Abstract
Objective
Alternative payment models such as accountable care organizations (ACO) hold provider groups accountable for an assigned patient population, but little is known about unassigned patients. We compared clinical and utilization profiles of patients attributable with those of patients not attributable to any provider group.
Study Design
Cross-sectional study of 2012 Medicare fee-for-service beneficiaries aged 21 and older.
Methods
We applied the Medicare Shared Savings Program attribution approach to assign beneficiaries to two mutually exclusive categories: attributable or unattributable. We compared attributable and unattributable beneficiaries according to: demographics, dual eligibility for Medicaid, nursing home residency, clinical comorbidities, annual service utilization, annual spending, and one- and two-year mortality. We estimated multivariate regression models describing correlates of attribution status.
Results
Most beneficiaries (88 percent) were attributable to a provider group. The remaining 12 percent were unattributable. Beneficiaries unattributable to any provider group were more likely to be younger, male, from a minority group, with disability as the basis for enrollment, and were more likely to live in high-poverty areas. Unattributable beneficiaries included three distinct subgroups: non-care-users, decedents, and those with healthcare service use but no qualifying evaluation and management visits. Many unattributable Medicare beneficiaries have minimal use of healthcare services, with the exception of a small subgroup of beneficiaries who die within the attribution year.
Conclusions
Attribution approaches that more fully capture unattributable low-service users and patients near the end of life should be reconsidered to reward population health efforts and improve end-of-life care.
Keywords: Accountable Care Organizations, Medicare, Medicare Shared Savings Program, Attribution to a provider group
Introduction
By holding networks of healthcare providers responsible for the total cost and quality of care for a designated population, the accountable care organization (ACO) model creates incentives to coordinate care across providers, to reduce unnecessary spending, and to improve the quality of care.1 The ACO model has grown steadily2 and by January 2017, Medicare held ACO contracts with 525 organizations serving over 10 million beneficiaries.3 Medicare ACO contract participants who meet quality benchmarks are eligible to share savings they generate.4,5 An ACO’s performance is evaluated based on Medicare methods of measurement and attribution. Researchers have evaluated outcomes of ACOs among attributable beneficiaries,6,7 but no study has described unattributable beneficiaries.
In Medicare ACO contracts, beneficiaries are attributed to organizations based on use of primary care services from eligible providers,8,9 so attribution can influence an organization’s performance under an ACO contract. Among organizations serving patients with complex clinical and psychosocial needs, for example, quality metrics may be hard to achieve, giving organizations incentives to avoid such patients. Conversely, ACO participants effectively managing population health may not achieve shared savings for patients who, appropriately, do not use primary care services eligible for attribution. ACOs have little financial incentive to deliver preventive care that might decrease the chance that healthy patients are attributed to an ACO.10 As advanced payment models mature to include downside risk, and as models like Comprehensive Primary Care expand, it is important to understand which patients are left out in order to develop regulations that encourage participation and improved care in new payment models.
To date, we have no information on the composition of patients who are not attributable to any provider group under Medicare Shared Savings regulations. This paper examines beneficiary characteristics associated with attribution and compares hospitalization, mortality, and spending across attributable and unattributable beneficiaries. Results from our analyses can guide policy on whether additional actions are necessary to adequately give provider participants incentives to improve population health, and to ensure that vulnerable beneficiaries—who may benefit the most from improved care coordination—are not excluded from new payment models.
Methods
Using Medicare claims and the Centers for Medicare & Medicaid Services (CMS) attribution rules for the most widely adopted model, the Medicare Shared Savings Program (MSSP),11 we categorized beneficiaries into two mutually exclusive groups: patients attributable to provider groups and patients who were not attributable to any provider group. We only included beneficiaries with full Parts A and B coverage, limiting the sample to beneficiaries for whom traditional, fee-for-service Medicare is the primary payer. We combined all beneficiaries attributable to provider organizations (i.e. MSSP, Pioneer ACO, non-ACO medical groups) into a single category because the purpose of our research was to investigate the characteristics of those “falling through the cracks” of the current attribution methodology. In 2012, Medicare ACO contracts included 32 Pioneer program participants and 114 MSSP participants (over the first performance period from April 2012 or July 2012 through December 2013) were responsible for over 2 million beneficiaries.12 While Pioneer program participants, which were responsible for nearly 700,000 beneficiaries in 2012,13 faced downside risk for spending, virtually all MSSP participants, 110, were in Track 1, eligible for upside savings only.12 We conducted cross-sectional analyses examining characteristics of beneficiaries according to their attribution status.
Beneficiary Attribution to Provider Organizations
Following MSSP’s 2-step attribution process, a beneficiary who has at least one face-to-face outpatient evaluation and management (E&M) visit is assigned to the provider group that has the highest allowed charges by primary care clinicians (general practice, family practice, internal medicine, and geriatric medicine practitioners) for those visits.11,14–16 Patients not seeing primary care clinicians are attributed based on visits to qualifying non-primary care clinicians (physicians in other specialties, nurse practitioners, physician assistants and clinical nurse specialists; see pages 22–23 of the referenced document).11 Beneficiaries who only received care from non-qualifying clinical providers (e.g. interventional cardiology or certified registered nurse), or in non-standard settings such as emergency departments (EDs) or no visits at all, are not able to be assigned to any provider group using current MSSP attribution rules and form our “unattributable” group.11
Data
For beneficiaries in Parts A and B aged 21 years or older, data were drawn from a 40 percent random sample of 2012 Medicare fee-for-service claims and 2012 to 2013 beneficiary summary files. Beneficiary summary files provided patient demographics, enrollment status and date of death. Attribution status, diagnoses, utilization and spending were obtained using claims. We chose 2012 because that is the most recent year of claims available without the suppression of claims indicating substance use disorder in research files17 and within the period of Medicare ACO implementation.
Long-term residence in nursing homes was determined from the 2012 Minimum Data Set (MDS). Patients’ ZIP codes were used to identify Hospital Referral Regions (HRRs) of residence; HRRs represent regional healthcare markets in the United States defined by the Dartmouth Atlas. We further used beneficiaries’ ZIP codes to identify rural and urban areas18 and whether they lived in high-poverty census tracts (≥20 percent of residents below the federal poverty line).19
Variable Descriptions
The main outcome of interest was whether a beneficiary was attributable to a provider group or not. We estimated correlations between attribution and beneficiary characteristics including age, sex, race and ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian or Pacific Islander, other race), residence in high-poverty census tracts or a rural area, dual eligibility for Medicaid, residence in long-term nursing home (at least 100 days in a nursing home according to MDS), disability as a basis of original Medicare entitlement, and date of death.
We used ICD-9 codes on claims to identify beneficiaries’ comorbid conditions in 2011 using hierarchical condition categories (HCCs) developed by CMS20 (see Appendix Table A1). Chronic conditions included dichotomous variables for coronary artery disease (CAD), cancer, cerebrovascular disease and stroke, congestive heart failure (CHF), connective tissue disease, chronic obstructive pulmonary disease (COPD), diabetes, hematologic/thrombotic disease, liver disease, Parkinson’s/Huntington’s, paralysis (not stroke), renal disease, peripheral vascular disease, dementia, and mental health conditions (severe mental illness, substance use disorder, and depression). We identified these conditions in 2011 to account for beneficiary diagnosis history and for their chronic nature and association with mortality and costs. We categorized patients with zero observed chronic conditions, multiple observed chronic conditions and those with unknown diagnoses in 2011 due to lack of medical claims. We reported utilization (ambulatory care sensitive condition, or ASC admissions; admissions to acute care or critical access hospitals; E&M visits, ED visits not leading to admission) and 2012 spending (Medicare payments to all providers, total and by category: inpatient, physician services, home health, and hospice).
To distinguish those unattributable because attribution rules may exclude end-of-life care, we examined mortality in 2012 and 2013 (among those alive January 1, 2013). This approach disentangles the mechanical relationship between attribution and death from clinical complexity.
Data Analysis
We compared characteristics of attributable and unattributable Medicare enrollees—based on a 40% random sample to minimize computing time—using multivariate logistic models. We modeled attribution status as a function of demographics, dual eligibility for Medicaid, nursing home residency, disability, measures of chronic condition history, and HRR fixed effects.
We considered attribution status of those with dual eligibility for Medicaid, over 10 million people as of 2016, due to their low socioeconomic status, complex clinical needs, and elevated medical spending.21–26
Results
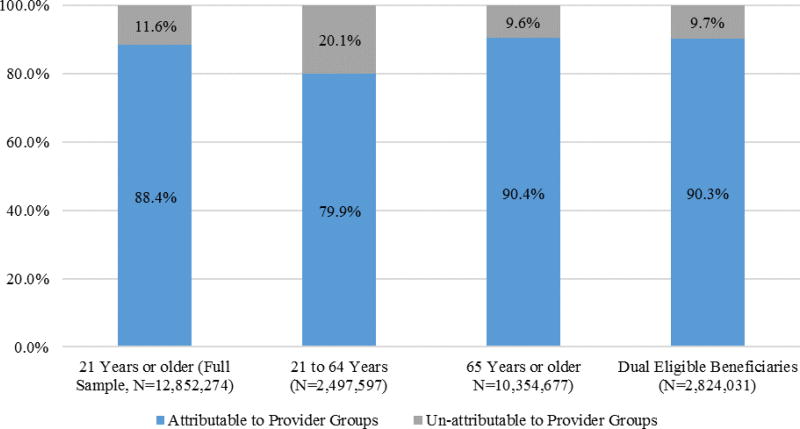
This study included about 13,000,000 fee-for-service Medicare beneficiaries in 2012. Most of these fee-for-service Medicare beneficiaries, 88 percent, were able to be attributed to a provider group, leaving 12 percent unattributable (Figure 1).
Figure 1.
Attribution Status of Fee-for-Service Medicare Beneficiaries in 2012 (21 years or older)
Compared with attributable beneficiaries, unattributable beneficiaries tended to be younger (65 versus 71 years of age), male (60 versus 43 percent), from a minority race or ethnic group (28 versus 18 percent), living in a high poverty area (25 versus 22 percent; Table 1), and less likely to be dually eligible for Medicaid but no less likely to reside in a rural area (Appendix Table A2). Unattributable beneficiaries had fewer observed chronic conditions and were less likely to have two or more chronic conditions in 2011 (5.3 versus 22.3 percent; Table 1) compared with attributable beneficiaries, however, people with no-service use or very few visits have no or fewer opportunities for diagnoses to be observed. Mortality was higher in the unattributable group in 2012 (6.6 versus 4.3 percent; Table 1) but lower in 2013 (2.8 versus 4.9 percent). More beneficiaries under age 65 were unattributable than those over age 65 (20 versus 10 percent; Figure 1). We did not report p-values of differences in variables’ means and proportions across attribution groups, which were all statistically significant (p-value<0.001) due to the large sample size.
Table 1.
Descriptive statistics Fee-for-Service Medicare Beneficiaries, 2012 (21 years or older)
| Attributable to Provider Groups |
Unattributable to Provider Groups |
|
|---|---|---|
| Variable | N=11,359,424(88.4%) | N=1,492,850(11.6%) |
| Demographic Characteristics | ||
| Age, mean, (SD), years | 71.4 (12.4) | 65.4 (14.0) |
| Age <65 years, % | 17.6 | 33.6 |
| Age 65-69 years, % | 22.5 | 31.4 |
| Age 70-74 years, % | 19.1 | 13.8 |
| Age 75-79 years, % | 15.3 | 8.3 |
| Age 80-84 years, % | 12.5 | 5.8 |
| Age ≥85 years, % | 13.1 | 7.2 |
| Gender, % | ||
| Male | 42.7 | 60.1 |
| Female | 57.3 | 39.9 |
| Race, % | ||
| Non-Hispanic white | 81.9 | 72.1 |
| Black | 9.2 | 13.7 |
| Hispanic | 5.2 | 8.9 |
| Asian/Pacific Islander | 2.2 | 2.8 |
| Other | 1.5 | 2.4 |
| Lives in High Poverty (above 20 percent) census tract, % | 21.8 | 25.1 |
| Dual Medicare and Medicaid Status, % | 22.5 | 18.3 |
| Dual Medicare and Medicaid Status if <65 years, %a | 57.7 | 32.1 |
| Dual Medicare and Medicaid Status if ≥65 years, %b | 14.9 | 11.3 |
| Nursing Home Resident (=1 if ≥100 days in Nursing Home), % | 3.8 | 0.6 |
| Clinical Condition History and Disability | ||
| Disabled, % | 24.5 | 35.8 |
| Disabled if <65 years, %a | 97.0 | 88.6 |
| Chronic Conditions based on Hierarchical Condition Categories (HCCs)-2011, %c | ||
| Coronary Artery Disease | 4.1 | 1.1 |
| Cancer | 2.9 | 0.9 |
| Cerebrovascular Disease & Stroke | 2.3 | 0.6 |
| Congestive Heart Failure | 8.3 | 2.2 |
| Connective Tissue Disease | 3.5 | 0.4 |
| Chronic Obstructive Pulmonary Disease | 9.5 | 2.3 |
| Dementia | 7.8 | 2.7 |
| Depression | 6.3 | 1.5 |
| Diabetes | 21.7 | 3.3 |
| Hematologic/Thrombotic Disease | 2.0 | 0.6 |
| Liver Disease | 0.6 | 0.2 |
| Parkinson’s/Huntington’s | 1.2 | 0.3 |
| Paralysis (not Stroke) | 0.5 | 0.2 |
| Renal Disease | 8.7 | 2.5 |
| Peripheral Vascular Disease | 5.3 | 1.0 |
| Severe Mental Illness | 5.4 | 2.9 |
| Substance Use Disorder | 1.1 | 0.7 |
| Zero Observed Chronic Conditions | 23.1 | 7.3 |
| Non Healthcare Service User-2011d | 23.3 | 63.2 |
| Unknown Chronic Condition History-2011e | 4.5 | 17.8 |
| Number of Observed Chronic Conditions based on HCCs-2011, mean, (SD) | 0.9(1.3) | 0.2(0.8) |
| Two or more Observed Chronic Conditions based on HCCs-2011, % | 22.3 | 5.3 |
| Healthcare Utilization (Count per person), mean, (SD)f | ||
| Hospitalizations for Ambulatory Sensitive Conditions (ASC) | 0.06(0.33) | 0.03(0.23) |
| Acute Care/Critical Access Hospital Hospitalizations | 0.34 (0.88) | 0.20(0.61) |
| Evaluation and Management (E&M) Visits | 9.19(8.17) | 0.0001(0.01) |
| Emergency Department (ED) Visits | 0.72(1.72) | 0.52(1.48) |
| Medicare Spending, $, mean, (SD)f,g | ||
| Total Spending | 10,406 (22,120) | 5,578(17,070) |
| Inpatient | 4,614(15,778) | 2,852(13,546) |
| Physician | 4,517(8,669) | 1,188(4,672) |
| Facility | 16(49) | 4(26) |
| Home Health Agency | 589(2,291) | 204(1,292) |
| Hospice | 315(3,055) | 1,259(6,580) |
| Durable Medical Equipment | 354(1,498) | 71(604) |
| Mortality, % | ||
| Death recorded in 2012 | 4.3 | 6.6 |
| Death recorded in 2013 conditional on survival in 2012h | 4.9 | 2.8 |
Notes: N=12,852,274. Percent may not sum exactly to one hundred due to rounding error
The sample is restricted to beneficiaries less than 65 years old (N=2,497,597).
The sample is restricted to beneficiaries aged 65 years or older (N=10,354,677).
These conditions include 2011 diagnoses for 419,853 beneficiaries without both Parts A & B coverage in 2011.
This identifies existing Medicare fee-for-service beneficiaries with no healthcare use in 2011 and therefore unknown chronic condition history.
This identifies new Medicare enrollees in 2012 and patients who may have missing diagnoses because they were not in both Parts A and B or were in Managed Care in 2011 and these plans have incomplete reporting.
The sample is restricted to those with health care service use (N=11,908,360).
Inpatient spending includes acute care and critical access hospitalization, skilled nursing facility, inpatient rehabilitation, and long term acute care facility spending; physician spending including procedure, evaluation and management, imaging, testing and other physician spending.
2013 mortality rate excludes 587,459 beneficiaries who died in 2012.
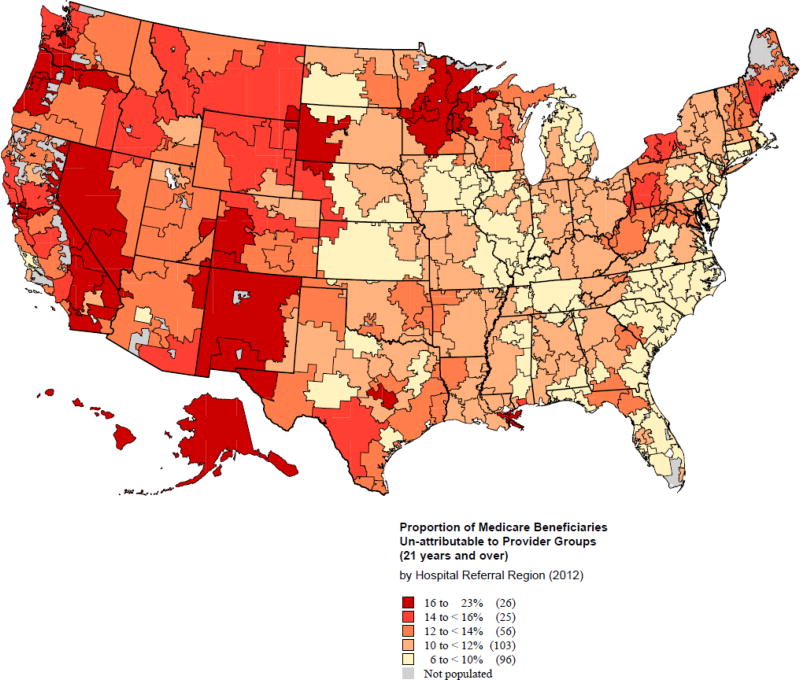
In terms of geographic distribution, the proportion of unattributable beneficiaries ranged from 6 to 22 percent across HRRs (Figure 2); HRRs with few unattributable beneficiaries (under 10 percent) were located primarily in parts of the Midwest, South, and Northeast regions.27 Although not shown, we examined 2011 physican supply in the market—a proxy for access—and found no evidence for differences across attribution categories.
Figure 2.
Proportion of Medicare Beneficiaries Un-attributable to Provider Groups in 2012 by Hospital Referral Region (HRR)
In the unattributable group, we identified a subpopulation with no healthcare service use or spending in 2012 (63 percent of the unattributable) and a group that died in 2012 (6 percent). The remaining beneficiaries (31 percent) used healthcare services, including primary care services that did not qualify for attribution (Table 2). Among the unattributable non-care-users in 2012, few, 3.6 percent had one or more reported diagnoses in 2011. However, spending averaged $9,000 in 2011 in this group, primarily from ED visits and hospitalization (not shown).
Table 2.
Descriptive statistics Un-attributable Fee-for-Service Medicare Beneficiaries, 2012 (21 years or older)
| Non-Users (No Claims) | Health Care Service Users | ||
|---|---|---|---|
| N=943,914(63.2%) | Decedents | Survivors | |
| Variable | N=82,057(5.5%) | N=466,879(31.3%) | |
| Demographic Characteristics | |||
| Age, mean, (SD), years | 64.9(12.8) | 78.2(12.9) | 64.3(15.4) |
| Gender, % | |||
| Male | 62.2 | 49.8 | 57.5 |
| Female | 37.8 | 50.2 | 42.5 |
| Race, % | |||
| Non-Hispanic white | 70.3 | 81.5 | 74.3 |
| Black | 13.7 | 10.7 | 14.3 |
| Hispanic | 10.3 | 4.8 | 6.9 |
| Asian/Pacific Islander | 3.2 | 1.6 | 2.3 |
| Other | 2.6 | 1.4 | 2.3 |
| Lives in High Poverty (above 20 percent) census tract, % | 24.9 | 24.7 | 25.4 |
| Dual Medicare and Medicaid Status, % | 14.0 | 23.7 | 26.0 |
| Dual Medicare and Medicaid Status <65 years, % a | 22.0 | 41.9 | 51.0 |
| Dual Medicare and Medicaid Status ≥65 years, % b | 10.0 | 20.9 | 12.0 |
| Nursing Home Resident (=1 if ≥100 days in Nursing Home), % | 0.1 | 6.2 | 0.5 |
| Clinical Condition History and Disability | |||
| Disabled, % | 34.8 | 23.5 | 40.1 |
| Disabled if <65 years, %a | 85.2 | 93.1 | 94.9 |
| Chronic Conditions based on based on Hierarchical Condition Categories (HCCs)-2011, %c | |||
| Coronary Artery Disease | 0.4 | 9.6 | 1.0 |
| Cancer | 0.2 | 13.0 | 0.3 |
| Cerebrovascular Disease & Stroke | 0.2 | 6.1 | 0.5 |
| Congestive Heart Failure | 0.5 | 24.6 | 1.7 |
| Connective Tissue Disease | 0.1 | 2.9 | 0.4 |
| Chronic Obstructive Pulmonary Disease | 0.7 | 21.7 | 2.3 |
| Dementia | 0.6 | 28.2 | 2.4 |
| Depression | 0.4 | 11.5 | 2.0 |
| Diabetes | 1.2 | 22.5 | 4.2 |
| Hematologic/Thrombotic Disease | 0.1 | 6.2 | 0.4 |
| Liver Disease | 0.1 | 2.2 | 0.2 |
| Parkinson’s/Huntington’s | 0.05 | 3.0 | 0.2 |
| Paralysis (not Stroke) | 0.05 | 1.5 | 0.2 |
| Renal Disease | 0.5 | 23.2 | 2.8 |
| Peripheral Vascular Disease | 0.3 | 10.2 | 0.9 |
| Severe Mental Illness | 0.5 | 4.9 | 7.3 |
| Substance Use Disorder | 0.2 | 2.4 | 1.3 |
| Zero Observed Chronic Conditions | 4.6 | 8.2 | 12.7 |
| Non Healthcare Service User-2011d | 70.0 | 20.4 | 57.1 |
| Unknown Chronic Condition History-2011e | 21.8 | 4.3 | 12.2 |
| Number of Observed Chronic Conditions based on HCCs-2011, mean, (SD) | 0.1(0.4) | 1.9(2.0) | 0.3(0.7) |
| Two or more Observed Chronic Conditions based on HCCs-2011, % | 1.4 | 48.4 | 5.7 |
| Healthcare Utilization (Count per person), mean, (SD)f | |||
| Hospitalizations for Ambulatory Sensitive Conditions (ASC) | _ | 0.09(0.34) | 0.02(0.20) |
| Acute Care/Critical Access Hospital Hospitalizations | _ | 0.57(0.82) | 0.13(0.54) |
| Evaluation and Management (E&M) Visits | _ | 0(0) | 0.0001(0.01) |
| Emergency Department (ED) Visits | _ | 0.63(0.98) | 0.51(1.55) |
| Medicare Spending, $, mean, (SD)f,g | |||
| Total Spending | _ | 18,914(32,186) | 3,235(11,125) |
| Inpatient | _ | 11,708(29,251) | 1,295(7,010) |
| Physician | _ | 1,950(4,522) | 1,054(4,686) |
| Facility | _ | 2(17) | 5(27) |
| Home Health Agency | _ | 399(1,436) | 170(1,262) |
| Hospice | _ | 4,785(9,583) | 639(5,675) |
| Durable Medical Equipment | _ | 69(485) | 72(622) |
| Mortality, % | |||
| Death recorded in 2012 | 1.7 | 100 | 0 |
| Death recorded in 2013 conditional on survival in 2012h | 2.1 | _ | 4.0 |
Notes: N=1,492,850. Percent may not sum exactly to one hundred due to rounding error.
The sample is restricted to beneficiaries less than 65 years old (N=501,243).
The sample is restricted to beneficiaries aged 65 years or older (N=991,607).
These conditions include 2011 diagnoses for 19,253 beneficiaries without both Parts A & B coverage in 2011.
This identifies existing Medicare fee-for-service beneficiaries with no healthcare use in 2011 and therefore unknown chronic condition history.
This identifies new Medicare enrollees in 2012 and patients who may have missing diagnoses because they were not in both Parts A and B or were in Managed Care in 2011 and these plans have incomplete reporting.
The sample is restricted to those with health care service use (N=548,936).
Inpatient spending includes acute care and critical access hospitalization, skilled nursing facility, inpatient rehabilitation, and long term acute care facility spending; physician spending including procedure, evaluation and management, imaging, testing and other physician spending.
This variable excludes 98,105 beneficiaries who died in 2012.
Unattributable user-decedents spent $19,000, on average (including $12,000 of inpatient spending and $5,000 of hospice spending, Table 2). More than half (60 percent) of the unattributable decedents died within the first three months of 2012 compared with 20 percent of decedents attributable to provider organizations (Appendix Table A3). Of the over 80,000 unattributable user-decedents, 67 percent had one or more chronic condition diagnoses in claims in 2011; dementia, CHF, renal disease, diabetes, and COPD were common.
The third group of unattributable beneficiaries (with healthcare use but no qualifying primary care visits) averaged over $3,000 annual spending, one third of the spending of attributable patients. Very few of these beneficiaries had non-qualifying E&M visits, (N=57) while others had other healthcare use in non-attribution settings. Of the unattributable non-decedent healthcare users, 31 percent had reported clinical conditions in the prior year with severe mental illness, diabetes, renal diseases, dementia, and COPD being most common.
In multivariate regression analyses, older beneficiaries and females were less likely to be unattributable (0.1 percentage point decrease per year of age and 4.5 percentage points less likely among females, p-value<0.001, Table 3). Minorities and those living in high-poverty census tracts were more likely to be unattributable. Nursing home residents and dual-eligible beneficiaries were less likely than others to be unattributable to provider groups (4.3 and 4.2 percentage points lower probability, respectively, p-value<0.001). Disability (as original reason for entitlement) was associated with a 4.5-percentage-point increase in the likelihood of being unattributable. Beneficiaries with zero observed chronic condition diagnoses and those with unknown chronic condition history (non-service users and not in both Parts A and B fee-for-service Medicare) in 2011 were more likely to be unattributable in 2012 relative to those with observed chronic conditions (2.4 and 20.4 to 22.3 percentage points higher probability, respectively, p-value<0.001).
Table 3.
Association between Attribution Status and Beneficiary Characteristics (40% Random Sample)
| Un-attributable to Provider Groups† | ||
|---|---|---|
| Variable | Marginal Effect | Std. Err. |
| Age | -0.001*** | (0.00001) |
| Female | -0.045*** | (0.0002) |
| Race Ethnicity | ||
| Black | 0.047*** | (0.0003) |
| Hispanic | 0.052*** | (0.0003) |
| Asian/Pacific Islander | 0.035*** | (0.0005) |
| Other Ethnicity | 0.026*** | (0.0006) |
| Lives in High Poverty (above 20 percent) Census Track | 0.012*** | (0.0002) |
| Dually Eligible | -0.042*** | (0.0002) |
| Nursing Home Resident | -0.043*** | (0.0010) |
| Disabled | 0.045*** | (0.0002) |
| Chronic Conditions-2011‡ | ||
| Zero Observed Chronic Conditions | 0.024*** | (0.0003) |
| Non Healthcare Service User (Unknown Chronic Conditions)-2011 | 0.204*** | (0.0002) |
| Unknown Chronic Condition History (New 2012 Fee-for-Service Medicare Enrollees and 2011 Managed Care Enrollees)-2011 | 0.223*** | (0.0003) |
| Observations | 12,852,274 | |
| Pseudo R-squared | 0.220 | |
Notes: A Logistic regression was estimated for the attribution measure. The marginal effects reported are the averages of marginal effects across the sample on the predicted probability of being unattributable.
Std. Err. Standard Error.
The outcome variable is a dichotomous variable for whether the beneficiary is unattributable to any provider group as opposed to being attributable to a provider group (either associated with a Medicare ACO or any other provider group). The coefficients represent the estimated marginal effects or changes in the predicted probability. HRR fixed effects are included. Standard errors are in parentheses.
The reference category is the group of patients with one or more observed chronic conditions.
Inference: *** p<0.001,
p<0.01,
p<0.05.
Since Medicaid coverage complements Medicare benefits for certain low-income adults, we explored how attribution status differed in the 22 percent of our sample dually eligible for Medicaid benefits. Although the dually eligible were less likely than other beneficiaries to be unattributable (Figure 1), results from multivariate analyses of dual-eligible enrollees were consistent with those found in the overall sample (Appendix Table A4) with two exceptions: Asian/Pacific Islander ethnicity and disability status (both associated with a decrease in the likelihood of being unattributable).
Discussion
Because existing evidence on ACOs focuses exclusively on attributable Medicare beneficiaries;8,15,28,29 we explored the excluded group of unattributable patients to understand how these omitted groups might influence performance in Medicare ACO programs.30 Using MSSP attribution methods, nearly 12 percent of beneficiaries were unattributable in 2012. This group was heterogeneous, comprised of decedents, beneficiaries using no services, and those using services ineligible for attribution under common methods. The majority of unattributable beneficiaries, had no encounter with the healthcare system at all, which may be due to financial and non-financial barriers that limit access to care.31,32 A small but costly group, 6 percent of unattributable beneficiaries, died in 2012; among them inpatient and hospice service use was common. The remaining 31 percent of unattributable beneficiaries had minimal healthcare use, largely for urgent or hospital-based services.
The distribution of unattributable beneficiaries aligned with regional spending patterns in Medicare.33 Beneficiaries living in low-cost regions such as Minnesota had higher proportions of unattributable beneficiaries. This pattern indicates that alternative payment models in these regions, ironically, may not reward organizations for their activities targeting healthier groups who use few services. For example, ACOs in low-cost regions were evaluated against lower financial benchmarks than ACOs in high-cost regions. Beginning in 2017, cost benchmark calculations were improved to integrate regional factors, making an ACO’s benchmark less reflective of its historical spending and more dependent on fee-for-service expenditures in its region.34 Two changes to attribution might capture more low-utilization beneficiaries. First, Track 3 of the MSSP, with 16 participating organizations in 2016, and the Next Generation ACO model—another demonstration project in the CMS Innovation Center—are currently piloting attestation, allowing beneficiaries to voluntarily choose an ACO,35–38 which would allow for accountability for preventive services in healthier populations with little or no observed utilization. Second, “sticky” attribution, or allowing utilization in a prior year for those with no use could capture more healthy patients and very sick individuals who use non-primary care settings due to death early in the year. While attestation has the benefit of permitting patients to choose, “sticky” attribution may be more feasible in existing programs since it can be done just using claims.
Facilitating the attribution of healthy patients and those with minimal use through any method may improve consistency and continuity of care for patients. Since ACOs are responsible for both cost and quality of care for assigned patients, attribution of healthy patients with no healthcare use and of those with some healthcare use but non-qualifying visits for current attribution purposes would reward providers for care delivered to these patients, without rewarding unnecessary care.
Attribution of non-users may more appropriately contribute to an ACO’s shared savings, which are conditional on meeting predetermined quality measure benchmarks, and reflect beneficiaries classified as at-risk populations for example in benchmark year(s) who currently do not use services.39,40 Moreover, financial benchmarks are calculated using attributed beneficiaries’ historical healthcare use. The current attribution methodology provides incentives for ACOs to bring in healthy patients for primary care visits but attribution of non-users through alternative methods may be warranted to appropriately reward providers without inducing potentially unnecessary primary care visits.41 A practical solution, addressing non-care-users with healthcare use in previous years, would be to assign them based on “sticky” attribution.
Furthermore, healthcare providers and policy makers should give greater attention to the consequential subpopulation of decedents (i.e. end-of-life patients) currently falling outside of the responsibility of organizations participating in ACOs. One-quarter of Medicare spending is devoted to care in the last year of life,42 so relative savings on end-of-life care contribute outsized savings to the Medicare program. There are also opportunities for improvements in quality and preference-based care. Organizations that deliver thoughtful end-of-life care, by using providers or settings not recognized by current attribution methods, are not rewarded for this high quality care. On the flip side, organizations that fail to coordinate end-of-life care, or deliver resource intensive care not aligned with patient preferences face no penalty. Additionally, higher hospice spending registered in unattributable decedents signals that involvement of hospice and palliative care in new payment initiatives may improve quality of care and potentially reduce costs43–45 for end-of-life patients.
Alternative attribution methods have different strengths and weaknesses. Retrospective attribution, currently used for most MSSPs and done at the end of the performance year, reflects actual care delivery. It removes patients who no longer receive care from an ACO and captures new patients, such as those aging into Medicare during the year (Appendix Table A5).6 However, with retrospective attribution, organizations cannot plan care for patients assigned to them only after care delivery. In contrast, prospective attribution removes this uncertainty which might facilitate active engagement with patients and can improve management of patient population health. However, prospective attribution is a forecast based on past healthcare use, and works best when care-seeking patterns are stable and therefore predictable over time. This attribution, although preferred by ACOs,46 may have important cost implications for ACOs if patient care patterns drastically change from one year to another since patients may see any Medicare provider. This type of attribution would also fail to capture patients that initiated care during the year (Appendix Table A5).
Most of the gaps in attribution rules we identified may be addressed with a hybrid attribution method. First, for most populations, the current assignment based on E&M visits is appropriate. Second, for those with limited healthcare use and the healthy, assignment would be based on attestation over a longer time period, therefore “rewarding” providers for early prevention and early detection care patterns. For those with serious illnesses, assignment could be broadened to include hospice and palliative care to encourage better end-of-life care (Appendix Table A5). Before patients with serious illness reach end of life, however, a first priority is to engage them in care. Providers could tailor care management and coordination to the needs of patients with chronic conditions that have accessed very little health care47 in order to improve quality and health outcomes.
Gender disparities were noted between attribution categories, with a disproportionately larger proportions of younger and male populations among the unattributable beneficiaries. This may signal gender differences in healthcare seeking behavior.48 The slightly higher proportion of those with dual eligibility for Medicaid attributable to a provider group (90 percent) may reflect greater coverage for out-of-pocket spending in this group, lowering a barrier to access. New initiatives, such as the CMS Financial Alignment Initiative, which integrates care across primary, acute, behavioral health and long-term care for dual eligible beneficiaries, offer another avenue to engage these patients.
This cross-sectional exploratory study in the first year of the ACO payment model has limitations. First, alternative methods to MSSP used in this study may result in slightly different distributions of beneficiaries across categories. However, MSSP is the largest Medicare ACO program. Second, a longitudinal study with additional years of data could examine trends in attribution of Medicare beneficiaries as provider groups gain knowledge and develop better strategies to manage attribution. Third, claims-based measures of patient illness cannot capture the illness burden for those without utilization. Thus, our results may reflect care seeking behavior, as we cannot attribute or observe clinical comorbidities for those with no or little healthcare utilization and for new enrollees in the preceding year.
The promise of ACO contracts to lower spending, improve quality of care, and ultimately, population health, can be enhanced by improving attribution of individuals to providers. By rewarding organizations for keeping patients healthy and out of resource-intensive care settings, and by appropriately capturing and rewarding compassionate care, such as hospice services, for patients approaching the end of life, Medicare’s continued development of advanced payment models has the potential to move us closer to the goal of improving population health.
APPENDIX
Table A1.
Definition of Chronic Conditions Based on Hierarchical Condition Categories (HCCs) developed by the Centers of Medicare & Medicaid Services. Categories chosen for chronic nature, association with mortality and costs.
| Hierarchical Condition Categories (HCC)/RxHCC/ICD-9 Label and Number | |
|---|---|
| CORONARY ARTERY DISEASE | HCC 81, HCC82, HCC83 |
| CANCER | HCC7, HCC8, HCC9 |
| CEBROVASCULAR DISEASE & STROKE | HCC96, HCC100(REMOVE ICD9 343.X), HCC95 |
| CONGESTIVE HEART FAILURE | HCC80 |
| CONNECTIVE TISSUE DISEASE | HCC38 |
| CHRONIC OBSTRUCTIVE PULMONARY DISEASE | HCC108 |
| DIABETES | HCC15, HCC16, HCC17, HCC18, HCC19 HCC119 |
| HEMATOLOGIC/ THROMBOTIC DISEASE | HCC44, RXHCC100 (REMOVE ICD9 343.X) |
| LIVER DISEASE | HCC25, HCC26, HCC27 |
| PARKINSON’S/HUNTINGTON’S | HCC73 |
| PARALYSIS (NOT STROKE) | HCC67, HCC68, HCC69 |
| RENAL DISEASE | HCC130, HCC131, HCC132 |
| SEVERE MENTAL ILLNESS* | HCC54, HCC55 (REMOVE e codes from HCC55) |
| PERIPHERAL VASCULAR DISEASE | HCC105 |
| DEPRESSION* | RxHCC62, ICD-9 296.2X, ICD-9 296.3X |
| DEMENTIA | RxHCC54, RxHCC55 |
| SUBSTANCE USE DISORDER* | HCC51, HCC52 |
Notes:
Severe Mental Illness= includes schizophrenia, bipolar disease, and depression; Depression= major depressive disorder, single episode; major depressive disorder, recurrent episode; depression Not Otherwise Specified and Not Elsewhere Classified; Substance Use Disorder= drug/alcohol dependence and drug/alcohol mental illness
Table A2.
Attribution Status by Rural-Urban Commuting Area (RUCA) Codes, 2010
| Rural Urban Commuting Codes | Observations, % | |||
|---|---|---|---|---|
| Metropolitan Areas: core, high commuting, and low commuting |
Micropolitan Areas: core, high commuting, low commuting |
Small Towns (core, high commuting, low commuting), Rural Areas, Not Coded Areas |
||
| 1, 1.1, 2, 2.1, 3 | 4, 4.1, 5, 5.1, 6 | 7, 7.1, 7.2, 8,8.1, 8.2, 9,
10, 10.1, 10.2, 10.3, 99 |
||
| Attributable to Provider Groups, % | 76.0 | 12.3 | 11.6 | 100 |
| Unattributable to Provider Groups, % | 76.9 | 11.5 | 11.7 | 100 |
Notes: N=12,852,274. “Not coded areas” are more likely to be less populated rural areas. Percent may not sum exactly to one hundred due to rounding error. Considering each row separately
-
Attributable beneficiaries in metropolitan areas: 76.03%Attributable beneficiaries in micropolitan areas: 12.33%Attributable beneficiaries in small towns and rural areas: 11.64%
-
Unattributable beneficiaries in metropolitan areas: 76.86%Unattributable beneficiaries in micropolitan areas: 11.47%Unattributable beneficiaries in small towns and rural areas: 11.66%
Table A3.
Distribution of Decedents by Attribution Status and Month of Death
| Month of Death 2012 | Attributable, % | Unattributable, % |
|---|---|---|
| 1 | 4.0 | 34.7 |
| 2 | 7.1 | 15.1 |
| 3 | 8.6 | 10.4 |
| 4 | 8.5 | 7.2 |
| 5 | 8.6 | 5.9 |
| 6 | 8.3 | 4.7 |
| 7 | 8.6 | 4.4 |
| 8 | 8.7 | 3.9 |
| 9 | 8.7 | 3.7 |
| 10 | 9.3 | 3.7 |
| 11 | 9.3 | 3.0 |
| 12 | 10.3 | 3.3 |
|
| ||
| Total, N, (%) | 489,354(100) | 98,105(100) |
Table A4.
Association between Attribution Status and Beneficiary Characteristics, Dual-Eligible Beneficiaries (40% Random Sample)
| Un-attributable to Provider Groups† | ||
|---|---|---|
| Variable | Marginal Effect | Std. Err. |
| Age | −0.001*** | (0.00001) |
| Female | −0.052*** | (0.0003) |
| Race Ethnicity | ||
| Black | 0.023*** | (0.0005) |
| Hispanic | 0.007*** | (0.0006) |
| Asian/Pacific Islander | −0.027*** | (0.0009) |
| Other Ethnicity | 0.002* | (0.0011) |
| Lives in High Poverty (above 20 percent) Census Track | 0.004*** | (0.0004) |
| Nursing Home Resident | −0.082*** | (0.0011) |
| Disabled | −0.028*** | (0.0005) |
| Chronic Conditions-2011‡ | ||
| Zero Observed Chronic Conditions | 0.027*** | (0.0006) |
| Non Healthcare Service User (Unknown Chronic Conditions)-2011 | 0.155*** | (0.0004) |
| Unknown Chronic Condition History (New 2012 Fee-for-Service Medicare Enrollees and 2011 Managed Care Enrollees) -2011 | 0.155*** | (0.0006) |
| Observations | 2,824,031 | |
| Pseudo R-squared | 0.191 | |
Notes: A Logistic regression was estimated for the attribution measure. The marginal effects reported are the averages of marginal effects across the sample on the predicted probability of being unattributable.
Std. Err. Standard Error.
The outcome variable is a dichotomous variable for whether the beneficiary is unattributable to any provider group as opposed to being attributable to a provider group (either associated with a Medicare ACO or any other provider group). The coefficients represent the estimated marginal effects or changes in the predicted probability. HRR fixed effects are included. Standard errors are in parentheses.
The reference category is the group of patients with one or more observed chronic conditions. Inference:
p<0.001,
p<0.01,
p<0.05.
Table A5.
Comparison of Attribution Methodologies-Fee for Service Medicare Beneficiaries (2011 – 2012)
| Attribution Categories
in 2011 |
Attribution
Categories in 2012 |
|||||
|---|---|---|---|---|---|---|
| Attributable to (ACO
and Non-ACO) Provider Groups |
Unattributable | Not in 2012 data | Observations, N, (%) | |||
| Health Care Service
Users |
||||||
| Non-Users | Decedents | Survivors | ||||
|
|
||||||
| Attributable to (ACO and Non-ACO) Provider Groups, N, (%) | 10,021,587(88.8) | 127,514(1.1) | 60,445(0.5) | 183,282(1.6) | 890,675(7.9) | 11,283,503(100) |
| Unattributable, N, (%) | 419,910(28.7) | 603,427(41.2) | 15,521(1.1) | 216,197(14.8) | 208,887(14.3) | 1,463,942(100) |
| Not in 2011 data, N, (%) | 917,927(76.2) | 212,973(17.7) | 6,091(0.5) | 674,000(5.6) | − | 1,204,391(100) |
|
| ||||||
| Observations, N | 11,359,424 | 943,914 | 82,057 | 466,879 | 1,099,562 | 13,951,836 |
Notes: Percent may not sum exactly to 100 due to rounding error.
Under prospective attribution (based on 2011 utilization), some of the 890,000 health care users in 2011 would be prospectively attributable to provider groups (ACOs and Non-ACOs) in 2012 even though they were no longer enrolled in 2012—those who have lost eligibility for enrollment in 2012. Beneficiaries who may have died in 2011, following the end of the attribution window (October-September) and production of the attribution list would be removed. Over 370,000 (127,514+60,445+183,282) unattributable beneficiaries in 2012 would be prospectively attributable beneficiaries to provider groups based on their utilization in 2011. About 920,000 new (Parts A & B) fee-for-service Medicare beneficiaries in 2012 attributable to provider groups in 2012 would be excluded from responsibility of provider groups.
Under the proposed hybrid attribution and focusing on 2011 and 2012 data above, over 10,000,000 beneficiaries would be attributable in 2012 based on retrospective attribution method. To that number would be added over 127,000 2012 unattributable health care non-users, 60,000 2012 unattributable decedents, and 183,000 2012 unattributable survivors who were attributable in 2011 under the long-term or “sticky” attribution recommendations. Attestation would permit attribution for additional 2012 unattributable patients, those who were new enrollees in 2012 and patients with some health care use in 2012 but who were unattributable in 2011. Finally, some of the remaining 2012 unattributable decedents would further be included by broadening assignment to hospice and palliative care.
Contributor Information
Mariétou H. Ouayogodé, The Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine at Dartmouth, Williamson Translational Research Building, Level 5, 1 Medical Center Drive, Lebanon, New Hampshire, 03756.
Ellen R. Meara, Dartmouth College, Hanover, New Hampshire, 03755; The Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine at Dartmouth, Williamson Translational Research Building, Level 5, 1 Medical Center Drive, Lebanon, New Hampshire, 03756.
Chiang-Hua Chang, The Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine at Dartmouth, Williamson Translational Research Building, Level 5, 1 Medical Center Drive, Lebanon, New Hampshire, 03756.
Stephanie R. Raymond, The Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine at Dartmouth, Williamson Translational Research Building, Level 5, 1 Medical Center Drive, Lebanon, New Hampshire, 03756
Julie P.W. Bynum, Geisel School of Medicine at Dartmouth; Hanover, New Hampshire, 03755; The Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine at Dartmouth, Williamson Translational Research Building, Level 5, 1 Medical Center Drive, Lebanon, New Hampshire, 03756.
Valerie A. Lewis, The Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine at Dartmouth, Williamson Translational Research Building, Level 5, 1 Medical Center Drive, Lebanon, New Hampshire, 03756.
Carrie H. Colla, The Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine at Dartmouth, Williamson Translational Research Building, Level 5, 1 Medical Center Drive, Lebanon, New Hampshire, 03756.
References
- 1.Bard M, Nugent M. Accountable Care Organizations: Your Guide to Design, Strategy, and Implementation. Health Administration Press; 2011. [Google Scholar]
- 2.Muhlestein D, Saunders R, McClellan M. Growth Of ACOs And Alternative Payment Models In 2017. Health Affairs Blog. 2017;2017 [Google Scholar]
- 3.NAACOS Joins CMS in Welcoming 525 ACOs in 2017! [press release] 2017. [Google Scholar]
- 4.McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of pioneer accountable care organizations. New England Journal of Medicine. 2015;372(20):1927–1936. doi: 10.1056/NEJMsa1414929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pyenson BS, Fitch KV, Iwasaki K, Berrios MM. The Two Medicare ACO Programs: Medicare Shared Savings and Pioneer—Risk/Actuarial Differences. Milliman Inc; New York, NY: 2011. [Google Scholar]
- 6.Lewis VA, McClurg AB, Smith J, Fisher ES, Bynum JP. Attributing patients to accountable care organizations: performance year approach aligns stakeholders’ interests. Health Affairs. 2013;32(3):587–595. doi: 10.1377/hlthaff.2012.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu J, Price M, Spirt J, et al. Patient Population Loss At A Large Pioneer Accountable Care Organization And Implications For Refining The Program. Health Affairs. 2016;35(3):422–430. doi: 10.1377/hlthaff.2015.0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McWilliams JM, Chernew ME, Dalton JB, Landon BE. Outpatient care patterns and organizational accountability in Medicare. JAMA Internal Medicine. 2014;174(6):938–945. doi: 10.1001/jamainternmed.2014.1073. [DOI] [PubMed] [Google Scholar]
- 9.Epstein AM, Jha AK, Orav EJ, et al. Analysis of early accountable care organizations defines patient, structural, cost, and quality-of-care characteristics. Health Affairs. 2014;33(1):95–102. doi: 10.1377/hlthaff.2013.1063. [DOI] [PubMed] [Google Scholar]
- 10.The Centers for Medicare and Medicaid Services. Guide to Quality Measurement for Accountable Care Organizations Starting in 2012: Agreement Period, Performance Year, and Reporting Period. 2012. [Google Scholar]
- 11.The Centers for Medicare and Medicaid Services. Shared Savings and Losses and Assignment Methodology Specifications. 2014. Medicare Shared Savings Program. [Google Scholar]
- 12.Services TCfMaM, ed2017. 2013 Shared Savings Program Accountable Care Organizations (ACO) PUF. [Google Scholar]
- 13.The Centers for Medicare and Medicaid Services. Performance Year 1. 2012. 2012. [Google Scholar]
- 14.The Centers for Medicare and Medicaid Services. Final Rule. Vol 76: Federal Register. 2011. Medicare Program; Medicare Shared Savings Program: Accountable Care Organizations; pp. 67802–67990. [PubMed] [Google Scholar]
- 15.McWilliams JM, Chernew ME, Zaslavsky AM, Landon BE. Post-Acute Care and ACOs—Who Will Be Accountable? Health Services Research. 2013;48(4):1526–1538. doi: 10.1111/1475-6773.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Centers for Medicare and Medicaid Services. Pioneer ACO Alignment and Financial Reconciliation Methods. 2014. [Google Scholar]
- 17.Research Data Assistance Center. [Accessed April 6, 2016];Redaction of Substance Abuse Claims. 2015 http://www.resdac.org/resconnect/articles/203.
- 18.United States Department of Agriculture Economic Research Service. [Accessed 10/12/17];Rural-Urban Commuting Area Codes. 2016 https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/.
- 19.United States Census Bureau. [Accessed April 7, 2016];How the Census Bureau Measures Poverty. 2016 https://www.census.gov/topics/income-poverty/poverty/about.html.
- 20.The Centers for Medicare and Medicaid Services. Medicare Managed Care Manual: Chapter 7- Risk Adjustment. 2014. [Google Scholar]
- 21.Medicare Payment Advisory Commission. Issues Affecting Dual-Eligible Beneficiaries: CMS's Financial Alignment Demonstration and The Medicare Shared Savings Programs. 2016. [Google Scholar]
- 22.Miller ME. Dual Eligible Beneficiaries and Care Coordination. 2010 https://www.nhpf.org/uploads/Handouts/Miller-slides_07-23-10.pdf.
- 23.Coughlin TA, Waidmann TA, Phadera L. Among dual eligibles, identifying the highest-cost individuals could help in crafting more targeted and effective responses. Health Affairs. 2012 doi: 10.1377/hlthaff.2011.0729. [DOI] [PubMed] [Google Scholar]
- 24.The Kaiser Commision on Medicaid and the Uninsured. The Diversity of Dual Eligible Beneficiaries: An Examination of Services and Spending for People Eligible for Both Medicaid and Medicare. 2012 https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7895-02.pdf.
- 25.Young K, Garfield R, Musumeci M, Clemans-Cope L, Lawton E. Medicaid's Role for Dual Eligible Beneficiaries. 2013 https://kaiserfamilyfoundation.files.wordpress.com/2013/08/7846-04-medicaids-role-for-dual-eligible-beneficiaries.pdf.
- 26.Medicare-Medicaid Coordination Office. Data Analysis Brief: Medicare-Medicaid Dual Enrollment from 2006 through 2013. 2014. [Google Scholar]
- 27.United States Census Bureau. [Accessed 10/31/2017];Regions. 2017. 2017 https://www.census.gov/geo/reference/webatlas/regions.html.
- 28.Colla CH, Lewis VA, Kao L-S, O’Malley AJ, Chang C-H, Fisher ES. Association between Medicare accountable care organization implementation and spending among clinically vulnerable beneficiaries. JAMA Internal Medicine. 2016;176(8):1167–1175. doi: 10.1001/jamainternmed.2016.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McWilliams JM, Landon BE, Chernew ME. Changes in health care spending and quality for Medicare beneficiaries associated with a commercial ACO contract. JAMA. 2013;310(8):829–836. doi: 10.1001/jama.2013.276302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luft HS. Becoming accountable—opportunities and obstacles for ACOs. New England Journal of Medicine. 2010;363(15):1389–1391. doi: 10.1056/NEJMp1009380. [DOI] [PubMed] [Google Scholar]
- 31.Fitzpatrick AL, Powe NR, Cooper LS, Ives DG, Robbins JA. Barriers to Health Care Access Among the Elderly and Who Perceives Them. American Journal of Public Health. 2004;94(10):1788–1794. doi: 10.2105/ajph.94.10.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medicare Payment Advisory Commission. A Data Book: Health Care Spending and the Medicare Program. 2017. [Google Scholar]
- 33.Skinner JS, Gottlieb DJ, Carmichael D. A New Series of Medicare Expenditure Measures by Hospital Referral Region: 2003–2008. The Dartmouth Atlas Project: The Dartmouth Institute for Health Policy and Clinical Practice; 2011. [PubMed] [Google Scholar]
- 34.The Centers for Medicare and Medicaid Services. Medicare Program. Medicare Shared Savings Program; Accountable Care Organizations—Revised Benchmark Rebasing Methodology, Facilitating Transition to Performance-Based Risk, and Administrative Finality of Financial Calculations. 2016;81:37950–38017. [PubMed] [Google Scholar]
- 35.Kocot SL, White R, McClellan M. Health Affairs Blog. Vol. 2017. Health Affairs; 2015. The Revised Medicare ACO Program: More Options … And More Work Ahead. [Google Scholar]
- 36.The Centers for Medicare and Medicaid Services. Medicare Shared Savings Program Final Rule Overview. 2015. [Google Scholar]
- 37.The Centers for Medicare and Medicaid Services. [Accessed 01/18/2017];2016 Medicare Shared Savings Program Organizations. 2016. 2017 https://data.cms.gov/ACO/2016-Medicare-Shared-Savings-Program-Organizations/5kdu-cnmy.
- 38.The Centers for Medicare and Medicaid Services. Next Generation ACO Model. 2016. [Google Scholar]
- 39.The Centers for Medicare and Medicaid Services. Medicare Shared Savings Program Quality Measure Benchmarks for the 2015 Reporting Year. 2015. [Google Scholar]
- 40.The Centers for Medicare and Medicaid Services. Guide to Quality Performance Scoring Methods for Accountable Care Organizations. 2012. [Google Scholar]
- 41.Morden NE, Colla CH, Sequist TD, Rosenthal MB. Choosing wisely—the politics and economics of labeling low-value services. New England Journal of Medicine. 2014;370(7):589–592. doi: 10.1056/NEJMp1314965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riley GF, Lubitz JD. Long-term trends in medicare payments in the last year of life. Health Services Research. 2010;45(2):565–576. doi: 10.1111/j.1475-6773.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith S, Brick A, O’Hara S, Normand C. Evidence on the cost and cost-effectiveness of palliative care: a literature review. Palliative Medicine. 2014;28(2):130–150. doi: 10.1177/0269216313493466. [DOI] [PubMed] [Google Scholar]
- 44.Smith G, Bernacki R, Block SD. The role of palliative care in population management and accountable care organizations. Journal of Palliative Medicine. 2015;18(6):486–494. doi: 10.1089/jpm.2014.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelley AS, Meier DE. The Role of Palliative Care in Accountable Care Organizations. The American Journal of Managed Care: The Palliative Care Special Issue. 2015;21(6):SP212–SP214. [Google Scholar]
- 46.The House Energy and Commerce Subcommittee on Health. 2017. MACRA and Alternative Payment Models: Developing Options for Value-based Care. [Google Scholar]
- 47.Blumenthal D, Anderson G, Fulmer T, Jha AK, Long P. Tailoring Complex-Care Management, Coordination, and Integration for High-Need, High-Cost Patients. National Academy of Medicine Perspectives. 2016 [Google Scholar]
- 48.Dunlop DD, Manheim LM, Song J, Chang RW. Gender and ethnic/racial disparities in health care utilization among older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57(4):S221–S233. doi: 10.1093/geronb/57.4.s221. [DOI] [PubMed] [Google Scholar]