Abstract
Background
Underlying coronary artery disease (CAD) is the primary cause of sudden cardiac death in masters athletes (>35 years). Preparticipation screening may detect cardiovascular disease; however, the optimal screening method is undefined in this population. The Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and the American Heart Association (AHA) Preparticipation Screening Questionnaire are often currently used; however, a more comprehensive risk assessment may be required. We sought to ascertain the cardiovascular risk and to assess the effectiveness of screening tools in masters athletes.
Methods
This cross-sectional study performed preparticipation screening on masters athletes, which included an ECG, the AHA 14-element recommendations and Framingham Risk Score (FRS). If the preparticipation screening was abnormal, further evaluations were performed. The effectiveness of the screening tools was determined by their positive predictive value (PPV).
Results
798 athletes were included in the preparticipation screening analysis (62.7% male, 54.6±9.5 years, range 35–81). The metabolic equivalent task hours per week was 80.8±44.0, and the average physical activity experience was 35.1±14.8 years. Sixty-four per cent underwent additional evaluations. Cardiovascular disease was detected in 11.4%, with CAD (7.9%) being the most common diagnosis. High FRS (>20%) was seen in 8.5% of the study population. Ten athletes were diagnosed with significant CAD; 90% were asymptomatic. A high FRS was most indicative of underlying CAD (PPV 38.2%).
Conclusion
Masters athletes are not immune to elevated cardiovascular risk and cardiovascular disease. Comprehensive preparticipation screening including an ECG and FRS can detect cardiovascular disease. An exercise stress test should be considered in those with risk factors, regardless of fitness level.
Keywords: cardiology prevention, cardiovascular, aging, athlete, sports
What are the new findings?
This is the first study to systematically analyse the effectiveness of European cardiovascular preparticipation screening recommendations.
We identified which screening tools had the highest likelihood of predicting coronary artery disease (CAD), where a high Framingham Risk Score (FRS), significant q-waves, exertional dyspnoea and a family history of premature CAD exhibited the highest positive predictive value (PPV).
Preparticipation screening in masters athletes detected 11.4% to have clinically significant cardiovascular disease (CVD) and 8.5% to have a high cardiovascular risk profile.
The physical examination generated high false-positives and a low PPV for detecting valvular disease.
How might it impact on clinical practice in the future?
Masters athletes are not immune to elevated cardiac risk and should be evaluated for cardiovascular risk factors (including lipid profile), family history of CAD and concerning symptoms, and undergo a resting ECG.
Masters athletes, regardless of fitness level, with a family history of premature CAD, abnormal ECG and cardiovascular risk factors may benefit from undergoing a maximal exercise stress test (EST).
Masters athletes with a high FRS, and abnormal EST, ECG or physical exam (ie, diastolic murmur or systolic click), should undergo clinically indicated examinations to confirm or exclude CVD, as recommended by a sports cardiologist.
In considering preparticipation screening of masters athletes, efforts to decrease false-positives include refining the criteria for follow-up and/or eliminating the physical examination, development of a masters athlete-specific ECG interpretation criteria, refinement of the questionnaire, and consideration of other screening modalities.
Introduction
Coronary artery disease (CAD) is the primary cause of sudden cardiac death (SCD) in athletes over 35 years old, 1–3 with symptoms preceding the event in only 12%–36% of cases.1 4 Although regular physical activity reduces cardiovascular and all-cause mortality,5 6 athletes may possess cardiovascular risk factors7–10 or underlying cardiovascular disease (CVD),7 9–11 putting them at heightened risk for SCD during periods of vigorous-intensity activity.12 The risk of SCD transiently increases during vigorous-intensity activity compared with mild or no physical activity, with the greatest risk in inactive men (74.1 relative risk) compared with those who exercise frequently (10.9 relative risk).13 Masters athletes (≥35 years old) are a growing population that require dedicated attention to identify at-risk individuals in order to ensure their safe participation in competitive sports, which often demand sustained vigorous to maximal intensity effort.
Preparticipation screening strategies have been proposed to mitigate the risk of sports-related SCD but have not been extensively evaluated in masters athletes. Self-administered surveys such as the Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and the American Heart Association (AHA)/American College of Sports Medicine Preparticipation Screening Questionnaire are often used, but not mandated.14 Many organisations such as the European Association of Cardiovascular Prevention and Rehabilitation (EACPR), the AHA and the IOC have suggested more comprehensive risk assessments for athletes who routinely engage in vigorous-intensity exercise.15–17 The percentage of masters athletes that undergo preparticipation screening is modest (24.6%–51.5%).18 Healthcare providers tend to refer athletes for preparticipation screening if they are older, or participating in long distance events, rather than considering their traditional cardiovascular risk factors (ie, hypertension, diabetes, hypercholesterolaemia, smoking history and family history).18 The Framingham Risk Score (FRS) is the risk calculator commonly used in Canada to evaluate one’s 10-year risk of having a cardiovascular event based on risk factors (age, sex, total cholesterol, high-density lipoprotein (HDL)-cholesterol, diabetes, smoking history, systolic blood pressure, whether or not medication is taken for blood pressure, and family history of CVD) and is not currently used in preparticipation screening of masters athletes.
Two studies have prospectively used preparticipation screening to detect CVD risk in masters athletes. Both studies used the EACPR recommendations16 and reported that the 12-lead ECG had the highest detection rate for CVD.7 10 There is no Canadian data examining cardiovascular risk and preparticipation screening in masters athletes. Moreover, there is considerable debate regarding the best approach. The purpose of this study was to evaluate the cardiovascular risk in masters athletes following current comprehensive preparticipation screening guidelines, and to assess the effectiveness of screening tools for detecting CVD in masters athletes. We hypothesised that 5% of masters athletes would fulfil the criteria for a diagnosis of CVD.
Methodology
Study design
This cross-sectional screening study included participants throughout British Columbia, Canada (April 2015–January 2016). Study participants were self-referred and recruited through television, radio, social media (Facebook, Twitter), sporting teams, clubs and organisations. Male and female recreational and competitive athletes aged ≥35 years old from a variety of sports were included.
All participants engaged in moderate to vigorous intensity physical activity, at least 3 days per week over the preceding 3 months. Individuals with previously known CAD, peripheral vascular disease, thoracic aortic aneurysm, inherited arrhythmias, congenital heart disease, valvular disease (moderate or greater stenosis or regurgitation), and those with missing documentation or those lost to follow-up were excluded from the analysis.
Athlete examination
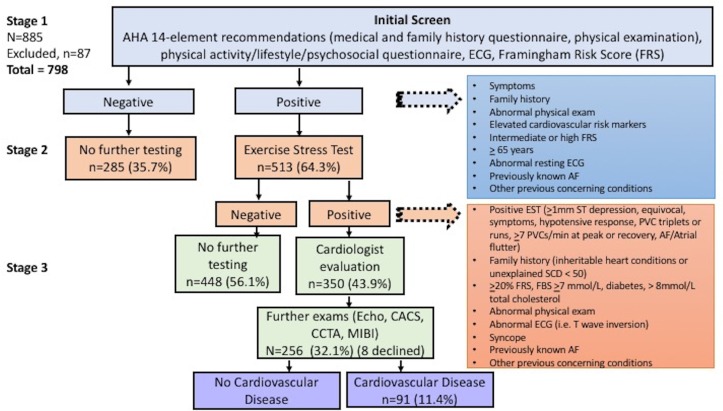
All participants were screened following the EACPR algorithm.16 The study had three stages: (1) initial screening test; (2) exercise stress test (EST); and (3) evaluation by a cardiologist and further examinations (figure 1). The results of the previous stages decided whether a participant proceeds to the next stage. The first two stages were the screening methods where performances of the participants were to be assessed, and the third stage was the reference standard. All participants underwent stage 1, which included the initial preparticipation screen (ie, anthropometrics, resting blood pressure and heart rate, modified version of the AHA 14-element recommendations (medical and family history, physical examination),19 resting ECG, FRS (including fasting lipid and glucose profile), and a lifestyle, physical activity and psychosocial questionnaire). Background information (age, sex, ethnicity, educational level, marital status, income range and occupation) was collected. All ECGs (Mortara Instrument, Milwaukee, Wisconsin) were interpreted by cardiologists with expertise in athlete ECG interpretation using the ‘Seattle Criteria’ (SI, BH).20 The physical examination was performed by cardiologists, cardiology fellows and internal medicine residents.
Figure 1.
Study algorithm. AF, atrial fibrillation; AHA, American Heart Association; CACS, coronary artery calcium score; CCTA, computed cardiac tomography angiography; Echo, echocardiogram; ECG, electrocardiogram;EST, exercise stress test; FBS, fasting blood sugar; MIBI, myocardial perfusion imaging; PVC, premature ventricular complex; SCD, sudden cardiac death.
Participants with abnormal history, physical examination and ECG, intermediate FRS (10%–19%), high FRS (>20%), markedly raised cardiovascular risk factor (fasting blood sugar ≥7.0 mmol/L, >8 mmol/L total cholesterol), history of diabetes, >65 years, and/or other previously concerning conditions were considered abnormal (positive) and underwent an EST (stage 2) (online supplementary material table 1). The intermediate FRS was included due to the recommendations of the Canadian Cardiovascular Society Guidelines for the Diagnosis and Treatment of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult,21 and the age criteria (≥65 years) was included as suggested by the AHA guidelines.17
bmjsem-2018-000370supp001.docx (104.1KB, docx)
Participants were evaluated by a cardiologist (stage 3) if their EST was abnormal or if their initial evaluation identified a positive family history (inheritable heart disease and/or unexplained SCD in a first-degree or second-degree relative <50 years), syncope, high FRS, abnormal ECG, abnormal physical examination and/or other previously concerning conditions (online supplementary material figure 1 ). The cardiologists (who have expertise in athlete evaluation) determined if subsequent examinations were necessary based on their clinical discretion. The final decision to undergo further examination (coronary artery calcium score, coronary CT angiography, cardiac catheterisation, echocardiogram, myocardial perfusion imaging (MIBI), cardiac myocardial resonance imaging, Holter monitoring, 24-hour ambulatory blood pressure monitor) was made collectively by the cardiologist and the patient (once the patient was fully informed of the risks and benefits of the test).
bmjsem-2018-000370supp002.jpg (849.2KB, jpg)
Physical activity exposure
The volume of physical activity was reported as metabolic equivalent task hours per week (MET-hour/week). The type and intensity of the activities were matched to the corresponding METs using the Ainsworth compendium of physical activities.22 Lifetime hours were based on a self-report of total years spent being physically active and were calculated as follows: average total hours of endurance, mixed and strength per week × 52 × training years (adapted from Brugger et al23) (online supplementary material table 2).
bmjsem-2018-000370supp003.docx (19.9KB, docx)
bmjsem-2018-000370supp004.docx (11.1KB, docx)
Outcome measures
Clinically significant CVD (ie, CAD, bicuspid aortic valve, mitral valve prolapse, high premature ventricular contraction (PVC) burden,24 aortic dilation,25 atrial fibrillation (AF), other concerning arrhythmias (ie, second-degree type II atrioventricular (AV) block), hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, dilated cardiomyopathy, coronary artery anomalies, inherited arrhythmias and myocarditis) was the primary outcome. Our secondary outcome was the positive predictive value (PPV) for each screening tool according to the type of CVD (CAD, valvular disease).
Statistical analysis
Continuous variables were expressed as mean and SD. Frequency tables were generated for all categorical data and reported as number of participants (n) and percentage (%). The PPV was calculated using the following formula:
We compared the screening methods using a weighted generalised score statistic. Bonferroni correction was applied for multiple comparisons. Statistical analysis was performed using SPSS V.23.0 software or Excel V.15.33 (Microsoft, Redmond, Washington). Those with missing data were not included in the sample size for the associated variable and the mean and percentage were adjusted accordingly.
Results
Study population
A total of 885 masters athletes were screened. Ten per cent (n=87) were excluded from the analysis (previous CVD n=43; did not meet physical activity requirements n=7; missing documentation n=11; lost to follow-up n=26). Participants were predominately male (62.7%) and Caucasian (87.7%), with a mean age of 54.6±9.5 years (range 35–81) (table 1). Participants took part in 23 sports and were representative of sports commonly played by masters athletes living in British Columbia (online supplementary material figure 2). The most common primary sports (most athletes participated in more than one sport) were running (34.2%), cycling (19.1%), hockey (10.9%), triathlon (9.5%) and rowing (4.3%). Four hundred and seventy-seven (59.8%) participants had completed a minimum of half or full marathon (18.2±23.8, range 1–176). Participants spent 35.1±14.8 years of their lives being active. Weekly time spent in moderate to vigorous physical activity was 7.6±4.3 hours and weekly training volume was 80.8±44.0 MET-hour/week.
Table 1.
Baseline characteristics
| Variables | |
| Number of athletes, n (%) | 798 |
| Male | 500 (62.7) |
| Female | 298 (37.3) |
| Age, years (SD) | 54.6 (9.5) |
| Ethnicity, n (%) | |
| Caucasian | 700 (87.7) |
| Asian/Asian Caucasian | 46 (5.8) |
| South Asian | 5 (0.6) |
| Aboriginal/Aboriginal Caucasian | 5 (0.6) |
| African/African Caucasian | 5 (0.6) |
| Other | 13 (1.6) |
| No response | 24 (3.0) |
| Height, cm (SD) | 173.2 (9.6) |
| Weight, kg (SD) | 75.0 (14.4) |
| Body mass index, kg/m2 (SD) | 24.8 (3.4) |
| Waist circumference, cm (SD) | 86.6 (10.4) |
| Systolic blood pressure, mm Hg (SD) | 123.5 (15.3) |
| Diastolic blood pressure, mm Hg (SD) | 76.0 (8.3) |
| Resting heart rate, bpm (SD) | 57.9 (9.4) |
| Marital status, n (%) | |
| Married/common law | 655 (82.1) |
| Separated/Divorced | 76 (9.5) |
| Single | 50 (6.3) |
| Widowed | 10 (1.3) |
| No response | 7 (0.9) |
| Educational level, n (%) | |
| Did not complete high school | 10 (1.2) |
| Completed high school | 49 (6.1) |
| Vocational/college/undergraduate | 427 (53.5) |
| Graduate/professional degree | 307 (38.5) |
| No response | 5 (0.6) |
| Average income, n (%) | |
| ≤$20 000 | 37 (4.6) |
| $20 001–$40 000 | 57 (7.1) |
| $40 001–$75 000 | 219 (27.0) |
| >$75 000 | 445 (55.8) |
| No response | 40 (5.0) |
| Level of competition, n (%) | |
| Recreational | 535 (67.0) |
| Competitive | 185 (23.2) |
| Elite (professional, provincial, national) | 78 (9.8) |
| Weekly training hours, mean (SD) | 10.9±6.4 |
| Weekly training volume, MET-hour/week, mean (SD) | 80.8±44.0 |
| Years physically active, mean (SD) | 35.1±14.8 |
| Known cardiovascular risk factors | |
| Medication use | |
| Antihypertensive | 61 (7.7) |
| Lipid-lowering | 18 (2.3) |
| Diabetic | 8 (1.0) |
| Current smokers/quit ≤2 years ago | 8 (1.0) |
| Former smoker | 199 (24.9) |
| Obesity | 65 (8.1) |
| Previous atrial fibrillation/flutter | 23 (2.8) |
| Ablation/cardioversion | 17 (2.1) |
| Previous cardiac examination in ≤18 months | 118 (14.8) |
| ECG only | 61 (7.6) |
| Lifestyle | |
| ≤3 servings of fruits/vegetables per day (n=791) | 365 (45.7) |
| ≥3 servings per week of red meat (n=795) | 191 (24.0) |
| ≥7 sedentary hours per day (n=697) | 281 (40.3) |
| Alcohol consumption (drinks/week) | |
| Abstainer/former drinker | 85 (10.6) |
| Less than 7 | 435 (58.8) |
| ≥7 to 14 | 179 (22.3) |
| ≥14 to 21 | 40 (5.0) |
| ≥21 | 13 (1.6) |
| Heavy drinkers | 154 (19.3) |
| No response | 3 (0.4) |
| Depression | |
| Depressed mood | 88 (11.0) |
| Depressed | 25 (3.1) |
| No response | 67 (8.4) |
Heavy drinker was defined as (≥5 drinks on one occasion, at least once a month).33 Obesity was defined as body mass index ≥30.33 Depressed mood (adapted from the short form DSM-IV CIDI questionnaire for depression) was defined as feeling sad/blue for >2 weeks. Depression was defined by a positive response to having felt sad, blue or depressed for 2 weeks or more in a row and a positive response to five of the seven additional questions.
bpm, beats per minute; DSM-IV CIDI, diagnostic and statistical manual of mental disorders (4th edition) compositive international diagnostic interview; MET-hour/week, metabolic equivalent task hours per week.
bmjsem-2018-000370supp005.jpg (510.1KB, jpg)
Cardiovascular risk
A small percentage were already on antihypertensive and lipid-lowering medication (7.7% and 2.3%, respectively), 1.0% had known diabetes, 1.0% were current smokers and 24.9% were former smokers (quit ≥2 years ago) (table 1). At screening, 222 (27.8%) had dyslipidaemia (low-density lipoprotein ≥3.5 or total cholesterol to HDL-cholesterol ratio ≥5.0 or self-reported use of a lipid-modifying medication), 54 (6.7%) had HDL <1.0 mmol/L, 182 (22.8%) were hypertensive (≥140 mm Hg systolic or ≥90 mm Hg diastolic or self-reported use of antihypertensive medication) and 105 (13.1%) had hyperglycaemia (fasting glucose >5.5 mmol/L). A total of 365 (45.7%) participants did not meet the recommended daily servings of fruits and vegetables and 154 (19.2%) were heavy drinkers (≥5 drinks on one occasion, at least once a month). Eleven per cent (n=88) reported a depressive mood and 27 (3.7%) met the criteria for depression.
Indications for follow-up and PPV for screening tests
The initial screen was abnormal in 513 participants (64.3%), subsequently leading to an EST (figure 1). A total of 350 (43.9%) participants met the criteria for a cardiologist evaluation, and 256 (32.1%) participants required further examinations which may have included echocardiogram, stress echocardiogram, Holter monitoring, 24-hour ambulatory blood pressure, coronary artery calcium score, coronary CT angiography and/or MIBI. The primary indicators for follow-up were an intermediate FRS (10%–19%) (n=196, 24.6%), >2/6 systolic murmur (n=92, 11.5%) and palpitations with exercise (n=86, 10.8%) (table 2). The most common abnormality that warranted further investigation on resting ECG was left axis deviation (n=36, 4.5%), but had a very low PPV (11.1%). Two hundred and ninety-seven (37.3%) participants had more than one indicator that warranted further investigations.
Table 2.
Indications for follow-up and positive predictive value for cardiovascular disease
| Indicator | n | Positive predictive value for CAD (%)‡ | Positive predictive value for valvular disease (%)* |
| AHA questionnaire | 147§ | 11.6 | 3.4 |
| Exertional dyspnoea | 26 | 23.1 | 0.0 |
| Exertional syncope/presyncope | 34 | 14.7 | 5.9 |
| Previously known AF, other† | 40 | 12.5 | 2.5 |
| Exertional chest pain | 34 | 11.8 | 0.0 |
| Palpitations with exercise | 86 | 8.1 | 3.5 |
| Exertional fatigue | 4 | 0.0 | 0.0 |
| Family history | 120§ | 10.8 | 5.8 |
| Family history of premature CAD (<50 years) | 52 | 15.4 | 7.7 |
| Family history of inheritable heart conditions | 48 | 10.4 | 6.3 |
| Family history of unexplained SCD | 32 | 6.3 | 0.0 |
| Physical examination | 122§ | 9.0 | 7.4 |
| Diastolic murmur | 1 | 0.0 | 100.0 |
| Systolic click | 22 | 9.1 | 18.2 |
| ≥2/6 systolic murmur | 92 | 9.8 | 4.3 |
| Abnormal second heart sound | 2 | 0.0 | 0.0 |
| Hypertension (≥180/110) | 2 | 0.0 | 0.0 |
| Irregular pulse | 8 | 0.0 | 0.0 |
| Abnormal resting 12-lead ECG | 98§ | 11.2 | 3.1 |
| Significant q-waves | 6 | 33.3 | – |
| Premature ventricular contractions | 11 | 27.3 | – |
| Complete RBBB | 12 | 16.7 | – |
| Left axis deviation | 36 | 11.1 | – |
| Right axis deviation | 12 | 8.3 | – |
| T-wave inversions | 13 | 7.7 | – |
| ST depression | 5 | 0.0 | – |
| Complete LBBB | 5 | 0.0 | – |
| Left atrial enlargement | 12 | 0.0 | – |
| Right axis enlargement | 2 | 0.0 | – |
| Prolonged QT interval | 1 | 0.0 | – |
| LVH + RVH | 1 | 0.0 | – |
| Atrial tachyarrhythmia (ie, atrial fibrillation) | 3 | 0.0 | – |
| Cardiovascular risk | |||
| Diabetes | 8 | 50.0 | 0.0 |
| FBS ≥7.0 | 5 | 40.0 | 0.0 |
| High FRS | 68 | 38.2 | 1.5 |
| ≥65 years | 130 | 13.8 | 0.8 |
| Intermediate FRS | 196 | 13.3 | 3.1 |
| Cholesterol >8 mmol/L | 2 | 0.0 | 0.0 |
| Total participants with abnormal findings | 513 |
*Pairwise comparisons between all five tests for valve disease were conducted. Statistical significance was not found between any of the tests.
†Other: AF/flutter (n=23), sick sinus syndrome (n=1), supraventricular tachycardia (n=1), potential athlete’s heart (n=2), dissection of vein in the neck (n=1), rheumatic heart disease (n=3), unconfirmed stroke (no documentation) (n=1), unconfirmed congestive heart failure (no documentation) (n=1), pulmonary embolism (n=2), unconfirmed myocarditis (no documentation) (n=2), epicardial cyst (n=1) and pulmonary oedema (n=1).
‡Pairwise comparisons between all five tests for CAD were conducted (p<0.00001 was observed in all comparisons). Statistical significance was found only for high FRS. Bonferroni correction has been applied for multiple comparisons and statistical significance was still evident.
§Select athletes had more than one abnormal indication within the given section.
Bold values indicate the total number of participants that had a positive response and the overall total positive predictive value for the respective section
AF, atrial fibrillation; AHA, American Heart Association; CAD, coronary artery disease; FBS, fasting blood sugar; FRS, Framingham Risk Score; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; RBBB, right bundle branch block; RVH, right ventricular hypertrophy; SCD, sudden cardiac death.
A high FRS (≥20%) was the single most statistically effective tool in predicting CAD (PPV=38.2%; p<0.00001) (table 2). Thirty-two (47.1%) individuals with a high FRS underwent coronary CT angiography, coronary artery calcium score, MIBI and/or catheterisation, and 26 (81.2%) were diagnosed with CAD thereafter (figure 2). An additional seven individuals underwent further testing (echocardiogram, Holter monitoring) and three were diagnosed with other types of CVD (AF, bicuspid aortic valve, second-degree type II AV block). Twenty-nine (42.6%) did not undergo further testing, but 8 (27.6%) initiated medications, 8 (24.1%) were already on appropriate medications and 13 (44.8%) did not initiate medication (cardiologist’s discretion or the participant declined).
Figure 2.
Clinical course of participants with high cardiovascular risk profile. *No CACS, CCTA, Cath and MIBI. AF, atrial fibrillation; AV, atrioventricular; BAV, bicuspid aortic valve; BP, blood pressure; Cath, cardiac catheterisation; CACS, coronary artery calcium score; CCTA, computed cardiac tomography angiography; CVD, cardiovascular disease; Echo, echocardiogram; FRS, Framingham Risk Score; MIBI, myocardial perfusion imaging.
In the detection of CAD, significant q-waves on resting ECG exhibited a 33.3% PPV, exertional dyspnoea had a PPV of 23.1% and a family history of premature CAD had a PPV of 15.4% (table 2). The ECG was abnormal in 4 (66.7%) of 6 participants diagnosed with AF (the remaining two cases of AF were detected at the time of their EST). The physical examination had a very low PPV (7.4%) for detecting valvular disease. A 2/6 systolic murmur exhibited a PPV of 4.3%, while a systolic click demonstrated a higher PPV of 18.2%. The physical examination did not detect 5 (45.5%) of 11 of those with mitral valve prolapse. All individuals diagnosed with bicuspid aortic valve had an abnormal physical examination.
CVD diagnosis: clinical characteristics
A total of 91 (11.4%) were diagnosed with clinically significant CVD, with 19 athletes (2.4%) having multiple diagnoses (table 3). Those individuals diagnosed with CVD were predominately male (88%), with a mean age of 60±8 years (range 38–76). The most common diagnosis was CAD (n=63; 7.9%). Twenty-nine (3.6%) participants had non-obstructive CAD and 34 (4.3%) had obstructive CAD. Symptoms were reported in only 27.0% of the participants diagnosed with CAD. Notably, the presence of angina to predict underlying disease was variable. None of the participants with obstructive CAD reported angina. Paradoxically, four participants with non-obstructive disease reported angina. Three (50%) athletes diagnosed with AF reported symptoms. Two individuals were identified with coronary artery anomalies; one was incidentally flagged by ECG and the other by a high FRS who subsequently had a coronary CT angiography with an incidental coronary artery anomaly and moderate CAD. Two athletes displayed T-wave inversions suggestive of hypertrophic cardiomyopathy on ECG, but declined subsequent evaluations. Two-thirds (40/63) of the participants diagnosed with CAD had a positive EST.
Table 3.
Clinical characteristics of participants diagnosed with cardiovascular disease
| Diagnosis | Sex | Age, mean (range) | Symptoms, n (%) | High FRS, n (%) | Abnormal EST, n (%) | METs achieved on EST, mean (range) |
| Mild CAD | 26 M, 3 F | 60 (38–74) | 9 (31.0) | 9 (31.0) | 18 (62.1) | 14.6 (10.7–24.1) |
| Moderate CAD | 23 M, 1 F | 61 (39–74) | 7 (29.2) | 12 (50.0) | 14 (58.3) | 14.0 (10.0–19.0) |
| Significant CAD | 9 M, 1 F | 63 (50–76) | 1 (10.0)* | 5 (50.0) | 8 (80.0) | 13.4 (7.0–18.7) |
| BAV | 3 M | 46 (39–57) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 16.7 (10.7–20.7) |
| MVP | 6 M, 5 F | 56 (42–68) | 4 (36.4) | 0 (0.0) | 4 (36.4) | 15.2 (10.9–18.6) |
| RHD | 1 F | 63 | 0 (0.0) | 0 (0.0 | 1 (100.0) | 14.1 |
| Arrhythmia (AF, second-degree type II AV block) | 7 M | 64 (56–73) | 3 (42.9) | 3 (42.9) | 7 (100.0) | 14.1 (9.8–18.6) |
| PVC burden | 7 M | 59 (50–66) | 1 (14.3) | 1 (14.3) | 4 (57.2) | 14.6 (9.6–19.3) |
| Probable HCM† | 1 M, 1 F | 40, 60 | 0 (0.00 | 0 (0.0) | 0 (0.0) | 17.2 (15–19.3) |
| Coronary artery anomaly | 2 M | 61, 62 | 0 (0.0) | 1 (50.0) | 2 (100.0) | 12.2 (10.4–14) |
| Inheritable heart disease | 3 M, 1 F | 56 (40–62) | 0 (0.0) | 1 (25.0) | 2 (50.0) | 14.7 (10.4–19.3) |
| Dilated aorta | 14 M, 2 F | 61 (42–74) | 7 (43.4) | 4 (25.0) | 4 (25.0) | 15.0 (10.1–18.6) |
19 participants had multiple diagnoses; therefore, some participants are represented twice.
*Symptom reported was palpitations.
†Both patients declined further testing.
AF, atrial fibrillation; AV, atrioventricular; CAD, coronary artery disease; BAV, bicuspid aortic valve; EST, exercise stress test; F, female; FRS, Framingham Risk Score; HCM, hypertrophic cardiomyopathy; M, male; METs, metabolic equivalent task; MVP, mitral valve prolapse; RHD, rheumatic heart disease; PVC, premature ventricular contraction.
Discussion
This is the first study to ascertain the cardiovascular risk in masters athletes in Canada. CVD and high cardiovascular risk profiles (FRS ≥20%) were found in 11.4% and 8.5% of athletes, respectively. Most participants (73.0%) diagnosed with CAD were asymptomatic and were unaware of their elevated cardiovascular risk. Compared with the Canadian general population, participants had a lower prevalence of risk factors (figure 3).
Figure 3.
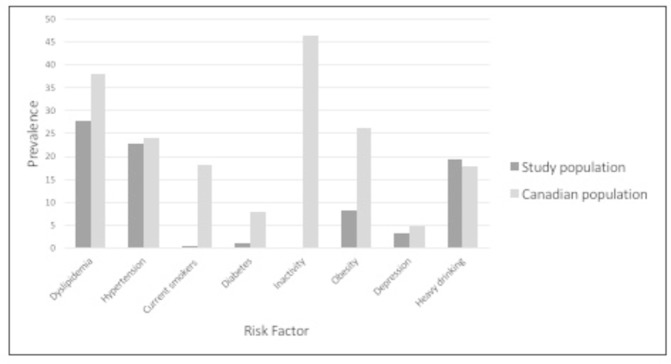
Prevalence of cardiovascular risk factors compared with the Canadian population. Data from the Canadian Health Measures Survey (CHMS) and Canadian Community Health Survey (CCHS) from a nationally representative sample of Canadians compared with the current population, aged >35. Data for those aged >35 were not available for all risk factors. The age and source for each risk factor were reported as follows: dyslipidaemia: aged 18–79, source: CHMS, 2012–2014; hypertension: aged 20–79, source: CHMS, 2012–2014; smoking (self-reported): aged 12–49, source: CHMS, 2012 and 2013; diabetes (diagnosed by a health professional): aged 12 and over, source: CCHS, 2015; inactivity: aged 12 and older, source: CCHS, 2014; obesity (directly measured): aged 18–79, source: CHMS, 2010; depression (major depressive episode in the last 12 months): aged 15 and older, source: CCHS, 2012; heavy drinking: aged 12 or older, source: CCHS, 2014. Dyslipidaemia was defined as having a low-density lipoprotein >3.5, or total cholesterol to high-density lipoprotein cholesterol ratio >5.0, or self-reported use of a lipid-modifying medication. Hypertension was defined as >140 mm Hg systolic or >90 mm Hg diastolic, or self-reported use of antihypertensive medication. Hyperglycaemia was defined as having a fasting glucose >5.5 mmol/L. Heavy drinker was defined as >5 drinks on one occasion, at least once a month. Obesity was defined as body mass index >30 (http://www.statcan.gc.ca).
The prevalence of CVD was higher in our study compared with previous prospective studies. Menafoglio et al10 studied 785 highly active individuals (>6 hours of physical activity per week) using the EACPR protocol and detected 22 individuals (2.8%) with significant CVD. One (0.1%) individual had CAD compared with 63 (7.9%) present in this study and 9 (1.1%) had valvular disease, similar to this study (n=15, 1.9%). Using the EACPR guidelines and an echocardiogram, Aagaard et al7 reported significant CVD (ie, long QT syndrome, AF, third-degree AV block) in 5.9% of participants, and none had CAD or valvular disease. The greater prevalence of CVD in our population compared with Menafoglio et al and Aagaard et al’s studies may be attributed to (1) higher mean age (54.6±9. vs 46.8±7.3 vs 51.5±5 years, respectively); (2) long-term activity habits (35.1±14.8 years vs 20.0±14.5 years, and novice runners, respectively); (3) more participants undergoing EST due to an intermediate FRS (24.6% vs 0% vs 0%, respectively); and/or (4) a greater number of secondary investigations. Over half of our population (63.3%) underwent EST compared with the 14.3% and 9.0% reported by Menafoglio et al and Aagaard et al, respectively. Aagaard et al included a more comprehensive onsite assessment by physicians (echocardiogram in all participants), which likely resulted in fewer participants proceeding to subsequent testing (ie, EST).
Current screening tools (AHA 14-element recommendations, ECG interpretation criteria) are not specific for use in masters athletes and may need to be modified.19 20 For example, the presence of a 2/6 systolic murmur had low clinical significance (4.3% PPV) and should not warrant further testing. The presence of a mid-systolic click was associated with a slightly better PPV (18.2%) for detecting valvular disease. The ECG demonstrated a low PPV for detecting CAD (11.1%), although q-waves (33.3%) and PVCs (27.3%) increased the PPV for CAD. Left axis deviation elicited the greatest number of follow-ups (n=36), but had a low PPV (11.2%). As such, the presence of isolated left axis deviation may not warrant further testing. The ECG also has an ability to detect asymptomatic electrical disease (ie, AF, long QT syndrome and Wolff-Parkinson-White syndrome). In the current study, the ECG detected three (50.0%) individuals with AF. Positive responses to the symptoms and family history of unexplained SCD elicited the greatest number of follow-ups, yet had a low detection rate for CAD (PPV 11.6% and 6.3%, respectively). Increasing the sensitivity of the questionnaire (ie, differentiating cardiogenic symptoms from non-cardiogenic, and reviewing in conjunction with the ECG and family history) or a more comprehensive review by an onsite physician with appropriate training would reduce the number of participants having to undergo subsequent testing, and thereby reduce false-positives.
In the present study, a positive EST detected CAD in 63.5%. This is consistent with the findings by Sofi et al,26 whereby the majority of participants diagnosed with CVD demonstrated an abnormality on the EST. While previous reports suggest that ECG changes are masked in individuals at low risk of CAD (ie, active, physically fit population) and in those who can achieve >10 METs,27 28 our study did not show this (table 3). However, 81.0% of those diagnosed with CAD had an intermediate or high FRS, which is known to increase the likelihood of a positive EST in men.29 Although the FRS is widely used and is a good predictor of one’s 10-year CVD risk, it may underestimate cardiovascular risk in masters athletes.11 30 31 Masters athletes have been shown to have a higher prevalence of coronary plaques (44.3% vs 22.2%) when compared with sedentary men who were similar in age, sex and low FRS.11 However, a distinction between the detection of CAD is different from myocardial infarction and death.
Using coronary CT angiography improves detection rates as shown by Braber et al,30 who identified CAD in 60 of 318 (18.9%, 95% CI 14.9% to 23.5%) asymptomatic participants, of whom 300 had a low cardiovascular risk and a normal sports medical examination. Although the implications of increased coronary plaques in masters athletes are unknown, research suggests that individuals with elevated cholesterol levels benefit from statin therapy, even in the setting of high fitness. Kokkinos et al32 reported a greater 10-year survival rate when high fitness was combined with statin therapy (fully adjusted HR 0.30; 0.21-0.41) versus no statin therapy (HR 0.53; 0.44–0.65) when compared with less fit individuals taking statins.
Limitations
A true prevalence of CVD cannot be reported because gold-standard cardiovascular examinations (ie, coronary artery calcium score, coronary CT angiography, echocardiogram) were not performed on all athletes. However, most athletes had an EST, which increased the likelihood of detecting those at risk by triggering subsequent examinations. In this study, the reference standard was the evaluation by cardiologists, who determined which cardiac evaluations were clinically indicated. Although gold-standard imaging tests that have sensitivities approaching 100% would be ideal to ascertain the prevalence of CVD, we did not perform these examinations on all participants because of the following reasons: (1) the current evidence for incorporating cardiac imaging into preparticipation screening is insufficient10; (2) cost; (3) time commitment; (4) potential radiation exposure; (5) testing a population of generally healthy asymptomatic low-risk patients is not clinically indicated; and (6) the potential psychological and insurance risks.
Additionally, since the reference standard was only available for those participants who went through all stages, the sample PPV for each individual screening method computed based on this set of biased samples might not estimate the population PPV, but rather the PPV of those that screened positive. However, we were able to assess the value of the screening test when it was reported positive, which may be of benefit in screening studies.
The reported cardiovascular risk may be overestimated due to selection bias. Participants with symptoms, previously known risk factors and/or known family history of CVD may have been more inclined to enrol in the study. Conversely, athletes who had a low FRS did not undergo further evaluations but may in fact have CVD as the FRS underestimates risk in masters athletes.
Our population was predominately Caucasian, limiting the generalisability of our results to other populations. Inclusion of a control group in a large, multisport, multiethnic, longitudinal study will be instrumental in determining whether preparticipation screening among masters athletes reduces morbidity, mortality and healthcare costs.
Conclusion
Despite their high fitness level, masters athletes can exhibit elevated cardiovascular risk and possess CVD. A high FRS (or elevated cardiac risk) was the best predictor of CAD in masters athletes and should be incorporated into the preparticipation screening algorithm. An ECG may be warranted to raise suspicion of and/or diagnose asymptomatic disease. Exercise stress testing may be indicated in masters athletes who are symptomatic, have a family history of premature CAD and/or have an intermediate or high FRS. The optimal method to screen masters athletes requires continued study to decrease the number of false-positive screens while having accessible and cost-effective screening tools to identify individuals potentially at risk.
Acknowledgments
We would like to thank Dr Anthony Della Siega, Dr Kevin Pistawka, Dr Dylan Stanger and Dr Farnoosh Ashraf for their help with athlete evaluation, and the numerous volunteers for their assistance with screening and data input. We would also like to thank GE Healthcare for their ongoing support of our research.
Footnotes
Contributors: BNM, JM, SI and DERW were responsible for the conception and design of the study. BNM and BAH conducted the analyses, which were checked by all coauthors. All authors contributed to the interpretation of the findings. BNM, JM, SI, DL, HN, CC, MM, BH and BAH all assisted with data collection over the study period. BNM wrote the first draft of the paper, which was critically revised by JM, AMDS and DERW. The final manuscript was approved by all authors. BNM is the study guarantor.
Funding: This study was supported by a grant from MITACs and CIHR (FRN 157930). VGH and UBC Hospital Foundation provided financial and material support.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The University of British Columbia Clinical Research Ethics Board approved this study (H15-00009).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: This is a 5-year study, so data collection is ongoing. The data come from the SportsCardiologyBC clinic, which will not be made available to other researchers.
References
- 1. Marijon E, Tafflet M, Celermajer DS, et al. Sports-related sudden death in the general population. Circulation 2011;124:672–81. 10.1161/CIRCULATIONAHA.110.008979 [DOI] [PubMed] [Google Scholar]
- 2. Risgaard B, Winkel BG, Jabbari R, et al. Sports-related sudden cardiac death in a competitive and a noncompetitive athlete population aged 12 to 49 years: data from an unselected nationwide study in Denmark. Heart Rhythm 2014;11:1673–81. 10.1016/j.hrthm.2014.05.026 [DOI] [PubMed] [Google Scholar]
- 3. Bohm P, Scharhag J, Meyer T. Data from a nationwide registry on sports-related sudden cardiac deaths in Germany. Eur J Prev Cardiol 2016;23:649–56. 10.1177/2047487315594087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marijon E, Uy-Evanado A, Reinier K, et al. Sudden cardiac arrest during sports activity in middle age. Circulation 2015;131:1384–91. 10.1161/CIRCULATIONAHA.114.011988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ 2006;174:801–9. 10.1503/cmaj.051351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Warburton DE, Bredin SS. Reflections on physical activity and health: what should we recommend? Can J Cardiol 2016;32:495–504. 10.1016/j.cjca.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 7. Aagaard P, Sahlén A, Bergfeldt L, et al. Preparticipation evaluation of novice, middle-age, long-distance runners. Med Sci Sports Exerc 2013;45:130–7. 10.1249/MSS.0b013e31826c5552 [DOI] [PubMed] [Google Scholar]
- 8. De Matos LD, Caldeira NA, Perlingeiro PS, et al. Cardiovascular risk and clinical factors in athletes: 10 years of evaluation. Med Sci Sports Exerc 2011;43:943–50. 10.1249/MSS.0b013e318203d5cb [DOI] [PubMed] [Google Scholar]
- 9. Shapero K, Deluca J, Contursi M, et al. Cardiovascular risk and disease among masters endurance athletes: insights from the Boston MASTER (Masters Athletes Survey To Evaluate Risk) Initiative. Sports Med Open 2016;2:29 10.1186/s40798-016-0053-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menafoglio A, Di Valentino M, Porretta AP, et al. Cardiovascular evaluation of middle-aged individuals engaged in high-intensity sport activities: implications for workload, yield and economic costs. Br J Sports Med 2015;49:757–61. 10.1136/bjsports-2014-093857 [DOI] [PubMed] [Google Scholar]
- 11. Merghani A, Maestrini V, Rosmini S, et al. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation 2017;136:126–37. 10.1161/CIRCULATIONAHA.116.026964 [DOI] [PubMed] [Google Scholar]
- 12. Goodman JM, Burr JF, Banks L, et al. The acute risks of exercise in apparently healthy adults and relevance for prevention of cardiovascular events. Can J Cardiol 2016;32:523–32. 10.1016/j.cjca.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 13. Albert CM, Mittleman MA, Chae CU, et al. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med 2000;343:1355–61. 10.1056/NEJM200011093431902 [DOI] [PubMed] [Google Scholar]
- 14. Warburton DER, Bredin SSD, Jamnik V, et al. Consensus on evidence-based preparticipation screening and risk stratification. Annual Review of Gerontology and Geriatrics 2016;36:53–102. 10.1891/0198-8794.36.53 [DOI] [Google Scholar]
- 15. The International Olympic Committee (IOC) consensus statement on periodic health evaluation of elite athletes: March 2009. J Athl Train 20092009;44:538–57. 10.4085/1062-6050-44.5.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borjesson M, Urhausen A, Kouidi E, et al. Cardiovascular evaluation of middle-aged/ senior individuals engaged in leisure-time sport activities: position stand from the sections of exercise physiology and sports cardiology of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 2011;18:446–58. 10.1097/HJR.0b013e32833bo969 [DOI] [PubMed] [Google Scholar]
- 17. Maron BJ, Araújo CG, Thompson PD, et al. Recommendations for preparticipation screening and the assessment of cardiovascular disease in masters athletes: an advisory for healthcare professionals from the working groups of the World Heart Federation, the International Federation of Sports Medicine, and the American Heart Association Committee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation 2001;103:327–34. 10.1161/01.CIR.103.2.327 [DOI] [PubMed] [Google Scholar]
- 18. Abbatemarco JR, Bennett C, Bell AJ, et al. Application of pre-participation cardiovascular screening guidelines to novice older runners and endurance athletes. SAGE Open Med 2016;4:205031211561613 10.1177/2050312115616136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maron BJ, Friedman RA, Kligfield P, et al. Assessment of the 12-lead electrocardiogram as a screening test for detection of cardiovascular disease in healthy general populations of young people (12-25 years of age): a scientific statement from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol 2014;64:1479–514. 10.1016/j.jacc.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 20. Drezner JA, Ackerman MJ, Anderson J, et al. Electrocardiographic interpretation in athletes: the ‘Seattle criteria’. Br J Sports Med 2013;47:122–4. 10.1136/bjsports-2012-092067 [DOI] [PubMed] [Google Scholar]
- 21. Anderson TJ, Grégoire J, Hegele RA. 2012 update of the Canadian cardiovascular society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013;29:151–67. 10.1016/j.cjca.2012.11.032 [DOI] [PubMed] [Google Scholar]
- 22. Ainsworth BE, Haskell WL, Herrmann SD. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575–81. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 23. Brugger N, Krause R, Carlen F, et al. Effect of lifetime endurance training on left atrial mechanical function and on the risk of atrial fibrillation. Int J Cardiol 2014;170:419–25. 10.1016/j.ijcard.2013.11.032 [DOI] [PubMed] [Google Scholar]
- 24. Sajadieh A, Nielsen OW, Rasmussen V, et al. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age >or=55 years. Am J Cardiol 2006;97:1351–7. 10.1016/j.amjcard.2005.11.067 [DOI] [PubMed] [Google Scholar]
- 25. Braverman AC, Harris KM, Kovacs RJ, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 7: aortic diseases, including marfan syndrome. a scientific statement from the American Heart Association and American College of Cardiology. JACC 2015;132:e303–9. [DOI] [PubMed] [Google Scholar]
- 26. Sofi F, Capalbo A, Pucci N, et al. Cardiovascular evaluation, including resting and exercise electrocardiography, before participation in competitive sports: cross sectional study. BMJ 2008;337:a346 10.1136/bmj.a346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pigozzi F, Spataro A, Alabiso A, et al. Role of exercise stress test in master athletes. Br J Sports Med 2005;39:527–31. 10.1136/bjsm.2004.014340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Freeman J, Froelicher V, Ashley E. The ageing athlete: screening prior to vigorous exertion in asymptomatic adults without known cardiovascular disease. Br J Sports Med 2009;43:696–701. 10.1136/bjsm.2008.054783 [DOI] [PubMed] [Google Scholar]
- 29. Gibbons LW, Mitchell TL, Wei M, et al. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. Am J Cardiol 2000;86:53–8. 10.1016/S0002-9149(00)00827-4 [DOI] [PubMed] [Google Scholar]
- 30. Braber TL, Mosterd A, Prakken NH, et al. Occult coronary artery disease in middle-aged sportsmen with a low cardiovascular risk score: The Measuring Athlete’s Risk of Cardiovascular Events (MARC) study. Eur J Prev Cardiol 2016;23:1677–84. 10.1177/2047487316651825 [DOI] [PubMed] [Google Scholar]
- 31. Tsiflikas I, Thomas C, Fallmann C, et al. Prevalence of subclinical coronary artery disease in middle-aged, male marathon runners detected by cardiac CT. Rofo 2015;187:561–8. 10.1055/s-0034-1399221 [DOI] [PubMed] [Google Scholar]
- 32. Kokkinos PF, Faselis C, Myers J, et al. Interactive effects of fitness and statin treatment on mortality risk in veterans with dyslipidaemia: a cohort study. Lancet 2013;381:394–9. 10.1016/S0140-6736(12)61426-3 [DOI] [PubMed] [Google Scholar]
- 33. Statistics Canada. Canadian health measures survey (2011-2015) and Canadian community health survey (2012-2015). http://www.statcan.gc.ca (accessed 1 Nov 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsem-2018-000370supp001.docx (104.1KB, docx)
bmjsem-2018-000370supp002.jpg (849.2KB, jpg)
bmjsem-2018-000370supp003.docx (19.9KB, docx)
bmjsem-2018-000370supp004.docx (11.1KB, docx)
bmjsem-2018-000370supp005.jpg (510.1KB, jpg)