Abstract
Chordoma is a rare malignant tumour of bone, the molecular marker of which is the expression of the transcription factor, brachyury. Having recently demonstrated that silencing brachyury induces growth arrest in a chordoma cell line, we now seek to identify its downstream target genes. Here we use an integrated functional genomics approach involving shRNA-mediated brachyury knockdown, gene expression microarray, ChIP-seq experiments, and bioinformatics analysis to achieve this goal. We confirm that the T-box binding motif of human brachyury is identical to that found in mouse, Xenopus, and zebrafish development, and that brachyury acts primarily as an activator of transcription. Using human chordoma samples for validation purposes, we show that brachyury binds 99 direct targets and indirectly influences the expression of 64 other genes, thereby acting as a master regulator of an elaborate oncogenic transcriptional network encompassing diverse signalling pathways including components of the cell cycle, and extracellular matrix components. Given the wide repertoire of its active binding and the relative specific localization of brachyury to the tumour cells, we propose that an RNA interference-based gene therapy approach is a plausible therapeutic avenue worthy of investigation.
Keywords: chordoma, brachyury, T, sarcoma, bone tumour, genetics, ChIP-seq, gene expression microarray, siRNA
Introduction
Chordoma is a rare malignant tumour that recapitulates the differentiation repertoire of notochordal cells. It not only has microscopic features resembling the embryonic notochord, but also expresses the transcription factor T (referred to as brachyury henceforth), the founding member of the T-box family of transcription factors, which is required for notochord development [1–4]. The view that chordomas show notochordal differentiation is also based on the vast majority of chordomas arising in the axial skeleton, the site of the embryonic notochord [4,5]. These observations provide a strong link between chordomas and the notochord.
Duplication of the brachyury locus is associated with susceptibility to developing familial chordoma [6], and amplification of brachyury is noted in approximately 5% of the sporadic form of the disease [7]. Also, mutations in the coding region of brachyury have not been identified in chordomas [6,8]. At a functional level, we and others have shown that in vitro suppression of brachyury halts cell proliferation in two different chordoma cell lines [7,9]. It has also been shown that overexpression of brachyury, in a cell line which does not express this protein or mRNA, results in enhanced proliferation, motility, and invasiveness [10]. Taken together, this evidence implicates brachyury in the pathogenesis of chordoma and suggests that it acts as an oncogene.
The mean overall survival of individuals with chordoma is 7 years from diagnosis [11,12]. The mainstay of treatment is surgery, although this can be supported with proton beam therapy [13]. The tumours are largely resistant to chemo- and radio-therapy, and although some of the new targeted therapies have shown evidence of tumour response, the results overall appear to have limited benefit [14,15].
Targets of brachyury have been identified in mesoderm during development of model organisms [16–19]. In view of brachyury being implicated in the pathogenesis of chordoma, we sought to identify its downstream targets, which have not been documented in humans, because in addition to providing insight into the pathogenesis of this tumour, we may also identify genes, which could be exploited therapeutically.
Materials and methods
Human samples
The chordoma samples were obtained from the RNOH Musculoskeletal Biobank (approved by the Cambridgeshire Research Ethics committee, Cambs, UK; Reference No: 09/H0304/78).
Cell culture and shRNA knockdown of brachyury in the U-CH1 cell line
The U-CH1 cell line (provided by Dr David Alcorta, Duke University, Durham, NC, USA through the Chordoma Foundation) was grown as a monolayer in 10 cm tissue culture dishes (Corning Life Sciences, Corning, NY, USA) coated with 0.1% gelatin (Sigma-Aldrich, Ayrshire, UK) as previously described [7,20]. The shRNA-mediated brachyury knockdown was achieved using a model previously established in our laboratory [7]. The U-CH1 cells were harvested the day before infection and plated at a density of 1 × 106 cells per 10 cm dish. The cultures were infected in triplicate with the V2LHS 153729 shRNA construct (pGIPZ™; Thermoscientific Open Biosystems, Chesterville, AL, USA), which targets the 3’UTR of human brachyury mRNA, at a multiplicity of infection of 1 in the presence of polybrene (10 μg/ml) (Sigma-Aldrich, St Louis, MO, USA). The chemotactic migration and adhesion assays are described in the Supplementary methods.
RNA extraction and q-RT-PCR analysis
Total RNA was extracted according to the manufacturer’s instructions using the Qiagen miRNeasy kit (Qiagen GmbH, Hilden, Germany) and quantified using Nanodrop® spectrophotometry (Thermoscientifc, Wilmington, DE, USA). Reverse transcription to cDNA was performed using the Applied Biosystems High Capacity RNA to cDNA reverse transcription mastermix kit (Applied Biosystems, CA, USA) using 100 ng of total RNA. q-RT-PCR was performed using Applied Biosystems Sybr® green chemistry with custom-designed primers for brachyury (Eurofins MWG-Operon, Germany), GAPDH, TGFA, AKR1B10, FGF1, EGF, HOPX, ETV1, and IL-8 (Invitrogen, Paisley, UK) (Supplementary Table 1). Detection was carried out using the Mastercycler® Realplex Eppendorf system (Eppendorf, Cambridge, Cambs, UK).
The mRNA expression level of brachyury served as validation of the efficacy of the knockdown of this gene in the U-CH1 cell line. The relative gene expression level was determined using the 2−ΔΔCT comparative method normalized to the housekeeping gene GAPDH [21]. Each bar graph corresponds to the mean ± standard deviation of three experiments performed in triplicate.
For confirmation of brachyury target expression in human chordoma samples, the 2−ΔCT method was employed, normalised to GAPDH expression [21]. These values were log2-transformed and used to generate the heatmap. Each datum point represents the mean expression derived from a minimum of two q-RT-PCR runs performed in duplicate. Both the heatmap and unsupervised hierarchical clustering were generated with GENE-E using Euclidian distance as a parameter [22].
Gene expression microarray
Total RNA was quantified using a Nanodrop 1000 Spectrophotometer and the Agilent Bioanalyser 2100 instrument. RNA samples with RNA integrity number (RIN) greater than 7 were chosen for hybridization by the in-house microarray facility–UCL Genomics. Total starting RNA material (175 ng) was prepared using the Applause WT-Amp ST System (NuGEN, San Carlos, CA, USA), and fragmentation and labelling of the cDNA were carried out using the NuGEN Encore Biotin Module. The fragmented and labelled cDNA (5 μg) was then hybridized to Affymetrix GeneChip® Human Exon 1.0 ST arrays (Affymetrix, Santa Clara, CA, USA) according to NuGEN instructions for 16 h at 45 °C. The arrays were washed and stained using the GeneChip Fluidics Station 450, and scanned using the Affymetrix GeneChip® Scanner. Expression Console 1.1 (Affymetrix) was used to assess quality metrics.
Differential gene expression analysis
Expression values were calculated from Affymetrix GeneChip® Human Exon 1.0 ST data using the aroma.affymetrix package for the R statistical programming language (http://www.aroma-project.org/). Expression summaries for each gene were calculated using a custom chip description file (CDF) that collects individual probes into probe sets for ENSEMBL genes (V13;http://brainarray.mbni.med.umich.edu/Brainarray/Database/CustomCDF/13.0.0/ensg. asp). Significance of differential expression was estimated using the limma package from Bioconductor [23]. ARACNE and gene set enrichment analysis are described in the Supplementary methods.
Cell proliferation and cell cycle assay
Cell proliferation was assessed using the Click-IT® Edu Alexa Fluor 647 system (Invitrogen). Briefly, cells were grown as a monolayer and labelled with 10 μM (final concentration) Edu overnight. Cells were permeabilized with the saponin-based solution, and Edu detection was performed using Alexa Fluor 647 azide dye chemistry. Cell cycle labelling was performed using propidium iodide (Sigma-Aldrich). Non Edu- and non-propidium iodide-containing cells were labelled with the Alexa Fluor 647 dye and served as the background control. The percentage of actively cycling cells and the cell cycle distribution phase were determined by flow cytometry using the CyAN-ADP™ flow instrument (Beckman Coulter Inc, High Wycombe, UK) using appropriate parameters and controls.
Chromatin immunoprecipitation and sequencing (ChIP-seq)
Three biologically independent ChIP experiments were performed. For each replicate, adherent U-CH1 cells (~2 × 106) were fixed in situ in the tissue culture flasks with 1% formaldehyde solution, which was quenched after 10 min by the addition of glycine (final concentration 0.125 M). Cells were then washed twice with 1× Dulbecco’s phosphate buffered saline (Sigma-Aldrich), harvested using a cell scraper (Sarstedt, NC, USA), centrifuged, and frozen in liquid nitrogen.
ChIP was carried out as previously described [24] using 5 μg of ChIP-grade anti-brachyury (N19) antibody (sc17743; Santa Cruz Biotechnology, Santa Cruz, CA, USA) [25]. A paired-end library suitable for sequencing was generated from each immunoprecipitated DNA sample, and a whole cell extract input sample (30 ng). The Illumina Paired-End DNA Sample Prep Kit (Illumina, San Diego, CA, USA) was used for end repair A-tailing and adaptor ligation according to the manufacturer’s instructions, except that the adaptor ligation reaction constituted a 50 μl reaction including 4 μl of a 1-in-100 dilution of paired-end adaptor oligonucelotide mix. The ligated products were amplified with Phusion HF polymerase enzyme (Finnzymes, Vantaa, Finland) using PCR primers PE 1.0 and 2.0 (Illumina) before size selection and sequencing. 54 bp paired-end sequencing was carried out on the Illumina Genome Analyser II platform. ChIP-seq data analysis is described in the Supplementary methods.
Immunohistochemistry
Immunohistochemistry was performed on chordoma tissue microarrays [8] with the Bond-Max Autostainer (Novocastra, Newcastle upon Tyne, UK) using the chromogen diaminobenzidine and the mouse monoclonal antibody against AKR1B10 (M01), clone1A6 (Abnova, Jhongli City, Taoyuan County, Taiwan). Sections were counterstained with haematoxylin. Incubation without a primary antibody was used as a negative control. Non-neoplastic lymphocytes and fibroblasts served as internal negative controls.
Accession codes
Raw ChIP-seq reads are stored under European Nucleotide Archive accession ERP000953 and Array Express accession E-ERAD-23. Microarray data are stored under accession E-MEXP3716 in the ArrayExpress database.
Results
Brachyury knockdown diminishes proliferation and migration by down-regulating genes involved in these processes
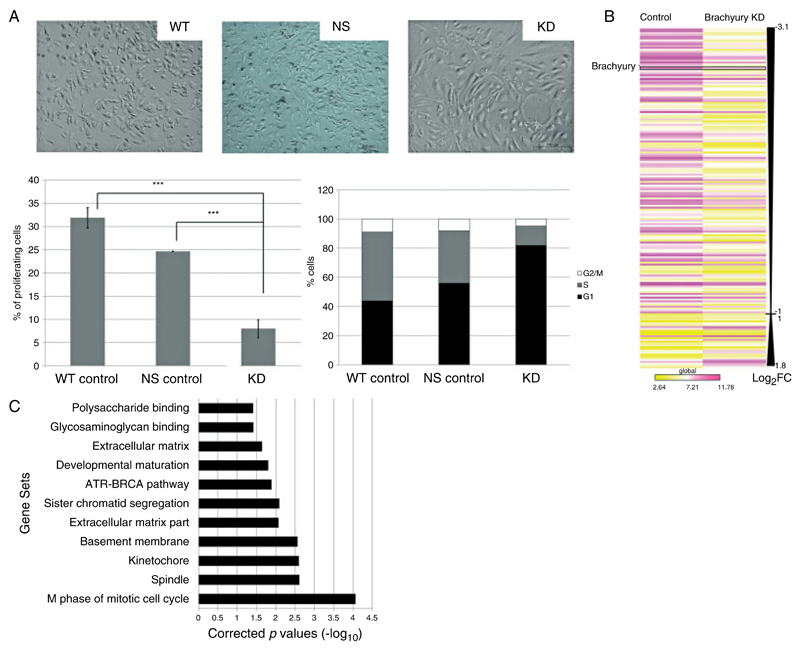
To identify brachyury-responsive genes, we used gene expression microarrays to assay gene expression changes on stable knockdown of brachyury in the human U-CH1 chordoma cell line. As previously reported [7], knockdown (KD) of brachyury resulted in the cells becoming large and flattened with extensive branching (Figure 1A). This was associated with growth arrest, as revealed using the Edu nucleoside analogue proliferation and cell cycle assay (Figure 1A). KD of brachyury was also associated with a significant reduction in chemotactic migration and a general trend towards reduced adhesion of U-CH1 cells in vitro (Supplementary Figure 1).
Figure 1.
Gene expression data derived from U-CH1 chordoma cells in which brachyury is silenced. (A) Phase contrast photomicrographs of U-CH1 cells showing the epithelioid, physaliphorous appearance characteristic of control wild-type (WT) and non-silencing (NS) vector-transfected chordoma cells contrasted with the spindled, flattened phenotype when brachyury is silenced. Bottom left (cell proliferation assay): bars represent the percentage of U-CH1 cells incorporated with Edu demonstrating a significant reduction in proliferation in the brachyury knockdown cells (KD) compared with both wild-type and non-silencing control cells (***p < 0.01, Student’s t-test). Bottom right (cell cycle analysis): a representative bar graph of the cell cycle profile with brachyury knockdown cells showing more than 80% of cells arrested in G1 phase. (B) Heatmap schematic of the global view of statistically significant differentially expressed genes (log2 fold change of ≥1) in brachyury knockdown U-CH1 cells reflecting a predominance of down-regulated genes. Brachyury is amongst the most down-regulated genes. A complete list of these genes may be found in Supplementary Table 2a. Yellow = lower expression; violet = higher expression; Log2FC = log2 fold change. Comparison between both groups using Student t-test significance p < 0.05. (C) GSEA of the microarray data demonstrates significant enrichment (p < 0.05) of biological pathway and gene ontology gene sets in the control cells compared with U-CH1 cells in which brachyury is knocked down.
Analysis of the microarrays and q-RT-PCR validation revealed brachyury to be among the most down-regulated genes (Figure 1B, Supplementary Table 2, and Supplementary Figure 2). Modular and functional gene annotation enrichment of the significantly differentially expressed genes (p < 0.01) was determined using GSEA [26]. This revealed enrichment for genes involved in regulation of the cell cycle (spindle, mitosis, sister chromatid segregation) (Figure 1C), in the production of extracellular matrix and cytokines, and in glycosaminoglycan binding, all of which are pertinent to the notochord and chordomas [1,27,28] (Figure 1A).
Brachyury binds to the canonical T-box in close proximity to genes
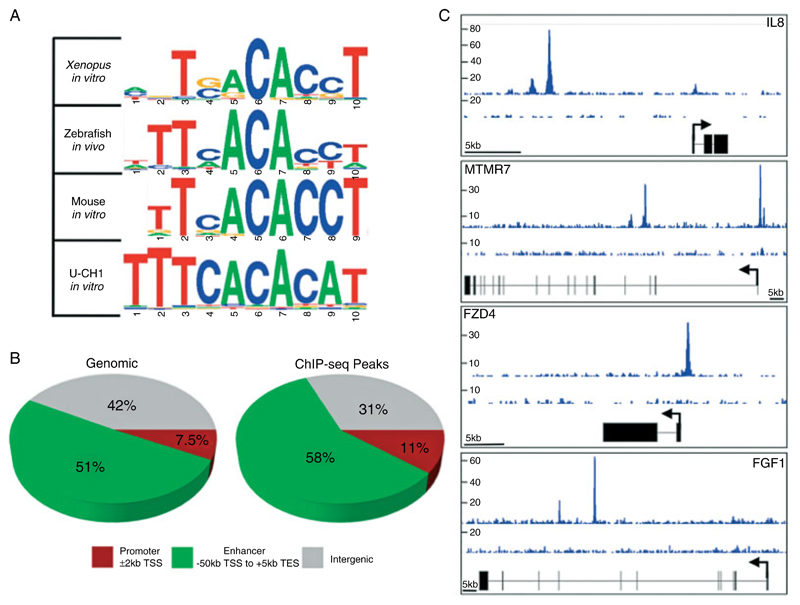
Microarray analysis cannot identify direct target genes. To this end, we performed ChIP-seq experiments to identify the binding sites of brachyury across the genome of U-CH1 cells. Studies in other organisms have revealed a conserved T-box binding site [16–18]. De novo motif searching within the ChIP-seq peaks identified a T-box binding site, which we found to be similar to that identified for mouse, Xenopus, and zebrafish brachyury orthologues (Figure 2A). This provides evidence that the identified binding regions reflect true brachyury binding sites in chordoma cells. Having established that the ChIP-seq approach identifies bona fide brachyury binding sites, we proceeded to analyse the results in an attempt to find the genomic regions where the protein localizes. This revealed 6420 reproducibly bound regions (ChIP-seq peaks) (Supplementary Table 3). The ChIP-seq peaks were significantly enriched in both promoter and enhancer regions as defined in the Materials and methods section (t-test, p ≤ 1 × 10−100, Figure 2B), suggesting that brachyury binding occurs in close proximity to genes. These ChIP-seq peaks provided a list of potential direct targets for cross-validation with our expression microarray data. Representative examples of ChIP-seq peaks are shown in Figure 2C.
Figure 2.
ChIP-seq data indicate brachyury binding to the conserved T-box site proximal to genes. (A) The discovered binding motif for brachyury in the U-CH1 chordoma cell line is the same as the published binding sites of Xenopus brachyury, mouse brachyury, and zebrafish Ntla. (B) Pie charts indicating simulated random (left) and real (right) distribution of ChIP-seq peaks. ChIP-seq peaks are significantly enriched within promoter and enhancer regions as defined (p = 2.10 × 10−101 and 7.13 × 10−110, respectively). Percentages in pie chart are rounded off to two significant figures. (C) Representative ChIP-seq peaks surrounding brachyury target genes. Sequence depth is indicated on the left and is representative of merged replicate ChIP-seq experiments.
Brachyury positively regulates the transcription of genes identified by ChIP-seq
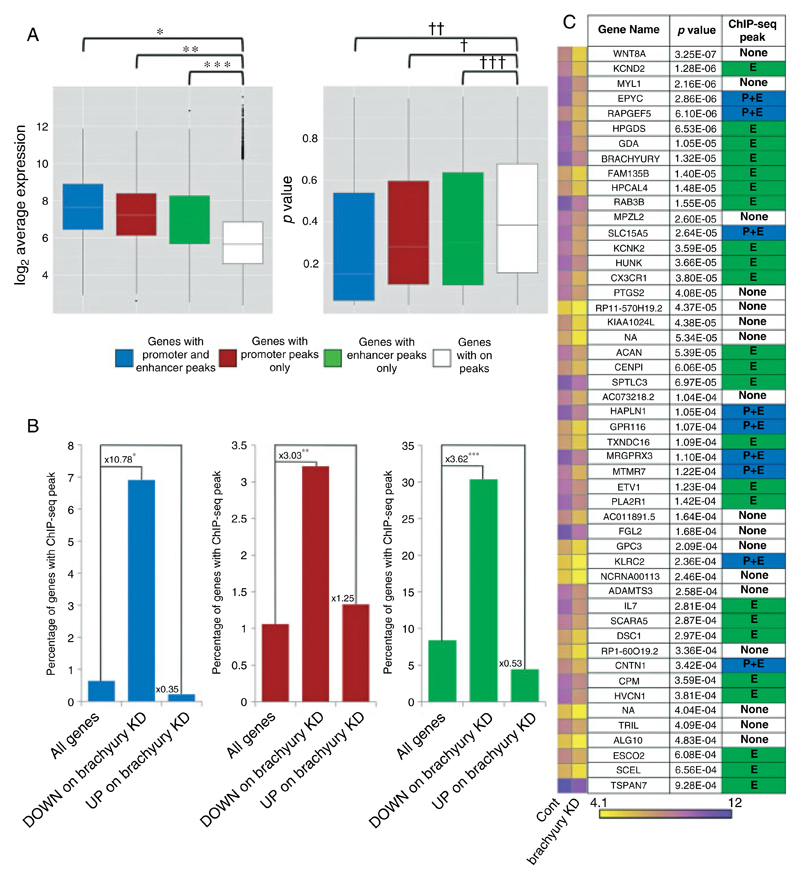
By integrating the gene expression microarray data with the ChIP-seq data, we sought to determine if the identified brachyury binding sites are functional. We classified the genes into those with both promoter and enhancer peaks, those with enhancer peaks only, and those with promoter peaks only: we refer to them collectively as ‘marked genes’ (Supplementary Table 4). All three categories of marked genes showed significantly higher gene expression in control U-CH1 cells than genes without ChIP-seq peaks (Figure 3A, left box-plot). We conclude that the ChIP-seq peaks are associated with transcriptionally active genes in U-CH1 cells. We next sought to determine whether the expression levels of these genes are brachyury-dependent. To do this, we compared the distribution of p values for differential expression of marked genes with that of unmarked genes. All categories of marked genes showed significantly lower p values for differential expression when brachyury was knocked down, compared with unmarked genes (p ≤ 3.47 × 10−5; Figure 3A, right box-plot). Marked genes are therefore likely to be direct transcriptional targets of brachyury. Additionally, this analysis identified regulatory regions (promoters and enhancers) through which brachyury is likely to act.
Figure 3.
ChIP-seq peaks are significantly associated with expressed genes that are down-regulated on knockdown of brachyury. (A) Box plots of gene expression for peak-marked and unmarked genes in control U-CH1 cells (left) and box plots of p value for differential expression on knockdown of brachyury for peak-marked and unmarked genes (right). Genes were categorized as described in the Materials and methods section. Genes with ChIP-seq peaks are more highly expressed than those without in control U-CH1 cells. They are also more likely to change in response to a change in expression on knockdown of brachyury. *p = 4.95 × 10−37; **p = 1.23 × 10−45; ***p = 1.09 × 10−210; †p = 3.47 × 10−5; ††p = 3.18 × 10−9; †††p = 9.42 × 10−16. (B) Graphs of the percentage of all genes and differentially expressed genes (p ≤ 0.01) with promoter and enhancer peaks, genes with only promoter peaks, and genes with only enhancer peaks. Genes down-regulated on knockdown of brachyury are significantly associated with ChIP-seq peaks. Up-regulated genes are not. *p = 8.46 × 10−85; **p = 3.58 × 10−7; ***p = 5.18 × 10−88. (C) Heatmap of expression for 50 genes down-regulated on brachyury knockdown (p ≤ 1 × 10−3, ≥2.5-fold change in expression). For each gene name, representative heatmap, p value, and ChIP-seq peaks are indicated.
We next asked whether brachyury positively or negatively regulates its target genes in cells in which brachyury expression is knocked down. We found that down-regulated genes (p ≤ 0.01) were significantly associated with brachyury-binding events (χ2, p ≤ 0.01), whereas up-regulated genes were not (p ≤ 0.01) (Figure 3B). In addition, GSEA indicated a significant association of ChIP-seq peaks with genes down-regulated as a result of brachyury knockdown [false discovery rate (FDR) q-val <0.001, FWER p < 0.001; Supplementary Figure 3]. In total, we identified 257 significantly down-regulated genes (p ≤ 0.01) with associated ChIP-seq peaks (Figure 3C and Supplementary Table 4) and propose that these genes are likely to be direct transcriptional targets of brachyury, whereas the other 366 differentially expressed genes, not associated with ChIP-seq peaks, represent genes whose expression is controlled indirectly by brachyury. Based on these results, we constructed a gene regulatory network (GRN) for brachyury in U-CH1 cells (Supplementary Figure 4).
The brachyury GRN in U-CH1 cells reflects transcriptional networks active in primary tumours
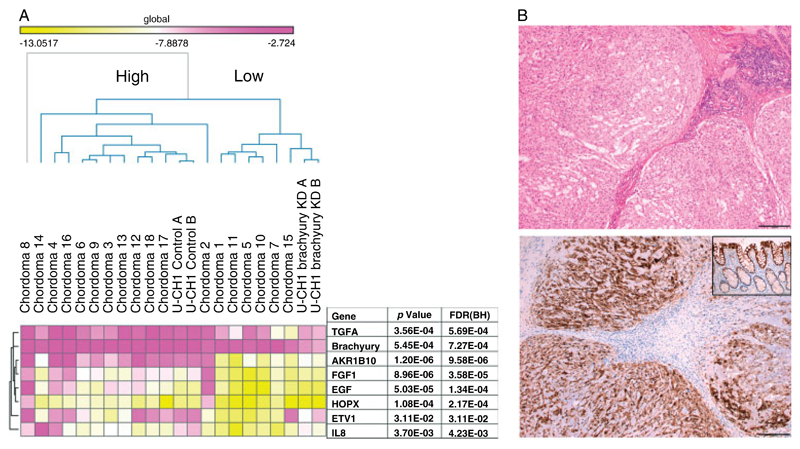
We asked if the proposed brachyury GRN in U-CH1 cells was representative of that in other chordoma cell lines and human chordoma samples. To address this, we employed ARACNE, an in silico target prediction software that can be used to infer transcriptional networks [29]. To predict brachyury targets, we analysed the previously published gene expression profiles of eight human primary chordomas and two chordoma cell lines (U-CH2 and K001) (Materials and methods section) [25]. From this, we identified 2660 putative brachyury targets. Of the direct targets and other differentially expressed genes (without peaks) identified from the U-CH1 cell line (see above), 99 direct targets (39%) and 64 other genes (17%) were common to the U-CH1 cell line, the primary tumours, and other cell lines (Supplementary Table 5). The overlap of the direct targets is greater than would be expected by chance (χ2, p < 0.002). To provide additional evidence that our ‘core’ gene set represented bona fide direct targets in primary tumours and in particular to validate the targets predicted from ARACNE, which is considered to function best when analysing large datasets [29], we performed q-RT-PCR assays on a new set of 18 chordomas, all of which express brachyury. Not only were targets of potential interest, selected on the basis of previous publications [8,30–34], found to be expressed in chordoma samples, but unsupervised hierarchical clustering revealed that higher levels of brachyury expression were found to be associated with higher mRNA levels of target genes (Figure 4A).
Figure 4.
Confirmation of the expression of core targets in chordoma samples. (A) Heatmap of q-RT-PCR mRNA expression data (selection of core targets) analysis of 18 chordoma samples and the U-CH1 cell line (Materials and methods section). Unsupervised hierarchical clustering demonstrates two distinct groups of chordomas (High and Low). p represents the significant difference in the expression between these two groups (t-test). Statistically higher expression levels of brachyury are associated with higher levels of expression of downstream targets and vice versa. KD = knockdown; FDR (BH) = false discovery rate (Benjamini–Hochberg method). (B) Light photomicrographs of a haematoxylin and eosin-stained section of a conventional chordoma (top panel) and a chordoma showing immunoreactivity for AKR1B10 (bottom panel). Scale bar = 200 μm. Inset: positive control, normal colonic tissue.
Additional validation was performed on aldo-keto reductase family 1 member B10, a gene identified as being indirectly regulated by brachyury in U-CH1. AKR1B10 is located on a putative chordoma susceptibility region [34] and has been shown to be a key antioxidant response element factor in renal cancer [35]. In view of its documented roles in cancer development and chemoresistance [36], in addition to its strong co-expression with brachyury in chordomas [25,32], we performed immunohistochemical analysis on a tissue microarray comprising 50 conventional chordomas and demonstrated unequivocal cytoplasmic expression in all cases (Figure 4B).
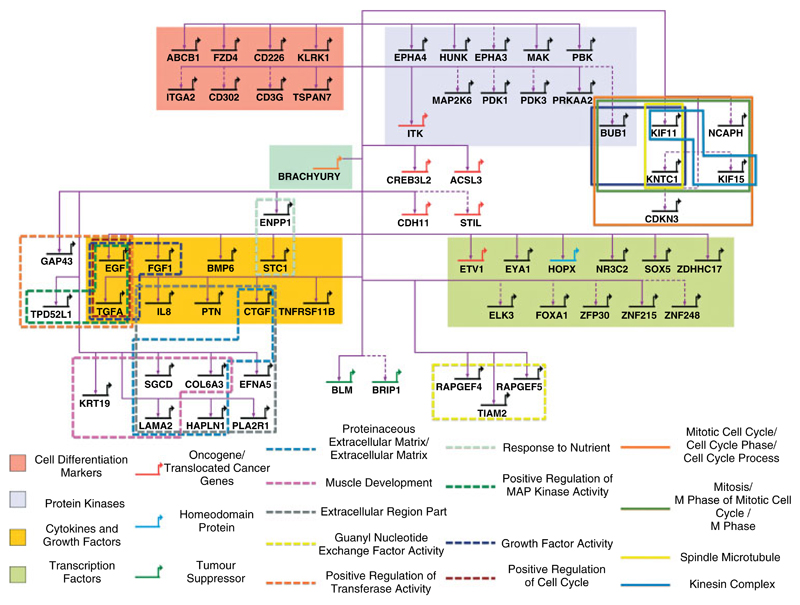
The core gene set derived from the integration of data from cell lines and primary disease allowed us to generate a putative GRN of brachyury target genes in chordoma samples (Figure 5). Gene ontology analysis of the core targets, using Broad’s Molecular Signature database [37], revealed enrichment for gene sets involved in regulation of the cell cycle. Other enriched gene sets of pathophysiological interest included those involved in the production of extracellular matrix and growth factor activity. These results are similar to those obtained from the gene expression microarray experiment generated from the U-CH1 cell line in which brachyury was silenced (Figure 1). Hence, the reproducibility of the data provides another level of confirmation that the function of the identified core set of target genes accurately reflects the global changes seen when brachyury is knocked down.
Figure 5.
Gene regulatory network for brachyury in chordoma. Genes identified by both U-CH1 study and ARACNE analysis of primary tumour data. Genes are categorized by terms enriched in GSEA (FDR < 0.05). Coloured background and coloured genes represent Broad Institute Molecular Signatures Database (MSigDB) v3.0 categories; genes in outlined boxes are categorized by Gene Ontology (GO) term. Solid lines connecting genes indicate brachyury binding within promoter and/or enhancer region and significant down-regulation on knockdown of brachyury (p ≤ 0.01) in U-CH1 cells; dotted lines indicate significant down-regulation on knockdown of brachyury (p ≤ 0.01). All genes were also identified in ARACNE analysis of primary tumour data.
Discussion
The identification of functional transcription binding sites is a prerequisite for understanding the complex regulatory dynamics and networks that govern the behaviour and phenotype of the cancer cell. Having integrated transcriptome data (GEM) from a chordoma cell line in which brachyury was silenced with genome-wide brachyury–DNA interactions (ChIP-seq) generated from the same cell line, we have identified, for the first time in humans, the chief transcriptional targets of brachyury. We show that a major subset of genes controlled by brachyury is involved in the regulation of cell cycle control, a hallmark for the sustained replicative potential of cancer cells. These data are supported by our in vitro experiments in which silencing of brachyury results in reduced cell proliferation. Results generated from ARACNE, despite being from a small number of datasets, are robust as these were sufficient to generate significant pairwise gene co-expression data which when overlapped with the in vitro results were validated using an independent set of human chordoma samples. Hence, the data add weight to the existing evidence that brachyury expression mediates an oncogenic effect in chordoma [7,9].
Brachyury and cell proliferation
During development, T-box genes, including brachyury, have recognized roles in cell fate decisions, tissue specification, and differentiation [18,38]. Specifically, brachyury is indispensable for development, as shown by the embryonic lethal murine T−/− mutant, and in ascidians it is involved in the regulation of cell division of notochordal cells [39–41]. In the context of cancer, melanomas and breast carcinoma are associated with TBX2 and TBX3 up-regulation; these genes act as transcriptional repressors of key senescent and proliferation checkpoints, including CDKN2A [42,43]. It is therefore of interest that chordomas also show loss of heterozygosity of CDKN2A in up to 70% of cases [44]. Conversely, TBX5 is a tumour suppressor and loss of its function in colorectal cancer results in cell proliferation [45]. In experiments complementary to those in our study, modification of brachyury expression has been shown to alter cell proliferation and migration in pancreatic cancer cells [10]. Our findings suggest that changes in T-box regulation may be more widespread in different types of cancer than hitherto appreciated.
Our experiments show that growth arrest is induced by silencing of brachyury. It is therefore of interest that a set of genes, including EGF, TGFA, and NUSAP1, are identified as brachyury targets in U-CH1 as these are known to regulate cell cycle progression (Supplementary Figure 4). NUSAP1 is of particular interest being a spindle checkpoint gene, exclusively expressed in proliferating cells and required for mitosis [46]. It is also noteworthy that NUSAP1 is a target of NKX2.1 and a member of a cluster of mitotic spindle checkpoint genes, along with BUB1, CDKN3, KIF15, and KIF11, which have been reported as representing a specific gene signature for a ‘proliferative’ subtype of T-acute lymphoblastic leukaemia [47], and that these same genes are also down-regulated as a consequence of silencing brachyury in U-CH1 cells (Figure 5). BUB1, a protein kinase, is overexpressed in many cancers [48]. It is a critical co-regulator of the spindle assembly checkpoint, and it is also a direct target of TBX2, together with a cohort of other cell cycle genes [49]. Furthermore, it is associated with spontaneous tumourigenesis through defects in chromosomal segregation in murine model [50,51]. These data argue that brachyury mediates its effect through regulation of mitotic spindle checkpoint genes.
Regulation of chemokines and growth factors and the dynamic interplay with extracellular matrix
It has previously been reported that a number of growth factor receptors including EGFR and FGFR are activated in chordomas, and this has not been adequately explained by the presence of mutations or structural variation [8,33,52]. It is therefore interesting to find that brachyury appears to orchestrate the expression of multiple growth factors and cytokines including CTGF, PTN, TGFA, EGF, FGF1, and BMP6 (Figure 5). Hence, the findings argue that activation of their respective pathways may be ligand-dependent.
Extracellular matrix (ECM) genes represent another major group of genes that are targeted by brachyury in chordoma (Figure 5). It is recognized that critical interactions between the ECM and components of the cell plasma membrane (adhesion molecules and receptor tyrosine kinases) determine the downstream transcriptional response of various cytokines through their receptors. EGFR is of particular interest as it forms a physical complex with ITGA2B1 [53], an integrin, which not only determines specificity of signalling but also controls cell proliferation, survival, and migration. It is therefore noteworthy that we found that brachyury indirectly regulates the expression of selected integrins (ITGA2 and ITGA10), ECM, and adhesion proteins (LAMA2, HAPLN1, OLFM4, PKP2, COL6A3, CNTNAP2, CHST4, MMP16, and ADAMTS3). Furthermore, connective tissue growth regulating factor (CTGF), which is required for ECM formation in development and is known to play a pivotal role in regulating the functions of integrins, signalling, and motility in the notochord, has been identified as a direct brachyury target in our study. This adds weight to the speculative relationship, posited by others, between the functions of brachyury and CTGF in notochordal cells [54,55]. These genetic targets are supported by the functional assays which revealed reduced chemotactic migratory ability and adhesion properties of cells in which brachyury is knocked down. The findings are further supported by previous reports of disorganization of the notochord, neural tube, and somites in T−/− murine embryos, and that brachyury has a role in the development of structural components of the notochordal sheath matrix in zebrafish [1,39]. Overall, the results are consistent with brachyury being a master regulator of a complex signalling network involved in controlling the growth of chordoma through its downstream transcriptional programme regulating proliferation, signalling, and migration.
Therapeutic implications of brachyury targets in chordoma
Our integrative functional approach has identified genes and pathways not previously associated with brachyury which may have important implications for stratified medicine. The finding that brachyury targets many growth factor ligands is consistent with the known activation of multiple tyrosine kinase receptors in chordoma. It may also account for the limited value of single targeted therapies in patients with chordoma, for example antagonists to EGFR, and ckit/PDGFR [13,14,56]. Given this background, and that chordoma has a poor prognosis with limited therapeutic options [57], we consider that targeting brachyury directly using RNA interference-mediated gene therapy, or other methodologies [58], has a biologically justifiable rationale.
Supplementary Material
The following supporting information may be found in the online version of this article.
(A) Chemotactic migration responses are abrogated in brachyury knockdown cells. (B) Adhesion of chordoma cells is reduced when brachyury is knocked down.
Validation of gene expression data.
GSEA indicates that ChIP-seq peaks are significantly associated with genes down-regulated on brachyury K knockdown.
Gene regulatory network for brachyury in U-CH1 cells.
Primer list.
Gene list— gene expression data.
ChIP peaks.
Integrated gene list of targets.
In silico targets.
In silico targets.
In silico targets.
Acknowledgment
We are grateful to the patients for donating their tissue for research and to all of the clinicians and support staff in the London Sarcoma Service who were involved in caring for the patients involved in this study. This research was generously supported by Skeletal Cancer Action Trust (SCAT), UK; The Rosetrees Trust, UK; The Pathological Society of Great Britain and Ireland (NPil); and the European Molecular Biology Laboratory. The research was also supported by the infrastructure and personnel of the Stanmore Musculoskeletal Research Programme and Biobank, UCLH/UCL Comprehensive Biomedicine Cancer Theme and UCL Experimental Medicine Programme. FCW is an MRC Career Development Fellow and Lister Institute Fellow. ACN is supported by the Lister Institute.
Abbreviations
- ChIP-seq
chromatin immunoprecipitation combined with massively parallel DNA sequencing
- GSEA
gene set enrichment analysis
- KD
knockdown
- WT
wild type
Footnotes
No conflicts of interest were declared.
Author contribution statement
AMF contributed to the experimental design and conceived the project. ACN performed bioinformatic analysis of ChIP-seq data, integration with GEM data, gene set enrichment analysis, and network generation. NiP performed shRNA knockdown, functional assays, ARACNE analysis, GSEA, and modular annotation for network generation and RT-qPCR analyses. CS was involved in the data analysis and reviewed the MS. SH performed bioinformatic analysis on GEM data and integration with the ChIP-seq data. NaP provided material for ChIP. DH and FB prepared the tumour samples and performed immunohistochemistry. RT reviewed the pathology. PF supplied bioinformatic platform support. FCW performed ChIP-seq experiments. The manuscript was written by NiP, ACN, FW, and AMF.
References
Note: References 59–69 are cited in the Supporting information to this article.
- 1.Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–2512. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- 2.Vujovic S, Henderson S, Presneau N, et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209:157–165. doi: 10.1002/path.1969. [DOI] [PubMed] [Google Scholar]
- 3.Salisbury JR, Deverell MH, Cookson MJ, et al. Three-dimensional reconstruction of human embryonic notochords: clue to the pathogenesis of chordoma. J Pathol. 1993;171:59–62. doi: 10.1002/path.1711710112. [DOI] [PubMed] [Google Scholar]
- 4.Heaton JM, Turner DR. Reflections on notochordal differentiation arising from a study of chordomas. Histopathology. 1985;9:543–550. doi: 10.1111/j.1365-2559.1985.tb02835.x. [DOI] [PubMed] [Google Scholar]
- 5.Tirabosco R, Mangham DC, Rosenberg AE, et al. Brachyury expression in extra-axial skeletal and soft tissue chordomas: a marker that distinguishes chordoma from mixed tumor/myoepithelioma/parachordoma in soft tissue. Am J Surg Pathol. 2008;32:572–580. doi: 10.1097/PAS.0b013e31815b693a. [DOI] [PubMed] [Google Scholar]
- 6.Yang XR, Ng D, Alcorta DA, et al. T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nature Genet. 2009;41:1176–1178. doi: 10.1038/ng.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Presneau N, Shalaby A, Ye H, et al. Role of the transcription factor T (brachyury) in the pathogenesis of sporadic chordoma: a genetic and functional-based study. J Pathol. 2011;223:327–335. doi: 10.1002/path.2816. [DOI] [PubMed] [Google Scholar]
- 8.Shalaby AA, Presneau N, Idowu BD, et al. Analysis of the fibroblastic growth factor receptor-RAS/RAF/MEK/ERK-ETS2/brachyury signalling pathway in chordomas. Mod Pathol. 2009;22:996–1005. doi: 10.1038/modpathol.2009.63. [DOI] [PubMed] [Google Scholar]
- 9.Hsu W, Mohyeldin A, Shah SR, et al. Generation of chordoma cell line JHC7 and the identification of Brachyury as a novel molecular target. J Neurosurg. 2011;115:760–769. doi: 10.3171/2011.5.JNS11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernando RI, Litzinger M, Trono P, et al. The T-box transcription factor Brachyury promotes epithelial–mesenchymal transition in human tumor cells. J Clin Invest. 2010;120:533–544. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMaster ML, Goldstein AM, Bromley CM, et al. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control. 2001;12:1–11. doi: 10.1023/a:1008947301735. [DOI] [PubMed] [Google Scholar]
- 12.Whelan J, McTiernan A, Cooper N, et al. Incidence and survival of malignant bone sarcomas in England 1979–2007. Int J Cancer. 2012;131:E508–E517. doi: 10.1002/ijc.26426. [DOI] [PubMed] [Google Scholar]
- 13.Park L, Delaney TF, Liebsch NJ, et al. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys. 2006;65:1514–1521. doi: 10.1016/j.ijrobp.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 14.Launay SG, Chetaille B, Medina F, et al. Efficacy of epidermal growth factor receptor targeting in advanced chordoma: case report and literature review. BMC Cancer. 2011;11:423. doi: 10.1186/1471-2407-11-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stacchiotti S, Longhi A, Ferraresi V, et al. Phase II study of imatinib in advanced chordoma. J Clin Oncol. 2012;30:914–920. doi: 10.1200/JCO.2011.35.3656. [DOI] [PubMed] [Google Scholar]
- 16.Tada M, Smith JC. T-targets: clues to understanding the functions of T-box proteins. Dev Growth Differ. 2001;43:1–11. doi: 10.1046/j.1440-169x.2001.00556.x. [DOI] [PubMed] [Google Scholar]
- 17.Hotta K, Takahashi H, Satoh N, et al. Brachyury-downstream gene sets in a chordate, Ciona intestinalis: integrating notochord specification, morphogenesis and chordate evolution. Evol Dev. 2008;10:37–51. doi: 10.1111/j.1525-142X.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 18.Morley RH, Lachani K, Keefe D, et al. A gene regulatory network directed by zebrafish No tail accounts for its roles in mesoderm formation. Proc Natl Acad Sci U S A. 2009;106:3829–3834. doi: 10.1073/pnas.0808382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans AL, Faial T, Gilchrist MJ, et al. Genomic targets of Brachyury (T) in differentiating mouse embryonic stem cells. PLoS One. 2012;7:e33346. doi: 10.1371/journal.pone.0033346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheil S, Bruderlein S, Liehr T, et al. Genome-wide analysis of sixteen chordomas by comparative genomic hybridization and cytogenetics of the first human chordoma cell line, U-CH1. Genes Chromosomes Cancer. 2001;32:203–211. doi: 10.1002/gcc.1184. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Luo B, Cheung HW, Subramanian A, et al. Highly parallel identification of essential genes in cancer cells. Proc Natl Acad Sci U S A. 2008;105:20380–20385. doi: 10.1073/pnas.0810485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wardle FC, Odom DT, Bell GW, et al. Zebrafish promoter microarrays identify actively transcribed embryonic genes. Genome Biol. 2006;7:R71. doi: 10.1186/gb-2006-7-8-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruderlein S, Sommer JB, Meltzer PS, et al. Molecular characterization of putative chordoma cell lines. Sarcoma. 2010;2010 doi: 10.1155/2010/630129. 630129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueda Y, Oda Y, Kawashima A, et al. Collagenous and basement membrane proteins of chordoma: immunohistochemical analysis. Histopathology. 1992;21:345–352. doi: 10.1111/j.1365-2559.1992.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 28.Smits P, Lefebvre V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development. 2003;130:1135–1148. doi: 10.1242/dev.00331. [DOI] [PubMed] [Google Scholar]
- 29.Basso K, Margolin AA, Stolovitzky G, et al. Reverse engineering of regulatory networks in human B cells. Nature Genet. 2005;37:382–390. doi: 10.1038/ng1532. [DOI] [PubMed] [Google Scholar]
- 30.Chi P, Chen Y, Zhang L, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010;467:849–853. doi: 10.1038/nature09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernando RI, Castillo MD, Litzinger M, et al. IL-8 signaling plays a critical role in the epithelial–mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71:5296–5306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson SR, Guiliano D, Presneau N, et al. A molecular map of mesenchymal tumors. Genome Biol. 2005;6:R76. doi: 10.1186/gb-2005-6-9-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shalaby A, Presneau N, Ye H, et al. The role of epidermal growth factor receptor in chordoma pathogenesis: a potential therapeutic target. J Pathol. 2011;223:336–346. doi: 10.1002/path.2818. [DOI] [PubMed] [Google Scholar]
- 34.Kelley MJ, Korczak JF, Sheridan E, et al. Familial chordoma, a tumor of notochordal remnants, is linked to chromosome 7q33. Am J Hum Genet. 2001;69:454–460. doi: 10.1086/321982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ooi A, Wong JC, Petillo D, et al. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell. 2011;20:511–523. doi: 10.1016/j.ccr.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Matsunaga T, Wada Y, Endo S, et al. Aldo-keto reductase 1B10 and its role in proliferation capacity of drug-resistant cancers. Front Pharmacol. 2012;3:5. doi: 10.3389/fphar.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberzon A, Subramanian A, Pinchback R, et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naiche LA, Harrelson Z, Kelly RG, et al. T-box genes in vertebrate development. Annu Rev Genet. 2005;39:219–239. doi: 10.1146/annurev.genet.39.073003.105925. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs-Cohen RJ, Spiegelman M, Bennett D. Abnormalities of cells and extracellular matrix of T/T embryos. Differentiation. 1983;25:48–55. doi: 10.1111/j.1432-0436.1984.tb01337.x. [DOI] [PubMed] [Google Scholar]
- 40.Herrmann BG. Expression pattern of the Brachyury gene in wholemount TWis/TWis mutant embryos. Development. 1991;113:913–917. doi: 10.1242/dev.113.3.913. [DOI] [PubMed] [Google Scholar]
- 41.Fujikawa T, Takatori N, Kuwajima M, et al. Tissue-specific regulation of the number of cell division rounds by inductive cell interaction and transcription factors during ascidian embryogenesis. Dev Biol. 2011;355:313–323. doi: 10.1016/j.ydbio.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 42.Vance KW, Carreira S, Brosch G, et al. Tbx2 is overexpressed and plays an important role in maintaining proliferation and suppression of senescence in melanomas. Cancer Res. 2005;65:2260–2268. doi: 10.1158/0008-5472.CAN-04-3045. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs JJ, Keblusek P, Robanus-Maandag E, et al. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19(ARF)) and is amplified in a subset of human breast cancers. Nature Genet. 2000;26:291–299. doi: 10.1038/81583. [DOI] [PubMed] [Google Scholar]
- 44.Hallor KH, Staaf J, Jonsson G, et al. Frequent deletion of the CDKN2A locus in chordoma: analysis of chromosomal imbalances using array comparative genomic hybridisation. Br J Cancer. 2008;98:434–442. doi: 10.1038/sj.bjc.6604130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu J, Ma X, Cheung KF, et al. Epigenetic inactivation of T-box transcription factor 5, a novel tumor suppressor gene, is associated with colon cancer. Oncogene. 2010;29:6464–6474. doi: 10.1038/onc.2010.370. [DOI] [PubMed] [Google Scholar]
- 46.Raemaekers T, Ribbeck K, Beaudouin J, et al. NuSAP, a novel microtubule-associated protein involved in mitotic spindle organization. J Cell Biol. 2003;162:1017–1029. doi: 10.1083/jcb.200302129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Homminga I, Pieters R, Langerak AW, et al. Integrated transcript and genome analyses reveal NKX2-1 and MEF2C as potential oncogenes in T cell acute lymphoblastic leukemia. Cancer Cell. 2011;19:484–497. doi: 10.1016/j.ccr.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Lewis TB, Robison JE, Bastien R, et al. Molecular classification of melanoma using real-time quantitative reverse transcriptase-polymerase chain reaction. Cancer. 2005;104:1678–1686. doi: 10.1002/cncr.21372. [DOI] [PubMed] [Google Scholar]
- 49.Vance KW, Shaw HM, Rodriguez M, et al. The retinoblastoma protein modulates Tbx2 functional specificity. Mol Biol Cell. 2010;21:2770–2779. doi: 10.1091/mbc.E09-12-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ricke RM, Jeganathan KB, van Deursen JM. Bub1 overexpression induces aneuploidy and tumor formation through Aurora B kinase hyperactivation. J Cell Biol. 2011;193:1049–1064. doi: 10.1083/jcb.201012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baker DJ, Jin F, Jeganathan KB, et al. Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell. 2009;16:475–486. doi: 10.1016/j.ccr.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dewaele B, Maggiani F, Floris G, et al. Frequent activation of EGFR in advanced chordomas. Clin Sarcoma Res. 2011;1:4. doi: 10.1186/2045-3329-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu X, Miyamoto S, Mekada E. Integrin alpha 2 beta 1-dependent EGF receptor activation at cell–cell contact sites. J Cell Sci. 2000;113:2139–2147. doi: 10.1242/jcs.113.12.2139. [DOI] [PubMed] [Google Scholar]
- 54.Erwin WM. The notochord, notochordal cell and CTGF/CCN-2: ongoing activity from development through maturation. J Cell Commun Signal. 2008;2:59–65. doi: 10.1007/s12079-008-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhar A, Ray A. The CCN family proteins in carcinogenesis. Exp Oncol. 2010;32:2–9. [PubMed] [Google Scholar]
- 56.Sleijfer S, Wagner AJ. The challenge of choosing appropriate end points in single-arm phase II studies of rare diseases. J Clin Oncol. 2012;30:896–898. doi: 10.1200/JCO.2011.40.6942. [DOI] [PubMed] [Google Scholar]
- 57.Dei Tos AP. Unveiling the molecular pathogenesis of chordoma: a new paradigm for molecular targeting of rare cancers. J Pathol. 2011;223:565–566. doi: 10.1002/path.2847. [DOI] [PubMed] [Google Scholar]
- 58.Palena C, Polev DE, Tsang KY, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13:2471–2478. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 59.Salasznyk RM, Williams WA, Boskey A, et al. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langmead B, Trapnell C, Pop M, et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goecks J, Nekrutenko A, Taylor J. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 2010;11:R86. doi: 10.1186/gb-2010-11-8-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giardine B, Riemer C, Hardison RC, et al. Galaxy: a platform for interactive large-scale genome analysis. Genome Res. 2005;15:1451–1455. doi: 10.1101/gr.4086505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu H, Handoko L, Wei X, et al. A signal–noise model for significance analysis of ChIP-seq with negative control. Bioinformatics. 2010;26:1199–1204. doi: 10.1093/bioinformatics/btq128. [DOI] [PubMed] [Google Scholar]
- 64.Pavesi G, Mereghetti P, Mauri G, et al. Weeder Web: discovery of transcription factor binding sites in a set of sequences from co-regulated genes. Nucleic Acids Res. 2004;32:W199–W203. doi: 10.1093/nar/gkh465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahony S, Benos PV. STAMP: a web tool for exploring DNA-binding motif similarities. Nucleic Acids Res. 2007;35:W253–W258. doi: 10.1093/nar/gkm272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Margolin AA, Nemenman I, Basso K, et al. ARACNE: an algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinformatics. 2006;7(Suppl 1):S7. doi: 10.1186/1471-2105-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Margolin AA, Wang K, Lim WK, et al. Reverse engineering cellular networks. Nature Protoc. 2006;1:662–671. doi: 10.1038/nprot.2006.106. [DOI] [PubMed] [Google Scholar]
- 68.Reich M, Liefeld T, Gould J, et al. GenePattern 2.0. Nature Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 69.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Chemotactic migration responses are abrogated in brachyury knockdown cells. (B) Adhesion of chordoma cells is reduced when brachyury is knocked down.
Validation of gene expression data.
GSEA indicates that ChIP-seq peaks are significantly associated with genes down-regulated on brachyury K knockdown.
Gene regulatory network for brachyury in U-CH1 cells.
Primer list.
Gene list— gene expression data.
ChIP peaks.
Integrated gene list of targets.
In silico targets.
In silico targets.
In silico targets.