Abstract
Polycystic Ovary Syndrome (PCOS) affects 10-15% of reproductive age women and is a recognized risk factor for nonalcoholic fatty liver disease (NAFLD). The more severe form of NAFLD, known as nonalcoholic steatohepatitis (NASH), results in liver inflammation with or without fibrosis, and is now a leading cause of cirrhosis. Ethnic differences are apparent in NAFLD, with higher prevalence in Hispanics, although the role of Hispanic ethnicity on risk for NAFLD/NASH in women with PCOS is not known. Objective: The aim of this study was to evaluate ethnic differences in the prevalence and risk of NAFLD/NASH in women with PCOS. Study Design: Among PCOS women followed in a large academic medical center the association of Hispanic ethnicity with elevated biomarkers of NASH, including plasma cytokeratin 18 (CK18) M30 fragments and/or ALT levels (n=303), was assessed. Prevalence of hepatic steatosis by Controlled Attenuation Parameter (CAP) imaging was also evaluated in a subset of PCOS women (n=35). Results: The median cohort age (n=303) was 28 years (IQR 8), and 15.5% (n=47) were Hispanic, the majority of whom reported white race (94%). Most Hispanic women had hepatic steatosis on imaging, which was markedly higher than in non-Hispanics (83% vs 24%, p=0.005). Approximately 17% of PCOS women had elevated ALT or elevated CK18, which was more common in Hispanics than non-Hispanics, at 34% vs 14%, respectively, p=0.002. On univariate analysis, Hispanic ethnicity was associated with two-fold higher odds of NASH (OR 2.0, 95% CI 1.0-3.9, p=0.038), and the association persisted after adjustment for HOMA-IR and waist circumference (AOR 3.1, 95% CI 1.1-8.9, p=0.034). Conclusion: NAFLD/NASH is an important condition to be considered by PCOS providers and Hispanic women with PCOS are a particularly high-risk group that may warrant routine screening.
Keywords: Fatty liver, Hispanic, Polycystic ovary syndrome, Nonalcoholic steatohepatitis
Introduction
Polycystic Ovary Syndrome (PCOS) affects 10-15% of reproductive age women, and is marked by increased risk of obesity, dyslipidemia, and insulin resistance [1,2]. Between 40-50% of PCOS women have hepatic steatosis on imaging [3,4], which may relate to their increased metabolic comorbidities. PCOS women without obesity or insulin resistance also have higher risk of NAFLD which may further relate to the role of elevated androgens on the development of hepatic steatosis [5]. Hepatic steatosis is part of the spectrum of non-alcoholic fatty liver disease (NAFLD), which is the most common cause of liver disease in the United States (U.S.), affecting one third of Americans [6,7]. NAFLD is an umbrella term that includes nonalcoholic steatohepatitis (NASH), the more severe form of NAFLD characterized by hepatic inflammation and/or fibrosis [8,9]. NASH develops in 5-10% of those with hepatic steatosis, though due to the sheer number of affected individuals, NASH will soon be the leading cause of cirrhosis and liver cancer in the U.S. [10,11].
Though women with PCOS have increased risk of hepatic steatosis, it is not clear whether women with PCOS, and especially women with PCOS of Hispanic ethnicity are at increased risk of NASH. Hispanic ethnicity is associated with increased prevalence of hepatic steatosis [12–14], and a potentially higher risk of NASH [14]. Therefore Hispanic women with PCOS may represent an at-risk group for this condition. NAFLD in PCOS, and its potential long-term morbidity and mortality remains under-recognized amongst reproductive health providers caring for this population [15]. As lifestyle and medical intervention can help to improve hepatic fibrosis [16,17], proper diagnosis and referral for treatment are of critical importance. The long-term health implications of NAFLD in PCOS may also extend beyond liver disease, as NAFLD is an independent risk factor for cardiovascular disease [18].
In the current study we aim to determine if ethnic differences in NAFLD/NASH are apparent in a population of women with PCOS. If ethnic differences in NASH are identified, these results could help to identify a subgroup of PCOS women at greatest risk for progressive liver disease, and support the need for NAFLD screening guidelines in women with PCOS. Moreover, these data may help with identifying those at greatest risk for long-term consequences of this disease, thereby helping to improve referral and subsequent treatment.
Methods
UCSF PCOS Cohort
This is a prospective cohort comprised of women ages 18-45 years seen in a tertiary-referral PCOS clinic at the University of California, San Francisco (UCSF). PCOS diagnosis is established by Rotterdam Criteria, defined as 2 of following 3 criteria:oligomennorhea, hyperandrogenism, and polycystic ovaries [19]. Oligomenorrhea is defined as < 8 menstrual cycles per year, hyperandrogenemia is defined as either a Modfied Ferriman-Gallwey Score (MFG) score >8 [20], by dermatologist exam and/or serum androgen levels above the upper limit of normal on screening labs, and polycystic ovaries are defined as > 12 follicles per ovary or ovarian volume >10 cc on transvaginal ultrasound. Discontinuation of hormonal medications, including oral contraceptives and/or spironolactone at least one month prior to their screening visit is required. Alternative diagnoses, including late-onset congenital adrenal hyperplasia, hypothyroidism, hyperprolactinemia, and functional hypothalamic amenorrhea were excluded by clinical and laboratory evaluation. Participants undergo comprehensive serologic and anthropomorphic evaluation for metabolic disease, as well as banking of serum, for research purposes, at the time of initial enrollment. Further details regarding the UCSF PCOS Cohort methodology are published elsewhere [21]. Institutional review board approval was obtained for the UCSF PCOS Cohort and its associated bio-repository, and informed consent from patients was obtained.
Study sample
The current study includes women enrolled in the UCSF PCOS Cohort between 2006-2015 with either available liver enzymes performed within 6 months of enrollment or available banked serum for cytokeratin 18 (CK18) M30 fragment measurements (n=303) (Figure 1). Women with heavy alcohol use defined as > 7 drinks per week at the time of study enrollment were excluded. No women in the cohort had known chronic viral hepatitis.
Figure 1.
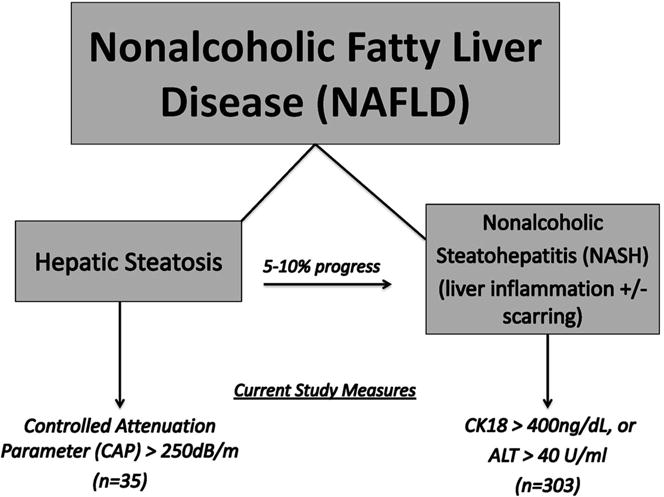
Study Definitions. NAFLD includes a spectrum of liver disease from simple steatosis, in one third of Americans, to nonalcoholic steatohepatitis (NASH) developing in up to 10% of patients with simple steatosis. In the current study, steatosis is defined as controlled attenuation parameter (CAP)>250 dB/m, and NASH defined as CK18>400 ng/dL and/or ALT>40 U/ml.
Predictor and outcome measures
The primary study predictor was self-reported Hispanic ethnicity, of any race. Race was defined as white, black (including mixed black races), Asian/Pacific Islander, and Native American. The primary study outcome was at least one elevated biomarker of NASH, including ALT > 40 U/L (approximately twice the upper limits of normal for women [22], and/or plasma cytokeratin 18 (CK18) M30 fragments > 400 ng/dL, which reflects CK18 levels that correlate with biopsy-confirmed NASH [23]. CK18 is a filament protein that is released from dying hepatocytes, hence its use as a biomarker to distinguish between NASH and simple hepatic steatosis [24].
TE-CAP
During a one-year pilot ancillary study from 2014-2015 we also consecutively enrolled new participants of the UCSF PCOS Cohort (n=35) to undergo a transient-elastrography (TE) with-controlled attenuation parameter (CAP) imaging study, for the evaluation of hepatic fibrosis and steatosis, respectively. TE-CAP (FibroScan®, Echosens, Paris, France) is a Food and Drug Administration (FDA)-approved, non-invasive method for evaluating hepatic fibrosis and steatosis that is validated in NAFLD [25]. Our secondary outcome was presence of steatosis by CAP defined as CAP score > 250 dB/m, given the high concordance of this threshold with biopsy-confirmed steatosis [26].
TE-CAP measurements were performed by a certified technician and values were considered “reliable” if the following criteria were met: (i) at least 10 valid test results obtained per device quality control; (ii) >60% success rate (i.e., number of valid tests divided by the number of total tests); (iii) the inter quartile range (IQR) of the last 10 valid test results was <30% of the median of last 10 test results.
Covariates
Covariates included patient age, self-reported race, metabolic co-factors including fasting serum lipids, body mass index, waist circumference, homeostatic model assessment of insulin resistance (HOMA-IR), and androgen levels. HOMA-IR was calculated as fasting insulin (mIU/l)*glucose (mg/dl)/405. Free androgen index (FAI) was calculated as total testosterone (nmol/liter) × 100/SHBG (nmol/liter).
Laboratory Assays
The majority of serum measurements were obtained at commercial laboratories as dictated by patient insurance. For select tests with known variability between clinical labs, banked serum from time of cohort enrollment was sent to a central facility at the University of Virginia Center for Research and Reproduction Ligand Assay and Analysis Core Laboratory. These measures included total testosterone, high sensitivity C-reactive protein (hs-CRP), sex hormone binding globulin (SHBG), glucose and insulin. Total testosterone was measured by RIA (Siemens); assay sensitivity: 10 ng/dL; intra-assay coefficient of variation (CV): 5.0% and inter-assay CV: 8.2%. High sensitivity CRP was measured by chemiluminescent immunoassay (Immulite 2000, Siemens); assay sensitivity: 0.2 mg/L; intra-assay CV: 4.2% and inter-assay CV: 7.5%. Glucose was measured by glucose oxidation methodology (Analox); (intra and inter-assay CV <2%). Caspase-cleaved cytokeratin 18 (CK 18) was also measured from banked sera from initial cohort enrollment using an M30-apoptosense ELISA kit (#10011, Peviva, Sweden) per the manufacturer’s specifications (intra and inter-assay CV <10%).
Statistical analysis
Participant characteristics were compared by ethnicity using chi-squared and Mann-Whitney tests where appropriate. Logistic regression was used to evaluate the association of ethnicity with serum markers of NASH. Multivariate analyses were performed using backward elimination, and adjusted for covariates with p <0.05. Analyses were performed using STATA 12.1 (Stata Corp, College Station, TX).
Results
The median age of the cohort (n=303) was 28 years (IQR 8). Most women reported white race (72%), followed by Asian (18%), and black (8%). Approximately 15.5% (n=47) were Hispanic, the majority of whom were white (94%). Metabolic parameters were more severe in Hispanic than non-Hispanic women, including greater waist circumference (36 vs 32 inches), triglycerides (103 vs 79 mg/dL), HOMA-IR (2.9 vs 1.8) and lower HDL (45 vs 57 mg/d), all p values ≤ 0.005. Clinical hyperandrogenism as assessed by the MFG score was more common in Hispanics (89% vs 74%, p=0.03), though serum androgen levels were similar between groups (Table 1).
Table 1.
Cohort Characteristics by Hispanic Ethnicity in Women with PCOS.
| n | Overall | Non Hispanic | Hispanic | p value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 303 | 28.2 (6.8) | 28.1 (8.0) | 28.5 (8.8) | 0.837 |
| Race (%): | |||||
| White | 72 | 68 | 93.6 | 0.004 | |
| Asian | 303 | 17.5 | 20.3 | 2.1 | |
| Black | 7.6 | 8.6 | 2.1 | ||
| Native American | 3 | 3.1 | 2.1 | ||
| Hispanic ethnicity | 303 | 15.5 | --- | --- | --- |
| Metabolic Factors | |||||
| Acanthosis (%) | 291 | 39.2 | 34.3 | 65.2 | <0.001 |
| Body mass index (kg/cm2) | 297 | 27.6 (10.7) | 26.0 (10.1) | 31.2 (9.8) | <0.001 |
| Waist circumference (in) | 280 | 33 (10) | 32 (10) | 36 (7) | <0.001 |
| Lipids (mg/dL): | |||||
| Total cholesterol | 265 | 181 (50) | 181 (50) | 184 (60) | 0.954 |
| LDL | 102 (44) | 102 (46) | 106 (44) | 0.898 | |
| HDL | 55 (21) | 57 (23) | 45 (20) | <0.001 | |
| Triglycerides | 81 (69) | 79 (62) | 103 (83) | 0.004 | |
| HOMA-IR | 178 | 1.96 (2.4) | 1.76 (2.2) | 2.90 (3.0) | 0.005 |
| CRP (mg/L) | 178 | 0.84 (4.4) | 0.73 (3.7) | 3.40 (6.2) | 0.013 |
| PCOS-Specific Factors | |||||
| Hirsutism (%) | 294 | 53.3 | 49 | 75.6 | 0.001 |
| Acne (%) | 304 | 64.8 | 63.4 | 70.5 | 0.369 |
| Androgenic alopecia (%) | 291 | 30.5 | 30.5 | 31.1 | 0.934 |
| Hyperandrogenism (%): | |||||
| Clinical | 287 | 76.2 | 73.9 | 88.9 | 0.03 |
| Biochemical | 62.4 | 61.9 | 72.1 | 0.2 | |
| Hormone measures (ng/dL) | |||||
| Total testosterone | 219 | 42.3 (51.6) | 42.4 (51.6) | 39.7 (45.9) | 0.995 |
| Androstenedione | 244 | 168 (126) | 180 (107) | 164 (126) | 0.278 |
| DHEAS | 275 | 240 (167) | 234 (164) | 268 (176) | 0.332 |
| Sex hormone binding globulin (SHBG) | 213 | 40.7 (42.5) | 42.9 (43.7) | 36.1 (20.9) | 0.062 |
| Free Androgen Index | 210 | 102.2 (189.1) | 100.7 (191.1) | 116.7 (195.5) | 0.450 |
| Medication use (history of prior use) (%) | |||||
| OCPs | 198 | 16.3 | 14.9 | 23.3 | 0.250 |
| Metformin | 16.8 | 17.5 | 13.3 | 0.439 | |
| Spironolactone | 5.2 | 6.2 | 0 | 0.161 | |
| Liver-Related Factors | |||||
| ALT (U/L) | 268 | 19 (17) | 19 (17) | 21 (21) | 0.047 |
| CK18 (ng/dL) | 185 | 286 (205) | 280 (137) | 307 (205) | 0.065 |
| CAP (dB/m) | 35 | 225 (87) | 211 (58) | 292 (51) | 0.016 |
Median (IQR) reported unless otherwise specified.
Approximately 17% of women had elevated ALT or elevated CK18, which was more common in Hispanics than non-Hispanics at 34% vs 14%, p=0.002 (Figure 2). Regarding individual values, Hispanic women were twice as likely to have ALT > 40 U/ml (22% vs 11%, p=0.046). Likewise, CK18 levels were also higher in Hispanic than non-Hispanic women, with 27% of Hispanic women having levels compatible with NASH as compared to 12% of non-Hispanic women, p=0.040. Importantly, the majority of Hispanic women had hepatic steatosis on imaging, which was markedly higher than non-Hispanics at 83% vs 23%, respectively, p=0.005 (Figure 3). Median liver stiffness was slightly higher in Hispanics (5.3 vs 4.2 kPA, p=0.099), although these values reflect minimal to no hepatic fibrosis for both groups.
Figure 2.
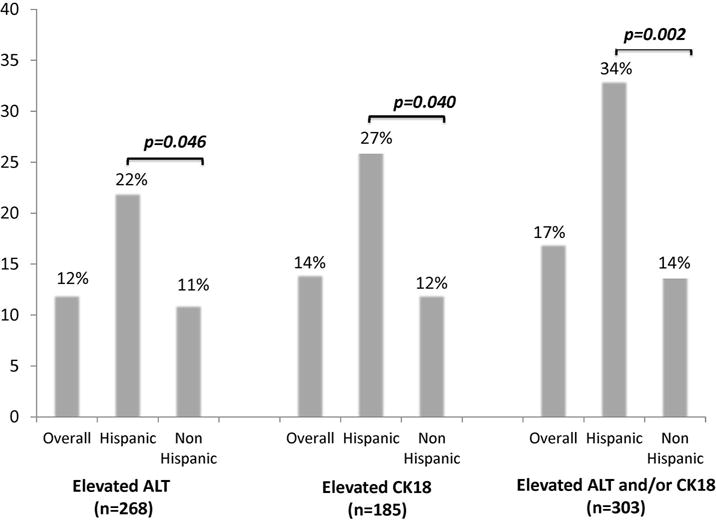
Prevalence of elevated NASH markers, by Hispanic ethnicity (n=303), defined as CK18>400 ng/dL and/or ALT>40 U/ml.
Figure 3.
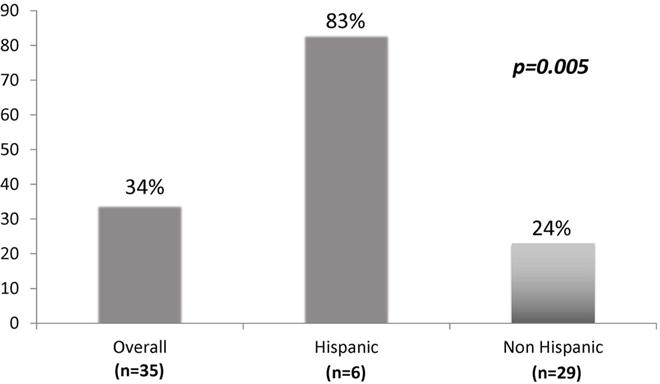
Prevalence of hepatic steatosis by Hispanic ethnicity (n=35), defined as controlled attenuation parameter (CAP)>250 dB/m.
Among the n=35 women in the TE-CAP sub-study, 5 were referred to hepatology for liver biopsy at the discretion of their reproductive endocrinologist. Biopsy was declined in one patient. Of the remaining 4, all had evidence of NASH, with stage 1/4 fibrosis in 3 patients and stage 2/4 in the fourth patient. Of those biopsied, two were Hispanic, one of whom had stage 2 fibrosis.
On univariate analysis Hispanic ethnicity was associated with a two-fold higher odds of elevated ALT or CK18 (OR 2.0, 95% CI 1.0-3.9, p=0.038). The strength of association increased further with adjustment for HOMA-IR and waist circumference (AOR 3.1, 95% CI 1.1-8.9, p=0.034) (Table 2).
Table 2.
Univariate and Multivariate Associations with NASH.
| Univariate | Multivariate* | |||
|---|---|---|---|---|
| Covariate | OR (95% CI) | p value | OR (95% CI) | p value |
| Hispanic ethnicity | 2.00 (1.0-3.9) | 0.038 | 3.10 (1.1-9.0) | 0.034 |
| White vs non white race | 1.23 (0.76-2.0) | 0.395 | ----- | ----- |
| Age (per year) | 1.02 (0.99-1.05) | 0.235 | ----- | ----- |
| Acanthosis | 1.06 (0.67-1.68) | 0.810 | ----- | ----- |
| BMI (per 5 kg/m2) | 1.28 (1.10-1.49) | 0.001 | ----- | ----- |
| Waist circumference (per inch) | 1.06 (1.02-1.10) | 0.002 | 0.95 (0.87-1.03) | 0.255 |
| LDL (per 10 mg/dL) | 1.02 (0.94-1.10) | 0.642 | ----- | ----- |
| HDL (per 5 mg/dL) | 0.91 (0.84-0.98) | 0.011 | ----- | ----- |
| Triglycerides (per 20 mg/dL) | 1.09 (1.0-1.17) | 0.027 | ----- | ----- |
| HOMA-IR (per unit) | 1.30 (1.13-1.50) | <0.001 | 1.40 (1.12-1.76) | 0.004 |
| Hirsutism | 0.85 (0.54-1.33) | 0.475 | ----- | ----- |
| Total testosterone (per ng/dL) | 1.0 (1.0-1.0) | 0.285 | ----- | ----- |
| Free androgen index (FAI) | 1.0 (1.0-1.0) | 0.624 | ----- | ----- |
Waist circumference maintained in final model given established association with NASH
Discussion
In an ethnically diverse cohort of women with PCOS, we found that Hispanics had a three-fold higher odds of NASH as defined by elevated serum markers compared to non-Hispanics after adjustment for other metabolic factors. Moreover, hepatic steatosis assessed by CAP was present in over one third of all women with PCOS, while present in the majority of Hispanic women. Most metabolic comorbidities including anthropomorphic measures, dyslipidemia, and insulin resistance were also worse in Hispanics. Thus, Hispanic women with PCOS are uniquely at high-risk for metabolic disease, and this risk extends to their increased risk of liver disease.
The prevalence of NAFLD is known to be higher in women with PCOS as compared to the general U.S. population, with prior data showing between 40-50% of PCOS women having ultrasound-confirmed steatosis [3,4]. This likely relates to overlapping metabolic risk factors including increased obesity and insulin resistance in PCOS [1,3,4]. While the current study did not identify an association with elevated androgens and NAFLD, prior data in larger cohorts have shown testosterone levels to be independently associated with visceral adiposity and hepatic fat [5,27], suggesting another potential mechanism exposing women with PCOS to increased risk. Despite the high prevalence of NAFLD in PCOS, the association between these conditions remains under-recognized by those on the front-lines of PCOS care. Indeed, a recent study reported that over 70% of obstetrics and gynecology (ObGyn), and 31% of reproductive endocrinology and infertility (REI) practitioners, did not know of the association between NAFLD and PCOS [15]. Referral and subsequent treatment of this condition can help to reduce the long-term consequences of liver disease, therefore greater awareness is needed amongst primary PCOS providers.
While women with PCOS are known to have higher risk of steatosis than non-PCOS controls, it is less clear whether they have increased risk of NASH, which is the more severe manifestation of NAFLD. NASH represents a down-stream sequelae of steatosis and is characterized by liver cell death and inflammation with or without liver fibrosis. Importantly, NASH is predicted to soon be the leading cause of cirrhosis in the U.S., and leading indication for liver transplantation [6,10,11]. Proper identification of patients at risk for NASH is therefore critical, as interventions that are employed in a timely fashion may prevent progressive scarring. A prior study found that 39% of women with PCOS compared to 3% of age- and BMI- matched non-PCOS controls had ALT levels ≥ 25 U/ml, suggestive of hepatic inflammation [4]. Only 4% of those PCOS women were Hispanic, with minimal available data on risk of NAFLD/NASH in PCOS women of Hispanic ethnicity. The plasma-borne caspase-generated CK18 is a filament protein that is released from dying hepatocytes, therefore can help to distinguish simple steatosis from NASH [28], with elevated levels independently predictive of NASH [24,29]. In the current study ~12% of women had CK18 levels compatible with the presence of NASH [23], though more than twice as many Hispanics (27%) reached these thresholds. Approximately 17% of our PCOS population had either elevated ALT and/or CK18, while more than a third of Hispanic women had these markers of NASH.
Lifestyle modification by way of diet and exercise is an integral part of NAFLD/NASH management [9]. Indeed loss of 7-10% of body weight has been shown to improve the amount of hepatic fat and result in regression of liver scarring [16,30]. For patients with biopsy-confirmed NASH, vitamin E has been shown to improve liver inflammation [31]. Though available FDA-approved medications for NASH are otherwise limited, there are burgeoning clinical trials underway in various stages of development, and promising treatments are anticipated to be on the market within the next few years [32]. Importantly, treatment for NASH is restricted to patients with biopsy-confirmed NASH [9], highlighting the importance of screening and early referral of high-risk patients.
The public health implications of NAFLD/NASH also extend beyond liver disease, as this condition is an independent risk factor for cardiovascular (CV) disease [18,33]. This association carries great importance in women with PCOS, in whom obesity, insulin resistance, and dyslipidemia are common [1]. Although controversial whether women with PCOS have increased risk for cardiovascular events [34,35], the potential added cardiovascular risk in women with PCOS and NAFLD warrants investigation. These findings are particularly pressing among Hispanic women with PCOS, given significantly greater metabolic comorbidities noted in the current study, including obesity, dyslipidemia, and insulin resistance. Moreover, many reproductive age women may only follow-up with general ObGyn providers, highlighting the need for greater awareness of prevalent general medical conditions in women with PCOS [15].
The clinical relevance of PCOS and NAFLD in Hispanics is also important given the growing number of Hispanic women in the U.S. According to the 2010 US Census Data, the Hispanic population grew by 43% between 2000-2010, or four times the growth in the total population [36]. By 2060, it is estimated that one in every three Americans will be Hispanic. The prevalence of hepatic steatosis is in non PCOS populations is also greater in Hispanics, with imaging-confirmed steatosis seen in 45% of Hispanic patients compared to 33% of non-Hispanics [37]. This disproportionate risk in part relates to genetic factors, including a higher prevalence of a polymorphism in the patinin-like phospholipase domain containing 3 (PNPLA3) gene, that leads to impaired lipid metabolism [38,39]. While prior data on the association of Hispanic ethnicity with NASH are conflicting [12,14,40], the current study highlights not only an extremely high prevalence of steatosis, but also higher prevalence of markers of NASH markers in Hispanic compared to non-Hispanic women with PCOS. Therefore in the context of PCOS, Hispanic ethnicity appears to accentuate this risk.
There are notable limitations of the current study. First, ALT and CK18 are imperfect markers of NASH, with ALT being normal in up to one third of individuals with NASH on biopsy [41]. However, ALT remains the first-line, non-invasive clinical test to assess forhepatic inflammation. CK18 is available only for research purposes, and has variable specificity for NASH depending on the cutoff employed [24], although the threshold used in the current study has been shown to correlate with histologically-confirmed disease [23]. We also lacked cross sectional imaging such as MRI, which has the highest sensitivity for detecting hepatic steatosis [42]. However, CAP is an FDA-approved ultrasound-based modality for characterizing the presence and severity of hepatic steatosis, and the threshold used to define steatosis has shown high correlation with histologically-confirmed steatosis [26]. In the current study there were too few women to evaluate ethnic differences in liver biopsy measures of NASH, however, existing data on biopsy-confirmed NASH in PCOS are currently quite limited. One series of 6 PCOS women confirmed the presence of NASH with fibrosis in all cases [43]. Though small in number, our data add to prior reports, as all biopsied women in the current study also had histologically-confirmed NASH, with evidence of early stage fibrosis. It is also important to note the young age of PCOS women presenting with existing fibrosis, who may have a head start in the development of progressive liver scarring.
Conclusion
In summary, Hispanic women with PCOS are a distinct high-risk group for metabolic disease including NAFLD/NASH. It is established that lifestyle modifications, as well as medical therapy in those with biopsy-confirmed NASH, can reduce NASH-associated morbidity and mortality, including reversal of fibrosis. Therefore increased awareness of NAFLD/NASH in PCOS, particularly among Hispanic women, may help to improve early diagnosis and treatment, prior to the development of long term cardiovascular and hepatic complications.
Acknowledgments
Sources of Funding
This work was supported by the University of California, San Francisco (UCSF) Resource Allocation Program (to HH) and by a pilot grant from the UCSF Liver Center, National Institute of Digestive Diseases and Kidney (NIDDK)P30 DK026743 (to MS). MS is supported by a K23 from the NIDDK (DK111944).
Footnotes
Disclosures
The authors have no conflicts of interest.
References
- 1.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16:347–363. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 2.Livadas S, Diamanti-Kandarakis E. Polycystic ovary syndrome: definitions, phenotypes and diagnostic approach. Front Horm Res. 2013;40:1–21. doi: 10.1159/000341673. [DOI] [PubMed] [Google Scholar]
- 3.Gambarin-Gelwan M, Kinkhabwala SV, Schiano TD, Bodian C, Yeh HC, et al. Prevalence of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Clin Gastroenterol Hepatol. 2007;5:496–501. doi: 10.1016/j.cgh.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Cerda C, Perez-Ayuso RM, Riquelme A, Soza A, Villaseca P, et al. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. J Hepatol. 2007;47:412–417. doi: 10.1016/j.jhep.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to non-hyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3709–3716. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 6.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 7.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, et al. Global Epidemiology of Non-Alcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence and Outcomes. Hepatol. 2015 doi: 10.1002/hep.28431. 2015. [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatol. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 10.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the US. Hepatol. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 11.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterol. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 12.Bambha K, Belt P, Abraham M, Wilson LA, Pabst M, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatol. 2012;55:769–780. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 14.Saab S, Manne V, Nieto J, Schwimmer JB, Chalasani NP. Nonalcoholic Fatty Liver Disease in Latinos. Clin Gastroenterol Hepatol. 2016;14:5–12. doi: 10.1016/j.cgh.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Dokras A, Saini S, Gibson-Helm M, Schulkin J, Cooney L, et al. Gaps in knowledge among physicians regarding diagnostic criteria and management of polycystic ovary syndrome. Fertil Steril. 2017;107:1380–1386. doi: 10.1016/j.fertnstert.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterol. 2015;149:367–378. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Berzigotti A, Albillos A, Villanueva C, Genesca J, Ardevol A, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The Sport Diet study. Hepatol. 2017;65:1293–1305. doi: 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 18.VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatol. 2015;62:773–783. doi: 10.1002/hep.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rotterdam EA-SPcwg. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 20.Martin KA, Chang RJ, Ehrmann DA, Ibanez L, Lobo RA, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. JCEM. 2008;93:1105–1120. doi: 10.1210/jc.2007-2437. [DOI] [PubMed] [Google Scholar]
- 21.Wang ET, Kao CN, Shinkai K, Pasch L, Cedars MI, Huddleston HG. Phenotypic comparison of Caucasian and Asian women with polycystic ovary syndrome: a cross-sectional study. Fertil Steril. 2013;100:214–218. doi: 10.1016/j.fertnstert.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 23.Malik R, Chang M, Bhaskar K, Nasser I, Curry M, et al. The clinical utility of biomarkers and the nonalcoholic steatohepatitis CRN liver biopsy scoring system in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:564–568. doi: 10.1111/j.1440-1746.2008.05731.x. [DOI] [PubMed] [Google Scholar]
- 24.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatol. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, et al. Performance Characteristics of Vibration-Controlled Transient Elastography for Evaluation of Non-Alcoholic Fatty Liver Disease. Hepatol. 2017;67:134–144. doi: 10.1002/hep.29489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jun BG, Park WY, Park EJ, Jang JY, Jeong SW, et al. A prospective comparative assessment of the accuracy of the FibroScan in evaluating liver steatosis. PLOS One. 2017;12:e0182784. doi: 10.1371/journal.pone.0182784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkar M, Wellons M, Cedars MI, VanWagner L, Gunderson EP, et al. Testosterone Levels in Pre-Menopausal Women are Associated With Nonalcoholic Fatty Liver Disease in Midlife. Am J Gastroenterol. 2017;112:755–762. doi: 10.1038/ajg.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterol. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 29.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, et al. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatol. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 30.Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255–266. doi: 10.1016/j.jhep.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. NEJM. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perazzo H, Dufour JF. The therapeutic landscape of non-alcoholic steatohepatitis. Liver Int. 2017;37:634–647. doi: 10.1111/liv.13270. [DOI] [PubMed] [Google Scholar]
- 33.Mirbagheri SA, Rashidi A, Abdi S, Saedi D, Abouzari M. Liver: an alarm for the heart? Liver. 2007;27:891–894. doi: 10.1111/j.1478-3231.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 34.Shroff R, Kerchner A, Maifeld M, Van Beek EJ, Jagasia D, et al. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrinol Metab. 2007;92:4609–4614. doi: 10.1210/jc.2007-1343. [DOI] [PubMed] [Google Scholar]
- 35.Wang ET, Ku IA, Shah SJ, Daviglus ML, Schreiner PJ, et al. Polycystic ovary syndrome is associated with higher left ventricular mass index: the CARDIA women’s study. J Clin Endocrinol Metab. 2012;97:4656–4662. doi: 10.1210/jc.2012-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Population estimates. United States Census Bureau; Accessed: October 2nd, 2017. [Google Scholar]
- 37.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatol. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 38.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatol. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 40.Kallwitz ER, Guzman G, TenCate V, Vitello J, Layden-Almer J, et al. The histologic spectrum of liver disease in African-American, non-Hispanic white, and Hispanic obesity surgery patients. Am J Gastroenterol. 2009;104:64–69. doi: 10.1038/ajg.2008.12. [DOI] [PubMed] [Google Scholar]
- 41.Torres DM, Harrison SA. NAFLD: Predictive value of ALT levels for NASH and advanced fibrosis. Nat Rev Gastroenterol Hepatol. 2013;10:510–511. doi: 10.1038/nrgastro.2013.138. [DOI] [PubMed] [Google Scholar]
- 42.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatol. 2013;58:1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Setji TL, Holland ND, Sanders LL, Pereira KC, Diehl AM, et al. Nonalcoholic steatohepatitis and nonalcoholic Fatty liver disease in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1741–1747. doi: 10.1210/jc.2005-2774. [DOI] [PubMed] [Google Scholar]


