Abstract
The mechanistic target of rapamycin (mTOR) network is an evolutionary conserved signaling hub that senses and integrates environmental and intracellular nutrient and growth factor signals to coordinate basic cellular and organismal responses such as cell growth, proliferation, apoptosis, and inflammation dependent on the individual cell and tissue. A growing list of evidence suggests that mTOR signaling influences longevity and aging. Inhibition of the mTOR complex 1 (mTORC1) with rapamycin is currently the only known pharmacological treatment that increases lifespan in all model organisms studied. This review discusses the potential mechanisms how mTOR signaling controls lifespan and influences aging-related processes such as cellular senescence, metabolism, and stem cell function. Understanding these processes might provide novel therapeutic approaches to influence longevity and aging-related diseases.
Introduction
The mechanistic (formerly mammalian) target of rapamycin (mTOR) is an evolutionary conserved serine-threonine kinase that senses and integrates a diverse set of environmental and intracellular signals, such as growth factors and nutrients to direct cellular and organismal responses [1]. The name TOR (target of rapamycin) is derived from its inhibitor rapamycin, which was initially isolated in the 1970s from a soil bacterium on Rapa Nui (Easter Island). Rapamycin, also known as sirolimus, forms a complex with FK506-binding protein 12 (FKBP12) and in this form inhibits the activity of mTOR. Rapamycin was first described as an anti-fungal drug and used to inhibit the growth of yeast, but was later found to potently decrease proliferation of T lymphocytes [1]. We now know that the role of mTOR goes far beyond proliferation and coordinates a cell-tailored metabolic program to control cell growth and many biological processes including aging, cellular senescence, and lifespan. Rapamycin is currently the only known pharmacological substance to prolong lifespan in all studied model organisms and the only one in mammals. This review focuses on the role of the mTOR network in aging-related processes and discusses underlying molecular mechanisms that are controlled by mTOR. We discuss recent studies that may allow us to better understand the clinical consequences of mTOR inhibition in rapamycin-treated individuals.
mTOR signaling
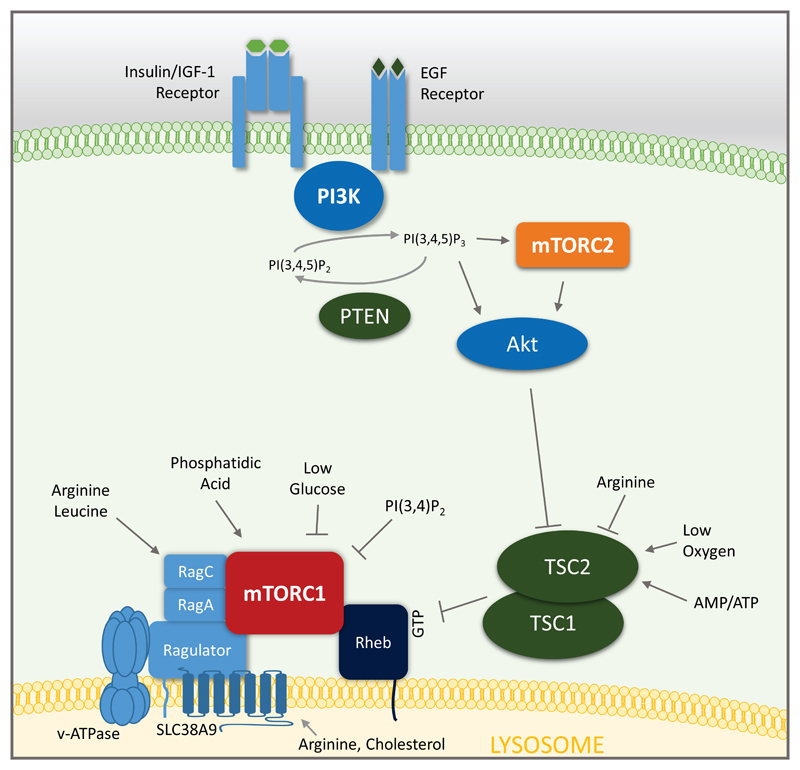
mTOR belongs to the phosphatidylinositol-3 kinases (PI3K)-related kinase (PIKK) family that is present as catalytic subunit in at least two protein complexes: mTOR complex 1 (mTORC1) and mTORC2 [1]. mTORC1 is composed of three core components: mTOR, Raptor (regulatory protein associated with mTOR), and mLST8 (mammalian lethal with Sec13 protein 8). In addition, mTORC1 comprises of DEPTOR (DEP domain containing mTOR interacting protein) and PRAS40 (proline-rich Akt substrate of 40 kDa), which are inhibitory subunits [1]. Numerous growth factor receptors such as the insulin receptor or the epidermal growth factor (EGF) receptor activate tyrosine kinase adaptor molecules at the cell membrane leading to the recruitment of the class I family of PI3K to the receptor complex (Figure 1). Following receptor engagement, PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate [PI(3,4,5)P2] to generate phosphatidylinositol-3,4,5-trisphosphate [PI(3,4,5)P3] that recruits and activates the serine-threonine kinase Akt (also known as protein kinase B) via phosphorylation on threonine 308 by phosphoinositide-dependent protein kinase 1 (PDPK1) [2,3]. mTORC2 is also activated by PI3K through PI(3,4,5)P3 [4] and phosphorylates Akt on serine 473, which is important for full activation and substrate specificity of Akt [3]. A main target of Akt is tuberous sclerosis 2 (TSC2). TSC2 forms a heterodimeric complex with TSC1 and inhibits mTORC1. Phosphorylation of TSC2 at threonine 1462 (Thr1462) by Akt blocks its GTPase-activating protein (GAP) activity for the small GTPase RAS homologue enriched in brain (Rheb), which therefore remains in a GTP-bound state and activates mTORC1 [1,3]. Thus, class I PI3K activation finally leads to mTORC1 activation through the inhibition of TSC2. Interestingly, the class II PI3K β (PI3KC2β) synthesizes phosphatidylinositol 3,4-bisphosphate [PI(3,4)P2] under growth factor-deprived conditions to inhibit mTORC1 on the lysosome [5].
Figure 1.
The mTOR signaling network. For details see text.
mTORC1 activation occurs on cellular organelles such as peroxisomes or lysosomes [6]. The activation of mTOR on lysosome is best understood: mTORC1 binds to a complex consisting of the v-ATPase, Ragulator, and SLC38A9 [1] (Figure 1). Our current understanding of full mTORC1 activation requires the presence of main nutrient and energy sources: amino acids, glucose, lipids, oxygen and a high ATP/AMP ratio [7]. mTORC1 senses amino acid sufficiency, especially leucine and arginine on the lysosome via Ras-related GTPases (Rag) and arginine by SLC38A9. In addition, arginine, independently of Rag family members, inhibits lysosomal localization of TSC2 to stimulate mTORC1 activity [8]. Low levels of glucose-6-phosphate decrease mTORC1 activity by stimulating its binding to hexokinase 2 (HK2). A direct interaction of the lipid molecule phosphatidic acid (PA) with mTORC1 is also assumed as precondition for mTORC1 activation. Oxygen deprivation stimulates expression of DDIT4 (DNA-damage-inducible transcript 4; also known as REDD1 or RTP801), which inhibits mTORC1 via TSC2 [2]. Thus, the presence of these nutrients and energy is supposed to be required for full mTORC1 activation. However, this concept has been established currently only in heavily transformed cancer cell lines such as HeLa cells and remains to be shown in primary cell types. Thus, it is possible that distinct cells require only specific signals to allow activation of mTORC1. Moreover, during homeostasis under non-proliferating conditions in vivo, most cells do not show active mTORC1 or mTORC2 signaling. This is in stark contrast to in vitro cell culture, where these pathways are always active under normal growth-promoting conditions. While rapamycin through FKBP12 directly inhibits mTORC1, mTORC2 is insensitivity to acute rapamycin treatment [9]. Like mTORC1, mTORC2 also contains mTOR and mLST8, but contains Rictor (rapamycin insensitive companion of mTOR), DEPTOR, as well as the regulatory subunits mSin1 and Protor1/2 [1]. Prolonged rapamycin treatment can abrogate mTORC2 signaling in some cells and in mice in vivo [9,10]; nevertheless the growth-promoting functions of mTOR inhibitors are thought to be mediated by mTORC1.
Identification of the mTOR network as lifespan regulator
The first indications that mTOR is a regulator of lifespan stem from experiments with the nematode C. elegans [11] and the fruit fly Drosophila melanogaster [12]. Afterwards, mTOR could also be linked to have a role in controlling lifespan in the yeast strain S. cerevisiae [13]. In this simple organism inhibition of mTOR with rapamycin doubled the lifespan (defined as survival time of non-dividing cells).. The interest in the mTOR network as regulator of aging and lifespan was strengthened by the finding that rapamycin extents the lifespan of genetically heterogeneous mice at three independent test locations by about 10-18% dependent on sex [14]. Interestingly, treatment was only started late when the mice were 600 days of age equivalent to roughly 60 years of age in a human person. This proposes that inhibition of mTOR in the elderly might be enough to prolong life. The findings were confirmed and extended in mice, in which rapamycin treatment started earlier [15]. However, they failed to substantially observe larger effects on longevity. However, the maximal lifespan extension seems to be dose-dependent [16]. A lot of other reports confirmed mTOR as lifespan regulator in mice [14,17–21]. Deleting the mTORC1 substrate S6K1 similarly increases lifespan, but only in female and not male mice [22]. These findings in total suggest an evolutionary conserved role of mTOR as longevity regulator. The lifespan-enhancing effects of mTOR inhibitors have been linked to mTORC1 inhibition, whereas inhibition of mTORC2 might even be detrimental, because mTORC2 controls insulin-mediated suppression of hepatic gluconeogenesis [10,23].
mTOR, lifespan, aging, and the mechanisms
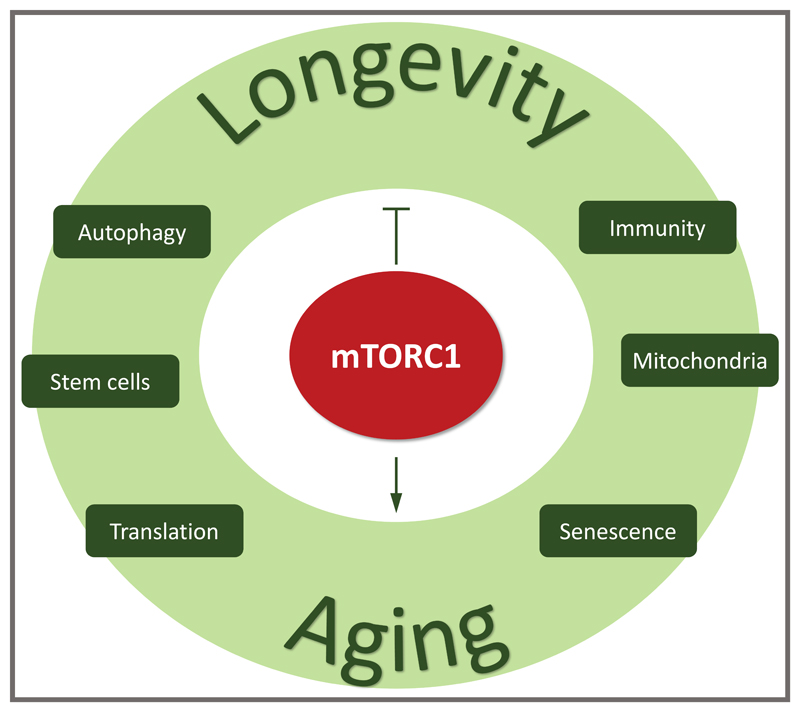
It is now accepted that mTOR inhibition increases lifespan, yet, the mechanism through which this occurs, is still uncertain. mTORC1 inhibition may not delay aging itself, but may delay age-related diseases [24–27]. However, many researchers directly link the longevity effects of mTOR inhibitors to a decrease in aging. Aging is generally characterized by a progressive loss of physiological integrity, which leads to impaired functions, and therefore increases vulnerability to death thus limiting lifespan [28]. Conserved hallmarks of aging have been recently proposed and include telomere attrition, epigenetic alterations, genomic instability, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication [28,29]. The mTOR network is known to regulate some of these aging hallmarks as described below. Ultimately, the prominence of mTORC1 signaling in aging likely reflects its exceptional capacity to regulate such a wide variety of key cellular functions (Figure 2).
Figure 2.
The role of mTORC1 for longevity and aging. The mechanisms how mTORC1 regulates longevity and aging.
mRNA translation
mTORC1 regulates cap-dependent and cap-independent translation of mRNAs by phosphorylating the translation inhibitors eIF4E-binding protein 1 (4E-BP1) and 4E-BP2, which then release eukaryotic translation initiation factor 4E (eIF4E). mTORC1 is particularly potent in promoting translation initiation of mRNAs containing a 5′ terminal oligopyrimidine tract (5′ TOP) or a pyrimidine-rich translational element (PRTE); many of those encode translation- and ribosomal-related proteins but also metabolism-related genes [30]. In addition, ribosomal protein S6 kinase 1 (S6K1) and S6K2 are two other well-characterized targets of mTORC1-mediated phosphorylation, which subsequently phosphorylate and activate the ribosomal protein S6 to stimulate protein translation [1]. Several studies suggest that a general decrease in mRNA translation caused by mTORC1 inhibition slows aging. Mechanistically, this is explained by reducing the accumulation of proteotoxic and oxidative stress [1,22]. Deletion of the translational regulator S6K1 prolongs lifespan in mammals, although a direct link to altered global translation has not been shown so far [22]. Hence, although counterintuitive at first thought, reducing mRNA translation might permit easier endogenous protein degradation or repair to preserve homeostasis when facing oxidative damage as well as protein aggregation during ageing [31].
Autophagy and Mitochondria
The starvation-induced degradation of cytosolic components known as autophagy is crucial in providing substrates for energy production under catabolic conditions of limited nutrient supply and removal of damaged organelles [32]. mTORC1 controls the Ser/Thr kinase ULK1, which regulates autophagosomal formation and autophagic flux [32]. mTORC1 actively suppresses autophagy by phosphorylating ULK1 and, accordingly, inhibition of mTORC1 induces autophagy. It has been suggested that autophagy might decline with age resulting in the accumulation of damaged proteins and organelles such as mitochondria, however, the underlying reason remains unclear. mTORC1 inhibition could slow aging by stimulating autophagy, which clears old and dysfunctional mitochondria (mitophagy), the accumulation of which are linked to aging and aging-related diseases [1]. Additionally, mTORC1 regulates mitochondrial functions [33]. On the one hand, mTORC1 controls the cellular energy metabolism by increasing glycolytic flux and simultaneously limiting oxidative phosphorylation (a process called aerobic glycolysis or Warburg effect in cancer cells). On the other hand, mTORC1 increases mitochondrial functions and stimulates mitochondrial biogenesis through PGC-1α and the transcription factor YY-1 in some cells [1]. It is important to preserve the function and number of mitochondria during aging, but the cumulative role of mTORC1 in these processes is complex and might depend on the individual tissue.
Stem cell and immune function
A decline of stem cell number and function might be a critical cause in age-related dysfunction of tissue homeostasis [34]. mTORC1 inhibition may preserve adult stem cell function in various tissues [31]. For example, treatment of old mice with rapamycin enhances intestinal stem cell function indirectly by reducing mTORC1 signaling in Paneth cells, which creates a better intestinal niche for stem cells [35]. In contrast, during calorie restriction, which enhances lifespan (see below), mTORC1 is induced in intestinal stem cells to allow their expansion [36]. Similarly, mTORC1 controls the adaptive transition of quiescent muscle stem cells from G0 arrest to an alert state that is important to respond to injury-induced systemic signals [37].
Optimal immune functions are evidently critical during aging for maintaining organismal fitness against pathogens, cancers, or other diseases. mTORC1 has many central and often divergent functions in the innate and adaptive immune system to enhance or limit inflammation or immune responses [2,38–40]. Importantly, mTORC1 inhibition is used as immunosuppressive therapy to limit T cell activation and prevent transplant rejection after organ transplantation. In contrast, inhibition of mTORC1 augments CD8+ T cell memory responses that are critical for viral defense [41]. The role and activity of mTORC1 in immune cells during aging is, however, not well studied. One study reported that an age-related decline in hematopoietic stem cell (HSC) function, which may contribute to anemia, poor vaccination, or enhanced tumorigenesis, is associated with an increased mTORC1 activity in HSCs from old mice [42]. Hence, rapamycin restores self-renewal and hematopoiesis of HSCs, which enables effective vaccination of old mice against a lethal challenge with influenza virus [42]. In summary, although mTORC1 inhibition clearly enhances overall lifespan, it may exert positive and negative functions on stem and immune cells that may differentially impact aging.
Cellular senescence
Cellular senescence is historically defined as an irreversible cell cycle exit, whilst preserving cell viability [34]. Cellular senescence has been suggested to function as a tumor suppressor mechanism and promotor of tissue remodeling after wounding [34,43]. However, senescent cells may also directly contribute to aging [28,34]. Senescent cells show marked changes in morphology including an enlarged size, irregular cell shape, prominent and sometimes multiple nuclei, accumulation of mitochondrial and lysosomal mass, increased granularity and highly prominent stress fibers that are accompanied by shifts in metabolism and a failure of autophagy. Interestingly, many of these phenotypes are regulated by mTORC1 in various cell types [44,45]. Senescent cells secrete proinflammatory and pro-oxidant signals, which can cause inflammation, and due to a suppression of apoptosis, they occupy key cellular niches [28,46]. Due to these mechanisms, senescent cells steadily accumulate with age and contribute to aging-related diseases and morbidity [34]. Hence, clearance of senescent cells improves aging-related disorders [47].
Senescence-associated secretory phenotype regulated by mTORC1
The secretion of proinflammatory mediators by senescent cells contributes to aging and has been termed senescence-associated secretory phenotype (SASP). Recent data identified a main role of mTORC1 to promote the SASP [48,49]. Rapamycin blunts the pro-inflammatory phenotype of senescent cells by specifically suppressing translation of the membrane-bound cytokine IL1A [48]. This reduction of IL1A diminishes transcription of inflammatory genes regulated by the proinflammatory transcription factors NF-κB. In parallel, mTOR controls translation of MK2, which in turn phosphorylates the RNA-binding protein ZFP36L1 during senescence. This phosphorylation of ZFP36L1 inhibits its ability to degrade transcripts of numerous SASP components. Thus, rapamycin activates ZFP36L1 to induce SASP component degradation [49]. The reason of mTORC1 activation in senescent cells might result from defects in amino acid and growth factor sensing [50]. Senescent human fibroblasts induced by stress, replicative exhaustion, or oncogene activation, show constitutive mTORC1 activation, which is resistant to serum and amino acid starvation. This is mediated in part by a depolarization of the plasma membrane resulting in a failure to abrogate growth factor signaling. Moreover, increased autophagy provide high amino acid levels to support mTORC1 activation [50]. Additionally, two proteins important for the DNA damage repair, O-6-methylguanine-DNA methyltransferase (MGMT) and N-myc downstream-regulated gene 1 (NDRG1) are negatively regulated by mTORC1 in senescent mice and cells [51]. These results in toto indicate that mTORC1 inhibitors inhibit the SASP through various mutually non-exclusive mechanisms.
Metabolic reprogramming in senescence
Despite maintaining a non-dividing state, senescent cells display a high metabolic rate [52]. Metabolic changes characteristic of replicative senescence often show a shift to glycolytic metabolism away from oxidative phosphorylation (which is also observed in proliferative cells), despite a marked increase in mitochondrial mass and markers of mitochondrial activity. This might stem from a rise in lysosomal pH as a consequence of proton pump failure, which leads to an inability to get rid of damaged organelles such as mitochondria caused by a failure of autophagy. Dysfunctional mitochondria not cleared by autophagy in senescent cells produce reactive oxygen species (ROS), which cause cellular damage including DNA damage. mTORC1 has been postulated as main driver of these metabolic changes [1], which are overlapping to the functions described above in the chapter “Autophagy and Mitochondria”. Hence, rapamycin treatment prevents metabolic stress and delays cellular senescence.
Calorie restriction
There is one other intervention besides mTOR inhibitors that extends lifespan in a lot of organisms: caloric restriction (CR). CR is defined as a reduction in nutrient intake without malnutrition. Because mTORC1 has an important role in sensing energy, nutrients and insulin intracellularly and on the organismal level [1,53], this fueled the speculation that the lifespan-enhancing effects of CR are at least partly mediated by decreased mTORC1 signaling. Indeed, CR-like regimens do not additionally lengthen the lifespan of yeast or worms when mTOR is inhibited, indicating overlapping mechanisms [12,13,54]. In contrast, mTORC1 inhibition and CR show additive effects on lifespan in flies [55]. In rhesus monkeys, CR has universal health benefits and but no clear-cut effects on survival [56]. Whether CR prolongs lifespan in human is currently unknown. In some animals including distinct strains of mice, CR does not always correlate with lifespan extension, although it consistently improves health [57]. Most CR studies (as well as mouse experiments in general) are designed in an ad libitum fashion; the animals can eat as much as they want. Hence, these studies may actually analyze more the effects of overfeeding, which is known to promote obesity-associated pathologies in our society [58].
Clinical use of mTOR inhibitors
The finding that inhibition of mTOR prolongs lifespan and postpones onset of age-associated diseases in mammals spurred the interest to develop mTOR inhibitors as drugs to augment human longevity. However, the side effect profile of mTOR inhibitors are a major cause of concern. Because pharmacologic inhibition of mTOR is an FDA-approved clinical principle, there is a wealth of information about the known side effects of mTOR inhibitors (Rapalogs) in humans [59,60]. Importantly, the introduction of mTOR inhibitors as a therapeutic regimen generated a new spectrum of side effects that resulted in 20-40% dropout rates in Phase III clinical trials [61]. The most common side effects are immunosuppression, hyperglycemia and dyslipidemia as well as interstitial pneumonitis [59]. Most of these side effects are dosage-dependent and may regress with lower dosage. Because mTOR inhibitors are mostly given in combination with other drugs such as steroids or proliferation inhibitors in already severe diseased patients, preventive mTOR inhibitor therapy in the general healthy population might be more tolerable. Moreover, the immunosuppressive properties of mTOR inhibitors for T cell responses might be particularly strong for graft-reactive alloantigens, but not for pathogen-specific responses such as infections [62]. Indeed, mTOR inhibitors might be future longevity drugs, because a clinical trial in elderly healthy humans receiving the mTORC1 inhibitor everolimus at a very low dose showed safety and even improved immune function [24]. Moreover, alternate dosing regimens may still promote longevity but reduce side effects [63,64].
Outlook
mTOR inhibitors are currently the only known pharmacological intervention that increases lifespan in all experimental animal models tested. This raises the prospect that someday it might be a possible to therapeutically increase lifespan, slow aging, or decrease aging-related diseases in humans. Nevertheless, we are currently only at the beginning of that journey. Further knowledge of the actual lifespan-enhancing effects of mTOR inhibition might generate more precise ways to promote longevity. For example, differential isoforms and splice variants exist for the individual mTOR pathway members, their role in normal cellular functions and for aging is currently poorly defined. Because RNA splicing is required for longevity in C. elegans downstream of the TOR pathway, this gap of knowledge should be closed [65]. Moreover, distinct mTORC1 and mTORC2 complexes may exist inside the cell at different locations that perform different functions that may differentially affect longevity [66]. In conclusion, defining the exact mechanisms and tissues, in which mTOR inhibition promotes longevity and reduces age-related diseases is of scientific and general but also of socioeconomic importance.
Acknowledgements
T.W. is supported by grants from the Austrian Science Fund (FWF) grant FWF-P27701-B20, the Else-Kröner-Fresenius-Stiftung (P2013_A149), the Foundation for Sarcoidosis Research (FSR), and the Herzfelder’sche Familienstiftung.
References
- 1.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017 Mar 9;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weichhart T, Hengstschläger M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015 Oct;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013 Feb;23:53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Liu P, Gan W, Chin YR, Ogura K, Guo J, Zhang J, et al. PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex. Cancer Discov. 2015 Nov;5:1194–1209. doi: 10.1158/2159-8290.CD-15-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marat AL, Wallroth A, Lo W-T, Müller R, Norata GD, Falasca M, et al. mTORC1 activity repression by late endosomal phosphatidylinositol 3,4-bisphosphate. Science. 2017 Jun 2;356:968–972. doi: 10.1126/science.aaf8310. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin D, Hall MN. TSC on the peroxisome controls mTORC1. Nat Cell Biol. 2013 Oct;15:1135–1136. doi: 10.1038/ncb2849. [DOI] [PubMed] [Google Scholar]
- 7.Jewell JL, Russell RC, Guan K-L. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013 Mar;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll B, Maetzel D, Maddocks OD, Otten G, Ratcliff M, Smith GR, et al. Control of TSC2-Rheb signaling axis by arginine regulates mTORC1 activity. elife. 2016 Jan 7;5 doi: 10.7554/eLife.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarbassov DD, Ali SM, Sengupta S, Sheen J-H, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006 Apr 21;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012 Mar 30;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003 Dec 11;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 12.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004 May 25;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaeberlein M, Powers RW, Steffen KK, Westman EA, Hu D, Dang N, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005 Nov 18;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 14.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009 Jul 16;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014 Jun;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson SC, Yanos ME, Bitto A, Castanza A, Gagnidze A, Gonzalez B, et al. Dose-dependent effects of mTOR inhibition on weight and mitochondrial disease in mice. Front Genet. 2015 Jul 22;6:247. doi: 10.3389/fgene.2015.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010 May;176:2092–2097. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos FJ, Chen SC, Garelick MG, Dai D-F, Liao C-Y, Schreiber KH, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012 Jul 25;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer KE, Gelfond JAL, Soto VY, Han C, Someya S, Richardson A, et al. Health Effects of Long-Term Rapamycin Treatment: The Impact on Mouse Health of Enteric Rapamycin Treatment from Four Months of Age throughout Life. PLoS ONE. 2015 May 15;10:e0126644. doi: 10.1371/journal.pone.0126644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurez V, Dao V, Liu A, Pandeswara S, Gelfond J, Sun L, et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell. 2015 Dec;14:945–956. doi: 10.1111/acel.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013 Sep 12;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selman C, Tullet JMA, Wieser D, Irvine E, Lingard SJ, Choudhury AI, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009 Oct 2;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamming DW, Mihaylova MM, Katajisto P, Baar EL, Yilmaz OH, Hutchins A, et al. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell. 2014 Oct;13:911–917. doi: 10.1111/acel.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014 Dec 24;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 25.Ross C, Salmon A, Strong R, Fernandez E, Javors M, Richardson A, et al. Metabolic consequences of long-term rapamycin exposure on common marmoset monkeys (Callithrix jacchus) Aging. 2015 Nov;7:964–973. doi: 10.18632/aging.100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue Q-L, Yang H, Li H-F, Abadir PM, Burks TN, Koch LG, et al. Rapamycin increases grip strength and attenuates age-related decline in maximal running distance in old low capacity runner rats. Aging. 2016 Apr;8:769–776. doi: 10.18632/aging.100929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bitto A, Ito TK, Pineda VV, LeTexier NJ, Huang HZ, Sutlief E, et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. elife. 2016 Aug 23;5 doi: 10.7554/eLife.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 6;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lees H, Walters H, Cox LS. Animal and human models to understand ageing. Maturitas. 2016 Nov;93:18–27. doi: 10.1016/j.maturitas.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012 Feb 22;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013 Jan 17;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deretic V. Autophagy in innate and adaptive immunity. Trends Immunol. 2005 Oct;26:523–528. doi: 10.1016/j.it.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol. 2014 Dec;36:79–90. doi: 10.1016/j.semcdb.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He S, Sharpless NE. Senescence in health and disease. Cell. 2017 Jun 1;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012 Jun 28;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Igarashi M, Guarente L. mTORC1 and SIRT1 Cooperate to Foster Expansion of Gut Adult Stem Cells during Calorie Restriction. Cell. 2016 Jul 14;166:436–450. doi: 10.1016/j.cell.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 37.Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert) Nature. 2014 Jun 19;510:393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sukhbaatar N, Hengstschläger M, Weichhart T. mTOR-Mediated Regulation of Dendritic Cell Differentiation and Function. Trends Immunol. 2016 Nov;37:778–789. doi: 10.1016/j.it.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Curr Opin Cell Biol. 2011 Dec;23:707–715. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009 Jul 2;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009 Nov 24;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014 Jul;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000 Nov 1;14:2712–2724. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Correia-Melo C, Marques FDM, Anderson R, Hewitt G, Hewitt R, Cole J, et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016 Apr 1;35:724–742. doi: 10.15252/embj.201592862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015 Dec;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016 Feb 11;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laberge R-M, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015 Aug;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015 Sep;17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carroll B, Nelson G, Rabanal-Ruiz Y, Kucheryavenko O, Dunhill-Turner NA, Chesterman CC, et al. Persistent mTORC1 signaling in cell senescence results from defects in amino acid and growth factor sensing. J Cell Biol. 2017 Jul 3;216:1949–1957. doi: 10.1083/jcb.201610113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dominick G, Bowman J, Li X, Miller RA, Garcia GG. mTOR regulates the expression of DNA damage response enzymes in long-lived Snell dwarf, GHRKO, and PAPPA-KO mice. Aging Cell. 2017 Feb;16:52–60. doi: 10.1111/acel.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nacarelli T, Sell C. Targeting metabolism in cellular senescence, a role for intervention. Mol Cell Endocrinol. 2016 Aug 31; doi: 10.1016/j.mce.2016.08.049. [DOI] [PubMed] [Google Scholar]
- 53.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004 Sep 9;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 54.Hansen M, Taubert S, Crawford D, Libina N, Lee S-J, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007 Feb;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 55.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010 Jan;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017 Jan 17;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, González-Reyes JA, et al. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 2016 Jun 14;23:1093–1112. doi: 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayflick L. Dietary restriction: theory fails to satiate. Science. 2010 Aug 27;329:1014–5. doi: 10.1126/science.329.5995.1014. author reply 1015. [DOI] [PubMed] [Google Scholar]
- 59.Pallet N, Legendre C. Adverse events associated with mTOR inhibitors. Expert Opin Drug Saf. 2013 Mar;12:177–186. doi: 10.1517/14740338.2013.752814. [DOI] [PubMed] [Google Scholar]
- 60.Duran I, Goebell P-J, Papazisis K, Ravaud A, Weichhart T, Rodriguez-Portal JA, et al. Drug-induced pneumonitis in cancer patients treated with mTOR inhibitors: management and insights into possible mechanisms. Expert Opin Drug Saf. 2014 Mar;13:361–372. doi: 10.1517/14740338.2014.888056. [DOI] [PubMed] [Google Scholar]
- 61.Groth CG, Bäckman L, Morales JM, Calne R, Kreis H, Lang P, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation. 1999 Apr 15;67:1036–1042. doi: 10.1097/00007890-199904150-00017. [DOI] [PubMed] [Google Scholar]
- 62.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, et al. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol. 2010 Aug 15;185:2004–2008. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arriola Apelo SI, Neuman JC, Baar EL, Syed FA, Cummings NE, Brar HK, et al. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell. 2016 Feb;15:28–38. doi: 10.1111/acel.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arriola Apelo SI, Pumper CP, Baar EL, Cummings NE, Lamming DW. Intermittent administration of rapamycin extends the life span of female C57BL/6J mice. J Gerontol A Biol Sci Med Sci. 2016 Jul;71:876–881. doi: 10.1093/gerona/glw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Razquin Navas P, Thedieck K. Differential control of ageing and lifespan by isoforms and splice variants across the mTOR network. Essays Biochem. 2017 Jul 15;61:349–368. doi: 10.1042/EBC20160086. [DOI] [PubMed] [Google Scholar]
- 66.Ebner M, Sinkovics B, Szczygieł M, Ribeiro DW, Yudushkin I. Localization of mTORC2 activity inside cells. J Cell Biol. 2017 Feb;216:343–353. doi: 10.1083/jcb.201610060. [DOI] [PMC free article] [PubMed] [Google Scholar]