Abstract
The mammalian target of rapamycin (mTOR) is a key signaling kinase associated with a variety of cellular functions including the regulation of immunological and inflammatory responses. Classical mTOR inhibitors such as rapamycin or everolimus are commonly used in transplant as well as cancer patients to prevent transplant rejection or cancer progression, respectively. Noninfectious drug-induced pneumonitis is a frequent side effect in mTOR-inhibitor-treated patients. Therefore, we tested the effects of the mTOR inhibitor everolimus and the novel dual PI3K/mTOR inhibitor NVP-BEZ235 in a murine lipopolysaccharide (LPS)-induced acute lung injury model. C57BL/6 mice were treated with either everolimus or NVP-BEZ235 on two consecutive days prior to intratracheal administration of LPS. LPS administration induced a significant increase in total cell, neutrophil and erythrocyte numbers in the bronchoalveolar lavage fluid. Histological examination revealed a serious lung injury as shown by interstitial edema, vascular congestion and mononuclear cell infiltration in these mice after 24 hours. Everolimus as well as NVP-BEZ235 did not noticeable affect overall histopathology of the lungs in the lung injury model. However, NVP-BEZ235 enhanced IL-6 and TNF-α expression after 24 hours. In contrast, everolimus did not affect IL-6 and TNF-α levels. Interestingly, both inhibitors reduced inflammatory cytokines in an LPS/oleic acid-induced lung injury model. In conclusion, the mTOR inhibitors did not worsen the overall histopathological severity, but they exerted distinct effects on proinflammatory cytokine expression in the lung depending on the lung injury model applied.
1. Introduction
The serine/threonine kinase mammalian target of rapamycin (mTOR) pathway belongs to a critical cellular signaling pathway that affects broad aspects of cellular functions including metabolism, growth, and survival [1,2]. mTOR complex 1 (mTORC1) is linked to the phosphatidylinositol-3 kinase (PI3K) via the serine/threonine kinase Akt [3]. The PI3K/Akt/mTOR pathway is best known for having important functions in regulating adaptive immune cell activation, proliferation, and survival [4,5]. Rapamycin (also called sirolimus) and everolimus, two prototypical inhibitors of mTORC1, have potent immunosuppressive and anti-tumor activities by preventing proliferation and cell-cycle progression [6,7]. Hence, inhibition of mTORC1 was introduced in clinical transplantation and is currently employed as an immunosuppressive treatment to ameliorate chronic allograft damage and prevent immunosuppression-associated malignancies [8,9]. The cell-cycle arrest induced by mTOR inhibitors might in part explain its potent anti-tumor action especially in cancers with an up-regulated PI3K/Akt/mTOR pathway including anti-metastatic and anti-angiogenic effects [10,11]. In addition, mTOR inhibitors have shown promising results in advanced clinical trials against certain malignancies like renal cell carcinoma, mantle cell lymphoma, and endometrial cancers [12,13].
Transplant patients and renal cell carcinoma patients treated with the mTOR inhibitors rapamycin or everolimus experience noninfectious drug-induced pneumonitis (DIP) [14–17]. Moreover, breast cancer patients treated with everolimus similarly develop DIP [18] suggesting that DIP is common to all mTOR inhibitors. The frequency of DIP varies from 3-30% depending on the study analysis and seems independent from drug trough levels. Histologically, the patients present with diffuse interstitial infiltrates and bronchoalveolar lavage as well as lung biopsy reveals features of lymphocytic alveolitis, lymphocytic interstitial pneumonitis, bronchoalveolar obliterans organizing pneumonia, focal fibrosis, pulmonary alveolar hemorrhage, or a combination thereof [19]. Bronchoalveolar fluid microbiological evaluation is negative for many bacteria, fungi, parasites, or viruses indicating a noninfectious origin of DIP [20]. However, the underlying molecular causes of this form of acute lung injury (ALI) induced by the mTOR inhibitors are unclear.
The pathogenesis of acute lung injury (ALI) is complex and may be triggered by air pollutants or environmental cues such as asbestos [21], nickel [22], or tobacco smoke [23] but also by chemotherapeutics. The pathology involves the recruitment of neutrophils, the expression of inflammatory cytokines, and the apoptosis of epithelial cells but also of neutrophils finally leading to a disruption of the alveolar epithelial lung barrier, pulmonary edema, and abnormalities in gas exchange [24,25]. Lung macrophages of the innate immune system play a key role for the pathogenesis of ALI. For example, in acid-induced lung injury, oxidized phospholipids generated in the lung induce injury via Toll-like receptor 4 (TLR4) activation on macrophages [26]. This induces the expression of proinflammatory cytokines such as TNF-α, IL-6 or IL-1β. In addition, the TLR4 ligand lipopolysaccharide (LPS) can induce ALI in the mouse [27], which is dependent on TLR4 [28]. These data together suggest that the innate immune system is critically involved in the pathogenesis of pneumonitis or ALI.
While mTOR inhibitors were initially thought to globally dampen innate immune responses [29] and thereby contribute to their potent immunosuppressive properties, we and others could recently show that inhibition of mTOR is able to promote expression of the proinflammatory cytokines IL-12, TNF-α, IL-6, and IL-1β after stimulation with the TLR4 ligand LPS [30–36]. Recently, it has been shown that activation of mTOR inhibits inflammatory responses and apoptosis in the lung of mice due to cigarette smoke or LPS [37–39]. Therefore, we speculated that inhibition of mTOR in the innate immune system might exaggerate an initial insult in the lung leading to augmented inflammatory responses and lung damage that may be at the basis of DIP.
Currently, everolimus has not been evaluated in a preclinical murine model of pneumonitis/ALI. Moreover, it is completely unknown whether novel active-site PI3K/mTORC1/2 inhibitors share the DIP-inducing activities with classical mTORC1 inhibitors. Therefore, we investigated the effects of everolimus and the PI3K/mTOR inhibitor NVP-BEZ235, which is currently being tested in multiple clinical trials for its antiproliferative effects in cancer [40], in a murine model of ALI.
2. Materials and Methods
2.1. Mice
8-10 week old C57BL/6JRj female mice were obtained from Janvier Labs, France. Mice were housed under specific pathogen-free conditions according to FELASA guidelines. All animal experiments were discussed and approved by the Ethics and Animal Welfare Committee of the University of Veterinary Medicine Vienna, conform to the guidelines of the national authority (the Austrian Federal Ministry of Science, Research and Economy) as laid down in the Animal Science and Experiments Act (Tierversuchsgesetz – TVG; refs BMWF-68.205/0159-II/3b/2013) and are performed according to the guidelines of FELASA and ARRIVE. All efforts were made to minimize suffering.
2.2. Materials
Escherichia coli O55:B5 LPS and oleic acid were purchased from Sigma-Aldrich. Everolimus and NVP-BEZ235 were provided by Novartis. Phospho-S6 (Ser240/244) and phospho-Akt (Ser473) were purchased from Cell Signaling Technology. TER-119-APC, CD4-APC (RM4-5), CD8a-PerCP (53-6.7), CD19-FITC (6D5), I-Ab-PE (AF6-120.1), CD11c-FITC (N418), and F4/80-APC (BM8) antibodies for flow cytometry were purchased from BioLegend.
2.3. Animal models of acute lung injury
Mice were pretreated with 200 µl everolimus (10 mg/kg/day), NVP-BEZ235 (20 mg/kg/day) [40,41], or placebo (PBS) by oral gavage on two consecutive days. One hour after the second application of the inhibitors or vehicle controls, mice were anaesthetized by intraperitoneal injection with ketasol/xylasol and then 50 µl LPS O55:B5 (10mg/kg) or saline were administered by intratracheal (i.t.) instillation. Six, 12, or 24 hours after the induction of model, mice were killed by cervical dislocation. In some experiments, mice received 100 µl of a 2% oleic acid solution in 0.1% BSA/PBS by intravenous application 30 minutes after the 50 µl LPS O55:B5 (10mg/kg) i.t. instillation.
2.4. Bronchoalveolar lavage and lung content
Bronchoalveolar lavage fluid (BALF) was obtained by cannulating the trachea and lavaging the lungs two times with 0.8 ml saline. BALF samples were pooled for each mouse and centrifuged at 350 xg at 4° C for 7 minutes. Supernatant was separated and stored at -80° C for determination of cytokines. The cell pellet was resuspended in 2% FCS/1x PBS. Total cell count was assessed after staining with trypan blue using a hemocytometer. For differential cell counting, the cells were centrifuged onto glass slides at 550 rpm for 5 minutes using a Shandon Cytospin 2. The cytospins were fixed with cold methanol and stained with 10% Giemsa. The number of neutrophils out of 200 total cells was determined based on morphology per high-power field by light microscopy.
2.5. Flow cytometry
BALF cells, single cell suspensions of the lung or spleen were analyzed by flow cytometry on a FACSCalibur. Therefore, cells were blocked with TrueStain fcX (anti-mouse CD16/32, bioLegend, clone 93), and stained with fluorescently-labeled primary antibodies.
2.6. Histology and Immunohistochemistry
Lungs were removed, placed in 4% paraformaldehyde for 24 h, and stored in ethanol before preparation of paraffin-embedded tissue blocks. Sections were cut and stained with hematoxylin and eosin (H&E) or with phospho-S6 (Ser240/244) and pAkt (Ser473) for immunohistochemical detection. Novocastra streptavidin-HRP (Leica) and AEC- high sensitivity substrat chromogen (Dako) were used for detection of primary antibodies.
2.7. Enzyme-linked immunosorbent assay (ELISA)
Mouse TNF-α, IL-6 and IL-12 ELISA kits (BioLegend-ELISA MAX Deluxe Sets) were used for analysis of mouse BALF supernatant and mouse blood serum according to the recommendation of the manufacturer.
3. Results
3.1. Establishment of a LPS-inducible acute lung injury model
We evaluated three different models to induce lung injury: intranasal (i.n.) administration of 100 ng LPS, intratracheal (i.t.) administration of 10 mg/kg LPS, and administration of 10 mg/kg LPS i.t. together with intravenous (i.v.) application of 100 µl of a 2% oleic acid (OA) solution. Six hours after the challenge we prepared cytospins of the BALF and analyzed neutrophil numbers. We could not detect significant numbers of neutrophils in the BALF of mice treated with either PBS or LPS i.n. (Figure 1 A,B). In contrast, we found high neutrophil influx into the BALF in mice treated either with LPS i.t. and with LPS i.t. and OA i.v. (Figure 1 A,B). In addition, we measured the production of TNF-α in the BALF by ELISA. We noticed strong production of TNF-α in the BALF of the mice treated with i.t. LPS or LPS/OA, whereas no TNF-α could be observed in mice treated with PBS or LPS i.n. (Figure 1 C).
Fig. 1.
Establishment of acute lung injury models. PBS, 100 ng LPS (dissolved in 50 μl PBS) (i.n.), 10 mg/kg LPS (i.t.), or 100 µl 2% oleic acid administrated intravenously 30 minutes after i.t. administration of 10 mg/kg LPS (LPS+OA). BALF was isolated after six hours. (A) Representative Giemsa stained cytospins of BALF. (B) Number of neutrophils from 200 counted cells in BALF (means ± SEM; n=5 mice per group; PBS: 3 mice). (C) TNF-α levels in BALF were quantified by ELISA. (means ± SEM; n=5 mice per group; PBS: 3 mice)
3.2. Histological lung damage is not influenced by everolimus and NVP-BEZ235 in LPS-induced ALI
We chose to evaluate the mTOR inhibitors initially in the model with i.t. LPS alone, because this model has already been used in a variety of studies to investigate the effects of rapamycin on ALI. We treated the mice with either placebo, everolimus or NVP-BEZ235 for two days before i.t. instillation of LPS or PBS as control for 24 hours. Mice treated with PBS did not exhibit a detectable histological lung damage (Figure 2A). In contrast, LPS-treated mice developed histopathologic evidence of lung injury, which was characterized by a strong thickening of the alveolar septum, infiltration of inflammatory cells, and hemorrhage (Figure. 2A). The histological injury was not observed homogenously throughout the lungs consistent with i.t. administration (data not shown). Everolimus and NVP-BEZ235 did not apparently modulate histopathological lung injury induced by LPS (Figure 2A). LPS i.t. administration resulted in a strong phosphorylation of the mTORC1 activation marker S6 in the cells of the alveolar endothelium after 24 hours, which was completely blocked by treatment with everolimus (Figure 2B). Interestingly, NVP-BEZ235 did only modestly affect alveolar S6 phosphorylation in the alveolar endothelium. In addition, we observed a strong staining for pS6 in bronchial cells already in the PBS-treated mice that was again reduced in the everolimus- but not NVP-BEZ235-treated mice (Figure 2B). We only detected a basal phosphorylation of Akt in some bronchial cells after 24 hours; however, neither LPS, everolimus, nor NVP-BEZ235 appreciable modulated pAkt in our experiments (Figure 2C).
Fig. 2.
Histological lung damage is not influenced by everolimus and NVP-BEZ235 in LPS-induced ALI. Female C57BL/6 mice were pretreated with placebo, everolimus, or NVP-BEZ235 on two consecutive days. One hour after the second inhibitor treatment, mice were challenged with i.t. LPS for 24 hours. (A) Formalin-fixed lung sections were stained with H&E. (B,C) Lung sections were stained for (B) pS6 or (C) pAKT. Original magnification x20. Representative examples of 5 mice per group.
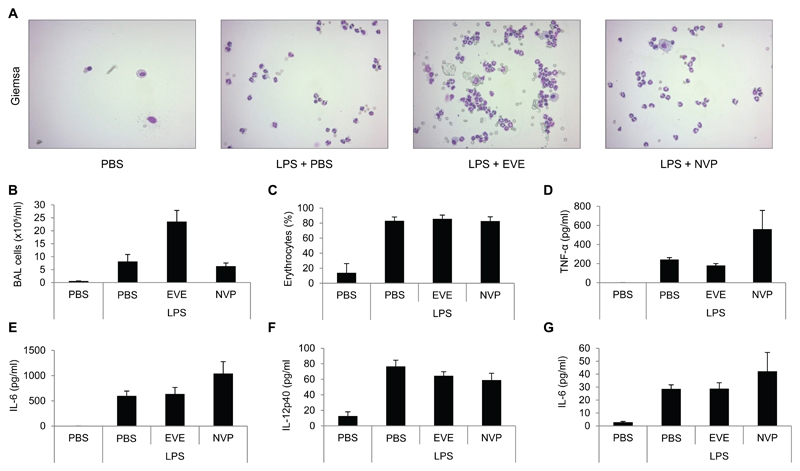
3.3. Everolimus and NVP-BEZ235 differentially affect neutrophil influx and proinflammatory cytokines
Next, we wanted to characterize cellular infiltration and cytokine expression in BALF in these mice. Mice treated with i.t. LPS alone developed a serious lung injury indicated by an influx of neutrophils and erythrocytes into the BALF after 24 hours (Figure 3A-C). Everolimus strongly enhanced the influx of neutrophils into the BALF, but did not change the numbers of erythrocytes (Figure 3A-C). In contrast, NVP-BEZ235 did not modulate either neutrophils or erythrocyte influx (Figure 3A-C). However, NVP-BEZ235 enhanced the expression of TNF-α and IL-6 but not IL-12 in the BALF compared to LPS-treated mice alone (Figure 3D-F). Everolimus did not influence cytokine expression in the lung of LPS-treated mice (Figure 3D-F). When we analyzed serum levels of these cytokines, we only detected measurable levels of IL-6 (Figure 3G). Again, NVP-BEZ235 enhanced serum IL-6 expression (Figure 3G). Moreover, we did not detect any gross systemic changes of either T-cells, B-cells, macrophages or dendritic cells in the spleen of the injured mice (data not shown).
Fig. 3.
Everolimus and NVP-BEZ235 differentially affect neutrophil influx and proinflammatory cytokines. Female C57BL/6 mice were pretreated with placebo (PBS), everolimus, or NVP-BEZ235. One hour after the second inhibitor treatment, mice were challenged with i.t. LPS for 24 hours. (A) Representative Giemsa-stained cytospins of BALF. (B) Total cell numbers in BALF. (C) Ter119 positive erythrocyte numbers in BALF were analyzed by flow cytometry. (D) TNF-α, (E) IL-6, and (F) IL-12p40 levels in BALF were quantified by ELISA. (G) IL-6 in serum was quantified by ELISA. means ± SEM; n= 7 mice per group.
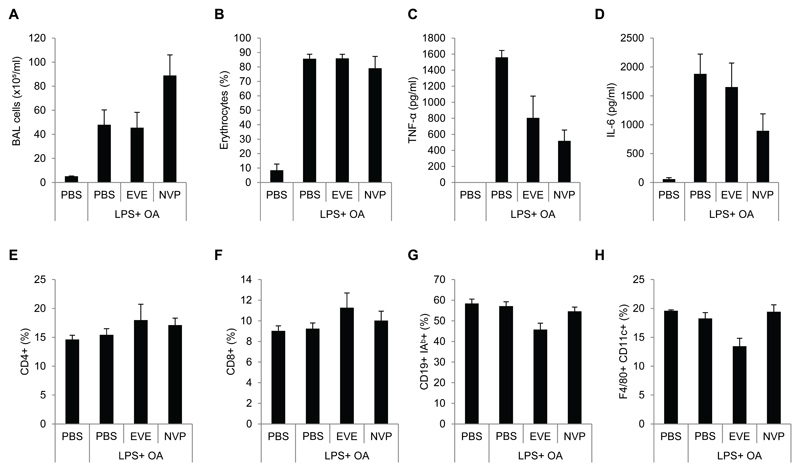
3.4. Evaluation of everolimus and NVP-BEZ235 in an LPS and oleic acid induced lung injury model
Finally, we investigated whether the response of the two inhibitors is dependent on the lung injury model applied. Therefore, we evaluated the impact of everolimus and NVP-BEZ235 in an ALI model, which includes the the i.v. administration of oleic acid together with i.t. administration of LPS. This model has been shown to exacerbate lung injury compared to LPS alone [42]. Interestingly, NVP-BEZ235 enhanced cell infiltration in the BALF of LPS/OA-treated mice, whereas everolimus had no effect (Figure 4A). Both inhibitors did not affect accumulation of erythrocytes in the BALF (Figure 4B). Interestingly, everolimus but also NVP-BEZ235 blocked expression of TNF-α as well as IL-6 in LPS/OA-treated mice (Figure 4C,D). We did not observe any major effects of the inhibitors in the distribution of major immune cells in the spleens of the mice (Figure 4E-H).
Fig. 4.
Evaluation of everolimus and NVP-BEZ235 in an LPS and oleic acid lung injury model. Female C57BL/6 mice were pretreated with placebo, everolimus, or NVP-BEZ235. One hour after the second inhibitor treatment, mice were challenged with i.t. LPS and after 30 minutes with oleic acid i.v. for 12 hours. (A) Total cell numbers in BALF. (B) Ter119 positive erythrocyte numbers in BALF were analyzed by flow cytometry. (C) TNF-α and (D) IL-6 levels in BALF were quantified by ELISA. (E-H) Spleens of the mice were analyzed for the indicated markers by flow cytometry. means ± SEM; n= 5 per group.
4. Discussion
The molecular causes of noninfectious mTOR-inhibitor induced pneumonitis are currently unclear, however important to understand to better manage or interfere with this side effect [17]. The elucidation of the molecular mechanisms might allow better management options for DIP and may also pave the way to uncouple the desired effects of mTOR inhibitors from their unwanted side effects. Transplant and cancer patients are currently treated with either rapamycin or everolimus. Noninfectious DIP is experienced with both inhibitors and occurs in about 3-30% of patients depending on the study analysis but probably independent from trough levels [14–17].
Previous studies have tried to elucidate the effects of the mTOR inhibitor rapamycin in murine models of ALI. Most studies only used the LPS-induced ALI model by i.t. administration of LPS and noticed an important role of mTOR during lung injury [38,43–45]. However, the effects of rapamycin were not uniformly similar in these studies. Rapamycin augmented LPS-induced lung injury and apoptosis in a study by Fielhaber et al [38]. In contrast, another study found that rapamycin decreased the severity of lung injury after i.t. LPS or Pam3Cys-Ser-(Lys)4 administration [43]. Rapamycin also ameliorated LPS-induced lung inflammation and T-cell activation in another report [44]. Another study found that rapamycin reduced the levels of inflammatory mediators but did not influence the overall severity and survival in the LPS-induced ALI model [45]. The effects of mTORC1 inhibitor in ALI might differ depending on the genetic background of the mice used (Balb/c versus C57BL/6). Moreover, the individual expression levels of inflammation-associated genes may also influence the subsequent response to mTOR inhibitors. For example, in mice that are deficient for Rtp801, which is a negative regulator of mTORC1, rapamycin strongly enhances LPS-induced lung inflammation [39]. Interestingly, environmental cues such as cigarette smoke promote activation of Rtp801 [37]. Hence, it can be envisioned that an individual expression pattern of Rtp801 or other genes in distinct mice may provide a basis, why mTORC1 inhibitors do not uniformly induce pneumonitis. In addition, the timing of the inhibitor treatment or the use of male vs. female mice might also account for different outcomes.
Everolimus, a derivative of rapamycin and the second major clinically used mTOR inhibitor, has not been evaluated in a murine model of ALI. Moreover, the novel active-site PI3K/mTOR inhibitor NVP-BEZ235 is currently in clinical trials and it is unknown whether it shares the DIP-inducing activities of classical mTOR inhibitors. Therefore, we analyzed the role of everolimus and NVP-BEZ3235 in two different murine models of ALI.
In our study, we treated 8-10 week C57BL/6 female mice with everolimus, NVP-BEZ235 or placebo by oral gavage on two consecutive days before induction of the murine ALI models. We found that i.t. LPS induced a significant increase in total cell, neutrophil and erythrocyte numbers in the bronchoalveolar lavage fluid. In addition, by histological examination, we observed a serious lung injury after 24 hours. We noticed in the LPS model that already PBS-treated animals showed activation of S6 in some bronchial cells. LPS strongly induced phosphorylation of S6 in the majority of alveolar cells after 24 hours, which was blocked by everolimus but not NVP-BEZ235. We did not detect activation of Akt 24 hours after LPS treatment. Moreover, we also did not detect appreciable levels of FoxP3-positive cells in the lung of the mice after 24 hours (data not shown). Everolimus and NVP-BEZ235 did not appreciably affect the histopathologic severity of the LPS-induced ALI model. In contrast, they exerted distinct effect on the proinflammatory cytokines TNF-α and IL-6 in the lung. After 24 hours both cytokines were strongly enhanced by treatment with NVP-BEZ235. Everolimus did not affect TNF-α or IL-6 levels in our LPS model, however everolimus strongly induced the infiltration of inflammatory cells into the BALF (Figure 3A,B). When we extended our results in a model using LPS and oleic acid, we observed that both inhibitors rather blocked proinflammatory cytokines after 12 hours. NVP-BEZ235-treated mice showed enhanced infiltration of neutrophils into the BALF in this model. Hence, we speculate that the immunomodulatory effects of mTOR inhibitors might depend on the ALI model applied.
5. Conclusion
In summary, our results provide evidence that everolimus and NVP-BEZ235 do not affect histopathologic signs of LPS or LPS/OA-induced lung injury. However, they exerted distinct immunomodulatory including proinflammatory responses depending on the injury model. These results advance our understanding of the effects of mTOR inhibitors in acute lung injury.
Acknowledgments
This study was supported by a research agreement from Novartis. T.W is supported by the Austrian Science Fund (FWF) grant FWF-P27701-B20, the Else-Kröner-Fresenius-Stiftung (P2013_A149), and the Herzfelder’sche Familienstiftung. We thank Sabine Hübner, Jan Tuckermann, Simona Saluzzo, and Sylvia Knapp for help with the initial setup of the ALI models.
References
- [1].Howell JJ, Ricoult SJ, Ben-Sahra I, Manning BD. A growing role for mTOR in promoting anabolic metabolism. Biochem Soc Trans. 2013;41:906–12. doi: 10.1042/BST20130041. [DOI] [PubMed] [Google Scholar]
- [2].Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- [3].Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–13. [PubMed] [Google Scholar]
- [4].Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–37. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4:313–9. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- [6].Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82:381–8. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- [7].Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–71. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- [8].Mulay AV, Hussain N, Fergusson D, Knoll GA. Calcineurin inhibitor withdrawal from sirolimus-based therapy in kidney transplantation: a systematic review of randomized trials. Am J Transplant. 2005;5:1748–56. doi: 10.1111/j.1600-6143.2005.00931.x. [DOI] [PubMed] [Google Scholar]
- [9].Shihab F, Christians U, Smith L, Wellen JR, Kaplan B. Focus on mTOR inhibitors and tacrolimus in renal transplantation: pharmacokinetics, exposure-response relationships, and clinical outcomes. Transpl Immunol. 2014;31:22–32. doi: 10.1016/j.trim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- [10].Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- [11].Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, et al. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317–23. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- [12].Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nature reviews Drug discovery. 2006;5:671–88. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- [13].Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- [14].Morelon E, Stern M, Kreis H. Interstitial pneumonitis associated with sirolimus therapy in renal-transplant recipients. N Engl J Med. 2000;343:225–6. doi: 10.1056/NEJM200007203430317. [DOI] [PubMed] [Google Scholar]
- [15].Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- [16].Saemann MD, Haidinger M, Hecking M, Horl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant. 2009;9:2655–61. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- [17].Duran I, Goebell PJ, Papazisis K, Ravaud A, Weichhart T, Rodriguez-Portal JA, et al. Drug-induced pneumonitis in cancer patients treated with mTOR inhibitors: management and insights into possible mechanisms. Expert opinion on drug safety. 2014 doi: 10.1517/14740338.2014.888056. [DOI] [PubMed] [Google Scholar]
- [18].Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pham PT, Pham PC, Danovitch GM, Ross DJ, Gritsch HA, Kendrick EA, et al. Sirolimus-associated pulmonary toxicity. Transplantation. 2004;77:1215–20. doi: 10.1097/01.tp.0000118413.92211.b6. [DOI] [PubMed] [Google Scholar]
- [20].Champion L, Stern M, Israel-Biet D, Mamzer-Bruneel MF, Peraldi MN, Kreis H, et al. Brief communication: sirolimus-associated pneumonitis: 24 cases in renal transplant recipients. Annals of internal medicine. 2006;144:505–9. doi: 10.7326/0003-4819-144-7-200604040-00009. [DOI] [PubMed] [Google Scholar]
- [21].Governa M, Amati M, Bellis D, Bichisecchi E, Santarelli L. Diagnosis of asbestos-related pleuropolmonary diseases. La Medicina del lavoro. 2006;97:463–74. [PubMed] [Google Scholar]
- [22].Kunimasa K, Arita M, Tachibana H, Tsubouchi K, Konishi S, Korogi Y, et al. Chemical pneumonitis and acute lung injury caused by inhalation of nickel fumes. Intern Med. 2011;50:2035–8. doi: 10.2169/internalmedicine.50.5557. [DOI] [PubMed] [Google Scholar]
- [23].Rao RN, Goodman LR, Tomashefski JF., Jr Smoking-related interstitial lung disease. Annals of diagnostic pathology. 2008;12:445–57. doi: 10.1016/j.anndiagpath.2008.10.001. [DOI] [PubMed] [Google Scholar]
- [24].Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2:403–11. doi: 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Martin TR, Hagimoto N, Nakamura M, Matute-Bello G. Apoptosis and epithelial injury in the lungs. Proc Am Thorac Soc. 2005;2:214–20. doi: 10.1513/pats.200504-031AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–49. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen H, Bai C, Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert review of respiratory medicine. 2010;4:773–83. doi: 10.1586/ers.10.71. [DOI] [PubMed] [Google Scholar]
- [28].Togbe D, Schnyder-Candrian S, Schnyder B, Doz E, Noulin N, Janot L, et al. Toll-like receptor and tumour necrosis factor dependent endotoxin-induced acute lung injury. International journal of experimental pathology. 2007;88:387–91. doi: 10.1111/j.1365-2613.2007.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weichhart T. Mammalian target of rapamycin: a signaling kinase for every aspect of cellular life. Methods Mol Biol. 2012;821:1–14. doi: 10.1007/978-1-61779-430-8_1. [DOI] [PubMed] [Google Scholar]
- [30].Katholnig K, Linke M, Pham H, Hengstschlager M, Weichhart T. Immune responses of macrophages and dendritic cells regulated by mTOR signalling. Biochem Soc Trans. 2013;41:927–33. doi: 10.1042/BST20130032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–31. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- [32].Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–43. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–77. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- [34].Ohtani M, Hoshii T, Fujii H, Koyasu S, Hirao A, Matsuda S. Cutting Edge: mTORC1 in Intestinal CD11c+CD11b+ Dendritic Cells Regulates Intestinal Homeostasis by Promoting IL-10 Production. J Immunol. 2012;188:4736–40. doi: 10.4049/jimmunol.1200069. [DOI] [PubMed] [Google Scholar]
- [35].Pan H, O'Brien TF, Zhang P, Zhong XP. The role of tuberous sclerosis complex 1 in regulating innate immunity. J Immunol. 2012;188:3658–66. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jiang Q, Weiss JM, Back T, Chan T, Ortaldo JR, Guichard S, et al. mTOR kinase inhibitor AZD8055 enhances the immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res. 2011;71:4074–84. doi: 10.1158/0008-5472.CAN-10-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yoshida T, Mett I, Bhunia AK, Bowman J, Perez M, Zhang L, et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat Med. 2010;16:767–73. doi: 10.1038/nm.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fielhaber JA, Carroll SF, Dydensborg AB, Shourian M, Triantafillopoulos A, Harel S, et al. Inhibition of mammalian target of rapamycin augments lipopolysaccharide-induced lung injury and apoptosis. J Immunol. 2012;188:4535–42. doi: 10.4049/jimmunol.1003655. [DOI] [PubMed] [Google Scholar]
- [39].Nadon AM, Perez MJ, Hernandez-Saavedra D, Smith LP, Yang Y, Sanders LA, et al. Rtp801 Suppression of Epithelial mTORC1 Augments Endotoxin-Induced Lung Inflammation. The American journal of pathology. 2014 doi: 10.1016/j.ajpath.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–63. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- [41].Liu TJ, Koul D, LaFortune T, Tiao N, Shen RJ, Maira SM, et al. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–10. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. American journal of physiology Lung cellular and molecular physiology. 2008;295:L379–99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lorne E, Zhao X, Zmijewski JW, Liu G, Park YJ, Tsuruta Y, et al. Participation of mammalian target of rapamycin complex 1 in Toll-like receptor 2- and 4-induced neutrophil activation and acute lung injury. American journal of respiratory cell and molecular biology. 2009;41:237–45. doi: 10.1165/rcmb.2008-0290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nakajima T, Lin KW, Li J, McGee HS, Kwan JM, Perkins DL, et al. T Cells and Lung Injury: Impact of Rapamycin. American journal of respiratory cell and molecular biology. 2014 doi: 10.1165/rcmb.2013-0171OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang L, Gui YS, Tian XL, Cai BQ, Wang DT, Zhang D, et al. Inactivation of mammalian target of rapamycin (mTOR) by rapamycin in a murine model of lipopolysaccharide-induced acute lung injury. Chinese medical journal. 2011;124:3112–7. [PubMed] [Google Scholar]