a) mPAP, (b) PVR, (c) WHO FC given in mean values, (d) NT-proBNP, (e) 6MWD, and (f) PAC. The asterisk indicates the significance level (*P < 0.05; **P < 0.001).
Response to treatment with a combination of targeted medication and BPA
The effects of BPA on hemodynamics, serum NT-proBNP, and exercise capacity in comparison with the assessment after at least three months of riociguat are presented in Table 3 and Fig. 3. The WHO FC improved in 34 (94.4%) patients and remained unchanged in two (5.6%) patients. The 6MWD after BPA improved by an average of 58 m (12.5%; range = 2–46%) compared with riociguat (riociguat = mean 409 ± 102 m vs. after BPA = 467 ± 95 m; P = 0.0001). Hemodynamic assessment showed significant improvement in mPAP and PVR. NT-proBNP was significantly decreased six months after BPA.
Complications of BPA
Twenty-seven procedure-related complications occurred during the 195 interventions (13.8% of all interventions). These adverse events were mostly caused by wire perforation of the pulmonary vasculature, resulting in parenchymal bleeding with mild hemoptysis in some cases. Seven patients developed reperfusion edema with clinical symptoms (coughing of frothy secretion, desaturation) and consolidations in chest X-ray during the post-procedural period of 6–24 h (3.6% of all interventions). Non-invasive ventilation, which is routinely used even after mild hemorrhage according to the local standard protocol, was performed in 11 patients. Invasive ventilation was not necessary. All of the patients were alive at the end of the observation period.
Discussion
There is incremental evidence from smaller and mid-sized case series that BPA exerts beneficial effects on pulmonary hemodynamics and physical capacity in inoperable CTEPH patients. The impact of administering riociguat, the only approved treatment for inoperable CTEPH, before BPA has not yet been elucidated. Here, we detail the changes in hemodynamics and exercise capacity induced by riociguat and describe the effects of treating with BPA in addition to this targeted medication. The main findings of this study are: (1) targeted medication with riociguat improves hemodynamics and physical capacity in inoperable CTEPH patients amenable to BPA; (2) the additional interventional treatment of these patients by BPA leads to further improvements; (3) the combination of riociguat and BPA is feasible and safe in this particular group of patients. These findings strengthen the evidence for the current recommendation by the guidelines.14
The changes in physical capacity and pulmonary hemodynamics under targeted medication are in line with published data.4 Based on the results of the CHEST-1 study, Ghofrani et al. reported a mean reduction in PVR of 226 (±248) dyn·s·cm–5 and WHO FC improved in 33% of the patients; in our present cohort, there was also a mean PVR reduction of 439 dyn·s·cm–5 and an improvement in WHO FC in 36.1% of the patients. Furthermore, there were no severe side effects observed and a discontinuation of the medication was not required in any patient; however, full dose acceptance was achieved in 77% of patients.
The outcome measures WHO FC, NT-proBNP, and 6MWD are associated with overall survival at baseline and follow-up,15 and 6MWD changes from baseline are clearly correlated with survival as reported in the CHEST-2 trial.6 In the current study, the 6MWD increased by a mean of 20 m after riociguat treatment and a further 58 m after BPA, clearly indicating the benefits of the sequential treatment with riociguat and BPA.
The changes in physical capacity and pulmonary hemodynamics after BPA in our cohort are comparable to the results of other European groups11,16 but are less distinct than those of Japanese observations.8–10 This has been discussed previously by our working group13 and may be due to an especially well-established program of PEA in Germany that is used less frequently in Japan; thus, some of the patients who underwent BPA in Japan would have been deemed operable in Germany. In our study, patients selected for BPA comprised only those with inoperable disease; hence, patient populations were not comparable. Furthermore, there are no clear data on the use of targeted pretreatment in CTEPH patients treated interventionally. As Lang et al. brought together in their very recent review,16 there is no consensus for the use of PAH therapies in CTEPH patients. Only one center has presented data from their BPA program in which 40% of the patients treated interventionally were on riociguat;17 the authors mainly described changes in MRI findings and did not discriminate hemodynamic changes before and during targeted medication and BPA. Just recently, Aoki et al. reported long-term results of their BPA program: PAH medical therapies were administered in 96% of all patients before BPA.18 However, the use of targeted medication was not standardized: only 17% of all patients received riociguat, which, in fact, represents the best standard of care according to evidence-based criteria; six months after the last intervention the proportion of patients undergoing any targeted treatment was decreased to 68%.18
Our results suggest that the combination of targeted medication with riociguat and BPA is an effective treatment for patients with inoperable CTEPH, leading to significant improvements in physical capacity (WHO FC, 6MWD) and pulmonary hemodynamics. These findings support the recommendations for the treatment of inoperable CTEPH patients available in the latest European guidelines.14 Decreasing PAPs before performing an interventional treatment may be a reasonable concept for avoiding severe complications. However, the observed complication rate is comparable to that of recently published trials.8–12,16,17 The higher rate of non-invasive ventilation can be explained by the routine use of short-term ventilation even after mild bleeding.
Some limitations to this study must be considered. This is a single-center study of an observational nature. Due to the highly selected population of CTEPH patients, there is no matched control group. However, our analysis reflects “real-world” treatment in an international reference center for CTEPH with high-volume surgical and interventional programs. In addition, this study is the first to investigate the sequential treatment with riociguat and BPA in inoperable CTEPH patients.
This is the first observation focusing on the sequential combination of riociguat and BPA in inoperable CTEPH patients. Despite it being a single-center experience, our study provides a realistic perspective on the management of CTEPH patients in a German referral center with an established surgical and interventional CTEPH program. The combination of targeted medical and interventional treatment shows positive effects on physical capacity and pulmonary hemodynamics in inoperable CTEPH patients. A RCT is required to confirm this concept.
Acknowledgments
The authors thank Elizabeth Martinson, PhD, from the KHFI Editorial Office for her assistance with language editing.
Conflict of interest
CBW has received speaker fees or consultant honoraria from Actelion, Bayer AG, BTG, MSD, and Pfizer. HAG has reported receiving fees for serving as a board member for Bellerophon Pulse Technologies, Medscape, OMT, UCB Celltech, and Web MD Global; receiving consultancy fees and fees for serving on a steering committee for Actelion Pharmaceuticals, Bayer, Gilead Sciences, GlaxoSmithKline, Merck, Novartis, and Pfizer; receiving lecture fees from Actelion Pharmaceuticals, Bayer, GlaxoSmithKline, Merck, Novartis, and Pfizer; and receiving grant support from Actelion Pharmaceuticals, Bayer, Novartis, and Pfizer. AR has received a research grant from Pfizer and speaker fees and/or honoraria from Servier, St. Jude Medical, Actelion and Novartis. EM has received speaker fees and/or honoraria for consultations from Actelion, Bayer, GSK, MSD, and Pfizer. SG has received speaker fees from Actelion, Bayer, GSK, and Pfizer. CL has received speaker fees from Abbott, Astra Zeneca, Bayer, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Elixir Medical, and Pfizer. MSDA, AB, MH, CWH, and SK have nothing to disclose.
Funding
This study was supported by the German Centre for Lung Research (DZL), Giessen, Germany. The funding agency had no influence on the collection of data, its analysis and interpretation, or in the right to approve or disapprove publication of the finished manuscript.
References
- 1.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 2.Jaïs X, D’Armini AM, Jansa P, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol 2008; 52: 2127–2134. [DOI] [PubMed] [Google Scholar]
- 3.Suntharalingam J, Treacy CM, Doughty NJ, et al. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest 2008; 134: 229–236. [DOI] [PubMed] [Google Scholar]
- 4.Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, D’Armini AM, Ghofrani HA, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J 2015; 45: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G, D'Armini AM, Ghofrani HA, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016; 4: 372–380. [DOI] [PubMed] [Google Scholar]
- 7.Feinstein JA, Goldhaber SZ, Lock JE, et al. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001; 103: 10–13. [DOI] [PubMed] [Google Scholar]
- 8.Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka M, Inami T, Hayashida K, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 756–762. [DOI] [PubMed] [Google Scholar]
- 10.Sugimura K, Fukumoto Y, Satoh K, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012; 76: 485–488. [DOI] [PubMed] [Google Scholar]
- 11.Andreassen AK, Ragnarsson A, Gude E, et al. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013; 99: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi Y, Miyagawa K, Nakayama K, et al. Balloon pulmonary angioplasty: an additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention 2014; 10: 518–525. [DOI] [PubMed] [Google Scholar]
- 13.Olsson KM, Wiedenroth CB, Kamp JC, et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J 2017; 49: 1602409. [DOI] [PubMed] [Google Scholar]
- 14.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 15.Ghofrani HA, Grimminger F, Grünig E, et al. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016; 4: 361–371. [DOI] [PubMed] [Google Scholar]
- 16.Lang I, Meyer BC, Ogo T, et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamasaki Y, Nagao M, Abe K, et al. Balloon pulmonary angioplasty improves interventricular dyssynchrony in patients with inoperable chronic thromboembolic pulmonary hypertension: a cardiac MR imaging study. Int J Cardiovasc Imaging 2017; 33: 229–239. [DOI] [PubMed] [Google Scholar]
- 18.Aoki T, Sugimura K, Tatebe S, et al. Comprehensive evaluation of the effectiveness and safety of balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension: long-term effects and procedure-related complications. Eur Heart J 2017; 38: 3152–3159. [DOI] [PubMed] [Google Scholar]